Abstract
Background:
Higher neuroticism and lower conscientiousness are risk factors for Alzheimer’s disease and related dementias, but the underlying neuropathological correlates remain unclear. Our aim was to examine whether personality traits are associated with amyloid and tau neuropathology in a new sample and meta-analyses.
Methods:
Participants from Baltimore Longitudinal Study of Aging (BLSA) completed the Revised NEO Personality Inventory and underwent amyloid (11C-labeled Pittsburgh Compound B) and tau (18F-flortaucipir) positron emission tomography.
Results:
Among cognitively normal BLSA participants, neuroticism was associated with higher (OR = 1.68, 1.20–2.34) and conscientiousness with lower (OR = 0.61, 0.44–0.86) cortical amyloid burden. These associations remained significant after accounting for age, sex, education, depressive symptoms, hippocampal volume, and APOE ε4. Similar associations were found with tau in the entorhinal cortex. Random-effect meta-analyses of 12 studies found higher neuroticism (N = 3015, r = .07, P=.008) and lower conscientiousness (N = 2990, r = −.11, P<.001) were associated with more amyloid deposition. Meta-analyses of 8 studies found higher neuroticism (N = 2231, r = .15, P<.001) and lower conscientiousness (N = 2206, r = −.14, P<.001) were associated with more tau pathology. The associations were moderated by cognitive status, with stronger effects in cognitively normal compared to heterogeneous samples, suggesting that the associations between personality and proteopathies are not phenomena that emerge with neuropsychiatric clinical symptoms.
Conclusions:
By aggregating results across samples, this study advances knowledge on the association between personality and neuropathology. Neuroticism and conscientiousness may contribute to resistance against amyloid and tau neuropathology.
Keywords: Personality, Alzheimer’s disease, amyloid, tau, neuropsychiatric disorders, meta-analysis
Introduction
Personality traits are defining features of a person’s psychological profile. The five major personality traits (neuroticism, extraversion, openness, agreeableness, and conscientiousness) emerge early in life, are fairly stable, and have a broad impact on important life outcomes, including neuropsychiatric disorders(1–4). In the health domain, a growing number of prospective studies have found that high neuroticism and low conscientiousness in cognitively normal (CN) adults predict who is at greater risk of developing Alzheimer’s disease (AD) and related dementias (ADRD)(5–10). These same traits also predict cognitive performance on standardized tests(11) and are associated with changes in cognitive and functional status, as rated by knowledgeable informants(12). These associations are robust across samples and extend across the lifespan(13). For example, personality traits assessed in adolescence predict cognitive function in middle-age(13) and dementia risk about 50 years later(14). Two postmortem studies indicate that personality traits may reduce the risk for clinical dementia by increasing resilience to AD neuropathology (e.g., high conscientiousness supports coping with AD pathology and delays clinical signs)(15, 16). It is similarly possible that personality modulates resistance(17) to AD neuropathology (e.g., high conscientiousness delays or even helps to avoid the development of AD pathology)(8, 18–21).
To better understand the mechanisms underlying the association between personality and risk of dementia, we investigate whether personality traits are associated with two defining markers of AD neuropathology: amyloid and tau deposition. Based on the literature on personality and dementia(9), our primary hypothesis is that high neuroticism and low conscientiousness are associated with greater amyloid and tau burden. We advance the same hypothesis for both biomarkers because amyloid and tau are interrelated and both are part of the cascade of AD neurodegeneration(22). To provide a more comprehensive assessment of the role of personality, we also report results for extraversion, openness, and agreeableness.
To test our hypothesis, we first examined the associations in a well-characterized sample of older adults from the Baltimore Longitudinal Study of Aging (BLSA) who completed a measure of personality and underwent amyloid and tau positron emission tomography (PET). We then conducted a systematic search of the literature to provide a quantitative synthesis of current evidence. The current literature is somewhat mixed, with some studies reporting associations between AD biomarkers and neuroticism(23–25), or conscientiousness(26), or neither(27). The meta-analytic approach in this context is essential because most studies to date have relied on relatively small sample sizes that are modestly powered to reliably detect the expected associations. We evaluated heterogeneity, publication bias, and tested two potential moderators: measure of neuropathology (postmortem vs. in vivo) and cognitive status (CN vs. CN + cognitively impaired individuals).
Materials and Methods
BLSA participants.
The BLSA (ClinicalTrials.gov: NCT00233272) is an ongoing longitudinal study of community-dwelling adults. Participants were from the BLSA neuroimaging substudy who underwent amyloid and tau PET. At enrollment into the substudy, all participants were free of dementia, stroke, bipolar illness, epilepsy, severe cardiac disease, severe pulmonary disease, and metastatic cancer. Personality data were available for all participants at the PET assessment or a previous visit. The personality assessment was within one year of the PET scan for 70% of participants; time ranged from −11.62 to 0.87 years (mean = −0.96, SD = 1.77) for the time between the personality and amyloid and from −11.62 to 0.86 years (mean = −1.33, SD = 1.96) for the time between the personality and tau imaging. For participants with multiple assessments, we selected the last available PET visit, which had concurrent amyloid and tau scans (the same day or within a few days for all participants except for one participant with the tau scan one year before and one participant one year after the amyloid scan). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study protocols were approved by local institutional review boards and all participants provided written informed consent before each visit.
Measures
Personality.
Participants completed the 240-item, self-report version of the Revised NEO Personality Inventory (NEO-PI-R)(28). Raw scores were standardized as T-scores (M = 50, SD = 10) using combined-sex norms(28). The NEO-PI-R factor structure in the BLSA shows high congruence with the normative structure (Tucker phi = 0.97─0.99), high internal consistency (α = 0.88─0.92), and high test-retest correlations (rtt = 0.78─0.85) over a mean interval of 10 years(29). In addition to BLSA studies, the reliability and validity of the NEO-PI-R (or briefer versions such as the NEO-Five-Factor Inventory) are supported by a large literature across clinical and non-clinical samples, self-report and observer rating methods, and across age groups, languages, and cultures(28–33).
PET imaging - Amyloid.
Amyloid was measured using 11C-Pittsburgh compound B (PiB) as described in the supplementary material. Briefly, scans were obtained over 70 minutes immediately following an intravenous bolus injection of approximately 15 mCi of 11C-PiB. Distribution volume ratio (DVR) images were computed in PET native space using the cerebellar gray matter as the reference region. The primary outcome was the mean cortical amyloid burden, calculated as the average of the DVR values in cingulate, frontal, parietal (including precuneus), lateral temporal, and lateral occipital cortical regions, excluding the sensorimotor strip. A mean cortical DVR threshold of 1.067, derived from a Gaussian mixture model, was used to categorize participants as PiB −/+(34).
PET imaging - Tau.
Tau was measured using 18F-AV-1451 (18F-flortaucipir) as previously described(34). Briefly, scans were obtained over 30 minutes starting 75 minutes after an intravenous bolus injection of approximately 10 mCi of 18F-flortaucipir. We computed 80–100 minutes standardized uptake value ratio (SUVR) images by dividing the partial volume corrected PET intensities by the mean within the inferior cerebellar gray matter. We computed the average SUVR in four regions of interest (ROIs) corresponding to the early stages of tau pathology: the entorhinal cortex (primary outcome), fusiform, inferior temporal gyrus, and hippocampus.
Clinical status.
The cognitive evaluation was based on a neuropsychological battery and clinical examination, including informant- and participant-structured interviews. Participants with a Clinical Dementia Rating(35) score ≥ 0.5 or Blessed Information-Memory-Concentration Test(36) ≥ 4 were reviewed through consensus conference. Mild cognitive impairment (MCI) was based on the Petersen criteria(37). Diagnosis of dementia was based on Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised)(38) criteria, and diagnosis of AD was based on the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria(39).
Statistical Analyses
We examined the association between each personality trait and the neuropathology markers using unadjusted correlations (Model 1), partial correlations with age, sex, and time between personality and imaging as covariates (Model 2), and education and depressive symptoms (Center for Epidemiological Studies-Depression score ≥16(40)) as additional covariates (Model 3). The primary outcomes were the mean cortical amyloid burden and entorhinal tau, respectively. Secondary outcomes presented in supplementary material were amyloid in the precuneus and tau in the fusiform, inferior temporal gyrus, and hippocampus. Separate logistic regressions were used to evaluate each personality trait as a predictor of PIB+, including the same covariates. We used z-scores to obtain odds ratios per 1 SD difference on the personality trait. In follow-up analyses, Apolipoprotein E (APOE) ε4 carrier status or hippocampal volume (adjusted for intracranial volume) were included as additional covariates in the logistic regressions and in the partial correlations between personality and entorhinal tau. In additional analyses, we included individuals with MCI or dementia.
Literature search and Meta-Analyses
The meta-analyses were prepared in line with the MOOSE guidelines for meta-analyses of observational studies. The protocol was not preregistered and the risk of bias of individual studies was not assessed. The PICO framework was used to form the research questions: Participants = human subjects; Intervention = no intervention/exposure, observational (cohort study); Comparison = level of personality traits (individual differences), and Outcome = amyloid and/or tau (in vivo or postmortem).
Eligibility criteria.
We included studies that measured at least one of the five personality traits and post-mortem or in-vivo (PET or CSF) measures of amyloid or tau. We had no exclusion criteria based on study design, type of population, publication status, or language of the article.
Systematic Literature Search.
A systematic literature search covering all years from inception up to 7 May 2021 was conducted using PubMed, PsycInfo, and Web of Science. We used the terms neuroticism OR extraversion OR openness OR agreeableness OR conscientiousness for personality, amyloid OR Aβ OR Pittsburgh Compound B OR PIB OR 18F-florbetapir OR 18F-Florbetaben OR 18F-Flutemetamol for amyloid, and tau or neurofibrillary tangles OR flortaucipir OR 18F-AV-1451 or 18F-T807 for tau. The reference lists of published articles were also screened. The literature search was conducted independently by two researchers (AT and DA). We screened the titles and keywords of each article for eligibility. Next, we screened abstracts and if an article seemed eligible, the full text was obtained. The full-text articles were then assessed for inclusion, and the data extracted from selected studies. Google Scholar was used to conduct a similar search and to identify additional studies through forward searches. We contacted study authors for effect estimates(41) and to clarify sample overlap(8).
Meta-Analyses:
Random-effect meta-analyses were based on reported correlation coefficients and sample sizes, or the exact p-values, or the t value (derived from beta/SE), and sample sizes. When multiple articles from the same cohort were identified, we used estimates from the largest sample and with PET over CSF(42). For consistency with PET, associations with CSF Aβ1–42 were reversed because CSF Aβ1–42 decreases with advancing neuropathology. When results were provided for multiple ROIs, we used global measures of amyloid deposition. For tau, we focused on the association with the entorhinal cortex because it is one of the first regions to manifest detectable elevated tau PET signal, and it is commonly reported in PET studies(24, 25, 34). Heterogeneity was quantified using Q, I2, and τ. Publication bias was evaluated by examining funnel plots, the Egger intercept, Kendall tau, and trim-and-fill method. We examined whether effect sizes differed between postmortem vs. in vivo measures and between samples of CN vs. CN + cognitively impaired individuals.
Results
BLSA
Descriptive statistics for demographics and other variables of interest are in Table 1 for the CN and full samples.
Table 1.
Demographic, clinical, imaging, and personality descriptive statistics of BLSA study participants.
| Amyloid | Tau | |||
|---|---|---|---|---|
|
| ||||
| CN (N=196) | Full (N=216) | CN (N=95) | Full (N=103) | |
| Age, years | 77.74 (8.56) | 78.55 (8.72) | 76.04 (8.59) | 76.85 (8.89) |
| Female | 101 (51.5%) | 107 (49.5%) | 55 (57.9%) | 58 (56.3%) |
| Black | 35 (17.9%) | 38 (17.6%) | 17 (17.9%) | 19 (18.4%) |
| Other | 10 (5.1%) | 10 (4.6%) | 6 (6.3%) | 6 (5.9%) |
| White | 151 (77.0%) | 168 (77.8%) | 72 (75.8%) | 78 (75.7%) |
| Education, years | 17.18 (2.39) | 17.24 (2.43) | 17.66 (2.43) | 17.60 (2.51) |
| Diagnosis: MCI^ | 0 (0%) | 13 (6%) | 0 (0%) | 6 (5.8%) |
| Diagnosis: Dementia^ | 0 (0%) | 5 (2.3%) | 0 (0%) | 0 (0%) |
| APOE e4 carrier | 56 (28.9%) | 63 (29.6%) | 26 (28.0%) | 29 (29.0%) |
| Hippocampus (cm3) | 7.29 (0.76) | 7.25 (0.80) | 7.34 (0.76) | 7.29 (0.77) |
| CESD ≥ 16 | 12 (6.1%) | 16 (7.4%) | 5 (5.3%) | 6 (5.8%) |
| Amyloid PiB+ | 57 (29.1%) | 72 (33.3%) | 21 (22.1%) | 26 (25.2%) |
| Amyloid mean cortical | 1.09 (0.18) | 1.11 (0.18) | 1.07 (0.15) | 1.11 (0.18) |
| Amyloid Precuneus | 1.17 (0.23) | 1.19 (0.24) | 1.15 (0.19) | 1.19 (0.24) |
| Tau Entorhinal | 1.03 (0.17) | 1.04 (0.18) | 1.03 (0.17) | 1.04 (0.18) |
| Tau Fusiform | 1.16 (0.22) | 1.16 (0.21) | 1.16 (0.22) | 1.16 (0.21) |
| Tau Inferior temporal gyrus | 1.30 (0.21) | 1.31 (0.20) | 1.30 (0.21) | 1.31 (0.20) |
| Tau Hippocampus | 1.31 (0.27) | 1.32 (0.29) | 1.31 (0.27) | 1.32 (0.29) |
| Neuroticism | 44.37 (8.65) | 44.74 (8.76) | 43.44 (9.19) | 43.76 (9.27) |
| Extraversion | 49.59 (10.12) | 49.63 (10.14) | 49.46 (11.79) | 49.43 (11.65) |
| Openness | 51.63 (9.93) | 51.07 (9.89) | 52.10 (10.03) | 51.33 (10.16) |
| Agreeableness | 54.46 (9.29) | 54.30 (9.18) | 55.19 (10.05) | 55.09 (10.07) |
| Conscientiousness | 52.33 (9.87) | 51.87 (10.08) | 54.12 (10.47) | 53.99 (10.49) |
Notes. CN = Cognitively normal. CESD = Center for Epidemiological Studies-Depression. MCI = Mild Cognitive Impairment. PiB = 11C-Pittsburgh compound B. In parenthesis are SD or percentages. Age at the time of personality assessment. APOE data was missing for 3 individuals. PiB statistics are distribution volume ratio (DVR) and tau are standardized uptake value ratio (SUVR).
The difference between CN and Full sample includes impaired participants, of whom two had impairment other than MCI or dementia.
Amyloid.
Unadjusted correlations (Model 1) and partial correlations accounting for age, sex, time between personality assessment and PET (Model 2), education, and depressive symptoms (Model 3) indicated that higher neuroticism and lower conscientiousness were associated with higher mean cortical amyloid deposition (Table 2; Figure S2; see Table S1 for precuneus). Neuroticism and conscientiousness each accounted for about 5% of the variance in the mean cortical amyloid deposition. Similar associations for neuroticism and conscientiousness were found with the non-parametric Kendall and Spearman rank correlations (ps < .01). As illustrated in Figure S2, the associations were most evident in the contrast between the PIB- and PIB+ groups, but also in PIB+ group. There was very low variability in the PIB- group and therefore no clear associations. In additional regression analyses, we found that neuroticism (p = .007) and conscientiousness (p = .046; but p = .063 when accounting for age and sex) interacted with PIB grouping in predicting mean cortical amyloid burden. Most important, relations between personality and PiB as a continuous measure were consistent with those obtained with logistic regression to predict the PiB+ group: A difference of 1 SD higher neuroticism and 1 SD lower conscientiousness were both associated with about 60% higher risk of PiB+. Adding APOE ε4 (to Model 3) had little effect on the associations of neuroticism (OR = 1.70, 1.17 – 2.48) and conscientiousness (OR = 0.66, 0.45 – 0.96) with risk of PiB+. Similarly, adding hippocampal volume (to Model 3) had little effect on the associations of neuroticism (OR = 1.70, 1.17 – 2.47) and conscientiousness (OR = 0.66, 0.45 – 0.94) with risk of PiB+. The associations were mostly unchanged in analyses that included individuals with cognitive impairment (Table 2). Among the other traits, higher openness was associated with lower risk of PiB+, but the effect was not consistently significant in models that accounted for education and depressive symptoms and with the continuous measure of mean cortical PiB (Table 2).
Table 2.
Associations between personality traits and amyloid and tau in BLSA study participants.
| PiB mean cortical | PiB+ | Entorhinal Tau | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| M1 | M2 | M3 | M1 | M2 | M3 | M1 | M2 | M3 | |
|
|
|
||||||||
| Cognitively | r | r | r | OR (95% CI) | OR (95% CI) | OR (95% CI) | r | r | r |
|
|
|
||||||||
| Normal (CN) | |||||||||
| Neuroticism | .24** | .26** | .24** | 1.68 (1.20–2.34)** | 1.69 (1.20–2.38)** | 1.72 (1.18–2.49)** | .18 | .18 | .24* |
| Extraversion | −.08 | −.04 | −.01 | .80 (.58–1.09) | .84 (.60–1.18) | .89 (.63–1.25) | −.20 | −.20 | −.24* |
| Openness | −.12 | −.13 | −.09 | .71 (.52–.98)* | .71 (.51–.99)* | .76 (.54–.1.07) | −.20* | −.21* | −.22* |
| Agreeableness | −.14* | −.14 | −.14 | .81 (.59–1.10) | .80 (.57–1.11) | .78 (.56–1.10) | −.12 | −.11 | −.15 |
| Conscientiousness | −.23** | −.20** | −.18* | .61 (.44–.86)** | .64 (.45–.91)* | .64 (.44–.93)* | −.28** | −.30** | −.42** |
| Full sample | |||||||||
| Neuroticism | .21** | .20** | .20** | 1.64 (1.21–2.22)** | 1.64 (1.19–2.25)** | 1.65 (1.16–2.35)** | .19 | .18 | .23* |
| Extraversion | −.07 | −.01 | .01 | .80 (.60–1.06) | .89 (.66–1.21) | .95 (.69–1.30) | −.20* | −.17 | −.20 |
| Openness | −.16* | −.14 | −.12 | .63 (.47–.85)** | .66 (.48–.91)* | .70 (.50–.97)* | −.23* | −.22* | −.23* |
| Agreeableness | −.12 | −.10 | −.11 | .80 (.60–1.07) | .81 (.59–1.11) | .81 (.59–1.12) | −.09 | −.08 | −.09 |
| Conscientiousness | −.22** | −.17* | −.16* | .57 (.42–.78)** | .62 (.45–.85)** | .63 (.45–.89)** | −.26** | −.25* | −.30** |
Notes. For CN, N = 196 for amyloid and N = 95 for tau. For full sample, N = 216 for amyloid and N = 103 for tau. M1 = No covariates; M2 = Includes age, sex, and time interval between personality and imaging; M3 = Includes M2 covariates, education and depressive symptoms. OR (95% CI) = Odds Ratios (95% Confidence Interval) from logistic regression with personality (z-scores) predicting risk of PiB+.
p<.05
p<.01.
Tau.
Both the unadjusted and partial correlations indicated that lower conscientiousness was associated with more tau in the entorhinal cortex, and explained 8% or more of the variance in tau (Table 2; see Table S1 for fusiform, inferior temporal gyrus, and hippocampus). The correlation coefficients were in the hypothesized direction for neuroticism but did not reach statistical significance except for the fully adjusted Model 3. The associations were essentially unchanged when either APOE (e.g., conscientiousness: r = −.42, P < .001) or hippocampal volume (e.g., conscientiousness: r = −.41, P < .001) was added as a covariate and were similar including individuals with cognitive impairment. Among the other traits, a notable finding was that higher openness was consistently associated with lower tau in the entorhinal cortex (R2 ~ 4%).
Meta-analyses
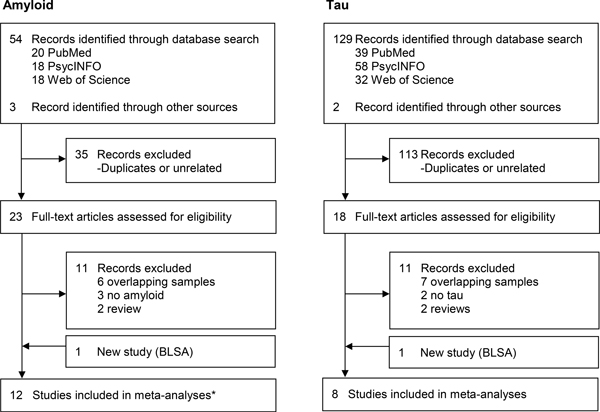
The flow chart of the literature search is in Figure 1. Table 3 presents the characteristics of the included samples. Two studies assessed AD pathology at autopsy and personality on average 9 and 30 years before death(15, 16). All other studies were essentially cross-sectional and assessed AD pathology with PET or CSF(23–27, 41, 43–45).
Figure 1.
Flow chart of literature search and selection.
Note. * Up to 12 studies included in the meta-analyses, but data came from 13 records as listed in Table 3.
Table 3.
Characteristics of samples included in the amyloid (top panel) and tau (bottom panel) meta-analysis.
| Study | Cohort or Institution, Country | N | Mean age | Female | AD biomarker | Cognitive status | Personality Measure |
|---|---|---|---|---|---|---|---|
| Amyloid | |||||||
| Terracciano et al 2013 | BLSA, US | 84 | 72.4 | 38% | Postmortem | mixed | NEO-PI-Ra |
| Snitz et al 2015 | U. of Pittsburgh, US | 92 | 81.2 | 49% | PET 11C-PiB | CN | NEO-FFI |
| Tautvydaite et al 2017 | Lausanne U., CH | 110 | ~70 | ~60% | CSF | CN + MCI | NEO-PI-R |
| Aschenbrenner et al 2020 | DIAN, International | 304 | 37.7 | 58% | PET 11C-PiB | mixed | IPIP-NEOb |
| Byun et al 2020 | KBASE, KR | 397 | 70.5 | 56% | PET 11C-PiB | CN + MCI | NEO-FFI |
| Giannakopoulos et al 2020 | U. of Geneva, CH | 65 | 74.2 | 63% | PET 18F-Florbetapir | CN | NEO-PI-Rc |
| Pichet Binette et al 2020 | PREVENT-AD, CA | 115 | 67.6 | 75% | PET [18F]NAV4694 | CN | BFI |
| Pichet Binette et al 2020 | DIAN, International | 117 | 34.6 | 55% | PET 11C-PiB | CN | IPIP-NEOb |
| Schultz et al 2020 | WashU, US | 128 | 66.7 | 61% | PET 18FAV-45 | CN | NEO-FFI |
| Yoon et al 2020 | BACS, US | 129 | 72.1 | 54% | PET 11C-PiB | CN | BFI |
| Graham et al 2021 | RUSH, US | 1375 | 89.6 | 68% | Postmortem | mixed | NEOd |
| Baena et al in press | COLBOS, CO | 20 | 35.7 | 60% | PET 11C-PiB | CN | NEO-FFI |
| Current study | BLSA, US | 196 | 78.6 | 50% | PET 11C-PiB | CN | NEO-PI-R |
| Tau | |||||||
| Terracciano et al 2013 | BLSA, US | 84 | 72.4 | 38% | Postmortem | mixed | NEO-PI-Ra |
| Tautvydaite et al 2017 | Lausanne U., CH | 110 | ~70 | ~60% | CSF | CN + MCI | NEO-PI-R |
| Aschenbrenner et al 2020 | DIAN, International | 304 | 37.7 | 58% | CSF | mixed | IPIP-NEO |
| Pichet Binette et al 2020 | PREVENT-AD, CA | 115 | 67.6 | 75% | PET 18F-flortaucipir | CN | BFI |
| Schultz et al 2020 | WashU, US | 128 | 66.7 | 61% | PET 18F-flortaucipir | CN | NEO-FFI |
| Graham et al 2021 | RUSH, US | 1375 | 89.6 | 68% | Postmortem | mixed | NEOd |
| Baena et al in press | COLBOS, CO | 20 | 35.7 | 60% | PET 18F-flortaucipir | CN | NEO-FFI |
| Current study | BLSA, US | 95 | 76.9 | 56% | PET 18F-flortaucipir | CN | NEO-PI-R |
Notes. BACS = Berkeley Aging Cohort Study; BLSA = Baltimore Longitudinal Study of Aging; COLBOS = Colombia-Boston Longitudinal Biomarker Study; DIAN = Dominant Inherited Alzheimer Network; KBASE = Korean Brain Aging Study; PREVENT-AD = EValuation of Experimental or Novel Treatments for AD; RUSH = Rush University, Memory and Aging Project and Religious Orders Study; U. = University; WashU = Knight Alzheimer Disease Research Center, Washington University; CA = Canada; CH = Switzerland; CO = Colombia; KR = South Korea; US = United States of America; PET = positron emission tomography; CSF = cerebrospinal fluid; 11C-PiB = 11C Pittsburgh compound B; MCI = Mild cognitive impairment; CN = Cognitively normal; mixed = CN, MCI and/or dementia; IPIP = International Personality Item Pool; BFI = Big Five Inventory; NEO-FFI = NEO-Five Factor Inventory; NEO-PI-R = NEO-Personality Inventory Revised.
The BLSA postmortem study (15) included 84 participants for neuroticism, extraversion, and openness and 59 for agreeableness and conscientiousness; there was no overlap between the previous BLSA postmortem (15) and current PET sample.
Effect estimates for neuroticism and conscientiousness were from (43) while for extraversion, openness and agreeableness were from (25).
Sixty-one 18F-Florbetapir and four 18F-Flutemetamol-PET.
Rush ROS participants completed 6 items for Extraversion and 12 for each of the other traits. Openness and agreeableness were not assessed in MAP (16).
Amyloid.
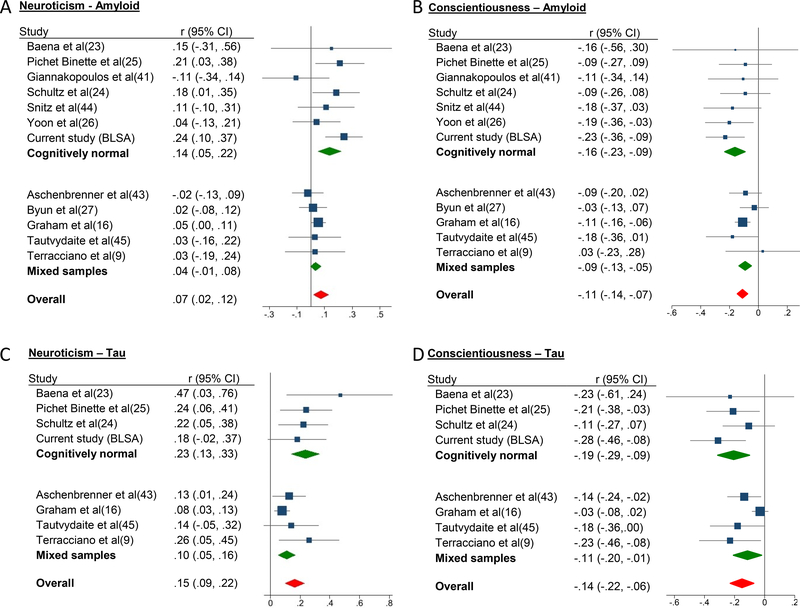
The meta-analysis of 12 studies (N = 3015) found that higher neuroticism was associated with higher amyloid burden (r = .07, P = .008)(Figure 2), low heterogeneity (I2 = 32%)(Table S2), and no evidence of publication bias (Table S3, Figure S3). Cognitive status was a significant moderator (meta-regression, Z = −2.57, P = .010): there was a stronger association in the CN (r = .14, P = .002) compared to mixed cognitive status samples (r = .04, P = .087). The results were similar in the postmortem vs. in vivo as well as CSF vs. PET studies.
Figure 2.
(a – d). Forest plots of the associations between personality traits and amyloid and tau in cognitively normal, mixed samples, and overall.
Note. Effect sizes are correlation coefficients with corresponding 95% confidence intervals. Results were similar if the current BLSA sample was excluded from the meta-analysis (Figure S4).
For conscientiousness, the meta-analysis of 12 studies (N = 2990) indicated that more conscientious individuals had lower amyloid burden (r = −.11, P < .001), low heterogeneity (I2 = 0%), and no evidence of publication bias. There were no significant moderators (meta-regression, P ≥ .10), but the association was stronger in CN (r = −.16, P < .001) compared to mixed cognitive status samples (r = −.09, P < .001). The results were similar in the postmortem vs. in vivo as well as CSF vs. PET studies.
There were no significant associations for extraversion (11 studies, N = 2431, r = .01, P = .55), openness (11 studies, N = 1675, r = −.04, P = .19), or agreeableness (11 studies, N = 1650, r = −.03, P = .19), and low heterogeneity (I2 ≤ 16%). Among the in vivo studies, higher openness was associated with lower amyloid (9 studies, r = −.08, P = .018).
Tau.
The meta-analysis of 8 studies (N = 2231) found that higher neuroticism was associated with more tau pathology (r = .15, P < .001; heterogeneity: I2 = 32%). Cognitive status was a significant moderator (Z = −2.33, P = .02) with a significantly stronger association in the CN (r = .23, P < .001) compared to mixed cognitive status samples (r = .10, P < .001). The results were similar in the postmortem and in vivo studies and CSF vs. PET. With only 8 studies, publication bias tests are not recommended(46), but we noted asymmetry in the funnel plot. The asymmetry was driven by one disproportionally large sample(16), which found a significant association (P = .0039), but smaller than the effects in the other samples. The difference may arise from clinical and methodological differences across studies or selective reporting.
For conscientiousness, the meta-analysis of 8 studies (N = 2206) found that more conscientious individuals had lower tau pathology (r = −.14, P < .001; heterogeneity: I2 = 47%). Associations were significantly stronger among studies that used CN (r = −.19, p < .001) compared to mixed cognitive status samples (r = −.11, P = .02)(meta regression, Z = −2.26, P = .02), and in vivo (r = −.17, P < .001) compared to postmortem assessment (r = −.09, P = .33)(meta regression, Z = −2.93, P = .003). Results were similar for CSF vs. PET studies. There was asymmetry in the funnel plot again due to the larger postmortem study(16).
There were no significant associations between tau and extraversion (7 studies, N = 1927, r = −.09, P = .17), openness (7 studies, N = 1171, r = −.14, P = .065), or agreeableness (7 studies, N = 1146, r = −.07, P = .15) with substantial heterogeneity (I2 ≥45%). Among the five in vivo studies, higher openness (r = −.22, P = .004) and extraversion (r = −.16, P = .009) were associated with lower tau pathology.
Discussion
In new data from the BLSA and meta-analyses, we found that high neuroticism and low conscientiousness were associated with higher amyloid and tau deposition. For example, 1 SD higher neuroticism or lower conscientiousness was associated with about 60% higher risk of being PiB+ in the BLSA. The pooled estimates indicated stronger associations for tau compared to amyloid. Further, we found a pattern of stronger associations in CN samples compared to samples inclusive of MCI and dementia. These patterns suggest that the associations are not emerging phenomena due to personality change with disease progression, as would be expected with reverse causality. Overall, the findings corroborate long-term prospective studies that personality predicts the risk of incident ADRD(5–10). Personality traits, which emerge early in life and are relatively stable throughout adulthood(47), may modulate ADRD risk by conferring resistance(17) against AD neuropathology (i.e., delaying or preventing its emergence).
The in vivo assessment of amyloid and especially tau is relatively recent, and most studies included in the meta-analyses were published in the last two years. Most studies were also based on relatively small samples, typically <200 individuals. Because of the limited power, these studies have often reported null findings, but the effects were generally in the same direction. For example, in the BLSA, the association between neuroticism and entorhinal tau was significant in some models but not in others, but the effect across models was consistent with the meta-analytic estimate. As such, the meta-analyses represent a major advance by achieving the required power to provide robust estimates of the associations between personality and neuropathology. The observed associations should be interpreted in the context of other ADRD risk factors. Current evidence, however, indicates that early-life cognitive ability or enrichment(48, 49), physical activity(50, 51), or vascular risk factors(51–54) are inconsistently associated with amyloid or tau deposition.
Neuroticism is a major risk factor for anxiety and mood disorders, as well as for behavioral and psychological symptoms of dementia(55, 56). While we limited our meta-analyses to personality traits, there is tentative evidence that depressive and anxiety symptoms are also associated with amyloid or tau(57–61). Late-life elevations in depressive symptoms may emerge during preclinical or prodromal AD(59, 62), and neuroticism may increase with amyloid and tau deposition as an early sign of preclinical AD. However, this latter hypothesis is less likely because (a) we found stronger associations in CN compared to mixed samples, (b) longitudinal data indicate that there are no increases in neuroticism in the preclinical phase of AD(63)[changes occur later with the onset(64) and progression of dementia(32, 33)], and (c) the associations of personality traits with amyloid and tau were independent of depressive symptoms. Future work is needed to disentangle the timing of these associations, and test whether neuroticism interacts with proteinopathies to increase the risk of depression and other behavioral and psychological symptoms of dementia. Future studies should also focus on the underlying mechanisms; a potential pathway is inflammation given the links of personality with inflammatory markers from younger ages(65), which over time may increase the risk of neuropathology(66, 67). Personality-linked differences in functional brain network connectivity(68) may also modulate the spread of neuropathology(69). Low neuroticism has been found to increase resilience(15, 16) and differences in network connectivity could partly explain how low emotional vulnerability helps maintain cognitive function despite neuropathologic changes(70). Recent evidence suggest that alterations of transcriptome of the frontal cortex, especially in modules related to tau pathology, may mediate the impact of neuroticism on cognitive decline and AD(71). Genetic factors may also play a role(72); genome-wide association studies (N = 449,484) of neuroticism(73) have found top hits (including an exonic nonsynonymous variant, P ~ 10−28) in the microtubule-associated protein tau (MAPT) gene, which is implicated in AD, frontotemporal dementia, and other tauopathies. The MAPT transgenic mouse model also displays abnormal fear-related behaviors(74).
We found consistent evidence that high conscientiousness is associated with lower risk of amyloid and tau neuropathology in both the BLSA and the meta-analyses. This finding is consistent with the evidence from prospective studies that consistently find high conscientiousness associated with lower risk of AD and related dementia(10). High conscientiousness is also associated with other measures of brain integrity, such as white matter fractional anisotropy(20). These associations are thought to arise in part from the healthier lifestyle of conscientious individuals, who tend to engage in more physical activity and avoid health risk behaviors, such as cigarette smoking(75, 76). Furthermore, conscientious individuals tend to have better sleep(77), better hearing(78), fewer chronic conditions such as diabetes and depression(56, 79), and spend more time in cognitively demanding activities, like studying, working, and reading(80, 81). Over time, this healthier profile and greater engagement in cognitive activities is likely to build cognitive reserve and reinforce compensation and optimization mechanisms that protect against AD neuropathology(82).
There were also significant associations between openness and the AD biomarkers in the BLSA, which were supported in the meta-analysis of the in vivo studies but not in the full meta-analyses. This again parallels the mixed findings from prospective studies linking low openness to dementia risk(10). It is of note that individuals with high openness tend to achieve higher education and engage in a variety of cognitively stimulating activities (e.g., watching less TV, more reading, more computer use)(80). The intrinsic interest in complex, diverse, and engaging activities is likely to partly explain the protective effects of openness.
Limitations and Future directions
While the meta-analysis included samples from four continents (North and South America, Europe, and Asia), a limitation of current work is the reliance on samples with high education and from high-income countries; ideally, future studies should include samples with lower education and income and from diverse communities that are at considerable risk for ADRD(84). Recent studies, however, found similar associations in samples from Colombia and Brazil(23, 85). Future studies could be further strengthened by using observer ratings as well as self-reported personality. More research is also needed to further understand the spatial specificity of these associations across the brain, especially for tau; the results for the entorhinal region were similar to those found for the fusiform and the inferior temporal gyrus, but not for the hippocampus region, which may be contaminated by choroid plexus biding (Table S1). For tau, there were only eight studies and there was asymmetry in the funnel plot, which could be due to selective reporting or methodological and substantive differences across studies(46). For amyloid, there was limited heterogeneity, despite methodological differences across studies that spanned post-mortem, imaging and CSF measures. The assessment of amyloid and tau has evolved rapidly in recent years, which may partly explain the limitations of the broad differences in study design, analytic approach, and reporting of findings. More methodological and reporting consistency will help future meta-analytic efforts and potentially explain some of the differences across studies.
In conclusion, the meta-analytic synthesis of current evidence found that neuroticism was a risk factor and conscientiousness was protective against amyloid and especially tau pathology burden; these associations were stronger in CN samples, which include preclinical AD. We and others(15, 16) had previously hypothesized that personality modulates the risk of clinical dementia mainly by providing resilience against the AD neuropathology, but these new findings support the hypothesis that personality traits may also confer resistance to neuropathology. Future longitudinal studies are essential to determine the temporal order of these associations and gain more insight into the underlying mechanisms.
Supplementary Material
KEY RESOURCES TABLE.
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | n/a | |||
| Bacterial or Viral Strain | n/a | |||
| Biological Sample | n/a | |||
| Cell Line | n/a | |||
| Chemical Compound or Drug | n/a | |||
| Commercial Assay Or Kit | n/a | |||
| Deposited Data; Public Database | Baltimore Longitudinal Study of Aging (BLSA), Humans, female and male | RRID:SCR_013148 | NIA/NIH | |
| Genetic Reagent | n/a | |||
| Organism/Strain | n/a | |||
| Peptide, Recombinant Protein | n/a | |||
| Recombinant DNA | n/a | |||
| Sequence-Based Reagent | n/a | |||
| Software; Algorithm | n/a | |||
| Transfected Construct | n/a | |||
| Other | n/a | |||
Acknowledgments
The authors gratefully acknowledge participants in the BLSA, Wendy Elkins and Alisa Bannerjee for their assistance with the BLSA neuroimaging study, and Dr. Duchek and Dr. Giannakopoulos for gracefully responding to our questions.
Funding:
The BLSA studies are supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health. The research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health (grant numbers R01AG068093, R01AG053297). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests: Authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data and materials availability:
The anonymized BLSA data is publicly accessible upon request at https://www.blsa.nih.gov. All data used in the meta-analysis are available in the main text or the supplementary materials.
References
- 1.McCrae RR, John OP (1992): An introduction to the Five-Factor Model and its applications. J Pers. 60:175–215. [DOI] [PubMed] [Google Scholar]
- 2.Chapman BP, Roberts B, Duberstein P (2011): Personality and longevity: knowns, unknowns, and implications for public health and personalized medicine. J Aging Res. 2011:759170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kendler KS, Gatz M, Gardner CO, Pedersen NL (2006): Personality and major depression: a Swedish longitudinal, population-based twin study. Archives of general psychiatry. 63:1113–1120. [DOI] [PubMed] [Google Scholar]
- 4.Association AP (2013): Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA (2007): Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Archives of general psychiatry. 64:1204–1212. [DOI] [PubMed] [Google Scholar]
- 6.Johansson L, Guo X, Duberstein PR, Hallstrom T, Waern M, Ostling S, et al. (2014): Midlife personality and risk of Alzheimer disease and distress: a 38-year follow-up. Neurology. 83:1538–1544. [DOI] [PubMed] [Google Scholar]
- 7.Kaup AR, Harmell AL, Yaffe K (2019): Conscientiousness is associated with lower risk of dementia among black and white older adults. Neuroepidemiology. 52:86–92. [DOI] [PubMed] [Google Scholar]
- 8.Duchek JM, Aschenbrenner AJ, Fagan AM, Benzinger TL, Morris JC, Balota DA (2019): The Relation Between Personality and Biomarkers in Sensitivity and Conversion to Alzheimer-Type Dementia. Journal of the International Neuropsychological Society.1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terracciano A, Sutin AR, An Y, O’Brien RJ, Ferrucci L, Zonderman AB, et al. (2014): Personality and risk of Alzheimer’s disease: New data and meta-analysis. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 10:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aschwanden D, Strickhouser JE, Luchetti M, Stephan Y, Sutin AR, Terracciano A (2021): Is personality associated with dementia risk? A meta-analytic investigation. Ageing Research Reviews. 67:101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caselli RJ, Dueck AC, Locke DE, Henslin BR, Johnson TA, Woodruff BK, et al. (2016): Impact of Personality on Cognitive Aging: A Prospective Cohort Study. Journal of the International Neuropsychological Society : JINS. 22:765–776. [DOI] [PubMed] [Google Scholar]
- 12.Sutin AR, Stephan Y, Terracciano A (2019): Self-Reported Personality Traits and Informant-Rated Cognition: A 10-Year Prospective Study. Journal of Alzheimer’s disease : JAD. 72:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutin AR, Stephan Y, Luchetti M, Aschwanden D, Sesker AA, O’Súilleabháin PS, et al. (2021): Self-reported and mother-rated personality traits at age 16 are associated with cognitive function measured concurrently and 30 years later. Psychological medicine.1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman BP, Huang A, Peters K, Horner E, Manly J, Bennett DA, et al. (2020): Association Between High School Personality Phenotype and Dementia 54 Years Later in Results From a National US Sample. JAMA psychiatry. 77:148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terracciano A, Iacono D, O’Brien RJ, Troncoso JC, An Y, Sutin AR, et al. (2013): Personality and resilience to Alzheimer’s disease neuropathology: a prospective autopsy study. Neurobiology of aging. 34:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham EK, James BD, Jackson KL, Willroth EC, Boyle P, Wilson R, et al. (2021): Associations Between Personality Traits and Cognitive Resilience in Older Adults. The Journals of Gerontology: Series B. 76:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arenaza-Urquijo EM, Vemuri P (2018): Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology. 90:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohrabi HR, Goozee K, Weinborn M, Shen K, Brown BM, Rainey-Smith SR, et al. (2020): Personality factors and cerebral glucose metabolism in community-dwelling older adults. Brain Structure and Function. 225:1511–1522. [DOI] [PubMed] [Google Scholar]
- 19.Jackson J, Balota DA, Head D (2011): Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiology of aging. 32:2162–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Booth T, Mottus R, Corley J, Gow AJ, Henderson RD, Maniega SM, et al. (2014): Personality, health, and brain integrity: the Lothian birth cohort study 1936. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 33:1477–1486. [DOI] [PubMed] [Google Scholar]
- 21.Simon SS (2021): Good News for Dementia Prevention: Multiple Behavioral Features Are Associated With Markers of Brain Pathology in the Preclinical Phase of Sporadic and Autosomal Dominant Alzheimer’s Disease. Biological Psychiatry. 89:739–741. [DOI] [PubMed] [Google Scholar]
- 22.Jack CR Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. (2013): Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. The Lancet Neurology. 12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baena A, Bocanegra Y, Torres V, Vila-Castelar C, Guzmán-Vélez E, Fox-Fuller JT, et al. (2021): Neuroticism Is Associated with Tau Pathology in Cognitively Unimpaired Individuals with Autosomal Dominant Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultz SA, Gordon BA, Mishra S, Su Y, Morris JC, Ances BM, et al. (2020): Association between personality and tau-PET binding in cognitively normal older adults. Brain imaging and behavior. 14:2122–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pichet Binette A, Vachon-Presseau É, Morris J, Bateman R, Benzinger T, Collins DL, et al. (2020): Amyloid and Tau Pathology Associations With Personality Traits, Neuropsychiatric Symptoms, and Cognitive Lifestyle in the Preclinical Phases of Sporadic and Autosomal Dominant Alzheimer’s Disease. Biol Psychiatry. 89:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon B, Baker SL, Korman D, Tennant VR, Harrison TM, Landau S, et al. (2020): Conscientiousness is associated with less amyloid deposition in cognitively normal aging Psychology and aging. 35:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byun MS, Jung JH, Sohn BK, Yi D, Lee JH, Jeon SY, et al. (2020): Neuroticism, conscientiousness, and in vivo Alzheimer pathologies measured by amyloid PET and MRI. Psychiatry and clinical neurosciences. 74:303–310. [DOI] [PubMed] [Google Scholar]
- 28.Costa PT Jr., McCrae RR (1992): Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- 29.Terracciano A, Costa PT Jr., McCrae RR (2006): Personality plasticity after age 30. Personality and Social Psychology Bulletin. 32:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCrae RR, Terracciano A, 78 Members of the Personality Profiles of Cultures Project (2005): Universal features of personality traits from the observer’s perspective: Data from 50 cultures. Journal of personality and social psychology. 88:547–561. [DOI] [PubMed] [Google Scholar]
- 31.Bagby RM, Costa PT Jr., McCrae RR, Livesley WJ, Kennedy SH, Levitan RD, et al. (1999): Replicating the five factor model of personality in a psychiatric sample. Pers Indiv Differ. 27:1135–1139. [Google Scholar]
- 32.Islam M, Mazumder M, Schwabe-Warf D, Stephan Y, Sutin AR, Terracciano A (2019): Personality Changes With Dementia From the Informant Perspective: New Data and Meta-Analysis. Journal of the American Medical Directors Association. 20:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terracciano A, Stephan Y, Luchetti M, Sutin AR (2018): Cognitive impairment, dementia, and personality stability among older adults. Assessment. 25:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziontz J, Bilgel M, Shafer AT, Moghekar A, Elkins W, Helphrey J, et al. (2019): Tau pathology in cognitively normal older adults. Alzheimer’s & dementia (Amsterdam, Netherlands). 11:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris JC (1997): Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. International psychogeriatrics / IPA. 9 Suppl 1:173–176; discussion 177–178. [DOI] [PubMed] [Google Scholar]
- 36.Blessed G, Tomlinson BE, Roth M (1968): The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. The British journal of psychiatry : the journal of mental science. 114:797–811. [DOI] [PubMed] [Google Scholar]
- 37.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999): Mild cognitive impairment: clinical characterization and outcome. Archives of neurology. 56:303–308. [DOI] [PubMed] [Google Scholar]
- 38.American Psychiatric Association (1987): Diagnostic and statistical manual of mental disorders (3rd ed., rev.). Washington, DC: Author. [Google Scholar]
- 39.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984): Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 34:939–944. [DOI] [PubMed] [Google Scholar]
- 40.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB (1997): Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and aging. 12:277–287. [DOI] [PubMed] [Google Scholar]
- 41.Giannakopoulos P, Rodriguez C, Montandon ML, Garibotto V, Haller S, Herrmann FR (2020): Less agreeable, better preserved? A PET amyloid and MRI study in a community-based cohort. Neurobiology of aging. 89:24–31. [DOI] [PubMed] [Google Scholar]
- 42.Mattsson N, Smith R, Strandberg O, Palmqvist S, Schöll M, Insel PS, et al. (2018): Comparing 18F-AV-1451 with CSF t-tau and p-tau for diagnosis of Alzheimer disease. Neurology. 90:e388–e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aschenbrenner AJ, Petros J, McDade E, Wang G, Balota DA, Benzinger TL, et al. (2020): Relationships between big-five personality factors and Alzheimer’s disease pathology in autosomal dominant Alzheimer’s disease. Alzheimer’s & dementia (Amsterdam, Netherlands). 12:e12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snitz BE, Weissfeld LA, Cohen AD, Lopez OL, Nebes RD, Aizenstein HJ, et al. (2015): Subjective Cognitive Complaints, Personality and Brain Amyloid-beta in Cognitively Normal Older Adults. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 23:985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tautvydaite D, Antonietti JP, Henry H, von Gunten A, Popp J (2017): Relations between personality changes and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology. J Psychiatr Res. 90:12–20. [DOI] [PubMed] [Google Scholar]
- 46.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. (2011): Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj. 343:d4002. [DOI] [PubMed] [Google Scholar]
- 47.Costa PT Jr., McCrae RR, Löckenhoff CE (2019): Personality Across the Life Span. Annual review of psychology. 70:423–448. [DOI] [PubMed] [Google Scholar]
- 48.Oveisgharan S, Wilson RS, Yu L, Schneider JA, Bennett DA (2020): Association of Early-Life Cognitive Enrichment With Alzheimer Disease Pathological Changes and Cognitive Decline. JAMA neurology. 77:1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu K, Nicholas JM, Collins JD, James SN, Parker TD, Lane CA, et al. (2019): Cognition at age 70: Life course predictors and associations with brain pathologies. Neurology. 93:e2144–e2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frederiksen KS, Gjerum L, Waldemar G, Hasselbalch SG (2019): Physical Activity as a Moderator of Alzheimer Pathology: A Systematic Review of Observational Studies. Current Alzheimer research. 16:362–378. [DOI] [PubMed] [Google Scholar]
- 51.Sperling RA, Donohue MC, Raman R, Sun C-K, Yaari R, Holdridge K, et al. (2020): Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA neurology. 77:735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vemuri P, Knopman DS, Lesnick TG, Przybelski SA, Mielke MM, Graff-Radford J, et al. (2017): Evaluation of Amyloid Protective Factors and Alzheimer Disease Neurodegeneration Protective Factors in Elderly Individuals. JAMA neurology. 74:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lane CA, Barnes J, Nicholas JM, Sudre CH, Cash DM, Malone IB, et al. (2019): Associations Between Vascular Risk Across Adulthood and Brain Pathology in Late Life: Evidence From a British Birth Cohort. JAMA neurology. 77:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Köbe T, Gonneaud J, Binette AP, Meyer P-F, McSweeney M, Rosa-Neto P, et al. (2020): Association of vascular risk factors with β-Amyloid peptide and tau burdens in cognitively unimpaired individuals and its interaction with vascular medication use. JAMA network open. 3:e1920780–e1920780. [DOI] [PubMed] [Google Scholar]
- 55.Sutin AR, Stephan Y, Luchetti M, Terracciano A (2018): Self reported personality traits are prospectively associated with proxy reported behavioral and psychological symptoms of dementia at the end of life. International journal of geriatric psychiatry. 33:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein DN, Kotov R, Bufferd SJ (2011): Personality and depression: explanatory models and review of the evidence. Annual review of clinical psychology. 7:269–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krell-Roesch J, Lowe VJ, Neureiter J, Pink A, Roberts RO, Mielke MM, et al. (2018): Depressive and anxiety symptoms and cortical amyloid deposition among cognitively normal elderly persons: the Mayo Clinic Study of Aging. International psychogeriatrics / IPA. 30:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holmes SE, Esterlis I, Mazure CM, Lim YY, Ames D, Rainey-Smith S, et al. (2016): β-Amyloid, APOE and BDNF Genotype, and Depressive and Anxiety Symptoms in Cognitively Normal Older Women and Men. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 24:1191–1195. [DOI] [PubMed] [Google Scholar]
- 59.Harrington KD, Lim YY, Gould E, Maruff P (2015): Amyloid-beta and depression in healthy older adults: a systematic review. The Australian and New Zealand journal of psychiatry. 49:36–46. [DOI] [PubMed] [Google Scholar]
- 60.Babulal GM, Roe CM, Stout SH, Rajasekar G, Wisch JK, Benzinger TL, et al. (2020): Depression is associated with tau and not amyloid positron emission tomography in cognitively normal adults. Journal of Alzheimer’s Disease. 74:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzales MM, Samra J, O’Donnell A, Mackin RS, Salinas J, Jacob M, et al. (2021): Association of Midlife Depressive Symptoms with Regional Amyloid-β and Tau in the Framingham Heart Study. Journal of Alzheimer’s Disease.1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donovan NJ, Locascio JJ, Marshall GA, Gatchel J, Hanseeuw BJ, Rentz DM, et al. (2018): Longitudinal Association of Amyloid Beta and Anxious-Depressive Symptoms in Cognitively Normal Older Adults. The American journal of psychiatry. 175:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terracciano A, An Y, Sutin AR, Thambisetty M, Resnick SM (2017): Personality Change in the Preclinical Phase of Alzheimer Disease. JAMA psychiatry. 74:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoneda T, Rush J, Graham EK, Berg AI, Comijs H, Katz M, et al. (2020): Increases in Neuroticism May Be an Early Indicator of Dementia: A Coordinated Analysis. The Journals of Gerontology: Series B. 75:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sutin AR, Terracciano A, Deiana B, Naitza S, Ferrucci L, Uda M, et al. (2010): High Neuroticism and low Conscientiousness are associated with interleukin-6. Psychological medicine. 40:1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGeer PL, McGeer EG (2013): The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta neuropathologica. 126:479–497. [DOI] [PubMed] [Google Scholar]
- 67.Hayley S, Hakim AM, Albert PR (2020): Depression, dementia and immune dysregulation. Brain : a journal of neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Passamonti L, Riccelli R, Indovina I, Duggento A, Terracciano A, Toschi N (2019): Time-resolved connectome of the five-factor model of personality. Scientific reports. 9:15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franzmeier N, Dewenter A, Frontzkowski L, Dichgans M, Rubinski A, Neitzel J, et al. (2020): Patient-centered connectivity-based prediction of tau pathology spread in Alzheimer’s disease. Science advances. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsvetanov KA, Gazzina S, Jones PS, van Swieten J, Borroni B, Sanchez Valle R, et al. (2021): Brain functional network integrity sustains cognitive function despite atrophy in presymptomatic genetic frontotemporal dementia. Alzheimer’s & Dementia. 17:500–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Jager CH, White CC, Bennett DA, Ma Y (2021): Neuroticism alters the transcriptome of the frontal cortex to contribute to the cognitive decline and onset of Alzheimer’s disease. Translational psychiatry. 11:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stephan Y, Sutin AR, Luchetti M, Caille P, Terracciano A (2018): Polygenic Score for Alzheimer Disease and cognition: The mediating role of personality. J Psychiatr Res. 107:110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagel M, Jansen PR, Stringer S, Watanabe K, de Leeuw CA, Bryois J, et al. (2018): Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nature genetics. 50:920–927. [DOI] [PubMed] [Google Scholar]
- 74.Cook C, Dunmore JH, Murray ME, Scheffel K, Shukoor N, Tong J, et al. (2014): Severe amygdala dysfunction in a MAPT transgenic mouse model of frontotemporal dementia. Neurobiology of aging. 35:1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hampson SE, Goldberg LR, Vogt TM, Dubanoski JP (2006): Forty years on: teachers’ assessments of children’s personality traits predict self-reported health behaviors and outcomes at midlife. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 25:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kekäläinen T, Terracciano A, Sipilä S, Kokko K (2020): Personality traits and physical functioning: a cross-sectional multimethod facet-level analysis. European Review of Aging and Physical Activity. 17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sutin AR, Gamaldo AA, Stephan Y, Strickhouser JE, Terracciano A (2020): Personality traits and the subjective and objective experience of sleep. International journal of behavioral medicine. 27:481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stephan Y, Sutin AR, Caille P, Terracciano A (2019): Personality and hearing acuity: Evidence from the Health and Retirement Study and the English Longitudinal Study of Ageing. Psychosomatic medicine. 81:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jokela M, Elovainio M, Nyberg ST, Tabak AG, Hintsa T, Batty GD, et al. (2014): Personality and risk of diabetes in adults: pooled analysis of 5 cohort studies. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 33:1618–1621. [DOI] [PubMed] [Google Scholar]
- 80.Rohrer JM, Lucas RE, Donnellan B, Schlegel R (2018): Only so many hours: Correlations between personality and daily time use in a representative German panel. Collabra: Psychology. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sutin AR, Aschwanden D, Stephan Y, Terracciano A (2021): The Association Between Facets of Conscientiousness and Performance- based and Informant- Rated Cognition, Affect, and Activities in Older Adults. J Pers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stern Y, Barnes CA, Grady C, Jones RN, Raz N (2019): Brain reserve, cognitive reserve, compensation, and maintenance: operationalization, validity, and mechanisms of cognitive resilience. Neurobiology of aging. 83:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noftle EE, Robins RW (2007): Personality predictors of academic outcomes: big five correlates of GPA and SAT scores. Journal of personality and social psychology. 93:116–130. [DOI] [PubMed] [Google Scholar]
- 84.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013): The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s & dementia. 9:63–75. e62. [DOI] [PubMed] [Google Scholar]
- 85.Capuano AW, Wilson RS, Leurgans SE, Sampaio C, Barnes LL, Farfel JM, et al. (2021): Neuroticism, negative life events, and dementia in older White and Black Brazilians. International journal of geriatric psychiatry. 36:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The anonymized BLSA data is publicly accessible upon request at https://www.blsa.nih.gov. All data used in the meta-analysis are available in the main text or the supplementary materials.