Abstract
Background:
There are conflicting data on the benefit of primary prevention implantable cardioverter-defibrillator (ICD) therapy in patients with ischemic versus non-ischemic cardiomyopathy (ICM/NICM).
Objectives:
We sought to determine the association of cardiomyopathy etiology with the likelihood of ventricular arrhythmias, appropriate ICD therapy and mortality.
Methods:
The study population comprised 4,803 patients with ICM (n=3,106) or NICM (n=1,697) with a primary prevention ICD enrolled in 5 randomized trials conducted between 1997 and 2017. The primary endpoint was sustained ventricular tachycardia ≥200 bpm or ventricular fibrillation (VT/VF). Secondary endpoints included appropriate ICD therapy and all-cause mortality. Differences in cause-specific mortality, including non-cardiac, sudden cardiac, and non-sudden cardiac, were also examined.
Results:
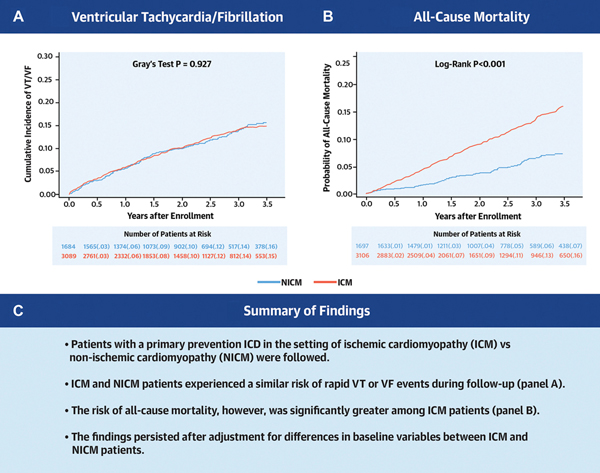
Patients with ICM were significantly older and had more comorbid conditions, whereas those with NICM had more advanced heart failure (HF) class at enrollment and were more often prescribed medical or cardiac resynchronization therapy for HF. Multivariate analysis showed that ICM vs. NICM had a similar risk of VT/VF events (HR=0.98 [95% CI 0.79–1.20]) and appropriate ICD therapy (HR=1.03 [95% CI 0.87–1.22]), whereas the risk of all-cause mortality was 1.8-fold higher among ICM vs. NICM patients (HR=1.84 [95%CI 1.42–2.38]), dominated by non-sudden cardiac mortality.
Conclusions:
Combined data from 5 landmark ICD clinical trials show that ICM patients experience a similar risk of life-threatening ventricular arrhythmic events but have an increased risk of all-cause mortality, dominated by non-sudden cardiac death, compared with NICM patients.
Keywords: Cardiomyopathies, Death, sudden, Defibrillators, implantable, Ventricular arrhythmias
CONDENSED ABSTRACT
We examined whether outcomes and potential benefits of primary prevention implantable cardioverter-defibrillator (ICD) therapy differ among individuals with ischemic versus non-ischemic cardiomyopathy (ICM/NICM). Among 4,803 patients with a primary prevention ICD enrolled in five prospective clinical trials, the risks of sustained rapid ventricular tachycardia or fibrillation and appropriate defibrillator therapy were similar in ICM versus NICM. Mortality, primarily related to non-sudden cardiac death, was substantially higher with ischemic cardiomyopathy. We conclude that individuals with NICM demonstrate similar arrhythmic burden but a lower incidence of death compared to those with ICM.
Implantable cardioverter-defibrillator (ICD) therapy is effective for the primary prevention of sudden cardiac death (SCD) among patients with both ischemic cardiomyopathy (ICM) and non-ischemic cardiomyopathy (NICM); however, data on the survival benefit of ICD placement are more robust for ICM patients. 1–8 Current multi-societal guidelines recommend ICD placement for primary prevention of SCD among patients with ICM or NICM in the setting of significantly reduced left ventricular ejection fraction (LVEF) with symptoms of heart failure (HF) despite guideline directed medical therapy. 9,10 While pooled analyses including randomized trials published in the early 2000s demonstrate benefits of ICD placement in the setting of NICM, 11–13 a more recent trial failed to demonstrate a mortality reduction in this population. 14 Consequently, the role of ICD therapy for primary prevention of SCD among patients with NICM who are receiving current guideline directed medical therapy has come under increased scrutiny. 15,16
The present study was designed to investigate potential differences in outcomes between patients with ICM versus NICM treated with ICD therapy for the primary prevention of SCD in a combined database of 5 landmark ICD trials. Specifically, we sought to evaluate differences in occurrence of ventricular arrhythmias, effectiveness and appropriateness of ICD therapy, and incidences of all-cause mortality, including sudden and non-sudden cardiac mortality, between these two groups.
METHODS
Study Population
The current study included patients with an ICD placed for primary prevention of SCD who were enrolled in one of the following randomized Multicenter Automatic Defibrillator Implantation Trials (MADIT) trials: MADIT-II 7, MADIT-II Risk (M2Risk) 7, MADIT- Cardiac Resynchronization Therapy (MADIT-CRT) 17, MADIT-Reduce Inappropriate Therapy (MADIT-RIT) 18, and the Ranolazine in High-Risk Patients with Implanted Cardioverter-Defibrillator (RAID) trial 19. While not a randomized trial of device implantation, The RAID trial was included in our analysis because it enabled inclusion of a large number of patients with NICM or ICM with a primary prevention ICD receiving contemporary HF therapies who underwent rigorous follow up with careful adjudication of clinical and arrhythmic endpoints. In the RAID trial, ranolazine was not associated with the incidence of ventricular arrhythmias or death among patients with either ICM or NICM. Patients with New York Heart Association (NYHA) functional class IV, unknown NYHA classification or unknown ICM status were excluded. The studies were conducted from July 1997 through January 2017. The design and results of these trials have been reported previously. MADIT-II randomized patients with prior myocardial infarction and LVEF ≤30% to ICD versus conventional (non-ICD) therapy, and M2Risk employed the same inclusion criteria as MADIT-II. MADIT-CRT randomized patients with ICM or NICM, a LVEF ≤ 30%, a QRS duration of ≤ 130 milliseconds, and NYHA functional class I or II symptoms to combined CRT plus defibrillator (CRT-D) therapy versus ICD alone. MADIT-RIT enrolled patients with ICM or NICM to receive an ICD with one of three programming configurations for primary prevention of SCD. The RAID trial enrolled primary prevention patients with de novo implant, primary prevention patients with past implant and history of VT/VF after implant, and secondary prevention patients. For the purpose of this analysis only de novo implant ICD patients were considered. RAID patients were randomized to treatment with ranolazine or placebo. Each study was approved by the institutional review boards at each enrolling site before participation. All patients provided informed consent before enrollment into the respective trials.
Clinical Definitions and Endpoints
The diagnosis of ICM required at least one of the following: (1) a documented history of prior MI (Q-wave or enzyme-positive); (2) a history of coronary revascularization, or; (3) documented coronary disease at angiography and history of angina or other coronary-related symptoms, such that the enrolling physician assessed the patient as having ICM. In all of the MADIT trials and in the RAID trial, all ICD therapies and arrhythmic events were reviewed and adjudicated by an independent committee of at least 2 experienced electrophysiologists. Criteria for adjudication were uniform across the trials. Appropriate ICD therapy was defined as therapy (either anti-tachycardia pacing or defibrillator shock) delivered for ventricular tachyarrhythmias. Adjudication of clinical end points and classification of causes of death in the trials included in our study was carried out by independent morbidity and mortality committees that were unaware of study-group assignments. The diagnosis of nonfatal HF events required signs and symptoms consistent with congestive HF that were responsive to intravenous decongestive therapy on an outpatient basis or an augmented decongestive regimen with oral or parenteral medications during an in-hospital stay.
The primary endpoint of the present study was the first occurrence of sustained ventricular tachycardia ≥200 bpm or ventricular fibrillation (VT/VF), both treated and monitored. Ventricular tachycardia was considered sustained if it persisted for at least 30 seconds or required termination. Prespecified secondary endpoints included all-cause mortality, any sustained VT ≥170 bpm or VF, and appropriate ICD therapy for VT/VF. Additional exploratory endpoints included the combined endpoint of HF or mortality, inappropriate defibrillator therapy including of shocks or anti-tachycardia pacing, and atrial fibrillation (AF) or supraventricular tachycardia (SVT). For classification of cause of death, SCD included those who 1) died suddenly and unexpectedly within 1 h of cardiac symptoms in the absence of progressive cardiac deterioration; 2) died unexpectedly in bed during sleep; and 3) died unexpectedly within 24 h after last being seen alive. Non-SCD was defined as patients who died of progressive cardiac failure or patients who did not meet the time criteria for sudden death.
Statistical analysis
Patient baseline characteristics were summarized as mean ± standard deviation for continuous variables and frequency (percentage) for categorical variables by etiology of cardiomyopathy (ICM versus NICM). Continuous variables were compared using the Wilcoxon rank-sum test, and categorical variables were compared using the chi-square test.
The cumulative incidences of arrhythmic outcomes and ICD therapy by patients with ICM and NICM were estimated using the Gray test. 20 To assess the association between arrhythmic outcomes and etiology of cardiomyopathy, Fine and Gray regression models, stratified by studies, were used for arrhythmia outcomes to account for the competing risk of death (21). From the list of potential covariates, stepwise variable selection method was performed on the primary endpoint, VT/VF, and covariates were included at the 0.05 level. The list of potential covariates was selected based on literature review and clinical judgement. Furthermore, variables that were not uniformly collected or available across all 5 trials were not included in the multivariate models. For example, serum creatinine at enrollment was not included in our model because the MADIT-RIT trial did not collect lab data. The final list of potential covariates consisted of: age at enrollment (year), sex, weight (kg), systolic blood pressure at enrollment (mmHg), previously smoked, diabetes mellitus, NYHA heart failure class, history of non-sustained ventricular arrhythmia requiring treatment, history of atrial arrhythmia requiring treatment, and LVEF (%). Furthermore, subgroup analyses and test of interaction between subgroups and ICM status were investigated for the primary outcome, VT/VF. The subgroups of interest were: age group (< or ≥65 years old), sex, LVEF (≤ or >25%), diabetes status, CRT-D, angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) therapy, and beta blocker therapy.
For all-cause mortality and composite outcome of HF and mortality, unadjusted Kaplan-Meier (KM) estimates were displayed by individuals with ICM and NICM, and the log-rank test was used to assess whether the differences between the outcome curves were statistically significant at p <0.05. Cox proportional hazard regression modeling, stratified by studies, was performed to assess the association between outcomes and ICM versus NICM. Similarly, stepwise variable selection method was performed for all-cause mortality to include variables at the 0.05 level. Final covariates used in the adjusted model included the following: age at enrollment, sex, diabetes, systolic blood pressure (mmHg), LVEF (%), and NYHA heart failure class. The reduced model was then applied for the combined endpoint of HF or mortality; thus, the same set of covariates was used for the mortality and HF or mortality models.
Lastly, adjusted curves for VT/VF and all-cause mortality based on the average values of the covariate vectors across ICM status were presented. In addition, the discriminative performance of the model for VT/VF and for all-cause mortality was quantified by Harrell’s concordance c-statistic.
All statistical tests were two-sided, and a p-value of <0.05 was considered statistically significant. Analyses were carried out with SAS software (version 9.4, SAS institute, Cary, North Carolina).
RESULTS
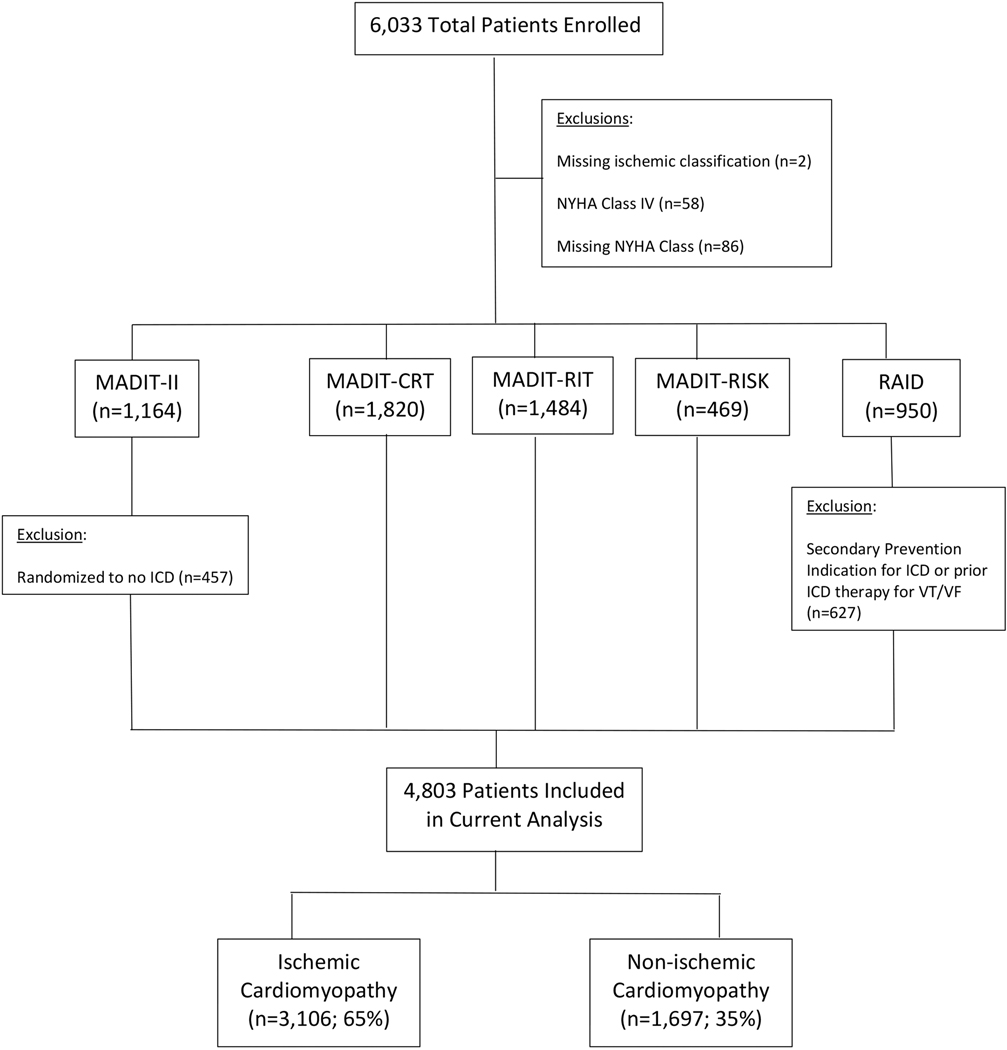
The study population comprised 4,803 patients enrolled in one of the four MADIT or RAID trials who had an ICD placed for primary prevention of sudden cardiac death (Figure 1). Baseline clinical and electrocardiographic characteristics are presented in Table 1. Compared to patients with NICM, those with ICM were significantly older, more often male and of white race, more likely to have comorbid medical illnesses including hypertension, diabetes, renal insufficiency and tobacco use, and were more often on antiplatelet or statin therapy. Patients with NICM were significantly more likely to have advanced HF symptoms (Class II or III), more likely to be taking HF medications at enrollment (including anti-angiotensin drugs, aldosterone, beta-blockers and diuretics), and were more likely to have a CRT-D device in place at enrollment.
Figure 1 –
Patient population included in the present study.
Table 1 –
Baseline Characteristics+ in Patients with Ischemic versus Non-ischemic Cardiomyopathy
| Non-Ischemic (n=1,697) | Ischemic (N=3,106) | P-value | |
|---|---|---|---|
| Age (years) | 61 ± 12 | 65 ± 10 | <0.001 |
| Female | 678 (40) | 458 (15) | <0.001 |
| White Race | 1306 (78) | 2734 (89) | <0.001 |
| Body Mass Index (Kg/m2) | 30 ± 7 | 29 ± 6 | 0.002 |
| Systolic BP (mm Hg) | 122 ± 18 | 123 ± 18 | 0.034 |
| Diastolic BP (mm Hg) | 73 ± 11 | 72 ± 11 | 0.007 |
| eGFR (ml/min/1.73 m2)* | 72 ± 20 | 67 ± 21 | <0.001 |
| LVEF (%) | 24 ± 6 | 25 ± 6 | <0.001 |
| Previous Smoker | 663 (41) | 1754 (58) | <0.001 |
| Current Smoker | 207 (13) | 482 (16) | 0.004 |
| Diabetes | 430 (26) | 1115 (36) | <0.001 |
| Hypertension | 1035 (61) | 2111 (68) | <0.001 |
| Myocardial Infarction | 29 (2) | 2621 (87) | <0.001 |
| Prior Revascularization (PCI or CABG) | 39 (2) | 2540 (82) | <0.001 |
| Prior Hospitalization for Heart Failure | 498 (30) | 1984 (65) | <0.001 |
| NYHA Class at Enrollment | <0.001 | ||
| Class I | 28 (2) | 702 (23) | |
| Class II | 1192 (70) | 1757 (57) | |
| Class III | 477 (28) | 647 (21) | |
| Atrial Fibrillation | 128 (17) | 127 (15) | 0.55 |
| QRS Duration (msec) | 159 ± 22 | 140 ±31 | <0.001 |
| Left Bundle Branch Block | 962 (77) | 857 (39) | <0.001 |
| Heart Rate (bpm) | 71 ± 12 | 69 ± 12 | <0.001 |
| CRT-D Present at Enrollment | 991 (58) | 976 (31) | <0.001 |
| Medications at Enrollment | |||
| ACE Inhibitor or ARB | 1582 (93) | 2767 (89) | <0.001 |
| Aldosterone Inhibitor | 690 (41) | 627 (26) | <0.001 |
| Amiodarone | 88 (5) | 203 (7) | 0.06 |
| Beta-Blocker | 1600 (94) | 2680 (86) | <0.001 |
| Calcium Channel Blocker | 88 (6) | 299 (10) | <0.001 |
| Diuretic | 1227 (72) | 2009 (65) | <0.001 |
| Statin | 772 (45) | 2384 (77) | <0.001 |
| Aspirin | 865 (51) | 2366 (76) | <0.001 |
| Clopidogrel | 43 (3) | 677 (30) | <0.001 |
Data represent as either number (%) or mean ± standard deviation.
Calculated via Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Creatinine Equation ACE - Angiotensin Converting Enzyme; ARB - Angiotensin Receptor Blocker; BP - Blood Pressure; CABG - Coronary Artery Bypass Graft Surgery; CRT-D - Cardiac Resynchronization Therapy-Defibrillator; eGFR - Estimated Glomerular Filtration Rate; LVEF - Left Ventricular Ejection Fraction; NYHA - New York Heart Association; PCI - Percutaneous Coronary Intervention
Risk of Arrhythmic Events by Etiology of Cardiomyopathy
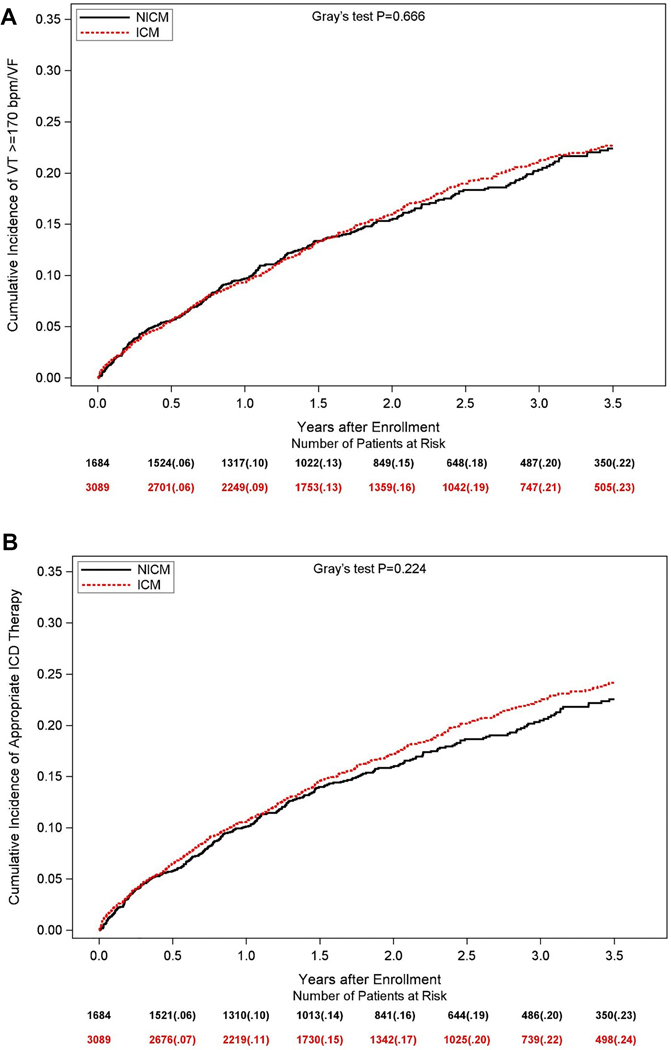
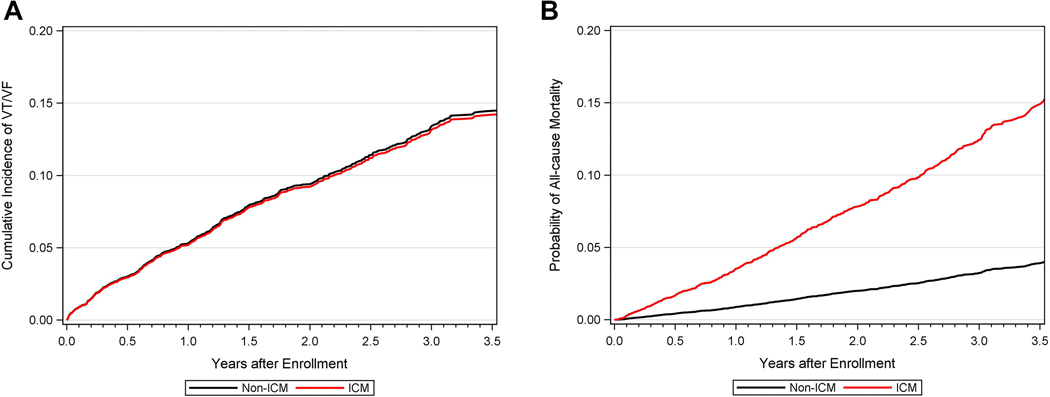
The observed cumulative incidence of the primary endpoint of life-threatening VT/VF at 3.5 years of follow-up was similar in ICM and NICM patients (15% and 16%, respectively; Gray’s p-value = 0.93 for the overall difference during follow-up [Central Figure, Panel A]). Similar findings were shown for the secondary endpoints of any VT ≥170 bpm/VF (23% and 22%, respectively; Gray’s p-value = 0.67 [Figure 2A]) and appropriate ICD therapy (24% and 23%, respectively; Gray’s p-value = 0.22 [Figure 2B]). Consistent with the univariate findings, multivariate analyses showed that the risk of all ventricular arrhythmic events was similar between ICM and NICM patients (Table 2 and Figure 3A). The discriminative performance of the model was quantified by Harrell’s concordance c-statistic for VT/VF was 0.64 (0.62, 0.66). In addition, we performed subgroup analyses to explore the relationship between VT/VF among individuals with ICM compared to those with NICM, and investigated interaction between subgroups and ICM status. None of the subgroups and interactions examined was associated with a significant difference in the likelihood of ventricular arrhythmias between individuals with ICM versus NICM (Supplemental Online Table 1).
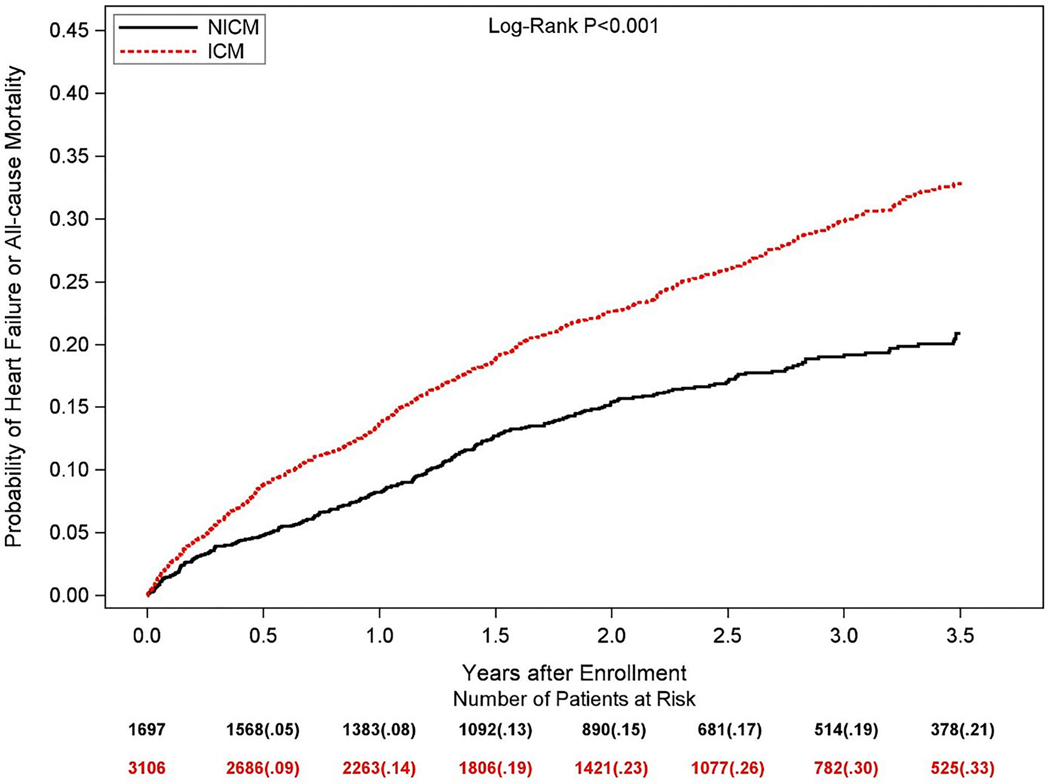
Central Figure –
(A) Observed cumulative incidences of ventricular tachycardia ≥200 bpm or ventricular fibrillation, and (B) unadjusted Kaplan-Meier estimates of the cumulative probabilities of all-cause mortality in patients with ischemic cardiomyopathy (ICM) and non-ischemic cardiomyopathy (NICM)
Figure 2 –
Observed cumulative incidences of (A) ventricular tachycardia ≥170 bpm or ventricular fibrillation, and (B) appropriate ICD therapy (anti-tachycardia pacing or shock) in patients with ischemic cardiomyopathy (ICM) and non-ischemic cardiomyopathy (NICM)
Table 2 –
Relative Risk for Adverse Outcomes for Patients with Ischemic versus Non-ischemic Cardiomyopathy
| Hazard Ratio | 95% CI | P-value | |
|---|---|---|---|
| A. Ventricular Arrhythmias | |||
| VT ≥2003 bpm or VF (unadjusted) | 0.96 | 0.79, 1.17 | 0.70 |
| VT ≥200 bpm or VF (adjusted) | 0.98 | 0.79, 1.20 | 0.82 |
| VT ≥170 bpm or VF (unadjusted) | 1.01 | 0.86, 1.19 | 0.88 |
| VT ≥170 bpm or VF (adjusted) | 1.01 | 0.85, 1.20 | 0.91 |
| Appropriate ICD Therapy (unadjusted) | 1.04 | 0.89, 1.22 | 0.60 |
| Appropriate ICD Therapy (adjusted) | 1.03 | 0.87, 1.22 | 0.73 |
| B. Other arrhythmic events | |||
| Inappropriate ICD Shock (unadjusted) | 0.74 | 0.56, 0.99 | 0.04 |
| Inappropriate ICD Shock (adjusted) | 0.81 | 0.59, 1.11 | 0.19 |
| Atrial Fibrillation or SVT (unadjusted) | 0.81 | 0.67, 0.97 | 0.02 |
| Atrial Fibrillation or SVT (adjusted) | 0.89 | 0.73, 1.09 | 0.26 |
| C. Adverse events | |||
| Mortality (unadjusted) | 2.06 | 1.62, 2.63 | <0.001 |
| Mortality (adjusted) | 1.84 | 1.42, 2.38 | <0.001 |
| HF or Mortality (unadjusted) | 1.56 | 1.35, 1.81 | <0.001 |
| HF or Mortality (adjusted) | 1.49 | 1.28, 1.75 | <0.001 |
All models compare ICM against with the reference group, NICM. “adjusted” indicated results after adjustment for clinical risk factors, as described in the Methods section.
CI - Confidence Interval; HF - Heart Failure; ICD - Implantable Cardiac Defibrillator; ICM - Ischemic Cardiomyopathy; NICM - Non-ischemic Cardiomyopathy; SVT - Supraventricular Tachycardia; VF - Ventricular Fibrillation; VT - Ventricular Tachycardia
Figure 3 –
Adjusted Cumulative Incidences of (A) Ventricular Tachycardia ≥200 bpm or Ventricular Fibrillation and (B) All-cause Mortality in Patients with Ischemic versus Non-ischemic Cardiomyopathy
Supraventricular arrhythmias, including atrial fibrillation or supraventricular tachycardia, occurred at significantly higher rates among patients with NICM compared to those with ICM (Supplemental Online Figure 1), although this difference was not statistically significant after adjustment for baseline variables (Table 2). Inappropriate ICD shocks were likewise more common among patients with NICM, but this difference also was not statistically significant after adjustment for baseline variables.
Risk of Mortality by Etiology of Cardiomyopathy
The Central Figure (Panel B) shows the unadjusted cumulative probability of all-cause mortality for patients with ICM versus NICM. At 3.5-years, the cumulative probability of death for patients with ICM was 16%, whereas the corresponding death rate was 7% in patients with NICM (log-rank p-value<0.001 for overall difference). In multivariate Cox proportional hazards regression analysis, patients with ICM maintained a 1.8-fold higher all-cause mortality compared to those with NICM (hazard ratio [HR] 1.84, 95% confidence interval [CI] 1.42 – 2.38), as demonstrated in Table 2 and Figure 3B. The discriminative performance of the model was quantified by Harrell’s concordance c-statistic for all-cause mortality was 0.69 (0.67, 0.72). Likewise, the risk of HF or mortality was significantly greater for patients with ICM when compared to those with NICM (Figure 4). Multivariate Cox proportional hazards regression analysis showed a persistent significantly higher risk of HF or all-cause mortality in patients with ICM when compared to those with NICM after adjustment for differences in baseline variables (Table 2).
Figure 4 –
Unadjusted Kaplan-Meier estimates of the cumulative probabilities of heart failure or all-cause mortality in patients with ischemic cardiomyopathy (ICM) and non-ischemic cardiomyopathy (NICM)
Because patients with NICM were significantly more likely than those with ICM to have a CRT-D present at enrollment, the interaction between CRT-D and cardiomyopathy type with respect to all-cause mortality was further examined. As indicated in Supplemental Appendix Table 2, the significant association between ICM and all-cause mortality persisted among patients with and without a CRT-D at enrollment (p-value for interaction = 0.88). Similarly, at 3.5 years, the unadjusted cumulative probability of non-sudden cardiac mortality in patients with ICM vs. NICM was 7% vs. 3% (p<0.001) for the entire population, 7% vs. 3% (p<0.001) for patients without CRT-D at enrollment, and minimally lower at 6% vs. 2% for patients with CRT-D (p<0.001).
Causes of Mortality
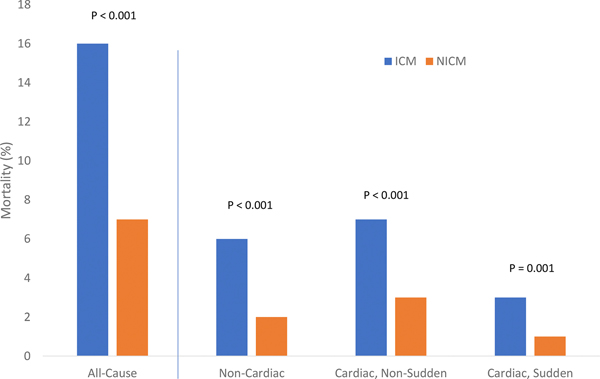
Mortality related to both cardiac and non-cardiac causes was significantly more frequent in patients with ICM compared to those with NICM. At 3.5 years, the unadjusted cumulative probability of cardiac mortality in individuals with ICM versus NICM was 9 vs. 4%, p<0.001, and mortality from non-cardiac causes was 6 vs. 2%, p<0.001 (Figure 5). When cardiac mortality was further assessed by cause, SCD at 3.5 years occurred in only 3 vs. 1% of ICM and NICM patients, respectively, whereas non-sudden cardiac mortality occurred in 7% and 3%, respectively.
Figure 5 –
Incidences of all-cause mortality and cause-specific mortality at 3.5-year follow-up in patients with ischemic cardiomyopathy (ICM) and non-ischemic cardiomyopathy (NICM)
DISCUSSION
Within this large and rigorously followed cohort of patients with left ventricular systolic dysfunction who underwent ICD implantation for the primary prevention of death, individuals with ICM and NICM demonstrated substantial and equal likelihoods of potentially fatal ventricular arrhythmic events. Nearly one in four patients with ICM and NICM received appropriate ICD therapy during follow-up. Patients with ICM, however, experienced a greater than two-fold increased risk of observed all-cause, cardiac and non-cardiac mortality compared to those with NICM.
While ICD therapy is effective in reducing the occurrence of SCD among patients with left ventricular dysfunction resulting from both ischemic and non-ischemic etiologies, the benefits of ICD placement for primary prevention indications are thought to vary according to cardiomyopathy type. Whereas individuals with ICM demonstrated consistent mortality benefit in large multicenter randomized trials of ICD versus conventional medical therapy performed in the early 2000s, 4,6,7 randomized trials examining ICD implantation for patients with NICM have yielded variable results. 1–3,5,8 However, meta-analyses of randomized trials have established an association between ICD therapy and reduced all-cause mortality among patients with NICM. 11–13 Accordingly, current guidelines provide a Class I indication for ICD implantation for the primary prevention of death for individuals with NICM with LVEF ≤35% and class II or III HF despite guideline directed medical therapy. 9,10 Importantly, the more contemporary Danish Study to Assess the Efficacy of ICDs in Patients with Non-Ischemic Systolic Heart Failure on Mortality (DANISH) failed to demonstrate a significant mortality benefit of ICD therapy compared to medical therapy alone among 1,116 patients with NICM and LVEF ≤35%. (14). DANISH may have been underpowered to assess the primary endpoint of all-cause mortality, and subgroup analysis did suggest benefit of ICD therapy among younger patients and those with lower N-terminal pro b-type natriuretic peptide (NT-proBNP) levels. Of note, NICM patients in our study were younger (61 vs 65 years) and had less severe HF (28 vs 45% with class III HF) than in DANISH.
Significant differences in demographic variables and comorbid conditions exist between patients with ischemic and non-ischemic cardiomyopathies, which may contribute to disparities in outcomes. 21,22 As expected, since ICM is a byproduct of advanced atherosclerotic coronary artery disease, individuals with ICM in our patient population were significantly older, more often male, and had more atherosclerotic risk factors and non-cardiac conditions than those with NICM. A significantly greater proportion of patients with NICM had CRT devices in place at study entry. Even after adjusting for differences in baseline characteristics, the likelihood of death remained significantly greater among individuals with ICM. Progressive pump failure and arrhythmic events are thought to represent the most common causes of death among individuals with HF, with death from non-cardiac conditions also contributing substantially. 23 ICD placement effectively reduces the likelihood of arrhythmic SCD, but is unlikely to prevent death related to progressive left ventricular dysfunction, non-cardiac conditions, or non-arrhythmic causes of sudden death such as pulmonary embolism or stroke. Within our cohort of patients with a primary prevention ICD in place, non-cardiac conditions were responsible for a substantial proportion of deaths among patients with both ICM and NICM, and SCD was uncommon irrespective of cardiomyopathy type.
ICD placement is expected to provide the greatest mortality benefit among patient groups who have a high risk of potentially lethal arrhythmic events, but a low risk of death related to non-arrhythmic causes such as progressive heart failure or non-cardiac conditions. Several authors have proposed the concept of an ICD benefit score that incorporates various clinical characteristics to predict the competing probabilities of potentially lethal ventricular arrhythmias versus non-arrhythmic mortality for individual patients, with the goal of calculating that patient’s likelihood of benefit from ICD placement. 24–26 Within our cohort, the occurrence of ventricular arrhythmias was equal between the ICM and NICM groups, while mortality related to non-arrhythmic causes was less frequent with NICM. While speculative and in need of confirmation in dedicated clinical trials, this observation supports the hypothesis that a potential “benefit” of ICD therapy may exist for appropriately selected patients with NICM.
Limitations
Our study has several potential limitations. First, the analysis was performed after combining patient data from several different trials encompassing a 20-year period during which medical treatment of HF and ICD programming have evolved. Nevertheless, the prevalence of beta-blocker therapy, angiotensin converting enzyme inhibitor or angiotensin receptor blockade use, and CRT-D use was substantial in our population, and the results were consistent in a subgroup analysis by HF treatment and in the more contemporary RAID trial. Second, the specific inclusion and exclusion criteria for each of the studies included in this analysis may limit the generalizability of our results; however, examination of patient level data from these rigorously conducted randomized trials permitted us to examine carefully adjudicated arrhythmic and clinical endpoints. In addition, even though we adjusted our multivariate models for relevant covariates, it is possible that unmeasured and unrecognized confounders may have been present that were not taken into consideration.
Conclusion
The present study identified significant differences in clinical outcomes between patients with ICM versus NICM treated with ICD placement for the primary prevention of SCD. While the risk of life-threatening ventricular arrhythmic events and appropriate ICD therapy were equivalent regardless of cardiomyopathy type, mortality rates were substantially greater among patients with ICM. These findings provide support for ongoing research aimed at further delineating which individuals with ICM and NICM are most likely to benefit from ICD therapy.
Supplementary Material
Supplemental Figure 1 – Observed cumulative incidence of atrial fibrillation (AF) or supraventricular tachycardia (SVT) in patients with ischemic cardiomyopathy (ICM) and non-ischemic cardiomyopathy (NICM)
Clinical Competencies:
While current guidelines recommend primary prevention ICD therapy for the prevention of sudden cardiac death among patients with significantly reduced left ventricular ejection fraction and symptoms of heart failure, some recent observations question the efficacy of such therapy among patients with non-ischemic cardiomyopathy.
Translational Outlook:
Our findings indicate that patients with non-ischemic cardiomyopathy experience a similar risk of life-threatening ventricular arrhythmic events as patients with ischemic cardiomyopathy but have a lower incidence of death related to cardiac and non-cardiac causes. These findings provide support for ongoing research aimed at further delineating which individuals with ICM and NICM are most likely to benefit from ICD therapy.
Acknowledgments
Funding information: Each of the MADIT trials were funded by an unrestricted research grant from Boston Scientific to the University of Rochester Medical Center, Rochester, NY. The RAID trial was funded by the National Institutes of Health (NIH) Grant U01HL096607.
Author disclosures: Craig R. Narins: None; Mehmet K. Aktas: Research grants from Boston Scientific and Medtronic; Anita Y. Chen: None; Scott McNitt: None; Fred S. Ling: None; Arwa Younis: None; Wojciech Zareba: Research grants from Boston Scientific, LevaNova, Biotronik, Gilead Sciences; Consulting: Medtronic, Abbott, Astra-Zeneca; James Daubert: Honoraria for: Events Committee, Data Safety Monitoring Board, Consulting, Advisory Boards and/or for lectures from Abbott, Biosense, Biotronik, Boston Scientific, Farapulse, Medtronic, Microport, Phillips, Vytronus and Zoll. Research grants from: Abbott and Medtronic; David T. Huang: Research support from Biosense Webster, Biotronik and Medtronic; Fellowship Program support from: Boston Scientific, Medtronic, Abbott, and Biotronik; Spencer Rosaro: Research grants from Medtronic, Biotronik and Spire Inc.; Valentina Kutyifa: Research grants from Boston Scientific, Biotronik, and ZOLL Inc., Spire Inc., and consultant agreements with Zoll Inc., and Biotronik; Ilan Goldenberg: Research grants from Boston Scientific, Zoll, Medtronic, Biosense-Webster, and Biotronik
ABBREVIATIONS LIST:
- ACEI
angiotensin converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- AF
atrial fibrillation
- CI
confidence interval
- CRT-D
cardiac resynchronization plus defibrillator therapy
- DANISH
Danish Study to Assess the Efficacy of ICDs in Patients with Non-Ischemic Systolic Heart Failure on Mortality
- HF
heart failure
- HR
hazard ratio
- ICD
implantable cardioverter-defibrillator
- ICM
ischemic cardiomyopathy
- KM
Kaplan-Meier
- LVEF
left ventricular ejection fraction
- MADIT
Multicenter Automatic Defibrillator Implantation Trial
- MI
myocardial infarction
- NICM
non-ischemic cardiomyopathy
- NYHA
New York Heart Association
- RAID
Ranolazine in High-Risk Patients with Implanted Cardioverter-Defibrillator trial
- SCD
sudden cardiac death
- SVT
supraventricular tachycardia
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bänsch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT). Circulation. 2002;105(12):1453–1458. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140–2150. [DOI] [PubMed] [Google Scholar]
- 4.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341(25):1882–1890. [DOI] [PubMed] [Google Scholar]
- 5.Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–2158. [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335(26):1933–1940. [DOI] [PubMed] [Google Scholar]
- 7.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. [DOI] [PubMed] [Google Scholar]
- 8.Strickberger SA, Hummel JD, Bartlett TG, et al. Amiodarone versus implantable cardioverter-defibrillator:randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia--AMIOVIRT. J Am Coll Cardiol. 2003;41(10):1707–1712. [DOI] [PubMed] [Google Scholar]
- 9.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72(14):e91–e220. [DOI] [PubMed] [Google Scholar]
- 10.Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). European Heart Journal. 2015;36(41):2793–2867. [DOI] [PubMed] [Google Scholar]
- 11.Anantha Narayanan M, Vakil K, Reddy YN, et al. Efficacy of Implantable Cardioverter-Defibrillator Therapy in Patients With Nonischemic Cardiomyopathy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. JACC Clin Electrophysiol. 2017;3(9):962–970. [DOI] [PubMed] [Google Scholar]
- 12.Golwala H, Bajaj NS, Arora G, Arora P. Implantable Cardioverter-Defibrillator for Nonischemic Cardiomyopathy: An Updated Meta-Analysis. Circulation. 2017;135(2):201–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stavrakis S, Asad Z, Reynolds D. Implantable Cardioverter Defibrillators for Primary Prevention of Mortality in Patients With Nonischemic Cardiomyopathy: A Meta-Analysis of Randomized Controlled Trials. J Cardiovasc Electrophysiol. 2017;28(6):659–665. [DOI] [PubMed] [Google Scholar]
- 14.Køber L, Thune JJ, Nielsen JC, et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016;375(13):1221–1230. [DOI] [PubMed] [Google Scholar]
- 15.Ganga HV, Maan A, Kevin Heist E. Should Primary Prevention ICDs Still Be Placed in Patients with Non-ischemic Cardiomyopathy? A Review of the Evidence. Curr Cardiol Rep. 2018;20(5):31. [DOI] [PubMed] [Google Scholar]
- 16.Kadakia RS, Link MS, Dominic P, Morin DP. Sudden cardiac death in nonischemic cardiomyopathy. Prog Cardiovasc Dis. 2019;62(3):235–241. [DOI] [PubMed] [Google Scholar]
- 17.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329–1338. [DOI] [PubMed] [Google Scholar]
- 18.Moss AJ, Schuger C, Beck CA, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367(24):2275–2283. [DOI] [PubMed] [Google Scholar]
- 19.Zareba W, Daubert JP, Beck CA, et al. Ranolazine in High-Risk Patients With Implanted Cardioverter-Defibrillators: The RAID Trial. J Am Coll Cardiol. 2018;72(6):636–645. [DOI] [PubMed] [Google Scholar]
- 20.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist. 1998;16:1141–1154. [Google Scholar]
- 21.Darma A, Nedios S, Kosiuk J, et al. Differences in predictors of implantable cardioverter-defibrillator therapies in patients with ischaemic and non-ischaemic cardiomyopathies. Europace. 2016;18(3):405–412. [DOI] [PubMed] [Google Scholar]
- 22.Shore S, Grau-Sepulveda MV, Bhatt DL, et al. Characteristics, Treatments, and Outcomes of Hospitalized Heart Failure Patients Stratified by Etiologies of Cardiomyopathy. JACC Heart Fail. 2015;3(11):906–916. [DOI] [PubMed] [Google Scholar]
- 23.Halliday BP, Gulati A, Ali A, et al. Sex- and age-based differences in the natural history and outcome of dilated cardiomyopathy. Eur J Heart Fail. 2018;20(10):1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barsheshet A, Moss A, Huang D, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol. 2012;59:2075–2079. [DOI] [PubMed] [Google Scholar]
- 25.Levy WC, Li Y, Reed SD, et al. Does the Implantable Cardioverter-Defibrillator Benefit Vary With the Estimated Proportional Risk of Sudden Death in Heart Failure Patients? JACC Clin Electrophysiol. 2017;3(3):291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeder HT, Shen C, Buxton AE, Haneuse SJ, Kramer DB. Joint Shock/Death Risk Prediction Model for Patients Considering Implantable Cardioverter-Defibrillators. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 – Observed cumulative incidence of atrial fibrillation (AF) or supraventricular tachycardia (SVT) in patients with ischemic cardiomyopathy (ICM) and non-ischemic cardiomyopathy (NICM)