Abstract
Background:
The effect of chronic graft-versus-host disease (cGVHD) on the risk of non-relapse mortality (NRM) and relapse has not been specifically studied in older adults, who are increasingly undergoing allogeneic hematopoietic cell transplantation (alloHCT) and surviving long-term to develop cGVHD. In this Center for International Blood and Marrow Transplant Research analysis, we tested our hypothesis that the risk of NRM was higher with the development of cGVHD, particularly among older adults (≥60 years).
Methods:
We included 4429 adults ≥40 years who received first HLA-matched peripheral blood alloHCT for acute myeloid leukemia or myelodysplastic syndrome between the years 2008-2017. We compared outcomes of 4 groups: older adults (≥60 years) and younger adults (40-59 years) with or without cGVHD to determine the effect of older age and cGVHD on various outcomes. We used Cox proportional hazard models to determine the risk of NRM, relapse and overall survival (OS). We treated cGVHD as a time-dependent covariate. Severity of cGVHD was based on the CIBMTR clinical definitions.
Results:
cGVHD was significantly associated with a higher risk of NRM and lower risk of relapse regardless of age. The risk of NRM was higher among older versus younger adults. Adults who developed cGVHD as a group had longer OS, compared to age-matched cohorts without cGVHD. Older adults had worse OS regardless of cGVHD. Among adults with cGVHD, clinically moderate or severe cGVHD was associated with a significantly higher risk of NRM and lower risk of relapse; severe cGVHD was associated with shorter OS, whereas mild and moderate cGVHD were associated with longer OS.
Conclusions:
Among both younger and older adults, the development of cGVHD was associated with a higher risk of NRM, lower risk of relapse and longer OS. Older adults had a higher risk of NRM but the increased risk of NRM associated with cGVHD did not differ based on age. Development of mild-moderate cGVHD offered the most favorable balance between minimizing NRM and decreasing relapses. The relapse risk was lowest for adults with severe cGVHD, but high NRM resulted in shorter OS. Developing strategies to avoid clinically severe cGVHD is critically important.
INTRODUCTION
Chronic graft-versus-host disease (cGVHD) is a major late complication of allogeneic hematopoietic cell transplantation (alloHCT). Chronic GVHD is associated with higher transplant-related morbidity, mortality, infectious complications, prolonged duration of immune suppression, and impaired patient-reported quality of life.1-4 Chronic GVHD represents a major obstacle to recovery and survival following alloHCT. Additionally, a 2015 CIBMTR study demonstrated an increasing incidence of cGVHD including moderate and severe cGVHD, in part related to an increase in number of older adults undergoing HCT, improvement in survival and use of peripheral blood stem cell grafts.5 Subsequent studies have demonstrated that an increasing number of adults, particularly in their 60s and 70s have undergone HCT in recent years,6 however, long-term health status of older transplant recipients is poorly studied. The incidence of cGVHD increases with age.7 The development of cGVHD and the use of immunosuppressive therapy may lead to a higher degree of non-relapse mortality (NRM) in older adults. Additionally, the effect of cGVHD on the risk of relapse has not been specifically studied in older adults.
In a prior study from the Chronic GVHD Consortium,8 older adults (≥60 years) with moderate or severe cGVHD were found to have comparable NRM and overall survival (OS) when compared to younger adults. In this Center for International Blood and Marrow Transplant Research (CIBMTR) analysis of adults transplanted more recently, we tested our hypothesis that the development of cGVHD was associated with a higher risk of NRM, and the increase in NRM with cGVHD was more pronounced among older adults, who may have greater burden of comorbidities and poor functional reserve. This study also aimed to compare the risk of disease relapse and OS of older and younger adults with versus without cGVHD. Understanding the impact of cGVHD on NRM and disease relapse could guide therapeutic strategies and provide useful prognostic information.
METHODS
Data Source
The CIBMTR maintains a database of blood and marrow transplants performed in 450 transplant centers in 47 countries. CIBMTR transplant centers are required to report data on all transplantations to a statistical center at the Medical College of Wisconsin. Centers provide yearly follow-up on survivors. Data quality and consecutive registration are ensured through computerized checks for discrepancies, physician reviews of submitted data, and onsite audits of participating centers.9
The CIBMTR collects Transplant Essential Data (TED) from all centers, and Comprehensive Report Form (CRF) data (detailed disease and pre-transplantation and post-transplantation clinical information) on a subset of registered patients selected for the CRF track via a weighted randomization scheme. Data are collected before transplantation, at 100 days and 6 months after transplantation, and annually thereafter or until death. The CIBMTR collects and maintains protected health information in its capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. This study utilized both TED and CRF report forms and was conducted in compliance with all applicable federal regulations pertaining to the protection of human research participants and was approved by the Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program. Participants provided written informed consent for use of their research data.
Patient Selection
The study included adults aged ≥40 years who had received first 8/8 matched related donor (MRD) or matched unrelated donor (MUD) peripheral blood alloHCT for acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) during the years 2008-2017. Patients who received calcineurin inhibitor-based combination for GVHD prophylaxis were included; the use of anti-thymocyte globulin (ATG) or alemtuzumab was considered acceptable for inclusion in the study. Cord blood, haploidentical, partially matched and mismatched unrelated donor transplants (n=3671) were excluded to increase homogeneity of the study population. Other reasons for exclusion included missing cGVHD status or date of onset (n=69), incomplete research forms (n=345), lack of consent (n=79), data from embargoed centers (n=246), GVHD prophylaxis such as ex-vivo T-cell depletion, CD34 selection, post-transplant cyclophosphamide, or single-agent calcineurin inhibitor (n=576), other missing data (n=451), not alive at 100 days post-HCT (n=710) and bone marrow grafts (n=427).
Study Definition and Outcomes
The CIBMTR defines the onset of cGVHD in relation to the timing of acute GVHD based on whether a patient never developed acute GVHD (de novo cGVHD), or acute GVHD was present within 2 weeks of cGVHD diagnosis (progressive cGVHD) or resolved greater than 2 weeks prior to onset of cGVHD (interrupted cGVHD).10 The severity of cGVHD used in this study is based on the best clinical judgement10 and differs from the NIH scoring.11 The severity is assessed during a longitudinal follow-up and reflects the maximum severity at any time.10 “Mild” cGVHD symptoms do not interfere substantially with function and do not progress once appropriately treated. “Moderate” cGVHD symptoms interfere somewhat with function despite appropriate therapy or are progressive through first-line systemic therapy. “Severe” cGVHD symptoms limit function substantially despite appropriate therapy or are progressive through second-line therapy.
The primary outcome of the study was NRM, defined as time from HCT to death without relapse. The secondary outcomes included relapse, disease free survival (DFS) and OS. Relapse was defined as time from HCT to the recurrence of disease. DFS was defined as time from HCT to death or relapse. OS was defined as time from HCT to death from any cause. The study also categorized the cause of death as relapse and specific causes of non-relapse mortality.
Statistical analysis
Patient-, disease-, transplant- and GVHD-related variables were compared between younger versus older adults (≥60 years). Variables analyzed included age, sex, Karnofsky performance score (KPS), race, hematopoietic cell transplantation comorbidity index (HCT-CI), disease type and disease risk index (DRI), donor/recipient sex match, conditioning regimens and intensity, use of ATG and alemtuzumab, GVHD prophylaxis, prior autologous transplant, year of transplant, acute GVHD grades, time to cGVHD, organ involvement, onset of cGVHD (de novo vs. progressive vs. interrupted), severity (mild vs. moderate vs. severe) and grade (limited vs. extensive)12 of cGVHD, KPS and platelet count at cGVHD diagnosis.
In univariate analysis, Kaplan Meier or cumulative incidence curves were plotted for NRM, relapse, and OS with appropriate adjustment for competing risks. In multivariable analysis, Cox proportional hazard models were used to determine the risk factors of non-relapse mortality, relapse and overall survival. All clinical variables known to potentially affect the endpoint of interest were tested first for the affirmation of the proportional hazard assumption. Factors violating the proportional hazards assumption were adjusted through stratification. Then the stepwise forward and backward selection procedures were used to determine the adjusted clinical variables (with a threshold of P=0.05 for both entry and retention in the model). The main comparison of this study was to determine the effect of older age and cGVHD on various outcomes by comparing the 4 groups: the older adults (≥60 years) with versus without cGVHD, and the younger adults (40-59 years) with versus without cGVHD, where the time to onset of cGVHD was treated as a time-dependent covariate. The interactions between cGVHD and patient age, baseline HCT-CI and disease risk index on OS were also examined and no significant interactions were detected. A subset analysis was also performed in adults aged 70 years and older with or without cGVHD. All P-values were raw and 2-sided. Statistical analyses were performed using SAS software version 9.4 (Cary, NC, USA).
RESULTS
Patient characteristics
We included 4429 adults ≥40 years who received first HLA-matched peripheral blood alloHCT using MRD (n=2156) or MUD (n=2273) for AML or MDS between the years 2008-2017. The incidence of cGVHD was 54% among MRD and 53% among MUD alloHCT recipients over a median follow-up of 73 months (Table 1). The median time to onset of cGVHD from HCT was 6 months for all groups. The risk of developing grade 2-4 acute GVHD, cGVHD, as well as moderate or severe cGVHD were comparable between younger and older adults (Table 2). The distribution of organ involvement was also comparable (Supplementary Table 1).
Table 1.
Characteristics of patients receiving first allo-HCT for AML or MDS between 2008-2017
| Characteristic | Age 40-59, MRD | Age 40-59, MUD | Age ≥60, MRD | Age ≥60, MUD | Total |
|---|---|---|---|---|---|
| No. of patients | 1184 | 969 | 972 | 1304 | 4429 |
| Age at HCT | |||||
| Median (min-max) | 53 (40-60) | 53 (40-60) | 65 (60-78) | 66 (60-83) | 60 (40-83) |
| Gender | |||||
| Male | 634 (54) | 529 (55) | 611 (63) | 826 (63) | 2600 (59) |
| Karnofsky score | |||||
| <90 | 492 (42) | 369 (38) | 453 (47) | 614 (47) | 1928 (44) |
| ≥90 | 692 (58) | 600 (62) | 519 (53) | 690 (53) | 2501 (56) |
| Disease | |||||
| AML | 768 (65) | 594 (61) | 344 (35) | 481 (37) | 2187 (49) |
| MDS | 416 (35) | 375 (39) | 628 (65) | 823 (63) | 2242 (51) |
| HCT-CI | |||||
| 0 | 339 (29) | 246 (25) | 199 (20) | 220 (17) | 1004 (23) |
| 1 | 167 (14) | 139 (14) | 119 (12) | 161 (12) | 586 (13) |
| 2 | 167 (14) | 135 (14) | 121 (12) | 178 (14) | 601 (14) |
| 3+ | 511 (43) | 449 (46) | 533 (55) | 745 (57) | 2238 (51) |
| Refined disease risk index | |||||
| Low | 51 (4) | 37 (4) | 9 (1) | 13 (1) | 110 (2) |
| Intermediate | 748 (63) | 531 (55) | 481 (49) | 560 (43) | 2320 (52) |
| High | 337 (28) | 337 (35) | 408 (42) | 602 (46) | 1684 (38) |
| Very high | 7 (1) | 14 (1) | 5 (1) | 10 (1) | 36 (1) |
| Missing | 41 (3) | 50 (5) | 69 (7) | 119 (9) | 279 (6) |
| Conditioning regimen intensity | |||||
| MAC | 840 (71) | 661 (68) | 237 (24) | 324 (25) | 2062 (47) |
| RIC | 294 (25) | 265 (27) | 627 (65) | 847 (65) | 2033 (46) |
| NMA | 50 (4) | 43 (4) | 108 (11) | 133 (10) | 334 (8) |
| ATG used | |||||
| No | 1082 (91) | 649 (67) | 841 (87) | 832 (64) | 3404 (77) |
| Yes | 102 (9) | 320 (33) | 131 (13) | 472 (36) | 1025 (23) |
| Alemtuzumab used | |||||
| No | 1175 (99) | 962 (99) | 960 (99) | 1280 (98) | 4377 (99) |
| Yes | 9 (1) | 7 (1) | 12 (1) | 24 (2) | 52 (1) |
| GVHD prophylaxis | |||||
| CNI + MMF +− other(s) (except post-CY) | 237 (20) | 238 (25) | 311 (32) | 449 (34) | 1235 (28) |
| CNI + MTX +− other(s) (except MMF, post-CY) | 838 (71) | 662 (68) | 583 (60) | 711 (55) | 2794 (63) |
| CNI + other(s) (except MMF, MTX, post-CY) | 109 (9) | 69 (7) | 78 (8) | 144 (11) | 400 (9) |
| Follow-up of survivors (months) - median (range) | 72 (3-145) | 96 (4-144) | 59 (3-144) | 73 (3-143) | 73 (3-145) |
AML acute myeloid leukemia, ATG anti-thymocyte globulin, cGVHD chronic GVHD, CNI calcineurin inhibitor, CY cyclophosphamide, GVHD graft-versus-host disease, HCT hematopoietic cell transplantation, HCT CI hematopoietic cell transplantation comorbidity index, MAC myeloablative conditioning, MDS myelodysplastic syndrome, MMF mycophenolate mofetil, MRD matched related donor, MTX methotrexate, MUD matched unrelated donor, NMA non-myeloablative conditioning, RIC reduced intensity conditioning
Table 2.
Risk of GVHD among patients receiving first allo-HCT for AML or MDS between 2008-2017
| Characteristic | Age 40-59, MRD | Age 40-59, MUD | Age ≥60, MRD | Age ≥60, MUD | Total |
|---|---|---|---|---|---|
| Grade 2-4 acute GVHD | |||||
| No | 754 (64) | 472 (49) | 595 (61) | 655 (50) | 2476 (56) |
| Yes | 416 (35) | 489 (50) | 370 (38) | 640 (49) | 1915 (43) |
| Missing | 14 (1) | 8 (1) | 7 (1) | 9 (1) | 38 (1) |
| Chronic GVHD | |||||
| No | 519 (44) | 417 (43) | 477 (49) | 646 (50) | 2059 (46) |
| Yes | 665 (56) | 552 (57) | 495 (51) | 658 (50) | 2370 (54) |
| Severity of cGVHD | |||||
| Mild | 250 (38) | 191 (35) | 164 (33) | 237 (36) | 842 (36) |
| Moderate | 218 (33) | 197 (36) | 177 (36) | 229 (35) | 821 (35) |
| Severe | 186 (28) | 156 (28) | 138 (28) | 185 (28) | 665 (28) |
| Missing | 11 (2) | 8 (1) | 16 (3) | 7 (1) | 42 (2) |
| Maximum grade of cGVHD | |||||
| Limited | 83 (12) | 60 (11) | 53 (11) | 80 (12) | 276 (12) |
| Extensive | 577 (87) | 492 (89) | 434 (88) | 577 (88) | 2080 (88) |
| Missing | 5 (1) | 0 (0) | 8 (2) | 1 (0) | 14 (1) |
| Onset of cGVHD | |||||
| Progressive | 119 (18) | 103 (19) | 89 (18) | 162 (25) | 473 (20) |
| Interrupted | 201 (30) | 248 (45) | 159 (32) | 280 (43) | 888 (37) |
| De novo | 293 (44) | 158 (29) | 209 (42) | 174 (26) | 834 (35) |
| Missing | 52 (8) | 43 (8) | 38 (8) | 42 (6) | 175 (7) |
| Karnofsky score at cGVHD diagnosis | |||||
| ≥90 | 235 (35) | 162 (29) | 140 (28) | 159 (24) | 696 (29) |
| <90 | 255 (38) | 215 (39) | 215 (43) | 319 (48) | 1004 (42) |
| Missing | 175 (26) | 175 (32) | 140 (28) | 180 (27) | 670 (28) |
| Platelet count at cGVHD diagnosis, x109/L | |||||
| <100 | 175 (26) | 174 (32) | 149 (30) | 227 (34) | 725 (31) |
| ≥100 | 461 (69) | 355 (64) | 311 (63) | 402 (61) | 1529 (65) |
| Missing | 29 (4) | 23 (4) | 35 (7) | 29 (4) | 116 (5) |
AML acute myeloid leukemia, cGVHD chronic GVHD, GVHD graft-versus-host disease, HCT hematopoietic cell transplantation, MDS myelodysplastic syndrome, MRD matched related donor, MUD matched unrelated donor
Non-relapse mortality
Our first hypothesis was that cGVHD was associated with an even higher risk of NRM in older compared to younger adults. A multivariate analysis demonstrated that both cGVHD and older age were associated with a significantly higher risk of NRM; however, the risks of NRM due to cGVHD and older age were additive but not synergistic (interaction between cGVHD and age on NRM was not statistically significant) (Table 3, Figure 1). Compared to adults aged 40-59 years without cGVHD, adults aged 40-59 years with cGVHD (HR 1.42, 95% CI 1.15-1.75, p=0.0009), adults aged ≥60 years without cGVHD (HR 1.52, 95% CI 1.23-1.87, p=0.0001), and adults aged ≥60 years with cGVHD (HR 1.97, 95% CI 1.60-2.42, p<0.0001) had higher risk of NRM. Other factors that were associated with a higher risk of NRM included acute GVHD grades 2-4, use of MUD, HCT CI ≥2, time from diagnosis to HCT ≥6 months, KPS <90, male recipients with female donors and recent years of transplant (Supplementary Table 2). More older adults (≥60 years) and those with higher comorbidity burden (e.g. HCT CI ≥3) were transplanted in recent years (data not shown), which likely explains an increase in NRM in recent years. No interactions were identified between cGVHD and other covariates such as patient age, HCT-CI or DRI for NRM, supporting that the increased risk of NRM with cGVHD was not affected by these factors.
Table 3.
Multivariate analysis results
| Factor | Count | Event | HR | 95% CI lower limit | 95% CI upper limit | P-value |
|---|---|---|---|---|---|---|
| Non-relapse mortality a | ||||||
| Patient age & cGVHD* | . | . | . | . | . | <.0001 |
| 40-59, no cGVHD | 935 | 165 | 1.00 | . | . | . |
| 40-59, cGVHD | 1216 | 285 | 1.42 | 1.15 | 1.75 | 0.0009 |
| >=60, no cGVHD | 1120 | 278 | 1.52 | 1.23 | 1.87 | 0.0001 |
| >=60, cGVHD | 1151 | 376 | 1.97 | 1.60 | 2.42 | <.0001 |
| Pairwise comparisons | ||||||
| ≥60, no cGVHD vs. 40-59, cGVHD | 1.07 | 0.88 | 1.30 | 0.4990 | ||
| ≥60, cGVHD vs. 40-59, cGVHD | 1.38 | 1.20 | 1.60 | <.0001 | ||
| ≥60, cGVHD vs. ≥60, no cGVHD | 1.29 | 1.10 | 1.53 | 0.0022 | ||
| Relapse b | ||||||
| Patient age & cGVHD* | . | . | . | . | . | <.0001 |
| 40-59, no cGVHD | 935 | 576 | 1.00 | . | . | . |
| 40-59, cGVHD | 1217 | 391 | 0.67 | 0.57 | 0.78 | <.0001 |
| >=60, no cGVHD | 1120 | 712 | 1.06 | 0.95 | 1.19 | 0.2840 |
| >=60, cGVHD | 1150 | 401 | 0.61 | 0.50 | 0.75 | <.0001 |
| Pairwise comparisons | ||||||
| ≥60, no cGVHD vs. 40-59, cGVHD | 1.59 | 1.39 | 1.83 | <.0001 | ||
| ≥60, cGVHD vs. 40-59, cGVHD | 0.92 | 0.77 | 1.09 | 0.3210 | ||
| ≥60, cGVHD vs. ≥60, no cGVHD | 0.58 | 0.49 | 0.68 | <.0001 | ||
| Disease free survival c | ||||||
| Patient age & cGVHD* | . | . | . | . | . | <.0001 |
| 40-59, no cGVHD | 935 | 741 | 1.00 | . | . | . |
| 40-59, cGVHD | 1217 | 676 | 0.87 | 0.78 | 0.98 | 0.0228 |
| >=60, no cGVHD | 1120 | 991 | 1.14 | 1.03 | 1.26 | 0.0086 |
| >=60, cGVHD | 1150 | 776 | 1.02 | 0.90 | 1.17 | 0.7305 |
| Pairwise comparisons | ||||||
| ≥60, no cGVHD vs. 40-59, cGVHD | 1.30 | 1.17 | 1.46 | <.0001 | ||
| ≥60, cGVHD vs. 40-59, cGVHD | 1.17 | 1.04 | 1.32 | 0.0093 | ||
| ≥60, cGVHD vs. ≥60, no cGVHD | 0.90 | 0.80 | 1.00 | 0.0554 | ||
| Overall survival d | ||||||
| Patient age & cGVHD* | . | . | . | . | . | <.0001 |
| 40-59, no cGVHD | 936 | 683 | 1.00 | . | . | . |
| 40-59, cGVHD | 1216 | 585 | 0.78 | 0.69 | 0.88 | <.0001 |
| >=60, no cGVHD | 1123 | 928 | 1.23 | 1.10 | 1.37 | 0.0004 |
| >=60, cGVHD | 1150 | 695 | 0.93 | 0.82 | 1.05 | 0.2506 |
| Pairwise comparisons | ||||||
| ≥60, no cGVHD vs. 40-59, cGVHD | 1.58 | 1.42 | 1.75 | <.0001 | ||
| ≥60, cGVHD vs. 40-59, cGVHD | 1.20 | 1.07 | 1.33 | 0.0011 | ||
| ≥60, cGVHD vs. ≥60, no cGVHD | 0.76 | 0.69 | 0.83 | <.0001 | ||
cGVHD chronic graft-versus-host disease
Adjusted for acute graft-versus-host disease grades, anti-thymocyte globulin use, donor type, graft-versus-host disease prophylaxis, hematopoietic cell transplantation comorbidity index, time from diagnosis to hematopoietic cell transplantation, Karnofsky performance score, sex match, year of transplant.
Adjusted for acute graft-versus-host disease grades, anti-thymocyte globulin use, conditioning regimen intensity, Karnofsky performance score, year of transplant.
Adjusted for acute graft-versus-host disease grades, anti-thymocyte globulin use, conditioning regimen intensity, donor type, hematopoietic cell transplantation comorbidity index, Karnofsky performance score, sex match, year of transplant.
Adjusted for acute graft-versus-host disease grades, disease, donor type, refined disease risk index, graft-versus-host disease prophylaxis, time from diagnosis to transplant, Karnofsky performance score, sex match.
Fig 1. Multivariate analysis of non-relapse mortality.
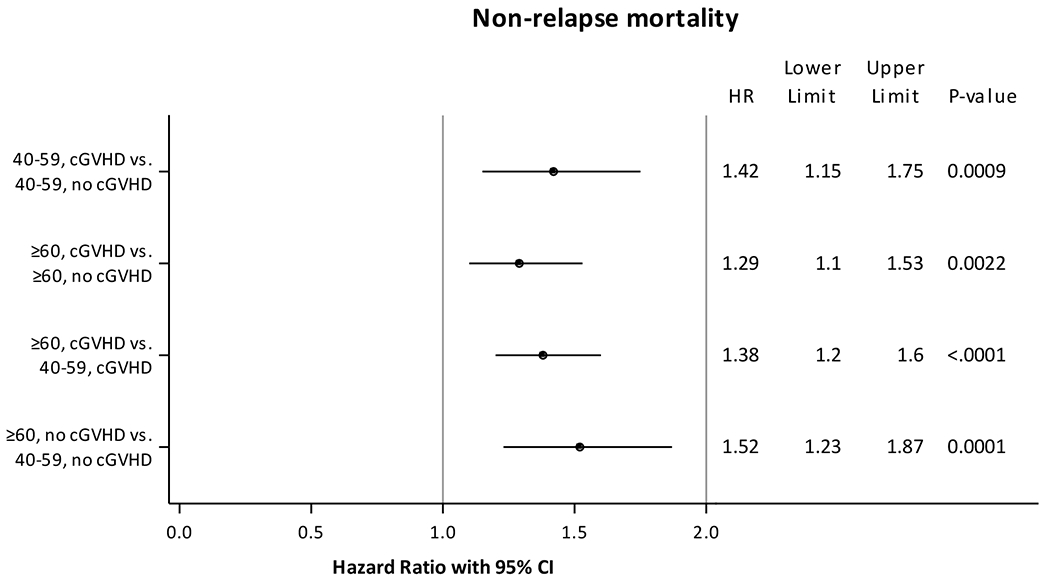
Adjusted for acute graft-versus-host disease grades, anti-thymocyte globulin use, donor type, graft-versus-host disease prophylaxis, hematopoietic cell transplantation comorbidity index, time from diagnosis to hematopoietic cell transplantation, Karnofsky performance score, sex match, year of transplant.
Relapse
Our second hypothesis was that the association of cGVHD with risk of relapse was not dependent on age, and this was confirmed by our results. Chronic GVHD regardless of age was associated with a significantly lower risk of relapse (Figure 2). Compared to adults aged 40-59 years without cGVHD, adults aged 40-59 years with cGVHD (HR 0.67, 95% CI 0.57-0.78, p<0.0001), and adults aged ≥60 years with cGVHD (HR 0.61, 95% CI 0.50-0.75, p<0.0001) but not adults aged ≥60 years without cGVHD (HR 1.06, 95% CI 0.95-1.19, p=0.28) had lower risk of relapse. Other factors that were associated with a lower risk of relapse included acute GVHD grades 2-4, lack of ATG use, myeloablative conditioning, KPS ≥90, year of transplant prior to 2015 (Supplementary Table 3). No significant interactions were identified between cGVHD and other covariates such as patient age, HCT-CI or DRI for relapse, and age itself was not associated with relapse.
Fig 2. Multivariate analysis of risk of relapse.
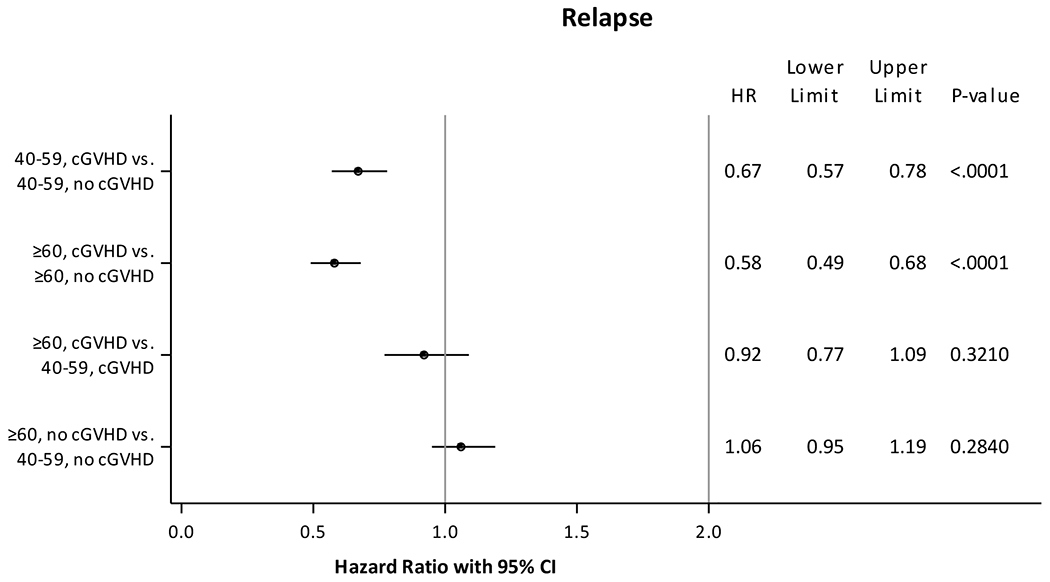
Adjusted for acute graft-versus-host disease grades, anti-thymocyte globulin use, conditioning regimen intensity, Karnofsky performance score, year of transplant.
Disease-free survival
Chronic GVHD was associated with a significantly higher probability of DFS, whereas older age was associated with a lower probability but these effects were independent and of approximately the same magnitude. Compared to adults aged 40-59 years without cGVHD, adults aged 40-59 years with cGVHD (HR 0.87, 95% CI 0.78-0.98, p=0.022) had a higher probability of DFS, whereas adults aged ≥60 years without cGVHD (HR 1.14, 1.03-1.26, 95% CI p=0.0086) had a lower probability of DFS, and adults aged ≥60 years with cGVHD (HR 1.02, 95% CI 0.90-1.17, p=0.73) had no statistical difference in DFS. Other factors that were associated with a lower probability of DFS included acute GVHD grades 3-4, ATG use, non-myeloablative conditioning, use of MUD, HCT CI ≥3, KPS <90, male recipients with female donors, and recent years of transplant (Supplementary Table 4). No significant interactions were identified between cGVHD and other covariates such as patient age, HCT-CI or DRI for DFS.
Overall survival
Results of the OS analysis were similar to DFS. Patients who developed cGVHD as a group had longer OS, compared to age-matched cohorts without cGVHD (Figure 3). Compared to adults aged 40-59 years without cGVHD, adults aged 40-59 years with cGVHD (HR 0.78, 95% CI 0.69-0.88, p<0.0001) had lower probability of mortality, whereas adults aged ≥60 years without cGVHD (HR 1.23, 95% CI 1.10-1.37, p=0.0004) had a higher risk of mortality, and adults aged ≥60 years with cGVHD (HR 0.93, 95% CI 0.82-1.05, p=0.25) had no statistical difference in risk of mortality. Other factors that were associated with a higher risk of mortality included acute GVHD grades 3-4, AML versus MDS, use of MUD, disease risk index other than low, KPS <90, and male recipients with female donors (Supplementary Table 5). No significant interactions were identified between cGVHD and other covariates such as patient age, HCT-CI or DRI for OS; the magnitudes of association for cGVHD and younger age with OS were similar.
Fig 3. Multivariate analysis of overall survival.
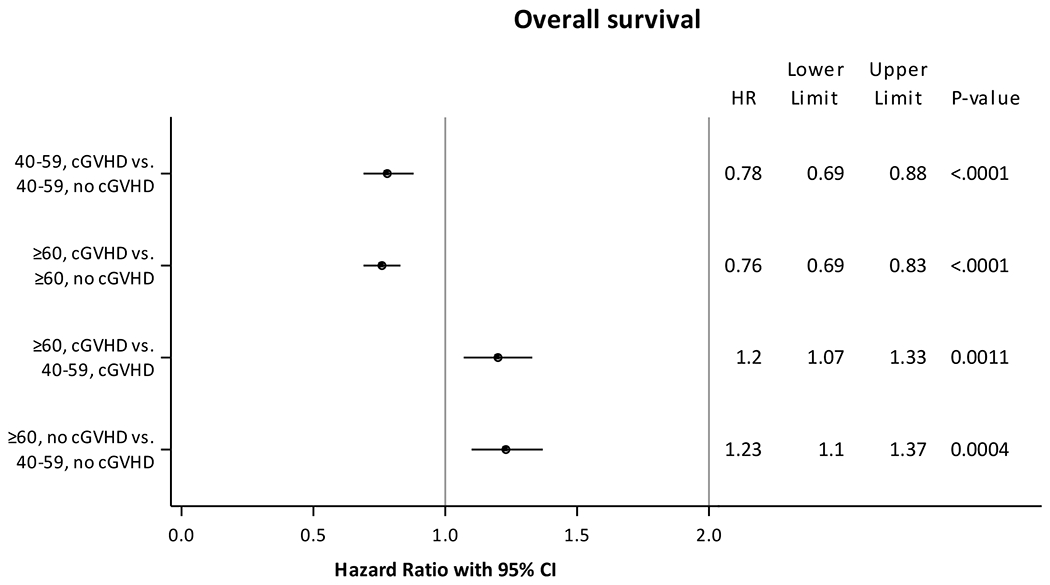
Adjusted for acute graft-versus-host disease grades, disease, donor type, refined disease risk index, graft-versus-host disease prophylaxis, time from diagnosis to transplant, Karnofsky performance score, sex match.
Association of chronic GVHD variables with various outcomes
Non-relapse mortality:
Among patients with cGVHD, those involving skin, eyes, mouth, or musculoskeletal involvement had a lower risk of NRM, whereas gastrointestinal (GI) or lung involvement was associated with a higher risk of NRM. Other factors associated with a higher risk of NRM included KPS <90, platelet count <100,000/μL, and moderate or severe cGVHD (Supplementary Table 6).
Relapse:
Patients with cGVHD involving eyes, mouth, lung or musculoskeletal involvement had a lower risk of relapse. Other factors associated with a lower risk of relapse included platelet count ≥100,000/μL, extensive versus limited cGVHD, de novo vs. progressive or interrupted cGVHD, and moderate or severe cGVHD (Supplementary Table 7).
Disease-free survival and Overall Survival:
Patients with cGVHD involving skin, eyes, liver, mouth, or musculoskeletal involvement had a higher probability of DFS and OS, whereas GI involvement was associated with a lower probability of DFS and OS (Supplementary Tables 8 and 9). Extensive versus limited cGVHD, KPS ≥90 and platelet count ≥100,000/μL were associated with a higher probability of DFS and OS. DFS was higher with de novo more than interrupted more than progressive cGVHD, and mild or moderate versus severe cGVHD. OS was higher with de novo or interrupted versus progressive cGVHD, and moderate more than mild more than severe cGVHD.
Subgroup analyses
Separate analyses were conducted for MRD and MUD HCT (data not shown). Among both younger and older adults (≥60 years) undergoing MRD or MUD HCT, within age-matched cohorts, the development of cGVHD was associated with a higher risk of NRM, lower risk of relapse and longer OS with the following exceptions. For the MRD subgroup, there was a lower probability of OS among younger adults with versus without cGVHD whereas OS was higher among older adults with versus without cGVHD. For the MUD subgroup, patients who developed cGVHD had longer OS, compared to age-matched cohorts without cGVHD. Chronic GVHD was associated with a significantly higher probability of DFS among older adults (≥60 years) who underwent MRD HCT; for other subgroups, statistically significant difference in DFS based on cGVHD were not noted among age-matched cohorts.
For the subgroup ≥70 years, the development of cGVHD was associated with a higher risk of NRM, lower risk of relapse, no statistical difference in DFS, and longer OS (Supplementary Table 10).
DISCUSSION
In this large CIBMTR analysis, we examined whether the characteristics and effects of cGVHD differ based on age. The risk of developing grade 2-4 acute GVHD, cGVHD, as well as moderate or severe cGVHD and the distribution of organ involvement were comparable between younger and older adults. We hypothesized that cGVHD would disproportionately increase NRM in older adults who have more comorbidities whereas relapse protection would not be associated with age. Although we did find that the decrease in relapse associated with cGVHD was similar between older and younger adults, contrary to our hypotheses, cGVHD did not disproportionately increase NRM in older adults compared to younger adults. . Results were qualitatively similar for the subgroups of MRD, URD and adults ≥70 years. In a Chronic GVHD Consortium study of patients with moderate or severe cGVHD, older adults aged ≥60 years (n=128), compared to middle-aged (n=279) and younger adults (n=115) had higher physical and functional limitations, but similar NRM and OS.8 Compared to the prior study, our study also had adults without cGVHD as controls and included a larger sample size providing greater power to detect any difference, which may explain the discrepancy in the study results.
The development of cGVHD was associated with a higher risk of NRM, lower risk of relapse, greater DFS and longer OS among both younger and older adults, compared to age-matched cohorts without cGVHD. Results were largely consistent when analyses were conducted separately for MRDs and MUDs, and in the subgroup ≥70 years. However, for the MRD subgroup, there was a lower probability of OS among younger adults with versus without cGVHD. Although the cause for this discrepancy is not clear, younger adults in the MRD subgroup frequently had intermediate disease risk index and underwent myeloablative alloHCT, hence the NRM associated with cGVHD likely outweighed the benefit from reduction in disease relapses.
A few registry studies have evaluated the effect of cGVHD on outcomes in the general transplant population without specifically looking at whether age magnified the effects.13-15 Older studies from the late 1990s and early 2000s did not find a benefit from cGVHD among patients with AML or MDS who undergo myeloablative alloHCT.13,14 Among patients who underwent RIC alloHCT, the risk of relapses were lower but a higher risk of NRM resulted in lower DFS and OS.14 In a European group for Blood and Marrow Transplantation (EBMT) study15 of AML and MDS patients who underwent RIC matched peripheral blood alloHCT between 2000 and 2009, the development of limited cGVHD was associated with a trend for a lower risk of relapse, no statistical difference in risk of NRM and higher OS, whereas patients with extensive cGVHD had a lower risk of relapse, higher NRM and no difference in OS.15 Our study from more recent years demonstrated an overall more favorable effect of cGVHD, as a result of reduction in relapses. These findings are consistent with the role of cGVHD in reducing risk of relapse in older individuals or those undergoing RIC alloHCT. The development of mild or moderate cGVHD, based on physician’s clinical judgement, offered the most favorable balance between minimizing NRM and decreasing relapse risk. Although the relapse risk was lowest for patients with severe cGVHD, the high NRM resulted in shorter OS, consistent with prior studies that have demonstrated poor outcomes of patients with severe cGVHD.16 Our study also highlighted that GI and lung involvement continue to pose challenges in management and still predict worse NRM. GI involvement did not correlate with a lower risk of relapse, consistent with a prior study that showed similar risk of relapses across different biomarker-based risk groups implicating that GI GVHD including the severity does not necessarily protect from relapse.17 GI involvement was associated with a worse NRM, DFS and OS, likely because GI involvement may result in malnutrition, or GI GVHD may represent concurrent acute and chronic GVHD.
The potential limitations of this study include possible inclusion of overlap syndrome among patients diagnosed with cGVHD early after HCT or those with GI involvement, and a lack of information on treatment of cGVHD and treatment responses. Treatment responses have a potential to alter the clinical course of cGVHD. The CIBMTR definition of severity of cGVHD is based on physician’s clinical judgement and differs from the NIH severity, a limitation like the EBMT study.15 While the intent of the CIBMTR definition is to define severe cGVHD as functional limitations due to cGVHD, it is possible that functional limitations as a result of treatment toxicities such as steroid-induced myopathy may influence physicians’ judgment regarding the severity of cGVHD. The study results may not be generalizable to cord blood, haploidentical, partially matched and mismatched unrelated donor transplants, which were excluded to increase homogeneity. Also, patients who received ATG or alemtuzumab were included but those who received GVHD prophylaxis such as ex-vivo T-cell depletion, CD34 selection, or post-transplant cyclophosphamide were excluded, limiting generalizability. Analyzing cGVHD as a time-varying covariate as we did significantly decreases but does not eliminate the potential bias introduced because patients must survive for a period of time to develop cGVHD. The study has several strengths. To improve homogeneity and overcome limitations of prior studies, we limited our analysis to matched donor transplantation undergoing peripheral blood stem cell transplant, the most common donor source in the recent years. The study also analyzed outcomes separately for patients older than 70 years, contributing knowledge to the literature for this subset of patients, who are increasingly undergoing transplant.6 Other strengths include a large sample size allowing adequate power, a more recent cohort of patients, and separate analyses for MRD versus MUD.
In conclusion, our results show that cGVHD in older adults is associated with similar increases in NRM, lower relapse rates and longer OS as in younger adults. Our hypothesis that cGVHD is tolerated worse in older adults was not confirmed, at least as measured by mortality. In addition, we showed that clinically severe cGVHD is the type that drives poor outcomes whereas clinically mild and moderate cGVHD are associated with some protection against relapse that could be beneficial. Based on these results, developing strategies to avoid clinically severe cGVHD is critically important. Future studies should compare the effect of cGVHD on quality of life and functional outcomes of older adults.
Supplementary Material
Highlights.
Increase in non-relapse mortality with chronic GVHD did not differ based on age.
Relapse reduction with chronic GVHD was similar between older and younger adults.
Overall survival increased with chronic GVHD in both older and younger adults.
Increased overall survival was observed with mild-moderate but not severe cGVHD.
Acknowledgments
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Accenture; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; Adienne SA; Allovir, Inc.; Amgen, Inc.; Astellas Pharma US; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Eurofins Viracor; ExcellThera; Fate Therapeutics; Gamida-Cell, Ltd.; Genentech Inc; Gilead; GlaxoSmithKline; Incyte Corporation; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karyopharm Therapeutics; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Magenta Therapeutics; Medac GmbH; Merck & Co.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncopeptides, Inc.; Orca Biosystems, Inc.; Pfizer, Inc.; Pharmacyclics, LLC; Sanofi Genzyme; Seagen, Inc.; Stemcyte; Takeda Pharmaceuticals; Tscan; Vertex; Vor Biopharma; Xenikos BV.
Conflicts of Interest
Dr. Bhatt reports consulting fees from Agios, Servier, Takeda, Rigel, Partner Therapeutics, Omeros, Genentech, Partnership for health analytic research, LLC, CSL Behring, grants and consulting fees from Abbvie and Incyte, grants from Jazz, Pfizer, National Marrow Donor Program, Tolero Pharmaceuticals and drug support for a clinical trial from Oncoceutics, outside the submitted work.
Dr. Maziarz reports participation with Novartis: Member of DSMB for REACH2 and REACH3 phase IIII studies assessing ruxolitinib for management of advanced acute and chronic GVHD, and advisory board from Incyte.
Dr. Liu reports research support from BMS and Karyopharm, advisory board for Agios, and presentation preparation from SITC.
Dr. Ganguly reports consulting fees from Kadmon, Astellas, Sanofi, Bristol Meyers Squibb, payment from Seattle Genetics, Daiichi Sankyo, KITE Pharma, participation on a board from Kadmon, BMS, Sanofi.
Dr. Murthy reports participation on a board from CRISPR Therapeutics.
Dr. Lee reports research funding from Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer, Syndax, and Takeda, and serves on a clinical trial steering committee for Novartis.
Dr. Modi reports participation on advisory board from Morphosys Seagen.
Dr. Hildebrandt reports research funding from Takeda, Jazz Pharmaceuticals, Parmacyclics, Incyte, AstraZeneca, consulting fees with Incyte, Jazz Pharmaceutical, Morphosys, Alexion Pharmaceuticals Kyropharm Therapeutics, support for attending meetings and/or travel from Jazz Pharmaceuticals, Astellas Pharma, Incyte, Falk Foundation, Incyte, Takeda, stock and other ownership interests with Bluebird Bio, Crispr Therapeutics, Scotts Miracle-Gro, CareTrust Reit, Sangamo Bioscience, Charlotte’s Web, Medical Properties Trust, Aetna, ANGI Homeservices, Axim Biotechnologies, Juno Therapeutics, Bristol-Myers Squibb/Medarex, Johnson & Johnson, Aimmune, Insys Therapeutics, Cardinal Health, Clovis Oncology, CVS Health, Pfizer, Vertex, Immunomedics. Cellectis, Celgene, Novartis, GW Pharmaceuticals, Endocyte, Kite (a Gilead company), Abbvie, IDEXX Laboratories, Procter & Gamble, Bayer, consulting or advisory role with Incyte, Jazz Pharmaceuticals, Pfizer, Morphosys, Alexion Pharmaceuticals, Seattle Genetics, Karyopharm Therapeutics, travel, accommodations, expenses from Falk Foundation, Takeda, Astellas Pharma, Incyte, Jazz Pharmaceuticals, Kite (a Gilead company) and Pfizer.
Dr. Hamilton reports consulting fees from Syndax, Equilium and participation on a data safety monitoring board or advisory board from Syndax or Equilium.
Data Use Statement
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee SJ, Flowers ME. Recognizing and managing chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program. 2008:134–141. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406–414. [DOI] [PubMed] [Google Scholar]
- 3.Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108(8):2867–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arai S, Arora M, Wang T, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21(2):266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muffly L, Pasquini MC, Martens M, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood. 2017;130(9):1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remberger M, Kumlien G, Aschan J, et al. Risk factors for moderate-to-severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2002;8(12):674–682. [DOI] [PubMed] [Google Scholar]
- 8.El-Jawahri A, Pidala J, Inamoto Y, et al. Impact of age on quality of life, functional status, and survival in patients with chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(9):1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horowitz M The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone Marrow Transplant. 2008;42 Suppl 1:S1–S2. [DOI] [PubMed] [Google Scholar]
- 10.CIBMTR Forms Instruction Manual. Q234-406: Chronic Graft vs. Host Disease (GVHD). Available at: https://www.cibmtr.org/manuals/fim/1/en/topic/f2100-q234-406. Accessed on February 21 2021.
- 11.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401 e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan KM, Shulman HM, Storb R, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57(2):267–276. [PubMed] [Google Scholar]
- 13.Boyiadzis M, Arora M, Klein JP, et al. Impact of Chronic Graft-versus-Host Disease on Late Relapse and Survival on 7,489 Patients after Myeloablative Allogeneic Hematopoietic Cell Transplantation for Leukemia. Clin Cancer Res. 2015;21(9):2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisdorf D, Zhang MJ, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transplant. 2012;18(11):1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron F, Labopin M, Niederwieser D, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia. 2012;26(12):2462–2468. [DOI] [PubMed] [Google Scholar]
- 16.Lazaryan A, Arora M. Evolving concepts in prognostic scoring of chronic GvHD. Bone Marrow Transplant. 2017;52(10):1361–1366. [DOI] [PubMed] [Google Scholar]
- 17.Levine JE, Braun TM, Harris AC, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol. 2015;2(1):e21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


