Abstract
Objective:
To identify predictors of changes in height, weight, and BMI in children with ADHD starting Central Nervous System (CNS) stimulants.
Study design:
230 medication-naïve children ages 5–12 with ADHD participated in a randomized trial evaluating the impact of CNS stimulants on growth over 30 months. This observational analysis focused on the 141 participants using study medication for ≥65 days in the first 6 months after starting medication. Biometric variables, ADHD and oppositional defiant disorder symptom scores at medication initiation and medication usage over the study were examined as predictors of changes in standardized height, weight, and BMI.
Results:
Mean changes in z-BMI, z-weight and z-height were negative throughout the study. The most consistent predictors of change in z-BMI, z-weight and z-height were percent days medicated and total medication exposure. Children with lower z-height and z-weight at medication initiation experienced greater z-BMI and z-weight reductions over the first 6 months on medication. Greater appetite suppression during dose optimization predicted greater reductions in z-weight over the entire study and greater reduction in z-height over the first 6 months on medication. Weight change correlated with height change. Behavioral symptoms did not predict changes in z-BMI, z-weight or z-height.
Conclusions:
How much and how often CNS stimulants are used predicts changes in z-BMI, z-weight, and z-height in children. Even smaller and lighter children may be at risk for z-weight and z-BMI decline. Parent ratings of appetite during dose titration may serve as feasible indicators of future weight and height change in children using CNS stimulants.
Trial registration Clinicialtrials.gov: NCT01109849
Keywords: ADHD, growth, CNS stimulants, children, weight loss
Central nervous system (CNS) stimulants are one of the most commonly prescribed pediatric medications.1–4 Side effects are a frequent reason parents are hesitant to use CNS stimulants and why patients discontinue them.5, 6 7, 8 Anorexia and weight loss are often seen with CNS stimulants9–11 and can lead to treatment discontinuation.1, 12 CNS stimulants are associated with a standard mean difference of 0.27 for height and 0.33 for weight. The largest impact on weight occurs during the first 6 months and height by months 24–30.13 The National Institute of Mental Health Multimodal Treatment of ADHD (MTA) study observed the most growth suppression in youth medicated prior to enrollment who consistently used medication over the next decade. The greatest slowing in height velocity occurred in the first two years of use.14 Growth velocity did not meaningfully rebound while taking CNS stimulants, suggesting that “catch-up” growth does not occur if medication is contiuned.15, 16
Identifying reliable indicators of weight loss and growth suppression could aid detection of children at greatest risk for clinically impactful decelerations with CNS stimulants and reassure families of children without identified risk factors. Weight and height deficits are correlated in some studies,17–19 suggesting that initial weight loss could predict growth. Results about the impact of dose and age are mixed.13, 14, 17. Besides the MTA, there has been limited examination of the impact of duration of exposure on growth. Some studies observed that premedication height and weight were inversely correlated with future suppression, leading a widely cited review on this topic to conclude that shorter or lighter youth are at lower risk for medication associated decelerations of height or weight velocity.17, 20, 21 One study observed that extended treatment with CNS stimulants led to a doubling in the percentage of children below 1.5 standard deviations for z-height, suggesting that shorter youth may develop meaningful suppression with CNS stimulants.22
Few treatment studies of ADHD were designed to measure growth, and most studies did not measure height at recurring intervals using standardized procedures.23 Additional limitations included not assessing pubertal status, short study duration, limited documentation of medication use and use of immediate release CNS stimulants which have been increasingly replaced by extended release (ER) versions.13, 17 The growth trajectories of 230 treatment naïve youth randomly assigned to behavior therapy or ER CNS stimulants were prospectively tracked over 30 months and the impact of caloric supplementation, drug holidays and increased monitoring on height, weight and body mass index (BMI) trajectory were assessed.24 This observational analysis examines predictors of standardized BMI, height and weight change for all participants recurrently using CNS stimulant medication in that study. The greatest declines in weight and height were hypothesized to occur in participants with the highest standardized height and weight scores at baseline, the largest exposure to study medication and for weight and BMI, the highest level of parent rated changes in appetite.
Methods
The funded study was designed to assess height and weight trajectories of children with ADHD using CNS stimulants and the effects of weight recovery treatments (WRT) on their growth.24 Participants were 230 children ages 5 to 12 meeting criteria for any Diagnostic and Statistical Manual of Mental Disorders(DSM)-IV ADHD subtype. Exclusion criteria were IQ < 70, BMI below the 5th percentile or above the 94th, use of CNS stimulants for over 30 days before enrollment, use of other psychotropics or medications that impair growth (eg, systemic oral steroids), autism spectrum disorder or medical contraindications to CNS stimulants. Consistent with the local area, 73% of the sample was Hispanic. Five participants (3.5%) previously used any CNS stimulants. ADHD was diagnosed using the Disruptive Behavior Disorders (DBD) Structured Interview,25 by masters-level or higher clinicians, combined with parent and teacher ratings.26 Psychiatric comorbidity was assessed by the NIMH Diagnostic Interview Schedule for Children IV, computerized version,27 with comorbid disorders allowed if ADHD was more impairing. Diagnoses were confirmed by two MD/PhD faculty.
Procedures
All procedures were approved by the Western Institutional Review Board and the study was registered at ClinicalTrials.gov (NCT01109849). Written consent was obtained from parents and assent from children ages seven plus. At baseline, participants were randomized to medication plus low intensity behavioral treatments (78%) or high intensity behavioral treatments without medication (22%) (Figure 1; available at www.jpeds.com). As planned, 180 were randomized to medication, with 165 taking at least one dose. All ADHD medications were prescribed through the study under open label conditions. This observational analysis included the 141 participants (61% of the original sample) using study medication for at least 65 days during the first six months after the date of first medication use. There were 24 participants who did not use sufficient medication to qualify for inclusion into this analysis. Participants were initially treated with OROS-Methylphenidate (MPH), starting at 18 mg with dose titrated every two weeks until optimized using parent and teacher ratings.26, 28, 29 Optimal dose was defined as a tolerable dose producing good home and school functioning with no meaningful room for improvement. Doses could be increased up to the FDA age maximum or 2mg/kg per day of MPH,10 whichever was lower. This optimization phase could last up to 12 weeks with a dose having to be stable for two consecutive visits to be optimized. If OROS-MPH was not efficacious or tolerable, alternative MPH (immediate release MPH, dexmethylphenidate ER or other sprinkle ER MPH capsules) or amphetamine products (immediate or ER mixed amphetamine salts or lisdexamfetamine) were prescribed. Study treatment lasted 30 months. After study month six, participants with persistent impairment could cross over to the other arm (e.g. medication or higher intensity behavior therapy). Of the 141 in this analysis, 21 (15%) were initially assigned to the behavior therapy arm and later crossed to medication. Once optimized, dose could be adjusted after 6 months of medication use if moderate or worse severity was scored on the Clinical Global Impressions Severity Scale (CGI-S),30 as long as the participant was not assigned to WRT.
Figure 1. Study Flowchart.
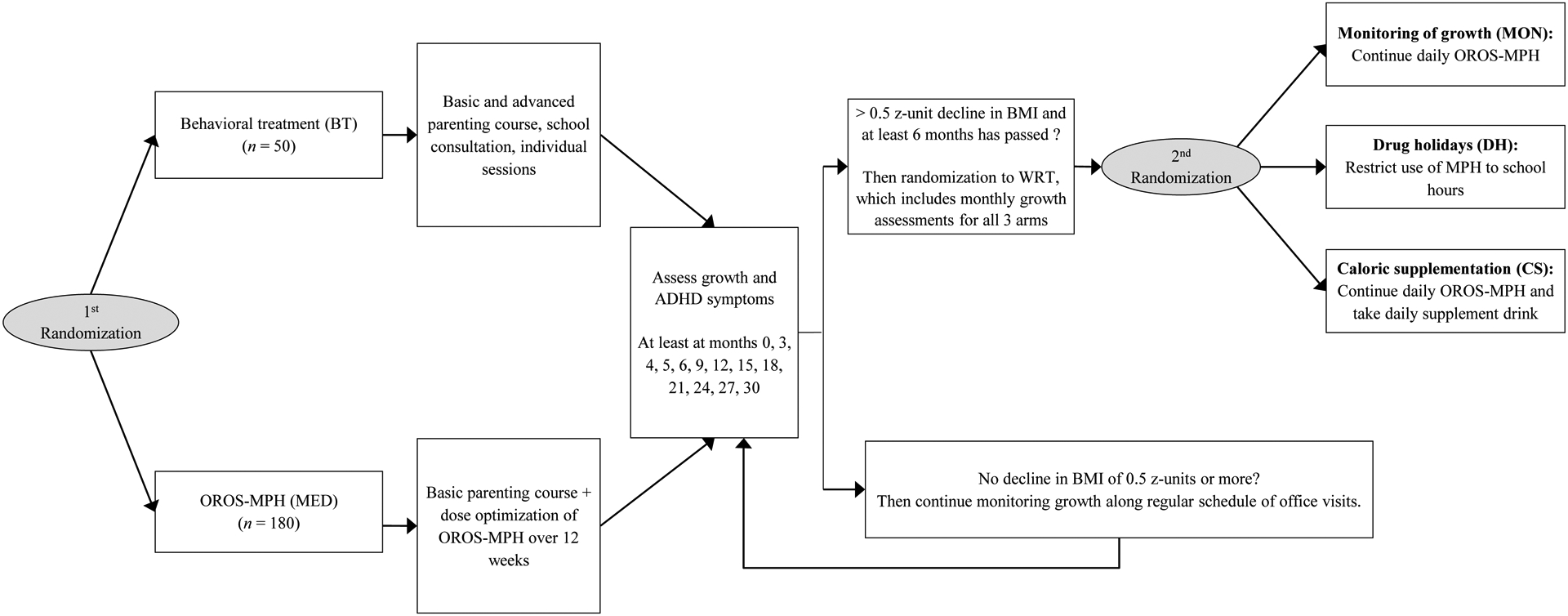
Note. BMI = body mass index, ADHD = Attention-Deficit/Hyperactivity Disorder, CNS + Central Nervous System, MPH = methylphenidate, kg = kilograms. Reprinted from “A Randomized Controlled Trial of Interventions for Growth Suppression in Children With Attention-Deficit/Hyperactivity Disorder Treated With Central Nervous System Stimulants,” by J.G. Waxmonsky, 2020, Journal of the American Academy of Child & Adolescent Psychiatry, 59, p. 1333. Copyright 2020 by the Elsevier. Reprinted with permission.
Height, weight, BMI, side effects and ADHD symptom ratings from parents were collected at every visit. For those starting medication at entry, assessments were completed at week 0, 2, 4, 6, 8, 10, 12, 16, 20, 24 and months 9, 12, 15, 18, 21, 24, 27, 30. The numbers of pills taken were recorded at each visit, and parents completed a monthly medication log. For those starting medication later, this assessment schedule began when medication was started (assessed every 3 months before that). After any six consecutive months on medication, participants with a >.5 z-unit decline in BMI were randomized to one of three WRTs.24 All participants in WRT had their stimulant dose capped and stayed in WRT until their BMI percentile returned to baseline or were cleared by study nutritionist to end WRT. During WRT, ratings were completed monthly. In this analysis, 69 (49%) participants met WRT criteria. For those never entering WRT, at least 18 assessment visits were completed over the 30 months of the study.
Predictor Variables
Table 1 lists examined predictors and Table 2 (available at www.jpeds.com) presents descriptive statistics. The only significant difference between the entire study population and the subset in this observational analysis was for marital status of the primary caregiver.
Table 1.
Descriptive Statistics for Variables
| Category | Variable | Proportion | Mean | SD | Range [Min, Max] | Percentage Missing |
|---|---|---|---|---|---|---|
| Independent variable | ||||||
| Female | 27 % | - | - | - | 0% | |
| Age in years at start of medication | 8.3 | 1.9 | [5.1, 15] | 1% | ||
| Started medication after AUXAL-projected age of slowest growth | 19 % | - | - | - | 1% | |
| Height (z) at start of medication | 0.04 | 0.96 | [−2.43, 3.04] | 2% | ||
| Weight (z) at start of medication | 0.34 | 0.89 | [−2.42, 2.60] | 1% | ||
| BMI (z) at start of medication | 0.46 | 0.85 | [−1.63, 2.24] | 2% | ||
| Midparental height in centimeters (measured or reported) | 172.9 | 7.6 | [153.1, 190.0] | 16% | ||
| Parent rating of hyperactivity/impulsivity symptoms of ADHD at study entry | 1.79 | 0.74 | [0, 3] | 6% | ||
| Parent rating of inattention symptoms of ADHD at study entry | 2.13 | 0.65 | [0.33, 3] | 6% | ||
| Parent rating of ODD symptoms at study entry | 0.98 | 0.69 | [0, 2.88] | 6% | ||
| Parent rating of loss of appetite at optimization was moderate/severe | 26 % | - | - | - | 6% | |
| Percent of days medicated in first 6 months after starting medication | 0.72 | 0.17 | [0.37, 1] | 0% | ||
| Percent of days medicated in first 12 months after starting medication | 0.68 | 0.17 | [0.28, 1] | 0% | ||
| Percent of days medicated from start of medication to last available visit | 0.63 | 0.19 | [0.14, 1] | 7% | ||
| Total MPH intake (kg) in first 6 months after starting medication | 2.82 | 1.08 | [0.18, 6.01] | 0% | ||
| Total MPH intake (kg) in first 12 months after starting medication | 5.54 | 2.41 | [0.48, 15.52] | 0% | ||
| Total MPH intake (kg) from start of medication to last available visit | 12.1 | 7.53 | [0, 44.68] | 0% | ||
| Dependent variable | ||||||
| Met criteria for Weight Recovery Treatment (WRT) | 49 % | - | - | - | 0 % | |
| Change in BMI (z) from start of medication to +6 months | −0.42 | 0.35 | [−1.70, 0.17] | 9 % | ||
| Change in BMI (z) from start of medication to +12 months | −0.36 | 0.31 | [−1.32, 0.28] | 13 % | ||
| Change in BMI (z) from start of medication to last available visit | −0.31 | 0.49 | [−1.78, 1.05] | 10 % | ||
| Change in weight (z) from start of medication to +6 months | −0.30 | 0.23 | [−0.96, 0.19] | 7 % | ||
| Change in weight (z) from start of medication to +12 months | −0.31 | 0.24 | [−0.92, 0.27] | 13 % | ||
| Change in weight (z) from start of medication to last available visit | −0.30 | 0.44 | [−1.58, 0.86] | 9 % | ||
| Change in height (z) from start of medication to +6 months | −0.05 | 0.12 | [−0.34, 0.33] | 9 % | ||
| Change in height (z) from start of medication to +12 months | −0.11 | 0.18 | [−0.66, 0.44] | 13 % | ||
| Change in height (z) from start of medication to last available visit | −0.16 | 0.39 | [−1.13, 1.10] | 10 % |
Note. SD = standard deviation, Min = minimum, Max = maximum, AUXAL = structural auxologic analysis model, BMI = body mass index, ADHD = Attention-Deficit/Hyperactivity Disorder, ODD = Oppositional Defiant Disorder, MPH = methylphenidate, kg = kilograms. Based on available data from N = 141 participants.
Table 2.
Characteristics at Study Entry of those Included versus. Excluded from Analyses
| Variable | Included in this sample (n = 141) | Excluded from this subsample (n = 88) |
|---|---|---|
| Child is female | 27 % | 26 % |
| Child age in years | 8.0 (1.9) | 8.1 (2.0) |
| Child is Hispanic | 72 % | 74 % |
| Child is Black | 10 % | 13 % |
| Primary caregiver is married* | 66 % | 52 % |
| Primary caregiver has BA | 53 % | 53 % |
| Number of ODD symptoms | 2.3 (2.2) | 2.2 (2.2) |
| Number of CD symptoms | 0.5 (0.9) | 0.4 (1.0) |
| Standardized height | 0.04 (0.98) | 0.12 (1.02) |
| Standardized weight | 0.31 (0.91) | 0.31 (0.88) |
| Standardized BMI | 0.43 (0.86) | 0.39 (0.82) |
Participants in the larger study were excluded from this analysis if they did not use medication for at least 6 months. Note. BA = Bachelor’s degree, ODD = Oppositional Defiant Disorder, CD = Conduct Disorder, BMI = body mass index. Number of ODD/CD symptoms is per parent report.
p<.05
Biometrics.
These included gender and standardized height, weight and BMI when starting medication. Height and weight were collected with using standardized protocol and calibrated stadiometer and scale.24 Structural auxologic analysis was used to estimate the age of minimum growth velocity for each child that marks the transition from the childhood to the adolescent growth phases. The large variation in the timing of the transition between growth phases is not accounted by z-scores and could confound associations between medication exposure and growth velocity in models assuming a uniform age of onset across participants.31 A binary predictor was created to indicate whether the child started medication before or after the estimated age of minimum growth. Of the 141 participants, 19% (27) had entered the adolescent growth phase before baseline and 53 (37.5%) more entered it during the study. Mid-parental height was calculated using measurements from all available biological parents. For biological fathers, 67/119 (56%) of collected heights were measured and 52 (44%) were estimated from parental report.
DSM symptoms.
Parents and teacher-rated symptoms of ADHD and oppositional defiant disorder (ODD) on the Disruptive Behavior Disorders Rating Scale prior to medication initiation were examined as predictor of weight, height and BMI trajectories.26 Predictors included the mean item response of items measuring impulsivity/hyperactivity, inattention, or ODD symptoms.
Side Effects:
Parents completed the Pittsburgh Side Effects Rating Scale (PSERS).32 It includes a specific item rating appetite suppression. PSERS was completed at every assessment, but only the first rating after dose optimization was examined as a predictor. Parent-rated “loss of appetite” was coded as being moderate or severe (versus none/mild) at this time point.
Medication.
Predictors were created measuring the percent of days medicated and (the cumulative amount of MPH consumed. Medication data was derived from the daily medication logs and expressed in milligrams of MPH equivalents using the conversion formulas in the MTA.33 Predictors were created separately for the first six months after starting medication and) the start of medication to end of follow-up (i.e., last available visit) to examine if different associations are seen as medication use becomes more chronic. The median interval between the date of first medication use and date of last medication use was 886 days (IQR 705-904). Frequency was defined as percent of days medication was used over the assessment period. Time intervals for medication use were chosen to match the time intervals used for growth measurements, as described below.
Dependent Variables
Entered WRT.
The first dependent variable was a binary indicator of whether the child ever entered WRT or not. To enter, participants must have lost > 0.5 z-units in BMI and been taking medication for at least 6 months. Participants who were above the 85th percentile for BMI at study entry had to lose > 1 z-unit of BMI and manifest raw weight loss to be assigned to WRT. Among the 141 participants, 69 (49%) participants entered WRT at any point during the study.
Change in z-BMI, z-weight, z-height across follow-up.
We computed variables estimating the change observed in the first 6 months after starting medication and from the start of medication to end of follow-up (i.e., last available visit). Each timeframe has its own advantages. The impact of CNS stimulants on weight has been reported to be maximal over the first 6 months of use and between 24–30 months after medication initiation for height velocity.13, 16 The protocol ensured that children did not receive any weight recovery treatments for the first 6 months of medication use. The timeframe from the start of medication to the end of follow-up was included to maximize the amount of data for each child, recognizing the associations may be more difficult to interpret given imperfect adherence to medication over time. For each timeframe, all measurements after the date of first medication use to the end of the assessment period were used, regardless of medication status at follow-up assessments.
For the 6-month window, we used height and weight measurements taken closest to the target duration, accepting those taken with ± 1.5 months of this target. A change score was computed by subtracting the height/weight/BMI measurement taken at medication initiation from the corresponding measurement taken approximately 6 months later, dividing that quantity by the number of days elapsed between the two measurements and multiplying by 180 to rescale back to change over 6 months. Table 1 reports descriptive statistics for these change scores.
Change in z-weight as a predictor of change in z-height.
To assess if initial weight changes are predictive of subsequent growth velocity, we included change in z-weight in the first 6 months of starting medication as a predictor of z-height change in the first 6 months after starting medication and from the start of medication to the end of follow-up (i.e., last available visit). We also included change in z-weight from medication start to the end of follow-up as a predictor of change in z-height over the entire follow-up period.
Analytic Plan
Missing data on predictors were rare except for midparental height (Table I). Data ranged from 87% to 100% complete for dependent variables. We estimated bivariate correlations between each predictor and each outcome in Mplus 8.34 To account for missing data, the model was estimated using full-information maximum likelihood and the biometric predictors listed in Table 1 were included as auxiliary variables.35, 36 As a sensitivity analysis, we re-estimated associations of predictors with WRT entry, excluding those with BMI > 85th percentile at baseline, as they had a different criterion for entry to WRT (n = 39 of 141). We also repeated the analyses of baseline biometrics predicting change in weight, height and BMI month 0 to 6 using percentage change in raw values over this timeframe versus change in z scores as even standardized units can become skewed at extreme range.37 Finally, we report results for the first 12-months after starting medication to produce estimates of annualized change in growth rates as these may allow for comparison with assessment periods employed in past reports17 and be a more readily interpretable metric for practicing clinicians. Procedures were identical to that used for the 6-month window.
Results
The mean dose at dose optimization was 22mg (6.1) MPH equivalents with a range of 10mg to 36mg per day. At last visit, mean dose for those using medication was 26.2mg (8.5) with range of 10 to 59mg. Of the 141 in this analysis, there were 131 (93%) participants with at least 12 months of medication use and 101 (71.6%) with at least 24 months of use. There were 118 (83.7%) that completed the final 30 month assessment regardless of medication status at endpoint. No child discontinued medication over growth concerns, but one child had medication stopped due to weight loss after assignment to drug holiday and caloric supplementation was not sufficient.
Table 3 reports the correlations of each predictor with each dependent variable. Only statistically significant associations are described below. Figure 2 shows the probability of entering WRT as a function of MPH exposure. Figure 3 shows how the correlation of changes in z-BMI, z-weight, and z-height with percent of days medicated and total exposure to MPH evolve over time.
Table 3.
Correlates of Entering Weight Recovery Treatment and Changes in z-BMI, z-Weight, and z-Height After Starting Methylphenidate
| Predictor | Entered Weight Recovery Treatment (WRT) | Δ z-BMI from 0 to 6 months | Δ z-BMI from 0 months to end of follow-up | Δ z-weight from 0 to 6 months | Δ z-weight from 0 months to end of follow-up | Δ z-height from 0 to 6 months | Δ z-height from 0 months to end of follow-up | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation (SE) | Sig. | Correlation (SE) | Sig. | Correlation (SE) | Sig. | Correlation (SE) | Sig. | Correlation (SE) | Sig. | Correlation (SE) | Sig. | Correlation (SE) | Sig. | |
| Biometrics | ||||||||||||||
| Female | +0.04 (0.08) | −0.02 (0.09) | −0.03 (0.09) | −0.04 (0.09) | −0.01 (0.09) | +0.16 (0.08) | +0.17 (0.09) | * | ||||||
| Age in years at start of medication | −0.13 (0.08) | +0.09 (0.09) | +0.20 (0.09) | * | +0.12 (0.09) | +0.19 (0.09) | * | +0.30 (0.08) | *** | +0.16 (0.09) | ||||
| Started medication after AUXAL-projected age of slowest growth | −0.09 (0.08) | +0.04 (0.09) | +0.12 (0.09) | +0.08 (0.09) | +0.18 (0.08) | * | +0.28 (0.08) | ** | +0.14 (0.09) | |||||
| Height (z) at start of medication | −0.20 (0.08) | * | +0.19 (0.08) | * | +0.14 (0.09) | +0.24 (0.08) | ** | +0.10 (0.09) | −0.07 (0.09) | −0.13 (0.09) | ||||
| Weight (z) at start of medication | −0.44 (0.07) | *** | +0.28 (0.08) | ** | +0.07 (0.09) | +0.28 (0.08) | *** | +0.05 (0.09) | +0.08 (0.09) | +0.00 (0.09) | ||||
| BMI (z) at start of medication | −0.45 (0.07) | *** | +0.20 (0.08) | * | −0.01 (0.09) | +0.16 (0.08) | −0.01 (0.09) | +0.15 (0.09) | +0.05 (0.09) | |||||
| Midparental height in centimeters (measured or reported) | −0.09 (0.09) | +0.06 (0.09) | +0.10 (0.09) | +0.11 (0.09) | +0.11 (0.09) | −0.10 (0.09) | −0.07 (0.09) | |||||||
| Biometric deltas | ||||||||||||||
| Change in weight (z) from start of medication to +6 months | - | - | - | - | - | +0.14 (0.09) | +0.20 (0.09) | * | ||||||
| Change in weight (z) from start of medication to last available visit | - | - | - | - | - | - | +0.42 (0.07) | *** | ||||||
| Symptoms | ||||||||||||||
| Parent rating of hyperactivity/impulsivity symptoms of ADHD at study entry | −0.08 (0.09) | −0.05 (0.09) | −0.06 (0.09) | −0.04 (0.09) | −0.05 (0.09) | −0.01 (0.09) | −0.08 (0.09) | |||||||
| Parent rating of inattention symptoms of ADHD at study entry | +0.06 (0.09) | +0.06 (0.09) | −0.09 (0.09) | +0.08 (0.09) | −0.07 (0.09) | +0.02 (0.09) | +0.02 (0.09) | |||||||
| Parent rating of ODD symptoms at study entry | −0.15 (0.08) | +0.03 (0.09) | −0.09 (0.09) | +0.03 (0.09) | −0.12 (0.09) | −0.07 (0.09) | −0.12 (0.09) | |||||||
| Medication | ||||||||||||||
| Parent rating of loss of appetite at optimization was moderate/severe | +0.10 (0.09) | −0.14 (0.09) | −0.14 (0.09) | −0.20 (0.09) | * | −0.19 (0.09) | * | −0.28 (0.08) | ** | −0.16 (0.09) | ||||
| Percent of days medicated in first 6 months after starting medication | +0.37 (0.07) | *** | −0.36 (0.07) | *** | - | −0.38 (0.07) | *** | - | −0.12 (0.09) | - | ||||
| Percent of days medicated from start of medication to last available visit | +0.32 (0.08) | *** | - | −0.38 (0.08) | *** | - | −0.40 (0.07) | *** | - | −0.26 (0.08) | ** | |||
| Total MPH intake (kg) in first 6 months after starting medication | +0.33 (0.07) | *** | −0.36 (0.08) | *** | - | −0.34 (0.08) | *** | - | +0.05 (0.09) | - | ||||
| Total MPH intake (kg) from start of medication to last available visit | +0.28 (0.08) | *** | - | −0.23 (0.09) | * | - | −0.30 (0.09) | *** | - | −0.32 (0.08) | *** | |||
Note. SE = standard error, AUXAL = structural auxologic analysis model, BMI = body mass index, ADHD = Attention-Deficit/Hyperactivity Disorder, ODD = Oppositional Defiant Disorder, MPH = methylphenidate, kg = kilograms.
Correlations are Pearson correlations. “Sig.” indicates statistical significance of correlation. All models were estimated using full-information maximum likelihood and included the 141 children who ever took medication. Auxiliary variables were female, age in years at study entry, entered study after AUXAL-projected age of slowest growth, z-height at study entry, z-weight at study entry, and z-BMI at study entry.
p < .05,
p < .01,
p < .001
Figure 2. Association Between Cumulative MPH Intake in First Six Months and Probability of Qualifying for Weight Recovery Treatment.

Note. MPH=methylphenidate. Line is model-estimated probability of entering weight recovery treatment (WRT) (from univariate logistic regression). Grey ribbon indicates 95% confidence interval. Dots are the observed proportion of children (i.e., empirical probability) in each quartile of cumulative dose that met WRT criteria (position along x-axis indicates mean dose within quintile). Data are from children ever taking medication (n = 141).
Figure 3. Association Between Medication History Parameters and Changes in z-BMI, z-Weight, and z-Height Across Follow-Up.

Note. BMI = body mass index, MPH = methylphenidate. Shows how the correlations between the medication use and change in body measurements evolve across time. Medication usage is calculated over the same interval as change in body measurements (e.g., leftmost red square in the left panel indicates correlation of change in z-BMI in the first 6 months of medication with the percent days medicated in first 6 months after starting medication). Percent days medicated and cumulative intake exhibit large correlations with changes in z-BMI and z-weight that are present at 6 months and persist through the end of follow-up. In contrast, the correlation of percent days medicated and cumulative intake with changes in z-height remains small at 6 months before growing over the follow up period.
Correlates of Entry to Weight Recovery Treatment
Children with greater z-height (r = −0.20), z-weight (r = −0.44), or z-BMI (r = −0.45) at medication start were less likely to enter WRT (p’s < .05). Children medicated on more days in the first 6 months of use (r = 0.37) or over the entire follow-up period (r = 0.32) were more likely to enter WRT (p’s <.001). Children using more MPH during the first 6 months (r = 0.33; Figure 2) or during the length of follow-up (r = 0.28) were more likely to enter WRT (p’s < .001).
Correlates of Changes in z-BMI
First 6 months after starting medication.
Children with greater z-height (r = 0.19), z-weight (r = 0.28), or z-BMI (r = 0.20) when starting medication exhibited less reduction in z-BMI. Children medicated on a greater percentage of days (r = −0.36) or using more total milligrams (r = −0.36) in the first 6 months on medication exhibited greater reduction in z-BMI (p’s < .05).
Start of medication to end of follow-up.
Older children exhibited less reductions in z-BMI (r = 0.20, p < .05). Children who were medicated on a greater percentage of days (r = −0.38) or used more total milligrams (r = −0.23) exhibited greater reduction in z-BMI (p’s < .05).
Correlates of Changes in z-Weight
First 6 months after starting medication.
Children with greater z-height (r = 0.24) or z-weight (r = 0.28) when starting medication exhibited less reduction in z-weight. Children whose parents rated them as experiencing moderate or severe loss of appetite during dose optimization exhibited greater reductions in z-weight (r = −0.20, P < .05). Children medicated on a greater percentage of days (r =−0.38) or using more milligrams (r = −0.34) in the first 6 months of medication use exhibited greater reduction in z-weight (p’s < .05).
Start of medication to end of follow-up.
Children who were older (r = 0.19, p < .05) or started medication after the projected age of slowest growth exhibited less reductions in z-weight (r = 0.18, p < .05). Children whose parents rated them as experiencing moderate or severe appetite loss during dose optimization exhibited greater reductions in z-weight (r = −0.19, p < .05). Children medicated on a greater percentage of days (r =−0.40) or using more milligrams (r = −0.30) exhibited greater reduction in z-weight (p’s < .001).
Correlates of Changes in z-Height
First 6 months after starting medication.
Older children (r = 0.30, p < .05) or those past the projected age of slowest growth exhibited less z-height reductions (r = 0.28, p < .05). Children experiencing moderate/severe appetite loss exhibited more decline in z-height (r = −0.28, p < .01).
Start of medication to end of follow-up.
Females exhibited less reductions in z-height (r = 0.17, p < .05). Children who were medicated on a greater percentage of days (r =−0.26) or used more milligrams (r = −0.32) over the study exhibited greater z-height reductions (p’s <.05).
Change in z-weight as predictor.
Children who exhibited less reductions in z-weight over the first 6 months of medication use exhibited less reductions in z-height over the entire follow-up period (r = 0.20, p < .05). Children who exhibited less reductions in z-weight over the full duration of follow-up exhibited less reductions in z-height over the same interval (r = 0.42, p < .001).
Sensitivity Analyses
Excluding those with entry BMIs ≥85th percentile who had modified WRT criteria produced small changes in the magnitude of predictors’ correlations (Table 4 and Figure 4; both available at www.jpeds.com). There was only one appreciable change: the correlation of BMI z-score prior to medication with BMI change over 6 months (r=−0.2, p<.05 vs r= 0.11, ns) was no longer significant.
Table 4.
Correlates of Entering Weight Recovery Treatment and Changes in z-BMI, z-Weight, and z-Height in the First 12 Months After Starting Methylphenidate
| Predictor | Δ z-BMI from 0 to 12 months | Δ z-weight from 0 to 12 months | Δ z-height from 0 to 12 months | |||
|---|---|---|---|---|---|---|
| Correlation (SE) | Sig. | Correlation (SE) | Sig. | Correlation (SE) | Sig. | |
| Biometrics | ||||||
| Female | −0.06 (0.09) | −0.09 (0.09) | +0.12 (0.09) | |||
| Age in years at start of medication | +0.22 (0.09) | * | +0.23 (0.09) | * | +0.25 (0.09) | ** |
| Started medication after AUXAL-projected age of slowest growth | +0.13 (0.09) | +0.18 (0.09) | * | +0.23 (0.08) | ** | |
| Height (z) at start of medication | +0.06 (0.09) | +0.12 (0.09) | −0.06 (0.09) | |||
| Weight (z) at start of medication | +0.01 (0.09) | +0.04 (0.09) | +0.08 (0.09) | |||
| BMI (z) at start of medication | −0.04 (0.09) | −0.05 (0.09) | +0.12 (0.09) | |||
| Midparental height in centimeters (measured or reported) | +0.06 (0.09) | +0.12 (0.09) | −0.04 (0.09) | |||
| Biometric deltas | ||||||
| Change in weight (z) from start of medication to +6 months | - | - | +0.28 (0.08) | ** | ||
| Change in weight (z) from start of medication to +12 months | - | - | +0.38 (0.08) | *** | ||
| Symptoms | ||||||
| Parent rating of hyperactivity/impulsivity symptoms of ADHD at study entry | −0.14 (0.09) | −0.15 (0.09) | −0.07 (0.09) | |||
| Parent rating of inattention symptoms of ADHD at study entry | +0.05 (0.09) | +0.04 (0.09) | −0.01 (0.09) | |||
| Parent rating of ODD symptoms at study entry | +0.07 (0.09) | +0.03 (0.09) | −0.10 (0.09) | |||
| Medication | ||||||
| Parent rating of loss of appetite at optimization was moderate/severe | −0.02 (0.09) | −0.10 (0.09) | −0.21 (0.09) | * | ||
| Percent of days medicated in first 12 months after starting medication | −0.37 (0.08) | *** | −0.37 (0.08) | *** | −0.24 (0.09) | ** |
| Total MPH intake (kg) in first 12 months after starting medication | −0.35 (0.08) | *** | −0.35 (0.08) | *** | −0.18 (0.09) | * |
Note. SE = standard error, AUXAL = structural auxologic analysis model, BMI = body mass index, ADHD = Attention-Deficit/Hyperactivity Disorder, ODD = Oppositional Defiant Disorder, MPH = methylphenidate, kg = kilograms.
Correlations are Pearson correlations. “Sig.” indicates statistical significance of correlation. All models were estimated using full-information maximum likelihood and included the 141 children who ever took medication. Auxiliary variables were female, age in years at study entry, entered study after AUXAL-projected age of slowest growth, z-height at study entry, z-weight at study entry, and z-BMI at study entry.
p < .05,
p < .01,
p < .001
Figure 4. Sensitivity Analysis 1: Excluding Children Above the 85th Percentile in BMI at Study Entry.

Note. AUXAL = structural auxologic analysis model, BMI = body mass index, ADHD = Attention-Deficit/Hyperactivity Disorder, ODD = Oppositional Defiant Disorder, MPH = methylphenidate, kg = kilograms. Compares correlations based on all children who took medication (black circles) with correlations based on only those with entry BMI below the 85th percentile (white circles). Pairs of correlations with a box around them differed in statistical significance (i.e., one above and one below a threshold of p < .05). As shown, excluding those with entry BMI above the 85th percentile generally had little impact on the magnitude or statistical significance of observed correlations.
When the percentage change in raw height/weight/BMI were used as outcomes versus standardized (z) scores, z-weight score when starting medication was significantly correlated with the 6-month change in height. Children of lower standardized weight at medication start experienced smaller height increases than heavier children at medication start (r=0.21, p<05). BMI at medication start predicted change in height over 6 months (r=0.18, p<.05).
Over 12 months of medication use (Table 4), percent days medicated (r’s =−0.24 to −0.38, p’s<.001) and total mg of medication (r’s=−0.18 to −0.35, p’s <.05) predicted change in z-BMI, z-weight and z-height. Age predicted all three change scores (r’s=0.22–0.25, p<.05). Starting medication after the age of minimal growth velocity predicted change in z-weight (r=0.18, p<.05) and z-height (r=0.23, p<.05). Children with less reductions in z-weight over the first 6 months of medication exhibited less z-height change over the first 12 months of use (r=.28, p<.01). Parent ratings of appetite at dose optimization predicted change in z-height (r=−0.21, p<.05).
Discussion
The aim of this analysis was to identify predictors of height, weight, and BMI change in medication naïve children with ADHD initiating CNS stimulants. Total MPH exposure was robustly correlated with changes in z-weight and BMI for each assessment period, for height over the study’s duration and for entry to weight recovery treatments. Older participants and those starting medication after the projected onset of the adolescent growth phase experienced less weight suppression over the study’s duration and less height suppression during the first 6 months on medication. Females experienced less height suppression than males. There was no evidence that children of short stature or low weight at medication initiation were at reduced risk for decelerations in weight or height velocity. Children with smaller standardized weights or heights at medication initiation experienced greater reductions in z-weight and BMI over the first 6 months of medication use. Parent ratings of appetite suppression proved to be useful indices of changes in weight and height velocity, but ADHD or ODD symptom severity did not predict changes in either.
Similar to prior work, there were robust associations with medication exposure and change in standardized weight and BMI.22, 38, 39 Associations emerged by month 6 and persisted for the study’s duration. Findings were not driven by overweight youth declining to healthier weights. As weight deficits early in care may lead to stopping treatment,20, 21 stabilizing weight may improve treatment adherence, which is often poor.40, 41 Although weight deficits may persist in childhood when medication is continued, a different trajectory emerges in adolescence. In the MTA, BMI declined when medication was started (mean entry age was 8.4). After year two, BMI increased in ADHD participants using medication, surpassing levels in non-ADHD controls.15 Other studies have reported similar patterns,42 and a meta-analysis reported strong links between childhood ADHD and higher BMI into adulthood.43 Given the appreciable morbidity risks with obesity,44, 45 it seems prudent to limit efforts to increase weight in children prescribed CNS stimulants to those with medically concerning weight loss or with suppressed weight velocities into adolescence.
In the MTA33 and here, frequency of medication use predicted growth velocity despite the large differences in medication exposure across the studies, likely due to the 16 year assessment period for the MTA versus 30 months for this study. Over 30-months in this study, youth assigned to WRT grew 1.4cm less than expected and 1.7cm less than ADHD youth not using medication.24 No evidence was found that drug holidays on non-school days increased growth velocity over a two year period, suggesting that medication cessation may be necessary to see meaningful growth accelerations over this time period. In the MTA, initiating medication at an early age and continuing it through adolescence had the greatest impact on height, with no evidence of meaningful catch-up growth while medication was used. Consistent users were 4.1cm shorter than negligible users and 3.3cm shorter than non-ADHD controls.15 Neither study could assess the impact of dosing schedules on growth beyond these comparisons. Behavioral therapies delay the onset of ADHD medication use46 and reduce the mean dose of medication needed.47 Therefore, behavioral therapies may be an effective means to preserve growth by reducing medication exposure, especially during young ages when medication impacts on growth may be greater.48
Most prior work assessing medication effects on weight and height has focused on dose measured as mg/kg/day and found mixed results.13, 17, 39, 49 In this protocol, all stimulant medication was dispensed though the study, enabling more precise estimates of actual medication exposure. Correlations with height, weight and BMI change were comparable when frequency of use or total medication exposure was the predictor. These results suggest that how often medication is used may be at least as impactful as the daily dose.
With their extended therapeutic duration, ER CNS stimulants give parents more opportunity to observe medication effects on appetite.50 Parent ratings after dose was optimized predicted weight change at month 6 with a trend for weight change at the last assessment. The association with height change was unexpected but is consistent with the theory that negative caloric balance contributes to the growth suppression with CNS stimulants.17, 39 This theory is further supported by significant correlations observed between changes in weight and height. Weight change over the study was the most robust predictor of height change. However, weight restoration did not lead to increased height velocity in this sample24 or in the MTA.15 Likewise, in adolescents with anorexia nervosa, weight restoration does not translate to improved height velocity.51 It appears that although appetite and weight loss may be predictive of a slowing in growth velocity, increasing weight is an insufficient means to accelerate growth.
We employed a brief public domain measure completed by parents to measure appetite loss and other side effects of CNS stimulants.32 Structured side effect ratings during dose optimization may be an inexpensive means to identify children at risk for concerning weight loss and even growth suppression. Parent ratings of appetite loss during early medication initiation could be used to identify children who should be preferentially targeted for early integration of behavioral therapies or other strategies to preserve growth. These ratings may be particularly valuable when direct assessment is challenging, such as during the current pandemic.52, 53
Prior reviews observed the greatest declines in height and weight velocity in the tallest and heaviest youth at medication initiation, suggesting that children of low weight or small stature may be at reduced risk.17 However, many studies enrolled chronically medicated youth. The greatest declines in growth velocity occur during the first 1–2 years of medication use,15, 54 with rates stabilizing but not recovering from year 3 onward. Mixing treatment naïve and treated children in one sample may blunt the degree of observed growth suppression and make it appear that shorter and lighter children are protected from growth suppression when they actually acquired it prior to entry.55 Our study eliminated the confound of prior medication status which may be why we failed to find a protective effect of short stature or low weight. We found that lighter and shorter youth were at increased risk for reductions in z-weight and z-BMI and were more likely to need WRT interventions. To be consistent with past work,17 we also measured change in height, weight and BMI using raw units. Results for change in weight were comparable, but low entry weight and BMI now predicted smaller height gains, which was not seen when z-units were the outcome. It is reassuring that studies of children with ADHD and short stature have not found diminished effect of growth hormone on height velocity when CNS stimulants are also used.56, 57 However, the results observed here suggest that children of small stature or low weight should be cautiously prescribed CNS stimulants and have their weight and height velocity routinely monitored. Nonstimulant options or behavioral treatments offer a potentially more tolerable initial treatment options for children already struggling to reach a medically appropriate weight or height.58–60
As expected, older age and being in the adolescent growth phase when starting medication were associated with larger gains in weight and height. This was likely because these participants would have experienced a period of faster growth during the assessment period. When measured using standardized or raw height, females experienced less declines in standardized height. They were more likely than males to be in the adolescent growth phase during the study, probably due to gender effects on the timing of the adolescent growth spurt.31 The majority of participants in this study and in other growth assessments of ADHD samples were male.13 Therefore, results should be applied with caution to females, especially in samples that are still growing, given the impact of gender on the timing of the adolescent growth spurt. For example, weight is more strongly correlated with pubertal onset in females than males,61 so correlations of weight and height changes may vary by gender. We examined whether symptom severity for ADHD/ODD would be predictive of change in weight or growth as it has been proposed that the two could be correlated.16, 49, 62 Symptom severity is an easily and frequently assessed metric in primary care, but we observed no consistent correlations with any symptom dimensions and auxologic outcomes.
After removal of participants with a BMI% >85th at study entry, all medication findings remained significant despite the reduced sample size. Several of the associations between biometric growth predictors and auxologic outcomes weakened. However, the weakening was minimal except for the correlation between premedication z-BMI and change in BMI over the first 6 months of medication use. Elevated BMI is more likely as children age and correlates with earlier pubertal onset, which may explain why excluding children between the 85th and 95th BMI % preferentially impacted correlations with BMI change.63
The primary limitation of this observational analysis is that participants were not protected by randomization as we included all participants regularly using medication over any 6-month period during the study regardless of their initial randomized assignments to medication or non-medication arms. Therefore, causal relationships between predictors and outcomes should not be assumed as it is possible the observed associations with medication and biometric outcomes could be due to other factors. Although 30 months is an extended duration for an ADHD treatment study and longer than many prospective ADHD trials assessing growth,13, 14, 17 it did not track participants long enough to assess the impact of the adolescent growth spurt and to see if height deficits persisted into adulthood. The MTA did track into adulthood, observing that acquired deficits persist as long as medication is consistently used.15 this study examined the impact of efforts to promote weight recovery on the change in growth and weight velocity in children prescribed CNS stimulants. Results may differ from routine clinical care where dose reductions may be more likely to occur before weight recovery efforts. Lastly, this study was run at a single research site, which may limit its generalizability.
In treatment naïve youth with ADHD change in weight was a significant predictor of both current and future growth velocity, and parent ratings of appetite suppression predicted weight change and initial height velocity. Adjusting the frequency of medication use or its dose may reduce the degree of acquired suppression in weight and height. In youth where height or weight are a preexisting concern, the efficacy and tolerability of CNS stimulant medication should be closely monitored after medication initiation to ensure a favorable risk to benefit ratio for continuing versus switching to alternative treatments.
Supplementary Material
Acknowledgments
Funded by NIMH (R01MH083692). The authors also received support from the National Institute on Drug Abuse (NIDA; T32 DA039772 [to WEP Jr]; R01DA034731, MH101096 [to W.E.P. Jr), the National Institute on Alcohol Abuse and Alcoholism (NIAAA; F31 AA026768 [to W.P., III]), the NIMH (MH80791 [to J.W.]; MH099030 [to WEP Jr]), the Institute of Education Sciences (IES; R324A180175 [to WEP Jr], R305A170523) and Shire Pharmaceuticals (to J.W.). Some study medication was donated by Janssen Pharmaceuticals.
They had no role in the design, analysis, interpretation or publication of this study. J.W. has received research funding from Supernus and Pfizer and served as a consultant for Adlon Therapeutics and Intracellular Therapies. W.E.P. has received funding from NIMH, NIAAA, NIDA, and IES. The other authors declare no conflicts of interest.
Abbreviations
- ADHD
Attention Deficit Hyperactivity Disorder
- CNS
Central Nervous System
- BMI
Body Mass Index
- WRT
Weight Recovery Treatment
- MG
Milligrams
- MTA
Multimodal Treatment of ADHD Study
- CM
centimeters
- KG
kilograms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this study were presented at the American Academy of Child and Adolescent Psychiatry conference, ≪ ≫, 2020, ≪ ≫.
Data sharing: Deidentified data will be made available that underlie results in this article from 6 to 36 months after publication for individual participant data meta-analysis for investigators who provide a methodologically sound proposal by emailing jwaxmonsky@pennstatehealth.psu.edu.
References
- 1.Pliszka S, AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2007;46:894–921. [DOI] [PubMed] [Google Scholar]
- 2.Hales CM, Kit BK, Gu Q, Ogden CL. Trends in Prescription Medication Use Among Children and Adolescents-United States, 1999–2014. JAMA 2018;319:2009–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics 2012;130:23–31. [DOI] [PubMed] [Google Scholar]
- 4.Wolraich ML, Hagan JF, Allan C, Chan E, Davison D, Earls M et al. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics 2019;144: e20192528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen DL, Hansen EH. Caught in a balancing act: parents’ dilemmas regarding their ADHD child’s treatment with stimulant medication. Qual Health Res 2006;16:1267–85. [DOI] [PubMed] [Google Scholar]
- 6.Rockhill CM. Editorial: A Spoonful of Injury Prevention Makes the ADHD Medicine Go Down. J Am Acad Child Adolesc Psychiatry 2020;59:920–922. [DOI] [PubMed] [Google Scholar]
- 7.Brinkman WB, Sucharew H, Majcher JH, Epstein JN. Predictors of Medication Continuity in Children With ADHD. Pediatrics 2018;14:e20172580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinkman WB, Simon JO, Epstein JN. Reasons Why Children and Adolescents With Attention-Deficit/Hyperactivity Disorder Stop and Restart Taking Medicine. Acad Pediatr. Apr 2018;18(3):273–280. doi: 10.1016/j.acap.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, Dittmann RW et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry 2013;54:227–46. [DOI] [PubMed] [Google Scholar]
- 10.Greenhill LL, Pliszka S, Dulcan MK. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41:26S–49S. [DOI] [PubMed] [Google Scholar]
- 11.National Instittute for Health and Care Excellence (NICE). ADHD: Diagnosis and Management (NG87). Accessed August 6, 2021. nice.org.uk/guidance/ng87 [PubMed]
- 12.Frank E, Ozon C, Nair V, Othee K. Examining why patients with attention-deficit/hyperactivity disorder lack adherence to medication over the long term: a review and analysis. J Clin Psychiatry 2015;76:e1459–68. [DOI] [PubMed] [Google Scholar]
- 13.Carucci S, Balia C, Gagliano A, Lampis A, Buitelaar JK, Danckaerts M et al. Long term methylphenidate exposure and growth in children and adolescents with ADHD. A systematic review and meta-analysis. Neurosci Biobehav Rev 2020;120: 509–525 [DOI] [PubMed] [Google Scholar]
- 14.Baweja R, Hale DE, Waxmonsky JG. Impact of CNS Stimulants for Attention-Deficit/Hyperactivity Disorder on Growth: Epidemiology and Approaches to Management in Children and Adolescents. CNS Drugs 2021. doi: 10.1007/s40263-021-00841-w [DOI] [PubMed] [Google Scholar]
- 15.Greenhill LL, Swanson JM, Hechtman L, Waxmonsky J, Arnold LE, Molina BS et al. Trajectories of Growth Associated With Long-Term Stimulant Medication in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry 2020;59:978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry 2007;46:1015–27. [DOI] [PubMed] [Google Scholar]
- 17.Faraone SV, Biederman J, Morley CP, Spencer TJ. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry 2008;47:994–1009. [DOI] [PubMed] [Google Scholar]
- 18.Poulton A Growth on stimulant medication; clarifying the confusion: a review. Arch Dis Child 2005;90:801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer T, Biederman J, Wilens T. Growth deficits in children with attention deficit hyperactivity disorder. Pediatrics 1998;102:501–6. [PubMed] [Google Scholar]
- 20.Faraone SV, Giefer EE. Long-term effects of methylphenidate transdermal delivery system treatment of ADHD on growth. J Am Acad Child Adolesc Psychiatry 2007;46:1138–1147. [DOI] [PubMed] [Google Scholar]
- 21.Faraone SV, Biederman J, Monuteaux M, Spencer T. Long-term effects of extended-release mixed amphetamine salts treatment of attention- deficit/hyperactivity disorder on growth. J Child Adolesc Psychopharmacol 2005;15:191–202. [DOI] [PubMed] [Google Scholar]
- 22.Landgren M, Nasic S, Johnson M, Lövoll T, Holmgren D, Fernell E. Blood pressure and anthropometry in children treated with stimulants: a longitudinal cohort study with an individual approach. Neuropsychiatr Dis Treat 2017;13:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haymond M, Kappelgaard AM, Czernichow P, Biller BM, Takano K, Kiess W. Early recognition of growth abnormalities permitting early intervention. Acta Paediatr 2013;102:787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waxmonsky JG, Pelham WE 3rd, Campa A, Waschbusch DA, Li T, Marshall R et al. A Randomized Controlled Trial of Interventions for Growth Suppression in Children With Attention-Deficit/Hyperactivity Disorder Treated With Central Nervous System Stimulants. J Am Acad Child Adolesc Psychiatry 2020;59:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelham W Disruptive Behavior Disorders Interview: Comprehensive Treatment of ADHD. . Buffalo, NY: 1998. [Google Scholar]
- 26.Pelham WE Jr., Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry 1992;31:210–8. [DOI] [PubMed] [Google Scholar]
- 27.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 2000;39:28–38. [DOI] [PubMed] [Google Scholar]
- 28.Fabiano GA, Pelham JWE, Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM et al. A practical measure of impairment: Psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples Journal of Clinical Child and Adolescent Psychology 2006;35:369–385. [DOI] [PubMed] [Google Scholar]
- 29.Loney J, Milich R. Hyperactivity, inattention, and aggression in clinical practice. vol 3. Advances in behavioral pediatrics JAI Press; 1982. [Google Scholar]
- 30.Guy W ECDEU Assessment manual for psychopharmacology. Washington DC: US Dept of Health, Education and Welfare; 1976. [Google Scholar]
- 31.Hermanusson M Auxology: An Update. Hormone Research in Pediatrics 2010;74:153–164. [DOI] [PubMed] [Google Scholar]
- 32.Pelham W Pharmacotherapy for children with ADHD. School Psych Rev 1993;22:199–227. [Google Scholar]
- 33.Swanson JM, Arnold LE, Molina BS, Sibley MH, Hechtman LT, Hinshaw SP et al. Young adult outcomes in the follow-up of the multimodal treatment study of attention-deficit/hyperactivity disorder: symptom persistence, source discrepancy, and height suppression. J Child Psychol Psychiatry 2017;58:663–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthen B, Muthen L, Asparouhov T. Regression and mediation analysis with MPLUS. Muthen & Muthen; 2016. [Google Scholar]
- 35.Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol 2009;60:549–76. [DOI] [PubMed] [Google Scholar]
- 36.Graham J Addingmissing-data-relevant variables to FIML-based structural equation models. Structural Equation Modeling 2003;10:80–100. [Google Scholar]
- 37.Chinchilli VM, McEnery PT, Chan JC. Statistical methods and determination of sample size in the Growth Failure in Children with Renal Diseases Study. J Pediatr 1990;116:S32–6. [DOI] [PubMed] [Google Scholar]
- 38.Powell SG, Frydenberg M, Thomsen PH. The effects of long-term medication on growth in children and adolescents with ADHD: an observational study of a large cohort of real-life patients. Child Adolesc Psychiatry Ment Health 2015;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poulton AS, Bui Q, Melzer E, Evans R. Stimulant medication effects on growth and bone age in children with attention-deficit/hyperactivity disorder: a prospective cohort study. Int Clin Psychopharmacol 2016;31:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charach A, Fernandez R. Enhancing ADHD medication adherence: challenges and opportunities. Curr Psychiatry Rep 2013;15:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gajria K, Lu M, Sikirica V, et al. Adherence, persistence, and medication discontinuation in patients with attention-deficit/hyperactivity disorder - a systematic literature review. Neuropsychiatr Dis Treat 2014;10:1543–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz BS, Bailey-Davis L, Bandeen-Roche K, Pollak J, Hirsch AG, Nau C et al. Attention deficit disorder, stimulant use, and childhood body mass index trajectory. Pediatrics 2014;133(4):668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akingbuwa WA, Hammerschlag AR, Jami ES, Allegrini AG, Karhunen V, Sallis H Genetic Associations Between Childhood Psychopathology and Adult Depression and Associated Traits in 42 998 Individuals: A Meta-analysis. JAMA Psychiatry 2020;77:715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maffeis C, Tatò L. Long-term effects of childhood obesity on morbidity and mortality. Horm Res 2001;55:42–5. [DOI] [PubMed] [Google Scholar]
- 45.Park MH, Falconer C, Viner RM, Kinra S. The impact of childhood obesity on morbidity and mortality in adulthood: a systematic review. Obes Rev 2012;13:985–1000. [DOI] [PubMed] [Google Scholar]
- 46.Coles EK, Pelham WE, Fabiano GA, Gnagy EM, Burrows-MacLean L, Wymbs BT et al. Randomized Trial of First-Line Behavioral Intervention to Reduce Need for Medication in Children with ADHD. J Clin Child Adolesc Psychol 2020;49(5):673–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelham WE, Burrows-MacLean L, Gnagy EM, Fabiano GA, Coles EK, Wymbs BT et al. A dose-ranging study of behavioral and pharmacological treatment in social settings for children with ADHD. J Abnorm Child Psychol 2014;42:1019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swanson J, Greenhill L, Wigal T, Kollins S, Stehli A, Davies M et al. Stimulant-related reductions of growth rates in the PATS. J Am Acad Child Adolesc Psychiatry 2006;45:1304–1313. [DOI] [PubMed] [Google Scholar]
- 49.Poulton AS, Melzer E, Tait PR, Garnett SP, Cowell CT, Baur LA et al. Growth and pubertal development of adolescent boys on stimulant medication for attention deficit hyperactivity disorder. Med J Aust 2013;198:29–32. [DOI] [PubMed] [Google Scholar]
- 50.Steingard R, Taskiran S, Connor DF, Markowitz JS, Stein MA. New Formulations of Stimulants: An Update for Clinicians. J Child Adolesc Psychopharmacol 2019;29:324–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Modan-Moses D, Yaroslavsky A, Pinhas-Hamiel O, Levy-Shraga Y, Kochavi B, Iron-Segev S et al. Prospective Longitudinal Assessment of Linear Growth and Adult Height in Female Adolescents With Anorexia Nervosa. J Clin Endocrinol Metab 2021;106(1):e1–e10. [DOI] [PubMed] [Google Scholar]
- 52.Cortese S, Asherson P, Sonuga-Barke E, Banaschewski T, Brandeis D, Buitelaar J ADHD management during the COVID-19 pandemic: guidance from the European ADHD Guidelines Group. Lancet Child Adolesc Health 2020;4:412–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortese S, Coghill D, Santosh P, Hollis C, Simonoff E. Starting ADHD medications during the COVID-19 pandemic: recommendations from the European ADHD Guidelines Group. Lancet Child Adolesc Health 2020;4:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poulton A, Cowell CT. Slowing of growth in height and weight on stimulants: a characteristic pattern. J Paediatr Child Health 2003;39:180–5. [DOI] [PubMed] [Google Scholar]
- 55.Poulton AS, Nanan R. Prior treatment with stimulant medication: a much neglected confounder of studies of growth in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2008;18:385–7. [DOI] [PubMed] [Google Scholar]
- 56.Rose SR, Reeves G, Gut R, Germak J. Attention-Deficit/Hyperactivity Disorder Medication Treatment Impact on Response to Growth Hormone Therapy: Results from the ANSWER Program, a Non-Interventional Study. J Pediatr 2015;167:1389–96. [DOI] [PubMed] [Google Scholar]
- 57.Frindik JP, Morales A, Fowlkes J, Kemp S, Thrailkill K, Lippe B et al. Stimulant medication use and response to growth hormone therapy: an NCGS database analysis. Horm Res 2009;72:160–6. [DOI] [PubMed] [Google Scholar]
- 58.Spencer TJ, Kratochvil CJ, Sangal RB, Saylor KE, Bailey CE, Dunn DW et al. Effects of atomoxetine on growth in children with attention-deficit/hyperactivity disorder following up to five years of treatment. J Child Adolesc Psychopharmacol 2007;17:689–700. [DOI] [PubMed] [Google Scholar]
- 59.Huss M, Dirks B, Gu J, Robertson B, Newcorn JH, Ramos-Quiroga JA. Long-term safety and efficacy of guanfacine extended release in children and adolescents with ADHD. Eur Child Adolesc Psychiatry 2018;27:1283–1294. [DOI] [PubMed] [Google Scholar]
- 60.Pelham WE, Fabiano GA, Waxmonsky JG, Greiner AR, Gnagy EM, Pelham WE III Treatment Sequencing for Childhood ADHD: A Multiple-Randomization Study of Adaptive Medication and Behavioral Interventions. J Clin Child Adolesc Psychol 2016;45:396–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction 2010;140:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mustillo S, Worthman C, Erkanli A, Keeler G, Angold A, Costello EJ. Obesity and psychiatric disorder: developmental trajectories. Pediatrics 2003;111:851–9. [DOI] [PubMed] [Google Scholar]
- 63.He Q, Karlberg J. Bmi in childhood and its association with height gain, timing of puberty, and final height. Pediatr Res 2001;49:244–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


