Abstract
Background and Purpose:
Delirium portends worse outcomes after intracerebral hemorrhage (ICH), but it is unclear if symptom resolution or post-acute care intensity may mitigate its impact. We aimed to explore differences in outcome associated with delirium resolution prior to hospital discharge, as well as the potential mediating role of post-acute discharge site.
Methods:
We performed a single-center cohort study on consecutive ICH patients over 2 years. Delirium was diagnosed according to DSM-5 criteria and further classified as “persistent” or “resolved” based on delirium status at hospital discharge. We determined the impact of delirium on unfavorable 3-month outcome (modified Rankin Scale 4–6) using logistic regression models adjusted for established ICH predictors, then used mediation analysis to examine the indirect effect of delirium via post-acute discharge site.
Results:
Of 590 patients (mean age 70.5±15.5 years, 52% male, 83% white), 59% (n=348) developed delirium during hospitalization. Older age and higher ICH severity were delirium risk factors, but only younger age predicted delirium resolution, which occurred in 75% (161/215) of ICH survivors who had delirium. Delirium was strongly associated with unfavorable outcome, but patients with persistent delirium fared worse (adjusted OR 7.3, 95% CI 3.3–16.3) than those whose delirium resolved (adjusted OR 3.1, 95% CI 1.8–5.5). Patients with delirium were less likely to be discharged to inpatient rehabilitation than skilled nursing facilities (adjusted OR 0.31, 95% CI 0.17–0.59), and post-acute care site partially mediated the relationship between delirium and functional outcome in ICH survivors, leading to a 25% reduction in the effect of delirium (without mediator: adjusted OR 3.0, 95% CI 1.7–5.6; with mediator: adjusted OR 2.3, 95% CI 1.2–4.3).
Conclusions:
Acute delirium resolves in most ICH patients by hospital discharge, which was associated with better outcomes than in patients with persistent delirium. The impact of delirium on outcomes may be further mitigated by post-acute rehabilitation.
Keywords: Intracerebral Hemorrhage, Delirium, Outcomes, Prognosis, Rehabilitation
Introduction
Delirium is known to occur frequently in patients with stroke,1–3 but the extent of its direct and indirect impact on outcomes is unclear. Pre-existing vulnerability related to cognitive reserve and frailty are important factors in delirium pathogenesis,4 and delirium is thought to be a marker of disease severity in non-neurologic critically ill patients,5 raising the possibility that worse outcomes may result solely from an epiphenomenon. However, stroke patients and those with other neurocritical illness face unique concerns: their outcomes are especially shaped by prognostication,6, 7 intensity of care,8, 9 and rehabilitation,10, 11 each of which may be influenced by the occurrence of delirium. For example, delirium in patients with intracerebral hemorrhage (ICH) may factor into prognostication and clinical decision-making leading to withdrawal of life-sustaining treatment (WLST),12 while the decreased attention and awareness that come with delirium are likely to limit participation in multi-disciplinary rehabilitation.13
Although there is currently no effective treatment for delirium after stroke, there are many clinically relevant questions that remain unanswered, each of which may influence patient outcomes. First, the frequency with which delirium resolves during the acute phase after stroke remains unknown, a necessary component to prognostication. Second, the lasting impact of delirium that is short-lived vs. persistent at hospital discharge is unclear, as is the potential benefit of post-acute rehabilitation in stroke patients who experience delirium. We therefore aimed to describe the rates of delirium incidence and resolution in a population of patients with ICH, and to test several sequential hypotheses: first, that delirium is associated with higher rates of unfavorable long-term outcome, but that outcomes differ based on whether delirium has resolved by the time of hospital discharge; second, that delirium is associated with decreased rates of discharge to post-acute inpatient rehabilitation facilities (IRF); and third, that the association between delirium and outcome is mediated by post-discharge IRF utilization, which has been linked with improved long-term functional status.11, 14
Methods
Study population
We performed a retrospective cohort study using data from the prospective ICH registry at our Comprehensive Stroke Center. We included consecutive patients who were determined to have a non-traumatic ICH by two attending neurocritical care and/or vascular neurologists over a 2-year period from February 2018 to February 2020. Patients diagnosed with ICH due to hemorrhagic conversion of a known ischemic stroke or a known intracranial malignancy at the time of admission were excluded from the registry. Additionally, patients who had no evidence of purposeful response to any stimulus for the duration of their hospitalization were considered persistently comatose and therefore excluded from this study. The use of data for this study was approved by our hospital’s Institutional Review Board, and the requirement for informed consent was waived. Reporting of data adhered to STROBE guidelines for observational studies (see Supplement). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data collection
We prospectively collected all data related to standard clinical stroke care in a REDCap15, 16 database (Vanderbilt University, Nashville, TN) as part of an ongoing institutional quality improvement project. These data included patient demographics, comorbidities, neuroimaging, and other diagnostic testing. Additionally, two attending neurologists with board certification in neurocritical care and/or vascular neurology prospectively adjudicated ICH-related clinical predictors and neuroimaging until consensus was achieved. These clinical predictors included ICH location, size (measured via the ABC/2 method17), and etiology. Modified Boston criteria18 were used to diagnose possible or probable cerebral amyloid angiopathy (CAA) via clinical history and available neuroimaging.
Delirium diagnosis
Delirium was diagnosed by an attending neurointensivist (MR) based on reference-standard DSM-5 criteria19: disturbances in attention and awareness (often accompanied by disturbances in other cognitive domains) that develop over a short period of time and tend to fluctuate, represent a change in function, and are due to an underlying medical condition or toxic/withdrawal syndrome. In many cases, delirium was established prospectively, either as part of a nested research study or in the course of standard clinical care. In all other cases, delirium was established retrospectively via detailed chart review (including physician documentation, nursing notes, and notes from physical, occupational, and speech therapists) using the same DSM-5 criteria, with established chart-based methods that have been previously described.12, 20 Specific considerations on distinguishing acute delirium symptoms from expected ICH-related cognitive deficits have also been previously described,21, 22 with information from multiple time points typically necessary to determine whether symptoms were fluctuating and out of proportion to patients’ expected deficits.
In addition to determining whether patients were “never delirious” or “ever delirious,” we also determined whether delirious patients had persistent symptoms meeting criteria for delirium at the time of hospital discharge (“persistent delirium”), or if their delirium resolved without further recurrence prior to discharge (“delirium resolution”). For those patients who did not have prospective assessments, we performed additional focused chart review from the 48 hours preceding hospital discharge to establish the presence or absence of delirium criteria from available documentation.
Outcomes
Functional outcome at 3 months was assessed with the modified Rankin Scale (mRS),23 which is performed by a certified assessor as part of our Comprehensive Stroke Center’s standard follow-up procedures. We defined our primary “unfavorable” outcome as death or moderate-to-severe disability (mRS score 4–6). Additional outcomes included delirium resolution in patients who experienced delirium, and post-acute discharge site (IRF vs. skilled nursing facility [SNF]) in patients who did not have WLST or in-hospital death.
Statistical analysis
We used standard descriptive statistics to report patient characteristics and rates of delirium incidence and resolution. Means and standard deviations (SD) were used to describe data that had a normal distribution, and medians and interquartile ranges (IQR) were used to describe non-normal data. We analyzed differences between continuous variables using t-tests or the Mann-Whitney U-test, as appropriate, and differences between categorical variables using the Chi-square test.
We performed multivariable logistic regression to identify risk factors associated with delirium occurrence among the entire patient cohort, with covariates including age, ICH features (hematoma size, infratentorial vs. supratentorial location, presence of intraventricular hemorrhage [IVH], and initial Glasgow Coma Scale [GCS] score), and comorbidities that had significant differences on univariate testing. We used similar multivariable logistic regression models to identify predictors of delirium resolution in patients who had delirium but not WLST or in-hospital death.
We then determined associations between delirium and unfavorable 3-month outcome in both the entire cohort and in the subgroup of patients who did not have WLST or in-hospital death using logistic regression models. In our primary analysis, we included patient demographics and established ICH predictors (hematoma size, infratentorial vs. supratentorial location, presence of IVH, and initial GCS score) as covariates, as well as a history of prior stroke or dementia given the importance of pre-existing reserve in delirium pathogenesis. In a secondary analysis, we also considered “persistent delirium” vs. “delirium resolution” as an additional covariate. Missing outcome data were handled with casewise deletion, and we reported baseline characteristics for patients with missing data using standard descriptive statistics.
Finally, we performed a mediation analysis to examine the indirect effect of delirium on 3-month outcomes via post-acute discharge site in patients who did not have WLST or in-hospital death. This analysis comprised three sequential logistic regression models: the first model determined associations between delirium and unfavorable 3-month outcome in this subgroup of patients, using the same model covariates as in the full cohort; the second model determined associations between delirium and discharge to IRF as opposed to SNF, and included insurance status as an additional covariate; and the third model revisited associations between delirium and unfavorable 3-month outcome, but now included post-acute discharge site as an additional covariate. As an additional consideration of the importance of pre-existing frailty, sensitivity analyses were performed in patients who had available pre-morbid mRS scores suggestive of independent functional status prior to their ICH (pre-morbid mRS score 0–2). Direct and indirect effects with odds ratios (OR) and bootstrapped confidence intervals (CI) were computed using the ‘ldecomp’ package24 in Stata/MP 16 (College Station, TX). All hypothesis-testing was two-sided and the threshold for significance was set at alpha = .05. Model fit diagnostics were performed using area under the receiver operating characteristic curve (AUROC) analysis and the Hosmer-Lemeshow (HL) goodness-of-fit test.
Results
Baseline characteristics & delirium risk factors
We identified 590 patients in our cohort, after excluding 75 patients who were persistently comatose throughout their hospitalization (Supplementary Figure). Mean age was 70.5 (SD 15.5) years, 52% (n=309) were male, 83% (n=490) were white, and 59% (n=348) developed delirium. Delirium was assessed prospectively in 26% of patients (n=156) and retrospectively in 74% of patients (n=434).
On univariate analysis, patients with delirium were older and more likely to have had a history of dementia or prior stroke. They also had a higher overall ICH severity with larger ICH volume and higher prevalence of IVH, were more likely to have ICH due to CAA, and less likely to have infratentorial ICH (Table 1). In a multivariable model (AUROC: 0.845; HL test: p=0.21), significant risk factors for delirium included initial GCS <13 (adjusted OR 13.4, 95% CI 5.6–32.3), larger ICH volume (adjusted OR 1.05 per cc, 95% CI 1.03–1.06), older age (adjusted OR 1.2 per decade, 95% CI 1.01–1.3), IVH (adjusted OR 3.3, 95% CI 2.1–5.2), and a history of dementia (adjusted OR 2.1, 95% CI 1.02–4.3).
Table 1.
Baseline characteristics, intracerebral hemorrhage (ICH) features, and outcomes for patients with and without delirium during their hospitalization.
| Delirium (n =348) | No delirium (n =242) | p-value | ||
|---|---|---|---|---|
| Univariate | Adjusted for demographics | |||
|
| ||||
| Demographics | ||||
|
| ||||
| Age, years, mean (SD) | 72.1 (15.5) | 68.3 (15.2) | 0.003 | - |
|
| ||||
| Male, n (%) | 181 (52%) | 127 (52%) | 0.91 | - |
|
| ||||
| White, n (%) | 285 (82%) | 205 (85%) | 0.37 | - |
|
| ||||
| Pre-morbid mRS score, median (IQR) * | 1 (0–3) | 0 (0–2) | 0.01 | 0.02 |
|
| ||||
| Comorbidities, n (%) | ||||
|
| ||||
| Atrial fibrillation | 73 (21%) | 66 (27%) | 0.18 | 0.49 |
|
| ||||
| Coronary artery disease | 66 (19%) | 25 (10%) | 0.004 | 0.03 |
|
| ||||
| Hypertension | 257 (74%) | 164 (68%) | 0.11 | 0.49 |
|
| ||||
| Diabetes mellitus | 83 (24%) | 57 (65%) | 0.93 | 0.88 |
|
| ||||
| Chronic kidney disease | 25 (7%) | 15 (6%) | 0.64 | 0.71 |
|
| ||||
| Dementia | 45 (13%) | 16 (7%) | 0.01 | 0.13 |
|
| ||||
| Prior stroke | 86 (25%) | 38 (16%) | 0.008 | 0.67 |
|
| ||||
| ICH characteristics | ||||
|
| ||||
| ICH volume, cc, mean (SD) | 28.0 (29.4) | 7.4 (12.6) | < 0.001 | < 0.001 |
|
| ||||
| Intraventricular hemorrhage, n (%) | 182 (52%) | 42 (17%) | < 0.001 | < 0.001 |
|
| ||||
| Infratentorial location, n (%) | 24 (7%) | 47 (19%) | < 0.001 | < 0.001 |
|
| ||||
| Initial GCS score <13, n (%) | 143 (41%) | 7 (3%) | < 0.001 | < 0.001 |
|
| ||||
| Adjudicated etiology, n (%)* | ||||
|
| ||||
| Hypertensive | 144 (42%) | 139 (57%) | < 0.001 | < 0.001 |
|
|
|
|||
| Cerebral amyloid angiopathy | 108 (32%) | 38 (16%) | < 0.001 | |
|
|
|
|||
| Other | 90 (26%) | 65 (27%) | 0.04 | |
|
| ||||
| Discharge outcomes | ||||
|
| ||||
| Hospital mortality, n (%) | 69 (20%) | 2 (1%) | < 0.001 | < 0.001 |
|
| ||||
| Discharged to hospice, n (%) | 64 (18%) | 2 (1%) | < 0.001 | < 0.001 |
|
| ||||
| Discharge mRS score, median (IQR) | 5 (4–5) | 3 (2–4) | < 0.001 | < 0.001 |
|
| ||||
| 3-month outcomes | ||||
|
| ||||
| Unfavorable 3-month outcome, n (%)* | 232 (71%) | 43 (20%) | < 0.001 | < 0.001 |
|
| ||||
| 3-month mRS score, median (IQR)* | 6 (3–6) | 2 (1–3) | < 0.001 | < 0.001 |
Note: Pre-morbid mRS scores were missing in 254 patients, etiology was unknown in 6 patients, and outcome data were missing in 44 patients
Abbreviations: SD, standard deviation; mRS, modified Rankin Scale; IQR, interquartile range; GCS, Glasgow Coma Scale
Pre-morbid mRS scores were available in 57% of patients (n=336). Among this group, patients with delirium had higher pre-morbid mRS scores compared to those without delirium (median [IQR] 1 [0–3] vs. 0 [0–2], p=0.01), and fewer patients with delirium were functionally independent prior to hospitalization as compared to patients without delirium (62% vs. 79%, p=0.001). In a subgroup analysis of patients with available pre-morbid mRS scores, higher mRS was a significant risk factor for delirium when added to our multivariable model (mRS >2: adjusted OR 3.5, 95% CI 1.8–6.8).
Delirium resolution
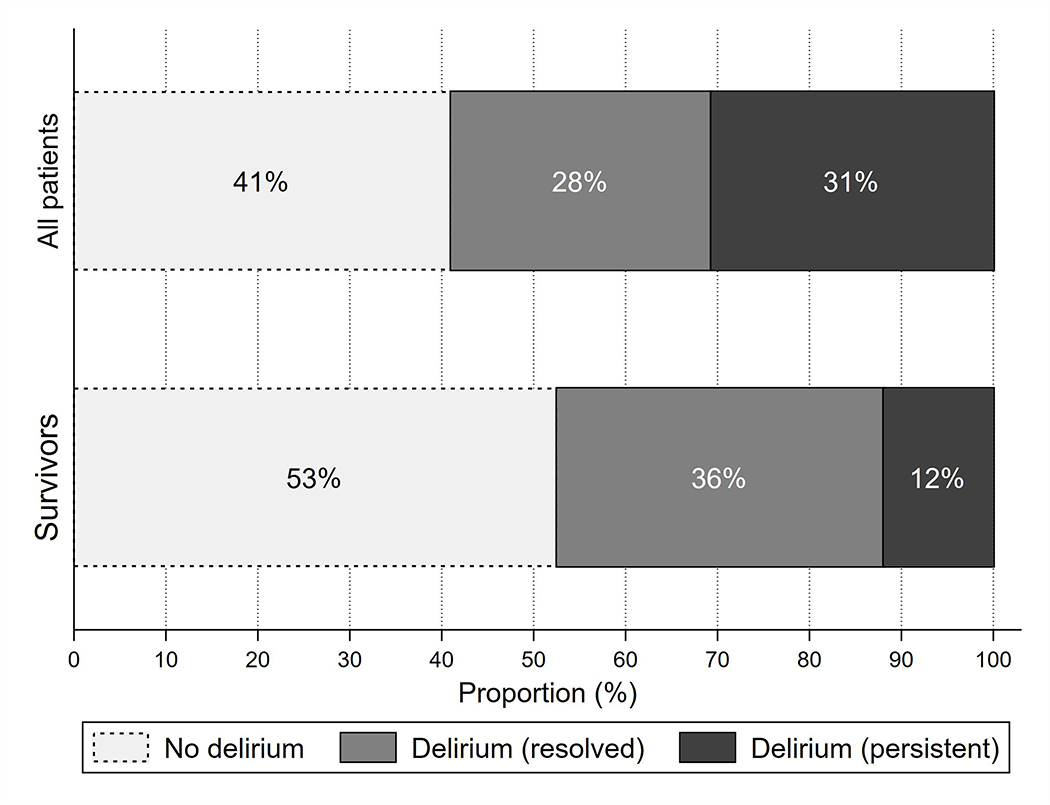
Among all patients who developed delirium, 48% (167/348) had delirium resolution by the time of hospital discharge. When considering only patients who did not have WLST or in-hospital death, 75% (161/215) were no longer delirious at hospital discharge (Figure 1). This rate was identical in both patients who were assessed prospectively (75% [52/69]) and those who were diagnosed retrospectively (75% [109/146]).
Figure 1.
Distribution of patients by delirium category (no delirium, delirium that has resolved by discharge, persistent delirium at discharge) in both the entire cohort and in patients without withdrawal of life-sustaining treatment or in-hospital death.
Patients who had persistent delirium at discharge were significantly older than those who had delirium resolution and had a higher prevalence of pre-existing dementia. However, ICH severity was not significantly different between the two groups (Table 2). In a multivariable model (AUROC: 0.719; HL test: p=0.35), older age remained the only significant demographic or ICH-related risk factor for persistent delirium (adjusted OR 1.5 per decade, 95% CI 1.1–1.9). In a subgroup analysis of patients with available pre-morbid mRS scores, higher mRS was also a significant risk factor for persistent delirium when added to our multivariable model (mRS >2: adjusted OR 2.2, 95% CI 1.1–4.7).
Table 2.
Baseline characteristics, intracerebral hemorrhage (ICH) features, and outcomes for ICH survivors with delirium during their hospitalization, stratified by delirium status at hospital discharge.
| Persistent delirium (n =54) | Delirium resolution (n =161) | p-value | ||
|---|---|---|---|---|
| Univariate | Adjusted for demographics | |||
|
| ||||
| Demographics | ||||
|
| ||||
| Age, years, mean (SD) | 74.7 (13.0) | 65.9 (16.7) | < 0.001 | - |
|
| ||||
| Male, n (%) | 19 (35%) | 89 (55%) | 0.01 | - |
|
| ||||
| White, n (%) | 42 (78%) | 123 (76%) | 0.84 | - |
|
| ||||
| Insurance status, n (%) | ||||
|
| ||||
| None | 6 (3%) | 10 (3%) | 0.97 | 0.56 |
|
| ||||
| Medicare/Medicaid | 64 (27%) | 87 (25%) | ||
|
| ||||
| Private insurance | 163 (68%) | 240 (69%) | ||
|
| ||||
| Other | 7 (3%) | 10 (3%) | ||
|
| ||||
| Pre-morbid mRS score, median (IQR) * | 2 (0–3) | 0 (0–3) | 0.006 | 0.07 |
|
| ||||
| Comorbidities, n (%) | ||||
|
| ||||
| Atrial fibrillation | 9 (17%) | 25 (16%) | 0.84 | 0.72 |
|
| ||||
| Coronary artery disease | 3 (6%) | 27 (17%) | 0.04 | 0.04 |
|
| ||||
| Hypertension | 40 (74%) | 117 (73%) | 0.84 | 0.52 |
|
| ||||
| Diabetes mellitus | 11 (20%) | 38 (24%) | 0.62 | 0.56 |
|
| ||||
| Chronic kidney disease | 4 (7%) | 10 (6%) | 0.10 | 0.81 |
|
| ||||
| Dementia | 12 (22%) | 17 (11%) | 0.03 | 0.48 |
|
| ||||
| Prior stroke | 11 (20%) | 20 (12%) | 0.15 | 0.34 |
|
| ||||
| ICH characteristics | ||||
|
| ||||
| ICH volume, cc, mean (SD) | 16.3 (18.8) | 21.0 (21.8) | 0.17 | 0.74 |
|
| ||||
| Intraventricular hemorrhage, n (%) | 27 (50%) | 77 (48%) | 0.78 | 0.46 |
|
| ||||
| Infratentorial location, n (%) | 5 (9%) | 11 (7%) | 0.56 | 0.45 |
|
| ||||
| Initial GCS score <13, n (%) | 20 (26%) | 42 (37%) | 0.09 | 0.03 |
|
| ||||
| Adjudicated etiology, n (%)* | ||||
|
| ||||
| Hypertensive | 22 (41%) | 72 (46%) | 0.21 | 0.87 |
|
|
|
|||
| Cerebral amyloid angiopathy | 21 (39%) | 41 (26%) | 0.70 | |
|
|
|
|||
| Other | 11 (20%) | 42 (27%) | 0.61 | |
|
| ||||
| Discharge outcomes | ||||
|
| ||||
| Hospital length of stay, days, median (IQR) | 8 (5–15) | 9 (5–16) | 0.47 | 0.98 |
|
| ||||
| Discharge mRS score, median (IQR) | 4 (4–5) | 4 (4–5) | 0.003 | 0.007 |
|
| ||||
| Discharge location, n (%) | ||||
|
| ||||
| Home (or assisted living facility) | 3 (7%) | 24 (15%) | < 0.001 | 0.10 |
|
|
|
|||
| Inpatient rehabilitation facility | 7 (13%) | 64 (40%) | 0.046 | |
|
|
|
|||
| Skilled nursing facility | 42 (78%) | 71 (44%) | 0.002 | |
|
|
|
|||
| Other | 2 (4%) | 2 (1%) | 0.47 | |
|
| ||||
| 3-month outcomes | ||||
|
| ||||
| Unfavorable 3-month outcome, n (%)* | 34 (71%) | 67 (46%) | 0.002 | 0.003 |
|
| ||||
| 3-month mRS score, median (IQR)* | 4 (3–6) | 3 (3–5) | < 0.001 | 0.001 |
Note: Pre-morbid mRS scores were missing in 19 patients, etiology was unknown in 6 patients, and outcome data were missing in 20 patients
Abbreviations: SD, standard deviation; mRS, modified Rankin Scale; IQR, interquartile range; GCS, Glasgow Coma Scale
Discharge outcomes
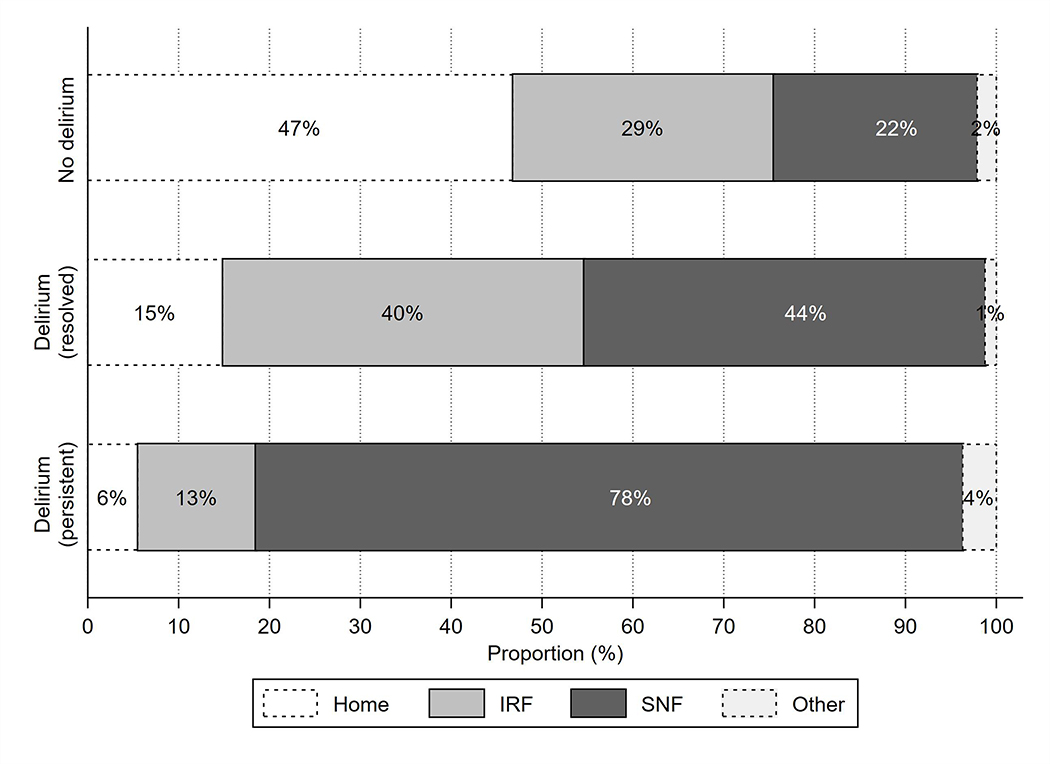
Among ICH patients without WLST or in-hospital death, patients with delirium had longer hospital length of stay compared to those without delirium (median [IQR] 8 [5–16] vs. 4 [2–6] days, p<0.001), but length of stay was similar in patients whose delirium resolved by discharge and those with persistent delirium (Table 2). At discharge, mRS scores were substantially higher in patients with delirium compared to those without delirium (Table 1), while they were statistically but not meaningfully different in patients with persistent delirium vs. delirium resolution (Table 2). Patients with delirium were less likely to be discharged to an IRF even if their delirium resolved, although those with persistent delirium had the lowest rates of IRF discharge (Figure 2).
Figure 2.
Distribution of post-acute discharge site (home, inpatient rehabilitation facility [IRF], skilled nursing facility [SNF], other) in intracerebral hemorrhage survivors stratified by delirium category.
In a multivariable model adjusting for demographics, insurance status, a history of dementia or prior stroke, and ICH severity (AUROC: 0.798; HL test: p=0.38), delirium was associated with significantly lower odds of discharge to IRF among ICH survivors (adjusted OR 0.31, 95% CI 0.17–0.59). Results were similar in a sensitivity analysis of patients with pre-morbid mRS 0–2 (adjusted OR 0.30, 95% CI 0.11–0.84). Among only patients who had delirium, those who had resolution of their delirium symptoms prior to discharge had substantially higher odds of discharge to IRF (adjusted OR 6.3, 95% CI 2.2–18.2) compared to patients with persistent delirium.
Stratification of 3-month outcomes
Outcomes at 3 months were available in 93% (n=546) of all patients in our cohort. Patients with missing outcome data had smaller ICH volumes, but other ICH characteristics, demographics, and delirium prevalence were not significantly different compared to patients who had outcome data available (Supplementary Table I).
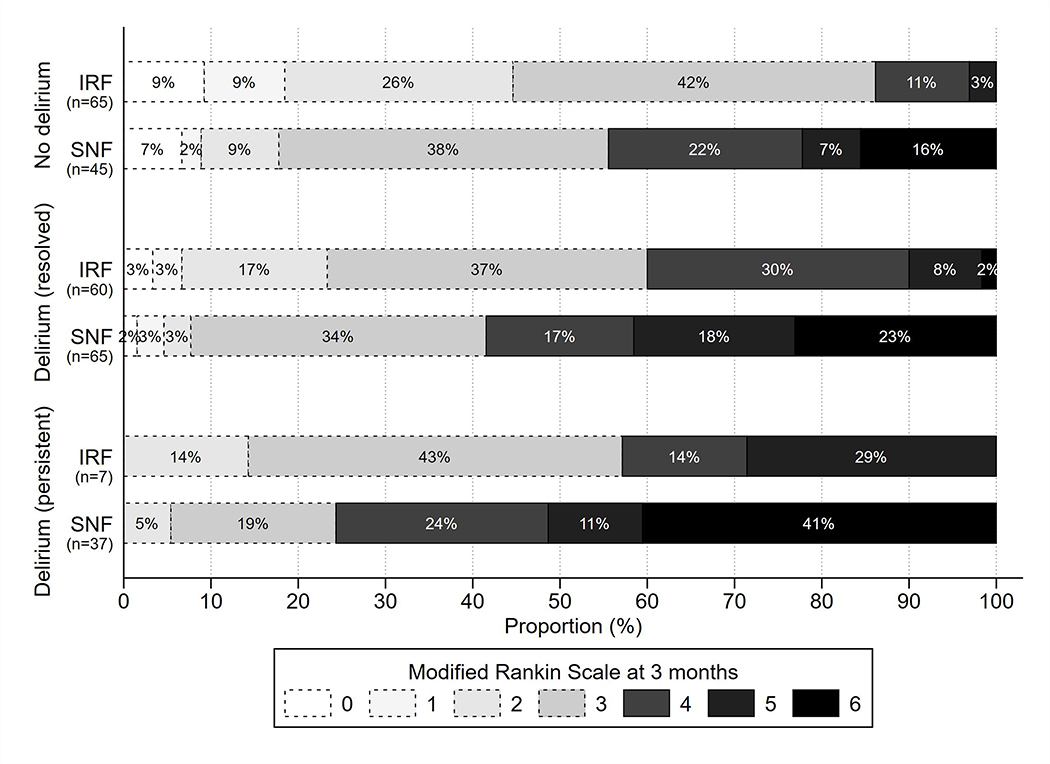
In univariate analyses, patients with delirium had a higher rate of unfavorable outcome compared to those without delirium, as well as higher 3-month mRS scores overall (Table 1). Meanwhile, patients who had delirium resolution had a lower rate of unfavorable outcome than patients with persistent delirium, as well as lower 3-month mRS scores overall (Table 2). Rates of unfavorable outcome were highest in patients who were discharged to SNF across all delirium categories (Figure 3).
Figure 3.
Distribution of 3-month modified Rankin Scale scores stratified by delirium category and post-acute discharge site (inpatient rehabilitation facility [IRF] vs. skilled nursing facility [SNF]). Bars with lighter shades and dotted outlines represent scores corresponding to favorable outcomes, and bars with darker shades and solid outlines represent scores corresponding to unfavorable outcomes.
In multivariable models that did not consider post-acute discharge site (AUROC: 0.839; HL test: p=0.74), delirium had a strong association with unfavorable outcome among the entire cohort of patients (adjusted OR 5.6, 95% CI 3.4–9.2). However, outcomes differed based on whether delirium was persistent (adjusted OR 24.2, 95% CI 11.5–51.0) or had resolved by the time of hospital discharge (adjusted OR 2.9, 95% CI 1.7–5.0). Among ICH patients without WLST or in-hospital death, there remained a significant association between delirium and unfavorable outcome (adjusted OR 3.8, 95% CI 2.2–6.5), with outcomes again differing based on whether delirium was persistent (adjusted OR 7.3, 95% CI 3.3–16.3) or had resolved by hospital discharge (adjusted OR 3.1, 95% CI 1.8–5.5). Results were similar in sensitivity analyses of patients with pre-morbid mRS 0–2 (any delirium: adjusted OR 4.3, 95% CI 1.7–10.9; persistent delirium: adjusted OR 32.3, 95% CI 5.4–192.5; resolved delirium: adjusted OR 3.3, 95% CI 1.3–8.7).
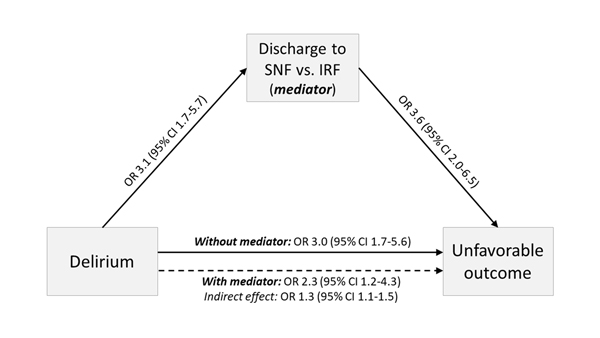
Among only those patients who were discharged to IRF or SNF, delirium had similar associations with unfavorable outcome (adjusted OR 3.0, 95% CI 1.7–5.6) when post-acute discharge site was not factored into the model as a covariate (AUROC: 0.711; HL test: p=0.41). However, the addition of discharge site to the model (AUROC: 0.740; HL test: p=0.83) partially mediated the impact of delirium via a 25% reduction in its effect on outcomes (adjusted OR 2.3, 95% CI 1.2–4.3), while SNF discharge itself also had a strong direct impact on unfavorable outcome (adjusted OR 3.6, 95% CI 2.0–6.5). Mediation analysis confirmed that delirium had a significant indirect effect on outcome via post-acute discharge site (adjusted OR 1.3, 95% CI 1.1–1.5; Figure 4), with similar estimates in a sensitivity analysis of patients with pre-morbid mRS 0–2 (adjusted OR 1.2, 95% CI 0.97–1.5) (Supplementary Table II).
Figure 4.
Results of mediation analysis examining the indirect effect of post-acute discharge site (skilled nursing facility [SNF] as opposed to inpatient rehabilitation facility [IRF]) on the relationship between delirium and unfavorable outcome.
Discussion
Delirium occurs frequently after ICH but does not preclude the possibility of a good outcome. Indeed, many patients with delirium in our cohort ultimately had favorable functional outcomes, but this appeared to depend on two primary factors: first, whether their acute delirium symptoms resolved prior to discharge; and second, whether they had the opportunity for post-acute rehabilitation after their hospitalization.
Many clinicians consider post-stroke delirium to be indicative of stroke severity, while there may also be disagreement about the potentially overlapping nature of acute cognitive changes in the context of expected stroke-related deficits. It is therefore both striking and reassuring to note that acute delirium symptoms eventually resolve in most ICH patients who do not have early limitations of care, with rates similar to those described for non-neurologic populations.25 At the very least, our findings should inform clinicians’ biases about the reversibility of acute cognitive symptoms in ICH patients as well as their prognostication practices, especially considering the potential impact of delirium on WLST after ICH.12 Further, while ICH severity appears to be a primary driving factor in the development of delirium, it does not appear to predict which patients will have delirium symptoms that persist beyond their hospitalization. If delirium does indeed play a role in decisions regarding intensity of care, the ability to accurately predict recovery from delirium should be a critical target for future ICH prognostication studies.
Importantly, ICH patients with delirium resolution had considerably higher rates of favorable outcome than patients who still had delirium at hospital discharge, suggesting that delirium resolution may be an important marker of long-term outcomes. This association between delirium resolution and functional recovery has been described in non-neurologic patients,26 but carries additional importance in stroke patients who depend on rehabilitation for their cognitive, motor, and functional recovery. As delirium is fundamentally a disorder of attention and awareness, it is reasonable to expect that delirious patients may not be able to fully participate with multi-disciplinary rehabilitation. Our finding that patients with persistent delirium had the lowest rates of discharge to IRF is therefore not entirely surprising. On the other hand, patients should theoretically have the potential to benefit from post-acute rehabilitation once their delirium symptoms resolve, yet we found those who had delirium resolution still had markedly lower rates of discharge to IRF than patients who did not have delirium. This suggests that there may be clinician bias against admitting patients with delirium to intensive rehabilitation settings. Although the potential benefits of IRF in this patient population are not well understood, some specialized rehabilitation centers have reported that as many as 10% of their patients have delirium at the time of IRF admission,13, 27 with a higher prevalence in neurologic patients compared to those with non-neurologic disorders. Further prospective studies are needed to explore the impact of IRF on post-stroke delirium, as there may be an opportunity for further functional improvement in patients whose recovery potential might otherwise be underestimated during the discharge planning process. It is also possible that early mobilization may be beneficial in mitigating delirium symptoms, and that some patients with delirium may warrant a therapeutic trial of intensive rehabilitation.
In support of this view, we found that the post-acute setting appears to be a vital branching point in determining long-term outcomes in ICH patients with delirium. In our study, those who had the opportunity for IRF had substantially lower rates of unfavorable outcome than those who were discharged to SNF, and even some patients who continued to have delirium at the time of hospital discharge were able to achieve favorable outcomes if they were discharged to IRF. Further, we found that the impact of delirium on outcomes was partly attributable to corresponding differences in IRF placement. This suggests that post-acute inpatient rehabilitation may mitigate some of the deleterious long-term effects of delirium in ICH patients, and that even patients with persistent delirium may still benefit from acute rehabilitation. Indeed, it is possible that patients with cognitive disturbances may derive additional benefit from the multi-disciplinary rehabilitation available in the IRF setting, with individualized care tailored to their cognitive needs and guided by specialized neurorehabilitation personnel. However, the potential benefits of rehabilitation may differ based on delirium subtype and on the interaction of delirium with stroke-related factors such as right vs. left hemispheric location and the presence of neglect, aphasia, or other focal cognitive deficits. Further studies should explore the role of rehabilitation in these subgroups.
Our study has several limitations. First, since we relied on data from a single center, there may be local and institutional factors that are not readily generalizable, including criteria for discharge to IRF or SNF based on regional resources. However, as the only tertiary-care referral center in our state, almost all patients with ICH who initially present to other hospitals in the state are subsequently transferred to our center. As a result, our cohort represents a near-statewide representation of all ICH patients during the time period studied. Second, there may have been some degree of residual confounding by indication, as patients with delirium who were discharged to IRF may have been considered better candidates for rehabilitation for reasons not captured by our model covariates. In reality, there were likely other confounding factors affecting the likelihood of discharge disposition, such as the extent of motor symptoms, inpatient complications and comorbidities other than delirium, non-delirium-related cognitive performance, and social determinants of health. Third, although many of our patients had prospective assessments for delirium, most were rated retrospectively, raising the possibility of misclassification in some cases. Fourth, we did not have data on the underlying causes of delirium for each patient, and outcomes may differ based on whether delirium is due to primary or downstream effects of the ICH itself, or from other unrelated causes. Finally, we did not have detailed data on pre-morbid cognitive impairment (e.g., using validated assessments such as the Informant Questionnaire on Cognitive Decline in the Elderly28) or on cognitive outcomes, and many patients may still be faced with long-term cognitive impairment as a result of their underlying ICH even if they experienced resolution of their acute delirium symptoms. These limitations underscore the critical importance of future prospective studies to examine the role of post-acute rehabilitation in improving functional and cognitive outcomes in patients with post-stroke delirium.
Conclusion
Although delirium is associated with worse outcomes after ICH, its effects are mitigated in patients whose delirium symptoms resolve prior to hospital discharge and in those who receive post-acute inpatient rehabilitation.
Supplementary Material
Acknowledgments
Sources of Funding: MER is supported by NIH grant R24AG054259 (NIDUS Pilot Grant Subaward) and the Rhode Island Foundation. LAD is supported by NIH grant R01AG058648.
Non-standard abbreviations
- ICH
Intracerebral hemorrhage
- WLST
Withdrawal of life-sustaining treatment
- IRF:
Inpatient rehabilitation facility
- SNF
Skilled nursing facility
- CAA
Cerebral amyloid angiopathy
- mRS
Modified Rankin Scale
- IVH
Intraventricular hemorrhage
- GCS
Glasgow Coma Scale
Footnotes
Conflict(s)-of-Interest/Disclosure(s): Dr. Wendell reports other funding from Biogen outside the submitted work. Dr. Stretz reports funding from Massachusetts General Hospital/BSC for his site’s participation in the Neuro AFib study. Dr Rudolph is an unpaid board member of the American Delirium Society.
References
- 1.Carin-Levy G, Mead GE, Nicol K, Rush R, van Wijck F. Delirium in acute stroke: Screening tools, incidence rates and predictors: A systematic review. Journal of neurology. 2012;259:1590–1599 [DOI] [PubMed] [Google Scholar]
- 2.Shi Q, Presutti R, Selchen D, Saposnik G. Delirium in acute stroke: A systematic review and meta-analysis. Stroke. 2012;43:645–649 [DOI] [PubMed] [Google Scholar]
- 3.Shaw RC, Walker G, Elliott E, Quinn TJ. Occurrence rate of delirium in acute stroke settings. 2019;50:3028–3036 [DOI] [PubMed] [Google Scholar]
- 4.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. The Lancet. 2014;383:911–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein Klouwenberg PMC, Zaal IJ, Spitoni C, Ong DSY, van der Kooi AW, Bonten MJM, et al. The attributable mortality of delirium in critically ill patients: Prospective cohort study. BMJ : British Medical Journal. 2014;349:g6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahuranec DB, Fagerlin A, Sánchez BN, Roney ME, Thompson BB, Fuhrel-Forbis A, et al. Variability in physician prognosis and recommendations after intracerebral hemorrhage. Neurology. 2016;86:1864–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCracken DJ, Lovasik BP, McCracken CE, Frerich JM, McDougal ME, Ratcliff JJ, et al. The intracerebral hemorrhage score: A self-fulfilling prophecy? Neurosurgery. 2018;84:741–748 [DOI] [PubMed] [Google Scholar]
- 8.Becker KJ, Baxter AB, Cohen WA, Bybee HM, Tirschwell DL, Newell DW, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001;56:766–772 [DOI] [PubMed] [Google Scholar]
- 9.Zahuranec DB, Brown DL, Lisabeth LD, Gonzales NR, Longwell PJ, Smith MA, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology. 2007;68:1651–1657 [DOI] [PubMed] [Google Scholar]
- 10.Deutsch A, Granger CV, Heinemann AW, Fiedler RC, DeJong G, Kane RL, et al. Poststroke rehabilitation. Stroke. 2006;37:1477–1482 [DOI] [PubMed] [Google Scholar]
- 11.Chan L, Sandel ME, Jette AM, Appelman J, Brandt DE, Cheng P, et al. Does postacute care site matter? A longitudinal study assessing functional recovery after a stroke. Archives of physical medicine and rehabilitation. 2013;94:622–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reznik ME, Moody S, Murray K, Costa S, Grory BM, Madsen TE, et al. The impact of delirium on withdrawal of life-sustaining treatment after intracerebral hemorrhage. Neurology. 2020: 10.1212/WNL.0000000000010738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bushi S, Barrett AM, Oh-Park M. Inpatient rehabilitation delirium screening: Impact on acute care transfers and functional outcomes. 2020;12:766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong I, Goodwin JS, Reistetter TA, Kuo Y-F, Mallinson T, Karmarkar A, et al. Comparison of functional status improvements among patients with stroke receiving postacute care in inpatient rehabilitation vs skilled nursing facilities. JAMA Network Open. 2019;2:e1916646–e1916646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The redcap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The abcs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305 [DOI] [PubMed] [Google Scholar]
- 18.Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diagnostic and statistical manual of mental disorders : Dsm-5. Fifth edition. Arlington, VA: : American Psychiatric Association, [2013]; 2013. [Google Scholar]
- 20.Pisani MA, Araujo KLB, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Critical Care. 2006;10:R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reznik ME, Drake J, Margolis SA, Moody S, Murray K, Costa S, et al. Deconstructing poststroke delirium in a prospective cohort of patients with intracerebral hemorrhage*. Read Online: Critical Care Medicine | Society of Critical Care Medicine. 2020;48:111–118 [DOI] [PubMed] [Google Scholar]
- 22.Reznik ME, Drake J, Margolis SA, Mahta A, Wendell LC, Thompson BB, et al. The authors reply. Critical care medicine. 2020;48:e636–e637 [DOI] [PubMed] [Google Scholar]
- 23.de Haan R, Limburg M, Bossuyt P, van der Meulen J, Aaronson N. The clinical meaning of rankin ‘handicap’ grades after stroke. Stroke. 1995;26:2027–2030 [DOI] [PubMed] [Google Scholar]
- 24.Buis ML. Direct and indirect effects in a logit model. The Stata Journal. 2010;10:11–29 [PMC free article] [PubMed] [Google Scholar]
- 25.Cole MG, Ciampi A, Belzile E, Zhong L. Persistent delirium in older hospital patients: A systematic review of frequency and prognosis. Age and Ageing. 2008;38:19–26 [DOI] [PubMed] [Google Scholar]
- 26.Kiely DK, Jones RN, Bergmann MA, Murphy KM, Orav EJ, Marcantonio ER. Association between delirium resolution and functional recovery among newly admitted postacute facility patients. The Journals of Gerontology: Series A. 2006;61:204–208 [DOI] [PubMed] [Google Scholar]
- 27.Oh-Park M, Chen P, Romel-Nichols V, Hreha K, Boukrina O, Barrett AM. Delirium screening and management in inpatient rehabilitation facilities. 2018;97:754–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorm AF. A short form of the informant questionnaire on cognitive decline in the elderly (iqcode): Development and cross-validation. Psychological Medicine. 1994;24:145–153 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.