Abstract
Vitamin D promotes a shift from a pro-inflammatory to a more tolerogenic immune state in allogeneic hematopoietic cell transplantation (HCT) recipients. The dominant mechanism responsible for this shift has not been elucidated. We took a multifaceted approach to evaluating the clinical and immunologic impact of low vitamin D levels in 53 HCT recipients. We used 28-plex flow cytometry for immunophenotyping, serum cytokine levels, T-cell cytokine production and T-cell whole genome transcription. The median day-30 vitamin D level was 20 ng/mL, and deficiency was common in younger patients undergoing myeloablative transplants. Low vitamin D levels were associated with a high CD8/Treg ratio; increased serum levels and T-cell production of proinflammatory cytokines; and a gene expression signature of unrestrained T-cell proliferation and epigenetic modulation through the PRC2/EZH2 complex. Immunophenotyping confirmed a strong association between high levels of vitamin D and an activated EZH2 signature, characterized by overexpression of ID3, which has a role in effector T-cell differentiation. Our findings demonstrate the critical role of vitamin D in modulating T-cell function in human GVHD and identify a previously undescribed interaction with EZH2 and ID3 which may impact effector differentiation and has implications to cell therapies and other forms of cancer immunotherapy.
Keywords: Vitamin D, Allogeneic hematopoietic cell transplantation, Acute graft-versus-host disease, T-cell function, Epigenetic regulation
Introduction
Acute graft-versus-host disease (GVHD) is a leading cause of morbidity and mortality in allogeneic hematopoietic cell transplant (HCT) recipients.(1) Despite standard prophylactic measures, the rates of GVHD remain high, with incidence rates ranging from 34% to 65% in the first 100 days post-transplant, and adversely impacts mortality.(2,3) Therefore, developing improved GVHD prevention strategies remains a critical goal for successful allogeneic HCT.
The pathogenesis of GVHD involves donor-derived T-cell recognition of host antigens as foreign, resulting in their activation, proliferation, migration and cytokine release, and the subsequent destruction of host tissues.(4) Vitamin D, a fat-soluble prohormone long recognized for its primary role in mediating calcium and bone mineral homeostasis, possesses various non-skeletal functions, including immune regulation. The biologically active form of vitamin D, 1,25(OH)2D3 (calcitriol), regulates the expression of hundreds of genes by binding to the vitamin D receptor, a member of the nuclear receptor family of transcription factors found in nearly all immune cells, including T lymphocytes, B lymphocytes, macrophages, and dendritic cells (DCs).(5,6) Vitamin D has been shown to have pleiotropic effects on the immune system but it is generally thought to promote a shift from a pro-inflammatory to a more tolerogenic immune state. Vitamin D appears to modulate the maturation and activation of DCs, augment regulatory T lymphocyte production, affect macrophage function, decrease T-cell proliferation and promote naïve CD4+ T lymphocyte polarization toward a Th2 immune response.(7–12) Several observational studies have identified associations between vitamin D deficiency and GVHD.(13) A prospective clinical trial demonstrated mitigation of the risk of chronic GVHD with vitamin D supplementation,(14) but the dominant mechanism that associates vitamin D deficiency with GVHD in humans has not been elucidated.
Given the important role of vitamin D in modulating alloreactive T cell responses and considering the high incidence of vitamin D deficiency in allogeneic HCT recipients,(15) we hypothesized that early vitamin D deficiency after allogeneic HCT predisposes patients to a higher risk of GVHD as the result of a pro-inflammatory state. We had a particular interest in skin GVHD as vitamin D has been shown to impact T cell homing to the skin.(16–18) We conducted a retrospective analysis to evaluate whether serum vitamin D concentrations on day 30 after allogeneic HCT were predictive of acute GVHD and specific organ involvement. We used a cohort of patients that were transplanted prior to implementation of routine vitamin D repletion. We chose to analyze day 30 levels to allow adequate time for the principal causes of vitamin D deficiency to manifest, such as malnutrition, malabsorption, and low sun exposure that are common in HCT patients during convalescence. In addition, we investigated the predominant mechanism of vitamin D’s effect on human GVHD using detailed immune profiling of post-transplant blood samples and gene expression analysis.
Patients and Methods
Patient Population
We retrospectively analyzed 53 adult patients with hematologic malignancies who underwent allogeneic HCT at the University of Pennsylvania between January 2008 and December 2012, for whom day-30 post-transplant peripheral blood samples had been banked. During that time frame, vitamin D levels were not routinely monitored, and routine supplementation was not implemented. Patients were randomly selected from a large bio-specimen repository of samples from allogeneic HCT recipients. Patients received standard tacrolimus-based GVHD prophylaxis without in vivo or ex vivo T-cell depletion. Patients did not receive oral vitamin D supplementation during their transplant. Patients who were administered total parenteral nutrition (TPN) received 5 mcg of ergocalciferol (vitamin D2) daily as part of the standard multi-vitamin infusion formula. The circulating form of vitamin D, 25OHD, was measured using a quantitative chemiluminescent immunoassay (ARUP Laboratories, Salt Lake City, UT). Vitamin D deficiency was defined as 25OHD <20 ng/mL and insufficiency as <30 ng/mL.(6)
Clinical Endpoints
The primary objective of the clinical analysis was to evaluate whether serum vitamin D concentrations on day 30 after allogeneic HCT were predictive of acute grade 2-4 GVHD. Secondary endpoints included time to organ-specific acute grade 2-4 GVHD, chronic GVHD and overall survival (OS). Acute GVHD and chronic GVHD were graded according to the modified Glucksberg criteria and National Institutes of Health consensus criteria, respectively.(19,20) Guidance published by the Mount Sinai Acute GVHD International Consortium was used to determine GVHD diagnosis and grading.(21) OS was defined as the time interval between date of HCT and death from any cause or for surviving patients, to last follow-up.
Immune Phenotyping
We characterized immune cell populations in peripheral blood mononuclear cell (PBMC) samples from all patients on day 30 post-HCT. The percentages and absolute numbers of cell populations carrying distinct phenotypic markers were analyzed by flow cytometry. A detailed panel using 25 surface markers was initially applied to 34 patients, selected for having the highest and lowest vitamin D levels in the dataset, with adjustments due to sample availability and quality. Samples from 23 patients were also analyzed for the intracellular expression of 10 transcription factors and 19 patients were analyzed for production of interferon (IFN)-γ, tumor necrosis factor (TNF)-α, IL-2, IL-17A and IL-4 using intracellular staining after activation with PMA and Ionomycin. Samples for these analyses were also selected from patients with the highest and lowest vitamin D levels based on availability. Briefly, cells were resuspended in fluorescence-activated cell sorting buffer (PBS and 0.5% BSA) and stained using Fixable Viability Dye NIR at 20°C for 15 minutes. Cell surface staining was performed for 30 minutes at 4°C and intracellular staining was performed using a fixation/permeabilization kit (eBioscience) according to manufacturer’s instructions. Antibodies are listed in Supplementary Table 1. Flow cytometry was conducted on BD Canto or Cytek Aurora and analyzed on FlowJo software.
Cytokine Analysis
Serum cytokine levels were measured by Milliplex Immunology Multiplex Assay (End Millipore) and analyzed by Bio-Plex 200 Systems (Bio-Rad). The cytokines examined were interleukin (IL)-1b, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and thymic stromal lymphopoietin. GVHD biomarkers were measured by enzyme-linked immunosorbent assay using kits from R&D Systems and MBL International.
Gene Expression Analysis
T lymphocytes from 16 samples including 8 with low (≤15 ng/mL) and high (≥30 ng/mL) vitamin D levels were isolated by negative selection using Magnetic Activated Cell Sorting (Miltenyi Biotech). RNA was extracted using RNeasy Mini kit (QIAGEN) and labeled using Agilent’s one-color labeling protocol. All steps from PBMC thawing to RNA extraction were conducted rapidly and on ice to minimize changes in gene transcription. Labeled cRNA was hybridized to Agilent 8x60 human gene expression arrays according to manufacturer instructions. Agilent software was used to assess raw signal intensity. One sample was identified as an outlier and was excluded from the analysis. Raw probe expression levels were normalized and probes measuring the same genes were averaged as previously described.(22)
Differentially expressed genes at a significance level of P ≤ 0.05 between low and high vitamin D samples were considered to be associated with vitamin D levels. Gene set enrichment analysis was performed against Gene Ontology annotation and the Broad Institute’s MSigDB gene sets using hyper-geometric function.(23) P-values were corrected using the false discovery rate (FDR) method.(24)
Statistical Analyses
Descriptive statistics were used to characterize distributions of variables. The cumulative incidence of acute grade 2-4 GVHD was determined using a Fine and Gray method, considering death and relapse as competing events. The Kaplan-Meier method was used to estimate OS and compare patients who had vitamin D concentration on day 30 after HCT above and below the median. The associations between vitamin D levels and other variables were conducted using the Pearson correlation coefficient and t-tests. We then used a landmark analysis to estimate the impact of day-30 vitamin D levels on subsequent clinical outcomes. A multivariable cumulative incidence analysis was performed, and the following variables were selected by backward elimination: GVHD prophylaxis, conditioning intensity, and degree of human leukocyte antigen (HLA) match. Student’s t-test and the Mann-Whitney test were used for parametric and non-parametric group comparisons, respectively. For the cytokine analyses, we log-transformed the observations to adjust for non-normality, and then analyzed using t-tests. A P-value of ≤ 0.05 was considered statistically significant. We then performed multivariable modeling to identify independent associations with vitamin D status in a concatenated dataset of clinical and immune phenotypic variables. Specifically, we used multivariate imputation by chained equations to generate 10 datasets in which missing data were imputed using the predictive mean matching method. We selected variables associated with vitamin D status in each of the 10 imputed datasets using the elastic net method for logistic regression models on 500 bootstrap samples. Stability of the selection was checked by selecting variables with different tuning parameters in the elastic net. In parallel, we also selected variables associated with vitamin D status using a random forest approach. Using the subset of variables selected with both approaches, we generated a final logistic regression model, using backward/forward selection based on the Akaike Information Criterion. Analyses were performed using Stata v13.1 (STATA Corp, College Station, TX) and R, using the cmprsk package for Fine and Gray models (https://CRAN.R-project.org/package=cmprsk), the mice package for imputation (https://www.jstatsoft.org/v45/i03/). the glmnet package for the elastic net method (http://www.jstatsoft.org/v33/i01/). the VSURF package for variable selection in random forests (https://CRAN.R-project.org/package=VSURF). and the MASS package for the final backward/forward selection process (The R Project for Statistical Computing, http://www.rproject.org). Prism 8.0 software (GraphPad) was used for the analysis of flow cytometry results. The study was approved by the Institutional Review Boards of the University of Pennsylvania and Columbia University Medical Center.
Results
Patient Characteristics
Patient and transplant characteristics are summarized in Table 1. The median follow-up was 49 months (range 1.5-62 months). The median day-30 post-HSCT vitamin D level was 20 ng/mL (range 6-50 ng/mL), reflecting vitamin D deficiency in half of the patients in the cohort. Vitamin D insufficiency (25OHD < 30 ng/mL) was present in 42/53 (79%) of the patients.
Table 1.
Patient Characteristics (N=53)
| Characteristic | Value |
|---|---|
|
| |
| Recipient age, median in years (range) | 55 (20 – 71) |
|
| |
| Recipient sex, male, n (%) | 28 (53) |
|
| |
| Donor age, median in years (range) | 40 (20 – 70) |
|
| |
| Donor sex, male, n (%) | 28 (53) |
|
| |
| Disease type, n (%) | |
| Myeloid | 33 (62) |
| Acute myeloid leukemia | 15 (28) |
| Myelodysplastic syndrome | 10 (19) |
| Primary myelofibrosis | 5 (9) |
| Chronic myelogenous leukemia | 3 (6) |
| Lymphoid | 20 (38) |
| Non-Hodgkin lymphoma | 8 (15) |
| Acute lymphoblastic leukemia | 6 (11) |
| Chronic lymphocytic leukemia | 2 (4) |
| Hodgkin lymphoma | 2 (4) |
| Multiple myeloma/plasma cell leukemia | 2 (4) |
|
| |
| Graft source, n (%) | |
| Peripheral blood stem cells | 44 (83) |
| Bone marrow | 9 (17) |
|
| |
| Donor type, n (%) | |
| Sibling | 23 (43) |
| Unrelated | 30 (57) |
|
| |
| HLA compatibility, n (%) | |
| 8/8 match | 43 (81) |
| 7/8 match | 10 (19) |
|
| |
| Conditioning intensity, n (%) | |
| Myeloablative | 25 (47) |
| Reduced intensity | 28 (53) |
|
| |
| GVHD prophylaxis, n (%) | |
| Tacrolimus/methotrexate | 41 (77) |
| Cyclosporine/methotrexate | 8 (15) |
| Othera | 4 (8) |
|
| |
| Pre-transplant albumin, median (range) | 4.0 (3.0 – 4.7) |
|
| |
| Day-30 albumin, median (range) | 3.6 (1.8 – 4.6) |
|
| |
| Required TPN post-transplant, n (%) | 22 (41) |
GVHD, graft-versus-host disease; HLA, human leukocyte antigen; TBI, total body irradiation; TPN, total parenteral nutrition.
Tacrolimus/sirolimus (n=3), mycophenolate mofetil/prednisone (n=1)
Clinical Predictors of Vitamin D Deficiency
We first examined whether day-30 vitamin D levels were associated with patient, disease, and transplant characteristics (Table 2). Surprisingly, vitamin D deficiency was more common in younger HCT recipients (P < 0.01) and there were lower vitamin D levels in patients who underwent myeloablative conditioning (MAC) without reaching statistical significance (P = 0.08). A depiction of vitamin D levels in relation to patient age and conditioning intensity is shown in Supplementary Figure 1A. Vitamin D deficiency was also more common in patients undergoing HCT for a lymphoid malignancy (P = 0.02) and in those that required TPN (P < 0.01). Moreover, a direct relationship was observed between day-30 albumin concentrations and vitamin D levels (P = 0.03), shown in Supplementary Figure 1B. We also explored the correlation between the intensity of the conditioning regimen and day-30 vitamin D levels, using several different cutoffs (25OHD <15 ng/mL, <20 ng/mL, and <30 ng/mL). Compared with RIC HCT recipients, a greater proportion of patients undergoing MAC were vitamin D insufficient at day-30 post HCT, defined as vitamin D levels <30 ng/mL (92% vs. 65%; P = 0.02).
Table 2.
Associations of Day-30 Vitamin D Levels with Disease and Transplant Characteristics a
| Variable | Pearson Correlation | P |
|---|---|---|
|
| ||
| Recipient age | 0.431 | 0.001 |
|
| ||
| Donor age | 0.010 | 0.48 |
|
| ||
| Baseline albumin | 0.102 | 0.47 |
|
| ||
| Day 30 albumin | 0.300 | 0.03 |
|
| ||
| Variable | Mean Day-30 Vitamin D Level (range) | P |
|
| ||
| Disease type | 0.02 | |
| Myeloid | 23.4 (8 – 50) | |
| Lymphoid | 17.6 (6 – 36) | |
|
| ||
| GVHD prophylaxis | 0.58 | |
| CSA/MTX | 20.6 (6 – 36) | |
| TAC/MTX | 22.0 (9 – 50) | |
|
| ||
| Donor source | 0.63 | |
| Sibling | 20.5 (6 – 50) | |
| Unrelated | 21.8 (9 – 45) | |
|
| ||
| Donor sex | 0.26 | |
| Male | 22.1 (9 – 37) | |
| Female | 20.2 (6 – 50) | |
|
| ||
| Recipient sex | 0.50 | |
| Male | 21.8 (9 – 50) | |
| Female | 20.5 (6 – 37) | |
|
| ||
| Conditioning | 0.08 | |
| Myeloablative | 18.5 (8 – 37) | |
| Reduced intensity | 23.6 (6 – 50) | |
|
| ||
| Graft type | 0.73 | |
| Peripheral blood | 21.5 (6 – 50) | |
| Bone marrow | 20.0 (9 – 37) | |
|
| ||
| HLA match | 0.65 | |
| 8/8 | 21.7 (6 – 50) | |
| 7/8 | 19.3 (10 – 34) | |
|
| ||
| TPN | 0.0009 | |
| Yes | 16.1 (6 – 37) | |
| No | 24.9 (9 – 50) | |
CSA, cyclosporine; GVHD, graft-versus-host disease; HLA, human leukocyte antigen; MTX, methotrexate; TAC, tacrolimus; TPN, total parenteral nutrition
Comparisons were performed after natural-log transformation of vitamin D levels. Significant P-values are highlighted in bold.
Vitamin D Deficiency on Day 30 after HCT Increases Risk of Acute GVHD
The cumulative incidence of acute grade 2-4 GVHD was 32.1% (95% confidence interval [CI], 21.4% to 45.5%) at day 100 and 50.9% (95% CI, 37.9% to 63.9%) at day 180 post-HCT. To assess whether day-30 vitamin D levels were predictive of acute grade 2-4 GVHD and organ-specific involvement, we used a landmark analysis starting on the day of serum vitamin D level measurement (approximately day 30) and examined the association between vitamin D deficiency and the outcome. A multivariable cumulative incidence analysis was used, with adjustment for significant covariates after backward elimination (GVHD prophylaxis regimen, conditioning intensity and HLA mismatch). We analyzed patients in two groups according to their median day-30 vitamin D level (<20 and ≥20 ng/mL), which coincidentally corresponded with the accepted cutoff that defines vitamin D deficiency.(6) This analysis is summarized in Supplementary Table 2.
We did not observe a significant association between day-30 vitamin D levels and acute grade 2-4 GVHD (HR 0.56; P = 0.11) in the entire cohort but saw a trend toward earlier GVHD in the vitamin D low group (Figure 1). We then evaluated whether day-30 vitamin D levels were associated with specific organ involvement in acute GVHD, particularly skin GVHD. We found an association between lower day-30 vitamin D levels and higher risk of acute skin GVHD (HR 0.32; P = 0.05). We did not detect a relationship between acute gastrointestinal (GI) (HR 0.96; P = 0.92) or hepatic GVHD (HR 0.76; P = 0.66) and day-30 vitamin D levels. Chronic GVHD and OS also showed no significant correlations with day-30 vitamin D levels (Supplementary Table 2).
Figure 1.
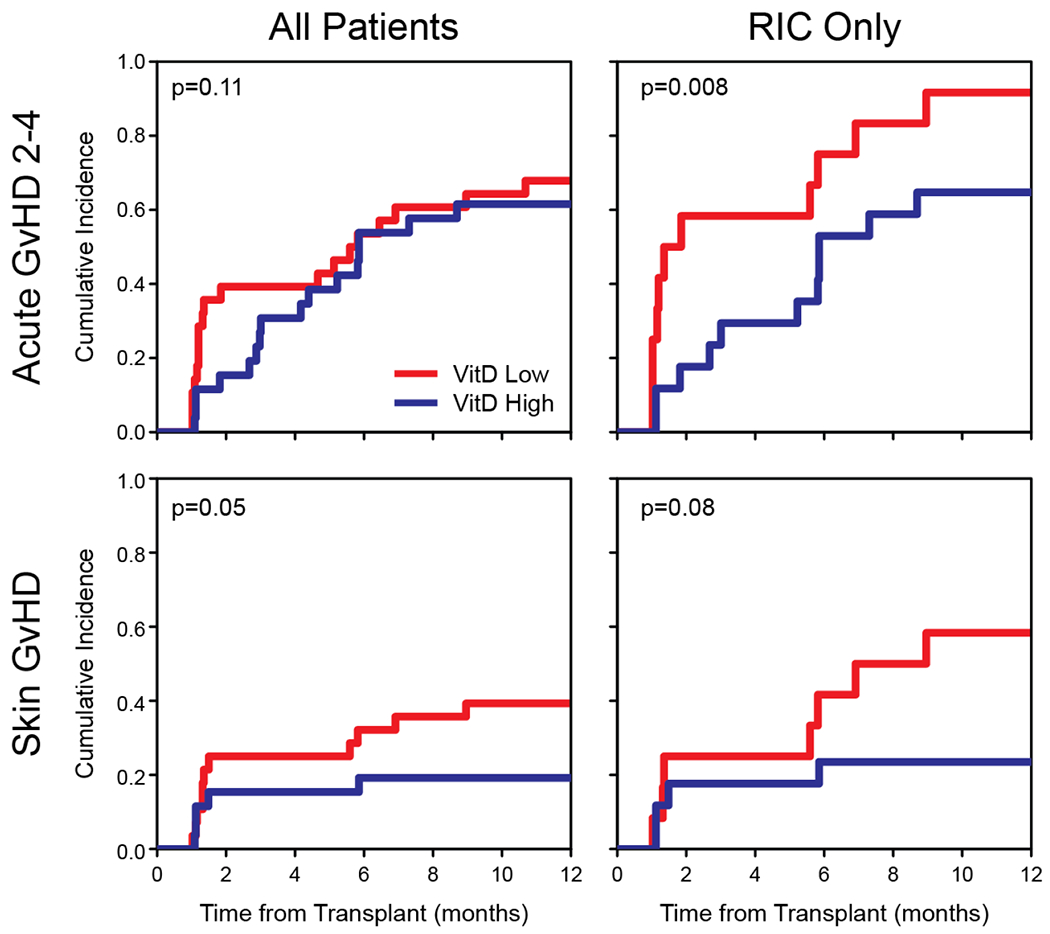
Cumulative incidence plots of acute grade 2 – 4 GVHD (A-B) and skin GVHD (C-D) according to day-30 vitamin D levels. Low vitamin D levels and high vitamin D levels were defined as 25OHD < 20 ng/mL and ≥ 20 ng/mL, respectively. Plots are displayed for all patients and reduced-intensity conditioning subset.
Since the majority of MAC patients were vitamin D deficient as opposed to the RIC subset, which had greater heterogeneity in vitamin D levels, we performed a separate analysis in the subset of RIC recipients (Figure 1); in this group, low vitamin D levels were significantly associated with a higher risk of acute grade 2-4 GVHD (HR 0.35; P = 0.008) and nearly all vitamin D low patients in this cohort developed acute GVHD, with the majority of cases occurring by day 60. Here again, the protection against skin GVHD was more dominant than other organs (HR 0.28; P = 0.08).
Vitamin D Deficiency After HCT is Associated with a Gene Expression Signature of Increased T-cell Proliferation, Dampened Interferon Response and Epigenetic Modulation
To determine the immune signature of vitamin D deficiency after HCT, we first performed whole transcriptome gene expression analysis with the goal of identifying underlying molecular mechanisms that are influenced by vitamin D and contribute to or protect against GVHD. Gene expression levels were measured from isolated T lymphocytes of 16 patients, 8 with high vitamin D (median 35 ng/mL, range 32-50) and 8 with low vitamin D (median 12.5 ng/mL, range 8-15) levels. We found 182 genes whose expression was correlated with vitamin D status, and 88 genes with anti-correlation with vitamin D (Supplementary Table 3). We then used a gene set enrichment analysis to identify the functionality of those genes, and the pathways that govern their expression (Figure 2A–B). All P-values noted in this section represent FDR correction.
Figure 2.
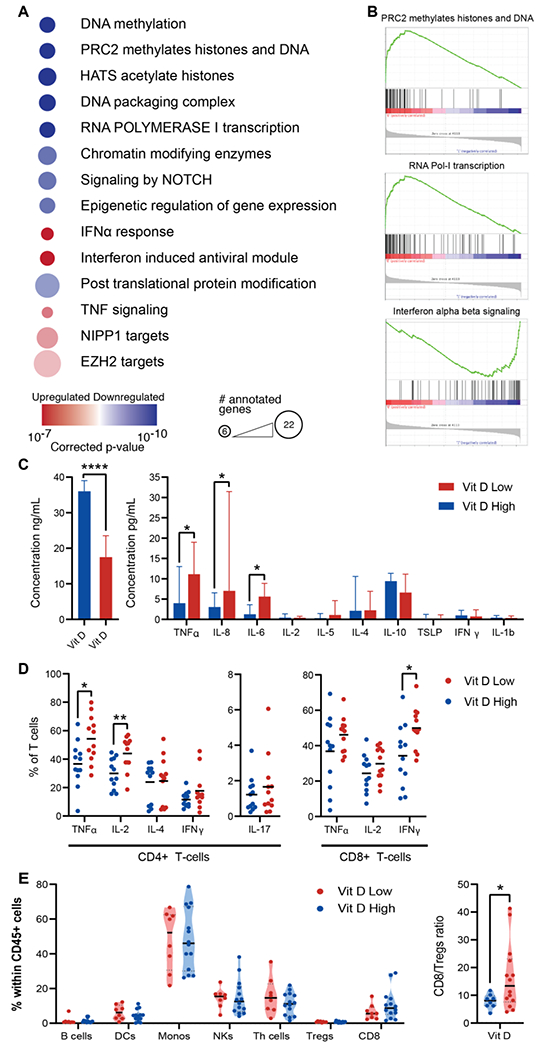
A. Gene set enrichment analysis of 182 correlated (upregulated) and 88 anti-correlated (downregulated) genes from a gene expression analysis of 8 patients with high and 8 patients with low vitamin D levels on day 30. Prominent gene sets from the Molecular Signature Database (MSigDB) v7.1 are listed in order of their adjusted p-values. Color signifies the direction of the correlation with vitamin D and size of each node indicates the number of annotated genes. B. Representative enrichment plots of prominent gene sets that are differentially expressed between the groups. C. Left – vitamin D levels in the vitamin D-hi and vitamin D-lo groups. Right - comparison of serum cytokine levels. D. Comparison of cytokine production in CD4+ and CD8+ T cells stimulated with PMA and lonomycin on day 30 according to vitamin D levels (above and below 30 ng/mL). E. Violin plots show the different proportions of major cell subsets out of the total of CD45+ cells in the peripheral blood between vitamin D-hi and vitamin D-lo patients. The CD8/Treg ratio is significantly lower for vitamin D-hi patients. Medians and interquartile ranges are plotted and comparisons use the Mann-Whitney U test. * P<0.05 ** P<0.01 **** P<0.0001
The genes with the strongest negative correlations with vitamin D levels involved DNA methylation, histone acetylation, chromosome packaging and RNA Polymerase I transcription (all with P ≤ 10−9), including 12 histone genes that are required for entry into S phase, indicating restrained T-cell proliferation in patients with adequate day-30 vitamin D levels. A highly significant association (P = 1.3x10−10) was with a gene set involving methylation by the Polycomb Repressive Complex 2 (PRC2), an epigenetic protein complex that regulates gene expression primarily by catalyzing trimethylation of histone H3 on lysine 27 (H3K27me3). These results indicate that normal vitamin D levels are important in restraining T-lymphocyte proliferation early after HCT and suggest an epigenetic mechanism for the protective effect against GVHD.
The genes that positively correlated with vitamin D levels matched the expression profile of a type I IFN response by enrichment analysis (lowest P value = 3.4x10−7) due to overexpression of several IFN-stimulated genes (IFI44, IFI44L, IRF7, MX1, MX2, TNFSF10, OAS1, TDRD7, TREX1, IFIH1, HERC6, PNPT1). While a type I IFN response is generally considered proinflammatory and critical for innate antiviral responses, its role in T cell responses after allogeneic HCT is more complex and seems protective against GVHD.(25,26) In addition, IF44L provides negative feedback regulation of the IFN response and may dampen inflammation.(27) Strikingly, one of the largest gene sets in the enrichment analysis contained 22 genes that are regulated by EZH2, a histone methyltransferase which is the primary catalytic subunit of PRC2 (P = 0.016).
Low Vitamin D Levels on Day 30 After HCT are Associated with Increased Inflammatory Cytokine Production
To assess the impact on the inflammatory environment on day 30 after HCT, we examined the associations between vitamin D levels and serum concentrations of major cytokines. We evaluated these associations using the clinical cutoffs for vitamin D insufficiency (25OHD <30 ng/mL) and deficiency (25OHD <20 ng/mL) and observed that vitamin D levels above 30 ng/mL correlated with lower levels of certain inflammatory cytokines (Figure 2C), whereas a cutoff of 20 ng/mL seemed insufficient to restrain cytokine levels (not shown). Specifically, serum levels of TNF-α were significantly increased in patients that were vitamin D insufficient (11.13 vs. 3.97 pg/mL; P = 0.02, Supplementary Figure 2). In addition, patients that were vitamin D insufficient had higher serum levels of IL-8 (7.02 vs. 3.04 pg/mL; P = 0.01) and IL-6 (5.62 vs. 1.27 pg/mL; P = 0.02). Because IL-8 is important for regulating migration and activation of neutrophils and is also regulated by them, we examined whether absolute neutrophil count (ANC) confounded this association, however, there was no difference in day-30 ANC between patients that had high versus low IL-8 levels (4.82 vs 3.26 THO/μL; p=0.27). In addition, we measured the day-30 serum concentrations of the known GVHD biomarkers, suppression of tumorigenicity 2 (ST2) and Regenerating islet-derived 3-alpha (REG3α).(28) The vitamin D-lo group had greater heterogeneity in biomarker levels with several patients exhibiting much higher levels than the vitamin D-hi group, however, statistical significance was not demonstrated (Supplementary Figure 3).
We further measured cytokine production in day-30 stimulated T-cells from 24 representative patients with the lowest (<15ng/mL) or highest (>25ng/mL) vitamin D levels. We found that patients with high vitamin D levels had lower production of IL-2 and TNF-α in CD4+ T-cells, and lower IFN-γ production in CD8+ T-cells (Figure 2D).
Increased ID3 and EZH2 Expression Characterizes T Cells from Patients with High Vitamin D Levels
To further investigate the mechanism by which high vitamin D levels restrain T cell proliferation and activation in post-transplant patients, we studied the expression of transcription factors known to be associated with the development of effector and memory T-cell populations, along with differentiation and activation surface markers. The gating strategy for major cell populations and multi-parameter data visualization using Uniform Manifold Approximation and Projection (UMAP) are shown in Supplementary Figure 4. Major cell populations had similar frequencies in vitamin D-hi and vitamin D-lo patients (Figure 2E, Supplementary Figure 5), however vitamin D-hi patients had a significantly lower ratio between CD8+ T-cells and regulatory T cells (Tregs). UMAPs showed clear separation between vitamin D-hi and vitamin D-lo patients (Figure 3A–C). In both groups there was a predominance of CD45RA-CCR7- effector memory cells that maintained high levels of expression of CD28 and CD27. Surprisingly, vitamin D-hi patients had significant expansion of cells expressing the DNA binding inhibitor ID3 with a subpopulation that also expressed high levels of EZH2 which has been associated with ID3 activation and silencing of several other transcription factors(29). High levels of ID3+ and EZH2+ T-cells were identified in CD8+, CD4+ conventional T cells (Tcons) and CD4+ Tregs and these cells were largely negative for other transcription factors that control T cell effector function (i.e., T-bet, RORγt, GATA-3, EOMES). In addition, the population of T-bet+ ID2+ cells, which are associated with enhanced effector differentiation(30–33), was similar in size but exhibited differences in phenotype – these cells largely retained CD27 and CD28 expression in vitamin D-hi patients, but had lower expression of these markers in vitamin D-lo patients, indicating a more differentiated memory phenotype.
Figure 3.
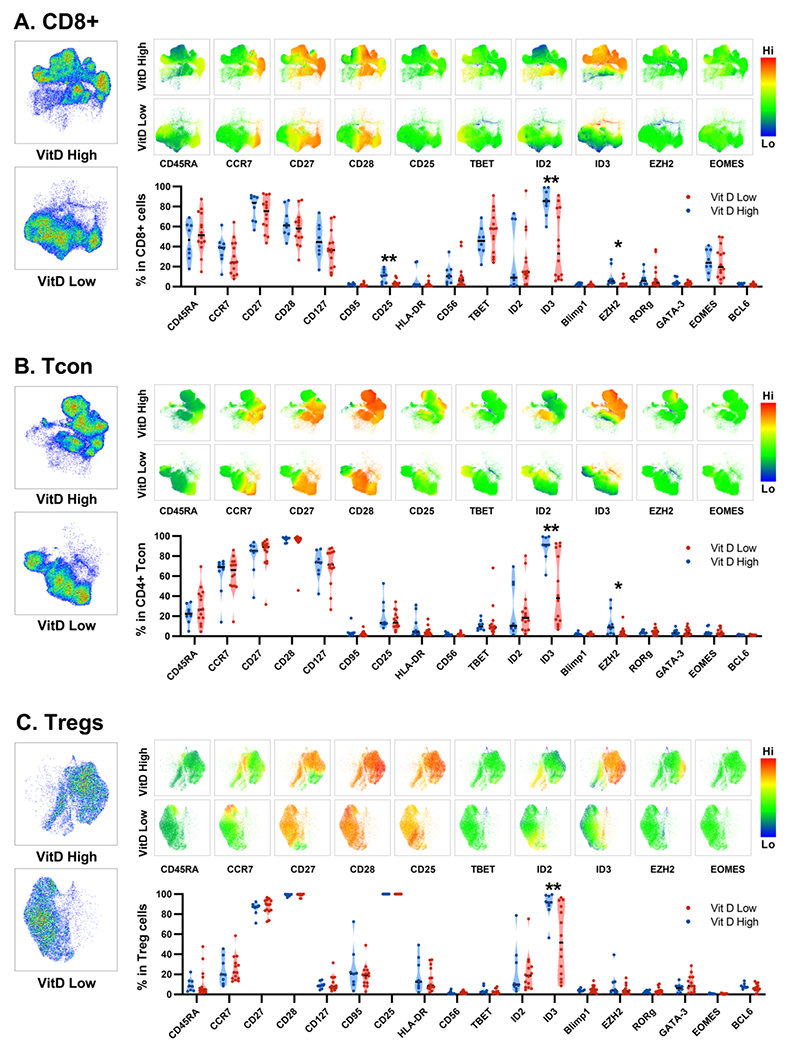
Flow cytometry data presented using UMAPS and specific markers displayed using heatmaps for vitamin D-hi vs. vitamin D-lo patients. Data presented separately for A. CD8+ T cells B. CD4+ conventional T cells (Tcons) and C. regulatory T cells (Tregs).
Vitamin D Levels Independently Correlate with ID3 Expression and are Associated with Expression of Trafficking Receptors
Our phenotypic and clinical data for vitamin D-hi and vitamin D-lo patients included a total of 132 immunological variables (Supplementary Table 4) and 7 demographic and clinical variables. To identify independent variables that are associated with vitamin D level, and account for clinical covariates, we used multivariable logistic regression. To maximize the number of observations and variables, we handled missing data by applying a validated imputation method that generated 10 imputed datasets. We performed variable selection on these datasets using elastic net and random forest methods, followed by a backward/forward selection procedure. We identified 6 variables that positively correlated (odds ratios > 1) with vitamin D levels in the majority of the datasets (Figure 4A). These variables included the expression of ID3 and Blimp1 in CD8+ T-cells, the proportion of CD4+FoxP3+ T-cells that were CD45RA+, and the levels of expression of CCR5 on CD8+ T-cells and cutaneous lymphocyte antigen (CLA) on CD4+ and CD8+CD45RO+ T-cells. ID3 exhibited the most striking differential expression, with 8 of 11 vitamin D-hi patients exhibiting at least 85% positivity within CD8+ T cells, but 9 of 12 vitamin D-lo patients being <42% (Figure 4B). There were no variables that correlated negatively with vitamin D status that met the selection criteria for the model. Although our model selected ID3+ CD8+ T-cells specifically, we found a highly significant correlation between ID3 expression levels in CD8+, CD4+ Tcons and Tregs as well as in subsets of effector memory T-cells within these populations (Supplementary Figure 6), implying that ID3 expression in all major T cell subsets is strongly correlated with vitamin D level. To test the robustness of these results, we conducted a principal component analysis (PCA) using ID3 expression in 6 populations (CD8+, CD8+ Tem, CD4+, CD4+ Tem, CD4+FoxP3+, CD4+FoxP3+ Tem) and found that they accounted for 98.9% of the variability in vitamin D status (Figure 4C).
Figure 4.
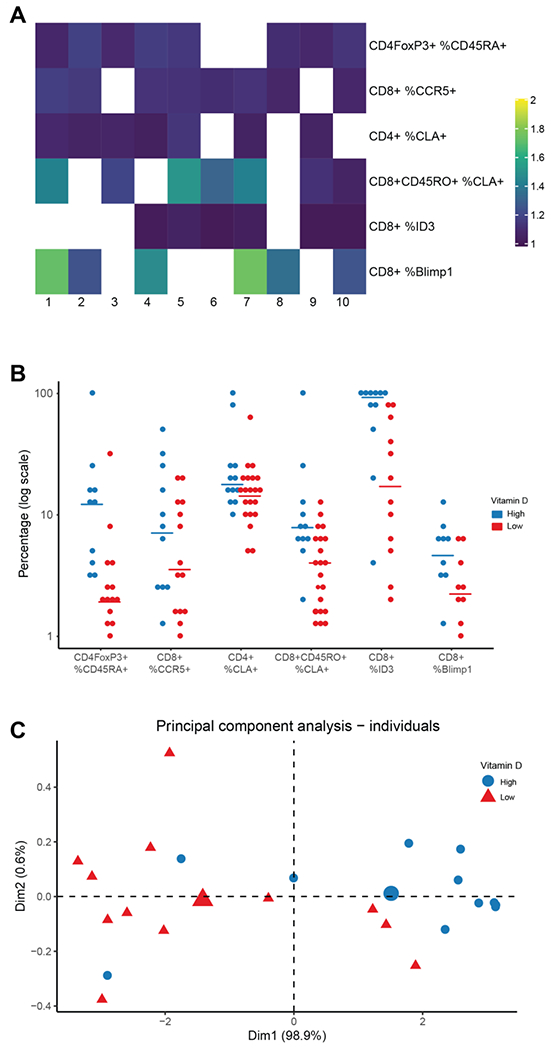
Multivariable logistic regression identifies 6 immunologic variables with a positive independent association with vitamin D-hi status. A. Heatmap of selected variables (lines) in 10 imputed data sets (columns). All OR are > 1. OR with lower bound of the confidence interval (CI) ≥ 0.95 are displayed. B. Plot of selected variables by vitamin D status displayed on log scale. Each dot corresponds to the value of one patient, lines correspond to median in each group. C. Principal Component Analysis of vitamin D status using 17 markers.
In summary, using samples from allogeneic HCT recipients, we demonstrate that adequate levels of vitamin D early post-transplant are associated with restrained T-cell proliferation and proinflammatory cytokine production, a favorable ratio of Tregs to CD8+ T cells, and overexpression of ID3, corresponding to an activated EZH2 signature.
Discussion
In this study, we found that vitamin D deficiency is frequent early after HCT. Vitamin D deficiency was more common in younger patients undergoing myeloablative conditioning and requiring TPN. Interestingly, low vitamin D levels on day 30 post-HCT were associated with a higher risk of acute GVHD in RIC recipients, demonstrating the potential impact that this nutritional deficiency may have on transplant outcomes. In addition, vitamin D deficiency early after HCT was associated with increased inflammatory cytokine production and a gene expression signature of cellular proliferation, histone methylation, and EZH2 activation. We identified a very strong association between vitamin D level and expression of ID3, a known target of EZH2. To the best of our knowledge, this study is the first to characterize the immunologic impact of vitamin D deficiency early after HCT and to identify an epigenetic mechanism that potentially contributes for the protective effect of vitamin D against GVHD.
Surprisingly, younger patients had a higher incidence of vitamin D deficiency in our cohort compared to older patients. Risk factors for vitamin D deficiency in non-HCT patients include inadequate exposure to ultraviolet radiation, malnutrition, obesity, and kidney disease,(6) however, in our cohort of HCT recipients who did not receive routine supplementation, younger age, MAC, and TPN were significant predisposing factors for vitamin D deficiency; these populations might benefit more than others from peri-transplant vitamin D repletion, a hypothesis that could be tested clinically.
We detected an inverse relationship between day-30 vitamin D levels and acute GVHD in RIC HCT recipients. This association is consistent with earlier preclinical reports in which vitamin D was found to mitigate T lymphocyte alloreactivity,(34,35) and several observational studies that were recently summarized.(13) Notably, we did not observe a correlation between vitamin D deficiency and acute GVHD in MAC HCT. This is likely because most MAC HCT recipients had significant vitamin D depletion by day 30 and the absence of variability in day-30 vitamin D levels precluded a meaningful analysis in this subset of patients. We also found a correlation between lower vitamin D levels specifically with acute cutaneous GVHD, consistent with reports that associated vitamin D specifically with cutaneous immunity(36).
Numerous mechanisms connect vitamin D with immunologic function,(37) but which of these mechanisms is primarily responsible for the protection against GVHD in humans has not been determined. To this end, we studied the immune signature associated with post-transplant vitamin D status by using multi-faceted profiling of peripheral blood samples with a focus on T-cell phenotype, function and gene expression. We identified several notable findings. First, vitamin D was associated with restrained proinflammatory cytokine production and T-cell proliferation. Specifically, we found an inverse association between vitamin D levels and production of TNF-α and IL-6 which have well-described roles in the pathophysiology of GVHD in animal models; therapeutic efficacy for TNF-α and IL-6 blockade has also been demonstrated in humans.(38–40) We also found elevated IL-8 levels in vitamin D deficiency, consistent with preclinical data in the non-HCT setting that demonstrated downregulation of IL-8 to be one of the anti-inflammatory mechanisms of Vitamin D.(41) Cytokine production in stimulated T cells also demonstrated an association between vitamin D status and IFN-γ and IL-2 production, which both have a complex role in GVHD pathogenesis.(42–45) These data suggest vitamin D plays an important role in regulating the cytokine milieu early after HCT.
In addition, we found that vitamin D was associated with a T-cell gene expression signature consistent with an interferon type I response, which in murine models was found to be favorable in terms of balancing the GVH and GVL responses.(25) A primary source of type I interferon is plasmacytoid dendritic cells, which are capable of suppressing GVHD.(46,47) It is known that the complex effects of vitamin D on dendritic cell maturation and activation skew them towards tolerogenic properties(48), although a direct impact of vitamin D on plasmacytoid dendritic cells has not been fully elucidated and is worthy of further investigation.
Finally, we identified a strong and novel association between vitamin D levels and ID3 expression in T cells. We previously demonstrated that ID3 expression is epigenetically controlled by EZH2 in murine T cells(29) and our current gene expression and flow cytometry data together suggest that a similar mechanism exists in humans and is impacted by vitamin D. This is of particular significance as inhibition of EZH2 is a promising therapeutic strategy to alleviate GVHD(49), and EZH2 has recently been identified as critical in maintaining the functional status of chimeric antigen receptor (CAR)-T cells.(50) Thus, the interaction between vitamin D and EZH2 may play an important role in various types of cancer immunotherapies. In addition, EZH2 has a known role in cancer pathogenesis and the first EZH2 inhibitor has already been improved as cancer therapy,(51–53) further underlining the need to decipher a potential interaction with vitamin D signaling.
EZH2 primarily impacts gene transcription in T cells after TCR activation.(49) In mice, EZH2 activates the transcription of ID3 which in turn guides memory development.(32,33) EZH2 also silences ID2, Blimp-1, and EOMES in antigen-experienced CD8+ T cells to curb effector differentiation. Elevated levels of ID2, Blimp-1, EOMES, and T-bet are associated with enhanced effector differentiation but decreased memory potential.(30–33,54) Based on our data, adequate levels of vitamin D are associated with ID3 expression, which may be required to restrain effector differentiation. This may explain why vitamin D-hi patients were relatively protected against GVHD.
The main limitations of our study include its single-center design, retrospective nature, and small sample size. Because our objective was to identify a biologic mechanism, the immunologic assays were performed on groups of patients with the highest and lowest vitamin D levels in our dataset, which may not have been perfectly matched on other variables. To overcome this limitation, we included demographic and clinical variables in the multivariable analysis and identified immunophenotypic variables that independently correlated with vitamin D status (Figure 4). Nevertheless, this is the first study to identify a strong association between vitamin D levels early post-transplant and ID3 expression in T cells and propose an epigenetic mechanism for the function of vitamin D. It is also the first human study to identify the role of EZH2-ID3 signaling in alloreactive T cells. Our findings provide rationale for investigating this pathway further in allogeneic HCT recipients. We also did not directly address the impact of vitamin D levels on antigen presenting cells and other cells and cytokines of the innate immune system that are known to be regulated by vitamin D but are more challenging to study without availability of fresh blood and tissue samples. Finally, we did not study the expression level of the vitamin D receptor in T cells or the potential impact of vitamin D on the antitumor response, which has been suggested by several studies.(55,56)
In summary, we conclude that vitamin D deficiency is frequent early after allogeneic HCT and predisposes patients to a higher risk of acute GVHD. The findings of our study demonstrate a novel association between vitamin D and EZH2-ID3 signaling as a potential mechanism that restrains proliferation and effector function of alloreactive T cells. These findings will require confirmation, ideally in a prospective trial of vitamin D repletion with appropriate correlative studies to measure the impact of this intervention.
Supplementary Material
Highlights.
Vitamin D deficiency is common after allogeneic HCT and is associated with GVHD, primarily skin.
Low vitamin D levels are associated with a high CD8/Treg ratio and elevated inflammatory cytokines.
Adequate levels of vitamin D are associated with an EZH2-ID3 signature that regulates T-cell differentiation.
Acknowledgements
The authors gratefully acknowledge the grant support of the National Institutes of Health (grants HL143424 to R.R., HL127351 to Y.Z., CA16520 to D.L.P. and R.H.V., AI093870 to A.J.Y., and CA013696 to the flow cytometry core at the Herbert Irving Comprehensive Cancer Center, Columbia University).
Financial support:
National Institutes of Health grants HL143424 to R.R., HL127351 to Y.Z., CA16520 to D.L.P. and R.H.V., AI093870 to A.J.Y., and CA013696 to the flow cytometry core at the Herbert Irving Comprehensive Cancer Center, Columbia University
Disclosures
Dr. Vonderheide reports consulting fees or honoraria from Medimmune and Verastem; and research funding from Fibrogen, Janssen, and Lilly. He is a member of the Lustgarten Therapeutics Advisory working group. He is an inventor on licensed patents relating to cancer cellular immunotherapy and cancer vaccines, and receives royalties from Children’s Hospital Boston for a licensed research-only monoclonal antibody. Dr. Porter reports consulting fees or honoraria from Novartis, Kite/Gilead, Incyte, Glenmark, Janssen and Adepcet Bio. He is an inventor on licensed patents to Tmunity and Novartis. He owns stock in Roche/Genentech. He is a Board Member at the National Marrow Donor Program. Dr. Reshef reports consulting fees or honoraria from Gilead, Novartis, BMS, Bayer, Synthekine, Atara, TScan and Regeneron. Other authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant 2020;26(8):e177–e82 doi 10.1016/j.bbmt.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 2012;119(1):296–307 doi 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reshef R, Saber W, Bolanos-Meade J, Chen G, Chen YB, Ho VT, et al. Acute GVHD Diagnosis and Adjudication in a Multicenter Trial: A Report From the BMT CTN 1202 Biorepository Study. J Clin Oncol 2021:JCO2000619 doi 10.1200/JCO.20.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet 2009;373(9674):1550–61 doi 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang CY, Leung PS, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol 2013;45(2):217–26 doi 10.1007/s12016-013-8361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357(3):266–81 doi 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood 2005;106(10):3490–7 doi 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 8.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol 2010;11(4):344–9 doi 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 9.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol 2000;164(9):4443–51. [DOI] [PubMed] [Google Scholar]
- 10.Kreutz M, Andreesen R. Induction of human monocyte to macrophage maturation in vitro by 1,25-dihydroxyvitamin D3. Blood 1990;76(12):2457–61. [PubMed] [Google Scholar]
- 11.Ferreira GB, Gysemans CA, Demengeot J, da Cunha JP, Vanherwegen AS, Overbergh L, et al. 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J Immunol 2014;192(9):4210–20 doi 10.4049/jimmunol.1302350. [DOI] [PubMed] [Google Scholar]
- 12.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol). J Clin Invest 1984;74(4):1451–5 doi 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soto JR, Anthias C, Madrigal A, Snowden JA. Insights Into the Role of Vitamin D as a Biomarker in Stem Cell Transplantation. Frontiers in immunology 2020;11:966 doi 10.3389/fimmu.2020.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caballero-Velazquez T, Montero I, Sanchez-Guijo F, Parody R, Saldana R, Valcarcel D, et al. Immunomodulatory Effect of Vitamin D after Allogeneic Stem Cell Transplantation: Results of a Prospective Multicenter Clinical Trial. Clin Cancer Res 2016;22(23):5673–81 doi 10.1158/1078-0432.CCR-16-0238. [DOI] [PubMed] [Google Scholar]
- 15.Hong S, Ferraro CS, Hamilton BK, Majhail NS. To D or not to D: vitamin D in hematopoietic cell transplantation. Bone Marrow Transplant 2020;55(11):2060–70 doi 10.1038/s41409-020-0904-7. [DOI] [PubMed] [Google Scholar]
- 16.Mebius RE. Vitamins in control of lymphocyte migration. Nat Immunol 2007;8(3):229–30 doi 10.1038/ni0307-229. [DOI] [PubMed] [Google Scholar]
- 17.Orgaz-Molina J, Buendia-Eisman A, Arrabal-Polo MA, Ruiz JC, Arias-Santiago S. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol 2012;67(5):931–8 doi 10.1016/j.jaad.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Peroni DG, Piacentini GL, Cametti E, Chinellato I, Boner AL. Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. Br J Dermatol 2011;164(5):1078–82 doi 10.1111/j.1365-2133.2010.10147.x. [DOI] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15(6):825–8. [PubMed] [Google Scholar]
- 20.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21(3):389–401 e1 doi 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant 2016;22(1):4–10 doi 10.1016/j.bbmt.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litvin O, Schwartz S, Wan Z, Schild T, Rocco M, Oh NL, et al. Interferon alpha/beta Enhances the Cytotoxic Response of MEK Inhibition in Melanoma. Mol Cell 2015;57(5):784–96 doi 10.1016/j.molcel.2014.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102(43):15545–50 doi 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storey JD, Tibshirani R. Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods Mol Biol 2003;224:149–57 doi 10.1385/1-59259-364-X:149. [DOI] [PubMed] [Google Scholar]
- 25.Robb RJ, Kreijveld E, Kuns RD, Wilson YA, Olver SD, Don AL, et al. Type I-IFNs control GVHD and GVL responses after transplantation. Blood 2011;118(12):3399–409 doi 10.1182/blood-2010-12-325746. [DOI] [PubMed] [Google Scholar]
- 26.Swimm A, Giver CR, DeFilipp Z, Rangaraju S, Sharma A, Ulezko Antonova A, et al. Indoles derived from intestinal microbiota act via type I interferon signaling to limit graft-versus-host disease. Blood 2018;132(23):2506–19 doi 10.1182/blood-2018-03-838193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeDiego ML, Martinez-Sobrido L, Topham DJ. Novel Functions of IFI44L as a Feedback Regulator of Host Antiviral Responses. J Virol 2019;93(21) doi 10.1128/JVI.01159-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartwell MJ, Ozbek U, Holler E, Renteria AS, Major-Monfried H, Reddy P, et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI insight 2018;3(16) doi 10.1172/jci.insight.124015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He S, Liu Y, Meng L, Sun H, Wang Y, Ji Y, et al. Ezh2 phosphorylation state determines its capacity to maintain CD8(+) T memory precursors for antitumor immunity. Nature communications 2017;8(1):2125 doi 10.1038/s41467-017-02187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat Immunol 2014;15(12):1104–15 doi 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 2012;338(6111):1220–5 doi 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji Y, Pos Z, Rao M, Klebanoff CA, Yu Z, Sukumar M, et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat Immunol 2011;12(12):1230–7 doi 10.1038/ni.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang CY, Best JA, Knell J, Yang E, Sheridan AD, Jesionek AK, et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat Immunol 2011;12(12):1221–9 doi 10.1038/ni.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenblatt J, Bissonnette A, Ahmad R, Wu Z, Vasir B, Stevenson K, et al. Immunomodulatory effects of vitamin D: implications for GVHD. Bone Marrow Transplant 2010;45(9):1463–8 doi 10.1038/bmt.2009.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joseph RW, Bayraktar UD, Kim TK, St John LS, Popat U, Khalili J, et al. Vitamin D receptor upregulation in alloreactive human T cells. Hum Immunol 2012;73(7):693–8 doi 10.1016/j.humimm.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 36.Yamanaka KI, Kakeda M, Kitagawa H, Tsuda K, Akeda T, Kurokawa I, et al. 1,24-Dihydroxyvitamin D(3) (tacalcitol) prevents skin T-cell infiltration. Br J Dermatol 2010;162(6):1206–15 doi 10.1111/j.1365-2133.2010.09692.x. [DOI] [PubMed] [Google Scholar]
- 37.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 2008;8(9):685–98 doi 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korngold R, Marini JC, de Baca ME, Murphy GF, Giles-Komar J. Role of tumor necrosis factor-alpha in graft-versus-host disease and graft-versus-leukemia responses. Biol Blood Marrow Transplant 2003;9(5):292–303. [DOI] [PubMed] [Google Scholar]
- 39.Couriel D, Saliba R, Hicks K, Ippoliti C, de Lima M, Hosing C, et al. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood 2004;104(3):649–54 doi 10.1182/blood-2003-12-4241. [DOI] [PubMed] [Google Scholar]
- 40.Rager A, Frey N, Goldstein SC, Reshef R, Hexner EO, Loren A, et al. Inflammatory cytokine inhibition with combination daclizumab and infliximab for steroid-refractory acute GVHD. Bone Marrow Transplant 2011;46(3):430–5 doi 10.1038/bmt.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dauletbaev N, Herscovitch K, Das M, Chen H, Bernier J, Matouk E, et al. Down-regulation of IL-8 by high-dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up-regulation of DUSP1. Br J Pharmacol 2015;172(19):4757–71 doi 10.1111/bph.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang YG, Dey BR, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest 1998;102(12):2126–35 doi 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy WJ, Welniak LA, Taub DD, Wiltrout RH, Taylor PA, Vallera DA, et al. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Invest 1998;102(9):1742–8 doi 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welniak LA, Blazar BR, Anver MR, Wiltrout RH, Murphy WJ. Opposing roles of interferon-gamma on CD4+ T cell-mediated graft-versus-host disease: effects of conditioning. Biol Blood Marrow Transplant 2000;6(6):604–12 doi 10.1016/s1083-8791(00)70025-5. [DOI] [PubMed] [Google Scholar]
- 45.Choi J, Ziga ED, Ritchey J, Collins L, Prior JL, Cooper ML, et al. IFNgammaR signaling mediates alloreactive T-cell trafficking and GVHD. Blood 2012;120(19):4093–103 doi 10.1182/blood-2012-01-403196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol 2008;9(11):1253–60 doi 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 2000;164(5):2405–11 doi 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 48.Bscheider M, Butcher EC. Vitamin D immunoregulation through dendritic cells. Immunology 2016;148(3):227–36 doi 10.1111/imm.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He S, Xie F, Liu Y, Tong Q, Mochizuki K, Lapinski PE, et al. The histone methyltransferase Ezh2 is a crucial epigenetic regulator of allogeneic T-cell responses mediating graft-versus-host disease. Blood 2013;122(25):4119–28 doi 10.1182/blood-2013-05-505180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber EW, Parker KR, Sotillo E, Lynn RC, Anbunathan H, Lattin J, et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science 2021;372(6537):eaba1786 doi 10.1126/science.aba1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 2012;492(7427):108–12 doi 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- 52.Italiano A, Soria JC, Toulmonde M, Michot JM, Lucchesi C, Varga A, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 2018;19(5):649–59 doi 10.1016/S1470-2045(18)30145-1 [DOI] [PubMed] [Google Scholar]
- 53.Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science 2012;338(6113):1465–9 doi 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 2012;12(11):749–61 doi 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruns H, Buttner M, Fabri M, Mougiakakos D, Bittenbring JT, Hoffmann MH, et al. Vitamin D-dependent induction of cathelicidin in human macrophages results in cytotoxicity against high-grade B cell lymphoma. Sci Transl Med 2015;7(282):282ra47 doi 10.1126/scitranslmed.aaa3230. [DOI] [PubMed] [Google Scholar]
- 56.Radujkovic A, Kordelas L, Krzykalla J, Beelen DW, Benner A, Lehners N, et al. Pretransplant Vitamin D Deficiency Is Associated With Higher Relapse Rates in Patients Allografted for Myeloid Malignancies. J Clin Oncol 2017;35(27):3143–52 doi 10.1200/JCO.2017.73.0085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


