Abstract
The lasso peptide benenodin-1, a naturally occurring and bacterially produced [1]rotaxane, undergoes a reversible zip tie-like motion under heat activation, in which a peptidic wheel stepwise translates along a molecular thread in a cascade of “tail/loop pulling” equilibria. Conformational and structural analyses of four translational isomers, in solution and in the gas phase, reveal that the equilibrium distribution is controlled by mechanical and non-covalent forces within the lasso peptide. Furthermore, each dynamic pulling step is accompanied by a major restructuring of the intramolecular hydrogen bonding network between wheel and thread, which affects the peptide’s physico-chemical properties.
Keywords: lasso peptides, molecular switches, natural products, rotaxane, Supramolecular chemistry
Graphical Abstract

Step-by-Step: The naturally occurring peptide [1]rotaxane benenodin-1 undergoes a shuttling cascade under thermal activation and can be switched between four different states in water. The mechanism of wheel translation is reminiscent of synthetically derived molecular shuttles. Switchable peptide rotaxanes pave the way for a new generation of functional mechanically interlocked architectures solely made from biomolecules.
In biomolecules, interactions of non-covalent nature, such as hydrogen bonding and ion-ion pairing, play key roles in determining structure, stability and function. For example, protein folding and conformational changes in proteins are largely governed by non-covalent interactions between multiple contacts within the molecules.[1]
Lasso peptides are a class of ribosomally synthesized and post-translationally modified peptides with a slipknot-like structure in which the C-terminal tail is threaded through an isopeptide-bonded N-terminal macrolactam ring. These intertwined natural products are highly interesting synthetic targets due to a broad spectrum of bioactivities, including exceptional antimicrobial and antiviral activities, and remarkable heat and protease resistance.[2] The kinetically stabilized [1]rotaxane structure is enzymatically formed and mechanically interlocked by bulky amino acid (aa) residues (“stoppers”) in the thread, above and below the macrolactam ring. Several attempts to artificially synthesize lasso peptides have failed.[3] It is assumed that these knotted peptides incorporate a complex network of intramolecular forces, which contribute to their structural and physicochemical properties.[2b, 4] A better understanding of the non-covalent interactions within the molecules and the effects of the mechanical bond on the structure-property relationship of lasso peptides will facilitate their total synthesis.
Although some lasso peptides can unthread into their branched‐cyclic counterparts at elevated temperatures, the nature of this first-order process is irreversible.[4] A special case of this dynamic process is the lasso peptide benenodin-1, which was recently discovered in our lab.[5] Heat activation of benenodin-1 does not lead to an unthreading of the lasso structure but results in an exchange between two threaded states. Herein, we continue our investigations on benenodin-1 and report that it equilibrates between a set of four threaded isomers. Switching between these isomers resembles a reversible zip tie-like motion. A cascade of so-called loop and tail pulling equilibria enables a reversible and stepwise translation of the lasso peptide’s ring along the peptidic thread. Conformational analysis of the translational isomers reveals that mechanical and non-covalent forces govern the potential energy surface of wheel shuttling.
Benenodin-1 is a lasso peptide encoded in the genome of α-proteobacterium Asticcacaulis benevestitus, which was isolated from a soil sample near the town of Vorkuta (Russia).[6] Heterologous expression in Escherichia coli yields a mixture of truncated [1]rotaxanes in which five or six aa are cleaved from the C-terminus. In the initially isolated state of benenodin-1 ΔC5 (1), the macrolactam wheel (8 aa, 25 atoms) resides between the lock residues Glu-14 and Gln-15. In the following, the notation Q15-1 is used, which indicates the lock residue in C-terminal direction and thus the location of the wheel on the thread (Figure 1a). We have previously shown that heating of Q15-1 leads to an exchange between two stable translational isomers, Q15-1 and K17-1, in which the ring can slip over the pseudostopper Gln-15 (EA = 27 kcal·mol−1 for Q15-1 → K17-1).[5] Thus, benenodin-1 is a naturally occurring peptide that acts as a switchable rotaxane, a so-called molecular shuttle[7].
Figure 1.

(a) Chemical structure of the isolated translational isomer Q15-1 of benenodin-1 ΔC5. The isopeptide-bonded macrolactam wheel is schematically shown as blue ring. (b) LC-MS trace (extracted ion chromatogram, m/z 1007.0) of isomer Q15-1 after heat activation for 12 h at 80 °C in water. (c) Schematic illustration of the cascade of loop/tail pulling equilibria which results in four different translational isomers. The sequence Gln-13, Glu-14 and Gln-15 serves as an array of pseudostoppers blocking the passage of the macrolactam wheel. The Ala-16 residue is not shown for convenience. (d) (Pseudo)stoppers in benenodin-1 and their experimentally determined molecular volumes.[8] (e) Schematic shuttling equilibrium of Q13F variant 3. The absence of translational isomer F13-3 indicates that Gln is a pseudostopper, whereas Phe is a stopper residue.
Based on aa side chain volumes[8], which serve as an approximation for steric demand of (pseudo)stoppers (see Supporting Information for DFT calculations and detailed discussion), we suspected that prolonged thermal activation at elevated temperatures gives access to more translational isomers. The macrolactam wheel can potentially slip over additional side chains and, thus, stepwise move along the molecular thread. For example, Glu-14 (69 Å3), the lock residue in the N-terminal direction of isomer Q15-1, has a similar steric demand as Gln-15 (84 Å3) and may also show pseudostopper character (Figure 1d, Table S4). Indeed, LC-MS and HPLC analysis of Q15-1 after heating for 12 h at 80 °C in water reveal the presence of four detectable species ([1+2H]2+, m/z 1007.0) with isotopic patterns corresponding to monomeric isomers of peptide 1 (Figure 1b and S5). Besides the previously identified isomers Q15-1 and K17-1 (9.2 and 8.9 min, respectively), two small signals with later retention times emerge under prolonged heat activation (11.4 and 12.1 min). We have already observed a broad peak with later retention time for the E14A variant of benenodin-1.[5] Continuous heating for additional 12 h at 80 °C or heating at 95 °C results in similar peak distributions indicating that equilibrium is reached (Table S5). Thus, a cascade of equilibria between interconvertible, threaded species is more likely than an irreversible accumulation of non-threaded side-products (i.e., the cyclic-branched form).
Based on these findings, we hypothesized that benenodin-1 could undergo a zip tie-like motion, in which the sizes of the lasso’s loop and tail are gradually altered in a cascade of “loop/tail pulling” equilibria[9] (Figure 1c). Taking the example of translational isomer K17-1, the wheel moves stepwise over an array of pseudostoppers and can thus reversibly hurdle along the molecular thread under thermal activation following the sequence K17-1 → Q15-1 → E14-1 → Q13-1 with loop sizes of 8, 6, 5, and 4 aa, respectively.
The putative translational isomers E14-1 and Q13-1 were identified by heating experiments using benenodin-1 variants T12F (2) and Q13F (3), which were generated by molecular cloning (see Supporting Information). Similar to Lys-17 (99 Å3), it is anticipated that Phe residues (131 Å3) are sterically demanding enough to serve as stoppers for the macrolactam wheel (Figure 1d). Accordingly, LC-MS analysis shows four translational isomers for variant 2 but only three isomers for variant 3 (Figure S2). We conclude that the Phe-13 residue blocks the passage of the macrolactam ring (E14-3 → F13-3) and makes an isomer F13-3 inaccessible (Figure 1e). Hence, the peak with the latest LC-MS retention time, the most hydrophobic species, can be identified as translational isomer E14-n, whereas the second latest peak can be addressed to the Q13-isomers. In summary, Gln and Glu residues serve as pseudostoppers, whereas the macrolactam wheel cannot overcome Lys and Phe residues, which are accordingly stoppers under given conditions.
These findings were further supported by enzymatic digestions using two different proteases. Carboxypeptidase digestion of peptide 1 in all four states shows that only translational isomers with a sterically accessible tail, E14-1 (6 aa) and Q13-1 (7 aa), are C-terminally cleaved yielding the corresponding ΔC6 species (Figure S3). The two isomers with shorter tails, Q15-1 (5 aa) and K17-1 (3 aa), are virtually unaffected. A similar picture emerges from a thermolysin digestion. Whereas Met-19 is preferably removed from isomers Q13-1, E14-1, and Q15-1, the short tail of K17-1 decelerates a reaction at this position. However, we noticed accelerated loop cleavage for isomer K17-1 between Ser-9/Ile-10 and Ile-10/Leu-11 (Figure S4), which is presumably enabled by the larger loop size (8 aa). The generated [2]rotaxane species also exist in a mixture of translational isomers. Thus, the general mechanism of wheel translation can be established in peptidic [2]rotaxanes and potentially in other mechanically interlocked peptides, such as catenanes.[10]
The presence of four stable threaded states in thermodynamic exchange raises a fascinating question: what interactions control the equilibrium distribution in the thermally activated shuttling cascade? In a first approximation, three major contributions can be taken into account: (i) random walk of the wheel, (ii) mechanical strain in a tightened loop, and (iii) non-covalent interactions, including intramolecular wheel/thread and intermolecular peptide/solvent interactions. Considering a molecular balance-type situation, it is apparent that the isomer distribution is not statistically given by a random walk of the wheel (Figure 2). Thus, the energy differences may result from mechanical strain and non-covalent interactions. Although the shortening of the loop to only 4 aa might be energetically disfavored, precise quantification of the generated strain is challenging. Examples of lasso peptides with loop sizes[11] similar to isomer Q13-1 show that these species are generally stable. Increased strain in a tight loop, however, may explain the absence of detectable amounts of a T12-1 isomer (Thr: 56 Å3).
Figure 2.

Simplified energy landscape showing the equilibrium distribution of benenodin-1 at 95 °C with K17-1 as globally most stable translation isomer in water. The one-dimensional pathway of wheel translation is defined by the peptidic thread. The inset displays the NMR solution structure (PDB code 5TJ1) of the Q15-1 isomer.
Studies on synthetic rotaxanes have shown that (co-)conformer ratios can be altered, if non-covalent interactions between interlocked components are affected by counter-ion and solvent effects.[12] Heat activation of [1]rotaxane 1 in different aqueous buffers, revealed that the equilibria depend on the molecular environment (Table S5). For example, in HEPES buffer (50 mM, pH 7.6), heat activation (80 °C, 24 h) results in an HPLC-peak ratio of 0.2 : 1.4 : 50.1 : 48.4 (Q13-1 / E14-1 / Q15-1 / K17-1), whereas heating in ultrapure water leads to a ratio of 0.5 : 5.2 : 36.3 : 58.0. In addition, we observed a significant dependence on the ionic strength of the solution and amplification of the buffer effects through equilibration at lower temperatures. Thus, we conclude that the shuttling cascade is, at least partly, governed by non-covalent interactions in the mechanically interlocked peptide. Although these experiments reveal rather small changes between the isomer’s energy differences across conditions (ΔΔG < ±1.0 kcal/mol), it is noteworthy that the shuttling cascade in peptide [1]rotaxane 1 is affected by its molecular environment.
Molecular dynamics (MD) simulations give further insight into potentially relevant intramolecular interactions. While the low abundance and interconversion (Figure S3) of isomers Q13-1 and E14-1 prevented NMR characterization, our previously reported solution NMR structures of Q15-1 and K17-1 are an excellent starting point.[5] MD derived distance histograms for cationic (Arg-6, Lys-17) and anionic (Glu-14, C-terminus) residues show that ion pair formation is dependent on the wheel’s position on the thread, most notably for ion pairs formed by Arg-6 or Lys-17 with the C-terminus (Figure S6). Furthermore, an inspection of the MD simulation trajectories reveals a major restructuring of the intramolecular hydrogen bond network (HBN) for each translational isomer (Figure S7 and Table S6). Hydrogen bond interactions between wheel and thread in other lasso peptides were previously shown, e.g., in hydrogen-deuterium exchange experiments by NMR[13]. A restructuring of the HBN between wheel and thread is reminiscent of co-conformational switching in synthetic rotaxanes.[14]
Tandem MS techniques have been used previously to verify the threaded structure of lasso peptides.[15] To further elucidate a restructuring of the HBN during wheel translation in benenodin-1, we conducted gas-phase fragmentation experiments of the four detectable translational isomers. Fragmentation patterns in collision-induced dissociation (CID) can be rationalized by the “mobile proton” model[16], in which proton traffic maps predict the probability of protonation, peptide bond cleavage, and subsequent fragmentation into b- and y-ions. The [1+2H]2+ ions bear two basic residues, Arg-6 and Lys-17, which sequester protons and hamper their migration. Proton transfer thus critically depends on a conducting HBN within the peptide.[16–17] Hence, different fragment ion intensities are expected for each translational isomer depending on the wheel’s position and the accompanying restructuring of the supporting HBN.
The box plots in figure 3 shows the relative b-ion intensities for the beam-type CID of the thread (b9–17) at normalized collision energy of 30 % (see Supporting Information for a detailed discussion). Particularly pronounced differences between the four isomers are observed for fragments b10 and b14. While fragment b10 shows small intensities for isomers K17-1, Q15-1 and E14-1, fragmentation is promoted in isomer Q13-1. Vice versa, fragment b14 is amplified in isomer K17-1. Another interesting trend can be seen for the b17 ion (Lys-17/Pro-18 cleavage). The N-terminal ProMet-fragment is, in all isomeric states, not fixed by a mechanical bond, and thus no [2]rotaxane intermediates[15a] are to be expected. Hence, the intensity differences presumably result from a spatial proximity effect of the wheel. The observed trends indicate that backbone fragmentation is either suppressed in the confining interior of the macrolactam ring[15b] and/or proximity to the rims partly promotes b-ion formation. Overall, the position of the mechanically bonded wheel alters the gas-phase fragmentation behavior of the lasso peptide. While it is known that adjacent aa[17] can facilitate site-specific fragmentation pathways, this is the first example that such an effect was observed through-space in translational isomers of a peptide rotaxane.
Figure 3.
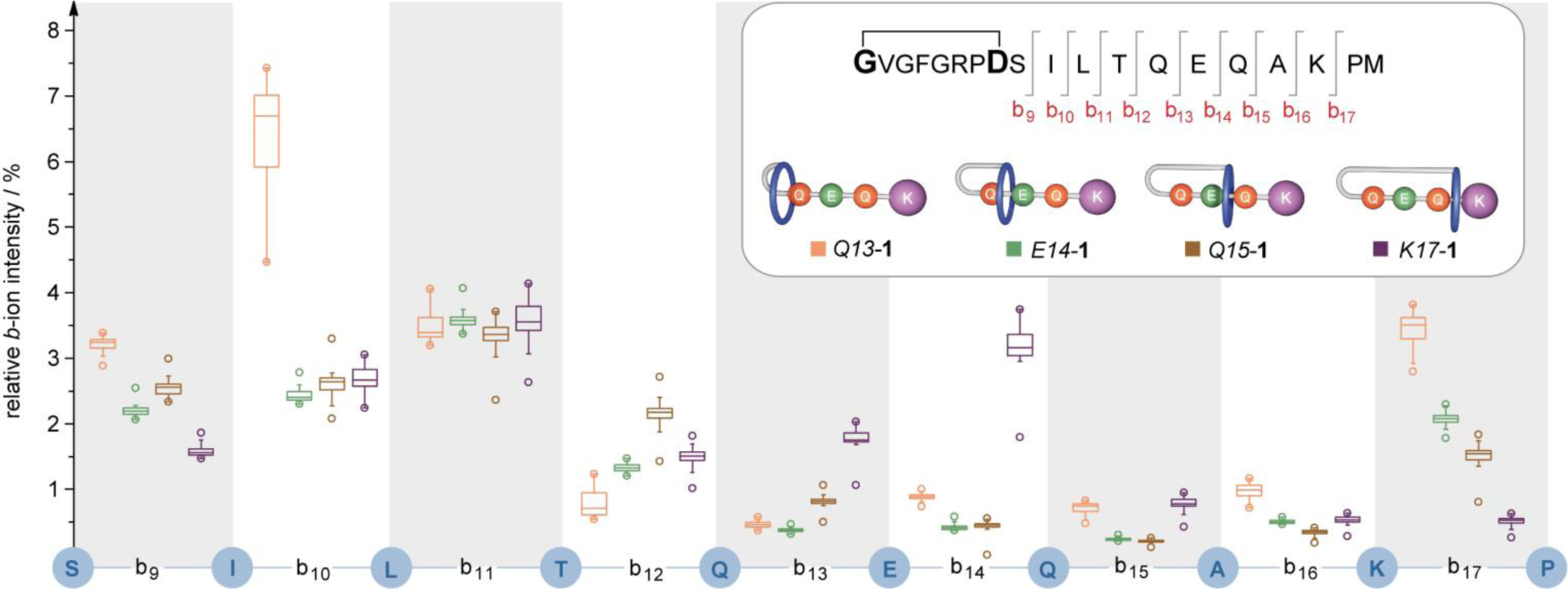
Box plot of relative b-ion intensities (+1 species) obtained from gas-phase fragmentation experiments of all four translational isomers of peptide 1: boxes range from 25–75 % and are given with corresponding median line, whiskers are determined by outliers within 1.5x interquartile range and circles represent the minimum and maximum values. The plot is based on 159 MS/MS spectra (see Supporting Information for details). Inset: structure and thread fragmentation of peptide 1 and the four translational isomers with corresponding colour code.
In conclusion, the lasso peptide benenodin-1 operates as a four-state molecular shuttle under heat activation. The wheel motion proceeds through a cascade of loop/tail pulling equilibria and results in four stable translational isomers. Conformational analysis demonstrates that putative mechanical strain and non-covalent forces govern the equilibrium distribution of the wheel and that each translation step is accompanied by a major restructuring of the hydrogen bond network within the [1]rotaxane. A better understanding of mechanical bonding and non-covalent interactions in lasso peptides will help to find ways to a total synthesis of these structurally sophisticated natural products, e.g., through new templating methods. Furthermore, molecular motion of large amplitude in mechanically interlocked compounds is the first step towards a new generation of molecular switches and machines solely made from biomolecules. While outstanding bioactivities of “static” lasso peptides are known, we envision that switchable and “shape-shifting” peptide rotaxanes may provide novel functions and perhaps yield new stimuli-responsive drugs.
Supplementary Material
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].Karshikoff A, Non-covalent Interactions In Proteins, Imperial College Press, London, UK, 2006. [Google Scholar]
- [2].(a) Maksimov MO, Pan SJ, Link AJ, Nat. Prod. Rep 2012, 29, 996–1006; [DOI] [PubMed] [Google Scholar]; (b) Hegemann JD, Zimmermann M, Xie X, Marahiel MA, Acc. Chem. Res 2015, 48, 1909–1919. [DOI] [PubMed] [Google Scholar]
- [3].(a) Ferguson AL, Zhang S, Dikiy I, Panagiotopoulos AZ, Debenedetti PG, Link AJ, Biophys. J 2010, 99, 3056–3065; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Waliczek M, Wierzbicka M, Arkuszewski M, Kijewska M, Jaremko L, Rajagopal P, Szczepski K, Sroczynska A, Jaremko M, Stefanowicz P, PLoS One 2020, 15, e0234901; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Martin-Gomez H, Tulla-Puche J, Org. Biomol. Chem 2018, 16, 5065–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hegemann JD, ChemBioChem 2020, 21, 7–18. [DOI] [PubMed] [Google Scholar]
- [5].Zong C, Wu MJ, Qin JZ, Link AJ, J. Am. Chem. Soc 2017, 139, 10403–10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vasilyeva LV, Omelchenko MV, Berestovskaya YY, Lysenko AM, Abraham WR, Dedysh SN, Zavarzin GA, Int. J. Syst. Evol. Microbiol 2006, 56, 2083–2088. [DOI] [PubMed] [Google Scholar]
- [7].Stoddart JF, Angew. Chem. Int. Ed 2017, 56, 11094–11125. [DOI] [PubMed] [Google Scholar]
- [8].Harpaz Y, Gerstein M, Chothia C, Structure 1994, 2, 641–649. [DOI] [PubMed] [Google Scholar]
- [9].Allen CD, Chen MY, Trick AY, Le DT, Ferguson AL, Link AJ, ACS Chem. Biol 2016, 11, 3043–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schröder HV, Zhang Y, Link AJ, Nat. Chem 2021, 13, 850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].(a) Xu F, Wu Y, Zhang C, Davis KM, Moon K, Bushin LB, Seyedsayamdost MR, Nat. Chem. Biol 2019, 15, 161–168; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cao L, Beiser M, Koos JD, Orlova M, Elashal HE, Schröder HV, Link AJ, J. Am. Chem. Soc 2021, 143, 11690–11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].(a) Gasa TB, Valente C, Stoddart JF, Chem. Soc. Rev 2011, 40, 57–78; [DOI] [PubMed] [Google Scholar]; (b) Fahrenbach AC, Bruns CJ, Cao D, Stoddart JF, Acc. Chem. Res 2012, 45, 1581–1592. [DOI] [PubMed] [Google Scholar]
- [13].Xie X, Marahiel MA, ChemBioChem 2012, 13, 621–625. [DOI] [PubMed] [Google Scholar]
- [14].Evans NH, Eur. J. Org. Chem 2019, 2019, 3320–3343. [Google Scholar]
- [15].(a) Jeanne Dit Fouque K, Lavanant H, Zirah S, Hegemann JD, Fage CD, Marahiel MA, Rebuffat S, Afonso C, Analyst 2018, 143, 1157–1170; [DOI] [PubMed] [Google Scholar]; (b) Zirah S, Afonso C, Linne U, Knappe TA, Marahiel MA, Rebuffat S, Tabet JC, J. Am. Soc. Mass Spectrom 2011, 22, 467–479. [DOI] [PubMed] [Google Scholar]
- [16].Paizs B, Suhai S, Mass. Spectrom. Rev 2005, 24, 508–548. [DOI] [PubMed] [Google Scholar]
- [17].Tsaprailis G, Somogyi Á, Nikolaev EN, Wysocki VH, Int. J. Mass. Spectrom 2000, 195–196, 467–479. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


