Abstract
Background:
Autologous stem cell transplantation (ASCT) is a standard of care for patients with chemosensitive, relapsed/refractory (R/R) classical Hodgkin lymphoma (cHL) and diffuse large B-cell lymphoma (DLBCL). While the clinical benefit of ASCT has traditionally been attributed solely to cytoreduction from intensive chemotherapy, ASCT has important immunogenic effects which may contribute to its anti-tumor efficacy and could provide a favorable immune environment for post-ASCT immune-based maintenance treatments. We previously reported clinical results of a phase II trial (NCT02362997) testing 8 doses of pembrolizumab maintenance therapy after ASCT for patients with R/R cHL or DLBCL. To clarify the impact of pembrolizumab on immune reconstitution, we compared the kinetics of peripheral blood immune cell recovery after ASCT for trial patients receiving pembrolizumab maintenance to those of a contemporaneous control cohort of similar patients undergoing ASCT without pembrolizumab maintenance.
Objective:
To characterize the impact of post-ASCT pembrolizumab maintenance therapy on immune reconstitution for patients with R/R DLBCL and cHL and to identify candidate biomarkers of efficacy and immune-related adverse events (irAEs).
Study design:
Peripheral blood mononuclear cell (PBMC) samples were prospectively collected 1–18 months after ASCT and analyzed by flow cytometry using a panel of fluorophore-conjugated monoclonal antibodies to identify B cells, natural killer (NK) cells, and various dendritic cell (DC) and T cell subsets.
Results:
A median of 5 (range 1–8) post-ASCT PB samples were collected from 144 patients (59 pembrolizumab, 85 control). Clinical characteristics of the two cohorts were similar. Compared to cHL patients, DLBCL patients (who all received anti-CD20 monoclonal antibody therapy before ASCT) had delayed CD19+ cell reconstitution which persisted for at least 18-month after ASCT. No other differences in immune reconstitution were observed based on lymphoma subtype. Post-ASCT pembrolizumab maintenance therapy was associated with 1) an elevation in circulating DCs (driven by higher levels of plasmacytoid and immature DCs) which persisted for the duration of pembrolizumab treatment, and 2) a significant reduction in PD-1+ T cells which persisted for 6–12 months after completion of pembrolizumab therapy. Despite the key role of T cells in mediating the effects of PD-1 blockade, pembrolizumab maintenance did not affect recovery of any T cell subsets. In an exploratory analysis, a higher baseline CD4+ TEMRA cell count (defined as CD3+ CD4+ CD45RA+ CD62L−) was associated with inferior PFS, but only among patients who received pembrolizumab maintenance (p=0.003). As continuous variables, lower absolute levels of NK cells (p=0.009), PD-1+ CD4+ T cells (p=0.005), and PD-1+ CD8+ T cells (p=0.005) before pembrolizumab initiation were each associated with a higher risk of grade 2+ irAEs.
Conclusion:
Post-ACST pembrolizumab maintenance therapy is associated with a persistent elevation in circulating DCs, but its impact on the reconstitution of other immune cells in peripheral blood appears limited. Our study suggests that early features of post-ASCT immune reconstitution could be associated with PFS and risk of irAE and warrant additional investigation.
Keywords: pembrolizumab, PD-1, immune reconstitution, autologous stem cell transplantation, Hodgkin lymphoma, Diffuse large B-cell lymphoma
Introduction
High-dose chemotherapy and autologous stem cell transplantation (ASCT) remain the standard of care for transplant-eligible patients with relapsed or refractory (R/R) classical Hodgkin lymphoma (cHL) and diffuse large B-cell lymphoma (DLBCL) who respond to salvage chemotherapy.1–3 The therapeutic benefit of ASCT has traditionally been attributed solely to cytoreduction from intensive chemotherapy; however, ASCT has important immunogenic effects which may also underlie some of its anti-tumor efficacy. High-dose chemotherapy results in increased antigen presentation and stimulation of the innate immune system and preclinical models suggest that high-dose chemotherapy and ASCT induces protective tumor-specific T cells that are associated with improved survival.4,5 Clinical studies of immune reconstitution after ASCT have demonstrated that early lymphocyte recovery is associated with improved OS for patients with NHL6, while higher levels of inhibitory cell populations, like M2 macrophages and myeloid derived suppressor cells, have been linked with impaired PFS.7
These findings suggest the need for additional analyses of immune reconstitution following ASCT and also support the investigation of immune-based consolidation or maintenance treatments after ASCT, with the goal of favorably manipulating the remodeling immune system. Pembrolizumab is an anti-PD-1 monoclonal antibody (mAb) that is FDA-approved for treatment of patients with R/R cHL based on high objective response rates (ORRs) in this disease.8,9 Pembrolizumab and other PD-1 mAbs have also been studied in DLBCL where PD-1 blockade is associated with infrequent responses in unselected patients.10,11 We previously reported results of a multicenter, non-randomized phase II trial that tested 8 doses of pembrolizumab maintenance after ASCT in 30 patients with cHL and 29 patients with DLBCL (including transformed indolent lymphoma [TIL] and primary mediastinal B-cell lymphoma [PMBL]). The cHL cohort achieved an 18-month PFS of 82%, meeting the primary endpoint, while the DLBCL cohort failed to achieve the study’s primary endpoint (18-month PFS 59%).12,13 The clinical trial included serial collection of peripheral blood (PB) samples for a planned analysis of immune reconstitution following ASCT. Patients undergoing ASCT for cHL or DLBCL during the same time period but outside of a clinical trial (and without pembrolizumab maintenance therapy) at Dana-Farber Cancer Institute/Brigham and Women’s Hospital (DFCI/BWH) were enrolled on a separate specimen collecting protocol. Among these patients, serial PB samples were collected at similar timepoints after ASCT and used as a control cohort.
Using samples from these patient cohorts, we characterized immune reconstitution following ASCT in a total of 144 patients with cHL and DLBCL. Our design allowed us to compare immune recovery between patients with cHL and DLBCL, and to assess the impact of pembrolizumab – both in HL where it is an effective agent and in DLBCL where its direct anti-tumor activity is limited. Finally, we determined if features of immune reconstitution were associated with either post-ASCT relapse/progression or (in pembrolizumab-treated patients) the incidence of immune-related adverse events (irAEs).
Methods
Patients
An open-label multicenter phase II trial of pembrolizumab maintenance therapy following ASCT was performed at 6 centers in the United States, as previously described (NCT02362997).12,13 Eligible patients were adults (age ≥ 18 years) with R/R cHL or DLBCL (including TIL and PMBCL) who had received ≤3 lines of therapy and had undergone ASCT with chemosensitive disease (defined as partial response or better to salvage therapy, per International Harmonization Project criteria).14 Study treatment consisted of pembrolizumab maintenance (200 mg intravenously every 3 weeks for 8 cycles) which was started within 60 days of ASCT (intended to start within 21 days of ASCT hospitalization discharge). Protocol approval for the pembrolizumab trials was obtained from the Institutional Review Board of each participating institution.
During the same period, we assembled a control cohort of patients who underwent ASCT for the same lymphoma types outside of a clinical trial at DFCI/BWH. Eligible patients met the following criteria: age ≥ 18 years; histologically confirmed diagnosis of DLBCL, TIL, PMBCL, or cHL; relapsed or refractory disease; and receipt of ASCT at DFCI/BWH between 6/1/2014 and 1/1/2018. Patients in the control cohort were eligible regardless of number of lines of therapy before ASCT. Finally, to establish an estimate of normal values for various immune cell subpopulations, PB specimens from 31 healthy donors were also analyzed. Written informed consent for sample collection was obtained from all patients before sample collection in accordance with the Declaration of Helsinki.
Stem cell collection and conditioning regimens
Stem cell mobilization and collection were performed with granulocyte-colony stimulating factor (G-CSF) +/− plerixafor according to the standard practices of participating centers. Among the pembrolizumab cohort, ASCT conditioning consisted of carmustine, etoposide, cytarabine, and melphalan (BEAM) in 56 patients and thiotepa, busulfan, and cyclophosphamide (TBC) in 3 patients. All patients in the control cohort received BEAM conditioning.
Restaging Assessments
In the pembrolizumab trial cohort, positron emission tomography (PET)/computed tomography (CT) scans were performed prior to initiation of pembrolizumab therapy, 10 weeks after starting pembrolizumab therapy, 22 weeks after pembrolizumab therapy and 12 and 18 months post-ASCT. In the control cohort, radiographic assessments after ASCT were at the discretion of treating physicians.
Peripheral blood collection and flow cytometry
Among pembrolizumab trial subjects, blood samples were collected prior to initiation of pembrolizumab maintenance (i.e. baseline sample), then 3 weeks, 6 weeks, 9 weeks, 15 weeks, and 21 weeks after treatment initiation, and then 12 and 18 months post-ASCT. Among subjects in the control cohort, PB samples were intended to be collected at similar time points, but the actual visit and laboratory assessment schedule for those patients was left to the discretion of their treating clinician since the patients were not on a clinical trial. Given the unavoidable variation in the exact time of sampling between patients, and for simplicity of analysis and reporting, we considered the following time points (all considered from the time of ASCT): 1-month (study baseline; occurring before initiation of pembrolizumab in the trial cohort), 2-months, 3-months, 6-months, 12-months, and 18-months. Samples were assigned to the closest specified time point.
Immunophenotypic analyses were performed with a panel of fluorophore-conjugated monoclonal antibodies, as previously described.15 B cells were defined as CD19+ cells. NK cells were defined as CD3−CD56+CD16+ as well as CD3−CD56+CD16−. Total dendritic cells were defined as LINEAGEnegHLA-DR+. Major T cell populations included: CD3+, CD3+CD4+, CD3+CD8+, T regulatory cells (Tregs) (CD3+CD4+CD25+), and conventional CD4 T cells (Tcons) (CD3+CD4+ minus CD3+CD4+CD25+) cells. Further analysis of T cells included identification of naive cells (CD45RA+ CD62L+), central memory (CM) cells (CD45RA− CD62L+), effector memory (EM) cells (CD45RA−CD62L−) and terminal effector memory (TEMRA) cells (CD45RA+ CD62L−) within each T cell population. We quantified the expression of PD-1 among all T cell subsets.
Statistical Analysis
For each time point, the absolute number of circulating cells for a given subset was compared between study and control patients using Wilcoxon rank-sum test; p-values were adjusted using the Benjamini-Hochberg method to account for multiplicity of testing. Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan-Meier (KM) method with Greenwood’s formula for variance estimation, and differences in survival between groups were assessed using the log-rank test. PFS was defined as the time from day 0 of ASCT to death from any cause, relapse, or progression, with patients censored at the last time seen alive and progression-free. OS was defined as the time from day 0 of ASCT to death from any cause, with patients censored at the last time seen alive. Median follow-up time was estimated using the reverse KM method. Descriptive statistics were used to summarize variables of interest. Univariable Cox proportional hazards regression were used to evaluate associations between prognostic factors and PFS or OS; hazard ratios (HRs), 95% confidence intervals (CIs), and Wald p-values were reported for covariates. All analyses were performed using R v4.0.2 and the package survival v3.2–11 for time-to-event analyses.
Results
Patient Characteristics and Sample Collection
Patient and transplant characteristics for the 144 patients are summarized in Table 1. The median age at transplant was 52 (range 20–77) with a lower median age for patients with cHL vs DLBCL (32 [21–63] vs 59 [27–77] years, p<0.001). Among patients with non-Hodgkin lymphoma (NHL), DLBCL NOS was the most frequent lymphoma subtype (67%) followed by TIL (20%), PMBL (8%), and T-cell histiocyte rich B-cell lymphoma (4%). Among cHL patients, nodular sclerosis was the most common subtype (82%). Patients received a median of 2 lines of therapy before ASCT and most patients achieved a complete response (CR) on pre-ASCT PET imaging (CR rate 85% for HL patients and 70% for DLBCL patients). Among patients with DLBCL, those in the pembrolizumab cohort were more heavily pretreated (38% vs 11% received 3 lines of therapy prior to ASCT). There were no other significant differences in baseline clinical features between the pembrolizumab and control cohorts for either cHL or DLBCL patients.
Table 1.
Baseline characteristics
| cHL | DLBCL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | No maintenance | Pembro maintenance | p- | No maintenance | Pembro maintenance | p- | |||
| n = | n = 31 (22) | n = 30 (21) | n = 54 (38) | n = 29 (20) | |||||
| Male | 89 | 19 (61) | 16 (53) | 0.61 ‡ | 33 (61) | 21 (72) | 0.34 ‡ | ||
| Age at ASCT | 0.97 † | 0.11 † | |||||||
| Median (range) | 52 | 32 (21 – 63) | 33 (20 – 69) | 60 (22 – 77) | 57 (22 – 76) | ||||
| Histology | 0.55 ‡ | 0.09 ‡ | |||||||
| cHL NOS | 32 | 2 (6) | 3 (10) | - | - | ||||
| Nodular Sclerosis | 23 | 27 (87) | 23 (77) | - | - | ||||
| Mixed Cellularity | 6 (4) | 2 (6) | 4 (13) | - | - | ||||
| DLBCL NOS | 56 | - | - | 39 (72) | 17 (59) | ||||
| TIL | 17 | - | - | 12 (22) | 5 (17) | ||||
| PMBL | 7 (5) | - | - | 2 (4) | 5 (17) | ||||
| TCHR | 3 (2) | - | - | 1 (2) | 2 (7) | ||||
| Number of systemic treatments prior to ASCT | 0.75 ‡ | 0.00 | |||||||
| Median (range) | 2 (1 – | 2 (1 – 5) | 2 (2 – 3) | 2 (2 – 3) | 2 (2 – 3) | ||||
| <= 2 | 115 | 24 (77) | 25 (83) | 48 (89) | 18 (62) | ||||
| 3 or more | 29 | 7 (23) | 5 (17) | 6 (11) | 11 (38) | ||||
| Primary refractory to first-line treatment | 0.31 ‡ | > | |||||||
| Yes | 53 | 13 (42) | 17 (57) | 15 (28) | 8 (28) | ||||
| Disease status at ASCT | 0.47 ‡ | 0.32 ‡ | |||||||
| CR | 114 | 25 (81) | 27 (90) | 40 (74) | 18 (62) | ||||
| PR | 23 | 6 (19) | 3 (10) | 14 (26) | 11 (38) | ||||
| Post-ASCT peripheral blood samples per patient | < | < | |||||||
| Median (range) | 5 (1 – | 4 (1 – 8) | 8 (1 – 8) | 3 (1 – 8) | 8 (1 – 8) | ||||
ASCT = auto ogous stem cell transplantation; cHL = classical Hodgkin lymphoma; DLBCL = diffuse large B-cell lymphoma; pembro = pembrolizumab; NOS = not other specified; TIL = transformed indolent lymphoma; PMBL = primary mediastinal B-cell lymphoma; TCHR = T cell histiocyte rich large B-cell lymphoma; CR = complete response; PR = partial response
Fisher’s exact test
Wilcoxon rank-sum test
A median of 5 (range 1–8) post-ASCT PB samples were collected from each study subject. More samples were available for analysis for trial patients than control patients (median 8 vs 3, p<0.001). The timing of sample collection for the two cohorts was similar with the exception of small differences in median time of sample collection between the pembrolizumab and control cohorts at the 2-month (median 61.5 [47–71] vs 58 [47–76] days, p=0.01), 6-month (median 167 [133–273] vs 197 [169–274] days, p<0.001), and 12-month timepoints (median 368 [278–392] vs 352 [281–395] days, p<0.001).
Immune Reconstitution: Total WBC, absolute lymphocyte count, B-cells, T-cells, and NK cells
The reconstitution of white blood cells (WBC), absolute lymphocytes, B-cells (CD19+), T-cells (CD3+), and NK cells is summarized in Figure 1. Recovery of WBC, absolute lymphocytes, and CD3+ cells was similar for pembrolizumab and control patients across all time points; however, for both cohorts, these cell counts remained below the interquartile range of healthy controls at all time points analyzed (Figure 1A–C). CD19+ B cell recovery followed the same trajectory in the pembrolizumab and control cohorts. Small, but statistically significant differences were observed in absolute CD19+ B cell counts in the pembrolizumab cohort compared to the control cohort at the 1-month (median 4.7 × 109 cells/L (0.0–79.8) vs 1.4 × 109 cells/L (0.0–62.4), p<0.001) and 3-month (median 20.9 × 109 cells/L (0.0–624.2) vs 1.0 × 109 cells/L (0.0–331.2), p<0.001) timepoints (Figure 1D). Compared to cHL patients, patients with DLBCL (who all received anti-CD20 monoclonal therapy prior to ASCT) had delayed CD19+ cell reconstitution which persisted through the 18-month timepoint (p<0.05 at all time points, data not shown). No differences between cHL and DLBCL patients were observed for other cell types. The absolute number of total NK cells was highest at the 1-month timepoint and then trended downward at subsequent timepoints. No significant differences in NK cell populations were observed between the pembrolizumab and control cohorts. In contrast to other cell populations, NK cell populations were similar in absolute number to healthy controls at all timepoints (Figure 1E).
Figure 1 –
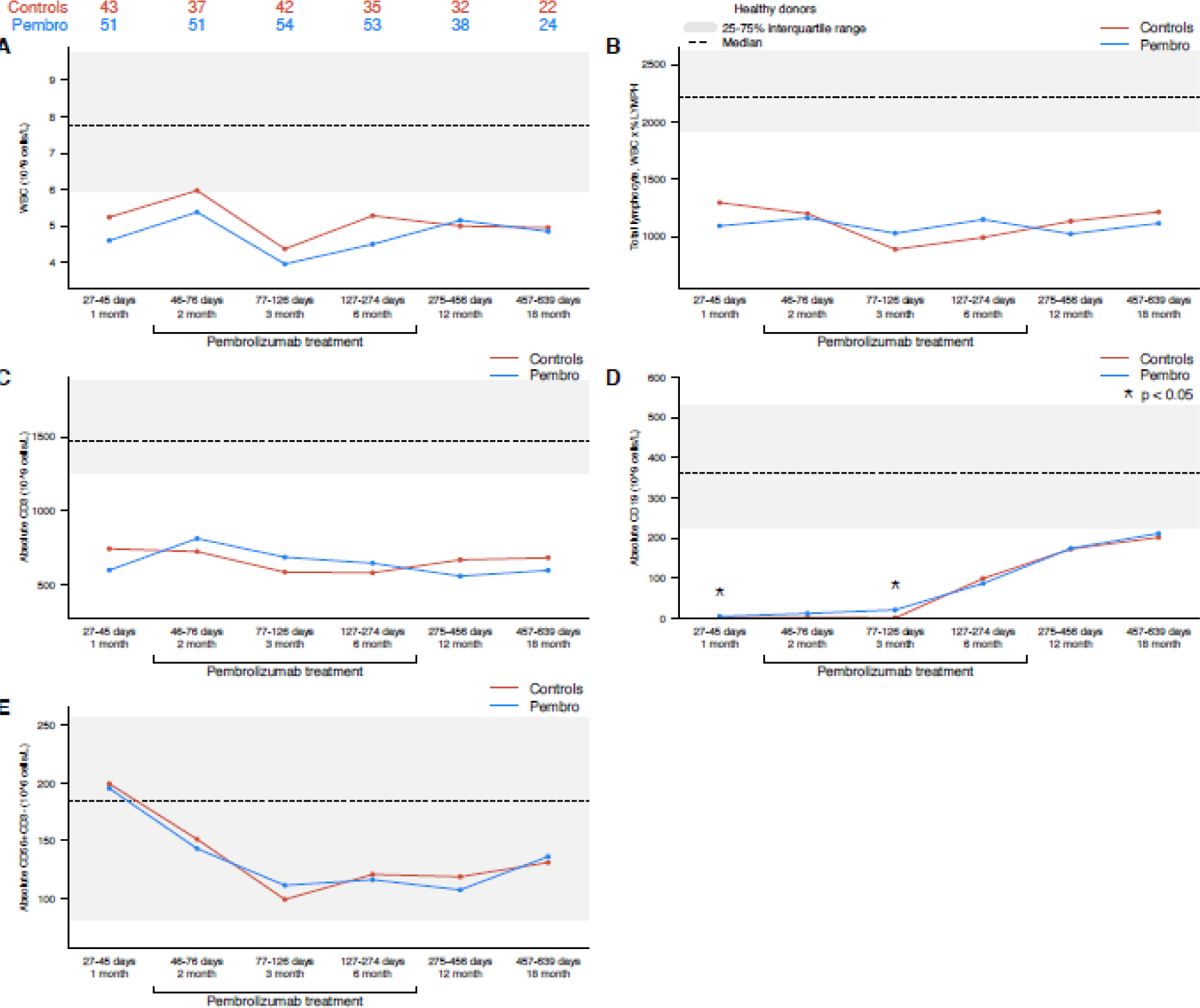
(A) Total WBC, (B) total lymphocyte, (C) CD3+, (D) CD19+, and (E) NK cells (pembrolizumab vs control cohort). The interquartile range of healthy control patients for each cell population is depicted as a gray bar, and the median value is noted with a dashed line.
Reconstitution of major T-cell populations
There were no significant differences in reconstitution of CD8+ T cells, CD4+ T cells, or Tregs (CD4+CD25+) between patients in the pembrolizumab and control cohorts (Figure 2A–C). Absolute numbers of CD4 Tcon cells at the 1-month baseline timepoint (collected before initiation of pembrolizumab) were lower in the pembrolizumab cohort (174.9 × 106 cells/L (24.8–791.6) vs 250.3 × 106 cells/L (50.5–1180.4), p=0.03); however, testing at subsequent time points (after initiation of pembrolizumab) revealed no significant differences in CD4 Tcon populations between the two cohorts. Absolute numbers of CD8+ T cells and Treg cells were similar to healthy controls at most timepoints, while numbers of CD4+ T cells and CD4+ T cons were significantly lower than healthy controls at all time points assessed.
Figure 2 –
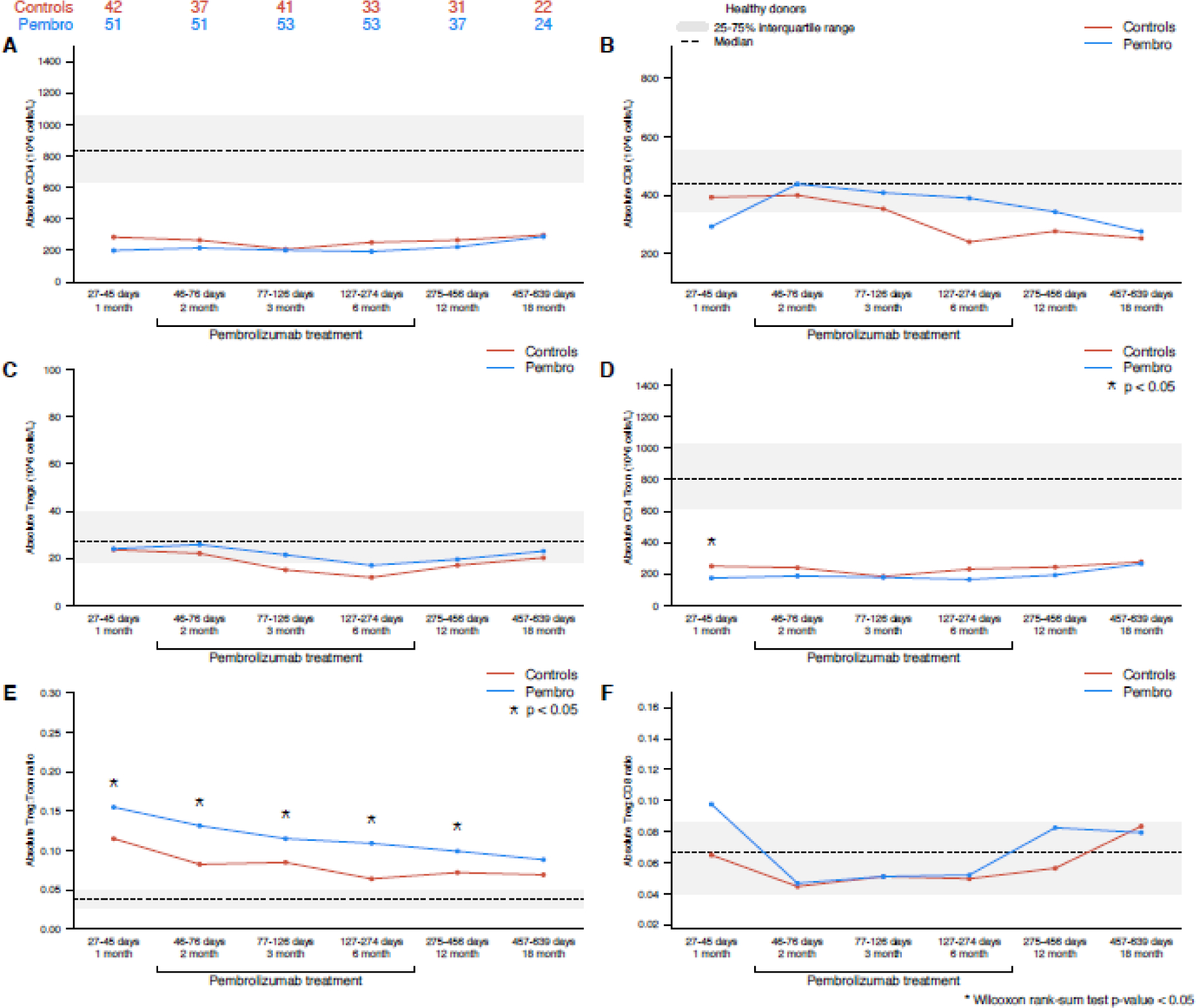
T cell subsets – (A) CD4+ T cells, (B) CD8+ T cells, (C) Tregs, (D) CD4 T con, (E) Treg:Tcon, (F) Treg:CD8 (pembrolizumab vs control cohort). The interquartile range of healthy control patients for each cell population is depicted as a gray bar, and the median value is noted with a dashed line.
Compared to the control cohort, the pembrolizumab cohort had a significantly higher Treg:Tcon ratio both prior to initiation of pembrolizumab (1-month timepoint 0.15 [0.02–2.25] vs 0.11 [0.00–0.28], p=0.009) and after pembrolizumab initiation at the 2-month (0.13 [0.02–0.37] vs 0.08 [0.01–0.52], p=0.016), 3-month (0.11 [0.01–0.48] vs 0.08 [0.01–0.39], p=0.019), 6-month (0.11 [0.02–0.45] vs 0.06 [0.01–0.18], p<0.001), and 12-month timepoints (0.10 [0.03–0.25] vs 0.07 [0.02–0.36], p=0.009) (Figure 2E). There were no significant differences in Treg:CD8 ratio between the two cohorts (Figure 2F). No differences between cHL and DLBCL patients were observed for these cell types.
Reconstitution of Naïve, Memory, and Effector T cells
The reconstitution of naïve, central memory (CM), effector memory (EM), and terminally differentiated effector (TEMRA) CD4+ and CD8+ T cells is summarized in Figure 3. Significant differences between the pembrolizumab and control cohorts were observed at the 1-month timepoint (for CM and naïve T cell subsets) and at the 18-month timepoint (for naïve T cells only); however, both of these timepoints fall outside of the pembrolizumab treatment period. No significant differences in any of these populations were observed during pembrolizumab treatment (i.e. 2-month, 3-month, and 6-month timepoints). In both cohorts, patients experienced incomplete reconstitution of naïve CD4+ T cells, naïve CD8+ T cells, and CD4+ TEMRA cells, even 18 months after ASCT. No differences between cHL and DLBCL patients were observed for these cell types.
Figure 3 –
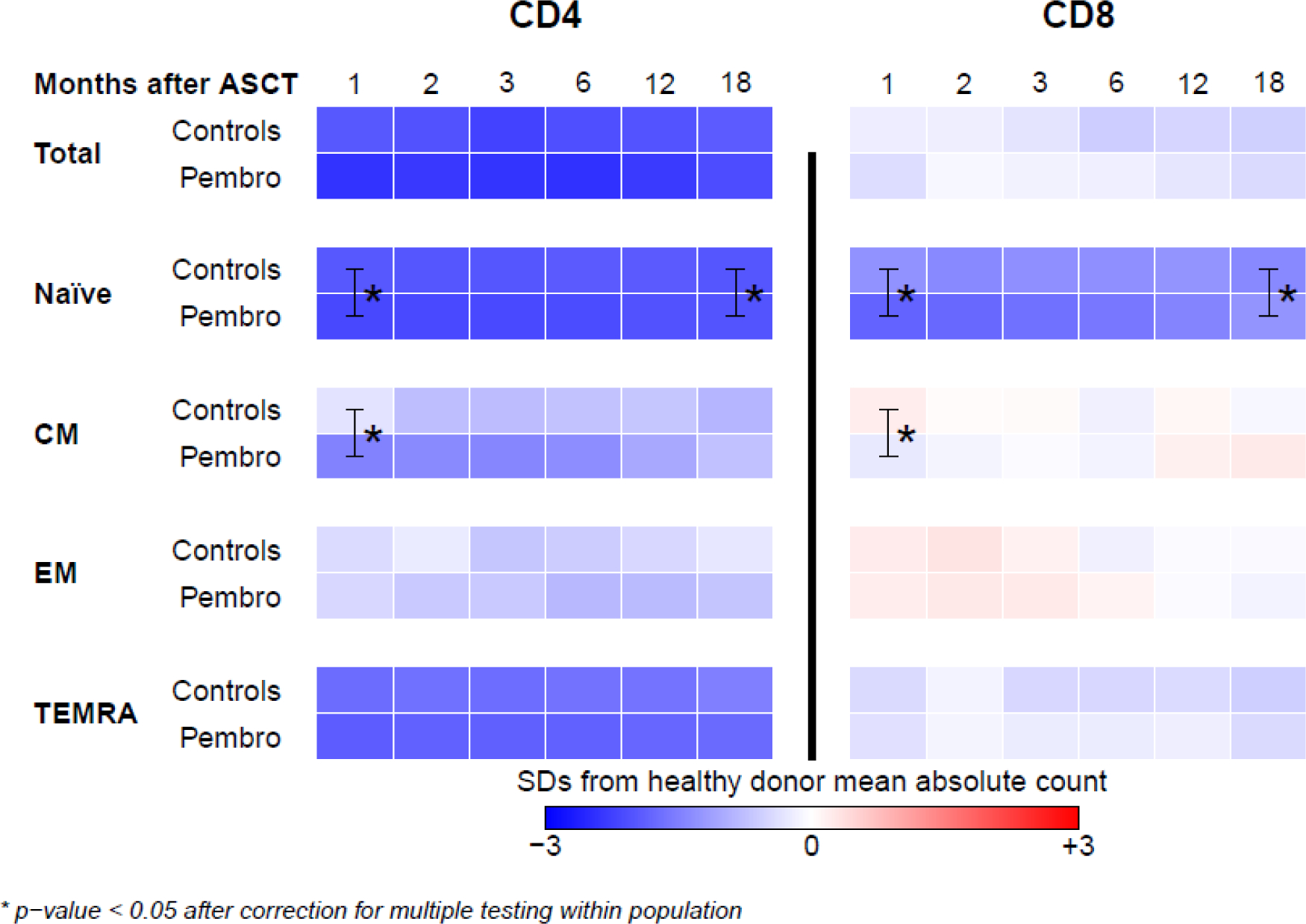
Heat map of naïve, central memory (CM), effector memory (EM), and terminally differentiated effector (TEMRA) CD4+ and CD8+ T cells (pembrolizumab vs control cohort). T cell subsets were quantified at multiple post-ASCT timepoints (1, 2, 3, 6, 12, and 18 months) among patients in the control and pembrolizumab cohorts. The absolute number of cells identified is displayed as a heatmap where blue, white, and red represent values less than, equal to, and greater than those of healthy donors. Significant differences between the control and pembrolizumab cohorts are marked with *. No significant differences between the two cohorts were observed during pembrolizumab treatment (i.e. at the 2, 3, and 6 month timepoints). Full recovery of multiple T cell subsets (represented in blue) was not achieved even 18 months following ASCT.
Reconstitution of circulating dendritic cells
The reconstitution of circulating dendritic cells (DCs) is summarized in Figure 4. Patients in the pembrolizumab cohort had significantly higher circulating total DC populations compared to the control cohort at 2-months (348 × 106/L [90 – 1470] vs 228 × 106/L [8 – 822], p=0.002), 3-months (236 × 106/L [36 – 1523] vs 157 × 106/L [5 – 555], p=0.008), and 6-months (211 × 106/L [32 – 583] vs 135 × 106/L [30 – 385], p=0.02), and 18-months (239 × 106/L [76 – 426] vs 190 × 106/L [38 – 488], p=0.03) (Figure 4A). These differences appeared to be driven by higher levels of plasmacytoid DCs (CD123+) (Figure 4B) and CD123−/CD11c− DCs (Figure 4D) in the pembrolizumab cohort, while myeloid DCs (CD11c +) (Figure 4C) were similar in both the control and pembrolizumab cohorts. No differences in DC reconstitution were observed between cHL and DLBCL patients.
Figure 4 –
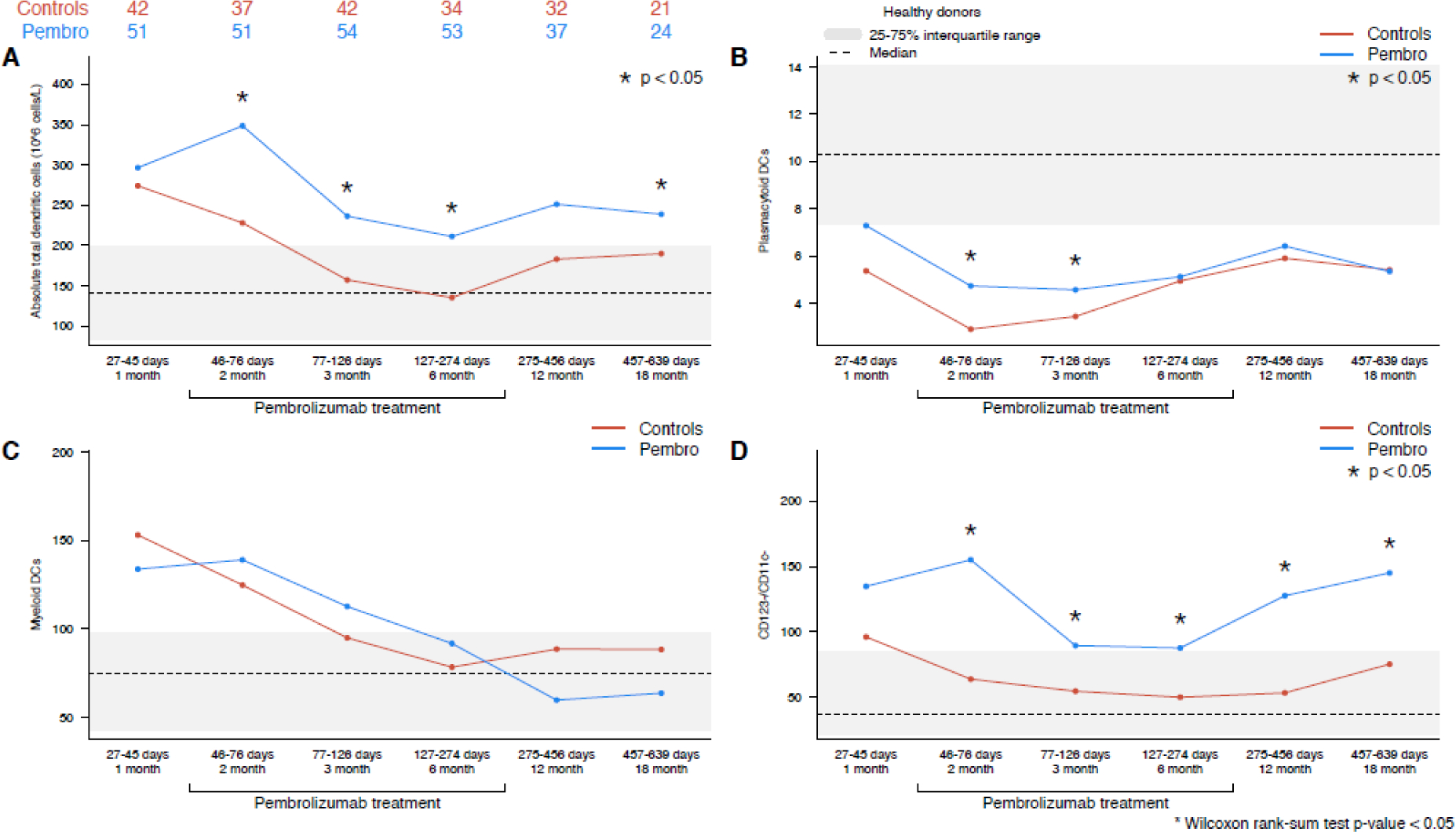
(A) Total circulating dendritic cells, (B) plasmacytoid DCs, (C) myeloid DCs, (D) CD123−/CD11c− DCs (pembrolizumab vs control cohort). The interquartile range of healthy control patients for each cell population is depicted as a gray bar, and the median value is noted with a dashed line.
Reconstitution of PD-1 expressing immune cell subsets
The absolute number of PD-1+ cells was quantified for all T cell populations. Post-ASCT pembrolizumab maintenance was associated with a sustained decrease in PD-1 positive CD4 T cells and PD-1 positive CD8 T cells (Figure 5). Lower levels of PD-1 positive cells were observed at the first timepoint after PD-1 initiation in the pembrolizumab cohort (2-months) and persisted beyond PD-1 discontinuation (which occurred approximately 6 months post-ASCT). Populations of PD-1 positive CD4+ and CD8+ T cells did not reach those of the control cohort until the 18-month timepoint. As pembrolizumab and the PD-1 mAb used for flow analyses both target the same epitope on the PD-1 receptor, PD-1 positive cell populations could be underreported due to competition from circulating pembrolizumab during the period of pembrolizumab treatment, but this blocking effect would be unlikely to persist for 12 months after treatment.
Figure 5 –
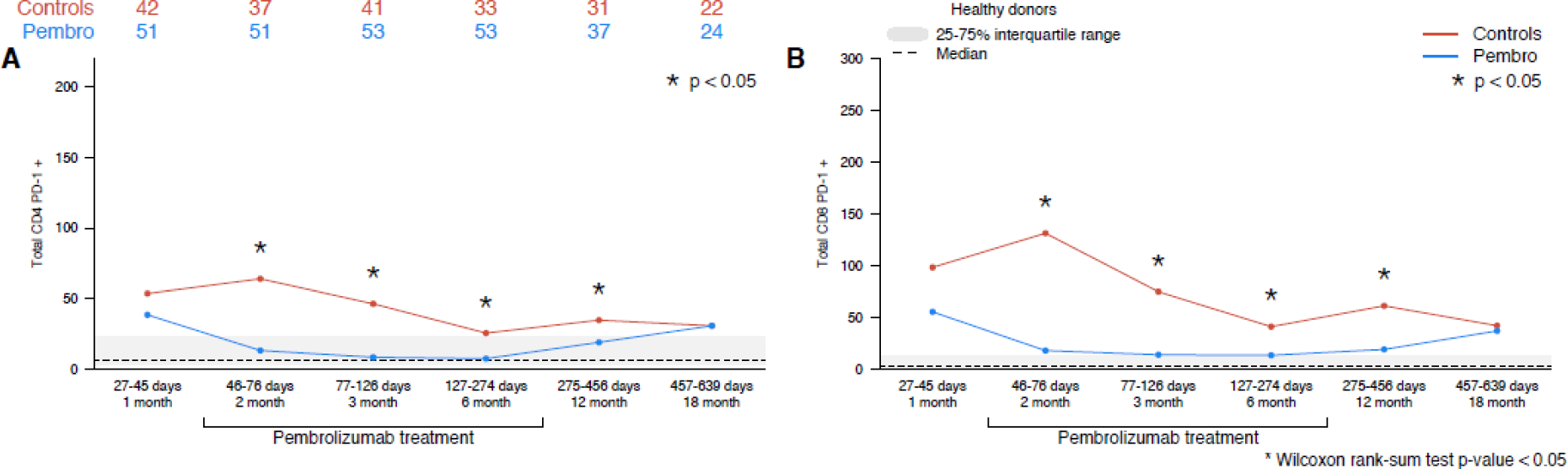
(A) PD-1+ CD4+, (B) PD-1+ CD8+ T cells (pembrolizumab vs control cohort). The interquartile range of healthy control patients for each cell population is depicted as a gray bar, and the median value is noted with a dashed line.
Impact of immune reconstitution on relapse and immune related adverse events
The median follow-up among survivors in both cohorts was 25 months (range 1–49 months). The 2-year PFS for cHL and NHL patients was 83% (95% CI: 74–93) and 58% (95% CI: 47–70), respectively. Among 43 patients who relapsed, the median time from ASCT to post-ASCT relapse was 7 months (range 2–32; 10 [3–18] months for cHL and 7 [2–32] months for DLBCL).
In an exploratory analysis, we analyzed associations between risk of relapse/progression and the immune cell subsets and ratios in Figures 1–5 at the 1- and 2-month post-ASCT timepoints (with a p value threshold for significance set at ≤ 0.01 to account for multiple comparisons). To identify immune features that predict relapse (and not merely co-occur with relapse), patients who progressed within 90 days of ASCT were excluded from this analysis. As a continuous variable, higher levels of CD4+ TEMRA cells at the 1-month time point (before pembrolizumab initiation in the trial cohort) were associated with an increased risk of relapse/progression (p=0.009). In a subgroup analysis, this effect was only observed for the pembrolizumab cohort (p=0.003) and not the control cohort (p=0.83). No other immune populations were associated with relapse in our exploratory analysis.
Among the 59 patients in the pembrolizumab trial cohort, we analyzed the same immune cell subsets and timepoints to explore associations between immune reconstitution and the development of grade 2 or higher irAEs (p value threshold of ≤ 0.01 to account for multiple comparisons). In total, 21 patients (36%) experienced a grade 2 or higher irAE with a median time of onset of 2 months (range 0.2 – 17 months) after pembrolizumab initiation. As continuous variables, lower absolute levels of NK cells (p=0.009), PD-1+ CD4+ T cells (p=0.005), and PD-1+ CD8+ T cells (p=0.005) before pembrolizumab initiation (1-month timepoint) were associated with a higher risk of grade 2+ irAEs.
Discussion
The design of our multi-cohort analysis allowed us to compare features of immune reconstitution after ASCT between patients with cHL and DLBCL and in the presence or absence of pembrolizumab maintenance therapy. We found that lymphoma subtype had little impact on immune reconstitution. Among patients with cHL and DLBCL, the only significant difference was a slower recovery of B cells in NHL patients, which was likely driven by universal prior treatment with CD20 mAbs in this group. While immune recovery was similar in DLBCL and cHL patients, we noted superior post-ASCT outcomes for cHL patients which could reflect inherent differences in chemosensitivity, higher rates of pre-ASCT PET negativity, and more frequent use of effective post-ASCT maintenance therapy among cHL patients.
Regardless of lymphoma subtype, we observed delayed recovery of multiple T cell subset (particularly CD4+ T cells) even 18 months after ASCT (Figure 3). Among patients who relapse after ASCT, delayed recovery of CD4+ T cells may impact both the efficacy and tolerability of post-ASCT immunotherapies including chimeric antigen receptor (CAR) T cell therapy, bispecific antibodies, and immune checkpoint agents. For example, increased CD4+ T cell diversity has been associated with higher complete response rates for HL patients receiving PD-1 blockade16, a higher percentage of Tregs has been correlated with decreased effectiveness of bispecific antibodies for patients with lymphoid malignancies17, and lower lymphocyte counts have been linked with inferior outcomes for patients with DLBCL receiving CAR T cell therapy.18
Post-ASCT pembrolizumab maintenance appeared to impact immune reconstitution, but its effects were limited to a small number of analyzed immune cell populations. Notably, there was no significant impact on B cell, T cell, or NK cell recovery. In contrast, patients receiving pembrolizumab had a significant increase in circulating DCs that persisted for the duration of pembrolizumab therapy. Studies analyzing changes in circulating immune cell populations after PD-1 blockade have primarily focused on changes in T cell numbers and gene expression profiles rather than on myeloid immune cells.19–23 Notably, most of these studies were performed in patients with solid tumors and none analyzed the impact of PD-1 blockade for patients recovering from high-dose conditioning and ASCT. A similar pattern of an early increase in circulating DCs was reported in trials of patients with multiple myeloma receiving the PD-L1 inhibitor, atezolizumab; however, circulating DC levels returned to baseline within a few weeks.24 In our study, the persistent elevation in circulating DCs was driven by higher numbers of plasmacytoid and CD123−/CD11c− DCs while recovery of myeloid DCs was not affected by PD-1 blockade. DCs that do not express CD123 or CD11c are relatively immature,25 but our flow cytometry panel did not include other markers that would help better characterize these cells and their functional activity.
Circulating DCs are an emerging biomarker across a number of malignancies.26,27 Newly diagnosed patients with cHL have reduced numbers of circulating DCs compared to healthy controls. Among cHL patients, high-risk clinical features (bulky disease, extranodal disease, B symptoms) have been associated with further reductions in some DC subsets.28 Circulating DC populations may have particular relevance in patients receiving PD-1 blockade. Patients with hepatocellular carcinoma and lung cancer receiving PD-1 blockade who had high baseline or persistently elevated levels of PD-L1 positive circulating DCs were more likely to progress on PD-1 therapy.29,30 Despite the significant changes in circulating DC populations induced by pembrolizumab in our study, neither baseline nor on-treatment circulating DC levels were associated with clinical outcomes.
Pembrolizumab maintenance was also associated with a higher Treg:CD4Tcon ratio in our study; however, this finding should be interpreted cautiously as a higher Treg:CD4Tcon ratio was observed at the 1-month timepoint before initiation of pembrolizumab in the trial cohort. Signaling through the PD-1/PD-L1 pathway appears to be important for conversion of type I T helper cells to Treg cells and maintenance of Treg populations31,32, and in other settings, pembrolizumab treatment has been associated with relative depletion of Treg cells.33,34 In our study, the observed elevation in Treg:CD4Tcon ratio could be due to baseline differences in the control and pembrolizumab cohorts. Future studies of post-ASCT PD-1 blockade should analyze the relative recovery of Tregs and CD4Tcons to confirm or refute this finding, as it could impact the efficacy of subsequent immune-based treatments.
We also identified persistent decreases in PD-1+ T cell populations after PD-1 blockade. Because pembrolizumab and the PD-1 mAb used for flow analyses target the same epitope on the PD-1 receptor, PD-1 positive cell populations could be underreported due to competition from circulating pembrolizumab. Even so, it is notable that the decreased levels of PD-1 positive T cells persists for 6–12 months after completion of pembrolizumab therapy. Given that pembrolizumab has a serum half-life of <4 weeks,35 the persistent depletion of PD-1 positive cells observed in our study suggests a sustained biologic effect of PD-1 blockade, which has also been suggested in a prior study.33
While our study was not designed to identify definitive predictive or prognostic markers, we identified several candidate biomarkers as part of an exploratory analysis. There was an association between lower levels of CD4+ TEMRA cells after ASCT and a lower risk of relapse/progression; however, the absolute levels of CD4+ TEMRA cells in both cohorts was very low and this association was only observed for patients receiving pembrolizumab maintenance therapy. Terminally differentiated CD4 T cells represent a relatively small T cell subset, and to our knowledge, CD4+ TEMRA cells have not previously been identified as prognostic marker for patients undergoing ASCT or receiving PD-1 blockade. While CD8+ T cells are considered the key immune effectors of PD-1 blockade for most cancers, CD4+ T cells likely play an outsized role in mediating PD-1 activity in cHL based on the frequent absence of MHC class I on RS cells,36 the importance of MHC II as a biomarker for response to PD-1 blockade,37 and the unique topography of cHL.38,39 Additional studies are needed to confirm the prognostic value of CD4+ TEMRA and to better understand the functional role that CD4+ TEMRA cells could potentially play in mediating responses to PD-1 blockade.
Our study is smaller than some others that have sought to identify predictors of irAEs, but our analysis provides hypothesis-generating data for lymphoma patients in this setting. We identified lower absolute levels of NK cells, PD-1+ CD4+ T cells, and PD-1+ CD8+ T cells before pembrolizumab initiation as potential predictors of irAEs with pembrolizumab after ASCT. PD-1 expression (which is a frequent marker of T cell exhaustion) has previously been associated with an inverse relationship with irAEs, while the relationship between NK cell populations/activity and irAEs was less clear in a recent large multi-omics analysis of predictors of irAEs.40 These findings also require validation in future studies.
Our study is one of the most detailed analyses of immune reconstitution following ASCT in patients with lymphoma, but it has several important limitations. There were minor differences in eligibility criteria between the pembrolizumab and control cohorts, and among patients with DLBCL, those treated on the pembrolizumab study were more likely to have received 3 lines of therapy before ASCT. While other baseline clinical features of the two cohorts were generally well-matched, we also acknowledge that comparison of on-trial and off-trial populations could result in unaccounted bias in patient selection. While we tried to align sample collection in the control and pembrolizumab cohorts, timing and frequency of PBMC collection were not uniform, which may have contributed to differences observed between these groups, including the significant differences we observed at the first post-ASCT timepoint. Pre-existing differences between the pembrolizumab and control cohorts may have limited our ability to identify subtle effects of pembrolizumab on immune reconstitution. The sample collection for our study was robust with a median of 5 samples collected for each subject; however, sample collection did not begin until approximately 1-month after ASCT, so we are unable to analyze the earliest features of immune reconstitution, which have had prognostic value in some prior studies.6,7
In summary, post-ACST pembrolizumab maintenance was associated with a persistent elevation in circulating DCs, driven by higher levels of plasmacytoid DCs and immature DCs. In contrast, pembrolizumab did not have a significant impact on B cell, T cell, or NK cell recovery. Finally, our study suggests that early features of post-ASCT immune reconstitution could be associated with PFS and risk of irAEs and warrant additional investigation.
Highlights.
Post-ACST pembrolizumab maintenance therapy does not impact recovery of T cells.
Pembrolizumab was associated with an elevation in circulating dendritic cells.
Features of post-ASCT immune reconstitution may be associated with PFS and irAEs.
Acknowledgments
This study was supported by Merck and NIH grant P01CA229092. PA and RWM would also like to acknowledge support from the Harold and Virginia Lash Grant Program. RWM would like to acknowledge support from an American Society for Transplantation and Cellular Therapy New Investigator Award and a Lymphoma Research Foundation Clinical Investigator Career Development Award.
Conflicts of interests
RWM – Consulting: Genmab. Research funding: Bristol Myers Squibb, Merck Robert Redd - none
EJ - none
JW - none
KM - none
CR - none
MN - none
AV - none
JRB – Consulting: Abbvie, Acerta/Astra-Zeneca, Beigene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Eli Lilly, Genentech/Roche, Janssen, MEI Pharma, Morphosys AG, Nextcea, Novartis, Pfizer, Rigel. Research funding: Gilead, Loxo/Lilly, SecuraBio, Sun, TG Therapeutics. Data safety monitoring committee: Invectys.
JLC - Consulting: Incyte, MorphoSys. Reseach Funding: Bayer, Abbvie
MSD - Consulting: AbbVie, Adaptive Biotechnologies, Ascentage Pharma, AstraZeneca, BeiGene, BMS, Celgene, Eli Lilly, Genentech, Janssen, Takeda, and TG Therapeutics. Research support: AbbVie, Ascentage Pharma, AstraZeneca, BMS, Genentech, MEI Pharma, Novartis, Surface Oncology, TG Therapeutics, and Verastem. Honoraria: Research to Practice and Aptitude Health.
DCF – none
EJ – Consulting: Syros, Takeda. Research funding: Acerta, Janssen, Novartis, Pharmacyclics.
CAJ - Consulting: Kite/Gilead, Novatis, BMS/Celgene, Precision Biosciences, Nkarta, bluebird bio, Epizyme, Lonza, Abbvie, Ipsen. Research funding: Kite/Gilead, Pfizer
AIK - none
ASL- none
SN- none
OOO - none
EMP - none
PBD - Advisory Board: Kite/ Gilead.
YN - Consulting: Affimed, Novonordisk. Resarch funding: Novartis, Biosecura, Astra-Zeneca, Affimed, Takeda.
RMJ - none
Y-BC– Consulting: Incyte, Magenta, Gamida Cell, Daiichi, Equilium, Celularity, Actinium
AFH – Consulting: Bristol Myers Squibb, Genentech, Merck, Seattle Genetics, AstraZeneca, Karyopharm, ADC Therapeutics, Takeda, Tubulis. Research funding: Bristol Myers Squibb, Genentech, Merck, Seattle Genetics, KiTE Pharma, Gilead Sciences, AstraZeneca, ADC Therapeutics.
PA - Consulting: Merck, BMS, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, Morphosys, Daiichi Sankyo, Miltenyi, Tessa, GenMab, C4, Enterome, Regeneron, Epizyme, Astra Zeneca, Genentech. Research funding (instutional): Merck, BMS, Affimed, Adaptive, Roche, Tensha, Otsuka, Sigma Tau, Genentech, IGM, Kite. Honoraria: Merck, BMS
JR – Consulting: Akron Biotech, Blackstone Life Sciences Advisors, Clade Therapeutics, Garuda Therapeutics, Immunitas Therapeutics, LifeVault Bio, Novartis, Rheos Medicines, Talaris Therapeutics and TScan Therapeutics. Research grant support: Amgen, Equillium, Novartis, and Kite/Gilead. Data safety monitoring committees: Avrobio.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540–1545. doi: 10.1056/NEJM199512073332305 [DOI] [PubMed] [Google Scholar]
- 2.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet (London, England). 1993;341(8852):1051–1054. http://www.ncbi.nlm.nih.gov/pubmed/8096958. Accessed April 23, 2019. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet (London, England). 2002;359(9323):2065–2071. doi: 10.1016/S0140-6736(02)08938-9 [DOI] [PubMed] [Google Scholar]
- 4.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. doi: 10.1016/j.immuni.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 5.Vuckovic S, Minnie SA, Smith D, et al. Bone marrow transplantation generates T cell–dependent control of myeloma in mice. J Clin Invest. 2018;129(1):106–121. doi: 10.1172/JCI98888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porrata LF, Gertz MA, Inwards DJ, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98(3):579–585. doi: 10.1182/blood.V98.3.579 [DOI] [PubMed] [Google Scholar]
- 7.Porrata LF, Inwards DJ, Ansell SM, et al. Autograft immune content and survival in non-Hodgkin’s lymphoma: A post hoc analysis. Leuk Res 2019;81:1–9. doi: 10.1016/j.leukres.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 8.Chen R, Zinzani PL, Fanale MA, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol. 2017;35(19):2125–2132. doi: 10.1200/JCO.2016.72.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. In: Journal of Clinical Oncology. Vol 34. ; 2016:3733–3739. doi: 10.1200/JCO.2016.67.3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansell SM, Minnema MC, Johnson P, et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J Clin Oncol. 2019;37(6):481–489. doi: 10.1200/JCO.18.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesokhin AM, Ansell SM, Armand P, Scott ECHA. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol. 2016;34(23):2698–2704. http://www.ncbi.nlm.nih.gov/pubmed/27269947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armand P, Chen Y-B, Redd RA, et al. PD-1 Blockade with Pembrolizumab for Classical Hodgkin Lymphoma after Autologous Stem Cell Transplantation. Blood. April 2019:blood.2019000215. doi: 10.1182/blood.2019000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frigault MJ, Armand P, Redd RA, et al. PD-1 blockade for diffuse large B-cell lymphoma after autologous stem cell transplantation. Blood Adv. 2020;4(1):122–126. doi: 10.1182/bloodadvances.2019000784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403 [DOI] [PubMed] [Google Scholar]
- 15.Alho AC, Kim HT, Chammas MJ, et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. 2016;127(5):646–657. doi: 10.1182/blood-2015-10-672345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cader FZ, Hu X, Goh WL, et al. A peripheral immune signature of responsiveness to PD-1 blockade in patients with classical Hodgkin lymphoma. Nat Med. 2020;26(9). doi: 10.1038/s41591-020-1006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duell J, Dittrich M, Bedke T, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia. 2017;31(10):2181–2190. doi: 10.1038/leu.2017.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vercellino L, Di Blasi R, Kanoun S, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(22). doi: 10.1182/bloodadvances.2020003001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das R, Verma R, Sznol M, et al. Combination Therapy with Anti–CTLA-4 and Anti–PD-1 Leads to Distinct Immunologic Changes In Vivo. J Immunol. 2015;194:950–959. doi: 10.4049/jimmunol.1401686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim CG, Hong MH, Kim KH, et al. Dynamic changes in circulating PD-1+CD8+ T lymphocytes for predicting treatment response to PD-1 blockade in patients with non-small-cell lung cancer. Eur J Cancer. 2021;143:113–126. doi: 10.1016/j.ejca.2020.10.028 [DOI] [PubMed] [Google Scholar]
- 21.Kim KH, Cho J, Ku BM, et al. The First-week Proliferative Response of Peripheral Blood PD-1 + CD8 + T Cells Predicts the Response to Anti-PD-1 Therapy in Solid Tumors. Clin Cancer Res. 2019;25(7):2144–2154. doi: 10.1158/1078-0432.ccr-20-4231 [DOI] [PubMed] [Google Scholar]
- 22.Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. doi: 10.1038/nature19330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peña-Asensio J, Calvo H, Torralba M, Miquel J, Sanz-De-villalobos E, Larrubia JR. Anti-pd-1/pd-l1 based combination immunotherapy to boost antigen-specific cd8+ t cell response in hepatocellular carcinoma. Cancers (Basel). 2021:1922. doi: 10.3390/cancers13081922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bar N, Costa F, Das R, et al. Differential effects of PD-L1 versus PD-1 blockade on myeloid inflammation in human cancer. JCI Insight. 2020;5(12):e129353. doi: 10.1172/jci.insight.129353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villani AC, Satija R, Reynolds G, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science (80- ). 2017;356(6335). doi: 10.1126/science.aah4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng Q, Qiu X, Zhang Z, et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat Commun. 2020;11(1):4835. doi: 10.1038/s41467-020-18570-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayoux M, Roller A, Pulko V, et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci Transl Med. 2020;12(534). doi: 10.1126/scitranslmed.aav7431 [DOI] [PubMed] [Google Scholar]
- 28.Galati D, Zanotta S, Corazzelli G, et al. Circulating dendritic cells deficiencies as a new biomarker in classical Hodgkin lymphoma. Br J Haematol. 2019;184(4):594–604. doi: 10.1111/bjh.15676 [DOI] [PubMed] [Google Scholar]
- 29.Hung YP, Shao YY, Lee JM, et al. Potential of circulating immune cells as biomarkers of nivolumab treatment efficacy for advanced hepatocellular carcinoma. J Chinese Med Assoc. 2021;84(2):144–150. doi: 10.1097/JCMA.0000000000000477 [DOI] [PubMed] [Google Scholar]
- 30.Riemann D, Schütte W, Turzer S, Seliger B, Möller M. High PD-L1/CD274 expression of monocytes and blood dendritic cells is a risk factor in lung cancer patients undergoing treatment with PD1 inhibitor therapy. Cancers (Basel). 2020;12(10):2966. doi: 10.3390/cancers12102966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amarnath S, Mangus CW, Wang JCM, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3(111):111–120. doi: 10.1126/scitranslmed.3003130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asano T, Kishi Y, Meguri Y, et al. PD-1 Signaling Has a Critical Role in Maintaining Regulatory T Cell Homeostasis; Implication for Treg Depletion Therapy By PD-1 Blockade. Blood. 2015;126(23):848. http://www.bloodjournal.org/content/126/23/848.abstract. Accessed May 2, 2016. [Google Scholar]
- 33.Merryman RW, Kim HT, Zinzani PL, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood. 2017;129(10):1380–1388. doi: 10.1182/blood-2016-09-738385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida K, Okamoto M, Sasaki J, et al. Anti-PD-1 antibody decreases tumour-infiltrating regulatory T cells. BMC Cancer. 2020;20(1):25. doi: 10.1186/s12885-019-6499-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Highlights of Prescribing Information - Pembrolizumab. US Food and Drug Administration. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125514lbl.pdf. Published 2014. Accessed May 2, 2016. [Google Scholar]
- 36.Roemer MGM, Advani RH, Redd RA, et al. Classical Hodgkin Lymphoma with Reduced 2M/MHC Class I Expression Is Associated with Inferior Outcome Independent of 9p24.1 Status. Cancer Immunol Res. 2016;4(11):910–916. doi: 10.1158/2326-6066.CIR-16-0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roemer MGM, Redd RA, Cader FZ, et al. Major Histocompatibility Complex Class II and Programmed Death Ligand 1 Expression Predict Outcome After Programmed Death 1 Blockade in Classic Hodgkin Lymphoma. J Clin Oncol. 2018;36(10):942–950. doi: 10.1200/JCO.2017.77.3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carey CD, Gusenleitner D, Lipschitz M, et al. Topological analysis reveals a PD-L1 associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood. September 2017:blood-2017–03-770719. doi: 10.1182/blood-2017-03-770719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cader FZ, Schackmann RCJ, Hu X, et al. Mass cytometry of Hodgkin lymphoma reveals a CD4+ regulatory T-cell-rich and exhausted T-effector microenvironment. Blood. 2018;132(8):825–836. doi: 10.1182/blood-2018-04-843714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jing Y, Liu J, Ye Y, et al. Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Nat Commun. 2020;11(1):4946. doi: 10.1038/s41467-020-18742-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


