Abstract
Vaccination is a modality that has been widely explored for the treatment of various diseases. To increase the potency of vaccine formulations, immunostimulatory adjuvants have been regularly exploited, and the stimulator of interferon genes (STING) signaling pathway has recently emerged as a remarkable therapeutic target. STING is an endogenous protein on the endoplasmic reticulum that is a downstream sensor to cytosolic DNA. Upon activation, STING initiates a series of intracellular signaling cascades that ultimately generate potent type I interferon-mediated immune responses. Both natural and synthetic agonists have been used to stimulate the STING pathway, but they are usually administered locally due to low bioavailability, instability, and difficulty in bypassing the plasma membrane. With excellent pharmacokinetic profiles and versatility, nanocarriers can address many of these challenges and broaden the application of STING vaccines. Along these lines, STING-inducing nanovaccines are being developed to address a wide range of diseases. In this review, we discuss the recent advances in STING nanovaccines for anticancer, antiviral, and antibacterial applications.
Keywords: STING, nanovaccine, immunotherapy, cancer, infectious disease
1. Introduction
Vaccination is an immunotherapeutic strategy focused on educating host immunity to fight off diseases. Professional antigen presenting cells (APCs) of the innate immune system are first activated in an immunostimulatory fashion, after which robust adaptive immunity against distinct antigenic targets can be achieved through a series of downstream signaling cascades. As prophylaxes, vaccines have experienced significant success and contributed largely to the eradication of major infectious diseases such as smallpox, polio, and measles (Henderson, 2011; Larson and Ghinai, 2011; Moss and Griffin, 2006). More recently, the development of therapeutic vaccines against cancer has shown much promise (Banchereau and Palucka, 2005; Hu et al., 2018; Melero et al., 2014). Unlike foreign pathogens, immune stimulation against marginally mutated cancer antigens is more challenging, so immune stimuli in the form of adjuvants are routinely included. On this front, compounds that can activate the stimulator of interferon genes (STING) signaling pathway have been particularly attractive due to their pivotal role in the cancer–immunity cycle and during pathogenic invasion (Ahn and Barber, 2019; Barber, 2015; Corrales et al., 2016; Hayman et al., 2021; Marinho et al., 2017; Zhu et al., 2019).
STING is a protein embedded in the endoplasmic reticulum and acts as a downstream sensor to detect cytosolic DNA (Chen et al., 2016a; Ishikawa and Barber, 2008). Healthy cells normally do not possess cytoplasmic DNA, but it can exist as a result of pathogenic infections, cellular damage, or tumorigenesis (Li and Chen, 2018). In the cytosol, DNA binds to cyclic GMP-AMP synthase (cGAS) to produce a cyclic dinucleotide (CDN) known as cyclic GMP-AMP (cGAMP) (Barber, 2015). The cGAMP molecule will subsequently bind to and activate STING, leading to the cellular production of type I interferons (IFNs) to help regulate immune activity. Type I IFNs are key cytokines that link the innate immune system with adaptive immunity, and they can stimulate immune cells to elicit potent antitumor and antiviral responses (Ivashkiv and Donlin, 2014). While largely deployed against cancer, applications of STING agonists for treating infections have emerged in the past years (Chattopadhyay and Hu, 2020; Gall et al., 2018; Guo et al., 2015; Sali et al., 2015). Here, we will review the development of STING-targeting nanoformulations in the battle against cancer and infectious diseases (Figure 1). We begin with background on the immunological functions of STING and introduce the wide range of different natural and synthetic STING-activating compounds. Then, we will discuss advantages of nanotechnology and review applications of STING nanovaccines against cancer, viral infection and bacterial infection.
Figure 1.
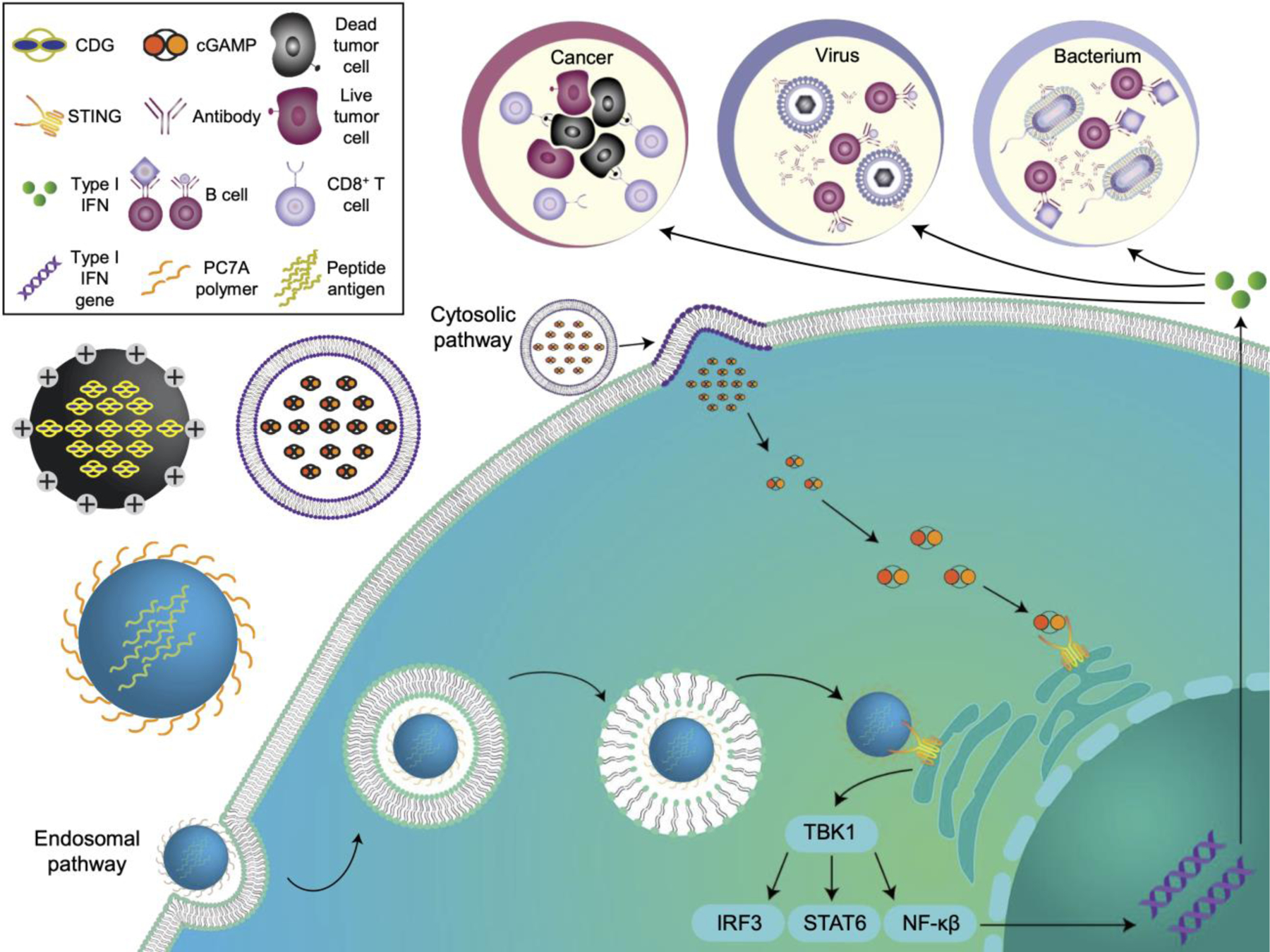
STING-activating nanovaccines against cancer, virus and bacterium. STING nanovaccines can be synthesized from various nanomaterials to deliver payloads intracellularly. After endocytosis, pH-responsive nanovaccines can escape from the endosome and engage with STING in the cytosol. In the cytosolic pathway, the nanovaccines can fuse directly with the plasma membrane to release the encapsulated payload into the cytosol. Once activated, STING complexes with TANK-binding kinase 1 (TBK1) and phosphorylates interferon regulatory factor 3 (IRF3), signal transducer and activator of transcription 6 (STAT6), or nuclear factor-κB (NF-κB) to stimulate the production of type I interferons (IFNs).
2. STING pathway and its immunological roles
The innate immune system can recognize specific pathogen-associated molecular patterns through pathogen recognition receptors (PRRs) (Kumar et al., 2011). STING is a PRR that senses pathogenic molecules in the cytosol and indirectly responds to cytosolic DNA through the recognition of CDNs (Barber, 2015). While some pathogens can produce CDNs that have a natural affinity for STING, detection of cytosolic DNA occurs indirectly through the cGAS-DNA sensing pathway. Interestingly, manganese ions (Mn2+) (Zhao et al., 2020b) and β-arrestin 2 (Zhang et al., 2020c) were recently found to engage with cGAS to initiate and enhance downstream synthesis of cGAMP. Upon proper binding, STING forms a complex with TANK-binding kinase 1 (TBK1) and migrates to the perinuclear Golgi. At the site, the complex phosphorylates transcription factors such as IFN regulatory factor 3 (IRF3), nuclear factor-κB (NF-κB), and signal transducer and activator of transcription 6 (STAT6), which subsequently induce the production of type I IFNs (Cheng et al., 2020). Cytokines such as type I IFNs act as signaling molecules that alert the rest of the body to pathogenic invasions or cellular damage and help recruit immune cells in response (Ivashkiv and Donlin, 2014). Type I IFNs, among which IFN-α and IFN-β are the most common, act as mediators that link innate immunity with the specialized immune subsets from the adaptive immune system.
Type I IFNs have critical functions in both the innate immune system and the adaptive branch of immunity. During the innate phase of an immune response, the cytokines can guide phagocytes to recognize specific pathogens and stimulate infected cells to inhibit pathogen replication or restrict intracellular bacterial growth (Yan and Chen, 2012). During viral infections, type I IFNs promote the proliferation and survival of natural killer (NK) cells (Martinez et al., 2008). In the adaptive immune system, these IFNs play vital roles in activating cytotoxic T lymphocytes (CTLs), which are responsible for the selective clearance of infected and cancerous cells (Iwasaki and Medzhitov, 2015). To accomplish this function, type I IFNs upregulate the expression of proteins associated with antigen presentation in APCs and increase the production of certain proinflammatory cytokines and chemokines (McNab et al., 2015). In addition, type I IFNs were found to enhance cross-presentation and increase dendritic cell (DC) accumulation in lymphatic tissues (Le Bon et al., 2006a; Rouzaut et al., 2010; Spadaro et al., 2012). They can also act directly on the specialized T cells and B cells by providing signals for proliferation, differentiation, clonal expansion, and survival (Brinkmann et al., 1993; Le Bon et al., 2006b; Marrack et al., 1999). While production of type I IFNs can be induced through multiple signaling pathways, STING is one of the most critical mechanisms for mounting an immune response against viruses and bacteria (Ahn and Barber, 2019; Chen et al., 2011).
Highlighting the importance of STING, one study demonstrated that expansion of CTLs and other immune subsets, such as plasma cells and follicular helper T cells, was significantly reduced when primed with DCs deficient in STING (Klarquist et al., 2014). Other studies have shown that host susceptibility to vesicular stomatitis virus and herpes simplex virus 1 (HSV-1) was significantly increased in STING knockout mice (Ishikawa and Barber, 2008; Ishikawa et al., 2009). In murine models, STING was key in activating T cells and stimulating antibody production in response to HSV-1 and DNA from Escherichia coli and Vibrio cholerae (Li et al., 2013). STING-induced expression of type I IFNs was identified as a central mediator of immune responses against varicella zoster virus, hepatitis B virus, chikungunya virus (CHIKV), human adenoviruses, and cytomegaloviruses, among many others (Anghelina et al., 2016; Gall et al., 2018; Guo et al., 2015; Kalamvoki et al., 2014; Kim et al., 2017; Lio et al., 2016; Sali et al., 2015; Stempel et al., 2019). In terms of antimicrobial immunity, bacteria are known to produce CDNs as part of their colonization process; thus, during intracellular invasion, bacterial CDNs will naturally bind to STING and trigger a robust type I IFN-mediated response (Whiteley et al., 2019). Several common bacteria such as Streptococcus pneumoniae, Listeria monocytogenes, Shigella flexneri, and Neisseria gonorrhoeae were found to trigger STING signaling in this fashion (Koppe et al., 2012; Marinho et al., 2017; Parker et al., 2011). In some bacteria such as Mycobacterium tuberculosis, STING activation was imperative for autophagy, an essential cellular process for degrading intracellular components and removing invasive microbes (Watson et al., 2012). STING-induced immunity can also occur during fungal and parasitic infections (Ahn and Barber, 2019; McNab et al., 2015; Sisquella et al., 2017).
More recently, the significance of the STING pathway for antitumor immunity was elucidated. STING activation was shown to induce cellular apoptosis (Tang et al., 2016), promote antigenic release (Lu et al., 2018), augment antigen presentation (Curran et al., 2016), enhance the priming and activation of CTLs (Jassar et al., 2005), improve T cell infiltration into tumor sites (Ohkuri et al., 2014), promote immune cell proliferation and survival (Tough et al., 1996), and assist in the recognition and killing of cancerous cells (Jing et al., 2019; Lirussi et al., 2017). Since tumor cells are derived from host cells, STING activation during anticancer immunity largely stems from the detection of self-DNA through the cGAS-DNA sensing pathway. Damaged self-DNA can leak out from the nucleus or mitochondria of apoptotic cells and subsequently be processed by immune cells. Once activated, STING can induce a powerful and antigen-specific immune response, thus propagating a positive feedback loop that drives anticancer immunity. Many STING agonists have been developed for cancer immunotherapy applications (Corrales et al., 2015; Corrales et al., 2017; Corrales et al., 2016; Fu et al., 2015; Kitai et al., 2017; Li et al., 2019; Woo et al., 2014; Xia et al., 2016). Overall, STING is involved in many aspects of the immune response and is an attractive target for vaccine-based immunotherapy. Selective activation of STING can program immune cells to recognize and target invasive pathogens or educate the host immune system to identify and eradicate cancerous cells. It is important to note that the STING pathway is independent of other pathogen-sensing pathways, such as toll-like receptor (TLR), nucleotide oligomerization domain (NOD)-like receptor, and retinoic acid-inducible gene-I (RIG-I)-like receptor pathways (Kawai and Akira, 2009). Ultimately, an in-depth understanding of the wide range of different compounds that can activate this signaling pathway is vital for engineering efficacious STING-targeted therapeutics.
3. Types of STING agonists
STING plays an indispensable role in anticancer, antiviral, and antibacterial immunity. Owing to their broad applicability and pivotal functions in immunity, many natural and synthetic STING agonists have been utilized in the design of more effective vaccines (Ding et al., 2020; Motedayen Aval et al., 2020; Wu et al., 2020). Better mechanistic understanding of the STING biological pathway has enabled the discovery of novel compounds and helped to elucidate their structure–activity relationship. Several STING agonists are currently being investigated in clinical trials (Motedayen Aval et al., 2020; Zhu et al., 2019). STING agonists can be subdivided into several classes, which include natural CDNs, CDN derivatives, flavonoids and xanthones, and other novel and unique compounds (Figure 2).
Figure 2.
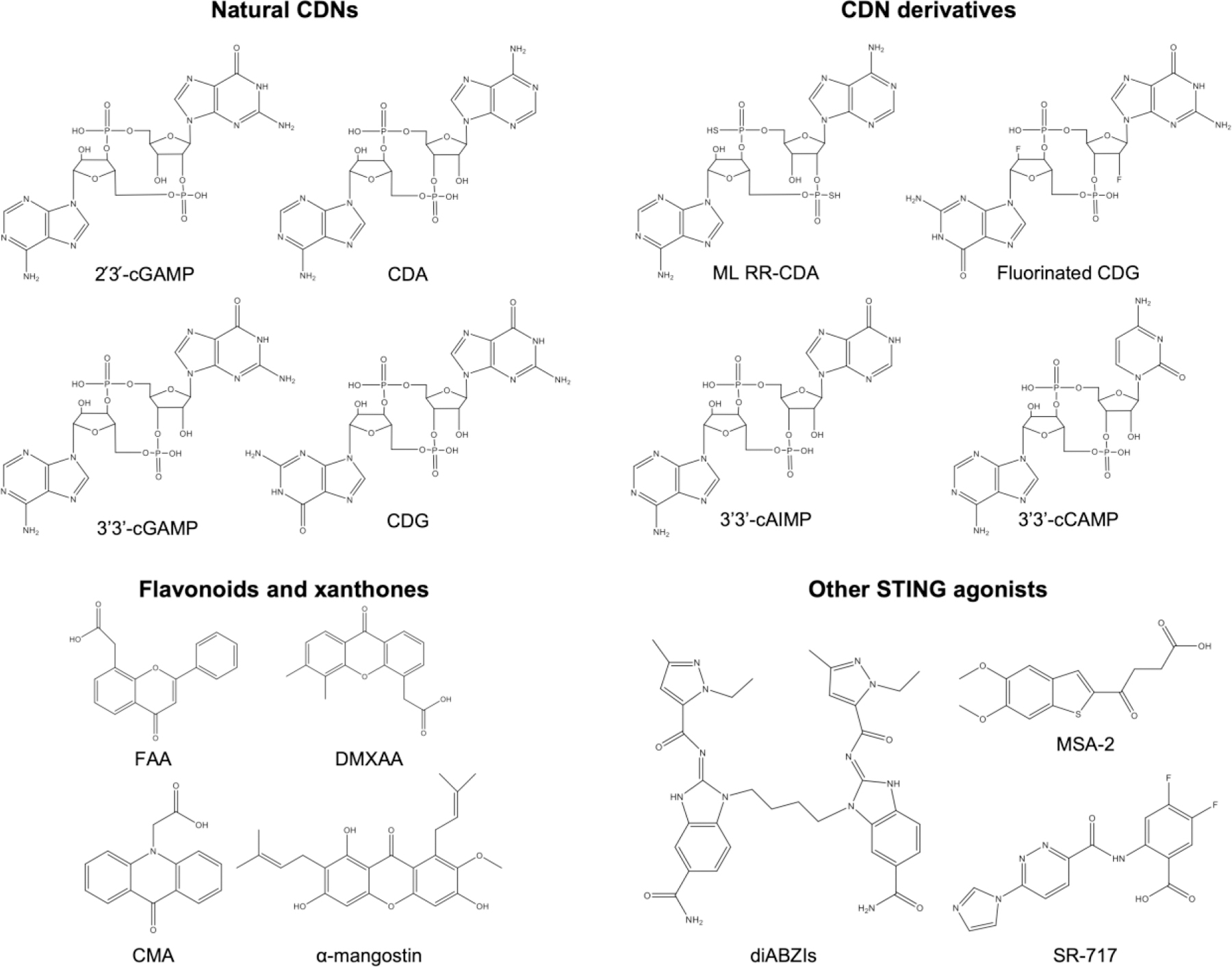
Chemical structures of various STING agonists. STING agonists can be generally divided into four main groups: natural cyclic dinucleotides (CDNs), derivatives of CDNs, flavonoids and xanthones, and other STING agonists.
3.1. Cyclic dinucleotides
CDNs are the most common STING agonists with innate binding affinity for STING (Danilchanka and Mekalanos, 2013). In the cGAS-DNA sensing pathway, mammalian cells synthesize noncanonical 2´3´-cGAMP with a mixed linkage (ML) upon detection of cytosolic DNA (Li and Chen, 2018). Canonical 3´3´-cGAMP with the classical 3´5´ phosphodiester bonds is naturally produced by bacteria (Diner et al., 2013). The potency of these two molecules for STING activation is indistinguishable; however, noncanonical 2´3´-cGAMP has a stronger binding interaction with the STING protein (Zhang et al., 2013). Alternate isoforms of cGAMP such as 3´2´-cGAMP and 2´2´-cGAMP have been evaluated as potential STING agonists, but no clear benefits were found for these synthetic molecules (Zhang et al., 2013). Besides the heterogenous cyclic nucleotide structure, natural homogenous analogs such as cyclic di-GMP (CDG) (Burdette et al., 2011; Madhun et al., 2011) and cyclic di-AMP (CDA) (Ebensen et al., 2011; Skrnjug et al., 2014) can likewise activate the STING pathway (Jin et al., 2011). CDG and CDA are second messengers naturally produced by bacteria and have vital biological roles in pathogenesis (Corrigan and Grundling, 2013; Romling et al., 2013). In particular, CDG was found to be a promising mucosal adjuvant by many researchers (Blaauboer et al., 2015; Ebensen et al., 2017; Madhun et al., 2011; Mansouri et al., 2016). Not surprisingly, noncanonical forms of 2´3´-cyclic di-GMP (ML CDG) and 2´3´-cyclic di-AMP (ML CDA) have been chemically synthesized. While the potency of 2´3´-cGAMP and 3´3´-cGAMP is minimal, ML CDG and ML CDA can actually induce higher levels of type I IFNs in cells when compared to their canonical counterparts (Corrales et al., 2015; Fu et al., 2015).
A better understanding of the molecular interactions between natural CDNs and STING has engendered several synthetic CDN derivatives with enhanced potency, binding affinity, and stability. One such derivative is cyclic AMP-IMP (cAIMP), which is fabricated by the replacement of the guanine nucleoside with inosine in cGAMP (Lioux et al., 2016). Stimulation with 3´3´-cAIMP outperformed 2´3´-cAIMP, and both molecules were found to elicit a stronger type I IFN response than 2´3´-cGAMP. Under the same conditions, the adjuvanticity of 3´2´-cAIMP and 2´2´-cAIMP were both lower than 2´3´-cGAMP, while the difference between the two analogs was indistinguishable. Homogenous cyclic di-IMP (CDI) can be synthesized by substituting the remaining adenosine nucleoside with inosine. As an adjuvant, CDI can stimulate mucosal immunity comparable to CDG (Libanova et al., 2010), but the advantage of using synthetic CDI as opposed to natural CDG is unclear. Through observing the chemical structure of CDNs, researchers have synthesized all possible forms of canonical CDNs with the four natural ribonucleotide bases: cytosine, guanine, adenine, and uracil (Wang et al., 2017). When evaluating the immunostimulatory capability of these synthetics, 3´3´-cCAMP was found to activate STING, but its ability to induce IRF expression in a RAW264.7 reporter cell line was inferior to naturally derived bacterial CDNs. Compared with the natural STING agonists, application of these novel synthetic CDN derivatives is less common.
The chemical structure of CDNs is two nucleotides connected by phosphodiester bonds in a cyclic fashion, but the phosphodiester bonds are susceptible to degradation by phosphodiesterases and nucleases (Kato et al., 2018). These enzymes are commonly found systemically and in host cells, and they act as a barrier that lowers the effectiveness of CDNs. To overcome this obstacle and increase the stability of CDNs, phosphorothioate diester linkages have been used to connect the nucleotides. While the immunostimulatory effects of canonical CDG linked by one or two (RR-CDG) phosphorothioate diester bonds were lower than native CDG in a mucosal setting (Yan et al., 2008), similar modifications on noncanonical cGAMP, CDG, and CDA (ML RR-CDA) have shown enhanced potency (Corrales et al., 2015). In the case of cAIMP, the potency of 3´3´-cAIMP with two phosphorothioate diester linkages was likewise improved when compared to 3´3´-cAIMP (Lioux et al., 2016). Besides phosphorothioate diester modifications, CDNs have been linked with thiourea, urea, carbodiimide, guanidinium, and triazole bonds (Fujino et al., 2014; Gaffney and Jones, 2014). Stability of CDNs can be further increased through fluorination, a technique commonly utilized in medicinal chemistry (Bohm et al., 2004; Cavaliere et al., 2017; Liu et al., 2008). Interestingly, fluorination at the 2´ or 3´ nucleotide sites not only increased stability in vivo, but it also significantly intensified biological activity and adjuvanticity (Corrales et al., 2015; Fu et al., 2015; Lioux et al., 2016; Smola et al., 2021; Wu et al., 2021; Zhang et al., 2020a). The phenomenon is attributed to the increased lipophilicity after fluorine modification, which allows the CDNs to better traverse the cell membrane.
3.2. Flavonoids and xanthones
A second major class of STING agonists are flavonoids and xanthones, which are polyphenolic compounds naturally found in plants. Flavonoids are plant metabolites that are commonly consumed for health benefits and structurally contain two phenol rings and a heterocyclic ring (Panche et al., 2016). Xanthones are molecules that are chemically similar to flavonoids, except the three cyclic rings are bound to one another. Flavone-8-acetic acid (FAA) is a flavonoid that was discovered through screening natural compounds, and it was shown to induce an immune-mediated antitumor response (Bibby et al., 1991). FAA was one of the earliest STING agonists to be uncovered prior to the discovery of the STING pathway, and recent findings have confirmed that FAA does initiate immunity through STING engagement (Zheng et al., 2020). The xanthone 5,6-dimethylxanthenone-4-acetic acid (DMXAA) is an adjuvant derived from FAA that exhibits increased potency (Philpott et al., 1995). A single intratumoral injection of DMXAA was shown to induce systemic tumor necrosis factor (TNF-α) secretion at levels similar to FAA, but with doses 10 times lower. Another xanthone compound, 10-carboxymethyl-9-acridanone (CMA), was found to have potent antiviral effects (Guo et al., 2015). Despite promising preclinical studies, all three compounds failed clinical trials because they were later found to react only to murine STING and were unable to activate the human STING pathway (Cavlar et al., 2013; Conlon et al., 2013; Kim et al., 2013). Nevertheless, not all flavonoid and xanthone derivatives are murine STING specific, as α-mangostin was shown to activate human STING to a greater extent than murine STING (Zhang et al., 2018b).
3.3. Other STING agonists
High throughput drug screening has emerged as a powerful strategy to identify novel inhibitors and agonists for various therapeutic targets (Bleicher et al., 2003; Dove, 2003; Macarron et al., 2011). Many novel STING agonists, including dispiro diketopiperzine (DSDP) (Liu et al., 2017a), 6-bromo-N-(naphthalen-1-yl)benzo[d][1,3]dioxole-5-carboxamide (BNBC) (Zhang et al., 2019a), G10 (Sali et al., 2015), and C11 (Gall et al., 2018), were discovered after screening libraries of small molecules using cell reporter systems. Treatment with DSDP and BNBC elicited higher IFN-β and interleukin (IL)-29 mRNA expression in human cell lines and significantly protected THF fibroblasts from infection by dengue virus, zika virus, and yellow fever virus in a prophylactic setting. It is important to note that both DSDP and BNBC are human-specific STING agonists and do not respond to murine STING. G10 and C11 were found to increase IFN-β release through IRF3, but not by NF-κB transcription. When THF cells were pre-exposed to G10, replication of alphaviruses such as CHIKV and venezuelan equine encephalitis virus (VEEV) were inhibited by more than three orders of magnitude. Treatment with C11 was able to inhibit replication of CHIKV, VEEV, ross river virus, mayaro virus, and o’nyong-nyong virus in a similar fashion. Another STING agonist is STING-mediated interferon-inducing and cytotoxic reagent, original (SINCRO), which has dual functionalities; the compound not only activates STING, but it also induces cellular apoptosis in cancerous cells through oxidative stress (Kimura et al., 2018). Upon intratumoral treatment, the cytocidal property of SINCRO helped to eradicate cancerous cells while concurrently activating immune cells to process tumor neoantigens in a synergistic manner.
STING agonists in free form are generally administered intratumorally, which is not always an option for cancer therapy. Dimetric amidobenzimidazole (diABZI) was identified as a STING inducer from a library of 1.8 million small molecules through a cGAMP competitive binding assay (Ramanjulu et al., 2018). Compared to 2´3´-cGAMP, diABZI is 400 times more potent for STING activation and can be safely administered systemically. In a CT26 colorectal cancer model, intravenous treatment with diABZI at 1.5 mg/kg completely eradicated tumors in 8 out of 10 mice. Several derivatives of amidobenzimidazole with comparable potency have been synthesized and identified by others (Xi et al., 2020). Another compound that can be administered systemically is SR-717, which was synthesized after screening approximately 100,000 small molecules for the induction of IRF in a THP-1 reporter cell line (Chin et al., 2020). SR-717 stimulated IFN genes at a level comparable to diABZI in vitro, but mice interperitoneally treated with SR-717 had plasma IFN-β levels roughly 60 times lower than with diABZI treatment. In a B16F10 melanoma model, daily intraperitoneal treatment with SR-717 at 30 mg/kg for a week was able to extend median survival from 22 days to 27 days. Benzothiophene oxobutanoic acid (MSA-2) is a compound recently identified from a library screening of 2.4 million molecules through the detection of IFN-β in a THP-1 reporter cell line (Pan et al., 2020). Treatment efficacy of MSA-2 in an MC38 tumor model was assessed after intratumoral, subcutaneous, or oral administration. While different treatment regimens and dosages were used for the three routes, complete tumor regression was achieved in 80% to 100% of all treated mice. At a 60 mg/kg dosage, administration by oral gavage achieved tumor bioavailability comparable to subcutaneous injections at 50 mg/kg. Oral delivery is particularly attractive for clinical translation and improves patient compliance due to the painless and simple administration process.
4. STING nanovaccines
While STING agonists have shown significant promise as vaccine adjuvants, limited bioavailability and efficacy have thwarted progress in clinical applications. On this front, nanoparticles are promising drug delivery carriers that can specifically localize drug payloads to increase efficacy and decrease nonspecific cytotoxicity (Couvreur, 2013). Nanoparticles are versatile materials with distinct advantages and can be broadly employed for many different applications. Over the past several years, a wide range of different nanoparticle formulations have been successfully developed to better facilitate the delivery of STING agonists into immune cells and increase their activity against cancers, viruses, and bacteria.
4.1. Advantages of nanovaccines
Nanotechnology can address many shortcomings of traditional vaccines through their unique size, shape, hydrophobicity, and surface properties. Nanovaccines can be fabricated to approximate the size of pathogens for improved cellular uptake and designed to efficiently drain into lymphatic tissues for antigen presentation (Gheibi Hayat and Darroudi, 2019; Luo et al., 2017a). Lymphatic drainage is heavily dependent on nanoparticle size, where smaller nanoparticles accumulate significantly better than their larger counterparts (Gao et al., 2015). In one study, activation of resident DCs in the lymph nodes with 100-nm polypropylene sulfide nanoparticles was only 10% as efficient as 25-nm nanoparticles (Reddy et al., 2007). The shape of nanomaterials is another factor that needs to be considered. Spherical nanoparticles, for example, are more likely to be taken up by immune cells (Gheibi Hayat and Darroudi, 2019), whereas particles with an ellipsoid shape can interface with the surface membrane in a superior fashion (Kroll et al., 2017b; Meyer et al., 2015). On the other end of the spectrum, unique structures such as nanorods and nanostars have high cytotoxicity due to their protruding structure (Lee et al., 2019). Modulating the hydrophobicity and electrostatic properties of nanoparticles can similarly affect cellular uptake. While cationic nanoparticles are readily phagocytosed and have poor pharmacokinetic profiles, anionic nanoparticles have lower nonspecific uptake and can be engineered to specifically target immune cell subsets (Luo et al., 2017a). Modification of the nanoparticle surface with antibodies, lipids, proteins, or synthetic compounds can bestow new functionalities, alter in vivo fate, and extend the therapeutic window of encapsulated payloads (Ai et al., 2020; Fang et al., 2018; Gheibi Hayat and Darroudi, 2019; Liu et al., 2017b; Luo et al., 2017a).
With a wide range of different materials for selection, nanoparticles can readily incorporate both hydrophobic and hydrophilic molecules for delivery (Kroll et al., 2019; Zhou et al., 2020a). Drugs encapsulated within nanoparticles have limited exposure to the external environment, which not only protects the payloads from systemic degradation, but also reduces unwanted cytotoxicity (Zhou et al., 2020c). As ideal drug delivery vehicles, nanocarriers are attractive candidates for vaccine development, especially due to their ability to colocalize antigens and adjuvants (Zhu et al., 2017). As immune cells process nanovaccines that contain both components, they not only receive an immunostimulatory activation signal, but also have an antigenic target to direct the activation against (Fischer et al., 2013). Furthermore, nanoparticles can be stimuli-responsive, which allows them to intelligently react to pH, chemical gradients, temperature, or reactive oxygen species (Cheng et al., 2013; Ganta et al., 2008; Motornov et al., 2010). Nanocarriers can also be engineered to respond to external manipulations, such as ultrasound, magnetic fields, or irradiation (Mura et al., 2013). An advantage of nanovaccines that can respond to changes in pH is their ability to escape from lysosomal degradation and enhance major histocompatibility complex (MHC) I-mediated antigen presentation (Kim et al., 2019). Traditional vaccines struggle with this process because a large majority of the antigenic material is degraded within the endosomes, while only a small portion is processed through endogenous cross-presentation pathways that lead to the activation CD8+ T cells (Joffre et al., 2012).
4.2. STING nanovaccines for cancer immunotherapy
The STING signaling pathway plays critical roles in the cancer–immunity cycle, and as such, many researchers have identified STING as a good therapeutic target to exploit for cancer treatment. On this front, an impressive number of STING nanovaccines have been developed to treat a wide range of different cancers.
4.2.1. Polymeric nanoparticles
Polymeric nanoparticles are commonly employed in the development of STING nanovaccines for cancer therapy due to their ease of synthesis, scalability, and biocompatibility. One study reported on a polymeric nanovaccine using antigen-loaded PC7A nanoparticles that enabled safe and effective delivery to the peripheral lymph nodes (Figure 3) (Luo et al., 2017b). When mixed with the model antigen ovalbumin (OVA), PC7A polymers naturally self-assembled into 29-nm nanoparticles. Besides OVA, PC7A nanoparticles can be formulated with personalized cancer neoantigen peptides for clinical applications (Wilhelm et al., 2021). In addition to its facile fabrication procedure, the platform also facilitated the loading of the peptide antigens onto MHC I to prime a strong CD8+ T cell response. While certain subsets of APCs can naturally present exogenous antigens on MHC I through natural cross-presentation mechanisms (Embgenbroich and Burgdorf, 2018), PC7A nanoparticles expedited the process through cytosolic delivery. Phagocytosed PC7A nanoparticles escaped from the endosome by the proton sponge effect, where the endosomes were ruptured from osmotic pressure buildup due to an influx of protons (Smith et al., 2019). Once the contents were released into the cytosol, the PC7A naturally bound to and activated the STING signaling pathway for potent immunity. PC7A nanoparticles loaded with the appropriate tumor antigens demonstrated effective tumor growth inhibition in B16-OVA, B16F10 melanoma, MC38 colon cancer, and human papilloma virus (HPV) TC-1 mouse models. Combination of the PC7A nanoparticles with anti-PD-1 immune checkpoint blockade (ICB) led to enhanced efficacy against both the B16-OVA and the TC-1 tumor models. The effectiveness of the treatment was immune mediated and resulted in long-term memory, as mice with previously eradicated TC-1 tumors were resistant to tumor rechallenge 82 days later.
Figure 3.
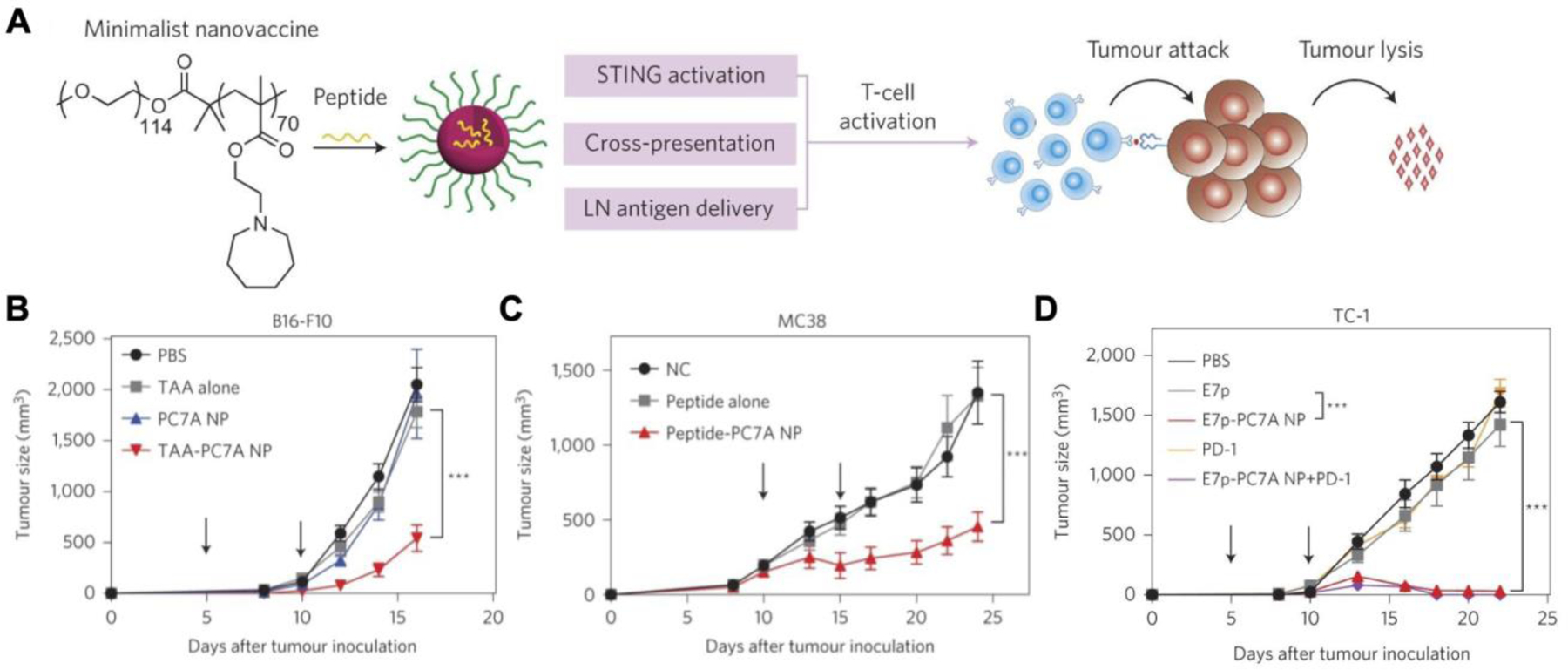
PC7A polymer nanovaccines for anticancer therapy. A) Neoantigen peptides mixed with PC7A polymers self-assemble into nanovaccines. Once administered, the PC7A nanovaccine can deliver the peptides to the cytosol, and the polymers can directly activate STING to generate antigen-specific T cells. B) Treatment with a PC7A nanovaccine loaded with tumor associated antigens (TAAs) slows the growth B16F10 melanoma tumors. C) A neoantigen peptide-loaded PC7A nanovaccine effectively controls tumor growth in an MC38 colorectal cancer model. D) Combination of anti-PD-1 checkpoint blockade with an antigen peptide-loaded PC7A nanovaccine inhibits tumor growth in a TC-1 model. Reproduced with permission (Luo et al., 2017b). Copyright 2017, Springer Nature.
In a subsequent study, PC7A nanovaccines were found to be ineffective against solid tumors once an immunosuppressive tumor microenvironment (TME) had been established (Luo et al., 2019). In combination with radiation therapy (RT), however, efficacy could be improved, and the growth of large tumors was controlled. The synergism was driven by the enhanced local antitumor immunity resulting from RT, while PC7A nanovaccines helped to elicit a systemic immune response. PC7A nanovaccines have also been combined with existing STING agonists such as 2´3´-cGAMP (Li et al., 2021). Since PC7A binds to STING in a uniquely different site, synergistic treatment of MC38 tumors with 2´3´-cGAMP, which was loaded in the nanovaccine, completely eradicated tumors in 4 out of 7 mice. In comparison, monotherapy with 2´3´-cGAMP rescued only 1 out of the 6 mice, while all mice undergoing PC7A treatment alone succumbed to the disease. In another study, a PC7A nanovaccine was used in a combinational approach to produce an in situ cancer vaccine (Patel et al., 2019). Rather than targeting just the STING pathway, PC7A was encapsulated in a polyplex core along with CpG oligodeoxynucleotides to concurrently stimulate the TLR9 pathway. Furthermore, the nanoparticle core was coated with bacteria-derived outer membrane vesicles as an additional broad spectrum immune stimulus. Maleimide functional groups were embedded onto the membrane surface to generate the final formulation. Rather than preloading antigens into the formulation, the maleimides captured tumor antigens in situ after local RT (Min et al., 2017). In murine B78 melanoma and NXS2 neuroblastoma models, the nanovaccine in combination with RT eliminated a significant portion of the treated tumors.
A more common application of polymeric nanoparticles is STING agonist delivery, an example being the utilization of biodegradable poly(beta-amino ester) (PBAE) nanoparticles to carry CDNs into the cytosol (Wilson et al., 2018). Like PC7A, the cationic nature of PBAE allows for direct cytosolic delivery through the proton sponge effect. Cellular uptake of RR-CDG-loaded PBAE nanoparticles was significantly higher in THP-1 human monocytes and RAW264.7 murine macrophages when compared to B16 cancer cells. The selectivity can be attributed to the versatility of PBAE, where endcap modifications of the polymer can result in vastly different cellular uptake profiles (Sunshine et al., 2009). In a B16 tumor model, the more potent ML RR-CDA-loaded PBAE nanoparticles controlled tumor growth significantly better than free CDN at the same dosage. When used in combination with anti-PD-1 checkpoint inhibitors, the nanoformulation achieved similar efficacy at a ten times lower dosage compared to free CDN alone. Other examples of delivery using polymeric nanocarriers include methoxy poly(ethylene glycol)-poly(lactide) nanoparticles to deliver α-mangostin (Zheng et al., 2018) and poly(l-glutamic acid)-g-methoxy poly(ethylene glycol) nanoparticles to deliver SN38 and induce DNA damage for indirect STING activation (Zhao et al., 2021).
Endosomolytic polymerosomes, which are amphiphilic polymers that assemble into a liposome-like structure, were developed to carry STING agonists directly to the cytosol (Shae et al., 2019; Wang-Bishop et al., 2020). The polymerosomes were composed of a hydrophilic core for high CDN loading, a pH-responsive vesicle membrane for endosomal escape, and an outer polyethylene glycol (PEG) shell for prolonged circulation. In an erythrocyte hemolysis assay, noncanonical cGAMP-loaded polymerosomes rapidly disassembled and induced hemolysis at lower pH, implying that the platform could facilitate the cytosolic delivery of cGAMP through endosomal disruption. Intratumoral treatment with the formulation in a B16F10 model elicited higher numbers of tumor-infiltrating CD4+ and CD8+ T cells when compared to free cGAMP. In a therapeutic setting, tumor growth was significantly controlled, with complete responses observed in 3 out of 9 mice. When used in combination with anti-PD-1 and anti-CTLA-4 ICBs, intratumoral injections of the nanoformulation resulted in profound tumor regression in both the treated tumor and a contralateral tumor. Combination with ICBs likewise boosted the potency of nanoparticles that were administered intravenously, resulting in strong antitumor responses.
Peptide neoantigens have been loaded into CDN-containing endosomolytic polymerosomes to strengthen vaccine efficacy through antigen–adjuvant colocalization (Shae et al., 2020). Upon cytosolic delivery, the nanoparticles boosted presentation of cancer neoantigens on MHC I to elicit stronger CD8+ T cell immunity. Mice treated with a formulation loaded with SIINFEKL, an MHC I-restricted peptide derived from OVA, had the highest number of OVA-specific CD8+ T cells compared to various controls. Similarly, mice treated with an MC38-specific cocktail loaded with either Reps1 or Adpgk peptide epitopes had the highest proportion of IFN-γ+TNF-α+CD8+ peripheral T cells upon ex vivo restimulation. In murine tumor studies, cocktail nanovaccine treatment with anti-PD-1 inhibited MC38 and B16F10 tumor growth. Another pH-responsive polymerosome platform employed a PEG block copolymer with poly(2-(diisopropanol amino) ethyl methacrylate) to deliver DMXAA and peptide antigens (Zhou et al., 2020b). The polymersomes had mannose as an additional DC-targeting moiety, which contributed to increased lymph node localization, higher DC uptake, better MHC I presentation, and stronger antigen-specific CD8+ T cell activation. In both a B16-OVA and a 4T1 orthotopic breast cancer model, subcutaneous vaccination at the tail base with the polymerosomes slowed tumor growth.
Instead of relying on the delivery of tumor antigens, STING agonists can also work synergistically with chemotherapy by utilizing antigens generated in situ (Figure 4). One such strategy involved the mixture of 2´3´-cGAMP-loaded hollow poly(lactic-co-glycolic acid) (PLGA) nanoshells with CT26 cells that were treated with irinotecan (CPT-11), a frontline chemotherapeutic (Chattopadhyay et al., 2020). When incubated with JAWS II murine DCs in vitro, the mixture induced significant upregulation of DC maturation markers and type I IFN release. In an animal study, CT26 tumor-bearing mice treated with the combined formulation had the slowest tumor growth, with 1 out of 8 mice becoming tumor-free. The strategy was tested in other animal models with different chemotherapy combinations, including B16 plus cisplatin and B16F10 plus doxorubicin (DOX) in combination with anti-CTLA-4. In the former, 5 out of 10 mice had a complete response to the therapy, whereas in the highly aggressive B16F10 model, only 1 out of 7 mice survived until day 90 despite the triple combination. In another example of chemoimmunotherapy with STING agonists, CDA was loaded with camptothecin (CPT) into nanotubes that self-assembled into a hydrogel structure (Wang et al., 2020a). The hydrogel encouraged the retention of CDA and CPT, allowing a gradual and continuous release of the vaccine components as a substitute for booster doses (Jiang et al., 2020a). A single intratumoral dose of the hydrogel resulted in strong efficacy in GL-261 glioblastoma, CT26, and 4T1 models.
Figure 4.
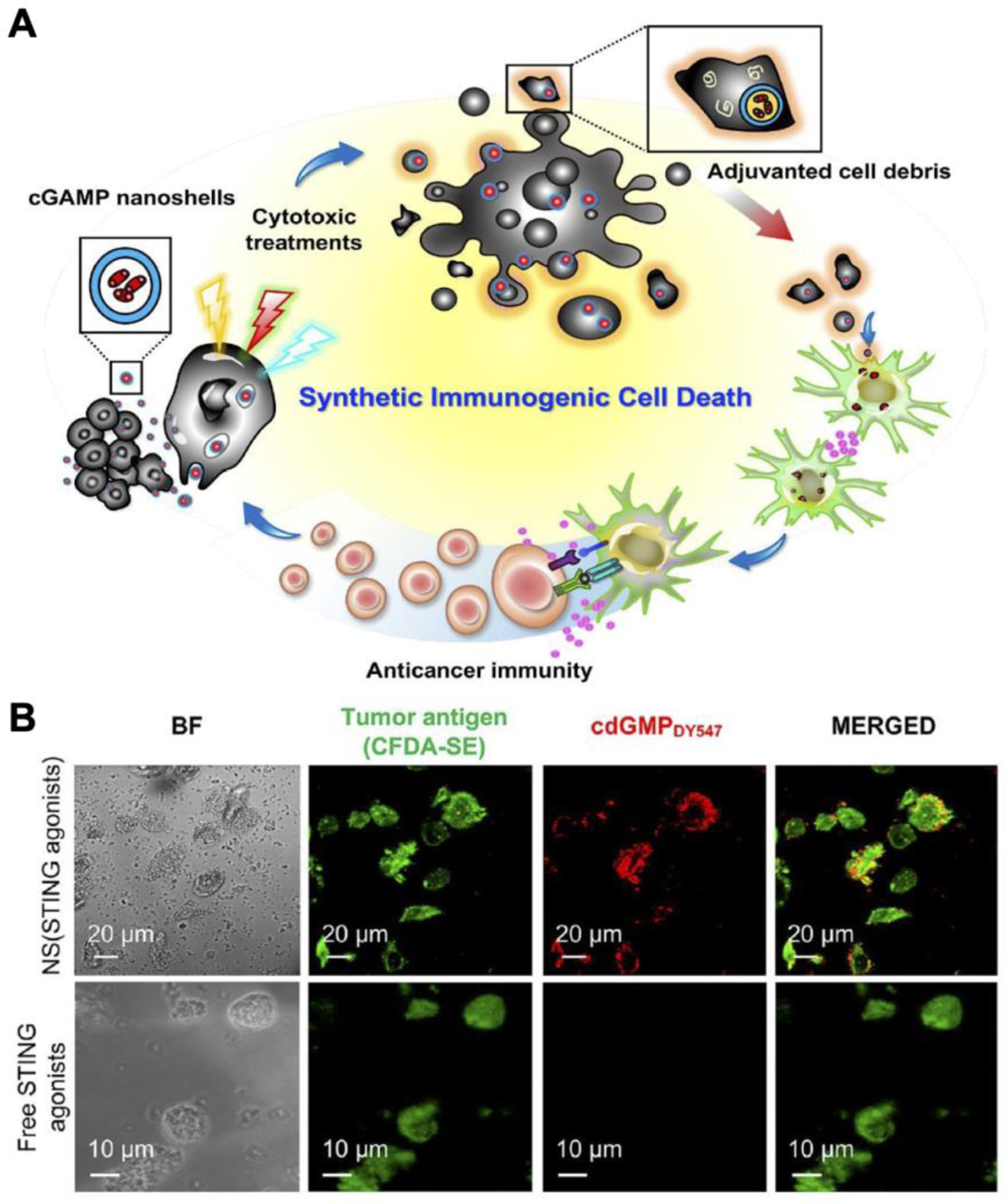
Nanoshells loaded with cGAMP to induce synthetic immunogenic cell death for chemoimmunotherapy. A) cGAMP-loaded nanoshells can be readily loaded into dying tumor cells treated with chemotherapeutics. Upon phagocytosis, nanoparticle-laden cancer cells can stimulate an antitumor immune response. B) Fluorescently labeled nanoshells are significantly colocalized with dead tumor cells compared to free CDG. Reproduced with permission (Chattopadhyay et al., 2020). Copyright 2020, American Chemistry Society.
4.2.2. Liposomes and lipid nanoparticles
Liposomes consist of phospholipids assembled into a spherical nanostructure and are one of the most popular drug delivery platforms. Due to their facile synthesis and biocompatibility, liposomes have been utilized to effectively deliver STING agonists. In one study, PEGylated phosphatidylcholine liposomes enhanced delivery of CDG into APCs and improved targeting into the draining lymph nodes (dLNs) (Hanson et al., 2015). Enhanced delivery of the liposomal formulation directly translated to better immune activation and therapeutic efficacy. EG.7-OVA tumor-bearing mice vaccinated with a mixture of the adjuvant-loaded nanoparticles and OVA on days 6, 13, and 20 had a nearly 3-fold higher number of OVA-specific CD8+ T cells when compared to vaccination with free CDG and OVA. Treatment using the nanoformulation effectively extended the median survival time from around 16 days to 29 days, while no noticeable efficacy was observed in mice treated with the free CDG mixture. Similar effects were seen in a B16F10 tumor model using the melanoma-specific gp100 antigen.
YSK05 is a pH-sensitive cationic synthetic lipid that promotes fusion with endosomal membrane (Sato et al., 2012). By incorporating the lipid into liposomes, YSK05-modified liposomes have been utilized for siRNA and CDG delivery (Miyabe et al., 2014). In RAW264.7 cells, a CDG-loaded formulation induced high levels of IFN-β secretion and upregulated the maturation markers CD80, CD86, and MHC I. Mice challenged with E.G7-OVA a week after immunization with the adjuvanted liposomes mixed with OVA showed a noticeable reduction in tumor growth. Antitumor efficacy in this model was primarily mediated by CTLs due to the high expression of SIINFEKL-MHC I on the tumor surface. Interestingly, antitumor efficacy of these liposomes was also observed in B16F10 cells with downregulated MHC I expression (Nakamura et al., 2015). Upon closer inspection, the immunity originated from the activation of NK cells. Splenocytes derived from mice 8 hours after intravenous immunization had a significantly smaller proportion of NK cells, indicating that they had been recruited away in response to CDG. In a B16F10 lung metastatic tumor model, mice treated with the formulation had obvious reduction in lung nodules, but such effects were abrogated in mice with NK cells depleted by anti-asialo GM1.
Another example employed cationic liposomes fabricated from cholesterol and 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) to treat B16F10 tumors (Koshy et al., 2017). The liposomes were loaded with 2´3´-cGAMP and coated with 5% or 10% PEG to improve stability (Figure 5). The cationic nature of the liposomes allowed binding to the cell membrane with high affinity and facilitated the endosomal release of cGAMP into the cytosol. Compared to free 2´3´-cGAMP and CpG controls, the liposomal formulations elicited better gene expression of Ifnb1, Cxcl10, Cxcl9, and Tnf in mice. In an orthotopic tumor model, mice intratumorally treated with free cGAMP and the liposomal formulations all had the same survival rate, but upon rechallenge 60 days later, the long-lasting immune memory elicited by the liposomes could be discerned. While 50% of the mice in the free cGAMP group succumbed to the rechallenge, 100% of the mice treated with the 2´3´-cGAMP-loaded liposomes coated with 10% PEG survived. Liposomes have also been leveraged to deliver canonical cGAMP against more aggressive tumor models, such as triple-negative breast cancer (TNBC) (Cheng et al., 2018). TNBC is especially resistant against existing treatments due to the absence of three common breast cancer markers. When used in combination with anti-PD-L1, treatment with the cGAMP-loaded liposomes effectively eradicated the tumors, leading to a 100% survival rate. In addition, an inhalable 2´3´-cGAMP-loaded liposomal formulation has been recently proposed as a treatment for metastatic lung cancer through the activation of pulmonary APCs (Liu et al., 2019c).
Figure 5.
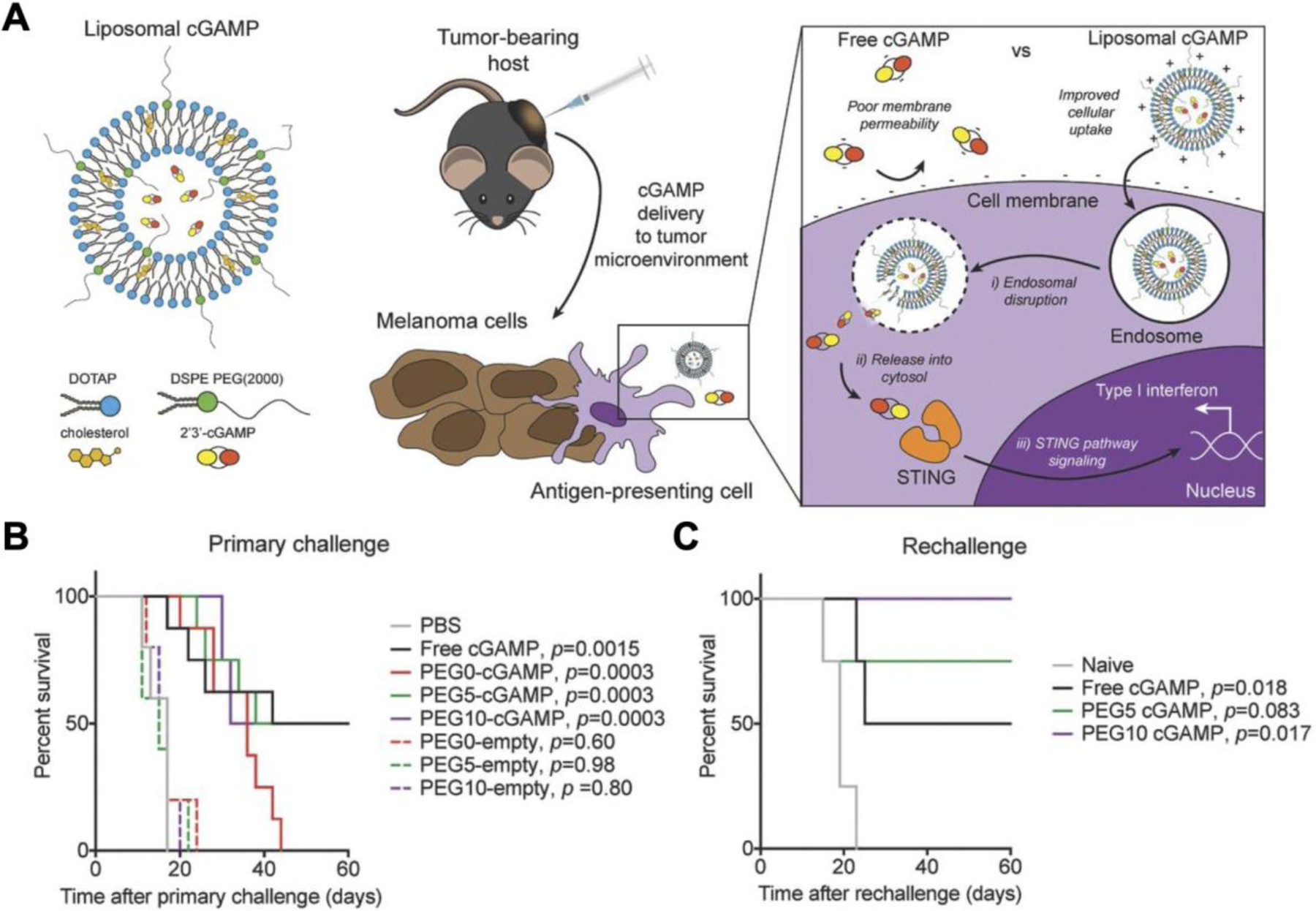
Cationic liposomes enhance cytosolic delivery of cGAMP for melanoma treatment. A) Noncanonical cGAMP is encapsulated inside liposomes fabricated from cholesterol and 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP). The liposomes more readily facilitate intracellular delivery of cGAMP to activate the STING pathway. B) Treatment with PEGylated liposomal formulations prolongs survival in a B16F10 melanoma model. C) After treatment with cGAMP-loaded liposomes, a significant portion of mice are protected from a tumor rechallenge. Reproduced with permission (Koshy et al., 2017). Copyright 2017, Wiley-VCH.
Lipid nanoparticles (LNPs) have been designed to directly activate STING without the need for a separate agonist payload. In one case, a lipid nanoformulation was screened from a library of ionizable lipid-like materials, and an optimal nanovaccine was identified from more than 1,000 formulations (Miao et al., 2019). The identified formulation was found to efficiently deliver mRNA into cells and in vivo, activate innate and adaptive immunity through the STING pathway, and potently induce anticancer immunity in a therapeutic setting (Figure 6). The STING activation was found to be due to the presence of unique cyclic amino head groups. In a B16-OVA model, a single dose of OVA-encoding mRNA-loaded LNPs prolonged survival and rescued 3 out of 11 mice. Using mRNA encoding for TRP2, a B16F10 antigen, three doses of the LNPs significantly retarded tumor growth, with more than 60% of the mice still alive on day 40. In contrast, all untreated mice bearing B16F10 tumors succumbed to the disease by day 25. Comparable therapeutic efficacy was observed with a TC-1 tumor model after a single dose of LNPs in combination with anti-PD-1.
Figure 6.
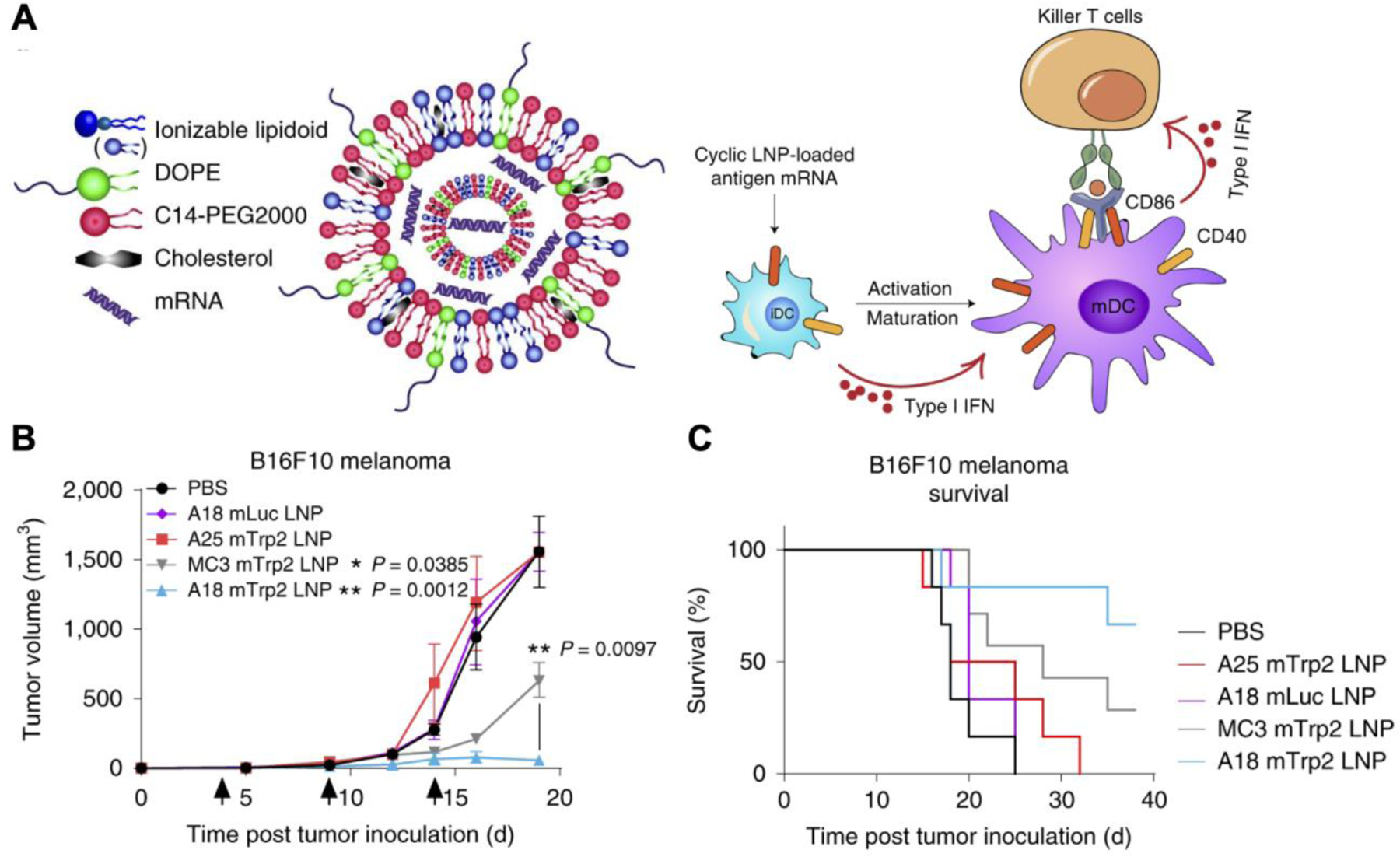
Liposomal mRNA vaccine directly activates the STING pathway to treat melanoma. A) Heterocyclic lipid nanoparticles (LNPs) are composed of an ionizable lipidoid for STING activation and mRNA encoding for tumor antigens. Cellular uptake of the LNPs induces dendritic cell (DC) maturation and potentiates STING-mediated immunity against tumor cells via type I interferon (IFN) production. B,C) A18 LNPs with mRNA encoding the melanoma antigen TRP2 control tumor growth (B) and extend survival (C) in a B16F10 melanoma model. Reproduced with permission (Miao et al., 2019). Copyright 2019, Springer Nature.
4.2.3. Inorganic nanoparticles
The toxicity of inorganic nanoparticles to humans and the environment is a common concern in the field of nanotechnology (Sengul and Asmatulu, 2020; Zhou et al., 2021). However, when employed carefully, the inherent properties of inorganic nanoparticles can be leveraged against cancerous cells. In one example, cationic silica nanoparticles were used to induce necrotic tumor cell death and locally deliver CDG for vaccination (An et al., 2018). To prepare the particles, negatively charged CDG was electrostatically complexed with the cationic, amine-modified silica nanoparticles. Intratumoral administration induced destruction of the tumor from the intrinsic cytotoxicity of the nanoparticles, and the dying cancer cells were subsequently phagocytosed by APCs activated with the CDG. Tumor tissues from treated mice had noticeable areas of necrosis. In a melanoma model, treatment with the CDG-loaded nanoformulation significantly inhibited tumor development in mice compared to free CDG and free CDG co-administered with unloaded silica nanoparticles. The latter control group demonstrated the importance of incorporating CDG with the nanoformulation, since free STING agonists have difficulty bypassing the plasma membrane and can rapidly diffuse out of the tumor and into the bloodstream. A single dose of the CDG-loaded nanoparticles rescued 3 out of 8 mice in a B16F10 model and helped to establish long-lasting immunity, as all mice survived a tumor rechallenge 60 days later.
Metal nanoparticles have been explored for treating aggressive cancers such as head and neck squamous cell carcinoma (HNSCC) (Tan et al., 2018). HNSCC is challenging to treat with ICBs and has low CTL responses. In addition, SOX2, a common HNSCC oncogene, was discovered to degrade STING through autophagy and inhibit STING-mediated IFN production. To combat HNSCC, a nanosatellite vaccine composed of iron oxide nanoparticle cores coated with a biodegradable copolymer was utilized to deliver 2´3´-cGAMP to tip the immune balance away from immunosuppression. The cores were attached with gold nanoparticles as satellites and contained peptide antigens conjugated onto the surface. The nanosatellites significantly enhanced intracellular delivery of cGAMP into the cytosol as measured by increased mRNA levels in IFNA4, IFNB1, ISG15, ISG54, CXCL9, and CXCL10. In addition, the expression of CD86 and MHC II increased in bone marrow-derived dendritic cells (BMDCs). In an engineered MOC2-E6/E7 mouse oral squamous cell carcinoma model, the nanosatellites achieved a reduction in tumor growth and increased survival when compared to free peptide antigens, free 2´3´-cGAMP, and anti-PD-L1.
Manganese nanoparticles themselves have the propensity to activate the STING pathway through the release of Mn2+ (Zhao et al., 2020b). The manganese does not activate STING directly, but rather induces cGAMP synthesis by binding to cGAS and concurrently promotes stronger binding affinity between cGAMP and STING (Wang et al., 2018). In an example work, amorphous porous manganese phosphate nanoparticles were loaded with DOX and coated with phospholipids (Hou et al., 2020). DOX is a common chemotherapeutic that not only eradicates tumor cells, but also promotes DNA damage to further enhance STING activation and generate immunogenic cell death (Casares et al., 2005), while phospholipid modification can help extend in vivo stability and circulation (Figure 7). It was shown that the formulation was pH responsive and could release DOX in response to the mildly acidic TME, thus preventing undesirable systemic drug exposure. In addition, phospholipase is overly expressed inside aggressive tumor cells, which further strengthened the specificity to the tumor site. In a 4T1 tumor model, intravenously administration of the DOX-loaded nanoparticles synergistically inhibited tumor growth compared to various controls. Tumor growth reduction was similarly seen in a distant tumor implanted post-treatment, indicating the generation of systemic antitumor immunity.
Figure 7.
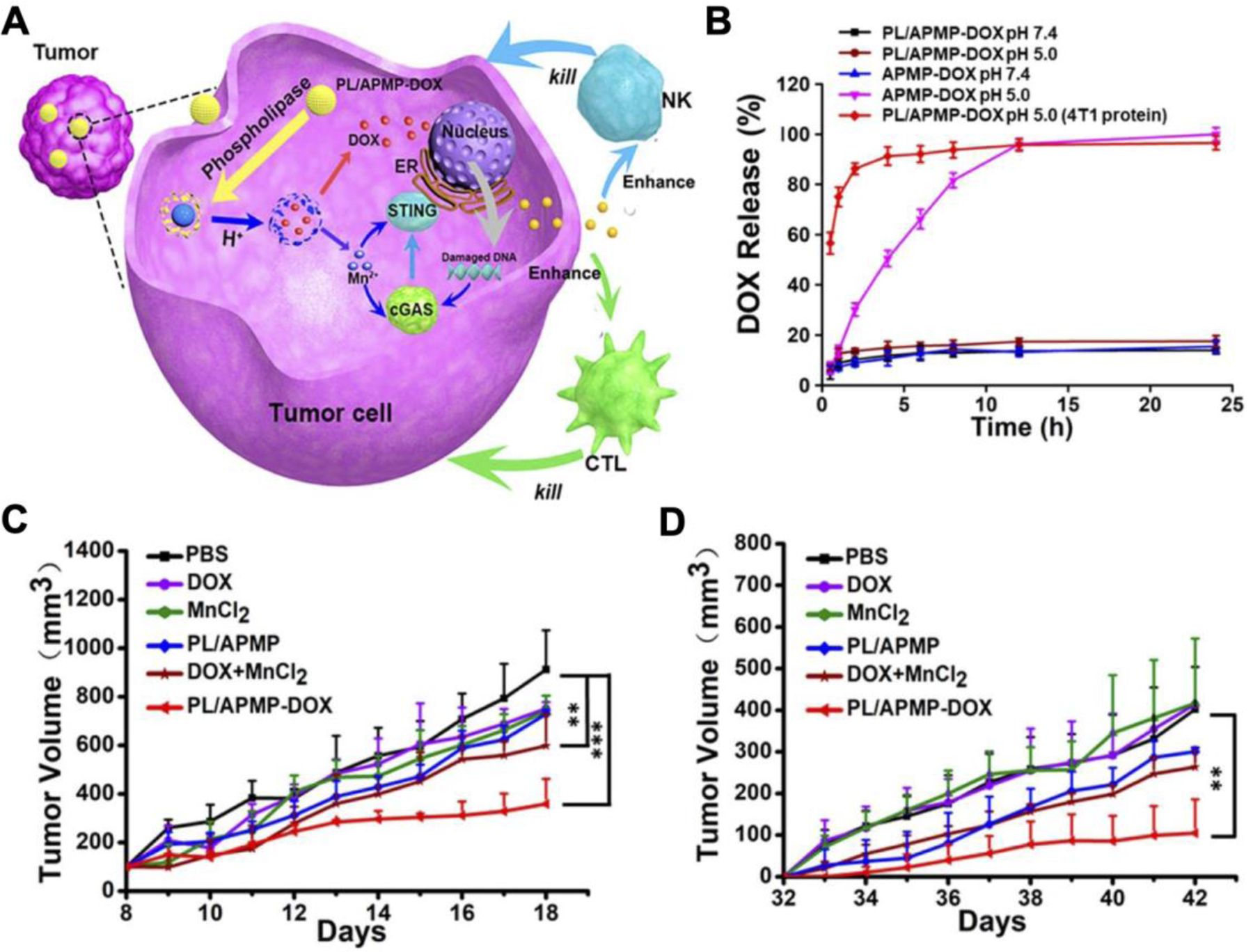
Manganese nanoparticles loaded with doxorubicin (DOX) for synergistic immunotherapy against 4T1 breast cancer. A) The degradation of DOX-loaded amorphous porous manganese phosphate nanoparticles coated with phospholipid (PL/APMP-DOX) is facilitated by phospholipase within tumor cells. DOX causes damaged DNA to release from the nucleus, while manganese ions (Mn2+) facilitate STING activation to promote immune responses mediated by natural killer (NK) cells and cytotoxic T lymphocytes (CTL). B) DOX is rapidly released at low pH and in the presence of phospholipase, which are significantly upregulated in cancerous cells. C,D) Intravenous treatment with PL/APMP-DOX reduces growth in both the primary tumor (C) and a distant secondary tumor (D) inoculated post-treatment. Reproduced with permission (Hou et al., 2020). Copyright 2020, American Chemistry Society.
4.2.4. Microparticles
Although microparticles have limited lymphatic drainage due to their large size (Reddy et al., 2007), the application of microparticles for cancer therapy have been reported due to their ability to carry more payload and release the cargo over an extended period of time. To avoid frequent administrations and address the issue of patient compliance, a PLGA microparticle platform was developed to release STING agonists with varying kinetics (Lu et al., 2020). The microparticles were fabricated by soft lithography and contained a hollow rectangular base filled with 3´3´-cGAMP, which was then sealed with another PLGA cap. Individual microparticles could load up to 4 nL of solution, but with alternating dry and fill cycles, up to 10 µg of cGAMP could be loaded into a single particle. The release kinetics were fine-tuned by varying the lactide to glycolide ratio or the average molecular weight of the polymers, and a combination injection of 3 different polymeric microparticles achieved a pulsed release on days 4, 8, and 12. In a B16F10 model, a single intratumoral injection of the cGAMP-loaded microparticles showed tumor inhibition on par with four separate administrations of soluble 3´3´-cGAMP. The advantage of a single dose, pulsed release formulation was clearly shown in a pancreatic cancer allograft model due to the high difficulty in performing multiple injections. Compared to a bolus injection of free cGAMP at the same dosage, treatment with cGAMP-loaded microparticles resulted in significantly smaller tumors and fewer metastatic nodules in the lungs.
Microparticles have also been used to co-deliver STING and TLR agonists to the lymph nodes. In an example, acetalated dextran microparticles were incorporated with resiquimod (R848), a TLR7/8 agonist, and 3´3´-cGAMP (Collier et al., 2018). The acetal groups provided the particles with pH sensitivity, which facilitated rapid release of the payload inside the lysosomes after cellular uptake. BMDCs treated with the microparticles stimulated higher levels of IL-6, TNF-α, IL-1β, and IFN-β compared to free cGAMP and R848, as well as a mixture of microparticles loaded individually with either cGAMP or R848. Overall, the results suggested that codelivery of the two adjuvants was more effective than delivering them separately. The acetalated dextran microparticles also outperformed PLGA microparticles at the same dosage due to more rapid degradation and release. Intramuscular vaccination with the formulation using OVA as the antigen produced the highest levels of antibody titers, even slightly outperforming the alum positive control. Splenocytes from mice vaccinated with the microparticles that were restimulated ex vivo also had the highest amounts of IL-2 and IFN-γ secretion. In a follow up study on the same platform, it was shown that microparticles loaded with only 3´3´-cGAMP had the best antitumor activity in B16F10 tumors compared to microparticles loaded individually with poly(I:C), murabutide, or imiquimod, which are agonists for TLR3, NOD2, and TLR4, respectively (Watkins-Schulz et al., 2019). The results highlighted the advantages of cGAMP as an adjuvant for antitumor immunotherapy when compared to other commonly employed adjuvant systems. Efficacy was also observed in a E0771 TNBC tumor model.
4.3. STING nanovaccines for infectious diseases
STING agonists have been largely exploited as adjuvants in anticancer vaccines due to the difficulty in achieving proper immune activation against tumor antigens that originate from endogenous proteins. In the context of infectious diseases, generating immunity against foreign pathogens generally does not require the use of sophisticated adjuvant systems. Nevertheless, adjuvants can be leveraged to help amplify the immune response and efficacy, and thus nanovaccines employing STING agonists have been developed against both viruses and bacteria.
4.3.1. Antiviral nanovaccines
Middle East respiratory syndrome coronavirus (MERS-CoV) is a respiratory virus closely related to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that caused the 2019 pandemic (de Wit et al., 2016). Treatments for both SARS-CoV-2 and MERS-CoV are limited (Zhang et al., 2020b; Zhou et al., 2021). As a measure to prevent future coronavirus pandemics, a hollow PLGA nanovaccine with CDG loaded inside and MERS-CoV receptor-binding domain (RBD) antigens on the surface was developed (Lin et al., 2019). The capsid-like nanoparticle structure mimics that of the actual virus and can evoke immune response similar to natural viruses (Figure 8). The polymeric shell contained an outer PEG layer for better pharmacokinetics and was acid-sensitive, allowing for burst release of the payload at lower pH values. Footpad injection of the formulation elevated IFN-β cytokine levels in the dLNs while no noticeable increase in TNF-α was found in the serum, which demonstrated the local immune priming capability of the nanoparticles while minimizing systemic inflammation. Subcutaneous vaccination on days 0 and 21 elicited significant antibody titers against the RBD antigen, and they remained elevated for at least 300 days. Higher numbers of functional CD4+IFN-γ+ T cells and CD44+CD62L+ central memory T cells were detected in the spleen, and more importantly, antigen-specific CD8+IFN-γ+ T cells were increased by nanoparticle vaccination. Profound protective efficacy was shown in a human DPP4 transgenic mice model, where prophylactic vaccination with the nanovaccine protected 100% of the mice from a lethal MERS-CoV challenge.
Figure 8.
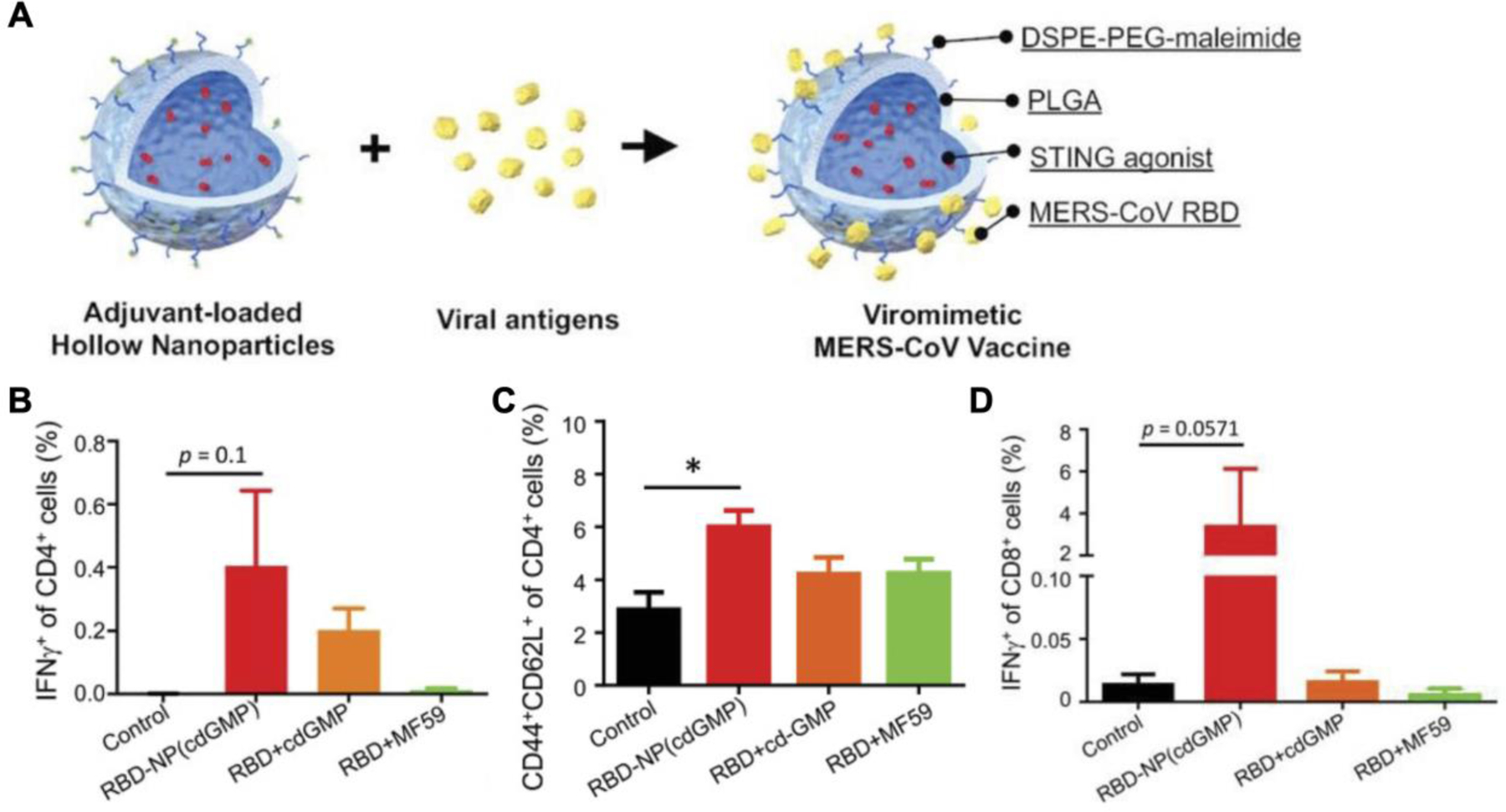
Virus-like particles loaded with CDG as a vaccine against Middle East respiratory syndrome coronavirus (MERS-CoV). A) Hollow PLGA nanoparticles are loaded with CDG as an adjuvant and engineered to display recombinant MERS-CoV antigens on the surface to mimic the structure of natural viruses. B-D) Vaccination with the viromimetic nanoparticles induces high proportions of CD4+IFN-γ+ functional T cells (B), CD4+CD44+CD62L+ central memory T cells (C), and CD8+IFN-γ+ effector T cells (D). Reproduced with permission (Lin et al., 2019). Copyright 2019, Wiley-VCH.
Polymeric nanovaccines have also been formulated against human immunodeficiency virus (HIV) (Aroh et al., 2017). Here, noncanonical cGAMP was loaded into PC7A polymeric nanoparticles, and the resulting formulation was able to inhibit replication of HIV-BaL, HIV-1 (IIIB), and HIV-1 (LAI) in peripheral blood mononuclear cells (PBMCs), a phenomenon that was not observed with the adjuvants poly(I:C), R848, and CpG. Efficacy appeared to be STING-specific, with type I IFNs playing a major role. More in-depth studies elucidated that monocyte-depleted PBMCs did not confer any protection, whereas B cell and NK cell depletion had minimal effects. Another STING-inducing nanovaccine for HIV treatment utilized liposomes as the carrier (Hanson et al., 2015). The formulation was loaded with CDG and included the membrane proximal external region (MPER) from HIV gp41 and gp120 tethered onto the surface. MPER is a lowly immunogenic antigen, so the incorporation of the STING agonist was used to help boost the immune response. Indeed, vaccination with the final formulation elicited 4-fold higher DC activation and 3-fold higher macrophage activation as compared to empty MPER liposomes administered with free CDG. Furthermore, a robust humoral response was generated while minimizing the induction of systemic inflammatory cytokines. However, despite the elevated antibody titer levels, sera from vaccinated mice failed to neutralize HIV.
Influenza virus is a highly mutative pathogen that mandates the development of new flu vaccines annually. To address this issue, several universal influenza vaccines have been proposed (Boyoglu-Barnum et al., 2021; Kanekiyo et al., 2019; Kanekiyo et al., 2013). One formulation utilized pulmonary surfactant biomimetic liposomes encapsulating 2´3´-cGAMP as a mucosal adjuvant (Wang et al., 2020b). When the liposomes were intranasally administered along with inactivated A/California/7/2009 (CA09) H1N1 virus, IgG antibodies in the serum and IgA antibodies in the bronchoalveolar lavage fluid (BALF) were elevated by 10,000-fold and 60-fold, respectively, compared to immunization with the inactivated virus alone. Mechanistic studies elucidated that the negatively charged nanoformulation was taken up by alveolar macrophages with the assistance of surfactant proteins A and D. Upon cytosolic release, cGAMP not only activated the alveolar macrophages, but was also transferred into alveolar epithelial cells through gap junctions for activation (Figure 9). In addition to humoral responses, the nanoparticles augmented cellular immunity by activating CD4+IFN-γ+ T cells and CD8+IFN-γ+ T cells. A major benefit of this approach was that the vaccine formulation conferred protection against the influenza virus as early as 2 days after immunization. Early immunity was not mediated by innate immune responses, as the nanoparticles alone lacked efficacy, but rather due to rapid induction of CD8+ T cells in both the lungs and BALF. Immunization provided broad protection against the seven heterosubtypic influenza viruses that were tested. Vaccinated mice had nearly 100% survival when challenged by the seven different substrains, whereas almost all unvaccinated mice succumbed to the infections.
Figure 9.
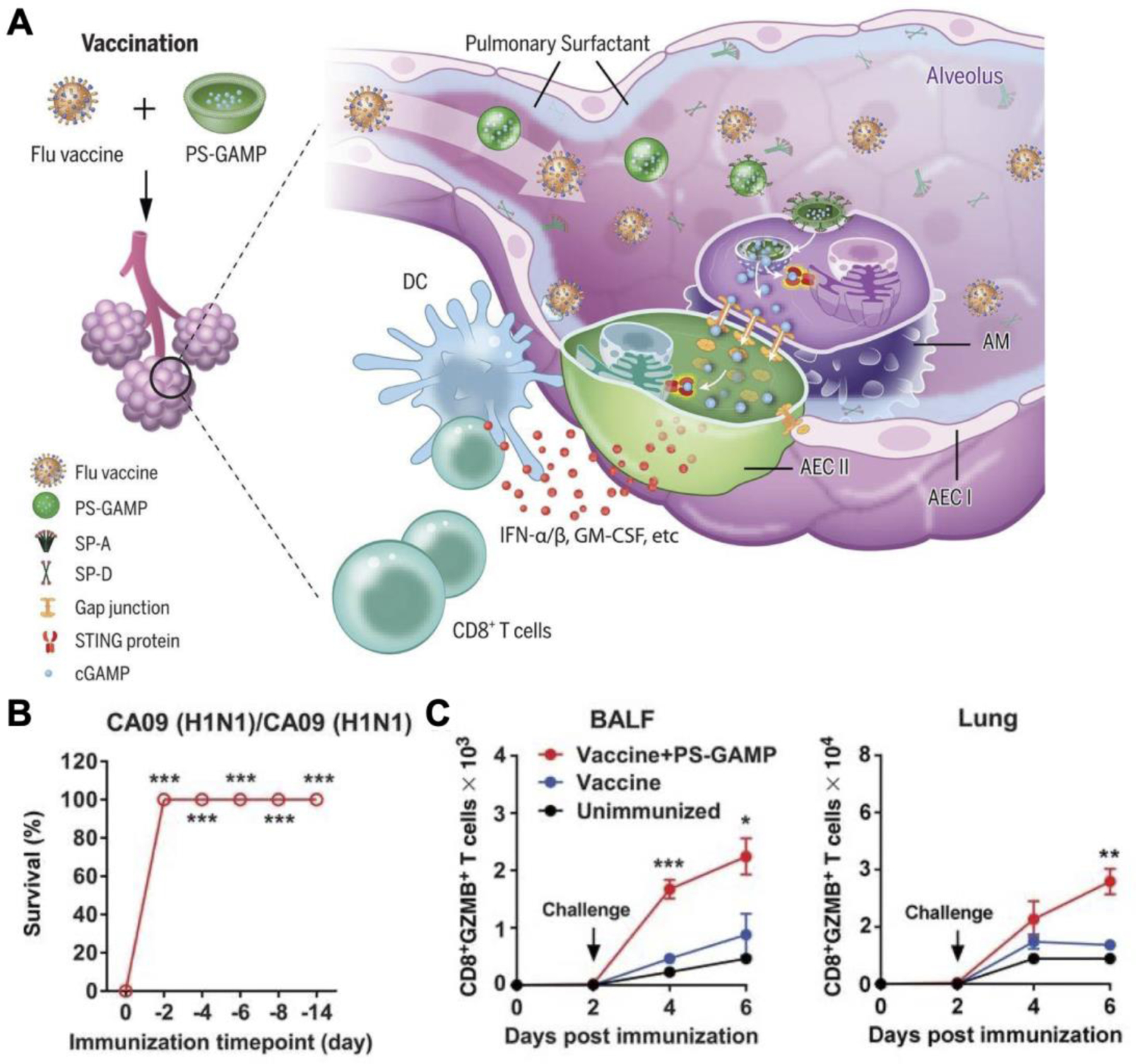
Biomimetic liposomal cGAMP co-administered with a flu vaccine to protect against influenza. A) Liposomes modified with biomimetic surfactants and loaded with cGAMP are mixed with inactivated influenza virus as a mucosal vaccine. The surfactant aids adjuvant transport across the epithelium and into alveolar macrophages to induce a rapid and broad immune response against different substrains of influenza. B) Immunized animals are rapidly protected from lethal viral challenges. C) The adjuvanted vaccine promotes high numbers of CD8+Granzyme B+ (GZMB) effector T cells in the bronchoalveolar lavage fluid (BALF) and the lungs shortly after immunization. Reproduced with permission (Wang et al., 2020b). Copyright 2019, American Association for the Advancement of Science.
Another influenza vaccine was developed to target the aging population, where vaccine potency tends to diminish due to immunosenescence (Ross et al., 2019). Polyanhydride nanoparticles loaded with the hemagglutinin and nucleoprotein antigens were synthesized through a double emulsion process. Concurrently, a pentablock copolymer micelle was fabricated to facilitate sustained antigenic release and promote drug delivery into the cytosol. While subcutaneous immunization with the two nanoparticles produced high levels of antibody titers and lowered viral loads in young mice, vaccine potency was significantly compromised in aged mice. Incorporation of RR-CDG into the formulation increased antibody titer levels by an order of magnitude and protected 60% of the animals from a lethal H1N1 challenge. Another unique platform against influenza consisted of acetalated dextran microparticles loaded with 3´3´-cGAMP (Junkins et al., 2018). For the antigenic target, HA protein derived from influenza strain A/Puerto Rico/8/1934 H1N1 (PR8) was adsorbed onto the microparticle surface. Intramuscular injection induced antibody titers 41-fold higher compared to free 3´3´-cGAMP, 600-fold higher than alum, and more than 5 orders of magnitude higher than HA alone. Furthermore, germinal center B cells in the dLNs and central memory CD4+ and CD8+ T cells in the spleen were significantly increased 14 days after immunization. The expanded immune cell populations helped to protect 12 out of 13 mice from a lethal challenge of PR8 influenza virus one month post vaccination, and all mice challenged seven months later.
4.3.2. Antibacterial nanovaccines
Even though CDNs originate from bacteria, the use of STING agonists in bacterial vaccines is much less common than with other applications. Polyanhydride nanoparticles loaded with F1-V antigens were combined with ML RR-CDG to treat pneumonic plague (Wagner et al., 2019). Polyanhydride nanoparticles are naturally immunostimulatory and have been frequently employed as adjuvant systems (Torres et al., 2011), so the combinatorial approach with ML RR-CDG presented a dual-adjuvant system to further bolster the immune response. The F1-V antigen is derived from the V antigen and the F1 capsule of Yersinia pestis, a gram-negative bacterium that is responsible for the plague. Subcutaneous administration of the combination nanovaccine showed higher antibody titers against the F1-V antigen and strongly protected the animals from a lethal dose of Y. pestis CO92 challenge. Immunization with the antigen-loaded nanoparticles alone did not confer sufficient protection. Interestingly, co-administration of ML RR-CDG with soluble F1-V antigens elicited similar levels of antibodies and protected approximately 90% of mice from the challenge. However, when the dose of the bacteria was increased by two-fold, almost all mice vaccinated with the soluble antigens succumbed to the disease, while mice vaccinated with the nanoparticles maintained complete protection. A single dose of the combination nanovaccine induced long-lasting immunity, with a 75% survival rate in mice challenged 182 days after vaccination.
4.4. Nanovaccines outlook
STING-activating nanovaccines have shown considerable promise as therapeutics against cancer and as prophylaxes against infectious pathogens. However, almost all the examples discussed have focused on a single antigenic target; as such, mutations and antigenic escape can render the vaccine formulations ineffective (Denamur and Matic, 2006; Petrova and Russell, 2018; van der Burg et al., 2016). Multivalent vaccines can circumvent this issue by producing immunity against a broad range of relevant antigens and have a higher possibility to completely eradicate or prevent the targeted disease (Angsantikul et al., 2015; Fang et al., 2015; Singh, 2021). Along these lines, cell membrane coating technology is an emerging biomimetic technique utilized to fabricate immunocompatible and multivalent nanovaccines (Fang et al., 2018; Hu et al., 2015; Hu et al., 2011). The membrane from live cells can be isolated and coated onto diverse substrates, including nanoparticles (Fang et al., 2014; Hu et al., 2013b; Wei et al., 2016), nanofibers (Chen et al., 2016b), micromotors (Esteban-Fernández de Ávila et al., 2018; Esteban-Fernandez de Avila et al., 2018; Wei et al., 2019a), and even two dimensional nanomaterials (Gong et al., 2019; Kumar et al., 2019), to bestow new biological functionalities. Cell membrane coating technology has proven successful with a wide range of different membrane sources, including immune cells (Thamphiwatana et al., 2017; Wei et al., 2018; Zhang et al., 2018a), stem cells (Bose et al., 2018; Gao et al., 2016a; Gao et al., 2016b), exosomes (Liu et al., 2019a; Yong et al., 2019; Zhao et al., 2020a), bacterial membranes (Chen et al., 2020a; Gao et al., 2015; Zhang et al., 2019b), and fusion membranes (Chen et al., 2020b; Dehaini et al., 2017; Liu et al., 2019b). Because the entire cellular membrane is employed, nanovaccines produced in this fashion are naturally multiantigenic and can evoke broad immune protection without the need for labor-intensive studies to identify and fully characterize individual antigens. Multivalent cell membrane-coated nanovaccines have been successfully implemented against cancer (Jiang et al., 2020b; Kroll et al., 2017a; Yang et al., 2018) and bacteria (Gao et al., 2015; Hu et al., 2013a; Wang et al., 2016; Wei et al., 2017; Wei et al., 2019b). Combined with STING agonists, these nanovaccines have the potential to effect more powerful, long-lasting, and multivalent immunity against various diseases. In addition, membrane-cloaked platforms have been engineered for effective cytosolic delivery through mechanisms such as endosomal escape (Zhuang et al., 2020) or direct fusion with the plasma membrane (Gong et al., 2021).
An example application of the cell membrane coating technology with STING agonists was very recently reported (Yang et al., 2021). The nanovaccine leveraged manganese dioxide nanoparticles and DiR, a photothermal agent, encapsulated inside B16F10 cancer cell membrane vesicles. Besides providing a multivalent source of tumor antigens, cancer cell membrane has natural homotypic targeting properties (Fang et al., 2014). Upon reaching the TME, the manganese dioxide nanoparticles, which can stimulate STING responses through Mn2+ release, were rapidly degraded by hydrogen peroxide and hydronium ions (Figure 10). Reaction of the manganese dioxide nanoparticles in the TME restored pH levels back to normal and generated oxygen to alleviate tumor hypoxia. The remaining DiR-loaded membrane vesicles were exploited for photothermal therapy to further enhance tumor killing and local antigen release. Intravenous administration of the nanoparticles resulted in the best antitumor efficacy in a primary tumor model when combined with laser irradiation. At the tumor site, a significant number of CD4+ and CD8+ T cells infiltrated into the tumor, while the number of CD4+CD25+Foxp3+ regulatory T cells was low. In a surgical resection recurrence model, large tumors were initially treated with the formulation before the primary tumor was removed in its entirety. When rechallenged 12 days later after surgery with a secondary tumor on the contralateral flank, 3 out of 5 mice that received the nanoformulation along with photothermal therapy did not exhibit tumor growth. Similar effects were observed in a bilateral tumor model where only the primary tumor was subjected to laser treatment. In a metastatic melanoma model, the treatment significantly inhibited tumor nodule formation in the lungs. The impressive results achieved by this biomimetic nanoplatform provides a glimpse into the rationale combination of cell membrane coating nanotechnology with STING agonists to achieve broad immune responses.
Figure 10.
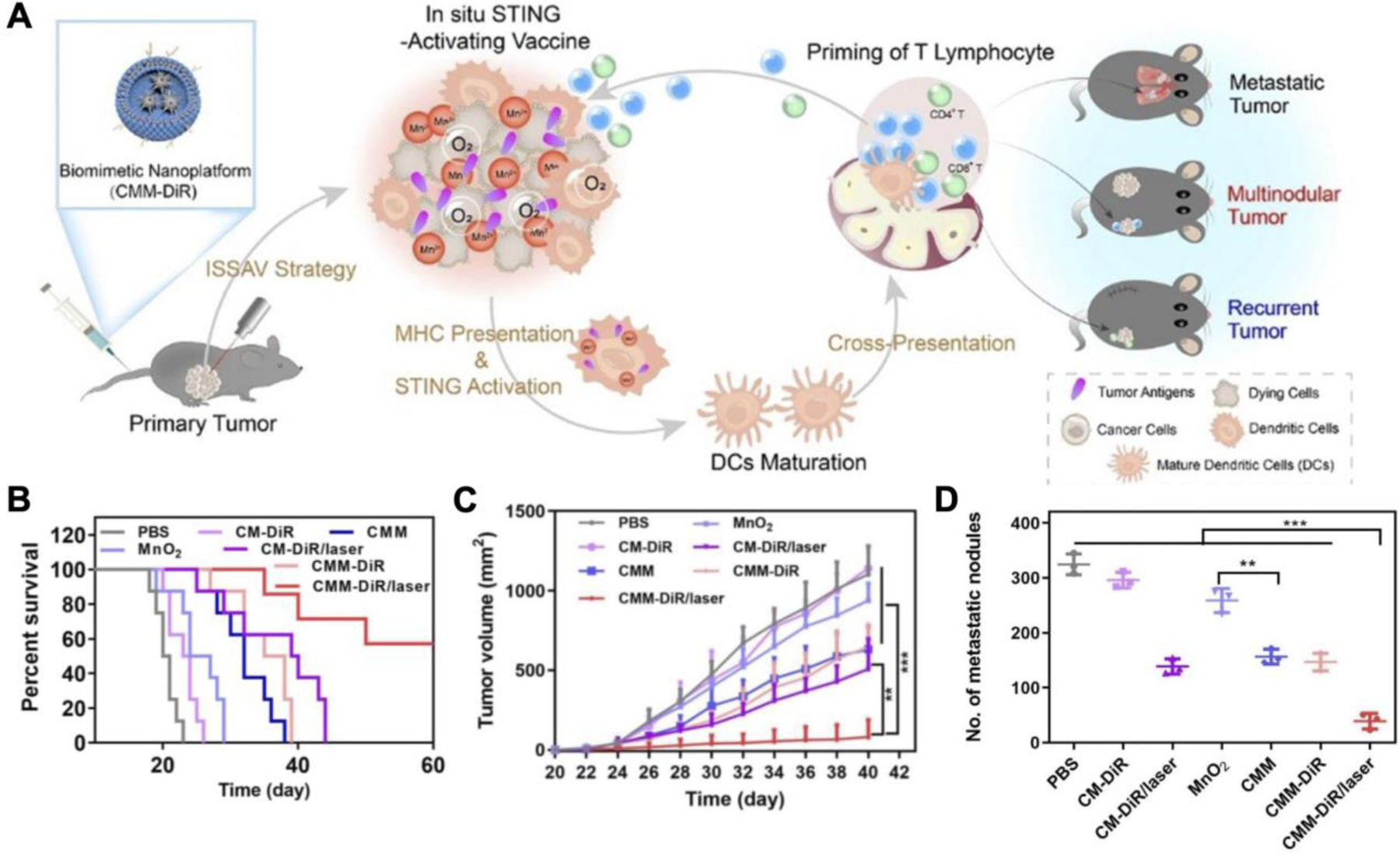
Cancer cell membrane-coated manganese dioxide (MnO2) nanoparticles loaded with DiR (CMM-DiR) as a multivalent nanovaccine platform. A) CMM-DiR preferentially accumulates at the tumor site after intravenous injection due to homotypic targeting. At the tumor, the MnO2 nanoparticles are rapidly degraded into manganese ions (Mn2+) for STING activation, while the remaining nanovesicles can undergo photothermal therapy to amplify antigenic release. Activated dendritic cells (DCs) can take up tumor neoantigens locally and trigger an antigen-specific immune response. B) Combination of CMM-DiR with laser treatment increases survival rates in a primary melanoma model. C) Growth of secondary tumors implanted after the removal of treated primary tumors is significantly controlled by the combinatorial therapy. D) Metastatic nodules in the lungs of mice intravenously challenged with B16F10 after primary tumor treatment are significantly reduced by the combination treatment. Reproduced with permission (Yang et al., 2021). Copyright 2021, Elsevier.
5. Conclusions
In this review, we have summarized the recent advances in nanovaccines that exploit the STING signaling pathway to combat cancer, viral infection, and bacterial infection. STING is a PRR with numerous roles in the cancer–immunity cycle and during pathogenic infections. Proper activation of the pathway can potently evoke type I IFN-mediated innate and adaptive immune responses, which can be leveraged in disease treatment. While research in the field is still at its infancy, a wide range of different STING agonists have been discovered and synthesized, greatly expanding the toolkit available for research and development. However, soluble agonists currently suffer from low bioavailability and have difficulty traversing the plasma membrane. On this front, nanoparticles have been utilized to more effectively deliver STING agonists for vaccine applications. Because STING is located in the cytosol, nanocarriers offer the ability for enhanced intracellular delivery while also improving safety. Nanovaccines applied in this fashion have demonstrated considerable potential against cancers and infectious diseases. Ultimately, continued research along these lines will lead to the development of innovative immunotherapeutic platforms that can reshape how we approach the clinical management of a wide range of diseases.
Acknowledgements
This work is supported by the National Institutes of Health under Award Number R01CA200574 and the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense under Grant Number HDTRA1–18–1–0014.
Footnotes
Declaration of Competing Interest
None.
References
- Ahn J, Barber GN, 2019. STING signaling and host defense against microbial infection. Exp. Mol. Med 51 (12), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai X, Wang S, Duan Y, Zhang Q, Chen MS, Gao W, Zhang L., 2020. Emerging approaches to functionalizing cell membrane-coated nanoparticles. Biochemistry 60 (13), 941–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An M, Yu C, Xi J, Reyes J, Mao G, Wei WZ, Liu H., 2018. Induction of necrotic cell death and activation of STING in the tumor microenvironment via cationic silica nanoparticles leading to enhanced antitumor immunity. Nanoscale 10 (19), 9311–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anghelina D, Lam E, Falck-Pedersen E., 2016. Diminished innate antiviral response to adenovirus vectors in cGAS/STING-deficient mice minimally impacts adaptive immunity. J. Virol 90 (13), 5915–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angsantikul P, Thamphiwatana S, Gao W, Zhang L., 2015. Cell membrane-coated nanoparticles as an emerging antibacterial vaccine platform. Vaccines 3 (4), 814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroh C, Wang Z, Dobbs N, Luo M, Chen Z, Gao J, Yan N., 2017. Innate immune activation by cGMP-AMP nanoparticles leads to potent and long-acting antiretroviral response against HIV-1. J. Immunol 199 (11), 3840–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Palucka AK, 2005. Dendritic cells as therapeutic vaccines against cancer. Nat. Rev. Immunol 5 (4), 296–306. [DOI] [PubMed] [Google Scholar]
- Barber GN, 2015. STING: infection, inflammation and cancer. Nat. Rev. Immunol 15 (12), 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby MC, Phillips RM, Double JA, Pratesi G., 1991. Anti-tumour activity of flavone acetic acid (NSC 347512) in mice--influence of immune status. Br. J. Cancer 63 (1), 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaauboer SM, Mansouri S, Tucker HR, Wang HL, Gabrielle VD, Jin L., 2015. The mucosal adjuvant cyclic di-GMP enhances antigen uptake and selectively activates pinocytosis-efficient cells in vivo. eLife 4, e06670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicher KH, Bohm HJ, Muller K, Alanine AI, 2003. Hit and lead generation: beyond high-throughput screening. Nat. Rev. Drug Discov 2 (5), 369–378. [DOI] [PubMed] [Google Scholar]
- Bohm HJ, Banner D, Bendels S, Kansy M, Kuhn B, Muller K, Obst-Sander U, Stahl M., 2004. Fluorine in medicinal chemistry. ChemBioChem 5 (5), 637–643. [DOI] [PubMed] [Google Scholar]
- Bose RJ, Kim BJ, Arai Y, Han IB, Moon JJ, Paulmurugan R, Park H, Lee SH, 2018. Bioengineered stem cell membrane functionalized nanocarriers for therapeutic targeting of severe hindlimb ischemia. Biomaterials 185, 360–370. [DOI] [PubMed] [Google Scholar]
- Boyoglu-Barnum S, Ellis D, Gillespie RA, Hutchinson GB, Park YJ, Moin SM, Acton OJ, Ravichandran R, Murphy M, Pettie D, Matheson N, Carter L, Creanga A, Watson MJ, Kephart S, Ataca S, Vaile JR, Ueda G, Crank MC, Stewart L, Lee KK, Guttman M, Baker D, Mascola JR, Veesler D, Graham BS, King NP, Kanekiyo M., 2021. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature 592, 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Geiger T, Alkan S, Heusser CH, 1993. Interferon alpha increases the frequency of interferon gamma-producing human CD4+ T cells. J. Exp. Med 178 (5), 1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE, 2011. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478 (7370), 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Metivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, Kroemer G., 2005. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med 202 (12), 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere A, Probst KC, Westwell AD, Slusarczyk M., 2017. Fluorinated nucleosides as an important class of anticancer and antiviral agents. Future Med. Chem 9 (15), 1809–1833. [DOI] [PubMed] [Google Scholar]
- Cavlar T, Deimling T, Ablasser A, Hopfner KP, Hornung V., 2013. Species-specific detection of the antiviral small-molecule compound CMA by STING. EMBO J 32 (10), 1440–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Hu CJ, 2020. Nanomedicinal delivery of stimulator of interferon genes agonists: recent advances in virus vaccination. Nanomedicine (Lond.) 15 (29), 2883–2894. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Liu YH, Fang ZS, Lin CL, Lin JC, Yao BY, Hu CJ, 2020. Synthetic immunogenic cell death mediated by intracellular delivery of STING agonist nanoshells enhances anticancer chemo-immunotherapy. Nano Lett 20 (4), 2246–2256. [DOI] [PubMed] [Google Scholar]
- Chen G, Bai Y, Li Z, Wang F, Fan X, Zhou X., 2020a. Bacterial extracellular vesicle-coated multi-antigenic nanovaccines protect against drug-resistant Staphylococcus aureus infection by modulating antigen processing and presentation pathways. Theranostics 10 (16), 7131–7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Sun H, You F, Sun W, Zhou X, Chen L, Yang J, Wang Y, Tang H, Guan Y, Xia W, Gu J, Ishikawa H, Gutman D, Barber G, Qin Z, Jiang Z., 2011. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell 147 (2), 436–446. [DOI] [PubMed] [Google Scholar]
- Chen Q, Huang G, Wu W, Wang J, Hu J, Mao J, Chu PK, Bai H, Tang G., 2020b. A hybrid eukaryotic–prokaryotic nanoplatform with photothermal modality for enhanced antitumor vaccination. Adv. Mater 32 (16), 1908185. [DOI] [PubMed] [Google Scholar]
- Chen Q, Sun L, Chen ZJ, 2016a. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol 17 (10), 1142–1149. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhang Q, Luk BT, Fang RH, Liu Y, Gao W, Zhang L., 2016b. Coating nanofiber scaffolds with beta cell membrane to promote cell proliferation and function. Nanoscale 8 (19), 10364–10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N, Watkins-Schulz R, Junkins RD, David CN, Johnson BM, Montgomery SA, Peine KJ, Darr DB, Yuan H, McKinnon KP, Liu Q, Miao L, Huang L, Bachelder EM, Ainslie KM, Ting JP, 2018. A nanoparticle-incorporated STING activator enhances antitumor immunity in PD-L1-insensitive models of triple-negative breast cancer. JCI Insight 3 (22), e120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R, Meng F, Deng C, Klok HA, Zhong Z., 2013. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 34 (14), 3647–3657. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Dai T, He X, Zhang Z, Xie F, Wang S, Zhang L, Zhou F., 2020. The interactions between cGAS-STING pathway and pathogens. Signal Transduct. Target. Ther 5 (1), 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin EN, Yu C, Vartabedian VF, Jia Y, Kumar M, Gamo AM, Vernier W, Ali SH, Kissai M, Lazar DC, Nguyen N, Pereira LE, Benish B, Woods AK, Joseph SB, Chu A, Johnson KA, Sander PN, Martinez-Pena F, Hampton EN, Young TS, Wolan DW, Chatterjee AK, Schultz PG, Petrassi HM, Teijaro JR, Lairson LL, 2020. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science 369 (6506), 993–999. [DOI] [PubMed] [Google Scholar]
- Collier MA, Junkins RD, Gallovic MD, Johnson BM, Johnson MM, Macintyre AN, Sempowski GD, Bachelder EM, Ting JP, Ainslie KM, 2018. Acetalated dextran microparticles for codelivery of STING and TLR7/8 agonists. Mol. Pharm 15 (11), 4933–4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon J, Burdette DL, Sharma S, Bhat N, Thompson M, Jiang Z, Rathinam VA, Monks B, Jin T, Xiao TS, Vogel SN, Vance RE, Fitzgerald KA, 2013. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J. Immunol 190 (10), 5216–5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, Metchette K, Dubensky TW Jr., Gajewski TF, 2015. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep 11 (7), 1018–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L, Matson V, Flood B, Spranger S, Gajewski TF, 2017. Innate immune signaling and regulation in cancer immunotherapy. Cell Res 27 (1), 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L, McWhirter SM, Dubensky TW Jr., Gajewski TF, 2016. The host STING pathway at the interface of cancer and immunity. J. Clin. Investig 126 (7), 2404–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan RM, Grundling A., 2013. Cyclic di-AMP: another second messenger enters the fray. Nat. Rev. Microbiol 11 (8), 513–524. [DOI] [PubMed] [Google Scholar]
- Couvreur P., 2013. Nanoparticles in drug delivery: past, present and future. Adv. Drug Deliv. Rev 65 (1), 21–23. [DOI] [PubMed] [Google Scholar]
- Curran E, Chen X, Corrales L, Kline DE, Dubensky TW Jr., Duttagupta P, Kortylewski M, Kline J., 2016. STING pathway activation stimulates potent immunity against acute myeloid leukemia. Cell Rep 15 (11), 2357–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilchanka O, Mekalanos JJ, 2013. Cyclic dinucleotides and the innate immune response. Cell 154 (5), 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, van Doremalen N, Falzarano D, Munster VJ, 2016. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol 14 (8), 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaini D, Wei X, Fang RH, Masson S, Angsantikul P, Luk BT, Zhang Y, Ying M, Jiang Y, Kroll AV, Gao W, Zhang L., 2017. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv. Mater 29 (16), 1606209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denamur E, Matic I., 2006. Evolution of mutation rates in bacteria. Mol. Microbiol 60 (4), 820–827. [DOI] [PubMed] [Google Scholar]
- Diner EJ, Burdette DL, Wilson SC, Monroe KM, Kellenberger CA, Hyodo M, Hayakawa Y, Hammond MC, Vance RE, 2013. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep 3 (5), 1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Song Z, Shen A, Chen T, Zhang A., 2020. Small molecules targeting the innate immune cGAS–STING–TBK1 signaling pathway. Acta Pharm. Sin. B 10 (12), 2272–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A., 2003. Screening for content--the evolution of high throughput. Nat. Biotechnol 21 (8), 859–864. [DOI] [PubMed] [Google Scholar]
- Ebensen T, Debarry J, Pedersen GK, Blazejewska P, Weissmann S, Schulze K, McCullough KC, Cox RJ, Guzman CA, 2017. Mucosal administration of cycle-di-nucleotide-adjuvanted virosomes efficiently induces protection against influenza H5N1 in mice. Front. Immunol 8, 1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebensen T, Libanova R, Schulze K, Yevsa T, Morr M, Guzman CA, 2011. Bis-(3’,5’)-cyclic dimeric adenosine monophosphate: strong Th1/Th2/Th17 promoting mucosal adjuvant. Vaccine 29 (32), 5210–5220. [DOI] [PubMed] [Google Scholar]
- Embgenbroich M, Burgdorf S., 2018. Current concepts of antigen cross-presentation. Front. Immunol 9, 1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban-Fernández de Ávila B, Angsantikul P, Ramírez-Herrera DE, Soto F, Teymourian H, Dehaini D, Chen Y, Zhang L, Wang J., 2018. Hybrid biomembrane–functionalized nanorobots for concurrent removal of pathogenic bacteria and toxins. Sci. Robot 3 (18), eaat0485. [DOI] [PubMed] [Google Scholar]
- Esteban-Fernandez de Avila B, Gao W, Karshalev E, Zhang L, Wang J., 2018. Cell-like micromotors. Acc. Chem. Res 51 (9), 1901–1910. [DOI] [PubMed] [Google Scholar]
- Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L., 2014. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett 14 (4), 2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang RH, Kroll AV, Gao W, Zhang L., 2018. Cell membrane coating nanotechnology. Adv. Mater 30 (23), 1706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang RH, Kroll AV, Zhang L., 2015. Nanoparticle-based manipulation of antigen-presenting cells for cancer immunotherapy. Small 11 (41), 5483–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer NO, Rasley A, Corzett M, Hwang MH, Hoeprich PD, Blanchette CD, 2013. Colocalized delivery of adjuvant and antigen using nanolipoprotein particles enhances the immune response to recombinant antigens. J. Am. Chem. Soc 135 (6), 2044–2047. [DOI] [PubMed] [Google Scholar]
- Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W, Sivick KE, Zeng Q, Soares KC, Zheng L, Portnoy DA, Woodward JJ, Pardoll DM, Dubensky TW Jr., Kim Y., 2015. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med 7 (283), 283ra252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino T, Okada K, Isobe H., 2014. Conformational restriction of cyclic dinucleotides with triazole-linked cyclophane analogues. Tetrahedron Lett 55 (16), 2659–2661. [Google Scholar]
- Gaffney BL, Jones RA, 2014. Synthesis of c-di-GMP analogs with thiourea, urea, carbodiimide, and guanidinium linkages. Org. Lett 16 (1), 158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall B, Pryke K, Abraham J, Mizuno N, Botto S, Sali TM, Broeckel R, Haese N, Nilsen A, Placzek A, Morrison T, Heise M, Streblow D, DeFilippis V., 2018. Emerging alphaviruses are sensitive to cellular states induced by a novel small-molecule agonist of the STING pathway. J. Virol 92 (6), e01913–01917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganta S, Devalapally H, Shahiwala A, Amiji M., 2008. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 126 (3), 187–204. [DOI] [PubMed] [Google Scholar]
- Gao C, Lin Z, Jurado-Sanchez B, Lin X, Wu Z, He Q., 2016a. Stem cell membrane-coated nanogels for highly efficient in vivo tumor targeted drug delivery. Small 12 (30), 4056–4062. [DOI] [PubMed] [Google Scholar]
- Gao C, Lin Z, Wu Z, Lin X, He Q., 2016b. Stem-cell-membrane camouflaging on near-infrared photoactivated upconversion nanoarchitectures for in vivo remote-controlled photodynamic therapy. ACS Appl. Mater. Interfaces 8 (50), 34252–34260. [DOI] [PubMed] [Google Scholar]
- Gao W, Fang RH, Thamphiwatana S, Luk BT, Li J, Angsantikul P, Zhang Q, Hu CM, Zhang L., 2015. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett 15 (2), 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheibi Hayat SM, Darroudi M., 2019. Nanovaccine: a novel approach in immunization. J. Cell. Physiol 234 (8), 12530–12536. [DOI] [PubMed] [Google Scholar]
- Gong C, Zhang X, Shi M, Li F, Wang S, Wang Y, Wang Y, Wei W, Ma G., 2021. Tumor exosomes reprogrammed by low pH are efficient targeting vehicles for smart drug delivery and personalized therapy against their homologous tumor. Adv. Sci, 2002787. [DOI] [PMC free article] [PubMed]
- Gong H, Chen F, Huang Z, Gu Y, Zhang Q, Chen Y, Zhang Y, Zhuang J, Cho YK, Fang RH, Gao W, Xu S, Zhang L., 2019. Biomembrane-modified field effect transistors for sensitive and quantitative detection of biological toxins and pathogens. ACS Nano 13 (3), 3714–3722. [DOI] [PubMed] [Google Scholar]
- Guo F, Han Y, Zhao X, Wang J, Liu F, Xu C, Wei L, Jiang JD, Block TM, Guo JT, Chang J., 2015. STING agonists induce an innate antiviral immune response against hepatitis B virus. Antimicrob. Agents Chemother 59 (2), 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ, 2015. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J. Clin. Investig 125 (6), 2532–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman TJ, Baro M, MacNeil T, Phoomak C, Aung TN, Cui W, Leach K, Iyer R, Challa S, Sandoval-Schaefer T, Burtness BA, Rimm DL, Contessa JN, 2021. STING enhances cell death through regulation of reactive oxygen species and DNA damage. Nat. Commun 12 (1), 2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DA, 2011. The eradication of smallpox--an overview of the past, present, and future. Vaccine 29 (4), D7–D9. [DOI] [PubMed] [Google Scholar]
- Hou L, Tian C, Yan Y, Zhang L, Zhang H, Zhang Z., 2020. Manganese-based nanoactivator optimizes cancer immunotherapy via enhancing innate immunity. ACS Nano 14 (4), 3927–3940. [DOI] [PubMed] [Google Scholar]
- Hu CM, Fang RH, Luk BT, Zhang L., 2013a. Nanoparticle-detained toxins for safe and effective vaccination. Nat. Nanotechnol 8 (12), 933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CM, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, Zhang L., 2015. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526 (7571), 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L., 2011. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. U.S.A 108 (27), 10980–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CMJ, Fang RH, Copp J, Luk BT, Zhang LF, 2013b. A biomimetic nanosponge that absorbs pore-forming toxins. Nat. Nanotechnol. 8 (5), 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Ott PA, Wu CJ, 2018. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol 18 (3), 168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Barber GN, 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455 (7213), 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ma Z, Barber GN, 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461 (7265), 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkiv LB, Donlin LT, 2014. Regulation of type I interferon responses. Nat. Rev. Immunol 14 (1), 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R., 2015. Control of adaptive immunity by the innate immune system. Nat. Immunol 16 (4), 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching LM, Kaiser LR, Albelda SM, 2005. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res 65 (24), 11752–11761. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Krishnan N, Heo J, Fang RH, Zhang L., 2020a. Nanoparticle-hydrogel superstructures for biomedical applications. J. Control. Release 324, 505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Krishnan N, Zhou J, Chekuri S, Wei X, Kroll AV, Yu CL, Duan Y, Gao W, Fang RH, Zhang L., 2020b. Engineered cell-membrane-coated nanoparticles directly present tumor antigens to promote anticancer immunity. Adv. Mater 32 (30), 2001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Hill KK, Filak H, Mogan J, Knowles H, Zhang B, Perraud AL, Cambier JC, Lenz LL, 2011. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J. Immunol 187 (5), 2595–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing W, McAllister D, Vonderhaar EP, Palen K, Riese MJ, Gershan J, Johnson BD, Dwinell MB, 2019. STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J. Immunother. Cancer 7 (1), 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre OP, Segura E, Savina A, Amigorena S., 2012. Cross-presentation by dendritic cells. Nat. Rev. Immunol 12 (8), 557–569. [DOI] [PubMed] [Google Scholar]
- Junkins RD, Gallovic MD, Johnson BM, Collier MA, Watkins-Schulz R, Cheng N, David CN, McGee CE, Sempowski GD, Shterev I, McKinnon K, Bachelder EM, Ainslie KM, Ting JP, 2018. A robust microparticle platform for a STING-targeted adjuvant that enhances both humoral and cellular immunity during vaccination. J. Control. Release 270, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamvoki M, Du T, Roizman B., 2014. Cells infected with herpes simplex virus 1 export to uninfected cells exosomes containing STING, viral mRNAs, and microRNAs. Proc. Natl. Acad. Sci. U.S.A 111 (46), E4991–E4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo M, Joyce MG, Gillespie RA, Gallagher JR, Andrews SF, Yassine HM, Wheatley AK, Fisher BE, Ambrozak DR, Creanga A, Leung K, Yang ES, Boyoglu-Barnum S, Georgiev IS, Tsybovsky Y, Prabhakaran MS, Andersen H, Kong WP, Baxa U, Zephir KL, Ledgerwood JE, Koup RA, Kwong PD, Harris AK, McDermott AB, Mascola JR, Graham BS, 2019. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat. Immunol 20 (3), 362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo M, Wei CJ, Yassine HM, McTamney PM, Boyington JC, Whittle JR, Rao SS, Kong WP, Wang L, Nabel GJ, 2013. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499 (7456), 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Nishimasu H, Oikawa D, Hirano S, Hirano H, Kasuya G, Ishitani R, Tokunaga F, Nureki O., 2018. Structural insights into cGAMP degradation by Ecto-nucleotide pyrophosphatase phosphodiesterase 1. Nat. Commun 9, 4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S., 2009. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol 21 (4), 317–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CG, Kye YC, Yun CH, 2019. The role of nanovaccine in cross-presentation of antigen-presenting cells for the activation of CD8(+) T cell responses. Pharmaceutics 11 (11), 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Park SK, Seo SW, Lee CH, Shin OS, 2017. STING is involved in antiviral immune response against VZV infection via the induction of type I and III IFN pathways. J. Investig. Dermatol 137 (10), 2101–2109. [DOI] [PubMed] [Google Scholar]
- Kim S, Li L, Maliga Z, Yin Q, Wu H, Mitchison TJ, 2013. Anticancer flavonoids are mouse-selective STING agonists. ACS Chem. Biol 8 (7), 1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Negishi H, Matsuda A, Endo N, Hangai S, Inoue A, Nishio J, Taniguchi T, Yanai H., 2018. Novel chemical compound SINCRO with dual function in STING-type I interferon and tumor cell death pathways. Cancer Sci 109 (9), 2687–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai Y, Kawasaki T, Sueyoshi T, Kobiyama K, Ishii KJ, Zou J, Akira S, Matsuda T, Kawai T., 2017. DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity. J. Immunol 198 (4), 1649–1659. [DOI] [PubMed] [Google Scholar]
- Klarquist J, Hennies CM, Lehn MA, Reboulet RA, Feau S, Janssen EM, 2014. STING-mediated DNA sensing promotes antitumor and autoimmune responses to dying cells. J. Immunol 193 (12), 6124–6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppe U, Hogner K, Doehn JM, Muller HC, Witzenrath M, Gutbier B, Bauer S, Pribyl T, Hammerschmidt S, Lohmeyer J, Suttorp N, Herold S, Opitz B., 2012. Streptococcus pneumoniae stimulates a STING- and IFN regulatory factor 3-dependent type I IFN production in macrophages, which regulates RANTES production in macrophages, cocultured alveolar epithelial cells, and mouse lungs. J. Immunol 188 (2), 811–817. [DOI] [PubMed] [Google Scholar]
- Koshy ST, Cheung AS, Gu L, Graveline AR, Mooney DJ, 2017. Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv. Biosyst 1 (1–2), 1600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll AV, Fang RH, Jiang Y, Zhou J, Wei X, Yu CL, Gao J, Luk BT, Dehaini D, Gao W, Zhang L., 2017a. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv. Mater 29 (47), 1703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll AV, Fang RH, Zhang L., 2017b. Biointerfacing and applications of cell membrane-coated nanoparticles. Bioconjugate Chem 28 (1), 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll AV, Jiang Y, Zhou J, Holay M, Fang RH, Zhang L., 2019. Biomimetic nanoparticle vaccines for cancer therapy. Adv. Biosyst 3 (1), 1800219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S., 2011. Pathogen recognition by the innate immune system. Int. Rev. Immunol 30 (1), 16–34. [DOI] [PubMed] [Google Scholar]
- Kumar S, Han JA, Michael IJ, Ki D, Sunkara V, Park J, Gautam S, Ha HK, Zhang LF, Cho YK, 2019. Human platelet membrane functionalized microchips with plasmonic codes for cancer detection. Adv. Funct. Mater 29 (30), 1902669. [Google Scholar]
- Larson HJ, Ghinai I., 2011. Lessons from polio eradication. Nature 473 (7348), 446–447. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Durand V, Kamphuis E, Thompson C, Bulfone-Paus S, Rossmann C, Kalinke U, Tough DF, 2006a. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J. Immunol 176 (8), 4682–4689. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Thompson C, Kamphuis E, Durand V, Rossmann C, Kalinke U, Tough DF, 2006b. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J. Immunol 176 (4), 2074–2078. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Ahn EY, Park Y., 2019. Shape-dependent cytotoxicity and cellular uptake of gold nanoparticles synthesized using green tea extract. Nanoscale Res. Lett 14 (1), 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Yi M, Qin S, Song Y, Chu Q, Wu K., 2019. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J. Hematol. Oncol 12 (1), 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Luo M, Wang Z, Feng Q, Wilhelm J, Wang X, Li W, Wang J, Cholka A, Fu YX, Sumer BD, Yu H, Gao J., 2021. Prolonged activation of innate immune pathways by a polyvalent STING agonist. Nat. Biomed. Eng [DOI] [PMC free article] [PubMed]
- Li T, Chen ZJ, 2018. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med 215 (5), 1287–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ, 2013. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341 (6152), 1390–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libanova R, Ebensen T, Schulze K, Bruhn D, Norder M, Yevsa T, Morr M, Guzman CA, 2010. The member of the cyclic di-nucleotide family bis-(3’, 5’)-cyclic dimeric inosine monophosphate exerts potent activity as mucosal adjuvant. Vaccine 28 (10), 2249–2258. [DOI] [PubMed] [Google Scholar]
- Lin LC, Huang CY, Yao BY, Lin JC, Agrawal A, Algaissi A, Peng BH, Liu YH, Huang PH, Juang RH, Chang YC, Tseng CT, Chen HW, Hu CJ, 2019. Viromimetic STING agonist-loaded hollow polymeric nanoparticles for safe and effective vaccination against Middle East respiratory syndrome coronavirus. Adv. Funct. Mater 29 (28), 1807616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio CW, McDonald B, Takahashi M, Dhanwani R, Sharma N, Huang J, Pham E, Benedict CA, Sharma S., 2016. cGAS-STING signaling regulates initial innate control of cytomegalovirus infection. J. Virol 90 (17), 7789–7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioux T, Mauny MA, Lamoureux A, Bascoul N, Hays M, Vernejoul F, Baudru AS, Boularan C, Lopes-Vicente J, Qushair G, Tiraby G., 2016. Design, synthesis, and biological evaluation of novel cyclic adenosine-inosine monophosphate (cAIMP) analogs that activate stimulator of interferon genes (STING). J. Med. Chem 59 (22), 10253–10267. [DOI] [PubMed] [Google Scholar]
- Lirussi D, Ebensen T, Schulze K, Trittel S, Duran V, Liebich I, Kalinke U, Guzman CA, 2017. Type I IFN and not TNF, is essential for cyclic di-nucleotide-elicited CTL by a cytosolic cross-presentation pathway. EBioMedicine 22, 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Tang L, Zhang X, Ma J, Sehgal M, Cheng J, Zhang X, Zhou Y, Du Y, Kulp J, Guo JT, Chang J., 2017a. A cell-based high throughput screening assay for the discovery of cGAS-STING pathway agonists. Antivir. Res 147, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhang W, Li Y, Chang J, Tian F, Zhao F, Ma Y, Sun J., 2019a. Microfluidic sonication to assemble exosome membrane-coated nanoparticles for immune evasion-mediated targeting. Nano Lett 19 (11), 7836–7844. [DOI] [PubMed] [Google Scholar]
- Liu P, Sharon A, Chu CK, 2008. Fluorinated nucleosides: synthesis and biological implication. J. Fluor. Chem 129 (9), 743–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WL, Zou MZ, Liu T, Zeng JY, Li X, Yu WY, Li CX, Ye JJ, Song W, Feng J, Zhang XZ, 2019b. Cytomembrane nanovaccines show therapeutic effects by mimicking tumor cells and antigen presenting cells. Nat. Commun 10 (1), 3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Crowe WN, Wang L, Lu Y, Petty WJ, Habib AA, Zhao D., 2019c. An inhalable nanoparticulate STING agonist synergizes with radiotherapy to confer long-term control of lung metastases. Nat. Commun 10 (1), 5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hardie J, Zhang X, Rotello VM, 2017b. Effects of engineered nanoparticles on the innate immune system. Semin. Immunol 34, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Concha-Benavente F, Shayan G, Srivastava RM, Gibson SP, Wang L, Gooding WE, Ferris RL, 2018. STING activation enhances cetuximab-mediated NK cell activation and DC maturation and correlates with HPV(+) status in head and neck cancer. Oral Oncol 78, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Miao L, Gao W, Chen Z, McHugh KJ, Sun Y, Tochka Z, Tomasic S, Sadtler K, Hyacinthe A, Huang Y, Graf T, Hu Q, Sarmadi M, Langer R, Anderson DG, Jaklenec A., 2020. Engineered PLGA microparticles for long-term, pulsatile release of STING agonist for cancer immunotherapy. Sci. Transl. Med 12 (556), eaaz6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Liu Z, Zhang X, Han C, Samandi LZ, Dong C, Sumer BD, Lea J, Fu YX, Gao J., 2019. Synergistic STING activation by PC7A nanovaccine and ionizing radiation improves cancer immunotherapy. J. Control. Release 300, 154–160. [DOI] [PubMed] [Google Scholar]
- Luo M, Samandi LZ, Wang Z, Chen ZJ, Gao J., 2017a. Synthetic nanovaccines for immunotherapy. J. Control. Release 263, 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, Du M, Huang G, Wang C, Chen X, Porembka MR, Lea J, Frankel AE, Fu YX, Chen ZJ, Gao J., 2017b. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol 12 (7), 648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarron R, Banks MN, Bojanic D, Burns DJ, Cirovic DA, Garyantes T, Green DV, Hertzberg RP, Janzen WP, Paslay JW, Schopfer U, Sittampalam GS, 2011. Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Discov 10 (3), 188–195. [DOI] [PubMed] [Google Scholar]
- Madhun AS, Haaheim LR, Nostbakken JK, Ebensen T, Chichester J, Yusibov V, Guzman CA, Cox RJ, 2011. Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine 29 (31), 4973–4982. [DOI] [PubMed] [Google Scholar]
- Mansouri S, Blaauboer S, Jin L., 2016. Mucosal vaccine adjuvant cyclic di-GMP induces STING-dependent pulmonary dendritic cell activation. J. Immunol 196 (1), 145.141. [Google Scholar]
- Marinho FV, Benmerzoug S, Oliveira SC, Ryffel B, Quesniaux VFJ, 2017. The emerging roles of STING in bacterial infections. Trends Microbiol 25 (11), 906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Kappler J, Mitchell T., 1999. Type I interferons keep activated T cells alive. J. Exp. Med 189 (3), 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Huang X, Yang Y., 2008. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. J. Immunol 180 (3), 1592–1597. [DOI] [PubMed] [Google Scholar]
- McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A., 2015. Type I interferons in infectious disease. Nat. Rev. Immunol 15 (2), 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, Mellstedt H., 2014. Therapeutic vaccines for cancer: an overview of clinical trials. Nat. Rev. Clin. Oncol 11 (9), 509–524. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Sunshine JC, Perica K, Kosmides AK, Aje K, Schneck JP, Green JJ, 2015. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small 11 (13), 1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao L, Li L, Huang Y, Delcassian D, Chahal J, Han J, Shi Y, Sadtler K, Gao W, Lin J, Doloff JC, Langer R, Anderson DG, 2019. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol 37 (10), 1174–1185. [DOI] [PubMed] [Google Scholar]
- Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, Chai S, Herring LE, Zhang L, Zhang T, DeSimone JM, Tepper JE, Vincent BG, Serody JS, Wang AZ, 2017. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol 12 (9), 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabe H, Hyodo M, Nakamura T, Sato Y, Hayakawa Y, Harashima H., 2014. A new adjuvant delivery system ‘cyclic di-GMP/YSK05 liposome’ for cancer immunotherapy. J. Control. Release 184, 20–27. [DOI] [PubMed] [Google Scholar]
- Moss WJ, Griffin DE, 2006. Global measles elimination. Nat. Rev. Microbiol 4 (12), 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motedayen Aval L, Pease JE, Sharma R, Pinato DJ, 2020. Challenges and opportunities in the clinical development of STING agonists for cancer immunotherapy. J. Clin. Med 9 (10), 3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motornov M, Roiter Y, Tokarev I, Minko S., 2010. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog. Polym. Sci 35 (1), 174–211. [Google Scholar]
- Mura S, Nicolas J, Couvreur P., 2013. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater 12 (11), 991–1003. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Miyabe H, Hyodo M, Sato Y, Hayakawa Y, Harashima H., 2015. Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma. J. Control. Release 216, 149–157. [DOI] [PubMed] [Google Scholar]
- Ohkuri T, Ghosh A, Kosaka A, Zhu J, Ikeura M, David M, Watkins SC, Sarkar SN, Okada H., 2014. STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol. Res 2 (12), 1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BS, Perera SA, Piesvaux JA, Presland JP, Schroeder GK, Cumming JN, Trotter BW, Altman MD, Buevich AV, Cash B, Cemerski S, Chang W, Chen Y, Dandliker PJ, Feng G, Haidle A, Henderson T, Jewell J, Kariv I, Knemeyer I, Kopinja J, Lacey BM, Laskey J, Lesburg CA, Liang R, Long BJ, Lu M, Ma Y, Minnihan EC, O’Donnell G, Otte R, Price L, Rakhilina L, Sauvagnat B, Sharma S, Tyagarajan S, Woo H, Wyss DF, Xu S, Bennett DJ, Addona GH, 2020. An orally available non-nucleotide STING agonist with antitumor activity. Science 369 (6506), eaba6098. [DOI] [PubMed] [Google Scholar]
- Panche AN, Diwan AD, Chandra SR, 2016. Flavonoids: an overview. J. Nutr. Sci 5, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D, Martin FJ, Soong G, Harfenist BS, Aguilar JL, Ratner AJ, Fitzgerald KA, Schindler C, Prince A., 2011. Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. mBio 2 (3), e00016–00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RB, Ye M, Carlson PM, Jaquish A, Zangl L, Ma B, Wang Y, Arthur I, Xie R, Brown RJ, Wang X, Sriramaneni R, Kim K, Gong S, Morris ZS, 2019. Development of an in situ cancer vaccine via combinational radiation and bacterial-membrane-coated nanoparticles. Adv. Mater 31 (43), 1902626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova VN, Russell CA, 2018. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol 16 (1), 47–60. [DOI] [PubMed] [Google Scholar]
- Philpott M, Baguley BC, Ching LM, 1995. Induction of tumour necrosis factor-alpha by single and repeated doses of the antitumour agent 5,6-dimethylxanthenone-4-acetic acid. Cancer Chemother. Pharmacol 36 (2), 143–148. [DOI] [PubMed] [Google Scholar]
- Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang SY, Tran JL, Moore P, Lehmann S, Eberl HC, Muelbaier M, Schneck JL, Clemens J, Adam M, Mehlmann J, Romano J, Morales A, Kang J, Leister L, Graybill TL, Charnley AK, Ye G, Nevins N, Behnia K, Wolf AI, Kasparcova V, Nurse K, Wang L, Puhl AC, Li Y, Klein M, Hopson CB, Guss J, Bantscheff M, Bergamini G, Reilly MA, Lian Y, Duffy KJ, Adams J, Foley KP, Gough PJ, Marquis RW, Smothers J, Hoos A, Bertin J., 2018. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 564 (7736), 439–443. [DOI] [PubMed] [Google Scholar]
- Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA, 2007. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol 25 (10), 1159–1164. [DOI] [PubMed] [Google Scholar]
- Romling U, Galperin MY, Gomelsky M., 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev 77 (1), 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross K, Senapati S, Alley J, Darling R, Goodman J, Jefferson M, Uz M, Guo B, Yoon KJ, Verhoeven D, Kohut M, Mallapragada S, Wannemuehler M, Narasimhan B., 2019. Single dose combination nanovaccine provides protection against influenza A virus in young and aged mice. Biomater. Sci 7 (3), 809–821. [DOI] [PubMed] [Google Scholar]
- Rouzaut A, Garasa S, Teijeira A, Gonzalez I, Martinez-Forero I, Suarez N, Larrea E, Alfaro C, Palazon A, Dubrot J, Hervas-Stubbs S, Melero I., 2010. Dendritic cells adhere to and transmigrate across lymphatic endothelium in response to IFN-alpha. Eur. J. Immunol 40 (11), 3054–3063. [DOI] [PubMed] [Google Scholar]
- Sali TM, Pryke KM, Abraham J, Liu A, Archer I, Broeckel R, Staverosky JA, Smith JL, Al-Shammari A, Amsler L, Sheridan K, Nilsen A, Streblow DN, DeFilippis VR, 2015. Characterization of a novel human-specific STING agonist that elicits antiviral activity against emerging alphaviruses. PLoS Pathog 11 (12), e1005324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Hatakeyama H, Sakurai Y, Hyodo M, Akita H, Harashima H., 2012. A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. J. Control. Release 163 (3), 267–276. [DOI] [PubMed] [Google Scholar]
- Sengul AB, Asmatulu E., 2020. Toxicity of metal and metal oxide nanoparticles: a review. Environ. Chem. Lett 18 (5), 1659–1683. [Google Scholar]
- Shae D, Baljon JJ, Wehbe M, Christov PP, Becker KW, Kumar A, Suryadevara N, Carson CS, Palmer CR, Knight FC, Joyce S, Wilson JT, 2020. Co-delivery of peptide neoantigens and stimulator of interferon genes agonists enhances response to cancer vaccines. ACS Nano 14 (8), 9904–9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shae D, Becker KW, Christov P, Yun DS, Lytton-Jean AKR, Sevimli S, Ascano M, Kelley M, Johnson DB, Balko JM, Wilson JT, 2019. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol 14 (3), 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., 2021. Eliciting B cell immunity against infectious diseases using nanovaccines. Nat. Nanotechnol 16 (1), 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisquella X, Ofir-Birin Y, Pimentel MA, Cheng L, Abou Karam P, Sampaio NG, Penington JS, Connolly D, Giladi T, Scicluna BJ, Sharples RA, Waltmann A, Avni D, Schwartz E, Schofield L, Porat Z, Hansen DS, Papenfuss AT, Eriksson EM, Gerlic M, Hill AF, Bowie AG, Regev-Rudzki N., 2017. Malaria parasite DNA-harbouring vesicles activate cytosolic immune sensors. Nat. Commun 8 (1), 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrnjug I, Rueckert C, Libanova R, Lienenklaus S, Weiss S, Guzman CA, 2014. The mucosal adjuvant cyclic di-AMP exerts immune stimulatory effects on dendritic cells and macrophages. PLoS One 9 (4), e95728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Selby LI, Johnston APR, Such GK, 2019. The endosomal escape of nanoparticles: toward more efficient cellular delivery. Bioconjugate Chem 30 (2), 263–272. [DOI] [PubMed] [Google Scholar]
- Smola M, Gutten O, Dejmek M, Kozisek M, Evangelidis T, Tehrani ZA, Novotna B, Nencka R, Birkus G, Rulisek L, Boura E., 2021. Ligand strain and its conformational complexity is a major factor in the binding of cyclic dinucleotides to STING protein. Angew. Chem. Int. Ed. Engl 133 (18), 10260–10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro F, Lapenta C, Donati S, Abalsamo L, Barnaba V, Belardelli F, Santini SM, Ferrantini M., 2012. IFN-alpha enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood 119 (6), 1407–1417. [DOI] [PubMed] [Google Scholar]
- Stempel M, Chan B, Juranic Lisnic V, Krmpotic A, Hartung J, Paludan SR, Fullbrunn N, Lemmermann NA, Brinkmann MM, 2019. The herpesviral antagonist m152 reveals differential activation of STING-dependent IRF and NF-kappaB signaling and STING’s dual role during MCMV infection. EMBO J 38 (5), e100983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine J, Green JJ, Mahon KP, Yang F, Eltoukhy AA, Nguyen DN, Langer R, Anderson DG, 2009. Small-molecule end-groups of linear polymer determine cell-type gene-delivery efficacy. Adv. Mater 21 (48), 4947–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YS, Sansanaphongpricha K, Xie Y, Donnelly CR, Luo X, Heath BR, Zhao X, Bellile E, Hu H, Chen H, Polverini PJ, Chen Q, Young S, Carey TE, Nor JE, Ferris RL, Wolf GT, Sun D, Lei YL, 2018. Mitigating SOX2-potentiated immune escape of head and neck squamous cell carcinoma with a STING-inducing nanosatellite vaccine. Clin. Cancer Res 24 (17), 4242–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CH, Zundell JA, Ranatunga S, Lin C, Nefedova Y, Del Valle JR, Hu CC, 2016. Agonist-mediated activation of STING induces apoptosis in malignant B cells. Cancer Res 76 (8), 2137–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamphiwatana S, Angsantikul P, Escajadillo T, Zhang Q, Olson J, Luk BT, Zhang S, Fang RH, Gao W, Nizet V, Zhang L., 2017. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc. Natl. Acad. Sci. U.S.A 114 (43), 11488–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MP, Wilson-Welder JH, Lopac SK, Phanse Y, Carrillo-Conde B, Ramer-Tait AE, Bellaire BH, Wannemuehler MJ, Narasimhan B., 2011. Polyanhydride microparticles enhance dendritic cell antigen presentation and activation. Acta Biomater 7 (7), 2857–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tough DF, Borrow P, Sprent J., 1996. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272 (5270), 1947–1950. [DOI] [PubMed] [Google Scholar]
- van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ, 2016. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Cancer 16 (4), 219–233. [DOI] [PubMed] [Google Scholar]
- Wagner DA, Kelly SM, Petersen AC, Peroutka-Bigus N, Darling RJ, Bellaire BH, Wannemuehler MJ, Narasimhan B., 2019. Single-dose combination nanovaccine induces both rapid and long-lived protection against pneumonic plague. Acta Biomater 100, 326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang-Bishop L, Wehbe M, Shae D, James J, Hacker BC, Garland K, Chistov PP, Rafat M, Balko JM, Wilson JT, 2020. Potent STING activation stimulates immunogenic cell death to enhance antitumor immunity in neuroblastoma. J. Immunother. Cancer 8 (1), e000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Guan Y, Lv M, Zhang R, Guo Z, Wei X, Du X, Yang J, Li T, Wan Y, Su X, Huang X, Jiang Z., 2018. Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity 48 (4), 675–687. [DOI] [PubMed] [Google Scholar]
- Wang C, Sinn M, Stifel J, Heiler AC, Sommershof A, Hartig JS, 2017. Synthesis of all possible canonical (3’−5’-linked) cyclic dinucleotides and evaluation of riboswitch interactions and immune-stimulatory effects. J. Am. Chem. Soc 139 (45), 16154–16160. [DOI] [PubMed] [Google Scholar]
- Wang F, Fang RH, Luk BT, Hu CJ, Thamphiwatana S, Dehaini D, Angsantikul P, Kroll AV, Pang Z, Gao W, Lu W, Zhang L., 2016. Nanoparticle-based antivirulence vaccine for the management of methicillin-resistant Staphylococcus aureus skin infection. Adv. Funct. Mater 26 (10), 1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Su H, Xu D, Dai W, Zhang W, Wang Z, Anderson CF, Zheng M, Oh R, Wan F, Cui H., 2020a. Tumour sensitization via the extended intratumoural release of a STING agonist and camptothecin from a self-assembled hydrogel. Nat. Biomed. Eng 4 (11), 1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li P, Yu Y, Fu Y, Jiang H, Lu M, Sun Z, Jiang S, Lu L, Wu MX, 2020b. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 367 (6480), eaau0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins-Schulz R, Tiet P, Gallovic MD, Junkins RD, Batty C, Bachelder EM, Ainslie KM, Ting JPY, 2019. A microparticle platform for STING-targeted immunotherapy enhances natural killer cell- and CD8(+) T cell-mediated anti-tumor immunity. Biomaterials 205, 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RO, Manzanillo PS, Cox JS, 2012. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 150 (4), 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Beltran-Gastelum M, Karshalev E, Esteban-Fernandez de Avila B, Zhou J, Ran D, Angsantikul P, Fang RH, Wang J, Zhang L., 2019a. Biomimetic micromotor enables active delivery of antigens for oral vaccination. Nano Lett 19 (3), 1914–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Gao J, Fang RH, Luk BT, Kroll AV, Dehaini D, Zhou J, Kim HW, Gao W, Lu W, Zhang L., 2016. Nanoparticles camouflaged in platelet membrane coating as an antibody decoy for the treatment of immune thrombocytopenia. Biomaterials 111, 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Gao J, Wang F, Ying M, Angsantikul P, Kroll AV, Zhou J, Gao W, Lu W, Fang RH, Zhang L., 2017. In situ capture of bacterial toxins for antivirulence vaccination. Adv. Mater 29 (33), 1701644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Ran D, Campeau A, Xiao C, Zhou J, Dehaini D, Jiang Y, Kroll AV, Zhang Q, Gao W, Gonzalez DJ, Fang RH, Zhang L., 2019b. Multiantigenic nanotoxoids for antivirulence vaccination against antibiotic-resistant gram-negative bacteria. Nano Lett 19 (7), 4760–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Zhang G, Ran D, Krishnan N, Fang RH, Gao W, Spector SA, Zhang L., 2018. T-cell-mimicking nanoparticles can neutralize HIV infectivity. Adv. Mater 30 (45), 1802233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley AT, Eaglesham JB, de Oliveira Mann CC, Morehouse BR, Lowey B, Nieminen EA, Danilchanka O, King DS, Lee ASY, Mekalanos JJ, Kranzusch PJ, 2019. Bacterial cGAS-like enzymes synthesize diverse nucleotide signals. Nature 567 (7747), 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J, Quinones-Perez M, Wang J, Wang X, Basava VS, Gao J., 2021. Antigen folding improves loading efficiency and antitumor efficacy of PC7A nanoparticle vaccine. J. Control. Release 329, 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DR, Sen R, Sunshine JC, Pardoll DM, Green JJ, Kim YJ, 2018. Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomedicine 14 (2), 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, Alegre ML, Gajewski TF, 2014. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41 (5), 830–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Zhao L, Han BB, Hu HG, Zhang BD, Li WH, Chen YX, Li YM, 2021. A novel STING agonist for cancer immunotherapy and a SARS-CoV-2 vaccine adjuvant. Chem. Commun 57 (4), 504–507. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Zhao L, Hu HG, Li WH, Li YM, 2020. Agonists and inhibitors of the STING pathway: potential agents for immunotherapy. Med. Res. Rev 40 (3), 1117–1141. [DOI] [PubMed] [Google Scholar]
- Xi Q, Wang M, Jia W, Yang M, Hu J, Jin J, Chen X, Yin D, Wang X., 2020. Design, synthesis, and biological evaluation of amidobenzimidazole derivatives as stimulator of interferon genes (STING) receptor agonists. J. Med. Chem 63 (1), 260–282. [DOI] [PubMed] [Google Scholar]
- Xia T, Konno H, Barber GN, 2016. Recurrent loss of STING signaling in melanoma correlates with susceptibility to viral oncolysis. Cancer Res 76 (22), 6747–6759. [DOI] [PubMed] [Google Scholar]
- Yan H, Wang X, KuoLee R, Chen W., 2008. Synthesis and immunostimulatory properties of the phosphorothioate analogues of cdiGMP. Bioorg. Med. Chem. Lett 18 (20), 5631–5634. [DOI] [PubMed] [Google Scholar]
- Yan N, Chen ZJ, 2012. Intrinsic antiviral immunity. Nat. Immunol 13 (3), 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Xu J, Xu L, Sun X, Chen Q, Zhao Y, Peng R, Liu Z., 2018. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano 12 (6), 5121–5129. [DOI] [PubMed] [Google Scholar]
- Yang X, Yang Y, Bian J, Wei J, Wang Z, Zhou Z, Li Z, Sun M., 2021. Converting primary tumor towards an in situ STING-activating vaccine via a biomimetic nanoplatform against recurrent and metastatic tumors. Nano Today 38, 101109. [Google Scholar]
- Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, Hakeem A, Hu J, Gan L, Santos HA, Yang X., 2019. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun 10 (1), 3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, You QD, Xu XL, 2020a. Targeting stimulator of interferon genes (STING): a medicinal chemistry perspective. J. Med. Chem 63 (8), 3785–3816. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Dehaini D, Zhang Y, Zhou J, Chen X, Zhang L, Fang RH, Gao W, Zhang L., 2018a. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol 13 (12), 1182–1190. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Honko A, Zhou J, Gong H, Downs SN, Vasquez JH, Fang RH, Gao W, Griffiths A, Zhang L., 2020b. Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett 20 (7), 5570–5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu B, Tang L, Su Q, Hwang N, Sehgal M, Cheng J, Ma J, Zhang X, Tan Y, Zhou Y, Duan Z, DeFilippis VR, Viswanathan U, Kulp J, Du Y, Guo JT, Chang J., 2019a. Discovery and mechanistic study of a novel human-stimulator-of-interferon-genes agonist. ACS Infect. Dis 5 (7), 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen ZJ, 2013. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell 51 (2), 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Lo C, Zhuang J, Angsantikul P, Zhang Q, Wei X, Zhou Z, Obonyo M, Fang RH, Gao W, Zhang L., 2019b. Inhibition of pathogen adhesion by bacterial outer membrane-coated nanoparticles. Angew. Chem. Int. Ed. Engl 58 (33), 11404–11408. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li M, Li L, Qian G, Wang Y, Chen Z, Liu J, Fang C, Huang F, Guo D, Zou Q, Chu Y, Yan D., 2020c. Beta-arrestin 2 as an activator of cGAS-STING signaling and target of viral immune evasion. Nat. Commun 11 (1), 6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sun Z, Pei J, Luo Q, Zeng X, Li Q, Yang Z, Quan J., 2018b. Identification of alpha-mangostin as an agonist of human STING. ChemMedChem 13 (19), 2057–2064. [DOI] [PubMed] [Google Scholar]
- Zhao J, Ma S, Xu Y, Si X, Yao H, Huang Z, Zhang Y, Yu H, Tang Z, Song W, Chen X., 2021. In situ activation of STING pathway with polymeric SN38 for cancer chemoimmunotherapy. Biomaterials 268, 120542. [DOI] [PubMed] [Google Scholar]
- Zhao L, Gu C, Gan Y, Shao L, Chen H, Zhu H., 2020a. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control. Release 318, 1–15. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ma Z, Wang B, Guan Y, Su XD, Jiang Z., 2020b. Mn(2+) directly activates cGAS and structural analysis suggests Mn(2+) induces a noncanonical catalytic synthesis of 2’3’-cGAMP. Cell Rep 32 (7), 108053. [DOI] [PubMed] [Google Scholar]
- Zheng J, Mo J, Zhu T, Zhuo W, Yi Y, Hu S, Yin J, Zhang W, Zhou H, Liu Z., 2020. Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Mol. Cancer 19 (1), 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Liu J, Faried A, Richard SA, Gao X., 2018. Novel chemically synthesized, alpha-mangostin-loaded nano-particles, enhanced cell death through multiple pathways against malignant glioma. J. Biomed. Nanotechnol 14 (11), 1866–1882. [DOI] [PubMed] [Google Scholar]
- Zhou J, Krishnan N, Jiang Y, Fang RH, Zhang L., 2021. Nanotechnology for virus treatment. Nano Today 36, 101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Kroll AV, Holay M, Fang RH, Zhang L., 2020a. Biomimetic nanotechnology toward personalized vaccines. Adv. Mater 32 (13), 1901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Hou B, Wang D, Sun F, Song R, Shao Q, Wang H, Yu H, Li Y., 2020b. Engineering polymeric prodrug nanoplatform for vaccination immunotherapy of cancer. Nano Lett 20 (6), 4393–4402. [DOI] [PubMed] [Google Scholar]
- Zhou S, Chen W, Cole J, Zhu G., 2020c. Delivery of nucleic acid therapeutics for cancer immunotherapy. Med. Drug Discov 6, 100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Zhang F, Ni Q, Niu G, Chen X., 2017. Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano 11 (3), 2387–2392. [DOI] [PubMed] [Google Scholar]
- Zhu Y, An X, Zhang X, Qiao Y, Zheng T, Li X., 2019. STING: a master regulator in the cancer-immunity cycle. Mol. Cancer 18 (1), 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Gong H, Zhou J, Zhang Q, Gao W, Fang RH, Zhang L., 2020. Targeted gene silencing in vivo by platelet membrane–coated metal-organic framework nanoparticles. Sci. Adv 6 (13), eaaz6108. [DOI] [PMC free article] [PubMed] [Google Scholar]


