Abstract
Since the discovery of insulin 100 years ago, we have seen considerable advances across diabetes therapies. The more recent advent of glucose-responsive automated insulin delivery has started to revolutionise the management of type 1 diabetes in children and adults. Evolution of closed-loop insulin delivery from research to clinical practice has been rapid, and multiple systems are now commercially available. In this review, we summarise key evidence on currently available closed-loop systems and those in development. We comment on dual-hormone and do-it-yourself systems, as well as reviewing clinical evidence in special populations such as very young children, older adults and in pregnancy. We identify future directions for research and barriers to closed-loop adoption, including how these might be addressed to ensure equitable access to this novel therapy.
Keywords: type 1 diabetes, closed loop insulin delivery, artificial pancreas, diabetes technology
1. Introduction
1.1. The need for closed-loop insulin delivery
The ground-breaking discovery of insulin 100 years ago revolutionised treatment of type 1 diabetes, a condition characterised by immune-mediated destruction of insulin-producing pancreatic beta-cells, which was previously universally fatal. While now treatable, this lifelong and incurable condition is associated with significant risks of both short- and long-term complications, high management burden and a reduced quality of life.
Over the last 100 years, there have been significant pharmacological and technological advances in diabetes therapy. Rapid- and long-acting insulin analogues were developed, which allowed more flexible dosing and lowered the risk of hypoglycaemia [1]. In the 1970s insulin pumps were developed, allowing for more precise insulin dosing with adjustable basal rates enabling users to accommodate diurnal changes in insulin needs [2]. Continuous glucose monitoring (CGM) devices, which provide near real-time subcutaneous glucose measurements, were developed in the 2000s and are now so accurate that they can replace finger prick blood glucose measurements [3]. While both insulin pumps and CGM devices have been shown to improve glycaemic control [4] and reduce the risk of diabetic ketoacidosis and severe hypoglycaemia [5], they rely on significant user-input and frequent insulin dosing adjustments. This is challenging as glucose levels are influenced by a variety of factors causing considerable day-to-day variability in insulin needs [6]. As a result, the majority of people with type 1 diabetes do not meet recommended glycaemic targets with these treatment options.[7]
To address this issue, the notion of glucose-responsive closed-loop automated insulin delivery was proposed. Hybrid closed-loop systems utilise a computer program (algorithm) that processes real-time sensor glucose information to automatically adjust insulin delivery via an insulin pump to bring glucose levels to a pre-specified target. While users are still required to administer prandial boluses, the majority of management decisions become automated. Following more than a decade of development and clinical trials, these systems are now available for use in clinical practice, and are beginning to transform the management of type 1 diabetes.
1.2. Closed-loop – how does it work?
While the idea of closed-loop insulin delivery was born in the 1960s [8], it was not possible to move forward from the initial bulky laboratory prototypes until the advent of smaller insulin pumps and reliable and accurate CGM systems. Commercial closed-loop system were preceded by insulin pumps, which automatically suspended insulin delivery when glucose levels were low (low glucose suspend feature) or predicted to be low (PLGS: predictive low glucose suspend feature). These systems were shown to reduce hypoglycaemia, but did not address the issue of hyperglycaemia [9–11].
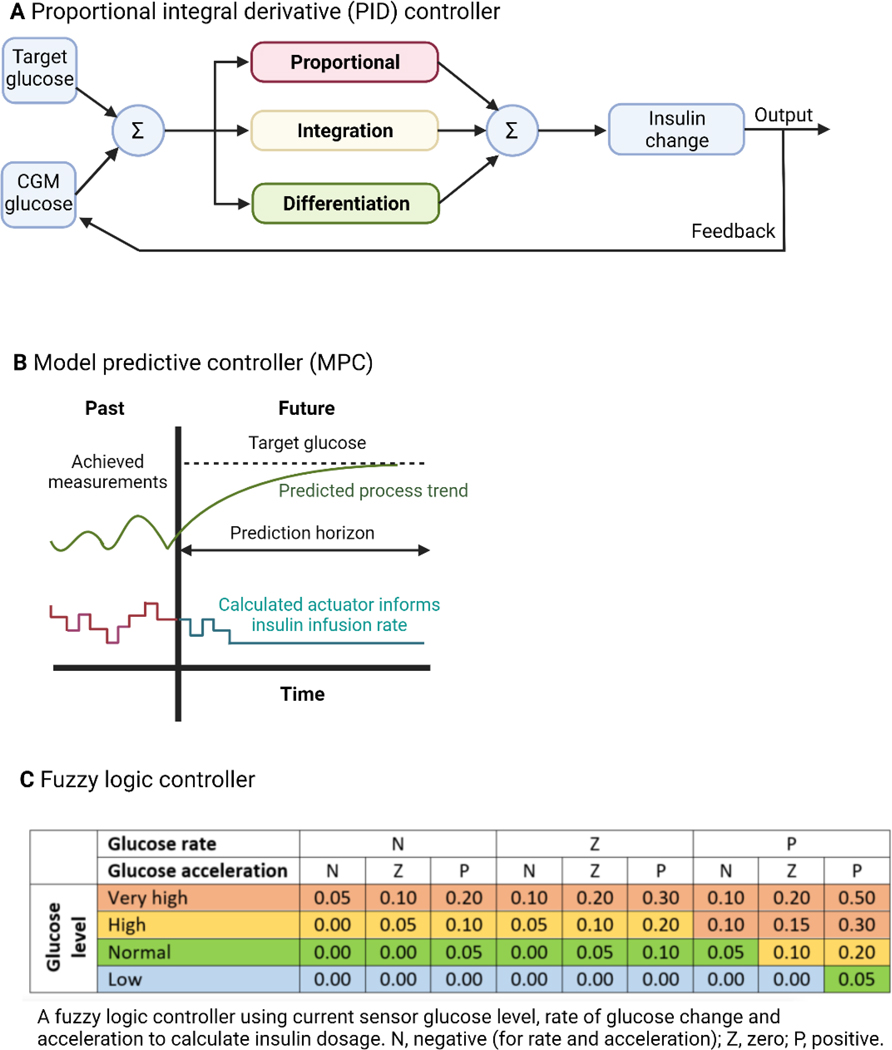
Closed-loop systems are more advanced in that the control algorithm automatically adjusts insulin delivery up and down in real-time based on sensor glucose levels, more closely replicating normal physiology. Three main types of closed-loop algorithms are used: proportional integral derivative (PID) controllers, model predictive control (MPC) algorithms, and the fuzzy logic approach [12]. PID controllers direct insulin doses based on the difference from target glucose at the current time point (proportional component), the rate of change in sensor glucose over time (derivative component) and the area under the curve between measured and target glucose levels including memory of prior controller-induced changes (integral component). All three components are weighted with a multiplier, which may be pre-determined or adjusted over time. All components are added together to inform modification of insulin delivery, thereby changing future sensor glucose values (Fig. 1). Most PID controllers also include active insulin estimates to restrict maximum insulin delivery and reduce the risk of hypoglycaemia. MPC algorithms predict glucose levels and simultaneously adjust insulin delivery whilst accommodating insulin absorption delays, active insulin, as well as diurnal and post-prandial variations in glucose levels. Predictions are made for a future period, and updated every 5–15 minutes with new information on sensor glucose levels, insulin variables, carbohydrate intake and other available data, such as exercise indicated by the user (Fig. 1). The MPC algorithm includes control strategies to minimise hypo- and hyperglycaemia. The fuzzy logic approach adjusts insulin delivery by applying approximate rules imitating the line of reasoning of diabetes practitioners. Sensor glucose data, including rate of change and acceleration, feed into the controller, and an insulin infusion rate is then determined (Fig. 1).
Figure 1. Commercially available hybrid closed-loop systems.
Control algorithms for closed-loop insulin delivery. Panel A is a diagram of a simple proportional integral derivative (PID) controller (adapted from Lal et al [79]); Panel B is a graphical representation of a simplified model predictive controller (MPC) (adapted from Wang et al [80]); Panel C is an example of a fuzzy logic controller using current sensor glucose level, rate of change and acceleration in sensor glucose (adapted from Mauseth et al [81]).
At present, fully closed-loop systems are limited by the pharmacokinetics and pharmacodynamics of available insulin analogues, including inhaled insulin,[13] as well as CGM accuracy in the extreme hypo- and hyperglycaemic ranges and during exercise. All available systems are therefore hybrid closed-loop systems where user-initiated prandial bolusing is required, but insulin delivery is automated at night-time and between meals.
2. Closed-loop insulin delivery systems in clinical research
2.1. Safety and efficacy evidence
Initial closed-loop studies assessed safety and feasibility in the inpatient setting only. This was followed by supervised camp studies and at-home studies at night-time only, eventually evolving to larger randomised controlled trials of 6 months or more in the unsupervised home setting. The most recent meta-analysis of outpatient randomised controlled trials of hybrid closed-loop insulin delivery in children and adults from 2018 analysed 41 studies, encompassing more than 1,000 people with type 1 diabetes [14]. Use of earlier hybrid closed-loop systems was associated with a significant increase in time in target glucose range 3.9 to 10.0mmol/L of 140 minutes per day (9.6 percentage point improvement) compared to control. Importantly, time in hypoglycaemia was reduced by 1.5 percentage points (approximately 20 minutes per day) with the use of closed-loop systems compared to control, with low incidence of severe hypoglycaemia events. Studies of more than 8 weeks’ duration demonstrated a reduction in HbA1c of around 0.3% (3.3mmol/mol), which is significant in conjunction with a reduction in hypoglycaemia in a cohort with low HbA1c at baseline. Hybrid closed-loop insulin delivery has also been shown to decrease hypoglycaemia in those with impaired hypoglycaemia awareness and during exercise.[15–17]
However, comparisons of efficacy between hybrid closed-loop systems across different studies should be interpreted cautiously given differences in study design, frequency of study visits, level of supervision, and baseline characteristics. Ideally, studies would include a higher proportion of technology-naïve participants with a diverse range of ethnic and socioeconomic backgrounds to improve generalisability of findings.
2.2. Special populations
Innovative methods of insulin delivery are particularly needed in vulnerable populations, where achieving optimal glycaemic control is especially challenging. This includes very young children, older adults and those who are pregnant.
Very young children have high variability of insulin requirements [6] and unpredictable eating and activity patterns, coupled with poor hypoglycaemia awareness [18]. Recently, data from a 4-month randomised trial showed significant improvements in glycaemic control with hybrid closed-loop insulin delivery compared to the current gold standard sensor-augmented pump therapy in very young children [20]. Only one closed-loop system is currently licensed for use in this age-group (CamAPS FX, CamDiab, Cambridge, UK). Limitations include the management of significant post-prandial hyperglycaemic excursions resulting from delayed bolusing, which is common in this age-group, mainly hampered by the pharmacology of current insulins [21]. Many young children have very low total daily insulin needs leading to higher variability of absorption with such small volumes [22].
Whilst older adults tend to have better glycaemic control than younger adults and children [7], they are at higher risk of hypoglycaemia unawareness and adverse outcomes related to severe hypoglycaemia, such as falls, fractures, seizures, cardiac arrhythmias and cognitive impairment [23]. As a result, treatment guidelines aim to minimise hypoglycaemia, accepting the trade-off in terms of higher HbA1c. The use of CGM in this population reduces time in hypoglycaemia and severe hypoglycaemia events, but the majority of older adults do not achieve the recommended <1% time in hypoglycaemia target. In a 4-month randomised crossover trial in adults aged 60 years and over, outcomes favoured closed-loop across all key CGM metrics, with a 6 percentage point increase in time in range, albeit with no significant difference in HbA1c [24].
Pregnancy is a particularly challenging time for those with type 1 diabetes as it is associated with a higher risk of maternal and neonatal complications [25] particularly when glycaemic control is suboptimal [26].The aim of management during pregnancy is to reduce fetal exposure to hyperglycaemia, whilst minimising maternal hypoglycaemia, necessitating tighter glycaemic targets. In a small 4-week randomised crossover study comparing closed-loop insulin delivery to sensor-augmented pump therapy in pregnancy, there was a significant reduction in hypoglycaemia of 1.1 percentage points, with a similar time in target range 3.5 to 7.8mmol/L [27]. Larger pivotal studies of closed-loop in pregnancy are under way (NCT04938557). Only one hybrid closed-loop system, CamAPS FX, is currently licensed for use in pregnancy.
2.3. Dual-hormone systems
Alongside insulin, the pancreas also produces glucagon, which is secreted in response to falling glucose levels. The glucagon response is often impaired in people with type 1 diabetes, so even if insulin delivery is suspended by a closed-loop system, hypoglycaemia may occur. Dual-hormone systems, which additionally deliver subcutaneous glucagon when hypoglycaemia is detected or predicted, may further protect from hypoglycaemia whilst potentially allowing for more aggressive insulin delivery in response to hyperglycaemia [28].There are multiple barriers to the use of dual-hormone systems, including the need for a second or dual chamber pump, a lack of stable formulations of glucagon at room temperature approved for chronic subcutaneous delivery, and the risk of gastrointestinal side-effects. Therefore, dual-hormone closed-loop system studies have been of shorter duration and in smaller cohorts than insulin-only closed-loop systems. The longest study in the remotely monitored home setting studied 43 participants over 11 days, comparing dual-hormone closed-loop therapy with conventional or sensor-augmented pump therapy. Results were promising with a reduction of 1.1mmol/L in mean sensor glucose (7.8mmol/L vs 9.0mmol/L), an increase in time in range (78% vs 62%), and less time in hypoglycaemia (0.6% vs 1.9%) favouring the dual-hormone system [29]. Two shorter studies comparing dual-hormone to insulin-only closed-loop systems showed a reduction in hypoglycaemia [30, 31], although one system achieved this at the expense of increased time in hyperglycaemia [31].
Another pancreatic hormone largely absent in people with type 1 diabetes is amylin, which is secreted alongside insulin and delays gastric emptying, thereby reducing postprandial hyperglycaemic excursions. Its synthetic analogue pramlintide has been trialled in inpatient dual-hormone closed-loop studies, and results have been promising with an increase in time in range compared to insulin-only systems [32]. Limitations of pramlintide dual-hormone systems are similar to those using glucagon. However, co-formulations with insulin are in development [33] and this may aid development of a fully closed-loop system where pre-meal bolusing is no longer required [34].
2.3. Other adjunctive therapies
Alongside glucagon and pramlintide, other adjunctive therapies have been introduced to optimise glycaemic control, and potentially offer additional therapeutic benefits such as renal protection. Glucagon-like peptide-1 (GLP-1) is an incretin hormone that increases satiety, slows gastric emptying and suppresses glucagon release, similar to pramlintide. Synthetic GLP-1 receptor agonists are used extensively in the management of type 2 diabetes. Studies in type 1 diabetes have shown modest improvement in glycaemic control, often accompanied by significant weight loss, however offset by gastrointestinal side-effects. [35, 36] Data on GLP-1 as an adjuvant in closed-loop therapy is limited, with a handful of small studies showing significant reductions in post-prandial hyperglycaemia.[37] However, given their side-effect profile, the benefits of GLP-1 use with closed-loop therapy remain unclear.
Another recent add-on therapy are Sodium-glucose co-transporter (SGLT2) inhibitors. SGLT2s lower plasma glucose by blocking renal reabsorption and increasing renal excretion of glucose in an insulin-independent manner. A large randomised trial in type 1 diabetes showed improvements in HbA1c, blood pressure and weight loss, however demonstrated an increased risk of diabetic ketoacidosis (DKA).[38] Similar to GLP-1 agonists, evidence for the use of SGLT2 inhibitors as an adjunct to closed-loop therapy is limited,[37] and the increased risk of DKA remains a concern.
3. Closed-loop insulin delivery systems in clinical practice
3.1. Commercially available closed-loop systems
There are five commercially available hybrid closed-loop systems licensed for use in children and adults, with varying minimum age for use. Figure 1 shows currently available systems, while Table 1 shows key randomised clinical studies.
Table 1.
Key randomised controlled trials of closed-loop insulin delivery using commercialised systems.
| Closed-loop system | Type of study | Comparator | Duration | Population | Baseline HbA1c | Glycaemic outcomes | Closed-loop usage | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| TIR | TBR | HbA1c | |||||||
|
| |||||||||
| 670G HCL [39] | Randomised, parallel | Usual care (MDI or pump) | 6 months | n=120 adults | 58mmol/mol [7.5%] | ↑ 15 percentage points to 70% [vs 55% control] | ↓ to 1.8% [vs 3.8% control] | ↓ to 7.0% [vs 7.4% control] | Median 89% |
| 670G HCL [41] | Randomised, parallel | Usual care (MDI or pump) | 6 months | n=172 children, adolescents and young adults (12–25 years) |
64mmol/mol [8.0%] |
↑ 6.7 percentage points to 63% [vs 56% control] | ↓ to 2.2% [vs 4.1% control] | ↓ to 7.5% [vs 7.6% control] | Not stated |
| 780G AHCL [42] | Randomised, crossover | 670G HCL | 3 months | n=113 adolescents and young adults (14–29 years) |
63mmol/mol [7.9%] |
↑to 67% [vs 63% 670G HCL] | ↔at 2.1% [vs 2.1% 670G HCL] | ↓ to 7.4% [vs 7.6% 670G HCL] | Median 75% [HCL] 86% [AHCL] |
| 780G AHCL [44] | Randomised, crossover | 670G PLGM | 4 weeks | n=59 children and adults (7–80 years) |
60mmol/mol [7.6%] |
↑ 13 percentage points to 70% [vs 60% PLGM] | ↓ to 2.1% [vs 2.5% PLGM] | Not measured due to short study duration | Mean 96% |
|
| |||||||||
| Control-IQ [47] | Randomised, parallel | SAP therapy | 6 months | n=168 (14+ years) |
57mmol/molbr] [7.4%] |
↑ 11 percentage points to 70% [vs 59% SAP] | ↓ to 1.6% [vs 2.3% SAP] | ↓ to 7.1% [vs 7.4% SAP] | Median 90% |
| Control-IQ [48] | Randomised, parallel | SAP therapy | 4 months | n=101 children (6–13 years) |
60 – 63mmol/mol [7.6 – 7.9%] |
↑ 11 percentage points to 67% [vs 55% SAP] | Similar between groups [1.6% vs 1.8% SAP] | ↓ to 7.0% [vs 7.6% SAP] but this change was not statistically significant | Median 93% |
|
| |||||||||
| Cambridge closed-loop [CamAPS FX] [56] | Randomised, crossover | HCL with faster insulin aspart (Fiasp) | 2 months | n=25 adults | 57mmol/mol [7.4%] | Not different between HCL with fiasp vs HCL with aspart [75% for both] | ↓ in HCL with fiasp vs aspart [2.4% vs 2.9%] | HbA1c not measured due to short study duration | Median 95% (Fiasp) and 96% (Aspart) |
| Cambridge closed-loop [CamAPS FX] [55] | Randomised, parallel | Insulin pump therapy | 6 months | n=46 children (6–18 years) |
67 to 68mmol/mol [8.2 – 8.3%] |
↑ 12 percentage points to 63% [vs 49% control] | Similar between groups [10.8% vs 6.3% control] | ↓ to 6.8% [vs 7.9% control] | Median 93% |
| Cambridge closed-loop [CamAPS FX] [20] |
Randomised, crossover | SAP therapy | 4 months | n=74 children (1–7 years) |
57mmol/mol [7.3%] |
↑ 9 percentage points to 72% (vs 63% control] | Similar between groups [4.9% vs 4.5% control] | ↓ to 6.6% [vs 7.0% control] | Median 95% |
|
| |||||||||
| DBLG1 [57] | Randomised, crossover | SAP therapy | 3 months | n=68 adults | 59mmol/mol [7.6%] | ↑ 9 percentage points to 69% [vs 59% SAP] | ↓ to 2.0% [vs 4.3% SAP] | HbA1c significantly decreased in HCL, absolute values not available | Median 84% |
| DBLG1 [59] | Randomised, crossover, non-inferiority | SAP therapy | 6 weeks | n=17 children (6–12 years) |
Not stated | ↑ to 66% [vs 59% SAP] | ↓ to 2.6% [vs 5.2% SAP] | HbA1c not measured due to short study duration | Not stated |
HCL – hybrid closed-loop. AHCL – advanced hybrid closed-loop. PLGM – predictive low glucose management. SAP – sensor-augmented pump. TIR – time in range (3.9–10.0mmol/L). TBR – time below range (<3.9mmol/L). HbA1c – glycated haemoglobin.
Medtronic 670G and 780G
The Medtronic 670G Hybrid Closed-loop System (Medtronic, Northridge, CA, USA) was the first commercially available closed-loop system in the USA and Europe, and is licensed from age 7 years. It comprises the 670G insulin pump with an embedded PID algorithm, communicating wirelessly with the Guardian sensor 3, which requires a minimum of 4–6 finger prick calibrations per day to remain in Auto Mode. Additional finger pricks may be required as this sensor is not licensed to replace finger prick blood glucose measurements. Accurate basal rates, insulin-to-carbohydrate ratios, insulin sensitivity setting, and active insulin time are required for set-up and to modulate insulin delivery appropriately. Basal insulin delivery is automatically determined by the algorithm and adjusted every 5 minutes according to sensor glucose values. Algorithm learning is based on total daily insulin dose of the previous days. The algorithm is treat-to-target, with a fixed target of 6.7mmol/L. An optional activity mode temporarily raises the target to 8.3mmol/L.
Initial studies were non-randomised single-arm studies, but recently a randomised controlled study compared the 670G system to usual care (either multiple daily injections or pump therapy without CGM) over 6 months in 120 adults with type 1 diabetes [39]. Time in target range increased by 15 percentage points in the closed-loop group compared to usual care (70% vs 55%) with a significant reduction in hypoglycaemia <3.9mmol/L (1.8% vs 3.8%). Closed-loop was used a median 89% of the time. There were a high number of severe hypoglycaemia events (8 in closed-loop and 7 in control group), but these were similar between interventions. [40]A randomised parallel study comparing the 670G system to usual care with insulin pump or multiple daily injection (MDI) therapy in 172 children, adolescents and young adults aged 12 to 25 years over 6 months showed a modest improvement in time in range of 7 percentage points with a significant reduction in hypoglycaemia <3.9mmol/L (2.2% vs 4.1%). [41] No severe hypoglycaemia or DKA events were reported.
The 780G Advanced Hybrid Closed-Loop (AHCL) is the next-generation system, with an enhanced PID algorithm with added fuzzy logic component embedded on a 780G insulin pump, using the same glucose sensor, but requiring fewer finger prick calibrations per day. This system has customisable glucose targets as well as giving automated correction boluses. A 3-month crossover study compared AHCL to the 670G HCL system in 113 adolescents and young adults with type 1 diabetes [42]. Glycaemic outcomes favoured the AHCL system, although time in hypoglycaemia was similar between interventions. Importantly, closed-loop usage was higher with AHCL, by addressing the issue of frequent closed-loop exits hampering real-life usability of the 670G HCL system [43]. A shorter randomised crossover study in a larger age range (7 to 80 years) compared the AHCL system to 670G with predictive low glucose management (PLGM) over 4 weeks [44]. Time in target range was higher (70% vs 55%) and time in hypoglycaemia lower in the AHCL period, with high mean closed-loop usage of 96%.
Tandem Control-IQ
The second hybrid closed-loop system to become commercially available in the USA and Europe is Tandem’s Control-IQ system (Tandem Diabetes Care, San Diego, CA, USA), licensed from age 6 years. It comprises the t:slim X2 insulin pump with embedded MPC algorithm, paired with a Dexcom G6 (Dexcom, San Diego, CA, USA) CGM. The Dexcom G6 sensor is factory-calibrated, requires no finger prick calibrations and is licensed to be used for diabetes treatment decisions. Similarly to the Medtronic systems, it requires accurate basal rates, insulin-to-carbohydrate ratios, and insulin sensitivity settings for set-up and to modulate insulin delivery appropriately. Active insulin time is non-adjustable at 5 hours. The treat-to-range (6.2 to 8.9mmol/L, non-adjustable) algorithm does not incorporate adaptive learning. The system delivers automated correction boluses and users can choose from an optional activity mode and a ‘sleep mode’, which lowers the target range to 6.2 to 6.7mmol/L.
Initial single-arm pilot studies demonstrated safety of the algorithm.[45, 46] The pivotal trial remains the largest and longest closed-loop trial to date, with 168 participants aged 14 to 71 years with type 1 diabetes, randomised to either Control-IQ hybrid closed-loop or sensor-augmented pump (SAP) therapy for 6 months [47]. The closed-loop group spent 2.6 extra hours per day in the target glucose range compared to SAP (time in target range 71% vs 59%) with significant reductions in time in hypoglycaemia and HbA1c favouring the closed-loop group. There were no severe hypoglycaemia or diabetic ketoacidosis events, and median closed-loop usage was high at 90%. A 4-month study randomised parallel study in children aged 6 to 13 years also comparing Control-IQ hybrid closed-loop to SAP therapy showed an increase in time in target range, but no significant difference in time in hypoglycaemia or HbA1c between groups, with median closed-loop usage of 93% [48]. Safety of Control-IQ has been evaluated in very young children aged 2 to 5 years in a short, single-arm, non-randomised study over 3 days, with no severe hypoglycaemia or DKA events reported [49]. However, longer randomised controlled studies are needed to evaluate efficacy of the system in this population.
CamAPS FX
In contrast to the pump-integrated Medtronic and Tandem systems, CamAPS FX (CamDiab, Cambridge, UK), which uses the control algorithm developed at the University of Cambridge, was the first regulatory approved inter-operable hybrid closed-loop app and is licensed in Europe from age 1 year. The algorithm currently runs on an unlocked Android smartphone, and communicates wirelessly with the Dana-i or Dana RS pumps (Sooil Development, Seoul, Korea) and the Dexcom G6 CGM, with the intention to connect to other insulin pumps and CGM devices in the future. Set-up requires total daily insulin dose, weight and insulin-to-carbohydrate ratios, while active insulin time and insulin sensitivity factors are calculated and adjusted automatically. The treat-to-target MPC algorithm is highly adaptive, taking into account total daily insulin as well as postprandial and diurnal insulin requirements. The glucose target is user-adjustable in 0.1mmol/L increments from 4.4 to 11.1mmol/L. There are optional activity and ‘boost’ modes, which make the make the algorithm more or less aggressive for the set time-period.
Studies have been conducted across a wide range of populations, including very young children, adolescents with suboptimal control and in pregnancy. Following early randomized inpatient studies [50, 51] and shorter studies in the home setting,[52, 53] the pivotal randomised parallel study included children, adolescents and adults with suboptimal glycaemic control (HbA1c >58mmol/mol [7.5%]) and compared Cambridge hybrid closed-loop algorithm to SAP therapy over 3 months. Closed-loop was beneficial across all glycaemic metrics, with an increase in time in target range of 11 percentage points [65% closed-loop vs 54% SAP] and a significant reduction in hypoglycaemia and HbA1c [54]. A prototype of the current CamAPS FX system using the Cambridge algorithm, but different hardware, was used with a median 71% closed-loop usage. In a more recent study comparing hybrid closed-loop with insulin pump therapy with or without glucose sensor in 133 children aged 6 to 18 years with suboptimal HbA1c (>7.0% [53mmol/mol]), those using CamAPS FX hybrid closed-loop with current hardware had a 15 percentage point improvement in time in target range, and a 1.1% (11.5mmol/mol) reduction in HbA1c at 6 months.[55] The Cambridge algorithm is the only closed-loop algorithm to have been evaluated in a longer randomised trial in very young children aged 1 to 7 years [19]. The 4-month crossover study compared hybrid closed-loop insulin delivery with sensor-augmented pump therapy in 74 very young children. Time in target range was consistent with closed-loop studies in older children and adults, and was 9 percentage points higher [72% closed-loop vs 63% SAP] in the closed-loop period, without increasing time in hypoglycaemia [20]. The CamAPS FX system has also been trialled with newer insulins. In a 2-month randomised crossover studies, 24 adults used hybrid closed-loop with faster insulin aspart and hybrid closed-loop with standard aspart in random order [56]. While time in range was consistent with previous studies at 75%, it was not different between groups. However, time in hypoglycaemia was significantly lower when hybrid closed-loop was used with faster insulin Aspart. Median closed-loop usage was high at 95%.
Diabeloop
The Diabeloop system uses an MPC algorithm known as DBLG1 (Diabeloop, Grenoble, France), which resides on a dedicated handset, and is commercially available in Europe for adults only. Similar to CamAPS FX, the system is inter-operable, currently used with the Accu-Check Insight insulin pump (Roche Diabetes Care, Basel, Switzerland) and Dexcom G6 CGM, but has been used with the Kaleido insulin patch-pump (ViCentra, Utrecht, Netherlands) in clinical trials. Set-up requires only total daily insulin dose, weight and insulin-to-carbohydrate ratios. The algorithm is adaptive, and includes five user-adjustable settings: the personal glucose target (pre-set at 6.1mmol/L), the hypoglycaemia threshold (pre-set at 3.9mmol/L), the reactivity in the hyperglycaemic range, the reactivity in the normoglycaemic range and the prandial insulin dose. Additionally there is an optional activity mode, which temporarily increases the glucose target.
In the pivotal trial, DBLG1 hybrid closed-loop was compared to SAP therapy in 68 adults over 3 months in a randomised crossover design [57]. Time in target range increased by 9 percentage points to 69% compared to 59% in the SAP group, and there was a significant reduction in time in hypoglycaemia (2.0% in closed-loop vs 4.3% in SAP group). Median closed-loop usage was 84%. Importantly, this study was analysed on a modified intention-to-treat basis and did not include 5 participants who withdrew. A longer, single-arm, non-randomised study showed the DLBG1 system was safe in 25 adults over 6 months, with no reported severe hypoglycaemia or DKA events [58]. Recently, results of a 6-week randomised crossover study comparing DBLG1 hybrid closed-loop to sensor-augmented pump therapy in 17 children aged 6 to 12 years, showed improvements in time in range and a reduction in time in hypoglycaemia.[59]
3.2. Closed-loop systems in development
Insulet’s Omnipod 5 hybrid closed-loop system (Insulet, Acton, MA, USA) has undergone pivotal trials, and is awaiting regulatory approval. The MPC algorithm resides on an individual screenless pod, which communicates wirelessly with the compatible glucose sensor Dexcom G6. User interaction is facilitated via an app on a locked-down Android phone, and/or the system-specific handheld receiver device. In the pivotal single-arm, non-randomised study, 112 children (age 6 years and over) and 128 adolescents and adults used the closed-loop system for 3 months [60]. There were two severe hypoglycaemia events not attributable to closed-loop in adults, one in a child and one DKA event due to an infusion site failure in a child. Median closed-loop usage was 96 to 97%. The system was recently evaluated in 80 very young children (age 2–5 years) in a non-randomised single-arm study with the same design, and no severe hypoglycaemia or DKA events were reported [61].
The Beta Bionics insulin-only iLet hybrid closed-loop system is based on an MPC algorithm, and comprises the iLet Bionic Pancreas System (Beta Bionics, Boston, MA, USA) and Dexcom G6 CGM. The system is in the pivotal trial stage (NCT04200313). Tidepool Loop is developing an interoperable automated glycaemic controller (iAGC), an app-based algorithm modelled on the do-it-yourself (DIY) diabetes community’s original Loop app, which can pair with an alternate controller-enabled (ACE) insulin pump and integrated CGM (iCGM). The Loop DIY automated insulin delivery system was evaluated in a recent observational study [62] and is awaiting US regulatory approval.
3.3. Do-It-Yourself (DIY) systems
In response to what was perceived as slow progress in commercial closed-loop system development, a community of people with type 1 diabetes and their families started to work together online to promote the development of open source artificial pancreas systems in 2013 using the hashtag ‘#WeAreNotWaiting’. The three main DIY hybrid closed-loop systems, OpenAPS, AndroidAPS and Loop, use open-source software that has no regulatory oversight or approval. DIY systems are used by around 1,500 people with type 1 diabetes worldwide [63]. User-driven optimisation is flexible and frequent, while systems are available at low-cost, making them an attractive option for people with type 1 diabetes. However, users need to have the know-how and skills to build and maintain their own systems with little assistance from healthcare professionals, who may struggle to support the use of these unregulated systems. Observational studies show improvements in glycaemic control, but no randomised controlled clinical trial data exists at present [63]. A 6-month randomised controlled parallel design study using a locked version of OpenAPS is currently under way in New Zealand, with results expected mid-2022.[64]
3.4. Real-life and qualitative data
Several high-quality qualitative studies have explored the impact of closed-loop insulin delivery on quality of life in children, adolescents, adults and in pregnancy [65–69]. Reported benefits of closed-loop insulin delivery across all populations include more flexible eating, feeling more ‘normal’ and being able to participate in a wider range of activities, improved sleep, and reduced burden of diabetes. Device burden, connectivity problems, high alarm frequency and lack of inter-operability were all identified as limitations of current closed-loop systems. As systems have only recently become commercially available, most qualitative studies include participants involved in closed-loop studies, possibly limiting generalisability.
Observational real-world studies have mirrored some of these findings. Two prospective observational studies of children, adolescents and adults using the 670G HCL system showed a steady decline of closed-loop use over time, with highest discontinuation rates among children and adolescents [43, 70]. The need for regular sensor calibrations and frequent closed-loop exits were the main reasons for discontinuation, underlining that addressing usability issues is key to optimising closed-loop usage and glycaemic outcomes. In a more recent retrospective real-world study, adolescent and adult users of Control-IQ, which uses a factory-calibrated sensor and had higher closed-loop usage in clinical trials, used closed-loop a median 94% of the time over 12 months and reported a positive impact on their quality of life and sleep quality after starting closed-loop insulin delivery. [71] Retrospective data on real-world use of the 780G AHCL system showed similarly high closed-loop usage, albeit over a shorter time period of mean 54 days. [72]
Limitations of hybrid closed-loop insulin delivery systems in the real-world setting are their ability to cope with stress, illness, exercise and missed meal boluses. During periods of stress or mild illness many systems are able to cope. Some systems, such as CamAPS FX, include a user-initiated ‘Boost’ function, which can be used during times of higher insulin needs.[73] At present, clinical guidance is to revert to manual mode, i.e. stop closed-loop insulin delivery, if ketones are present as current systems may not be able to cope with the extremes of glucose levels and higher insulin requirements. Additionally, significant and prolonged hyperglycaemia with ketosis is frequently indicative of a mechanical insulin delivery problem, which cannot be resolved by the algorithm on its own. With regards to physical activity, the majority of commercially available systems now include an ‘exercise mode’, where users are able to announce exercise and the algorithm responds by reducing insulin delivery and/or increasing the glucose target.[21, 73] However, users are still required to plan for exercise and intake of small amounts of carbohydrate during activity may still be required to prevent hypoglycaemia.[12] Lastly, to maximise benefits of hybrid closed-loop therapy, pre-meal bolusing and accurate carbohydrate counting remains important, but adds to management burden.
4. Looking ahead
4.1. Fully closed-loop systems
While hybrid closed-loop systems improve glycaemic control and improve quality of life, challenges remain, particularly in the daytime when the necessity for increased user interaction around mealtimes and exercise still creates burden. A fully closed-loop system would alleviate these issues, but the pharmacokinetics and pharmacodynamics of available insulins and limitations to current dual-hormone systems are barriers to implementation. Studies of fully closed-loop insulin-only systems have shown that time in target range is lower without meal announcements [74] and risk of delayed post-prandial hypoglycaemia may be higher, even when used with ultra-rapid acting insulin analogues [75, 76].
4.2. Healthcare provider training
Healthcare providers are key gatekeepers of advanced diabetes technology, so appropriate education and training is key in facilitating rollout and access to closed-loop systems. Increased availability of online training materials and virtual workshops is improving access to training, but providing sufficient working hour time to complete relevant modules is vital. Given the increasing number of available closed-loop systems, tools utilising practical frameworks that identify key concepts for each closed-loop system, such as the CARES paradigm [77], will help healthcare professionals in setting appropriate expectations of system capabilities, and when adjusting settings for treatment optimisation in the clinical setting. Closed-loop users require less healthcare professional input than standard pump users following an initial adjustment period [78], reducing burden on clinical teams in the longer-term.
4.3. Equitable access to closed-loop therapy
The biggest barrier to closed-loop therapy for most people with type 1 diabetes is access. Due to its novelty, insurance coverage and reimbursement is poorly established, and CGM and insulin pumps are too expensive for many to self-fund. Already this is leading to growing disparities in diabetes management, particularly for those from lower socio-economic backgrounds. Increasingly studies show that those with suboptimal glycaemic control stand to benefit most from closed-loop [70], and that healthcare providers are inappropriately biased towards those with higher educational attainment in terms of candidacy for closed-loop therapy [78]. Robust health-economic analyses need to be incorporated into clinical and real-world studies to show long-term cost-effectiveness, ultimately aiding reimbursement for this novel therapy.
5. Conclusion
The last 100 years have seen enormous advances across diabetes therapies, and the advent of diabetes technologies has revolutionised the management of type 1 diabetes. It is clear that closed-loop insulin delivery improves glycaemic outcomes in the clinical trial and real-world settings across different populations, whilst importantly reducing burden and improving quality of life. The speed of innovations is challenging for healthcare professionals in terms of keeping up to date with training and providing best possible support to people with diabetes, who will have an increasing variety of commercially available closed-loop systems to choose from. The focus of future developments should be to further reduce burden whilst retaining, and ideally optimising efficacy. Adequate reimbursement strategies are vital to ensure equitable access to closed-loop therapy across the diabetes population.
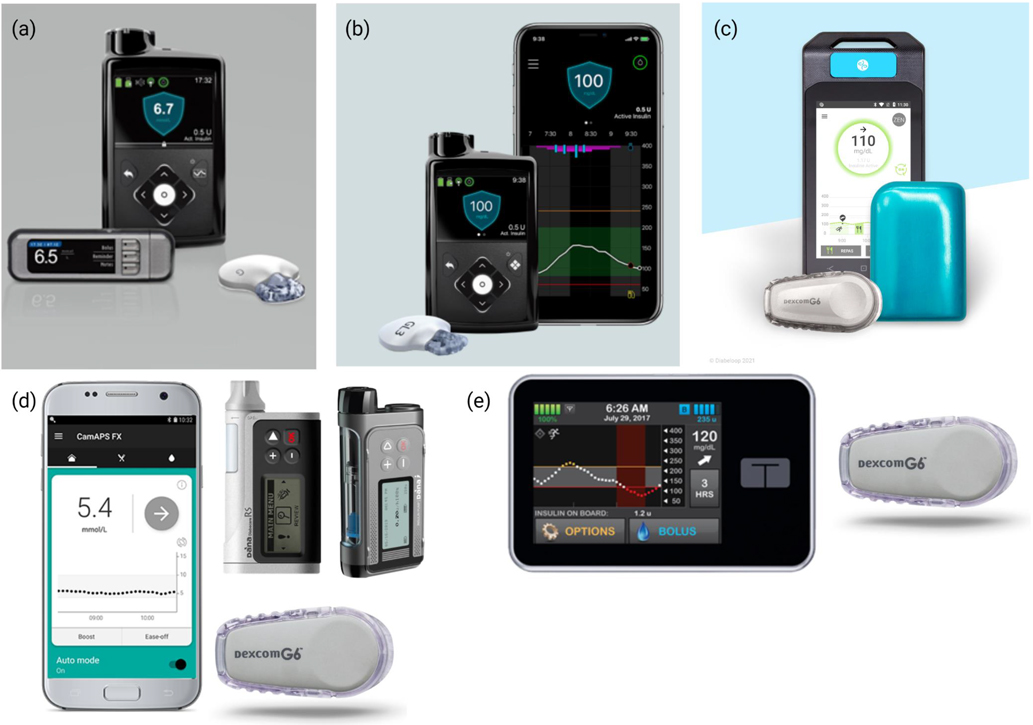
Figure 2. Commercially available hybrid closed-loop systems.
(a) Medtronic 670G Hybrid Closed Loop System with Guardian Link 3 transmitter and the COUNTOUR NEXT LINK blood glucose meter and (b) Medtronic 780G Advanced Hybrid Closed Loop System (reproduced with permission of Medtronic, Inc.) (c) DBLG1 hybrid closed-loop system with Kaleido insulin patch-pump and Dexcom G6 CGM (Dexcom, San Diego, CA, USA) (reproduced with permission of Diabeloop SA) (d) CamAPS FX algorithm with Dexcom G6 sensor and Dana Diabecare RS and Dana-i insulin pumps (Sooil Development, Seoul, Korea) (reproduced with permission of CamDiab Ltd) (e) Tandem t:slim X2 insulin pump (Tandem Diabetes Care, San Diego, CA, USA) paired with a Dexcom G6 Sensor (Dexcom, San Diego, CA, USA) (reproduced with permission).
Highlights.
Type 1 diabetes is a lifelong condition with high management burden
Glucose-responsive automated insulin delivery improves glycaemic outcomes and quality of life
Multiple closed-loop systems are commercially available, offering increased choice for users
Working towards a fully closed-loop system and addressing barriers to adoption should be the focus of future research
Role of the funding source:
Supported by the National Institute of Health Research Cambridge Biomedical Research Centre; Efficacy and Mechanism Evaluation National Institute for Health Research (14/23/09); National Institute of Diabetes and Digestive and Kidney Diseases (UC4DK108520); JDRF; The Leona M. & Harry B. Helmsley Trust (#2016PG-T1D046); National Institute for Health Research Cambridge Biomedical Research Centre; and European Union Horizon 2020 research and innovation programme (grant agreement no. 731560).
The views expressed are those of the authors and not necessarily those of the funders. No sponsor had any role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Footnotes
Declaration of Interest: RH reports having received speaker honoraria from Eli Lilly and Novo Nordisk, serving on advisory panel for Eli Lilly and Novo Nordisk; receiving personal fees from BBraun and Abbott Diabetes Care; patents related to closed-loop insulin delivery, shareholder and director at CamDiab. JW has no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Drucker DJ. Transforming type 1 diabetes: the next wave of innovation. Diabetologia. 2021;64:1059–65. [DOI] [PubMed] [Google Scholar]
- [2].Pickup JC. Is insulin pump therapy effective in Type 1 diabetes? Diabet Med. 2019;36:269–78. [DOI] [PubMed] [Google Scholar]
- [3].Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Thabit H, Prabhu JN, Mubita W, Fullwood C, Azmi S, Urwin A, et al. Use of factory-calibrated real-time continuous glucose monitoring improves time in target and HbA1c in a multiethnic cohort of adolescents and young adults with type 1 diabetes: The MILLENNIALS study. Diabetes Care. 2020;43:2537–43. [DOI] [PubMed] [Google Scholar]
- [5].Tauschmann M, Hermann JM, Freiberg C, Papsch M, Thon A, Heidtmann B, et al. Reduction in diabetic ketoacidosis and severe hypoglycemia in pediatric type 1 diabetes during the first year of continuous glucose monitoring: A multicenter analysis of 3,553 subjects from the DPV Registry. Diabetes Care. 2020;43:e40–e2. [DOI] [PubMed] [Google Scholar]
- [6].Dovc K, Boughton C, Tauschmann M, Thabit H, Bally L, Allen JM, et al. Young children have higher variability of insulin requirements: observations during hybrid closed-loop insulin delivery. Diabetes Care. 2019;42:1344–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther. 2019;21:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Boughton CK, Hovorka R. Is an artificial pancreas (closed-loop system) for Type 1 diabetes effective? Diabet Med. 2019;36:279–86. [DOI] [PubMed] [Google Scholar]
- [9].Forlenza GP, Li Z, Buckingham BA, Pinsker JE, Cengiz E, Wadwa RP, et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care. 2018;41:2155–61. [DOI] [PubMed] [Google Scholar]
- [10].Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369:224–32. [DOI] [PubMed] [Google Scholar]
- [11].Battelino T, Nimri R, Dovc K, Phillip M, Bratina N. Prevention of hypoglycemia with predictive low glucose insulin suspension in children with type 1 diabetes: A randomized controlled trial. Diabetes Care. 2017;40:764–70. [DOI] [PubMed] [Google Scholar]
- [12].Boughton CK, Hovorka R. New closed-loop insulin systems. Diabetologia. 2021;64:1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Galderisi A, Cohen N, Calhoun P, Kraemer K, Breton M, Weinzimer S, et al. Effect of Afrezza on glucose dynamics during HCL treatment. Diabetes Care. 2020;43:2146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee MH, Vogrin S, Paldus B, Jayawardene D, Jones HM, McAuley SA, et al. Glucose and counterregulatory responses to exercise in adults with type 1 diabetes and impaired awareness of hypoglycemia using closed-loop insulin delivery: A randomized crossover study. Diabetes Care. 2020;43:480–3. [DOI] [PubMed] [Google Scholar]
- [16].Tagougui S, Taleb N, Rabasa-Lhoret R. The benefits and limits of technological advances in glucose management around physical activity in patients type 1 diabetes. Front Endocrinol (Lausanne). 2018;9:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Burckhardt MA, Abraham MB, Dart J, Smith GJ, Paramalingam N, O’Dea J, et al. Impact of hybrid closed loop therapy on hypoglycemia awareness in individuals with type 1 diabetes and impaired hypoglycemia awareness. Diabetes Technol Ther. 2021;23:482–90. [DOI] [PubMed] [Google Scholar]
- [18].Sherr JL. Closing the loop on managing youth with type 1 diabetes: Children are not just small adults. Diabetes Care. 2018;41:1572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tauschmann M, Allen JM, Nagl K, Fritsch M, Yong J, Metcalfe E, et al. Home use of day-and-night hybrid closed-loop insulin delivery in very young children: A multi-center, 3-week, randomized trial. Diabetes Care. 2019;42:594–600. [DOI] [PubMed] [Google Scholar]
- [20].Fuchs J, Allen J, Boughton C, Wilinska M, Thankamony A, de Beaufort C, et al. 57th EASD Annual Meeting of the European Association for the Study of Diabetes: OP 41 Building the evidence base for new devices; 233 Cambridge hybrid closed-loop in very young children with type 1 diabetes: a multinational 4-month randomised trial. Diabetologia. 2021;64:S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fuchs J, Hovorka R. Benefits and challenges of current closed-loop technologies in children and young people with type 1 diabetes. Front Pediatr. 2021;9:679484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ruan Y, Elleri D, Allen JM, Tauschmann M, Wilinska ME, Dunger DB, et al. Pharmacokinetics of diluted (U20) insulin aspart compared with standard (U100) in children aged 3–6 years with type 1 diabetes during closed-loop insulin delivery: a randomised clinical trial. Diabetologia. 2015;58:687–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pratley RE, Kanapka LG, Rickels MR, Ahmann A, Aleppo G, Beck R, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: A randomized clinical trial. JAMA. 2020;323:2397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McAuley SA, Vogrin S, Trawley S, Colman PG, Fourlanos S, Grills C, et al. 212-OR: Closed-loop increases time-in-range in older adults with type 1 diabetes compared with sensor-augmented pump therapy: A randomized crossover trial. Diabetes. 2021;70:212–or. [Google Scholar]
- [25].Macintosh MC, Fleming KM, Bailey JA, Doyle P, Modder J, Acolet D, et al. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: population based study. BMJ. 2006;333:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Murphy HR, Bell R, Cartwright C, Curnow P, Maresh M, Morgan M, et al. Improved pregnancy outcomes in women with type 1 and type 2 diabetes but substantial clinic-to-clinic variations: a prospective nationwide study. Diabetologia. 2017;60:1668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stewart ZA, Wilinska ME, Hartnell S, O’Neil LK, Rayman G, Scott EM, et al. Day-and-night closed-loop insulin delivery in a broad population of pregnant women with type 1 diabetes: A randomized controlled crossover trial. Diabetes Care. 2018;41:1391–9. [DOI] [PubMed] [Google Scholar]
- [28].Peters TM, Haidar A. Dual-hormone artificial pancreas: benefits and limitations compared with single-hormone systems. Diabet Med. 2018;35:450–9. [DOI] [PubMed] [Google Scholar]
- [29].El-Khatib FH, Balliro C, Hillard MA, Magyar KL, Ekhlaspour L, Sinha M, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. The Lancet. 2017;389:369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Castellanos LE, Balliro CA, Sherwood JS, Jafri R, Hillard MA, Greaux E, et al. Performance of the insulin-only iLet Bionic Pancreas and the bihormonal iLet using Dasiglucagon in adults with type 1 diabetes in a home-use setting. Diabetes Care. 2021;44:e118–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wilson LM, Jacobs PG, Ramsey KL, Resalat N, Reddy R, Branigan D, et al. Dual-hormone closed-loop system using a liquid stable glucagon formulation versus insulin-only closed-loop system compared with a predictive low glucose suspend system: An open-label, outpatient, single-center, crossover, randomized controlled trial. Diabetes Care. 2020;43:2721–9. [DOI] [PubMed] [Google Scholar]
- [32].Haidar A, Tsoukas MA, Bernier-Twardy S, Yale JF, Rutkowski J, Bossy A, et al. A novel dual-hormone insulin-and-pramlintide artificial pancreas for type 1 diabetes: A randomized controlled crossover trial. Diabetes Care. 2020;43:597–606. [DOI] [PubMed] [Google Scholar]
- [33].Andersen G, Meiffren G, Famulla S, Heise T, Ranson A, Seroussi C, et al. ADO09, a co-formulation of the amylin analogue pramlintide and the insulin analogue A21G, lowers postprandial blood glucose versus insulin lispro in type 1 diabetes. Diabetes Obes Metab. 2021;23:961–70. [DOI] [PubMed] [Google Scholar]
- [34].Majdpour D, Tsoukas MA, Yale JF, El Fathi A, Rutkowski J, Rene J, et al. Fully automated artificial pancreas for adults with type 1 diabetes using multiple hormones: Exploratory experiments. Can J Diabetes. 2021. [DOI] [PubMed] [Google Scholar]
- [35].Dimitrios P, Michael D, Vasilios K, Konstantinos S, Konstantinos I, Ioanna Z, et al. Liraglutide as adjunct to insulin treatment in patients with type 1 diabetes: A systematic review and meta-analysis. Curr Diabetes Rev. 2020;16:313–26. [DOI] [PubMed] [Google Scholar]
- [36].Johansen NJ, Dejgaard TF, Lund A, Schlüntz C, Frandsen CS, Forman JL, et al. Efficacy and safety of meal-time administration of short-acting exenatide for glycaemic control in type 1 diabetes (MAG1C): a randomised, double-blind, placebo-controlled trial. The Lancet Diabetes & Endocrinology. 2020;8:313–24. [DOI] [PubMed] [Google Scholar]
- [37].Srinivasan S, Ekhlaspour L, Cengiz E. Adjunctive therapies to optimize closed-loop glucose control. J Diabetes Sci Technol. 2021;15:1243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dandona P, Mathieu C, Phillip M, Hansen L, Tschope D, Thoren F, et al. Efficacy and safety of Dapagliflozin in patients with inadequately controlled type 1 diabetes: The DEPICT-1 52-week study. Diabetes Care. 2018;41:2552–9. [DOI] [PubMed] [Google Scholar]
- [39].McAuley SA, Lee MH, Paldus B, Vogrin S, de Bock MI, Abraham MB, et al. Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: A randomized, controlled trial. Diabetes Care. 2020;43:3024–33. [DOI] [PubMed] [Google Scholar]
- [40].Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, Shulman DI, Bailey TS, Bode BW, et al. Safety evaluation of the MiniMed 670G System in children 7–13 Years of age with type 1 diabetes. Diabetes Technol Ther. 2019;21:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Abraham MB, de Bock M, Smith GJ, Dart J, Fairchild JM, King BR, et al. Effect of a hybrid closed-loop system on glycemic and psychosocial outcomes in children and adolescents with type 1 diabetes: A randomized clinical trial. JAMA Pediatr. Published online October 11, 2021. doi: 10.1001/jamapediatrics.2021.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bergenstal RM, Nimri R, Beck RW, Criego A, Laffel L, Schatz D, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet. 2021;397:208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care. 2019;42:2190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Collyns OJ, Meier RA, Betts ZL, Chan DSH, Frampton C, Frewen CM, et al. Improved glycemic outcomes with Medtronic MiniMed Advanced Hybrid Closed-Loop delivery: Results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care. 2021;44:969–75. [DOI] [PubMed] [Google Scholar]
- [45].Brown S, Raghinaru D, Emory E, Kovatchev B. First look at control-IQ: a new-generation automated insulin delivery system. Diabetes Care. 2018;41:2634–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Anderson SM, Raghinaru D, Pinsker JE, Boscari F, Renard E, Buckingham BA, et al. Multinational home use of closed-loop control is safe and effective. Diabetes Care. 2016;39:1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381:1707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, et al. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med. 2020;383:836–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ekhlaspour L, Schoelwer MJ, Forlenza GP, Deboer MD, Norlander L, Hsu L, et al. Safety and performance of the Tandem t:slim X2 with Control-IQ automated insulin delivery system in toddlers and preschoolers. Diabetes Technol Ther. 2020;23:384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schierloh U, Wilinska ME, Pit-Ten Cate IM, Baumann P, Hovorka R, De Beaufort C, et al. Lower plasma insulin levels during overnight closed-loop in school children with type 1 diabetes: Potential advantage? A randomized cross-over trial. PLoS One. 2019;14:e0212013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hovorka R, Kumareswaran K, Harris J, Allen JM, Elleri D, Xing D, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ. 2011;342:d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bally L, Thabit H, Kojzar H, Mader JK, Qerimi-Hyseni J, Hartnell S, et al. Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. The Lancet Diabetes & Endocrinology. 2017;5:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tauschmann M, Allen JM, Wilinska ME, Thabit H, Acerini CL, Dunger DB, et al. Home use of day-and-night hybrid closed-loop insulin delivery in suboptimally controlled adolescents with type 1 diabetes: A 3-week, free-living, randomized crossover trial. Diabetes Care. 2016;39:2019–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tauschmann M, Thabit H, Bally L, Allen JM, Hartnell S, Wilinska ME, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392:1321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fuchs J, Boughton CK, Allen JM, Wilinska ME, Tauschmann M, Denvir L, et al. 214-OR: Cambridge hybrid closed-loop in children and adolescents with T1D: A multicentre six-month randomised trial. Diabetes. 2021;70. [Google Scholar]
- [56].Boughton CK, Hartnell S, Thabit H, Poettler T, Herzig D, Wilinska ME, et al. Hybrid closed-loop glucose control with faster insulin aspart compared with standard insulin aspart in adults with type 1 diabetes: A double-blind, multicentre, multinational, randomized, crossover study. Diabetes Obes Metab. 2021;23:1389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Benhamou PY, Franc S, Reznik Y, Thivolet C, Schaepelynck P, Renard E, et al. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digit Health. 2019;1:e17–e25. [DOI] [PubMed] [Google Scholar]
- [58].Amadou C, Franc S, Benhamou PY, Lablanche S, Huneker E, Charpentier G, et al. Diabeloop DBLG1 closed-loop system enables patients with type 1 diabetes to significantly improve their glycemic control in real-life situations without serious adverse events: 6-month follow-up. Diabetes Care. 2021;44:844–6. [DOI] [PubMed] [Google Scholar]
- [59].Kariyawasam D, Morin C, Casteels K, Tallec CL, Godot C, Sfez A, et al. 98-LB: Diabeloop DBL4K hybrid closed-loop system improves time-in-range without increasing time-in-hypoglycemia in children aged 6–12 years. Diabetes. 2021;70. [Google Scholar]
- [60].Brown SA, Forlenza GP, Bode BW, Pinsker JE, Levy CJ, Criego AB, et al. Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care. 2021;44:1630–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sherr J, Bode BW, Forlenza GP, Laffel LM, Brown SA, Buckingham BA, et al. 70-OR: Evaluation of the Omnipod 5 Automated Insulin Delivery System in very young children with type 1 diabetes (T1D). Diabetes. 2021;70. [Google Scholar]
- [62].Lum JW, Bailey RJ, Barnes-Lomen V, Naranjo D, Hood KK, Lal RA, et al. A real-world prospective study of the safety and effectiveness of the Loop open source automated insulin delivery system. Diabetes Technol Ther. 2021;23:367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jennings P, Hussain S. Do-it-yourself artificial pancreas systems: A review of the emerging evidence and insights for healthcare professionals. J Diabetes Sci Technol. 2019;14:868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Burnside M, Lewis D, Crocket H, Wilson R, Williman J, Jefferies C, et al. CREATE (Community deRivEd AutomaTEd insulin delivery) trial. Randomised parallel arm open label clinical trial comparing automated insulin delivery using a mobile controller (AnyDANA-loop) with an open-source algorithm with sensor augmented pump therapy in type 1 diabetes. J Diabetes Metab Disord. 2020:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rankin D, Kimbell B, Hovorka R, Lawton J. Adolescents’ and their parents’ experiences of using a closed-loop system to manage type 1 diabetes in everyday life: qualitative study. Chronic Illn. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lawton J, Blackburn M, Rankin D, Allen JM, Campbell FM, Leelarathna L, et al. Participants’ experiences of, and views about, daytime use of a day-and-night hybrid closed-loop system in real life settings: Longitudinal qualitative study. Diabetes Technol Ther. 2019;21:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Farrington C, Stewart Z, Hovorka R, Murphy H. Women’s experiences of day-and-night closed-loop insulin delivery during type 1 diabetes pregnancy. J Diabetes Sci Technol. 2018;12:1125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Barnard KD, Wysocki T, Ully V, Mader JK, Pieber TR, Thabit H, et al. Closing the loop in adults, children and adolescents with suboptimally controlled type 1 diabetes under free living conditions: A psychosocial substudy. J Diabetes Sci Technol. 2017;11:1080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Musolino G, Dovc K, Boughton CK, Tauschmann M, Allen JM, Nagl K, et al. Reduced burden of diabetes and improved quality of life: Experiences from unrestricted day-and-night hybrid closed-loop use in very young children with type 1 diabetes. Pediatr Diabetes. 2019;20:794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Berget CM, Vigers Laurel H., Frohnert Tim, Pyle Brigitte I., Wadwa Laura, Driscoll R. Paul, Forlenza Kimberly A., Gregory P. Six months of hybrid closed loop in the real-world: An evaluation of children and young adults using the 670G system. Pediatr Diabetes. 2020;21:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Breton MD, Kovatchev BP. One year real-world use of the Control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther. 2021;23:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Da Silva J, Lepore G, Battelino T, Arrieta A, Castaneda J, Grosman B, et al. Real-world performance of the MiniMed 780G System: First report of outcomes from 4’120 users. Diabetes Technol Ther. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Leelarathna L, Choudhary P, Wilmot EG, Lumb A, Street T, Kar P, et al. Hybrid closed-loop therapy: Where are we in 2021? Diabetes Obes Metab. 2020:1–6. [DOI] [PubMed] [Google Scholar]
- [74].Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31:934–9. [DOI] [PubMed] [Google Scholar]
- [75].Dovc K, Piona C, Yesiltepe Mutlu G, Bratina N, Jenko Bizjan B, Lepej D, et al. Faster compared with standard insulin aspart during day-and-night fully closed-loop insulin therapy in type 1 diabetes: A double-blind randomized crossover trial. Diabetes Care. 2020;43:29–36. [DOI] [PubMed] [Google Scholar]
- [76].Cameron FM, Ly TT, Buckingham BA, Maahs DM, Forlenza GP, Levy CJ, et al. Closed-loop control without meal announcement in type 1 diabetes. Diabetes Technol Ther. 2017;19:527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Messer LH, Berget C, Forlenza GP. A clinical guide to advanced diabetes devices and closed-loop systems using the CARES paradigm. Diabetes Technol Ther. 2019;21:462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kimbell B, Rankin D, Ashcroft NL, Varghese L, Allen JM, Boughton CK, et al. What training, support, and resourcing do health professionals need to support people using a closed-loop system? A qualitative interview study with health professionals involved in the Closed Loop from Onset in type 1 Diabetes (CLOuD) trial. Diabetes Technol Ther. 2020;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lal RA, Ekhlaspour L, Hood K, Buckingham B. Realizing a closed-loop (artificial pancreas) system for the treatment of type 1 diabetes. Endocr Rev. 2019;40:1521–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wang Y, Fang M, Jiang X, Bequette BW, Xie H. Intensive insulin therapy for critically ill subjects based on direct data-driven model predictive control. Journal of Process Control. 2014;24:493–503. [Google Scholar]
- [81].Mauseth R, Hirsch IB, Bollyky J, Kircher R, Matheson D, Sanda S, et al. Use of a “fuzzy logic” controller in a closed-loop artificial pancreas. Diabetes Technol Ther. 2013;15:628–33. [DOI] [PubMed] [Google Scholar]