SUMMARY
The mucosa of the body of the stomach (i.e., the gastric corpus) employs two overlapping, depth-dependent mechanisms to respond to injury. Superficial injury heals via surface cells with histopathological changes like foveolar hyperplasia. Deeper, usually chronic, injury/inflammation, most frequently induced by the carcinogenic bacteria H pylori, elicits glandular histopathological alterations, initially manifesting as pyloric (also known as pseudopyloric) metaplasia. In this pyloric metaplasia, corpus glands become antrum (pylorus)-like with loss of acid-secreting parietal cells (atrophic gastritis), expansion of foveolar cells, and reprogramming of digestive enzyme-secreting chief cells into deep antral gland-like, mucous cells. Following acute parietal cell loss, chief cells can reprogram through an orderly, stepwise progression (paligenosis) initiated by IL-13-secreting innate lymphoid cells (ILC2s). First, massive lysosomal activation helps mitigate reactive oxygen species (ROS) and remove damaged organelles. Second, mucus and wound-healing proteins (e.g. TFF2) and other transcriptional alterations are induced, at which point the reprogrammed chief cells are recognized as mucus-secreting Spasmolytic Polypeptide Expressing Metaplasia (SPEM) cells. In chronic severe injury, glands with pyloric metaplasia can harbor both actively proliferating SPEM cells and eventually intestine-like cells. Gastric glands with such lineage confusion (mixed incomplete intestinal metaplasia and proliferative SPEM) may be at particular risk for progression to dysplasia and cancer. A pyloric-like pattern of metaplasia after injury also occurs in other gastrointestinal organs including esophagus, pancreas and intestines, and the paligenosis program itself seems broadly conserved across tissues and species. Here, we discuss aspects of metaplasia in stomach, incorporating data derived from animal models and work on human cells and tissues in correlation with diagnostic and clinical implications.
The response to Injury in the stomach.
The stomach comprises two anatomical areas. The corpus is defined by epithelium harboring abundant acid secreting parietal cells.1 The antrum in most mammals is characterized by epithelium harboring mucus secreting glands without parietal cells (although in humans some parietal cells are also present in the antrum).1 Antrum and corpus are also distinguished by enteroendocrine cells with ghrelin-secreting cells in corpus and gastrin-producing in antrum.1 Gastric contents can harbor ingested pathogens and mutagens, and gastric juice is caustic owing to acidity (~pH 2) and abundant pepsin (a digestive enzyme).2 Thus, it is not surprising that the stomach has evolved protective mechanisms that vary in rapidity and complexity according to the nature of the injury. Generally, there are two broad categories of response based on the depth of injury.3 Upon superficial damage, when surface cells are damaged or denuded, foveolar mucous cells rapidly migrate to close wounds4, 5 and, moreover, can rapidly be regenerated from stem cells located within the isthmus of corpus glands. When this proliferative response is over-exuberant, it can lead to foveolar hyperplasia.
In contrast, deeper damage triggers metaplasia of the entire epithelium that serves to reduce endogenous production of caustic substance. Acid-secreting parietal cells die and pepsin-secreting chief cells are reprogrammed acutely into mucin-secreting, wound-healing cells. Mucus-secreting neck cells and foveolar surface mucus cells also expand during the metaplastic response, likely arising from mucous neck and isthmal progenitor cells.6 Deep and superficial damage can be concomitant. For example, a deep erosion from the surface or full-thickness damage (ulceration) can occur secondary to non-steroidal anti-inflammatory agents or H. pylori-induced inflammation and acid damage. Such broader damage can be repaired by coordinated foveolar hyperplasia and metaplasia. The end result of all of these responses is to minimize the release of caustic substances and create a focus of mucus-secreting cells that provide protection and promote healing (Figure 1). In acute injury, all such changes are reversible, at least in the short term, upon resolution of the cause of injury.
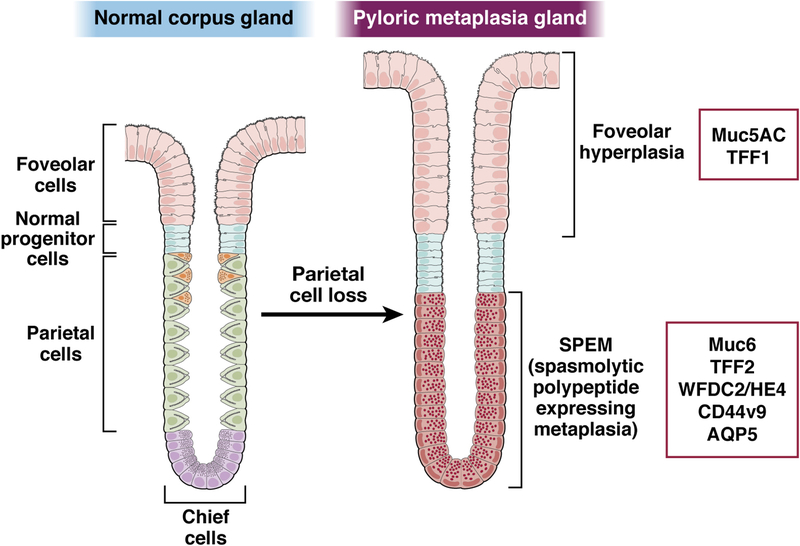
Figure 1: Markers of pyloric metaplasia.
Pyloric metaplasia evolves in the corpus of the stomach following parietal cell loss with the establishment of SPEM cells at the base of glands and often foveolar hyperplasia luminal to the SPEM lineages. Foveolar hyperplasia is marked by expansion of cells expressing Muc5AC and TFF1. SPEM is identified by cells expressing chief cell markers in low abundance plus induced AQP5, MUC6, TFF2, WFDC2/HE4 and CD44v9.
Metaplasia as a response to injury
Metaplasia means the replacement of normal cells with lineages more representative of another region, from a different developmental stage, or from a different organ entirely. In the gastrointestinal tract, a metaplastic lesion commonly comprises an array of mucus-secreting cells that arise to provide protection and healing following severe damage.7
In the stomach, two principal types of metaplasia are prominent. One, variously described as pyloric or pseudopyloric metaplasia, has been recognized for over a century by histopathologists who noted that tissue sections of patients with atrophic gastritis (i.e. inflammation with loss of parietal cells) in the body of the stomach also had altered digestive enzyme-secreting chief cells.8–11 Namely, the chief cell zone in the base of the glands becomes occupied with mucous cells displaying a deep antral or Brunner’s gland phenotype, such that atrophic gastric units resemble the normal antral or pyloric units (Figure 1).1, 12–15 Histopathologists from the Nineteenth century had also recognized that intestine-like cells could be found in the stomach, although their occurrence was so common in that era that many considered them a normal aspect of gastric microanatomy (see e.g. this 1912 textbook16).9, 13 Intestinal metaplasia is most prominently identified by the presence of MUC2-secreting goblet cells, but it can also include absorptive lineages, Paneth cells and enteroendocrine cells.17, 18 Interest in intestinal metaplasia over the past 60 years or so has completely overwhelmed recognition of pyloric metaplasia. The reason for this may be because most diagnostic histopathological examination of stomach tissue came to be via endoscopic biopsy, and such small amounts of randomly oriented, often superficial tissue does not allow examination of differentiation across the entire gastric epithelium, including the deepest portion of glands where which define pyloric metaplasia. Also, it was noted that gastric cancers often harbor intestine-like cells, consistent with intestinal metaplasia harboring the cancer cell of origin.19 Pelayo Correa most famously synthesized observations about gastritis, atrophy, and metaplasia into a theory of stepwise development of gastric cancer with an intestinal metaplasia intermediate playing a central role.17
In the last two decades, however, interest in pyloric-type metaplasia has resurged, with an inflection point occurring when it was recognized that the metaplastic cells at the base of these lesions express spasmolytic polypeptide (TFF2). TFF2 induction marked such metaplastic cells (known as Spasmolytic Polypeptide-expressing Metaplasia; SPEM) as ectopic, spurring the search for the cells of origin (Figure 1).20 We argue it is most useful to define pyloric metaplasia as the entire surface-to-gland-base alteration that occurs during atrophic gastritis, whereas SPEM is specifically the TFF2+/MUC6+/PGCLow mucus cell lineage that arises at the gland base. Thus, a pyloric metaplasia lesion exhibits parietal cell loss and replacement of basal chief cells by SPEM cells. Additionally, pyloric metaplasia can harbor other lineage abnormalities including foveolar hyperplasia and mucous neck cell census changes (Figure 1). Because SPEM cells at the base of corpus glands are seen only in the setting of parietal cell loss and pyloric metaplasia, pyloric metaplasia can be diagnosed by the presence of SPEM lineages. However, SPEM cells are only one part of the lesion.
For over a century, pathologists have noted pyloric metaplasia is a response to numerous acute and chronic gastric mucosal injuries including diphtheria infection, gastric peptic ulcers/erosions, and associated with autoimmune and Helicobacter pylori-induced gastritis.21, 22 Pyloric metaplasia can also develop outside the stomach in duodenal ulcers, cholecystitis, Crohn’s disease and pancreatitis.23–27 Work with animal models and high-resolution genomic and transcriptomic analyses of metaplastic and cancerous tissue has supported a central role for pyloric metaplasia and SPEM lineages as precursor lesions for gastric cancer,20, 28–30 a concept also proposed by pioneering gastric pathologists of the 19th and early 20th century, including Ménétrier.15, 21, 22, 31 It remains unclear how corpus pyloric metaplastic glands differ from normal antral glands, specifically whether SPEM cells in pyloric metaplasia differ from actual deep antral cells. SPEM markers, including TFF2, MUC6, AQP5 and CD44v9, are also expressed in deep antral gland cells,32, 33 although some markers (e.g. HE4, also known as WFDC2) may be unique to SPEM cells.34 It is clear, however, from both old and new literature that pyloric metaplasia is at least as important as intestinal metaplasia as a harbinger and precursor for so-called intestinal type cancers.
The relationship between pyloric metaplasia and intestinal metaplasia remains unclear. SPEM cells can rapidly emerge (even as rapidly as 48 hours in mice) in response to severe, deep glandular injury.35–39.40, 41 Hybrid lesions between normal chief and SPEM cells at the bases of corpus glands are easily identifiable in inflamed human corpus, indicating a potentially dynamic process.20, 29, 42 However, in human pyloric metaplasia, SPEM cells are commonly non-proliferative, indicating they can also be a chronic mucosal adaptation.42–45 Multiple lines of evidence argue that intestinal metaplasia arises from pyloric metaplasia.46 For one, intestinal metaplasia often arises in pre-existing atrophic pyloric metaplastic stomach,13 and cells of hybrid phenotype have been noted both historically47 and via more modern immunological approaches.29, 43 Moreover, in H. pylori-infected gerbils, intestinal metaplasia similarly arises within pre-existing pyloric metaplastic lesions.35 Likewise, when constitutively-active mutant Kras(G12D) is induced in chief cells, TFF3 and other intestinal phenotypes emerge only after SPEM cells have emerged.48
In addition to metaplasia, repair can also induce increased proliferation of normal cells, i.e. hyperplasia. Expansion of normal surface mucous cells (foveolar hyperplasia) increases surface mucus secretion (Figure 1). Foveolar hyperplasia can be isolated, but it often also occurs in pyloric metaplasia as SPEM cells emerge at gland bases.49
Thus, the stomach can mount a two-compartment response to injury with isthmal stem cells fueling foveolar hyperplasia and expansion of neck cells and chief cells at the gland base fueling the development of SPEM. 45, 50, 51 Even in homeostasis, two recent papers showed that constitutive proliferation of stem and progenitor cells in the isthmus fuels foveolar, parietal, and mucous neck cell replacement during homeostasis. Basal chief cells are long-lived and replaced infrequently either by forward differentiation of mucous neck cells52 or by autoduplication.50, 53
In addition to foveolar hyperplasia, mucous neck cells in the mid-portions of the glands can expand. Such mucous neck cell hyperplasia can occur acutely after parietal cell loss induced by DMP-77740 and also in more chronic mouse models of pyloric metaplasia.54, 55 It is not clear how much of such mucous neck cell hyperplasia is due to more rapid proliferation of neck cells versus arrest of mucous neck cell differentiation into chief cells or even contribution of chief cells to mucous neck cells.56 Mucous neck and chief cells seem to be remarkably plastic after injury.57, 58
Even though metaplastic lineages, by definition, replace normal lineages, the changes may be reversible. Indeed, it seems likely that pyloric metaplasia evolved as an acute repair mechanism to recruit additional progenitor cells, while increasing production of protective (e.g. mucins) and wound-healing (e.g. TFF2) factors.7 Such rapid tissue restitution is indeed what happens after parietal cell-toxic drug (e.g. DMP-777) administration in rodents.59, 60 Only when inflammation is chronic does metaplasia manifest as a stable, potentially precancerous lesion. Transient, successful metaplasia likely occurs all the time and is not noted clinically. Indeed, we have noticed, in otherwise normal stomachs, occasional glands with focal SPEM cells.1
Even chronic metaplasia may reverse. In both mutant Kras-expressing metaplastic mice and H. pylori-infected gerbils, treating animals with MEK inhibitors both arrests the frankly metaplastic cells and facilitates the return of normal gastric lineages from normal, rather than metaplastic progenitors.48, 61 Thus, metaplastic cells may create a milieu that inhibits, but does not eliminate, normal progenitor cells from the corpus.
The origin of SPEM lineages in the stomach.
SPEM cells were first distinguished over 20 years ago when TFF2-expressing cells were observed in the bases of atrophic glands in the human corpus (TFF2 is usually confined to the mucous neck cells).20 Subsequently, SPEM was noted in rodent models including the H. felis-infected mouse54, 62, 63 and in rats and mice treated with the parietal cell toxic drug DMP-777.59, 60, 64 Further studies demonstrated the induction of SPEM with other parietal cell-toxic agents including L635, a molecular cousin of DMP-777,40, 60 and high doses of the drug tamoxifen.39, 65, 66 The induction of SPEM lineages by these parietal cell toxic agents is rapid, synchronous, and the entire corpus of the stomach converts to pyloric metaplasia with SPEM cells at the base of every gastric unit. All the mentioned drugs act as protonophores, likely inducing metaplasia by poisoning proton-secreting parietal cells, though injury/repair after each drug varies slightly.65 For example, DMP-777 induces SPEM without substantial infiltration of the mucosa by inflammatory cells, likely because the drug also acts as a neutrophil elastase inhibitor.60 L635 does not affect neutrophil elastase,60 kills parietal cells quickly, and induces robust inflammation.40 Tamoxifen induces intermediate inflammation.39 Despite variation in obvious inflammatory infiltrates, innate immune defense and cytokines are nonetheless likely critical for SPEM induction, no matter the injury. Together, drug-based models have allowed detailed analysis of the mechanisms required for induction of SPEM, because parietal cell loss and metaplasia induction are so rapid.
Early studies using DMP-777 demonstrated that SPEM cells harbored both zymogen granules (normally found in mature chief cells) and mucin granules (of the type normally found in neck cells)34 suggesting that SPEM cells might derive from chief cells.67 To test this, mice expressing both a lineage reporter and a tamoxifen-inducible cre recombinase knocked into the Mist1 (Bhlha15) promoter were used to trace chief cell fate after metaplasia induction. Such lineage-mapping studies depend on the predominant expression of Mist1 in mature, exocrine secretory cells (e.g. acinar cells of pancreas or salivary glands).29, 40, 68 Indeed, MIST1 is required expressly for terminal maturation of exocrine cells.69 Although both authors’ labs have confirmed the initial findings using a variety of reporter alleles, the original studies employed Mist1-CreERT2;RosaR26R-LSL-LacZ mice to mark chief cells with β-galactosidase expression, and then induce SPEM.40 In all cases of SPEM induction, acute via DMP-777 and L635 treatment as well as chronic via H. felis infection, SPEM cells marked with β-galactosidase expression derived from chief cells.40 Thus, SPEM cells emerged largely via cellular plasticity of chief cells.
Subsequent studies have demonstrated that chief cells have the capacity to reprogram into SPEM, although as chief cells age over 60 days, their ability to give rise to SPEM declines.70 Moreover, we and multiple other laboratories, using a variety of lineage markers, have demonstrated such chief cell plasticity using various lineage-tracing approaches71–73 and single-cell RNA-sequencing,57 corroborated by identification of cells in the bases of human gastric glands showing hybrid phenotypes between those of SPEM and chief cells.29 Chief cell DNA can even be loaded with nucleotide analogs (BrdU and EdU) and traced into SPEM cell DNA.56
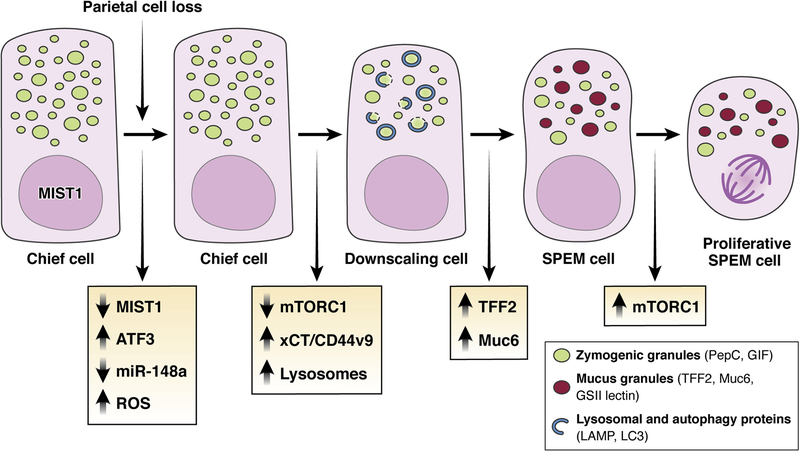
We have proposed that chief cells become SPEM cells via discrete stages that are reproduced consistently in all cells (Figure 2). Moreover, progression from stage to stage can be inhibited by drugs or genetic mutations, leading to arrest of cells at different points in the reprogramming process. The cellular program that governs how mature cells can become proliferative, regenerative cells seems to be conserved across tissues and species, with cells of diverse phenotypes having access to the same pathways, just as apoptosis is similar from cell type to cell type.34, 40, 74–76 This program (designated as paligenosis) involves an initial stage in which the massive rough endoplasmic reticulum and secretory apparatus in the chief cell, including much of the complement of large zymogenic granules is recycled via extensive lysosome activation and autophagy (Figure 2).45, 75 The first stage depends on the early-response transcription factor ATF3, which, in turn, upregulates expression of the lysosome and autophagy trafficking protein RAB7B.77 The next stage involves the induction of the pathognomonic TFF2 as a component of MUC6-containing mucous granules. Even before autodegradative pathways are activated, one of the earliest events is that chief cells en route to SPEM decrease expression of Mist1.29, 70, 78, 79 Downregulation of Mist1 helps dismantle mature cell architecture, given the role of Mist1 in promoting the expression of Rab proteins and ubiquitin ligases that maintain the elaborate secretory architecture of chief cells (Figure 2).68, 69 Loss of miR-148a, which is strongly expressed in mature chief cells, is also an early event in the reprogramming process.78
Figure 2: Evolution of SPEM through transdifferentiation/paligenosis.
Following parietal cell loss, chief cells rapidly down-regulate expression of the chief-cell-architecture master regulating transcription factor Mist1 and the micro-RNA miR-148a. mTORC1 is downregulated, and reactive oxygen species (ROS) increase. Loss of MIST1, decrease of mTORC1, and ROS facilitate expansion of lysosomal elements to remove damaged ROS-producing organelles and consume excess zymogen granules. Next, cellular ROS-adaptive mechanisms such as xCT/CD44v9 are increased. Management of ROS and downscaling of zymogen granules are required before the next stage: up-regulation of TFF2 and MUC6 expression and the assembly of mucus-secreting granules that define the reprogramming into a SPEM cell phenotype. Continued damage and inflammation can promote the progression of SPEM into a more proliferative lineage and transition to this stage requires re-induction of mTORC1.
Concomitant with upregulation of TFF2 and specific mucins in the second stage of paligenosis, chief cells en route to SPEM upregulate CD44 variant 9 (CD44v9),41 which can stabilize the xCT cystine transporter.32 Either knockout of xCT or inhibition of xCT with sulfasalazine arrests paligenosis at a step distal to the down-regulation of Mist1.41 Blockade of xCT leads to increased reactive oxygen species that chief cells cannot compensate for, causing apoptosis. Similarly, paligenosis can also be arrested by a blockade of lysosomal pathways through deletion of a gene required for lysosomal function (Gnptab) or inhibition of mTORC.45 Loss of lysosome function traps cells in the first stage, before induction of metaplastic genes. Blockade of mTORC1 traps cells in the CD44v9+/TFF2+/Mucin-expressing state preventing cell cycle reentry (Figure 2).
SPEM is associated with upregulation of a number of genes associated with unwinding DNA, as well as monitoring DNA damage via proteins, including MCM genes along with IFRD1, DDIT4, and p53.34, 53, 75 These genes help rearrange and monitor the genome as an altered transcriptome fuels redifferentiation towards mucus-secretion and even cell cycle reentry. Different inducers of SPEM cause varying degrees of proliferation among resulting SPEM cells with chronic SPEM mostly mitotically quiescent.43, 56, 75
Though evidence largely indicates that the bulk of SPEM in an acute setting derives from chief cell reprogramming, a role for mucous neck cells or isthmal progenitors has not been ruled out. Indeed, we acknowledge that Hayakawa and Wang have suggested that SPEM actually arises from isthmal progenitor cells.80 Many of the conclusions of these investigators depended on the results of experiments in which Lgr5 driven expression of DTR was used to ablate chief cells. Because such DTR-mediated ablation did not alter SPEM induction, they concluded that chief cells were not a source for SPEM. In contrast, Barker and colleagues, who also used Lgr5 to express DTR, did not observe complete ablation of chief cells, and concluded that SPEM was indeed derived from chief cells.71 Moreover, several studies have shown that SPEM can arise in the absence of any proliferation, which would preclude the 1–2 isthmal stem cells per gland giving rise to 10+ SPEM cells within a few days.40, 42, 45, 70
More recently, the Hayakawa group has suggested that acute parietal cell loss leads to apoptosis among chief cells marked with GPR30 or Mist1 driver mice and that not all such chief cells make it to SPEM cells.81 These results don’t actually conflict with data from other laboratories, as varying degrees of injury cause varying degrees of conversion of chief cells to SPEM cells, depending on the age of mice and of chief cells.70 Moreover, as mentioned above, apoptosis of a small cohort of cells has been noted after SPEM induction, with many more dying if the process is disrupted by drugs or mutations that affect completion of paligenosis.40, 45, 70, 75, 77 Some differences in results may be due to differences in the various fluorescent LSL-Rosa26R reporter strains. We have observed that Confetti mouse labeling of chief cells is lost following induction of acute parietal cell loss, while mapping with mTmG or YFP strains is not (data not shown). Moreover, mapping with mTmG is scanter than mapping with Ai9 or YFP reporter strains. The hybrid CAG promoter used in the Confetti mouse seems to dampen its induction. As an increase in DNMT1 is noted early on in chief cell paligenosis,78 silencing of the CAG promoter during the reprogramming process can lead to artifactual loss of lineage mapping. These findings indicate that interpretation of lineage mapping in plasticity scenarios must consider silencing of reporter expression.
In the final analysis, much of the problems in interpreting lineage mapping studies of SPEM induction have accrued from lack of a consensus, non-tamoxifen-inducible chief cell-specific reporter allele. However, Caldwell, et al.82 recently described that the Gastric Intrinsic Factor (GIF)-rtTA mouse allele drives doxycycline-inducible reporter expression only in chief cells in the corpus with no detectable expression in isthmal progenitor cells. Treatment of GIF-rtTA;tetO-CreERT2;Rosa-nTnG mice with L635 induced SPEM from GFP-marked chief cell-derived lineages.82 In addition, these studies demonstrated that GRP30 protein-expressing chief cells also showed transdifferentiation into SPEM cells, presenting conclusive evidence that SPEM can evolve from chief cells.82 We would also note that in other studies, where long-lived cells were labeled by a BrdU pulse followed by 3-month chase and rapidly dividing cells were labeled with a short EdU pulse, BrdU was largely confined to chief cells before acute SPEM induction, whereas EdU labeled isthmal cells. After SPEM induction, the EdU-labeled populations included foveolar and neck cells but not SPEM cells. SPEM cells were largely BrdU-positive.56 There was no substantial loss of BrdU-labeled cells after SPEM induction, consistent with chief cells becoming SPEM cells without significant death. The GIF-rtTA allele lines should allow further studies as to the origins of SPEM in longer term and more chronic models.
One population in the stomach that seems non-plastic and does not appear to contribute to SPEM is the parietal cell lineage. We see no contribution of mature, existing parietal cells to SPEM or pyloric metaplasia.39 Parietal cell progenitors can expand when glands are cultivated ex vivo,83 or when expression of pro-proliferative genes is forced;84, 85 but in vivo in mice and humans chronic atrophic gastritis means actual parietal cell loss (i.e. death rather than reprogramming or some sort of plasticity).
Kras activation and the generation of pyloric gland phenotypes.
Controversy has emerged over studies examining the role of Ras activation driving the evolution of SPEM and intestinal metaplasia. Expression of phospho-ERK is observed in SPEM in the setting of Helicobacter infection.61, 86 Induction of active KRAS in chief cells was examined with low-dose tamoxifen-induction of Kras(G12D) expression using Mist1-CreERT2;LSL-KrasG12D (Mist1-Kras) mice discussed above.48 SPEM developed in the stomach by one month after induction of active Kras indicating the existence of a SPEM-associated pyloric metaplasia (SA-PM, Figure 3). TFF3-expressing intestinal metaplasia developed by 3 months of induction followed by development of dysplasia by 4 months after KrasG12D induction.43, 48 Lineage tracing showed that the metaplastic cells were derived from cells originally expressing Mist1 (i.e. chief cells). Importantly, treatment of Mist1-Kras mice with a MEK inhibitor at 3 months after active Kras induction arrested metaplasia and triggered metaplastic cell extrusion from the mucosa by recrudescence of normal gastric lineages (parietal cells). Lineage mapping demonstrated that the new, normal gastric lineages were not derived from SPEM cells, but rather from normal progenitor cells that had lain dormant within the metaplastic mucosa.48 Similarly, MEK inhibitor treatment in Mongolian gerbils infected with H. pylori also showed reversal of metaplasia and reappearance of normal gastric lineages.61 Recent investigations have also shown that H. pylori infection of Mist1-Kras mice can accelerate dysplasia.87
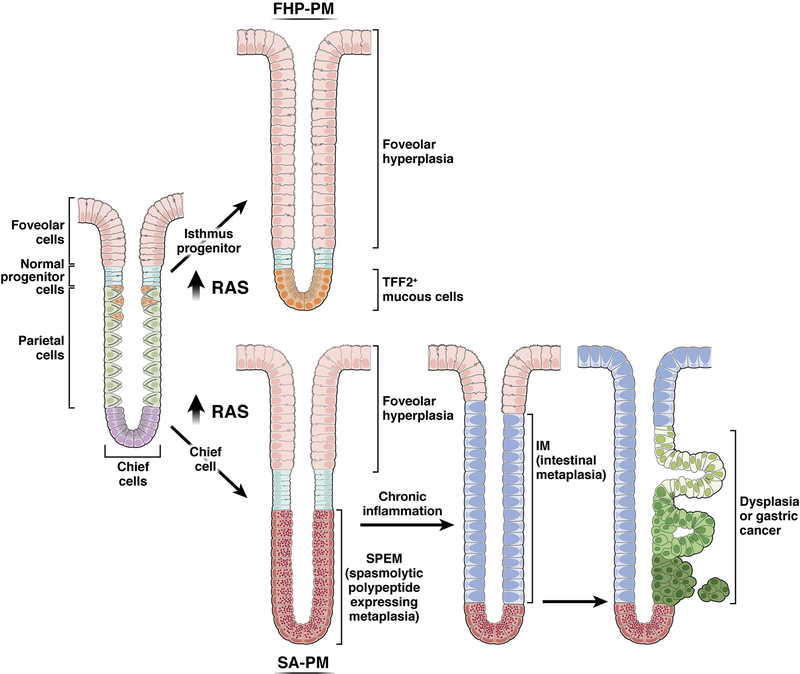
Figure 3: The divergent effects of Ras activation in isthmal progenitor cells and chief cells establish two types of pyloric metaplasia.
Increases in Ras activation in isthmal progenitor cells in transgenic mice and in the setting of Ménétrier’s disease leads to massive foveolar hyperplasia (foveolar hyperplasia predominant pyloric metaplasia, FHP-PM) with preferential differentiation of foveolar cells, and to a lesser extent mucous neck cells, over parietal cells. In contrast, activation of Ras in chief cells leads initially to the development of SPEM from reprogramming of chief cells (SPEM-associated pyloric metaplasia, SA-PM). Continuously active Ras expression can lead to intestinal metaplasia and dysplasia, establishing the full range of pre-neoplastic lineages associated with the development of intestinal type gastric cancer. Note: the intestinal metaplasia depicted here would be of the “incomplete type.” The figure is not meant to show definitively which exact cells are the ones fueling the expansion of dysplastic/neoplastic cells, but evidence does point to lineages at the interface of intestinal metaplasia arising from pyloric metaplasia/SPEM as the figure depicts.
In contrast to the previous work indicating a role for Ras in promoting SPEM from chief cells, it has been suggested that SPEM accrues from activation of Ras signaling in isthmal progenitor cells.80 The laboratories of both authors of the current review article have done extensive lineage mapping using Mist1-CreERT2 to drive various reporters. To a varying degree, we do observe rare cells (less than 1 per 20 gastric units) above the basal chief cell zone that mark as expressing activated nuclear Cre within a few days.70 Moreover, no investigators have published data that MIST1 protein is expressed in isthmal cells. While one must take into account the ability of Kras mutant stem cells to expand through crypt fission,88 it is nevertheless our view that the rare population of Mist1-marked cells outside the chief cell zone cannot on their own account for the majority of SPEM seen in the Mist1-Kras mice
Nevertheless, it is also clear that mouse models suggest that activation of Ras in isthmal cells can result in a gland phenotype that shows the characteristics of a pyloric gland, albeit with a vastly predominant expansion of the foveolar compartment (Figure 3). Activation with mutant Kras directly in isthmal progenitor populations results in a marked foveolar hyperplasia response (Figure 3). This pattern is observed in mice in which Kras is driven either by elements of the Runx1 promoter (eR1-Kras), or by the Iqgap3 promoter (both of which have predominant isthmal progenitor expression)73, 89 It is also seen in mice in which Kras is driven by the Bmi1 promoter or the Lrig1 promoter, which show only isthmal progenitor Kras expression with no mutant Kras induced in chief cells.90, 91 In all of these mouse models, a small number of TFF2-expressing cells are observed at the bases of glands. Whether they are SPEM cells or some derivative of mucous neck cells that phenocopies deep antral gland cells has not been thoroughly examined. This overall pattern therefore does phenocopy a pyloric gland morphology and could be considered as foveolar hyperplasia-predominant pyloric metaplasia (FHP-PM, Figure 3). Importantly, this same gland phenotype, with mostly foveolar hyperplasia and a small number of TFF2-positive cells at their bases,92 is similar to that observed in the stomachs of patients with Ménétrier’s disease. The etiology of Ménétrier’s disease, characterized by massive foveolar hyperplasia, is TGF-alpha/EGFR driven activation of ERK, the upstream activator of Ras.93, 94 Thus, the Ménétrier’s disease pathology phenocopies activation of Ras in isthmal progenitor cells of mice. This FHP-PM or Ménétrier’s phenotype is often associated with expression of the antral transcription factor Pdx1, confirming a pyloric-type metaplasia.89, 91, 92 This type of pyloric metaplasia appears to arise by a switch in lineage allocation such that only foveolar and mucous neck cells arise from the isthmal progenitors with parietal cell generation curtailed. Treatment of Ménétrier’s disease patients with antibodies that block the EGF receptor, causes normalization of the production of parietal cells versus surface cells.94 In sum, in FHP-PM, unlike the situations discussed above where chief cells fuel SPEM, the isthmal progenitor zone may be the source of the basal, TFF2-expressing population. Thus, there seem to be two different pathways toward pyloric metaplasia: one potentially fueled more by isthmal progenitors, and the other fueled more by chief cells. It is notable that the isthmal Ras induction mouse models and Ménétrier’s disease itself do not incite significant inflammation, so the relationship of these lesions to cancer induction remains unclear.
Immune cell regulation of metaplasia induction and progression.
In humans and in rodents, H. pylori induction of metaplasia proceeds through a chronic inflammatory response in two linked phases. Chronic H. pylori infection causes chronic inflammation and parietal cell death, producing atrophic gastritis. Helicobacter-induced parietal cell loss requires intact T and B cell responses and IFN-gamma95 and does not occur in immunocompromised mice (e.g. SCID or Rag1−/− mice).96 In humans, as corpus glands lose parietal cells, pyloric metaplasia develops,42 which is consistent with animal models that indicate that parietal cell loss is required for, and almost always is sufficient to induce chief cells to reprogram to SPEM. Other studies have shown that myeloid-derived suppressor cells (MDSCs) are also required for the development of oxyntic atrophy and SPEM.97 Interestingly, B and T cells are not directly involved in the process of SPEM induction. Rather, both L635-induced and adrenalectomy-induced metaplasia models have revealed that M2-polarized macrophages promote SPEM induction and progression towards more proliferative metaplasia.98, 99
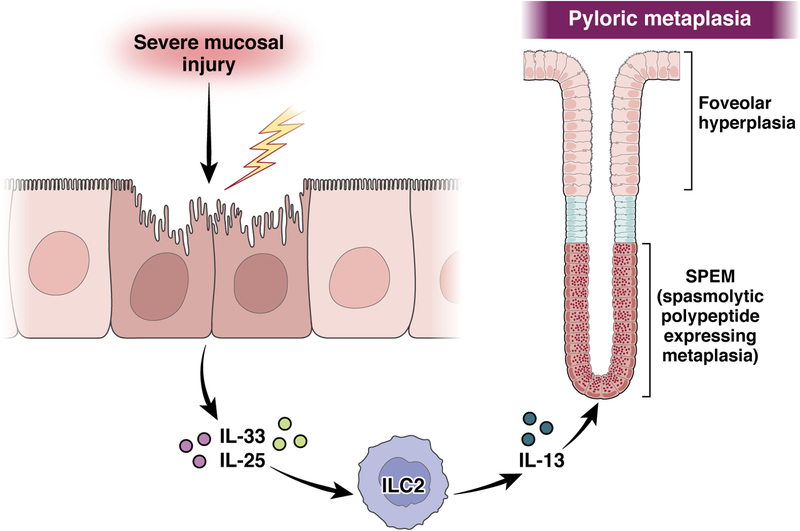
Furthermore, a cascade of intrinsic mucosal cytokines from IL-33-release leading to release of IL-13 is required for induction of SPEM (Figure 4).100, 101 IL-13 in particular appears to be acting on chief cells directly through intrinsic IL13RA1 receptors.100, 102 The key intermediary in this pathway is the class of type two innate lymphoid cells, ILC2s, that are intrinsic to the gastric mucosa.103 During homeostasis, the ILC2s can regulate isthmal progenitor function in the corpus mucosa,80 but with induction of acute parietal cell loss, ILC2s expand and promote the induction of SPEM through release of IL-13 and perhaps other cytokines and growth factors, including amphiregulin and IL-4.103 Ablation of ILC2s prevents the induction of SPEM following acute parietal cell loss.103, 104 Thus, ILC2s are key intrinsic regulators of the early response to severe injury in the stomach. It remains unclear whether IL-13 or other ILC2-derived peptides promote maintenance or progression of metaplastic lineages, especially during chronic injury and inflammation.
Figure 4: Intrinsic immune cells coordinate the initiation of SPEM.
Severe damage in the corpus mucosa leads to the release of IL-33 from surface mucous cells and IL-25 from tuft cells, both of which stimulate the release of IL-13 from ILC2s. IL-13 in turn is required for initiation of the reprogramming of chief cells into SPEM cells and the development of pyloric metaplasia.
Induction of parietal cell loss is also associated with up-regulation of DCLK1-expressing sensory tuft cells in the gastric mucosa.105, 106 Tuft cells express IL-25, another stimulator of ILC2 release of IL-13 (Figure 4).107 Thus, the injured gastric mucosa has coordinated the response of intrinsic immune and sensory elements to orchestrate the response to severe injury. Importantly, resolution of injury and return of normal gastric lineages leads to a rapid loss of tuft cells from the gastric muocsa.105
Delineating the immune cell subsets that cause metaplasia can inform our investigations into gastric adenocarcinoma tumorigenesis. For example, chronic administration to rats of DMP-777 for over a year causes long term oxyntic atrophy and pyloric metaplasia but not dysplasia or cancer, likely because DMP-777 does not cause a marked immune response.60 Similarly, patients who have autoimmune gastritis (pernicious anemia), and thus have chronic oxyntic atrophy due to antibodies to parietal cell-specific proteins (e.g. H+-K+-ATPase), develop severe chronic atrophic gastritis with pyloric as well as intestinal metaplasia.108, 109 However, in the absence of concomitant H. pylori infection, they show a lower risk for adenocarcinoma than those with diffuse atrophic gastritis and metaplasia secondary to H. pylori infection. Indeed, they have a much higher risk for developing tumors of the enterochromaffin-like cell (ECL) leading to multifocal carcinoid tumors.110, 111 It is possible that the reason for the differing adenocarcinoma risk is the type of inflammation stimulated by H. pylori and not simply loss of parietal cells alone, with autoimmune gastritis patients having far fewer mucosal M2-macrophages than patients with gastric adenocarcinoma.44 Thus, development of adenocarcinoma is linked not only loss of parietal cells and response of chief cells, but also to the presence of a milieu with pro-neoplastic macrophages.
Diagnosing SPEM and incomplete vs. complete intestinal metaplasia in sections.
Traditional pathology terms for various lesions are general and not cell-lineage-specific: “mucinous metaplasia”, “foveolar hyperplasia”, “pyloric metaplasia” and “incomplete” and “complete intestinal metaplasia”. As our ability to observe multiple morphological and molecular phenotypes within individual cells increases, we should be able to make more specific diagnoses. However, single markers can also be misleading, as heterogeneous cells can express the same staining phenotype. For example, SPEM lineages are increasingly identified by either histochemical Diastase-PAS staining, where SPEM cells stain as pink rather than the carmine-stained foveolar cells, as well as immunostaining for TFF2. But both of these stains also label mucous neck cells in normal corpus mucosa, so confusion can be present in the setting of mucous neck cell hyperplasia. Similarly, after 14 days of DMP-777 treatment, extensive TFF2-staining cells are observed along half the length of the gland. However, by lineage mapping, only TFF2-expressing cells at the bases of glands are derived from chief cells,40 and long-term nucleotide-labeling studies suggest similar zone-specific patterns of proliferation and reprogramming.56 Thus, it may be better to think of the pattern of gastric units characterized by foveolar cells (often hyperplastic) on top, hyperplastic TFF2+ mucous neck cells in the middle and SPEM cells at the base as “pyloric metaplasia” and dissect each cell lineage within the metaplastic unit as a separate entity, each with its own etiology. In short, it is best to use multiple markers to characterize injury-induced lesions.
We have discussed how intestinal metaplasia likely arises from pyloric metaplasia. However, intestinal metaplasia is not monolithic. In some cases (often referred to as complete or small intestine-like metaplasia), entire intestinal units occur in the stomach with Paneth cells, crypt-base-columnar cells, and enterocytes all arranged in the crypt to villus organization typical of small intestine. In contrast, incomplete intestinal metaplasia has been dubbed “colonic”, although, in our experience, most so-called incomplete intestinal metaplasia is simply more disorganized, harboring immature goblet cells or glands with hybrid gastric and intestinal morphologies. In fact, a common pattern in incomplete metaplasias is to observe mixed, immature intestine-like cells in the superficial portion of the glandular unit with basal cells having characteristics of SPEM cell differentiation in a pattern resembling Barrett’s esophagus with so-called specialized epithelium.33, 112 Recent studies have shown that AQP5-expressing SPEM cells are present at the bases of glands with incomplete, but not complete, intestinal metaplasia.33 Incomplete intestinal metaplasia in humans has been associated with the highest risk for gastric adenocarcinoma, while complete intestinal metaplasia is associated with a lower risk.113 Thus, complete intestinal metaplasia in the stomach, as is also seen in Barrett’s esophagus, may represent a successful endpoint for evolution of a protective metaplasia.30 In contrast, incomplete intestinal metaplasia, with mixtures of gastric and less mature intestinal goblet lineages indicating a milieu with lineage confusion, is associated with higher risk of dysplasia and cancer, both in stomach and in esophagus in the setting of Barrett’s metaplasia.112, 114
Insights into the evolution of metaplasia towards dysplasia.
It is likely that many mature cells in most tissues have access to a conserved program of paligenosis, like the one that turns chief into SPEM cells, to execute a regenerative, healing response to tissue injury.76 Pyloric metaplasia can emerge in a gland-by-gland pattern from the corpus and incisura in both humans1, 78, 115 and in rodent models.74, 116 Thus, the metaplastic response to injury is organized to repair localized insults to the mucosa in the harsh environment of the gastric mucosa. In the case of more expansive local injuries, such as focal ulcerations, SPEM induction mobilizes regenerative cells surrounding the entire circumference of an ulcer.6, 117, 118 Thus, in general, pyloric metaplasia likely evolved as a transient source of regenerative, reparative cells that either redifferentiate back into mature cells or are replaced by normal gastric lineages upon resolution of mucosal injury. However, chronic injury or inflammation can make metaplasia persist, spread, and even transform into other types of metaplasia or dysplasia.
As mentioned, the animal models for following development of intestinal metaplasia and other steps involving progression of pyloric metaplasia to other lesions are relatively limited.119, 120 However, the metaplasia that develops in Mist1-Kras mice phenocopies incomplete intestinal metaplasia with an absence of Paneth cells or CCK-expressing enteroendocrine cells and the presence of basal AQP5-expressing SPEM cells.48 Interestingly, recent studies have shown that the gene Trop2 (Tacstd2), an intronless gene encoding a membrane protein of uncertain function, is upregulated in Mist1-Kras mice beginning at 3 months after Kras(G12D) and is augmented further at four months, concomitant with emergence of dysplastic glands.43 In human tissues, TROP2 is upregulated in incomplete intestinal metaplasia, but not in complete intestinal metaplasia.43 TROP2 is prominently expressed in human dysplasia, but no TROP2 expression is observed in either mouse or human SPEM lineages. Knockdown of Trop2 in mouse dysplastic gastroids leads to reduced organoid growth.43 As noted above, AQP5-expresing SPEM cells are present at the bases of incomplete intestinal metaplasia glands.33 Overall, thus, there may be a connection between the lineage confusion associated with incomplete intestinal metaplasia and progression to dysplasia.
Other experimental models have also shed light on progression of metaplasia and dysplasia. Organoids grown from corpus epithelial cells (gastroids) of Mist1-Kras mice, for example, adopt intestinalized growth patterns.121 In contrast to such gastroids (termed Meta3 since the cultures were begun 3 months after induction of mutant Kras), 4-month, Meta4 gastroids express increased Trop2 and harbor stem cells that may propagate dysplastic clones.43, 121 A more quiescent stem cell population was triple positive for markers CD44, CD133 and CD166. A separate, actively proliferating Meta4 stem cell population was positive for CD133 and CD166, but negative for CD44. The populations classified by these two patterns of proliferation and gene expression could interconvert, and proliferation of the double-positive stem cells was inhibited by MEK inhibitor treatment.121 The data show how discrete populations of cells with behavior and gene expression patterns consistent with progenitors seen in dysplasia and cancer can emerge ultimately from chief cells forced to express mutant Kras. Moreover, such dysplastic stem cells may be precursors of progressive carcinogenesis.
As discussed above, metaplasia can originate and spread from individual gland units, and, even in a phenotypically normal stomach from an organ donor, individual glands with SPEM can be found within a field of normal glands.1 Similarly, individual glands with the features of incomplete intestinal metaplasia can be identified in a field of complete intestinal metaplasia and normal gastric glands.33 Thus, adenocarcinoma may originate from single glands of metaplasia, especially from glands with lineage confusion and mixtures of SPEM and incomplete intestinal lineages. Accordingly, single glands of incomplete intestinal metaplasia have been shown to expand through gland fission, spawning pre-neoplastic gland islands.122 This scenario is compatible with the observed pattern of field cancerization in patients with extensive metaplasia, with frequent metachronous lesions found in patients after they have undergone endoscopic mucosal resection for early (Stage I) cancers. Such patients with early cancers demonstrate a 2–4% per year incidence of metachronous cancers despite complete resection and H. pylori eradication treatment.123, 124 Thus, therapy for gastric cancer must also confront the fact that there may be diffuse individual glands with neoplastic potential throughout the stomach.
Although, we are still dissecting how pyloric metaplasia evolves into intestinal metaplasia of differing phenotypes or progresses to cancer, there does seem to be an order to how pyloric metaplasia itself spreads within the stomach. It first occurs in humans usually at junctional zones between antrum and corpus (and likely cardia and corpus). It then spreads over decades into the corpus to eventually include the whole corpus/fundus. In endemic H. pylori regions, the transformation occurs over the 5 or so decades from the first appearance of atrophic gastritis and metaplasia in children.125,126 Mouse models of H. pylori and pyloric metaplasia recapitulate these patterns.3, 74 The prevailing hypothesis is that the bacteria, which initially can persist only in the antrum, may trigger pyloric metaplasia in the corpus to progressively convert the whole stomach into a hospitable niche for their continued growth.74, 87, 125
It has long been a mystery why many gastric cancers seem to originate in the antrum even though the extent of atrophic gastritis/metaplasia in the corpus is the major risk factor. However, the seeming antral origin of gastric cancers has to be scrutinized, given that the antral histological appearance may be due to metaplastic conversion of mucosa that had been corpus decades earlier. Common sites for tumors are along borders between corpus- and antral-type mucosae. Interestingly, in our own experience inducing multiple rounds of paligenosis along with mutagenesis in a mouse model, the invasive tumors invariably arose in regions surrounded by non-tumor epithelial cells that exhibited antral histology on one side and corpus histology on the other.127 Thus, one way to explain tumors arising in these antral or antral-corpus junctional zones is that those are the regions where metaplastic injuries have occurred the longest, because the initial metaplasia in children begins in corpus epithelium bordering the antrum. In those initial regions of pyloric metaplasia, decades of cycles of paligenotic cell reprogramming and redifferentiation and repair may occur. Such cycles would allow the acquisition, storage, and eventual unmasking of serial mutations, putting cells at risk for developing dysplasia with uncontrolled growth. This pattern of mutation accumulation has been called the cyclical hit model of tumorigenesis.128
A common gastrointestinal tract metaplasia/tumorigenesis phenotype.
Pathologists and investigators have mostly approached tumorigenesis and metaplasia within the silos of their particular tissue of interest. We who study SPEM/pyloric metaplasia in the stomach focus on the molecular-morphological patterns in the stomach. Barrett’s esophagus is studied separately, as is acinar-to-ductal metaplasia in the pancreas, and proliferative ductular changes in cirrhosis are studied by those interested in hepatocellular tumorigenesis. However, there is emerging evidence that all such processes may be similar and that each of these metaplasias arises as mature cells in each tissue invoke similar gene programs to converge on a shared regenerative phenotype.76 All of these lesions have certain common characteristics, including that they secrete wound-healing substances (mucins, TFFs), and express common, progenitor-associated transcription factors (e.g. SOX9 and nuclear YAP1), as well as markers of proliferating or progenitor cells (e.g. CD44). Interestingly, as we mentioned earlier, H. pylori can induce the transition from the complex architecture of the gastric corpus gland units to a simpler pyloric-like gland metaplasia. The normal pylorus is composed of a majority of mucus-secreting cells, comprising many cells positive for TFF1 or TFF2, as well as AQP5, CD44 and SOX9.33, 129
Similarly, acinar-ductal metaplasia in the pancreas is characterized by acinar cells (the digestive-enzyme-secreting, exocrine cousins of gastric chief cells) downscaling and reprogramming to become mucin-expressing, SOX9+ cells that can proliferate to regenerate pancreatic damage.130, 131 The precancerous lesions known to pathologists as Pancreatic intraepithelial neoplasias (PaNINs) arise from such metaplastic acinar cell changes, and can progress to pancreatic ductal adenocarcinoma in a similar sequence to chief cells undergoing reprogramming into SPEM and eventually progressing to gastric adenocarcinoma.132 Accordingly, recent single cell transcriptomes have noted the presence of mucus secretory lineages in the setting of pancreatitis that show strong similarity to SPEM or antral/pyloric glands.133–135 Similarly, the ulcer-associated cell lineage (UACL), originally identified by Sir Nicholas Wright in the context of intestinal mucosal damage in Crohn’s disease, represents a recapitulation of the pyloric gland structure to provide local injury response.136, 137 Recent transcriptomic analysis of UACL lesions has revealed that these lesions showed similarity to SPEM lineages within a pyloric metaplasia lesion.138, 139 Other studies have highlighted the plasticity of Paneth cells. Paneth cells, like chief and acinar cells, are MIST1-expressing secretory cells and display plasticity that may contribute to a metaplasia that also resembles the pylorus.140–142 Pyloric-like metaplastic changes have long been known to arise after chronic acid exposure in the setting of duodenal peptic ulcers.143 Similarly, the gall bladder can undergo pyloric-like metaplastic changes.144
The prominent example of Barrett’s metaplasia in the esophagus appears to be a specialized case. While in other GI tract locations, pyloric metaplasia lineages are likely derived from the plasticity of resident cell lineages, in the case of Barrett’s epithelium, the origin of the initial change to replace normal squamous epithelium with pyloric-like epithelium is unclear. In perhaps the most accepted theory, the origin of the columnar epithelium in Barrett’s accrues from the most proximal glands of the fundus. These glands,145 which vary in abundance in humans, are pyloric-like and known as cardia-type mucosa. Every mammal appears to have one or two pyloric type glands at the gastroesophageal squamocolumnar junction.145 It is thought that cells from these cardia glands can migrate proximally to replace squamous esophageal cells in the face of severe damage via refluxed acid and bile.112, 146–148 Interestingly, proximal cardia glands vary widely in abundance among the human population, and autopsy and biopsy series of children have shown the naïve cardia may be only a few millimeters. Thus, some have proposed that most, if not all, of what composes an adult cardia is actually metaplastic.149, 150 That would indicate that the normal corpus glands have been replaced over time by insult (either with H. pylori or acid reflux) with pyloric metaplasia.145 Whatever the etiology, the initial metaplasia in Barrett’s is likely to involve the replacement of squamous epithelia with pyloric type epithelium with goblet and other intestinal cell phenotypes arriving on the scene later.
Thus, one can make the case that multiple types of metaplastic changes, known by organ-specified names, may all be related manifestations of pyloric metaplasia. However, similar, mucin-rich, proliferative, simple glandular/duct-like growth patterns also characterize the epithelial cells of the developing, embryonic gastrointestinal tract. Indeed, the stomach mucosa, itself, is first pyloric-like with fundic-type chief and parietal cells maturing only after 6 months of gestation in humans21 and postnatally in mice.151, 152 Thus, pyloric metaplasia might also be considered a reversion to the embryonic pattern. Similarly, it has been noted that acinar-ductal metaplasia is considered more a return to an embryonic pattern of pancreas organization with scant resemblance to adult pancreatic duct-lining epithelial cells.153, 154 Regardless of whether the lineage changes in the injured gastrointestinal mucosa are described as a pyloric metaplasia or a return to an embryonic phenotype, it is clear that mechanisms of mucosal cell plasticity have much more in common than previously thought, and we might learn much about repair and common routes to tumorigenesis by studying them as a common mechanism.
Conclusions and prospectives: Is metaplasia good or bad?
Several lines of evidence indicate that pyloric metaplasias are typically benign, regenerative adaptations to injury that can be transient, if the injury is temporary. Even if injury and the reactive metaplasia become chronic, in most cases, the changes are benign. The vast majority of patients with Barrett’s esophagus never develop dysplasia or cancer, and complete intestinal metaplasia in the stomach also seems to be benign.12, 30 Thus, most metaplastic processes during chronic injury may be successful, adaptive mucosal responses. The establishment of protective lineages in the mucosa prevents further damage and may allay deleterious symptoms, as in the case of gastroesophageal reflux.
On the other hand, in both the esophagus and the stomach, the risk of neoplasia appears to correlate with the presence of metaplastic lineages that fail to complete their transitions to endpoint metaplasia, but are rather caught in between gastric and intestinal lineages. This type of lineage confusion may represent the true pre-neoplastic field cancerization scenario. In both incomplete intestinal metaplasia and mixed lineage Barrett’s epithelium, these pre-neoplastic lesions may represent isolated single or small groups of glands within a field of otherwise benign complete intestinal or columnar metaplasia. Such scenarios can account for the multifocality of cancerous lesions within a field of metaplastic esophageal or gastric mucosa. It certainly accounts for the high incidence of metachronous lesions in the stomach of patients following endoscopic resection of Stage I intestinal type gastric cancers,123, 124, 155 even after concomitant eradication of H. pylori.156, 157 Therefore, the vast majority of metaplastic lesions in both the esophagus and stomach appear to be protective, and future focus should rely on identification and treatment of patients with incomplete intestinal metaplasia in the stomach and the equivalent mixed metaplasia (“specialized epithelium”) in the esophagus, who are truly at higher risk of developing cancer. Targeting early metaplastic lineages for reprogramming could be an effective approach in scenarios with chronic pyloric and intestinal metaplasia.
ACKNOWLEDGMENTS
JRG is supported by grants from a Department of Veterans Affairs Merit Review Award IBX000930, NIH RO1 DK101332 and DOD CA160479, and a Cancer UK Grand Challenge Award 29075. JCM is supported by NIH awards: R01DK094989, R01DK105129, R01DK110406, P30 DK052574, P30 CA09182, R01CA239645, R01CA246208, and the BETRNet (U54CA163060).
Footnotes
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Choi E, Roland JT, Barlow BJ, et al. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut 2014;63:1711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engevik AC, Kaji I, Goldenring JR. The Physiology of the Gastric Parietal Cell. Physiol Rev 2020;100:573–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saenz JB, Mills JC. Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer. Nat Rev Gastroenterol Hepatol 2018;15:257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aihara E, Medina-Candelaria NM, Hanyu H, et al. Cell injury triggers actin polymerization to initiate epithelial restitution. J Cell Sci 2018;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aihara E, Hentz CL, Korman AM, et al. In vivo epithelial wound repair requires mobilization of endogenous intracellular and extracellular calcium. J Biol Chem 2013;288:33585–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engevik AC, Feng R, Choi E, et al. The Development of Spasmolytic Polypeptide/TFF2-Expressing Metaplasia (SPEM) During Gastric Repair Is Absent in the Aged Stomach. Cell. Molec. Gastroent. Hepatol. 2016;2:605–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldenring JR. Pyloric metaplasia, pseudopyloric metaplasia, ulcer-associated cell lineage and spasmolytic polypeptide-expressing metaplasia: reparative lineages in the gastrointestinal mucosa. J Pathol 2018;245:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt A Untersuchungen über das menschliche Magenepithel unter normalen und pathologischen Verhältnissen. Archiv für pathologische Anatomie und Physiologie und für klinische Medicin 1896;143:477–508. [Google Scholar]
- 9.Chuma M Zur normalen u. patholog. Histologie der Magenschleimhaut. Virchows Archiv für pathologische Anatomie und Physiologie und für klinische Medizin 1923;247:236–277. [Google Scholar]
- 10.Kalima T Pathologisch-anatomische Studien iiber die Gastritis des Ulcusmagens nebst einigen Bemerkungen zur Pathogenese und pathologischen Anatomie des Magengeschwiirs. Arch. Klin. Chir. 1924;128:20–108. [Google Scholar]
- 11.Bonne C, Hartz PH, Klerks JV, et al. Morphology of the Stomach and Gastric Secretion in Malays and Chinese and the Different Incidence of Gastric Ulcer and Cancer in These Races. Am J Cancer 1938;33:265–279. [Google Scholar]
- 12.Graham DY, Zou WY. Guilt by association: intestinal metaplasia does not progress to gastric cancer. Curr Opin Gastroenterol 2018;34:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faber K Gastritis and Its Consequences. New York: Oxford University Press, 1935. [Google Scholar]
- 14.Störk O Über gastritis chronica. Wien. kl. Wchnsch. 1922. [Google Scholar]
- 15.Mathieu A État de la muqueuse de l’estomac dans le cancer de cet organe. . Arch. Gén. Méd. 1889;163:402–420. [Google Scholar]
- 16.Schaffer EA. Text-book of microscopic anatomy. LONDON LONGMANS GREEN AND CO; 1912. [Google Scholar]
- 17.Correa P A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 18.Stemmermann GN, Hayashi T. Intestinal metaplasia of the gastric mucosa: a gross and microscopic study of its distribution in various disease states. J Natl Cancer Inst 1968;41:627–34. [PubMed] [Google Scholar]
- 19.Morson BC. Intestinal metaplasia of the gastric mucosa. Br J Cancer 1955;9:365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab.Invest. 1999;79:639–646. [PMC free article] [PubMed] [Google Scholar]
- 21.GUISS LW, EWART ST FW. CHRONIC ATROPHIC GASTRITIS AND CANCER OF THE STOMACH. Archives of Surgery 1943;46:823–843. [Google Scholar]
- 22.Ewald CA, Manges M. The diseases of the stomach. New York: Appleton, 1897. [Google Scholar]
- 23.Geboes K, Van den Oord JJ, Rutgeerts P, et al. Immunohistochemical identification of lysozyme in pseudopyloric gland metaplasia in Crohn’s disease. Hepatogastroenterology 1983;30:158–60. [PubMed] [Google Scholar]
- 24.Oddsson E, Binder V, Thorgeirsson T, et al. A prospective comparative study of clinical and pathological characteristics in Icelandic and Danish patients with gastric ulcer, duodenal ulcer, and X-ray negative dyspepsia. II. Histological results. Scand J Gastroenterol 1978;13:489–95. [DOI] [PubMed] [Google Scholar]
- 25.Lambert R Chronic gastritis. A critical study of the progressive atrophy of the gastric mucosa. Digestion 1972;7:83–126. [DOI] [PubMed] [Google Scholar]
- 26.Lee FD. Pyloric Metaplasia in the Small Intestine. J Pathol Bacteriol 1964;87:267–77. [DOI] [PubMed] [Google Scholar]
- 27.Wright NA. Aspects of the biology of regeneration and repair in the human gastrointestinal tract. Philos Trans R Soc Lond B Biol Sci 1998;353:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halldorsdottir AM, Sigurdardottrir M, Jonasson JG, et al. Spasmolytic polypeptide-expressing metaplasia (SPEM) associated with gastric cancer in Iceland. Dig Dis Sci 2003;48:431–41. [DOI] [PubMed] [Google Scholar]
- 29.Lennerz JKM, Kim S, Oates EL, et al. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia and carcinoma. Amer. J. Pathol. 2010;177:1514–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham DY, Rugge M, Genta RM. Diagnosis: gastric intestinal metaplasia - what to do next? Curr Opin Gastroenterol 2019;35:535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menetrier P Cancer. In: Gilbert A, Thoinot L, eds. Nouveau Traité de Médicine et de Thérapeutique. Paris: Librairie J.B.Ballière et Fils, 1908. [Google Scholar]
- 32.Wada T, Ishimoto T, Seishima R, et al. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci 2013;104:1323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S, Jang B, Min J, et al. Upregulation of AQP5 defines spasmolytic polypeptide-expressing metaplasia (SPEM) and progression to incomplete intestinal metaplasia. Cell Molec Gastroenterol. Hepataol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nozaki K, Ogawa M, Williams JA, et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008:511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshizawa N, Takenaka Y, Yamaguchi H, et al. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest 2007;87:1265–1276. [DOI] [PubMed] [Google Scholar]
- 36.Nomura S, Baxter S, Yamaguchi T, et al. Spasmolytic polypeptide expressing metaplasia (SPEM) to pre-neoplasia in H. felis-infected mice. Gastroenterology 2004;127:582–594. [DOI] [PubMed] [Google Scholar]
- 37.Nomura S, Yamaguchi H, Wang TC, et al. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild type and gastrin deficient mice. Amer.J.Physiol. 2004;288:G362–G375. [DOI] [PubMed] [Google Scholar]
- 38.Weis VG, Sousa JF, LaFleur BJ, et al. Heterogeneity in mouse SPEM lineages identifies markers of metaplastic progression. Gut 2013;62:1270–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huh WJ, Khurana SS, Geahlen JH, et al. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 2012;142:21–24.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nam KT, Lee H-J, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 2010;139:2028–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer AR, Engevik AC, Willet SG, et al. Cystine/Glutamate Antiporter (xCT) Is Required for Chief Cell Plasticity After Gastric Injury. Cell Mol Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radyk MD, Burclaff J, Willet SG, et al. Metaplastic Cells in the Stomach Arise, Independently of Stem Cells, via Dedifferentiation or Transdifferentiation of Chief Cells. Gastroenterology 2018;154:839–843.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riera KM, Jang B, Min J, et al. Trop2 is upregulated in the transition to dysplasia in the metaplastic gastric mucosa. Journal of Pathology 2020;251:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeong S, Choi E, Petersen CP, et al. Distinct metaplastic and inflammatory phenotypes in autoimmune and adenocarcinoma-associated chronic atrophic gastritis. United European Gastroenterol J 2017;5:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willet SG, Lewis MA, Miao ZF, et al. Regenerative proliferation of differentiated cells by mTORC1-dependent paligenosis. EMBO J 2018;37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldenring JR, Nam KT, Wang TC, et al. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 2010;138:2207–10, 2210.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gosset A, Masson P. Cancer Intestinal de l’Estomac. Press Médicale; 1923:225–228. [Google Scholar]
- 48.Choi E, Hendley AM, Bailey JM, et al. Expression of Activated Ras in Gastric Chief Cells of Mice Leads to the Full Spectrum of Metaplastic Lineage Transitions. Gastroenterology 2016;150:918–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nomura S, Baxter T, Yamaguchi H, et al. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology 2004;127:582–94. [DOI] [PubMed] [Google Scholar]
- 50.Han S, Fink J, Jorg DJ, et al. Defining the Identity and Dynamics of Adult Gastric Isthmus Stem Cells. Cell Stem Cell 2019;25:342–356 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miao ZF, Adkins-Threats M, Burclaff JR, et al. A Metformin-Responsive Metabolic Pathway Controls Distinct Steps in Gastric Progenitor Fate Decisions and Maturation. Cell Stem Cell 2020;26:910–925.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat.Rec. 1993;236:297–313. [DOI] [PubMed] [Google Scholar]
- 53.Miao ZF, Sun JX, Adkins-Threats M, et al. DDIT4 Licenses Only Healthy Cells to Proliferate During Injury-induced Metaplasia. Gastroenterology 2021;160:260–271.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang TC, Goldenring JR, Dangler C, et al. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–689. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen TL, Khurana SS, Bellone CJ, et al. Autoimmune gastritis mediated by CD4+ T cells promotes the development of gastric cancer. Cancer Res 2013;7:2117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burclaff J, Willet SG, Saenz JB, et al. Proliferation and Differentiation of Gastric Mucous Neck and Chief Cells During Homeostasis and Injury-induced Metaplasia. Gastroenterology 2020;158:598–609.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bockerstett KA, Lewis SA, Noto CN, et al. Single-Cell Transcriptional Analyses Identify Lineage-Specific Epithelial Responses to Inflammation and Metaplastic Development in the Gastric Corpus. Gastroenterology 2020;159:2116–2129.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bockerstett KA, Lewis SA, Wolf KJ, et al. Single-cell transcriptional analyses of spasmolytic polypeptide-expressing metaplasia arising from acute drug injury and chronic inflammation in the stomach. Gut 2020;69:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nomura S, Yamaguchi H, Ogawa M, et al. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 2005;288:G362–75. [DOI] [PubMed] [Google Scholar]
- 60.Goldenring JR, Ray GS, Coffey RJ, et al. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology. 2000;118:1080–1093. [DOI] [PubMed] [Google Scholar]
- 61.Yang Q, Yasuda T, Choi E, et al. MEK Inhibitor Reverses Metaplasia and Allows Re-Emergence of Normal Lineages in Helicobacter pylori-Infected Gerbils. Gastroenterology 2019;156:577–581 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang TC, Dangler CA, Chen D, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 2000;118:36–47. [DOI] [PubMed] [Google Scholar]
- 63.Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow-derived cells. Science 2004;306:1568–71. [DOI] [PubMed] [Google Scholar]
- 64.Ogawa M, Nomura S, Car BD, et al. Omeprazole treatment ameliorates oxyntic atrophy induced by DMP-777. Dig Dis Sci 2006;51:431–9. [DOI] [PubMed] [Google Scholar]
- 65.Manning EH, Lapierre LA, Mills JC, et al. Tamoxifen Acts as a Parietal Cell Protonophore. Cell Mol Gastroenterol Hepatol 2020;10:655–657.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keeley TM, Horita N, Samuelson LC. Tamoxifen-Induced Gastric Injury: Effects of Dose and Method of Administration. Cell Mol Gastroenterol Hepatol 2019;8:365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Neal RL, Nam KT, LaFleur BJ, et al. Human epididymis protein 4 is upregulated in gastric and pancreatic adenocarcinomas. Hum Pathol 2013;44:734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramsey VG, Doherty JM, Chen CC, et al. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 2007;134:211–22. [DOI] [PubMed] [Google Scholar]
- 69.Lo HG, Jin RU, Sibbel G, et al. A single transcription factor is sufficient to induce and maintain secretory cell architecture. Genes Dev 2017;31:154–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weis VG, Petersen CP, Weis JA, et al. Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am J Physiol Gastrointest Liver Physiol 2016;312:G67–G76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leushacke M, Tan SH, Wong A, et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol 2017;19:774–786. [DOI] [PubMed] [Google Scholar]
- 72.Stange DE, Koo BK, Huch M, et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 2013;155:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsuo J, Kimura S, Yamamura A, et al. Identification of Stem Cells in the Epithelium of the Stomach Corpus and Antrum of Mice. Gastroenterology 2017;152:218–231. [DOI] [PubMed] [Google Scholar]
- 74.Saenz JB, Vargas N, Mills JC. Tropism for Spasmolytic Polypeptide-Expressing Metaplasia Allows Helicobacter pylori to Expand Its Intragastric Niche. Gastroenterology 2019;156:160–174 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miao ZF, Lewis MA, Cho CJ, et al. A Dedicated Evolutionarily Conserved Molecular Network Licenses Differentiated Cells to Return to the Cell Cycle. Dev Cell 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mills JC, Sansom OJ. Reserve stem cells: Differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal 2015;8:re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Radyk MD, Spatz LB, Peña BL, et al. ATF3 induces RAB7 to govern autodegradation in paligenosis, a conserved cell plasticity program. . EMBO Reports 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shimizu T, Sohn Y, Choi E, et al. Decrease in MiR-148a Expression During Initiation of Chief Cell Transdifferentiation. Cell Mol Gastroenterol Hepatol 2020;9:61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Capoccia BJ, Jin RU, Kong YY, et al. The ubiquitin ligase Mindbomb 1 coordinates gastrointestinal secretory cell maturation. J Clin Invest 2013;123:1475–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hayakawa Y, Ariyama H, Stancikova J, et al. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell 2015;28:800–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hata M, Kinoshita H, Hayakawa Y, et al. GPR30-Expressing Gastric Chief Cells Do Not Dedifferentiate But Are Eliminated via PDK-Dependent Cell Competition During Development of Metaplasia. Gastroenterology 2020;158:1650–1666 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caldwell B, Meyer AR, Weis JA, et al. Chief cell plasticity is the origin of metaplasia following acute injury in the stomach mucosa. Gut 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miao ZF, Adkins-Threats M, Burclaff JR, et al. A Metformin-Responsive Metabolic Pathway Controls Distinct Steps in Gastric Progenitor Fate Decisions and Maturation. Cell Stem Cell 2020;26:910–925 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shimada S, Mimata A, Sekine M, et al. Synergistic tumour suppressor activity of E-cadherin and p53 in a conditional mouse model for metastatic diffuse-type gastric cancer. Gut 2012;61:344–53. [DOI] [PubMed] [Google Scholar]
- 85.Kim TH, Shivdasani RA. Notch signaling in stomach epithelial stem cell homeostasis. J Exp Med 2011;208:677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khurana SS, Riehl TE, Moore BD, et al. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem 2013;288:16085–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Brien VP, Koehne AL, Dubrulle J, et al. Sustained Helicobacter pylori infection accelerates gastric dysplasia in a mouse model. Life Sci Alliance 2021;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Snippert HJ, Schepers AG, van Es JH, et al. Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep 2014;15:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsuo J, Douchi D, Myint K, et al. Iqgap3-Ras axis drives stem cell proliferation in the stomach corpus during homoeostasis and repair. Gut 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi E, Means AL, Coffey RJ, et al. Active Kras Expression in Gastric Isthmal Progenitor Cells Induces Foveolar Hyperplasia but Not Metaplasia. Cell Mol Gastroenterol Hepatol 2019;7:251–253 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoshioka T, Fukuda A, Araki O, et al. Bmi1 marks gastric stem cells located in the isthmus in mice. J Pathol 2019;248:179–190. [DOI] [PubMed] [Google Scholar]
- 92.Nomura S, Settle SH, Leys CM, et al. Evidence for repatterning of the gastric fundic epithelium associated with Menetrier’s disease and TGFalpha overexpression. Gastroenterology 2005;128:1292–305. [DOI] [PubMed] [Google Scholar]
- 93.Nomura S, Settle SH, Leys C, et al. Evidence for repatterning of the gastric fundic epithelium associated with Menetrier’s disease and TGFa overexpression. Gastroenterology 2005;128:1292–1305. [DOI] [PubMed] [Google Scholar]
- 94.Burdick JS, Chung E, Tanner G, et al. Treatment of Menetrier’s disease with a monoclonal antibody against the epidermal growth factor receptor. N Engl J Med 2000;343:1697–701. [DOI] [PubMed] [Google Scholar]
- 95.Osaki LH, Bockerstett KA, Wong CF, et al. Interferon-gamma directly induces gastric epithelial cell death and is required for progression to metaplasia. J Pathol 2019;247:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smythies LE, Waites KB, Lindsey JR, et al. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol 2000;165:1022–9. [DOI] [PubMed] [Google Scholar]
- 97.Ding L, Hayes MM, Photenhauer A, et al. Schlafen 4-expressing myeloid-derived suppressor cells are induced during murine gastric metaplasia. J Clin Invest 2016;126:2867–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Petersen CP, Weis VG, Nam KT, et al. Macrophages Promote Progression of Spasmolytic Polypeptide-Expressing Metaplasia Following Acute Loss of Parietal Cells. Gastroenterology 2014;146:1727–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Busada JT, Ramamoorthy S, Cain DW, et al. Endogenous glucocorticoids prevent gastric metaplasia by suppressing spontaneous inflammation. J Clin Invest 2019;129:1345–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petersen CP, Meyer AR, De Salvo C, et al. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut 2018;67:805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Salvo C, Pastorelli L, Petersen CP, et al. Interleukin 33 Triggers Early Eosinophil-Dependent Events Leading to Metaplasia in a Chronic Model of Gastritis-Prone Mice. Gastroenterology 2021;160:302–316.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Noto CN, Hoft SG, Bockerstett KA, et al. IL-13 acts directly on gastric epithelial cells to promote metaplasia development during chronic gastritis. Cell Mol Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meyer AR, Engevik AC, Madorsky T, et al. Group 2 Innate Lymphoid Cells Coordinate Damage Response in the Stomach. Gastroenterology 2020;159:2077–2091 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Busada JT, Peterson KN, Khadka S, et al. Glucocorticoids and Androgens Protect From Gastric Metaplasia by Suppressing Group 2 Innate Lymphoid Cell Activation. Gastroenterology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi E, Petersen CP, Lapierre LA, et al. Dynamic expansion of gastric mucosal doublecortin-like kinase 1-expressing cells in response to parietal cell loss is regulated by gastrin. Am J Pathol 2015;185:2219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saqui-Salces M, Keeley TM, Grosse AS, et al. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem Cell Biol 2011;136:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.von Moltke J, Ji M, Liang HE, et al. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016;529:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neumann WL, Coss E, Rugge M, et al. Autoimmune atrophic gastritis--pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol 2013;10:529–41. [DOI] [PubMed] [Google Scholar]
- 109.Toh B, Gleeson PA, Simpson RJ, et al. The 60- to 90-kDa parietal cell autoantigen associated with autoimmune gastritis is a b subunit of the gastric H+/K+-ATPase (proton pump). Proc.Natl.Acad.Sci.,USA 1990;87:6418–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Petersson F, Borch K, Franzen LE. Prevalence of subtypes of intestinal metaplasia in the general population and in patients with autoimmune chronic atrophic gastritis. Scand J Gastroenterol 2002;37:262–6. [DOI] [PubMed] [Google Scholar]
- 111.Hughes JW, Muegge BD, Tobin GS, et al. High-Risk Gastric Pathology and Prevalent Autoimmune Diseases in Patients with Pernicious Anemia. Endocr Pract 2017;23:1297–1303. [DOI] [PubMed] [Google Scholar]
- 112.Lavery DL, Nicholson AM, Poulsom R, et al. The stem cell organisation, and the proliferative and gene expression profile of Barrett’s epithelium, replicates pyloric-type gastric glands. Gut 2014;6:1854–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gonzalez CA, Sanz-Anquela JM, Companioni O, et al. Incomplete type of intestinal metaplasia has the highest risk to progress to gastric cancer: results of the Spanish follow-up multicenter study. J Gastroenterol Hepatol 2016;31:953–8. [DOI] [PubMed] [Google Scholar]
- 114.Ota H, Katsuyama T, Nakajima S, et al. Intestinal metaplasia with adherent Helicobacter pylori: a hybrid epithelium with both gastric and intestinal features. Hum Pathol 1998;29:846–50. [DOI] [PubMed] [Google Scholar]
- 115.Hebbel R The topography of chronic gastritis in otherwise normal stomachs. Am J Pathol 1949;25:125–41. [PMC free article] [PubMed] [Google Scholar]
- 116.Shimizu T, Choi E, Petersen CP, et al. Characterization of progressive metaplasia in the gastric corpus mucosa of Mongolian gerbils infected with Helicobacter pylori. J Pathol 2016;239:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bertaux-Skeirik N, Wunderlich M, Teal E, et al. CD44 variant isoform 9 emerges in response to injury and contributes to the regeneration of the gastric epithelium. J Pathol 2017;242:463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aihara E, Matthis AL, Karns RA, et al. Epithelial Regeneration After Gastric Ulceration Causes Prolonged Cell Type Alterations. Cell. Molec. Gastroent. Hepatol. 2016;2:625–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mutoh H, Hakamata Y, Sato K, et al. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem Biophys Res Commun 2002;294:470–9. [DOI] [PubMed] [Google Scholar]
- 120.Silberg DG, Sullivan J, Kang E, et al. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology 2002;122:689–96. [DOI] [PubMed] [Google Scholar]
- 121.Min J, Vega PN, Engevik AC, et al. Heterogeneity and dynamics of active Kras-induced dysplastic lineages from mouse corpus stomach. Nat Commun 2019;10:5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gutierrez-Gonzalez L, Graham TA, Rodriguez-Justo M, et al. The clonal origins of dysplasia from intestinal metaplasia in the human stomach. Gastroenterology 2011;140:1251–1260 e1–6. [DOI] [PubMed] [Google Scholar]
- 123.Pimentel-Nunes P, Mourao F, Veloso N, et al. Long-term follow-up after endoscopic resection of gastric superficial neoplastic lesions in Portugal. Endoscopy 2014;46:933–40. [DOI] [PubMed] [Google Scholar]
- 124.Kosaka T, Endo M, Toya Y, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center retrospective study. Dig Endosc 2014;26:183–91. [DOI] [PubMed] [Google Scholar]
- 125.Ricuarte O, Gutierrez O, Cardona H, et al. Atrophic gastritis in young children and adolescents. J Clin Pathol 2005;58:1189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.El-Zimaity HMT, Ota H, Graham DY, et al. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer. 2002;94:1428–1436. [DOI] [PubMed] [Google Scholar]
- 127.Miao ZF, Sun JX, Adkins-Threats M, et al. DDIT4 Licenses Only Healthy Cells to Proliferate During Injury-induced Metaplasia. Gastroenterology 2021;160:260–271 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saenz JB, Burclaff J, Mills JC. Modeling Murine Gastric Metaplasia Through Tamoxifen-Induced Acute Parietal Cell Loss. Methods Mol Biol 2016;1422:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tan SH, Swathi Y, Tan S, et al. AQP5 enriches for stem cells and cancer origins in the distal stomach. Nature 2020;578:437–443. [DOI] [PubMed] [Google Scholar]
- 130.Kopp JL, von Figura G, Mayes E, et al. Identification of Sox9-Dependent Acinar-to-Ductal Reprogramming as the Principal Mechanism for Initiation of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2012;22:737–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Prevot PP, Simion A, Grimont A, et al. Role of the ductal transcription factors HNF6 and Sox9 in pancreatic acinar-to-ductal metaplasia. Gut 2012;61:1723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Means AL, Meszoely IM, Suzuki K, et al. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development 2005;132:3767–76. [DOI] [PubMed] [Google Scholar]
- 133.Tosti L, Hang Y, Debnath O, et al. Single nucleus and in situ RNA sequencing reveals cell topographies in the human pancreas. Gastroenterology 2020. [DOI] [PubMed] [Google Scholar]
- 134.Schlesinger Y, Yosefov-Levi O, Kolodkin-Gal D, et al. Single-cell transcriptomes of pancreatic preinvasive lesions and cancer reveal acinar metaplastic cells’ heterogeneity. Nat Commun 2020;11:4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ma Z, Lytle N, Novak SW, et al. METAPLASIA-INDUCED EPITHELIAL HETEROGENEITY DIRECTS PANCREATIC TISSUE INJURY AND RECOVERY. Gastroenterology 2021;160:S–62. [Google Scholar]
- 136.Wright NA, Poulsom R, Stamp GW, et al. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J.Pathol. 1990;162:279–284. [DOI] [PubMed] [Google Scholar]
- 137.Wright NA, Pike C, Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990;343:82–85. [DOI] [PubMed] [Google Scholar]
- 138.Thorsvik S, van Beelen Granlund A, Svendsen TD, et al. Ulcer-associated cell lineage expresses genes involved in regeneration and is hallmarked by high neutrophil gelatinase-associated lipocalin (NGAL) levels. J Pathol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meditskou S, Grekou A, Toskas A, et al. Pyloric and foveolar type metaplasia are important diagnostic features in Crohn’s disease that are frequently missed in routine pathology. Histol Histopathol 2019:18167. [DOI] [PubMed] [Google Scholar]
- 140.Jones JC, Brindley CD, Elder NH, et al. Cellular Plasticity of Defa4(Cre)-Expressing Paneth Cells in Response to Notch Activation and Intestinal Injury. Cell Mol Gastroenterol Hepatol 2019;7:533–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu S, Tong K, Zhao Y, et al. Paneth Cell Multipotency Induced by Notch Activation following Injury. Cell Stem Cell 2018;23:46–59 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hayakawa Y, Tsuboi M, Asfaha S, et al. BHLHA15-positive Secretory Precursor Cells Can Give Rise to Tumors in Intestine and Colon in Mice. Gastroenterology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chang CC, Pan S, Lien GS, et al. Relationship of duodenal ulcer recurrence to gastric metaplasia of the duodenal mucosa and duodenal bulb deformity. J Formos Med Assoc 2001;100:304–8. [PubMed] [Google Scholar]
- 144.Esendagli G, Akarca FG, Balci S, et al. A Retrospective Evaluation of the Epithelial Changes/Lesions and Neoplasms of the Gallbladder in Turkey and a Review of the Existing Sampling Methods: A Multicentre Study. Turk Patoloji Derg 2018;34:41–48. [DOI] [PubMed] [Google Scholar]
- 145.O’Neil A, Petersen CP, Choi E, et al. Unique Cellular Lineage Composition of the First Gland of the Mouse Gastric Corpus. J Histochem Cytochem 2017;65:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Srivastava S, Liew MS, McKeon F, et al. Immunohistochemical analysis of metaplastic non-goblet columnar lined oesophagus shows phenotypic similarities to Barrett’s oesophagus: a study in an Asian population. Dig Liver Dis 2014;46:170–5. [DOI] [PubMed] [Google Scholar]
- 147.Xian W, Ho KY, Crum CP, et al. Cellular origin of Barrett’s esophagus: controversy and therapeutic implications. Gastroenterology 2012;142:1424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang X, Ouyang H, Yamamoto Y, et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell 2011;145:1023–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Burclaff J, Willet SG, Saenz JB, et al. Proliferation and Differentiation of Gastric Mucous Neck and Chief Cells During Homeostasis and Injury-induced Metaplasia. Gastroenterology 2020;158:598–609 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kilgore SP, Ormsby AH, Gramlich TL, et al. The gastric cardia: fact or fiction? Am J Gastroenterol 2000;95:921–4. [DOI] [PubMed] [Google Scholar]
- 151.Ogias D, Rattes IC, Hosoya LYM, et al. Neonatal- maternal separation primes zymogenic cells in the rat gastric mucosa through glucocorticoid receptor activity. Sci Rep 2018;8:9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Keeley TM, Samuelson LC. Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin-interacting protein 1-related-deficient mice. Am J Physiol Gastrointest Liver Physiol 2010;299:G1241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Greer RL, Staley BK, Liou A, et al. Numb regulates acinar cell dedifferentiation and survival during pancreatic damage and acinar-to-ductal metaplasia. Gastroenterology 2013;145:1088–1097 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miyatsuka T, Kaneto H, Shiraiwa T, et al. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev 2006;20:1435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Choi MK, Kim GH, Park do Y, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc 2013;27:4250–8. [DOI] [PubMed] [Google Scholar]
- 156.Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N Engl J Med 2018;378:1085–1095. [DOI] [PubMed] [Google Scholar]
- 157.Lee YC, Chiang TH, Chou CK, et al. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016;150:1113–1124 e5. [DOI] [PubMed] [Google Scholar]