Abstract
Background:
Biomarkers that can risk-stratify children with influenza virus lower respiratory infection may identify patients for targeted intervention. Early elevation of alveolar-related proteins in the bloodstream in these patients could indicate more severe lung damage portending worse outcomes.
Methods:
We used a mouse model of human influenza infection and evaluated relationships between lung pathophysiology and surfactant protein D (SP-D), SP-A, and Club cell protein 16 (CC16). We then measured SP-A, SP-D and CC16 levels in plasma samples from 94 children with influenza-associated acute respiratory failure (PICFLU cohort), excluding children with underlying conditions explaining disease severity. We tested for associations between levels of circulating proteins and disease severity including the diagnosis of acute respiratory distress syndrome (ARDS), mechanical ventilator, intensive care unit and hospital days, and hospital mortality.
Results:
Circulating SP-D showed a greater increase than SP-A and CC16 in mice with increased alveolar-vascular permeability following influenza infection. In the PICFLU cohort, SP-D was associated with moderate-severe ARDS diagnosis (p=0.01) and with mechanical ventilator (r =0.45, p=0.002), ICU (r=0.44, p=0.002), and hospital days (r = 0.37, p=0.001) in influenza infected children without bacterial coinfection. Levels of SP-D were lower in children with secondary bacterial pneumonia (p=0.01) and not associated with outcomes. CC16 and SP-A levels did not differ with bacterial coinfection and were not consistently associated with severe outcomes.
Conclusions:
SP-D has potential as an early circulating biomarker reflecting degree of lung damage caused directly by influenza virus infection in children. Secondary bacterial pneumonia alters SP-D biomarker performance.
Keywords: influenza virus, ARDS, surfactant proteins, pediatric, murine
INTRODUCTION
The management of children with life-threatening influenza pneumonia might be improved by identifying biomarkers that correlate with pulmonary damage, facilitating targeted treatment stratified by risk. Surfactant proteins (SP) and other secretory proteins in the lung have important homeostatic and immune defense roles. Leakage into the bloodstream from damaged alveoli could provide a readout of viral- or inflammatory-mediated lung damage. Secretory lung proteins may provide information distinct from systemic cytokines, which can have multiple cellular sources both in and outside of the infected lung.
Pulmonary surfactant is made of surface tension-reducing lipids such as dipalmitoylphosphatidylcholine, and surfactant proteins. The hydrophilic proteins SP-D and SP-A belong to the collectin family, which bind microbial carbohydrates to facilitate pathogen clearance by phagocytes as part of the innate immune system and can be directly bacteriocidal1-3. SP-A modulates inflammation by regulating macrophage production of nitric oxide while SP-D can block proinflammatory mediator production by alveolar stromal cells 2,4. SP-D is protective against influenza infection in vitro exerting antiviral activity by binding to mannose-rich glycans on hemagglutinin (HA) and neuraminidase (NA) proteins on the influenza virus surface 5-8. CC16 is a member of the secretoglobulin family and also plays an immunomodulatory role in the lung by maintaining epithelial integrity 9. SP-D, SP-A, and CC16 are therefore viable candidate biomarkers for assessing degree of pulmonary injury.
Elevated levels of surfactant proteins have been measured in the bloodstream in a variety of lung diseases including acute infection, acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease (COPD), and idiopathic pulmonary fibrosis (IPF) 10-15. Serum levels were increased in ARDS across multiple studies in adults and children 12,16-22 and positively correlated with duration of mechanical ventilation 14. In the case of influenza virus infection, Delgado et. al. reported that hospitalized influenza virus-infected adult patients with serum levels of SP-D ≥250 ng/mL had an increased risk of mortality 23. Reports showed SP-A and CC16 were elevated in influenza-infected adult outpatients 24,25. The prognostic value of these proteins in children with influenza virus infection is unclear 19,26.
An optimal biomarker would have robust mechanistic justification, and would be easily measured in the circulation following lung damage, be associated with the degree of respiratory failure, and be predictive of time to recovery. We first used a murine model to understand the mechanistic relationship of SP-D, SP-A and CC16 with lung injury. We then tested the association of these protein levels in peripheral blood of children with laboratory confirmed influenza virus lower respiratory tract infection admitted to the pediatric intensive care unit (PICU), assessing their relationship with disease severity and clinical outcomes.
MATERIALS AND METHODS
Murine influenza infection model
We used a well-characterized non-lethal infection model of young (6-8 week old) female Balb/c mice inoculated intranasally with 1000 pfu influenza A/PuertoRico/8/34 H1N1 virus (PR8) in 50 ul Dulbecco’s Modified Eagle Medium (DMEM) or DMEM alone. Mice were anesthetized by intraperitoneal injection of Ketamine (75-80 mg/kg) and Xylazine (7.5-15 mg/kg), and maintained on an appropriate heating pad. Once anesthesia was confirmed, mice were manually restrained and the inoculum was administered via hand pipette. Mice were elevated on a vertical stand for a few minutes post-delivery to ensure the inoculum did not leak out. Following inoculation, mice were monitored until fully recovered from anesthesia and breathing normally. Mice were weighed daily as a measure of morbidity. Blood was collected through cardiac puncture under a surgical plane of anesthesia (confirmed by the absence of hind-toe pinch reflex). Mice were euthanized by thoracotomy under isoflurane. Bronchioalveolar lavage (BAL) was performed post-euthanasia by instilling a total of 1 ml of PBS into the lung by syringe and subsequently withdrawing fluid for collection, with recovery of approximately 900 ul BAL. Following centrifugation for 5 min at 3000rpm, BAL supernatants were harvested for ELISA. Lungs were collected following BAL and weighed as a metric of lung inflammation, and weights were correlated with severity and distribution of findings by histopathology (data not shown), as has been described for other influenza models 27,28. Increased albumin concentration in BAL fluid is a measure of increased alveolar-vascular permeability and lung edema, and correlates increased BAL fluid total protein or IgM in this model. Since ~90% of the PBS instilled into the lung was consistently recovered, the residual PBS did not confound lung weight measurements. Lungs were snap frozen using dry ice and stored at −80 °C to preserve RNA integrity prior to isolation. RNA was then isolated for quantitative RT-PCR (qRT-PCR). Plasma was collected for ELISA. Studies were approved by the Institutional Animal Care and Use Committee at Genentech, Inc. and were conducted in compliance with the regulations of the Association for Assessment and Accreditation of Laboratory Animal Care.
Mouse lung viral load.
Lungs were homogenized in 2 mL DMEM in M-tubes (gentleMACS, Miltenyi Biotec) and RNA isolated from 100 uL in duplicate using the Qiagen RNeasy Mini Kit per manufacturer’s instructions. Equivalent volumes of lung homogenates were used to achieve equal tissue loading into the cDNA synthesis and PCR reactions. cDNA was synthesized with the SuperScript III First Strand RT Kit (Invitrogen) and qPCR performed using primers and probe specific for influenza A M1 gene (details in the online supplement). Reverse transcription was performed on purified influenza A/PuertoRico/8/1934 virus RNA (ViraPur) with a known 50% tissue culture infective dose (TCID50) concentration to generate a standard curve for qRT-PCR assays. Lung viral load was quantitated by extrapolation of unknown Ct values from the A/PR/8/1934 standard curve, using the average of the Ct values from both lung eluate samples for final TCID50-equivalent genomes calculation.
Clinical samples
SP-D, SP-A, and CC16 were measured in plasma samples collected within 72 hours of PICU admission from children (≤18 years old) with influenza virus lower respiratory tract infection. Children were enrolled at 24 sites across the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Pediatric Intensive Care Influenza (PICFLU) Network from March 2009 through May 2016. All had confirmed influenza A or B virus by PCR on a respiratory sample and available biobanked plasma samples. Children with chronic severe respiratory, cardiac, immune or other disorders that could predispose to severe influenza virus infection were excluded. Additional details of the PICFLU study methods were previously published 29. The institutional review board for human studies approved the protocol (Immune Response to Severe Influenza Infection in Children, protocol #X08-11-0534) and informed written consent was obtained from at least one parent or guardian. Blood was drawn from indwelling catheters or from venipuncture when clinical laboratory draws were scheduled. Blood volumes taken for research purposes were minimal and adjusted downward for patient age.
The Pediatric Risk of Mortality (PRISM) III Score was used to assess illness severity upon admission to the PICU 30. No pediatric-specific definition for moderate to severe acute respiratory distress syndrome (ARDS) was available at the time of initial study enrollment and we wanted to reflect diffuse disease, so the Berlin Criteria were used with acute onset of hypoxia (ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) of ≤ 200) with bilateral infiltrates on chest radiograph and no evidence of left heart failure 31. Vasopressor-dependent septic shock was defined as receiving dopamine greater than 5 μg/kg/min and/or any epinephrine or norepinephrine 32. Bacterial co-infections were defined as the growth of an organism from the endotracheal, blood, and/or pleural fluid within 72 hours of PICU admission, as previously described 33.
Biomarker measurements
The ELISA measurements were performed as per manufacturer’s protocol. Mouse ELISAs. Mouse SP-D (Cat # 028248) and SP-A (Cat # 028239) from USBiological Life Sciences and mouse CC16 (Cat# EKU03200) ELISA was purchased from Biomatik. Mouse albumin ELISA (Cat# E90-134) was from Bethyl Laboratories, Inc. Human SP-A, SP-D and CC16 were also measured by ELISAs (BioVendor). Human ELISAs. The plasma sample collected closest to study enrollment was measured using the following assays. Human SP-D (Cat # RD194059101) SP-A (Cat# RD191139200R) and CC16 (Cat# RD191022200) kits were obtained from BioVendor and ELISA measurements were performed as per manufacturer’s protocol. Plasma was diluted 11-fold for SP-D measurement and diluted 25-fold for CC16 measurement, and the final OD was measured at 450 nm using Synergy 2 plate reader from BioTek. Proteins are reported in absolute concentrations (pg/mL or ng/mL plasma).
Statistical analysis
Statistical tests were performed on log10 transformed clinical data for a normal distribution. Unpaired Student’s t test or one-way ANOVA with Holm-Sidak’s multiple comparisons test were performed where indicated, comparing uninfected (day 0) with 8 infected time points. Non-parametric Spearman rank correlation coefficients were calculated for continuous variables using Prism and R. Reported p values are unadjusted for testing of multiple biomarkers. Area under the receiver operating characteristics curves (AUROC) were calculated using Prism software and the biomarker cutoff was calculated using Youden’s index J (J = sensitivity + specificity-1 is maximized).
RESULTS
SP-D correlation with lung injury
We first evaluated the relationships between disease correlates and plasma or lung surfactant protein levels in a murine influenza virus infection model. As shown in Figure 1, influenza A viral loads were detectable in the lung between days 2-11, peaked at day 4, and were no longer detectable by day 14 post-infection (Figure 1A). The concentration of bronchoalveolar lavage (BAL) albumin, a metric of alveolar-vascular permeability, began increasing at day 4 and was significantly elevated between days 7-14 (Figure 1B). Increased lung weight, which correlates with influenza-associated inflammation as assessed by histopathology 28, had slightly later kinetics, with elevation observed between days 9-28 (Figure 1C). Body weight loss, a metric of overall morbidity, was observed between days 4-11, reached the nadir at day 9 and returned to baseline levels by day 14 (Figure 1D).
Figure 1.
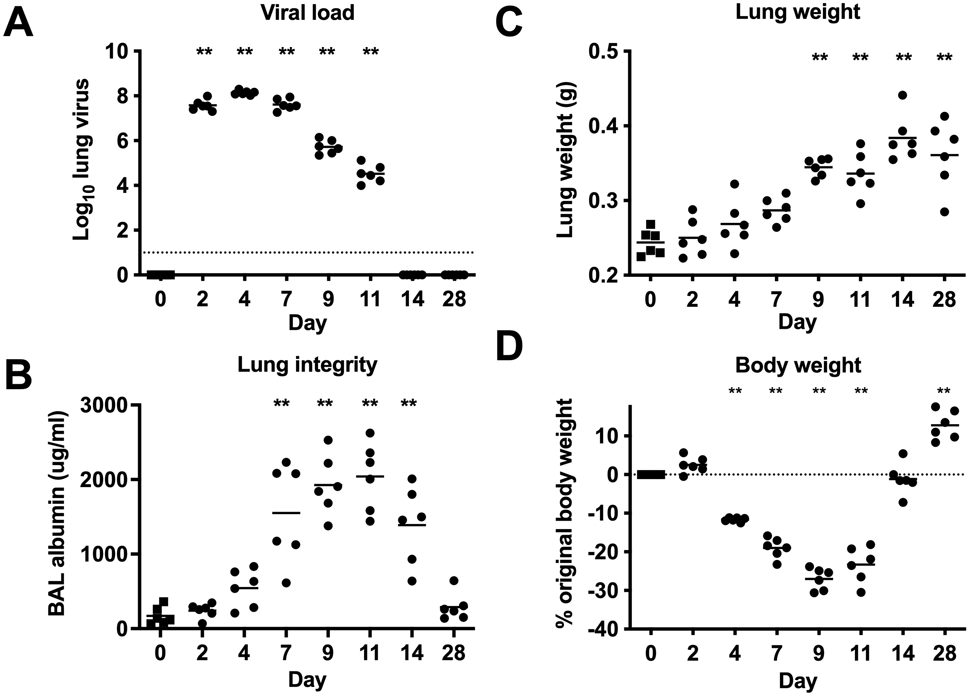
Relationships between viral load, lung integrity and inflammation, and morbidity in the mouse influenza A virus PR8 infection model. A. Lung viral load quantified by qRT-PCR; viral genomes are shown as Log10 TCID50 equivalent copies/genomes. Assay detection limit indicated by dotted line. B. BAL albumin concentration as an indication of lung integrity. C. Lung weight as an indication of lung inflammation. D. Body weight loss as a measure of overall morbidity. Body weight at time of infection is indicated by dotted line. Means are shown for n=6 mice per time point. ** p<0.01 (One-way ANOVA, Day 0 (uninfected) compared with 8 infected time points, adjusted p values indicated). Data are representative of 2 independent experiments. Day 0 = uninfected.
We evaluated how changes in circulating surfactant proteins levels correlated with the observed kinetics of viral load (days 2-11), morbidity (weight loss between days 4-11), followed by compromised lung integrity (days 7-14). SP-D, SP-A, and CC16 could be detected in mouse plasma and BAL. SP-D showed the largest increased plasma concentration following influenza virus infection, with a >6-fold increase from pre-infection levels, which peaked on day 4 and remained significantly elevated through day 9 (Figure 2A). Plasma levels of SP-A and CC16 modestly decreased at day 2 and increased <2-fold at later time points (days 14-28) subsequent to maximal lung permeability (Figure 2A). The murine model permitted assessment of how viral infection could alter the pool of surfactant protein expressed in the lung, by quantifying BAL protein levels. BAL SP-D levels were rapidly increased by day 2 following influenza virus infection, with a 10-fold elevation in BAL by day 4 and remaining significantly elevated through day 28 (Figure 2B). In contrast, CC16 protein levels were 4-fold lower in BAL between days 2-11 (Figure 2B). SP-A protein expression in BAL also decreased at day 2 but returned to pre-infection levels at subsequent time points (Figure 2B).
Figure 2.
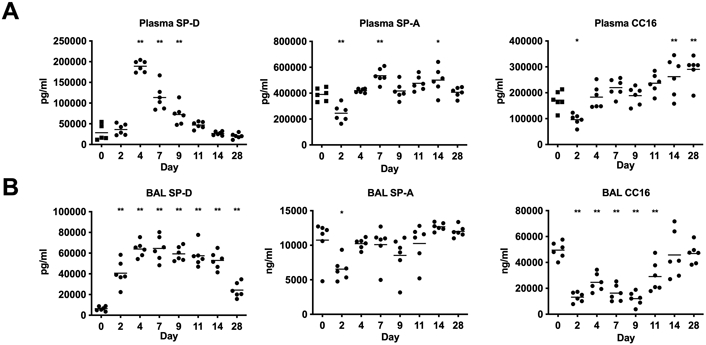
Surfactant protein levels in mice infected with influenza virus in 2A. Plasma and 2B. Bronchoalveolar lavage (BAL). Means are shown for n=6 mice per time point. * p<0.05, ** p<0.01 (One-way ANOVA, Day 0 (uninfected) compared with 8 infected time points, adjusted p values indicated). Data are representative of 2 independent experiments.
SP-D in pediatric influenza infection
The characteristics of the 94 children admitted to the PICU with influenza A or B virus-related lower respiratory tract infection are listed in Table 1. The majority (78%) were infected with influenza A virus and over half tested positive for the 2009 pandemic H1N1 strain. The median duration from the start of symptoms to presentation was 3 days (interquartile range 2, 5 days). There were 6 deaths (4%) and almost half of the children (48%) met ARDS criteria. Baseline viral load in tracheal aspirates or nasopharyngeal swabs were not associated with poor clinical outcomes (p>0.05, data not shown). A large proportion of the children were also diagnosed with bacterial co-infection (52%, n=49), most commonly from Staphylococcus aureus (n=33), with other bacteria listed at the bottom of Table 1. Children with influenza virus and bacterial co-infection were more severe in their illness upon admission as assessed by PRISM III score and were more frequently on extracorporeal membrane oxygenation (ECMO) support compared to children with a low suspicion of bacterial coinfection (Table 1). Additional viruses were detected in 19 (20%) of patients, and did not differ by bacterial co-infection status and were not associated with higher illness severity.
Table 1.
Characteristics, clinical course and complications of critically ill children infected with influenza virus with or without a bacterial co-infection (n=94)
| All Patients n=94 |
Influenza - No Bacterial Co- infection n=45 |
Influenza - Bacterial Co- infection † n=49 |
P-value | |
|---|---|---|---|---|
| Male | 60 (63.8) | 29 (64.4) | 31 (63.3) | 0.9 |
| Hispanic Ethnicity | 19 (20.2) | 10 (22.2) | 9 (18.4) | 0.6 |
| Race | 0.7 | |||
| White | 70 (74.5) | 32 (71.1) | 38 (77.6) | |
| Black | 17 (18.1) | 9 (20.0) | 8 (16.3) | |
| Mixed/Other | 7 (7.5) | 4 (8.9) | 3 (6.1) | |
| Age, years | 6.6 (2.5, 12.0) | 5.7 (2.1, 9.9) | 8.2 (3.3, 12.6) | 0.1 |
| Baseline Health Status | ||||
| Previously Healthy | 58 (61.7) | 23 (51.1) | 35 (71.4) | 0.04 |
| Asthma/Asthma Medications | 18 (19.2) | 12 (26.7) | 6 (12.2) | 0.08 |
| Other Underlying Respiratory Disorder | 5 (5.3) | 3 (6.7) | 2 (4.1) | 0.6 |
| Other Underlying Condition | 20 (21.3) | 12 (26.7) | 8 (16.3) | 0.2 |
| Influenza Virus Type | ||||
| Influenza A | 73 (77.7) | 37 (82.2) | 36 (73.5) | 0.3 |
| Influenza A(H1N1)pdm09 | 38 (40.4) | 20 (44.4) | 18 (36.7) | |
| Influenza A H3N2 | 32 (34.0) | 15 (33.3) | 17 (34.7) | |
| Influenza A Seasonal H1 | 1 (1.1) | 0 (0) | 1 (2.0) | |
| Influenza A Other | 2 (2.1) | 2 (4.4) | 0 (0) | |
| Influenza B | 22 (23.4) | 9 (20.0) | 13 (26.5) | 0.5 |
| Time to Presentation, days | n=91 3 (2, 5) | n=45 2 (1, 4) | n=46 3 (2, 5) | 0.2 |
| Secondary Complications | ||||
| Extracorporeal Life Support | 16 (17.0) | 2 (4.4) | 14 (28.6) | 0.002 |
| Moderate-Severe ARDS | 45 (47.9) | 18 (40.0) | 27 (55.1) | 0.1 |
| Shock Requiring Vasopressors | 54 (57.5) | 22 (48.9) | 32 (65.3) | 0.1 |
| Illness Severity & Outcomes | ||||
| PRISM III Score | 7.5 (3.0, 16.0) | 5 (3, 9) | 11 (5, 21) | 0.002 |
| Duration Mechanical Ventilation, hours | 137.4 (67.5, 271.0) | 133.5 (90.0, 213.0) | 143 (62, 356) | 0.7 |
| Duration PICU stay, hours | 192.5 (103.0, 340.0) | 174 (113, 297) | 236 (103, 389) | 0.3 |
| Hospital Mortality | 6 (6.4) | 1 (2.2) | 5 (10.2) | 0.2 |
| Ventilator Free Days | 22.2 (15.2, 25.2) | 22.3 (18.5, 24.3) | 22.0 (12.8, 25.4) | 0.8 |
Bacterial infections MRSA (n=12), MSSA (n=21), Group A Streptococcus (n=3), Pneumococcus (n=2), Haemophilus influenzae (n=2), Other (n=9)
Data are number (%) of children or median (interquartile range).
PRISM III Score: Pediatric Risk of Mortality Score; ARDS: acute respiratory distress syndrome
Ventilator Free Days: Days alive and free of mechanical ventilation up to 28 days
SP-D was the only biomarker associated with presence of bacterial co-infection (p=0.007), with bacterially co-infected children having a lower median level of SP-D than non-coinfected patients (Figure 3). Therefore, only for SP-D we evaluated associations separately by co-infection status. Strong associations between plasma SP-D and clinical outcomes were observed only in influenza virus-infected children without diagnosed bacterial co-infection (Figure 4 and Table S1). SP-D correlated with admission PRISM III severity score and the median level was higher in children diagnosed with moderate-severe ARDS (Figure 4A-B). Clinical outcome associations included longer durations of mechanical ventilation, ICU stay, and hospitalization (Figure 4C-E). Only one child without bacterial coinfection died, precluding evaluation of mortality. In contrast, none of the associations between SP-D and clinical severity were significant in children with bacterial co-infection (Figure 4F-J, Table S1). We calculated area under the receiver operating characteristics curves (AUROC) and biomarker cutoffs that maximize combined sensitivity and specificity were calculated for AUROC p<0.05. Similar SP-D cutoffs predicted death (entire cohort: 125 ng/mL SP-D, AUROC=0.76, p=0.03) or identified ARDS (entire cohort: 134 ng/mL, AUROC=0.62, p=0.04; no bacterial co-infection: 162 ng/mL, AUROC 0.71, p=0.02). SP-D concentrations in this range also identified shock and ECMO with maximal sensitivity and specificity in the entire cohort.
Figure 3.
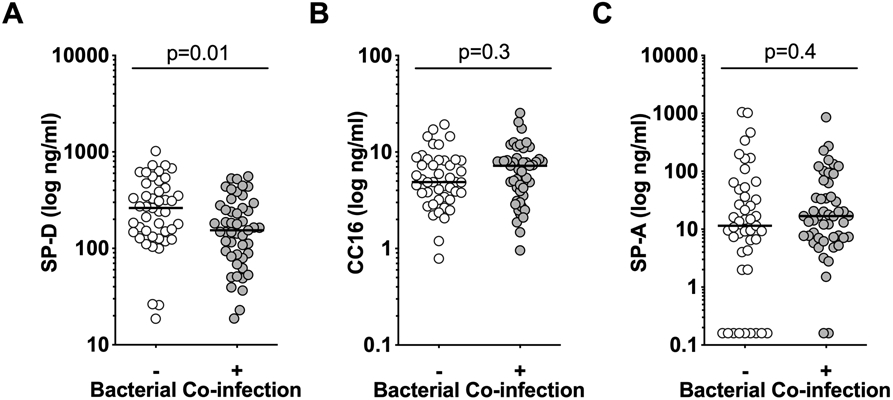
Median levels of SP-D (3A), but not CC16 (3B) or SP-A (3C) differ in influenza infected children with (n=49) and without (n=45) bacterial co-infection using unpaired t test.
Figure 4.
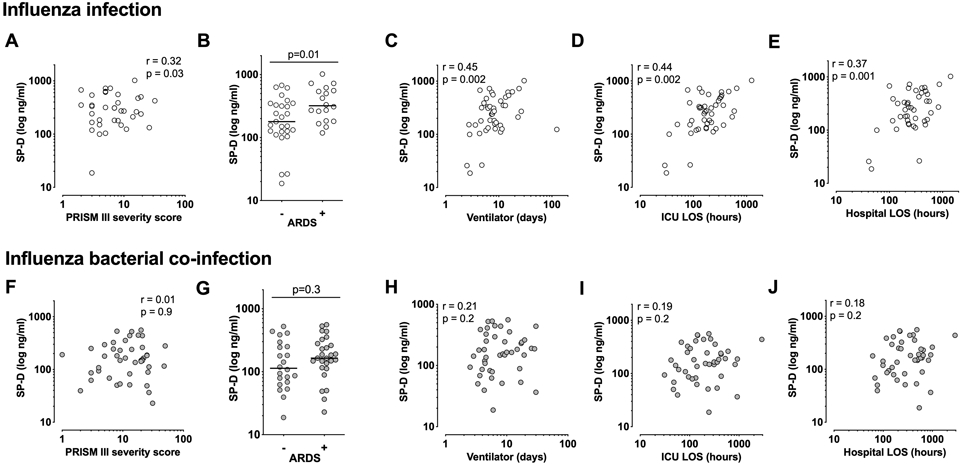
SP-D is associated with clinical severity only in PICFLU pediatric influenza virus-infected patients without bacterial co-infection. Panels A-E: Influenza-infected children without bacterial co-infection (n=45, 1 death); Panels F-J: Influenza-bacterial co-infection (n=49, 5 deaths). PRISM III admission disease severity score (4A, 4F) and acute respiratory distress syndrome (ARDS) (4B, 4G). Outcomes include days of mechanical ventilator support in survivors (4C, 4H), hospital length of stay (LOS) in hours (4D, 4I), pediatric intensive care unit (ICU) hours (4E, 4J), Spearman correlation (r) and p values from unpaired t tests are indicated.
Levels of CC16 in plasma samples collected within the first few days of PICU admission in the PICFLU cohort did not differ by bacterial coinfection (Figure 3B). They were weakly associated with diagnosis of ARDS (p=0.04), which usually was present upon PICFLU admission or by day 2, and admission illness severity PRISM III score (r=0.38, p=0.006). Elevated CC16 was not prognostic in survivors for duration of ventilation, hospitalization, or days in PICU (Table S1). CC16 plasma levels were highest in the 6 children (6.4% of cohort) that died (AUROC=0.87, 95% CI 0.76-0.98, p=0.002). Five of the six children who did not survive were co-infected with methicillin-resistant S. aureus and were supported on ECMO for lung failure from necrotizing pneumonia.
Serum SP-A levels were not significantly associated with any metric of clinical severity in the entire population or subdivided by bacterial co-infection status (Table S1). SP-D, SP-A and CC16 levels were not associated with viral strain (Table S1), gender, or ethnicity.
DISCUSSION
Murine and pediatric data confirm that SP-D is a potential biomarker for the degree of lung damage caused by influenza virus. In the non-lethal mouse model, SP-D showed the largest magnitude of increase between days 4-9 compared with SP-A and CC16; this increase coincided with compromised lung integrity and increased inflammation and viral load. The decreases in SP-A and CC16 at day 2, and increases on or after day 14, were inconsistent with increases in serum levels reflecting leakage from lung epithelial cells following infection. In the mice, we measured protein levels from the lungs, which is not possible to accurately measure in the children. The lung pool of SP-D showed the largest increase following viral infection compared with SP-A and CC-16. In the children with influenza critical illness, only circulating SP-D was a prognostic biomarker for prolonged clinical course. Elevated plasma SP-D was associated with moderate-severe ARDS and was prognostic for longer durations of mechanical ventilation, hospitalization and ICU stay. These associations were not due to increased influenza viral load and were attenuated in patients with secondary bacterial pneumonia. Elevated CC16 was associated with overall degree of lung injury and disease severity in the cohort, but was more strongly associated with bacterial coinfection.
Circulating surfactant proteins have been associated with acute lung injury 11,12,17 and chronic lung diseases such as COPD 34, but not specifically in influenza critical illness. Our murine results are consistent with other murine studies showing elevated pulmonary levels of SP-D in the BAL of influenza virus-infected mice at day 3 35 and increased BAL and serum protein levels in mice following LPS or bleomycin exposure 36. SP-D was similarly observed to be more associated with ARDS and respiratory disease severity than CC16 in intubated patients in a large clinical trial (PASS). In PASS, high serum SP-D predicted a lower likelihood of ventilated patients to recover respiratory function within 28 days 37. Although mortality is lower in children with influenza pneumonia compared to that adults with lung failure, our findings are consistent in the association with prolonged clinical course. High serum SP-D is also associated with severity of other viral lung infections such as bronchiolitis resulting from respiratory syncytial virus (RSV) infection, a common cause of hospitalization in infants 38. The association of higher CC16 with ARDS is consistent with observations in adult populations 17,39-41.
This study also highlights the importance of diagnostic microbiological cultures, as bacterial co-infection confounded the prognostic value of SP-D for severe clinical outcomes when the subgroups were analyzed separately. Comparing lung and serum SP-D levels in mouse models of influenza virus infection with and without bacterial co-infection could provide insight on how co-infection could impact the extent of lung damage or loss of SP-D-producing cells, however mice are more resistant to bacteria commonly coinfecting children. Measurement of SP-D in larger clinical cohorts with a spectrum of bacterial strains is necessary to evaluate how differences in clinical severity could explain the relationship between serum SP-D levels and bacterial co-infection observed in this cohort. Recently, Dahmer et al. reported that SP-D was not associated with pediatric ARDS in a cohort of children with acute respiratory failure, one third with pneumonia caused by a variety of pathogens 26, consistent with data from this study that SP-D was not significantly associated with ARDS when influenza-bacterial co-infections were included. Their study used a definition of pediatric ARDS with unilateral infiltrates, whereas our study required bilateral lung disease. They also reported that SP-D was associated with ARDS severity 26, suggesting that the association of elevated SP-D with poor prognosis observed in influenza virus infection extends to other etiologies.
Strengths of this study include a rigorously characterized pediatric cohort that was free of preexisting severe underlying lung disease. The cohort was recruited from multiple U.S. centers. Despite this, the cohort is of moderate size, including only 94 children. Influenza-related death was uncommon, but almost half of the children had moderate-severe ARDS and 17% were supported on extracorporeal life support. Although the data are consistent for SP-D for influenza-infected children and mice without bacterial coinfection, we did not have a mouse model of influenza-bacterial coinfection for reasons outlined above. We unfortunately were unable to accurately measure the more hydrophobic surfactant proteins SP-B and SP-C in mouse or human samples, thus limiting our ability to evaluate other potentially relevant surfactant protein biomarkers 20. Future studies could compare the prognostic value of SP-D with other potential biomarkers of lung injury, such as sRAGE. Because biomarker levels were not associated with viral strain, ethnicity, or gender in univariate analyses, we did not adjust analyses for these variables. We could not assess the relationship between SP-D and body-mass index as many children were too young and height was often missing 42.
In summary, both murine and human data support SP-D as a potential biomarker of the degree of lung damage from influenza virus. Aside from supportive care and influenza antivirals, there are no therapies shown to improve outcomes of patients with severe influenza-associated respiratory failure. Given that a future influenza pandemic is inevitable, SP-D may be a useful biomarker for stratifying patients by disease severity for interventional trials to test future therapies.
Supplementary Material
Funding:
This study was funded by Genentech Inc. (Rosenberger) and by the National Institutes of Health (NIH AI084011, AI154470 Randolph).
Acknowledgments
The following PICFLU Investigators at the study sites critically reviewed the initial study proposal and all modifications, and enrolled and collected data on the patients in this study, and critically reviewed the results of the study and their implications: Children’s of Alabama, Birmingham, AL (Michele Kong, MD); Arkansas Children’s Hospital, Little Rock, AR (Ronald C. Sanders Jr., MD, Glenda Hefley, RN, MNsc); Phoenix Children’s Hospital, Phoenix, AZ (David Tellez, MD); Banner Children’s/Diamond Children’s Medical Center, Tucson, AZ (Katri Typpo, MD); Children’s Hospital of Los Angeles, Los Angeles, CA (Barry Markovitz, MD); UCSF Benioff Children’s Hospital Oakland, Oakland, CA (Heidi Flori, MD, Natalie Cvijanovich, MD); Children’s Hospital of Orange County, Orange, CA (Nick Anas, MD [deceased], Adam Schwarz, MD, Ofelia Vargas-Shiraishi, BS, CCRC); UCSF Benioff Children’s Hospital San Francisco, San Francisco, CA (Anil Sapru, MD, Patrick McQuillen, MD); Children’s Hospital Colorado, Aurora, CO (Angela Czaja, MD, Peter Mourani, MD); Holtz Children’s Hospital, Miami, FL (Gwenn McLaughlin, MD); Children’s Healthcare of Atlanta at Egleston, Atlanta, GA (Matthew Paden, MD, Keiko Tarquinio, MD, Cheryl L. Stone, RN); Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL (Bria M. Coates, MD); The University of Chicago Medicine Comer Children’s Hospital, Chicago, IL (Juliane Bubeck Wardenburg,MD, PhD, Neethi Pinto, MD); Boston Children’s Hospital, Boston, MA (Adrienne G. Randolph, MD, MSc, Anna A. Agan, MPH, Margaret Newhams, MPH); Children’s Hospital and Clinics of Minnesota, Minneapolis, MN (Stephen C. Kurachek, MD); St. Louis Children’s Hospital, St. Louis, MO (Allan Doctor, MD, Mary Hartman, MD); Children’s Hospital of Nebraska, Omaha, NE (Edward Truemper, MD, Sidharth Mahapatra, MD, Machelle Dawson, RN, BSN, MEd, CCRC); Golisano Children’s Hospital, Rochester, NY (Kate Ackerman, MD, L. Eugene Daugherty, MD); Nationwide Children’s Hospital, Columbus, OH (Mark W. Hall, MD, Lisa Steele, RN, BSN, CCRN); Penn State Children’s Hospital, Hershey, PA (Neal J. Thomas, MD, Debra Spear, RN); Children’s Hospital of Philadelphia, Philadelphia, PA (Julie Fitzgerald, MD, Scott Weiss, MD, Jenny L. Bush, RNC, BSN, Kathryn Graham, BA); Dell Children’s Medical Center of Central Texas, Austin, TX (Renee Higgerson, MD, LeeAnn Christie, RN); Texas Children’s Hospital, Houston, TX (Laura L. Loftis, MD, Nancy Jaimon, RN, MSN-Ed); Children’s Hospital of Wisconsin, Milwaukee, WI (Rainer Gedeit, MD, Kathy Murkowski, RRT, CCRC).
References
- 1.Ikegami M, Na C-L, Korfhagen TR, Whitsett JA. Surfactant protein D influences surfactant ultrastructure and uptake by alveolar type II cells. Am J Physiol Cell Mol Physiol 2005;288(3):L552–L561. [DOI] [PubMed] [Google Scholar]
- 2.Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc 2007;4(3):252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.H W, A K, S W, L S, A W, JH F, KS K, FX M. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest 2003;111(10):1589–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardai SJ, Xiao Y-Q, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By Binding SIRPα or Calreticulin/CD91, Lung Collectins Act as Dual Function Surveillance Molecules to Suppress or Enhance Inflammation. Cell 2003;115(1):13–23. [DOI] [PubMed] [Google Scholar]
- 5.Hawgood S, Brown C, Edmondson J, Stumbaugh A, Allen L, Goerke J, Clark H, Poulain F. Pulmonary collectins modulate strain-specific influenza a virus infection and host responses. J Virol 2004;78(16):8565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeVine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol 2001;167(10):5868–73. [DOI] [PubMed] [Google Scholar]
- 7.Hillaire MLB, Haagsman HP, Osterhaus ADME, Rimmelzwaan GF, van Eijk M. Pulmonary surfactant protein D in first-line innate defence against influenza A virus infections. J Innate Immun 2013;5(3):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KBM, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: Structure, function and receptors. Mol Immunol 2006;43(9):1293–1315. [DOI] [PubMed] [Google Scholar]
- 9.HERMANS C, BERNARD A. Lung Epithelium–specific Proteins. Am J Respir Crit Care Med 1999;159(2):646–678. [DOI] [PubMed] [Google Scholar]
- 10.Determann RM, Millo JL, Waddy S, Lutter R, Garrard CS, Schultz MJ. Plasma CC16 levels are associated with development of ALI/ARDS in patients with ventilator-associated pneumonia: a retrospective observational study. BMC Pulm Med 2009;9(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 2003;58(11):983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene KEE, Wright JRR, Steinberg KPP, Ruzinksi JTT, Caldwell E, Wong WBB, Hull W, Whitsett JAA, Akino T, Kuroki Y, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med 1999;160(6):1843–50. [DOI] [PubMed] [Google Scholar]
- 13.Spoorenberg SMC, Vestjens SMT, Rijkers GT, Meek B, van Moorsel CHM, Grutters JC, Bos WJW. YKL-40, CCL18 and SP-D predict mortality in patients hospitalized with community-acquired pneumonia. Respirology 2017;22(3):542–550. [DOI] [PubMed] [Google Scholar]
- 14.Hermans C, Bernard A. Lung epithelium-specific proteins: Characteristics and potential applications as markers. Am J Respir Crit Care Med 1999;159(2):648–678. [DOI] [PubMed] [Google Scholar]
- 15.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS. Acute respiratory distress syndrome. Nat Rev Dis Prim 2018;5(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, Brower RG, Standiford TJ, Martin TR, Matthay MA, et al. Prognostic and Pathogenetic Value of Combining Clinical and Biochemical Indices in Patients With Acute Lung Injury. Chest 2010;137(2):288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Determann RM, Royakkers AA, Haitsma JJ, Zhang H, Slutsky AS, Ranieri VM, Schultz MJ. Plasma levels of surfactant protein D and KL-6 for evaluation of lung injury in critically ill mechanically ventilated patients. BMC Pulm Med 2010;10(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishizaka A, Matsuda T, Albertine KH, Koh H, Tasaka S, Hasegawa N, Kohno N, Kotani T, Morisaki H, Takeda J, et al. Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Physiol Cell Mol Physiol 2004;286(6):L1088–L1094. [DOI] [PubMed] [Google Scholar]
- 19.Imai T, Takase M, Takeda S, Kougo T. Serum KL-6 levels in pediatric patients: Reference values for children and levels in pneumonia, asthma, and measles patients. Pediatr Pulmonol 2002;33(2):135–141. [DOI] [PubMed] [Google Scholar]
- 20.Bersten AD, Hunt T, Nicholas TE, Doyle IR. Elevated Plasma Surfactant Protein-B Predicts Development of Acute Respiratory Distress Syndrome in Patients with Acute Respiratory Failure. Am J Respir Crit Care Med 2001;164(4):648–652. [DOI] [PubMed] [Google Scholar]
- 21.Park J, Pabon M, Choi AMK, Siempos II, Fredenburgh LE, Baron RM, Jeon K, Chung CR, Yang JH, Park C-M, et al. Plasma surfactant protein-D as a diagnostic biomarker for acute respiratory distress syndrome: validation in US and Korean cohorts. BMC Pulm Med 2017;17(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, Ware LB. Distinct Molecular Phenotypes of Direct vs Indirect ARDS in Single-Center and Multicenter Studies. Chest 2015;147(6):1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delgado C, Krötzsch E, Jiménez-Alvarez LA, Ramírez-Martínez G, Márquez-García JE, Cruz-Lagunas A, Morán J, Hernández C, Sierra-Vargas P, Avila-Moreno F, et al. Serum Surfactant Protein D (SP-D) is a Prognostic Marker of Poor Outcome in Patients with A/H1N1 Virus Infection. Lung 2015;193(1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar Y, Liang C, Limmon GV., Liang L, Engelward BP, Ooi EE, Chen J, Tannenbaum SR. Molecular Analysis of Serum and Bronchoalveolar Lavage in a Mouse Model of Influenza Reveals Markers of Disease Severity That Can Be Clinically Useful in Humans Hartl D, editor. PLoS One 2014;9(2):e86912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukushi M, Yamashita M, Miyoshi-Akiyama T, Kubo S, Yamamoto K, Kudo K. Laninamivir Octanoate and Artificial Surfactant Combination Therapy Significantly Increases Survival of Mice Infected with Lethal Influenza H1N1 Virus Guan Y, editor. PLoS One 2012;7(8):e42419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahmer MK, Flori H, Sapru A, Kohne J, Weeks HM, Curley MAQ, Matthay MA, Quasney MW, Bateman ST, Berg MD, et al. Surfactant Protein D Is Associated With Severe Pediatric ARDS, Prolonged Ventilation, and Death in Children With Acute Respiratory Failure. In: Chest. Vol. 158. Elsevier Inc.; 2020. p. 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CJ S, P V, JL M, R B, PC D, PG T. Compromised respiratory function in lethal influenza infection is characterized by the depletion of type I alveolar epithelial cells beyond threshold levels. Am J Physiol Lung Cell Mol Physiol 2013;304(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao GK, Prell RA, Laing ST, Burleson SCM, Nguyen A, McBride JM, Zhang C, Sheinson D, Halpern WG. In Vivo Assessment of Antibody-Dependent Enhancement of Influenza B Infection. Toxicol Sci 2019. Feb 22. [DOI] [PubMed] [Google Scholar]
- 29.Randolph AG, Yip W-K, Kaitlynn Allen E, Rosenberger CM, Agan AA, Ash SA, Zhang Y, Bhangale TR, Finkelstein D, Cvijanovich NZ, et al. Evaluation of IFITM3 rs12252 Association With Severe Pediatric Influenza Infection. J Infect Dis 2017;02115:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 1996;24(5):743–52. [DOI] [PubMed] [Google Scholar]
- 31.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: The Berlin definition. JAMA -J Am Med Assoc 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 32.Hall MW, Geyer SM, Guo C-Y, Panoskaltsis-Mortari A, Jouvet P, Ferdinands J, Shay DK, Nateri J, Greathouse K, Sullivan R, et al. Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med 2013;41(1):224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randolph AG, Xu R, Novak T, Newhams MM, Bubeck Wardenburg J, Weiss SL, Sanders RC, Thomas NJ, Hall MW, Tarquinio KM, et al. Vancomycin Monotherapy May Be Insufficient to Treat Methicillin-resistant Staphylococcus aureus Coinfection in Children With Influenza-related Critical Illness. Clin Infect Dis 2019;68(3):365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lock-Johansson S, Vestbo J, Sorensen GL. Surfactant protein D, Club cell protein 16, Pulmonary and activation-regulated chemokine, C-reactive protein, and Fibrinogen biomarker variation in chronic obstructive lung disease. Respir Res 2014;15:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reading PC, Morey LS, Crouch EC, Anders EM. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol 1997;71(11):8204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaunsbaek MQ, Rasmussen KJ, Beers MF, Atochina-Vasserman EN, Hansen S. Lung Surfactant Protein D (SP-D) Response and Regulation During Acute and Chronic Lung Injury. Lung 2013;191(3):295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen J-US, Itenov TS, Thormar KM, Hein L, Mohr TT, Andersen MH, Løken J, Tousi H, Lundgren B, Boesen HC, et al. Prediction of non-recovery from ventilator-demanding acute respiratory failure, ARDS and death using lung damage biomarkers: data from a 1200-patient critical care randomized trial. Ann Intensive Care 2016;6(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawasaki Y, Endo K, Suyama K, Sato M, Ito M, Hashimoto K, Hosoya M. Serum SP-D levels as a biomarker of lung injury in respiratory syncytial virus bronchiolitis. Pediatr Pulmonol 2011;46(1):18–22. [DOI] [PubMed] [Google Scholar]
- 39.Lin J, Zhang W, Wang L, Tian F. Diagnostic and prognostic values of Club cell protein 16 (CC16) in critical care patients with acute respiratory distress syndrome. J Clin Lab Anal 2018;32(2):e22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ware LB, Koyama T, Zhao Z, Janz DR, Wickersham N, Bernard GR, May AK, Calfee CS, Matthay MA. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care 2013;17(5):R253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lesur O, Langevin S, Berthiaume Y, Légaré M, Skrobik Y, Bellemare J-F, Lévy B, Fortier Y, Lauzier F, Bravo G, et al. Outcome value of Clara cell protein in serum of patients with acute respiratory distress syndrome. Intensive Care Med 2006;32(8):1167–1174. [DOI] [PubMed] [Google Scholar]
- 42.Ward SL, Dahmer MK, Weeks HM, Sapru A, Quasney MW, Curley MAQ, Liu KD, Matthay MA, Flori HR. Association of patient weight status with plasma surfactant protein D, a biomarker of alveolar epithelial injury, in children with acute respiratory failure. Pediatr Pulmonol 2020;55(10):2730–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


