Abstract
Supplementation with antioxidant carotenoids is a therapeutic strategy to protect against age-related macular degeneration (AMD); however, the transport mechanism of carotenoids from the liver to the retina is still not fully understood. Here, we investigate if HDL serves as the primary transporter for the macular carotenoids. ApoA-I, the key apolipoprotein of HDL, was genetically deleted from BCO2 knockout (Bco2−/−) mouse, a macular pigment mouse model capable of accumulating carotenoids in the retina. We then conducted a feeding experiment with a mixed carotenoid chow (lutein:zeaxanthin:β-carotene=1:1:1) for one month. HPLC data demonstrated that the total carotenoids were increased in the livers but decreased in the serum, retinal pigment epithelium (RPE)/choroids, and retinas of ApoA-I−/−/Bco2−/− mice compared to Bco2−/− mice. In detail, ApoA-I deficiency caused a significant increase of β-carotene but not lutein and zeaxanthin in the liver, decreased all three carotenoids in the serum, blocked the majority of zeaxanthin and β-carotene transport to the RPE/choroid, and dramatically reduced β-carotene and zeaxanthin but not lutein in the retina. Furthermore, surface plasmon resonance spectroscopy (SPR) data showed that the binding affinity between ApoA-I and β-carotene>>zeaxanthin>lutein. Our results show that carotenoids are transported from the liver to the eye mainly by HDL, and ApoA-I may be involved in the selective delivery of macular carotenoids to the RPE.
Keywords: HDL, carotenoids, ApoA-I, zeaxanthin, β-carotene, age-related macular degeneration
Introduction
Age-related macular degeneration (AMD) is a common cause of blindness in developed countries [1–3]. The content of carotenoids in the human retina is inversely correlated with the development of AMD [4–7]. Supplementation with carotenoids can increase retinal carotenoids and significantly reduces AMD risk [8–10]. Unlike plants, humans do not have the enzymes to synthesize carotenoids and have to obtain these antioxidants from the diet [11, 12]. Therefore, it is important to understand the transport mechanism of carotenoids. Carotenoids are believed to be transported from the liver to the eye by lipoproteins [13–16]. But it is still not clear which lipoprotein is responsible for carotenoid transport from the liver to the retina.
Genetic and biological studies indicate that HDL is likely the major transporter for macular carotenoids. Genome-wide association studies (GWAS) have reported that many gene variants of HDL-cholesterol transporters and receptors, such as HDL apolipoprotein A-I (ApoA-I), SR-BI, ABCA1, CETP, LCAT, and LIPC, are significantly associated with the level of carotenoids in the human retina [1, 17, 18]. HDL deficiency caused by a mutated ABCA1 transporter in Wisconsin hypoalpha mutant (WHAM) chickens dramatically reduced their retinal carotenoids [19]. Blocking the activity of SR-BI by either antibody or RNAi decreased carotenoid uptake by cultured ARPE-19 cells [20, 21].
Similar to HDL cholesterol, the uptake of macular carotenoids is a selective transport process. There are more than 600 carotenoids in nature, ~50 in the human diet, and ~20 detectable in the human serum, but only two carotenoids, lutein and zeaxanthin, can be taken up by the human retina [5, 22]. Similarly, HDL carries various lipid species and selectively transports cholesterol ester (CE) to the liver through reverse cholesterol transport. In contrast, the LDL particles are entirely internalized into cells by the endocytosis process [23, 24]. Therefore, the transport of macular carotenoids occurs likely through the HDL pathway but not the LDL pathway.
ApoA-I is the main apolipoprotein of HDL, accounting for around 70% of the HDL proteins [24, 25]. ApoA-I protein is expressed mainly in the liver, with a small amount produced in the intestine. It facilitates efflux of excessive free cholesterol in the peripheral tissues to HDL through ABCA1, while cholesterol ester (CE) influxes into hepatic cells through SR-BI when HDL approaches the liver [26]. The composition of lipids and apolipoproteins of HDL is altered in the ApoA-I knockout mice, significantly reducing HDL-mediated reverse cholesterol transport [25]. This indicates that ablation of ApoA-I dramatically changes the structure and function of HDL.
In this manuscript, we investigate if the disruption of HDL caused by ApoA-I deletion impairs carotenoid transport in mice deficient in the enzyme β-carotene oxygenase 2 (Bco2), a mouse model capable of accumulating carotenoids in their retinas [27–29]. We first crossed ApoA-I knockout (ApoA-I−/−) mice with Bco2 knockout (Bco2−/−) mice to generate ApoA-I /Bco2 double knockout (ApoA-I−/−/Bco2−/−) mice. We then carried out a carotenoid feeding experiment in which ApoA-I−/−/Bco2−/− and Bco2−/− mice were fed with a mixed carotenoid chow for one month. Carotenoids were then extracted from the liver, serum, retinal pigment epithelium (RPE)/choroids, and retina of these mice and analyzed by HPLC. We further investigate the binding affinity between the recombinant human ApoA-I protein and various carotenoids by surface plasmon resonance (SPR) spectroscopy.
Materials and Methods
1. Materials
Tetrahydrofuran (THF), butylated hydroxytoluene (BHT), polyvinylpyrrolidone, dimethyl sulfoxide (DMSO), and methanol were purchased from Sigma Chemicals (Saint Louis, MO, USA). Organic solvents such as ethyl acetate, hexane, dichloromethane, and N, N-diisopropylethylamine (DIPEA) were obtained from Thermo Fisher (Waltham, MA, USA). Carotenoid standards zeaxanthin and meso-zeaxanthin were provided by DSM Nutritional Products, Ltd. (Kaiseraugst, Switzerland), while lutein was from Kemin Health (Des Moines, IA, USA). β-Carotene and astaxanthin standards were purchased from Sigma Chemicals (Saint Louis, MO, USA). Recombinant human ApoA-I was purchased from Sino Biological (#10686-H07E; Wayne, PA, USA), while the IRBP protein was a gift from Dr. Federico Gonzalez-Fernandez at State University of New York Buffalo. Recombinant human StARD3 protein was prepared in our lab as previously described [30]. ApoA-I antibody was from Abnova (# H335-D01P; Walnut, CA, USA), and actin antibody was from Sigma-Aldrich (#A2066; Saint Louis, MO, USA). Goat anti-rabbit IgG conjugated to HRP was from Alpha Diagnostic International (# 20320; San Antonio, TX, USA).
2. Mice breeding and maintenance
To generate ApoA-I−/−/Bco2−/− mice, germline knockout mice of ApoA-I were purchased from the Jackson Laboratories (#002055, Bar Harbor, Maine) and crossed with Bco2−/− mice. The Bco2−/− mouse line was a gift from Dr. Johannes von Lintig of the Case Western Reserve University and maintained at the animal facility of the Moran Eye Center, University of Utah. The ApoA-I−/−/Bco2−/− mice were identified from the offspring by genotyping using the following PCR primers. Two forward primers: 5’ –CCT TCT ATC GCC TTC TTG ACG- 3’ and 5’-TCT GGT CTT CCT GAC AGG TAG G-3’ with a common reverse primer: 5’-GTT CAT CTT GCT GCA TAC G-3’ were used to determine the ApoA-I mutated gene and wild type gene, respectively. Accordingly, the ApoA-I mutated gene yielded a ~250 bp PCR product, while the WT gene produced a ~169 bp PCR product. As before, the Bco2 mutated gene was identified with the following primers: 5’-TTT CTA GTC TAG ATT CGT GTA CC-3’ and 5’-CAG TCA CGA CGT TGT AAA AC-3’, while the Bco2 wild type gene was determined using this pair of primers: 5’-ATA CAA TCA TTG GTT TGA TGG AA-3’ and 5’-TAG GTG GCT CAA ACC TTG ACA-3’. The Bco2 wildtype (WT) gene and mutated gene produced a ~212 and a ~790 bp PCR product, respectively.
3. Western blots
We also validated the status of ApoA-I protein in the ApoA-I−/−/Bco2−/− and Bco2−/− mice by western blot. 50 μL of serum was collected from each animal, and then 50 μL of 2xSDS-PAGE loading buffer was added to the serum sample. After a 40-second sonication on ice, the samples were boiled in water for 5 minutes. Next, 30 μl of the protein sample was loaded on 4–15% gradient SDS–PAGE for separation and then transferred onto 0.45 μm nitrocellulose membrane. After blocking with 5% nonfat dried milk, the membranes were incubated with primary antibody overnight at 4 °C, then with secondary antibodies for 2 hours. The dilution ratios were 1:1000 and 1:2000, respectively. The membranes were developed using ECL Plus Western blot detection reagents from GE HealthCare (Lafayette, CO, USA).
4. Carotenoid-feeding experiments
Twenty 6–8- week-old ApoA-I−/−/Bco2−/− mice and twenty 6–8-week- old Bco2−/− mice were used in the carotenoid-feeding experiments. To improve carotenoids’ bioavailability, all of the mice were fed with a vitamin A-deficient chow (AIN-93) for four weeks. Subsequently, they were given a chow containing mixed carotenoids for another four weeks. This chow was customized by an animal feed company, TestDiet (Richmond, IN, USA). The DSM beadlet carotenoids lutein, zeaxanthin, and β-carotene were mixed at a ratio of 1:1:1 and incorporated into a rodent base chow from TestDiet (Richmond, IN, USA). The final content of total carotenoids was 1 g carotenoids/kg chow. ~2.6 mg of carotenoid were given to each mouse per day, while the total carotenoid dosage was 12 mg/day in the AREDS2 clinical trial [31]. All procedures were approved by the appropriate Institutional Animal Care and Use Committees and were carried out according to National Institutes of Health guidelines.
5. Carotenoid extraction and analysis by HPLC
After four weeks of carotenoid feeding, all three carotenoids in the livers, serums, RPE/choroids, and retinas of the ApoA-I−/−/Bco2−/− and Bco2−/− mice were extracted and analyzed using HPLC. 0.1–0.2g of homogenized liver sample was taken individually from animals, while each sample of RPE/choroid and retina contained tissues from five animals. These samples then were homogenized by a BioSpec bead beater homogenizer (Bartlesville, OK, USA) and extracted with 1mL tetrahydrofuran (THF) containing 0.1% butylated hydroxytoluene (BHT) by a 30-min sonication in ice-cold water as reported [32]. After three extractions, all three extracts were combined and evaporated to dryness under vacuum at room temperature.
Each serum sample (~200 μL) was collected from individual animals, but serum carotenoids were extracted with a different protocol. In detail, we first added 400 μL ethanol containing 0.1% of BHT to the serum samples to precipitate the proteins and then added 1mL ethyl acetate to extract the carotenoids. Following a 30-second vortex, the samples were centrifuged at 2,000 × g for 5 minutes at room temperature, and the supernatants were collected. This extraction step was repeated two more times. The supernatants were then combined and dried down as above.
To perform the HPLC analysis, hexane and water were added to the dried residue of each sample, vortexed for 30 seconds, centrifuged at 2000 × g for 5 minutes at room temperature, and the hexane phase was collected. This extraction sequence was conducted three times, and the combined hexane phases were dried down to yield the carotenoid samples for analysis. The carotenoid samples were re-dissolved into the HPLC mobile phase and injected into the HPLC system, in which a silica-based nitrile bonded column (25 cm length × 4.6 mm internal diameter with 5-μm spherical particle from Regis Chemical, Morton Grove, IL, USA) was employed. The eluent contained 75% hexanes, 25% dichloromethane, 0.3% methanol, and 0.1% N, N-diisopropylethylamine (DIPEA). The column flow rate, monitoring wavelength, and column temperature were set to 1 mL/min, 445 nm, and 25 °C during each HPLC run, respectively.
6. Carotenoid-binding assay
Surface plasmon resonance (SPR) spectroscopy assays were conducted at 25°C using a fully automated SensiQ Pioneer optical biosensor equipped with carboxyl modified sensor chips (Pall ForteBio, Fremont, CA, USA). Using this platform, a protein surface and an unmodified reference surface were prepared for simultaneous analysis. Proteins were immobilized using a standard amine coupling method. Carotenoids (lutein, zeaxanthin, meso-zeaxanthin, β-carotene, and astaxanthin) were prepared in 100% DMSO and then diluted to a 5% working dilution (1–100 μM) in running buffer (10 mM phosphate-buffered saline, 0.1% polyvinylpyrrolidone, and 5% DMSO, pH 7.4). Carotenoids were injected using Onestep® (Taylor dispersion) injection method. A concentration gradient was created by slowly diffusing the analyte in the running buffer. Buffer blanks were injected for double referencing purposes. Each carotenoid concentration was injected three times, and assays were performed at 25°C. Data were collected at 10 Hz. For affinity determination, SPR response data (sensorgrams) were zeroed at the beginning of each injection and double referenced. Equilibrium binding constants were determined by using Qdat® analysis software (Pall ForteBio, Fremont, CA, USA).
7. Statistical analysis
Carotenoid contents of the livers, serum, and ocular tissue of the mice were analyzed using ANOVA and t-tests. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). The bars on the graphs represent means with ± SD. Two-tailed P < 0.05 was considered significant.
Results
1. Generation of ApoA-I/Bco2 double knockout (ApoA-I−/−/Bco2−/−) mice
We first bred ApoA-I−/−mice with Bco2−/− mice to create ApoA-I−/−/Bco2−/− mice. The genotyping results demonstrated that both the ApoA-I and Bco2 genes had been deleted in the ApoA-I−/−/Bco2−/− mice, while in the Bco2−/− mice, only the Bco2 gene was knocked out (Figure 1 A, B). We further examined the expression of the ApoA-I protein in both the ApoA-I−/−/Bco2−/− and Bco2−/− mice by western blot. Figure 1C shows that a ~25kDa protein band was detected in the serum of the Bco2−/− mice, but no protein band was detected in the ApoA-I−/−/Bco2−/− mice. These data demonstrated that the ApoA-I−/−/Bco2−/− mouse line had been successfully established. The ApoA-I−/−/Bco2−/− mice and Bco2−/− mice were used in the following carotenoid feeding experiments.
Figure 1. Genotyping and western blot results of the ApoA-I−/−/Bco2−/− mice and the Bco2−/− mice.
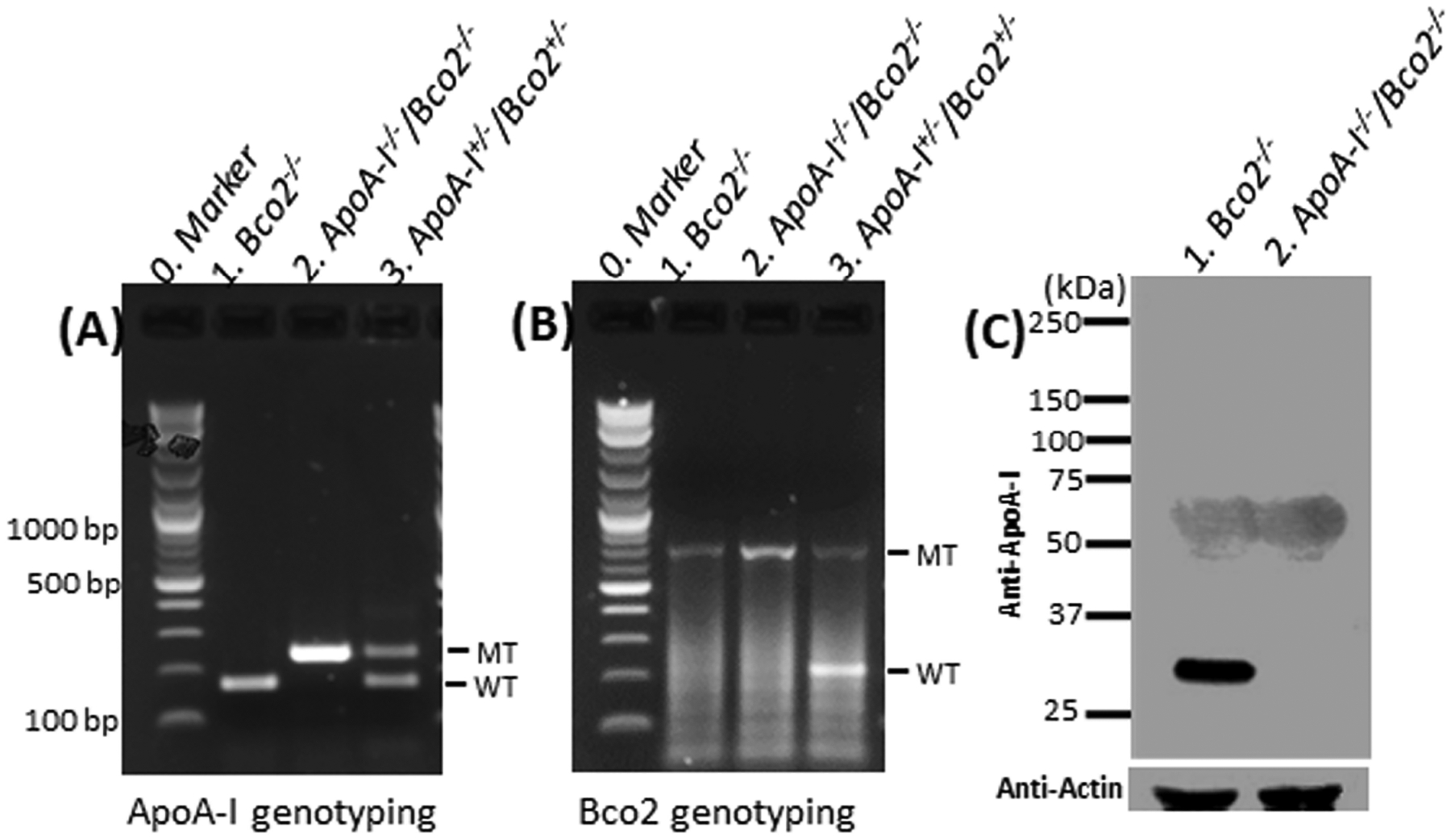
(A) Genotyping results of mutated (MT) and wild type (WT) ApoA-I genes of the mouse by PCR. The PCR products of mutated and wild type Apo A-I genes are ~ 250 bp and 169 bp, respectively. (B) Genotyping results of MT and WT mouse Bco2 genes by PCR. The PCR product of the mutated Bco2 gene is ~790 bp, while that of the wild type gene is ~212 bp. (C) Western blot. A ~25Kd protein band was detected in the Bco2−/− mice but not in the ApoA-I−/−/Bco2−/− mice. The data of PCR and western blot demonstrated that the ApoA-I−/−/Bco2−/− mouse line had been successfully generated.
2. Total carotenoid alteration caused by ApoA-I deficiency in the Bco2−/− mice.
Next, we conducted carotenoid-feeding experiments. Twenty ApoA-I−/−/Bco2−/− mice and twenty Bco2−/− mice were fed with a chow containing three carotenoids, lutein, zeaxanthin, and β-carotene at a ratio of 1: 1: 1 for one month. To obtain comprehensive insight into HDL’s role in carotenoid transport, we first investigated the effects of ApoA-I deficiency on the contents of total carotenoids in the Bco2−/− mice. Figure 2 demonstrates that the total carotenoids in the livers of the ApoA-I−/−/Bco2−/− mice increased to ~150% of the Bco2−/− mice, while the total carotenoids of the serum, RPE/choroids, and the retina in the ApoA-I−/−/Bco2−/− mice reduced to about 27%, 30%, and 55% of the Bco2−/− mice, respectively.
Figure 2. The contents of total carotenoids detected in the liver, serum, RPE/choroids, and retina of the ApoA-I−/−/Bco2−/− mice and the Bco2−/− mice by HPLC.
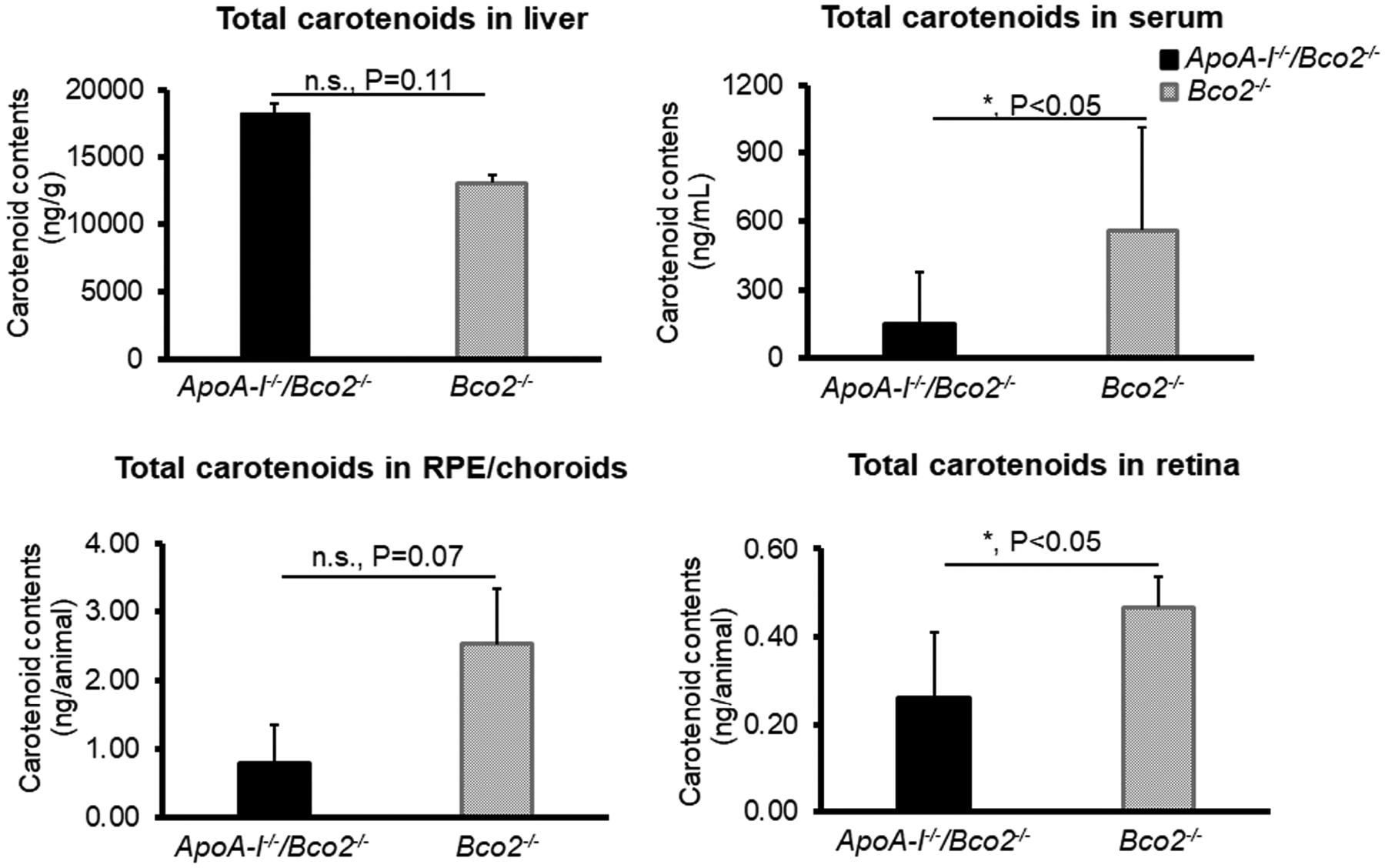
The level of total carotenoids in the liver of ApoA-I−/−/Bco2−/− mice was increased but reduced in serum, RPE/choroids, and retina relative to the Bco2−/− mice, suggesting HDL participates in the carotenoid transport from the liver to the eye. Twenty ApoA-I−/−/Bco2−/− mice and twenty Bco2−/− mice were used in the feeding experiments. Total carotenoids consist of lutein, zeaxanthin, and β-carotene. The carotenoids of the liver and serum were extracted from individual animals, while the ocular tissues of five animals were pooled together for carotenoid extraction. *, P<0.05. Error bars represent standard deviation.
3. Individual carotenoid alteration caused by ApoA-I deficiency in the Bco2−/− mice.
Subsequently, we further analyzed the influences of ApoA-I deficiency on the status of individual carotenoids relevant to the eye. Lutein and zeaxanthin are the dietary carotenoids highly concentrated in the human retina, while β-carotene is the precursor of 11-cis-retinal, the chromophore of the visual pigment rhodopsin. Carotenoids are packed on HDL and/or VLDL in the liver and secreted into the blood to be transported to the eye and other peripheral tissues.
Figure 3 shows the carotenoid levels of lutein, zeaxanthin, and β-carotene in the livers of ApoA-I−/−/Bco2−/− and Bco2−/− mice. The levels of lutein and zeaxanthin in the liver of the ApoA-I−/−/Bco2−/− mice were not significantly changed compared with the Bco2−/− mice. Interestingly, the β-carotene in the livers of ApoA-I−/−/Bco2−/− increased to 150% of the levels in Bco2−/− mice. In addition, we can see that β-carotene is about 5 to 15 times higher than lutein and zeaxanthin in both the ApoA-I−/−/Bco2−/− and the Bco2−/− mice.
Figure 3. The contents of lutein, zeaxanthin, and β-carotene detected in the livers of the ApoA-I−/−/Bco2−/− mice and the Bco2−/− mice by HPLC.
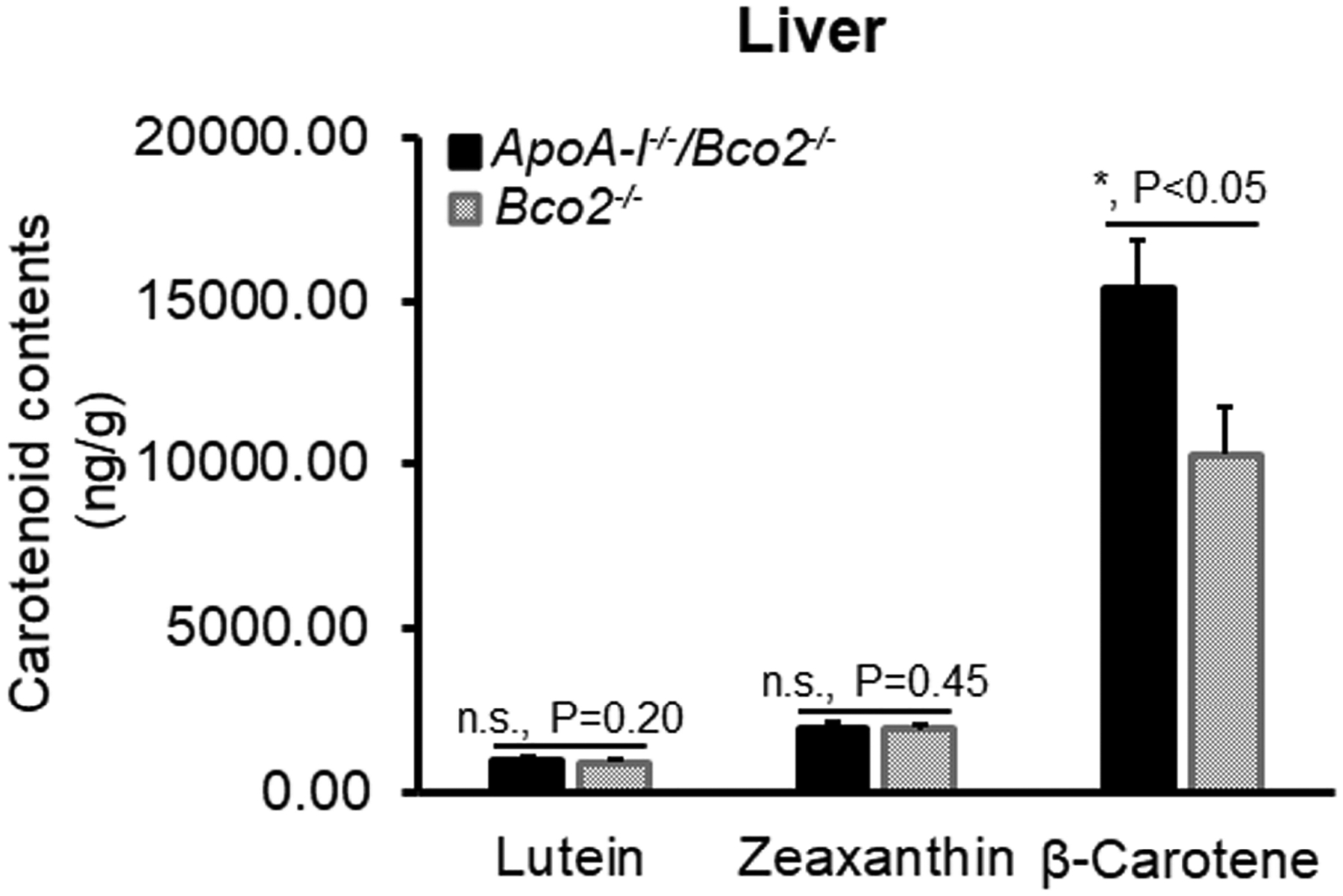
The levels of all three carotenoids in the liver of the ApoA-I−/−/Bco2−/− mice were increased compared with the Bco2−/− mice. The contents of β-carotene in both mice are 5 to 10 times higher than lutein and zeaxanthin, indicating that β-carotene can preferentially accumulate in the liver. The ratio of lutein:zeaxanthin:β-carotene is 1.0: 2.1: 11.7 in the Bco2−/− mice, while it becomes 1.0: 2.0: 15.6 in the ApoA-I−/−/Bco2−/− mice. Carotenoids were extracted from twenty liver samples of the ApoA-I−/−/Bco2−/− mice and twenty liver samples of the Bco2−/− mice. *, P<0.05. Error bars represent standard deviation.
Figure 4 demonstrates the levels of three individual carotenoids in the serum of the ApoA-I−/−/Bco2−/− and the Bco2−/− mice. Lutein, zeaxanthin, and β-carotene of ApoA-I−/−/Bco2−/− mice were significantly reduced to 30%, 19%, and 37% of the levels in Bco2−/− mice, indicating that HDL may serve as the primary transporter for all three carotenoids. ApoA-I deficiency impacted serum zeaxanthin more than the other two carotenoids, implying that HDL may have a higher capacity for acquiring zeaxanthin from the liver.
Figure 4. The contents of lutein, zeaxanthin, and β-carotene detected in the serum of the ApoA-I−/−/Bco2−/− mice and the Bco2−/− mice by HPLC.
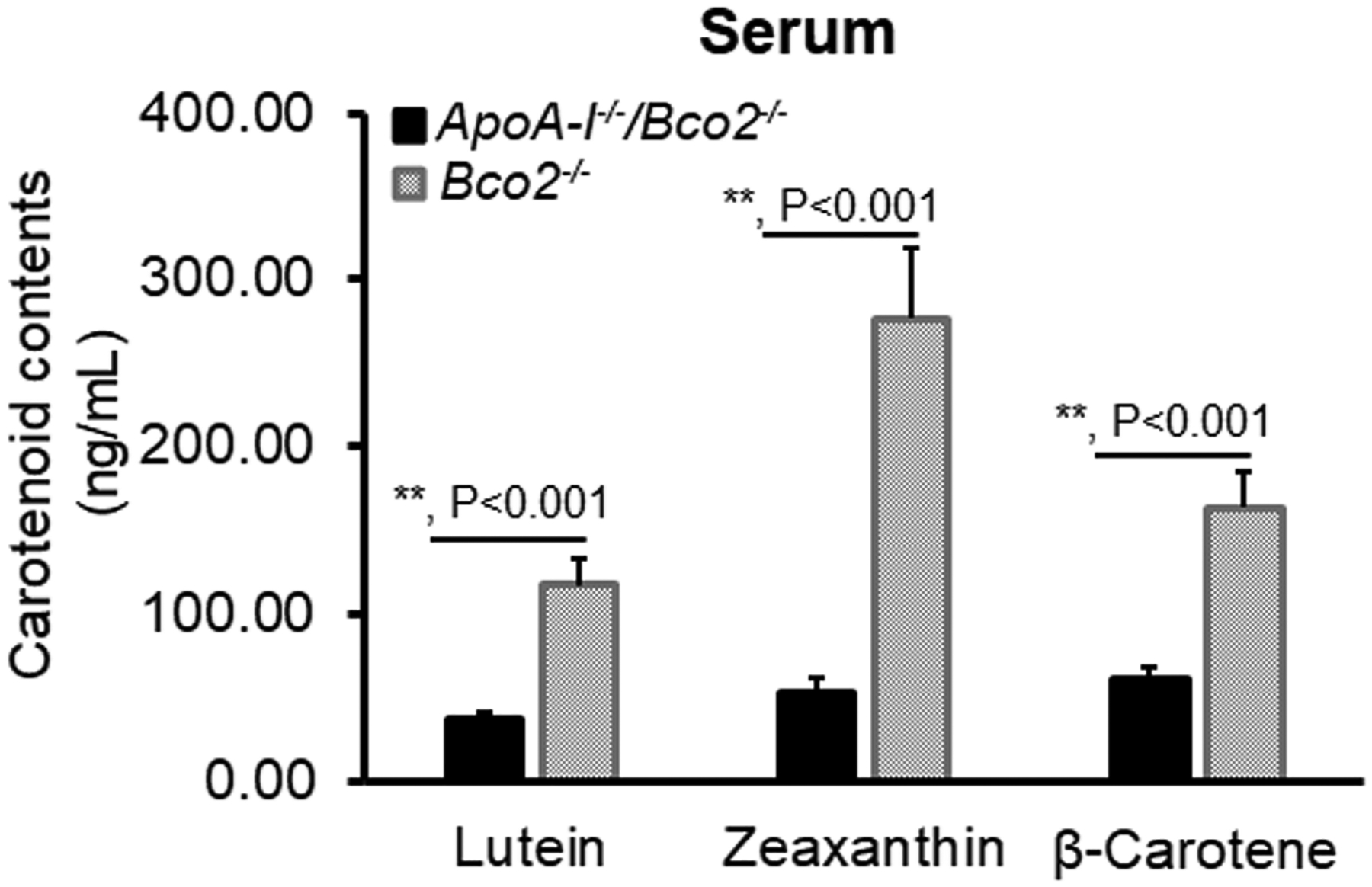
The levels of all three carotenoids in the serum of the ApoA-I−/−/Bco2−/− mice were significantly reduced relative to the Bco2−/− mice, suggesting all three carotenoids were secreted to HDL to be transported to the eye. The ratio of lutein:zeaxanthin:β-carotene is 1.0: 2.4: 1.4 in the Bco2−/− mice, while it becomes 1.0: 1.5: 1.7 in the ApoA-I−/−/Bco2−/− mice. Carotenoids were extracted from twenty serum samples of ApoA-I−/−/Bco2−/− mice and twenty serum samples of the Bco2−/− mice. **, P<0.001. Error bars represent standard deviation.
Figure 5 exhibits the levels of three carotenoids in the RPE/choroids of the ApoA-I−/−/Bco2−/− and the Bco2−/− mice. It demonstrates that ApoA-I deletion reduced about 34% of lutein, 66% of zeaxanthin, and 84% of β-carotene in the RPE/choroids of the Bco2−/− mice. This result reveals that ApoA-I has more impact on the transport of zeaxanthin and β-carotene than lutein, implying that HDL may be responsible for the selective transport of zeaxanthin and β-carotene to the RPE.
Figure 5. The contents of lutein, zeaxanthin, and β-carotene detected in the RPE/choroids of the ApoA-I−/−/Bco2−/− mice and the Bco2−/− mice by HPLC.
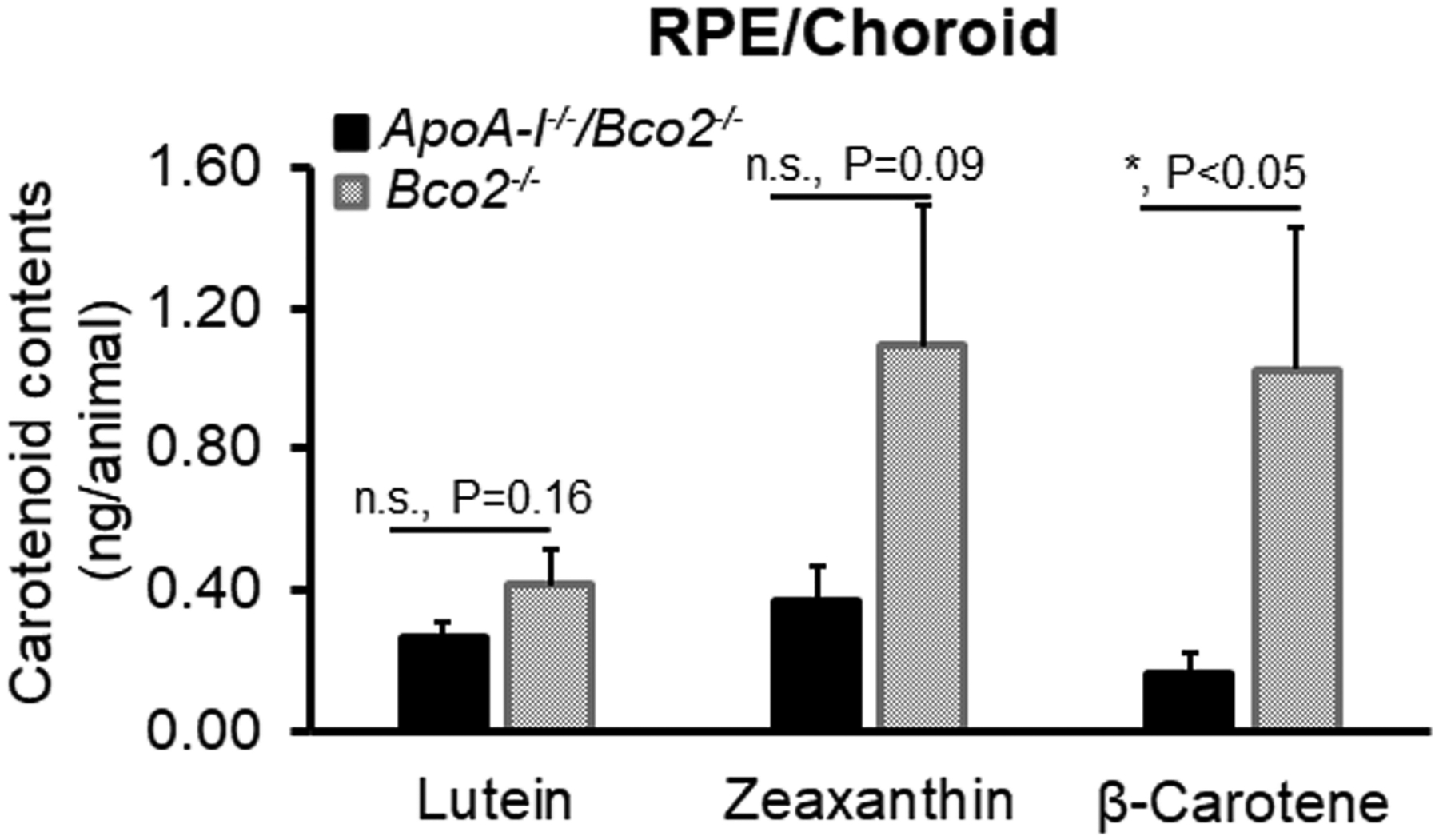
The levels of all three carotenoids in the RPE/choroids of ApoA-I−/−/Bco2−/− mice were decreased relative to the Bco2−/− mice. It also showed that ApoA-I deficiency resulted in more reduction of zeaxanthin and β-carotene than lutein, suggesting that zeaxanthin and β-carotene are transported to RPE/choroids by HDL. In contrast, lutein is probably delivered by LDL. The ratio of lutein:zeaxanthin:β-carotene is 1: 2.6: 2.5 in the Bco2−/− mice, while it becomes 1.0: 1.4: 0.6 in the ApoA-I−/−/Bco2−/− mice. Carotenoids were extracted from four RPE/choroid samples pooled from twenty ApoA-I−/−/Bco2−/− double knockout mice and four RPE/choroid samples pooled from twenty Bco2−/− mice (5 pairs of RPE/choroids/sample). *, P<0.05. Error bars represent standard deviation.
Figure 6 shows that the level of β-carotene in the retina of the ApoA-I−/−/Bco2−/− decreased to 31% of the Bco2−/− mice, while lutein and zeaxanthin only reduced to 70% and 60%, respectively. It appears that ApoA-I may be involved in the retinal transport of β-carotene and zeaxanthin more than lutein. In addition, we also observed that more zeaxanthin was deposited in the retina than the other two carotenoids.
Figure 6. The contents of lutein, zeaxanthin, and β-carotene detected in the retinas of the ApoA-I−/−/Bco2−/− mice and the Bco2−/− mice by HPLC.
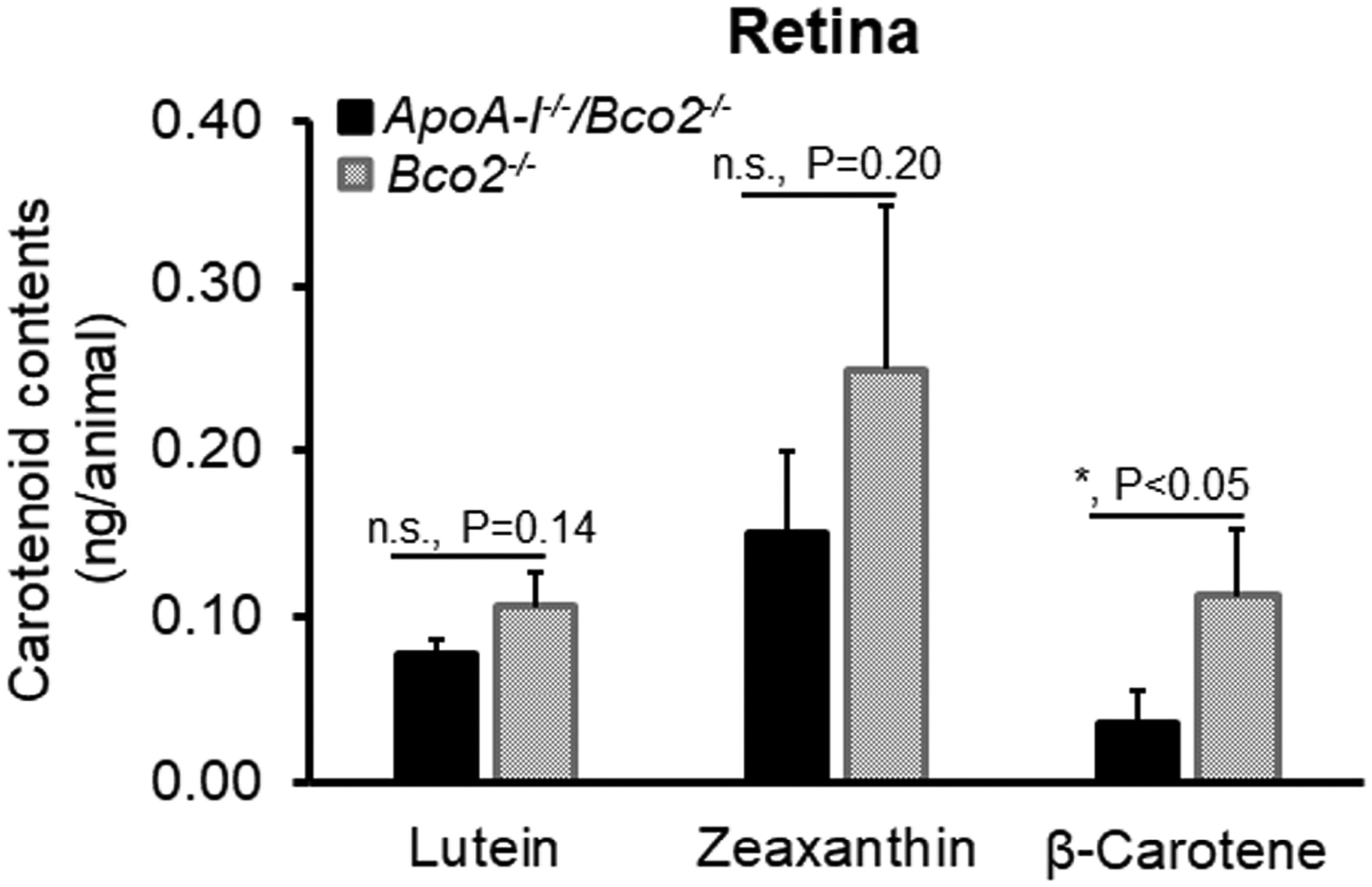
The levels of all three carotenoids in the retinas of ApoA-I−/−/Bco2−/− mice were reduced compared with the Bco2−/− mice. ApoA-I deficiency caused a dramatic reduction of β-carotene but not lutein and zeaxanthin, suggesting that β-carotene is transported from RPE/choroids to the retina by HDL. In contrast, lutein and zeaxanthin are probably delivered by LDL. The ratio of lutein:zeaxanthin:β-carotene is 1.0: 2.4: 1.1 in the Bco2−/− mice, while it becomes 1.0: 2.0: 0.5 in the ApoA-I−/−/Bco2−/− mice. Carotenoids were extracted from four retina samples pooled from twenty ApoA-I−/−/Bco2−/− double knockout mice and four retina samples pooled from twenty Bco2−/− mice (5 pairs of retinas/sample). *, P<0.05. Error bars represent standard deviation.
4. Binding affinity between ApoA-I protein and various carotenoids.
To further investigate whether ApoA-I protein directly contributes to the selective uptake of the macular carotenoids, we examined the binding affinity between the recombinant human ApoA-I protein and various carotenoids using SPR spectroscopy (Table 1). The KD values between ApoA-I and β-carotene, zeaxanthin, and lutein are 0.33 μM, 5.6 μM, and 12.14 μM, respectively. This demonstrates that binding affinities between ApoA-I and three eye-related carotenoids are β-carotene> zeaxanthin ≈ meso-zeaxanthin >lutein, as smaller KD values represent higher binding affinity. ApoA-I binds β-carotene and zeaxanthin stronger than lutein. This probably explains why Apo A-I deficiency causes β-carotene and zeaxanthin reduction in the RPE/choroid and retina more than lutein. We also found that the binding affinity between ApoA-I and β-carotene is much stronger than that of lutein and its binding protein, StARD3. The binding affinities between ApoA-I and xanthophyll carotenoids (lutein and zeaxanthin) are weaker than the retinal transport protein IRBP.
Table 1.
The binding affinities between carotenoid-related proteins and various carotenoids determined by surface plasmon resonance (SPR) spectroscopy
| Carotenoids | Human IRBP (μM) | StARD3 (μM) | Human ApoA-I (μM) |
|---|---|---|---|
| Lutein | 1.06 ± 0.20 | 0.45±0.03 | 12.14±0.11 |
| Zeaxanthin | 1.64 ± 0.50 | 2.60±0.01 | 5.62±0.34 |
| meso-Zeaxanthin | 1.85 ± 0.30 | 1.80±0.40 | 7.90±0.20 |
| β-Carotene | 0.92 ± 0.10 | 2.10±0.05 | 0.33±0.03 |
| Astaxanthin | 1.64 ± 0.06 | 1.50±0.20 | 1.52±0.01 |
Note: Data presented in this table are mean KD±SD. The smaller value of KD stands for the higher binding affinity. Each test was repeated three times.
Discussion
In this work, we investigated the role of HDL in carotenoid transport using mouse model experiments. We found that HDL is the primary transporter to deliver carotenoids from the liver to the RPE, and ApoA-I may be involved in the selective transport of carotenoids to the retina. Our data provide new insights into the transport mechanism of carotenoids.
It remains unclear how carotenoids are transported from the liver to the serum. After digestion of the food matrix, carotenoids are packed onto chylomicrons in the intestine and then transported to the liver, where they are repacked to lipoproteins and secreted into the blood to be delivered to peripheral tissues such as the eye [13, 14]. However, it is unknown on which lipoprotein carotenoids are packed in the liver before secretion into the blood. LDL has often been considered a candidate due to the hydrophobic feature of carotenoids. Another opinion is that xanthophylls and non-xanthophyll carotenoids are packed onto HDL and LDL, respectively. According to the carotenoid composition in these human serum lipoproteins, the xanthophylls lutein and zeaxanthin are mainly associated with HDL, while β-carotene is located primarily in LDL [21, 33]. In this study, we found that the deletion of ApoA-I significantly reduced 70% of lutein, 80% of zeaxanthin, and 60% of β-carotene in the serum of the Bco2−/− mice (Figure 4); meanwhile, the levels of all three carotenoids were increased in their livers (Figure 3). Our results demonstrate that lutein, zeaxanthin, and β-carotene are loaded mainly onto the HDL and secreted into the blood, suggesting that HDL may serve as the major transporter to carry carotenoids from the liver to the eye and other peripheral tissues.
Macular carotenoid uptake from the bloodstream to the RPE is believed to occur through the HDL pathway. Genetic studies have revealed that both the key apolipoproteins and the cell surface receptors of HDL are significantly associated with macular pigment optical density (MPOD), an index for the carotenoid contents in the human retina. HDL receptor SR-BI exists in the human and mouse RPE, and inhibition of SR-BI function by antibody or siRNA treatment dramatically reduced carotenoids in cultured ARPE-19 cells [20]. More direct evidence demonstrated that HDL addition in the medium of ARPE-19 cells facilitated the transport of carotenoids into the cells [21]. In the same study, the authors also compared the carotenoid delivery abilities between HDL and LDL. They found that HDL preferentially delivers zeaxanthin, whereas LDL is more involved in the transport of lutein and β-carotene. Our data revealed that HDL was responsible for 66% of zeaxanthin, 34% of lutein, and 84% of β-carotene transported into the RPE/choroid of Bco2−/− mice (Figure 5). Thus, our animal feeding experiments showed similar results to the cell culture assays that showed that HDL can selectively deliver zeaxanthin. Interestingly, our animal data indicated that HDL may be more involved in the transport of β-carotene, which is different from the cell culture assays. This could be ascribed to the difference between the two experimental systems. However, our SPR results revealed the binding affinities between ApoA-I and β-carotene>>zeaxanthin>lutein, which might in part explain the animal feeding data.
HDL is unlikely to play a key role in the transport of lutein and zeaxanthin from the RPE to the retina. Compared to the Bco2−/− mice, the lutein, zeaxanthin in the retina of ApoA-I−/−/Bco2−/− mice only reduced 30% and 40%, respectively (Figure 6). Therefore, we suggest that macular carotenoids are transported from RPE to the retina mainly through mechanisms different from the HDL pathway, probably by the LDL pathway or diffusion. This is not a surprise. After all, most ApoA-I proteins are expressed in the liver and the intestine but not in the eye.
ApoA-I may contribute to the selective uptake of zeaxanthin from the bloodstream to the RPE as it binds zeaxanthin tighter. Meanwhile, we found that the preferential accumulation of β-carotene in the liver may also contribute to the selective uptake of macular carotenoids. This is because hepatic β-carotene is about 15 to 30 times higher than lutein and zeaxanthin in both the ApoA-I−/−/Bco2−/− mice and the Bco2−/− mice when fed the three carotenoids in equal proportions (Figure 3). A similar accumulation pattern was also seen in a previous feeding experiment [28]. Moreover, the human hepatic content of β-carotene has been reported to be about 30 times higher than that of xanthophylls [32, 34]. All of these lines of evidence suggest that the preferential accumulation of β-carotene in the liver over lutein and zeaxanthin may result in less β-carotene release into the blood, thereby causing the selective delivery of xanthophylls to the eye. Of course, to completely understand this selective uptake process, many other factors, such as HDL receptor SR-BI, carotenoid cleavage enzymes BCO1 and BCO2, and specific carotenoid-binding proteins GSTP1 and StARD3 [20, 27, 30, 33, 35, 36], should be taken into account, too.
In conclusion, we investigated the role of HDL in the transport of three eye-related carotenoids in mouse models for the first time. Our results demonstrated that carotenoids are transported from the liver to the eye mainly by HDL, and ApoA-I may be involved in the selective delivery of macular carotenoids. These data may enhance our understanding of macular carotenoid transport and provide new insight into AMD prevention by carotenoids.
Highlights:
HDL is the primary carotenoid transporter in mice.
Zeaxanthin and β-carotene are taken up into the RPE mainly through the HDL pathway.
Apo A-I binds zeaxanthin and β-carotene much stronger than lutein.
Acknowledgments:
This work was supported by the BrightFocus Foundation M201606, by NIH grants EY-11600, EY-14800, and by unrestricted departmental funds from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors have no conflict of interest.
References
- [1].Montezuma SR, Sobrin L, Seddon JM, Review of genetics in age related macular degeneration, Semin Ophthalmol 22(4) (2007) 229–40. [DOI] [PubMed] [Google Scholar]
- [2].Aronow ME, Chew EY, Age-related Eye Disease Study 2: perspectives, recommendations, and unanswered questions, Curr Opin Ophthalmol 25(3) (2014) 186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Beatty S, Boulton M, Henson D, Koh HH, Murray IJ, Macular pigment and age related macular degeneration, The British journal of ophthalmology 83(7) (1999) 867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE, Macular pigment in donor eyes with and without AMD: a case-control study, Investigative ophthalmology & visual science 42(1) (2001) 235–40. [PubMed] [Google Scholar]
- [5].Krinsky NI, Landrum JT, Bone RA, Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye, Annual review of nutrition 23 (2003) 171–201. [DOI] [PubMed] [Google Scholar]
- [6].Krinsky NI, Johnson EJ, Carotenoid actions and their relation to health and disease, Mol Aspects Med 26(6) (2005) 459–516. [DOI] [PubMed] [Google Scholar]
- [7].Bernstein PS, Zhao DY, Wintch SW, Ermakov IV, McClane RW, Gellermann W, Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients, Ophthalmology 109(10) (2002) 1780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. , Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group, Jama 272(18) (1994) 1413–20. [PubMed] [Google Scholar]
- [9].Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE, A one year study of the macular pigment: the effect of 140 days of a lutein supplement, Experimental eye research 65(1) (1997) 57–62. [DOI] [PubMed] [Google Scholar]
- [10].Nolan JM, Stack J, O’Connell E, Beatty S, The relationships between macular pigment optical density and its constituent carotenoids in diet and serum, Investigative ophthalmology & visual science 48(2) (2007) 571–82. [DOI] [PubMed] [Google Scholar]
- [11].Eroglu A, Harrison EH, Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids, Journal of lipid research 54(7) (2013) 1719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].von Lintig J, Hessel S, Isken A, Kiefer C, Lampert JM, Voolstra O, Vogt K, Towards a better understanding of carotenoid metabolism in animals, Biochimica et biophysica acta 1740(2) (2005) 122–31. [DOI] [PubMed] [Google Scholar]
- [13].Loane E, Nolan JM, O’Donovan O, Bhosale P, Bernstein PS, Beatty S, Transport and retinal capture of lutein and zeaxanthin with reference to age-related macular degeneration, Surv Ophthalmol 53(1) (2008) 68–81. [DOI] [PubMed] [Google Scholar]
- [14].Li B, Vachali P, Bernstein PS, Human ocular carotenoid-binding proteins, Photochem Photobiol Sci 9(11) (2010) 1418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang W, Connor SL, Johnson EJ, Klein ML, Hughes S, Connor WE, Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration, The American journal of clinical nutrition 85(3) (2007) 762–9. [DOI] [PubMed] [Google Scholar]
- [16].Harrison EH, Mechanisms of Transport and Delivery of Vitamin A and Carotenoids to the Retinal Pigment Epithelium, Molecular nutrition & food research 63(15) (2019) e1801046. [DOI] [PubMed] [Google Scholar]
- [17].Meyers KJ, Johnson EJ, Bernstein PS, Iyengar SK, Engelman CD, Karki CK, Liu Z, Igo RP Jr., Truitt B, Klein ML, Snodderly DM, Blodi BA, Gehrs KM, Sarto GE, Wallace RB, Robinson J, LeBlanc ES, Hageman G, Tinker L, Mares JA, Genetic determinants of macular pigments in women of the Carotenoids in Age-Related Eye Disease Study, Investigative ophthalmology & visual science 54(3) (2013) 2333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].SanGiovanni JP, Neuringer M, The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: promise of molecular genetics for guiding mechanistic and translational research in the field, The American journal of clinical nutrition 96(5) (2012) 1223S–33S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Connor WE, Duell PB, Kean R, Wang Y, The prime role of HDL to transport lutein into the retina: evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter, Investigative ophthalmology & visual science 48(9) (2007) 4226–31. [DOI] [PubMed] [Google Scholar]
- [20].During A, Doraiswamy S, Harrison EH, Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism, Journal of lipid research 49(8) (2008) 1715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thomas SE, Harrison EH, Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins, Journal of lipid research 57(10) (2016) 1865–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, Nolan JM, Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease, Progress in retinal and eye research 50 (2016) 34–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Santamarina-Fojo S, Gonzalez-Navarro H, Freeman L, Wagner E, Nong Z, Hepatic lipase, lipoprotein metabolism, and atherogenesis, Arteriosclerosis, thrombosis, and vascular biology 24(10) (2004) 1750–4. [DOI] [PubMed] [Google Scholar]
- [24].Mahley RW, Innerarity TL, Rall SC Jr., Weisgraber KH, Plasma lipoproteins: apolipoprotein structure and function, Journal of lipid research 25(12) (1984) 1277–94. [PubMed] [Google Scholar]
- [25].Temel RE, Walzem RL, Banka CL, Williams DL, Apolipoprotein A-I is necessary for the in vivo formation of high density lipoprotein competent for scavenger receptor BI-mediated cholesteryl ester-selective uptake, The Journal of biological chemistry 277(29) (2002) 26565–72. [DOI] [PubMed] [Google Scholar]
- [26].Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M, Identification of scavenger receptor SR-BI as a high density lipoprotein receptor, Science 271(5248) (1996) 518–20. [DOI] [PubMed] [Google Scholar]
- [27].Li B, Vachali PP, Gorusupudi A, Shen Z, Sharifzadeh H, Besch BM, Nelson K, Horvath MM, Frederick JM, Baehr W, Bernstein PS, Inactivity of human beta,beta-carotene-9’,10’-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment, Proceedings of the National Academy of Sciences of the United States of America 111(28) (2014) 10173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li B, Vachali PP, Shen Z, Gorusupudi A, Nelson K, Besch BM, Bartschi A, Longo S, Mattinson T, Shihab S, Polyakov NE, Suntsova LP, Dushkin AV, Bernstein PS, Retinal accumulation of zeaxanthin, lutein, and beta-carotene in mice deficient in carotenoid cleavage enzymes, Experimental eye research 159 (2017) 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li B, Rognon GT, Mattinson T, Vachali PP, Gorusupudi A, Chang FY, Ranganathan A, Nelson K, George EW, Frederick JM, Bernstein PS, Supplementation with macular carotenoids improves visual performance of transgenic mice, Archives of biochemistry and biophysics 649 (2018) 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li B, Vachali P, Frederick JM, Bernstein PS, Identification of StARD3 as a lutein-binding protein in the macula of the primate retina, Biochemistry 50(13) (2011) 2541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Age-Related G Eye Disease Study 2 Research, Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial, Jama 309(19) (2013) 2005–15. [DOI] [PubMed] [Google Scholar]
- [32].Khachik F, de Moura FF, Zhao DY, Aebischer CP, Bernstein PS, Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models, Investigative ophthalmology & visual science 43(11) (2002) 3383–92. [PubMed] [Google Scholar]
- [33].Shyam R, Vachali P, Gorusupudi A, Nelson K, Bernstein PS, All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids, Archives of biochemistry and biophysics 634 (2017) 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schmitz HH, Poor CL, Wellman RB, Erdman JW Jr., Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue, The Journal of nutrition 121(10) (1991) 1613–21. [DOI] [PubMed] [Google Scholar]
- [35].Lindqvist A, Andersson S, Cell type-specific expression of beta-carotene 15,15’-mono-oxygenase in human tissues, J Histochem Cytochem 52(4) (2004) 491–9. [DOI] [PubMed] [Google Scholar]
- [36].Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS, Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye, The Journal of biological chemistry 279(47) (2004) 49447–54. [DOI] [PubMed] [Google Scholar]


