Abstract
Background:
The systemic inflammatory response (SIRS) drives late morbidity and mortality after injury. The α7 nicotinic acetylcholine receptor (α7nAchR) expressed on immune cells regulates the vagal anti-inflammatory pathway that prevents an overwhelming SIRS response to injury. Non-specific pharmacologic stimulation of the vagus nerve has been evaluated as a potential therapeutic to limit SIRS. Unfortunately, the results of clinical trials have been underwhelming. We hypothesized that directly targeting the α7nAchR would more precisely stimulate the vagal anti-inflammatory pathway on immune cells and decrease gut and lung injury after severe burn.
Methods:
C57BL/6 mice underwent 30% total body surface area steam burn. Mice were treated with an intraperitoneal injection of a selective agonist of the α7nAchR (AR-R17779) at 30 minutes post-burn. Intestinal permeability to 4kDa FITC-Dextran was measured at multiple time-points post-injury. Lung vascular permeability was measured 6 hours after burn injury. Serial behavioral assessments were performed to quantify activity levels.
Results:
Intestinal permeability peaked at 6 hours post-burn. AR-R17779 decreased burn-induced intestinal permeability in a dose-dependent fashion (p<0.001). There was no difference in gut permeability to 4kDa FITC-Dextran between sham and burn injured animals treated with 5 mg AR-R17779. While burn injury increased lung permeability 10-fold, AR-R17779 prevented burn-induced lung permeability with no difference compared to sham (p<0.01). Post-injury activity levels were significantly improved in burned animals treated with AR-R17779.
Conclusion:
Directly stimulating the α7nAchR prevents burn-induced gut and lung injury. Directly targeting the α7nAChR that mediates the cholinergic anti-inflammatory response may be an improved strategy compared to non-specific vagal agonists.
Level of Evidence:
Level IV, Therapeutic
Keywords: Vagus nerve, cholinergic anti-inflammatory, lung, alpha7 nicotinic acetylcholine, intestine
Background:
Multi-organ failure resulting from the systemic inflammatory response (SIRS) to injury is the leading cause of late complications and death after severe trauma and burn injury (1, 2). The innate immune response to severe injury results in the rapid mobilization of immune cells recruited to tissue injury sites (3). While this mobilization of monocytes/macrophages has an important role in propagating the inflammatory response through pro-inflammatory cytokine release and further immune cell recruitment, compensatory anti-inflammatory mechanisms in these cells are required to restrain the inflammation and prevent deleterious effects on the host. Therapeutically modulating this inflammatory response to resolve tissue injury without end-organ dysfunction has remained elusive in treating severely injured patients.
The vagus nerve senses the inflammatory state of the host and transmits an anti-inflammatory reflex by stimulating the alpha-7 nicotinic acetylcholine receptor (α7nAChR) on immune cells (4). This cholinergic anti-inflammatory pathway is critical in obtaining inflammatory homeostasis in the host and limiting organ failure after severe infection or injury (5). Cholinergic agonists or vagal nerve signaling fail to attenuate the pro-inflammatory response to injury and infection in knockout mice that lack the α7nAChR (CHRNA7 KO), confirming its importance in mediating cholinergic control of inflammation (6, 7). In animal models, agonists of the α7nAChR have been demonstrated to improve survival after administration of LPS and improve organ injury in models of severe burn and trauma injury (8–12).
Despite their success in preclinical models, the protective effects of non-specific vagal agonists in modulating inflammation in the clinical setting have been largely disappointing (13). The drug AR-R17779 ((−)-spiro[1-azabicyclo[2.2.2]octane-3,5’-oxazolidin-2’-one) has been developed as a highly selective agonist of the α7nAchR (14). Here, we hypothesized that post-injury treatment with AR-R17779 would attenuate burn-induced organ injury. More directly targeting the α7nAchR that mediates the cholinergic anti-inflammatory pathway may improve the clinical application of vagal agonists aimed at limiting the SIRS response to injury.
Methods:
Severe Burn Model
Male C57BL/6J mice weighing 18 – 22 grams were purchased from The Jackson Laboratory (JAX #000664). Prior to burn injury, mice were treated with inhaled isoflurane for general anesthesia. The dorsal fur was clipped before placement in a template estimating 30% total body surface area prior to exposure to steam from boiling water for 7 seconds as we have previously described (15, 16). Control mice were placed under general anesthesia, underwent fur clipping, but were not exposed to steam burn. All mice, including sham, were then given a subcutaneous injection of resuscitation fluid (100uL 12.6 ug/mL buprenorphine with 1.4 mL 0.9% normal saline) and placed in their respective cages on a warm water recirculating pad. A separate cohort of animals received an intraperitoneal injection of the selective α7nAchR AR-R17779 immediately post-burn at the concentration of 0.2, 1, or 5 mg/kg in resuscitation fluid. Control animals underwent injection of vehicle immediately after injury. All mice were exposed to 12-hour light-dark cycles and were given free access to food and water prior to the experimental protocol. The order animals selected for sham vs. burn and control vs. AR-R17779 was randomized for each study. Animals were excluded if they did not survive the first 4 hours after burn injury. All animal experiments were approved by the University of California San Diego Institutional Animal Care and Use Committee (IACUC). All experimental details are reported per ARRIVE guidelines.
Gut CHRNA7 Expression
Ileum and jejunum were harvested (n=4–6 per group) and homogenized using Lysing Matrix D tubes and FastPrep-120 homogenizer (MP Biomedical, cat#6913–100) followed by lysis in 1 ml trizol reagent. Total RNA purification was performed according to manufacture instructions using the Direct-zol™ RNA Miniprep Plus (Zymo Research, Tustin, CA), including the DNAase treatment. CHRNA7 cDNA was generated using iScript RT supermix (BioRad, cat#1708840) according to manufacture instructions. qRT-PCR was performed using primers for mouse CHRNA7 forward 5’- CGTGGGCCTCTCTGTAGTGG-3′, reverse 5’- CTGGAGTTGGGGCACAGTGC-3′; and mouse GAPDH (Qiagen QuantiTect #QT01658692) using a CFX96 thermal cycler (BioRad) and SsoAdvance SybrGreen (#172–5271) 2X master mix (BioRad). Relative gene expression was calculated by comparing the thermal cycle number (Ct) to that of GAPDH.
Gut Permeability
Gut permeability assay was performed following the protocol published by Mateer et al. (17). In brief, intestinal sacs harvested from ileum or jejunum measuring 5cm in length from sham or burn mice (n=5–8/group) were injected with 300 μl of 1 mg/ml 4 kDa FITC-Dextran (Sigma, 46944). Intestinal sacs were placed into a 15 ml conical tube filled with 10 ml of PBS in a 37°C water bath. Samples of 100 μl volume were collected in duplicate from the fluid surrounding the intestinal sac and placed into a 96 well plate (Corning, CLS3631) at 0, 30, 60, 90, and 120 minutes time points with replacement of 200 μl fresh PBS after each aliquot was removed. To measure apparent permeability, a standard curve of log dilutions for FITC-Dextran was calculated. Cumulative concentration versus time was plotted to calculate the slope, which was then normalized by the area of the intestinal tissue sac utilized. Gut permeability was the primary outcome and sample size was estimated based on our prior experience and powered to detect a 2-fold increase in permeability between burn and burned animals treated with AR-R17779
Lung Permeability
To assess lung vascular permeability, retro-orbital injection of 100 μL (50mg/ml) 70 kDa FITC-Dextran (Sigma, FD70–1G) was performed following sham or 6 hours post-burn injury (n=4/group) (18). At 30 minutes following injection of FITC-Dextran, the heart was perfused by injection of 5ml heparinized saline before resection of the right lobe of the lung. The lung was placed in 2ml PBS and spun for 5 minutes at 10,000 xg. The supernatant was removed and placed in a fresh tube to measure fluorescence intensity in duplicate using an Omega FLUOStar Reader (BMG Labtech, Cary, NC).
Behavioral Assessments
Following general anesthesia, animals were returned to their cage. During recovery their health was monitored and recorded on a Surgical Post -Op sheet for the first hour and at 2, 4, and 6 hours post-burn. Activity scoring was assessed by an evaluator blinded to treatment with AR-R17779 vs. vehicle. Animals were evaluated for activity (1=active, 0=stationary), posture (1=normal, 0=hunched), dehydration (1=not dehydrated, 0=dehydrated), and pelage (1=smooth, 0=rough) with a maximum score of 4 for the best behavioral assessment to a score of 0 for the worse behavioral assessment, as previously described (19).
Statistical Analysis
Data are expressed as the mean ± standard deviation. One-way analysis of variance or Student’s t-test was performed where appropriate. Statistical significance was determined based on p < 0.05.
Results:
Burn-induced intestinal permeability peaks at 6 hours post-injury.
We compared intestinal permeability at multiple time points following 30% TBSA burn to determine the timing of peak gut permeability to 4kDa FITC-Dextran. Compared to sham, intestinal permeability was increased after burn injury, with no difference in the degree of intestinal permeability when comparing the jejunum and ileum (Figure 1A). Next, we measured intestinal permeability at multiple time points following injury, finding that gut permeability peaked at 6 hours post-burn in this model (Figure 1B).
Figure 1: Gut permeability peaks at 6 hours post-burn injury.
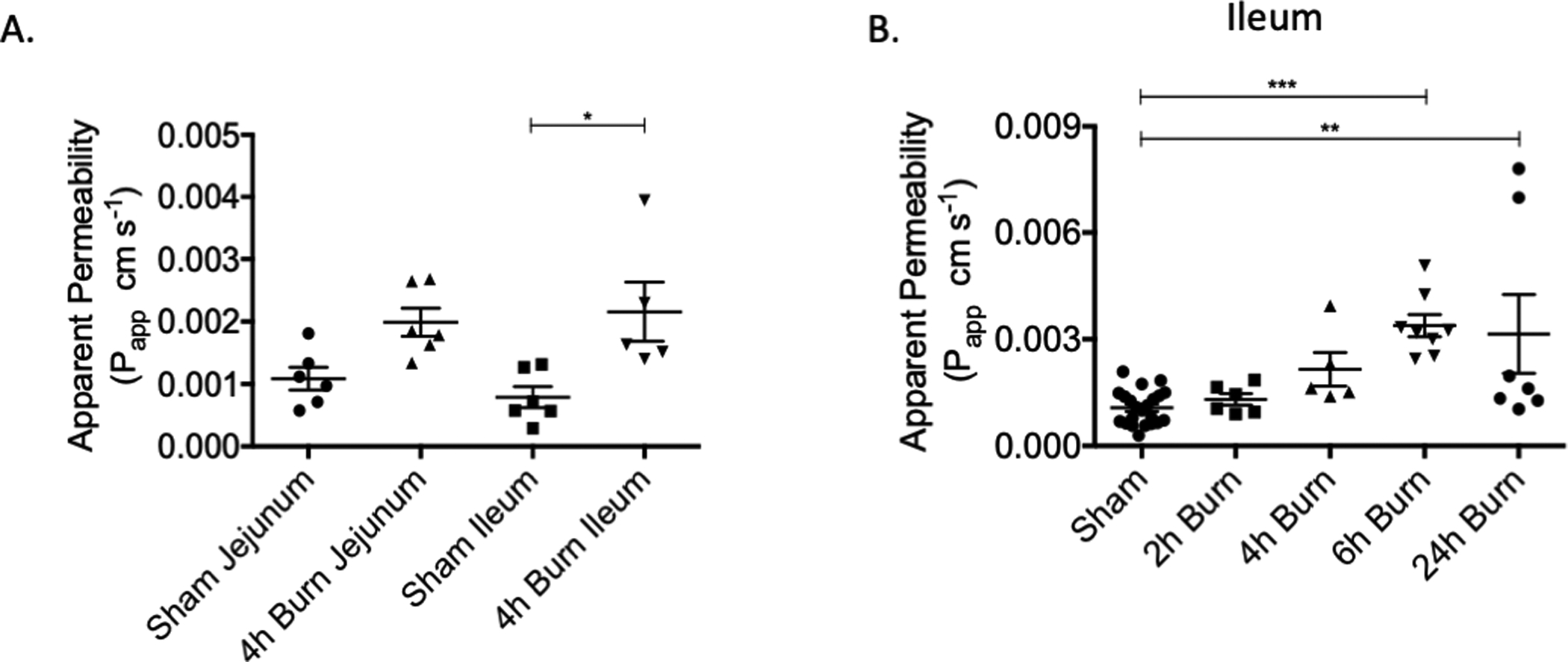
Ex vivo intestinal permeability to 4kDa FITC-Dextran was measured at 6 hours following 30% TBSA burn injury. (A) Burn-induced gut permeability was increased in the ileum, there was no difference in permeability based on location in the intestine. (B) Gut permeability time course demonstrating that burn-induced gut permeability peaks at 6 hours post-injury. N= 5–7 animals per group. * p< 0.05, ** p< 0.01, ***p< .001.
The pharmacologic vagal agonist AR-R17779 provides dose-dependent protection against burn-induced gut permeability.
As the CHRNA7 gene encodes the α7nAChR that has been shown to mediate cholinergic anti-inflammatory signaling, we confirmed the presence of CHRNA7 gene expression in the gut. While CHRNA7 was widely expressed in the gut, we found no difference in relative gene expression when comparing the jejunum and ileum (Figure 2A). To understand the effects of AR-R17779 on burn-induced gut permeability, we performed a dose-response curve. We found a dose-dependent decrease in burn-induced gut permeability after treatment with AR-R17779 (Figure 2B). We selected the mid-range dose (5mg/ml) for subsequent studies.
Figure 2: AR-R17779 provides dose-dependent protection against burn-induced gut permeability.
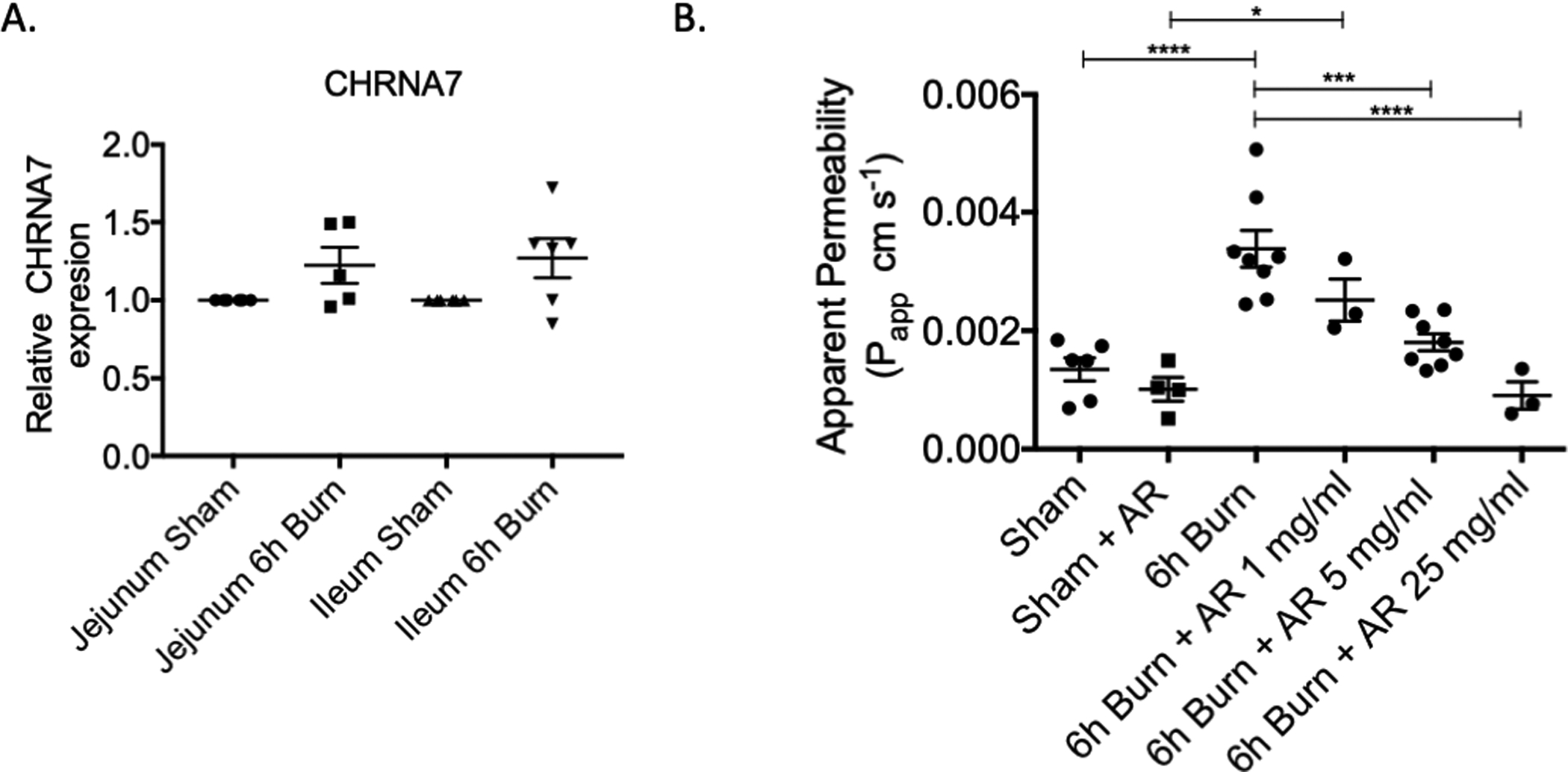
(A) CHRNA7 gene expression measured by PCR. There was no difference in CHRNA7 gene expression when comparing jejunum vs. ileum or in sham vs. burn injury, n=4–6 / group. (B) Changes in burn-induced gut permeability were assessed after escalating doses of AR-R17779. There was a dose-dependent decrease in gut permeability after treatment with AR-R17779, n= 3–8 animals per group. * p< 0.05, ***p< 0.001, ****p< 0.0001.
AR-R17779 prevents burn-induced lung vascular permeability.
Severe burn is associated with lung inflammation and acute lung injury responsible for significant morbidity during recovery from injury. We assessed the effects of AR-R17779 (5mg/ml) on lung vascular permeability at 6 hours following burn (Figure 3). Severe burn increased lung permeability nearly 10-fold compared to sham. Conversely, treatment with AR-R17779 prevented burn-induced lung injury, with lung permeability similar to sham.
Figure 3: Injection of AR-R17779 decreases lung vascular permeability after severe burn.
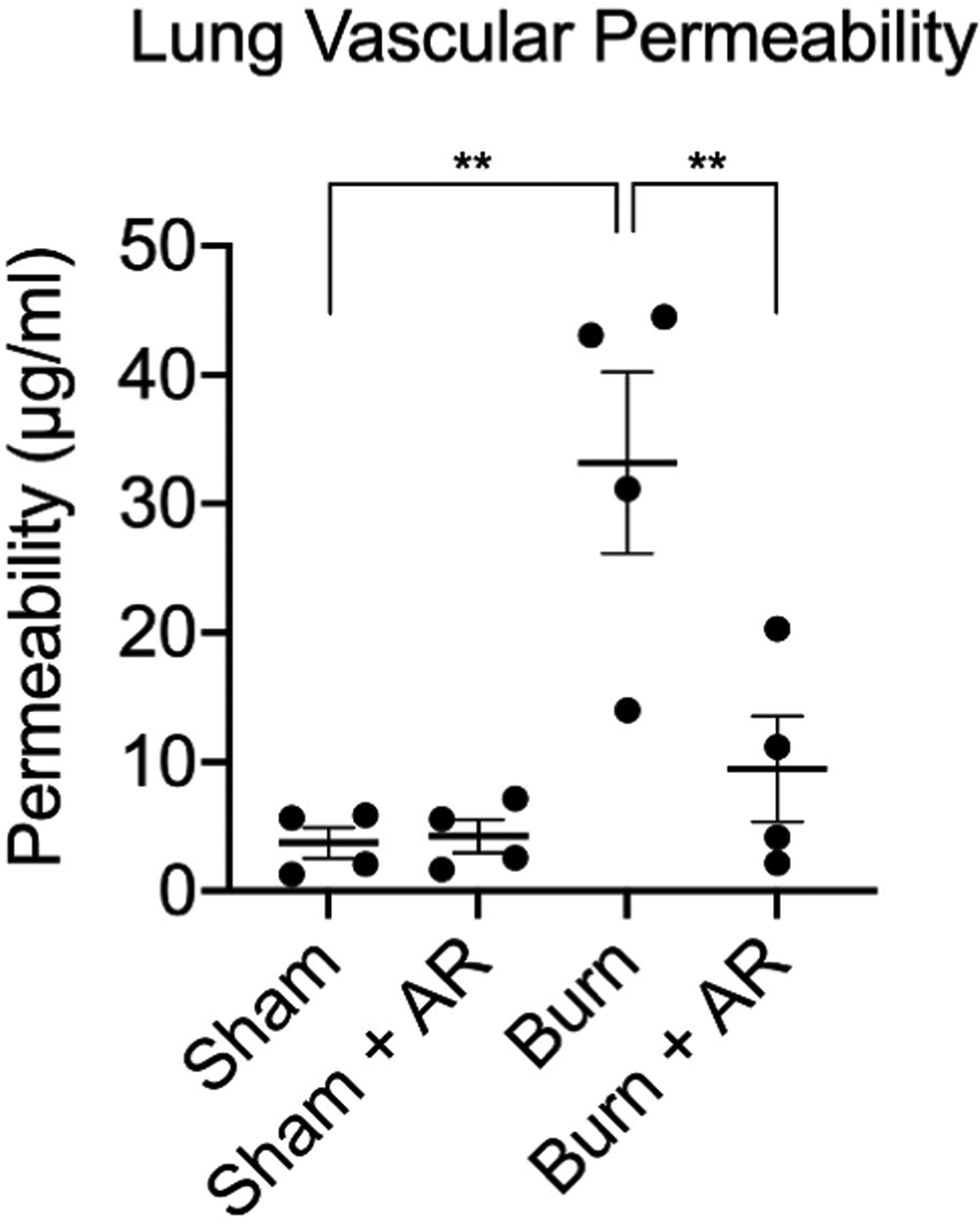
Lung vascular permeability was measured 6 hours following severe burn injury. AR-R17779 prevents burn-induced lung permeability. N= 4 animals per group. ** p<0.01 using one-way ANOVA.
Treatment with AR-R17779 improves activity levels after severe burn injury.
Behavioral assessments were performed at multiple early time points following burn injury to determine whether treatment with AR-R17779 improved activity levels (Figure 4). Behavioral scores correlated with the improved gut and lung permeability measurements seen in animals treated with AR-R17779. While still lower than sham animals, activity in burn injured animals treated with AR-R17779 was significantly improved. We concluded that AR-R17779 improves physiologic recovery following severe burn injury.
Figure 4: Treatment with AR-R17779 improves activity after severe burn injury.
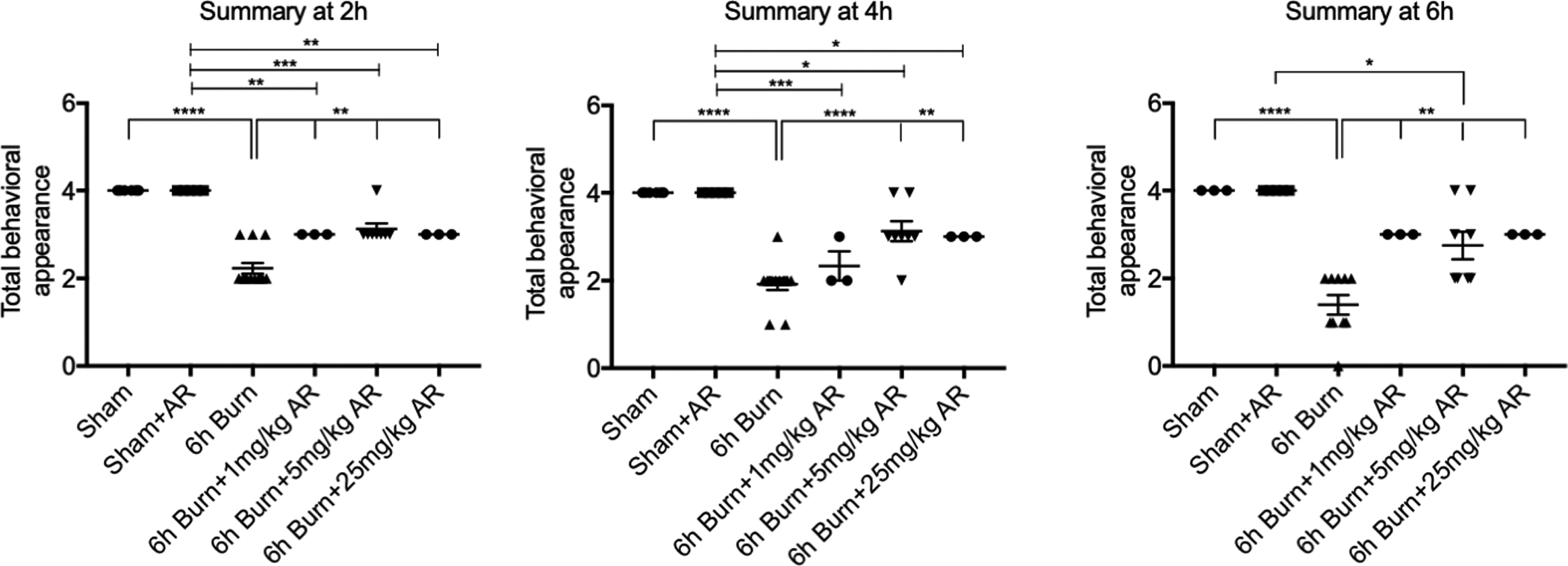
Behavioral assessments were performed at multiple early time points following burn injury. Treatment with AR-R17779 improved activity levels compared to burn alone. N= 3–8 animals per group. * p< 0.05, ** p< 0.01, ***p< 0.001, ****p< 0.0001.
Discussion:
An overwhelming SIRS response to severe injury can lead to organ dysfunction and potentially multi-organ failure associated with significant morbidity and mortality (20). We stimulated the cholinergic anti-inflammatory pathway to attenuate burn-induced organ injury by administering a selective α7nAChR as an adjunct to resuscitation fluid. We demonstrated that AR-R17779 prevented burn-induced gut and lung injury associated with improved behavioral activity during recovery. Directly targeting the α7nAChR may be a useful pharmacologic strategy to improve the efficacy of vagal therapeutics that target the SIRS response to injury in the clinical setting.
Following injury, early gut barrier breakdown leads to a gut inflammatory response that is believed to drive SIRS and acute lung injury (21). Attenuating inflammation along this gut-lung axis is believed to have the potential to prevent an uncontrolled inflammatory response that can have deleterious effects on the host. Direct vagus nerve stimulation (VNS) has been shown to prevent gut permeability and lung inflammation in a model of severe burn injury (22). Interestingly, the protective effects of VNS on lung injury were lost after the abdominal vagus nerve was severed, demonstrating that VNS-induced gut barrier protection drives the lung response along this gut-lung axis (23). This study utilized a pharmacologic agonist of the α7nAChR, widely expressed on macrophages and enteric glial cells in the intestine (6, 10), rather than direct stimulation of the efferent vagus nerve. While α7nAChR agonists are known to prevent gut inflammation in injury models, it is unclear whether the decrease in burn-induced lung injury here is solely due to secondary effects via the gut-lung axis versus direct protective effects on the lung itself (6, 24).
Several non-specific agonists of the cholinergic anti-inflammatory pathway have been studied in both preclinical models and have been trialed in human clinical studies to a limited extent. Vagal agonists have been extremely effective in limiting inflammation in animal models of sepsis (25), trauma/hemorrhagic shock (26), burns (8), inflammatory bowel disease (27) and post-operative ileus (28). We have previously utilized a cholinergic agonist in this same burn model, demonstrating decreased gut and lung injury (10). Unfortunately, these preclinical successes have not translated to a translational therapeutic as the individual response to vagal agonists has been highly variable and limited the success of human studies (29). Healthy subjects treated with the oral cholinergic agonist GTS-21 before exposure to endotoxin did not significantly alter inflammatory cytokine levels compared to placebo (13). Nicotine, another non-specific agonist of the cholinergic anti-inflammatory pathway, did not significantly alter inflammatory cytokine levels in healthy humans treated with intravenous endotoxin (30). While Semapimod (CNI-1493) was effective in reducing inflammation in animal studies, a trial of patients with moderate to severe Crohn’s disease, treatment with the vagal agonist Semapimod (CNI-1493) did not improve disease severity compared to placebo; however, cumulative dosing did result in improved disease activity in a subset of patients (31–33).
Initial mechanistic studies evaluating the cholinergic anti-inflammatory pathway suggested a spleen-dependent pathway that required activation of the α7nAChR in splenic macrophages to limit systemic cytokine release (34). More recently, it has been demonstrated that the vagal anti-inflammatory pathway prevents gut injury via a spleen-independent mechanism (6, 10). Recently, a liver-brain-gut axis has been characterized as a mediator of gut inflammatory homeostasis (35). Therefore, directly targeting the receptor that mediates the cholinergic anti-inflammatory pathway may alter multiple afferent and efferent signaling pathways that mediate the cholinergic anti-inflammatory response.
This study is not the first to evaluate the potential for AR-R17779 to limit inflammation after an acute insult. A prior in vitro study using intestinal epithelial cells and pulmonary microvascular epithelial cells found that AR-R17779 decreased cytokine levels after hypoxia-reoxygenation compared to vehicle controls (36). AR-R17779 has also been shown to limit post-operative ileus in an animal model of intestinal manipulation and was associated with decreased pro-inflammatory cytokine production from peritoneal macrophages (37). Conversely, a previous animal study using a stroke model demonstrated that treatment with AR-R17779 did not prevent cerebral ischemia-reperfusions injury (38). The effects of binding to different receptors than just the α7nAChR need to also be considered. AR-R17779 is reported to be a highly selective agonist of the α7nAChR, with in vitro studies demonstrating 96% efficacy for binding the α7nAChR (14). AR-R17779 is also an agonist of the α4β2 nAChR that plays an important role in inhibiting the transduction of pain signals and mediates pain perception (39, 40). These potential secondary effects of AR-R17779 may be advantageous in the post-injury period and should be the topic of future study.
We acknowledge several limitations in utilizing this mouse model of burn injury to recapitulate the human response to burn and the difficulty in simulating the critical care that is provided to human patients in Burn Centers. In this animal model, we resuscitate animals with a single bolus fluid resuscitation post-injury which differs from the Parkland Formula that is utilized in the clinical setting. Although we do not measure continuous urine output nor endpoints of resuscitation in this model, we believe the burn injured mice are adequately resuscitated based on the behavioral scores seen in mice treated with AR-R17779 who received identical fluid resuscitation as control mice. Second, we evaluated gut barrier function by measuring ex vivo intestinal permeability to low molecular weight FITC-Dextran but are aware that this is just one assay and does not fully evaluate the complex functions of the gut barrier after burn injury. Finally, we also recognize the limitations in correlating the inflammatory response in mice with the inflammatory response seen in humans based on their well described differences in immune response (41). Future studies using “humanized” mice (15, 16) that better recapitulate the human immune system will be helpful in further supporting the clinical, translational potential of AR-R17779.
These results suggest that the selective α7nAChR agonist AR-R17779 may limit distant organ injury after severe burn. While these promising preclinical results do not guarantee success in human studies, we postulate that more directly targeting the α7nAChR that mediates the cholinergic anti-inflammatory response may improve the efficacy of this approach compared to prior, non-specific vagal agonists. Studies in humans will be needed to assess individual variability in response to cholinergic agonists and the effects of the human variant gene CHRFAM7A that inhibits ligand binding to the α7nAChR (15, 42).
Supplementary Material
Acknowledgments:
The authors would like to recognize Ann-Marie Hageny, Emelie Amburn, and Olga Cohen for their excellent technical assistance.
Funding:
Research supported by a grant from the National Institutes of Health (5R01GM121530-05, TWC)
Footnotes
This manuscript was presented as an oral podium presentation at the 80th Annual Meeting of AAST and Clinical Congress of Acute Care Surgery, September 29-October 2, 2021. Atlanta, Georgia
The authors have no disclosures to report.
Conflicts of Interest: The authors report no conflicts of interest.
References:
- 1.Peiseler M, Kubes P. Macrophages play an essential role in trauma-induced sterile inflammation and tissue repair. Eur J Trauma Emerg Surg. 2018;44(3):335–49. [DOI] [PubMed] [Google Scholar]
- 2.Maier RV. Pathogenesis of multiple organ dysfunction syndrome--endotoxin, inflammatory cells, and their mediators: cytokines and reactive oxygen species. Surg Infect (Larchmt). 2000;1(3):197–204; discussion −5. [DOI] [PubMed] [Google Scholar]
- 3.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–9. [DOI] [PubMed] [Google Scholar]
- 5.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, Michel K, Tracey KJ, Schemann M, Boesmans W, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63(6):938–48. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–8. [DOI] [PubMed] [Google Scholar]
- 8.Costantini TW, Bansal V, Krzyzaniak M, Putnam JG, Peterson CY, Loomis WH, Wolf P, Baird A, Eliceiri BP, Coimbra R. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol. 2010;299(6):G1308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costantini TW, Bansal V, Peterson CY, Loomis WH, Putnam JG, Rankin F, Wolf P, Eliceiri BP, Baird A, Coimbra R. Efferent vagal nerve stimulation attenuates gut barrier injury after burn: modulation of intestinal occludin expression. J Trauma. 2010;68(6):1349–54; discussion 54–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costantini TW, Krzyzaniak M, Cheadle GA, Putnam JG, Hageny AM, Lopez N, Eliceiri BP, Bansal V, Coimbra R. Targeting alpha-7 nicotinic acetylcholine receptor in the enteric nervous system: a cholinergic agonist prevents gut barrier failure after severe burn injury. Am J Pathol. 2012;181(2):478–86. [DOI] [PubMed] [Google Scholar]
- 11.Krzyzaniak M, Peterson C, Loomis W, Hageny AM, Wolf P, Reys L, Putnam J, Eliceiri B, Baird A, Bansal V, et al. Postinjury vagal nerve stimulation protects against intestinal epithelial barrier breakdown. J Trauma. 2011;70(5):1168–75; discussion 75–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu X, Costantini T, Lopez NE, Wolf PL, Hageny AM, Putnam J, Eliceiri B, Coimbra R. Vagal nerve stimulation protects cardiac injury by attenuating mitochondrial dysfunction in a murine burn injury model. J Cell Mol Med. 2013;17(5):664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kox M, Pompe JC, Gordinou de Gouberville MC, van der Hoeven JG, Hoedemaekers CW, Pickkers P. Effects of the alpha7 nicotinic acetylcholine receptor agonist GTS-21 on the innate immune response in humans. Shock. 2011;36(1):5–11. [DOI] [PubMed] [Google Scholar]
- 14.Mullen G, Napier J, Balestra M, DeCory T, Hale G, Macor J, Mack R, Loch J 3rd, Wu E, Kover A, et al. (−)-Spiro[1-azabicyclo[2.2.2]octane-3,5’-oxazolidin-2’-one], a conformationally restricted analogue of acetylcholine, is a highly selective full agonist at the alpha 7 nicotinic acetylcholine receptor. J Med Chem. 2000;43(22):4045–50. [DOI] [PubMed] [Google Scholar]
- 15.Costantini TW, Chan TW, Cohen O, Langness S, Treadwell S, Williams E, Eliceiri BP, Baird A. Uniquely human CHRFAM7A gene increases the hematopoietic stem cell reservoir in mice and amplifies their inflammatory response. Proc Natl Acad Sci U S A. 2019;116(16):7932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costantini TW, Meads M, Dang X, Coimbra R, Torbett BE, Baird A, Eliceiri BP. The Response to Burn Injury in Mice With Human Hematolymphoid Systems. Annals of surgery. 2016;263(1):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mateer SW, Cardona J, Marks E, Goggin BJ, Hua S, Keely S. Ex Vivo Intestinal Sacs to Assess Mucosal Permeability in Models of Gastrointestinal Disease. J Vis Exp 2016(108):e53250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, Ingber DE, Sukhatme VP. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem. 2007;282(33):23910–8. [DOI] [PubMed] [Google Scholar]
- 19.Van Landeghem L, Blue RE, Dehmer JJ, Henning SJ, Helmrath MA, Lund PK. Localized intestinal radiation and liquid diet enhance survival and permit evaluation of long-term intestinal responses to high dose radiation in mice. PLoS One. 2012;7(12):e51310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauaia A, Moore FA, Moore EE. Postinjury Inflammation and Organ Dysfunction. Crit Care Clin. 2017;33(1):167–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520–8. [DOI] [PubMed] [Google Scholar]
- 22.Reys LG, Ortiz-Pomales YT, Lopez N, Cheadle G, de Oliveira PG, Eliceiri B, Bansal V, Costantini TW, Coimbra R. Uncovering the neuroenteric-pulmonary axis: vagal nerve stimulation prevents acute lung injury following hemorrhagic shock. Life Sci. 2013;92(13):783–92. [DOI] [PubMed] [Google Scholar]
- 23.Krzyzaniak MJ, Peterson CY, Cheadle G, Loomis W, Wolf P, Kennedy V, Putnam JG, Bansal V, Eliceiri B, Baird A, et al. Efferent vagal nerve stimulation attenuates acute lung injury following burn: The importance of the gut-lung axis. Surgery. 2011;150(3):379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arora S, Dev K, Agarwal B, Das P, Syed MA. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology. 2018;223(4–5):383–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–62. [DOI] [PubMed] [Google Scholar]
- 26.Kojima M, Gimenes-Junior JA, Chan TW, Eliceiri BP, Baird A, Costantini TW, Coimbra R. Exosomes in postshock mesenteric lymph are key mediators of acute lung injury triggering the macrophage activation via Toll-like receptor 4. FASEB J. 2018;32(1):97–110. [DOI] [PubMed] [Google Scholar]
- 27.de Araujo A, de Lartigue G. Non-canonical cholinergic anti-inflammatory pathway in IBD. Nat Rev Gastroenterol Hepatol. 2020;17(11):651–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6(8):844–51. [DOI] [PubMed] [Google Scholar]
- 29.Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016;113(29):8284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittebole X, Hahm S, Coyle SM, Kumar A, Calvano SE, Lowry SF. Nicotine exposure alters in vivo human responses to endotoxin. Clin Exp Immunol. 2007;147(1):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dotan I, Rachmilewitz D, Schreiber S, Eliakim R, van der Woude CJ, Kornbluth A, Buchman AL, Bar-Meir S, Bokemeyer B, Goldin E, et al. A randomised placebo-controlled multicentre trial of intravenous semapimod HCl for moderate to severe Crohn’s disease. Gut. 2010;59(6):760–6. [DOI] [PubMed] [Google Scholar]
- 32.The F, Cailotto C, van der Vliet J, de Jonge WJ, Bennink RJ, Buijs RM, Boeckxstaens GE. Central activation of the cholinergic anti-inflammatory pathway reduces surgical inflammation in experimental post-operative ileus. Br J Pharmacol. 2011;163(5):1007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Grishin AV, Ford HR. Experimental Anti-Inflammatory Drug Semapimod Inhibits TLR Signaling by Targeting the TLR Chaperone gp96. J Immunol. 2016;196(12):5130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203(7):1623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teratani T, Mikami Y, Nakamoto N, Suzuki T, Harada Y, Okabayashi K, Hagihara Y, Taniki N, Kohno K, Shibata S, et al. The liver-brain-gut neural arc maintains the Treg cell niche in the gut. Nature. 2020;585(7826):591–6. [DOI] [PubMed] [Google Scholar]
- 36.Tarras SL, Diebel LN, Liberati DM, Ginnebaugh K. Pharmacologic stimulation of the nicotinic anti-inflammatory pathway modulates gut and lung injury after hypoxia-reoxygenation injury. Surgery. 2013;154(4):841–7; discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 37.The FO, Boeckxstaens GE, Snoek SA, Cash JL, Bennink R, Larosa GJ, van den Wijngaard RM, Greaves DR, de Jonge WJ. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology. 2007;133(4):1219–28. [DOI] [PubMed] [Google Scholar]
- 38.Hammarlund ME, Darsalia V, Mjornstedt F, Pattanaik B, Mallard C, Rocha-Ferreira E, Patrone C, Johansson ME. The selective alpha7 nicotinic acetylcholine receptor agonist AR-R17779 does not affect ischemia-reperfusion brain injury in mice. Biosci Rep. 2021;41(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabri O, Meyer PM, Graf S, Hesse S, Wilke S, Becker GA, Rullmann M, Patt M, Luthardt J, Wagenknecht G, et al. Cognitive correlates of alpha4beta2 nicotinic acetylcholine receptors in mild Alzheimer’s dementia. Brain. 2018;141(6):1840–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bagdas D, Ergun D, Jackson A, Toma W, Schulte MK, Damaj MI. Allosteric modulation of alpha4beta2* nicotinic acetylcholine receptors: Desformylflustrabromine potentiates antiallodynic response of nicotine in a mouse model of neuropathic pain. Eur J Pain. 2018;22(1):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan T, Williams E, Cohen O, Eliceiri BP, Baird A, Costantini TW. CHRFAM7A alters binding to the neuronal alpha-7 nicotinic acetylcholine receptor. Neurosci Lett. 2019;690:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


