Abstract
Purpose:
Commensal microbiome secretes various metabolites that can exert important effects on the host immunity and inflammation and can alter cellular functions. However, little is known regarding the effect of microbiome on corneal immunity and genetic expression. The purpose of this study is to describe the effect of diet-induced gut dysbiosis on corneal immunity and corneal gene expression after wounding.
Methods:
This study is approved by the Animal Care and Use of the University of Illinois. Six-week-old female C57BL6 mice were fed on a normal chow diet (ND), isocaloric low-fat control diet (LFD), or a 21% milk high-fat diet (HFD) for six weeks. 2mm corneal epithelial debridement was performed (n=10). Fecal samples from mice were used for microbial diversity analysis (n>3). Immunofluorescence staining of corneal wholemount tissue post-debridement was used to visualize immune cell distribution. RNA Seq was performed on tissue samples from corneas following debridement.
Results:
Mice fed differing diets had significant alterations in gut microbial diversities. After corneal debridement, HFD mice experienced delayed wound healing in comparison to LFD mice and ND mice groups. However, fecal transplantation led to normalization of wound closure rates. Increased γδTCR staining was observed in the LFD group, and decreased LY6G was observed in HFD group (p<0.05). Gene Ontology terms of differentially expressed genes included response to external stimulus, cell proliferation, migration, adhesion, defense response and leukocyte migration. Top over-represented pathways included ECM-receptor interaction, Cytokine-cytokine receptor interaction, Focal adhesion and Leukocyte trans-endothelial migration.
Conclusions:
Gut microbial dysbiosis alters corneal immune cell distribution, corneal response to injury, and genes related to epithelial function and corneal immunity.
Keywords: Microbiome, Corneal healing, Gut dysbiosis
Introduction
The gut microbiome plays a critical role in our health and disease states(1–6). Recently, researchers demonstrated that, under natural conditions, the human neonatal gut microbiome develops in an orchestrated fashion under the nutritional, immunological, hormonal and prebiotic effect of food and the external environment(7). The gut microbiome is closely associated with the growth of the body by controlling the secretion of hormones and plays an important function in immune development by regulating the maturation of the immune system(8). Several recent studies have also shown that the changes in the gut microbiome can directly affect ocular surface health(9–13). Notably, germ-free mice were found to develop Sjögren-like keratoconjunctivitis(14). However, the effect of the gut microbiome on specifically corneal epithelial regeneration and healing is still an understudied area of medicine.
Several recent studies also suggest that the microbiome plays an important role in the development of the cornea by changing the immune status in the cornea. For example, gut dysbiosis due to antibiotic over-usage may lead to changes in macrophage distribution in the cornea, leading to decreased corneal size and alterations in murine corneal development (15). The environmental and diet-induced triggers of immune system alteration, which lead to changes in corneal epithelial cell function and corneal wound healing, remain unknown. However, current research largely agrees that the gut microbiome is heavily influenced by diets, and in particular, a Western style high-fat diet rapidly and reproducibly alters the gut microbiome(16). Given this knowledge, the gut microbiome likely impacts corneal wound healing, and the inflammatory state that occurs in the setting of corneal injury plays an important role in epithelial progenitor cell function.
In this study, we aim to evaluate the effect of the gut microbiome on corneal epithelial cell function and wound healing in a diet-induced gut dysbiosis model in mice. Our central hypothesis proposes that host microbiome-induced changes in specific immune cells in the cornea can affect corneal response to injury and regeneration. We thus firstly evaluate how differing diets change gut microbial profile in mice; we subsequently evaluate how diet-induced changes may affect murine corneal response to injury and corneal immunity.
Materials and Methods
Animals
Specific-pathogen-free female C57BL/6J mice without eye disease, aged 6 weeks and 12 weeks, were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA). Six-week-old mice were fed either normal chow rodent diet (ND) (Teklad global 2019 rodent diet), sterile isocaloric low-fat diet (LFD) (TD08485, Envigo), or sterile 21% milk Western Style high-fat diet (HFD) (TD88137, Envigo) from 6 weeks of age for 6 weeks. Mice from each group were measured for weight, fasting blood glucose levels, and tear production using phenol red thread test at the end of the feeding period at 12 weeks. All animal protocols were approved by the Animal Care Committee of the University of Illinois at Chicago (UIC). All animals were treated in accordance with the Association for Research in Vision and Ophthalmology’s Statement for the Use of Animals in Ophthalmology and Vision Research and the guidelines of the Animal Care Committee at the University of Illinois at Chicago.
16S rRNA Gene Sequencing
Fecal samples from each group of mice were collected at the end of the feeding period. Diversity and abundance of the gut microbiota of ND, LFD and HFD mice were analyzed with 16S rRNA gene sequencing. DNA of the gut microbiota from stool of at least 3 mice per group was obtained by the QIAamp DNA Stool Mini Kit (Qiagen, Germantown, MD). Then, DNA samples was sent to the UIC Genomics Core for 16rRNA gene sequencing.
Corneal Epithelial Wound Healing Model
The right eyes of mice fed ND, LFD and HFD for 6-weeks were subjected to a 2mm epithelial debridement surgery without causing injury to the underlying stroma. The left eyes were untouched and served as controls. Mice were anesthetized with intraperitoneal injections of a combination of ketamine, xylazine and proparacaine ophthalmic solution as a local anesthesia. A 2-mm trephine (Integra, Elmhurst, IL) was used to mark the area for the debridement, and an Algerbrush burr (Algerbrush II, Sigmapharmaceuticals, North Liberty, IA) was used to gently detach the epithelium as previously described(17,18). To minimize damage to the underlying basement membrane and increase the consistency of the procedure, the procedure was performed carefully by a single corneal surgeon with direct visualization under a microscope in a blinded fashion. In addition, we also recognize that the usage of proparacaine prior to corneal injury may also alter corneal response to injury(19,20). However, proparacaine was used in both control and experimental groups. Fluorescein-stained murine corneas were then imaged at time 0-, 16-, 24-, 28-, 32- and 48-hours after debridement to calculate rate of epithelial wound closure.
Fecal microbial transplantation
Twelve-week-old female mice fed ND served as fecal microbial transplantation (FMT) donors. Donor mice were placed in empty autoclaved cages and allowed to defecate. Following collection of a minimum of twelve fecal pellets using individual sterile toothpicks, pellets were promptly placed in 2 mL round-bottom tubes containing 800 μL autoclaved, filtered water (Milli-Q, EMD Millipore, Billerica, MA) and homogenized for 1 min. Homogenates were then passed through a 30 μm pore-size nylon filters to remove large particulate and fibrous matter. Fresh fecal slurries were then be pooled and diluted to a volume of 2.5 mL(21). Fecal transplantation from pooled samples of five donor mice to 12-week old mice fed HFD were performed by a gavage according to published method in literature(15). This was performed once every three days for three times. Subsequently, the right eyes of mice were subjected to 2mm epithelial debridement.
Quantitative PCR
Using Invitrogen High-Capacity RNA-to-cDNA Kit (Life Technologies, Grand Island, NY, USA), RNA isolated from debrided corneas (at least 200 ng per sample) was reverse transcribed into cDNA. cDNA samples were diluted 25-fold to 500 microliters. Quantitative real-time PCR (Q-PCR) was performed using Invitrogen Power SYBR Green Master Mix according to manufacturer’s protocol (Life Technologies). The expressions of candidate genes were normalized against glyceraldehyde-3-phosphate-dehydrogenase (GAPDH). Differential expression analysis was performed using the ddCt method(22) ANOVA (StatPlus, Version 6, AnalystSoft Inc., Walnut, CA) was used to analyze the statistical significance of Q-PCR data. Test results with P < 0.05 were declared statistically significant.
Immunostaining and Qualitative Analysis
It has previously been shown that vessel dilatation and neutrophil accumulation reached a peak at 12 to 18 hours after corneal epithelial injury (23). Thus, after 18 hours from debridement surgery, mice were euthanized, and the eyes were enucleated. Tissues (n>3 per group) were fixed in 4% paraformaldehyde for 1 hour and the globes were dissected in phosphate-buffered saline (PBS) under a dissecting microscope, leaving the corneas with complete limbi. After washing three times with PBS, corneas were blocked in 2% bovine serum albumin for 15 minutes and permeabilized with 0.15 Triton X-100/2% bovine serum albumin for 1 hour. The corneas were then incubated with the following antibodies; anti-Ki67(Invitrogen 42569880, Carlsband, CA), rat anti-mouse Ly6G (BD Biosciences 565369, San Jose, CA), and hamster anti-mouse γδTCR (Invitrogen 17571181). At least three replicate corneal samples were imaged for each marker. Z stack corneal images were analyzed using ImageJ (Version 1.51, https://imagej.nih.gov/ij/) and subsequent manual counting of positively stained cells confirmed accuracy. Immunofluorescent cells at central, paracentral, peripheral and limbal regions (each presumed to occupy ¼ of corneal radius) of the cornea were counted. Wound edge is defined as occupying two adjacent 20x microscopic fields from the ridge of proliferating epithelial cells.
Isolation of RNA and Illumina Sequencing
Eighteen hours after surgery, murine corneas were isolated. Corneas were disintegrated with TissueRupter (Qiagen, Valencia, CA, USA). Qiagen RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA) was used to extract total RNA. Agilent Technologies 2100 Bioanalyzer and Nanodrop Spectrophotometer were used to check RNA quantity and integrity. At least 100 ng of total RNA with a minimum RNA integrity number of 8 per sample was sent to the UIC Genomics Core to perform bulk RNA-Seq. Total RNA was extracted with rRNA depletion. cDNA libraries were multiplexed with three samples per lane and loaded onto flow cell lanes. Paired-end sequencing-by-synthesis of 51-nucleotide length was performed on Illumina NovaSeq 6000 sequencing system (Illunima Inc, San Diego, CA, USA).
Analysis of RNA-Seq Data
Quality control of the RNA-Seq reads was performed using FastQC. Low quality bases were removed using Cutadapt. After quality control, more than 97% reads remained. The RNA-Seq reads were aligned to the mm10 mouse reference genome using RNA STAR (version 2.7)(24). The overall read mapping rate ranges 95–98%. Differential gene expression was calculated by DESeq2. Genes were considered to be differentially expressed with absolute log-fold >2 and false discovery rate <0.05. The R package (Version 4.0) was used for data visualization and subsequent analysis. Gene ontology enrichment analysis was performed with Goseq with KEGG pathway analysis for finding significant pathways involved(25).
Pathway Analysis
A data set containing differentially expressed gene identifiers and corresponding expression values were uploaded onto Ingenuity Pathways Analysis (IPA) software (Redwood, CA, USA) for pathway and network analyses. These differentially expressed gene identifiers were overlaid onto a global molecular network developed from information contained in the Ingenuity Pathway Knowledge Base, which was derived from known gene interactions published in scientific literature.
Statistical Analysis
All experiments were repeated a minimum of three independent times. As much as possible, all data were collected and analyzed in a masked fashion. T-test/ANOVA were used for comparing means of continuous data (P < 0.05 significant) and Chi squared/Fisher’s exact for categorical data. Alpha and beta diversities were calculated using R.
Results
Animal Characteristics
Specific-pathogen-free female C57BL/6J mice without eye disease, aged 6 weeks were fed normal chow rodent diet (ND), sterile isocaloric low-fat diet (LFD) or sterile 21% milk Western Style high fat diet (HFD) from 6 weeks of age (Figure 1) for 6 weeks. Mice from each group were measured for weight, fasting blood glucose levels, and tear production using phenol red thread test at the end of the feeding period at 12 weeks. Figure 1B illustrates that statistically significant increased weight (p<0.05) was observed in the HFD group after feeding (ND 20.0±1.7g, LFD 20.0±0.7g, HFD 25.0±2.6g, n≥10). However, no statistically significant changes in fasting blood glucose levels (ND 119±38 mg/dL, LFD 128±12 mg/dL, HFD 127±30 mg/dL, n≥10) and phenol red tear production tests (ND 3.5±0.5mm, LFD 3.3±mm, HFD 3.6±0.5mm, n≥10) were observed among the different groups of mice.
Figure 1.
Diets and mice characteristics. A) Composition of normal chow diet (ND), low fat diet (LFD) and high fat diets (HFD). B) Bar plot demonstrating mice weight and fasting blood glucose levels in mice on different diets (n≥10 per group). No significant difference in fasting blood glucose levels were found (ND 119±38 mg/dL, LFD 128±12 mg/dL, HFD 127±30 mg/dL). Mice on HFD showed significant weight gain (ND 20.0±1.7g, LFD 20.0±0.7g, HFD 25.0±2.6g). (*p<0.01, ND=normal chow diet, LFD=low fat diet, HFD=high fat diet).
Diet induced changes in gut microbial profile
Significant changes in the gut microbial profile were observed after 6 weeks of dietary changes. Figure 2 demonstrates 16S rRNA gene sequencing results of fecal specimens. Figure 2A shows the relative abundance of major bacterial phyla and classes, and Figure 2B shows heatmap of relative abundance of different bacterial groups. (In Figure 2C, non-metric multidimensional scaling (NMDS) based on a Bray-Curtis dissimilarity matrix was shown (Adonis R2=0.87525, p<0.001). In Figure 2D, dissimilarity in alpha diversity (Kruskal-Wallis chi-squared = 7.4364, df = 2, p-value = 0.02) was shown. Alpha diversity showed that the ND group had the lowest Shannon index, whereas the LFD group had the highest Shannon index. In Figure 2E, LEfSe (Linear discriminant analysis Effect Size) predicts organisms most likely to explain differences between groups of mice. According to LEfSe result, Muribaculaceae most likely explains changes observed in ND group; Lachnospiraceae most likely explains changes observed in the LFD group, and Akkermansiaceae most likely explains changes observed in the HFD group. The exact functions of these bacterial groups are still uncertain and are currently under investigation.
Figure 2.
16S rRNA sequencing of fecal specimens. A). Relative abundance of major bacterial phyla and classes. B). Heatmap demonstrating relative abundance of different bacterial groups. (light yellow=lower abundance, Dark red = higher abundance) (ND=Normal chow diet, HFD=High fat diet, LFD=Low fat diet). C). Non-metric multidimensional scaling (NMDS) based on a Bray-Curtis dissimilarity matrix showing dissimilarity of bacterial population depending on diets (Adonis R2=0.87525, p<0.001). D) Box plot showing dissimilarity in alpha diversity (Kruskal-Wallis chi-squared = 7.4364, df = 2, p-value = 0.02). E) LEfSe (Linear discriminant analysis Effect Size) predicting organisms most likely to explain differences between groups of mice. According to LEfSe result, Muribaculaceae most likely explains changes observed in ND group; Lachnospiraceae most likely explains changes observed in the LFD group, and Akkermansiaceae most likely explains changes observed in the HFD group.
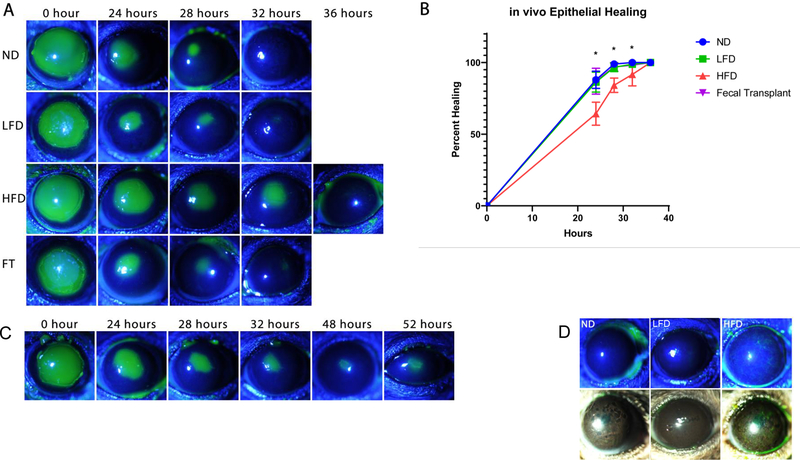
In vivo epithelial healing
Differences in the rate of corneal epithelial healing after injury were observed in the groups of mice after 6 weeks of dietary changes. Figure 3 shows in vivo epithelial healing in mice fed different diets after a 2mm central corneal debridement (n=10 for each group). Fluorescein staining demonstrated that ND and LFD groups healed after 32 hours whereas HFD group exhibited delayed epithelial healing. In addition, although the rate of healing was similar in the ND and LFD groups, healed epithelium appeared thinner in the LFD group when compared to the ND group. Figure 3B shows a line graph demonstrating significant delay in epithelial healing in mice fed HFD.
Figure 3.
in vivo corneal epithelial healing after debridement injury. A) in vivo epithelial healing in mice fed different diets after 2mm central corneal debridement (n=10). Fluorescein staining demonstrating that ND and LFD groups healed after 32 hours whereas HFD group showed delayed epithelial healing. Healed epithelium appears thinner in LFD group compare to ND group. Epithelial healing normalized after fecal transplantation from ND to HFD mice. B) Graph showing significant delay in epithelial healing in mice fed HFD (ND=Normal chow diet, HFD=High fat diet, LFD=Low fat diet, FT=Fecal transplantation from ND to HFD, *p <0.05). C) Example of fluorescein staining pattern of murine cornea showing persistent epithelial defect in 20% of mice fed HFD. D) fluorescein staining and slit lamp photography showing whorl-like epitheliopathy in 20% of mice on HFD and thin healed central epithelium in LFD mice.
Fecal Transplantation
Female mice fed ND served as FMT donors. Fecal transplantation from donor mice (12-week-old HFD C57 mice) was performed by a 200-μL gavage according to published method in literature(15). This was performed once every three days for three times. Subsequently, the right eye of each mouse was subjected to 2mm epithelial debridement. Corneas were imaged to measure rate of wound closure. Figure 3 shows that the delayed rate of epithelial healing in mice fed HFD normalized after fecal transplantation to that of mice fed ND. Figure 3C shows an example of the fluorescein staining pattern of murine cornea, showing persistent epithelial defects in 20% of mice fed HFD. Figure 3D further demonstrates the fluorescein staining pattern in a whorl-like epitheliopathy in 20% of mice on HFD. These findings in HFD mice are suggestive of limbal stem cell deficiency.
qPCR analysis
Current research has shown that vessel dilatation and neutrophil accumulation reach a peak at 12–18 hours after injury(23). Thus, after 18 hours from surgery, mice were euthanized, the corneal epithelia were isolated, and qPCR was performed. Figure 4 demonstrates relative expression of inflammatory markers and epithelial progenitor cell markers for ND, LFD, HFD and fecal transplant groups (n=3). Of note, IL1b and IL6 were found to be elevated in the HFD group in comparison to the ND group; however, TNF-alpha was noted to be decreased in the HFD group in comparison to the ND group (p<0.05). The P63 gene supports epithelial stem cell proliferation and regulation, and δNP63 expression was found to be the highest in the limbal region of the cornea(26,27). In addition, ABCG2, a member of the ATP binding cassette (ABC) transporters, has been proposed as a universal marker for stem cells and was found to identify a population of clonogenic limbal epithelial cells(28). Putative stem cell markers δNP63 and ABCG2 were found to be decreased in the HFD group in comparison to ND and LFD groups (p<0.05). Moreover, fecal transplant increased the expression of ABCG2 (p<0.05). Consistent with our previous observation in figure 3D that a portion of HFD mice showed whorl-like epitheliopathy and persistent epithelial defect, which are early findings of limbal stem cell deficiency, these qPCR results provide further evidence towards the possibility that a high fat diet exerts a detrimental effect on corneal limbal epithelial cells.
Figure 4.
qPCR demonstrating relative expression of inflammatory markers and epithelial progenitor cell markers for ND, LFD, HFD and fecal transplant groups (n=3). Expression values are relative to ND (set to 1) for each marker. Of note, IL1b and IL6 were found to be elevated in the high fat diet group in comparison to the ND group, however, TNF-alpha was noted to be decreased in the HFD group in comparison to the ND group (p<0.05). Putative stem cell markers δNP63 and ABCG2 were found to be decreased in the HFD group in comparison to ND and LFD groups (p<0.05). Fecal transplant increased the expression of ABCG2 (p<0.05).
Immunostaining of cell proliferation marker Ki67
After 18 hours following the initial surgery, immunostaining was performed. Figure 5 demonstrates Ki67 immunostaining of murine corneal epithelium at 20x magnification. Figure 5B illustrates higher rates of epithelial cell proliferation in ND at limbal cornea, which explains the observed faster rate of wound closure in ND mice (P<0.05). No statistically significant rate of wound closure was observed in ND and LFD mice in vivo. We found that the rate of proliferation of LFD groups at wound edge is higher in LFD in comparison to HFD. It is possible that the higher proliferation rate at the wound edge helped LFD mice to heal; however, in LFD mice, healed epithelium appeared thinner in comparison to that of ND mice. It is likely that, in LFD mice, the epithelium was closed, but additional time was required to regenerate multiple epithelial layers.
Figure 5.
Immunostaining of Ki67. A) Ki67 immunostaining of ND, LFD and HFD murine corneal epithelium at wound edge, peripheral and limbal corneas at 20x magnification. B) Graph demonstrating higher rates of epithelial cell proliferation in ND at limbal cornea explain faster rate of wound closure in ND mice (*P<0.05). No statistically significant rate of wound closure was observed in ND and LFD mice. LFD mice showed relatively higher proliferation at wound edge but lower at periphery.
Immunostaining of immune cell markers
We subsequently performed staining of each cornea after 18 hours of injury with immune cell markers. We focused on Ly6G+ and γδTCR+ immune cells, as these cells have previously been shown to play a role in epithelial wound closure(23,29,30). Figure 6 shows Ly6G immunostaining of murine cornea epithelium at 20x magnification demonstrating Ly6G+ neutrophil infiltration upon injury. Of note, Ly6G+ neutrophils were shown to help wounds to close(29,30). Graph 6B illustrates that in the corneas of ND mice, a higher number of Ly6G+ cells was found at the central cornea than at the wound edge. Thus, it is plausible that higher concentrations of Ly6G+ cells at the central cornea and wound edge helped ND mice epithelial wounds to close.
Figure 6.
Immunostaining of Ly6G. A) Ly6G immunostaining of ND, LFD and HFD murine corneas at wound edge, central and peripheral corneal epithelium at 20x magnification demonstrating Ly6G positive neutrophil infiltration. B) Graph demonstrating ND murine corneas with higher Ly6G+ cells at central cornea and wound edge (*P<0.05).
Figure 7 demonstrates the staining of murine corneal epithelium with anti- γδTCR marker. Graph 7B demonstrates increased γδTCR+ cells in the peripheral cornea of LFD mice. As γδT cells were shown to help wound closure, increase in γδT cells in peripheral corneal of LFD mice may help epithelial wound to close in these mice.
Figure 7.
Immunostaining of γδTCR. A) anti-γδTCR staining of ND, LFD and HFD murine corneal epithelium at the central and peripheral corneal epithelium. B) Graph demonstrating increased γδTCR+ cells in the peripheral cornea of LFD mice and decreased staining in the central corneas of HFD mice (*P<0.05).
RNA seq analysis of murine corneas upon injury
Eighteen hours after surgery, murine corneas were isolated, and bulk RNA seq was performed on whole murine corneas (n=3 for ND, HFD and LFD mice). Figure 8 demonstrates RNA seq analysis of LFD, ND and HFD murine corneas. Figure 8A shows STAR align scores. For all samples, approximately 80% of the reads were uniquely mapped. Figure 8C demonstrates principal component analysis (PCA) showing unique clustering of LFD, ND and HFD samples. Figure 8C shows a heatmap demonstrating changes in gene expression of murine corneal samples depending on mice diets.
Figure 8.
RNA seq analysis of LFD, ND and HFD murine corneas. A) STAR align scores demonstrating approximately 80% of the reads were uniquely mapped. B) Heatmap showing changes in gene expression of murine corneal samples depending on mice diets. C) PCA analysis showing clustering of LFD, ND and HFD samples.
DESeq2 differential gene expression analyses revealed that, when comparing all groups, 3158 genes were differentially expressed, and 225 of these genes had a greater than two-fold change in expression. Gene Ontology(GO) terms of differentially expressed genes included response to external stimulus, cell proliferation, migration, adhesion, defense response, immune system process and leukocyte migration. The most over-represented KEGG pathways included ECM-receptor interaction, cytokine-cytokine receptor interaction, focal adhesion, hematopoietic cell lineage and leukocyte trans-endothelial migration.
Ingenuity pathway analysis (IPA) was used to further analyze RNA seq data and identify key molecules and signaling pathways involved. Figure 9 shows graphical summaries of potential significantly altered pathways and genes related to diet-induced gut dysbiosis and corneal healing. In Figure 9, genes with concentration change of greater than 2-fold and p<0.05 among all three groups were included in the analysis. Of note, TP53, which belongs to the family of p63 molecules, were found to be an upstream regulator in the IPA analysis. It was predicted that TP53 activity was inhibited in the HFD group with statistically significant activation z-score of −4.685. In figure 9A comparing all three groups, cell functions that were found to be significantly altered include adhesion of immune cells, differentiation of mononuclear leukocytes. In comparing ND vs LFD groups (Supplemental figure A), IPA shows that activation of neutrophils, mitosis of epithelial cell lines, and growth of connective tissue were all found be relatively inactivated in the LFD group. This is consistent with our immunostaining results showing increased Ly6G+ neutrophil expression in the ND samples. In comparing ND and HFD groups (Supplemental figure B), significantly involved pathways include agranulocyte and granulocyte adhesion and diapedesis, and activation of granulocytes. In comparing LFD and HFD groups (Supplemental figure C), significantly involved pathways include mobilization of phagocytes, proliferation of connective tissue cells, and DNA damage checkpoint regulation. In addition, many pathways lead to inhibition of TP53 in the HFD group in comparison to the LFD group.
Figure 9.
IPA graphical summary of potential significantly altered pathways and genes related to diet induced gut dysbiosis and corneal healing. Genes with FC >2 and p<0.05 amongst all three groups were included in the analysis. (blue = decreased expression in HFD group, red = increased expression in HFD group)
Discussion
In this study, we observed phenotypic changes in corneal epithelial response to injury in a diet-induced gut dysbiosis model in mice. We also observed that high-fat-diet-induced gut dysbiosis can affect genetic expression of the cornea upon injury. We conclude that high-fat-diet-induced gut microbial dysbiosis alters corneal immune cell distribution, corneal response to injury and genes related to epithelial function and corneal immunity.
An emerging area of research focuses on the link between the eye and the human microbiome(5,31–33) indicating that the gut microbiome and diet play an important role in the regulation of various ocular conditions, including glaucoma, uveitis, and age-related macular degeneration(10–12,31). It was recently suggested that gut dysbiosis, or a disturbance in the microbial community of the gut, due to various causes including dietary changes (high-fat diet), antibiotics, etc., may lead to decreased corneal epithelial healing(15,34,35). Several recent studies also suggested that the microbiome plays an important role in the development of the cornea by changing the immune status in the cornea. For example, gut dysbiosis due to antibiotic over-usage may lead to changes in macrophage distribution in the cornea, leading to decreased corneal size and abnormalities in murine corneal development (15,34). However, at the present time, the environmental and diet/lifestyle triggers of immunity that lead to changes in corneal epithelial cellular function remain largely unknown. It is likely that microbes both on, and distal to, the ocular surface (i.e. gut microbiome) contribute to the inflammatory state that occurs in the setting of corneal injury and may affect corneal epithelial healing. However, a systematic evaluation of the effect of the microbiome on corneal healing and immunity is lacking(13,32,36). Furthermore, clinical and experimental studies have revealed that diet is one of the most consistent and predictable ways of reshaping the gut microbiome(16,37). Lack of adequate nutrition has been linked to dysfunctional microbiome and dysbiosis in infants, for example. Recent research has focused on the influence of Western style high fat diet consumption on gut microbial composition. Diet-induced changes in gut microbiota and resulting metabolic perturbations also depend on the fat content, as a milk fat-based diet contains high saturated fatty acid sources similar to the Western style diets. High fat diet-induced obesity is also associated with low-grade chronic inflammation, which may negatively affect corneal wound healing(34). In this study, we systematically investigated the role of the Western style high fat diet-induced gut dysbiosis on corneal response to injury and inflammation. We observed that dietary changes including a low fat and a high fat diet can both consistently alter gut microbial profile in mice. These finding are consistent with previous studies on the effect of diets on gut microbiome (16,37). In addition, Hargrave et al. recently noted that corneal dysfunction precedes the onset of hyperglycemia after feeding mice on HFD(38). This study supports our findings that an alternative mechanism other than hyperglycemia may lead to changes in corneal dysfunction after HFD feeding. In our study, we found that HFD feeding altered gut microbial profiles, but interestingly, fecal transplantation led to relative normalization of corneal function in HFD mice.
Increasing scientific evidence has also recently demonstrated that human-microbial interaction may lead to changes in epithelial progenitor cell function and healing(39,40). For example, in germ-free mice, skin epithelium regeneration is accelerated, and skin wound closure is scarless(41). In addition, in the gut epithelium, microbes were found to produce factors which regulate key pathways involved in epithelial progenitor cell differentiation including the Wnt/β-catenin signaling pathway(42). Various gut microbial catabolites were also found to exert anti-inflammatory, ant-oxidative and anti-toxic effects in the systemic circulation, leading to changes in tissue in distant sites such as the epithelium(43). These recent discoveries demonstrate that in addition to regulating the immune status and the inflammatory state of the body, microbes are likely directly involved in epithelial cell biological function. In this study, as we observed in the RNA Seq analysis, various ontological categories related to epithelial cell function were found to be significantly altered in the diet induced gut dysbiosis model, including cell proliferation, migration and adhesion.
In addition, immune cells in the cornea play a central role in many corneal inflammatory and repair processes(44,45,23). For example, neutrophils were noted to play a dual and important role for corneal epithelial healing(18,29,30,30,46–49). Excessive accumulation of neutrophils at the time of epithelial division, which is essential to wound repair, may cause a disruption to corneal wound healing(49), However, it was also found that there are two waves of neutrophil emigrations in response to corneal epithelial injury, and early leukocyte emigration in the first 18 hours post corneal epithelial injury appears to promote re-epithelialization(18). In addition, neutrophil depletion worsened epithelial healing in wild type C57 mice(30). In this study we observed that both HFD and LFD lead to a decrease in the number of neutrophils in the cornea at 18 hours upon injury, leading decreased rate corneal healing in the HFD group and thinning in the healed corneal epithelium in both HFD and LFD groups, highlighting the important potential contributing role of early neutrophil infiltration in corneal response to injury in the setting of dietary changes.
A tissue resident T cell population, γδT cells have recently been implicated to play a role in microbial-host interaction in a variety of tissue types connecting the gut microbiome and epithelial barrier functions of the skin and intestinal mucosa(50–54). Previously, skin γδT cells were found to play a major role in wound repair by producing cytokines, and these cells proliferate in response to damaged and stressed keratinocytes (55–58). Depletion of epidermal γδT cells were found to impede wound healing(56). These T cells were also recently found to serve therapeutic purposes in clinical studies (55–58). Previously, it was shown that a commensal bacterium on the ocular surface induced production of IL-17 by γδT cells(50). γδT cells were also found to play a critical role by producing IL-17 and IL-22 to recruit neutrophils and promote epithelial cell division, respectively(59–61). In this study, we found that the microbiome may affect cell distribution of γδT cells in the cornea, which may lead to changes in the rate of corneal wound healing. Specifically, it appears that in low fat diet mice, there is an increase in γδT cell distribution in the both the central and peripheral cornea upon injury in comparison to high fat diet mice, thus leading to improved rate of corneal epithelial healing. In our RNA seq analysis, we noted a significant change in IL-17A expression (RPKM ND of 612 vs. LFD of 595 vs. HFD of 458, adjusted p<0.01). This shows that the expression of IL-17A is significantly lower in HFD mice. This result is consistent with our immunofluorescence result showing that HFD mice had lower γδTCR expression, especially in comparison to LFD mice. In the future, we aim to investigate whether γδTCR+ lymphocytes from mice with a conventional microbiome can facilitate corneal wound healing in germ-free mice and aid in establishing a causal relationship between γδTCR+ lymphocyte distribution and epithelial phenotypes.
We further observed in this study that diet-induced gut dysbiosis may affect the genetic expression of the murine cornea upon injury. GO terms of differentially expressed genes included response to external stimulus, cell proliferation, migration, adhesion, defense response, immune system process, leukocyte migration, etc. From this analysis, it is evident that diet-induced changes can significantly impact immunity and cell function, including cell proliferation, migration, adhesion, etc. Top over-represented KEGG pathways included ECM-receptor interaction, cytokine-cytokine receptor interaction, focal adhesion, hematopoietic cell lineage, leukocyte trans-endothelial migration, linoleic acid metabolism and folate metabolism. Many of these pathways are involved in epithelial cell functions, response to the underlying extracellular matrix and immune cell functions. We hypothesize that diet-induced changes in the gut microbial profile in mice potentially lead to changes in secreted microbial metabolites which then elicit changes in function of corneal immunity and wound healing. For example, linoleic acid and folic acid metabolic processes were over-represented in the KEGG pathway analyses. In the future, to establish a direct causal relationship between diet-induced gut dysbiosis and corneal response to injury, we aim to transfer fecal microbiome to germ-free mice and observe germ-free murine corneal responses to injury and perform metabolomics studies.
In this study, the effect of conjunctival commensals on wound healing was not specifically explored; however, this is an important area that will need to be explored in future studies. It is well known that the ocular surface is paucibacterial, and neutrophils were found to play an important role in controlling ocular paucibacteriality, thus protecting the ocular surface by preventing commensal bacterial overgrowth(62). However, commensals on the conjunctival surface were found to be protective for the cornea. For example, ocular commensals were found to protect against corneal infection by driving an IL-17 response from mucosal γδT cells(50). In addition, the presence of ocular commensals significantly increased the concentrations of immune effectors in the tear film, including secretory IgA and complement protein exertion a protective role in the cornea against infections(13). As a future study, we aim to explore the role of diet-induced changes on conjunctival and lid margin commensals and on corneal wound healing.
Supplementary Material
Financial Support:
This work was supported by the grant NIH K12EY021475, P30EY001792, Eversight Eye Bank Research grant and an unrestricted departmental grant from the Research to Prevent Blindness.
Footnotes
Conflict of Interest:
The authors have no relevant financial/conflicting interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Sherwin E, Rea K, Dinan TG, Cryan JF. A gut (microbiome) feeling about the brain. Curr Opin Gastroenterol. 2016. Mar;32(2):96–102. [DOI] [PubMed] [Google Scholar]
- 2.Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. 2016. 21;6:30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016. Dec 1;167(6):1469–1480.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012. Jul 5;487(7405):104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett C, Knight R, Gordon JI. The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature. 2007. Oct 18;449(7164):804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsiao WWL, Metz C, Singh DP, Roth J. The Microbes of the Intestine: An Introduction to Their Metabolic and Signaling Capabilities. Endocrinol Metab Clin North Am. 2008. Dec;37(4):857–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez-Bello MG, Godoy-Vitorino F, Knight R, Blaser MJ. Role of the microbiome in human development. Gut. 2019. Jun 1;68(6):1108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016. Feb 19;351(6275):854–7. [DOI] [PubMed] [Google Scholar]
- 9.Wen X, Hu X, Miao L, Ge X, Deng Y, Bible PW, et al. Epigenetics, microbiota, and intraocular inflammation: New paradigms of immune regulation in the eye. Progress in Retinal and Eye Research. 2018;64:84–95. [DOI] [PubMed] [Google Scholar]
- 10.Astafurov K, Elhawy E, Ren L, Dong CQ, Igboin C, Hyman L, et al. Oral Microbiome Link to Neurodegeneration in Glaucoma. PLOS ONE. 2014. Sep 2;9(9):e104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Cho K-S, Vu THK, Shen C-H, Kaur M, Chen G, et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat Commun. 2018. 10;9(1):3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu LJ, Liu J. Human Microbiota and Ophthalmic Disease. Yale J Biol Med. 2016. Sep 30;89(3):325–30. [PMC free article] [PubMed] [Google Scholar]
- 13.Kugadas A, Christiansen SH, Sankaranarayanan S, Surana NK, Gauguet S, Kunz R, et al. Impact of Microbiota on Resistance to Ocular Pseudomonas aeruginosa-Induced Keratitis. PLOS Pathogens. 2016. Sep 22;12(9):e1005855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C, Zaheer M, Bian F, Quach D, Swennes AG, Britton RA, et al. Sjögren-Like Lacrimal Keratoconjunctivitis in Germ-Free Mice. Int J Mol Sci [Internet]. 2018. Feb 13 [cited 2020 Feb 2];19(2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5855787/ [DOI] [PMC free article] [PubMed]
- 15.Liu J, Wu M, He J, Xiao C, Xue Y, Fu T, et al. Antibiotic-Induced Dysbiosis of Gut Microbiota Impairs Corneal Nerve Regeneration by Affecting CCR2-Negative Macrophage Distribution. Am J Pathol. 2018. Dec;188(12):2786–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014. Jan;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam FW, Phillips J, Landry P, Magadi S, Smith CW, Rumbaut RE, et al. Platelet Recruitment Promotes Keratocyte Repopulation following Corneal Epithelial Abrasion in the Mouse. PLOS ONE. 2015. Mar 16;10(3):e0118950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Burns AR, Smith CW. Two Waves of Neutrophil Emigration in Response to Corneal Epithelial Abrasion: Distinct Adhesion Molecule Requirements. Invest Ophthalmol Vis Sci. 2006. May 1;47(5):1947–55. [DOI] [PubMed] [Google Scholar]
- 19.Dass BA, Soong HK, Lee B. Effects of proparacaine on actin cytoskeleton of corneal epithelium. J Ocul Pharmacol. 1988;4(3):187–94. [DOI] [PubMed] [Google Scholar]
- 20.Liu JC, Steinemann TL, McDonald MB, Thompson HW, Beuerman RW. Topical bupivacaine and proparacaine: a comparison of toxicity, onset of action, and duration of action. Cornea. 1993. May;12(3):228–32. [DOI] [PubMed] [Google Scholar]
- 21.Ericsson AC, Personett AR, Turner G, Dorfmeyer RA, Franklin CL. Variable Colonization after Reciprocal Fecal Microbiota Transfer between Mice with Low and High Richness Microbiota. Front Microbiol [Internet]. 2017. Feb 23 [cited 2019 Sep 3];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5322181/ [DOI] [PMC free article] [PubMed]
- 22.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001. Dec 1;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Burns AR, Byeseda Miller S, Smith CW. CCL20, γδ T cells, and IL-22 in corneal epithelial healing. FASEB J. 2011. Aug;25(8):2659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013. Jan 1;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biology. 2010. Feb 4;11(2):R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan S, Sudha B, Krishnakumar S. Isoforms of p63 in corneal stem cells cultured on human amniotic membrane. Biologicals. 2010. Sep 1;38(5):570–6. [DOI] [PubMed] [Google Scholar]
- 27.Wang D-Y, Cheng C-C, Kao M-H, Hsueh Y-J, Ma DHK, Chen J-K. Regulation of Limbal Keratinocyte Proliferation and Differentiation by TAp63 and ΔNp63 Transcription Factors. Invest Ophthalmol Vis Sci. 2005. Sep 1;46(9):3102–8. [DOI] [PubMed] [Google Scholar]
- 28.de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li D-Q. ABCG2 Transporter Identifies a Population of Clonogenic Human Limbal Epithelial Cells. Stem Cells. 2005;23(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahu SK, Mittal SK, Foulsham W, Li M, Sangwan VS, Chauhan SK. Mast Cells Initiate the Recruitment of Neutrophils Following Ocular Surface Injury. Invest Ophthalmol Vis Sci. 2018. Apr;59(5):1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrazzo G, Bellner L, Halilovic A, Li Volti G, Drago F, Dunn MW, et al. The Role of Neutrophils in Corneal Wound Healing in HO-2 Null Mice. PLoS One [Internet]. 2011. Jun 17 [cited 2020 Oct 8];6(6). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3117875/ [DOI] [PMC free article] [PubMed]
- 31.Grover M, Kashyap PC. Germ free mice as a model to study effect of gut microbiota on host physiology. Neurogastroenterol Motil. 2014. Jun;26(6):745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C, Zaheer M, Bian F, Quach D, Swennes AG, Britton RA, et al. Sjögren-Like Lacrimal Keratoconjunctivitis in Germ-Free Mice. Int J Mol Sci. 2018. Feb 13;19(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baim AD, Movahedan A, Farooq AV, Skondra D. The microbiome and ophthalmic disease. Exp Biol Med (Maywood). 2018. Nov 21;1535370218813616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Burns AR, Rumbaut R, Smith W. Diet-induced obesity in mice is accompanied by reduced corneal wound healing. Invest Ophthalmol Vis Sci. 2014. Apr 30;55(13):5161–5161. [Google Scholar]
- 35.Li Z, Hargrave A, Xue Y, Hanlon SD, Burns AR, Smith CW. Short-term high fat diet feeding reduces corneal wound healing in mice. Invest Ophthalmol Vis Sci. 2017. Jun 23;58(8):847–847. [Google Scholar]
- 36.Wang C, Schaefer L, Bian F, Yu Z, Pflugfelder SC, Britton RA, et al. Dysbiosis Modulates Ocular Surface Inflammatory Response to Liposaccharide. Invest Ophthalmol Vis Sci. 2019. Oct 1;60(13):4224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J, Chang EB. Exploring gut microbes in human health and disease: Pushing the envelope. Genes Dis. 2014. Dec;1(2):132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hargrave A, Courson JA, Pham V, Landry P, Magadi S, Shankar P, et al. Corneal dysfunction precedes the onset of hyperglycemia in a mouse model of diet-induced obesity. PLoS One. 2020;15(9):e0238750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson TR, Gómez BI, McIntyre MK, Dubick MA, Christy RJ, Nicholson SE, et al. The Cutaneous Microbiome and Wounds: New Molecular Targets to Promote Wound Healing. Int J Mol Sci [Internet]. 2018. Sep 11 [cited 2020 Sep 4];19(9). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6164292/ [DOI] [PMC free article] [PubMed]
- 40.Zevin AS, Xie IY, Birse K, Arnold K, Romas L, Westmacott G, et al. Microbiome Composition and Function Drives Wound-Healing Impairment in the Female Genital Tract. PLOS Pathogens. 2016. Sep 22;12(9):e1005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canesso MCC, Vieira AT, Castro TBR, Schirmer BGA, Cisalpino D, Martins FS, et al. Skin wound healing is accelerated and scarless in the absence of commensal microbiota. J Immunol. 2014. Nov 15;193(10):5171–80. [DOI] [PubMed] [Google Scholar]
- 42.Moossavi S Location-specific effect of microbiota and MyD88-dependent signaling on Wnt/β-catenin pathway and intestinal stem cells. Gut Microbes. 2014. Jan 1;5(1):11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nature Communications. 2018. Aug 17;9(1):3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jamali A, Kenyon BM, Ortiz G, Bayraktutar BN, Sendra VG, Hamrah P. Topical Adoptive Transfer of Plasmacytoid Dendritic Cells for Corneal Wound Healing. Methods Mol Biol. 2021;2193:159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eslani M, Putra I, Shen X, Hamouie J, Tadepalli A, Anwar KN, et al. Cornea-Derived Mesenchymal Stromal Cells Therapeutically Modulate Macrophage Immunophenotype and Angiogenic Function. STEM CELLS. 2018;36(5):775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest. 2019. Jun 17;129(7):2629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillipson M, Kubes P. The Healing Power of Neutrophils. Trends Immunol. 2019. Jul;40(7):635–47. [DOI] [PubMed] [Google Scholar]
- 48.Xiao C, Wu M, Liu J, Gu J, Jiao X, Lu D, et al. Acute tobacco smoke exposure exacerbates the inflammatory response to corneal wounds in mice via the sympathetic nervous system. Commun Biol. 2019;2:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Burns AR, Smith CW. Lymphocyte function-associated antigen-1-dependent inhibition of corneal wound healing. Am J Pathol. 2006. Nov;169(5):1590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.St. Leger AJ, Desai JV, Drummond RA, Kugadas A, Almaghrabi F, Silver P, et al. An Ocular Commensal Protects against Corneal Infection by Driving an Interleukin-17 Response from Mucosal γδ T Cells. Immunity. 2017. Jul 18;47(1):148–158.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nielsen MM, Witherden DA, Havran WL. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nature Reviews Immunology. 2017. Dec;17(12):733–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chien Y, Meyer C, Bonneville M. γδ T Cells: First Line of Defense and Beyond. Annu Rev Immunol. 2014. Mar 21;32(1):121–55. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y, Xu C, Wu D, Wang Z, Wu P, Li L, et al. γδ T Cells: Crosstalk Between Microbiota, Chronic Inflammation, and Colorectal Cancer. Front Immunol [Internet]. 2018. Jun 26 [cited 2020 Sep 7];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6028700/ [DOI] [PMC free article] [PubMed]
- 54.Wu M, Liu J, Li F, Huang S, He J, Xue Y, et al. Antibiotic-induced dysbiosis of gut microbiota impairs corneal development in postnatal mice by affecting CCR2 negative macrophage distribution. Mucosal Immunol. 2020;13(1):47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, et al. A role for skin gammadelta T cells in wound repair. Science. 2002. Apr 26;296(5568):747–9. [DOI] [PubMed] [Google Scholar]
- 56.Havran WL, Jameson JM. Epidermal T Cells and Wound Healing. J Immunol. 2010. May 15;184(10):5423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva-Santos B, Mensurado S, Coffelt SB. γδ T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer. 2019;19(7):392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y-S, Chen I-B, Pham G, Shao T-Y, Bangar H, Way SS, et al. IL-17–producing γδ T cells protect against Clostridium difficile infection. J Clin Invest. 2020. May 1;130(5):2377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Z, Burns AR, Rumbaut RE, Smith CW. γδ T Cells Are Necessary for Platelet and Neutrophil Accumulation in Limbal Vessels and Efficient Epithelial Repair after Corneal Abrasion. The American Journal of Pathology. 2007. Sep 1;171(3):838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Z, Burns AR, Han L, Rumbaut RE, Smith CW. IL-17 and VEGF Are Necessary for Efficient Corneal Nerve Regeneration. Am J Pathol. 2011. Mar;178(3):1106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Byeseda SE, Burns AR, Dieffenbaugher S, Rumbaut RE, Smith CW, Li Z. ICAM-1 Is Necessary for Epithelial Recruitment of γδ T Cells and Efficient Corneal Wound Healing. Am J Pathol. 2009. Aug;175(2):571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu X, Kugadas A, Smith-Page K, Lamb J, Lin T, Ru Y, et al. Neutrophil L-Plastin Controls Ocular Paucibacteriality and Susceptibility to Keratitis. Front Immunol [Internet]. 2020. [cited 2021 Jul 12];11. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2020.00547/full [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.