Abstract
Cell membrane-coated nanoparticles (CMCNP), which involve coating a core nanoparticle (NP) with cell membranes, have been gaining attention due to their ability to mimic the properties of the cells, allowing for enhanced delivery and efficacy of therapeutics. Two CMCNP systems comprised of an acetalated dextran-based NP core loaded with curcumin (CUR) coated with cell membranes derived from pulmonary epithelial cells were developed. The NP were approximately 200 nm and their surface charges varied based on their coating, where CMCNP systems exhibited negative surface charge like natural cell membranes. The NP were smooth, spherical, and homogeneous with distinct coatings on their cores. Minimal in vitro toxicity was observed for the NP and controlled release of CUR was observed. The CMCNP internalized into and translocated across an in vitro pulmonary epithelial monolayer significantly more than the control NP. Blocking endocytosis pathways reduced the transcytosis of NP, indicating a relationship between endocytosis and transcytosis. These newly developed CMCNP have the potential to be used in pulmonary drug delivery applications to potentially enhance NP internalization and transport into and across the pulmonary epithelium.
Keywords: Cell membrane-coated nanoparticle, pulmonary epithelium, lung cells, nanoparticle transcytosis, air-blood barrier
Graphical Abstract

1. Introduction
Advances in nanotechnology have allowed for many advantages in drug delivery applications including targeted delivery, enhanced stability and bioavailability, and controlled release of therapeutics. Aerosol administration of NP to the lungs has attracted increasing attention owing to the large alveolar surface area available for drug adsorption, presence of a thin surfactant layer in the alveolar region, and plentiful capillary blood vessels present that provide passive drug and NP transport to the blood stream (Chow et al., 2007; Ehrmann et al., 2020; Liu et al., 2020). Direct delivery of therapeutics to the lungs in comparison to systemic delivery typically reduces the overall dose requirement and systemic side effects. Despite these advantages, the pulmonary route presents physiological barriers such as mucus, surfactant, and the air-blood barrier, and nanotechnology can be utilized to overcome these barriers to allow for more effective and targeted delivery of therapeutics.
NP properties such as size, morphology, surface charge, and surface chemistry have been shown to impact the interaction of NP with cells and tissues (Abedin et al., 2018; des Rieux et al., 2005; Maeda et al., 2000; Oroojalian et al., 2020; Pan et al., 2020; Sohaebuddin et al., 2010). For example, the Hanes group pioneered poly(ethylene glycol) (PEG)-coated NP that can penetrate mucus from patients with cystic fibrosis more efficiently than other NP systems (Lai et al., 2009; Nance et al., 2012; Wang et al., 2008). Other studies have shown that PEG molecular weight, density, and conformation affect NP internalization and transcytosis into lymphatic endothelial cells (McCright et al., 2020; Saucier-Sawyer et al., 2015; Tehrani et al., 2019). In pulmonary drug delivery applications, NP need to cross the air-blood barrier to reach systemic circulation for the potential treatment of systemic diseases. Despite the many studies evaluating the impact of NP properties, a better understanding of NP internalization and transport pathways can aid in developing more effective drug delivery systems, especially for pulmonary applications.
Cell membrane-coated NP (CMCNP) are a newer class of biomimetic NP that combine the unique properties of cell membranes with the functionality of synthetically engineered NP systems (He et al., 2020; Liu et al., 2019; Luk and Zhang, 2015; Zou et al., 2020). CMCNP have been developed for applications in drug delivery, diagnosis, imaging, and detoxification, however, few studies with these systems looked at their specific interactions with cells. To date, immune cells such as red blood cells (RBC), platelets, macrophages, cancer cells, and bacterial cells have been the main types of cells used to develop CMCNP to enable applications to NP beyond those traditionally associated with nanomedicine. For example, RBC-coated NP exhibit longer circulation times, reduced reticuloendothelial system clearance, and evasion of immune cells (Fang et al., 2018), while platelet-coated NP have been used for targeting atherosclerosis plaque and collagen in arthritis, and reduction in the uptake of NP by macrophages (He et al., 2018; Hu et al., 2015; Wei et al., 2018).
While CMCNP have been used in many therapeutic applications, little work has been done in the realm of pulmonary drug delivery or utilizing epithelial cells. Here we developed lung epithelial-based CMCNP for the first time. Six NP systems with different surface properties were developed and characterized to elucidate how NP surface properties impact NP internalization and transcytosis in an in vitro pulmonary epithelial model. We hypothesized that CMCNP would internalize into and transport through an epithelial monolayer more effectively than other NP systems. Curcumin (CUR)-loaded acetalated dextran NP were coated with cell membranes, polymers, or lipids to provide varying surface properties such as composition and charge. Pharmacological inhibitors were used to block endocytosis pathways to determine the impact of NP surface properties on their internalization and transport into cells. We confirmed that lung epithelial CMCNP both internalized into and transported across a pulmonary epithelial monolayer more efficiently than the polymer- and lipid-coated NP systems. The studies demonstrate the potential use of lung epithelial CMCNP in pulmonary drug delivery by bridging the properties of natural membrane components and synthetic nanomaterials.
2. MATERIALS AND METHODS
2.1. Materials
Dextran from Leuconoctoc mesenteroides (9000–11000 MW), pyridinium p-toluenesulfonate (PPTS, 98%), poly(ethylene glycol) methyl ether (mPEG, Mn 5000), D-α-tocopherol succinate (vitamin E succinate, 1210 IU/g), N,N’-dicyclohexyl-carbodiimide (DCC, 99%), 4-(dimethylamino) pyridine (DMAP, 99%), 2-methoxypropene (2-MOP, 97%), dichloromethane (DCM, >99.8%), triethylamine (TEA, ≥ 99%), Chlorpromazine hydrochloride and anhydrous dimethyl sulfoxide (DMSO, ≥ 99.9%) were obtained from Sigma-Aldrich (St. Louis, MO). Genistein and dynasore were obtained from Tocris Bioscience (Bristol, UK). Polyvinyl alcohol (PVA, 88% hydrolyzed, average MW 22,000), and curcumin (CUR) were obtained from Acros Organics (Geel, Belgium). Iodine, potassium iodide, boric acid, ferric chloride hexahydrate and ammonium thiocyanate were obtained from Sigma-Aldrich (St. Louis, MO). Ethanol (anhydrous, ASC/USP grade) was obtained from Pharmco-AAPER (Brookfield, CT). 1, 2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-dioleoyl-3-trimethylammonium-propane (chloride salt, DOTAP) were obtained from Avanti Polar Lipids (Alabaster, AL). Lecithin from soybean was obtained from Bioworld (Dublin, OH).
Dulbecco’s Modified Eagle Medium (DMEM), Penicillin-Streptomycin (Pen-Strep), and Fungizone® were obtained from Life Technologies (Norwalk, CT). Corning™ RPMI 1640 medium with L-glutamine, trypsin-EDTA, sodium pyruvate, Dulbecco’s phosphate buffered saline (PBS), and Invitrogen™ NanoOrange™ Protein Quantification kit were obtained from Fisher Scientific (Waltham, MA). Biotium CellBrite Fix 640 was obtained from Biotium (Fremont, CA). Fetal bovine serum (FBS) was obtained from Atlanta Biologics (Flowery Branch, GA). Paraformaldehyde (32%, formaldehyde, aqueous solution) was purchased form Electron Microscopy Sciences (Hatfield, PA, USA). RIPA lysis buffer system was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). A549 and H441 cell lines were obtained from ATCC (Manassas, VA). CD47 and anti-α tubulin antibodies (catalog # ab218810 and ab7291, respectively) were purchased from Abcam (Cambridge, UK).
2.2. Synthesis of Acetalated Dextran (Ac-Dex)
Ac-Dex was synthesized as previously described with minor modifications (Broaders et al., 2009), where 1 g of lyophilized dextran (9–11 kDa) and 30 mg of pyridinium p-toluenesulfonate were dissolved in 10 ml anhydrous DMSO. The resulting solution was reacted with 7.5 ml of 2-methoxypropene (2-MOP) under nitrogen gas for 3 hours and quenched with 1 ml of triethylamine (TEA). The mixture was precipitated in basic water (water and TEA, pH 10), vacuum filtered, frozen overnight and lyophilized (−50°C, 0.05 mbar) for 24 hours.
2.3. Synthesis of Vitamin E Poly(ethylene glycol) (VP5k)
VP5k was synthesized as previously described with some minor modifications (Mert et al., 2012), where 1.95 g of vitamin E succinate and 22 g of mPEG were dissolved in 60 ml of DCM. 0.84 g of DCC and 45 mg of DMAP were added to the solution. The reaction mixture was stirred at room temperature overnight, vacuum filtered (0.45 μm), and concentrated under reduced pressure via a rotor evaporator (IKA-RV, Wilmington, NC) to obtain a crude product. The resulting product was dissolved in DI water at 5% (w/v) and centrifuged twice at 19,802×g for 30 minutes. The filtrate was vacuum filtered (0.22 μm) each time, frozen overnight and lyophilized (−50°C, 0.05 mbar) for 72 hours to yield the final product.
2.4. Nuclear Magnetic Resonance (NMR) Analysis of Ac-Dex
The cyclic-to-acyclic (CAC) ratio of acetal and amount of total acetal coverage was confirmed by 1H NMR spectroscopy (Bruker 300 MHz NMR, MA). The hydrolysis of one cyclic acetal group produces one acetone whereas one acyclic group produces one acetone and one methanol. From the normalized integration peaks related to acetone, methanol and the carbon ring of dextran, the CAC ratio of acetal coverage and degrees of total acetal coverage per 100 glucose molecules were determined.
2.5. Preparation of Curcumin-Loaded Nanoparticles
Curcumin (CUR)-loaded polyvinyl alcohol-coated nanoparticles (PVA NP) were prepared via single emulsion and solvent evaporation, where 40 mg Ac-Dex and 1 mg CUR were dissolved in 1 ml DCM forming the organic phase. After, 6 ml of 3% PVA in PBS (aqueous phase) was added to the organic phase and sonicated (Q500 sonicator, Qsonica, Newton, CT, USA) for 60 seconds with 1 second on/off pulse at 70% amplitude (100% amplitude = 120 μm). The resulting emulsion was transferred to a 40 ml spinning solution of 0.3% PVA in PBS and stirred for 3 hours to evaporate the organic solvent and allow for hardening of the nanoparticles. The final solution was centrifuged twice at 19,802×g for 20 minutes at 4°C. The nanoparticles were re-dispersed in 0.1% PVA in PBS, frozen overnight and lyophilized (−50°C, 0.05 mbar) for 24 hours.
CUR-loaded VP5k-coated nanoparticles (PEG NP) were prepared via nanoprecipitation. 40 mg Ac-Dex and 1 mg CUR were dissolved in 2 ml of ethanol and added dropwise to 40 ml of 1.5% (w/v) VP5k solution. The resulting suspension was stirred for 3 hours for removal of ethanol and hardening of the particles, and the final solution was centrifuged twice at 19,802×g for 20 minutes at 4°C. The nanoparticles were re-dispersed in 0.1% (w/v) VP5k, frozen overnight and lyophilized (−50°C, 0.05 mbar) for 24 hours.
CUR-loaded lipid-coated nanoparticles (DPPC NP and DOTAP NP) were prepared as previously described, with minor modifications (Zhang et al., 2008). 30 mg Ac-Dex and 0.5 mg CUR was dissolved in 6 mL of acetone (5 mg/mL). Lecithin (5 mg) and DPPC (1 mg) or DOTAP (3 mg) were dissolved in a 4% ethanol aqueous solution. The solution was heated to 65°C to ensure that all lipids were in liquid phase. The resulting Ac-Dex/acetone solution was added to the aqueous preheated lipid solution dropwise under gentle stirring. The mixed solution was vortexed vigorously for 3 minutes followed by gentle stirring for 4 hours at room temperature to allow for the removal of acetone and hardening of nanoparticles. The final solution was centrifuged twice at 19,802×g for 20 minutes at 4°C. The nanoparticles were re-dispersed in DI water, frozen overnight, and lyophilized for 24 hours.
2.6. Cell Culture Preparation
Lung adenocarcinoma cells (A549 and H441) cultured in DMEM and RPMI, respectively, were supplemented with 10% (v/v) FBS, Pen-Strep (100 U/ml penicillin, 100 μg/ml streptomycin), and Fungizone (0.5 μg/ml amphotericin B, 0.41 μg/ml sodium deoxycholate) at standard conditions (37°C and 5% CO2 at saturated humidity).
2.7. Preparation of Cell Membrane-Coated Curcumin-Loaded Nanoparticles
Two cell membrane-coated, CUR-loaded nanoparticle systems (A549 NP and H441 NP) were prepared as previously described, with minor modifications (Fang et al., 2014). To harvest the cell membranes, cells were grown to confluency, detached with trypsin-EDTA, and washed 3 times with 1X PBS after centrifugation at 500×g. The cells were suspended in a hypotonic lysis buffer containing 20 mM Tris-HCl and 1X PBS and were disrupted using a Dounce homogenizer. The entire cell solution was subjected to 30 passes through the homogenizer before spinning down at 3,200×g for 5 minutes. The supernatant was saved, and the pellet was resuspended in hypotonic lysis buffer and subjected to another 30 passes and spun down again. The supernatants were pooled and centrifuged at 20,000×g for 20 minutes, after which the pellet was discarded, and the supernatant was centrifuged again at 200,000×g for 2 hours. The final pellet was collected and used as the purified cell membrane.
Uncoated, CUR-loaded nanoparticles (NP) were prepared via nanoprecipitation as described previously. To prepare the cell membrane vesicles, the purified membrane materials were physically extruded through 400 nm polycarbonate membrane for 11 passes. The resulting vesicles were then coated onto the CUR-loaded NP by co-extruding the vesicles and NP through a 200 nm polycarbonate membrane. The resulting solution was centrifuged at 20,000×g for 20 minutes at 4°C. The nanoparticles were re-dispersed in DI water, frozen overnight, and lyophilized for 24 hours.
2.8. Scanning Electron Microscopy (SEM) Analysis of Nanoparticles
The shape and surface morphology of the NP were evaluated using scanning electron microscopy (SEM) with a Zeiss SIGMA VP Field Emission-Scanning Electron Microscope (Germany). NP were dispersed in distilled water (10 mg/ml), and the suspension was dropped onto aluminum SEM stubs and dried at room temperature. The samples were sputter coated with a thin film (15 nm) of gold/palladium alloy in a BIO-RAD system at 20 μA for 30 seconds under argon gas. Images were captured at 5 kV.
2.9. Transmission Electron Microscopy (TEM) Analysis of Nanoparticles
The core and shell structures of the NP were evaluated using transmission electron microscopy (TEM) with a JEOL JEM-2100F (Peabody, MA). NP were dispersed in distilled water (1 mg/ml), and the suspension was dropped onto 300 square mesh Cu grid and dried overnight at room temperature.
2.10. Particle Size, Size Distribution, and Zeta Potential Analysis
The size, size distribution (polydispersity index, PDI), and zeta potential of the NP were measured by dynamic light scattering (DLS) using a Malvern Nano Zetasizer (Malvern Instruments, Worcestershire, UK). The NP were suspended in distilled water (0.3 mg/ml), vortexed, and sonicated for 30 seconds. All measurements were performed in triplicate and at 25°C.
2.11. Curcumin Loading and Encapsulation Efficiency Analysis
Curcumin (CUR) loading of the NP was determined via fluorescence spectroscopy at 420/520 nm with a Biotek Cytation 3 plate reader (BioTek Instruments Inc., Winooski, VT). NP samples were dissolved in DMSO (1 mg/ml) and CUR concentration was quantified via comparison with a standard curve of CUR in DMSO. CUR loading and encapsulation was calculated using following equation:
2.12. Curcumin and Nanoparticle Stability Analysis
Curcumin (CUR) and nanoparticle stability were evaluated using a Cytation 3 plate reader (BioTek, Winooski, VT, USA). NP and free CUR dissolved in DMSO (final DMSO concentration < 0.05 % v/v) were suspended in 1X PBS and RPMI media supplemented with 10% fetal bovine serum. 200 μl of 1 mg/ml NP solution was added to a 96 well plate. Fluorescence intensity (excitation/emission: 420/520) of the curcumin was recorded in every 30 minutes for 24 hours. Initial fluorescence intensity was considered as control, and the relative stability index was measured using the following equation:
2.13. PVA Quantification
The amount of PVA on the surface of NP was determined using a previously described method with some minor modifications (Shah et al., 2019a). NPs were suspended in distilled water (1 mg/ml). 400 μL of the NP solution, 300 μL of an aqueous iodine solution (1.25% iodine and 2.5% potassium iodide), and 1.5 ml of 4% boric acid solution were mixed for 30 minutes at room temperature. The absorbance of the samples was measured at a wavelength of 630 nm.
2.14. Lipid Quantification
The amount of lipid present on the surface of the NPs was determined using Stewart’s method (Stewart, 1980), where 2.7 g of ferric chloride hexahydrate and 3 g of ammonium thiocyanate were dissolved in 100 ml of distilled water. NP were dissolved in chloroform (1 mg/ml) and were mixed with the ammonium ferrothiocyanate solution via vortexing for 10 minutes at a 2:1 (v/v) ratio. After mixing, the solution was allowed to separate, and the phospholipid content was measured using the absorbance of the samples at a wavelength of 460 nm.
2.15. Protein Quantification and Western Blot Analysis
The amount of protein present in the purified cell membrane material and cell membrane-coated NP was extracted using RIPA lysis buffer (phenylmethylsulfonyl fluoride, sodium orthovanadate, protease inhibitor cocktail, and 1x lysis buffer) and determined using a NanoOrange® Protein Quantification Kit. To extract the protein, cells were trypsinized and washed with ice cold PBS. RIPA lysis buffer was added to cells and CMCNP at a concentration of 1 ml/ 1×107 cells and 1 ml/mg CMCNP, respectively. Samples were agitated for 30 minutes at 4°C followed by a centrifugation at 19,802 ×g at 4°C. The supernatant was collected and stored at −80°C for further analysis. For quantification, samples were suspended in a 1x NanoOrange® working solution at 0.5 mg/ml. The samples were incubated at 90–93°C for 10 minutes and cooled to room temperature for 20 minutes under light protected conditions, then 200 μl of the samples were added to a 96 well plate. Fluorescence intensity of the samples was measured using Cytation 3 plate reader (BioTek, Winooski, VT, USA) at excitation and emission wavelength of 485 nm and 590 nm, respectively. Western blot analysis was performed on the purified cell membrane material and cell membrane-coated NP with primary antibodies against CD47 and anti-α tubulin as the loading control.
2.16. In Vitro Drug Release from Nanoparticles
The in vitro release profiles of curcumin from the nanoparticle systems were determined via a release study of NP suspended (1 mg/ml) in 1X PBS with 0.5% (w/v) of Tween® 20. The suspension was incubated at 37°C and 200 rpm. At various time points (0 to 24 hours), NP samples were centrifuged at 14000 rpm for 5 minutes at 4°C to isolate NP. 200 μl of the supernatant was removed and replaced with same amount of fresh modified PBS at each time point. The fluorescence intensity of the withdrawn samples was analyzed using Cytation 3 plate reader (BioTek, Winooski, VT, USA) at excitation and emission wavelengths of 420 nm and 520 nm, respectively.
2.17. In Vitro Cytotoxicity of Curcumin and Nanoparticles
The in vitro cytotoxicity of free CUR dissolved in DMSO (final DMSO concentration < 0.05 % v/v) and CUR-loaded NP was evaluated via a resazurin assay and H441 cells. Cells were trypsinized and plated at 10,000 cells/well in flat-bottomed 96 well plates and incubated overnight. Cells exposed to 0.05 % v/v DMSO served as the control. The following day, the cells were exposed to differing NP concentrations for 24 hours and free CUR for 24 and 48 hours and 20 μl of resazurin solution (60 μM) was then added to each well and was incubated for 3 hours to allow viable cells to convert resazurin to resorufin. The fluorescence intensity of the viable cells (via resorufin) was detected at excitation and emission wavelengths of 520 nm and 590 nm, respectively. Wells containing cells and resazurin without particles were considered the control.
2.18. Nanoparticle In Vitro Cellular Internalization and Transport
In vitro cellular internalization of the NP systems into H441 cells was determined using confocal/fluorescence microscopy and quantified using a Cytation 3 plate reader. For confocal microscopy cells were seeded on 35 mm glass bottom petri dish at a concentration of 500,000 cells/dish and incubated overnight. Cells were exposed to 2 ml of different NP systems (1 mg/ml) and incubated for 4 hours. NPs were removed and washed twice with 1X PBS, stained with CellMask™ deep red plasma membrane (0.5 ul/ml in 1X PBS), and fixed with 4% paraformaldehyde in 1X PBS. Images were taken at 100X magnification. For cell uptake studies, cells were trypsinized and plated at 20,000 cells/well in flat-bottomed 96 well plates and incubated overnight. The following day, 100 μl of the endocytosis inhibitors chlorpromazine hydrochloride (20 μg/mL), genistein (50 μg/mL) or dynasore (20 μg/mL) was added to the cells and incubated for 30 minutes. The cells were then washed twice with 1X PBS, exposed to 100 μl of differing NP systems (1 mg/mL), and incubated for 4 hours. NP were then removed, and the cells were washed twice with 1X PBS. Cells were fixed with 4% paraformaldehyde for 10 minutes and rinsed with 1X PBS. The fresh 1X PBS was replaced, and cells were immediately analyzed using Cytation 3 plate reader by measuring the fluorescence signal of each sample and calculating the amount of CUR and subsequently amount of NP loaded into the cells.
For the transport studies, H441 cells were trypsinized and seeded at 1.25 × 10^5 cells/well on the apical side of a Transwell. To confirm the confluency, transepithelial electrical resistance (TEER) was measured prior to exposure to NP. Once the TEER values were steady transport studies were conducted. Initially, the culture medium was replaced with of the inhibitors used in the uptake studies and allowed to incubate on the cells for 30 minutes. Cells were then washed with 1X PBS, and 0.5 ml of 50 μM free CUR dissolved in DMSO (final DMSO concentration < 0.05 % v/v) or 1 mg/mL of nanoparticle with similar CUR loading were suspended in culture medium and added to the apical side of the Transwell. Nanoparticle transport across the cell monolayers was determined by sampling the basolateral solution at different time points (up to 24 hours), and fresh culture medium was added as replacement at each time point. After 24 hours TEER values were measured to understand the effect of exposure to NP and inhibitors. 100 μl of DMSO was added to each sample to allow for the degradation of nanoparticle and release of curcumin and CUR solubilization. The amount of CUR or NP in the basolateral solution was quantified by fluorescence detection as described previously. The amount of free CUR transported across the Transwell both with and without cells was also evaluated.
2.20. Statistical Analysis
All measurements were performed in at least triplicate, and values were presented as mean ± standard deviation. The statistical significance of the results was determined using Student’s t tests, where p < 0.05 (*) was considered statistically significant.
3. RESULTS AND DISCUSSION
Two cell membrane coated nanoparticle (CMCNP) systems were developed that were comprised of a curcumin (CUR)-loaded acetalated dextran NP core coated with two different pulmonary epithelial cell lines (H441 and A549) as seen in Figure 1. Several control NP systems were used including two lipid-coated NP systems and two polymer-coated systems. CMCNP have been used in drug delivery, disease targeting and treatment, immune modulation, and detoxification (Liu et al., 2019), and this project adds to that body of work by showing that lung epithelial CMCNP are capable of internalizing into and translocating across an in vitro pulmonary epithelial cell monolayer significantly more than the lipid- and polymer-coated NP systems, which could be beneficial in pulmonary drug delivery applications.
Figure 1.
Schematics of (Top) Formation of cell membrane-coated nanoparticles (CMCNP) from core acetalated dextran (Ac-Dex) nanoparticles (NP) loaded with curcumin (CUR) and isolated pulmonary cell membranes. (Bottom) The six NP systems formulated and characterized with their respective NP coatings and surface properties, including two CMCNP systems, two lipid-coated NP systems, and two polymer-coated NP systems. H441 and A549 = lung epithelial cell membranes, DPPC = dipalmitoylphosphatidylcholine, DOTAP = 1,2-dioleoyl-3-trimethylammonium-propane, PEG = polyethylene glycol, and PVA = polyvinyl alcohol.
Acetalated dextran (Ac-Dex), which was used as the biodegradable polymer in the core NP, is an acid-sensitive, biodegradable, dextran-based polymer that has been extensively used in drug delivery applications (Broaders et al., 2009). Curcumin (CUR) was used as a model therapeutic due to its hydrophobic nature and fluorescence, allowing for facile detection. H441 and A549 epithelial cells were chosen for the development of the CMCNP due to their ubiquitous use in pulmonary drug delivery applications. To examine the effect of interactions between NP and the negatively-charged cell membrane, lipid-coated NP coated with anionic dipalmitoylphosphatidylcholine (DPPC) and cationic 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) were developed as control NP systems, where DPPC is naturally present in cellular membranes (Jobe, 1993). Polyethylene glycol (PEG) and polyvinyl alcohol (PVA) were used for the polymer-coated NP systems. The stealth property of PEG is known to enhance the penetration of NP in mucus in the lungs (Mert et al., 2012), so we evaluated the interactions of PEG-coated NP with pulmonary cells. PVA-coated NP were served as controls since PVA is widely used to coat NP for drug delivery applications, as it provides NP stability via the minimization of electrostatic interactions.
Figure 1 illustrates the formation of CMCNP and the NP systems used in this study. CMCNP were synthesized via the coextrusion of Ac-Dex NP (core) and cell membrane vesicles (shell/coating). The resulting NP systems were approximately 200 nm in diameter and exhibited low polydispersity index (PDI), indicating size homogeneity (Table 1). CMCNP were slightly larger than the other NP, which could be due to the presence of the cell membrane coating. It has been shown that the hydrodynamic size of NP measured via dynamic light scattering can be substantially larger than their geometric diameter (Chernyshev et al., 2015), which was the case in this study (Figure 2). Scanning electron microscopy showed the NP systems to be spherical, smooth, and homogeneous in size. The surface charge (ζ potential) of the NP systems was evaluated and PVA NP were neutrally charged, PEG NP were slightly negative, DOTAP NP were highly positively charged, DPPC NP were highly negatively charged, and the H441 NP and A549 NP systems were negatively charged, which matches previously reported results (Beck-Broichsitter et al., 2014; Fang et al., 2014; Mohamed et al., 2019; Shah et al., 2019a; Wang and Meenach, 2016). The surface charge of the NP systems served as an initial confirmation for the presence of the appropriate coating for each system.
Table 1.
Characteristics of curcumin (CUR)-loaded nanoparticle (NP) systems, including NP surface coating properties, diameter, polydispersity index (PDI), zeta (ζ) potential, CUR loading, and CUR encapsulation efficiency (EE). Data represents the mean ± standard deviation (n = 3).
| NP System | Surface Coatings | Diameter (nm) | PDI | ζ Potential (mV) | CUR Loading (μg CUR/mg NP) | EE (%) |
|---|---|---|---|---|---|---|
| H441 NP | Cell Membrane | 226 ± 1 | 0.23 ± 0.04 | −24.0 ± 1.9 | 11.1 ± 0.4 | 45 ± 2 |
| A549 NP | Cell Membrane | 211 ± 5 | 0.14 ± 0.04 | −24.8 ± 1.1 | 8.6 ± 0.5 | 35 ± 2 |
| DPPC NP | Anionic Lipid | 124 ± 1 | 0.19 ± 0.03 | −44.6 ± 1.0 | 16.6 ± 1.0 | 68 ± 4 |
| DOTAP NP | Cationic Lipid | 195 ± 1 | 0.10 ± 0.01 | 49.5 ± 2.5 | 18.7 ± 0.2 | 76 ± 1 |
| PEG NP | Stealth Polymer | 177 ± 1 | 0.18 ± 0.02 | −13.7 ± 1.3 | 12.8 ± 0.2 | 52 ± 1 |
| PVA NP | Neutral Polymer | 175 ± 1 | 0.22 ± 0.04 | −5.4 ± 1.0 | 19.2 ± 0.2 | 78 ± 1 |
Figure 2.
(Top and Middle) Scanning electron microscopy (SEM) images of nanoparticle (NP) systems (scale bar = 500 nm). (Bottom) transmission electron microscopy (TEM) micrographs of NP systems (scale bar = 100 nm).
The NP coating presence was further confirmed using several methods. Evaluation via Stewart’s method confirmed the presence of lipid in the DPPC NP and DOTAP NP formulations (Stewart, 1980) (Table A.1), and PVA was present on the surface of PVA NP, as determined using a spectrophotometric method based on blue iodine-PVA complexation (Procházková et al., 2014). Transmission electron microscopy confirmed the presence of coatings on the NP as seen in Figure 2. The presence of cell membrane protein in the CMCNP formulations was determined using extracted proteins from purified cell membranes in comparison to the NP formulations using a NanoOrange™ protein quantitation kit. The presence of CD47, a cell membrane protein present in A549 and H441 cells(Lee et al., 2012), was confirmed by Western blot analysis in proteins extracted from A549 NP and H441 NP, respectively, further confirming the presence of the cell membrane coatings (Figure 3A).
Figure 3.
Characterization of nanoparticle (NP) systems. (A) Total protein was extracted from the nanoparticle (NP) or purified cell membrane and Western blot analysis was performed to detect CD47, a cell membrane protein present in A549 and H441 epithelial cells. Anti-α tubulin was used as the specificity and loading control. Stability of nanoparticles in (B) PBS and (C) RPMI media with 10% FBS over 24 hours, where the presence of curcumin (CUR) in the NP was determined using fluorescence spectroscopy. (D) In vitro release profiles of CUR from the NP systems over 24 hours. (E) Relative cell viability (%) after incubation of 1 or 4 mg/ml of nanoparticles (NP) with H441 cells for 24 hours. Data represents the mean ± standard deviation (n = 3).
The stability of the NP systems in PBS and cell media was evaluated via florescence spectroscopy (Figure 3B/C). The initial increase of florescence is likely from an initial burst release of CUR from the NP. Free CUR was not stable in PBS or media, whereas the NP were generally stable in both for up to 24 hours, indicating the necessary stability for cell studies.
CUR was successfully encapsulated into the NP systems, as indicated by the CUR loading and encapsulation efficiency data (Table 1). In vitro CUR release profiles (Figure 3D) show that 15–30% of total CUR was released from the NP systems within 24 hours with a burst release for up to 2 hours. The burst release of CUR from PVA NP and PEG NP was higher than that of the other NP systems, which is likely due to an increased presence of CUR on the NP surfaces. Ultimately, these data show that the NP systems can provide controlled release of poorly water-soluble compounds such as CUR.
The cytotoxicity of free CUR was evaluated (Figure A.2), showing that free CUR is more toxic to H441 cells at concentrations below 50 μM after 24 hours. These data were used to design the in vitro evaluation of the NP, where 1 mg/ml of NP with the highest CUR loading (19 μg CUR/mg NP for PVA NP) corresponds to 50 μM of CUR. Based on this data, 1 and 4 mg/ml NP concentrations were chosen for the NP toxicity studies (Figure 3E). No significant toxicity was observed for cells exposed to the NP systems (p > 0.13 against control). It has been shown that cell growth is enhanced by Ac-Dex NP as dextran is released into the medium during NP degradation, acting as a nutrition source for the cells (Torrico Guzmán et al., 2019), which could explain the higher viability in cells exposed to the higher NP concentration at 4 mg/ml.
We hypothesized that coating Ac-Dex NP with epithelial cell membranes would increase their internalization into and transport across pulmonary epithelial cells in vitro. Thus, internalization of the NP systems into H441 cells in presence and absence of endocytosis inhibitors was evaluated (Figure 4). These data revealed that both the surface coating of the NP and presence of inhibitors played an important role in NP internalization. Without endocytosis inhibitors, the CMCNP systems H441 NP and A549 NP internalized significantly more into H441 cells than the other NP systems. DOTAP NP internalized more than the other non-CMCNP systems, whereas PVA NP internalization was minimal. Positively charged DOTAP NP were internalized significantly more than negatively charged DPPC NP, despite the natural presence of DPPC in cell membranes, indicating charge-dependent NP internalization. Confocal imaging qualitatively confirms the internalization of NP into H441 cells, where the NP internalized within the cytoplasm (Figures 4 and A.2).
Figure 4.
Evaluation of nanoparticles (NP) internalization into H441 cells including (Top) fluorescence quantification of NP internalization with respect to the number of cells analyzed and (Bottom) representative confocal images of the internalization of NP in H441 cells, where the NP fluoresce green due to curcumin and the red is the cellular membrane (scale bar = 50 μm). Cells were exposed to NP and endocytosis-related inhibitors chloropromazine hydrochloride (CPZ), genistein (GS), and dynasore (DS) for 4 hours, where NP without inhibitors served as the control. With respect to the naming convention ‘A549’ corresponds to A549 NP, whereas A549 CPZ corresponds to A549 NP plus CPZ, as an example. *p < 0.05 and **p < 0.01, mean ± standard deviation, n = 3.
Endocytosis inhibitors were used to elucidate the potential endocytosis pathways of the NP into pulmonary epithelial cells. It has been reported that 100–200 nm polymeric NP can be internalized into cells and are often capable of avoiding macrophage clearance (Yin Win and Feng, 2005). In this study, chlorpromazine hydrochloride (CPZ), genistein (GS), and dynasore (DS) were used to block clathrin-, caveolae-, and dynamin-mediated endocytosis pathways, respectively (Le and Nabi, 2003; Macia et al., 2006; Stuart and Brown, 2006; Yao et al., 2002). In the presence of CPZ, the internalization of H441 NP, A549 NP, and DOTAP NP was significantly reduced, suggesting that these NP may undergo clathrin-mediated endocytosis. In presence of GS, H441 NP, A549 NP, and PEG NP internalization was significantly reduced, indicating possible caveolae-mediated endocytosis. NP internalization was inhibited by DS for the CMCNP systems and PEG NP, indicating dependence on dynamin-mediated endocytosis. The internalization of DPPC NP was not impacted by the presence of endocytosis inhibitors, whereas PEG NP internalization decreased in the presence of GS and DS, indicating possible caveolae- and dynamin-dependent endocytosis. PVA NP were minimally internalized and did not show endocytosis pathway dependency. Overall, these data show that the presence of a pulmonary epithelial cell membrane coating, regardless of the cell line (e.g., A549 versus H441), significantly increases the uptake of NP into pulmonary epithelial cells in comparison lipid-coated and polymer-coated NP systems, where the presence of proteins in the CMCNP are likely the driving force behind the enhanced internalization.
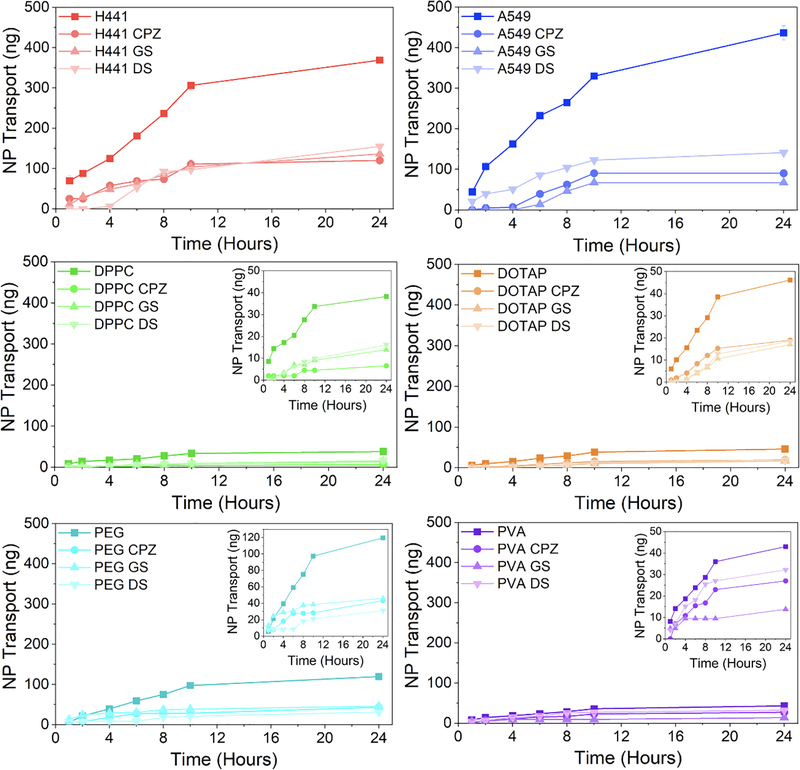
Next, we evaluated the transport of the NP across an in vitro H441 monolayer. Prior to evaluating NP transport, the transport of free CUR with and without cells was evaluated (Figure 5A/B). The transport of free CUR without cells was significantly higher than with cells. The 4-orders of magnitude difference in CUR transport rates indicated that CUR transport was significantly inhibited by the presence of cells in comparison to the Transwell. The transport rate of free CUR across the cell monolayer was significantly lower than the transport rates of all NP systems by at least two orders of magnitude. Due to the limited transport of free CUR across the cell monolayer, it was assumed that CUR-loaded NP crossing the cell monolayer was measured rather than the transport of free CUR. Cell monolayer confluency was confirmed prior to transport studies by measuring the transepithelial electrical resistance (TEER) (Figure A.3), and the values were similar to our previous results (approximately 50 Ω•cm2 after 12 days) (Shah et al., 2019b; Torrico Guzmán et al., 2019). The impact of the NP and endocytosis inhibitors on cell monolayer integrity was evaluated, where TEER increased upon exposure to NP and in the presence of inhibitors after 24 hours, indicating that neither the NP nor inhibitors had an adverse effect on cell monolayer integrity.
Figure 5.
Transport mass of free CUR across a Transwell (A) without cells and (B) with the presence of a cell monolayer over 24 hours. (C) Nanoparticle (NP) mass transport with respect to time, and (D) transport flux of NP systems across an H441 cell monolayer in Transwells after 24 hours. *p < 0.01 and **p < 0.001 for NP in comparison to all other samples, mean ± standard deviation, n = 3.
The transport over time and flux of the NP systems across a pulmonary epithelial monolayer was evaluated to determine the impact of NP coating (Figure 5C/D). The CMCNP systems (A549 NP and H441 NP) exhibited significantly more NP mass transport and higher transport flux in comparison to the other NP systems. PEG NP exhibited higher transport flux in comparison to PVA NP, DPPC NP, and DOTAP NP. Overall, these data show that coating NP with pulmonary epithelial cells increases the transport of the NP across a pulmonary epithelial monolayer, regardless of the cell line used in the coating. Furthermore, with the transport flux of the DPPC NP being so low, we hypothesize that the presence of cell-specific proteins is the driving force behind the increased transport of the CMCNP.
The impact of endocytosis inhibitors on NP transcytosis was evaluated with the same inhibitors used in the internalization study (Figures 6 and 7). The transport flux of NP across the cell monolayer was significantly lower for all NP systems in the presence of inhibitors, except for PVA NP, which did not see a decrease in transport when exposed to DS. For the CMCNP systems, H441 NP and A549 NP, exposure to the three inhibitors significantly reduced NP transcytosis, indicating dependence on clathrin-, caveolae- and dynamin-mediated endocytosis pathways. Initially H441 NP and A549 NP exhibited opposite trends, where H441 NP transcytosis was inhibited by DS and A549 NP transcytosis was inhibited by GS, indicating that H441 NP transcytosis may initially be more dependent on dynamin-mediated transport, whereas A549 NP transcytosis is more dependent on clathrin- and caveolae-mediated transport.
Figure 6.
Transport of nanoparticle systems across a H441 cell monolayer with and without the presence of the inhibitors chloropromazine (CPZ), genestein (GS), and dynasore (DS) over 24 hours. 500 μl of NP at 1 mg/ml concentration was used for each sample. Data represents the mean ± standard deviation (n = 3).
Figure 7.
Transport rates of nanoparticle systems across an H441 cell monolayer with and without the presence of the inhibitors chloropromazine hydrochloride (CPZ), genestein (GS), and dynasore (DS) over 24 hours. (*p < 0.05 and **p < 0.01 for control versus inhibitor samples, mean ± standard deviation, n = 3).
DPPC NP transcytosis was initially inhibited by CPZ, GS, and DS, whereas after 4 hours transcytosis was more readily inhibited by CPZ. DOTAP NP transcytosis showed similar dependency on all three endocytosis pathways. For the first two hours PEG NP transcytosis was not inhibited by GS, which was the opposite of DS and CPZ, indicating a change in transcytosis pathway dependency over time. This correlates with previous studies showing that PEG NP transcytosis is dependent on multiple endocytosis pathways (Tehrani et al., 2019). PVA NP transcytosis was impacted less by the inhibitors than the other systems, with GS having the most significant effect. Overall, these results indicate that NP transport is likely due to multiple endocytosis pathways for the various systems.
Due to the differing impact the transcytosis inhibitors had on NP internalization versus transcytosis, we did a comparison of these two parameters to allow for an indirect comparison of NP transport efficiency (Figure A.4). While the CMCNP systems (H441 NP and A549 NP) exhibited both higher NP internalization and transport, their transport efficiency was less than the polymer-coated NP (PEG NP and PVA NP) and greater than the lipid-coated NP (DPPC NP and DOTAP NP). As indicated in Figure A.4(D), the CMCNP and lipid-coated NP internalized more efficiently than they transported across the cell monolayer, and the opposite was true for the polymer-coated NP. These results can be attributed to the NP coatings with respect to both content and surface charge. DOTAP NP internalized more than they were trancytosed in comparison to the other NP, which is likely because of the interaction of the negatively charged NP with the positively charged extracellular membrane. Once internalized, DOTAP NP are potentially unable to penetrate the negatively charged intracellular membrane. Overall, the CMCNP systems exhibit the potential to both internalize into pulmonary cells and cross a pulmonary cell monolayer.
4. CONCLUSIONS
Overall, this work demonstrates that NP surface coatings are crucial in terms of dictating the interactions of NP with epithelial cell monolayers with respect to both cellular internalization and transcytosis. We found that CMCNP systems exhibited significantly higher endocytosis and transcytosis in comparison to the other NP systems studied. We also showed that inhibiting endocytic pathways affected the transport of NP across an epithelial monolayer, indicating that NP transcytosis is likely related to endocytosis mechanisms. Before drawing any firm conclusions, further studies are required to understand these complex mechanisms.
Supplementary Material
HIGHLIGHTS.
Core polymer nanoparticles were fabricated with pulmonary epithelial cell membranes
Cell membrane-coated nanoparticles (CMCNP) are newer biomimetic materials
Cell membrane nanoparticles combine nanomaterial functionality and cell versatility
CMCNP internalized into pulmonary epithelial cells more than other NP systems
CMCNP more effectively trancytosed across epithelial cells than other NP
ACKNOWLEDGMENTS
The authors thank the Rhode Island Consortium of Nanoscience and Nanotechnology (RIN2) for SEM and XRD access. Research was made possible using confocal microscopy, ultracentrifugation, and NMR available through the Rhode Island Institutional Development Award (IDeA) Network of Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103430.
Funding Sources
Research reported in this publication was partially supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under grant number R01HL148727, R01HL146498 (YZ), and National Institute of General Medical Sciences under grant number P20GM103652 (YZ). This material is also based in part upon work supported by the National Science Foundation under grant numbers 1508868 and 1828057.
Abbreviations:
- CMCNP
Cell membrane-coated nanoparticles
- NP
Nanoparticle
- CUR
curcumin
- PEG
poly(ethylene glycol)
- RBC
red blood cell
- Ac-Dex
Acetalated dextran
- PPTS
pyridinium p-toluenesulfonate
- mPEG
poly(ethylene glycol) methyl ether
- DCC
N,N’-dicyclohexyl-carbodiimide
- DMAP
4-(dimethylamino) pyridine
- 2-MOP
2-methoxypropene
- DCM
dichloromethane
- TEA
triethylamine
- DMSO
dimethyl sulfoxide
- DMEM
Dulbecco’s Modified Eagle Medium
- Pen-Strep
Penicillin-Streptomycin
- PBS
phosphate buffered saline
- FBS
Fetal bovine serum
- VP5k
PEG derivative DPPC, dipalmitoylphosphatidylcholine
- DOTAP
1,2-dioleoyl-3-trimethylammonium-propane
- PVA
polyvinyl alcohol
- PDI
polydispersity index
- EE
encapsulation efficiency
- SEM
Scanning electron microscopy
- TEM
transmission electron microscopy
- CPZ
chloropromazine hydrochloride
- GS
genistein
- DS
dynasore
- TEER
transepithelial electrical resistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abedin MR, Umapathi S, Mahendrakar H, Laemthong T, Coleman H, Muchangi D, Santra S, Nath M, Barua S, 2018. Polymer coated gold-ferric oxide superparamagnetic nanoparticles for theranostic applications. Journal of Nanobiotechnology 16, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck-Broichsitter M, Ruppert C, Schmehl T, Günther A, Seeger W, 2014. Biophysical inhibition of pulmonary surfactant function by polymeric nanoparticles: Role of surfactant protein B and C. Acta Biomater. 10, 4678–4684. [DOI] [PubMed] [Google Scholar]
- Broaders KE, Cohen JA, Beaudette TT, Bachelder EM, Frechet JM, 2009. Acetalated dextran is a chemically and biologically tunable material for particulate immunotherapy. Proc. Natl. Acad. Sci. U. S. A. 106, 5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyshev VS, Rachamadugu R, Tseng YH, Belnap DM, Jia Y, Branch KJ, Butterfield AE, Pease LF, Bernard PS, Skliar M, 2015. Size and shape characterization of hydrated and desiccated exosomes. Anal. Bioanal. Chem. 407, 3285–3301. [DOI] [PubMed] [Google Scholar]
- Chow AH, Tong HH, Chattopadhyay P, Shekunov BY, 2007. Particle engineering for pulmonary drug delivery. Pharm. Res. 24, 411–437. [DOI] [PubMed] [Google Scholar]
- des Rieux A, Ragnarsson EG, Gullberg E, Preat V, Schneider YJ, Artursson P, 2005. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium. Eur. J. Pharm. Sci. 25, 455–465. [DOI] [PubMed] [Google Scholar]
- Ehrmann S, Schmid O, Darquenne C, Rothen-Rutishauser B, Sznitman J, Yang L, Barosova H, Vecellio L, Mitchell J, Heuze-Vourc’h N, 2020. Innovative preclinical models for pulmonary drug delivery research. Expert Opinion on Drug Delivery 17, 463–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang RH, Hu C-MJ, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L, 2014. Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery. Nano Letters 14, 2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang RH, Kroll AV, Gao W, Zhang L, 2018. Cell Membrane Coating Nanotechnology. Advanced Materials 30, 1706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Li R, Liang J, Zhu Y, Zhang S, Zheng Z, Qin J, Pang Z, Wang J, 2018. Drug targeting through platelet membrane-coated nanoparticles for the treatment of rheumatoid arthritis. Nano Research 11, 6086–6101. [Google Scholar]
- He Z, Zhang Y, Feng N, 2020. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: A review. Materials Science and Engineering: C 106, 110298. [DOI] [PubMed] [Google Scholar]
- Hu C-MJ, Fang RH, Wang K-C, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, 2015. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe AH, 1993. Pulmonary surfactant therapy. N. Engl. J. Med. 328, 861–868. [DOI] [PubMed] [Google Scholar]
- Lai SK, Wang Y-Y, Hanes J, 2009. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Advanced drug delivery reviews 61, 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le PU, Nabi IR, 2003. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J. Cell Sci. 116, 1059. [DOI] [PubMed] [Google Scholar]
- Lee G, Zhu M, Ge B, Potzold S, 2012. Widespread expressions of immunoglobulin superfamily proteins in cancer cells. Cancer Immunol. Immunother. 61, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Guan J, Qin L, Zhang X, Mao S, 2020. Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Discovery Today 25, 150–159. [DOI] [PubMed] [Google Scholar]
- Liu Y, Luo J, Chen X, Liu W, Chen T, 2019. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications. Nano-Micro Letters 11, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk BT, Zhang L, 2015. Cell membrane-camouflaged nanoparticles for drug delivery. J. Control. Release 220, 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T, 2006. Dynasore, a Cell-Permeable Inhibitor of Dynamin. Dev. Cell 10, 839–850. [DOI] [PubMed] [Google Scholar]
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K, 2000. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release 65, 271–284. [DOI] [PubMed] [Google Scholar]
- McCright J, Skeen C, Yarmovsky J, Maisel K, 2020. Dense poly(ethylene glycol) coatings maximize nanoparticle transport across lymphatic endothelial cells. bioRxiv, 2020.2008.2001.232249. [Google Scholar]
- Mert O, Lai SK, Ensign L, Yang M, Wang YY, Wood J, Hanes J, 2012. A poly(ethylene glycol)-based surfactant for formulation of drug-loaded mucus penetrating particles. J. Control. Release 157, 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A, Kunda NK, Ross K, Hutcheon GA, Saleem IY, 2019. Polymeric nanoparticles for the delivery of miRNA to treat Chronic Obstructive Pulmonary Disease (COPD). Eur. J. Pharm. Biopharm. 136, 1–8. [DOI] [PubMed] [Google Scholar]
- Nance EA, Woodworth GF, Sailor KA, Shih T-Y, Xu Q, Swaminathan G, Xiang D, Eberhart C, Hanes J, 2012. A Dense Poly(Ethylene Glycol) Coating Improves Penetration of Large Polymeric Nanoparticles Within Brain Tissue. Sci. Transl. Med. 4, 149ra119–149ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroojalian F, Charbgoo F, Hashemi M, Amani A, Yazdian-Robati R, Mokhtarzadeh A, Ramezani M, Hamblin MR, 2020. Recent advances in nanotechnology-based drug delivery systems for the kidney. J. Control. Release 321, 442–462. [DOI] [PubMed] [Google Scholar]
- Pan A, Jakaria MG, Meenach SA, Bothun GD, 2020. Radiofrequency and Near-Infrared Responsive Core–Shell Nanostructures Using Layersome Templates for Cancer Treatment. ACS Applied Bio Materials 3, 273–281. [DOI] [PubMed] [Google Scholar]
- Procházková L, Rodríguez-Muñoz Y, Procházka J, Wanner J, 2014. Simple spectrophotometric method for determination of polyvinylalcohol in different types of wastewater. Int. J. Environ. Anal. Chem. 94, 399–410. [Google Scholar]
- Saucier-Sawyer JK, Deng Y, Seo Y-E, Cheng CJ, Zhang J, Quijano E, Saltzman WM, 2015. Systemic delivery of blood-brain barrier-targeted polymeric nanoparticles enhances delivery to brain tissue. J. Drug Target. 23, 736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N, Gupta S, Wang Z, Meenach S, 2019a. Enhancement of macrophage uptake via phosphatidylserine-coated acetalated dextran nanoparticles. Journal of Drug Delivery Science and Technology 50. [Google Scholar]
- Shah NK, Wang Z, Gupta SK, Le Campion A, Meenach SA, 2019b. Sustained release of a model water-soluble compound via dry powder aerosolizable acetalated dextran microparticles. Pharm. Dev. Technol. 24, 1133–1143. [DOI] [PubMed] [Google Scholar]
- Sohaebuddin SK, Thevenot PT, Baker D, Eaton JW, Tang L, 2010. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fibre Toxicol. 7, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JCM, 1980. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 104, 10–14. [DOI] [PubMed] [Google Scholar]
- Stuart AD, Brown TDK, 2006. Entry of Feline Calicivirus Is Dependent on Clathrin-Mediated Endocytosis and Acidification in Endosomes. J. Virol. 80, 7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani SF, Bernard-Patrzynski F, Puscas I, Leclair G, Hildgen P, Roullin VG, 2019. Length of surface PEG modulates nanocarrier transcytosis across brain vascular endothelial cells. Nanomedicine: Nanotechnology, Biology and Medicine 16, 185–194. [DOI] [PubMed] [Google Scholar]
- Torrico Guzmán EA, Sun Q, Meenach SA, 2019. Development and Evaluation of Paclitaxel-Loaded Aerosol Nanocomposite Microparticles and Their Efficacy Against Air-Grown Lung Cancer Tumor Spheroids. ACS Biomaterials Science & Engineering 5, 6570–6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-Y, Lai SK, Suk JS, Pace A, Cone R, Hanes J, 2008. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angewandte Chemie (International ed. in English) 47, 9726–9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Meenach SA, 2016. Synthesis and Characterization of Nanocomposite Microparticles (nCmP) for the Treatment of Cystic Fibrosis-Related Infections. Pharm. Res. 33, 1862–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Ying M, Dehaini D, Su Y, Kroll AV, Zhou J, Gao W, Fang RH, Chien S, Zhang L, 2018. Nanoparticle Functionalization with Platelet Membrane Enables Multifactored Biological Targeting and Detection of Atherosclerosis. ACS Nano 12, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D, Ehrlich M, Henis YI, Leof EB, 2002. Transforming Growth Factor-β Receptors Interact with AP2 by Direct Binding to β2 Subunit. Mol. Biol. Cell 13, 4001–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Win K, Feng S-S, 2005. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 26, 2713–2722. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chan JM, Gu FX, Rhee J-W, Wang AZ, Radovic-Moreno AF, Alexis F, Langer R, Farokhzad OC, 2008. Self-Assembled Lipid−Polymer Hybrid Nanoparticles: A Robust Drug Delivery Platform. ACS Nano 2, 1696–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Wang B, Wang C, Wang Q, Zhang L, 2020. Cell membrane-coated nanoparticles: research advances. Nanomedicine 15, 625–641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.