Abstract
Nucleic acid sequence capture extraction was coupled with LightCycler PCR amplification and product detection using real-time fluorescence for rapid, definitive detection of Mycobacterium bovis in lymph node specimens from 38 cattle with bovine tuberculosis lesions. PCR amplification of sequence-captured DNA using both a conventional heating block thermocycler and a LightCycler thermocycler was compared with culture and histopathological analyses. Conventional PCR enabled detection of 26 of 28 culture-positive specimens (93%) in approximately 9 h, and the LightCycler PCR detected 20 of 28 culture-positive specimens (71%) in only 30 min. Specific confirmation of Mycobacterium tuberculosis complex DNA was achieved by LightCycler PCR amplification using Syb Green 1 and an M. tuberculosis complex-specific Cy5-labeled fluorescence resonance energy transfer probe. The system described here enabled rapid and specific laboratory confirmation of bovine tuberculosis, and this is the first report of the detection of M. bovis in tissues using LightCycler PCR. The fluorescence technology used in the study has potential to allow development of a high-throughput molecular diagnostic test for bovine tuberculosis.
Mycobacterium bovis, a member of the Mycobacterium tuberculosis complex, is the causative agent of bovine tuberculosis. This zoonotic disease continues to have considerable economic and public health implications (18, 19). National eradication programs employ tuberculin testing and slaughter strategies (17). Culture is used commonly to confirm infection in postmortem specimens from cattle slaughtered following a positive skin test reaction. Although culture is considered to be the “gold standard” for confirming tuberculosis, this procedure may take several weeks (15).
Consequently, rapid nucleic acid amplification techniques, including the PCR- and transcription-mediated amplification, have been applied to detect M. bovis directly in clinical specimens (1, 2, 15, 28; S. Roring et al., unpublished data). While PCR amplifications have enabled detection of nonviable mycobacteria (15, 16, 28), they are not as sensitive as culture. In comparison to M. tuberculosis in human sputa, which often contain large numbers of bacilli (28), bovine tuberculous tissues are associated with few bacilli. Extraction of mycobacterial DNA from sputa was considered to be less difficult than extraction from tissues, and consequently, improvements in nucleic acid extraction were recommended to increase sensitivity of PCR detection of M. bovis in tissue (6).
Mycobacterial DNA extraction efficiency has been improved with the development of a nucleic acid sequence capture procedure, which has enabled detection of mycobacteria in paucibacillary forms of tuberculosis (3, 16). Sequence capture PCR has been used successfully for simultaneous detection and strain typing of M. bovis from BACTEC cultures (22) and more recently from bovine lymph node tissue (23). These studies were performed in conventional heating block thermocyclers (HBTC) with end point detection of PCR products by agarose gel electrophoresis. Automation of the sequence capture PCR procedure should facilitate its use in routine diagnosis of M. tuberculosis in clinical specimens (3, 16).
Rapid-cycle PCR amplifications, using an air thermocycler (ATC), have increased the rapidity of M. tuberculosis detection by decreasing the amplification time (6, 13) and thus may assist automation. Additionally, alternative PCR product detection systems have the potential to improve automation, as traditional methods of detection may be laborious and time-consuming (12, 20).
Fluorimeter-based closed-tube PCR assays permit the continual monitoring of accumulating fluorescently labeled PCR products, termed real-time fluorescence (11). Rapid-cycle PCR in conjunction with fluorimeter-based closed-tube PCR assays have provided a rapid and sensitive method for identification and quantification of PCR products (21, 29). Real-time fluorescence has been used to detect M. tuberculosis in sputum using the TaqMan system (8). In this study, sequence capture is combined with rapid-cycle PCR and real-time fluorescence involving the use of an M. tuberculosis complex-specific fluorescence resonance energy transfer (FRET) probe in a LightCycler LC32 (Biogene Ltd., Kimbolton, United Kingdom) to detect M. bovis in bovine tissues.
MATERIALS AND METHODS
Biological material.
A bovine field isolate of M. bovis, characterized by restriction fragment length polymorphism analysis (24), was cultured in Middlebrook 7H9 broth (Difco Laboratories). Serial dilutions of culture broth (10−3 to 10−8) were made in sterile saline and subcultured on 7H9 agar plates. Aliquots of the culture dilution series (400 μl) were stored at −20°C and used for the determination of sequence capture PCR efficiency at a later date.
M. bovis genomic DNA was extracted from a second, similarly characterized bovine field isolate, and the IS6110 gene copy number was estimated from DNA concentrations determined by A260s. Serial dilutions of M. bovis genomic DNA (2.5 × 104 to 2.5 × 10−1 IS6110 genome equivalents) were used as positive PCR controls.
Lymph nodes were collected from 38 cattle exhibiting lesions at slaughter. These cattle were either intradermal skin test positive or identified as having lesions during routine meat inspection. Specimens were subjected to histopatholgical examination (5) and to microbiological decontamination prior to culture (26). Aliquots of the latter specimens were cultured both on slopes of Lowenstein-Jensen (L-J) solid medium (Media for Mycobacteria; Sully, South Glamorgan, United Kingdom) and in BACTEC 12B liquid medium (Becton Dickinson, Oxford, United Kingdom). Residual decontaminated tissue was stored at −20°C for sequence capture PCR. L-J slope cultures were monitored for bacterial growth after 28 days, and BACTEC cultures were examined up to 84 days postinoculation. The presence of acid-fast bacilli in BACTEC cultures was confirmed by Ziehl-Neelsen staining and microscopic examination (25). Isolates from L-J slopes were confirmed as M. tuberculosis complex using the Accuprobe (Gen-Probe, San Diego, Calif.).
DNA extractions.
DNA sequence capture PCR, as previously described (16) and modified (23), was applied to homogenates of the 38 decontaminated bovine lymph nodes with the following additional modifications. In brief, suspensions of decontaminated tissue homogenate (500 μl) were transferred to screw-cap microcentrifuge tubes containing 500 μl of 0.1-mm-diameter zirconium beads (Biospec Products Inc., Bartlesville, Okla.). Samples were centrifuged and washed as described previously (23). The pellet and beads were resuspended in 500 μl of TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid]–100 mM Tris-HCl (pH 7.4)–50 mM EDTA–150 mM NaCl and incubated in a sonicating water bath for 15 min. Samples were agitated in a FastPrep Bio 101 bead shaker (Savant Instruments Inc., Holbrook, N.Y.) at 6 m/s for 45 s. Proteinase K (Sigma, Poole, Dorset, United Kingdom) was added to a final concentration of 3 mg/ml, and the mixture was incubated at 50°C for 18 h. Samples were shaken in the FastPrep Bio 101 as before. Aliquots of proteinase K-treated homogenates (500 μl) were denatured at 100°C for 15 min and immediately transferred to ice for a further 5 min. The biotinylated capture oligonucleotides CapDRa (5′biotin-AAAAAGGTTTTGGGTCTGACGAC) and CapDRa (5′biotin-AAAAACCGAGAGGGGACGGAAAC) [Genosys Biotechnologies (Europe), Pampisford, Cambs., United Kingdom] were used to capture the DR region of the M. tuberculosis complex (16). Capture oligonucleotides (2.5 pmol of each) in 3.75 M NaCl solution were added to homogenates to a final concentration of 1 M NaCl, mixed well, and hybridized at 42°C with gentle agitation for 3 h. Following hybridization, 50 μg of streptavidin M-280 Dynal beads (Dynal, Oslo, Norway) was added, mixed well, and incubated at 36°C with gentle agitation for 2 h. The streptavidin M-280 Dynal beads were separated from the supernatant and washed using a magnetic bead separator (6 by 1.5 ml) (Stratagene, La Jolla, Calif.) in 750 μl of wash buffer (10 mM Tris-HCl [pH 7.4], 1 mM EDTA) and then in sterile deionized H2O. Magnetic beads were resuspended in 25 μl of sterile deionized H2O and stored at −20°C prior to PCR amplification. Immediately prior to amplification, captured mycobacterial DNA was released from magnetic beads by heat treatment at 100°C for 5 min. Following a brief centrifugation, the resulting supernatant was subjected to the appropriate amplification.
PCR amplification.
Sequence-captured mycobacterial nucleic acid was subjected to rapid thermal cycling and continuous monitoring of PCR products in a LightCycler LC32 (Biogene Ltd.) and to conventional PCR amplification using an HBTC (model 480; Perkin-Elmer, Warrington, United Kingdom) (HBTC-PCR). Oligonucleotides specific for the M. tuberculosis complex IS6110 sequence (9) (Genosys) were used for the amplifications. A standard PCR protocol (LC-PCR) utilizing the LC32 instrumentation was adopted only after extensive modification and optimization of previously reported PCR conditions (9). Optimal magnesium ion and oligonucleotide concentrations were determined along with cycling parameters such as denaturation, annealing and elongation temperatures, incubation periods, and temperature transition rates. LC32 instrumentation variables were optimized as directed by the manufacturers.
Standard LightCycler protocol.
After optimization, the following standard LC-PCR protocol was applied to all specimens. A commercial PCR master mixture (Bio/Gene Ltd.), containing 2.5 U of Taq polymerase, 250 μM deoxynucleoside triphosphates, and 3 mM MgCl2, was pretreated with Taq-Start antibody (7 μl) (Sigma). The PCR mixture consisted of pretreated PCR master mixture supplemented with IS6110-specific oligonucleotides (500 nM), Syb Green 1 (1/60,000 dilution) (Biogene Ltd.), lambda DNA (5 pg/μl), and Cy5 3′-labeled FRET probe LCP (100 nM) together with 2.5 μl of target DNA in a final reaction volume of 10 μl. The FRET probe LCP (5′GCCCAGGTCGACACATAGG3′-Cy5), specific for the M. tuberculosis complex, was designed using OLIGO 5 primer design software (Biogene Ltd.) and to the specifications described by Biogene Ltd.
The PCR mixture (3-μl aliquot) was applied to the top of a glass capillary reaction vessel (part 1720; Biogene Ltd.) which was filled by pulse centrifugation in a microcentrifuge. Conditions for cycling were 94°C for 45 s, followed by 50 cycles of 94°C for 0 s, 62°C for 0 s, and 74°C for 10 s; fluorescence was monitored at the end of every 74°C step. The amplification program was followed by a melting program of 45°C for 10 s and then 45 to 95°C at a transition rate of 0.2°C/s with continuous monitoring of fluorescence. The temperature transition rate for all cycling steps was 20°C per s except for those between 62 and 74°C, where a transition rate of 1°C per s was used. The gain on the F1 channel photometric detector was routinely set at 64.
HBTC protocol.
HBTC-PCR included either 2.5 μl of sequence-captured magnetic beads or a 5-μl inoculum of either genomic DNA or heat-inactivated M. bovis. A PCR master mix (50-μl final volume) contained 2.5 U of Taq polymerase (Sigma), a 500 nM concentration of each of the IS6110 primers, 250 μM deoxynucleoside triphosphates, and 1.75 mM MgCl2, with a mineral oil overlay (Sigma). Conditions for cycling were 94°C for 5 min, followed by 30 cycles of 94°C for 1 min, 68°C for 2 min, and 74°C for 1 min. Amplification mixtures were incubated for a further 7 min at 74°C, with a final 4°C soak.
For reamplification, 5 μl of first-round PCR product was subjected to a second round of PCR amplification using the same PCR conditions. The 123-bp PCR product of IS6110 was identified by 2% (wt/vol) agarose gel electrophoresis with TAE buffer (40 mM Tris-acetate, 1 mM EDTA).
RESULTS
Optimization of light cycler reactions. (i) Preliminary experiments.
PCR amplifications and monitoring of fluorescence emission during PCR were performed using a LightCycler LC32. An amplification program, recommended by the LC32 manufacturer, was modified so that annealing temperatures similar to those previously published for this primer set were used (9). Preliminary experimentation using positive controls of heat-killed mycobacterial suspension together with negative controls of water established suboptimal reaction conditions of 3 mM magnesium (range of 2 to 5 mM tested in 1 mM increments), annealing temperature of 60°C (range of 60 to 66oC tested in 1°C increments), 500 μM primer (100 and 500 nM tested), and 100 nM LCP (10, 50, and 100 nM tested).
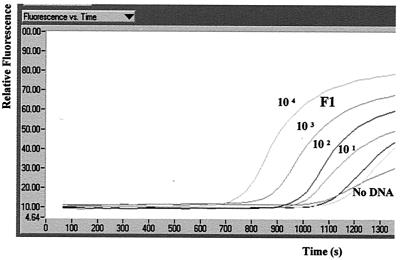
Run profile software graphically presented relative fluorescence versus time during the amplification program. Fluorescence signal acquisition, graphically displayed as a curve, increased in value with time as product was synthesized. Reaction mixtures containing M. bovis genomic DNA exhibited an exponential increase in signal after 20 cycles, while negative controls demonstrated a slow signal acquisition only after 40 cycles (Fig. 1).
FIG. 1.
Screen capture image (CorelDRAW; Corel Corporation, Ottawa, Ontario, Canada) of run profile analysis demonstrating the accumulation of F1 fluorescence during PCR of 10-fold serial dilutions of M. bovis genomic DNA representing 2.5 × 104 to 2.5 × 10−1 IS6110 gene copies using Syb Green 1 only.
Linking a melt cycle program to the amplification program facilitated the identification of amplification products. The continuous monitoring of fluorescence emissions during the slow denaturation step in the melt program permitted a precise calculation of the melting temperatures (Tms) of all PCR products. Melting-curve software (Idaho Technologies Inc.) converted fluorescence versus temperature to the rate of change of fluorescence emissions versus temperature (−dF/dT versus temperature), termed peak analysis. PCR product purity was demonstrated by determining the Tms of amplified products. Adjustment of points to average to 7 was found to smooth curves, aiding interpretation by reducing noise without compromising resolution. Fluorescence channel F1 detected emissions from the intercalation of the dye Syb Green 1 with all double-stranded nucleic acid PCR products. PCRs with M. bovis genomic DNA exhibited a peak at 89°C, while negative controls were characterized by a peak at 86°C. The identities of PCR products were confirmed by gel electrophoresis and ethidium bromide staining of LC32 reaction vessel contents. A 123-bp IS6110-specific PCR product was associated with the 89°C peak, while a smaller primer artifact was associated with the 86°C peak. LC-PCR amplifications of serial dilutions of M. bovis genomic DNA, over 4 log units (104 to 101 IS6110 gene copies), demonstrated a sensitivity of 101 gene copies (Fig. 2).
FIG. 2.
Screen capture (CorelDRAW) of F1 melting-peak analysis of 10-fold serial dilutions of M. bovis genomic DNA representing 2.5 × 104 to 2.5 × 10−1 IS6110 gene copies using Syb Green 1. Each trace graphically displays the rate of change of F1 fluorescence emissions versus temperature; a peak represents the Tm of the PCR product. The IS6110 123-bp species displays a Tm of 89°C, while a primer artifact displays a peak at 86°C.
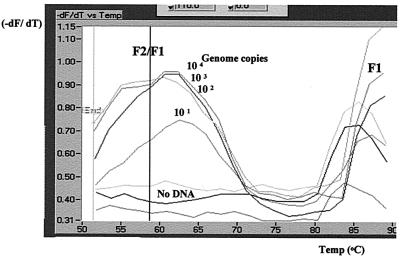
Fluorescence channel F2 detected emissions from Cy5-labeled FRET LCP stimulated by resonance energy donated by intercalated Syb Green 1. Fluorescent emissions from the melt program, monitored by both F1 and F2 optics, (F2/F1) after peak analysis exhibited a peak between 60 and 65°C for reaction mixtures containing M. bovis genomic DNA, while negative controls displayed no such peak (Fig. 3).
FIG. 3.
Screen capture (CorelDRAW) of F2 melting-peak analysis of an M. bovis genomic DNA dilution series representing 2.5 × 104 to 2.5 × 10−1 IS6110 gene copies using FRET probe LCP and Syb Green 1. The Tm of each PCR product is identified as a peak; each trace represents the rate of change of F2/F1 fluorescence emissions versus temperature. The IS6110 123-bp species displays a Tm of 60 to 65°C, while the primer artifact displays none. The F1 emissions are responsible for the F1 peaks at the right side of the graph.
(ii) Improved reproducibility and sensitivity.
In preliminary experiments, amplifications performed on consecutive days were found to lack reproducibility (data not shown). Positive control run profiles demonstrated inconsistencies in signal acquisition. This lack of reproducibility of the LC-PCR was attributed to Syb Green 1 instability. Revised protocols from the manufacturer (Biogene Ltd.) required that Syb Green 1 be stored at −20°C, in an undiluted form, for storage of up to 1 year without loss of activity. Once diluted 1/1,000 in the dilution buffer supplied by the manufacturer, Syb Green 1 must be stored in the dark at 4°C for no more than 1 month and shaken vigorously for 1 min immediately prior to use.
Several modifications were made to the PCR master mixture recommended by the manufacturer (Biogene Ltd.) to improve sensitivity. Commercial master reaction mixtures (500-μl aliquots) were pretreated with Taq-Start antibody (7 μl) (Sigma) incubated at room temperature for 20 min prior to storage at −20°C. Pretreatment, while reducing background in the negative control, delayed the onset of exponential signal in positive controls. Lambda DNA was added to the master mixture to a final concentration of 47.5 pg/μl in order to improve the specificity of PCRs. Storage of positive control reaction mix in the dark at room temperature for prolonged periods (24 h) was not detrimental to either specificity or sensitivity. The FRET LCP was stored in aliquots at 10 μM at −20°C and diluted to 1 μM immediately prior to use. The temperature transition rate of the cycle program, between annealing and extension temperatures, was modified to 1°C/s. The melt program was modified to include a 15-s hold at 45°C prior to the commencement of the melt program with a temperature transition rate of 0.2°C/s.
The preliminary protocol was optimized using positive controls of heat-killed mycobacterial suspension and purified genomic mycobacterial DNA and negative controls of water, establishing reaction conditions of 3 mM magnesium (2 to 5 mM tested), 62°C annealing temperature (60 to 68°C tested), 500 μM primer concentration (100 to 500 nM tested), and 100 nM LCP (10 to 100 nM tested) using the cycling conditions stated in Materials and Methods.
Sensitivity.
LightCycler PCR amplifications using the standard protocol reproducibly detected 25 copies of the M. bovis IS6110 gene DNA with melt peak analysis (dF/dT versus temperature) of both F1 and F2 emissions (Fig. 2 and 3). LC-PCR amplification of sequence-captured M. bovis culture dilution series detected 36 bacilli using the F1 channel and 360 bacilli using the F2 channel. A single round of HBTC-PCR amplification of the same dilution series of M. bovis IS6110 gene DNA as used for the LC-PCR amplifications reproducibly detected 625 gene copies. A second round of HBTC amplification, however, was required in order to detect 62 gene copies. Two rounds of HBTC amplifications were also required to detect 36 sequence-captured M. bovis bacilli.
Clinical specimens.
Lymph node tissues were cultured and examined histopathologically. Of the 38 lymph node specimens from animals with lesions, 13 were from cattle which were skin test positive (Table 1), and 11 of these 13 specimens were culture positive. Twenty-seven of the 38 specimens were culture positive using both L-J slope and BACTEC media (Table 1). All culture isolates were confirmed as M. tuberculosis complex by Accuprobe (Gen-Probe) (data not shown). Three were BACTEC cultured positive after 7 days, and the remaining 24 were positive after 14 days. Histopathological examination identified 26 bovine tuberculosis-positive specimens. Five of the 28 culture-positive specimens were negative by histopathology, and 3 of the 10 culture-negative specimens (Table 1) were positive by histopathology. One specimen, identified with morphology typical of actinomycetes by histopathology, was BACTEC culture negative; however, this specimen was L-J culture positive after 28 days.
TABLE 1.
Histopathological and bacteriological status of tissue specimens and performance of HBTC-PCR and LC-PCR for detecting M. bovis in tissue
| Culture result | Specimen no. | Skin test result | Histopathology | Days to positive BACTEC culture | HBTC-PCR resultd | LC-PCR result |
|---|---|---|---|---|---|---|
| Positive | 2 | + | + | 14 | − | − |
| 3 | + | + | 14 | +++ | − | |
| 4 | + | + | 14 | ++ | − | |
| 5 | + | + | 14 | +++ | + | |
| 6 | N/Aa | + | 14 | + | + | |
| 8 | N/A | + | 7 | +++ | + | |
| 9 | N/A | + | 14 | +++ | + | |
| 12 | N/A | + | 14 | +++ | + | |
| 19 | + | + | 14 | +++ | + | |
| 20 | + | − | 14 | +++ | + | |
| 22 | + | + | 14 | +++ | + | |
| 23 | N/A | + | 14 | + | + | |
| 24 | N/A | + | 14 | +++ | + | |
| 25 | N/A | + | 14 | + | − | |
| 26 | N/A | + | 14 | +++ | + | |
| 31 | N/A | − | 14 | ++ | + | |
| 32 | N/A | + | 7 | + | − | |
| 33 | + | + | 14 | + | + | |
| 36 | N/A | + | 14 | +++ | + | |
| 38 | N/A | + | 14 | + | − | |
| 39 | N/A | + | 7 | +++ | + | |
| 41 | N/A | + | 14 | − | − | |
| 42 | + | − | 14 | ++ | + | |
| 43 | + | −b | —c | +++ | + | |
| 44 | + | − | 7 | ++ | + | |
| 46 | N/A | + | 14 | +++ | + | |
| 49 | N/A | + | 14 | + | − | |
| 50 | N/A | + | 14 | ++ | + | |
| Negative | 1 | + | − | − | − | |
| 7 | N/A | + | − | − | ||
| 18 | N/A | + | − | − | ||
| 21 | N/A | −b | − | − | ||
| 27 | N/A | −b | − | − | ||
| 28 | N/A | −b | − | − | ||
| 29 | N/A | −b | − | − | ||
| 34 | + | − | − | − | ||
| 47 | N/A | −b | − | − | ||
| 48 | N/A | + | − | − |
N/A, animals were not skin tested prior to analysis.
Actinomyces infection.
A few colonies grew on L-J slope media.
+++, strong band; ++, medium band; +, weak band.
Sequence-captured nucleic acid derived from decontaminated homogenates was subjected to PCR amplification utilizing both LC32 and HBTC methodologies. DNA representing 10% of that sequence captured was amplified in either system. Using HBTC, M. bovis was detected in 26 (93%) of the 28 culture-positive tissue specimens (Table 2 and Fig. 4a). LC-PCR detected M. bovis in 20 (71%) of the 28 culture-positive tissue specimens (Table 3 and Fig. 4b). All of the culture-negative specimens were PCR negative in both the HBTC- and LC-PCRs (Table 1). There was also good agreement between the LC- and HBTC-PCR results; 13 of 14 (93%) of the HBTC strong positives, 4 of 5 (80%) of the HBTC moderate positives, and 3 of 7 (43%) of the HBTC weak positives were positive by LC-PCR. In addition, the two culture-positive HBTC-PCR-negative specimens were also negative by LC-PCR.
TABLE 2.
Comparison of HBTC-PCR with culture results for the detection of M. bovis in tissue
| Culture result | No. of specimens HBTC-PCR:
|
|
|---|---|---|
| Positive | Negative | |
| Positive | 26 | 2 |
| Negative | 0 | 10 |
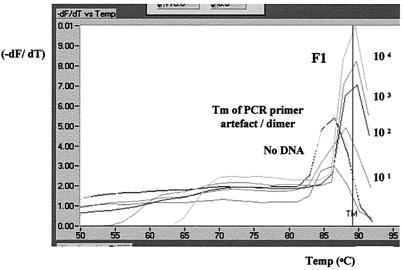
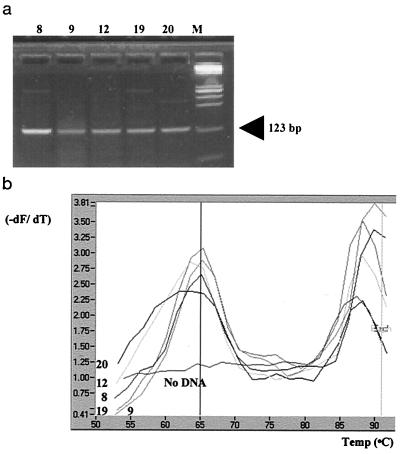
FIG. 4.
(a) Agarose gel electrophoresis of HBTC-PCR-amplified DNAs from lymph nodes from animals with bovine tuberculosis lesions. Amplification products from five specimens listed in Table 1 are displayed. The size of the amplified target DNA is shown. (b) Screen capture (CorelDRAW) of F2 melting peak analysis of LC-PCR amplification of DNAs from lymph nodes from five animals with bovine tuberculosis lesions (Table 1).
TABLE 3.
Comparison of LC-PCR with culture results for the detection of M. bovis in tissue
| Culture result | No. of specimens LC-PCR:
|
|
|---|---|---|
| Positive | Negative | |
| Positive | 20 | 8 |
| Negative | 0 | 10 |
DISCUSSION
This work is the first reported application of sequence capture PCR and real-time fluorescence detection of M. bovis in clinical tissue. The benefits of target enrichment and removal of inhibitory substances attributed to sequence capture were combined successfully with the alternative PCR product detection technology offered by real-time fluorescence. Several nucleic acid amplification-based techniques have become accessible to clinical mycobacteriological laboratories in recent years. However, poor performance in paucibacillary situations and high costs have limited their widespread application (20). Requirement of a high degree of operator skill to perform and interpret molecular analyses has also hindered adoption of molecular methods in diagnostic laboratories (10). The application of PCR to disease diagnosis is dependent on specificity, sensitivity, ease of use, and high sample throughput (3, 16, 20). In this study improved DNA extraction and fluorescent detection methodologies were used to examine these criteria and to facilitate PCR diagnosis of M. bovis in bovine tuberculosis specimens.
Culture of M. bovis from clinical specimens has been regarded as the standard against which other M. bovis diagnostic methodologies have been measured (15). However, culture of M. bovis is slow, and incubation periods of several weeks may be required (15, 28). Radiometric culture (BACTEC) permits more rapid detection of mycobacteria; however, minimum incubation periods of several days are still required (14). In this study, only 4 of the 28 culture-positive specimens were BACTEC culture positive within 7 days. A further 23 were culture positive within 14 days. The remaining specimen was BACTEC culture negative and exhibited only poor growth on L-J slopes. The inability to culture mycobacteria from tissue specimens has previously been associated with sample autolysis or microbial contamination (15, 28), which may account for the latter result.
Histopathological examination, used commonly for routine diagnosis of bovine tuberculosis, permits rapid identification of lesions but may not differentiate between those caused by M. bovis and by other mycobacterial or closely related agents (7). In addition to this lack of specificity, the sensitivity of microscopic examination is limited (31). The results of this study indicated the limitations of histopathological analysis, as only 82% (23 of 28) of the culture-positive specimens were positive by histopathological analysis. The five histopathologically negative specimens were both culture and PCR positive. Three histopathologically positive specimens were negative by both culture and PCR, suggesting that these histopathology results may represent false positives.
Limited sensitivity has also been associated with molecular technologies for mycobacterial diagnosis (16). PCR has been applied widely to the detection of M. tuberculosis in sputa and bronchiolar lavages from human tuberculosis patients with success but not so for bovine tuberculosis, where the bacterial load is substantially less (8, 28).
The application of sequence capture and conventional PCR amplification methodologies in this study demonstrated M. bovis detection sensitivities comparable to those of culture. This is in agreement with other sequence capture studies (3, 16, 23). Ninety-three percent of culture-positive specimens were detected using two rounds of PCR amplification in the HBTC followed by analysis of the PCR products by agarose gel electrophoresis. This was comparable to a sensitivity of 91% achieved in earlier PCR studies (1, 28). PCR amplification and product detection, using HBTC, took approximately 9 h, which was much shorter than the period required for detection by culture.
Further reductions in the PCR detection time for M. tuberculosis have been achieved using an ATC (6, 13). In these studies, air thermal cycling used small-volume glass capillaries and high-velocity heated air to generate temperature transition rates in excess of 10°C/s and culminated in amplification times of less than 30 min. In addition, the use of reduced reaction volumes associated with an ATC resulted in a substantial reduction in reagent costs (6). Until recently, PCR amplification and product analysis have been sequential procedures. The LC32 used in this study measures real-time fluorescence, combining the rapid-cycle capabilities of the ATC with continual fluorimetric detection of accumulating PCR products.
Interpretation of fluorimetric emissions generated by Syb Green 1 was confirmed initially by agarose gel electrophoresis analysis of PCR products evacuated from the capillary reaction vessels and resolved on agarose gels, as was the case in earlier studies utilizing fluorescent detection systems (27). The presence of the expected 123-bp PCR product along with PCR artifacts established that the molecular enzymology of the LC32 had resulted in the synthesis of the predicted IS6110 PCR product. The sensitivity achieved in this report with the IS6110 target was 25 IS6110 gene copies, which was comparable to that of previous studies (16). Accumulation of fluorescence may not equate to specific PCR product synthesis, as Syb Green 1 detects all double-stranded DNA, including PCR artifacts (21, 27).
In this present study PCR product differentiation was achieved, in most cases, by analyzing the melting curve characteristics of the PCR products. Melting curves have previously differentiated multiple PCR products in concordance with those products identified by gel electrophoresis. PCR products with Tms differing by only 2°C have been distinguished (21). Again in the present study, the 123-bp IS6110-specific product exhibited a Tm of 89°C, while the PCR primer artifact displayed a Tm of 86°C. Primer artifacts occasionally generated shoulder effects on F1 signal melt peaks of specific PCR products, making their interpretation difficult. Primer artifacts with Tms similar to those of specific products have been reported in other studies (27, 30). These reports suggested that melt curve analysis alone is not definitive in identifying specific PCR products.
Sequence-specific detection using FRET hybridization probes (specific oligonucleotide probes labeled with fluorescent dyes) has been reported. Several FRET formats have been developed (12). One format, hybridization probes, which employs two single fluorescence-labeled oligonucleotides that hybridize to adjacent regions of target DNA, has been used in conjunction with LightCycler instrumentation (4). In the present study, because the target was small and GC rich, only a single-oligonucleotide FRET probe was used as a hybridization probe. Resonance energy, received from Syb Green 1 intercalated between the oligonucleotide and PCR product, was transferred to the Cy5 fluorophore attached to the oligonucleotide's 3′ terminus. The broad emission spectrum of the fluorescence of Syb Green 1 overlaps that of Cy5, but the melt curve analysis compensates by dividing the F2 signal by the F1 signal. This FRET probe enabled detection of an M. tuberculosis complex target which exhibited a Tm of 60 to 65°C. A sensitivity of 25 IS6110 gene copies, similar to that achieved using the DNA dilution series and Syb Green 1 only, was demonstrated. The FRET probe circumvented the previously mentioned difficulties with interpretation of the F1 signal melt peak. Few studies have used this single-probe approach, but those that have report a greater sensitivity than with the double-probe format (4).
The Cy5-labeled FRET hybridization probe LCP described in this study detected M. bovis genomic DNA in decontaminated tissue homogenates of 20 of the 28 culture-positive specimens. This sensitivity of detection was lower than the 26 of 28 culture-positive specimens detected by PCR in the HBTC. The reduced sensitivity with the FRET probe was attributed in part to the perturbation of fluorescent signals by the red iron oxide present in the sequence capture magnetic beads. This perturbation was alleviated by detachment of captured mycobacterial DNA from magnetic beads by heat treatment at 100°C for 5 min and centrifugation prior to amplification. This treatment resulted in significant improvements in signal interpretation. PCR in the HBTC was probably more sensitive than that in the LC32 due to the second round of amplification employed in the HBTC. It is clear that the benefits of PCR reamplification need to be balanced against the associated increased risk of sample cross contamination and increased assay time. Further optimization of the LC32 system is expected to elevate the sensitivity to that of HBTC. This study demonstrated the rapidity of PCR diagnosis compared to culture. Rapid-cycle PCR and fluorimetric detection technologies have reduced detection time further to several minutes and would allow rapid screening of tissue specimens for the presence of M. bovis. The same fluorescent technology should facilitate development of a high-throughput molecular diagnostic assay and provide a more practical approach for confirmation of tuberculosis directly from clinical specimens.
ACKNOWLEDGMENTS
We are grateful to D. Brittain for the provision of M. bovis genomic DNA and to all of the staff in the tuberculosis diagnostic laboratory and histopathology laboratory for their technical assistance.
REFERENCES
- 1.Aranaz A, Liébana E, Mateos A, Vidal D, Domingo M, Dominguez L. Direct detection of M. bovis from tissue samples. Improvement of a DNA extraction method for PCR amplification. In: Griffin F, de Lisle G, editors. Tuberculosis in wildlife and domestic animals. Dunedin, New Zealand: University of Otago Press; 1995. pp. 60–63. [Google Scholar]
- 2.Bollo E, Guarda F, Capucchio M T, Galietti F. Direct detection of Mycobacterium tuberculosis complex in tissue specimens from cattle through identification of specific rRNA sequences. J Vet Med Ser B. 1998;45:395–400. doi: 10.1111/j.1439-0450.1998.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 3.Brugière O, Vokurka M, Lecossier D, Mangiapan G, Amrane A, Milleron B, Mayaud C, Cadranel J, Hance A J. Diagnosis of smear-negative pulmonary tuberculosis using sequence capture polymerase chain reaction. Am J Respir Crit Care Med. 1997;155:1478–1481. doi: 10.1164/ajrccm.155.4.9105098. [DOI] [PubMed] [Google Scholar]
- 4.Cane P A, Cook P, Ratcliffe D, Mutimer D, Pillay D. Use of real-time PCR and fluorimetry to detect lamivudine resistance-associated mutations in hepatitis B virus. Antimicrob Agents Chemother. 1999;43:1600–1608. doi: 10.1128/aac.43.7.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassidy J P, Bryson D G, Neill S D. Tonsillar lesions in cattle naturally infected with Mycobacterium bovis. Vet Rec. 1999;144:139–142. doi: 10.1136/vr.144.6.139. [DOI] [PubMed] [Google Scholar]
- 6.Chapin K, Lauderdale T-L. Evaluation of a rapid air thermal cycler for detection of Mycobacterium tuberculosis. J Clin Microbiol. 1997;35:2157–2159. doi: 10.1128/jcm.35.8.2157-2159.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lisle G W, Yates G F, Collins D M. Paratuberculosis in farmed deer: case reports and DNA characterisation of isolates of Mycobacterium paratuberculosis. J Vet Diagn Investig. 1993;5:567–571. doi: 10.1177/104063879300500411. [DOI] [PubMed] [Google Scholar]
- 8.Desjardin L E, Chen Y, Perkins M D, Teixeira L, Cave M D, Eisenach K D. Comparison of the ABI 7700 system (TaqMan) and competitive PCR for quantification of IS6110 DNA in sputum during treatment of tuberculosis. J Clin Microbiol. 1998;36:1964–1968. doi: 10.1128/jcm.36.7.1964-1968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenach K D, Cave M D, Bates J H, Crawford J T. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis. 1990;161:977–981. doi: 10.1093/infdis/161.5.977. [DOI] [PubMed] [Google Scholar]
- 10.Friedman C R, Stoeckle M Y, Johnston W D, Riley L W. Double-repetitive-element PCR method for subtyping mycobacteria tuberculosis clinical isolates. J Clin Microbiol. 1995;33:1382–1384. doi: 10.1128/jcm.33.5.1383-1384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higuchi R, Dollinger G, Walsh S, Griffith R. Simultaneous amplification and detection of specific-DNA sequences. Bio/Technology. 1992;10:413–417. doi: 10.1038/nbt0492-413. [DOI] [PubMed] [Google Scholar]
- 12.Isaksson A, Landegren U. Accessing genomic information: alternatives to PCR. Curr Opin Biotechnol. 1999;10:11–15. doi: 10.1016/s0958-1669(99)80003-2. [DOI] [PubMed] [Google Scholar]
- 13.Kearns A M, Freeman R, Steward M, Magee J G. A. rapid polymerase chain reaction technique for detecting M. tuberculosis in a variety of clinical specimens. J Clin Pathol. 1998;51:922–924. doi: 10.1136/jcp.51.12.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirihara J M, Hillier S L, Coyle M B. Improved detection times for Mycobacterium avium and Mycobacterium tuberculosis with the BACTEC radiometric system. J Clin Microbiol. 1985;22:841–845. doi: 10.1128/jcm.22.5.841-845.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liébana E, Aranaz A, Mateos A, Vilafranca M, Gomez-Mampaso E, Tercero J C, Alemany J, Suarez G, Domingo M, Dominguez L. Simple and rapid detection of Mycobacterium tuberculosis complex organisms in bovine tissue samples by PCR. J Clin Microbiol. 1995;33:33–36. doi: 10.1128/jcm.33.1.33-36.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangiapan G, Vokurka M, Schouls L, Cadranel J, Lecossier D, van Embden J, Hance A J. Sequence capture-PCR improves detection of mycobacterial DNA in clinical specimens. J Clin Microbiol. 1996;34:1209–1215. doi: 10.1128/jcm.34.5.1209-1215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neill S D. Mycobacterium bovis infection in cattle and its control in developed countries. In: Griffin F, de Lisle G, editors. Tuberculosis in wildlife and domestic animals. Dunedin, New Zealand: University of Otago Press; 1995. pp. 183–186. [Google Scholar]
- 18.Neill S D, Pollock J M, Bryson D B, Hanna J. Pathogenesis of Mycobacterium bovis infection in cattle. Vet Microbiol. 1994;40:41–52. doi: 10.1016/0378-1135(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 19.O'Reilly L M, Daborn C J. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuberc Lung Dis. 1995;76(Suppl. 1):1–46. doi: 10.1016/0962-8479(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 20.Pfyffer G E. Nucleic acid amplification for mycobacterial diagnosis. J Infect. 1999;39:21–26. doi: 10.1016/s0163-4453(99)90097-x. [DOI] [PubMed] [Google Scholar]
- 21.Ririe K M, Rasmussen R P, Wittwer C T. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem. 1997;245:154–160. doi: 10.1006/abio.1996.9916. [DOI] [PubMed] [Google Scholar]
- 22.Roring S, Hughes M S, Beck L-A, Skuce R A, Neill S D. Rapid diagnosis and strain differentiation of Mycobacterium bovis in radiometric culture by spoligotyping. J Clin Microbiol. 1998;61:71–80. doi: 10.1016/s0378-1135(98)00167-9. [DOI] [PubMed] [Google Scholar]
- 23.Roring S, Hughes M S, Skuce R A, Neill S D. Simultaneous detection and strain differentiation of Mycobacterium bovis directly from bovine tissue specimens by spoligotyping. Vet Microbiol. 2000;74:227–236. doi: 10.1016/s0378-1135(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 24.Skuce R A, Brittain D, Hughes M S, Neill S D. Differentiation of Mycobacterium bovis isolates from animals by DNA typing. J Clin Microbiol. 1996;34:2469–2474. doi: 10.1128/jcm.34.10.2469-2474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steven A, Frances R J. Micro-organisms. In: Bancroft J D, Stevens A, editors. Theory and practice of histological techniques. 4th ed. New York, N.Y: Churchill and Livingston; 1996. pp. 291–308. [Google Scholar]
- 26.Strong B E, Kubica G P. Isolation and identification of Mycobacterium tuberculosis, a guide for the level II laboratory. Atlanta, Ga: Centers for Disease Control; 1987. pp. 43–88. [Google Scholar]
- 27.Stürzenbaum S R. Transfer RNA reduces the formation of primer artefacts during quantitative PCR. BioTechniques. 1999;27:50–52. doi: 10.2144/99271bm08. [DOI] [PubMed] [Google Scholar]
- 28.Wards B J, Collins D M, de Lisle G W. Detection of Mycobacterium bovis in tissues by polymerase chain reaction. Vet Microbiol. 1995;43:227–240. doi: 10.1016/0378-1135(94)00096-f. [DOI] [PubMed] [Google Scholar]
- 29.Wittwer C T, Ririe K M, Andrew R V, David D A, Gundry R A, Balis U J. The LightCycler™: a microvolume multisample fluorimeter with rapid temperature control. BioTechniques. 1997;22:176–181. doi: 10.2144/97221pf02. [DOI] [PubMed] [Google Scholar]
- 30.Woo T H S, Patel B K C, Smythe L D, Symonds M L, Norris M A, Weyant R S, Dohnt M F. Identification of Leptospira inadia by continuous monitoring of fluorescence during rapid cycle PCR. Syst Appl Microbiol. 1998;21:89–96. doi: 10.1016/S0723-2020(98)80011-8. [DOI] [PubMed] [Google Scholar]
- 31.Yeager H, Jr, Lacy J, Smith L R, LeMaistre C A. Quantitative studies of mycobacterial populations in sputum and saliva. Am Rev Respir Dis. 1967;95:998–1004. doi: 10.1164/arrd.1967.95.6.998. [DOI] [PubMed] [Google Scholar]