Abstract
Background:
Examine the long-term outcomes among children newly diagnosed with cancer treated on dexrazoxane-containing clinical trials.
Methods:
P9404 (acute lymphoblastic leukemia/lymphoma [ALL]), P9425 and P9426 (Hodgkin lymphoma), P9754 (osteosarcoma), and Dana-Farber Cancer Institute 95–01 (ALL) enrolled 1,308 patients between 1996–2001: 1,066 were randomized (1:1) to doxorubicin±dexrazoxane and 242 (from P9754) were non-randomly assigned to receive dexrazoxane. Trial data were linked with the National Death Index, the Organ Procurement and Transplantation Network, Pediatric Health Information Systems (PHIS), and Medicaid. Osteosarcoma survivors from the Childhood Cancer Survivor Study (CCSS; n=495, no dexrazoxane) served as a comparator in sub-analyses. Follow-up events were assessed using cumulative incidence, Cox regression, and Fine-Gray methods.
Results:
In randomized trials (cumulative prescribed doxorubicin dose 100–360 mg/m2; median follow-up 18.6 years), dexrazoxane was not associated with relapse (hazard ratio [HR] 0.84, 95% CI 0.63–1.13), second cancers (HR 1.19, 95% CI 0.62–2.30), all-cause mortality (HR 1.07, 95% CI 0.78–1.47), or cardiovascular mortality (HR 1.45, 95% CI 0.41–5.16). Among P9754 patients (all DRZ-exposed; cumulative doxorubicin 450–600 mg/m2; median follow-up 16.6–18.4 years), no cardiovascular deaths or heart transplantation occurred. The 20-year heart transplantation rate among CCSS osteosarcoma survivors (mean doxorubicin 377±145 mg/m2) was 1.6% (versus 0% in P9754; p=0.13). Among randomized patients, serious cardiovascular outcomes (cardiomyopathy, ischemic heart disease, stroke) ascertained by PHIS/Medicaid occurred less commonly with dexrazoxane (5.6%) versus without (17.6%; p=0.02), although cardiomyopathy rates alone did not differ (4.4% versus 8.1%; p=0.35).
Conclusions:
Dexrazoxane did not appear to adversely impact long-term mortality, event-free survival, or second cancer risk.
Keywords: adolescent, cancer survivors, cardiotoxicity, child, second malignancy, survivorship
Precis:
Extended follow-up from pediatric dexrazoxane-containing trials suggest no adverse impact of dexrazoxane on all-cause mortality or second cancer risk. Dexrazoxane was associated with a potential reduction in serious cardiovascular outcomes.
INTRODUCTION
Anthracycline-related cardiotoxicity has been recognized as a significant contributor to adverse outcomes among childhood cancer patients.1,2 In the acute setting, compromised cardiac function has been independently associated with reduced event-free and overall survival.3 Among long-term survivors, those exposed to higher doses of anthracyclines and radiotherapy affecting the heart, along with other risk factors, may have a >10% chance of developing clinical heart failure by age 50.4,5
Effective strategies to minimize anthracycline-related cardiotoxicity are limited.2,6 Presently, dexrazoxane (DRZ) remains the only Food and Drug Administration (FDA) approved drug for this indication, specifically covering usage among metastatic breast cancer patients who have received 300 mg/m2 of doxorubicin with plans for further doxorubicin doses.7 The FDA granted DRZ orphan drug designation status for preventing cardiomyopathy in pediatric cancer patients based on short- and intermediate-term studies that suggest decreased cardiotoxicity.8,9
We previously showed, with a median follow-up of 12.6 years, that among 1000 patients treated on Children’s Oncology Group (COG) randomized trials of DRZ, that DRZ status was not associated with any adverse impact on overall and event-free survival, nor second cancer mortality.10 However, given continued uncertainty regarding long-term efficacy and side effects from DRZ, we present updated long-term mortality and second cancer data with median follow-up of nearly 20 years, enhanced with new data on heart transplantation and other cardiovascular morbidity among pediatric cancer patients enrolled on DRZ-containing clinical trials.
METHODS
Patients and Clinical Trial Data
Eligible patients for this analysis were treated on COG trials P9404 (acute lymphoblastic leukemia/lymphoma [ALL]),11 P9425 and P9426 (Hodgkin lymphoma),12,13 and P9754 (localized osteosarcoma),14 plus Dana-Farber Cancer Institute (DFCI) ALL Consortium’s 95–01 trial (high-risk arm; Table 1).15 Cumulative prescribed doxorubicin doses ranged from 100 to 600 mg/m2 across these trials. No other anthracyclines were given as upfront treatment. Except for P9754, where all patients were assigned DRZ, the other four trials featured 1:1 randomization to doxorubicin with or without DRZ. In all trials, DRZ was prescribed as an intravenous bolus before each doxorubicin dose in a DRZ:doxorubicin dose ratio of 10:1.
TABLE 1.
Overview of the analytic study population
| Trial | Histology | Therapy tested | Cumulative prescribed doxorubicin dose, mg/m2 | DRZ statusa | Cumulative prescribed etoposide dose, mg/m2 | No. eligible enrolled overall | No. US residentsb | No. Treated at PHIS sitec |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| DFCI 95–01 high risk arm | Pre-B/T-cell ALL | DFCI 87–01 treatment backbone, addition of high-dose methotrexate, E. Coli vs Erwinia Asparaginase; cranial RT 18 Gy | 300 | 1:1 random-ization | 0 | 58d | 58 | 58 |
| P9404 | T-cell ALL | DFCI 87–01 treatment backbone, ±high-dose methotrexate; cranial RT 18 Gy | 360 | 1:1 random-ization | 0 | 537 | 489 | 163 |
| P9425 | Intermediate/advanced risk Hodgkin lymphoma | Response-adapted, 3–5 cycles DBVE +PC; involved region RT 21 Gy | 180–300 | 1:1 random-ization | 1125–1875 | 216 | 183 | 51 |
| P9426 | Low risk Hodgkin lymphoma | Response-adapted, 2–4 cycles DBVE; involved field RT 25.5 Gy | 100–200 | 1:1 random-ization | 1000–2000 | 255 | 230 | 79 |
| P9754 | Localized osteosarcoma | MAP or MAP/ifosfamide, with either doxorubicin or ifosfamide/etoposide intensification if standard response | 450–600 | All received DRZ | 0 –1500 | 242e | 217 | 90 |
| CCSS | Osteosarcomaf | N/A | 40–1170g | No DRZ | N/A | 495 | 495 | N/A |
ALL, acute lymphoblastic leukemia/lymphoma; CCSS, Childhood Cancer Survivor Study; DBVE, doxorubicin, bleomycin, vincristine, etoposide; DFCI, Dana-Farber Cancer Institute; DRZ, dexrazoxane; Gy, Gray; MAP, methotrexate, doxorubicin, cisplatin; N/A, not applicable; PC, prednisone, cyclophosphamide; PHIS, Pediatric Health Information System; RT, radiotherapy
In all trials, DRZ was given as an intravenous bolus before each doxorubicin dose in a DRZ:doxorubicin dose ratio of 10:1
Only these individuals were linked with the National Death Index (through December 2017), Organ Procurement and Transplantation Network (through March 2019), and Medicaid claims (through December 2010)
Data available through December 2018
Due to regulatory reasons, only patients directly enrolled at DFCI (and not at consortium sites) were available for this analysis; the overall trial enrolled 219 patients on the high-risk arm
If limited to 5-year survivors, n=144
Initial disease stage information not collected, but cohort is limited to ≥5-year survivors by design
CCSS doses are received doses, with median 379 mg/m2 (interquartile range 288–455)
Overall, 1,308 patients were enrolled between 1996–2001 across 167 institutions (n=1172 from 154 US sites). All five trials ended accrual by 2001. Data availability for P9404, P9425, and P9426 were as of June 2013, while P9754 data were as of June 2016, and 95–01 data were as of October 2018. Due to regulatory reasons, 95–01 patients available for this analysis were limited to those enrolled directly at DFCI. Clinical trial data included original cancer relapse and progression status, any new cancers, all-cause and cause-specific mortality (e.g., death from the original cancer, second cancer, or other causes including cardiovascular). The institutional review boards at the National Institutes of Health and participating COG sites approved the study procedures. All patients/guardians consented to participate in the original clinical trials.
National Death Index (NDI)
COG and DFCI data from US sites (name, birthdate, sex, race, state of residence, last follow-up date, and known death date [if applicable]) were linked to the NDI to determine all-cause and cause-specific mortality through December 2017. Because unique identifiers were not available, NDI performed probabilistic matching per their standard protocol.16,17 NDI provided information on an underlying cause of death and up to 20 contributing causes, coded using the International Classification of Diseases (ICD), version 9 (before 1999) or version 10 (1999-onwards).
COG/DFCI and NDI data were combined to form 6 cause-of-death categories: original tumor (with/without history of relapse), second cancer, cardiovascular, other treatment-related toxicity, other cause not elsewhere classified, and unknown. Three investigators (EC, CS, LV) blinded to DRZ status reviewed death records and determined the probable cause of death by consensus. When COG/DFCI and NDI data were discordant, preference was typically given to COG/DFCI data. For each death, a primary cause was assigned, and if relevant, secondary and tertiary causes also were assigned.
Solid Organ Transplantation
Similar identifiers from COG and DFCI were also provided to the United Network for Organ Sharing (UNOS), which conducted probabilistic linkage with Organ Procurement and Transplantation Network (OPTN) data, available as of March 2019. The OPTN collects data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by members of the Network beginning in 1987. Two investigators (DD, EC), blinded to DRZ status, reviewed all potential matches (n=11) and accepted four as true.
Other Data Sources for Health Outcomes
Clinical trial data also were linked with the Pediatric Health Information System (PHIS), which includes administrative billing data for inpatient, ambulatory surgery, emergency department, and observation unit encounters for 50 children’s hospitals. Specifically, trial patients from 33 PHIS hospitals were probabilistically linked on treating hospital, birthdate, sex, and cancer diagnosis/treatment start date from 1998 to 2018.18 Linked patients were further verified by death date (if applicable). Inpatient and ambulatory claims data from the Centers for Medicare and Medicaid Services (CMS; i.e., 2001–2010 CMS Medicaid Analytic eXtract (MAX) files) were linked with clinical trial data to identify patients in a similar manner.
Based on ICD-9/10 diagnosis and procedure codes and unique patient identification numbers available in both PHIS and MAX, we defined a priori the following hard cardiovascular endpoints (i.e., cardiomyopathy, ischemic heart disease, and stroke), along with valvular disease, other vascular outcomes, arrhythmia, and other contributing cardiovascular risk factors (i.e., hypertension, dyslipidemia, diabetes, and kidney disease) for each linked patient (Supplemental Table 1). Codes for acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) also were ascertained a priori.
Osteosarcoma Comparison Group
As P9754 did not have a DRZ unexposed comparison group, anthracycline-exposed osteosarcoma patients from the Childhood Cancer Survivor Study (CCSS) cohort diagnosed between 1970 and 1986 and without DRZ exposure (n=495) were used as comparators. CCSS had completed a linkage with OPTN data from December 2013.19 As CCSS is limited to 5-year survivors, we limited P9754 patients to those linked with OPTN who also survived a minimum of 5-years from diagnosis (n=144) in comparisons with CCSS.
Statistical Analysis
We examined the cumulative incidence (at 15-years follow-up) and hazard ratios (HR) for all-cause mortality, cause-specific mortality, second cancer risk, and relapse by DRZ assignment. For all-cause mortality, we used Kaplan-Meier methods to estimate cumulative incidence and Cox proportional hazards models to estimate the HR. For the other outcomes, we used methods that accounted for competing events (death due to any cause for relapse; death due to other causes for cause-specific mortality).20,21 As NDI data were only available through 2017, we set December 31, 2017 as the date of last follow-up for our mortality analyses; patients enrolled from non-US COG sites (n=136) were censored on their COG date of last contact. COG did not report any instances of relapse nor deaths beyond 2017 as most participants had ceased active follow-up many years earlier. In our analysis of relapse risk, patients were censored at their last COG contact date. For second cancer risk, those who died of a second cancer after their last COG contact date were assigned a date three-quarters of the way between last contact and death. Those without a second cancer were censored at their last COG contact date. For organ transplantation risk, March 31, 2019 was the date of last follow-up. Proportionality of Cox models was assessed via Schoenfeld residuals, and for competing risks models, by testing the interaction of DRZ with analysis time. Gray’s test was used to determine the significance of differences in the cumulative incidence functions for heart transplantation/waitlist between groups.22 Alpha was set at 0.05 and all tests were two-tailed. Data were analyzed with R (R Foundation for Statistical Computing), SAS (version 9.3, SAS Institute), and Stata (version 16, StataCorp).
RESULTS
Among leukemia and lymphoma patients treated on the four DRZ randomized trials (n=1,066), characteristics were similar by DRZ status, with both groups having a median time since cancer diagnosis of 18.6 years (interquartile range [IQR] 16.6–19.9; Table 2). Median follow-up among osteosarcoma patients treated on P9754 (n=242) was shorter at 16.6 years (IQR 4.8–17.4), but these patients were of similar age at last follow-up (median 28.1 years, IQR 20.4–32.2) as leukemia and lymphoma patients (median 28.7 years, IQR 23.0–33.7).
TABLE 2.
Demographic and clinical characteristics of patients treated on dexrazoxane-containing clinical trials
| Characteristics (%) | DRZ-randomized trialsa |
DRZ non-randomized trialb N=242 | |
|---|---|---|---|
| No DRZ-assigned N=524 | DRZ-assigned N=542 | ||
|
| |||
| Female | 163 (31.1) | 172 (31.7) | 103 (42.5) |
| Race/ethnicity | |||
| White, non-Hispanic | 341 (74.0) | 348 (74.8) | 156 (67.0) |
| Black | 90 (19.5) | 77 (16.6) | 40 (17.2) |
| Hispanic | 19 (4.1) | 22 (4.7) | 24 (10.3) |
| Other | 11 (2.4) | 18 (3.9) | 13 (5.6) |
| Unknown | 63 | 77 | 9 |
| Cancer diagnosis | |||
| ALL | 287 (54.8) | 308 (56.8) | - |
| Hodgkin lymphoma | 237 (45.2) | 234 (43.2) | - |
| Osteosarcoma | - | - | 242 (100.0) |
| Median age at diagnosis, years (IQR) | 12.4 (7.5–15.3) | 12.3 (7.5–15.4) | 13.9 (10.9–16.2) |
| Mean doxorubicin dose, mg/m2 (SD) | 281 (94) | 285 (91) | 524 (75) |
| Any cranial radiotherapyc | 287 (54.8) | 308 (56.8) | 0 |
| Any radiotherapy to the heart | 178 (34.0) | 167 (30.8) | 0 |
| Mean prescribed field dose, Gray (SD) | 22.9 (2.4) | 23.0 (2.4) | 0 |
| Median age at last follow-up, years (IQR)d | 29.0 (23.1–33.5) | 28.3 (23.0–34.0) | 28.1 (20.4–32.2) |
ALL, acute lymphoblastic leukemia/lymphoma; DRZ, dexrazoxane; IQR, interquartile range; SD, standard deviation
P9404, P9425, P9426, and DFCI 95–01 (high-risk arm)
P9754, osteosarcoma; all DRZ-assigned
ALL patients on these trials were assigned to receive 18 Gy
Death, last known follow-up date (non-US patients), or December 31, 2017 (US patients)
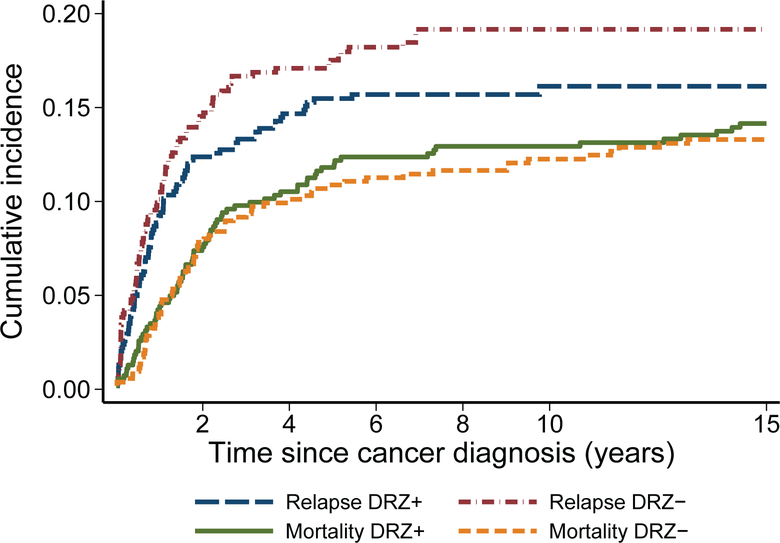
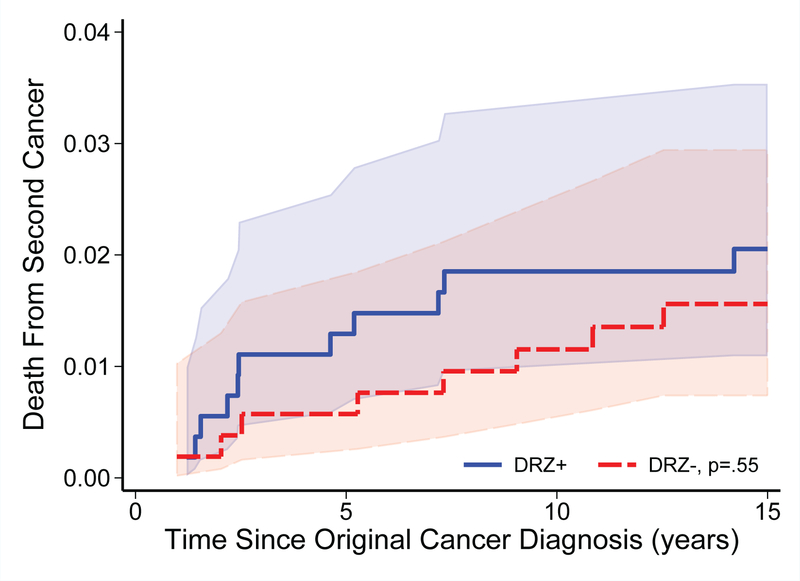
With extended follow-up of leukemia and lymphoma patients, 153 deaths were noted (14.4%) in the four DRZ randomized trials, including 11 deaths identified via the NDI and not previously reported by participating sites. The cumulative relapse and all-cause mortality rates remained similar between DRZ arms, now up to 15 years from cancer diagnosis (Figure 1). There also remained no significant differences in second cancers, second cancer mortality, or other cause-specific mortality risks (Table 3; Figure 2). Among second cancers, AML/MDS was the most common (n=14 of 36 cancers; n=12 of 22 cancer deaths; Supplemental Table 2). The risk of AML/MDS associated with DRZ was HR 1.73 (95 CI% 0.58–5.15), p=0.33. Additional models that adjusted for other topoisomerase inhibitor exposures (i.e., prescribed etoposide and doxorubicin doses) showed similar results (data not shown). There was no clear evidence of statistical interaction between etoposide dose and DRZ (p=0.17). DRZ also was not associated with a differential risk of death related to any cardiovascular cause (n=10 overall; DRZ-assigned, n=6; HR 1.45, 95% CI 0.41–5.16).
FIGURE 1.
Cumulative incidence of relapse and all-cause mortality among patients treated on randomized clinical trials of dexrazoxane (P9404, 9425, 9426, and DFCI 95–01) by dexrazoxane (DRZ) status.
TABLE 3.
Long-term relapse and second cancer risks, causes of death, and solid organ transplantation among patients treated on dexrazoxane-containing clinical trials
| Outcome (%) | DRZ-randomized trialsa |
DRZ non-randomized trialb N=242 | ||
|---|---|---|---|---|
| No DRZ-assigned N=524 | DRZ-assigned N=542 | HR (95% CI)c | ||
|
| ||||
| Relapse/progression | 96 (18.3) | 85 (15.7) | 0.84 (0.63–1.13) | 92 (38.0) |
| Original cancer mortality | 55 (10.5) | 54 (10.0) | 0.95 (0.65–1.38) | 65 (26.9) |
| Second cancer, all | 16 (3.1) | 20 (3.7) | 1.19 (0.62–2.30) | 2 (0.8) |
| Second cancer mortality | 10 (1.9) | 12 (2.2) | 1.17 (0.51–2.70) | 1 (0.4) |
| All-cause mortality | 73 (13.9) | 80 (14.8) | 1.07 (0.78–1.47) | 69 (28.5) |
| Other cause-specific mortality | ||||
| Cardiovasculard | 1 (0.2) | 1 (0.2) | - | 0 |
| Other toxicity | 3 (0.6) | 8 (1.5) | 2.59 (0.69–9.76) | 1 (0.4) |
| Other specified | 4 (0.8) | 4 (0.7) | 0.96 (0.24–3.85) | 1 (0.4) |
| Unknown | 0 | 1 (0.2) | - | 0 |
| Solid organ transplantation | ||||
| Heart | 1 (0.2) | 0 | - | 0 |
| Other organ | 1 (0.2) | 2 (0.4)e | - | 0 |
DRZ, dexrazoxane
P9404, P9425, P9426, and DFCI 95–01 (high-risk arm)
P9754, localized osteosarcoma; all DRZ-assigned
No DRZ-assigned as referent
If secondary cardiovascular contributing causes of death included, then 4 (no DRZ-assigned) and 6 (DRZ-assigned) patients affected (HR 1.45, 95% CI 0.41–5.16); no patient on P9754 had a primary or secondary cardiovascular cause of death
Includes one waitlisted patient not yet transplanted
FIGURE 2.
Cumulative mortality (with shaded 95% confidence intervals) from second cancers among patients treated on randomized clinical trials of dexrazoxane (P9404, 9425, 9426, and DFCI 95–01) by dexrazoxane (DRZ) status. Numbers at risk for mortality shown in Figure 1.
Among osteosarcoma patients treated on P9754, all assigned to DRZ, 69 deaths were recorded (28.5%), including four not previously known to COG (Table 3). While the 15-year cumulative mortality was 28.7% (95% CI 23.1–34.5), this was all from the original tumor, with only one patient dying of secondary cancer (AML). No cardiovascular-related deaths were recorded, even among the 110 patients who were prescribed a cumulative doxorubicin dose of 600 mg/m2.
When OPTN data were evaluated, among US patients treated on the four randomized leukemia and lymphoma trials, only three patients were found to have received solid organ transplants (one kidney, lung, and heart), 9–13 years after initial cancer diagnosis (Table 3). The heart transplant recipient was not assigned to DRZ. An additional patient was waitlisted for a kidney/pancreas transplant. No US-based osteosarcoma patient treated on P9754 (all DRZ-assigned) received any solid organ transplant or was waitlisted after a median follow-up of 18.4 years (IQR 17.8–18.8) despite mean prescribed doxorubicin dose of 524±75 mg/m2. In contrast, among CCSS osteosarcoma patients (n=495; no DRZ; mean doxorubicin dose 377±145 mg/m2), five received a heart transplant and an additional three were waitlisted after a median follow-up of 32.5 years (IQR 28.7–36.5). Affected CCSS survivors had received a median doxorubicin dose of 509 mg/m2, and all events occurred within 20 years of cancer diagnosis, corresponding to a 20-year cumulative incidence of 1.6% (95% CI 0.8–3.0) versus 0% for P9754 (p=0.13).
Finally, 233 (20.3% of eligible US patients) were linked with PHIS (n=81) and MAX (n=181; n=29 linked to both PHIS and MAX) datasets with a median age 18 years (IQR 14–21) at last follow-up. Compared with those not matched, the matched group was less likely to be White non-Hispanic (48.9% versus 67.0%; p<0.001) and younger at cancer diagnosis (median age 10.8 versus 13.2 years; p<0.001; Supplemental Table 3). Among matched patients, because P9754 osteosarcoma patients were non-randomly assigned to receive DRZ, DRZ-assigned patients were on average older, prescribed higher doxorubicin doses, and were less likely to have received cranial radiation compared with patients not assigned to receive DRZ. However, among PHIS/MAX-matched patients on randomized DRZ trials, demographic and clinical characteristics did not differ by DRZ status.
Cardiomyopathy rates ascertained by PHIS/MAX were lower among DRZ-assigned patients versus those without DRZ (3.8–4.4% versus 8%), although differences were not statistically significant (p=0.20–0.35; Table 4). However, collectively, hard cardiovascular endpoints (cardiomyopathy, ischemic heart disease, stroke) occurred less commonly among randomized DRZ-assigned patients versus patients without DRZ (5.6% versus 17.6%; p=0.02). This difference by DRZ status became more pronounced when osteosarcoma patients non-randomly assigned to DRZ were included (4.4% versus 17.6%; p=0.002). Patients not assigned to DRZ appeared to have more related cardiovascular risk factors present (27.0% versus 12.6–14.4%; p=0.01–0.05). However, there was no difference in the proportion of patients who had secondary AML/MDS by DRZ status (p=0.76 to >0.99). Among PHIS/MAX-matched patients, median observation time was similar by DRZ status (no DRZ: 660 days, IQR 221–2834; DRZ-assigned: 676 days, IQR 295–2833; p=0.77).
TABLE 4.
Health outcomes among patients matched to Pediatric Health Information System and Medicaid data
| Outcome (%) | DRZ-randomized trialsa |
P-value | All DRZ- assigned N=159b | P-valuec | |
|---|---|---|---|---|---|
| No DRZ-assigned N=74 | DRZ-assigned N=90 | ||||
|
| |||||
| Hard cardiovascular endpoints | 13 (17.6) | 5 (5.6) | 0.02 | 7 (4.4) | 0.002 |
| Cardiomyopathy | 6 (8.1) | 4 (4.4) | 0.35 | 6 (3.8) | 0.20 |
| Ischemic heart disease | 3 (4.1) | 0 | 0.09 | 0 | 0.03 |
| Stroke | 7 (9.5) | 1 (1.1) | 0.02 | 1 (0.6) | 0.002 |
| Other endpoints | |||||
| Valve disease | 4 (5.4) | 2 (2.2) | 0.41 | 6 (3.8) | 0.73 |
| Other vascular disease | 1 (1.4) | 3 (3.3) | 0.63 | 3 (1.9) | 1.00 |
| Arrhythmia | 13 (17.6) | 5 (5.6) | 0.02 | 10 (6.3) | 0.01 |
| Related cardiovascular risk factors | 20 (27.0) | 13 (14.4) | 0.05 | 20 (12.6) | 0.01 |
| Hypertension | 12 (16.2) | 10 (11.1) | 0.37 | 15 (9.4) | 0.19 |
| Dyslipidemia | 2 (2.7) | 0 | 0.20 | 1 (0.6) | 0.24 |
| Diabetes | 6 (8.1) | 2 (2.2) | 0.14 | 4 (2.5) | 0.08 |
| Kidney disease | 7 (9.5) | 4 (4.4) | 0.23 | 5 (3.1) | 0.06 |
| Acute myeloid leukemia/myelodysplasia | 4 (5.4) | 7 (7.8) | 0.76 | 8 (5.0) | >0.99 |
DRZ, dexrazoxane
P9404, P9425, P9426, and DFCI 95–01
Same as above but also P9754 where all patients were DRZ-assigned
Comparison between no DRZ-assigned (n=74) versus all DRZ-assigned, including P9754 (n=159); all p-values based on Fisher’s exact test
DISCUSSION
After more than 15 years follow-up, childhood cancer patients treated on clinical trials with or without DRZ had equivalent rates of long-term relapse, second cancers, and all-cause mortality by DRZ status. Among those treated with DRZ, rates of serious heart disease appeared exceedingly low, including no patient known to have required a heart transplant. While the comparison of heart transplantation rates with a historic osteosarcoma group was not statistically significant, no osteosarcoma patient treated with DRZ and upwards of 600 mg/m2 of doxorubicin received or was listed for heart transplantation after 20 years. Linkage with PHIS and Medicaid data included a disproportionate number of racial/ethnic minority survivors who tend to be under-represented in prospective follow-up studies. In that linkage, although cardiomyopathy rates were not significantly different, we observed an overall lower rate of serious cardiovascular disease among DRZ-assigned patients compared with those treated without DRZ.
Meta-analyses featuring primarily adult breast cancer patients treated on DRZ-randomized trials have shown that DRZ is not associated with reduced oncologic efficacy, and is instead associated with a >50% reduction in clinical and subclinical heart failure risk.6,7 Meta-analyses of pediatric patients, including earlier published data from randomized trials in this analysis also support DRZ not interfering with overall and event-free survival.9,10 Short and intermediate-term (i.e., 3–5 years from diagnosis) data from randomized and non-randomized studies also suggest that DRZ may also be cardioprotective in children.9,23,24 This includes follow-up from some of the trials analyzed in this study which showed that children randomized to receive DRZ had less frequent blood markers of cardiomyocyte injury25 and pathologic ventricular remodeling compared with children treated without DRZ.26,27
However, these and other previously published studies were based on <10 years follow-up. Our current analysis with 15–20 years follow-up may represent the most extensive follow-up reported for both pediatric and adult cancer patients treated with DRZ. A population-based study of heart transplant risk in childhood cancer survivors from the United Kingdom found that affected survivors (n=43; <0.1% of children diagnosed with cancer over a 35-year period) had received a median anthracycline dose of 400–450 mg/m2 and all patients were listed for transplant before 20 years.28 Data from the CCSS reported a cumulative incidence of being listed for a heart transplant (n=62 survivors) at 35 years after initial cancer diagnosis of 0.49% (95% CI 0.36–0.62), with a median time to heart transplantation of 17 years.19 While bone cancer (osteosarcoma and Ewing sarcoma) contributed the largest proportion of CCSS cases (24%), 19% of cases were Hodgkin lymphoma survivors, followed by non-Hodgkin lymphoma (16%) and acute lymphoblastic leukemia (13%). Although our analysis’ follow-up duration was shorter than these studies, no DRZ-assigned patient in our analysis experienced heart failure requiring transplantation after a median follow-up of 18 years.
Given that heart transplantation represents the most severe cardiomyopathy cases, the actual proportion of patients (regardless of DRZ status) with cardiomyopathy is greater, although those with subclinical changes may not be ascertained by the data sources available to this study. In our PHIS/MAX linkage, we observed a greater number of more serious cardiovascular outcomes (including cardiomyopathy) among patients without DRZ compared with those assigned to DRZ. However, as DRZ is thought primarily to mitigate risk of anthracycline-related cardiomyopathy, it is not entirely clear why ischemic heart disease and stroke rates differed by DRZ status. Data on the natural history of anthracycline-related cardiomyopathy in childhood cancer survivors are limited.1,2 In the general population, heart failure is a well-established risk factor for subsequent stroke and strongly associated with ischemic heart disease.29,30 Given the small number of events, we could not perform analyses adjusting for related cardiovascular risk factors, which appeared to be more common among patients who did not receive DRZ versus DRZ-assigned patients. It is possible that this imbalance may contribute to the greater number of serious cardiovascular events seen among DRZ-exposed patients. The reliance on administrative data may have also introduced some degree of misclassification, although these should not be differential by DRZ status.
There has also been concern that DRZ, a topoisomerase inhibitor, increases the risk of new cancers among other toxicities.31 Initial reports from P9425 and P9426, both Hodgkin lymphoma trials, suggested a possible increased risk of new cancers, particularly secondary AML/MDS.32 However, with further follow-up and additional deaths attributed to second cancers, we did not observe any significant difference in new cancer risk by DRZ status. Given that secondary AML/MDS typically occur within 10 years of topoisomerase exposure and are associated with high mortality,33 it is unlikely that these findings will shift with further follow-up. We also did not observe a difference in AML/MDS rates in our PHIS/MAX linkages. However, DRZ has been associated with increased myelosuppression, infections, and related complications like typhlitis in some prior Hodgkin lymphoma trials, especially when combined with a more intense treatment backbone.12,13
Our results have some limitations. Patients enrolled in other countries represent approximately 10% of the study population and could not be assessed by our administrative datasets. However, we have no reason to suspect that patients enrolled outside the US would differ in their outcomes, especially on the randomized trials where the primary comparison is by DRZ assignment. While all US patients were eligible for our NDI and OPTN linkages, the PHIS/MAX linkage only ascertained the status of 20% of US patients. However, the likelihood of being linked also should not vary by randomization. Over-representation of racial/ethnic minority patients by the PHIS/MAX linkage is also a strength as that population is often under-represented in follow-up studies, where participation bias may be an issue.34
In summary, extended follow-up from pediatric DRZ-containing trials continue to suggest no adverse impact of DRZ on second cancer risk or all-cause mortality. DRZ was associated with a potential reduction in serious cardiovascular outcomes. Prospective ascertainment of cardiac function in patients treated on these clinical trials is ongoing (COG protocol ALTE11C2, clinicaltrials.gov NCT01790152). Those future results may determine DRZ’s cardioprotective efficacy more definitively among survivors of childhood cancer.
Supplementary Material
Acknowledgements:
The data reported were supplied by UNOS as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the authors and should not be seen as an official policy of or interpretation by the OPTN, the National Institutes of Health, or the US Government. The authors thank the Childhood Cancer Survivor Study for providing data access.
Funding Support:
US National Institutes of Health (P30 CA21765, R01 CA211996, U10 CA098543, U10 CA098413, U10 CA180886, U10 CA180899, U10 CA095861, U24 CA55727, UG1 CA189955), the St. Baldrick’s Foundation, the Leukemia & Lymphoma Society, and the American Lebanese Syrian Associated Charities.
Footnotes
Conflicts of Interest: The authors report no relevant conflicts of interest.
REFERENCES
- 1.Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128: 1927–1995. [DOI] [PubMed] [Google Scholar]
- 2.Armenian SH, Armstrong GT, Aune G, et al. Cardiovascular disease in survivors of childhood cancer: insights into epidemiology, pathophysiology, and prevention. J Clin Oncol. 2018;36: 2135–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Getz KD, Sung L, Ky B, et al. Occurrence of treatment-related cardiotoxicity and its impact on outcomes among children treated in the AAML0531 clinical trial: a report from the Children’s Oncology Group. J Clin Oncol. 2019;37: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow EJ, Chen Y, Kremer LC, et al. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015;33: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Chow EJ, Oeffinger KC, et al. Traditional cardiovascular risk factors and individual prediction of cardiovascular events in childhood cancer survivors. J Natl Cancer Inst. 2020;112: 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2011: CD003917. [DOI] [PubMed] [Google Scholar]
- 7.Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35: 893–911. [DOI] [PubMed] [Google Scholar]
- 8.Orphan drug designations and approvals. US Food and Drug Administration. Available from URL: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=441314 [accessed 09/01/2021].
- 9.Shaikh F, Dupuis LL, Alexander S, Gupta A, Mertens L, Nathan PC. Cardioprotection and second malignant neoplasms associated with dexrazoxane in children receiving anthracycline chemotherapy: a systematic review and meta-analysis. J Natl Cancer Inst. 2016;108. [DOI] [PubMed] [Google Scholar]
- 10.Chow EJ, Asselin BL, Schwartz CL, et al. Late mortality after dexrazoxane treatment: a report from the Children’s Oncology Group. J Clin Oncol. 2015;33: 2639–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asselin BL, Devidas M, Wang C, et al. Effectiveness of high dose methotrexate in T-cell lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a randomized study by the Children’s Oncology Group (POG 9404). Blood. 2011;118: 874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood. 2009;114: 2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tebbi CK, Mendenhall NP, London WB, et al. Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2012;59: 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz CL, Wexler LH, Krailo MD, et al. Intensified chemotherapy with dexrazoxane cardioprotection in newly diagnosed nonmetastatic osteosarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2016;63: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95–01 for children with acute lymphoblastic leukemia. Blood. 2007;109: 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics. National Death Index user’s guide. Hyattsville, MD, 2013. [Google Scholar]
- 17.Rogot E, Sorlie P, Johnson NJ. Probabilistic methods in matching census samples to the National Death Index. J Chronic Dis. 1986;39: 719–734. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Hall M, Fisher BT, et al. Merging Children’s Oncology Group data with an external administrative database using indirect patient identifiers: a report from the Children’s Oncology Group. PLoS One. 2015;10: e0143480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dietz AC, Seidel K, Leisenring WM, et al. Solid organ transplantation after treatment for childhood cancer: a retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2019;20: 1420–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed. New York: Wiley, 2002. [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94: 496–509. [Google Scholar]
- 22.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16: 1141–1154. [Google Scholar]
- 23.Kim H, Kang HJ, Park KD, et al. Risk factor analysis for secondary malignancy in dexrazoxane-treated pediatric cancer patients. Cancer Res Treat. 2019;51: 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Getz KD, Sung L, Alonzo TA, et al. Effect of dexrazoxane on left ventricular systolic function and treatment outcomes in patients with acute myeloid leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2020;38: 2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351: 145–153. [DOI] [PubMed] [Google Scholar]
- 26.Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11: 950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asselin BL, Devidas M, Chen L, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: a report of the Children’s Oncology Group randomized trial Pediatric Oncology Group 9404. J Clin Oncol 2016;34: 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levitt G, Anazodo A, Burch M, Bunch K. Cardiac or cardiopulmonary transplantation in childhood cancer survivors: an increasing need? Eur J Cancer. 2009;45: 3027–3034. [DOI] [PubMed] [Google Scholar]
- 29.Adelborg K, Szepligeti S, Sundboll J, et al. Risk of stroke in patients with heart failure: a population-based 30-year cohort study. Stroke. 2017;48: 1161–1168. [DOI] [PubMed] [Google Scholar]
- 30.Lee DS, Gona P, Albano I, et al. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail. 2011;4: 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasinoff BB, Hellmann K, Herman EH, Ferrans VJ. Chemical, biological and clinical aspects of dexrazoxane and other bisdioxopiperazines. Curr Med Chem. 1998;5: 1–28. [PubMed] [Google Scholar]
- 32.Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol. 2007;25: 493–500. [DOI] [PubMed] [Google Scholar]
- 33.Winer ES. Secondary acute myeloid leukemia: a primary challenge of diagnosis and treatment. Hematol Oncol Clin North Am. 2020;34: 449–463. [DOI] [PubMed] [Google Scholar]
- 34.Bhatia S, Gibson TM, Ness KK, et al. Childhood cancer survivorship research in minority populations: a position paper from the Childhood Cancer Survivor Study. Cancer. 2016;122: 2426–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.