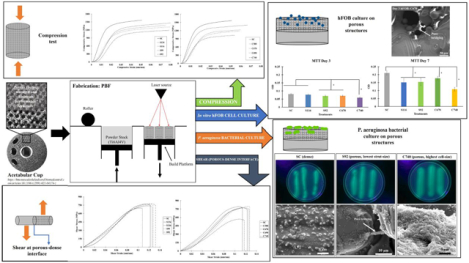
Abstract
Mechanical properties of porous metal coatings in load-bearing implants play a critical role in determining the in vivo lifetime. However, there is a knowledge gap in measuring the shear strength of porous metal coatings at the porous-dense interface. This study evaluated pore morphology dependence and strut-size on compression, shear deformation, and in vitro response of additively manufactured porous Ti6Al4V structures. Selective laser melting (SLM)-based additive manufacturing (AM) technique was used to process two types of structures with honeycomb cell design- one with constant cell-size of ~470 μm with mean strut-size varying from 92 to 134 μm, and denoted as strut-size variation (SSV); and the other with a constant strut-size of ~135 μm with mean cell-size varying from 580 to 740 μm, denoted as cell-size variation (CSV). It was observed that under compressive loading, changes in elastic modulus were more sensitive to variations in strut-size over cell-size. Under shear loading at the porous-dense interface, strength enhancement and material hardening were observed in both SSV and CSV samples due to pore-collapsing. Our results show that for hexagonal cell designs, shear behavior is more sensitive to variations in cell-size over strut-size, although elastic modulus is more sensitive to changes in strut-size for porous metallic structures. From in vitro hFOB analysis, it was observed that pore size of 670 μm demonstrated the highest osteoblast cell viability among porous structures with evidence of pore-bridging by cells. P. aeruginosa bacterial culture showed that bacterial cell viability was higher for porous structures than dense Ti, with evidence of pore-bridging by bacterial cells.
Keywords: Shear strength, Ti6Al4V, Implants, Porous coating, Compressive strength, Pseudomonas aeruginosa
Graphical Abstract
1. Introduction
Surface porosity on metallic implants has been proven to enhance osseointegration in orthopedic and dental applications in vivo [1]. Additive manufacturing (AM) offers the freedom of designing desired pore morphology to achieve the required porosity on implant material in a single operation. The strength of such designed porous structures depends on overall volume fraction porosity, pore-morphology, pore size, and pore-pore connectivity [2], [3]. Since implants are under a multi-axial loading environment in vivo, evaluating porous metal coatings' strength under compressive and shear loading is essential. A weak interface between the porous coating and the dense substrate can be a problem, as evidenced by many coated implants' recalls over the years by implant manufacturers [4], [5]. Studies have been conducted on compression behavior of additively manufactured porous structures, but there is limited understanding related to shear deformation at the dense-porous interface of porous coatings, which is the focus of the current study using additively manufactured porous Ti6Al4V structures as a function of cell-size and strut-size variations.
Titanium and its alloys have found widespread use in the field of load-bearing implants [6]. Due to their excellent biocompatibility, fatigue, corrosion resistance, and high strength to weight ratio combined with low elastic modulus, Ti6Al4V is a popular choice for metallic implants [7]–[9]. Ti6Al4V is extensively used in total hip arthroplasty (THA) and total knee arthroplasty (TKA), along with various dental and spinal implants [10]. Selective laser melting (SLM) and electron beam melting (EBM)-based AM techniques are generally used to print Ti6Al4V implants since these processes offer good print resolution along with ease of printing complex designs [11]. Surface properties also play an essential role in surrounding bone tissue integration and, thus, reduce patient recovery time [12], [13]. Porosities on the surface of an implant promote nutrient exchange and surrounding host-tissue anchoring [14]. Recommended pore sizes for porous metal coatings typically range between 300 and 800μm for bone tissue ingrowth [15]. With the aid of AM, we can control both pore morphology and pore size to achieve such porosity. Titanium, a bio-inert material, does not actively react with surrounding tissue in vivo, leading to implant loosening and scar tissue formation [16]. Researchers are working towards fabricating porous Ti coatings on bulk Ti6Al4V implants by various processes such as diffusion bonding of Ti foams [17], sintered Ti microspheres [18], plasma spray coatings [19], and electrophoretic deposition using TiH2 suspension [20]. Stryker (Mahwah, NJ) has developed a highly porous open 3D surface to be used on the acetabular shell for hip replacement to enhance biological fixation strength to bone, Fig. 1a, marketed by the name 'Tritanium' [21]–[23]. Similar structures can also be found in other commercial biomedical devices, as shown in Fig. 1b–d [24]–[26]. The strength of such porous coatings depends on both pore volume and pore morphology.
Fig. 1.
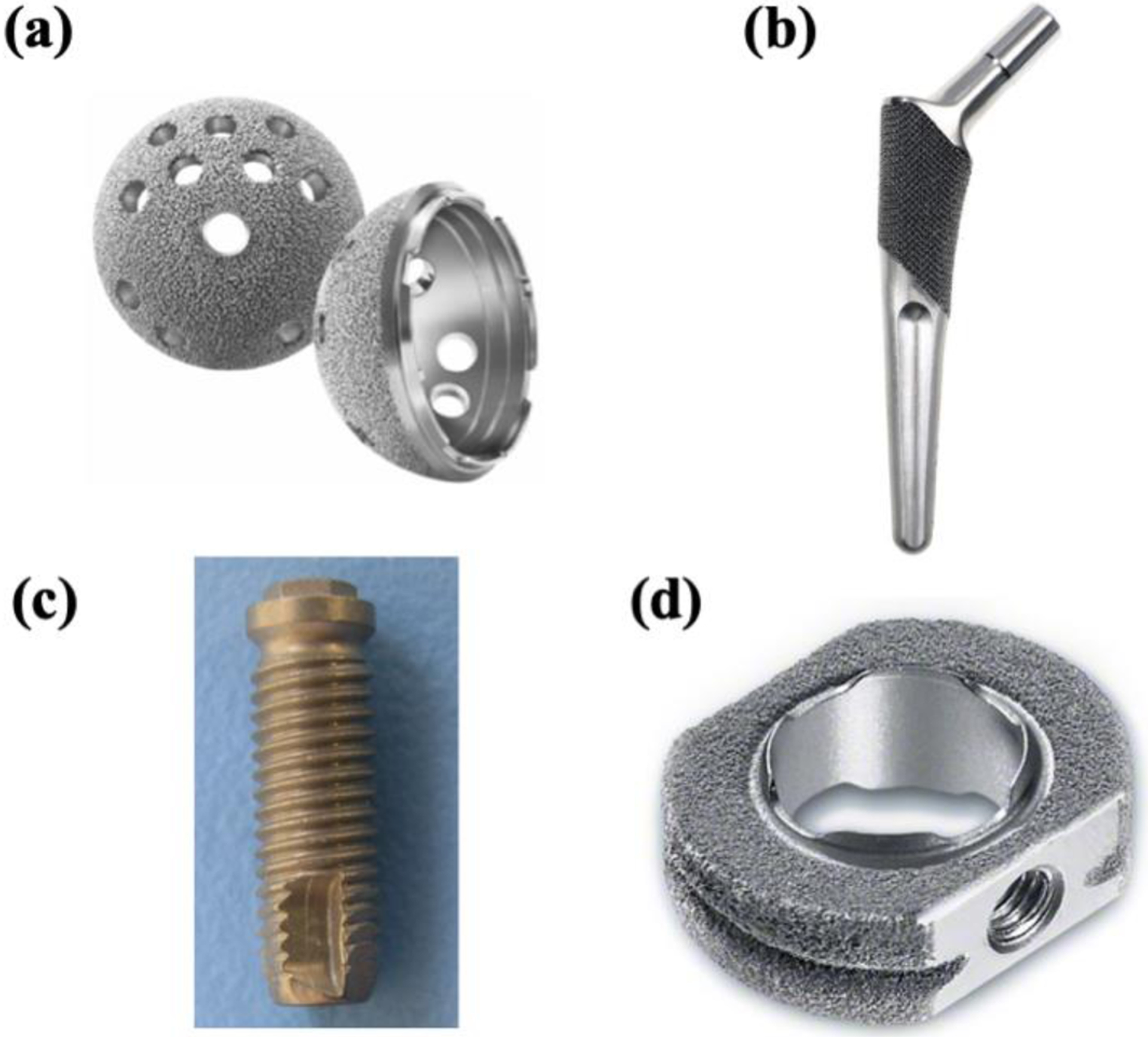
Porous Ti coated biomedical implants (a) Tritanium acetabular shell [21], (b) Hipstem [27], (c) Dental Screw [28], and (d) Spacer for cervical interbody fusion [29].
Researchers have studied the compression behavior of porous structures processed via AM. Balla et al. showed that for directed energy deposition (DED)-based AM of commercially pure titanium (CpTi) porous structures with similar designed and randomly-generated pore-volume where designed porosity showed a higher elastic modulus and higher yield strength [3]. Ahmadi et al. varied the apparent density of SLM printed Ti6Al4V diamond lattice structures from 0.105–0.36 by varying the strut length and diameter ratio and found corresponding elastic modulus ranging between 0.37 to 4.24 GPa, and yield strength between 8.20 to 99.64 MPa [30]. Choy et al. demonstrated compressive properties of SLM printed Ti6Al4V cubic and honeycomb lattice cell porous structures with functionally graded material by varying strut-size from 350 to 1200 μm within a structure and compared against structures with uniform strut-size of 900 to 1000 μm. A gradual layerwise failure was observed in functionally gradient structures while abrupt failure with uniform strut size [31]. Parthasarathy et al. demonstrated interlayer shear evaluation of Ti6Al4V porous designs with a varying designed strut (450 and 800 μm) and cell-sizes (1000–2000 μm) fabricated via EBM. A decrease in shear modulus from 328 MPa to 201 MPa was observed with an increase in resulting porosity from 49.75% to 70.32%, respectively [32]. Bartolomeu et al. demonstrated interlayer shear bond strength evaluation for NiTi, Ti6Al4V, NiTi-Ti6Al4V multi-material porous, cellular structures fabricated via SLM and observed the shear strengths to be 25.5 MPa, 47.1 MPa, and 33.2 MPa, respectively [33]. Although recent studies have shown compressive and shear deformation of AM processed porous metals, there is a knowledge gap of the same under shear deformation, particularly at the porous-dense interface relevant for porous metal coatings. The weakest point on the porous metal-coated load-bearing implant surface, for example, is the porous-dense interface. The performance of such porous metal coatings will depend on both compressive and shear properties since implants' surface is always under a multi-axial loading environment in vivo [19].
Along with its mechanical integrity, evaluating the biological performance of the porous coatings is also essential. As mentioned before, surface porosities play a vital role in enhancing osseointegration by providing anchor sites on the implant surface for surrounding bone-tissue integration. Taniguchi et al. printed porous titanium structures using the SLM technique and observed from an in vivo rabbit study that the structure with a pore size of 632 μm showed better fixation ability and rapid bone ingrowth [34]. Ouyang et al. confirmed that pore size of 650 μm showed the best performance on the proliferation of MC3T3 osteoblast cells and bone regeneration and ingrowth in an in vivo rabbit study for SLM fabricated Ti structures [35].
Bacterial infections caused in implants are another concern. Evaluating the effect of surface morphology on bacterial inhibition is essential. Domínguez-Trujillo et al. investigated the effect of porosity on bacterial growth for Ti structures with E. coli, MRSA, and P. aeruginosa, and observed that the bacterial activity in porous structures was higher in comparison to the dense surface due to increased surface area resulting from pore-walls [36]. On the other hand, nanoscale roughness on the Ti surface is proven to inhibit bacterial viability. Stolzoff et al. showed that rough nano surfaces on Ti surface with RMS roughness values between 29.6–31.6 nm demonstrated antibacterial ability on S. aureus bacterial cells with a reduction in bacterial colony-forming units factor of 10 compared to that of plain Ti used as control treatment [37]. Cao et al. showed that pocket-type TiO2 nanostructures on the Ti surface inhibited S. epidermis bacterial cells [38].
This study's objective was to understand pore morphology's influence on compression and shear deformation of porous structures of Ti6Al4V, as well as osteoblast and bacterial cell viability. We have used SLM- based AM to fabricate structures with different pore morphologies by varying strut- and cell-sizes. We have measured the effects of variation in strut and cell-sizes of porous structures on mechanical properties such as elastic modulus, compressive yield strength, and shear strength. Compressive behavior was observed for completely porous structures, whereas shear tests were carried out at the interface of porous metal-coated dense samples. Fracture surfaces were observed under a scanning electron microscope to understand different failure modes. Porous structures were subjected to in vitro osteoblast and Pseudomonas aeruginosa bacterial culture to evaluate the effect of pore morphologies on osteoblast and bacterial viability, respectively.
2. Materials and methods
2.1. Design strategy: Strut-size variation (SSV) and cell-size variation (CSV)
Samples were designed in 3DXpert CAD Software (3D Systems, Rock Hill, SC). Porous structures were designed with honeycomb as a primary unit cell, as shown in Fig. 2a. As shown in Fig. 2e–f, two groups of samples were fabricated: average strut-size decreasing from 134 to 92 μm with increasing porosity with constant cell-size (~470 μm) denoted as 'Strut-Size Variation (SSV)' group, and average cell-size increasing with increasing porosity from 580 to 740 μm with constant strut-size (~135 μm) denoted as 'Cell-Size Variation (CSV)' group. Each SSV and CSV group had 5 treatments and a control treatment SC, designed as dense. All treatment notations are reported in Table 1. Three types of samples were designed: 3.1 mm diameter and ~11 mm height cylinders with a porous-dense interface for shear strength testing as shown in Fig. 2b, 7mm diameter and ~15mm height porous cylinders for compression testing, as shown in Fig. 2c, and 7mm diameter porous disk with 2.25 mm thickness built on top of the dense disk for in vitro characterization, Fig. 2d. Dense samples, treatment SC, were designed and processed for compression and shear strength testing.
Fig. 2.
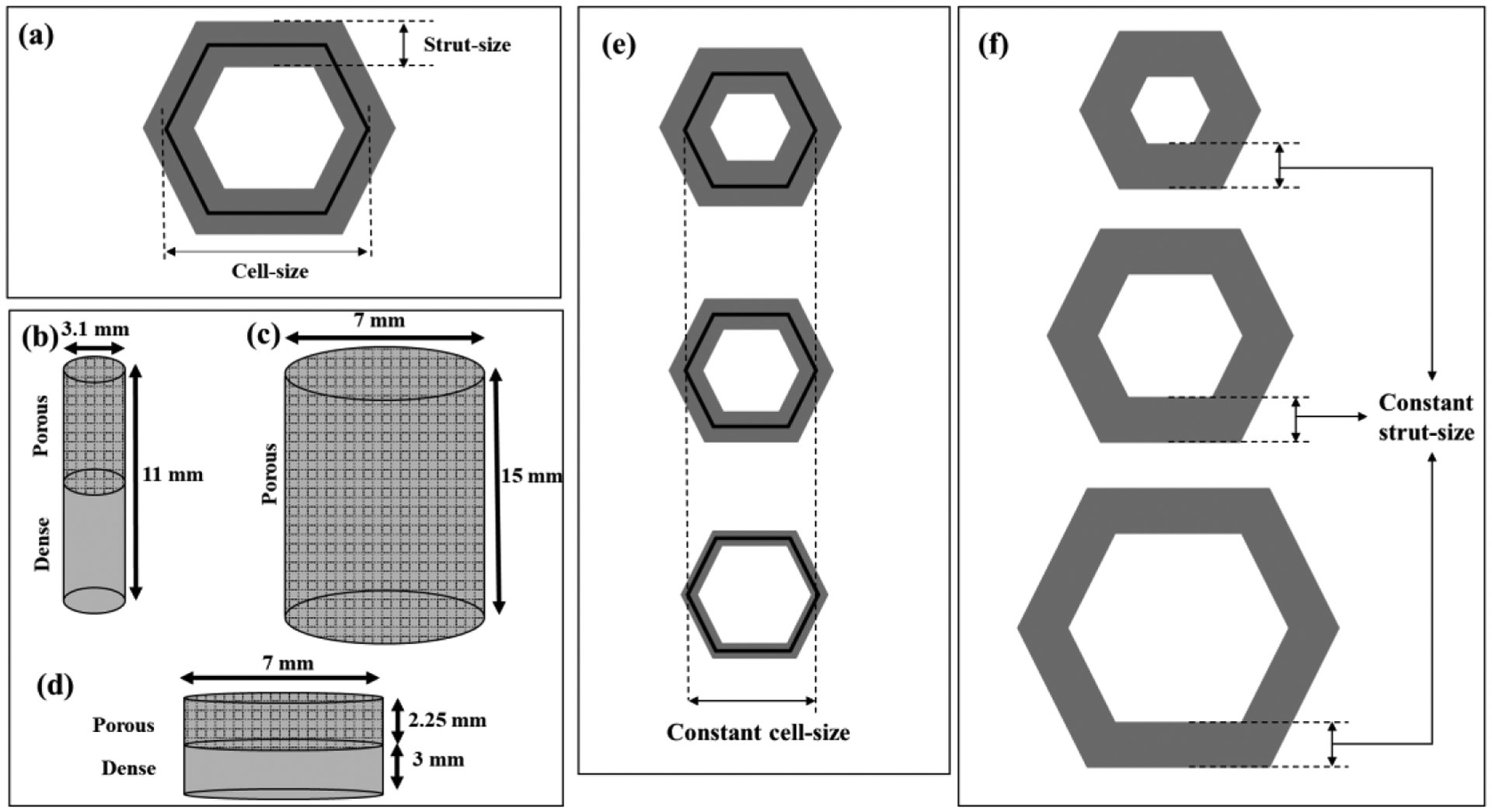
(a) 2D top view of single honeycomb cell showing strut and cell sizes; (b) Sample for the shear test with the porous-dense interface; (c) Porous sample for compression test; (d)Porous disk printed over dense for in vitro test; (e) Variation in strut-size with cell-size constant (SSV), and (f) variation in cell-size with strut-size constant (CSV).
Table 1.
Measurements of strut- and cell-sizes of as-printed samples under an optical microscope and their corresponding measured porosities.
| SSV: Strut-Size Variation | CSV: Cell-Size Variation | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | Strut-size (μm) | Cell-size (μm) | % Measured Porosity | Treatment | Strut-size (μm) | Cell-size (μm) | % Measured Porosity |
| SC | - | - | 3.2 ± 0.3 | SC | - | - | 3.2 ± 0.3 |
| S134 | 134 ± 26 | 500 ± 50 | 6.8 ± 0.4 | C580 | 137 ± 29 | 580 ± 80 | 7.5 ± 0.2 |
| S116 | 116 ± 17 | 450 ± 60 | 8.7 ± 0.2 | C670 | 139 ± 9 | 670 ± 50 | 12.6 ± 0.3 |
| S99 | 99 ± 8 | 460 ± 70 | 11.4 ± 0.2 | C690 | 132 ± 6 | 690 ± 60 | 16.2 ± 0.6 |
| S92 | 92 ± 5 | 480 ± 20 | 14.3 ± 0.2 | C740 | 130 ± 7 | 740 ± 40 | 18.8 ± 0.4 |
2.2. Powder bed system operation and as-printed sample characterization
Samples were built on a powder bed fusion system (3D Systems ProX® DMP 200, Rock Hill, SC) with a 300W fiber laser and wavelength λ=1070 nm. The system has a supply chamber for powder storage, a stage with a build plate from 3D Systems beneath the laser head, and a roller. The wiper beneath the roller carries the powder from the supply, and the roller spreads it evenly onto the build plate, wherein the laser melts areas on the build plate according to the CAD design. With each layer, the supply moves up to supply powder to the roller for the next layer, and the stage moves down according to the desired layer thickness. The print chamber was enclosed in an argon atmosphere with O2 < 500ppm to limit the build structure's oxidation. Ti6Al4V powders used were procured from 3D Systems (LaserForm® Ti6Al4V ELI, Gr.23). Powders were spherical and were sieved to obtain a powder particle size of < 63 μm. The build plate used was a 2.5 cm thick CpTi plate. A laser power of 180 W with a layer thickness of 30 and scan speed of 1600 mm/s was used for all dense and porous structures. The hexagonal cell scan strategy was used to print dense parts, whereas the strip scan strategy was used to print porous parts. The melt-pool owing to laser melting for each strip was ~100 μm, as reported by 3D Systems. The laser power of 180 watts with a layer thickness of 30 μm and a scan speed of 1600 mm/s were used. A sidestep of 70 μm was used to print the samples.
An image of the build plate with samples just after the powder bed operation is shown in Supplemental Fig. S1. Samples were cut from the build plate using a band saw and ground using 120 grit SiC paper to make the opposite faces of the cylinders parallel. Samples were then sonicated in DI water followed by ethanol sonication twice, each for 30 minutes at room temperature, to remove loose powders from inside the pores and clean the outer surfaces. Shear samples with a porous-dense interface and compression samples are shown in Fig. 3b and Fig. 3c, respectively.
Fig. 3.

Images of (a) shear test samples and (b) compression test samples after cutting off the build plate and cleaning for loose powders.
The top part of the as-printed samples was observed under an optical microscope to measure printed strut and cell-size and reported in Table 1. Each sample was tested at 3 distinctly visible regions for strut and cell-size. The strut-size measured was the visible portion of the struťs diameter, whereas the cell-size measured as the distance between the two farthest corners of the hexagon, as shown in Fig. 2a. Nomenclature for the SSV and CSV groups were assigned as follows: for SSV group treatments, the letter 'S' followed by the mean strut-size observed in μm, and similarly, for CSV group treatments, the letter 'C' followed by the mean cell-size observed in μm. For example, S134 represents SSV treatment with a mean strut-size of 134 μm, and C580 represents CSV group treatment with a mean cell-size of 580 μm. Porosity was measured by taking the porous sample's measured volume to its theoretical volume of dense Ti6Al4V. The bulk density of Ti6Al4V was considered to be 4.43 g/cm3. Measured bulk porosity values for all treatments in SSV and CSV groups are presented in Table 1.
2.3. Compressive and shear strength measurements and failure analysis
Samples were subjected to compression testing on Instron servohydraulic testing machine (600DXS, Grove City, Pennsylvania). Samples with a diameter of 7mm and a height of ~15 mm were tested, according to ASTM E9–19. A crosshead displacement rate of 1.3mm/min was used for all samples, and corresponding stress and strain values were recorded. Each treatment had at least three replicates tested. Samples were observed up to a 0.05–0.08 mm/mm strain. Elastic modulus was calculated from the linear region's slope, and the compressive yield strength was calculated using a 0.2% strain offset method.
Samples with 3.1 mm diameter and 12 mm height were subjected to shear strength test at the interface of the dense-porous region using a single-shear test device shown in Supplemental Fig. S2 and Fig. S3 developed in our lab as per the procedure described in references 39 and 40 [39], [40]. The nature of the shear test carried out is tensile shear rather than torsional shear. A porous-dense interface was positioned at the center of the shear device. The test was carried out on Instron servohydraulic testing machine (600DXS, Grove City, PA) in tension mode using an external 1360 kg load cell to measure the load and an extensometer to precisely measure the strain. A crosshead displacement rate of 0.3 mm/min was used for all samples. Each treatment had at least three replicates tested. Samples were sheared until fracture, and the corresponding load vs. extension data was recorded. The linear region slope for normalized stress-strain data was used for the 'shear modulus' measurement. Shear yield strength was calculated from normalized stress-strain data using a 0.2% strain offset method. Maximum shear strength was evaluated as maximum strength from normalized stress-strain data. Fracture surfaces from shear tested samples were observed under Scanning Electron Microscope (SEM, Apreo, Thermo Scientific, MA, USA) was used to image at a porous-dense interface for both the porous and the dense part of each fractured sample. No etching was done on fracture surfaces for microstructural imaging.
2.4. In vitro hFOB study
Samples were subjected to an in vitro analysis to study the effect of pore morphology on bone-cell materials interaction with human fetal osteoblast (hFOB) (American Type Culture Collection (ATCC), Manassas, VA) cells for 3 and 7 days of culture. The sample schematic used for this study is shown in Fig. 2d. The intermediate and highest porosity treatments from each SSV and CSV group, i.e., S116, S92, and C670, C740, respectively, were used for the in vitro analysis, along with the dense control treatment SC. Samples were sterilized by autoclaving at 121°C for 60 min. Cells were seeded onto the top surface of the as-printed samples (initial seeding density: 20,000/well) in 24 well-plates. Ham's F12 medium and Dulbecco's Modified Eagle's Medium (DMEM/F12, Sigma, St. Louis, MO) mixture along with 2.5mM L-glutamine was used as the nutrient growth medium and was mixed with 10% fetal bovine serum (ATCC, Manassas, VA) along with 0.3 mg/ml G418 sulfate salt (Sigma Aldrich). 2 ml of this media was used per well-plate, and replaced with fresh media every 2 days throughout the experiment. The well-plates were incubated at 34°C under a 5% CO2 environment in an incubator.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay was performed to evaluate cell viability. A 5mg/ml solution of MTT (Sigma Aldrich) salt in phosphate buffer saline (PBS) was prepared and sterilized through a 0.2 μm pore filter paper using a filter sterilization unit. Each treatment had 3 replicate samples, and triplicate measurements from each sample were assessed for MTT assay. A fixative solution consisting of 2% paraformaldehyde / 2% glutaraldehyde added to 0.1M phosphate buffer was used to fix the samples for SEM. 2 ml of this fixative solution was added to each well and maintained at 4°C overnight. Two replicates for each treatment were used. The samples were then rinsed thrice with a phosphate buffer to eliminate any residue of the fixative solution. Following this, 2% osmium tetraoxide (OsO4) was added to the samples and left at room temperature for 2 hours. The samples were thrice rinsed in ddH2O and subjected to serial dehydration using ethanol (30%, 50%, 70%, 90%, and thrice with 100%) followed by critical drying using hexadimethyldisilazane (HMDS). The samples were gold coated, and their surface was imaged in a Scanning Electron Microscope (SEM, Apreo, Thermo Scientific, MA, USA) for the surface morphology of the cells.
2.5. P. aeruginosa bacterial culture
Freeze-dried P. aeruginosa (Carolina Biological, NC) was rehydrated using rehydration media followed by subsequent dilutions for 0.5McFarland standard OD measurement. Calculations were made from the OD of the subsequent dilution. Sample schematics used for bacterial culture were similar to those used for hFOB in vitro cell culture (Fig. 2d). The highest strut-size from SSV and highest cell-size from CSV, i.e., S92 and C740, respectively, were used as treatments, and SC was used as the control treatment. Each treatment had 3 replicates for bacterial colony count and 2 for SEM characterization. P. aeruginosa was seeded on the as-printed surface of the autoclave sterilized samples at an initial count of 106 CFU/ml. After 36 hrs, the bacterial colony count samples were ultrasonicated for 30 mins and vortexed for 10s in 0.1M PBS, and 1μl of the respective solutions were streaked on cetrimide agar plate (Pseudosel agar, Fisher Scientific, NH). After 24 hrs of incubation, bacterial colonies on the agar plates were counted. SEM characterization samples were preserved in 2 ml of fixative solution and dehydrated as described in section 2.4 after 36 hrs of culture and observed under a Scanning Electron Microscope (SEM, Apreo, Thermo Scientific, MA, USA) for the surface morphology of cells.
2.6. Statistical analysis
All values reported in this paper are represented in mean ± standard deviations as error bars. Values for SSV and CSV groups were separately subjected to a statistical ANOVA performed on JMP®, Version14 (SAS Institute Inc., Cary, NC, 1989–2019) for treatments. Tukey-Kramer correction simulation was used for pairwise comparison of means among treatments with α = 0.05, and a P value less than α was marked significant. ANOVA tables for all treatments and tests and corresponding P value comparison tables for Tukey-Kramer simulations have been provided in Table S1–14.
3. Results
3.1. Characterization of pore morphologies
Measured strut and cell-size values and corresponding measured bulk porosities are reported in Table 1. Optical images and strut-/cell-size measurements for S92 and C740 are shown in Supplemental Fig. S4. For SSV treatments S134, S116, S99, and S92, cell-sizes were observed to be similar, between 450–500 μm, and mean strut-sizes were 134 μm (S134), 116 μm (S116), 99 μm (S99), and 92 μm (S92). Similarly, for CSV treatments C580, C670, C690, and C740, strut-size was similar, between 130–139 μm, and mean cell-sizes were 580 μm (C580), 670 μm (C670), 690 μm (C690), and 740 μm (C740). Dense, i.e., SC, had a residual bulk porosity of 3.18%. For the SSV, a decrease in average strut-size from S134 to S92 increased bulk porosity from 6.8% to 14.3%. Similarly, an increase in average cell-size from C580 to C740 increased bulk porosity from 7.5% to 18.8% for the CSV.
3.2. Compressive deformation behavior of porous samples
Compression stress-strain plots from raw data for SSV and CSV treatments are presented in supplemental data, Fig. S5. Compressive elastic modulus values for SSV and CSV groups are plotted in Fig. 4. The elastic modulus for dense SC was observed to be 109.7 GPa. For the SSV, elastic modulus decreased with a decrease in strut-size, while elastic modulus decreased with increasing cell-size for the CSV. From dense SC to porous S134 the elastic modulus reduced from 109.7 to 72.1 GPa, i.e., a 34.3% reduction for a porosity difference of 3.6%. For C580 with an elastic modulus of 87.3 GPa, this reduction was observed to be 20.41% for a porosity difference of 4.3%. With further reduction in strut-size, elastic modulus values for S116, S99, and S92 were observed to be 66.4 GPa, 61.5 GPa, and 50.9 GPa, respectively. For the CSV group, with further increase in cell-size, elastic modulus values for C670, C690, and C740 were 67.8 GPa, 63.2 GPa, and 65.2 GPa, respectively. A pairwise comparison of treatment means for elastic modulus was done separately for SSV and CSV groups. Significantly different means with P-value < 0.05 are represented in Fig. 4 with an '*', and ANOVA tables and corresponding P values for pairwise comparisons are shown in the supplemental data, Tables S1–2, respectively. Treatment means under the same bracket are statistically similar.
Fig. 4.
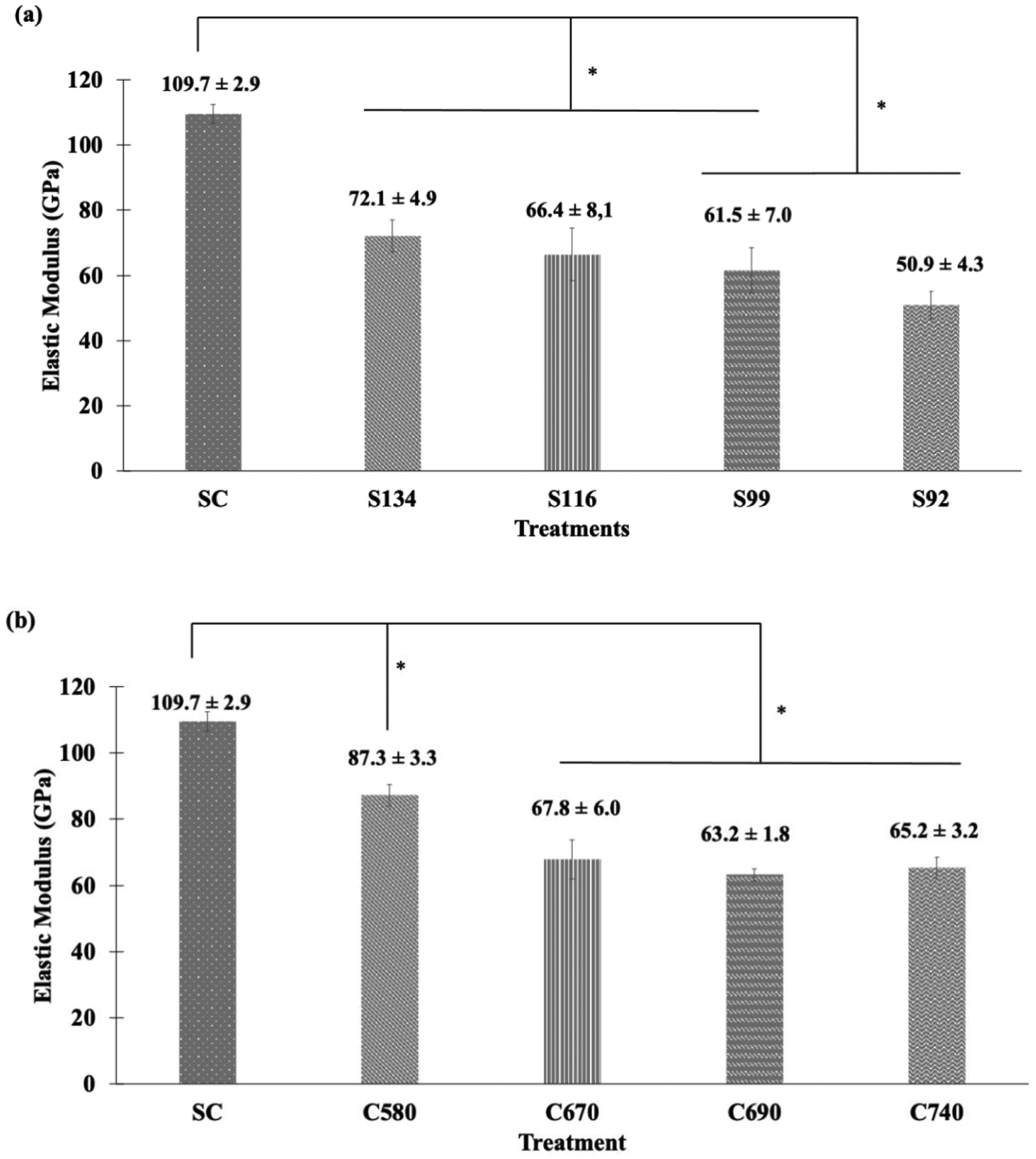
Modulus of Elasticity for (a) SSV and (b) CSV groups; the boxed area in (a) indicates a sharp decrease in modulus values. Statistical analyses were done using one-way ANOVA for n=3 and α = 0.05. Pairwise comparison of means among treatments with P-value < 0.05 has been considered significantly different and marked with an '*'. Treatments under the same bracket are not significantly different.
For the SSV, elastic modulus values for SC were significantly different from S134, S116, S99, and S92. Also, the elastic modulus of S134 and S116 was different from that for S92. For the CSV, SC's elastic modulus was significantly different from C580, C670, C690, and C740. Also, the elastic modulus for C580 was significantly different from C670, C690, and C740. Comparing S134 and C580, the modulus of elasticity for C580, 72.1 GPa, was higher than that of S134, 87.3 GPa, despite C580 having higher porosity of 7.5% than S134 with a porosity of 6.8%. Comparing S99 and C670, the modulus of elasticity value of 67.8 GPa (C670) was comparable to 61.5 GPa (S99), despite C670 having higher porosity of 12.6% than S99 with a porosity of 11.4%. Comparing S92, C690, and C740, the elastic modulus of 50.9 GPa (S92) was lower than 63.2 GPa (C690) and 65.2 GPa (C740) despite S92 having lower porosity.
Compressive yield strengths for SSV and CSV groups are plotted in Fig. 5. Compressive yield strength for dense SC was observed to be 1103 MPa. For the SSV group, compressive yield strength decreased with decreasing strut-size from 1064 MPa for S134 to 910 MPa for S92. Compressive yield strength for the CSV group decreased with increased cell size from 1024 MPa for C580 to 774 MPa for C740. A pairwise comparison of treatment means for compressive yield strength was done separately for SSV and CSV groups. Significantly different means with P-value < 0.05 are represented in Fig. 5 with an '*', and the ANOVA table and corresponding P values for pairwise comparisons are shown in the supplemental data, Tables S3–4. Treatment means under the same bracket are not significantly different. For the SSV group, compressive yield strength for SC was significantly different from S116, S99, and S92. Also, compressive yield strength for S134 was significantly different from that for S99 and S92. For the CSV group, compressive yield strength for all treatments was significantly different from each other. Comparing SSV and CSV groups, it was observed that compressive yield strength decreased with increasing porosity irrespective of the design strategy.
Fig. 5.
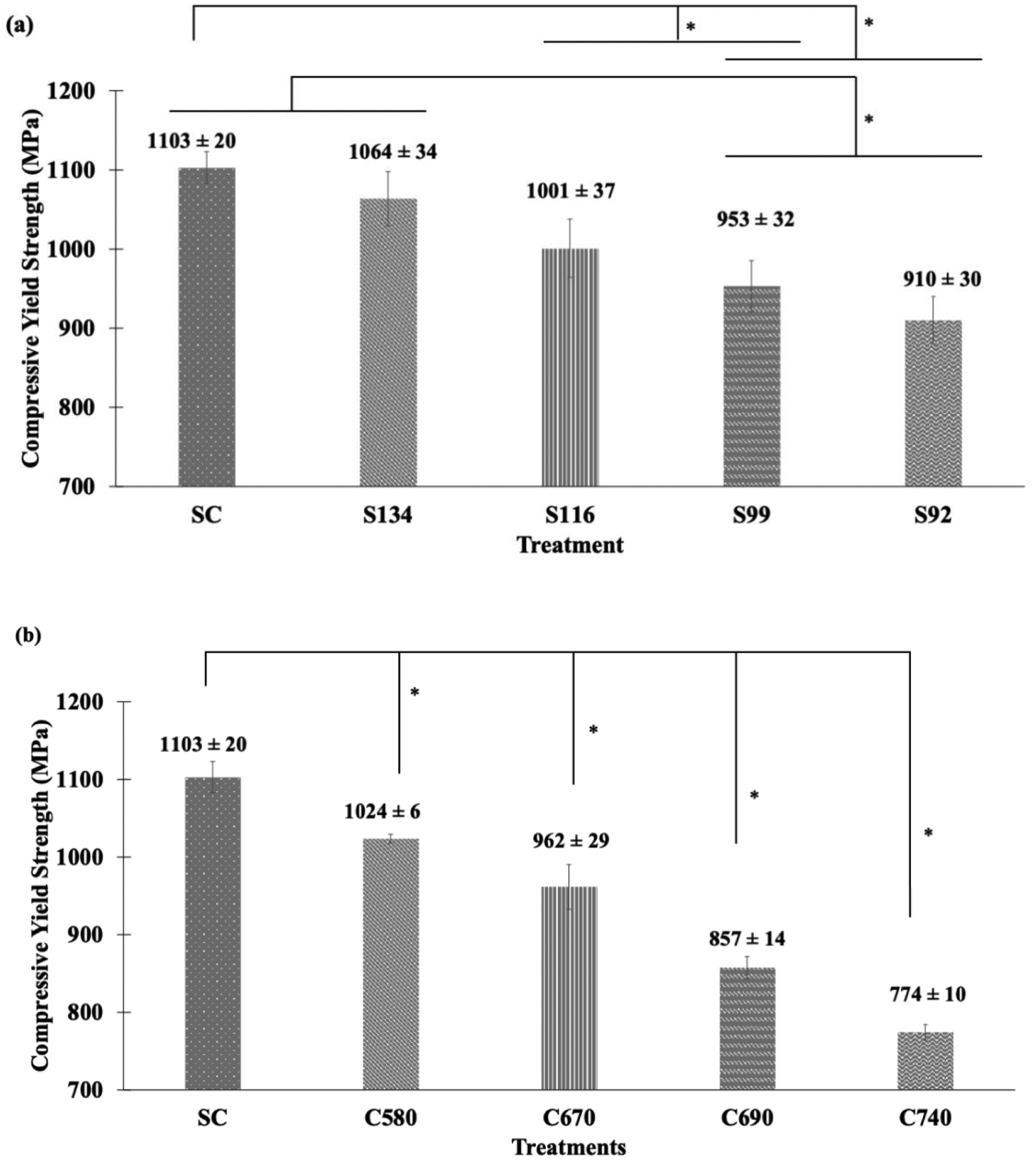
Compressive yield strength for (a) SSV and (b) CSV porous structures. Statistical analyses were done using one-way ANOVA for n=3 and α = 0.05. Pairwise comparison of means among treatments with P-value < 0.05 has been considered significantly different and marked with an '*'. Treatments under the same bracket are not significantly different.
3.3. Shear deformation behavior of porous samples
Shear stress-strain plots from raw data for SSV and CSV are shown in the supplemental data, Fig. S6. Shear modulus values for SSV and CSV groups are plotted in Fig. 6. Shear modulus for SC was observed to be 9.61 GPa. For the SSV group, shear modulus decreased with a reduction in strut-size and ranged from 9.06 GPa for S134 to 6.92 GPa for S92. The shear modulus for the CSV decreased with cell size and ranged from 7.83 GPa for C580 to 6.54 GPa for C740. A pairwise comparison of treatment means for shear modulus was done separately for SSV and CSV groups. Significantly different means with P-value < 0.05 are represented in Fig. 6 with an '*'. The ANOVA table and corresponding P values for pairwise comparisons are shown in the supplemental data, Tables S5–6, respectively. Treatment means under the same bracket are statistically similar. For the SSV group, the shear modulus for SC was significantly different from S116, S99, and S92. Also, the shear modulus for S134 was significantly different from S99 and S92. For the CSV group, the shear modulus for SC was significantly different from C580, C670, C690, and C740. Moreover, the shear modulus for C580 was significantly different from C690 and C740. Comparing the shear modulus of SSV and CSV groups, the shear modulus decreased with increasing porosity irrespective of the design strategy.
Fig. 6.

Shear Modulus for (a) SSV and (b) CSV treatments. Statistical analyses were done using one-way ANOVA for n=3 and α = 0.05. Pairwise comparison of means among treatments with P-value < 0.05 has been considered significantly different and marked with an '*'. Treatments under the same bracket are not significantly different.
Shear yield strengths for SSV and CSV are plotted in Fig. 7. Shear yield strength for SC was 678 Mpa, and for S134 and S116 were 664 MPa and 658 MPa, respectively. With a further decrease in strut-size, an increase in shear yield strength was observed for S99 and S92 with 725 MPa and 743 MPa, respectively. For the CSV group, shear yield strength increased for C580 (763 MPa) compared to dense SC. With a further increase in cell-size, for C670, shear yield strength was 779 MPa. Shear yield strength decreased with an increase in cell-size, from 739 MPa (C690) to 630 MPa (C740). A pairwise comparison of treatment means for shear yield strength was done separately for SSV and CSV groups. Significantly different means with P-value < 0.05 are represented in Fig. 7 with an '*', and ANOVA table corresponding P values for pairwise comparisons are shown in the supplemental data, Table S7–8, respectively. For the SSV group, shear yield strength for all treatments was found to be statistically similar. For the CSV group, the shear yield strength for SC was different from C670. Also, the shear yield strengths for C580, C670, and C690 were different from C740.
Fig. 7.

Shear yield strength for (a) SSV and (b) CSV treatments, boxed areas show an unusual change in values. Statistical analyses were done using one-way ANOVA for n=3 and α = 0.05. Pairwise comparison of means among treatments with P-value < 0.05 has been considered significantly different and marked with an '*'. No statistically significant difference was observed among the mean values of treatments in (a), and treatments under the same bracket are not significantly different.
Maximum shear strength values for SSV and CSV are plotted in Fig. 8. The maximum shear strength for SC was 741 MPa. Within the SSV, a decrease in strut size from S134 to S116 resulted in a decrease in maximum shear strength from 726 MPa to 712 MPa. However, with a further decrease in strut-size for S99 and S92, maximum shear strength increased to 748 MPa and 752 MPa, respectively. These strength values were comparable to that of SC control treatment. In the CSV group, this maximum shear strength enhancement for SC was observed for C580, 772 MPa for C580, and it further increased to 790 MPa for C670. For C690, the maximum shear strength was 762 MPa, and it dropped to 634 MPa for C740. A pairwise comparison of treatment means for maximum shear strength was done separately for SSV and CSV groups. Significantly different means with P-value < 0.05 are represented in Fig. 8 with an '*'. The ANOVA table and corresponding P values for pairwise comparisons are shown in the supplemental data, Tables S9–10, respectively. For the SSV group, maximum shear strength for all treatments was found to be statistically similar. For the CSV group, maximum shear strength C740 was significantly different from all other treatments.
Fig. 8.

Maximum shear strength for (a) SSV and (b) CSV treatments, boxed areas show an unusual change in values. Statistical analyses were done using one-way ANOVA for n=3 and α = 0.05. Pairwise comparison of means among treatments with P-value < 0.05 has been considered significantly different and marked with an '*'. No statistically significant difference was observed among the mean values of treatments in (a), and treatments under the same bracket are not significantly different.
3.4. Failure analysis at the porous-dense interface
Deviation at the porous-dense interface from circular to elliptical/oval cross-section was observed during shear testing, Fig. 9c. The ovality at fracture surface, i.e., the ratio of larger to a smaller axis, Fig. 9c, should be one for a circular cross-section, which was the case for an untested sample. It was observed that ovality increased during shear testing with increasing porosity for SSV and CSV, Fig. 9a–b. For the SSV, elliptical behavior was observed to increase for S116 and S99. For CSV, elliptical behavior was prominent in all treatments, C580 to C740. Pairwise comparison of treatment means for the ovality at the porous-dense fracture interface was done separately for SSV and CSV, and significantly different means with P-value < 0.05 is represented in Fig. 9a–b with an '*', and ANOVA table and corresponding P values for pairwise comparisons are shown in the supplemental data, Tables S11–12, respectively.
Fig. 9.

The ovality at the fracture interface from shear tests for (a) SSV and (b) CSV group treatments. A higher value indicates higher pore collapsing at the interface (c) Schematic of circular to elliptical cross-sectional fracture surface of the shear sample at a porous-dense interface. Statistical analyses were done using one-way ANOVA for n=3 and α = 0.05. Pairwise comparison of means among treatments with P-value < 0.05 has been considered as significantly different and marked with an '*' in (a) and (b). Treatments under the same bracket are not significantly different.
For the SSV, the ovality for S92 was significantly higher than all other treatments, and the ovality for S99 was statistically higher than that for SC and S134. For the CSV, the ovality at fracture interface for C690 and C740 was significantly different from SC. Fig. 10 shows microstructures of fracture surfaces for both SSV and CSV. All microstructures show features indicating brittle failure at the porous-dense interface with striation-like features.
Fig. 10.

SEM images of fracture surface of the interface from shear tests: (a) SC, (b) S134, (c) S116, (d) S99, (e) S92, (f) C580, (g) C670, (h) C690, (i) C740. Elongated striation morphologies are marked with white arrows.
3.5. Effect of pore morphology on hFOB cells
MTT assay and SEM morphological analysis were performed for SC, S116, S92, C670, and C740 treatments after 3 and 7 days of culturing hFOB cells to understand the effects of pore morphology on cellular proliferation and attachment. For the porous treatments, structures were designed and printed on top of the dense structure, with a porous part height of 2.25 mm (Fig. 2d) spanning 4.5 cells (S116, S92), 3.6 cells (C670), and 3 cells (C740) along with the height. The cells were plated on an as-printed surface for all treatments. MTT assay shows osteoblast viability and proliferation as a function of optical density for all treatments, Fig. 11. Pairwise comparison of treatment means for the same was done separately for SSV and CSV groups, and significantly different means with P-value < 0.05 is represented in Fig. 11 with an '*', and ANOVA table and corresponding P values for pairwise comparisons are shown in the supplemental data, Tables S13–14, respectively. It was observed that the highest MTT values were observed for control SC and the lowest for C740 after 3 and 7 days of culture. MTT value for S116 for day 3 was found higher than that for S92 and C670. After 7 days of culture, it was found that the MTT value for C670 was higher than that for S116 and S92. SEM micrographs show well-attached osteoblasts with good surface area coverage for both 3 and 7 day time points. The presence of filopodial extensions of osteoblast cells was observed for control SC after 7 days of culture, as shown in Fig. 12b. Pore-bridging by osteoblast cells was observed for S92 treatment for both day 3 and 7 time points and C670 for day 3 timepoint with a high surface coverage of cells over the pores for day 7. No evidence of pore-bridging was observed for C740 treatment.
Fig. 11.

MTT assay for in vitro biological response after (a) 3 and (b) 7 days of hFOB culture. Statistical analyses were done using one-way ANOVA for n=9 and α = 0.05. Pairwise comparison of means among treatments with P-value < 0.05 has been considered significantly different and marked with an '*', and treatments under the same bracket are not significantly different.
Fig. 12.
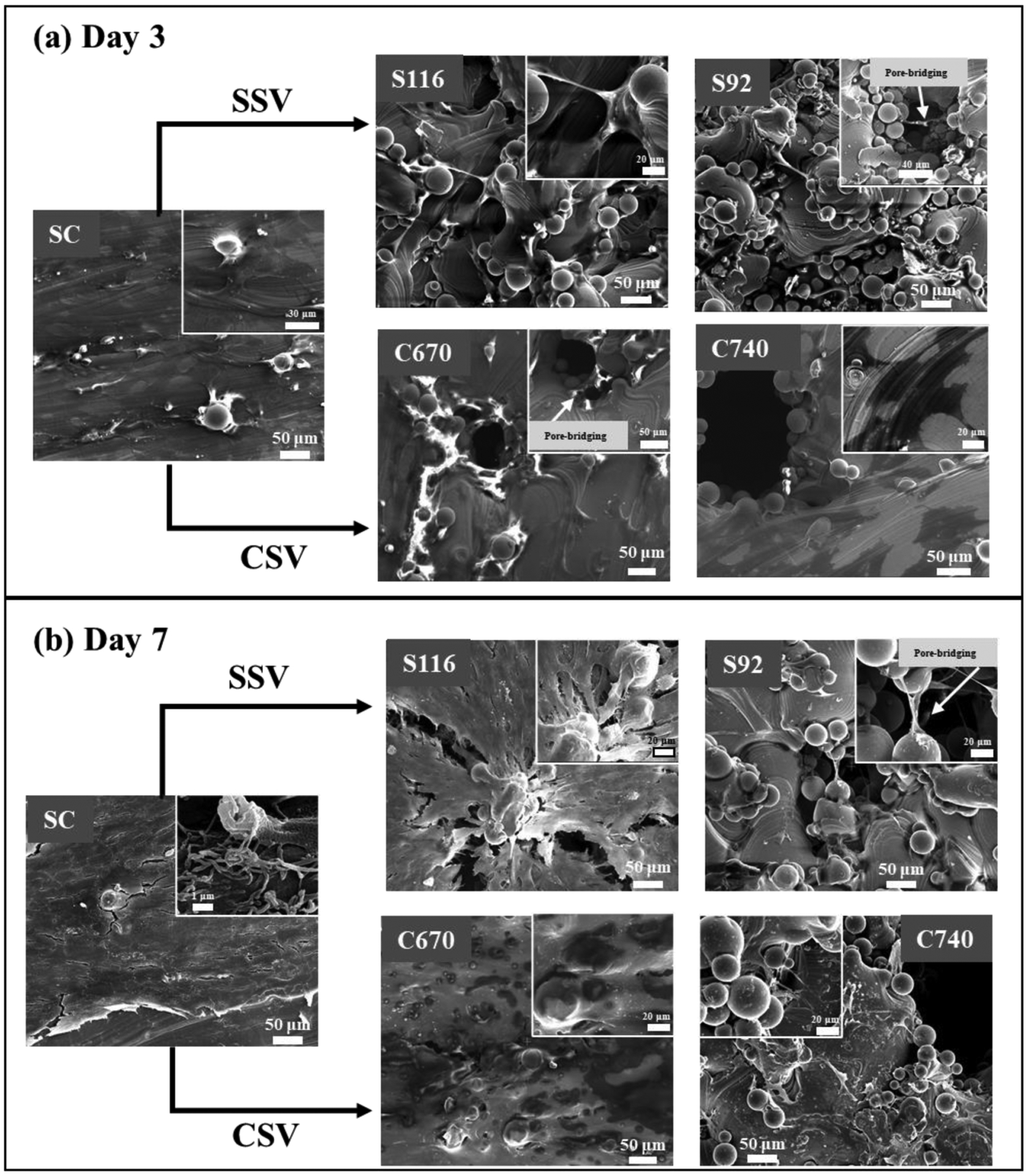
SEM micrographs for in vitro biological response after (a) 3 and (b) 7 days of hFOB culture. Pore-bridging was observed for S92 for both days 3 and 7 and C670 for day 3.
3.6. Effect of pore morphology on P. aeruginosa bacterial cells
Cetrimide agar plate, Pseudosel agar is a selective medium used to culture P. aeruginosa. P. aeruginosa secretes a pigment pyoverdine which can be seen as bright fluorescent green in UV light on cetrimide agar. Bacterial colonies formed on cetrimide agar plate were counted after 36 hrs of culture. An average colony count of 61 CFU per agar plate was counted for control SC treatment, Fig. 13a. However, for S92 and C740, extremely dense bacterial colonies were observed, Fig. 13b–c, which could not be counted. The bacterial viability observed for treatments S92 and C740 from agar plate was much higher than for control SC treatment. SEM micrographs show well-spread bacterial cells for all three treatments, Fig. 13d–f. Pore-bridging by P. aeruginosa bacterial cells was observed in S92, Fig. 13e, but no pre-bridging was observed in C740.
Fig. 13.
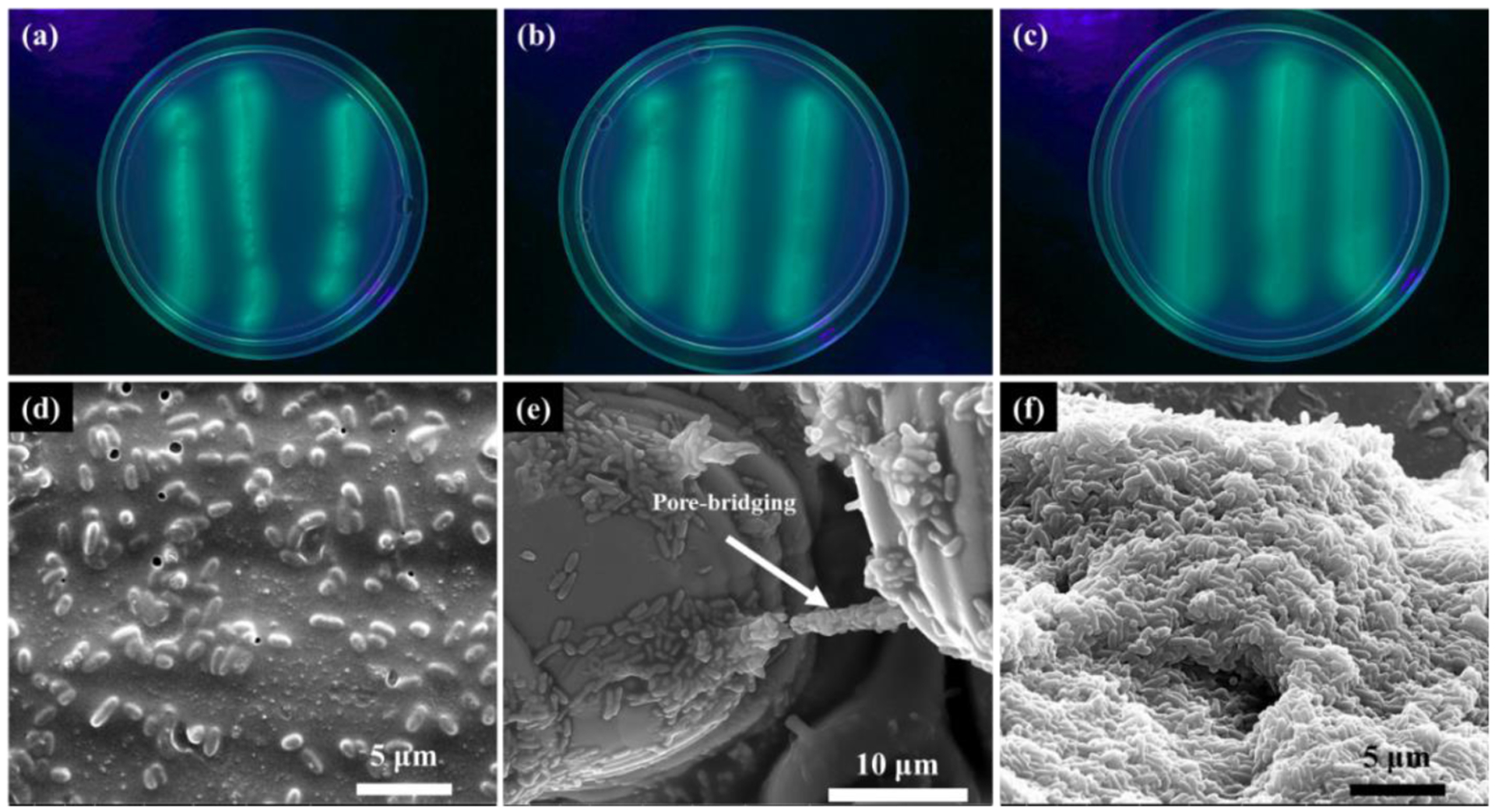
Cetrimide agar plates streaked with 1μl of P. aeruginosa bacterial solution after 36 hrs of culture surface of on (a) SC, (b) S92, (c) C740, and SEM micrographs after 36 hrs of bacterial culture on the surface of (d) SC, (e) S92, and (c) C740. Evidence of pore-bridging by bacterial cells was observed for S92.
4. Discussion
4.1. The behavior of lattice structure under compression and shear loading
The elastic modulus for SC was observed to be 109.7 GPa which is comparable to the elastic modulus of 118 GPa for Ti6Al4V printed via SLM with 0.8% residual porosity [41]. Elastic modulus is expected to decrease with increasing volume fraction porosity by either decreasing strut-size or increasing cell-size [27], [28]. The elastic modulus reduced by 34.3% from SC to S134. However, a reduction in elastic modulus from SC to C580 was found only 20.4%, despite C580 having higher porosity than S134. Hence, variation in strut-size on elastic modulus is more prominent than in cell size variation. Comparing S99 and C670, elastic modulus reduced by 44.9% from SC to S99 with a reduction in strut-size, whereas the modulus reduced by 38.2% from SC to C670 with an increase in cell-size. Despite S99 having a lower porosity than C670, a larger reduction in elastic modulus for S99 was observed compared to C670. Comparing S92 with C740, a reduction in elastic modulus from SC to S92 was 53.6%, while SC to C740 was 40.6%. Although volume porosity for C740 is higher than S92, reduction in elastic modulus was higher for S92 than C740. Overall, our results indicate that elastic modulus is more sensitive to variation in strut-size than in cell-size. Although Gibson and Ashby model suggests that elastic modulus depends on overall porosity of the parts, we have observed the effect of the pore structure on elastic modulus [42]. Maheo et al. have also observed deviation from Ashby and Gibson model for PolyHIPE foams where the elastic modulus increased with cell diameter for samples with similar porosity [43]. Further experiments are needed to validate such results with different materials.
A compressive stress-strain plot for an S92 sample is shown in Fig. 14 with optical images before and after the test. The samples were tested only till significant plastic deformation and not until failure. It was observed that the pores were longitudinal and parallel before the compression test. Post-compression, a clear bulging, can be observed at the center with longitudinal pores non-parallel and no crack propagation along with the longitudinal pores. The total longitudinal plastic strain from the stress-strain plot is 6.7%. Maximum bulging at the center resulted in a change in diameter from 7.16 ± 0.20 mm to 7.40 mm, resulting in a lateral plastic strain of 3.4%. For ideal deformation under compression, i.e., bulging throughout the sample, and considering the Poisson's ratio of dense Ti6Al4V to be 0.34, the lateral plastic strain should be 2.3%, corresponding to the 3.4% lateral strain at the center of the sample due to non-uniform bulging. Güden et al. demonstrated a compression test of sintered Ti6Al4V by powder compaction and reported similar stress-strain characteristics till failure, where the maximum strength was reached at a strain of 0.157 mm/mm for a 34% porous sample [44]. Researchers have studied the deformation behavior of additively manufactured porous Ti6Al4V under compression, which showed ductile [38] and brittle layer-wise failure [41, 42] behavior in the plastic region.
Fig. 14.

Compression stress-strain plot of S92, with optical images of the sample cut longitudinally and laterally, prior- and post-compression test.
SC's compressive yield strength was observed to be 1103 MPa, comparable to the yield strength value of 1040 ± 13 MPa for Ti6Al4V printed using SLM with a residual porosity of 0.8% reported by Fousová et al. [41]. Compressive yield strength is expected to decrease with decreasing strut-size or increasing cell-size because of increased volume fraction porosity [28], [31]. In this study, no effect of design strategy was observed on compressive yield strength. Compressive yield strength decreased with increasing porosities for all treatments irrespective of the design strategy. The densification effect and related strength enhancement during post-plastic region due to collapsing of cells in porous designs [29] have not been observed in compression tests reported in this study as the designed pores were columnar along the axis of compression.
It is expected for shear modulus, yield, and the maximum shear strength to decrease with increased pore volume. Given that shear modulus decreased with increasing porosity for all treatments irrespective of the design strategy, anomalous behavior in shear stress-strain plots was observed in S99, S92, C580, C670, C690, and C740 treatments. Supplemental Fig. S7a shear stress-strain plot for S116 displays a linear elastic region followed by yielding and brittle failure. However, in S99, Supplemental Fig. S7b, the graph curves upwards followed by yielding and fracture, indicating hardening at the porous-dense interface due to pore-collapsing. It is not clear at which point the sample starts yielding for S99. This phenomenon is observed due to localized pore-collapsing and densification at the porous-dense interface where the sample was sheared. Köhnen et al. studied similar densification and strength enhancement phenomena due to cells collapsing under compression in porous metals [29]. Due to pore-collapsing and hardening, shear yield and maximum strength values for S99 were comparable to control SC despite the former having higher porosity. It is to be noted that shear yield strengths were evaluated by a 0.2% strain offset method and were found close to its maximum shear strength due to upward curving of shear stress-strain plot, Supplemental Fig. S7b, Fig. S8b, and Fig. S9a–b. Shear strength enhancement can also be validated by changing shape at the porous-dense cross-sectional interface from circular to elliptical, Fig. 9a. The ovality at the fracture interface increased from S116 to S99 to S92, showing a higher elliptical shape for S99 and S92, indicating pore-collapsing at the porous-dense interface. SEM images at the porous-dense interfaces for S99 and S92, Fig.13e–f, show striation-like features that indicate pore collapsing at the interface. In the CSV group, enhancement in shear strengths was observed for the first treatment in the CSV group, i.e., C580, Supplemental Fig. S8b. However, control SC did not show any shear strength enhancement, Supplemental Fig. S8a. CSV treatments with higher cell-size displayed shear strength enhancement even with lower porosity values compared to SSV. Ovality at the fracture interface for CSV increased right from the first treatment, C580, and was highest for C690 and C740. This indicates variation in cell size having a higher effect on shear strengths than variation in strut-size. It is worth noting that shear strength values for all CSV treatments, except C740, were comparable to the control SC. Shear strength was found similar for C670 and C690, with an increase in cell-size. Further increase in cell-size for C740 displayed pore-collapsing and densification but an early failure resulting in the lowest shear strength, Supplemental Fig. S9b.
4.2. Effect of pore structure on hFOB and P. aeruginosa bacterial cells
MTT values from in vitro hFOB study showed the highest values for control SC with as-printed rough surface compared to other treatments. A possible explanation for this is the penetration of cells into the porous structures, and sonication for performing MTT assay might not have captured osteoblast cells into the MTT solution. MTT values for S116 were statistically higher than those for C670 for day 3, but the reverse was observed for day 7. At the same time, bridging of pores by osteoblast cells was observed for S92 and C670 for day 3 timepoint, Fig. 12a. The lowest MTT values were observed for C740 for both time points with no evidence of pore-bridging. Hence, a cell-size of 670 μm is optimal for surface coating on an orthopedic implant application. This result corroborates with those presented by Taniguchi et al. and Ouyang et al. They reported a pore size of 632 and 650 μm, respectively, showing the best biological performance from an in vivo rabbit study SLM fabricated Ti structures [34] [35].
P. aeruginosa is a gram-negative bacteria causing infection at the dental implant site. P. aeruginosa culture for 36 hrs showed higher bacterial viability for porous structures S92 and C740 than for dense SC treatment. Evidence of pore-bridging by bacterial cells was observed in S92, but not in C740, which indicates that a cell-size of 740 μm is high for pore-bridging by bacterial cells. Nanoscale roughness on the Ti surface has been known to efficiently inhibit bacterial growth to a great extent [37], [38]. However, no such bacterial inhibition was observed in our study with an increase in specific surface area for porous treatment S92 and C740 compared to SC; bacterial viability increased for the former with pore-bridging by bacterial cells S92. This bacterial behavior was corroborated by a study by Domínguez-Trujillo et al. in which porosity in Ti resulted in increased P. aeruginosa bacterial viability [36]. Our results show that porosity on the surface of metal implants enhances osseointegration and increases the risk of bacterial infection in dental implants.
Most orthopedic implants with porous metal coatings have a coating thickness of ~1 mm [15], [47]. Cell-sizes in this study typically range from 500 to 800 μm, accommodating 1 to 3 layers in the desired coating thickness. With hexagonal cellular architecture used in this study, the desired coating volume-porosity of 50% can be achieved, having the last outer layer open. From the results of this study, we can get an insight into design issues for appropriate cell and strut-sizes, ensuring maximum strength under mechanical loading conditions and higher biocompatibility while minimizing metal coating failures at the interface resulting in longer implant life in vivo.
5. Conclusions
Porous honeycomb Ti6Al4V unit cell designs were printed using SLM-based AM. Two groups of samples were printed - one with a constant cell-size of ~470 μm and mean strut-size varying from 92 to 134 μm denoted as SSV, and the other group with a constant strut-size of ~134 μm and mean cell-size was varying from 580 to 740 μm denoted as CSV. Reduction in elastic modulus from SC to S92 (porosity difference 11.1%) was 53.6%, higher than that from SC to C740 (porosity difference 15.6%), 40.6%. Such results emphasize that changes in elastic modulus are more sensitive to strut-size over cell-size. Compression yield strength and shear modulus decreased with increasing porosities irrespective of the design strategy indicating no preferential influence of strut or cell-size variations. Shear strength evaluation at a porous-dense interface showed increasing shear strength due to local pore-collapsing and densification at the porous-dense interface. For SSV, this phenomenon was observed when strut-size was reduced to 99 μm for S99. However, for CSV, such shear strength enhancement was observed right from the first treatment in CSV group C580 and higher cell-size samples, indicating that shear strength is more sensitive to changes cell-size over strut-size. For C740 treatment with the highest cell-size of 740 μm, pore-collapsing was seen, followed by early failure resulting in lower shear strength. A cell-size of 670 μm showed better osteoblast cell viability compared to other porous structures with evidence of pore-bridging by cells. P. aeruginosa bacterial culture showed that higher bacterial viability for porous structures over dense SC treatment. Thus, a pore size of 670 μm is optimal for orthopedic implants' porous metal coating applications.
Supplementary Material
Highlights.
Porous Ti6Al4V structures were fabricated using metal additive manufacturing.
Both cell- and strut sizes were varied.
Elastic modulus is found to be more sensitive to strut-size over cell-size.
Shear behavior is found to be more sensitive to cell-size over strut-size.
A cell-size of 670 μm showed better in vitro performance on hFOB cells.
No effect of pore-morphology was observed on P. aeruginosa bacteria.
6. Acknowledgments
Authors acknowledge financial support from the National Science Foundation under grant # CMMI 1934230 (PI- Bandyopadhyay). The authors also acknowledge financial support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR067306. The authors are also grateful for financial assistance from JCDREAM (Seattle, WA) for a capital equipment grant to purchase the powder bed-based metal 3D Printer at WSU. The content is solely the authors' responsibility and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- SSV
Strut-size Variation
- CSV
Cell-size Variation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
7. References
- [1].Bose S, Roy M, and Bandyopadhyay A, “Recent advances in bone tissue engineering scaffolds,” Trends Biotechnol, vol. 30, no. 10, pp. 546–554, Oct. 2012, doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bandyopadhyay A, Krishna BV, Xue W, and Bose S, “Application of Laser Engineered Net Shaping (LENS) to manufacture porous and functionally graded structures for load bearing implants,” J. Mater. Sci. Mater. Med, vol. 20, no. 1, p. 29, Jun. 2008, doi: 10.1007/s10856-008-3478-2. [DOI] [PubMed] [Google Scholar]
- [3].Balla VK, Bose S, and Bandyopadhyay A, “Understanding compressive deformation in porous titanium,” Philos. Mag, vol. 90, no. 22, pp. 3081–3094, Jul. 2010, doi: 10.1080/14786431003800891. [DOI] [Google Scholar]
- [4].“Class 2 Device Recall Zimmer 4.1 Trabecular Metal.” https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?id=108235 (accessed May 27, 2020).
- [5].“Class 2 Device Recall Zimmer Trabecular Metal Modular Acetabular System.” https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=102997 (accessed May 27, 2020).
- [6].Elias CN, Lima JHC, Valiev R, and Meyers MA, “Biomedical applications of titanium and its alloys,” JOM, vol. 60, no. 3, pp. 46–49, Mar. 2008, doi: 10.1007/s11837008-0031-1. [DOI] [Google Scholar]
- [7].Amin Yavari S et al. , “Fatigue behavior of porous biomaterials manufactured using selective laser melting,” Mater. Sci. Eng. C, vol. 33, no. 8, pp. 4849–4858, Dec. 2013, doi: 10.1016/j.msec.2013.08.006. [DOI] [PubMed] [Google Scholar]
- [8].Kasemo B, “Biocompatibility of titanium implants: Surface science aspects,” J. Prosthet. Dent, vol. 49, no. 6, pp. 832–837, Jun. 1983, doi: 10.1016/0022-3913(83)90359-1. [DOI] [PubMed] [Google Scholar]
- [9].Bandyopadhyay A, Shivaram A, Tarafder S, Sahasrabudhe H, Banerjee D, and Bose S, “In Vivo Response of Laser Processed Porous Titanium Implants for Load-Bearing Implants,” Ann. Biomed. Eng, vol. 45, no. 1, pp. 249–260, Jan. 2017, doi: 10.1007/s10439016-1673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bose S, Ke D, Sahasrabudhe H, and Bandyopadhyay A, “Additive manufacturing of biomaterials,” Prog. Mater. Sci, vol. 93, pp. 45–111, Apr. 2018, doi: 10.1016/j.pmatsci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bandyopadhyay A and Bose S, Additive Manufacturing, Second Edition. CRC Press, 2019. [Google Scholar]
- [12].Schwarz MLR, Kowarsch M, Rose S, Becker K, Lenz T, and Jani L, “Effect of surface roughness, porosity, and a resorbable calcium phosphate coating on osseointegration of titanium in a minipig model,” J. Biomed. Mater. Res. A, vol. 89A, no. 3, pp. 667–678, 2009, doi: 10.1002/jbm.a.32000. [DOI] [PubMed] [Google Scholar]
- [13].Bandyopadhyay A, Shivaram A, Mitra I, and Bose S, “Electrically polarized TiO2 nanotubes on Ti implants to enhance early-stage osseointegration,” Acta Biomater, vol. 96, pp. 686–693, Sep. 2019, doi: 10.1016/j.actbio.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mour M et al. , “Advances in Porous Biomaterials for Dental and Orthopaedic Applications,” Materials, vol. 3, no. 5, Art. no. 5, May 2010, doi: 10.3390/ma3052947. [DOI] [Google Scholar]
- [15].Kalita VI, Komlev DI, Komlev VS, and Radyuk AA, “The shear strength of threedimensional capillary-porous titanium coatings for intraosseous implants,” Mater. Sci. Eng. C, vol. 60, pp. 255–259, Mar. 2016, doi: 10.1016/j.msec.2015.11.033. [DOI] [PubMed] [Google Scholar]
- [16].Nishiguchi S, Nakamura T, Kobayashi M, Kim H-M, Miyaji F, and Kokubo T, “The effect of heat treatment on bone-bonding ability of alkali-treated titanium,” Biomaterials, vol. 20, no. 5, pp. 491–500, Mar. 1999, doi: 10.1016/S0142-9612(98)90203-4. [DOI] [PubMed] [Google Scholar]
- [17].Hamilton B, Oppenheimer S, Dunand DC, and Lewis D, “Diffusion Bonding of Ti-6Al-4V Sheet with Ti-6Al-4V Foam for Biomedical Implant Applications,” Metall. Mater. Trans. B, vol. 44, no. 6, pp. 1554–1559, Dec. 2013, doi: 10.1007/s11663-013-9942-5. [DOI] [Google Scholar]
- [18].Amigó V, Salvador MD, Romero F, Solves C, and Moreno JF, “Microstructural evolution of Ti–6Al–4V during the sintering of microspheres of Ti for orthopedic implants,” J. Mater. Process. Technol, vol. 141, no. 1, pp. 117–122, Oct. 2003, doi: 10.1016/S0924-0136(03)00243-7. [DOI] [Google Scholar]
- [19].Kalita VI and Gnedovets AG, “Plasma Spraying of Capillary Porous Coatings: Experiments, Modeling, and Biomedical Applications,” Plasma Process. Polym, vol. 2, no. 6, pp. 485–492, 2005, doi: 10.1002/ppap.200500023. [DOI] [Google Scholar]
- [20].Braem A, Mattheys T, Neirinck B, Schrooten J, der Biest OV, and Vleugels J, “Porous Titanium Coatings Through Electrophoretic Deposition of TiH2 Suspensions,” Adv. Eng. Mater, vol. 13, no. 6, pp. 509–515, 2011, doi: 10.1002/adem.201100011. [DOI] [Google Scholar]
- [21].Muth J, Poggie M, Kulesha G, and Michael Meneghini R, “Novel Highly Porous Metal Technology in Artificial Hip and Knee Replacement: Processing Methodologies and Clinical Applications,” JOM, vol. 65, no. 2, pp. 318–325, Feb. 2013, doi: 10.1007/s11837012-0528-5. [DOI] [Google Scholar]
- [22].Yoshioka S et al. , “Comparison of a highly porous titanium cup (Tritanium) and a conventional hydroxyapatite-coated porous titanium cup: A retrospective analysis of clinical and radiological outcomes in hip arthroplasty among Japanese patients,” J. Orthop. Sci, vol. 23, no. 6, pp. 967–972, Nov. 2018, doi: 10.1016/j.jos.2018.06.018. [DOI] [PubMed] [Google Scholar]
- [23].Levine B, “A New Era in Porous Metals: Applications in Orthopaedics,” Adv. Eng. Mater, vol. 10, no. 9, pp. 788–792, 2008, doi: 10.1002/adem.200800215. [DOI] [Google Scholar]
- [24].Rodriguez M, “Total Hip Replacement,” BioMedtrix. https://biomedtrix.com/total-hipreplacement/ (accessed May 23, 2020). [Google Scholar]
- [25].Civantos A, Martínez-Campos E, Ramos V, Elvira C, Gallardo A, and Abarrategi A, “Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants,” ACS Biomater. Sci. Eng, vol. 3, no. 7, pp. 1245–1261, Jul. 2017, doi: 10.1021/acsbiomaterials.6b00604. [DOI] [PubMed] [Google Scholar]
- [26].“CeSPACE® Titanium.” https://www.bbraun.com/en/products/b/cespace-titanium.html (accessed May 23, 2020).
- [27].Van Bael S et al. , “The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds,” Acta Biomater, vol. 8, no. 7, pp. 2824–2834, Jul. 2012, doi: 10.1016/j.actbio.2012.04.001. [DOI] [PubMed] [Google Scholar]
- [28].Cheng XY et al. , “Compression deformation behavior of Ti–6Al–4V alloy with cellular structures fabricated by electron beam melting,” J. Mech. Behav. Biomed. Mater, vol. 16, pp. 153–162, Dec. 2012, doi: 10.1016/j.jmbbm.2012.10.005. [DOI] [PubMed] [Google Scholar]
- [29].Köhnen P, Haase C, Bültmann J, Ziegler S, Schleifenbaum JH, and Bleck W, “Mechanical properties and deformation behavior of additively manufactured lattice structures of stainless steel,” Mater. Des, vol. 145, pp. 205–217, May 2018, doi: 10.1016/j.matdes.2018.02.062. [DOI] [Google Scholar]
- [30].Ahmadi SM et al. , “Mechanical behavior of regular open-cell porous biomaterials made of diamond lattice unit cells,” J. Mech. Behav. Biomed. Mater, vol. 34, pp. 106–115, Jun. 2014, doi: 10.1016/j.jmbbm.2014.02.003. [DOI] [PubMed] [Google Scholar]
- [31].Choy SY, Sun C-N, Leong KF, and Wei J, “Compressive properties of functionally graded lattice structures manufactured by selective laser melting,” Mater. Des, vol. 131, pp. 112–120, Oct. 2017, doi: 10.1016/j.matdes.2017.06.006. [DOI] [Google Scholar]
- [32].Parthasarathy J, Starly B, and Raman S, “A design for the additive manufacture of functionally graded porous structures with tailored mechanical properties for biomedical applications,” J. Manuf. Process, vol. 13, no. 2, pp. 160–170, Aug. 2011, doi: 10.1016/j.jmapro.2011.01.004. [DOI] [Google Scholar]
- [33].Bartolomeu F, Costa MM, Alves N, Miranda G, and Silva FS, “Additive manufacturing of NiTi-Ti6Al4V multi-material cellular structures targeting orthopedic implants,” Opt. Lasers Eng, vol. 134, p. 106208, Nov. 2020, doi: 10.1016/j.optlaseng.2020.106208. [DOI] [Google Scholar]
- [34].Taniguchi N et al. , “Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment,” Mater. Sci. Eng. C, vol. 59, pp. 690–701, Feb. 2016, doi: 10.1016/j.msec.2015.10.069. [DOI] [PubMed] [Google Scholar]
- [35].Ouyang P et al. , “Hydromechanical mechanism behind the effect of pore size of porous titanium scaffolds on osteoblast response and bone ingrowth,” Mater. Des, vol. 183, p. 108151, Dec. 2019, doi: 10.1016/j.matdes.2019.108151. [DOI] [Google Scholar]
- [36].Domínguez-Trujillo C et al. , “Bacterial behavior on coated porous titanium substrates for biomedical applications,” Surf. Coat. Technol, vol. 357, pp. 896–902, Jan. 2019, doi: 10.1016/j.surfcoat.2018.10.098. [DOI] [Google Scholar]
- [37].Stolzoff M et al. , “Decreased bacterial growth on titanium nanoscale topographies created by ion beam assisted evaporation,” Int. J. Nanomedicine, vol. 12, pp. 1161–1169, Feb. 2017, doi: 10.2147/IJN.S119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cao Y et al. , “Nanostructured titanium surfaces exhibit recalcitrance towards Staphylococcus epidermidis biofilm formation,” Sci. Rep, vol. 8, no. 1, p. 1071, Dec. 2018, doi: 10.1038/s41598-018-19484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Onuike B and Bandyopadhyay A, “Bond strength measurement for additively manufactured Inconel 718- GRCop84 copper alloy bimetallic joints,” Addit. Manuf, vol. 27, pp. 576–585, May 2019, doi: 10.1016/j.addma.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Onuike B and Bandyopadhyay A, “Functional bimetallic joints of Ti6Al4V to SS410,” Addit. Manuf, vol. 31, p. 100931, Jan. 2020, doi: 10.1016/j.addma.2019.100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fousová M, Vojtěch D, Kubásek J, Jablonská E, and Fojt J, “Promising characteristics of gradient porosity Ti-6Al-4V alloy prepared by SLM process,” J. Mech. Behav. Biomed. Mater, vol. 69, pp. 368–376, May 2017, doi: 10.1016/j.jmbbm.2017.01.043. [DOI] [PubMed] [Google Scholar]
- [42].Gibson LJ, “Cellular Solids,” MRS Bull, vol. 28, no. 4, pp. 270–274, Apr. 2003, doi: 10.1557/mrs2003.79. [DOI] [Google Scholar]
- [43].Maheo L et al. , “Elastic behavior of multi-scale, open-cell foams,” Compos. Part B Eng, vol. 44, no. 1, pp. 172–183, Jan. 2013, doi: 10.1016/j.compositesb.2012.06.006. [DOI] [Google Scholar]
- [44].Güden M, Çelik E, Hızal A, Altındiş M, and Çetiner S, “Effects of compaction pressure and particle shape on the porosity and compression mechanical properties of sintered Ti6Al4V powder compacts for hard tissue implantation,” J. Biomed. Mater. Res. B Appl. Biomater, vol. 85B, no. 2, pp. 547–555, 2008, doi: 10.1002/jbm.b.30978. [DOI] [PubMed] [Google Scholar]
- [45].Kadkhodapour J, Montazerian H, Darabi A. Ch., Zargarian A, and Schmauder S, “The relationships between deformation mechanisms and mechanical properties of additively manufactured porous biomaterials,” J. Mech. Behav. Biomed. Mater, vol. 70, pp. 28–42, Jun. 2017, doi: 10.1016/j.jmbbm.2016.09.018. [DOI] [PubMed] [Google Scholar]
- [46].Yang L, Harrysson O, West H, and Cormier D, “Compressive properties of Ti–6Al–4V auxetic mesh structures made by electron beam melting,” Acta Mater, vol. 60, no. 8, pp. 3370–3379, May 2012, doi: 10.1016/j.actamat.2012.03.015. [DOI] [Google Scholar]
- [47].Van Bael S, Kerckhofs G, Moesen M, Pyka G, Schrooten J, and Kruth JP, “Micro-CT-based improvement of geometrical and mechanical controllability of selective laser melted Ti6Al4V porous structures,” Mater. Sci. Eng. A, vol. 528, no. 24, pp. 7423–7431, Sep. 2011, doi: 10.1016/j.msea.2011.06.045. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


