Abstract
Methotrexate (MTX) is widely employed for children with cancer, but is also associated with persistent cognitive deficits among survivors. The present study investigated the mechanisms behind long-term cognitive dysfunction after juvenile animals are treated with MTX. Male and female Long-Evans rats were treated with a combination of 6 systemic doses (0.5 mg/kg/dose intraperitoneally) and 4 intrathecal doses (1 mg/kg) beginning at post-natal age 3 weeks, a schedule designed to mimic repeated exposure given to children with leukemia. Behavioral testing was conducted at 60-61 weeks of age, followed by analysis of brain histolopathology. This MTX regimen had no acute toxicity and no effect on growth. The spatial memory and visual memory deficits observed at 13 and 17 weeks of age persisted 1 year after MTX exposure in both females and males. Significantly decreased cell proliferation and increased hippocampal microglial activation were observed in MTX-treated females when compared to the controls, with a similar trend in the male groups. In addition, MTX treatment significantly increased the number of TUNEL positive cells in the periventricular area. Our study demonstrates that a clinically relevant regimen of systemic and intrathecal MTX induces persistent deficits in cognition, lasting approximately 1 year after the last injection. The mechanisms behind MTX-induced deficits are likely multifactorial, including suppression of neurogenesis, microglial activation, and increased brain cell apoptosis. Our study suggests female and male animals differ in susceptibility to MTX-induced neurotoxicity and provides insights for developing therapeutic approaches to prevent treatment related cognitive impairment among children with ALL.
Keywords: Antifolate, neurotoxicity, neurocognitive deficits, survivorship, neurocognitive function
1. Introduction
Curative chemotherapy can cause persistent organ dysfunction among a substantial subset of childhood cancer survivors (Hudson, Mertens et al. 2003, Gibson, Mostoufi-Moab et al. 2018). Chemotherapy-induced cognitive impairment (CICI) is one example of these adverse sequelae of cancer therapy, which can be detected in 30-60% of childhood cancer survivors, with a sensitivity depending on the depth of neurocognitive testing (Hardy, Embry et al. 2017, Krull, Hardy et al. 2018). CICI leads to impaired school and occupational performance relative to siblings or healthy controls (Kirchhoff, Krull et al. 2011, Nathan, Henderson et al. 2018), as well as diminished health-related quality of life (Kunin-Batson, Kadan-Lottick et al. 2014, van der Plas, Noakes et al. 2020). Inter-subject variability in susceptibility to CICI may relate to environmental factors (e.g. poverty, diet, gut microbiome) (Raver, Blair et al. 2013, Peng, Yam et al. 2020, Ciernikova, Mego et al. 2021) or inherited host differences (Cole, Finkelstein et al. 2015, Wang, Yin et al. 2017, Tan, Lim et al. 2019). Most studies of children with acute lymphoblastic leukemia (ALL), the most common childhood cancer, also indicate a sex difference, with girls being more susceptible to CICI than boys (Krull, Hardy et al. 2018).
Methotrexate is a chemotherapeutic agent that is central to curative regimens for children and adults with a wide variety of cancer types, including ALL, non-Hodgkin lymphoma, breast cancer, and osteosarcoma. However, it is also considered one of the agents most responsible for CICI (Duffner, Armstrong et al. 2014). As a folate antagonist and inhibitor of dihydrofolate reductase, its on-target antineoplastic activity results from limiting synthesis of nucleotides. The off-target neurotoxic side effects of methotrexate, in contrast, appear to be caused by multiple pathways (Cole and Kamen 2006), including inducing neuroinflammation (Ahles and Saykin 2007) and oxidative stress (Ataie, Sabetkasaei et al. 2010, Aluise, Miriyala et al. 2011), altering neurotransmission (Vijayanathan, Ali et al. 2011), inhibiting neural stem cell proliferation (Seigers, Schagen et al. 2009), and damaging both endothelial surfaces (Brouwer, Postma et al. 2013) and myelinated tracts (Sleurs, Lemiere et al. 2019, Zhou, Zhuang et al. 2019).
Animal models have been useful for dissecting these mechanisms responsible for neurotoxic side effects caused by methotrexate as well as other commonly used chemotherapeutic agents, such as cisplatin, fluorouracil, and doxorubicin (Seigers and Fardell 2011). These models demonstrate that both the spectrum of observed cognitive deficiencies and the underlying pathophysiology varies with the class of the chemotherapy agent studied, the dose, schedule, and route of administration, as well as the age at which the animals are exposed. However, many of these studies used animals of a single sex, precluding a direct comparison of the impact of chemotherapy between male and female animals. Furthermore, most report short-term toxicity observed within a few weeks of drug exposure, while clinical studies note that childhood cancer survivors exhibit cognitive deficits well into adulthood, decades later. We hypothesize that our model can be useful for understanding persistent CICI by following chemotherapy-exposed animals into adulthood, and explaining the observed difference between male and female patients in clinical toxicity, by exploring differences between the sexes in susceptibility to CICI and in the induced pathology.
We have previously studied the impact of methotrexate when given to juvenile animals on a schedule and dosing regimen designed to mimic treatment regimens used to cure children with ALL (Wen, Maxwell et al. 2018). Compared to sham-treated controls, MTX-treated animals exhibit impairment of cognitive flexibility, visual recognition, and spatial pattern memory that persist at least two months after the last drug exposure, when the animals had recovered from any acute toxicity of drug exposure. We also observed structural changes in white matter, decreased proliferation of neural progenitors, and altered microglial activation relative to sham-treated controls, supporting the hypothesis that these mechanisms contribute to the observed cognitive impairment. In this report, we further characterize the long-term impact of methotrexate exposure to juvenile animals, analyzing cognitive function and neuropathology in animals more than one year following completion of the methotrexate treatment regimen. In addition, by including animals of both sexes, we are able to highlight differences between male and female animals in susceptibility to CICI.
2. Materials and methods
2.1. Animals and reagents
Long Evans rats were purchased from Charles River Laboratories (Wilmington, MA) at 2 weeks of age and were habituated to the vivarium for one week before the experiments. Forty animals were treated with MTX and 40 controls were given sham injections of saline; both groups included approximately equal numbers of males and females. Rats were housed in same-sex groups of two or three with a 12/12 hours light/dark cycle and ad lib. access to food (LabDiet 5053) and water. All experiments were approved by the Animal Institute and Use Committee of Rutgers University and were conducted following the “Guide for the Care and Use of Laboratory Animals”. The NC3R's ARRIVE guidelines were followed in the conduct and reporting of all experiments described here.
Methotrexate (USP grade) was purchased from Hospira (Lake Forest, IL) and phosphate buffered saline (PBS) was purchased from VWR (Radnor, PA). All injected solutions were sterilized by filtering through 0.22 μm syringe filters (Millipore, Billerica, MA).
2.2. Injection schedule and CSF collection
As previously published, a methotrexate treatment schedule was designed to approximate the methotrexate repeatedly given to children treated for ALL over a 2 to 3 year period, while their brains are continuing to develop. Briefly, the treatment regimen includes repeated doses of systemic (intraperitoneal) and intraventricular injections at doses calculated to approximate serum and cerebrospinal fluid concentrations similar to those observed among children being treated for ALL. The detailed injection schedule is illustrated in Figure 1A. Briefly, rats received six intraperitoneal (IP) injections (0.5 mg/kg methotrexate or PBS) at 3, 4, 7, and 8 weeks of age. Four intrathecal injections were given within a two-week period from 5 to 6 weeks of age (1 mg/kg methotrexate or artificial cerebrospinal fluid [aCSF]).
Figure 1. Injection and behavioral test schedule and growth curve.
(A) indicated our injection and behavioral test schedule. (B) Growth curve of control and methotrexate-treated animals. Both control and methotrexate treated animals were weighed at 3, 8, 11, 21, 34 and 60 weeks of age, respectively. The number of animals in the control and methotrexate treated groups is 5-20 (F), 5-20 (M) and 5-19 (F), 5-21 (M), respectively.
Intrathecal (IT) injections were carried out as previously described (Li, Vijayanathan et al. 2010) by transcutaneous cisterna magna puncture with a 25-gauge butterfly needle, with inhaled isoflurane anesthesia (2-5%). Correct insertion of the needle was verified by outflow of clear cerebrospinal fluid (CSF), which was collected in isovolumetric amounts (i.e., volume of CSF removed was equivalent to volume of drug to be administered) prior to IT injection. Methotrexate (1 mg/kg) or artificial CSF (aCSF; Na+ 150 mM, K+ 3 mM, Ca2+ 1.4 mM, Mg2+ 0.8 mM, P 1.0 mM, and Cl− 155mM, in double distilled water) was then manually injected through the same needle over one minute. All animals were monitored for signs of acute toxicity under direct visualization for one hour after injection and subsequently on a daily basis for evidence of abnormal behaviors. Artificial CSF was purchased from Basi (West Lafyette, Indiana).
2.3. Behavioral Testing Schedule
Behavioral assessments of cognitive function were conducted at three time points (Figure 1A) to assess subacute effects (at 12-14 and 16-18 weeks of age; 1-2 month after the last methotrexate exposure) and long-term consequences (at 59-61 weeks of age; approximately 1 year after the last methotrexate exposure). The battery included the following: open field (OF), object placement (OP) and object recognition (OR)(Ennaceur and Meliani 1992), conducted as previously published (Li, Vijayanathan et al. 2010, Thomsen, Gulinello et al. 2017) and described briefly below.
2.3.1. Open field
The open field test was used to evaluate locomotor activity and thigmotaxis, an indicator of anxiety-like behavior (Treit and Fundytus 1988). The assay was carried out in an arena (69 × 69 x 60 cm) with visual cues for 6 minutes. Total track length, center track length, center time, and center entries were recorded and analyzed by Viewer III software (Biobserve, Bonn, Germany).
2.3.2. Object Placement and Object Recognition Tasks
Both the OP and OR test rely on the innate preference of rodents to preferentially explore novel environmental stimuli. Intact pattern recognition in the object placement test is indicated by a preference for an object that has been moved to a novel location. Intact recognition memory in the novel object recognition test is indicated by a preference for a novel object over the familiar one previously encountered. Briefly, during training, animals are exposed to a pair of identical objects. After a defined retention interval in their home cages (20 minutes for OP and 120 minutes for OR), rats were presented with one unmoved and one relocated object (OP) or one old and one novel object (OR) in a testing trial. Total object exploration time were recorded manually. Exploration was defined as any physical contact with the object (sniffing, whisking, or touching). Data from subjects with less than 4 seconds of total exploration time were excluded from analysis (less than 2% of subjects). A discrimination index (DI) was calculated as the difference in time exploring the novel object compared to the familiar one, divided by the total time spent in both of the objects. Discrimination index above 0 indicates preference to the novel object (intact memory), while DI below 0 indicates non-preference. In addition, the proportion of animals with intact memory (individuals with DI >0) was calculated for each group. All animals were exposed to equal conditions regarding time of training, testing, and period of retention interval. The tester was blinded to treatment conditions. The objects were previously validated for similar valance and exploration.
2.4. Organ and sample collection
The animals were anesthetized with 2-5% isoflurane. The heart was exposed and blood was collected from the apex of the heart and kept on ice. Animals were then extensively perfused with ice cold PBS via a butterfly needle inserted into the left ventricle of the heart. Brains were then extracted and dissected into left and right hemispheres. The left hemisphere of the brain was fixed in 4% paraformaldehyde 24 hours at 4 °C, followed by PBS washes at 4 °C, then transferred to 70% ethanol at 4 °C, and sent to the CINJ Histopathology Core facility to be embedded in paraffin for the sagittal sections. Sections were collected from the midline in 5 um thickness. The hippocampus was dissected from the right hemisphere of the brains, snapped frozen in liquid nitrogen and transferred to a −80°C for storage. Blood samples were centrifuged at 4°C x 10,000 rpm for 10 minutes. Serum was collected and stored at −80°C until analysis.
2.5. Immunohistochemistry and Immunofluorescence
Staining for Iba-1, glial fibrillary acidic protein (GFAP), Ki67, myelin basic protein (MBP), and adenomatous polyposis coli (APC) were performed on 5 um paraffin sections. Briefly, sections were blocked with 20% normal horse serum with 0.1%-0.5% Triton X-100 and incubated overnight at 4 °C with primary antibodies: rabbit anti-rat Iba-1(1:250, WAKO Chemicals, Mountain View, CA), mouse anti-rat GFAP (1:500, Millipore, Billerica, MA), rabbit anti-rat Ki67 (1:200, Abcam, Cambridge, MA), mouse anti-rat MBP (1:500, Millipore), and mouse anti-rat APC (1:200, Abcam), followed by secondary antibodies, donkey anti-mouse Alexa Fluor 488 (1:600, Invitrogen, Carlsbad, California) or donkey anti-rabbit Alexa Fluor 594 (1:600, Invitrogen), respectively, at room temperature for 1 hr. Sections were imaged using a fluorescence microscope (Keyence BZ-X700). One section was used from each animal. For each location, one representative image was taken. For Iba-1 and GFAP staining, a representative image was taken from each section from the cortex, dentate gyrus (DG) of the hippocampus, and corpus callosum. For MBP and APC staining, a representative image was taken, focused on the DG. The fluorescence intensity of Iba-1, GFAP and MBP was then quantified by Image J. The mean fluorescence intensity in control group was set as 1, and the fold changes of fluorescence intensity of each animal in MTX group was calculated accordingly, referred as “relative intensity”. For Ki67 staining, the number of Ki67+ cells were counted manually in each section. For APC staining, the number of APC+ cells were counted manually in each representative image. All quantifications were done by an investigator who remained blinded to the treatment conditions.
2.6. TUNEL staining
TUNEL staining was performed using an in-situ cell death detection (Fluorescein) kit (Roche Diagnostic, Indianapolis, IN). Briefly, sections were incubated with 20 ug/mL proteinase K for 30 minutes at room temperature, followed by TUNEL mixture solution at 37°C for an hour. Slides were imaged using a fluorescence microscope (Keyence BZ-X700). The number of TUNEL positive cells in the prefrontal and frontal cortex, hippocampus, corpus callosum, and periventricular areas were counted manually by an investigator who remained blinded to the treatment conditions.
2.7. Analysis of serum blood urea nitrogen (BUN) and creatinine
Serum BUN and creatinine were analyzed using BUN colorimetric detection kit (Arbor Assays, An Arbor, MI) and creatinine colorimetric assay kit (Cayman Chemical Companies, Ann Arbor, MI), respectively, following manufacture’s protocols.
2.8. Statistics
Data were analyzed by D’Agostino-Pearson test to assess whether the data was normally distributed. For two group comparisons, mean discrimination indexes were compared with GraphPad Prism 9.02 (GraphPad Software, San Diego, CA) using two-tailed t-tests (for normally distributed data) or Mann-Whitney U test (non-normally distributed data). Contingencies were analyzed by Chi-Square test. Variances were evaluated by Brown-Forsythe test. For multiple group comparisons, data were analyzed using one-way ANOVA test (normally distributed data with equal variances), Welch ANOVA (normally distributed data without equal variances) or Kruskal-Wallis test (non-normally distributed data). For all statistical tests, a probability value less than 0.05 was considered to be statistically significant.
3. Results
3.1. There were no differences in the weight, general locomotor activity, anxiety-like behavior or kidney function after methotrexate treatment
Administration of methotrexate to juvenile rats at this dose and schedule did not alter weight gain compared to the controls (Figure 1B). At 12 and 16 weeks of age, the results for open field were similar to what we have published previously (Wen, Maxwell et al. 2018), with no significant differences between the methotrexate-treated and control group. Similarly, at 59 weeks of age, general locomotor activity was not altered by methotrexate treatment. Total track length was similar between methotrexate-treated and control group at three time points (data not shown). There were no differences in thigmotaxis, an anxiety behavior, as indicated by the comparable center track length (data not shown), center time (data not shown) and center entries (data not shown) between the two groups at three time points. In addition, there were no differences in serum BUN and creatinine levels (data not shown), and BUN/creatinine ratios (data not shown) between the two groups at 1 year of age, indicating that MTX had no deleterious impact on renal function.
3.2. Methotrexate-treated juvenile animals displayed persistent impairment in spatial memory at 1 year old
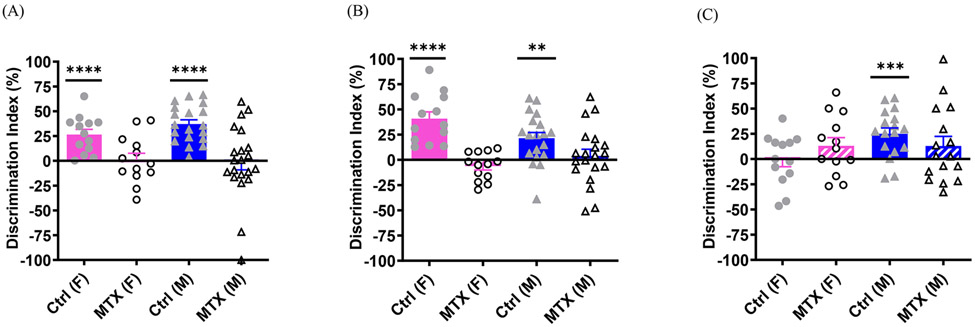
At 13 and 17 weeks of age, both methotrexate-treated female and male animals displayed spatial memory impairment in the object placement test, while both control females and males maintained intact cognition, demonstrated by one sample t-test (Figure 2A, control (F), t(12)=5.057, p=0.0003; control (M), t(18)=8.626, p<0.0001; 2B, control (F), t(12)=6.049, p<0.0001; control (M), t(18)=3.779, p=0.0014 ). This result was similar as what we have published previously (Wen, Maxwell et al. 2018). At 60 weeks of age, methotrexate-treated rats displayed persistent spatial memory deficits in the object placement test, as both males and females in the methotrexate group failed to attain a discrimination index significantly higher than chance, demonstrated by one sample t-test (Figure 2C). In contrast, control male animals displayed intact spatial memory (Figure 2C, t(16)=4.370, p=0.0005). Interestingly, the control females also showed cognitive dysfunction at 60 weeks of age, indicated by the mean discrimination index lower than zero (Figure 2C). In addition, a greater proportion of methotrexate-treated male animals did not reliably prefer the displaced object (7 of 16 [56.25%] had discrimination index <0) in the object placement test compared to the control males (2of 17 [17.65%]; Chi-square(1)=4.251, df=1, p=0.04). However, a similar proportion of methotrexate-treated and control females failed this test (control vs MTX : 30.77% vs 38.46%) .
Figure 2. The spatial memory deficits induced by methotrexate treatment.
Object placement tests were conducted at 13 (A), 17 (B) and 60 (C) weeks of age, respectively. The number of animals in the control and methotrexate treated groups is 13 (F), 19 (M) and 13 (F), 21 (M), respectively, for (A), 13 (F), 19 (M) and 13 (F), 20 (M) for (B) and 13 (F), 17 (M) and 13 (F), 16 (M) for (C). In all the figures, * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001, and **** indicates to p<0.0001.
3.3. Methotrexate-treated juvenile animals displayed persistent deficits in visual memory at 1 year old
At 14 and 18 weeks of age, both methotrexate-treated female and male animals displayed visual memory deficits in the object recognition test, while controls of both sexes showed intact cognition, demonstrated by one sample t-test. (Figure 3A, control (F), t(12)=5.701, p<0.0001; control (M), t(18)=5.065, p<0.0001; 3B, control (F), t(12)=3.512, p=0.0043; control (M), t(18)=3.320, p=0.0038), and this replicated our previous study (Wen, Maxwell et al. 2018).
Figure 3. The visual memory impairment induced by methotrexate treatment.
Object recognition tests were conducted at 14 (A), 18 (B) and 61 (C) weeks of age, respectively. The number of animals in the control and methotrexate treated groups is 13 (F), 19 (M) and 13 (F), 20 (M), respectively, for (A), 13 (F), 19 (M) and 13 (F), 20 (M) for (B) and 13 (F), 17 (M) and 13 (F), 18 (M) for (C).
At 1 year old, both control females and males obtained a discrimination index significantly higher than chance, indicated by one sample t-test (Figure 3C, control (F), t(12)=2.660, p=0.0208; control (M), t(16)=4.349, p=0.0005). In contrast, methotrexate-treated females and males both displayed a discrimination index that did not differ significantly from chance, indicating cognitive deficits. However, a similar percentage of methotrexate-treated female and male animals failed tests compared to the control females and males when conducting a Chi-Square test (control (F) vs MTX (F): 15.38% vs 30.76%; control (M) vs MTX (M): 17.65% vs 33.33 %). When females and males in both groups were combined, both control and methotrexate-treated group demonstrated intact visual memory, indicated by one sample t-test (Control: t(29)=5.047, p<0.0001; MTX: t(30)=2.387, p=0.0235).
3.4. Female methotrexate-treated animals displayed reduced number of proliferative cells in the brain at 66 weeks of age
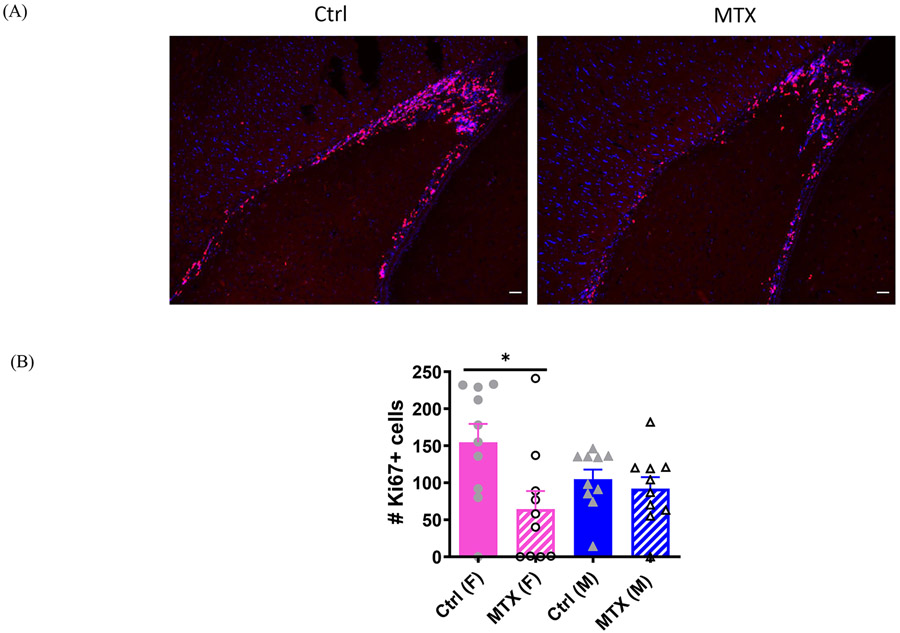
At 66 weeks of age, Ki67+ cells were predominantly located in the rostral migratory stream (RMS) and subventricular zone (SVZ). When both sexes were combined, methotrexate-treated animals displayed a significant reduction in neurogenesis compared to the controls (control:129.75±20.88 vs MTX:78.25±20.50, t(38)=2.489, p=0.0173). However, when separated by sex, the difference in the number of Ki67 positive cells was significant only among the female animals (Figure 4A,B, Kruskal-Wallis(3,36)=8.533, p=0.0362; control (F) vs MTX (F), z(19)=2.813, p=0.0294), while no significant differences were observed between the methotrexate-treated and control males (Figure 4B).
Figure 4. Ki 67 staining in brain sections of control and methotrexate treated animals.
A representative image of Ki67 staining in the periventricular area (lateral ventricle) of control and methotrexate treated female animals is shown in (A). The quantitation of the number of Ki67+ cells in a brain sagittal section is shown in (B). Ki67+ cells are located predominantly in the subventricular zone and rostral migratory stream. The number of animals in the control and methotrexate treated groups is 10 (F), 10 (M) and 10 (F), 10 (M), respectively. The scale bars in all the figures refer to 100 um. The scale bars in all the figures refer to 100 μm.
3.5. Methotrexate-treated juvenile animals demonstrated increased number of apoptotic cells in the periventricular area at 66 weeks of age
At 66 weeks of age, TUNEL positivity was observed primarily in the periventricular area including the subventricular zone and rostral migratory stream (Figure 5A), with relatively few apoptotic cells in the cortex, hippocampus, and corpus callosum. When both sexes were combined, MTX-treated animals displayed significantly more TUNEL+ cells in the periventricular areas compared to the controls (control: 8.1±3.0 vs MTX: 21.8±6.9, t(38)=2.497, p=0.017, Figure 5B). No significant differences were observed in the prefrontal and frontal cortex, hippocampus, or corpus callosum (data not shown). An increase in TUNEL+ cells was seen in both female and male MTX-treated animals compared to controls, although the within-sex differences were not statistically significant (Figure 5A, C).
Figure 5. TUNEL staining in brain sections of control and methotrexate treated animals.
A representative image of TUNEL staining in the periventricular area (lateral ventricle) of control and methotrexate-treated male animals is shown in (A). The quantitation of the number of TUNEL+ cells in this area is shown in (B) when sexes are combined. TUNEL+ cells are predominantly located in the subventricular zone and rostral migratory stream. (C) indicates the quantitation of the number of TUNEL + cells in each group when we separate sexes. The number of animals in the control and methotrexate treated groups is 10 (F), 10 (M) and 10 (F), 10 (M), respectively.
3.6. Female methotrexate-treated animals demonstrated increased microglial activation but not astrocyte activation (astrogliosis) in the hippocampus at 66 weeks of age
We analyzed microglial activation in three areas of the brain: cortex, hippocampus, and corpus callosum. No significant differences were observed in Iba-1 staining in the cortex or corpus callosum. However, significantly elevated microglial activation was seen in the DG area of hippocampus after methotrexate treatment (control: 1.00±0.11 vs MTX: 2.51±0.37, t(29)=4.015, p=0.0004). When the groups were split by sex, we found that microglial activation was significantly upregulated in the DG in the methotrexate-treated females compared to the control females (Figure 6A,B, Kruskal-Wallis(3,27)=15.14, p=0.0017; z(14)=3.658, p=0.0015), while methotrexate-treated males displayed identical Iba-1 staining pattern compared to the control males (Figure 6B). There were no significant differences in astrogliosis, indicated by GFAP staining, in the cortex (Supplemental figure1A, D), hippocampus (Supplemental figure1B, E) or corpus callosum (Supplemental figure1C, F).
Figure 6. Iba-1 staining in brain sections of control and methotrexate treated animals.
A representative image of Iba-1 staining in the hippocampus of control and methotrexate-treated female animals is shown in (A). The quantitation of the intensity of Iba-1 staining in the hippocampus is shown in (B). The Iba-1 relative intensity refers to the fold changes of fluorescence intensity in MTX group compared to the controls by setting the mean fluorescence intensity in control group as 1. The number of animals in the control and methotrexate treated groups is 8 (F), 7 (M) and 8 (F), 8 (M), respectively.
3.7. Methotrexate treatment did not affect the number of oligodendrocytes and myelination in the hippocampus at 66 weeks of age
APC is a marker for oligodendrocytes that synthesize myelin sheath, and MBP is a marker for myelin. APC and MBP staining demonstrated the number of oligodendrocytes (supplemental figure 2A-C) and myelin synthesis (supplemental figure 2B-D) in the hippocampus were not affected after methotrexate treatment.
4. Discussion
While many components of treatment for childhood ALL can be associated with neurotoxicity, the antifolate drug methotrexate is thought to be the chemotherapeutic agent most responsible for cognitive deficits that persist into the survivorship years (Duffner, Armstrong et al. 2014, Foster, Zheng et al. 2021, van der Plas, Modi et al. 2021). We have previously shown that MTX administered to juvenile rats in a regimen mimicking therapy for childhood leukemia causes memory impairments that last through adolescence, at least 8 weeks after the last dose (Wen, Maxwell et al. 2018). In this report, we extend those findings, by demonstrating that the deficits persist into adulthood more than one year following the final MTX injection, along with corresponding neuropathological changes including decreased neurogenesis, increased apoptosis, and microglial activation.
In most studies of childhood leukemia survivors, girls exhibit more frequent cognitive deficits than boys (van der Plas, Modi et al. 2021), even though childhood ALL is more frequent among boys (Esparza and Sakamoto 2005) and boys are exposed to more chemotherapy as they are treated for a year longer than girls on some current protocols (Teachey, Hunger et al. 2021). In this current study, we were able to describe sex-related differences in susceptibility to MTX-related cognitive deficits and neuropathology, which may prove to be a starting point toward understanding the observed disparity between male and female leukemia survivors. First, we note a MTX-independent difference between sexes in age-related cognitive function. Among the control females, spatial memory (object placement task) was intact at 13 and 17 weeks of age, but impaired at 1 year, while control males retained intact spatial memory through this late time point. This observation is consistent with previous literature showing a decline in spatial memory in one and two-year old female rats (Markowska 1999), as well as clinical data indicating increased deficits in older women with Alzheimer’s disease (Li and Singh 2014) or stroke (Bushnell, Chaturvedi et al. 2018) than in men. Both sets of data are consistent with the known impact of changes in sex hormones on cognitive function in older adulthood (Gurvich, Hoy et al. 2018).
In our animal model, we found evidence supporting three components of the multifactorial mechanism of MTX-induced cognitive dysfunction, including the inhibition of neurogenesis, increased neuroinflammation, and apoptotic cell death. Notably, there were sex differences in susceptibilities to these MTX-induced changes. Our data showed that methotrexate treatment significantly suppressed neurogenesis, with a greater magnitude of differences in females compared to males. Additionally, TUNEL staining demonstrated that there were increased apoptotic cells in the periventricular area in the methotrexate-treated group with a greater fold change in females as well. This may be attributed to the role of androgen, which has shown to stimulate cell proliferation and protect cell from apoptosis (Nguyen, Jayaraman et al. 2010). Therefore, manipulating neurogenesis and neuron apoptosis by antipsychotic drugs (Apple, Fonseca et al. 2017) or neural stem cell transplant (Acharya, Martirosian et al. 2015), may be a potential therapeutic approach to alleviate the neurotoxicity of chemotherapy.
Microglia, as the main immune cell in the brain, plays an important role in maintaining optimal CNS function. In response to inflammatory or infectious damage, activated anti-inflammatory microglia (M1 phenotype) eliminate damaged structures and initiate the repair process. However, microglial activation status depends on the timing and the specific type of inflammatory milieu. Excessive inflammation in the brain can stimulate microglia to secrete more cytokines (M2 phenotype), resulting in more inflammation, tissue damage, and scar formation (Colonna and Butovsky 2017). We previously showed that MTX-treated rats demonstrated suppressed microglial activation at 10 weeks of age, with the trend gone at 20 weeks of age (Wen, Maxwell et al. 2018). In this study, at 1 year of age, methotrexate-treated animals displayed enhanced microglial activation in the hippocampus with greater fold change in females. Similarly, Gilbson et al reported that when MTX was administered intraperitoneally at 100 mg/kg weekly for three consecutive weeks in mice, increased microglial activation persisted for at least 6 months in white matter (Gibson, Nagaraja et al. 2019). However, Berlin et al. found that total microglia cell populations were suppressed in response to methotrexate with a different regimen (Berlin, Lange et al. 2020). Interestingly, sex differences also play a role in microglial activation. Evidence has suggested that a pro-inflammatory milieu develops with age in females (Nissen 2017). At 12-14 months of age, female wild-type mice have increased microglial activation compared to the males (Biechele, Franzmeier et al. 2020). In a CNS injury model, male mice have more resting or anti-inflammatory microglia, while female mice have more inflammatory phenotype (Villapol, Loane et al. 2017). In addition, Gilbson et al. demonstrated depletion of microglia by PLX5622, a colony-stimulating factor 1 receptor (CSF1R) inhibitor have shown to normalize oligodendroglial lineage dynamics, protect myelin microstructure, and improve cognition after MTX treatment (Gibson, Nagaraja et al. 2019).
Ongoing studies in our lab will help determine how these histopathological alterations remain more than 1 year after MTX exposure. There are several possible mechanisms that may account for this. First, methotrexate may compromise the blood brain barrier (BBB). By preventing remethylation of homocysteine to methionine, MTX exposure predictably leads to an increase in homocysteine in blood, cerebrospinal fluid, and brain parenchyma (Li, Vijayanathan et al. 2010, Wen, Maxwell et al. 2018). Through induction of oxygen radical formation, homocysteine is toxic to vascular endothelium, leading to increased permeability of the BBB in an animal model of Alzheimer’s disease (Kamath, Chauhan et al. 2006, Ehrlich and Humpel 2012) as well as in patients with amyotrophic lateral sclerosis (Wu, Yang et al. 2020). Interestingly, our histopathological findings were mostly located in the periventricular area and hippocampus where BBB is the most fragile (Ueno, Akiguchi et al. 2000, Wen, Doerner et al. 2015). Once the BBB is compromised, chronic neuroinflammation may occur, leading to subsequent cognitive dysfunction (Wen, Doerner et al. 2015). Second, methotrexate may cause permanent neuron damage. Although there were no differences in the number of oligodendrocytes and myelin sheath synthesis between methotrexate and control group in our study, Gibson et al. have demonstrated the myelin microstructure damage and alteration of oligodendroglial lineage with a different chemotherapy regimen (Gibson, Nagaraja et al. 2019).
5. Conclusion
This study illustrates that methotrexate can induce cognitive impairment and histopathological changes including depressed neurogenesis, increased brain cell apoptosis, and enhanced hippocampal microglial activation that persist into adulthood, 1 year after drug exposure. In addition, the observed sex differences in cognitive function and neuropathology begin to explain the clinical observation that girls are more susceptible to chemotherapy induced cognitive impairment. These findings may provide insights for developing therapeutic approaches to alleviate the neurotoxicity of MTX for children with ALL.
Supplementary Material
Supplemental figure 1. GFAP staining in brain sections of control and methotrexate treated animals. A representative image of GFAP staining in the cortex, hippocampus (mostly DG area) and corpus callosum of control and methotrexate-treated female animals is shown in (A-C). The quantitation of the intensity of GFAP staining in the cortex, hippocampus and corpus callosum is shown in (D), (E) and (F), respectively. The GFAP relative intensity refers to the fold changes of fluorescence intensity in MTX group compared to the controls by setting the mean fluorescence intensity in control group as 1. The number of animals in the control and methotrexate treated groups for cortex is 10 (F), 10 (M) and 10 (F), 10 (M), respectively. For hippocampus, 10 (F), 8 (M) and 7 (F), 9 (M), respectively. For corpus callosum, 8 (F), 8 (M) and 10 (F), 10 (M), respectively.
Supplemental figure 2. APC and MBP staining in the hippocampus of control and methotrexate treated animals. A representative image of APC and MBP staining in the hippocampus of control and methotrexate-treated female animals is shown in A and D, respectively. The quantitation of APC staining is shown in (B), when we separate sexes, and (C) when sexes are combined. The quantitation of MBP staining is shown in (E), when we separate sexes, and (F) when sexes are combined. The number of animals used in APC staining in the control and methotrexate treated groups is 5 (F), 5 (M) and 5 (F), 4 (M), respectively. And for MBP staining, 9 (F), 6 (M) and 7 (F), 6 (M), respectively.
Highlights.
A clinically relevant regimen of systemic and intrathecal MTX administered to juvenile animals induces persistent deficits in cognition, lasting into adulthood, more than 1 year after the last drug exposure.
Pathologic examination demonstrated suppression of neurogenesis, microglial activation, and increased brain cell apoptosis.
Differences between male and female animals suggest the sexes differ in susceptibility to components of MTX-induced neuropathology.
Acknowledgements:
This work was supported by NIH/NCI R01-CA182284 and NIH T32 GM135141, Embrace Kids Foundation and Hugs for Brady Foundation. The authors have no conflict of interests.
Footnotes
CRediT author statement
The corresponding author (Cole) is responsible for ensuring that the descriptions are accurate and agreed by all authors.
Jing Wen, Conceptualization, Methodology, Investigation, Writing, Reviewing and Editing
Chadni Patel, Investigation, Writing, Reviewing and Editing
Frank Diglio, Methodology, Investigation, Writing, Reviewing and Editing
Kayla Baker, Investigation, Reviewing and Editing
Gregory Marshall, Investigaion, Reviewing and Editing
Shengguo Li, Investigation, Supervision, Reviewing and Editing
Peter D. Cole, Conceptualization, Methodology, Supervision, Funding Acquisition, Writing, Reviewing and Editing
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya MM, Martirosian V, Chmielewski NN, Hanna N, Tran KK, Liao AC, Christie LA, Parihar VK and Limoli CL (2015). "Stem cell transplantation reverses chemotherapy-induced cognitive dysfunction." Cancer Res 75(4): 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA and Saykin AJ (2007). "Candidate mechanisms for chemotherapy-induced cognitive changes." Nat Rev Cancer 7(3): 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluise CD, Miriyala S, Noel T, Sultana R, Jungsuwadee P, Taylor TJ, Cai J, Pierce WM, Vore M, Moscow JA, St Clair DK and Butterfield DA (2011). "2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-α release: Implications for the reactive oxygen species-mediated mechanisms of chemobrain." Free Radical Biology and Medicine 50(11): 1630–1638. [DOI] [PubMed] [Google Scholar]
- Apple DM, Fonseca RS and Kokovay E (2017). "The role of adult neurogenesis in psychiatric and cognitive disorders." Brain Res 1655: 270–276. [DOI] [PubMed] [Google Scholar]
- Ataie A, Sabetkasaei M, Haghparast A, Moghaddam AH and Kazeminejad B (2010). "Neuroprotective effects of the polyphenolic antioxidant agent, Curcumin, against homocysteine-induced cognitive impairment and oxidative stress in the rat." Pharmacol Biochem Behav 96(4): 378–385. [DOI] [PubMed] [Google Scholar]
- Berlin C, Lange K, Lekaye HC, Hopland K, Phillips S, Piao J and Tabar V (2020). "Long-term clinically relevant rodent model of methotrexate-induced cognitive impairment." Neuro Oncol 22(8): 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biechele G, Franzmeier N, Blume T, Ewers M, Luque JM, Eckenweber F, Sacher C, Beyer L, Ruch-Rubinstein F, Lindner S, Gildehaus FJ, von Ungern-Sternberg B, Cumming P, Bartenstein P, Rominger A, Hoglinger GU, Herms J and Brendel M (2020). "Glial activation is moderated by sex in response to amyloidosis but not to tau pathology in mouse models of neurodegenerative diseases." J Neuroinflammation 17(1): 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer CA, Postma A, Hooimeijer HLH, Smit AJ, Vonk JM, van Roon AM, van den Berg MP, Dolsma WV, Lefrandt JD and Bink-Boelkens M (2013). "Endothelial damage in long-term survivors of childhood cancer." Journal of clinical Oncology 31(31): 3906–3913. [DOI] [PubMed] [Google Scholar]
- Bushnell CD, Chaturvedi S, Gage KR, Herson PS, Hurn PD, Jimenez MC, Kittner SJ, Madsen TE, McCullough LD, McDermott M, Reeves MJ and Rundek T (2018). "Sex differences in stroke: Challenges and opportunities." J Cereb Blood Flow Metab 38(12): 2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciernikova S, Mego M and Chovanec M (2021). "Exploring the Potential Role of the Gut Microbiome in Chemotherapy-Induced Neurocognitive Disorders and Cardiovascular Toxicity." 13(4): 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PD, Finkelstein Y, Stevenson KE, Blonquist TM, Vijayanathan V, Silverman LB, Neuberg DS, Sallan SE, Robaey P and Waber DP (2015). "Polymorphisms in Genes Related to Oxidative Stress Are Associated With Inferior Cognitive Function After Therapy for Childhood Acute Lymphoblastic Leukemia." Journal of Clinical Oncology 33(19): 2205–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PD and Kamen BA (2006). "Delayed neurotoxicity associated with therapy for children with acute lymphoblastic leukemia." Mental Retardation & Developmental Disabilities Research Reviews 12(3): 174–183. [DOI] [PubMed] [Google Scholar]
- Colonna M and Butovsky O (2017). "Microglia Function in the Central Nervous System During Health and Neurodegeneration." Annu Rev Immunol 35: 441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffner PK, Armstrong FD, Chen L, Helton KJ, Brecher ML, Bell B and Chauvenet AR (2014). "Neurocognitive and neuroradiologic central nervous system late effects in children treated on Pediatric Oncology Group (POG) P9605 (standard risk) and P9201 (lesser risk) acute lymphoblastic leukemia protocols (ACCL0131): a methotrexate consequence? A report from the Children's Oncology Group." J Pediatr Hematol Oncol 36(1): 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffner PK, Armstrong FD, Chen L, Helton KJ, Brecher ML, Bell B and Chauvenet AR (2014). "Neurocognitive and Neuroradiologic Central Nervous System Late Effects in Children Treated on Pediatric Oncology Group (POG) P9605 (Standard Risk) and P9201 (Lesser Risk) Acute Lymphoblastic Leukemia Protocols (ACCL0131): A Methotrexate Consequence? A Report From the Children’s Oncology Group." Journal of Pediatric Hematology/Oncology 36(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich D and Humpel C (2012). "Chronic vascular risk factors (cholesterol, homocysteine, ethanol) impair spatial memory, decline cholinergic neurons and induce blood-brain barrier leakage in rats in vivo." J Neurol Sci 322(1-2): 92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A and Meliani K (1992). "A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. non-spatial working memory." Behav Brain Res 51(1): 83–92. [DOI] [PubMed] [Google Scholar]
- Esparza SD and Sakamoto KM (2005). "Topics in pediatric leukemia--acute lymphoblastic leukemia." MedGenMed 7(1): 23. [PMC free article] [PubMed] [Google Scholar]
- Foster R, Zheng DJ, Netson-Amore KL and Kadan-Lottick NS (2021). "Cognitive Impairment in Survivors of Pediatric Extracranial Solid Tumors and Lymphomas." J Clin Oncol 39(16): 1727–1740. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L, Woo PJ, Barres BA, Liddelow S, Vogel H and Monje M (2019). "Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment." Cell 176(1-2): 43–55 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson TM, Mostoufi-Moab S, Stratton KL, Leisenring WM, Barnea D, Chow EJ, Donaldson SS, Howell RM, Hudson MM and Mahajan A (2018). "Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–99: a report from the Childhood Cancer Survivor Study cohort." The Lancet Oncology 19(12): 1590–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvich C, Hoy K, Thomas N and Kulkarni J (2018). "Sex Differences and the Influence of Sex Hormones on Cognition through Adulthood and the Aging Process." Brain Sci 8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy KK, Embry L, Kairalla JA, Helian S, Devidas M, Armstrong D, Hunger S, Carroll WL, Larsen E, Raetz EA, Loh ML, Yang W, Relling MV, Noll RB and Winick N (2017). "Neurocognitive Functioning of Children Treated for High-Risk B-Acute Lymphoblastic Leukemia Randomly Assigned to Different Methotrexate and Corticosteroid Treatment Strategies: A Report From the Children’s Oncology Group." Journal of Clinical Oncology 35(23): 2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, Yeazel M, Recklitis CJ, Marina N and Robison LRJJ (2003). "Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study." 290(12): 1583–1592. [DOI] [PubMed] [Google Scholar]
- Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE and Wagner DD (2006). "Elevated levels of homocysteine compromise blood-brain barrier integrity in mice." Blood 107(2): 591–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff AC, Krull KR, Ness KK, Armstrong GT, Park ER, Stovall M, Robison LL and Leisenring W (2011). "Physical, Mental, and Neurocognitive Status and Employment Outcomes in the Childhood Cancer Survivor Study Cohort." Cancer Epidemiology Biomarkers & Prevention 20(9): 1838–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull KR, Hardy KK, Kahalley LS, Schuitema I and Kesler SR (2018). "Neurocognitive Outcomes and Interventions in Long-Term Survivors of Childhood Cancer." Journal of Clinical Oncology 36(21): 2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin-Batson A, Kadan-Lottick N and Neglia JP (2014). "The contribution of neurocognitive functioning to quality of life after childhood acute lymphoblastic leukemia." Psycho-Oncology 23(6): 692–699. [DOI] [PubMed] [Google Scholar]
- Li R and Singh M (2014). "Sex differences in cognitive impairment and Alzheimer's disease." Front Neuroendocrinol 35(3): 385–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Vijayanathan V, Gulinello M and Cole PD (2010). "Intrathecal methotrexate induces focal cognitive deficits and increases cerebrospinal fluid homocysteine." Pharmacol Biochem Behav 95(4): 428–433. [DOI] [PubMed] [Google Scholar]
- Markowska AL (1999). "Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle." J Neurosci 19(18): 8122–8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PC, Henderson TO, Kirchhoff AC, Park ER and Yabroff KR (2018). "Financial Hardship and the Economic Effect of Childhood Cancer Survivorship." Journal of Clinical Oncology 36(21): 2198–2205. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Jayaraman A, Quaglino A and Pike CJ (2010). "Androgens selectively protect against apoptosis in hippocampal neurones." J Neuroendocrinol 22(9): 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen JC (2017). "Microglial Function across the Spectrum of Age and Gender." Int J Mol Sci 18(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Yam PP-Y, Yang LS, Sato S, Li CK, Cheung YTJC and Reviews M (2020). "Neurocognitive impairment in Asian childhood cancer survivors: a systematic review." 1–15. [DOI] [PubMed] [Google Scholar]
- Raver CC, Blair C and Willoughby M. J. D. p. (2013). "Poverty as a predictor of 4-year-olds' executive function: new perspectives on models of differential susceptibility." 49(2): 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigers R and Fardell JE (2011). "Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research." Neuroscience and biobehavioral reviews 35(3): 729–741. [DOI] [PubMed] [Google Scholar]
- Seigers R, Schagen SB, Coppens CM, van der Most PJ, van Dam FS, Koolhaas JM, Buwalda B, Seigers R, Schagen SB, Coppens CM, van der Most PJ, van Dam FSAM, Koolhaas JM and Buwalda B (2009). "Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats." Behavioural Brain Research 201(2): 279–284. [DOI] [PubMed] [Google Scholar]
- Sleurs C, Lemiere J, Radwan A, Verly M, Elens I, Renard M, Jacobs S, Sunaert S, Deprez S and Uyttebroeck A (2019). "Long-term leukoencephalopathy and neurocognitive functioning in childhood sarcoma patients treated with high-dose intravenous chemotherapy." Pediatric Blood & Cancer 0(0): e27893. [DOI] [PubMed] [Google Scholar]
- Tan CJ, Lim SWT, Toh YL, Ng T, Yeo A, Shwe M, Foo KM, Chu P, Jain A and Koo S.-L. J. M. n. (2019). "Replication and meta-analysis of the association between BDNF Val66Met polymorphism and cognitive impairment in patients receiving chemotherapy." 56(7): 4741–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Hunger SP and Loh ML (2021). "Optimizing therapy in the modern age: differences in length of maintenance therapy in acute lymphoblastic leukemia." Blood 137(2): 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen AM, Gulinello ME, Wen J, Schmiegelow K and Cole PD (2017). "Liposomal Cytarabine Induces Less Neurocognitive Dysfunction Than Intrathecal Methotrexate in an Animal Model." Journal of Pediatric Hematology/Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D and Fundytus M (1988). "Thigmotaxis as a test for anxiolytic activity in rats." Pharmacol Biochem Behav 31(4): 959–962. [DOI] [PubMed] [Google Scholar]
- Ueno M, Akiguchi I, Hosokawa M, Kotani H, Kanenishi K and Sakamoto H (2000). "Blood-brain barrier permeability in the periventricular areas of the normal mouse brain." Acta Neuropathol 99(4): 385–392. [DOI] [PubMed] [Google Scholar]
- van der Plas E, Modi AJ, Li CK, Krull KR and Cheung YT (2021). "Cognitive Impairment in Survivors of Pediatric Acute Lymphoblastic Leukemia Treated With Chemotherapy Only." J Clin Oncol 39(16): 1705–1717. [DOI] [PubMed] [Google Scholar]
- van der Plas E, Noakes TLS, Butcher DT, Weksberg R, Galin-Corini L, Wanstall EA, Te P, Hopf L, Guger S and Hitzler JJPR (2020). "Cognitive and behavioral risk factors for low quality of life in survivors of childhood acute lymphoblastic leukemia." 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayanathan V, Ali N, Gulinello M and Cole PD (2011). "Persistent cognitive deficits, induced by intrathecal methotrexate, are associated with elevated CSF concentrations of excitotoxic glutamate analogs and can be reversed by an NMDA antagonist." Behavioural Brain Research 225(2): 491–497. [DOI] [PubMed] [Google Scholar]
- Villapol S, Loane DJ and Burns MP (2017). "Sexual dimorphism in the inflammatory response to traumatic brain injury." Glia 65(9): 1423–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Yin J, Miller AH and Xiao C (2017). "A systematic review of the association between fatigue and genetic polymorphisms." Brain, Behavior, and Immunity 62: 230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Doerner J, Weidenheim K, Xia Y, Stock A, Michaelson JS, Baruch K, Deczkowska A, Gulinello M, Schwartz M, Burkly LC and Putterman C (2015). "TNF-like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/lpr mice." J Autoimmun 60: 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Maxwell RR, Wolf AJ, Spira M, Gulinello ME and Cole PD (2018). "Methotrexate causes persistent deficits in memory and executive function in a juvenile animal model." Neuropharmacology 139: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Yang X, Li X, Wang H and Wang T (2020). "Elevated cerebrospinal fluid homocysteine is associated with blood-brain barrier disruption in amyotrophic lateral sclerosis patients." Neurol Sci 41(7): 1865–1872. [DOI] [PubMed] [Google Scholar]
- Zhou C, Zhuang Y, Lin X, Michelson AD and Zhang A (2019). "Changes in neurocognitive function and central nervous system structure in childhood acute lymphoblastic leukaemia survivors after treatment: a meta - analysis." British Journal of Haematology. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. GFAP staining in brain sections of control and methotrexate treated animals. A representative image of GFAP staining in the cortex, hippocampus (mostly DG area) and corpus callosum of control and methotrexate-treated female animals is shown in (A-C). The quantitation of the intensity of GFAP staining in the cortex, hippocampus and corpus callosum is shown in (D), (E) and (F), respectively. The GFAP relative intensity refers to the fold changes of fluorescence intensity in MTX group compared to the controls by setting the mean fluorescence intensity in control group as 1. The number of animals in the control and methotrexate treated groups for cortex is 10 (F), 10 (M) and 10 (F), 10 (M), respectively. For hippocampus, 10 (F), 8 (M) and 7 (F), 9 (M), respectively. For corpus callosum, 8 (F), 8 (M) and 10 (F), 10 (M), respectively.
Supplemental figure 2. APC and MBP staining in the hippocampus of control and methotrexate treated animals. A representative image of APC and MBP staining in the hippocampus of control and methotrexate-treated female animals is shown in A and D, respectively. The quantitation of APC staining is shown in (B), when we separate sexes, and (C) when sexes are combined. The quantitation of MBP staining is shown in (E), when we separate sexes, and (F) when sexes are combined. The number of animals used in APC staining in the control and methotrexate treated groups is 5 (F), 5 (M) and 5 (F), 4 (M), respectively. And for MBP staining, 9 (F), 6 (M) and 7 (F), 6 (M), respectively.