Abstract
Established roles for PI3K and MAPK signaling pathways in tumorigenesis has prompted extensive research towards the discovery of small-molecule inhibitors as cancer therapeutics. However, significant compensatory regulation exists between these two signaling cascades, leading to redundancy among survival pathways. Consequently, initial clinical trials aimed at either PI3K or MEK inhibition alone have proven ineffective and highlight the need for development of targeted and innovative therapeutic combination strategies. We designed a series of PI3K inhibitor derivatives wherein a single morpholine group of the PI3K inhibitor ZSTK474 was substituted with a variety of 2-aminoethyl functional groups. Analogs with pendant hydroxyl or methoxy groups maintained low nanomolar inhibition towards PI3Kα, PI3Kγ, and PI3Kδ isoforms in contrast to those with pendant amino groups which were significantly less inhibitory. Synthesis of prototype PI3K/MEK bifunctional inhibitors (6r, 6s) was guided by the structure-activity data, where a MEK-targeting inhibitor was tethered directly via a short PEG linker to the triazine core of the PI3K inhibitor analogs. These compounds (6r, 6s) displayed nanomolar inhibition towards PI3Kα, δ, and MEK (IC50 ~105 to 350 nM), and low micromolar inhibition for PI3Kβ and PI3Kγ (IC50 ~1.5 to 3.9 μM) in enzymatic inhibition assays. Cell viability assays demonstrated superior anti-proliferative activity for 6s over 6r in three tumor-derived cell lines (A375, D54, SET-2), which correlated with inhibition of downstream AKT and ERK1/2 phosphorylation. Compounds 6r and 6s also demonstrated in vivo tolerability with therapeutic efficacy through reduction of kinase activation and amelioration of disease phenotypes in the JAK2V617F mutant myelofibrosis mouse cancer model. Taken together, these results support further structure optimization of 6r and 6s as promising leads for combination therapy in human cancer as a new class of PI3K/MEK bifunctional inhibitors.
Keywords: Bifunctional MEK/PI3K inhibitor, ZSTK474, PI3K isoform inhibition
Graphical Abstract

Introduction
The PI3K and MAPK signaling pathways play crucial regulatory roles in many aspects of cell growth and survival under normal and pathological conditions [1–4]. Aberrant activation of these signaling cascades due to somatic mutations in key pathway components leads to uncontrolled kinase activity in many human cancers including leukemia, melanoma, breast, ovarian, lung and prostate cancer [5–7]. The broad implications of these pathways in tumorigenesis has been supportive of extensive drug discovery efforts towards development of small-molecule inhibitors for cancer therapy. However, increasing evidence has shown that regulatory cross-talk between these two pathways can lead to induction of therapeutic resistance through redundant and compensatory signaling mechanisms [2, 8–12]. Advancements in targeted combination therapy and innovative treatment strategies are thus urgently needed.
Differential regulation of PI3K and MAPK signal transduction has implied that simultaneous co-targeting of these two pathways may provide a viable alternative strategy for more effective cancer therapy [4, 13, 14]. Synergistic tumor growth suppression using PI3K and/or mTOR inhibitors administered in combination with MEK inhibitors has been demonstrated in many preclinical trials, confirming the effectiveness of this strategy [10, 15–18]. There is currently an intense focus in the pharmaceutical industry towards the discovery of single-agent, multifunctional drugs for modulation of multiple biological targets [19, 20]. In principle, co-targeting multiple pathways with a single agent, rather than two separate molecules, has several advantages; simpler dosing schedules, more uniform pharmacokinetics and pharmacodynamics for inhibition of desired targets, and the potential for reduced toxicity.
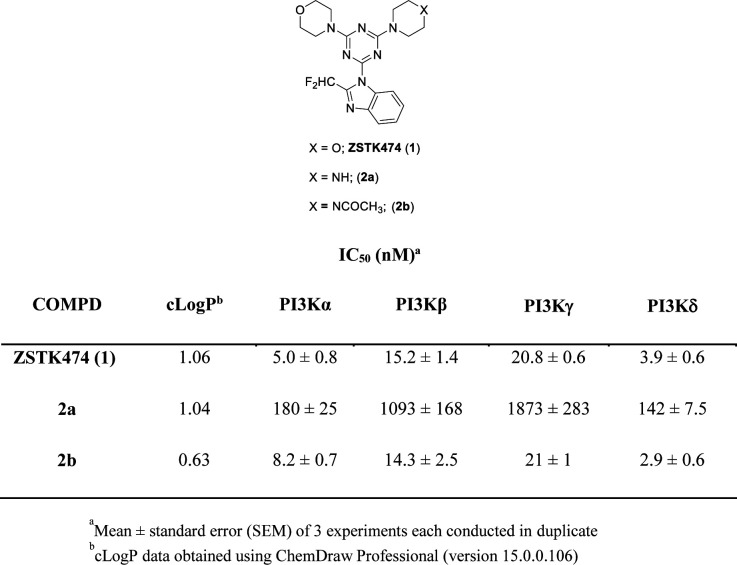
As an approach to targeted multi-kinase inhibition, we previously reported a series of prototypic, single-entity, small-molecule PI3K/MEK bifunctional inhibitors capable of simultaneous inhibition of these two dominant KRAS effector pathways [21–23]. We chose the ATP-competitive, pan-Class I PI3K inhibitor ZSTK474 [24] (1; Figure I) as the targeting moiety for these studies based on its superior PI3K isoform inhibition profile and synthetic considerations which permitted its covalent linking to a MEK targeting inhibitor. The PI3K family of enzymes comprises three major categories (Class I, II and III, respectively) based on their structure and substrate specificity. Class I PI3Ks are further subdivided into two subclasses: Class IA (comprising the PI3Kα, β, δ isoforms) activated by receptor tyrosine kinases and Class IB (PI3Kγ isoform) activated by G-protein coupled receptors [25, 26]. In particular, pan-Class I PI3K inhibitors such as ZSTK474, which belong to the “flat conformation inhibitor” category, do not display PI3K isoform selectivity as they are excluded from access to the specificity pocket in the active site of the enzyme [27, 28].
Fig. 1.
PI3K Isoform Inhibition of ZSTK474 and piperazine substituted analogs.
It is well established that of the two equivalent morpholine groups in ZSTK474, only one is involved in binding interactions at the PI3K enzyme active site [27, 29]. Analysis of the literature showed that structure-activity relationship (SAR) studies of ZSTK474 analogs having morpholine group replacements were not fully explored [29–32]. In the present study we focused on developing a new series of PI3K/MEK bifunctional inhibitors where the MEK targeting moiety was directly tethered by a PEG linker to the 1,3,5-triazine core of ZSTK474. Accordingly, we focused on conducting an initial SAR evaluation of ZSTK474 analogs containing a variety of 2-aminoethyl containing functional groups as a replacement for morpholine towards linker optimization. Two representative analogs from this series of PI3K ligands based on their PI3K inhibition profile were selected as templates for conjugation to the MEK inhibitor analog PD0316684 for development of prototype PI3K/MEK bifunctional inhibitors. The SAR and preliminary biological investigation of these new PI3K analogs and PI3K/MEK bifunctional kinase inhibitors are disclosed in this report.
1. Results and Discussion
2.1. Synthesis and PI3K Isoform Inhibition of ZSTK474 Analogs
In previous studies we observed that substitution of a single morpholine group in ZSTK474 with piperazine to give analog 2a (Figure 1) resulted in a 36-fold reduction in its PI3Kα and PI3Kδ inhibition and a >70-fold reduction in PI3Kβ and PI3Kγ inhibition highlighting the sensitivity of this region towards oxygen replacement [21]. However, N-acetylation of 2a to give 2b restored its PI3K isoform inhibition profile (IC50 = 2.9 to 21 nM) similar to that of ZSTK474 (IC50 = 3.9 to 20.8 nM) (Figure 1) [21]. To further investigate the role of the morpholine oxygen in these compounds on PI3K inhibition, a series of ZSTK474 derivatives having a variety of 2-aminoethyl functionalities as morpholine group replacements were synthesized for additional SAR exploration. Synthesis of the key secondary amine intermediates (3, 4) were carried out as shown in Scheme 1. In brief, reaction of 2-bromoethanol in excess 2-methoxyethylamine afforded 3 in 82% yield after chromatographic purification. Similarly, reaction of Boc-aminoxy-(PEG)3 bromide in excess ethanolamine afforded 4 in 46% yield.
Scheme 1.

Synthetic route for key intermediates.
Synthesis of compounds 6a – 6k were conducted as shown in Figure 2 by condensation of the 4-chloro-1,3,5 triazine derivative 5 [30] with appropriate amine derivatives (or terminal Boc-protected amines) in refluxing acetone. TFA catalyzed Boc-deprotection of analogs 6h - 6k provided compounds 6l – 6o in 75 – 93% yield after chromatography. Compounds 6l and 6m were reacted with acetic anhydride to give the corresponding terminal acetamide analogs 6p and 6q, respectively, in 84 – 96% yields. Bifunctional inhibitors 6r and 6s (Figure 3) were obtained in 63% and 82% isolated yields, respectively, by reaction of 6n and 6o with perfluorophenyl 3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)benzoate [33] (7) using previously reported methods [23]. All new compounds gave analytical and spectroscopic data consistent with their structures.
Fig. 2.
Synthesis of ZSTK474 analogs.
Fig. 3.
Synthesis and MEK and PI3K Inhibition Data for Bifunctional Inhibitors 6r and 6s.
The inhibition data for new ZSTK474 analogs are reported in Table 1. ZSTK474 (1) displays potent nanomolar inhibition towards all Class 1 PI3K isoforms, most notably towards PI3Kα (IC50 = 5.0 nM) and PI3Kδ (IC50 = 3.9 nM). Analogs having ethanolamine (6a) or diethanolamine (6b) as morpholine replacements retained high inhibition towards PI3Kα (9.9 nM and 3.7 nM, respectively) but showed reduced PI3Kδ (2.5-fold decrease) and PI3Kβ inhibition (5-fold decrease) in comparison to ZSTK474. Interestingly, the potency of these compounds towards PI3Kγ either improved (6b; 14.6 nM) or was slightly lowered (2.5-fold) in the case of 6a. Replacement of the hydroxy group in 6a with methoxy to give 6c led to a 14-fold loss in PI3Kβ inhibitory potency whereas its loss of inhibition towards the other isoforms were more modest (3 – 4 fold lower).
Table 1.
In vitro PI3K Isoform Inhibition Data for ZSTK474 analogs.
| IC50 (nM)a | |||||
|---|---|---|---|---|---|
| COMPD | cLogPb | PI3Kα | PI3Kβ | PI3Kγ | PI3Kδ |
|
| |||||
| ZSTK474 | 1.06 | 5.0 ± 0.8 | 15.2 ± 1.4 | 20.8 ± 0.6 | 3.9 ± 0.6 |
| 6a | 0.96 | 9.9 ± 1.2 | 71 ± 8 | 54 ± 1 | 8.1 ± 1.7 |
| 6b | 0.28 | 3.7 ± 1.0 | 74 ± 5.7 | 14.6 ± 1.4 | 9.9 ± 0.4 |
| 6c | 1.72 | 20 ± 3 | 208 ± 15 | 64 ± 5 | 17 ± 1 |
| 6d | 1.04 | 5.1 ± 1.1 | 136 ± 6.4 | 30.7 ± 0.9 | 8.9 ± 0.5 |
| 6e | 1.25 | 148 ± 22 | 627 ± 174 | 869 ± 11 | 46.7 ± 3.3 |
| 6f | 1.38 | 12.4 ± 0.7 | 787 ± 106 | 22.3 ± 0.7 | 60 ± 3.4 |
| 6g | 0.92 | 20 ± 0.4 | 431 ± 83 | 67 ± 2.8 | 26 ± 4.9 |
| 6l | 1.03 | 292 ± 31 | 2117 ± 444 | 771 ± 63 | 291 ± 32 |
| 6m | 1.34 | 248 ± 24 | 2900 ± 375 | 681 ± 90 | 135 ± 19 |
| 6n | 0.46 | 89.3 ± 9.5 | 1867 ± 245 | 502 ± 20 | 12.6 ± 1.0 |
| 6o | −0.22 | 10.9 ± 0.75 | 1085 ± 143 | 137 ± 21 | 8.6 ± 1.2 |
| 6p | 0.92 | 16.6 ± 1.4 | 199 ± 12 | 54 ± 1.5 | 9.5 ± 2.4 |
| 6q | 1.32 | 10.3 ± 1.0 | 404 ± 63 | 55.2 ± 11.5 | 11.7 ± 2.2 |
Mean ± standard error (SEM) of 3 experiments each conducted in duplicate
cLogP data obtained using ChemDraw Professional (version 15.0.0.106)
The monomethoxylated analog (6d) showed a similar inhibition profile as 6b although its potency towards PI3Kβ and PI3Kγ was 2-fold lower. However, substitution of a single hydroxyl in 6b with a dimethylamino group (analog 6e) led to a significantly lowered inhibition towards the PI3Kα, PI3Kβ and PI3Kγ isoforms (30 – 40 fold) as compared to ZSTK474. Similarly, morpholine replacement in ZSTK474 with either the 3-aminopropanoic acid or 3-amino-N-methylpropanamide substituent to give 6f and 6g, respectively, significantly lowered its PI3Kβ inhibition (30 – 52-fold), although its effects were less detrimental towards the remaining PI3K subtypes (2.5 – 15 fold lower). Analogs with 2-aminoethylamine or 2-N-methylethylamine substituents (compounds 6l, 6m) displayed uniformly poor inhibition across all PI3K isoforms (32 – 190-fold lower). However, N-acetylation of the pendant amine groups in compounds 6l and 6m to give 6p and 6q, respectively, led to a significant (7- to 30-fold) improvement in inhibition towards all four PI3K isoforms as seen previously for the acetylated analog 2b compared to 2a.
2.2. Synthesis and PI3K/MEK Inhibition of Bifunctional Inhibitors
Analogs 6a – 6d were selected as templates for the development of potential MEK/PI3K bifunctional inhibitors based on their superior PI3K inhibition potency and structural similarity to ZSTK474. Accordingly, analogs containing the aminoxy PEG linker intermediates 6n and 6o were synthesized and converted to the corresponding bifunctional inhibitor analogs 6r and 6s, respectively, along with potency for PD0316684 (Figure 3). The reduced MEK1 inhibition potency for 6r and 6s as compared to PD0316684 is most likely due to less favorable steric and/or electronic effects at the MEK1 allosteric pocket due to the extended PEG linker attachment. We have observed this behavior previously for similarly related compounds (21–23).
Aminoxy intermediates 6n and 6o showed highest inhibition potency towards PI3Kδ (IC50 = 12.6 and 8.6 nM, respectively), followed by PI3Kα (IC50 = 89.3 and 10.9 nM, respectively) and PI3Kγ (IC50 = 502 and 137 nM, respectively) and were least potent towards PI3Kβ (1.1 – 1.9 μM). Bifunctional inhibitors 6r and 6s displayed nanomolar inhibition towards PI3Kα (IC50 = 130 nM and 107 nM, respectively) and PI3Kδ (IC50 = 236 nM and 137 nM, respectively) with low micromolar inhibition (IC50 = 1.5 to 3.9 μM) for PI3Kβ and PI3Kγ (Figure 3). Compounds 6r and 6s also displayed inhibition in the nanomolar range towards MEK1 (IC50 = 124 nM and 352 nM, respectively). In comparison, the structurally similar allosteric MEK inhibitor PD0316684 displayed an IC50 for MEK1 inhibition of 3.2 ± 0.1 nM under the same assay conditions. The PI3Kα, PI3Kδ and MEK inhibition potencies of 6r and 6s and their isoform selectivity ratios over PI3Kβ, PI3Kγ were comparable to that of our previously reported MEK/PI3K bifunctional inhibitors, encouraging further biological evaluation [21].
2.3. Molecular docking of PI3K/MEK Bifunctional Inhibitors
A detailed molecular docking analysis of the p110δ/ZSTK474 crystal structure complex has been reported by Berndt and coworkers [27]. These studies showed that one of the morpholines of ZSTK474 assumes a chair conformation wherein its oxygen makes a key hydrogen bond interaction with the hinge Val828 backbone amide in the catalytic domain whereas, the second morpholine group assumes a twisted half-chair conformation and extends out of the ATP binding site. Additionally, the benzimidazole N-3 atom hydrogen bonds with the primary amine of Lys779 [27]. We performed molecular docking analysis on ZSTK474 and the potent inhibitor analog 6a at the ATP pocket of PI3Kδ for comparison of their respective binding modes. The docked structure of ZSTK474 (yellow) and the superposed structures of ZSTK474 and 6a (pink) within the ATP pocket are shown in Panels A and B, respectively (Figure 4). As seen in these figures, analog 6a superimposes closely with ZSTK474 replicating both hydrogen bond contacts with Val 828 and Lys779 as displayed by ZSTK474. Similarly, these hydrogen bond contacts were also maintained at the PI3Kδ ATP site for the PI3K/MEK bifunctional inhibitor 6r (purple) which superimposes closely with 6a (Panel C, Figure 4). The docked structure of 6r at the MEK1 allosteric site (Panel D of Figure 4) was evaluated using the MEK1 crystal structure in complex with the structurally similar MEK inhibitor CH4987655 [34]. As shown, 6r (orange) superimposes closely with CH4987655 (teal) showing interactions typically observed for these benzhydroxamate class of MEK inhibitors including hydrogen bonding of the hydroxamate oxygen with Lys97 and an electrostatic interaction of the iodine atom with the backbone carbonyl oxygen of Val127.
Fig. 4.
Docked structures of ZSTK474, 6a and 6r at PI3Kδ catalytic site (PDB code 2WXL) and superposed CH4987655 and 6r at MEK1 allosteric pocket (PDB code 3ORN). (A) Binding mode of ZSTK474 to PI3Kδ. (B) Overlay of docked ZSTK474 (yellow) and 6a (pink) at PI3Kδ catalytic site. (C) Overlay of docked 6a (pink) and 6r (purple) at PI3Kδ catalytic site. MEK1 binding substructure is exposed to solvent (orange; bottom center) (D) Overlay of docked CH4987655 (teal) and 6r (orange) at MEK1 allosteric site. PI3Kδ binding substructure is exposed to solvent (purple; right). Atom denotation: white (hydrogen) blue (nitrogen) red (oxygen) fluorine (green) iodine (purple). Hydrogen bonds are displayed as hashed lines with contact distances reported in angstroms (Å).
2.4. Cellular viability and in vitro functional activity of Bifunctional Inhibitors
The anti-proliferative activities of ZSTK474, 6r and 6s were evaluated against human A375 melanoma, D54 glioblastoma and SET-2 leukemia tumor cell lines with known activated MAPK and PI3K signaling pathways using a fluorometric assay. As shown in Figure 5, both inhibitors showed a dose dependent decrease in cell viability (70 – 90%), which was most pronounced in SET-2 tumor cells. The anti-proliferative activity of 6s (IC50 = 7.0 μM to 9.9 μM) was superior to that of 6r (IC50 = 10.7 μM to 17.3 μM) in all tested cell lines.
Fig. 5.
Inhibition of proliferation of tumor-derived cell lines (A) A375, (B) D54 and (C) SET-2 by 6r or 6s. Table depicts mean inhibition of proliferation IC50 values ± SEM for various cells (A375, D54, SET-2) treated for 72 h with indicated concentrations of 6r or 6s.
aData are compiled from 3 independent experiments each conducted in duplicate.
To compare the functional activity of bifunctional inhibitors 6r and 6s, cellular MEK1 and PI3K inhibition was measured by monitoring changes in phosphorylation of AKT and ERK1/2, respectively, in the three human tumor-derived cell lines: A375 (melanoma), D54 (glioblastoma) and SET-2 (megakaryoblast/leukemia). Cells were treated for 30 min with 6r or 6s (10 μM) in the presence of PDGF (excluded for SET-2 cells because these cells constitutively activate both MAPK and PI3K pathways) and cell lysates were subjected to western blot analysis (Figure 6A). Quantification of the western blot image data was performed to further evaluate the effect of 6r and 6s on suppression of pAKT and pERK1/2 (Figure 6B). These data showed that inhibition of 6r on pERK phosphorylation (66 – 85%) was significantly higher than its inhibition of pAKT phosphorylation (22 – 29%) for all tested cell types. In comparison, 6s displayed a more robust reduction than 6r of both pERK (87 – 96%) and pAKT (55 – 71%) across all cell types tested. (Figure 6C). Compound 6s was found to be significantly better than 6r for inhibition of pAKT in A375 and SET-2 cells while both compounds had similar potency across all cell types for pERK1/2 inhibition (Figure 6C).
Fig. 6.
Functional activity of 6r and 6s targets in tumor-derived cell lines. (A) Tumor-derived cell lines, A375, D54, and SET-2, were treated for 30 min with 6r (10 μM) or 6s (10 μM) combined with PDGF (50 ng/ml, excluded for SET-2) and evaluated by Western blot analysis for phosphorylation of AKT (S473) and ERK1/2 (T202/Y204). (B) Quantification of western blot image data from tumor-derived cells (A375, D54, SET-2) after treatment with 6r and 6s. Individual measurements along with the mean relative density of pERK and pAKT protein expression is reported relative to control pERK and pAKT expression normalized by total ERK and AKT, respectively. Density of phosphorylated protein was analyzed by ImageJ program. Data (mean ± SEM) are compiled from 3 independent experiments. Statistics were analyzed using ordinary one-way ANOVA multiple comparisons. * p< 0.05, ** p< 0.01, *** p< 0.001, **** p<0.0001, ns (not significant). (C) Table shows the percentage (expressed as % of control) of protein expression inhibition in A375, D54 and SET-2 cells after treatment with compounds 6r and 6s. Data (mean ± SEM) are compiled from 3 independent experiments.
These studies confirm that 6r and 6s suppress pAKT and pERK1/2 levels, which are critical components of the PI3K and MEK signaling pathways, respectively. Additionally, these studies demonstrate a correlation of the functional activity of 6r and 6s on pAKT and pERK1/2 levels, which corresponds with the tumor cell proliferation data (Figure 5). The results from three independent tumor cell types also confirm that both 6r and 6s are cell permeable and interact with intended biochemical targets and across three distinctly different tumor types.
2.5. In vivo Evaluation of Bifunctional Inhibitors in a Myelofibrosis Mouse Model
The human megakaryoblastic SET-2 cells harboring the JAK2-V617F driver mutation of human Myelofibrosis (MF) constitutively activate both ERK1/2 and AKT pathways which are down regulated upon treatment with 6r and 6s. MF is a hematologic neoplasm with marked involvement of secondary lymphoid organs leading to pronounced splenomegaly due to over-production of blood cells shifting from bone marrow to the spleen. We used the JAK2V617F MF mouse model to evaluate therapeutic efficacy of 6r and 6s by monitoring compound tissue levels, kinase target inhibition along with spleen volume changes.
The in vivo metabolic profile of 6r and 6s in selected tissues (blood, spleen, bone marrow) were evaluated using LC/MS/MS at approximately 4 h following oral dosage administration of 325 mg/kg in mice (n=4 per compound). Selected independently-synthesized metabolite standards were also evaluated to confirm the metabolic degradation profiles for 6r and 6s. Metabolism of compound 6r occurs primarily via degradation at the PI3K and MEK linker attachment to give the active metabolites MV-187 (6a) and PD0316684 and MV4–6 (Figure 7A). Metabolism of 6s occurred primarily via hydrolysis of the benzhydroxamate moiety to form MV5–46 (6o), which is then further metabolized to MV5–32 (6b) and MV4–187 (6a) (Figure 7B). Quantification of tissue-specific compound levels (ng/g) for spleen, blood and bone marrow are also provided in the corresponding graphs (Figure 7A, B). Tissue levels of 2,000 to 8,000 ng/g for 6r and 6s translates approximately to 2 – 9 μM, similar to the values observed in SET-2 cell-based assays which demonstrated significant down-modulation of pAKT and pERK with reduced proliferation (Fig. 5, 6).
Fig 7.
In vivo plasma metabolic degradation profiles of orally dosed (A) 6r and (B) 6s following compound administration (325 mg/kg) revealing potent levels of parent and biologically active metabolites. Data (mean ± SEM) are compiled from 4 independent mice.
2.6. In vivo potency of Bifunctional Inhibitors in a myelofibrosis mouse model
We analyzed disease status of mice in 6r and 6s treated and vehicle groups by magnetic resonance imaging (MRI). Reduction in spleen volume as measured by anatomic magnetic resonance imaging (MRI) is used to measure treatment efficacy in clinical trials of therapies for patients with MF. We assessed efficacy of treatment with 6r and 6s over time with abdominal MRI scanning of the mouse MF model (Figure 8A). Treatment with 6r, 6s and vehicle was initiated after transplantation of JAK2V617F hematopoietic stem cells (HSCs) when MRI showed enlargement of spleen volumes (~ 4 weeks following transplantation). At 4 days following daily treatment with 325 mg/kg of 6r or 6s, spleen volumes determined by MRI were significantly reduced as compared with vehicle treated mice (Figure 8B). Following administration of the day 5 dose, animals were euthanized, and excised spleens were weighed to compare with MRI volumetric measurements. Spleen weights decreased by 35% and 53% in 6r and 6s treated animals over vehicle, respectively.
Fig 8.
Quantification of mouse spleen volumes of the JAK2V617F transgenic MF mouse model using MRI to assess in vivo therapeutic potency for reversal of the splenomegaly MF phenotype. (A) Coronal MR images from mouse abdominal scans obtained at days 0 and 4 post-treatment initiation reveal spleen anatomy at the indicated day of treatment. Spleen regions were delineated manually by visual inspection to allow for quantification of spleen volumes following treatment with vehicle, 6r or 6s. Presented images were selected to represent approximately the largest spleen cross section at each time point. (B) MRI-determined spleen volume measurements at 0 and 4 days after treatment initiation (left panels; open and light shaded bar graphs). Mice were euthanized at the end of 5 days of treatment and tissues were harvested for analysis to allow for spleens weights to be compared with MRI volumetric findings (right; dark shaded bar graphs). Data represent the mean ± s.d., n=5 (vehicle) and n=4 (6r and 6s). Statistics were analyzed using Welch’s t-test. * p< 0.05, ** p< 0.01, *** p< 0.001.
Spleens from mice with MF treated with vehicle showed markedly elevated levels of active ERK1/2 and AKT as quantified by western blot for pAKT and pERK1/2 (Figure 9). In comparison, treatment with 6r and 6s for 5 days significantly reduced phosphorylated ERK1/2 and AKT levels (Figure 9), showing that both compounds could inhibit constitutive signaling from JAK2V617F to PI3K and MAPK pathways. Both compounds were found to reduce aberrant signaling but it was noted that on average, 6s appeared to be more potent than 6r.
Fig 9.
Evaluation of MAPK and PI3K signaling molecules from spleens excised from the JAK2V617F transgenic MF mouse model. (A) Western blots from exemplar mouse spleen extracts taken from 2 animals from each group consisting of vehicle, 6s and 6r treated animals. Mean fluorescence intensity values from flow cytometry with intracellular staining for (B) pAKT (pS473) and (C) pERK1/2 (pT202/pY204) from isolated splenocytes from MF mice after 5 days p.o. treatment with vehicle, 6r or 6s at 325 mg/kg. Data represent the mean ± s.e.m., n=6 (vehicle), and n=4 (6r) and n=4 (6s). Statistics were analyzed using Welch’s t-test. * p< 0.05, ** p< 0.01, ns (not significant).
3. Summary and Conclusions
In previous reports we detailed the biological evaluation of prototype single-agent PI3K/MEK bifunctional inhibitors incorporating structural analogs of the ATP-competitive, pan-Class I, PI3K inhibitor ZSTK474 and a MEK inhibitor [21–23]. Several of these inhibitor candidates showed strong in vitro and in vivo inhibition towards their respective kinase targets and were effective at inhibition of tumor growth in mouse xenograft models of human cancer after oral administration [21, 23, 35]. The success of this strategy prompted the investigation of alternate linker variants wherein the MEK targeting moiety was directly tethered via a PEG linker to the 1,3,5 triazine core of ZSTK474 analogs. In general, this panel of ZSTK474 derivatives show that acyclic group substitution was least detrimental to PI3Kα and PI3Kδ potency and most detrimental towards PI3Kβ potency when compared to ZSTK474. Analogs bearing pendant hydroxy or methoxy substituents (6a – 6d) mimicking the morpholine oxygen were well tolerated affording a PI3K inhibition profile similar to ZSTK474. In contrast, pendant amino substituents (6l, 6m) displayed poor inhibition across all PI3K isoforms (32 – 145-fold reduction), however, their acetylated counterparts (6p, 6q) displayed a similar inhibition profile towards PI3Kα,γ,δ as that of compounds 6a – 6d. The pendant amino groups in analogs 6l and 6m (pKa = 9.2 – 9.3) are predicted to be protonated at physiological pH. It is possible that the weak PI3K inhibition of analogs 6l and 6m is a consequence of the aminoethyl side chains assuming an unfavorable conformational preference due to interaction of the protonated amino group with the electron-rich nitrogen of the 1,3,5-triazine moiety [36]. Consequently, loss of protonation capability by acetylation of the pendant amine could explain the improved inhibition of analogs 6p and 6q. An alternate possibility is that the protonated forms of 6l and 6m produces a conformational change of the enzymes active site, which could also affect its enzymatic inhibition.
A complementary objective of our SAR study was to evaluate the role of morpholine replacement on the compounds’ isoform selectivity. ZST474 does not display PI3K isoform selectivity as it does not access the specificity pocket of the enzymes active site [27, 28]. Our SAR studies show patterns of selectivity towards PI3Kα and/or PI3Kδ (e.g. analogs 6f, 6n, 6o) within this compound series suggesting the possibility of improvement of isoform selectivity in ZSTK474 analogs by suitable structural replacements of the second morpholine group which resides at the entrance to the ATP pocket. Given that the PIK3CA gene (encoding the p110α catalytic subunit of Class I PI3Ks) is one of the most commonly mutated genes in solid tumors, the high degree of p110α pathway deregulation compared to other PI3K isoforms also emphasizes potential for PI3Kα isoform specific inhibitors as therapeutics for human cancer [11, 37].
Based on our SAR data, two candidate MEK/PI3K bifunctional inhibitors (6r, 6s) modeled on the favorable PI3K inhibition profile of analogs 6a – 6d, were synthesized for further biological investigation. Bifunctional inhibitors 6r and 6s displayed MEK, PI3Kα and PI3Kδ inhibition values in the low nanomolar range (107 – 352 nM) and PI3Kβ and PI3Kγ inhibition in the low micromolar range (1.5 – 3.9 μM), which were comparable to that of our previously reported MEK/PI3K bifunctional inhibitors [21]. The in vitro cell viability studies for 6r and 6s showed a dose-dependent decrease (70 – 90%) for both compounds in three different cell lines. Western blot analysis of cell lysates confirmed that both 6r and 6s suppress pERK1/2 and pAKT levels, which are critical components of the MEK and PI3K signaling pathways, respectively. These studies also confirm that both compounds are cell permeable and interact with their intended kinase targets. Furthermore, the treatment study showed that 6r and 6s multi-functional kinase inhibitors were potent in a MF mouse cancer model as demonstrated by a reduction in a disease phenotype, splenomegaly. Analysis of tissues obtained from mice revealed efficacious levels in target organs such as spleen and bone marrow following oral administration while maintaining potent levels of 6r and 6s.
It is also notable that attachment of the MEK linker group directly to the triazine core of ZSTK474 results in a 100–132-fold greater inhibition potency for PI3Kα and PI3Kδ over PI3Kβ and PI3Kγ. Reports have emphasized the particular importance of PI3Kα isoform specific inhibitors as therapeutics for human cancer due to the high degree of p110α pathway deregulation compared to the other PI3K isoforms [11, 37]. The observed selectivity of these second generation bifunctional PI3K/MEK inhibitors could provide a therapeutic advantage for effective tumor therapy through the specific targeting of PI3K isoforms.
Finally, targeting of MEK remains an important strategy for cancer treatment however the effectiveness of monofunctional inhibitors has been hampered by acquired resistance necessitating development and evaluation of novel combination strategies [38]. Advancing multifunctional inhibitor strategies supports further structure optimization of 6r and 6s as promising leads for investigation as a new class of MEK/PI3K bifunctional inhibitors.
4. Experimental section
4.1. Chemistry
Chemical syntheses involving air or moisture sensitive reagents and solvents were conducted under a positive pressure of nitrogen in oven-dried glassware. Diethanolamine, 1-Boc-ethylenediamine and 1-Boc-1-methyl-ethylenediamine were obtained from Combi-Blocks Inc., San Diego, CA. 2-(2-Dimethylaminoethylamino)ethanol was purchased from Matrix Scientific, Columbia, SC. Beta-alanine and 3-amino-N-methylpropanamide hydrochloride were obtained from Enamine Ltd., Monmouth, NJ. 2,2-dimethyl-4-oxo-3,6,9,12,15-pentaoxa-5-azaoctadecan-18-bromide (t-Boc-aminoxy-PEG3-bromide) and tert-butyl (2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)ethoxy)carbamate (t-Boc-aminoxy-PEG3 amine) were purchased from Broadpharm, San Diego, CA. Key compound intermediates 1,3,5-triazine analog (5) [30] and perfluorophenyl 3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)benzoate (7) [33] were synthesized as previously reported. All other chemical reagents and anhydrous solvents were obtained from Aldrich Chemical Co., Milwaukee, WI and used without additional purification. Column chromatography was performed on silica gel 60 (230 – 400 mesh ASTM) purchased from EMD Millipore, Billerica, MA. Thin-layer chromatography (TLC) was performed using Analtech silica gel GF Uniplates (250 micron). TLC plates were visualized after development with ultraviolet (UV) light and treatment with phosphomolybdic acid reagent. 1H and 13C NMR spectra were recorded on Varian instruments at 400 and 126 MHz, respectively, in CDCl3 or CD3OD as solvent with tetramethylsilane (TMS) as internal standard. Chemical shifts (δ) and coupling constants (J) are reported in parts per million (ppm) and Hertz (Hz), respectively. High resolution mass spectral analyses were performed at the Department of Chemistry, University of Michigan, using a VG-70–250-S mass spectrometer for electron impact (EI) and chemical ionization (DCI) modes, a Waters Autospec Ultima instrument with an electrospray interface for electrospray ionization (ESI) mode or a Waters Tofspec-2E run in reflectron mode. HPLC was performed using a Waters Breeze HPLC System (Waters Corporation, Milford, MA) equipped with a Waters 2487 Dual Wavelength Absorbance Detector. HPLC analysis was conducted at ambient temperature on a Waters XSELECT CSH C-18 column (4.6 × 250 mm), 5μ particle, with 0.1% TFA in H2O (A) and 0.1% TFA in CH3CN (B) solvent mixtures at a flow rate of 1 mL/min with UV absorbance monitored at 254 and 280 nm. HPLC runs were conducted using a 25 min linear gradient elution of either 10% B (initial) to 90% B (Method A), 30% B (initial) to 90% B (Method B) or 50% B (initial) to 90% B (Method C). All biologically tested compounds were demonstrated to have >96% chemical purity by reversed-phase gradient HPLC analysis.
4.1.1. 2-((2-methoxyethyl)amino)ethan-1-ol 3
A solution of 2-bromoethanol (375 mg, 3.0 mmol) in THF (5 mL) was added dropwise to a solution of 2-methoxyethylamine (10 ml, 8.04 g, 107 mmol) in THF (20 mL) and stirred at ambient temperature for 24 h. The mixture was concentrated in vacuo and the product isolated by flash chromatography (solvent gradient of 5 – 12% CH3OH in DCM with 1% added NH4OH) to give 320 mg (82%) as a yellow oil. 1H NMR (CDCl3): δ 3.65 (t, 2H, J = 5.3 Hz), 3.50 (t, 2H, J = 5.2 Hz), 3.37 (s, 3H), 2.79 (m, 4H), 2.33 (br s, 2H). HRMS (ESI+): m/z calculated for C5H14NO2 (M + H+), 120.1019. Found 120.1021. HPLC (Method A): tR = 15.3 min (95%).
4.1.2. tert-butyl((1-hydroxy-6.9,12-trioxa-3-azatetradecan-14-yl)oxy)carbamate 4
A solution of Boc-aminoxy-(PEG)3 bromide (1.0 g, 2.7 mmol) in THF (5 mL) was added dropwise to a solution of ethanolamine (10 ml, 10.12g, 166 mmol) in THF (20 mL) and stirred at ambient temperature for 24 h. The mixture was concentrated in vacuo, the residue diluted with DCM (200 mL), extracted successively with saturated NaHCO3 solution, brine, H2O and dried (Na2SO4). The product was isolated by flash chromatography (solvent gradient of 5 – 12% CH3OH in DCM with 1% added NH4OH) to give 436 mg (46%) as a colorless oil. 1H NMR (CDCl3): δ 4.03 – 4.01 (m, 2H), 3.73 – 3.62 (m, 14H), 2.85 – 2.80 (m, 4H), 2.67 – 2.63 (br s, 2H), 1.48 (s, 9H). HRMS (ESI+): m/z calculated for C15H33N2O7 (M + H+), 353.2282. Found 353.2289.
4.2. General procedure for preparation of compounds 6a – 6k
A mixture of the 1,3,5-triazine analog 5 (1.0 equiv.), requisite primary amine (2.0 equiv.) and Na2CO3 (2.2 equiv.) in anhydrous acetone (unless otherwise noted) was refluxed under a nitrogen atmosphere and the reaction was monitored for completion by TLC analysis. The crude reaction mixture was diluted with EtOAc, the organic layer washed with brine, water and dried (Na2SO4) and the product purified by silica gel flash chromatography.
4.2.1. 2-((4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)amino)ethan-1-ol 6a
The reaction was conducted with ethanolamine to give the title compound as a white solid in 90% yield after chromatography (solvent gradient of 2 – 5% CH3OH in DCM with 1% added NH4OH). 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 8.55 (d, J = 8.0 Hz, 0.6H), 8.42 (d, J = 8.0 Hz, 0.4H), 8.05 – 7.65 (m, 3H), 7.50 – 7.39 (m, 2H), 4.78 – 4.75 (m, 1H), 3.78 (br m, 4H), 3.68 (br m, 4H), 3.58 – 3.54 (m, 2H), 3.47 – 3.39 (m, 2H); 13C NMR (126 MHz, DMSO-d6): δ 165.86, 165.35, 164.57, 164.25, 161.37, 161.13, 146.01, 141.29, 133.04, 132.95, 125.77, 125.58, 124.21, 120.60, 120.50, 116.62, 116.04, 108.69, 108.59, 65.83, 59.79, 59.34, 43.63, 43.14, 43.05. HRMS (ESI+): m/z calculated for C17H20N7F2O2 (M + H+), 392.1641. Found 392.1643. HPLC (Method B): tR = 10.4 min (99.9%).
4.2.2. 2,2’-((4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)azanediyl)bis(ethan-1-ol) 6b
The reaction was conducted with diethanolamine to give the title compound as a white foam in 81% yield after chromatography (solvent gradient of 2 – 8% CH3OH in DCM with 1% added NH4OH). 1H NMR (400 MHz, DMSO-d6, 25 °C): δ 8.38 (d, J = 8.0 Hz, 1H), 7.83 (d, J = 7.6 Hz, 1H), 7.76 (t, J = 52.8 Hz, 1H), 7.49 – 7.39 (m, 2H), 4.84 (t, J = 5.2 Hz, 1H), 4.78 (t, J = 5.2 Hz, 1H), 3.79 – 3.75 (m, 6H), 3.74 – 3.69 (m, 10H); 13C NMR (126 MHz, DMSO-d6): δ 164.63, 164.26, 161.08, 145.92, 141.31, 133.00, 125.81, 125.77, 124.26, 120.63, 115.92, 110.49, 108.60, 65.97, 65.88, 65.87, 65.78, 58.94, 58.29, 50.65, 50.59, 50.44, 43.76, 43.66, 43.52. HRMS (ESI+): m/z calculated for C19H24N7F2O3 (M + H+), 436.1903. Found 436.1902. HPLC (Method A): tR = 15.3 min (97.7%).
4.2.3. 4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-N-(2-methoxyethyl)-6-morpholino-1,3,5-triazin-2-amine 6c
The reaction was conducted with 2-methoxyethylamine to give the title compound as a white solid in 91% yield after chromatography (solvent gradient of 20 – 60% EtOAc in hexane). 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 8.56 (d, J = 8.0 Hz, 0.6H), 8.41 (d, J = 8.0 Hz, 0.4H), 8.06 – 7.64 (m, 3H), 7.50 – 7.38 (m, 2H), 3.78 (m, 4H), 3.68 (m, 4H), 3.55 – 3.47 (m, 4H), 3.28 and 3.26 (2s, 3H); 13C NMR (126 MHz, DMSO-d6): δ 165.83, 165.32, 164.55, 164.28, 161.37, 161.19, 146.06, 141.33, 141.31, 133.06, 125.78, 125.63, 124.26, 120.65, 120.53, 116.68, 116.02, 108.73, 108.62, 70.55, 70.15, 65.92, 65.83, 58.00, 57.90, 43.68. HRMS (ESI+): m/z calculated for C18H22N7F2O2 (M + H+), 406.1798. Found 406.1792. HPLC (Method B): tR = 15.0 min (98.5%).
4.2.4. 2-((4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)(2-methoxyethyl)amino)ethan-1-ol 6d
The reaction was conducted with 2-((2-methoxyethyl)amino)ethan-1-ol to give the title compound as a white foam in 92% yield after chromatography (solvent gradient of 2 – 4% CH3OH in DCM with 1% added NH4OH). 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 8.38 (d, J = 8.4 Hz, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.76 (t, JHF = 53.2 Hz, 0.5H), 7.75 (t, JHF = 52.8 Hz, 0.5H), 7.49 – 7.39 (m, 2H), 4.80 (t, J = 5.0 Hz, 0.5H), 4.73 (t, J = 5.4 Hz, 0.5H), 3.86 – 3.62 (m, 14H), 3.58 (t, J = 5.8 Hz, 2H), 3.27 (s, 3H); 13C NMR (126 MHz, DMSO-d6): δ 164.67, 164.33, 161.20, 145.99, 141.37, 133.05, 125.90, 125.83, 124.36, 124.34, 120.73, 116.00, 115.94, 110.56, 108.67, 106.78, 70.01, 69.49, 65.96, 58.96, 58.33, 58.23, 50.37, 50.09, 47.65, 47.35, 43.80, 40.10. HRMS (ESI+): m/z calculated for C20H26N7F2O3 (M + H+), 450.2060. Found 450.2061. HPLC (Method B): tR = 13.5 min (97.2%).
4.2.5. 2-((4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)(2-(dimethylamino)ethyl)amino)ethan-1-ol 6e
The reaction was conducted with 2-(2-dimethylamino-ethylamino)ethanol to give the title compound as a white foam in 63% yield after chromatography (solvent gradient of 2 – 4% CH3OH in DCM with 1% added NH4OH). 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 8.43 – 8.37 (m, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.77 (t, JHF = 53.2 Hz, 0.5H), 7.76 (t, JHF = 52.8 Hz, 0.5H), 7.50 – 7.39 (m, 2H), 5.06 (br s, 1H), 3.82 – 3.65 (m, 14H), 2.51 (t, overlaps with DMSO, J = 7.2 Hz, 2H), 2.20 (s, 3H), 2.19 (s, 3H); 13C NMR (126 MHz, DMSO-d6): δ 164.55, 164.51, 164.26, 164.17, 161.18, 161.08, 145.92, 141.30, 132.97, 125.80, 125.67, 124.30, 124.25, 120.65, 120.62, 115.97, 115.91, 108.61, 65.92, 59.07, 58.45, 56.83, 56.32, 50.48, 50.46, 49.81, 46.37, 45.37, 43.73. HRMS (ESI+): m/z calculated for C21H29N8F2O2 (M + H+), 463.2376. Found 463.2379. HPLC (Method A): tR = 11.8 min (96%).
4.2.6. 3-((4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)amino)propanoic acid 6f
The reaction was conducted with 3-aminopropanoic acid and Na2CO3 (4.2 equiv) in a mixture of acetone:H2O (1:1) to give the title compound as a white solid in 78% yield after chromatography (solvent gradient of 30 – 75% EtOAc in hexane with 1% added acetic acid). 1H NMR (500 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 12.26 (s, 1H), 8.53 (d, J = 8.5 Hz, 0.6H), 8.44 (d, J = 8.2 Hz, 0.4H), 8.05 – 7.68 (m, 3H), 7.47 – 7.39 (m, 2H), 3.77 (m, 4H), 3.68 (m, 4H), 3.61 – 3.40 (m, 2H), 2.56 (m, 2H); 13C NMR (126 MHz, DMSO-d6): δ 194.40, 172.89, 165.65, 165.18, 164.56, 164.56, 164.25, 161.15, 146.02, 141.31, 125.78, 125.62, 124.26, 120.53, 116.61, 116.14, 65.81, 43.68, 36.48, 33.45. HRMS (ESI+): m/z calculated for C18H20N7F2O3 (M + H+), 420.1590. Found 420.1591. HPLC (Method A): tR = 16.9 min (99.8%).
4.2.7. 3-((4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)amino)-N-methylpropanamide 6g
The reaction was conducted with 3-amino-N-methylpropanamide hydrochloride and Na2CO3 (4.2 equiv.) to give the title compound as a cream solid in 84% yield after chromatography (solvent gradient of 2 – 8% CH3OH in DCM with 1% added NH4OH). 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 8.54(d, J = 7.8 Hz, 0.6H), 8.45 (d, J = 7.8 Hz, 0.4H), 8.03 – 7.69 (m, 4H), 7.49 – 7.38 (m, 2H), 3.77 (br m, 4H), 3.69 (br m, 4H), 3.62 – 3.51 (m, 2H), 2.58 – 2.55 (m, 3H), 2.39 (t, J = 7.0 Hz, 2H); 13C NMR (126 MHz, DMSO-d6): δ 170.63, 170.58, 165.63, 165.17, 164.58, 164.24, 161.13, 146.02, 141.31, 133.03, 125.84, 125.62, 124.25, 120.52, 116.62, 116.22, 108.70, 65.94, 43.69, 35.19, 34.84, 25.43. HRMS (ESI+): m/z calculated for C19H23N8F2O2 (M + H+), 433.1907. Found 433.1909. HPLC (Method B): tR = 9.7 min (99.8%).
4.2.8. tert-butyl (2-((4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)amino)ethyl)carbamate 6h
The reaction was conducted with tert-butyl (2-aminoethyl)carbamate to give the title compound as a white solid in 75% yield after chromatography (solvent gradient of 2 – 5% CH3OH in DCM with 1% added NH4OH). 1H NMR (500 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 8.53 (d, J = 8.2 Hz, 0.6H), 8.43 (d, J = 8.2 Hz, 0.4H), 7.98 – 7.92 (m, 1H), 7.87 – 7.71 (m, 2H), 7.51 – 7.39 (m, 2H), 6.94 – 6.87 (m, 1H), 3.83 – 3.77 (m, 4H), 3.68 (br m, 4H), 3.43 – 3.34 (m, 2H), 3.17 – 3.11 (m, 2H), 1.36 and 1.34 (2s, 9H); 13C NMR (126 MHz, DMSO-d6): δ 165.87, 165.41, 164.55, 164.23, 161.47, 161.16, 155.63, 145.98, 141.31, 133.03, 125.88, 125.60, 124.26, 120.54, 116.55, 116.22, 108.67, 77.67, 65.87, 54.89, 43.74, 28.20, 28.09. HRMS (ESI+): m/z calculated for C22H29N8F2O3 (M + H+), 491.2325. Found 491.2324. HPLC (Method B): tR = 17.5 min (98.8%).
4.2.9. tert-butyl (2-((4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)amino)ethyl)(methyl)carbamate 6i
The reaction was conducted with tert-butyl (2-aminoethyl)(methyl)carbamate to give the title compound 6i as a white foam in 93% yield after chromatography (solvent gradient of 20 – 50% EtOAc in hexane). 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 8.52 (d, J = 8.0 Hz, 0.6H), 8.39 (d, J = 8.0 Hz, 0.2H), 8.08 – 7.74 (m, 3H), 7.48 – 7.39 (m, 2H), 3.78 (br m, 4H), 3.68 (br m, 4H), 3.42 (br m, 4H), 2.81 (s, 3H), 1.34 – 1.21 (m, 9H); 13C NMR (126 MHz, DMSO-d6): δ 165.36, 164.53, 161.43, 161.14, 154.79, 145.93, 141.28, 133.01, 125.57, 124.25, 120.54, 116.48, 110.52, 108.63, 106.74, 78.19, 65.93, 65.82, 43.72, 27.80, 27.66, 27.59. HRMS (ESI+): m/z calculated for C23H31N8F2O3 (M + H+), 505.2482. Found 505.2484. HPLC (Method C): tR = 12.2 min (97.9%).
4.2.10. tert-butyl (2-(2-(2-(2-((4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)amino)ethoxy)ethoxy)ethoxy)ethoxy)carbamate 6j
The reaction was conducted with tert-butyl (2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)ethoxy)carbamate to give the title compound as a colorless oil in 94% yield after chromatography (solvent gradient of 2 – 4% CH3OH in DCM with 1% added NH4OH). 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 9.96 (br s, 1H), 8.54 (d, J = 8.2 Hz, 0.6H), 8.41 (d, J = 8.0 Hz, 0.4H), 8.05 – 7.64 (m, 3H), 7.50 – 7.38 (m, 2H), 3.78 – 3.68 (m, 10H), 3.59 – 3.45 (m, 14H), 1.36 (s, 9H); 13C NMR (126 MHz, DMSO-d6): δ 165.83, 165.33, 164.55, 164.27, 161.36, 161.18, 156.08, 141.31, 139.69, 133.05, 132.95, 125.79, 125.63, 124.30, 124.26, 120.65, 120.53, 116.65, 116.05, 108.71, 79.55, 74.74, 69.72, 69.59, 69.08, 68.59, 67.99, 65.87, 65.82, 65.79, 28.01. HRMS (ESI+): m/z calculated for C28H41N8F2O7 (M + H+), 639.3061. Found 639.3067. HPLC (Method C): tR = 9.8 min (95.4%).
4.2.11. tert-butyl ((3-(4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)-1-hydroxy-6,9,12-trioxa-3-azatetradecan-14-yl)oxy)carbamate 6k
The reaction was conducted with tert-butyl ((1-hydroxy-6,9,12-trioxa-3-azatetradecan-14-yl)oxy)carbamate to give the title compound as a white foam in 93% yield after chromatography (solvent gradient of 2 – 4% CH3OH in DCM with 1% added NH4OH). 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 9.96 (br s, 1H), 8.39 (d, J = 8.0 Hz, 1H), 7.83 (d, J = 7.6 Hz, 1H), 7.76 (t, JHF = 52.8 Hz, 0.5H), 7.75 (t, JHF = 53.0 Hz, 0.5H), 7.48 (t, J = 8.4 Hz, 1H), 7.41 (t, J = 7.6 Hz, 1H), 4.79 (t, J = 5.2 Hz, 0.5H), 4.73 (t, J = 5.2 Hz, 0.5H), 3.86 – 3.63 (m, 18H), 3.53 – 3.45 (m, 10H), 1.37 (s, 9H); 13C NMR (126 MHz, DMSO-d6): δ 164.63, 164.28, 161.08, 156.10, 145.94, 141.32, 132.95, 125.84, 124.29, 120.65, 115.94, 110.50, 108.62, 79.57, 74.75, 74.74, 69.93, 69.80, 69.77, 69.71, 68.38, 68.03, 67.80, 65.83, 65.81, 58.91, 58.30, 54.91, 50.32, 50.07, 47.76, 47.50, 43.76, 28.02. HRMS (ESI+): m/z calculated for C30H45N8F2O8 (M + H+), 683.3323. Found 683.3325. HPLC (Method B): tR = 15.7 min (98.3%).
4.3. General procedure for preparation of compounds 6l – 6o
A solution of the carbamate analog 6h, 6i or 6j (0.5 mmol) in dichloromethane (5 ml) was treated dropwise at 0 °C (ice bath) with a solution of 50% TFA (7.4 g, 5 ml, 65.3 mmol) in DCM (5 ml) and stirred at 0 °C until completion by TLC analysis. The reaction mixture was poured on to crushed ice and the pH of the aqueous layer was adjusted to 10 by slow addition of saturated aqueous NaHCO3 solution. The aqueous layer was extracted thrice with EtOAc, the combined organic layers washed successively with brine, water and dried (Na2SO4) and the product purified by silica gel flash chromatography.
4.3.1. N1-(4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)ethane-1,2-diamine 6l
The reaction was conducted with compound 6h to give the title compound as a white solid in 75% yield after chromatography (solvent gradient of 4 – 10% CH3OH in DCM with 1% added NH4OH). 1H NMR (500 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 8.54 (d, J = 8.0 Hz, 0.6H), 8.42 (d, J = 8.0 Hz, 0.4H), 8.00 – 7.69 (m, 3H), 7.51 – 7.39 (m, 2H), 3.77 (br m, 4H), 3.68 (br m, 4H), 3.39 – 3.32 (m, 2H), 2.73 (t, J = 6.5 Hz, 2H), 1.92 (br s, 2H); 13C NMR (126 MHz, DMSO-d6): δ 165.87, 165.41, 164.55, 164.23, 161.47, 161.16, 155.63, 145.98, 141.31, 133.03, 125.88, 125.60, 124.26, 120.54, 116.55, 108.65, 77.71, 65.87, 54.89, 43.64, 28.20. HRMS (ESI+): m/z calculated for C17H21N8F2O (M + H+), 391.1801. Found 391.1805. HPLC (Method A): tR = 11.4 min (99.1%).
4.3.2. N1-(4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)-N2-methylethane-1,2-diamine 6m
The reaction was conducted with compound 6i to give the title compound as a white foam in 90% yield after chromatography (solvent gradient of 4 – 10% CH3OH in DCM with 1% added NH4OH). 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 8.54 (d, J = 8.0 Hz, 0.6H), 8.43 (d, J = 8.0 Hz, 0.4H), 8.03 – 7.66 (m, 3H), 7.50 – 7.39 (m, 2H), 3.77 (br m, 4H), 3.68 (br m, 4H), 3.48 – 3.41 (m, 2H), 2.69 (t, J = 6.4 Hz, 2H), 2.32 and 2.30 (2s, 3H); 13C NMR (126 MHz, DMSO-d6): δ 165.88, 165.43, 164.65, 164.32, 161.50, 161.21, 151.55, 141.39, 133.12, 125.85, 125.67, 124.31, 120.59, 116.68, 116.15, 108.75, 65.97, 65.88, 50.56, 50.28, 43.72, 35.75. HRMS (ESI+): m/z calculated for C18H23N8F2O (M + H+), 405.1957. Found 405.1958. HPLC (Method A): tR = 11.6 min (99.9%).
4.3.3. N-(2-(2-(2-(2-(aminooxy)ethoxy)ethoxy)ethoxy)ethyl)-4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-amine 6n
The reaction was conducted with compound 6j to give the title compound as a colorless oil in 93% yield after chromatography (solvent gradient of 2 – 4% CH3OH in DCM with 1% added NH4OH). 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 8.55 (d, J = 8.0 Hz, 0.6H), 8.42 (d, J = 8.0 Hz, 0.4H), 8.06 – 7.65 (m, 3H), 7.51 – 7.39 (m, 2H), 5.94 (s, 2H), 3.78 (m, 4H), 3.68 (m, 4H), 3.60 – 3.44 (m, 16H); 13C NMR (126 MHz, DMSO-d6): δ165.82, 165.32, 164.53, 164.26, 161.35, 161.17, 146.03, 141.30, 133.04, 132.92,125.79, 125.63, 124.25, 120.63, 120.52, 116.64, 116.05, 108.70, 108.60, 74.12, 69.79, 69.59, 69.07, 68.58, 65.86, 43.79, 43.68. HRMS (ESI+): m/z calculated for C23H33N8F2O5 (M + H+), 539.2536. Found 539.2534. HPLC (Method A): tR = 13.3 min (98.4%).
4.3.4. 14-(aminooxy)-3-(4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)-6,9,12-trioxa-3-azatetradecan-1-ol 6o
A solution of the carbamate derivative 6k (0.5 mmol) in dioxane (3 ml) was treated dropwise at 0 °C (ice bath) with a solution of 20% H2SO4 (1.15 g, 0.63 ml, 11.8 mmol) in dioxane (3 ml) and stirred at 0 °C until completion by TLC analysis. The reaction mixture was poured on to crushed ice and the pH of the aqueous layer was adjusted to 10 by slow addition of saturated aqueous NaHCO3 solution. The aqueous layer was extracted thrice with EtOAc, the combined organic layers washed successively with brine, water and dried (Na2SO4). The product was purified by silica gel flash chromatography (solvent gradient of 2 – 6% CH3OH in DCM with 1% added NH4OH) to give the title compound 6o as a colorless oil in 55% yield. 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 8.39 (d, J = 8.4 Hz, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.77 (t, JHF = 52.0 Hz, 0.5H), 7.76 (t, JHF = 52.0 Hz, 0.5H), 7.50 – 7.40 (m, 2H), 5.94 (br s, 1H), 4.79 (m, 0.5H), 4.72 (m, 0.5H), 3.99 – 3.63 (m, 16H), 3.60 – 3.43 (m, 12H); 13C NMR (126 MHz, DMSO-d6): δ 165.07, 164.69, 161.52, 154.62, 146.17, 141.77, 133.42, 126.29, 124.73, 121.09, 116.39, 109.05, 74.58, 74.56, 72.36, 70.29, 70.16, 69.00, 68.82, 68.24, 66.33, 59.35, 58.74, 50.80, 50.52, 48.18, 47.95, 44.18, 21.71, 15.76. HRMS (ESI+): m/z calculated for C25H37N8F2O6 (M + H+), 583.2799. Found 583.2801. HPLC (Method A): tR = 12.8 min (96.6%).
4.4. General procedure for preparation of compounds 6p and 6q
A mixture of the amine derivative (1.0 equiv.) and pyridine (1.1 equiv.) in DCM was cooled to 5 °C (ice bath) and treated with acetic anhydride (1.1 equiv.). The reaction mixture was warmed to rt and monitored for completion by TLC analysis. The reaction mixture was concentrated in vacuo and the residue was partitioned between DCM and aqueous 0.1 N HCl solution. The organic layer was washed successively with saturated aqueous NaHCO3 solution, brine, water and dried (Na2SO4) and the product purified by silica gel flash chromatography.
4.4.1. N-(2-((4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)amino)ethyl)acetamide 6p
The reaction was conducted with compound 6l to give the title compound as a white solid in 84% yield after chromatography (solvent gradient of 1 – 5% CH3OH in DCM with 1% added NH4OH). 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 8.54 (d, J = 8.4 Hz, 0.6H), 8.42 (d, J = 8.4 Hz, 0.4H), 8.02 – 7.67 (m, 4H), 7.51 – 7.39 (m, 2H), 3.82 – 3.77 (m, 4H), 3.69 (m, 4H), 3.45 – 3.36 (m, 2H), 3.26 – 3.22 (m, 2H), 1.80 and 1.78 (2s, 3H); 13C NMR (126 MHz, DMSO-d6): δ 169.38, 165.86, 165.46, 164.55, 164.23, 161.46, 161.17, 146.25, 146.00, 141.32, 133.03, 125.93, 125.62, 124.27, 120.53, 116.60, 116.15, 110.56, 108.67, 106.78, 65.87, 43.64, 40.38, 40.31, 38.47, 38.02, 22.67. HRMS (ESI+): m/z calculated for C19H23N8F2O2 (M + H+), 433.1907. Found 433.1905. HPLC (Method A): tR = 15.0 min (99.9%).
4.4.2. N-(2-((4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)amino)ethyl)-N-methylacetamide 6q
The reaction was conducted with compound 6m to give the title compound as a white foam in 96% yield after chromatography (solvent gradient of 1 – 5% CH3OH in DCM with 1% added NH4OH). 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 8.51 (d, J = 8.0 Hz, 0.6H), 8.45 – 8.39 (m, 0.4H), 8.12 – 7.64 (m, 3H), 7.50 – 7.39 (m, 2H), 3.77 (m, 4H), 3.68 (m, 4H), 3.54 – 3.47 (m, 4H), 2.98, 2.83 and 2.80 (m, 3H), 1.97 – 1.91 (m, 3H); 13C NMR (126 MHz, DMSO-d6): δ 170.11, 169.91, 169.46, 165.88, 165.49, 164.55, 164.26, 161.45, 161.19, 146.01, 141.30, 132.98, 125.83, 125.65, 124.29, 124.26, 120.60, 120.57, 116.48, 116.16, 110.55, 108.67, 65.86, 49.15, 49.04, 46.65, 46.30, 43.88, 43.71, 43.60, 36.60, 36.49, 32.82, 21.72, 20.96. HRMS (ESI+): m/z calculated for C20H25N8F2O2 (M + H+), 447.2063. Found 447.2067. HPLC (Method A): tR = 16.1 min (99.3%).
4.5. General procedure for preparation of compounds 6r and 6s
A solution of the requisite aminoxy derivative 6n or 6o (0.5 mmol) in anhydrous DMF (1 ml) was treated dropwise with an equimolar portion of perfluorophenyl 3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)benzoate (7) in DMF (1 ml) and stirred at ambient temperature until completion by TLC analysis. The reaction mixture was partitioned between DCM and brine, the organic extract washed with H2O, dried (Na2SO4) and the product purified by silica gel flash chromatography.
4.5.1. N-(2-(2-(2-(2-((4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)amino)ethoxy)ethoxy)ethoxy)ethoxy)-3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)benzamide 6r
Reaction of aminoxy derivative 6n and ester 7 provided the title compound as a white foam in 63% yield after chromatography (solvent gradient of 2 – 5% CH3OH in DCM with 1% added NH4OH). 1H NMR (400 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 11.84 (br s, 1H), 8.73 (br s, 1H), 8.55 (d, J = 8.0 Hz, 0.6H), 8.41 (d, J = 8.8 Hz, 0.4H), 8.04 – 7.77 (m, 3H), 7.64 – 7.33 (m, 5H), 7.21 – 7.14 (m, 1H), 6.67 – 6.61 (m, 1H), 3.89 (m, 2H), 3.77 (m, 4H), 3.68 (m, 4H), 3.58 – 3.47 (m, 14H); 13C NMR (126 MHz, DMSO-d6): δ 166.28, 165.78, 165.00, 164.72, 161.81, 161.63, 153.95, 151.97, 146.49, 141.76, 133.62, 133.49, 126.24, 126.08, 125.13, 125.01, 124.75, 124.71, 124.19, 124.02, 121.09, 120.98, 120.40, 117.11, 116.52, 110.63, 109.17, 70.23, 70.16, 70.04, 69.53, 69.05, 68.61, 66.30. HRMS (ESI+): m/z calculated for C36H38N9F5IO6 [M + H+]: 914.1904. Found: 914.1903. calculated for [M + Na+]: 936.1724. Found: 936.1721. HPLC (Method C): tR = 18.1 min (99.4%).
4.5.2. N-((3-(4-(2-(difluoromethyl)-1H-benzo[d]imidazol-1-yl)-6-morpholino-1,3,5-triazin-2-yl)-1-hydroxy-6,9,12-trioxa-3-azatetradecan-14-yl)oxy)-3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)benzamide 6s
Reaction of aminoxy derivative 6o and ester 7 provided the title compound as a white foam in 82% yield after chromatography (solvent gradient of 2 – 5% CH3OH in DCM with 1% added NH4OH). 1H NMR (500 MHz, DMSO-d6, 25 °C): (mixture of rotamers) δ 11.83 (br s, 1H), 8.71 (br s, 1H), 8.38 (d, J = 8.0 Hz, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.76 (t, JHF = 53.0 Hz, 0.5H), 7.75 (t, JHF = 53.0 Hz, 0.5H), 7.55 (d, J = 10.5 Hz, 1H), 7.49 – 7.33 (m, 4H), 7.17 (m, 1H), 6.64 (m, 1H), 4.78 (m, 0.5H), 4.71 (m, 0.5H), 3.89 – 3.73 (m, 9H), 3.68 – 3.65 (m, 9H), 3.56 – 3.45 (m, 10H); 13C NMR (126 MHz, DMSO-d6): δ 164.67, 164.32, 161.13, 153.55, 151.58, 151.56, 145.98, 141.37, 133.20, 133.01, 125.84, 124.33, 123.80, 123.63, 120.70, 120.01, 116.00, 110.27, 108.65, 70.05, 69.97, 69.78, 68.43, 68.23, 67.84, 65.92, 58.96, 58.35, 50.37, 50.12, 47.79, 47.55, 43.78, 43.75. HRMS (ESI+): m/z calculated for C38H42N9F5IO7 [M + H+]: 958.2167. Found: 958.2165. calculated for [M + Na+]: 980.1986. Found: 980.1984. HPLC (Method C): tR = 16.7 min (97.6%).
4.6. Molecular modeling studies
Docking models of selected compounds were obtained using software from Schrödinger Inc. (Schrödinger, LLC, New York, NY). The X-ray crystal structure of PI3Kδ bound to ZSTK474 (PDB code 2WXL) and MEK1 bound to CH4987655 (PDB code 3ORN) were chosen based on ligand similarity to the compound series and prepared using the Protein Preparation Wizard in Maestro (Protein Preparation Wizard, Schrödinger, LLC, New York, NY). The protein structure was used to generate receptor grids centered on the native ligand docking site using OPLS3 with a maximum ligand length of 36 Å due to the variation among ligand sizes. All inhibitor ligands were built and prepared for docking in Maestro using LigPrep 3.8 (LigPrep, Schrödinger, LLC, New York, NY). The docking procedures were performed using Glide 7.1 in standard precision mode with default parameters and no constraints. Generated poses for each protein-ligand structure were filtered by docking score of ≤ −4.0 and curated manually, with top poses of inhibitors selected based on their chemical and positional alignment with the native ligand ZSTK474, and overall docking score (≤ −5.4 for all poses presented).
4.7. In vitro biological studies
4.7.1. In vitro PI3K and MEK inhibition assays.
Quantitation of PI3K lipid kinase inhibition was conducted by Life Technologies (Madison, WI) with the purified human enzyme using the fluorescence-based Adapta™ TR-FRET assay protocol (PI3K) and. Assay catalog numbers are as follows: PI3kα: 1014; PI3Kβ: 973; PI3Kγ: 1017; PI3kδ: 976. Quantitation of MEK1 kinase inhibition was conducted by Life Technologies (Madison, WI) using the LanthaScreen binding assay protocol (Inactive MAP2K1 (MEK1) human enzyme with ATP; Assay catalog number: 2415). Assays were conducted with a range of inhibitor concentrations (0.1 nM – 10 μM) and reported data are the average of three experiments each conducted in duplicate. Inhibition data were reported as mean ± standard error of mean (SEM).
4.7.2. Cell viability studies
Human A375, D54 and SET-2 cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in RPMI 1640 medium (Life Technologies, Inc. Grand Island, NY) or DMEM (Life Technologies, Inc. Grand Island, NY) supplemented with 10% or 20% (SET-2) fetal bovine serum (FBS, Hyclone, Logan, UT) and maintained in a humidified incubator over 5% CO2 at 37 °C. Growing cells were treated with inhibitor compounds when they reached approximately 70 – 90% confluence. Bifunctional inhibitors 6r and 6s were evaluated for their anti-proliferative activity against human A375 melanoma, D54 glioblastoma and SET-2 leukemia cell lines using a CellTiter-Fluor cell viability assay (Promega, Madison WI) with ZSTK474 as a positive control. Cells were cultured in DMEM or RPMI1640 supplemented with 10% or 20% fetal bovine serum (FBS). Cells were suspended in complete media and seeded into 96-well plates (approximately 500 – 1,000 cells per well) and incubated over 5% CO2 at 37 °C for 24 h. Compounds at the indicated final concentration (0.1 – 20 μM) were added to the culture medium and cell cultures were incubated for an additional 72 h. All assay procedures were followed according to the manufacturer’s protocol. Resulting fluorescence was measured by Envision multi-plate reader (Perkin Elmer). Assays were performed in triplicate per cell line with duplicate wells for each experiment. Anti-proliferative activity expressed as IC50 (50% inhibitory concentration) was calculated using Graphpad Prism 7.0a software and reported as the mean ± standard error (SEM).
4.7.3. Western Blot analysis and quantification
A375 and D54 cell lines were grown in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. SET-2 cells were grown in RPMI supplemented with 20% FBS and 1% penicillin/streptomycin. Cells were lysed on ice for 20 min in RIPA lysis buffer containing protease inhibitor cocktail (IBI Scientific, Dubuque, IA) and phosphatase inhibitor cocktails (Thermo Scientific, Waltham, MA). Proteins from cell lysates were separated by SDS-PAGE chromatography, transferred to PVDF membranes and probed with the indicated primary antibodies. Antibodies recognizing pAKT (S473), pERK1/2 (T202/Y204). AKT and ERK1/2 were purchased from Cell Signaling Technology (Danvers, MA. HRP conjugated anti-rabbit secondary antibody was purchased from Thermo Scientific. Western blots were visualized with SuperSignal West Pico PLUS chemiluminescent substrate (Thermo Scientific, Waltham, MA) and pictured with Bio-Rad Chemi-Doc imaging system.
ImageJ software version 1.53c (NIH, USA) was used to select and determine the background-subtracted density of the bands in all blots. For background subtraction, the same volume of areas from the background near the bands were selected. For normalization density, phosphorylated ERK1/2, and phosphorylated AKT was normalized with the corresponding ERK1/2 and AKT density. Error bars represent the standard errors of the mean for three blot experiments.
4.8. In vivo biological and therapeutic studies
4.8.1. Myelofibrosis mouse model.
Conditional JAK2V617F knock-in mice and Mx1-Cre mice were purchased from Jackson Laboratory. The JAK2V617F knock-in mouse was crossed to the Mx1-Cre mouse to generate Mx1-Cre+/−; JAK2V617Ffl/−. Total bone marrow (BM) cells isolated from Mx1-Cre+/−; JAK2V617Ffl/− donor mice were mixed with WT C57BL/6 BM cells in a ratio of 1:9 (WT:mutant) and five million cells of mixed BM were transplanted into lethally irradiated C57BL/6 female recipients. Transplanted recipients were injected with 100 mg of polyI:C (Sigma-Aldrich, St. Louis, MO, USA) two times 2 weeks after BMT to express JAK2V617F gene.
4.8.2. Mouse therapy study with 6r and 6s, western blotting and flow cytometry analysis.
Development of MF was evaluated by spleen volumes using MRI beginning 1 week following polyI:C injection. Mice were grouped so that each group had an approximately equal number of mice with small and large spleen sizes for 6r, 6s, or vehicle and treated with 325 mg/kg of each drug daily by oral gavage for 5 days. For evaluation of efficacy of each drug in spleen and BM, we collected spleen tissues and BM after sacrifice. For western blot analysis, we isolated total protein from tissues using a RIPA buffer by homogenizer. Concentration of isolated total protein was calculated using the BCA protein assay kit (Thermo Scientific., Rockford, IL, USA). A total 30 μg of protein was used for SDS-PAGE. Anti-pERK1/2 and anti-pAKT antibodies were purchased from Cell Signaling (Danvers, MA, USA), and HRP-conjugated anti-β-actin antibody (Biolegend, San Diego, CA, USA) was used for internal loading control. For flow cytometry analysis, BM cells were collected and stained with APC-conjugated anti-pERK1/2 and APC-conjugated anti-pAKT antibodies (BD, San Jose, CA, USA) intracellularly. A LSRII fortessa instrument (BD, San Jose, CA, USA) was used for all flow cytometry studies. Mean fluorescence intensities of pERK1/2 and pAKT were calculated by the Flowjo software (BD, San Jose, CA, USA).
4.8.3. Mouse spleen MRI studies
MRI was performed on a Bruker BioSpin 7T MRI scanner console running ParaVision 6.0 interfaced with a 30 cm diameter horizontal-bore Magnex 7T (300 MHz 1H frequency) equipped with a 1H 72 mm transmit/receive coil. A 2D multi-slice FLASH sequence was used to obtain abdominal scans for spleen volume imaging. Acquisition parameters were as follows: repetition time/echo time (TR/TE) = 600/8 ms, 4 averages, flip angle = 30°, slice thickness = 0.4 mm, field of view = 40 × 30 mm2, 40 slices, matrix = 256 × 128, total acquisition time = 154 s. Respiratory gating was achieved using triggering through a small animal monitoring system with pneumatic pillow device (SA Instruments, NY) to reduce motion artifact. Spleen volumes were obtained from the multi-slice MR images. Organ boundaries visualized in each slice were electronically outlined by using image processing software (MATLAB, Natick, MA). The number of organ pixels were converted to an area by multiplication by the factor [(field of view)2/(matrix)2]. The total organ volume was calculated as the summed area on all slices, multiplied by the slice thickness. Data processing and analysis was performed using in-house software written in Matlab 2016 (The Mathworks, Inc., Natick, MA).
4.8.4. In vivo tissue levels of 6r, 6s and metabolites
Quantitative analysis by liquid chromatography tandem mass spectrometry (LC-MS/MS) was used for analysis of compounds 6r and 6s, and their metabolites, MV5–46, MV5–32, MV4–187, PD0316684, PD315209, using an ACQUITY UPLC H-Class (Waters Corp.) coupled to a SCIEX 5500+ QTRAP mass spectrometer (AB Sciex) against standard curves generated from purified compounds and internal standard (Aripiprazole-d8; Cerilliant Corp.) spiked in equivalent biological matrices. Standards and compound extraction from biological fluids or tissues was performed using similar protocols. Briefly, 200 μl acetonitrile was added to each sample which depending upon sample weight, yielded a final concentration of 75 – 90% acetonitrile in whole blood, bone marrow, or spleen samples for compound extraction. Sample extraction was performed by shaking and/or bead rupture using a Precellys Evolution tissue homogenizer (Bertin Technologies SAS). Extracted samples were centrifuged at 10,000 × g, supernatant transferred to autosampler vials, and then separated by reverse-phase chromatography using an ACQUITY BEH C18 column (50 mm × 2.1 mm i.d., 1.7 μm) flowing binary mobile phase gradients of 0.1% formic acid:water and 0.1% formic acid:acetonitrile (Optima LC-MS Grade; Fisher Scientific) at 0.6 mL/min, optimized to achieve baseline separation of all compound groups. Optimization of MS and MS/MS parameters for MRM quantitation and maximum sensitivity of individual compounds was performed using SCIEX Analyst software (AB Sciex). Internal standard calibration curves were used to determine sample analyte concentrations through linear regression and weighting average of 1/X, with all curves displaying a correlation coefficient r2 ≥ 0.99 and a linear dynamic range of 0.02 ng/mL to 160 ng/mL across all tissues and compounds. All LC-MS/MS acquisitions were performed with an independent standard curve for analyte groups and for each individual run.
Supplementary Material
HIGHLIGHTS.
ZSTK474 analogs with pendant acyclic groups attached to a single morpholine group.
Optimal PI3K isoform inhibition was found with pendant hydroxyl or methoxy groups.
Prototype PI3K/MEK bifunctional inhibitors (6r, 6s) were synthesized.
Demonstrated anti-proliferative activity in tumor-derived cell lines.
Achieved therapeutic activity in mouse cancer model with reduced kinase activation.
Acknowledgments
We wish to acknowledge valuable discussions concerning H-1 NMR spectra interpretation of compound rotamers with Dr. Scott Larsen and Dr. Jeffrey Zwicker of the Department of Medicinal Chemistry at the University of Michigan.
Funding
Funded in part by grants R35CA19770, U24CA237683, R01CA238023, and P30CA04659229 from the National Institutes of Health.
Abbreviations
- MEK
Allosteric Mitogen-Activated Protein Kinase
- PI3K
Phosphatidylinositol 3-Kinase
- AKT
Protein Kinase B
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- SAR
structure-activity relationship
- KRAS
Kirsten rat sarcoma
- pAKT
phosphorylated AKT
- pERK
phosphoERK
- PEG
polyethylene glycol
- PDGF
platelet-derived growth factor
- cLogP
calculated log P
- MF
myelofibrosis
- DIEA
N,N-diisopropylethylamine
- DMEM
Dulbecco’s Modified Eagles Medium
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bromberg-White JL, Andersen NJ, Duesbery NS, MEK genomics in development and disease, Brief Funct Genomics, 11 (2012) 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jokinen E, Koivunen JP, MEK and PI3K inhibition in solid tumors: rationale and evidence to date, Ther Adv Med Oncol, 7 (2015) 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F, Mazzarino MC, Donia M, Fagone P, Malaponte G, Nicoletti F, Libra M, Milella M, Tafuri A, Bonati A, Basecke J, Cocco L, Evangelisti C, Martelli AM, Montalto G, Cervello M, McCubrey JA, Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging, Aging (Albany NY), 3 (2011) 192–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE, Piccart-Gebhart MJ, Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer, Cancer Treat Rev, 39 (2013) 935–946. [DOI] [PubMed] [Google Scholar]
- [5].Yaeger R, Corcoran RB, Targeting Alterations in the RAF-MEK Pathway, Cancer Discov, 9 (2019) 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ryan MB, Corcoran RB, Therapeutic strategies to target RAS-mutant cancers, Nat Rev Clin Oncol, 15 (2018) 709–720. [DOI] [PubMed] [Google Scholar]
- [7].Samuels Y, Waldman T, Oncogenic mutations of PIK3CA in human cancers, Curr Top Microbiol Immunol, 347 (2010) 21–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, Basecke J, Stivala F, Donia M, Fagone P, Malaponte G, Mazzarino MC, Nicoletti F, Libra M, Maksimovic-Ivanic D, Mijatovic S, Montalto G, Cervello M, Laidler P, Milella M, Tafuri A, Bonati A, Evangelisti C, Cocco L, Martelli AM, McCubrey JA, Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health, Oncotarget, 2 (2011) 135–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Castellano E, Downward J, RAS Interaction with PI3K: More Than Just Another Effector Pathway, Genes Cancer, 2 (2011) 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Burmi RS, Maginn EN, Gabra H, Stronach EA, Wasan HS, Combined inhibition of the PI3K/mTOR/MEK pathway induces Bim/Mcl-1-regulated apoptosis in pancreatic cancer cells, Cancer Biol Ther, 20 (2019) 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fruman DA, Rommel C, PI3K and cancer: lessons, challenges and opportunities, Nat Rev Drug Discov, 13 (2014) 140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP, Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer, J Clin Invest, 118 (2008) 3065–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Temraz S, Mukherji D, Shamseddine A, Dual Inhibition of MEK and PI3K Pathway in KRAS and BRAF Mutated Colorectal Cancers, Int J Mol Sci, 16 (2015) 22976–22988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Poulikakos PI, Solit DB, Resistance to MEK inhibitors: should we co-target upstream?, Sci Signal, 4 (2011) pe16. [DOI] [PubMed] [Google Scholar]
- [15].Peng X, Liu Y, Zhu S, Peng X, Li H, Jiao W, Lin P, Zhang Z, Qiu Y, Jin M, Wang R, Kong D, Co-targeting PI3K/Akt and MAPK/ERK pathways leads to an enhanced antitumor effect on human hypopharyngeal squamous cell carcinoma, J Cancer Res Clin Oncol, 145 (2019) 2921–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y, Chirieac LR, Kaur R, Lightbown A, Simendinger J, Li T, Padera RF, Garcia-Echeverria C, Weissleder R, Mahmood U, Cantley LC, Wong KK, Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers, Nat Med, 14 (2008) 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chang Q, Chen E, Hedley DW, Effects of combined inhibition of MEK and mTOR on downstream signaling and tumor growth in pancreatic cancer xenograft models, Cancer Biol Ther, 8 (2009) 1893–1901. [DOI] [PubMed] [Google Scholar]
- [18].Chapuis N, Tamburini J, Green AS, Vignon C, Bardet V, Neyret A, Pannetier M, Willems L, Park S, Macone A, Maira SM, Ifrah N, Dreyfus F, Herault O, Lacombe C, Mayeux P, Bouscary D, Dual inhibition of PI3K and mTORC1/2 signaling by NVP-BEZ235 as a new therapeutic strategy for acute myeloid leukemia, Clin Cancer Res, 16 (2010) 5424–5435. [DOI] [PubMed] [Google Scholar]
- [19].Gossage L, Eisen T, Targeting multiple kinase pathways: a change in paradigm, Clin Cancer Res, 16 (2010) 1973–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Heffron TP, Ndubaku CO, Salphati L, Alicke B, Cheong J, Drobnick J, Edgar K, Gould SE, Lee LB, Lesnick JD, Lewis C, Nonomiya J, Pang J, Plise EG, Sideris S, Wallin J, Wang L, Zhang X, Olivero AG, Discovery of Clinical Development Candidate GDC-0084, a Brain Penetrant Inhibitor of PI3K and mTOR, ACS Med Chem Lett, 7 (2016) 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Van Dort ME, Galban S, Nino CA, Hong H, Apfelbaum AA, Luker GD, Thurber GM, Atangcho L, Besirli CG, Ross BD, Structure-Guided Design and Initial Studies of a Bifunctional MEK/PI3K Inhibitor (ST-168), ACS Med Chem Lett, 8 (2017) 808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Van Dort ME, Galban S, Wang H, Sebolt-Leopold J, Whitehead C, Hong H, Rehemtulla A, Ross BD, Dual inhibition of allosteric mitogen-activated protein kinase (MEK) and phosphatidylinositol 3-kinase (PI3K) oncogenic targets with a bifunctional inhibitor, Bioorg Med Chem, 23 (2015) 1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Van Dort ME, Hong H, Wang H, Nino CA, Lombardi RL, Blanks AE, Galban S, Ross BD, Discovery of Bifunctional Oncogenic Target Inhibitors against Allosteric Mitogen-Activated Protein Kinase (MEK1) and Phosphatidylinositol 3-Kinase (PI3K), J Med Chem, 59 (2016) 2512–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kong D, Yamori T, ZSTK474 is an ATP-competitive inhibitor of class I phosphatidylinositol 3 kinase isoforms, Cancer Sci, 98 (2007) 1638–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maira SM, Voliva C, Garcia-Echeverria C, Class IA phosphatidylinositol 3-kinase: from their biologic implication in human cancers to drug discovery, Expert Opin Ther Targets, 12 (2008) 223–238. [DOI] [PubMed] [Google Scholar]
- [26].Andrews S, Stephens LR, Hawkins PT, PI3K class IB pathway, Sci STKE, 2007 (2007) cm2. [DOI] [PubMed] [Google Scholar]
- [27].Berndt A, Miller S, Williams O, Le DD, Houseman BT, Pacold JI, Gorrec F, Hon WC, Liu Y, Rommel C, Gaillard P, Ruckle T, Schwarz MK, Shokat KM, Shaw JP, Williams RL, The p110 delta structure: mechanisms for selectivity and potency of new PI(3)K inhibitors, Nat Chem Biol, 6 (2010) 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Miller MS, Thompson PE, Gabelli SB, Structural Determinants of Isoform Selectivity in PI3K Inhibitors, Biomolecules, 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gamage SA, Giddens AC, Tsang KY, Flanagan JU, Kendall JD, Lee WJ, Baguley BC, Buchanan CM, Jamieson SMF, Shepherd PR, Denny WA, Rewcastle GW, Synthesis and biological evaluation of sulfonamide analogues of the phosphatidylinositol 3-kinase inhibitor ZSTK474, Bioorg Med Chem, 25 (2017) 5859–5874. [DOI] [PubMed] [Google Scholar]
- [30].Rewcastle GW, Gamage SA, Flanagan JU, Frederick R, Denny WA, Baguley BC, Kestell P, Singh R, Kendall JD, Marshall ES, Lill CL, Lee WJ, Kolekar S, Buchanan CM, Jamieson SM, Shepherd PR, Synthesis and biological evaluation of novel analogues of the pan class I phosphatidylinositol 3-kinase (PI3K) inhibitor 2-(difluoromethyl)-1-[4,6-di(4-morpholinyl)-1,3,5-triazin-2-yl]-1H-benzimidazole (ZSTK474), J Med Chem, 54 (2011) 7105–7126. [DOI] [PubMed] [Google Scholar]
- [31].Zhou Z, Zhang Y, Yan N, Wang Y, Sun T, Design, Synthesis and Biological Evaluation of Novel 1,3,5-triazine Derivatives as Potent Antitumor Agents, Medicinal Chemistry, 5 (2015) 345–350. [Google Scholar]
- [32].Elmenier FM, Lasheen DS, Abouzid KAM, Phosphatidylinositol 3 kinase (PI3K) inhibitors as new weapon to combat cancer, Eur J Med Chem, 183 (2019) 111718. [DOI] [PubMed] [Google Scholar]
- [33].Barrett SD, Biwersi CH, Kaufman MD, Tecle H, Warmus JS US 6,960,614B2,, Oxygenated esters of 4-Iodophenyaminobenzhydroxamic acids., in, 2005.
- [34].Isshiki Y, Kohchi Y, Iikura H, Matsubara Y, Asoh K, Murata T, Kohchi M, Mizuguchi E, Tsujii S, Hattori K, Miura T, Yoshimura Y, Aida S, Miwa M, Saitoh R, Murao N, Okabe H, Belunis C, Janson C, Lukacs C, Schuck V, Shimma N, Design and synthesis of novel allosteric MEK inhibitor CH4987655 as an orally available anticancer agent, Bioorg Med Chem Lett, 21 (2011) 1795–1801. [DOI] [PubMed] [Google Scholar]
- [35].Galban S, Apfelbaum AA, Espinoza C, Heist K, Haley H, Bedi K, Ljungman M, Galban CJ, Luker GD, Dort MV, Ross BD, A Bifunctional MAPK/PI3K Antagonist for Inhibition of Tumor Growth and Metastasis, Mol Cancer Ther, 16 (2017) 2340–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bartholomew D, Six Membered Rings with Two or More Heteroatoms and Fused Carbocyclic Derivatives, in: Comprehensive Heterocyclic Chemistry II, Pergamon, 1996, pp. 575–636. [Google Scholar]
- [37].Madsen RR, Vanhaesebroeck B, Semple RK, Cancer-Associated PIK3CA Mutations in Overgrowth Disorders, Trends Mol Med, 24 (2018) 856–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang C, Wang H, Zheng C, Liu Z, Gao X, Xu F, Niu Y, Zhang L, Xu P, Research progress of MEK1/2 inhibitors and degraders in the treatment of cancer, Eur J Med Chem, 218 (2021) 113386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.