Abstract
BACKGROUND AND AIMS:
The gastrointestinal (GI) tract extracts nutrients from ingested meals while protecting the organism from infectious agents frequently present in meals. Consequently, most animals conduct the entire digestive process within the GI tract while keeping the luminal contents entirely outside the body, separated by the tightly sealed GI epithelium. Therefore, like the skin and oral cavity, the GI tract must sense the chemical and physical properties of the luminal contents to optimize digestion. Specialized sensory enteroendocrine cells (EECs) in GI epithelium interact intimately with luminal contents. A subpopulation of EECs express the mechanically gated ion channel Piezo2 and are developmentally and functionally like the skin’s touch sensor, that is, the Merkel cell. We hypothesized that Piezo2+ EECs endow the gut with intrinsic tactile sensitivity.
METHODS:
We generated transgenic mouse models with optogenetic sensors in EECs and Piezo2 conditional knockouts. We used a range of reference standard and novel techniques from single cells to living animals, including single-cell RNA sequencing and opto-electrophysiology, opto-organ baths with luminal shear forces, and in vivo studies that assayed GI transit while manipulating the physical properties of luminal contents.
RESULTS:
Piezo2+ EECs have transcriptomic features of synaptically connected mechanosensory epithelial cells. EEC activation by optogenetics and forces led to Piezo2-dependent alterations in colonic propagating contractions driven by intrinsic circuitry, with Piezo2+ EECs detecting the small luminal forces and physical properties of the luminal contents to regulate transit times in the small and large bowel.
CONCLUSIONS:
The GI tract has intrinsic tactile sensitivity that depends on Piezo2+ EECs and allows it to detect the luminal forces and physical properties of luminal contents to modulate physiology.
Keywords: Enteroendocrine Cell, Neuroepithelial Connection, Mechanosensitivity, Ion Channels, GI Physiology
The somatosensory senses of touch and taste have evolved to critically assess the physical properties of meals, frequently guides ingestion.1 The skin, tongue, and oral cavity use specialized epithelial mechanosensory cells and sensory structures to determine a meal’s physical composition, including hardness, texture, and viscosity.2 Although ingestion takes minutes, digestion takes hours, so most interactions with food products occur post-prandially. The gut keeps the digestive process outside the body and has developed a remarkable ability to determine the physical properties of the luminal contents,3 such as size and shape of luminal particles, by modulating motility4 and sensation.3 Consequently, diets that manipulate physical properties, such as with insoluble fiber, form a bedrock of treatments in diseases ranging from gastrointestinal (GI) sensory and motility disorders,3,5 to metabolic disorders including obesity6 and diabetes,7 to developmental disorders like autism.8
To physically interact with luminal contents effectively, the gut developed a broad range of mechanosensory circuits composed of diverse mechanosensory cells.9,10 The gut epithelium, which comes in direct contact with luminal contents, harbors specialized sensory enteroendocrine cells (EECs) that are developmentally and functionally similar to the specialized epithelial cells in the oral cavity and skin that contribute to the sensations of taste11 and touch.12 The EECs sense an impressive range of luminal stimuli—chemicals, such as nutrients and bacterial metabolites13; spices and odorants14; and forces.15,16 The striking similarities between the Merkel cells in the skin and a subset of EECs led to a hypothesis of their being of common ancestry.17 We recently found a population of mechanically sensitive EECs in the small bowel that, like Merkel cells, contain the mechanically gated ion channel Piezo2.15,16 Merkel cells and mechanosensing EECs have common developmental pathways18,19; both detect mechanical forces through the mechanosensitive ion channel Piezo2,16,20 are electrically excitable,21 and release the neurotransmitter serotonin (5-HT)22,23 to initiate local and systemic responses.23
However, although the role of Merkel cells is well understood, mechanosensitive EEC roles in GI functions remain unresolved.24,25 Removing mucosal serotonin leaves mechanically induced contractions mostly intact,26,27 but alters the pellets’ physical properties,28 suggesting their role in luminal mechanosensing. We hypothesized that Piezo2 EECs are the gut’s tactile sensors. Using a range of techniques and models, we demonstrate that the Piezo2 EECs contribute to the gut’s ability to sense forces and physical properties of luminal contents to regulate critical digestive functions.
Methods
Full methods are available in the Supplementary Material.
Results
Piezo2+ Epithelial Cells Are Enteroendocrine Cells Involved in Neuroepithelial Communication
We were motivated by the recent discoveries that mechanosensitive epithelial cells in the skin and the gut expressed the mechanosensitive ion channel Piezo2.16,20,29 We wanted to determine the populations of Piezo2+ gut mucosal cells. We generated Piezo2tomato mice (Piezo2cre/+:: Rosa26tm9(CAG-tdTomato)Hze/+), in which Piezo2-expressing cells were lineage-traced (Figure 1A). We verified the model by taking a colon cross-section where we saw tomato+ cells present in the tunica muscularis, intracrypt, and sporadic epithelial cells (Figure 1B). From colon mucosa, we sorted 1,522,111 ± 142,606 single cells and found that 0.5% ± 0.2% were tomato+ (n = 3, Figure 1C and Supplementary Figure 1). We performed single-cell RNA sequencing on tomato+ cells and found several populations (Figure 1D), but only 3 were actively expressing Piezo2 (Figure 1E), suggesting that other populations expressed Piezo2 transiently, potentially during development.30,31 Based on the clusters’ enriched genes, we classified the Piezo2+ populations as innate immune, lymph endothelium, and EECs (Figure 1E and F).
Figure 1.
Transcriptional profile of the epithelial Piezo2 enteroendocrine cell subpopulation. (A) Strategy to enrich Piezo2+ cells for single-cell RNA sequencing (scRNAseq). Lineage-traced cells from Piezo2tomato colon mucosa: tomato+ if ever expressed Piezo2 (red) and some continued to express Piezo2 (red/blue). Tomato+ cells were sorted by fluorescence-activated cell sorting (FACS) and examined by scRNAseq. (B) Representative epifluorescence micrograph of Piezo2tomato colon showing tomato+ structures and cells: &myenteric plexus, #intracrypt, and *sporadic epithelial cells. Red line shows mucosal peeling plane. (C) FACS sorting of dissociated cells from Piezo2tomato colonic mucosa showing tomato+ cell population (from total mean ± SEM, 1,522,111 ± 142,606 cells, tomato+ were 0.2%−0.9%, mean ± SEM, 0.5% ± 0.2%, n = 3). (D) t-distributed stochastic neighbor embedding (tSNE) plot of 10 tomato+ populations. (E) tSNE plot of tomato+ subpopulations showing 3 clusters that actively express Piezo2. (F) Heatmap showing top cluster defining genes and Piezo2 expression across clusters (bottom strip). (G) Epithelial Piezo2+ population expresses EEC-specific developmental factors, a range of chemo- and olfactory receptors signal transduction ion channels, signaling and synaptic molecules, active zone, neuroepithelial communication, and post-synaptic receptors. (H) Pseudotime plot of the Piezo2+ enterochromaffin cell population showing that from root state (cyan), Piezo2 cells segregate into the following 3 states: Piezo2loTph1hi, Piezo2hiTph1hi, and Piezo2hiTph1lo. (I) Comparing transcriptomes of the Piezo2hi to Piezo2lo populations suggests that Piezo2hi is an electrically excitable synaptically connected EEC subpopulation. (J) Colonic organoids from Piezo2tomato mice show tomato+ cells (magenta). Scale bars: 50 μm (top), 10 μm (bottom). (K) FACS of dissociated Piezo2tomato colonic organoids. (I) Quantitative polymerase chain reaction of FAC sorted organoid-derived Piezo2tomato epithelial cells showing they are EECs enriched in Chga, Tph1, and Piezo2. (M) Piezo2 EECs are a unique population of synaptically connected electrically active mechanosensory EECs. Piezo2 epithelial cells are defined by EEC transcription factors (NeuroD1, Insm1, and Nkx2-2) and enriched in receptors for mechanical forces (Piezo2), and metabolites (Olfr78, Ffar2 [short-chain fatty acids]), and signal transduction ion channels (Scn3a, Cacna1h, Cacna1a). Synaptic vesicles (Chga/b, Snap25, Syt7) contain neurotransmitters, especially serotonin (Tph1), and less so others (secretin [Sct], substance P [Tac1], Glp-1 [Gcg and Pcsk1], Pyy, and Somatostatin [Sst]). They are enriched in molecules that form a presynaptic active zone (Pclo, Rimbp2, and Rim2), but also post-synaptic receptors (glutamate [Grin3a]).
The only Piezo2+ epithelial population (Vil1+) was EECs (Figure 1D and Supplementary Figure 2), and it expressed Piezo2 most densely compared with all sequenced cells (Figure 1E). Piezo2+ EECs expressed well-described EEC transcription factors (Insm1, Neurod1, Nkx2.2)32-34 and signaling molecules known to be synthesized and released by EECs (most 5-HT via Tph1, secretin Sct, substance P via Tac1, and some expressed somatostatin Sst, peptide YY Pyy, and GLP-1 via Pcsk1).35,36 In addition to high expression of Piezo2, Piezo2+ EECs expressed other EEC receptors, described previously, for bacterial metabolites (Ffar2, Olfr78)37,38 and hormones (Igf2r), as well as ion channels critical for signal transduction (Scn3a, Cacna1a, and Cacna1d)13,21 (Figure 1G). Our findings not only support the emerging theme that EECs co-express a range of signaling molecules,36,39 but also suggest that the variety of released signaling molecules in response to luminal forces may allow multifaceted control of a range of complex physiologic processes within the gut and systemically.23
Piezo2+ EECs expressed markers of neuroepithelial communication, including molecules relevant for synapses and synapse formation, neuroepithelial communication, and even postsynaptic receptors (Figure 1G).13,40 We proceeded to examine the heterogeneity of this population using pseudotime analysis41 and found that the Piezo2+ EEC population was divided into the following 2 subpopulations: Piezo2hi and Piezo2lo (Figure 1H). The Piezo2hi population was highly enriched in Gene Ontology cellular components related to electrical excitability and synaptic connectivity (Figure 1I) and Piezo2lo was not. When we compared the presynaptic machinery between Piezo2hi EECs and Merkel cells,42 we found a significant overlap of >30% (DynaVenn, P < .05) (Supplementary Figure 3). We confirmed our findings of epithelial Piezo2+ EECs in organoids from the Piezo2tomato mice (Figure 1J), where we found a tomato + epithelial population (Figure 1K and J). When enriched by fluorescence-activated cell sorting, Piezo2 population accounted for 1.1% of total cells (Figure 1K). This population was consistent with Piezo2 EECs through enrichment in both Piezo2 and classical EEC markers (Figure 1L). In all, the Piezo2+hi EEC population have a genetic landscape to be involved in communication between specialized mechanosensory epithelial cells and sensory neurons, similar to Merkel cells and sensory neurons in the skin (Figure 1M).
Mechanosensitive Enteroendocrine CellsReaChR Require Piezo2 for 5-HT Release
EECs are proposed to communicate with intrinsic primary afferent neurons, which regulate motility and secretion, which are critical aspects of digestion23 (Figure 2A). To selectively stimulate EECs, we integrated the red-shifted channelrhodopsin (ReaChR).43 Then we found that Piezo2+ EECs densely and selectively express NeuroD1 (Supplementary Figure 2), which is a “late” transcription factor in EEC development.44 Therefore, to express ReaChR specifically in EECs, we generated EECReaChR (NeuroD1Cre/+::Rosa26LSL-ReaChR-mCitrine/+) mice. We found that EECReaChR mice had a scattered population of ReaChR+ (Figure2A and B) cells in the GI epithelium (Figure 2A, B, E, and H and Supplementary Figure 4). Through colocalization with chromogranin A (Cga) immunolabeling (Figure 2A and C), a maker of EECs45 (Figure 2A and D), we determined that ReaChR+ cells were EECs and via Piezo2 immunolabeling we found that 40% ± 3% were Piezo2+ (Figure 2H-J, n = 4). As we expected in the colon, where 5-HT+ enterochromaffin cells comprise the largest EEC population,46 the majority of the EECReaChR cells were 5-HT+ (Figure 2A, E-G). NeuroD1+ EECs are mechanosensitive Piezo2+ EECs (Figure 2A and J),16,29 and indeed, EECReaChR (Figure 2A and H) colocalized with Piezo2 immunofluorescence (Figure 2A and I).
Figure 2.
EECReaChR cells in the GI tract are EECs that are predominately enterochromaffin cells that express a red light–gated ion channel ReaChR. (A) Model where Piezo2 and ReaChR are expressed by EECs, and activation of EECs by force or red-light releases 5-HT to enteric neurons that control GI physiologic functions. (B–J) Projection of confocal stack images of EECReaChR mouse colon with transgenic ReaChR expression (magenta in panels B, E, and H) colocalizing with immunofluorescence of EEC marker CgA (green in panels C and D), 5-HT (green in panels F and G), and Piezo2 (green in panels I and J). Nuclei counterstained with 4′,6-diamidino-2-phenylindole (blue). Lower panels expanded from within white rectangles. Scale bars = 20 μm. (B) ReaChR expression (magenta), (C) CgA immunofluorescence (green) and (D) colocalization. (E) ReaChR expression (magenta), (F) 5-HT immunofluorescence (green) and (G) colocalization. (H) ReaChR expression (magenta), (I) Piezo2 immunofluorescence (green) and (J) colocalization.
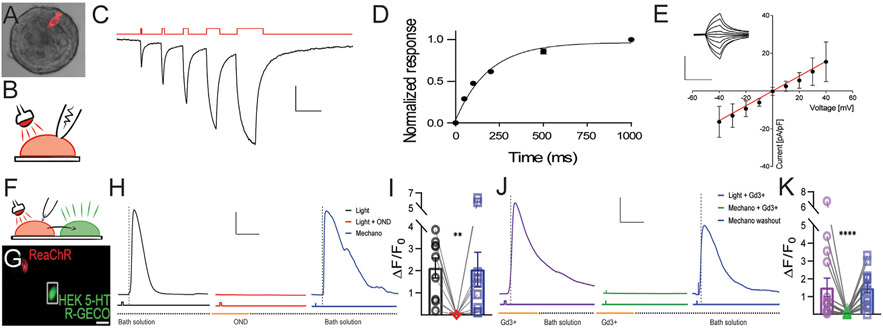
Optogenetics allows selective stimulation of excitable cell populations.47 EECs are electrically excitable with hyperpolarized resting potentials compared with other epithelial cells.13,21 Therefore, we asked whether EECReaChR cells can be activated by both optical43 and mechanical stimuli to drive 5-HT release.16 We generated EECReaChR colonoids, which contained a population of fluorescent cells that were morphologically EECs (Figure 3A) and made primary cultures for whole-cell voltage-clamp electrophysiology to examine optically induced currents (Figure 3B). We voltage-clamped the EECReaChR cells at a holding potential of −70 mV and used 625 nm optical stimuli to find robust duration-dependent inward opto-currents only in the fluorescent EECReaChR cells (Figure 3C and D) that had linear current–voltage relationships consistent with nonselective cation conductance (Figure 3E). The EEC opto-currents had kinetics consistent with previous reports,43 and when heterologously expressed ReaChR in HEK-293 cells (Supplementary Figure 5).
Figure 3.
Optical and mechanical stimulation activate single EECReaChR cells. (A) Projection of a confocal stack overlayed with DIC of live 3-dimensional colonic organoid derived from EECReaChR mouse with ReaChR (red) channel and transmitted light (gray). (B) Experimental setup showing an organoid-derived primary EEC under whole-cell voltage clamp electrophysiology and stimulated with red light. (C) Representative optically induced whole-cell currents with voltage-clamped electrophysiology. Scale bars = ordinate, 50 pA, abscissa, 1 second. (D) Normalized current dose-duration curve of EECReaChR cell. (E) Current–voltage relationship (I/V) of peak current in EECReaChR cell. Scale bars = ordinate, 200 pA, abscissa, 600 milliseconds. (F) Experimental setup showing mechanical or optogenetic stimulation of EECReaChR cell (red) leading to 5-HT release that is detected and reported by the 5-HT biosensor. (G) Confocal stack image showing EECReaChR cell (red) co-cultured with 5-HT biosensor (5HT3R/R-GECO, green). Scale bar = 20 μm. (H) Representative 5-HT biosensor fluorescence (ΔF/F0) traces after EECReaChR activation by light (black), block by 5HT3R inhibitor ondansetron (OND, 0.1 μM, red), and mechanical stimulation (blue). Stimulation period in the line below trace. Scale bars = ordinate, 0.5ΔF/F0, abscissa, 30 seconds. (I) Average 5-HT biosensor responses to EECReaChR light stimulation (black), with OND (red), and with mechanical stimulation (blue) (individual data points paired, and bars showing mean ± SEM, n = 9, paired t test). (J) Representative 5-HT biosensor fluorescence (ΔF/F0) traces after EEC activation with light in the presence of Piezo2 blocker Gd3+ (blue) mechanical stimulation with Gd3+ (red) and mechanical stimulation in washed control bath solution (blue). Stimulation period in the line below trace. Scale bars = ordinate, 0.5ΔF/F0, abscissa, 30 seconds. (K) Average 5-HT biosensor response to EECReaChR red-light stimulation with Gd3+ (purple) mechanical stimulation with Gd3+ (green) and washout control mechanical stimulation (blue) (mean ± SEM, n = 15, paired t test). **P < .01, ****P < .0001.
We wondered whether the optical and mechanical stimulation of EECReaChR cells induce 5-HT release, which is critical for neuroepithelial transmission.13 We constructed 5-HT biosensors from HEK-293 cells transfected with a genetically modified 5-HT–gated ion channel (5-HT3R) and a red-fluorescent calcium indicator (R-GECO) (Figure 3F and G).16 The 5-HT biosensors alone did not respond to optical stimuli, but responded to 1 μM 5-HT (Supplementary Figure 5). In co-cultured organoid-derived EECReaChR cells with 5-HT biosensors (Figure 3F and G), we found that in 67% of EECReaChR cells, optical stimulation led to the activation of 5-HT biosensors (n = 9, Figure 3H and I), and ondansetron (0.1 μM), a 5HT3R blocker, inhibited biosensors’ optical responses (Figure 3H and I). We wanted to test whether the optically sensitive EECReaChR cells are mechanosensitive EECs. We stimulated EECReaChR mechanically by direct membrane displacement and found that 67% of mechanically stimulated EECReaChR cells released 5-HT (n = 9, Figure 3H and I). Next, we used Gd3+, a Piezo2 channel blocker,48 which inhibited the mechanosensitive, but not optical, response (Figure 3J and K). These data showed that EECReaChR are mechanosensitive EECs that rely on Piezo2 for 5-HT release, and optogenetic stimulation can bypass pharmacological Piezo2 block.
Piezo2 Enteroendocrine Cells Adjust Motility in Response to Small Luminal Forces
EEC activation is proposed to control propulsive motility.23 However, EEC roles in GI motility have been disputed24,25 because previous studies have not directly activated EECs.26,28 We investigated EEC roles in regulating contractions using the EECReaChR model with optical stimulation in the organ bath (Figure 4A) using optogenetic stimulation protocols optimized in single EECs (Figure 3). To ask whether EECs influence propagating contractions, we used a low-volume intraluminal stimulus in colonic organ bath to induce repetitive contractions (Figure 4A). Optogenetic EECReaChR colon stimulation increased the frequency of propagating contractions (Figure 4B and C), but did not significantly alter contraction force, duration, or propagation (Supplementary Figure 6). The increases in frequency in response to light stimulation were inhibited by 5HT3R and 5HT4R blockade (Supplementary Figure 7).
Figure 4.
Optical stimulation of EECReaChR increases colonic propagating contraction frequency. (A) Experimental setup with organ bath used to measure optically stimulated (λ) propagating contractions through oral (ΔT1) and aboral (ΔT2) tension transducers and aboral pressure (ΔP) sensor. (B) Representative traces (ΔT1, ΔT2, ΔP) showing baseline, optical stimulation (2Hz, 40% duty cycle, 10 seconds, 90 seconds start to start), and recovery contraction frequency in EECReaChR colon. Gray dashed line at peak of each tension trace (from ΔT1 to ΔT2) follows contraction propagating along the tissue. Optical stimulation shown between T1 and T2 traces (red). (C) Average propagating contraction frequency during baseline (gray), optical stimulation (red), and recovery (blue) in EECWT and EECReaChR experimental colons (mean ± SEM, EECWT n = 6, EECReaChR n = 14, paired t test) (ΔT1 scale bars = ordinate, 0.5 mN, abscissa, 1 minute; ΔT2 scale bars = ordinate, 1 mN, abscissa, 1 minute; and ΔP scale bars = ordinate, 5 cmH2O, abscissa, 1 minute). **P < .01, ***P < .001.
We wondered whether mechanosensitive EECs altered the contraction frequency in response to luminal mechanical stimuli (Figure 5A). To abolish EEC mechanosensitivity, we used a conditional Piezo2 knockout from EECs (Piezo2CKO) using the gut epithelium-specific Vil1-Cre driver (Villin1cre/+ ::Piezo2f/f).16 We found no difference in resting colonic contraction frequency between Piezo2CKO and Piezo2WT mice (Figure 5B and D). We then asked whether EEC mechanical stimulation could change colonic contraction frequency. We used luminal shear stress, a physiologically relevant stimulus for mucosal contribution to colonic motility. We found that stimulation with small shear forces (10 mL/h) increased the contraction frequency in Piezo2WT mice, but failed to influence contraction frequency in Piezo2CKO mice (Figure 5B-E). In contrast, higher-shear forces that we predicted to affect both mucosa and tunica muscularis (25 mL/h) increased contraction frequency in both Piezo2CKO and Piezo2WT mice (Figure 5F). Although similar, the responses in Piezo2CKO tissue, unlike in Piezo2WT, were highly variable (Figure 5F and Supplementary Figure 8). These data suggest that although Piezo2-dependent EEC mechanosensitivity is not required for colonic motility, it is sufficient to regulate the contraction frequency, especially to small intraluminal mechanical stimuli.
Figure 5.
Mechanosensitive EEC activation by small luminal forces increases colonic contraction frequency. (A) Experimental setup to measure luminal shear (black arrow in the lumen) induced changes in colonic motility reported by a tension transducer (ΔT1). (B) Contractions intervals, displayed as black ticks, in Piezo2WT colon basal and intraluminal shear (small force: 10 mL/h and large force: 25 mL/h, for 10 seconds, 90 seconds start to start). (C) Contraction frequency during baseline, intraluminal, and recovery in Piezo2WT colon (mean ± SEM, 10 mL/h, n = 12; 25 mL/h, n = 6, paired t test). (D) Contractions intervals, displayed as red ticks, in Piezo2CKO colon at rest and with intraluminal shear flow (small force: 10 mL/h and large force: 25 mL/h, 10 seconds, 90 seconds start to start). (E) Contraction frequency during baseline, intraluminal flow, and recovery in Piezo2CKO colon (mean ± SEM, 10 mL/h, n = 10; 25 mL/h n = 7, paired t test). (F) Percent increase contraction frequency of Piezo2WT and Piezo2CKO colon from baseline to shear (mean ± SEM, Piezo2WT n = 6, Piezo2CKO n = 7, 1-tailed t test). (G) Experimental organ bath set up to measure luminal flow (black arrow in lumen) and 625 nm red-light optogenetic (2 Hz, 40% duty cycle) induced changes in colonic motility through a tension transducer (ΔT1). (H) Contraction intervals of tension traces showing basal, luminal flow (10 mL/h) baseline, flow (10 mL/h), and optogenetic stimulation with flow (2 Hz, 40% duty cycle and 10 mL/h) frequency in the presence of luminal Gd3+ in EECWT (top) and EECReaChR (bottom) colon. (I) Contraction frequency of EECWT and EECReaChR colon during baseline, intraluminal flow, baseline with Gd3+, Gd3+ intraluminal flow, and Gd3+ flow with optogenetic stimulation (mean ± SEM, EECWT n = 6, EECReaChR n = 7, paired t test). *P < .05, **P < .01, ***P < .001, ****P < .0001.
We then asked whether optical and mechanical stimulation in EECReaChR colons produced similar responses. The organ bath setup was modified to introduce a fiber optic red light for optogenetic stimulation (Figure 5G). We found that 10 mL/h intraluminal flow increased colonic contraction frequency in both EECReaChR and EECWT mice (Figure 5H). We then added luminal Gd3+ to inhibit Piezo2 channels and found that Piezo2 blockage did not affect propagating contractions without mechanical stimulation and shear stress (10 mL/h) responses were diminished. Finally, we combined optical and mechanical stimulation while blocking the epithelial Piezo2 channels and found that in EECReaChR tissue the diminished response to shear stress can be rescued by optical stimulation (Figure 5I). These data showed that colonic mechanosensitive Piezo2+ EECs adjust contraction frequency in response to small luminal forces generated by the movement of contents.
Mechanosensitive Enteroendocrine Cells Detect Physical Properties of Luminal Contents to Regulate Gut Motility
Next, we asked whether Piezo2-dependent EEC mechanosensitivity influences GI motility in vivo. Considering our in vitro data showing that mechanosensitive EECs directly regulate colonic motility, we tested the hypothesis that Piezo2-dependent EEC mechanosensitivity regulates gut transit (Figure 6A). To determine whole-gut transit, we used a carmine red gavage protocol with remote monitoring of pellet production (Figure 6B).49 Piezo2CKO mice had slowed whole-gut transit by 29% compared with Piezo2WT mice (timetotal Piezo2CKO 295 ± 15 minutes vs Piezo2WT 211 ± 26 minutes; *P < .05) (Figure 6B).
Figure 6.
Mechanosensitive Piezo2 EECs are regulators of tactile sensing by the luminal gut. (A) In vivo assays to determine the role of Piezo2 EECs in the gut: whole-gut transit (red), gastric emptying (blue), small intestine (yellow), and colon (purple). (B) Whole-gut transit time for Piezo2WT and Piezo2CKO (mean ± SEM, n = 12, n = 28, unpaired t test). (C) Gastric emptying T1/2 was not different between Piezo2CKO and Piezo2WT mice (mean ± SEM, n = 5, n = 7, unpaired t test). (D) Top row, small bowel transit in Piezo2WT vs Piezo2CKO for liquids. Bottom row, small bowel transit for 390 μm fluorescent beads (n = 8, n = 6, unpaired t test). Spectral analysis of fluorescence deconstructed by frequency, showing the dominant frequencies of the signa (scale bar = 0.1). (E) Piezo2WT (left) and Piezo2CKO (right) pellet length and width, with image of pellets comparing size (mean ± SEM, n = 40, n = 71, unpaired t test) (scale bar = 2.0 mm). (F) Distal colonic transit of Piezo2WT and Piezo2CKO mice in 1 mm, 2 mm, and 3 mm beads (mean ± SEM, unpaired t test). *P < .05, **P < .01.
We compared gastric emptying between Piezo2CKO and Piezo2WT mice using the validated 13C-octanoic acid breath test50,51 and found no differences (Figure 6C and Supplementary Figure 9). We were not surprised because the rodent stomach expresses Vil1 sparsely.52 Then we tested small bowel transit using liquid rhodamine isothiocyanate dextran gavage and profiling the small bowel fluorescence distribution between Piezo2CKO and Piezo2WT.53 We found no differences in the fluorescence distribution between the groups when using liquids (geometric center [GC], GCCKO_liquid = 4.9 ± 0.43, GCWT_liquid = 4.4 ± 0.38; P > .05) (Figure 6D). We asked whether small particulates would have a different distribution in the small bowel. We gavaged small (390 μm) fluorescent beads and found drastic changes compared with liquid only in Piezo2WT, with contents being spread out through the entire length of the small bowel (Figure 6D, bottom black). Intriguingly, although GCs between Piezo2WT and Piezo2CKO were not different (GCCKO_solid = 5.4 ± 0.33, GCWT_solid = 5.6 ± 0.43; P > .05) (Figure 6D), the 2 models’ distribution was remarkably different (Figure 6D, right panels). Although Piezo2WT distributed the beads across small bowel length, widening the spectral density peak, the Piezo2CKO small bowel continued to treat the luminal contents like liquids, showing up as a single peak (Figure 6D, right panels).
We proceeded to examine the colon. We looked at the fecal pellets’ dimensions because of previous findings that Tph1 knockout altered fecal pellet dimensions, suggesting a mechanosensory dysfunction.28 Interestingly, we found that pellets had a significantly larger diameter in Piezo2CKO mice compared with Piezo2WT (2.3 ± 0.005 mm vs 2.1 ± 0.005 mm; P < .05), but were of similar length (Piezo2CKO 5.74 ± 0.017 mm vs Piezo2WT 5.53 ± 0.027 mm; P > .05) (Figure 6E). These results suggested that Piezo2 EECs are critical regulators of pellet size. To test the hypothesis that pellet size altered colonic transit and contributed to slower whole-gut transit slowing in Piezo2CKO compared with Piezo2WT, we used colonic bead expulsion assay.54 We found that when comparing colonic transit times between different-sized beads, there was no difference in the smallest bead size (1 mm: Piezo2CKO 39.3 ± 1.9 seconds vs Piezo2WT 42.3 ± 5.7 seconds) (Figure 6F). As the pellet size grew, Piezo2CKO transit time was significantly delayed compared with Piezo2WT (2 mm: Piezo2CKO 47.4 ± 1.2 seconds vs Piezo2WT 33.6 ± 0.86 seconds; P < .05; 3 mm: Piezo2CKO 41.3 ± 1.4 seconds vs Piezo2WT 26.9 ± 1.0 seconds; P < .05). These results showed that the Piezo2 EECs are a critical element of the gut’s intrinsic mechanosensory system that detects luminal forces and discriminates particle sizes to regulate gut motility.
Discussion
The Gut’s Epithelial Tactile Receptors
The central finding in this study is that a population of epithelial Piezo2+ EECs serve as gut touch receptors and endow the gut with an intrinsic tactile sensitivity that regulates GI tract function and transit. The gut, like skin, is in constant contact with the external world and, therefore, it must determine the physical makeup of luminal contents. We found that a subpopulation of Piezo2 EECs was enriched in mechanisms involved in forming and maintaining synaptic contacts. Based on the shared developmental transcription factors, neurotransmitters, and synaptic connectivity, Piezo2+ EECs join the ranks of other Piezo2+ specialized epithelial mechanosensing cells—Merkel cells in the skin,20,42 hair cells in the ear,55 and neuroepithelial bodies in the lung.56 However, skin touch and hearing are well-defined senses with a clear function, but how might gut touch receptors work, and what do they do?
EECs are highly diverse in their expression of receptors, neurotransmitters, and hormones across the length of the gut and along the crypt–villus axis.35,39 Like other EEC subpopulations, Piezo2+ EECs express G-protein–coupled receptors potentially to complement and tune the mechanosensory responses based on the state of luminal contents, like microbiome.13,29 For example, EECs are implicated in diseases such as irritable bowel syndrome,57 in which there are alterations in motility and visceral mechanosensory dysfunction58 and our results raise a possibility that diet-related shifts in microbial metabolites may alter GI motility through alteration of mechanosensitive EECs.
The Piezo2+ EECs also expressed a range of genes that produce neurotransmitters critical for sensory transduction. Most were 5-HT–producing enterochromaffin cells through the expression of Tph1, but subpopulations also express neuropeptides, including substance P, secretin, PYY, and somatostatin. Some of these signaling molecules stimulate motility (5-HT)23,59,60; others inhibit it (Pyy and Sst),61 while the roles of others in GI motility are still unclear and likely segment dependent (substance P, secretin). Consequently, the regional repertoire of Piezo2+ EECs may define their impact on physiology. While Piezo2 EECs are present along the entire gut length,15,16,29 there are fewer Piezo2 EECs in the proximal small bowel, where the bulk of nutrient absorption occurs, than in the distal small bowel, where ileal brake relies on Pyy and Glp1 release to slow the escape of nutrients and solids from the absorptive area.15 Similarly, Piezo2 EECs were significantly denser in the distal colon and rectum than in the more proximal areas,29 and may contribute to the regulation of transit of contents through the colon. Indeed, the combination of surface receptors and chemical coding by Piezo2 EECs may underlie some regional complexities in GI physiology.
Gut Touch Receptors Regulate Gut Motility
The roles of EECs in gut motility remain unclear.24,25 Although EECs are epithelial, unlike other epithelial cell types, and like other neuroepithelial sensory cells,62 they have hyperpolarized resting potentials and are electrically excitable.21 Therefore, to get at the role of these cells in physiology, we made a novel mouse model where EECs were optogenetic (EECReaChR), allowing us, for the first time, to definitively assay EEC roles in GI physiology. We found that EECReaChR had robust dose–stimulus photocurrents, and optogenetic stimulation drove 5-HT release, as did mechanical stimulation in the same EECs. The ability to overcome Piezo2 block by optical stimulation not only confirmed the necessity of Piezo2 for mechanically induced 5-HT, but established a platform to study EEC contributions to physiology.
Therefore, we used optogenetic EECReaChR and conditional gut epithelial Piezo2 knockout (Piezo2CKO) models to determine the physiologic roles of Piezo2 EECs. Because luminal forces and physical makeup of contents tune EEC hormone release63 and regulate motility,64 we focused on this function. We found that the optical stimulation of EECReaChR in the colon most consistently increased the frequency of propagating contractions. Our findings are consistent with a model where mechanosensitive EECs regulate propagating contractions via serotonin receptors,60 but they are not necessary to initiate them.26 In turn, colonic contraction frequency regulates gut transit and may contribute to bowel disturbances and abdominal pain in patients with irritable bowel syndrome65 and slow transit constipation.
Gut Touch Senses Small Luminal Forces and Physical Properties of Contents
Small luminal forces increased colonic contraction frequency in a Piezo2 EEC-dependent fashion because direct optical stimulation could rescue epithelial Piezo2 blockade. However, larger forces increased contractile frequency independently of epithelial Piezo2, likely by engaging the mechanosensors that reside deeper in the gut wall—pacemaker cells66 or enteric neurons.67 Therefore, our study suggests that, similar to the layered extrinsic mechanosensory innervation in the skin68 and gut,69 there is an intrinsic multilayer mechanosensory circuitry built directly into the gut wall.
The skin and oral cavity sense food texture using tactile sensing. We wondered whether Piezo2 EECs adjusted gut motility in response to the physical makeup of luminal contents. Piezo2CKO had a delay in whole-gut transit, suggesting that GI motor function depends on Piezo2 EECs. To explore regional changes in motility, we independently tested motor function in the stomach, small bowel, and colon. The small bowel is the longest segment of the GI tract and has a critical life-sustaining function of digesting meals and extracting nutrients. The dictum is that mechanical digestion takes place in the stomach,70 and the small bowel is responsible for chemical digestion. Yet, the small bowel frequently contains particulates, and studies in humans showed that although transit times of solids and liquids through the small bowel are similar, the small bowel’s length ensures the appropriate distribution of particulates, presumably for mechanical digestion.71,72 Bolus distribution was similar between Piezo2WT and Piezo2CKO. However, the addition of insoluble particulates led to dramatic redistribution across the entire small bowel, possibly by segmenting activity required to physically digest the remaining solids. The small bowel transit time between Piezo2CKO and Piezo2WT for particulates was not altered, supporting previous findings that transit times between solids and liquids in the human small bowel are similar.71 Remarkably, however, Piezo2CKO led to a complete loss of the ability of the small bowel to redistribute the particulate contents. These results suggest that the gut tactile system regulates the distribution of small bowel contents and possibly segmenting activity in the small bowel.
We found that the colon relied on the gut touch system to respond to the physical sizes of contents, and loss of tactile sensing eliminated the size-dependent acceleration of colon transit. Interestingly, the epithelial 5-HT knockout led to a change in the physical size of fecal pellets,28 which, based on the current findings, is due to Piezo2+ EECs. These findings imply that the diseases may disrupt the gut touch system, such as slow transit constipation, where 5-HT deficiency was described previously,60,73 and slowed gastric emptying,60 and therapy involves targeting 5-HT pathways.74 Although constitutive knockout of epithelial 5-HT alone did not delay whole-gut transit in some studies,75 we found delays in whole-gut transit in Piezo2CKO mice compared with Piezo2WT. We may reconcile this apparent discrepancy in our finding that Piezo2+ EECs release other neurotransmitters in addition to 5-HT, which may provide physiologic redundancy. Therefore, the most effective approach to targeting this system may be via upstream receptor blockade rather than regulation of signaling molecule release or downstream receptors.
Our findings suggest that the gut touch sensors likely collaborate with other mechanosensory mechanisms deeper in the gut wall, likely the enteric76 and extrinsic nervous system.9,10,69 Small particulates mainly engage gut touch, while larger particles may engage both mechanosensors and speed up transit. When we eliminated gut touch mechanosensors, small bowel and colon lost their ability to determine the physical makeup of luminal contents, and discriminate particulate size, respectively. Neuro-epithelial circuits form between EECs and extrinsic sensory neurons,77 and possibly between EECs and intrinsic primary afferent neurons.9 Studies suggest that both intrinsic primary afferent neurons67 and extrinsic sensory neurons69 are mechanosensitive. An attractive hypothesis is that the gut touch system may be akin to somatosensory touch, where the epithelial Merkel cell forms a synapse with an Aβ neuron, and cooperative mechanosensitivity is required for touch sensing.20,62 The wiring of the gut touch circuitry and how it communicates with other intrinsic and extrinsic mechanosensors requires further investigation.
The mechanisms of how the gut feels luminal forces and the physical nature of its contents remained unclear. We described a novel intrinsic gut tactile sensing mechanism that requires specialized epithelial mechanosensors akin to Merkel cells in the skin and oral cavity.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
Enteroendocrine cells (EECs) are epithelial sensors of luminal nutrients, microbial metabolites, and mechanical forces. The roles of mechanically sensitive EECs that express mechanogated ion channel Piezo2 remain poorly understood.
NEW FINDINGS
Piezo2+ EECs may be synaptically connected epithelial sensory cells, and their activation by light (optogenetics) of forces leads to modulation of colonic contraction propagation frequency and an inability to detect small luminal forces and physical sizes of particulates, suggesting that the gastrointestinal (GI) tract has an intrinsic tactile sensitivity that we termed gut touch.
LIMITATIONS
The mechanisms of gut touch require further investigation, and these findings require translation to humans in health and disease.
IMPACT
GI epithelial mechanosensory EECs mediate gut touch, which contributes to the regulation of GI motility and therefore may play essential roles in functional and motility disorders.
Acknowledgments
The authors thank Peter Strege for help with the graphics; Gary Stoltz, Denika Kerska, and Tiffany Cassmann for technical assistance; Bob Highet for engineering; Simon J. Gibbons for constructive feedback; and Lyndsay Busby for administrative assistance. Anthony J. Treichel and Isabelle Finholm Q7 contributed equally to this work.
Funding
This work was supported by the Mayo Clinic Department of Medicine K2R Award, National Institutes of Health grants DK123549, AT010875, NS118790 (Arthur Beyder), and DK052766 (Gianrico Farrugia, Arthur Beyder).
Abbreviations used in this paper:
- EEC
enteroendocrine cell
- GC
geometric center
- GI
gastrointestinal
- ReaChR
red-shifted channelrhodopsin
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://doi.org/10.1053/j.gastro.2021.10.026.
Conflicts of interest
The authors disclose no conflicts.
CRediT Authorship Contributions
Anthony Treichel, BS (Data curation: Equal).
Isabelle Finholm, BS (Data curation: Equal; Writing – original draft: Lead; Writing – review & editing: Lead).
Kaitlyn Knutson, BS (Data curation: Supporting).
Alcaino Constanza, PhD (Data curation: Supporting).
Sara Whiteman, BS (Data curation: Supporting).
Matthew Brown, BS (Data curation: Supporting).
Aleksey Matveyenko, PhD (Data curation: Supporting).
Andrew Wegner, BS (Data curation: Supporting).
Halil Kacmaz, MD (Data curation: Supporting).
Arnaldo Mercado-Perez, BS (Data curation: Supporting).
Gabriella B. Gajdos, MS (Data curation: Supporting)
Tamas Ordog, MD (Data curation: Supporting).
Madhusudan Grover, MBBS (Data curation: Supporting).
Joseph Szurszewski, PhD (Data curation: Supporting).
David Linden, PhD (Data curation: Supporting).
Gianrico Farrugia, MD (Data curation: Supporting).
Arthur Beyder, MD, PhD (Data curation: Supporting; Writing – review & editing: Equal).
References
- 1.Dominy NJ, Yeakel JD, Bhat U, et al. How chimpanzees integrate sensory information to select figs. Interface Focus 2016;6:20160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moayedi Y, Duenas-Bianchi LF, Lumpkin EA. Somato-sensory innervation of the oral mucosa of adult and aging mice. Sci Rep 2018;8:9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebden JM, Blackshaw E, D’Amato M, et al. Abnormalities of GI transit in bloated irritable bowel syndrome: effect of bran on transit and symptoms. Am J Gastroenterol 2002;97:2315–2320. [DOI] [PubMed] [Google Scholar]
- 4.McIntyre A, Vincent RM, Perkins AC, et al. Effect of bran, ispaghula, and inert plastic particles on gastric emptying and small bowel transit in humans: the role of physical factors. Gut 1997;40:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karamanolis G, Tack J. Nutrition and motility disorders. Best Pract Res Clin Gastroenterol 2006;20:485–505. [DOI] [PubMed] [Google Scholar]
- 6.Desmarchelier C, Ludwig T, Scheundel R, et al. Diet-induced obesity in ad libitum-fed mice: food texture overrides the effect of macronutrient composition. Br J Nutr 2013;109:1518–1527. [DOI] [PubMed] [Google Scholar]
- 7.Nojima K, Ikegami H, Fujisawa T, et al. Food hardness as environmental factor in development of type 2 diabetes. Diabetes Res Clin Pract 2006;74:1–7. [DOI] [PubMed] [Google Scholar]
- 8.Huxham L, Marais M, van Niekerk E. Idiosyncratic food preferences of children with autism spectrum disorder in England. S Afr J Clin Nutr 2019;34(2):1–7. [Google Scholar]
- 9.Furness JB, Rivera LR, Cho H-J, et al. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol 2013; 10:729–740. [DOI] [PubMed] [Google Scholar]
- 10.Spencer NJ, Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol 2020;17:338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes 2008;15:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treichel AJ, Farrugia G, Beyder A. The touchy business of gastrointestinal (GI) mechanosensitivity. Brain Res 2018;1693:197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellono NW, Bayrer JR, Leitch DB, et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 2017;170:185–198.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latorre R, Sternini C, De Giorgio R, et al. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil 2016; 28:620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Knutson K, Alcaino C, et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol 2017; 595:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alcaino C, Knutson K, Treichel AJ, et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci U S A 2018; 115:E7632–E7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkelmann RK. The Merkel cell system and a comparison between it and the neurosecretory or APUD cell system. J Invest Dermatol 1977;69:41–46. [DOI] [PubMed] [Google Scholar]
- 18.Wright MC, Reed-Geaghan EG, Bolock AM, et al. Unipotent, Atoh1+ progenitors maintain the Merkel cell population in embryonic and adult mice. J Cell Biol 2015; 208:367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerbe F, van Es JH, Makrini L, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol 2011; 192:767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo SH, Ranade S, Weyer AD, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature 2014; 509:622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers GJ, Tolhurst G, Ramzan A, et al. Electrical activity-triggered glucagon-like peptide-1 secretion from primary murine L-cells. J Physiol 2011;589:1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cote F, Thevenot E, Fligny C, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A 2003;100:13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 2013;10:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith TK, Gershon MD. CrossTalk proposal: 5-HT is necessary for peristalsis. J Physiol 2015;593:3225–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer NJ, Sia TC, Brookes SJ, et al. CrossTalk opposing view: 5-HT is not necessary for peristalsis. J Physiol 2015;593:3229–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology 2010;138:659–670, 670. e1-e2. [DOI] [PubMed] [Google Scholar]
- 27.Nicholas S, Spencer NJ. Peristalsis and fecal pellet propulsion do not require nicotinic, purinergic, 5-HT3, or NK3 receptors in isolated guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 2010;298:G952–G961. [DOI] [PubMed] [Google Scholar]
- 28.Heredia DJ, Gershon MD, Koh SD, et al. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol 2013;591:5939–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billing LJ, Larraufie P, Lewis J, et al. Single cell transcriptomic profiling of large intestinal enteroendocrine cells in mice – Identification of selective stimuli for insulin-like peptide-5 and glucagon-like peptide-1 co-expressing cells. Mol Metab 2019;29:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pardo-Pastor C, Rubio-Moscardo F, Vogel-Gonzalez M, et al. Piezo2 channel regulates RhoA and actin cyto-skeleton to promote cell mechanobiological responses. Proc Natl Acad Sci U S A 2018;115:1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMillin MJ, Beck AE, Chong JX, et al. Mutations in PIEZO2 cause Gordon syndrome, Marden-Walker syndrome, and distal arthrogryposis type 5. Am J Hum Genet 2014;94:734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutoh H, Fung BP, Naya FJ, et al. The basic helix-loophelix transcription factor BETA2/NeuroD is expressed in mammalian enteroendocrine cells and activates secretin gene expression. Proc Natl Acad Sci U S A 1997; 94:3560–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai S, Loomis Z, Pugh-Bernard A, et al. Nkx2.2 regulates cell fate choice in the enteroendocrine cell lineages of the intestine. Dev Biol 2008;313:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gierl MS, Karoulias N, Wende H, et al. The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic beta cells and intestinal endocrine cells. Genes Dev 2006;20:2465–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haber AL, Biton M, Rogel N, et al. A single-cell survey of the small intestinal epithelium. Nature 2017;551:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grun D, Lyubimova A, Kester L, et al. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature 2015;525(7568):251–255. [DOI] [PubMed] [Google Scholar]
- 37.Nohr MK, Pedersen MH, Gille A, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 2013;154:3552–3564. [DOI] [PubMed] [Google Scholar]
- 38.Fleischer J, Bumbalo R, Bautze V, et al. Expression of odorant receptor Olfr78 in enteroendocrine cells of the colon. Cell Tissue Res 2015;361:697–710. [DOI] [PubMed] [Google Scholar]
- 39.Beumer J, Artegiani B, Post Y, et al. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat Cell Biol 2018;20:909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bohorquez DV, Shahid RA, Erdmann A, et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest 2015;125:782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trapnell C, Cacchiarelli D, Grimsby J, et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol 2014;32:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffman BU, Baba Y, Griffith TN, et al. Merkel cells activate sensory neural pathways through adrenergic synapses. Neuron 2018;100:1401–1413.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin JY, Knutsen PM, Muller A, et al. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci 2013;16:1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li HJ, Kapoor A, Giel-Moloney M, et al. Notch signaling differentially regulates the cell fate of early endocrine precursor cells and their maturing descendants in the mouse pancreas and intestine. Dev Biol 2012;371:156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engelstoft MS, Lund ML, Grunddal KV, et al. Research resource: a chromogranin A reporter for serotonin and histamine secreting enteroendocrine cells. Mol Endocrinol 2015;29:1658–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erspamer V. Occurrence and distribution of 5-hydroxytryptamine (enteramine) in the living organism. Z Vitam Horm Fermentforsch 1957;9:74–96. [PubMed] [Google Scholar]
- 47.Boyden ES, Zhang F, Bamberg E, et al. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 2005;8:1263–1268. [DOI] [PubMed] [Google Scholar]
- 48.Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science 1989;243:1068–1071. [DOI] [PubMed] [Google Scholar]
- 49.Kacmaz H, Alto A, Knutson K, et al. A simple automated approach to measure mouse whole gut transit. Neurogastroenterol Motil 2020:e13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Creedon CT, Verhulst PJ, Choi KM, et al. Assessment of gastric emptying in non-obese diabetic mice using a [13C]-octanoic acid breath test. J Vis Exp 2013:e50301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller KE, Bajzer Z, Hein SS, et al. High temporal resolution gastric emptying breath tests in mice. Neurogastroenterol Motil 2018:e13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rutlin M, Rastelli D, Kuo WT, et al. The Villin1 gene promoter drives cre recombinase expression in extra-intestinal tissues. Cell Mol Gastroenterol Hepatol 2020; 10:864–867.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woting A, Blaut M. Small intestinal permeability and gut-transit time determined with low and high molecular weight fluorescein isothiocyanate-dextrans in C3H mice. Nutrients 2018;10:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raffa RB, Mathiasen JR, Jacoby HI. Colonic bead expulsion time in normal and mu-opioid receptor deficient (CXBK) mice following central (ICV) administration of mu- and delta-opioid agonists. Life Sci 1987; 41:2229–2234. [DOI] [PubMed] [Google Scholar]
- 55.Wu Z, Grillet N, Zhao B, et al. Mechanosensory hair cells express two molecularly distinct mechanotransduction channels. Nat Neurosci 2016;20:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nonomura K, Woo SH, Chang RB, et al. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 2017;541:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology 2009;136:1979–1988. [DOI] [PubMed] [Google Scholar]
- 58.Major G, Pritchard S, Murray K, et al. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology 2017;152:124–133.e2. [DOI] [PubMed] [Google Scholar]
- 59.Melo CGS, Nicolai EN, Alcaino C, et al. Identification of intrinsic primary afferent neurons in mouse jejunum. Neurogastroenterol Motil 2020;32:e13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei L, Singh R, Ha SE, et al. Serotonin deficiency is associated with delayed gastric emptying. Gastroenterology 2021;160:2451–2466.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen J, Phillips SF, Sarr MG, et al. PYY and GLP-1 contribute to feedback inhibition from the canine ileum and colon. Am J Physiol 1995;269:G945–G952. [DOI] [PubMed] [Google Scholar]
- 62.Maksimovic S, Nakatani M, Baba Y, et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 2014;509:617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schonfeld J, Evans DF, Wingate DL. Effect of viscous fiber (guar) on postprandial motor activity in human small bowel. Dig Dis Sci 1997;42:1613–1617. [DOI] [PubMed] [Google Scholar]
- 64.Hebden JM, Blackshaw PE, Perkins AC, et al. Small bowel transit of a bran meal residue in humans: sieving of solids from liquids and response to feeding. Gut 1998; 42:685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chey WY, Jin HO, Lee MH, et al. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol 2001;96:1499–1506. [DOI] [PubMed] [Google Scholar]
- 66.Farrugia G Ionic conductances in gastrointestinal smooth muscles and interstitial cells of Cajal. Annu Rev Physiol 1999;61:45–84. [DOI] [PubMed] [Google Scholar]
- 67.Kunze WA, Clerc N, Furness JB, et al. The soma and neurites of primary afferent neurons in the guinea-pig intestine respond differentially to deformation. J Physiol 2000;526(Pt 2):375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abraira VE, Ginty DD. The sensory neurons of touch. Neuron 2013;79:618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brierley SM, Jones RC 3rd, Gebhart GF, et al. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 2004;127:166–178. [DOI] [PubMed] [Google Scholar]
- 70.Meyer JH, Ohashi H, Jehn D, et al. Size of liver particles emptied from the human stomach. Gastroenterology 1981;80:1489–1496. [PubMed] [Google Scholar]
- 71.Malagelada JR, Robertson JS, Brown ML, et al. Intestinal transit of solid and liquid components of a meal in health. Gastroenterology 1984;87:1255–1263. [PubMed] [Google Scholar]
- 72.Bennink R, Peeters M, Van den Maegdenbergh V, et al. Evaluation of small-bowel transit for solid and liquid test meal in healthy men and women. Eur J Nucl Med 1999; 26:1560–1566. [DOI] [PubMed] [Google Scholar]
- 73.El-Salhy M, Norrgard O, Spinnell S. Abnormal colonic endocrine cells in patients with chronic idiopathic slow-transit constipation. Scand J Gastroenterol 1999; 34:1007–1011. [DOI] [PubMed] [Google Scholar]
- 74.Bouras EP, Camilleri M, Burton DD, et al. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology 2001;120:354–360. [DOI] [PubMed] [Google Scholar]
- 75.Heredia DJ, Dickson EJ, Bayguinov PO, et al. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 2009;136:1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith TK, Bornstein JC, Furness JB. Interactions between reflexes evoked by distension and mucosal stimulation: electrophysiological studies of guinea-pig ileum. J Auton Nerv Syst 1991;34:69–75. [DOI] [PubMed] [Google Scholar]
- 77.Kaelberer MM, Buchanan KL, Klein ME, et al. A gut-brain neural circuit for nutrient sensory transduction. Science 2018;361(6408):eaat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.