Abstract
Inhalation of the fungus Alternaria alternata is associated with an increased risk of allergic asthma development and exacerbations. Recent work in acute exposure animal models suggests that A. alternata-induced asthma symptoms, which include inflammation, mucus overproduction and airway hyperresponsiveness, are due to A. alternata proteases that act via protease-activated receptor-2 (PAR2). However, because other active components present in A. alternata may be contributing to asthma pathophysiology through alternative signaling, the specific role PAR2 plays in asthma initiation and maintenance remains undefined. Airway epithelial cells provide the first encounter with A. alternata and are thought to play an important role in initiating the physiologic response. To better understand the role for PAR2 airway epithelial signaling we created a PAR2-deficient human bronchial epithelial cell line (16HBEPAR−/−) from a model bronchial parental line (16HBE14o−). Comparison of in vitro physiologic responses in these cell lines demonstrated a complete loss of PAR2 agonist (2at-LIGRL-NH2) response and significantly attenuated protease (trypsin and elastase) and A. alternata responses in the 16HBEPAR−/− line. Apical application of A. alternata to 16HBE14o− and 16HBEPAR2−/− grown at air-liquid interface demonstrated rapid, PAR2-dependent and independent, inflammatory cytokine, chemokine and growth factor basolateral release. In conclusion, the novel human PAR2-deficient cell line allows for direct in vitro examination of the role(s) for PAR2 in allergen challenge with polarized human airway epithelial cells.
Keywords: Allergen, Alternaria alternata, inflammatory cytokines, airway epithelium
INTRODUCTION
Alternaria alternata is a common fungus associated with allergic asthma and asthma exacerbations [1–4]. Hallmarks of allergic asthma include airway hyperreactivity, airway remodeling, mucus overproduction and inflammation [5,6]. PAR2 is a G protein-coupled receptor (GPCR) that is activated following proteolytic cleavage of the extracellular amino-terminus [7]. The resulting tethered ligand directly interacts with the receptor to initiate Gαq and β-arrestin-dependent signaling pathways [8]. Participation of A. alternata alkaline serine protease (AASP) and host protease-activated receptor-2 (PAR2) in the development of the asthma response have been elucidated using animal models [9–12]. Additionally, it has been suggested that alternative components from A. alternata can also contribute to allergen-induced response [13,14].
The airway epithelium that lines the conducting airways is uniquely positioned to encounter allergens such as A. alternata and thus, may provide a therapeutic target for asthma inhibition to elicit cellular responses that contribute to asthma [15–17]. It has been shown in vitro that airway epithelial exposure to A. alternata results in production of various cytokines, chemokines and inflammatory mediators that help form the innate immune response [14,18–22]. While these studies mostly suggest a role for PAR2 in the cellular response, they are limited by the numerous active signaling components in A. alternata [23,24] and the common use of cells such as BEAS-2B and A549 that lack key polarization features of the in vivo airway epithelium. The lack of commercially available, highly specific PAR2 inhibitors, and the inability to separate PAR2-dependent from PAR2-independent signaling in in vitro airway epithelium have been further obstacles in developing a clear understanding of PAR2-associated responses. Consequently, specific roles for airway epithelial-expressed PAR2 in allergic asthma initiation and pathophysiology are not fully understood.
Our goal is to better define the central role for human airway epithelial PAR2 in shaping the physiological and inflammatory response triggered by asthma-associated allergen exposure. In this report we address the issue of distinguishing PAR2-specific from non-specific A. alternata-induced airway epithelial signaling and select cytokine/chemokine/growth factor release by using CRISPR-Cas9 technology to create a human bronchial epithelial cell line devoid of PAR2 (16HBEPAR2−/−) along with parental, PAR2-expressing cells challenged with allergen in the presence or absence of an antagonist. Development of the 16HBEPAR2−/− cell line paired with antagonism studies allows for novel in vitro experiments in a human bronchial cell line that forms a functional, polarized epithelial layer.
MATERIALS AND METHODS
Materials
Alternaria alternata filtrate was purchased as a lyophilized powder from Stallergenes Greer Laboratories (Lenoir, NC). Filtrate was resuspended in Hanks Balanced Saline Solution additionally buffered with 25 mM HEPES (pH, 7.4; HBSS) and stored at 2.5 mg/mL protein at −20°C until use. Protease concentration was determined prior to use; activity of the filtrate used in these experiments was comparable to 0.5 μg/mL trypsin as determined with a commercial protease assay kit (Sigma Cat # PF-0100). Potent and specific PAR2 agonist [2-aminothiazol-4-yl-LIGRL-NH2 (2-at-LIGRL-NH2)] and antagonist C391 were produced in our laboratory as described [25–27]. Trypsin was purchased from Sigma (Cat#T6567) and human neutrophil elastase from Worthington Biochemical (Cat# LS003703; Lakewood, NJ). Unless listed below, all other chemicals/components were of Molecular Biology or higher grades and purchased from Fisher Scientific (Pittsburgh, PA), ThermoFisher Scientific (Watham, MA), Sigma-Aldrich (Burlington, MA), or VWR (West Chester, PA).
Cell culture
PAR2-expressing cells and the parental cells for CRISPR-Cas9 editing in this study are the 16HBE14o− cell line, SV40-transformed human bronchial epithelial cells [[28]; California Pacific Medical Center Research Institute (San Francisco, CA, USA)]. 16HBE14o− cells were passaged and grown in Minimal Essential Medium with Earle’s Salts supplemented with glutamax (Life Technologies), penicillin/streptomycin and 10% FBS (MEM) as described in [25,26]. Cells were then transferred to appropriate matrix-coated culture-ware for experiments.
Guide RNA (gRNA) design for Protease-activated receptor-2 gene (F2RL1) targeting
We identified and extracted the sequence of F2RL1 from the genomic sequence of human chromosome 5 (Ensembl, Transcript ID: ENST00000296677.4) and designed a gRNA to match the 5’ end of the protein sequence with the gRNA design tool (http://crispr.mit.edu) for off-target screening. The selected gRNA sequence for knockout was located in exon 1 (reverse strand GAGAGAGGCTGCTAGCAGGA; score 52; Figure 1).
Figure 1: Design of the CRISPR strategy to target the F2RL1 gene.
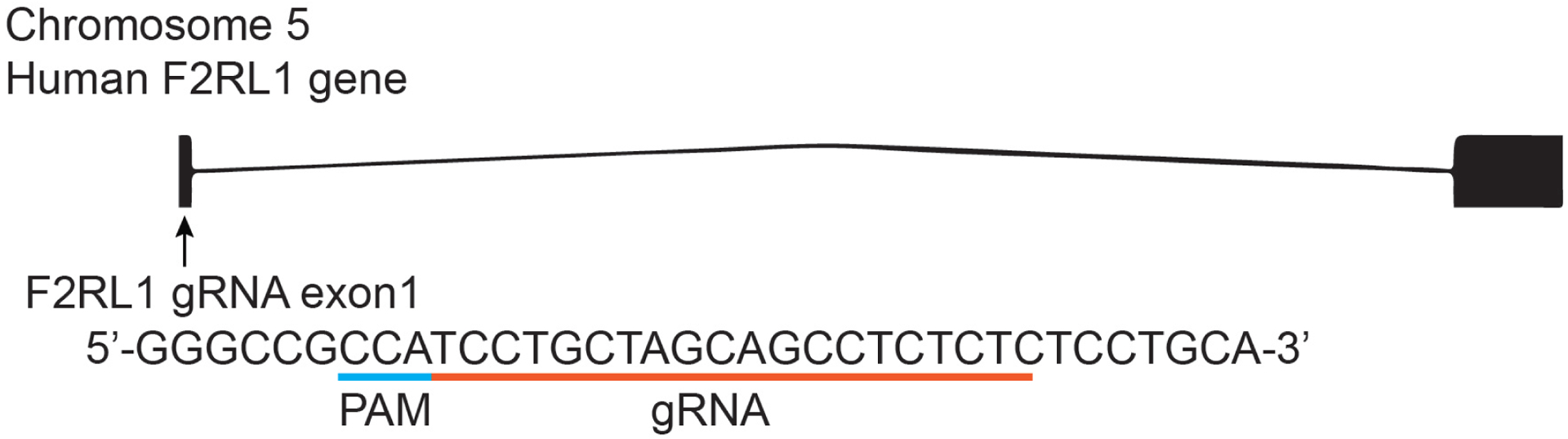
The designed gRNA from the reverse strand sequence targeted on exon 1 in F2RL1 is indicated by the green line oriented from 5’ to 3’. The protospacer adjacent motif (PAM) sequence is indicated by a blue line. The gRNA pairs with its DNA target, located 37 bp downstream of the translation initiation codon ATG, followed by a 5’NGG sequence (PAM). Cas9 catalyzes a double stranded cleavage on the genomic DNA 3 bp before the PAM sequence.
Cloning of gRNAs
We used the pl-CRISPR.EFS.PAC plasmid [(Addgene Cat#57828, Cambridge, MA) [29]] that allows for simultaneous expression of the Cas9 enzyme with the gRNA and Puromycin N-acetyltransferase (PAC) for selection. The plasmid was cut using BsmBI restriction enzyme (Thermo Fisher Scientific Cat# FD0454, Waltham, MA, USA) according to manufacturer instructions. The digested plasmid was extracted from a 1% agarose gel (Thermo Fisher Scientific, Cat#K0691). Oligonucleotides were designed according to [30] for the cloning of exon 1 targeting gRNA (F2RL1 forward: 5’- CACCGAGAGAGGCTGCTAGCAGGA-3’; F2RL1 reverse: 5’- AAACTCCTGCTAGCAGCCTCTCTC-3’; Eurofins Scientific, Brussels, Belgium). A 10 μM solution of forward and reverse oligonucleotides was annealed in a thermocycler using the following protocol: 37°C for 30 min, 95°C for 5 min with a cooling to 25°C at a rate of 5°C/min. After cooling, 100 ng of the digested pL-CRISPR.EFS.PAC plasmid was set up for ligation with 50 nM of the annealed oligonucleotides at 22°C for 5 min using Rapid DNA ligation Kit (Thermo Fisher Scientific Cat#K1422). The ligation products were transformed into E. coli DH5α competent bacteria (New England Biolabs Cat#C2987, Ipswich, MA) according to manufacturer instructions. The integrity of all plasmids was confirmed by Sanger sequencing (Eurofins). A 100% efficiency was observed for the insertion of gRNA sequences into the pL-CRISPR.EFS.PAC plasmid. Plasmids were then purified from E. coli DH5α using the NucleoBond® Xtra Maxi kit (Macherey, Cat# 740414, Nagel, Germany).
Transfection, positive selection and clonal selection
Cells (8 × 105) were seeded on matrix-coated 12-well plates. Confluency of ~80% was reached within 24 hrs. Cells were transfected with purified plasmid following manufacturer protocol (Thermo Fischer Scientific Cat # L3000001) and returned to the incubator for three days of growth in MEM supplemented with 4 μg/mL puromycin (Sigma-Aldrich Cat #P8833, St. Louis Missouri). At 10 days, and thereafter, cells were fed with MEM without puromycin. Once full confluency of positively transfected cells was reached, single cells/well were transferred onto a matrix-coated 96-well plate. One day later, wells with a surviving single cell were verified. Verified wells were fed and monitored until confluency was established before transfer onto T25 flasks.
In vitro impedance-based assay for functional testing and physiological response
High-capacity in vitro physiological response was accomplished with the xCELLigence Real Time Cell Analyzer multi-plate unit (RTCA-MP; ACEA Biosciences, San Diego, CA) and methods adapted from [31]. For reference, PAR2 activation results in an increase in impedance, represented as a Cell Index, whereas cell toxicity and/or death is associated with a decrease in Cell Index [31,32]. For our experiments, an initial background recording was taken of matrix-coated E-plates (ACEA, BioSciences) containing MEM alone. Media was replaced with 16HBE14o− or 16HBEPAR−/− cells in MEM with reduced FBS (5%) onto matrix-coated E-plates. Cells were allowed to grow overnight (37°C and 5% CO2) to establish full adhesion when ligand, protease or allergen was applied to the cells and physiological responses recorded every min for up to 4 hrs. FBS-free media was used for all protease experiments. Cell responses were collected in quadruplicate. To allow for the comparison of readings, cell responses were normalized at a time just prior to treatment and data is presented without SEM (e.g., supplemental Figure S2 in [31]).
Cytokine secretion and detection
Matrix-coated 24-well Transwell filters (Corning Costar Cat #3472) were seeded with MEM (100 μL) containing 1 × 105 airway epithelial cells with 600 μL full MEM added below the filter. Apical (100 μL) and basolateral media was replaced every other day until day 5 where apical media was aspirated, and cells were grown at air-liquid interface. Upon establishment of proper transepithelial resistance (> 200 Ω*cm2 for all filters) MEM was replaced with serum free-MEM overnight. Cells were then apically treated with control HBSS, 10μg/100 μL A. alternata or A. alternata supplemented with 1 μmole of the PAR2 antagonist C391 [25]. Basolateral medium (100 μL) was recovered and replaced with serum-free MEM immediately prior to apical application (time 0) and at 4 (data not shown) and 24 hrs. Samples were spun at 250× g for 5 min and stored at −20°C until evaluation. Cytokine concentrations in thawed cell culture supernatants were determined using the MAGPIX® (Luminex Corp Cat #MAGPIX-XPONENT, Austin, TX) and appropriate multiplex plates following manufacturer protocols (Millipore Sigma Cat# HTH17MAG-14K, Burlington, MA and Millipore Sigma Cat# HCYTOMAG-60K). Multiplex data were analyzed with MA Analyst v5.0 (Millipore/VigeneTech) using 5-parameter (5PL) logistic curve fitting.
Statistics
A Students t-test was performed on experiments with only two groups. One way ANOVA was performed with a Tukey post-hoc test for experiments with more than 2 groups. For experiments that were monitored over time, a Two-way ANOVA was performed. For all statistical tests p ≤ 0.05 was considered significant.
RESULTS
In vitro physiological responses to the potent, selective PAR2 agonist, 2-aminothiazol-4-yl-LIGRL-NH2 (2at-LIGRL-NH2) were monitored in 16HBE14o− and 16HBEPAR−/− cell lines (Figure 2). Responses to 2at-LIGRL-NH2 in 16HBE14o− cells were similar to published results [26,31], with a sharp increase in Cell Index that peaked after 30 min and remained elevated throughout the experiment and a desensitizing effect at the highest concentrations of 2at-LIGRL-NH2 tested (i.e., > 1 μM; Figure 2A). The EC50 [112 nM with 95% Confidence Interval (95CI) of 80 – 157 nM] and the relatively fast recovery time reflect a change in temperature to 37°C used here from the room temperature used previously [26]. In contrast, the 16HBEPAR−/− cells did not display a 2at-LIGRL-NH2-induced cellular response throughout the concentration range tested (Figure 2B). The lack of a physiological response to 2at-LIGRL-NH2 confirms the functional ablation of PAR2 signaling in this cell line.
Figure 2: The in vitro physiological responses of human bronchial epithelial cells following addition of select PAR2 activators.
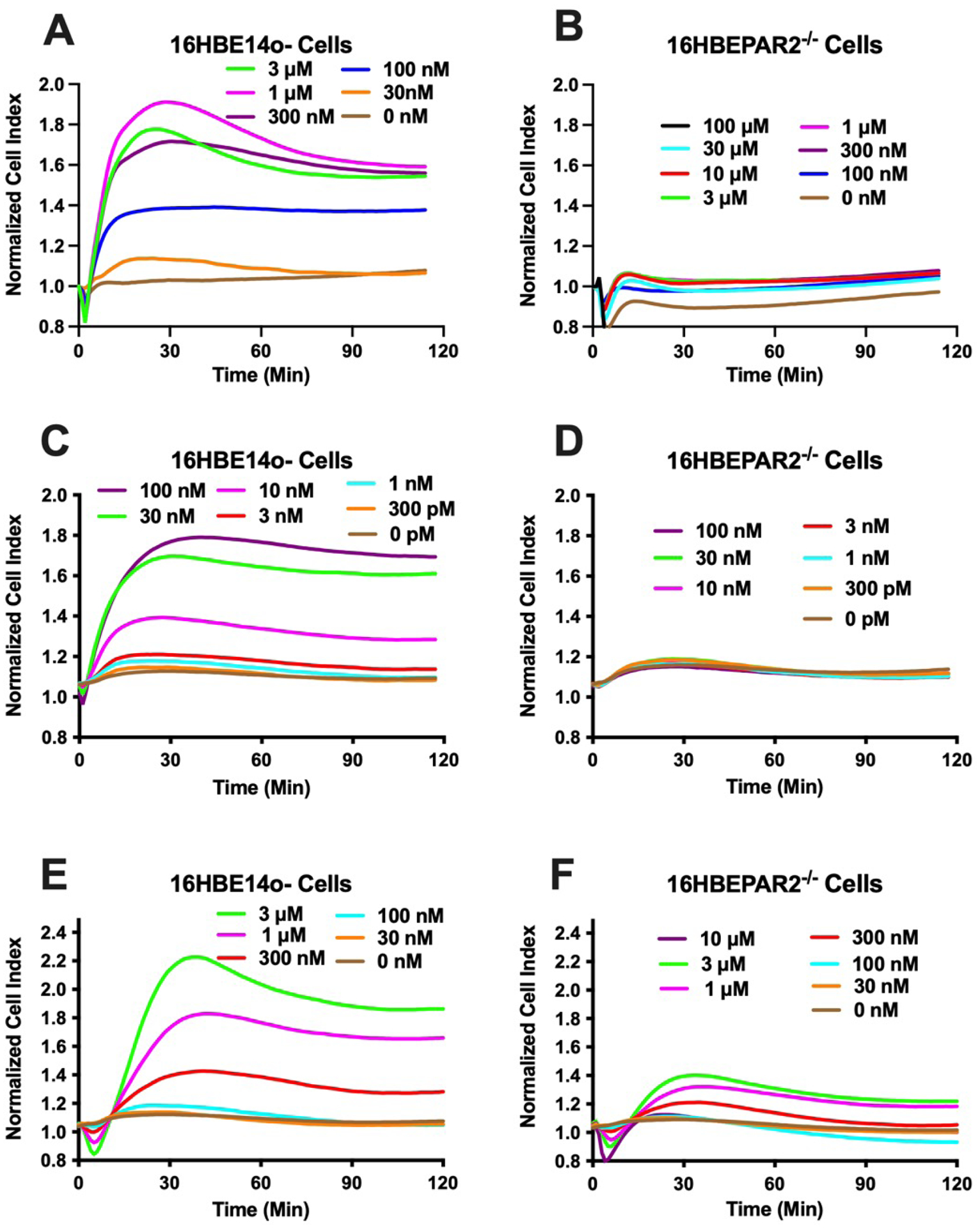
Each panel (A - F) represents the impedance responses (Cell Index) measured each minute following the addition of PAR2 agonist. Agonist concentrations were chosen to reflect full responses in 16HBE14o− cells and reduced in ½ log steps Traces represent the average of four experiments. Concentration-dependent response to 2at-LIGRL-NH2 for 16HBE14o− (A) or 16HBEPAR2−/− (B) cells. Concentration-dependent response to trypsin for 16HBE14o− (C) 16HBEPAR2−/− (D) cells. Concentration-dependent response to elastase for 16HBE14o− (E) 16HBEPAR2−/− (F) cells. The lack (2at-LIGRL-NH2; trypsin)- or severely reduced (elastase)-induced physiological responses in the 16HBEPAR2−/− cell line demonstrate a necessity for PAR2 expression for in vitro physiological response.
Because PAR2 can be activated by several trypsin-like serine proteases [8], and this type of activation is what occurs in vivo, we repeated the sensitive in vitro physiological assays with trypsin or elastase to evaluate concentrations that could provide PAR2-specific responses when apically exposed to airway epithelial cells (Figure 2C, 2E). Both trypsin (EC50 = 14 nM; 95CI = 11 – 17 nM) and elastase (EC50 = 300 nM, 95CI: 240 – 370 nM) displayed concentration-dependent activation of 16HBE14o− cells. The responses to trypsin and to elastase were largely absent in 16HBEPAR2−/− cells, although the elastase did induce a small physiological response (Figure 2D, 2F). These experiments demonstrate that low concentrations of trypsin and elastase primarily act at PAR2 to initiate signaling and subsequent in vitro physiological responses in airway epithelial cells.
While we have shown that Alternaria alkaline serine protease (AASP) is an important driver of asthma-like symptoms in animal models [11,12]. A. alternaria filtrates contain a variety of defined and undefined components [23,24] that may also contribute to cellular signaling (e.g., [14,33]). The comparison of A. alternata filtrate-induced signaling in 16HBE14o− and 16HBEPAR2−/− cells with the extremely sensitive RTCA-MP in vitro physiological output allowed us to more directly investigate PAR2-dependent and PAR2-independent signaling in human bronchial epithelial cells. Addition of A. alternata filtrate to 16HBE14o− cells resulted in an initial decrease in Cell Index followed by an increase that was similar to the PAR2 agonist and protease responses shown above, albeit on a reduced scale (Figure 3A). The full agonist response for A. alternata occurred at the lowest concentration tested, 30 ng/mL. Higher A. alternata concentrations elicited a secondary loss of Cell Index response indicative of PAR2 desensitization, interference with PAR2 signaling and/or activation of alternative signaling pathways. The physiological response induced by A. alternata filtrate in 16HBEPAR−/− cells resulted in a much different pattern. The initial dip in Cell Index was significantly reduced and the distinct peak in Cell Index induced by A. alternata in 16HBE14o− cells was largely absent across all concentrations measured (Figure 3B). Increases in Cell Index elicited by A. alternata were still evident, showing that the filtrate contains a PAR2-independent signaling component to establish the physiological response.
Figure 3: PAR2-dependent in vitro physiological response to A. alternata.
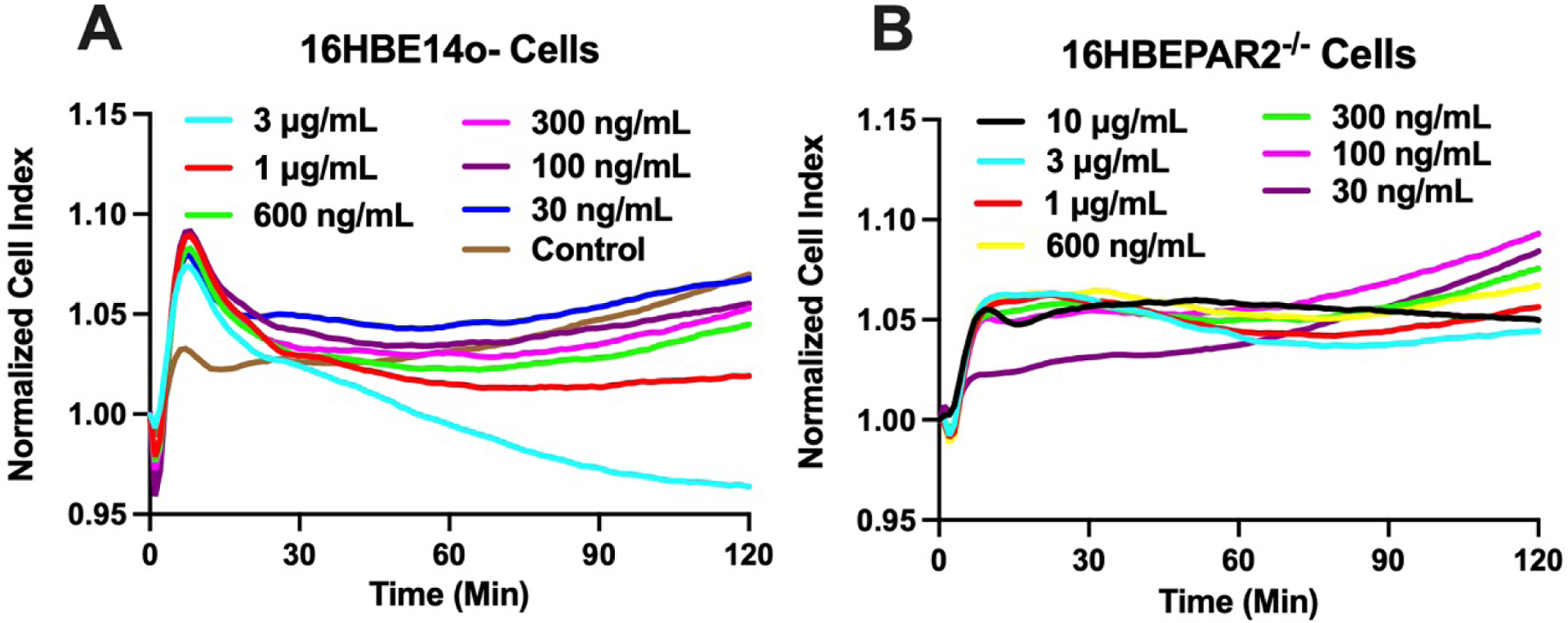
16HBE14o− (A) or 16HBEPAR−/− cells (B) were treated with 30 ng/mL – 10 μg/mL A. alternata filtrate and Cell Index was measured every minute for 2 hrs. A. alternata induced a distinctly different response in the PAR2-expressing parental cell line (16HBE14o−). Notably, the initial dip in signaling followed by a rapid peak and recovery was missing in the PAR2-ablated cell line (16HBEPAR2−/−). Traces are mean, normalized values from 6 experiments.
An advantage of the 16HBE14o− cell line over more commonly used human airway epithelial cell lines (e.g., A549 or BEAS-2B) is the ability to grow at air-liquid interface in an epithelial layer that separates apical from basolateral membrane and thus, better represents the in vivo condition [34–36]. To better understand how PAR2 signaling contributes to A. alternata-induced inflammation, we compared A. alternata-induced basolateral secretions in 16HBE14o− and 16HBEPAR−/− cells in combination with a potent PAR2 antagonist, C391 [12,25]. A. alternata filtrate induced significant IL-6 and IL-8 secretion in 16HBE14o− cells at 24 hr and this secretion was significantly reduced by PAR2 antagonism with C391 (Figure 3A – B). In the absence of PAR2, secreted IL-6 and IL-8 was significantly lower after the serum starvation step and did not significantly increase with 24 hr exposure to A. alternata. Apical A. alternata exposure resulted in a similar increase in RANTES and IP-10 secretion in 16HBE14o− cells, with significant increases at 24 hrs that were significantly reduced by C391 (Figure 3C – D). However, the 16HBE14o− cells also showed significant increase in these cytokines in the control HBSS application, suggesting an additional constitutive secretion pathway. This was apparent in the 16HBEPAR2−/− cells where secretion of both RANTES and IP-10 was increased at the 24 hr time point, albeit with a much lower final concentration than that observed in the 16HBE14o− cells. TNF-α exhibited a similar PAR2-dependent and independent secretion with the additional reduction PAR2-dependent secretion in response to overnight serum removal (Figure 4E). Growth factor (VEGF and PDGF) secretion was also affected by PAR2 (Figure 4F – G). In the PAR2-expressing 16HBE14o− cells, both VEGF and PDGF showed increased secretion at 24 hrs that was fully inhibited by C391. While A. alternata did not alter VEGF secretion, PDGF was increased at the 24 hr time point in the 16HBEPAR2−/− cells. These results suggest that PAR2 signaling in the airway epithelium is required for full cytokine/chemokine/growth factor release following A. alternata exposure and that it plays an essential role coordinating an acute inflammatory response to allergen exposure.
Figure 4: PAR2-mediated A. alternata-induced secretion.
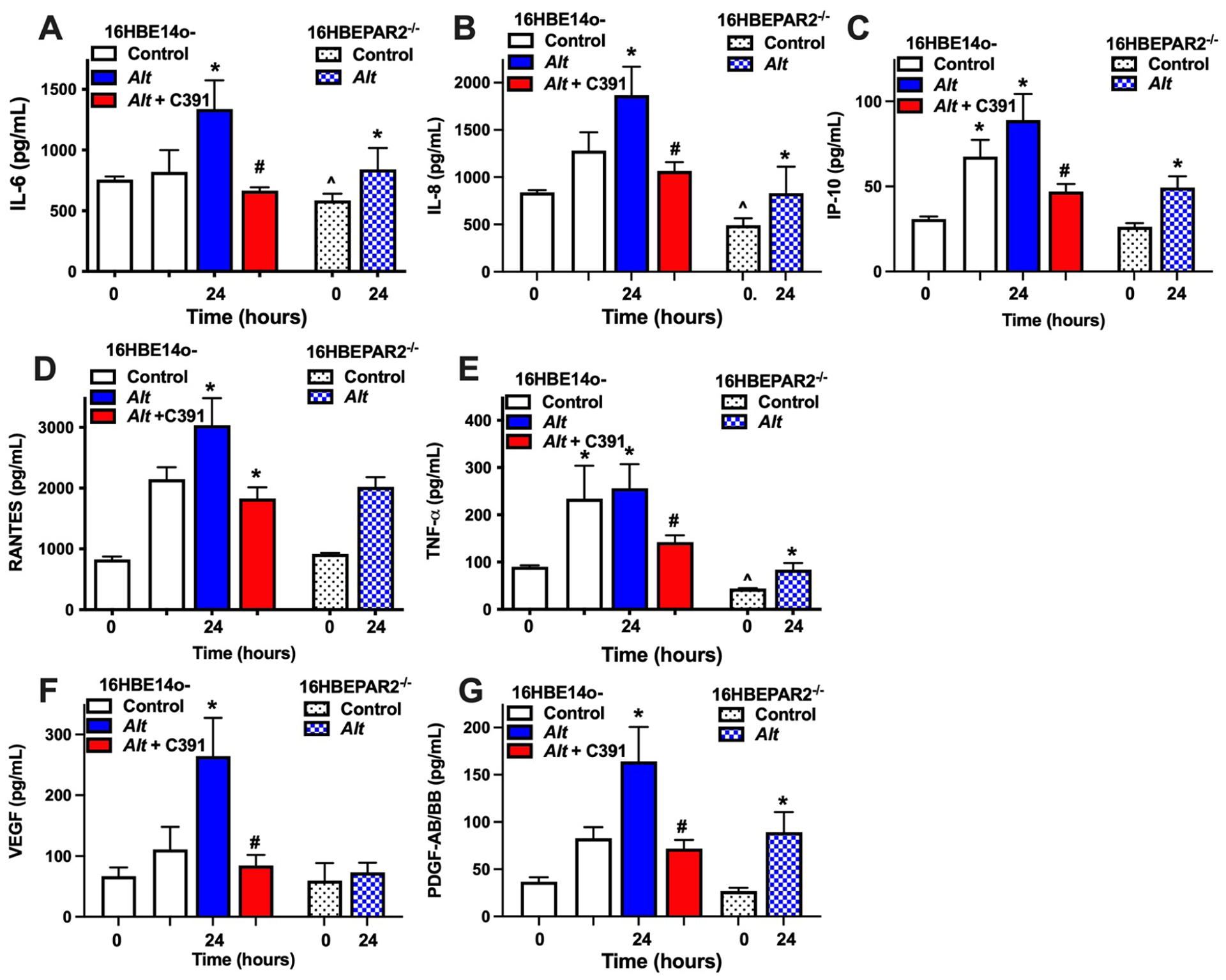
(A - G) Polarized human bronchial epithelial cells expressing PAR2 (16HBE14o−; solid columns) or lacking PAR2 (16HBEPAR2−/−; shaded columns) were grown at air-liquid interface, serum-starved overnight (time 0) and then apically challenged with HBSS (control, white columns), A. alternata (blue columns), or A. alternata with the PAR2 antagonist C391 (red columns). Basolateral cytokine concentration was measured just before apical treatment (t = 0) and at 24 hrs post-addition. PAR2 is necessary for full response to A. alternata (see text for details). Statistical differences (p < 0.05; one-way ANOVA with a Tukey post-test) are as follows: * indicates difference from corresponding time 0; # indicates difference with C391 treatment to matching time point; ^ indicates difference between 16HBE14o− and 16HBEPAR2−/− at time 0. Experiments with 16HBE14o− cells n = 4 ≤ n ≤ 11; experiments with 16HBEPAR2−/− n = 3.
DISCUSSION
In this study, we present a newly developed PAR2-deficient human bronchial epithelial cell line (16HBEPAR−/−) as a novel tool to better define the role of PAR2 in the initial physiological responses to acute A. alternata exposure. Our studies are in line with several others that demonstrate A. alternata-induces cytokine/chemokine response in human airway epithelial cells [18,20–22,33,37,38]. 16HBE14o− cells have been used to highlight the need for polarized epithelium to study A. alternata in vitro (e.g., [22]), the accompanying polarized 16HBEPAR2−/− can provide further distinct advantage in the study of physiologic properties that require epithelial layers. The noted findings that serum starvation resulted in altered baseline secretions between 16HBE14o− and 16HBEPAR2−/− cells also point to an ill-defined role for airway epithelial associated proteases in PAR2 signaling (e.g., [39]). Human airway epithelial PAR2 signaling has also been associated with ion channel function [40–42] and cell migration [43,44]; 16HBEPAR2−/− cells provide a novel tool to better define the role for PAR2 in these physiologic endpoints in concert with, or distinct from, A. alternata exposure.
Several allergens have been shown to express PAR2-cleaving proteases and induce asthma-associated inflammatory responses in animal and cellular models. These include house dust mite [45,46]; German cockroach frass [47–50] in addition to A. alternata [11,12,51]. Like A. alternata, these asthma-associated allergens contain a variety of microbe-associated molecular factors (e.g., glycoproteins, lipopolysaccharides, peptidoglycans and double-stranded DNA) that can initiate cellular signaling in the airway via host pattern recognition receptors (PRRs). In the case of A. alternata, it has been suggested by some researchers that the inflammatory response is independent of PAR2 [13,14]. Significantly we and others have noted the variation of protease in both homemade and commercially available A. alternaria extracts; similar to in vitro studies where PAR2-independent responses were noted (e.g., [14,33]), differences in protease activity can partially explain these results. As noted (e.g., [51]) and demonstrated with the 16HBEPAR2−/− cells in this report, even with protease-expressing A. alternata, there is distinct signaling and cytokine secretion that is independent of PAR2 expression. Nevertheless, our data shows a clear action on human PAR2 by A. alternata using multiple assays.
We conclude that PAR2 in the airway epithelium is an important receptor to initiate physiologic changes invoked by A. alternata, and thus is a viable target for PAR2 drug development in the reduction of inflammation associated with allergic asthma. The 16HBEPAR2−/− cells developed here will be a useful tool for characterizing how other allergens may act via human PAR2 to promote airway responses that are involved in asthma pathology.
Highlights:
Alternaria alternata actions on human airway epithelial cells depend on protease-activated receptor-2 (PAR2) signaling
Alternaria alternata cytokine, chemokine and growth factor is shaped by PAR2 activation
PAR2-deficient human airway epithelial cells create a model for studying PAR2 in airway disease
ACKNOWLEDGEMENTS
We would like to express our gratitude to the Heddwen Brooks laboratory, specifically Megan Sylvester and Joshua Uhlorn for their assistance in developing the splenocyte harvesting protocol.
GRANTS
This work was funded in part through grants from the National Institute of Health [grant numbers NS098826 (SB, TJ, GD, JV); AI140257 (SB); HL160424 (KAD, SB)]. CMR was an American Physiological Society William H Townsend Porter Pre-doctoral Fellow. HVS is an Undergraduate Biology Research Program Student.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Drs. Kathryn A DeFea, Theodore J. Price, Gregory Dussor, Josef Vagner and Scott Boitano are all involved in PARMedics, a company that specializes in the development of protease-activated receptor-2 ligands. None of the work in this manuscript originated from the company nor is protected by the company. On behalf of my colleagues, I am stating that there is no conflict of interest in the creation and eventual publication of this manuscript.
REFERENCES
- [1].Halonen M, Stern DA, Wright AL, Taussig LM, Martinez FD, Alternaria as a major allergen for asthma in children raised in a desert environment, Am J Respir Crit Care Med 155 (1997) 1356–1361. [DOI] [PubMed] [Google Scholar]
- [2].Salo PM, Arbes SJ Jr., Sever M, Jaramillo R, Cohn RD, London SJ, Zeldin DC, Exposure to Alternaria alternata in US homes is associated with asthma symptoms, J Allergy Clin Immunol 118 (2006) 892–898. 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pulimood TB, Corden JM, Bryden C, Sharples L, Nasser SM, Epidemic asthma and the role of the fungal mold Alternaria alternata, J Allergy Clin Immunol 120 (2007) 610–617. 10.1016/j.jaci.2007.04.045. [DOI] [PubMed] [Google Scholar]
- [4].Liu J, Li J, Yin J, Clinical relevance of Alternaria alternata sensitization in patients within type 2-high and type 2-low asthma, Int Immunopharmacol 101 (2021) 108333. 10.1016/j.intimp.2021.108333. [DOI] [PubMed] [Google Scholar]
- [5].Martinez FD, Vercelli D, Asthma, Lancet 382 (2013) 1360–1372. 10.1016/S0140-6736(13)61536-6. [DOI] [PubMed] [Google Scholar]
- [6].Hammad H, Lambrecht BN, The basic immunology of asthma, Cell 184 (2021) 1469–1485. 10.1016/j.cell.2021.02.016. [DOI] [PubMed] [Google Scholar]
- [7].Ossovskaya VS, Bunnett NW, Protease-activated receptors: contribution to physiology and disease, Physiol Rev 84 (2004) 579–621. 10.1152/physrev.00028.2003 84/2/579 [pii]. [DOI] [PubMed] [Google Scholar]
- [8].Ramachandran R, Noorbakhsh F, Defea K, Hollenberg MD, Targeting proteinase-activated receptors: therapeutic potential and challenges, Nat Rev Drug Discov 11 (2012) 69–86. 10.1038/nrd3615. [DOI] [PubMed] [Google Scholar]
- [9].Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, Lloyd CM, Alternaria-derived serine protease activity drives IL-33 mediated asthma exacerbations, J Allergy Clin Immunol (2014) 583–592. 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boitano S, Flynn AN, Sherwood CL, Schulz SM, Hoffman J, Gruzinova I, Daines MO, Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2, Am J Physiol Lung Cell Mol Physiol 300 (2011) L605–614. ajplung.00359.2010 [pii] 10.1152/ajplung.00359.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yee MC, Nichols HL, Polley D, Saifeddine M, Pal K, Lee K, Wilson EH, Daines MO, Hollenberg MD, Boitano S, DeFea KA, Protease-activated Receptor-2 Signaling through beta-Arrestin-2 Mediates Alternaria Alkaline Serine Protease-induced Airway Inflammation, Am J Physiol Lung Cell Mol Physiol 315 (2018) L1042–L1057. 10.1152/ajplung.00196.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rivas CM, Yee MC, Addison KJ, Lovett M, Pal K, Ledford JG, Dussor G, Price TJ, Vagner J, DeFea KA, Boitano S, Novel proteinase-activated receptor-2 (PAR2) antagonist C391 inhibits Alternaria-induced human airway epithelial signaling in vitro and asthma indicators in acute exposure murine models, Br J Pharmacol (2021). 10.1111/bph.15745. [DOI] [PubMed] [Google Scholar]
- [13].Denis O, Vincent M, Havaux X, De Prins S, Treutens G, Huygen K, Induction of the specific allergic immune response is independent of proteases from the fungus Alternaria alternata, Eur J Immunol 43 (2013) 907–917. 10.1002/eji.201242630. [DOI] [PubMed] [Google Scholar]
- [14].Daines M, Zhu L, Pereira R, Zhou X, Bondy C, Pryor BM, Zhou J, Chen Y, Alternaria induces airway epithelial cytokine expression independent of protease-activated receptor, Respirology 25 (2020) 502–510. 10.1111/resp.13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hammad H, Lambrecht BN, Barrier Epithelial Cells and the Control of Type 2 Immunity, Immunity 43 (2015) 29–40. 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- [16].Gras D, Chanez P, Vachier I, Petit A, Bourdin A, Bronchial epithelium as a target for innovative treatments in asthma, Pharmacol Ther 140 (2013) 290–305. 10.1016/j.pharmthera.2013.07.008. [DOI] [PubMed] [Google Scholar]
- [17].Wiesner DL, Klein BS, Lung epithelium: barrier immunity to inhaled fungi and driver of fungal-associated allergic asthma, Curr Opin Microbiol 40 (2017) 8–13. 10.1016/j.mib.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kauffman HF, Tomee JF, van de Riet MA, Timmerman AJ, Borger P, Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production, J Allergy Clin Immunol 105 (2000) 1185–1193. S0091674900380599 [pii]. [DOI] [PubMed] [Google Scholar]
- [19].Shin SH, Lee YH, Jeon CH, Protease-dependent activation of nasal polyp epithelial cells by airborne fungi leads to migration of eosinophils and neutrophils, Acta Otolaryngol 126 (2006) 1286–1294. [DOI] [PubMed] [Google Scholar]
- [20].Kouzaki H, O’Grady SM, Lawrence CB, Kita H, Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2, J Immunol 183 (2009) 1427–1434. jimmunol.0900904 [pii] 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Matsuwaki Y, Wada K, White T, Moriyama H, Kita H, Alternaria fungus induces the production of GM-CSF, interleukin-6 and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2, Int Arch Allergy Immunol 158 Suppl 1 (2012) 19–29. 10.1159/000337756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Leino MS, Loxham M, Blume C, Swindle EJ, Jayasekera NP, Dennison PW, Shamji BW, Edwards MJ, Holgate ST, Howarth PH, Davies DE, Barrier disrupting effects of alternaria alternata extract on bronchial epithelium from asthmatic donors, PLoS One 8 (2013) e71278. 10.1371/journal.pone.0071278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kustrzeba-Wojcicka I, Siwak E, Terlecki G, Wolanczyk-Medrala A, Medrala W, Alternaria alternata and its allergens: a comprehensive review, Clin Rev Allergy Immunol 47 (2014) 354–365. 10.1007/s12016-014-8447-6. [DOI] [PubMed] [Google Scholar]
- [24].Gabriel MF, Postigo I, Tomaz CT, Martinez J, Alternaria alternata allergens: Markers of exposure, phylogeny and risk of fungi-induced respiratory allergy, Environ Int 89–90 (2016) 71–80. 10.1016/j.envint.2016.01.003. [DOI] [PubMed] [Google Scholar]
- [25].Boitano S, Hoffman J, Flynn AN, Asiedu MN, Tillu DV, Zhang Z, Sherwood CL, Rivas CM, DeFea K, Vagner J, Price TJ, The novel PAR2 ligand C391 blocks multiple PAR2 signaling pathways in vitro and in vivo, Br J Pharmacol 172 (2015) 4535–4545. 10.1111/bph.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Flynn AN, Tillu DV, Asiedu MN, Hoffman J, Vagner J, Price TJ, Boitano S, The protease-activated receptor-2-specific agonists 2-aminothiazol-4-yl-LIGRL-NH2 and 6-aminonicotinyl-LIGRL-NH2 stimulate multiple signaling pathways to induce physiological responses in vitro and in vivo, J Biol Chem 286 (2011) 19076–19088. 10.1074/jbc.M110.185264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Boitano S, Flynn AN, Schulz SM, Hoffman J, Price TJ, Vagner J, Potent Agonists of the Protease Activated Receptor 2 (PAR2), J Med Chem 54 (2011) 1308–1313. 10.1021/jm1013049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gruenert DC, Finkbeiner WE, Widdicombe JH, Culture and transformation of human airway epithelial cells, Am J Physiol 268 (1995) L347–360. [DOI] [PubMed] [Google Scholar]
- [29].Heckl D, Kowalczyk MS, Yudovich D, Belizaire R, Puram RV, McConkey ME, Thielke A, Aster JC, Regev A, Ebert BL, Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing, Nat Biotechnol 32 (2014) 941–946. 10.1038/nbt.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F, Genome engineering using the CRISPR-Cas9 system, Nat Protoc 8 (2013) 2281–2308. 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Flynn AN, Hoffman J, Tillu DV, Sherwood CL, Zhang Z, Patek R, Asiedu MN, Vagner J, Price TJ, Boitano S, Development of highly potent protease-activated receptor 2 agonists via synthetic lipid tethering, Faseb J 27 (2013) 1498–1510. 10.1096/fj.12-217323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sherwood CL, Boitano S, Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine, Respir Res 17 (2016) 57. 10.1186/s12931-016-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].O’Grady SM, Patil N, Melkamu T, Maniak PJ, Lancto C, Kita H, ATP release and Ca2+ signalling by human bronchial epithelial cells following Alternaria aeroallergen exposure, J Physiol 591 (2013) 4595–4609. 10.1113/jphysiol.2013.254649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wan H, Winton HL, Soeller C, Gruenert DC, Thompson PJ, Cannell MB, Stewart GA, Garrod DR, Robinson C, Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen Der p 1, Clin Exp Allergy 30 (2000) 685–698. cea820 [pii]. [DOI] [PubMed] [Google Scholar]
- [35].Ehrhardt C, Kneuer C, Fiegel J, Hanes J, Schaefer UF, Kim KJ, Lehr CM, Influence of apical fluid volume on the development of functional intercellular junctions in the human epithelial cell line 16HBE14o−: implications for the use of this cell line as an in vitro model for bronchial drug absorption studies, Cell Tissue Res 308 (2002) 391–400. [DOI] [PubMed] [Google Scholar]
- [36].Sweerus K, Lachowicz-Scroggins M, Gordon E, LaFemina M, Huang X, Parikh M, Kanegai C, Fahy JV, Frank JA, Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma, J Allergy Clin Immunol 139 (2017) 72–81 e71. 10.1016/j.jaci.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Asokananthan N, Graham PT, Fink J, Knight DA, Bakker AJ, McWilliam AS, Thompson PJ, Stewart GA, Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells, J Immunol 168 (2002) 3577–3585. [DOI] [PubMed] [Google Scholar]
- [38].Ramu S, Menzel M, Bjermer L, Andersson C, Akbarshahi H, Uller L, Allergens produce serine proteases-dependent distinct release of metabolite DAMPs in human bronchial epithelial cells, Clin Exp Allergy 48 (2018) 156–166. 10.1111/cea.13071. [DOI] [PubMed] [Google Scholar]
- [39].Pawar NR, Buzza MS, Antalis TM, Membrane-Anchored Serine Proteases and Protease-Activated Receptor-2-Mediated Signaling: Co-Conspirators in Cancer Progression, Cancer Res 79 (2019) 301–310. 10.1158/0008-5472.CAN-18-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kunzelmann K, Schreiber R, Konig J, Mall M, Ion transport induced by proteinase-activated receptors (PAR2) in colon and airways, Cell Biochem Biophys 36 (2002) 209–214. 10.1385/CBB:36:2-3:209. [DOI] [PubMed] [Google Scholar]
- [41].Kunzelmann K, Sun J, Markovich D, Konig J, Murle B, Mall M, Schreiber R, Control of ion transport in mammalian airways by protease activated receptors type 2 (PAR-2), Faseb J 19 (2005) 969–970. 10.1096/fj.04-2469fje. [DOI] [PubMed] [Google Scholar]
- [42].Cho HJ, Choi JY, Yang YM, Hong JH, Kim CH, Gee HY, Lee HJ, Shin DM, Yoon JH, House dust mite extract activates apical Cl(−) channels through protease-activated receptor 2 in human airway epithelia, J Cell Biochem 109 (2010) 1254–1263. 10.1002/jcb.22511. [DOI] [PubMed] [Google Scholar]
- [43].Ewen D, Clarke SL, Smith JR, Berger C, Salmon G, Trevethick M, Shute JK, The role of protease-activated receptors PAR-1 and PAR-2 in the repair of 16HBE 14o(−) epithelial cell monolayers in vitro, Clin Exp Allergy 40 (2010) 435–449. CEA3453 [pii] 10.1111/j.1365-2222.2010.03453.x. [DOI] [PubMed] [Google Scholar]
- [44].Tsai CC, Chou YT, Fu HW, Protease-activated receptor 2 induces migration and promotes Slug-mediated epithelial-mesenchymal transition in lung adenocarcinoma cells, Biochim Biophys Acta Mol Cell Res 1866 (2019) 486–503. 10.1016/j.bbamcr.2018.10.011. [DOI] [PubMed] [Google Scholar]
- [45].de Boer JD, Van’t Veer C, Stroo I, van der Meer AJ, de Vos AF, van der Zee JS, Roelofs JJ, van der Poll T, Protease-activated receptor-2 deficient mice have reduced house dust mite-evoked allergic lung inflammation, Innate Immun 20 (2014) 618–625. 10.1177/1753425913503387. [DOI] [PubMed] [Google Scholar]
- [46].Davidson CE, Asaduzzaman M, Arizmendi NG, Polley D, Wu Y, Gordon JR, Hollenberg MD, Cameron L, Vliagoftis H, Proteinase-activated receptor-2 activation participates in allergic sensitization to house dust mite allergens in a murine model, Clin Exp Allergy 43 (2013) 1274–1285. 10.1111/cea.12185. [DOI] [PubMed] [Google Scholar]
- [47].Page K, Ledford JR, Zhou P, Dienger K, Wills-Karp M, Mucosal sensitization to German cockroach involves protease-activated receptor-2, Respir Res 11 (2010) 62. 1465-9921-11-62 [pii] 10.1186/1465-9921-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Page K, Role of cockroach proteases in allergic disease, Curr Allergy Asthma Rep 12 (2012) 448–455. 10.1007/s11882-012-0276-1. [DOI] [PubMed] [Google Scholar]
- [49].Day SB, Ledford JR, Zhou P, Lewkowich IP, Page K, German cockroach proteases and protease-activated receptor-2 regulate chemokine production and dendritic cell recruitment, J Innate Immun 4 (2012) 100–110. 10.1159/000329132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nadeem A, Alharbi NO, Vliagoftis H, Tyagi M, Ahmad SF, Sayed-Ahmed MM, Proteinase activated receptor-2-mediated dual oxidase-2 up-regulation is involved in enhanced airway reactivity and inflammation in a mouse model of allergic asthma, Immunology 145 (2015) 391–403. 10.1111/imm.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Velez-Del-Burgo P Sanchez E. Sunen, Martinez J, Postigo I, Purified Native and Recombinant Major Alternaria alternata Allergen (Alt a 1) Induces Allergic Asthma in the Murine Model, J Fungi (Basel) 7 (2021). 10.3390/jof7110896. [DOI] [PMC free article] [PubMed] [Google Scholar]


