SUMMARY
Although overwhelming plasma membrane integrity loss leads to cell lysis and necrosis, cells can tolerate a limited level of plasma membrane damage, undergo ESCRT-III-mediated repair, and survive. Here, we find that cells which undergo limited plasma membrane damage from the pore-forming actions of MLKL, GSDMD, perforin, or detergents experience local activation of PKCs through Ca2+ influx at the damage sites. S660-phosphorylated PKCs subsequently activate the TAK1/IKKs axis and RelA/Cux1 complex to trigger chemokine expressions. We observe that in late-stage cancers, cells with active MLKL show expression of CXCL8. Similar expression induction is also found in ischemia-injured kidneys. Chemokines generated in this manner are also indispensable for recruiting immune cells to the dead and dying cells. This plasma membrane integrity-sensing pathway is similar to the well-established yeast cell wall integrity signaling pathway at molecular level, and this suggests an evolutionary conserved mechanism to respond to the cellular barrier damage.
In brief
Cells can tolerate sub-lethal plasma membrane pore-forming damage by activating repair mechanisms. Wang et al. show that S660 p-PKCs can sense pore-forming damage by detecting local Ca2+ influx, resulting in chemokine production. This pathway works in parallel with the yeast cell wall integrity pathway and occurs in multiple pathological conditions.
Graphical Abstract

INTRODUCTION
Plasma membrane (PM) integrity is key for cell survival and normal cellular function. Overwhelmingly loss of PM integrity leads to cell necrosis and lysis, whereas modest levels of membrane damage are tolerable as they can be repaired by ESCRT-III and other cellular machineries (Andrews and Corrotte, 2018; Jimenez et al., 2014).
ESCRT-III can repair various forms of PM damage, triggered by laser light, detergent (e.g., digitonin), pore-forming toxins (e.g., Streptolysin O), or perforin (PFN) released from cytotoxic lymphocytes (Jimenez et al., 2014). It can also repair the PM damage induced by the pore-forming molecules that function in regulated necrosis (Gong et al., 2017b; Rühl et al., 2018). For example, upon phosphorylation by RIPK3, phosphorylated-mixed lineage kinase domain-like protein (pMLKL) can directly target and disrupt the PM, resulting in necroptosis (Sun et al., 2012; Wang et al., 2014). Similarly, the protease-activated gasdermin family proteins function as the pore-forming executors for pyroptosis (Kayagaki et al., 2015; Shi et al., 2015). ESCRT-III can repair both pMLKL- and gasdermin (N terminus)-mediated PM pores by direct assembly and action at the damaged PM sites. Hence, neither necroptotic nor pyroptotic cells are committed to die when engaging the PM pore-forming damage; on the contrary, they can survive if the repair capacity is not overwhelmed (Gong et al., 2017b; Lieberman et al., 2019; Rühl et al., 2018).
In this work, we show that, if some necrotic cells could manage to stay alive, in these “survivors,” the sub-lethal PM pore-forming was sufficient to initiate a sophisticated signal transduction network, which led to the production of chemokines and cytokines. Using their C2 domain, the conventional PKCs sense and translocate to sites of PM damage by detecting the Ca2+ influx associated with such pores. After becoming locally active, PKCs triggered the TAK1/IKKs axis and the RelA/Cux1 complex for chemokine expression, which were crucial for macrophage migration toward the dead and dying cells and inflammation during peritonitis. We also provide in vivo evidence indicating that similar alterations in expression occur in late-stage cancers and kidneys post-ischemia-reperfusion injury (IRI).
RESULTS
The general pattern of intracellular responses to sub-lethal PM pore formation
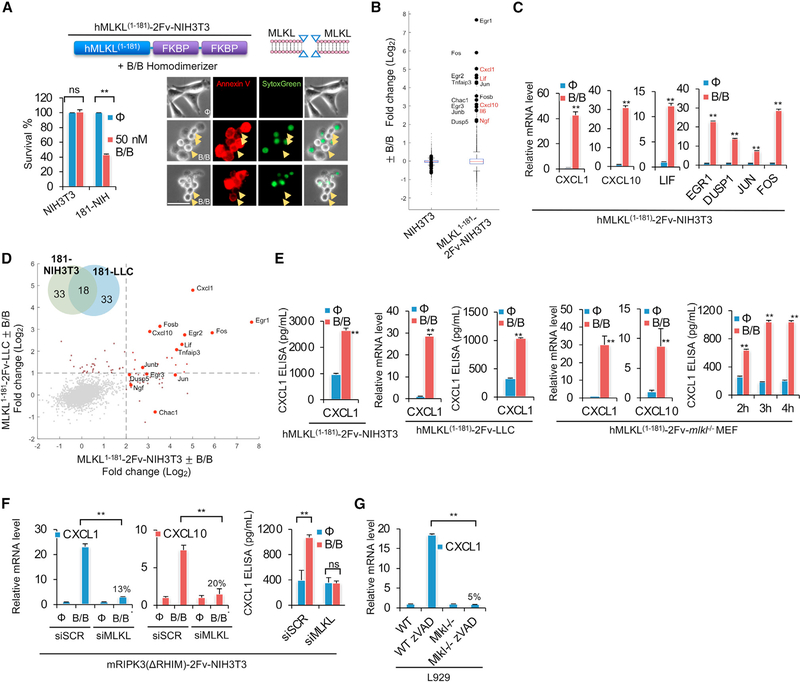
To induce sub-lethal PM damage, we fused human MLKL N terminus (1–181 aa) with a tandem FK506-binding domain of FKBP (Fv) and stably transduced it into NIH3T3 cells (Figure 1A). The oligomerization of hMLKL1–181-2xFv by the homodimerizer AP20187 (hereafter referred to as “B/B,” which binds to Fv domains) could initiate PM damage via MLKL-mediated pore formation. The concentration of B/B can control the intensity of MLKL-mediated PM damage. 50–100 nM B/B led to sub-lethal membrane damage to some cells, which rounded up and labeled with annexin V+ (Figure 1A) but could stay intact for hours without complete loss of membrane integrity (PI− or SytoxGreen−, (Gong et al., 2017b). Note that after MLKL activation, PS externalization and annexin V labeling occurs prior to cell lysis; thus, annexin V is not an apoptosis specific marker, but it also marks necroptotic cells (Gong et al., 2017b). Some of the annexin V+ SytoxGreen− cells could even survive and reattach to the culture dishes 7 h after B/B treatment and form clones in a long-term clonogenic survival assay (Figure S1A), further suggesting that some cells that transiently experienced the MLKL-mediated PM pore formation finally survived. hMLKL1–181-2xFv-mediated PM damage was independent the MLKL kinase RIPK3, as (1) the NIH3T3 cells we employed did not express RIPK3 (Figure S1B); (2) they could not undergo necroptosis induced by TNF+zVAD (±Lcl161) unless murine RIPK3 was reconstituted (Figure S1C); (3) the MLKL 1–181aa truncation lacked the RIPK3-binding domain and phosphorylation sites and (4) human MLKL cannot be engaged by murine RIPK3 (Sun et al., 2012).
Figure 1. The transcriptional inductions upon MLKL-mediated sub-lethal PM damage.
(A) Representative images of 50-nM-B/B-treated (1 h) hMLKL1–181-2Fv-NIH3T3 (181-NIH) cells, stained by SytoxGreen and annexin V. Arrows indicated cells that were rounded up and annexin V+ but still resistant to SytoxGreen staining. Scale bar, 50 μm. Cell survival was measured by CellTiter-Glo.
(B) RNA-seq analysis of NIH3T3 and hMLKL1–181-2Fv-NIH3T3 (±100 nM B/B, for 1 h). The y axis is log2 fold change (FC). Each red label shows a secreted protein (cytokines or chemokines).
(C) qRT-PCR qualification of gene production in hMLKL1–181-2Fv-NIH3T3 cells ±100 nM B/B, for 1 h (qRT-PCR).
(D) RNA-seq analysis of hMLKL1–181-2Fv-NIH3T3 and hMLKL1–181-2Fv-LLC (±100 nM B/B, for 1 h). Venn diagram shows the overlapped transcription in 181-NIH3T3 and 181-LLC cells upon sub-lethal MLKL damage.
(E) qRT-PCR and ELISA qualification of chemokine production in hMLKL1–181-2Fv-NIH3T3, hMLKL1–181-2Fv-LLC, and hMLKL1–181-2Fv-mlkl−/− MEF cells ± 100 nM B/B for 1 h (qRT-PCR) or 3 h (ELISA).
(F) qRT-PCR and ELISA qualification of chemokine production in mRIPK3(ΔRHIM)-2Fv-NIH3T3 ± 100 nM B/B for 1 h (qRT-PCR) or 3 h (ELISA) and transfected with indicated siRNAs.
(G) qRT-PCR qualification of cxcl1 production in WT and mlkl−/− L929 cells treated with 30-μM zVAD for 7 h.
For all qRT-PCR, ELISA, and CellTiter-Glo quantification, data are presented as mean of triplicate samples. In all cases, error bars were SD. Unpaired t test was performed between B/B-treated and control groups. For RNA-seq analysis, data are presented as the mean of duplicate samples. **p < 0.01.
After sub-lethal PM damage by B/B addition, we profiled mRNA expression by mRNA-seq. Compared with the parental NIH3T3 cells, where we found only marginal transcriptional changes after B/B treatment, we observed a unique pattern of gene expression within 1 h of treatment in hMLKL1–181-2xFv-NIH3T3 cells (Figure 1B). The chemokine cxcl1, cxcl10, and cytokine lif were among the most highly induced transcripts (Figure 1B, secretory proteins are labeled in red). We also observed increased expression of transcriptional regulators, such as egr1/2/3, fos, fosb, jun, and junb. Several dual-specificity phosphatase genes, including dusp-1, −5, −6, and −8, were also upregulated (Table S1). The transcriptional changes of several genes were verified by qPCR and CXCL1 secretion was confirmed by ELISA (Figures 1C and 1E). By performing the same mRNA-seq analysis in MLKL1–181-2xFv-expressing Lewis lung carcinoma (LLC) cells, we found an expression pattern upon B/B-induced transcripts that overlapped with that of NIH3T3 (Figure 1D; Table S1). Notably, the LLC cells we used did not express functional RIPK3 (Figures S1B and S1C). CXCL1 expression and secretion was confirmed by qPCR and ELISA, respectively (Figure 1E). We also stably transduced Mlkl−/− MEF cells with MLKL1–181-2xFv or MLKL1−140-2xFv-Venus. Upon B/B addition, similar chemokine production was observed (Figures 1E and S1D). We confirmed the transcriptional changes were from the damaged, yet intact, cells by performing expression analysis of the sorted SytoxGreen− cells or annexin V+ SytoxGreen− cells after B/B treatment and compared them with non-treated cells. We observed a similar expression pattern in both cases (Figure S1E).
One possibility was that the detachment from the culture dish during MLKL PM damage might induce this transcriptional reprogramming. To test this, we treated cells with trypsin to promote detachment. Treatment with 0.25% trypsin for 5 min was sufficient to detach all cells. However, we did not observe the induction of the signature genes cxcl1, egr1, or fos by qPCR (Figure S1F). Therefore, the effect appeared to be caused by the pore-forming activity of the MLKL fragment, rather than cell detachment.
A similar expression pattern was also induced by the endogenous MLKL phosphorylated by RIPK3. It is known that RIPK3 can activate cytokine expression (e.g., IL-6) during necroptosis (Orozco et al., 2019). However, this effect of RIPK3 requires the engagement of RIPK1 via RHIM motifs in both molecules (Yatim et al., 2015). Since the focus was the RHIM independent and pMLKL-mediated transcriptional activation, we employed RIPK3–2Fv-NIH3T3 and RIPK3(ΔRHIM)-2Fv-NIH3T3 cells. They both respond to dimerizer B/B and phosphorylate MLKL, thus initiating PM damage and necroptosis (Yatim et al., 2015). As reported previously, 45 min after dimerization, we observed necroptosis proceeding with a robust cxcl1 and cxcl10 induction only in RIPK3–2Fv-expressing NIH3T3 cells and not in NIH3T3 cells expressing RIPK3(ΔRHIM)-2Fv-NIH3T3 (Figure S1G). These data suggested that early-stage (40–45 min) transcriptional activation was mediated by RIPK3-RHIM-motif-mediated RIPK1 engagement. However, at later time points (from 60 min post-B/B addition and beyond) RIPK3(ΔRHIM)-2Fv elicited cxcl1 and cxcl10 induction, as robustly as in RIPK3–2Fv cells (Figures S1G and S1H), which was suppressed by silencing MLKL (Figure 1F). This suggests that rather than RIPK1 engagement, chemokine production from RIPK3(ΔRHIM)-2Fv-expessing NIH3T3 was triggered by pMLKL pore-forming activity.
To determine if more physiological stimuli could trigger a similar cytokine expression profile, we employed L929 cells, which undergo necroptosis upon addition of the caspase inhibitor zVAD (Wu et al., 2011). We found that cxcl1, cxcl10, lif, tnfaip3, egr1, jun, and fos were upregulated in zVAD stimulated annexin V+ SytoxGreen− L929 cells (Figure S1I). We also found that treated cells could reattach and spread out on the culture dish if the zVAD was washed out, survive, and generate colonies over the long term (Figure S1J). Further, these gene inductions upon zVAD-induced necroptosis were entirely abolished by MLKL ablation (Figures 1G and S1K). Thus, during necroptosis in L929 cells, MLKL pore forming was required for the induction of these genes.
Gasdermin family proteins can also form PM pores upon activations by protease cleavages, including Gasdermin D (GSDMD). When the GSDMD N-terminal (1–276 aa) sequence was fused with the tamoxifen-responsive estrogen receptor hormone-binding domain (HBD*), treatment of 4-hydroxytamoxifen (4-OHT) led to direct PM damage in the absence of additional signals (Figures 2A and 2B; Chen et al., 2016). Similar to hMLKL1–181-2xFv, a sub-lethal GSDMD activation (by optimized 4-OHT concentration) induced PM damage and elicited a PM damage response, featuring cxcl1, lif, cxcl10, fos, and egr1 in both NIH3T3 and LLC cells (Figures 2C–2E; Table S1). Cxcl1 and other transcripts were verified by qPCR and secretion was confirmed by ELISA (Figures 2D and S2A). Cells that round-up upon 4-OHT treatment could reattach to the culture dishes and spread out 7 h after 4-OHT withdraw and subsequently form clones in a clonogenic survival assay (Figure S2B). We confirmed that the gene induction occurred in cells that maintained PM integrity by sorting SytoxGreen− cells and assessing mRNA levels by qPCR (Figure S2C).
Figure 2. The general patterns of the cellular response to sub-lethal PM damage.
(A) Titration of 4-OHT to achieve an optimal dose of GSDMD-mediated sub-lethal PM damage. Cell death was measured by CellTiter-Glo.
(B) Representative images of 50-nM 4-OHT-treated (1 h) mGSDMD1-276-HBD-NIH3T3 cells, stained by SytoxGreen. Arrows indicated cells that were rounded up but still resistant to SytoxGreen staining. Scale bar, 50 μm.
(C) RNA-seq analysis of LLC and mGSDMD1-276-HBD*-LLC ± 50 nM 4-OHT, for 2.5 h. The y axis is log2 fold change (FC). Each red label showed a secreted protein (cytokines or chemokines).
(D) qRT-PCR qualification of chemokine production in NIH3T3, mGSDMD1-276-HBD-NIH3T3, LLC, and mGSDMD1-276-HBD*-LLC cells ± 50 nM 4-OHT, for 2.5 h.
(E) RNA-seq analysis of mGSDMD1-276-HBD-NIH3T3 and mGSDMD1-276-HBD-LLC (±50 nM 4-OHT, for 2.5 h). Venn diagram shows the overlapped transcription in GD-NIH3T3 and GD-LLC cells upon sub-lethal GSDMD PM damage.
(F) Titration of digitonin to achieve an optimal dose of detergent-mediated sub-lethal PM damage (3 h treatment). Cell death was measured by CellTiter-Glo.
(G) Representative images of 55-μg/mL digitonin-treated (3 h) NIH3T3 cells, stained by SytoxGreen. Arrows indicate cells that were rounded up but still resistant to SytoxGreen staining. Scale bar, 50 μm.
(H and I) qRT-PCR and ELISA qualification of chemokine/cytokine production in NIH3T3 ± digitonin (50, 52, and 55 μg/mL), for 3.5 h.
(J) RNA-seq analysis of NIH3T3 and LLC (±55 μg/mL digitonin, for 3 h). Venn diagram shows the overlapped transcription in NIH3T3 and LLC cells upon sub-lethal digitonin PM damage.
(K) Representative images and qRT-PCR analysis of the hMLKL1–181-2Fv-NIH3T3 ± PFN, for 2.5 h. Cells were stained with SytoxGreen. Arrows indicate cells that were rounded up but still resistant to SytoxGreen staining. Scale bar, 100 μm.
(L–N) Venn diagram shows the overlapped genes that were upregulated among all three types of sub-lethal PM damage in NIH3T3 cells. All 31 overlapped genes are listed in (M), and the pathway enrichment results are listed in (N).
For all qRT-PCR, ELISA, and CellTiter-Glo, data are presented as mean of triplicate samples. In all cases, error bars were SD. Unpaired t test was performed between PM-damaged and control-treated groups. For RNA-seq analysis, data are presented as the mean of duplicate samples. **p < 0.01.
Chemical detergents, such as digitonin can generate sub-lethal PM integrity loss when applied at a low concentration (Figures 2F and 2G). Similar to GSDMD- and MLKL-treated cells, the rounded up digitonin-treated cells could reattach to the culture dishes and spread out after digitonin was removed for 7 h and later form clones in a clonogenic survival assay (Figure S2D). Both NIH3T3 cells and LLC exhibited the upregulation of several transcripts with sub-lethal digitonin-induced PM damage, including egr1 and cxcl1 (Figures 2H–2J and S2E; Table S1). We also confirmed that the gene induction occurred in the intact cells by sorting out the SytoxGreen− cells as mentioned earlier (Figure S2F). Another detergent, CHAPS, also activated CXCL1 and CXCL10 expression and secretion (Figure S2G).
PFN is a glycoprotein with PM pore formation activity. It is mainly produced by natural killer (NK) cells and cytotoxic T lymphocytes to neutralize the target infected or cancer cells. It is also used to partially permeabilize cells for protein delivery (Thiery et al., 2010). In fact, the architecture of the gasdermin pores shares mechanistic similarities with pores formed by PFN (Ruan et al., 2018). We observed similar expression inductions when cells were challenged with sub-lethal-level PFN (Figure 2K).
The transcriptional change induced by the active form of MLKL, gasdermins, and digitonin overlapped. In NIH3T3 cells 31 transcripts were shared across all conditions (Figures 2L and 2M; Table S1). Similar overlap was also observed in LLC cells (Table S1). Given that all cellular challenges involved PM pore-forming damage, the joint transcriptional activation appeared to be caused by the sub-lethal PM integrity loss. Thus, we name it the plasma membrane integrity (PMI) response. To continue decoding the signal transduction pathway leading to cxcl1 production, we refer to it as the PMI signaling pathway.
The PMI response is a “cell-autonomous” process
We tested whether the PMI response was a “cell-autonomous” process or triggered by biomolecules (such as danger-associated molecular patterns, DAMPs) released from the neighboring lysed cells. First, we transferred the cell-free culture supernatants from the B/B-MLKL damaged cells and found that the supernatants failed to induce cxcl1 or cxcl10 in NIH3T3 cells (Figure S2H, positive control, digitonin stimulation).
We co-cultured NIH3T3 cells (which did not respond to B/B and were mCherry−) with MLKL1–181-2xFv-NIH3T3 (mCherry+) overnight. Cells were then stimulated with B/B for 1.5 h and separated by flow cytometry. A robust PMI response was present only in mCherry+ cells (Figures S2I and S2J).
It has recently been shown that NINJ1 can mediate PM rupture (dispensable to and downstream of PM pore formation) for the release of large molecular weight DAMPs, such as HMGB1 or LDH (Kayagaki et al., 2021). Therefore, we tested the role of NINJ1 in PMI responses. NINJ1 silencing by siRNA did not suppress but slightly promoted PMI responses (Figure S2K), suggesting that a role for DAMPs in PMI signaling is unlikely. NINJ1 silencing might also help pore-damaged cells sustain their structure without moving to uncontrollable PM collapse and thus could prolong cell survival to generate slightly more transcripts (Figure S2K).
Taken together, these data suggested that DAMPs released by dying/dead cells had very limited, if any, capacity to induce PMI-associated chemokine or cytokine production in bystander cells. PMI signaling appears to be a “cell-autonomous” process.
PMI-mediated chemokine and cytokine production via RelA/Cux1 and NF-κB signaling
To dissect the PMI signaling network, we applied gene set enrichment analysis to our overlapping 31 genes (Subramanian et al., 2005), which revealed an enrichment of TNF/NF-κB, KRAS-MAPK, and several other pathways among the Hallmark pathways enriched in this gene list (Figure 2N). Additional analysis of pore formation universally induced genes in LLC cells (10 genes, 7 of which overlap with the NIH3T3, p value = 1.16e−16, hypergeometric test) also showed similar enrichment for TNF/NF-κB and growth factor signaling (Table S1). Analysis of protein-protein interactions and complexes among this set of 31 genes likewise implicated Jun and TNF signaling (Figure S2L; Table S1, using the MIPPIE database of mouse protein-protein interactions; Alanis-Lobato et al., 2020). Thus, we first tested whether NF-κB signaling was an essential component of the PMI signaling network. We observed RelA (NF-κB p65 subunit) phosphorylation and nuclear translocation upon MLKL membrane damage (Figures 3A and 3B). Using a CFP reporter regulated by a minimal promoter with an NF-κB responsive element (Jutz et al., 2016), we further confirmed NF-κB activation (CFP expression, Figures 3C and S3A–S3C). Taken together, our results show that PMI signaling robustly induces NF-κB activation.
Figure 3. PMI-mediated cytokine/chemokine productions via RelA/Cux1 and NF-κB signaling.
(A) Immunoblotting of RelA/p65 and pRelA in 100-nM-B/B-treated hMLKL1–181-2Fv-NIH3T3 cells (30 min).
(B) Representative RelA/p65 immunostaining images of 100 nM B/B-treated, NIH3T3, or hMLKL1–181-2Fv-NIH3T3 cells (45 min). Scale bar, 100 μm. Percentages of cells with RelA nuclear translocation of total cells counted are shown in the bottom right corner. ~300 cells were analyzed in each group.
(C) NIH3T3, hMLKL1–181-2Fv-NIH3T3, and hMLKL1–181-2Fv-LLC cells were stably transduced with CFP driven by NF-κB minimal promoter. ~3 × 105 cells were seeded into culture plates overnight before the assay. After 100 nM B/B treatment for 6–8 h, CFP+ cells were counted by the software Gen5 using the microscope Lionheart. Representative images are presented in Figure S3A.
(D) Immunoblotting of RelA/p65 and actin in hMLKL1–181-2Fv-NIH3T3 cells transfected with indicated siRNAs for 72 h. Only siRNA #2 and #3 showed robust knockdown efficacy. siSCR, scramble siRNA.
(E) Cell survival of hMLKL1–181-2Fv-NIH3T3 cells after 100-nM 1-h B/B treatments with siRNA. Cell survival was measured by CellTiter-Glo.
(F and G) ELISA and qRT-PCR quantification of cxcl1 and cxcl10 in hMLKL1–181-2Fv-NIH3T3 cells followed by the addition of 100-nM B/B for 1 h and transfected with indicated siRNAs (3.5 h for ELISA).
(H) RNA-seq analysis of WT or RelA−/− hMLKL1–181-2Fv-NIH3T3 cells ± 100-nM B/B treatment for 1 h.
(I) qRT-PCR quantification of cxcl1, cxcl10, egr1, and fos in hMLKL1–181-2Fv-NIH3T3 cells followed by the addition of 100 nM B/B for 1 h and transfected with indicated siRNAs.
For all qRT-PCR, ELISA, CellTiter-Glo, and microscopy image quantification, data are presented as mean of triplicate samples. For RNA-seq analysis, data are presented as the mean of duplicate samples. In all cases, error bars were SD. Unpaired t test was performed between PM damaged scramble siRNA (siSCR) and RelA or CUX1 siRNA-treated groups. *p < 0.05, **p < 0.01. Fold changes were labeled as percentage next to the bar graph.
To identify which PMI-induced genes were regulated by NF-κB, we compared a previously published dataset on the response of NIH3T3 cells with TNF-α (a typical NF-κB signaling inducer) and the transcriptome change caused by MLKL-mediated PM damage (Figure S3D; Orozco et al., 2019). Chemokines cxcl1 and cxcl10, the cytokine lif, and tnfaip3 were upregulated in both cases, whereas the intracellular transcription factors egr1 and fos were only induced by PM damage (Figure S3D; Table S1). This suggested that NF-κB signaling probably regulated chemokines, cytokines, and tnfaip3, whereas egr1, fos, and others were not NF-κB targets. It also suggested that MLKL-mediated PM-damage-induced PMI response was fundamentally different from RIPK3-RHIM-RIPK1-induced transcriptional changes, which largely overlapped with the TNF-α-induced response (Orozco et al., 2019). We also found that in the context of PM damage, chemokine production did not rely on new protein translation as cycloheximide did not block cxcl1 transcriptional induction (Figure S3E).
Next, we used RelA/p65 siRNA and CRISPR to silence or ablate RelA/p65. RelA loss of function (siRNA #2 and #3 and CRISPR-cas9-mediated ablation, two independent clones) dramatically suppressed cxcl1, cxcl10, and lif expression in both MLKL- and digitonin-induced PMI responses (Figures 3D–3H and S3F–S3J). This effect was also confirmed by ELISA (Figures 3F, S3G, and S3I). In contrast, in RelA−/− cells induction of egr1, jun, fos, and dusp1 was largely unaffected (Figures 3H and S3K). The extent of PM damage and the consequent cell viability induced by MLKL or digitonin was unaffected by RelA ablation or silencing (Figures 3E and S3H).
We noted that only a subset of TNF-induced RelA targets (such as cxcl1 and cxcl10) were induced by PM damage (Figure S3D, TNF induced). Recent studies showed that in the promoters of several chemokines, including cxcl1, there is a putative cut-like homeobox 1 (CUX1) NF-κB-binding motif, which was not found elsewhere in the genome (Slowikowski et al., 2020). Consistently, we found that siRNA knockdown of CUX1 reduced PMI-mediated cxcl1 and cxcl10 production but not egr1 or fos expression (Figures 3I and S3L).
Upstream of the RelA-CUX1 complex, we found the involvements of TAK1, IKK complex, and IκBα degradation for cxcl1 induction upon MLKL-, GSDMD-, and digitonin-mediated PM damage (Figure S4). Using inhibitors listed in Figure S4A, we found cxcl1 induction was significantly suppressed upon various PM pore formation (Figures S4B, S4C, and S4E–S4G). Using siRNA against TAK1 and IKKγ, we further strengthened our findings (Figures S4I and S4K). None of these knockdowns or inhibitor treatments altered MLKL-induced PM damage or cell survival or lysis (Figures S4D and S4H). We also found a similar role of NF-κB signaling axis in CXCL1 productions in L929 cells undergoing zVAD-induced necroptosis (Figures S4L–S4N). Nec-1s, a necroptosis inhibitor was used as a positive control to block MLKL phosphorylation and pMLKL-induced CXCL1 secretion.
MAPK signaling pathway is activated upon PM integrity loss
The second module of upregulated genes, including fos and egr1, was broadly associated with “pro-growth” and MAPK signaling (Figure 2N). Many of them were immediate early genes (IEGs) known for their rapid response to growth factors. Serum response factor (SRF) is a transcription factor downstream of MAPK signaling and a well-established regulator of IEGs. The promoters of IEGs usually have serum response element (SRE) sequences (Lee et al., 2010).
To test the involvement of the MAPK pathway and transcription factor SRF, we first probed MAPK activation (indicated by phosphorylation) and p-Erk nuclear translocation. We observed an increase in p-SRF, p-MEK, p-Erk, and p-P90, as well as p-Erk nuclear translocation upon MLKL- and digitonin-induced PM damage (Figures 4A–4C and S5A). SRF silencing diminished egr1 or fos upregulation but not cxcl1 or cxcl10 induction (Figures 4D–4F). As srf is an essential gene, we were unable to ablate it by CRISPR-cas9.
Figure 4. MAPK signaling pathway was activated upon PM integrity loss.
(A and B) Representative pErk1/2 immunostaining images of 100-nM B/B and 50-μg/mL digitonin-treated hMLKL1–181-2Fv-NIH3T3 cells (30 min). Scale bar, 50 μm. Percentages of cells with pErk1/2 nuclear translocation of total cells counted were shown in (B). 50~100 cells were analyzed in each group.
(C) Immunoblotting of MAPK pathway components and actin in 100-nM-B/B-treated, hMLKL1–181-2Fv-NIH3T3 cells (30 min).
(D–F) qRT-PCR quantification of egr1, fos, cxcl1, and cxcl10 in hMLKL1–181-2Fv-NIH3T3 cells followed by the addition of 100-nM B/B for 1 h (D) or 50 μg/mL digitonin for 2 h (E) and transfected with indicated siRNAs. The efficiency of SRF siRNA silencing is shown in (F).
(G) qRT-PCR quantification of genes in hMLKL1–181-2Fv-NIH3T3 cells followed by the addition of 100-nM B/B for 1 h and transfected with indicated siRNAs.
(H) qRT-PCR quantification of egr1 in indicated cells followed by the addition of 100-nM B/B for 1 h, 50-nM 4-OHT for 2 h, or 50-μg/mL digitonin for 2 h. MEK inhibitors PD 0325901 (2.5 μM) or Trametinib (50 nM) were added 15 min before the PM damage.
For all qRT-PCR and microscopy image quantification, data are presented as mean of triplicate samples. In all cases, error bars were SD. Unpaired t test was performed between PM damaged scramble siRNA (siSCR) or Dimethyl sulfoxide (DMSO) (Φ)-treated and siRNA/inhibitors-treated groups. *p < 0.05, **p < 0.01. Fold changes were labeled as percentage next to the bar graph.
In partnership with SRF, the ternary complex factors (TCFs) (SAP1 [also known as Elk-4], Elk-1, and Net [also known as Elk3 or SAP2]) link transcription to MAPK signaling. Although TCFs are highly homologous and partially redundant, their structures reveal distinctive preferences in DNA binding (Vickers et al., 2004). By binding to different TCFs, SRF may exhibit specificity in transcriptional activation. We found upon MLKL-mediated PM damage, only SAP2, rather than SAP1 or Elk1 silencing partially repressed egr1 upregulation (Figure 4G). Similar to SRF, SAP2 silencing could partially suppress fos induction but had no effects on cxcl1 induction (Figure 4G). We found similar results on digitonin-induced PMI response (Figure S5B).
Consistent this model, MEK inhibitors, PD-325901 or Trametinib, reduced egr1 induction by PM damage (~80%–90% inhibition, Figure 4H). cxcl1 and cxcl10 were only slightly affected or unaffected by these inhibitors (Figure S5C).
In very specified immune cell subsets, such as macrophages and dendritic cells, PM pore-forming toxins can lead to NLRP3 inflammasome activation (Muñoz-Planillo et al., 2013). However, the cell lines employed here, such as NIH3T3, LLC, and L929 cells, did not express the key components, such as NLRP3 and ASC (Figure S5D, LPS primed RAW264.7, and bone-marrow-derived macrophages (BMDM) were used as positive control). Thus, our data suggest that, in a broad spectrum of cellular context, the PMI signaling can engage the NF-κB and MAPK pathways to drive a coherent transcriptional program (outlined in Figure S5E). The NF-κB pathway and RelA/Cux1 complex led to chemokine and cytokine expression, whereas the MAPK pathway and SRF/SAP2 complex controlled the induction of IEGs.
The action of ESCRT-III machinery is critical to avoid total lysis upon PM damage. Without ESCRT-III, cells (e.g., L929) could not survive long after necroptosis was initiated (Murai et al., 2018), which might limit the chemokine productions. Similarly, we found fewer zVAD stimulated L929 cells survived when ESCRT complex components (ESCRT-III component CHMP4B and ESCRT-I component TSG101) were silenced (Figure S5F). Notably, cells staying as annexin V+ SytoxGreen− status (sub-lethal subsets) were significantly decreased to only ~60% of scramble silenced cells (Figure S5G). As expected, we found that ESCRT deficiency resulted in decreased cxcl1 induction in these cells (Figure S5H). Similar effects were observed in B/B stimulated MLKL1–181-2xFv-NIH3T3 cells (Figures S5I and S5J). Note that as ESCRT-III has many critical functions in other cellular process, we were not able to completely ablate them by CRISPR-cas9.
Ca2+ influx is required for PMI signaling initiation
We tested whether extracellular TNF was required for PMI signaling activation. We found that although TNFR-Fc effectively neutralized extracellular TNF, as shown by NF-κB-controlled CFP expression (Figures 5A and 5B), it had no effect on cxcl1 and cxcl10 induction upon activation of MLKL (Figure 5C). Thus, extracellular TNF is unlikely to be the trigger of PMI signaling. This was also consistent with our RNA-seq data that showed TNF- and PM-damage-induced transcriptional changes are distinct (Figure S3D).
Figure 5. Ca2+ influx was required for PMI signaling initiation.
(A) Representative images of 10 ng/mL TNF or 0.5 μg/mL TNFR1-Fc-treated hMLKL1–181-2Fv-NIH3T3 cells that were stably transduced with CFP driven by NF-κB minimal promoter. Cells were treated for 12 h. Scale bar, 1,000 μm. ~3 × 105 cells were seeded into culture plates overnight before the assays.
(B) Quantification of CFP+ cells in (A).
(C) qRT-PCR quantification of cxcl1 and cxcl10 in hMLKL1–181-2Fv-NIH3T3 cells followed by the addition of 100-nM B/B for 1 h ± 0.5 μg/mL TNFR1-Fc.
(D) RNA-seq analysis of NIH3T3 or hMLKL1–181-2Fv-NIH3T3 cells ± 100 nM B/B treatment for 1 h, either cultured in regular serum-free DMEM or Ca2+-free DMEM. Note that genes in the upper box (such as egr1) were partially inhibited by Ca2+ depletion, whereas genes in the bottom box were almost suppressed to the basal level. Cell survival of hMLKL1–181-2Fv-NIH3T3 cells after the indicated treatment was shown in the bottom, measured by SytoxGreen staining. SytoxGreen− cells were counted as survivors.
(E) qRT-PCR quantification of cxcl1, cxcl10, and lif in NIH3T3 or hMLKL1–181-2Fv-NIH3T3 cells in the same conditions in (D).
(F) qRT-PCR quantification of cxcl1 and cxcl10 in hMLKL1–181-2Fv-NIH3T3 cells followed by the addition of 10 ng/mL TNF for 1 h.
(G) qRT-PCR quantification of cxcl1 in hMLKL1–181-2Fv-LLC cells after 100-nM 2-h B/B treatments with regular or Ca2+-free DMEM plus 20 μM Ca2+ chelator BAPTA-AM.
(H and I) qRT-PCR and ELISA quantification of cxcl1, cxcl10, and lif in NIH3T3 or hMLKL1–181-2Fv-NIH3T3 cells with or without 20 μMCa2+ chelator BAPTA-AM in regular DMEM.
(J) Cell survival of hMLKL1–181-2Fv-NIH3T3 cells in (H, 1 h post B/B). Cell survival was measured by CellTiter-Glo.
(K and L) qRT-PCR and ELISA quantification of cxcl1, cxcl10, and egr1 in mGSDMD1-276-HBD*-LLC cells followed by 50-nM 4-OHT for 2 h (qRT-PCR) or 3 h (ELISA) with regular or Ca2+-free DMEM plus 20 μM Ca2+ chelator BAPTA-AM.
(M) Cell survival of GSDMD1-276-HBD*-LLC cells followed by the addition of 50-nM 4-OHT for 2 h with regular (Ca) or Ca2+-free DMEM plus 20 μM Ca2+ chelator BAPTA-AM (no Ca). Cell survival was measured by CellTiter-Glo.
(N) qRT-PCR quantification of cxcl1, cxcl10, and lif in hMLKL1–181-2Fv-NIH3T3 cells after 1 μg/mL ionomycin treatments for 3 h.
For BAPTA-AM treatment, cells were precultured with the chelator for 20–30 min before PM damage was induced. For all qRT-PCR, CellTiter-Glo, and ELISA quantification, data are presented as mean of triplicate samples. For RNA-seq analysis, data are presented as the mean of duplicate samples. In all cases, error bars were SD. Unpaired t test was performed between PM damaged scramble siRNA (siSCR) or DMSO (Φ)-treated and siRNA/inhibitors/Ca2+ depletion-treated groups. *p < 0.05, **p < 0.01. Fold changes were labeled as percentage next to the bar graph.
We had reported a Ca2+ influx upon MLKL activation, immediately prior to (but not required for) phosphatidylserine externalization (Gong et al., 2017a). We tested whether the intracellular Ca2+ is critical for PMI responses. The depletion of extracellular Ca2+ is sufficient to block intracellular Ca2+ increase upon MLKL action (Gong et al., 2017a). Using RNA-seq, we found that extracellular Ca2+ depletion blocked most of the gene induction (Figures 5D and 5E; Table S1), including chemokines and LIF. As a control, Ca2+ depletion did not affect TNF-induced cxcl1 and cxcl10 production nor the overall survival rate within the time window we assayed (Figures 5D and 5F). We found similar inhibition by Ca2+ removal of cxcl1 induction and CXCL1 production upon GSDMD1-276-HBD* and MLKL1–181-2xFv PM damage (Figures 5G–5M). We also found that BAPTA-AM treatment in normal DMEM was sufficient to achieve similar PMI signaling inhibition, such as extracellular Ca2+ depletion (Figures 5H–5J).
The Ca2+ ionophore ionomycin induces Ca2+ influx to the same level as those seen in MLKL PM damaged cells (Gong et al., 2017a). However, ionomycin-enforced Ca2+ influx was insufficient to cause robust chemokine expression (Figure 5N, see further on). Thus, although a change in intercellular Ca2+ is required for the PMI, it is insufficient to trigger this response.
Ca2+ may come directly through the broken PM pores. This local Ca2+ influx leads ESCRT-III to be recruited to the injured sites (Scheffer et al., 2014). Moreover, as a small fraction of MLKL and GSDMD also target ER (Li et al., 2020; Wang et al., 2014). Therefore, it is possible that pore-formation-induced ER damage might induce [Ca2+]ER depletion, which could trigger Ca2+ influx through Ca2+ release activated Ca2+ (CRAC) channels on the PM. This process usually called store-operated Ca2+ entry (SOCE) (Hogan and Rao, 2015). We hypothesize that it would be possible that CRAC channels amplify the Ca2+ influx and/or maintain high Ca2+ level.
We first assessed the STIM1 location upon PM damage. STIM1 is an ER located protein and can detect [Ca2+]ER drop with its EF-hand domain. When [Ca2+]ER drops, STIM1 clusters and forms “puncta.” STIM1 relocates to near the PM and clusters, where it activates Orai1, a structural component of the CRAC channels. Upon MLKL activation, we observed STIM1 “puncta” formation in a sub-fraction of cells (Figure S6A).
Downstream of MLKL activation, Ca2+ influx happened with PS externalization (Gong et al., 2017a). Thus, B/B-treated annexin V+ cells had high [Ca2+]i as monitored by the calcium sensor cytosolic GCaMP3 (Gong et al., 2017a). We found that, compared with annexin V− cells, B/B-treated annexin V+ showed higher [Ca2+]i (indicated by GCaMP3), which was partially suppressed in STIM1 or Orai1 silenced B/B-treated annexin V+ cells (Figures S6B and S6C). Using siRNA, we found that PMI-mediated CXCL1 production was largely suppressed (~60%, Figures S6D and S6E). CRAC inhibition did not affect cell survival rates (Figure S6F). Taken together, CRAC channels were activated during PM integrity loss and contributed to chemokine inductions by magnifying or maintaining high intercellular Ca2+.
Note that RelA targets, such as cxcl1, were more sensitive to Ca2+ depletion than were the non-RelA targets, such as egr1. It appeared that CRAC channel inhibition, which only partially decreased intracellular Ca2+, blocked cxcl1 induction more efficiently than egr1 (Figures S6D and S6E). Similar effects were also recorded in GSDMD1-276-HBD*-LLC cells (Figure 5K). The different requirements of intracellular Ca2+ for the chemokine productions and IEG inductions (such as egr1) suggest that the Ca2+ sensors involved in these two pathways are distinct.
S660-phosphorylated PKCs detect and translocate to the pore-forming sites specific for chemokine production
Within the cells, intracellular Ca2+ concentration is detected by many protein sensors. Among all of them, we hypothesized that conventional PKCs (cPKCs, α, β, and γ) could be critical because (1) Pkc1 (the only yeast homolog of PKC) is a component in yeast cell wall integrity (CWI) pathway (see discussion; Levin, 2005), (2) cPKCs can bind Ca2+ with their C2 domain (Kohout et al., 2002), and (3) cPKCs have been suggested to play a role in NF-κB activation (Lilienbaum and Israel, 2003).
In general, PKCs are regulated by several phosphorylation events in vivo, including autophosphorylation on T641 and the subsequent autophosphorylation on S660. S660-autophosphorylated PKCs maintain catalytic competence and change their subcellular localization to membrane structures (Keranen et al., 1995). We performed immune staining for S660 p-PKCs in Mlkl−/− MEF expressing MLKL1−140-2xFv-Venus, where we found a strong PM translocation upon pore formation (Figure 6A). S660 p-PKCs accumulated at the cell-cell tight junction sites, which were also the major sites of MLKL PM targeting (Samson et al., 2020). A closer look revealed that S660 p-PKCs were specifically recruited to the sites where MLKL targeted and the ESCRT-III component CHMP4B assembled on PM (Gong et al., 2017b) (Figure 6B). Similar S660 p-PKCs translocation was observed with MLKL1–181-2xFv, GSDMD1-276-HBD*, digitonin, TNF-α+zVAD-induced necroptosis, and LPS+ATP-induced pyroptosis (Figures 6C and S7A–S7F). Differences in the percentage of S660 p-PKCs translocation (which is defined as the percentage of cells with visually scorable p-PKCs PM association of total cells in each image captured) were likely due to the time at which cells were fixed for staining, although we cannot rule out a variation due to different forms of PM damage. However, translocation of S660 p-PKCs required pore-forming action as it was abolished in Mlkl−/− HT-29 cells (Figures 6C, S7D, and S7E). We further found that S660 p-PKCs were enriched in the 0.5% Triton X-100 insoluble membrane fractions (including PM) of MLKL-damaged MLKL1–181-2xFv-NIH3T3 cells, confirming the immune-staining results (Figure 6D). Note that this antibody could not distinguish different PKC isoforms. The translocation pattern of S660 p-PKCs was also different from the STIM1 “puncta.”
Figure 6. S660 PKCs translocated to PM pore-forming sites to initiate chemokine productions.
(A and B) Images of hMLKL1−140-2Fv-mlkl−/− MEF cells treated with or without 10-nM B/B for 20 min, stained with S660 p-PKC. Scale bar, 100 μm(A) or 10 μm(B). hMLKL1−140-2Fv was fused with Venus tag and CHMP4B was fused with mCherry tag. Arrowheads indicated S660 p-PKC enriched at the tight conjugations. Values were (cells with p-PKC PM translocated)/(total cell counted) in (A). Zoomed in boxes in (B) were shown on the right.
(C) S660 p-PKC translocation percentage of mGSDMD1-276-HBD*-NIH3T3 + 50 nM 4-OHT for 30 min; + 50 μg/mL digitonin for 30 min; WT or mlkl−/− HT-29 cells treated with 20-ng/mL TNF, 20-μM Lcl161, and 100-μM zVAD for 4.5 h; L929 cells treated with 20-ng/mL TNF and 100-μM zVAD for 2.5 h; LPS primed (2 μg/mL for 6 h) ASC-expressing RAW264.7 cells followed by 3-mM ATP for 20 min. Values were (cells with PM translocated p-PKC)/(total cell counted). Representative images and non-treated controls are shown in Figures S6A–S6F. Each dot indicated the percentage of cells with visually scorable p-PKCs PM association of total cells in each image captured.
(D) hMLKL1–181-2Fv-NIH3T3 ± 50 nM B/B cells were either directly lyased by SDS-loading buffer (total lysates) or first dissolved by 0.5% Triton X-100 buffer then collected the insoluble fraction and dissolved by SDS loading buffer. Shown was western blot against indicated proteins. hMLKL1–181-2Fv had a C terminus HA tag and thus was blotted with anti-HA.
(E) Images of GFP-tagged C1 or C2 domain of PKCγ upon ± 100 nM B/B or 50 μg/mL digitonin for 20 min. Values were (cells with PM translocated C1 or C2)/(total cell counted). Scale bar, 30 μm.
(F) S660 p-PKC translocation percentage of hMLKL1–181-2Fv-NIH3T3 +50 nM B/B ± Ca2+. Representative images and non-treated controls were shown in Figure S6A. Each dot indicated the percentage of cells with visually scorable p-PKCs PM association of total cells in each image captured.
(G) S660 p-PKC translocation percentage and representative images of hMLKL1–181-2Fv-NIH3T3 + 50-nM B/B ± 2.5-μM Go6976. Scale bar, 30 μm.
(H) qRT-PCR quantification induction of cxcl1 in hMLKL1–181-2Fv-NIH3T3 ± 100-nM B/B treatment for 1 h (qRT-PCR) or 3 h (ELISA) with 5-μM Go6976 or 1-μM Go6983. PKC inhibitors were added 15 min before the PM damage.
(I) qRT-PCR quantification of cxcl1, cxcl10, and lif in hMLKL1–181-2Fv-NIH3T3 transfected with indicated siRNAs.
(J) RNA-seq analysis of hMLKL1–181-2Fv-NIH3T3 cells ± 100-nM B/B treatment for 1 h, co-transfected with scramble or PKCα and β siRNA.
(K) qRT-PCR quantifications induction of genes in hMLKL1–181-2Fv-NIH3T3 transiently transfected with GFP or dominant-negative (DN) forms of PKCs ± 100-nM B/B treatment for 1 h.
For all qRT-PCR, FACS, and CellTiter-Glo quantification, data are presented as mean of triplicate samples. In all cases, error bars were SD. Unpaired t test was performed between PM damaged scramble siRNA (siSCR) or DMSO (Φ)-treated and siRNA/inhibitors-treated groups. *p < 0.05, **p < 0.01, ***p < 0.001. Fold changes are labeled as percentage next to the bar graph.
Using either C1 domain (35–152 aa) or C2 domain (170–260 aa) of PKCγ fused with GFP (Codazzi et al., 2001; Oancea et al., 1998), we found only the Ca2+-binding C2 domain translocated to PM when cells were damaged by activated MLKL or digitonin (Figures 6E and S7G). Moreover, translocation of S660 p-PKCs was diminished when extracellular Ca2+ was depleted (Figures 6F and S7A), similar to Ca2+-mediated ESCRT-III translocation (Gong et al., 2017b). Altogether, these data suggest that, similar to ESCRT-III, cPKCs are specifically activated at the PM pore-forming sites and are guided by the local Ca2+ influx.
As PKCs are highly redundant, we first used PKC inhibitors to examine whether PKC activation is required for the PMI response. The inhibitors Go6976 (cPKC specific), Go6983 (pan-PKC), or bisindolylmaleimide I (Bis-I, pan-PKC) exhibited robust inhibition of cPKC activation (shown as S660 staining), cxcl1 production, and NF-κB-responsive CFP reporter expression (Figures 6G, 6H, S7H, and S7I). Similar results were also observed on zVAD stimulated L929 cells in the presence of the PKC inhibitors (Figures S7K–S7M). We then used siRNA to silence PKCα and β, and we found a significant (~80%) suppression of expression of cxcl1, cxcl10, and lif upon MLKL-induced PM damage (Figures 6I and 6J; Table S1). Neither PKC inhibitors nor silencing of PKCs affected MLKL PM damage and cell survival (Figures S7H, S7J, and S7M). We also found that only genes regulated by RelA (e.g., cxcl and lif), but not SRF (e.g., egr1, fos, and fosb), were suppressed by silencing of PKCs (Figure 6J). The kinase inactive forms of PKCs can act as a dominant-negative (DN) condition (Soh and Weinstein, 2003). We found cells that were transiently co-transfected with conventional PKCs-DN mutants (K368R of PKCα, K371R of PKCβ I, and K380R of PKCγ) showed defects in upregulating chemokines, but not egr1, fos, and jun (Figure 6K). Taken together, cPKCs are locally activated at the PM pore-forming sites and act on NF-κB to regulate the production of chemokines and cytokines, but they are dispensable for MAPK pathway regulation in the PMI. Thus, S660 p-PKCs can function as a PM pore-forming sensor that translates PM damage into chemokine and cytokine induction (see graphical abstract).
Since ESCRT-III and S660 p-PKCs colocalized to the PM damage sites, we further tested whether there would be any molecular-level cross talk between ESCRT-III and PKCs. We found that PKC inhibitors had no effects on ESCRT-III PM translocation upon MLKL-mediated pore forming (Figure S7N). Further, phorbol myristate acetate (PMA), which could robustly activate PKCs, indicated by S660 p-PKCs immunostaining (Figure S7O), did not promote ESCRT-III PM translocation (Figure S7N). PMA, but not Ca2+ ionophores (such as ionomycin), had been reported as an efficient inducer for LIF (Lotz et al., 1992). We confirmed the LIF induction but found that neither reagent (nor added together) could induce chemokine productions (Figure S7P). In our experiments, ionomycin alone could not induce PKC activation, either (Figure S7O). Taken together, our data suggested that, although ESCRT-III and PMI signaling were directly downstream of PM pore forming, we did not find evidence indicating a molecular link between these two pathways. Also, PKC activation alone was not sufficient to elicit chemokine production.
The physiological consequences of the PMI response
We asked whether PMI responses occur in vivo. Phosphorylation of MLKL has been observed in some late-stage tumors (Jiao et al., 2018). Many tumors can evolve mechanisms that evade apoptosis and at least partially inhibit casaspe-8 (such as by mutations), which under certain conditions allow necroptotic signaling activation (He et al., 2017). Meanwhile, reduction in RIPK3 expression often occurs gradually during tumor growth (Koo et al., 2015; Najafov et al., 2018). It is possible that in late-stage tumors MLKL is only partially phosphorylated. Hence, in certain tumors, cells might survive from a low level of pMLKL PM damage (due to incomplete activation) and produce chemokines via PMI signaling.
To test this, we first used digitonin to induce PM damage in a panel of human tumor cell lines as a “proof of principle.” RNA-seq analysis (pancreatic cancer cell line BxPC3 and a colon cancer cell line HT-29) showed digitonin induced robust transcriptional upregulation of a wide range of genes (Figures 7A–7C; Table S1). Genes that were highly activated in murine cells were also present, including egr1, jun, fos, dusps, and several chemokines. Besides cxcl1, the most dominant chemokines in PM damaged human cells also included cxcl8 and cxcl2 (confirmed by qPCR, Figures 7A–7C, and S8A; Table S1). We also stimulated HT-29 cells with TNF-α+zVAD+Smac mematic (Lcl-161), which induced necroptosis. We sorted the annexin V+ SytoxGreen− cells (suffering the PM pore forming) and compared them with DMSO- or TNF-α-alone-treated cells. Similar to digitonin, we found that cxcl8, cxcl2 jun, and fos were induced (Figure S8B). Note that the sorted annexin V+ SytoxGreen− cells could reattach to culture dished and formed colonies days later, indicating at least some of these cells were able to repair the PM damage and eventually survive (Figure S8C). Further, we probed stage-IV pancreatic and breast cancer patient biopsies by fluorescent immunostaining. We found that pMLKL+ cells were co-stained with CXCL8 (Figures 7D and S8D), suggesting that PMI signaling occurs in late-stage solid tumors at single-cell resolution.
Figure 7. PMI in physiological and pathological conditions.
(A) RNA-seq analysis of BxPC3 and HT-29 cells ± 40-μg/mL digitonin treatment for 2 h. The overlapped genes are shown in the Venn diagram.
(B) Log2 fold change of the selected genes upregulated in PM damaged BxPC3 and HT-29 cells.
(C) qRT-PCR quantification of chemokine expression in BxPC3 and HT-29 cell lines ± 40-μg/mL digitonin treated for 2 h.
(D) Sections of stage IV pancreatic cancers were stained with anti-human pMLKL (S358), anti-CXCL8, and DAPI. (n = 2). Zoomed in boxes in (D) were shown on the right.
(E) Microarray quantification of the mRNA levels of different mice kidney tissues in an IRI model (database, GEO: GSE52004).
(F) RAW 264.7 macrophage cells migrated toward cell-free supernatants from living or 100-nM-B/B-treated hMLKL1–181-2Fv-NIH3T3 (WT or RelA−/−) cultures. 10-ng/mL CXCL1 or LIF was added to RelA−/−supernatants. All supernatants were FBS free. ~8 × 105 FBS-starved RAW cells were added to the upper well, and after 18 h of migration, the transwell was fixed by the methanol-crystal violet solution. Scale bar, 50 μm. *p < 0.05, **p < 0.01, one-way ANOVA was applied.
(G) ~3.5 million B/B-treated hMLKL1–181-2Fv-NIH3T3 (WT or RelA−/−) were injected (i.p.) into C57BL/6 animals. 16 h post-injection, peritoneal wash was collected and infiltrated immune cells were analyzed and quantified by FACS. 5 mice in each group.
For qRT-PCR quantification (D), data are presented as mean of triplicate samples. In all cases, error bars were SD. For RNA-seq analysis (B), data are presented as the mean of duplicate samples. Unpaired t test was performed except in (A). *p < 0.05, **p < 0.01. Fold changes are labeled as percentage next to the bar graph.
Another pathological situation in which cells are pMLKL+ without cell lysis is in kidneys that suffer from IRI during the procedure of kidney transplantation. Compared with pre-procedure, kidneys post-IRI exhibited large numbers of pMLKL+ cells (in some cases, more than 80%; Gong et al., 2017b). Via animal models, it was shown that ablation of RIPK3 or MLK can protect kidney functions following IRI (Chen et al., 2018).
By re-analyzing public microarray data from animal models, where tissues such as nephron, kidney interstitial cell populations, vascular endothelium, and macrophages/monocytes were identified by EGFP reporters (Liu et al., 2014), we found that the gene induction pattern post-IRI was similar to that observed in cells undergoing sub-lethal PM damage (Figure 7E, actin B (actb) as an example of a house-keeping gene). By re-analyzing the published whole-kidney mRNA sequencing after IRI (Liu et al., 2017), we also observed that genes with rapid induction at 4 h post-IRI (identified as modules I and II) highly overlapped with genes upregulated in various conditions of PM damaged NIH3T3 cells (e.g., 31 of 50 genes upregulated in MLKL-damaged NIH3T3 were also present in kidneys 4 h after IRI, Figure S8E). Meanwhile, genes with a high induction 2~3 days after IRI (module III) did not overlap with PMI targets (Figure S8E). By re-analyzing public single-nucleus RNA sequencing (snRNA-seq) data from IRI kidneys (Kirita et al., 2020), we found cells enriched in Lrp2Hi clusters (proximal tubule) exhibited cxcl1 increase at 4 h post-IRI (Figure S8F). By re-analyzing RNA-seq data from human kidney samples undergoing ischemia-reperfusion stress during the transplantation procedure (Gong et al., 2017b), we also found significantly higher cxcl8, cxcl2, and lif expression in post-procedure samples (Figure S8G). These correlative studies provide in vivo evidence that PMI may occur in kidneys after IRI injuries.
Since the outcomes of PMI include production of chemokines and cytokines, we wondered whether PMI signaling could modulate immune responses. Dead and dying cells usually attract macrophages (Green, 2019). Therefore, we used WT and RelA−/− MLKL1–181-2xFv-expressing NIH3T3 cells to test the role of PMI signaling in macrophage migration by performing a transwell assay (Figure 7F). Only supernatants from PM damaged (+B/B) WT, but not RelA−/− cells, attracted macrophages. CXCL1 addition, but not LIF, mostly corrected the defects (Figure 7F). These results demonstrated that chemokines, such as CXCL1, provided by PMI signaling, promote macrophage migration to the damaged cell. The components passively released from dead RelA−/− cells (such as DAMPs) were insufficient to do so.
To test the pathological role of the PMI signaling, we extended the in vitro transwell assays to a mouse model for sterile peritonitis triggered by dying cells i.p. injection, where chemokines, such as CXCL1, play important roles (Chen and Nunez, 2010). Injecting MLKL1–181-2xFv-NIH3T3 cells damaged by B/B addition could sufficiently elicit peritonitis and inflammation, evidenced by massive monocytes (CD11b+F4/80−Ly6C+Ly6G−), macrophages (CD11b+F4/80+Ly6C−Ly6G−), dendritic cells (MHC+CD11b+CD11c+ and MHC+CD103+CD11c+), and neutrophiles (CD11b+F4/80−Ly6C−Ly6G+) peritoneal cavity infiltration. However, inflammation was significantly less when the animals were challenged by B/B-pretreated RelA−/− hMLKL1–181-2xFv-NIH3T3 cells, which could not generate PMI chemokines or cytokines (Figure 7G). Further, macrophages and monocytes recruited by WT cells displayed lower M2 polarity markers, such as CD163 and CD206, also suggesting a more inflammatory environment. This result extended the biological influence of PM integrity loss to the immune system. Thus, PM pore formation can function as an innate immune activation pattern.
DISCUSSION
Here, we formulated a general model of cellular response to sub-lethal PM purtabations. We found that the transcriptional PM damage response is universal—observed in multiple forms of PM damage and in several cells, including LLC, L929, MEF, and numerous human cell lines, solid tumors, and stressed kidney tissues. PM damage is sufficient to trigger chemokine expression, independent of the upstream signaling (such as RIPK3, caspases, or inflammasome components). S660-phosphorylated cPKCs can detect the pore-forming activity and signal to the NF-κB-mediated production of chemokines and cytokines. These, in turn, influence macrophage migration, and probably other immune events. Therefore, PM integrity loss can be functionally characterized as an innate immune activation signal in which S660-phosphorylated cPKCs can be characterized as a pattern recognition receptor. Sub-lethal PM damaged also activated MAPK pathway and the SRF/SAP2 transcription factors for IEG expression, such as egr1 and fos. The sensor for MAPK pathway is still unknown.
Yeast (such as Saccharomyces cerevisiae) have both a cell wall and a cell membrane. Sodium dodecyl sulfate (SDS), a common detergent that permeates cell membranes and cell walls, is one of the stimuli that can activate a signaling pathway similar to PMI signaling, the “CWI” pathway, which can lead to growth restriction and repair promotion (Smits et al., 1999). The downstream CWI signaling pathway comprises a small G-protein Rho1, which activates the Pkc1-MAP kinase cascade and the ensuing transcriptional changes (Figure S5E; Levin, 2005). Our data systematically revealed that the mammalian PMI signaling parallels to the CWI in yeast (Figure S5E). Although the final gene expression outputs are distinct, both CWI and PMI use PKC kinase cascades to transduce the signals. Collectively, it suggests that the recognition of cell barrier integrity and the signaling transduction modes are evolutionarily conserved.
In yeast, CWI sensors are transmembrane proteins Wsc1–3, Mid2, and Mtl1 (Levin, 2005). Wsc1–3 sensors are the transmembrane proteins with an N-terminal domain cysteine-rich WSC domain. This unique domain architecture is also present in human polycystin 1 (PKD1). PKD1 consists of a voltage-gated ion channel (VGIC) fold that interacts with PKD2 to form the domain-swapped, yet noncanonical, transient receptor potential (TRP) channel architecture, which can function as a cation channel, including inward Ca2+ conductance (Shen et al., 2016; Su et al., 2018). Whether PKD1–2 cation channel is involved in mammalian PMI initiation is yet untested. Intriguingly, PKD1 mutations are associated with autosomal dominant polycystic kidney disease, a severe kidney disorder.
Although Ca2+ is necessary for PMI activation, its influx is not sufficient to induce this response. Ca2+ accumulation elicited by ionophores (ionomycin or A23187) can increase the intracellular level to the same extent as does activated MLKL (Gong et al., 2017a), whereas PMI responses were not observed upon treatment with this ionophore (Figure 5N). It is possible that other ions may also function for PMI signaling transduction. One possibility would be K+ efflux, which also accompanies PM damage and plays a role in PM-damage-induced NLRP3 activation (Muñoz-Planillo et al., 2013). Local lipid composition changes at the pore-forming sites may also contribute to the PMI responses by modulating PKC activation level.
Ca2+ regulates ESCRT-III-mediated cell repair and survival, as well as PMI signaling. In general, in the absence of Ca2+, cell death moved faster due to less repair activity, upon PM pore formation. We found similar effects in a longer time course, but during the short window we tested (usually 1~2 h), the cell survival difference with or without Ca2+ was not significant when we triggered the dimerization of the N terminus of MLKL or GSDMD. However, we could not find such time window that digitonin-induced cell death was similar in the absence or presence of Ca2+. Without Ca2+, digitonin-induced cell death was too fast and massive; thus, we did not test whether digitonin elicited PMI required Ca2+. Also, for PFN-induced pore forming, as PFN also requires Ca2+ for its activity (Thiery et al., 2010), we did not test the role of Ca2+ in PFN-induced PMI response, either.
This study also sheds light on the non-cell-death functions of the pore-forming proteins, such as MLKL. IRI kidneys and late-stage solid tumors can contain pMLKL+ cells that remain intact (Gong et al., 2017b). Meanwhile, these cells appear to produce chemokines and/or IEGs for growth and repair (Figure 7E). These data suggest that the possibility that one of the functions of MLKL, besides cellular killing, could be to activate the PMI signaling pathway and consequently modulate the immune microenvironments to promote repair.
Taken together, we provide evidence of the mammalian PMI response. Yeast CWI and mammalian PMI appear to be conserved signaling pathways with homology in several components. PMI has also evolved new functions to generate immune modulators. Thus, PM integrity loss is a cell-recognizable innate immune activation pattern in which S660 p-PKCs serve as a pattern recognition receptor.
Limitations of the study
One limitation for our study is that we did not find out how MAPK pathway during PM damage was initiated. The PM pore-forming sensors identified in this study, S660 p-cPKCs, were dispensable for MAPK controlling transcripts, such as egr1 and fos. Second, S660 p-cPKCs was not sufficient to activate chemokine or cytokine productions. Other unknown molecules must be involved in PM pore formation sensing and PMI signaling initiation, together with p-PKCs. This will be our future research direction.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
As Lead Contact, Yi-Nan Gong is responsible for all reagent and resource requests. Please contact Yi-Nan Gong (yngong@pitt.edu) with requests and inquiries.
Materials availability
All requests for non-commercial reagents (plasmids, cell lines, etc.) will be fulfilled under a completed MTA with University of Pittsburgh.
Data and code availability
The RNAseq data for PM damage-induced responses generated in this study were uploaded to GEO: GSE166538.
No original code was developed in this study.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell lines
NIH3T3, L929, MEF, RAW264.7 macrophages, HEK293T, Panc 3.27, BxPC3, HeLa, HCC2935 and their derivatives were maintained at 37°C, 5% v/v CO2 in a humidified incubator in DMEM (GIBCO) supplemented with 10% FBS, 2 mM L-glutamine (GIBCO), 200 U/mL penicillin-–streptomycin (GIBCO) and 50 μg/mL plasmocin (Invivogen). HT-29 cells were cultured using the same conditions except McCoy’s 5A media (GIBCO).
RIPK3–2Fv-NIH3T3 and RIPK3(ΔRHIM)-2Fv-NIH3T3 cells were previously described (Yatim et al., 2015). The hMLKL1−181-2Fv-NIH3T3, hMLKL1−181-2Fv-LLC, hMLKL1−181-2Fv-MEFMlkl−/−, mGSDMD1-276-HBD*-LLC and mGSDMD1-276-HBD*-NIH3T3 cell lines were generated by retroviral transduction and mCherry sorting. Briefly, HEK293T cells were transfected with the indicated plasmid and pCL-Ampho packaging plasmid by using Lipofectamine™ 2000 (Life Technologies) for 48–72 hr. Target cells were infected with filtered virus containing supernatants from packaging cells supplemented with 6.6 μg/mL polybrene. Infected cells were spun at 3,000 rpm for 90 min before put back to the incubator. Stable transductions were selected at 72 hr post infection by FACS. To induce sub-lethal PM damage, B/B, 4-OHT, Digitonin (all dissolved in DMSO), or PFN (gift from Dr. Judy Lieberman) were added to cell cultures at the concentrations indicated in the figure legends.
The MEF(Mlkl−/−) (with or without MLKL1−140-2xFv-Venus and CHMP4B-mCherry) and L929(Mlkl−/−) cell lines were described previously (Gong et al., 2017b). hMLKL1−181-2Fv-NIH3T3 (RelA−l−/−)) cells were generated by CRSPR/Cas9 plasmid pSpCas9(BB)-2A-GFP (PX458). Targeting guide sgRNAs were listed in Table S2. After transfection, GFP positive cells were collected by FACS and subjected to single cell culture. RelA deleted colonies were verified by western blot.
Mouse models
For sterile peritonitis model, ~3.5 million cells damaged by B/B were injected into C57BL/6J (JAX 000664) mice i.p. At 16 h after challenge, the numbers of neutrophils, macrophages, and dendritic cells were in the peritoneum were evaluated by flow cytometry. All animal experiments were performed under protocols approved by University of Pittsburgh Animal Care.
Human Stage IV cancer samples
The human samples were stored and processed at UPMC Hillman Cancer Center, The Institute for Precision Medicine and the Tissue and Research Pathology/Pitt Biospecimen Core shared resource. Once tissue was retrieved, each sample was placed in a 2×4 Bitran bag labeled with TP #, organ, laterality, and specimen status. If there was no further processing, each sample was then weighed and entered into BIOS. All 2×4 bags were then placed into a larger 3×6 Bitran bag labeled with the TP #. The 3×6 bag was then placed directly into the −80°C freezer per the specimen location documented in BIOS or the logbook.
METHOD DETAILS
Plasmids
NF-κB driven CFP was obtained from Addgene (118094). GSDMDN-ter-HBD* was PCR amplified from the constructs using pBOB backbone (from Jiahuai Han Laboratory, (Chen et al., 2016)) and inserted into pMSCV-IRES-mCherry backbone (Addgene, 52114). GCaMP3 was cloned into pLZRS vector. All sgRNAs used in this study was cloned into pSpCas9(BB)-2A-GFP (PX458). GFP tagged PKCγ-C1 domain (21190) and -C2 domain (21215), PKC dominant-negative (DN) forms (21235, 21239 and 16381) were obtained from Addgene. We used pMIG-II (52107, addgene) as the GFP control. For PKC-DN forms related experiments, cells were transfected (lipofectamine 2000) with GFP+empty vector control (pCDNA3.1) or GFP+PKC-DN (1:2). Then GFP+ cells were sorted by FACS at 24 hr post transfection for the following up PM damage and transcriptional analysis.
siRNA
All of the smart-pool siRNA were purchased from Dharmacon (Table S2). siRNA transfection was performed by Lipofectamin RNAiMax (Life technology) on NIH3T3 cells or INTERFERin on L929 cells (Polyplus). RNAi efficiency was assessed by realtime PCR at 60 hr post transfection or western blot at 72 hr post transfection.
Flow cytometric quantifications
All in vitro experiments assessed by flow cytometry included at least triplicate cultures for each condition. Cells were trypsinized, resuspended in serum-free DMEM and stained with Annexin V, Alexa Fluor™ 647 (1:1000, Thermo Fisher) and ~50 nM SytoxGreen (Thermo Fisher), for 3–5 min. For sterile peritonitis model, peritoneal wash (lavage) was collected. Cells were pelleted by centrifugation and subjected to FACS staining. To count the absolute number of immune infiltrations, counting beads (C36950, Thermo Fisher) were used as a reference.
Cells were analyzed by flow cytometry using BD Accuri™ C6 systems and LSR Fortessa systems (BD). The percentages of differently labeled cells were calculated by FlowJo software (Tree Star).
Real-time PCR
Total RNA for real-time PCR was extracted and purified using the Purelink RNA Mini Kit (Thermo Fisher). Reverse transcription reactions were performed with M-MLV reverse transcriptase (Life Technology), following the standard protocol using random hexamers (NEB). Real-time PCR was performed with PowerUp™ SYBR™ Green Master Mix labeling in 7500 Fast Real-Time PCR System (Thermo Fisher). PCR conditions were 50°C for 2 min, 95°C for 10 min, and 43 cycles of 95°C for 15 s and 60°C for 1 min. mRNA expression was normalized against β-actin, allowing comparison of mRNA levels. Primers used in this study are listed in Table S2.
Lionheart analysis
For Lionheart analysis, cells were first seeded into 6, 12, or 24-well plates for overnight incubation. Sometimes, 50 nM SytoxGreen (Thermo Fisher) plus the indicated PM damage stimuli were added, and the cells were moved into a Lionheart automatic live cell imaging system (https://www.biotek.com/products/imaging-microscopy-automated-cell-imagers/lionheart-fx/). Cells were imaged overtime and the SytoxGreen labeled, or CFP expressed cells (counted as dead cells or NF-κB activation, respectively) were quantified by the Gen5 software (https://www.biotek.com/products/software-robotics/).
When percentages are shown, total cell number was quantified at the end of each course of treatment, using 200 μg/ml digitonin (Sigma-Aldrich) to permeabilize all cells which were stained with 50 nM SytoxGreen. Data were then expressed as a percentage of SytoxGreen+ cells to total cell numbers. At least three replicates were performed with for each condition.
Lionheart microscopy
Cells were plated on 2-well or 4-well glass chamber slides (Thermo Fisher) coated with fibronectin (100 μg/mL in PBS, Millipore). Cells were maintained in complete media at 37°C and 5% CO2 in an environmentally controlled chamber. After PM damage, cells were fixed cells in 4% paraformaldehyde-PBS 10~20 min at room temperature, permeabilized with 0.3% Triton X-100 in 1X PBS, blocked in 3% BSA in 1X PBS, stained with primary antibodies for 2–4 hr (room temperature) to overnight at 4°C, followed by staining with 2° antibodies (1 hr) and DAPI stain. Samples were imaged using Lionheart microscope (Biotek).
Cytokine and chemokine detection
Supernatants from treated cells were collected. Cell debris was removed by centrifugation at 6,000 rpm for 1 min. Samples were diluted with the dilution buffer provided in each ELISA kit, including murine CXCL1 (MKC00B, R&D). All experiments were performed in triplicate or more.
Western blotting
Cells were lysed with loading buffer (50 mM Tris pH=6.8, 2% SDS and 10% glycerol) and denatured by boiling. Protein concentration was then determined by the BCA assay (Thermo Fisher) and systematically normalized before SDS-PAGE. Following the transfer of proteins to nitrocellulose (Thermo Fisher), immunodetection was performed using the indicated primary and peroxidase-coupled secondary antibodies. Proteins were visualized by enhanced chemiluminescence (ECL, Thermo Fisher).
Histology and immunostaining of cancer samples
Frozen sections (5 μm) were fixed with 4% paraformaldehyde-PBS 20 min at room temperature, permeabilized with 0.3% Triton X-100 in 1X PBS, blocked in 3% BSA (or 10% FBS) in 1X PBS, stained with primary antibodies for overnight at 4°C, followed by staining with 2° antibodies (1 hr) and DAPI stain. Samples were covered with coverglasses and imaged using Lionheart microscope (Biotek). S358 pMLKL monoclonal antibody (Abcam, EPR9514, 1:500) and human IL-8 (CXCL8) Monoclonal Antibody (3IL8-H10, Thermo Fisher, 1:1000) were used to perform the immunofluorescence staining. Samples were imaged using Lionheart microscope (Biotek).
Transwell chamber assay
The Transwell migration assays were performed in general protocol. Briefly, 24 hr before this assay, RAW264.7 cells were cultured in FBS-free DMEM for serum starvation. Next, RAW264.7 cells (0.8 × 106/well) were plated on the Transwell filters with 8 μm pores (Corning). On the bottom well, 600 μL of cell-free/FBS-free culture supernatants from either living or B/B treated (4 hr) hMLKL1−181-2Fv-NIH3T3 cells were added. 18 h later, filters were fixed and stained with 0.5% crystal violet in 25% methanol. Nonmigrating cells on the upper side of the filters were removed with a cotton swab, and cells on the underside of the filters were imaged by Lionheart. To quantify cell motility, cells stained positively with crystal violet in the middle of each filter were counted, and three independent filters were analyzed.
RNAseq
Total RNA was extracted from culture cells using Purelink RNA Mini Kit (Thermo Fisher) and submitted for library construction to The Health Sciences Sequencing Core at Children’s Hospital of Pittsburgh using Illumina Truseq with poly A selection. Libraries were sequenced on a NextSeq-500 at 1×75 read length. Reads were aligned to the mouse mm10 transcriptome or the human hg19 transcriptome (both refseq annotation) using Salmon-0.7.2. Reads were summed across isoforms to focus on gene level analysis. Samples were collected in technical duplicate, with the expectation of the si-PKC samples where one duplicate failed during library construction. There was excellent agreement between all duplicates (R=>0.99), which we therefore averaged for subsequent analysis. Genes with >0.1 TPM in all samples from a particular comparison were kept for subsequent analysis, for comparisons between cell lines we used only those genes that passed this threshold in both lines. We computed fold change between each control sample and its treated equivalent and identified genes that showed a greater than 2–4 fold increase in expression (as noted in each figure).
Gene set enrichments among upregulated genes (overlapping dataset) were calculated using the Molecular-Signatures-Database/GSEA using the Hallmark gene sets using the fgsea package using the “fora” function. Enrichments were also calculated using the DAVID web interface and KEGG, UP and Reactome annotations using default settings.
The NIH3T3 TNF treated dataset was downloaded from SRA/GEO (Accession GSM3940136, samples: SRR9677614–7). The raw fastq files were analyzed with Salmon as described above, duplicate samples were averaged.
IRI mouse treatment, microarray and RNAseq analysis
Microarray data was downloaded as a tab delimited datafile from GEO: GSE52004. Values for individual genes were recovered and compared before and after treatment for the indicated genes. P-values were calculated using a two-sided t-test with a BH-correction for multiple hypothesis testing.
For the equivalent analysis of RNAseq data from IRI treatment mice (GEO: GSE98622), the FPKM values were extracted from Table S1 for gene clusters I, II, and III (the acute response clusters. From this data we computed the average fold change for each gene and displayed these values as a heatmap. The gene list was then aligned by gene symbol to our NIH3T3 RNAseq datasets and fold changes for each gene in joint datasets for groups I,II,II were displayed. Genes that appeared in both datasets were compared and overlap between genes we note as upregulated under different treatment conditions that were also in groups I or II were identified, p value of overlap was computed using a hypergeometric test.
Protein-protein interaction analysis
A protein-protein interaction analysis was run using the integrated MIPPIE database and the MIPPIE server (http://cbdm-01.zdv.unimainz.de/~galanisl/mippie/network.php). This analysis was run with default parameters using only experimental data for “physical association”.
snRNAseq analysis
Single cell RNAseq data (collected on the 10x platform from Nuclei) was downloaded from GEO: GSE139107, gene read counts (as computed by study authors) were downloaded for the IRIsham (datasets: IRIsham1b1, IRIsham1b2, IRIsham2, IRIsham3) and 4hr-post-IRI conditions (IRI4h1, IRI4h2, IRI4h3), cells with 150–8000 genes detected were accepted for subsequent analysis. Data were loaded in Seurat-V4 and the seven samples were normalized, scaled, and integrated (IntegrateData function) using default Seurat settings. We then visualized the dataset using the UMAP (PCA reduction, dimensions 1–30) on the integrated dataset. We then split the data into sham and treated groups and used FeaturePlot to display the UMAPed data with Cxcl1 or Lrp2.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical tests were done using Microsoft Excel and Prism. The statistical test used p values that were indicated within each figure. Generally, for comparison between two individual groups, standard student t test was used. For comparison of more than two groups, standard one-way ANOVA was used. The number of repeats of each experiment was indicated within each figure as well. Shown in each group indicated replicates of independent wells from one or several multiple well plate(s). Replicates representing the key experiments performed in a different batch(s) were summarized in Table S3. Data are presented as means ± SD or SEM (indicated within each figure). p values no more than 0.05 were considered statistically significant.
No methods were applied to determine whether the data met assumptions of the statistical approach used. Normal distributions were assumed. No strategy for randomization and/or stratification method was applied in this study.
Supplementary Material
Key resources table.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Actin | Santa Cruz | sc-1616 |
| ASC | Cell Signaling | 67824S |
| CXCL8 | Thermo Fisher | M801 |
| Erk1/2 | Cell Signaling | 4695 |
| MEK1/2 | Cell Signaling | 4694 |
| NLRP3 | Cell Signaling | 15101S |
| P65 | Cell Signaling | 8242 |
| pErk1/2 | Cell Signaling | 4370 |
| pMEK1/2 | Cell Signaling | 3958 |
| pMLKL (S358) | Abcam | ab187091 |
| pP65 | Cell Signaling | 3033 |
| pP90 | Cell Signaling | 11989 |
| pPKC (S660, pan) | Cell Signaling | 9371 |
| pSRF | Cell Signaling | 4261 |
| RIPK3 | Cell Signaling | 95702 |
| SRF | Cell Signaling | 5147 |
| Alexa Fluor 700 anti-mouse CD11c | Thermo Fisher | 56–0114-82 |
| APC anti-mouse CD163 | Thermo Fisher | 17–1631-82 |
| Brilliant Violet 421 anti-mouse Ly-6G | BioLegend | 127627 |
| Brilliant Violet 510 anti-mouse Ly-6C | BioLegend | 128033 |
| Brilliant Violet 650 anti-mouse CD45 | BioLegend | 103151 |
| Brilliant Violet 711 anti-mouse CD206 | BioLegend | 141727 |
| Brilliant Violet 785 anti-mouse/human CD11b | BioLegend | 101243 |
| FITC anti-mouse F4/80 | Thermo Fisher | 11–4801-82 |
| PE anti-mouse CD103 | Thermo Fisher | 12–1031-82 |
| PE/Cyanine7 anti-mouse CD80 | BioLegend | 104734 |
| PerCP-eFluor 710 anti-mouse MHC Class II (I-A/I-E) | Thermo Fisher | 46–5321-80 |
| Biological Samples | ||
| Human stage IV cancer samples | This paper | Institute for Precision Medicine and the Pitt Biospecimen Core |
| Organisms/Strains | ||
| C57BL/6J | JAX | 000664 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| 4-OHT | Sigma | H6278 |
| 5Z-7-Oxozeaenol | Caymanchem | 253863–19-3 |
| Annexin V-Alexa Fluor-647 | Thermo Fisher | A23204 |
| B/B homodimerizer | Clontech | 635059 |
| BAPTA-AM | Tocris | 2787 |
| Bisindolylmaleimide I | Caymanchem | 13298 |
| BMS345541 | Caymanchem | 16667 |
| CAPE | Calbiochem | 211200 |
| Cycloheximide | Sigma | C-7698 |
| Digitonin | TCI America | TCD0540 |
| Go6976 | Caymanchem | 13310 |
| Go6983 | Caymanchem | 13311 |
| ionomycin | Calbiochem | 11932 |
| Lcl-161 | Medchemexpress | HY-15518 |
| Lipofectamine 2000 | Invitrogen | 11668019 |
| Lipofectamine RNAiMAX | Invitrogen | 13778075 |
| MG132 | Caymanchem | 10012628 |
| mTNF | Peprotech | 315–01A |
| PD 0325901 | Caymanchem | 13034 |
| Phorbol 12-myristate 13-acetate | Sigma | 79346 |
| Recombinant Mouse CXCL1/GRO alpha/KC/CINC-1 Protein | Novusbio | NBP2–35153 |
| Recombinant Mouse LIF Protein | Novusbio | NBP2–35147 |
| SytoxGreen | Invitrogen | S7020 |
| TNFR1-Fc | ENZO | ALX-522–013-C050 |
| TPCA-1 | Caymanchem | 15115 |
| Trametinib | Caymanchem | 16292 |
| YM-58483 | Selleckchem | YM-58483 |
| zVAD-fmk | APExBIO | A1902 |
| Critical Commercial Assays | ||
| CellTiter-Glo™ Luminescent Cell Viability Assay Kit | Promega | G7572 |
| Mouse CXCL1/KC DuoSet ELISA | R&D systems | DY453–05 |
| Mouse CXCL1/KC Quantikine ELISA Kit | R&D systems | MKC00B |
| Zombie NIR Fixable Viability Kit | BioLegend | 423105 |
| Deposited Data | ||
| RNA seq data for PM damaged cells | This study | GEO: GSE166538 |
| Human kidney biopsies RNA-seq | (Gong et al., 2017b) | https://www.obvibase.com/#token/cHIuv8Hv4Cdb/r/hka6j0CQmzwp |
| Mouse kidney biopsies microarray | (Liu et al., 2014) | GEO: GSE52004 |
| mRNA array data for B/B induced hMLKL1–181-2Fv-NIH 3T3 cells | (Gong et al., 2017b) | GEO: GSE85660 |
| Mouse kidney biopsies RNAseq | (Liu et al., 2017) | GEO: GSE98622 |
| Mouse kidney biopsies single nuclear RNAseq | (Kirita et al., 2020) | GEO: GSE139107 |
| TNF induced transcriptome change in NIH3T3 cells | (Orozco et al., 2019) | GEO: GSE134234 |
| Experimental Models: Cell Lines | ||
| RIPK3–2Fv-NIH3T3 | (Yatim et al., 2015) | N/A |
| RIPK3(ΔRHIM)-2Fv-NIH3T3 | (Yatim et al., 2015) | N/A |
| hMLKL1–140-2Fv-Venus-MEFMlkl−/−-CHMP4B-mCherry | (Gong et al., 2017b) | N/A |
| hMLKL1–181-2Fv-NIH3T3 | (Gong et al., 2017b) | N/A |
| hMLKL1–181-2Fv-LLC | This study | N/A |
| hMLKL1–181-2Fv-MEFMlkl-/- | This study | N/A |
| L929Mlkl-/- | (Gong et al., 2017b) | N/A |
| GSDMDNter-HBD*-NIH3T3 | This study | N/A |
| GSDMDNter-HBD*-LLC | This study | N/A |
| GCaMP3- hMLKL1–181-2Fv-NIH3T3 | (Gong et al., 2017b) | N/A |
| Raw264.7-ASC | This study | N/A |
| HT-29 Mlkl-/- | (Gong et al., 2017b) | N/A |
| Oligonucleotides | ||
| siRNAs, sgRNAs and Primers were list in Table S2. | ||
| Recombinant DNA | ||
| NF-κB driven CFP | Addgene | 118094 |
| hMLKL1–181-2Fv | (Gong et al., 2017b) | |
| GSDMDNter-HBD* | This study | |
| STIM1-CFP | Addgene | 19755 |
| pSpCas9(BB)-2A-GFP (PX458) | Addgene | 48138 |
| GFP-pcDNA3-PKCgamma-cys1Acys1B | Addgene | 21190 |
| GFP-C2gamma | Addgene | 21215 |
| PKC alpha DN | Addgene | 21235 |
| PKC beta I DN | Addgene | 16381 |
| PKC gamma DN | Addgene | 21239 |
| Software and Algorithms | ||
| Gen5 | BioTek | https://www.biotek.com/products/software-robotics-software/gen5-microplate-reader-and-imager-software/ |
| FlowJo | FlowJo LLC | https://www.flowjo.com/ |
| NIS-Elements | Nikon | https://www.microscope.healthcare.nikon.com/products/software/nis-elements/nis-elements-advanced-research |
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| Salmon-0.7.2 | Academic, Kingsford Lab | Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14(4):417–419. doi:10.1038/nmeth.4197 https://salmon.readthedocs.io/en/latest/salmon.html |
| Seurat-V4 | Academic, Satija Lab | https://satijalab.org/seurat/ |
| R-4.0.3 | The R Foundation for Statistical Computing | https://www.r-project.org/foundation/ |
| Matlab-2019B | mathworks | https://www.mathworks.com/ |
| Molecular-Signatures-Database/GSEA | Broad/UCSC | http://www.gsea-msigdb.org/gsea/msigdb/index.jsp#citing_msigdb |
| GSEA Implemented in R as fgsea package | Sergushichev lab | https://bioconductor.org/packages/release/bioc/html/fgsea.html |
Highlights.
Sub-lethal plasma membrane pore-forming damage induces chemokine production
S660 p-PKCs sense the pore-forming damage by detecting the local Ca2+ influx
Plasma-membrane-damage-induced chemokine production occurs in pathological conditions
This pathway works in parallel to the yeast cell wall integrity pathway
ACKNOWLEDGMENTS
This study was generously supported by start-up funding from Hillman Cancer Center (Y.-N.G. and J.S.-O.) and in part by awards number P30CA04790, R21CA259457, and DP2GM146320 from the National Institutes of Health (Y.-N.G.) and NCI grant R00CA207727 (J.S.-O.). The human tumor samples were supported by the Institute for Precision Medicine and the Pitt Biospecimen Core. We thank John Rich Chaillet (Pitt) and Douglas R. Green (St. Jude) for invaluable comments and editing. We thank Zheng-Gang Liu’s group (NCI) for generous experimental support, Judy Lieberman and Zhibin Zhang (Boston Children’s Hospital) for providing perforin, and Liming Sun (CAS), Susu Duan (Sanofi) and Zhen Li (Ghent University) for the help on data visualization and helpful discussion.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.devcel.2021.12.015.
REFERENCES
- Alanis-Lobato G, Möllmann JS, Schaefer MH, and Andrade-Navarro MA (2020). MIPPIE: the mouse integrated protein-protein interaction reference. Database (Oxford) 2020. 10.1093/database/baaa035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews NW, and Corrotte M (2018). Plasma membrane repair. Curr. Biol. 28, R392–R397. 10.1016/j.cub.2017.12.034. [DOI] [PubMed] [Google Scholar]
- Chen GY, and Nuñez G (2010). Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837. 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Fang Y, Wu J, Chen H, Zou Z, Zhang X, Shao J, and Xu Y (2018). RIPK3-MLKL-mediated necroinflammation contributes to AKI progression to CKD. Cell Death Dis 9, 878. 10.1038/s41419-018-0936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, He W-T, Hu L, Li J, Fang Y, Wang X, Xu X, Wang Z, Huang K, and Han J (2016). Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res 26, 1007–1020. 10.1038/cr.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codazzi F, Teruel MN, and Meyer T (2001). Control of astrocyte Ca(2+) oscillations and waves by oscillating translocation and activation of protein kinase C. Curr. Biol. 11, 1089–1097. 10.1016/s0960-9822(01)00326-8. [DOI] [PubMed] [Google Scholar]
- Gong Y-N, Guy C, Crawford JC, and Green DR (2017a). Biological events and molecular signaling following MLKL activation during necroptosis. Cell Cycle 16, 1748–1760. 10.1080/15384101.2017.1371889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y-N, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, Linkermann A, and Green DR (2017b). ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell 169, 286–300.e16. 10.1016/j.cell.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR (2019). The coming decade of cell death research: five riddles. Cell 177, 1094–1107. 10.1016/j.cell.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G-W, Günther C, Thonn V, Yu Y-Q, Martini E, Buchen B, Neurath MF, Stürzl M, and Becker C (2017). Regression of apoptosis-resistant colorectal tumors by induction of necroptosis in mice. J. Exp. Med. 214, 1655–1662. 10.1084/jem.20160442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PG, and Rao A (2015). Store-operated calcium entry: mechanisms and modulation. Biochem. Biophys. Res. Commun. 460, 40–49. 10.1016/j.bbrc.2015.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao D, Cai Z, Choksi S, Ma D, Choe M, Kwon H-J, Baik JY, Rowan BG, Liu C, and Liu Z-G (2018). Necroptosis of tumor cells leads to tumor necrosis and promotes tumor metastasis. Cell Res 28, 868–870. 10.1038/s41422-018-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, and Perez F (2014). ESCRT machinery is required for plasma membrane repair. Science 343, 1247136. 10.1126/science.1247136. [DOI] [PubMed] [Google Scholar]
- Jutz S, Leitner J, Schmetterer K, Doel-Perez I, Majdic O, Grabmeier-Pfistershammer K, Paster W, Huppa JB, and Steinberger P (2016). Assessment of costimulation and coinhibition in a triple parameter T cell reporter line: simultaneous measurement of NF-κB, NFAT and AP-1. J. Immunol. Methods 430, 10–20. 10.1016/j.jim.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Kornfeld OS, Lee BL, Stowe IB, O’Rourke K, Li Q, Sandoval W, Yan D, Kang J, Xu M, et al. (2021). NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136. 10.1038/s41586-021-03218-7. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. (2015). Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671. 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- Keranen LM, Dutil EM, and Newton AC (1995). Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr. Biol. 5, 1394–1403. 10.1016/s0960-9822(95)00277-6. [DOI] [PubMed] [Google Scholar]
- Kirita Y, Wu H, Uchimura K, Wilson PC, and Humphreys BD (2020). Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc. Natl. Acad. Sci. USA 117, 15874–15883. 10.1073/pnas.2005477117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout SC, Corbalán-García S, Torrecillas A, Goméz-Fernandéz JC, and Falke JJ (2002). C2 domains of protein kinase C isoforms alpha, beta, and gamma: activation parameters and calcium stoichiometries of the membrane-bound state. Biochemistry 41, 11411–11424. 10.1021/bi026041k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo G-B, Morgan MJ, Lee D-G, Kim W-J, Yoon J-H, Koo JS, Kim SI, Kim SJ, Son MK, Hong SS, et al. (2015). Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res 25, 707–725. 10.1038/cr.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-M, Vasishtha M, and Prywes R (2010). Activation and repression of cellular immediate early genes by serum response factor cofactors. J. Biol. Chem. 285, 22036–22049. 10.1074/jbc.M110.108878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE (2005). Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69, 262–291. 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Deng M, Loughran PA, Yang M, Lin M, Yang C, Gao W, Jin S, Li S, Cai J, et al. (2020). LPS induces active HMGB1 release From hepatocytes Into exosomes Through the coordinated activities of TLR4 and caspase-11/GSDMD signaling. Front. Immunol. 11, 229. 10.3389/fimmu.2020.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J, Wu H, and Kagan JC (2019). Gasdermin D activity in inflammation and host defense. Sci. Immunol. 4. 10.1126/sciimmunol.aav1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienbaum A, and Israël A (2003). From calcium to NF-kappa B signaling pathways in neurons. Mol. Cell. Biol. 23, 2680–2698. 10.1128/MCB.23.8.2680-2698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Krautzberger AM, Sui SH, Hofmann OM, Chen Y, Baetscher M, Grgic I, Kumar S, Humphreys BD, Hide WA, and McMahon AP (2014). Cell-specific translational profiling in acute kidney injury. J. Clin. Invest. 124, 1242–1254. 10.1172/JCI72126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Kumar S, Dolzhenko E, Alvarado GF, Guo J, Lu C, Chen Y, Li M, Dessing MC, Parvez RK, et al. (2017). Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight 2. 10.1172/jci.insight.94716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M, Moats T, and Villiger PM (1992). Leukemia inhibitory factor is expressed in cartilage and synovium and can contribute to the pathogenesis of arthritis. J. Clin. Invest. 90, 888–896. 10.1172/JCI115964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, and Núñez G (2013). K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153. 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai S, Yamaguchi Y, Shirasaki Y, Yamagishi M, Shindo R, Hildebrand JM, Miura R, Nakabayashi O, Totsuka M, Tomida T, et al. (2018). A FRET biosensor for necroptosis uncovers two different modes of the release of DAMPs. Nat. Commun. 9, 4457. 10.1038/s41467-018-06985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafov A, Zervantonakis IK, Mookhtiar AK, Greninger P, March RJ, Egan RK, Luu HS, Stover DG, Matulonis UA, Benes CH, and Yuan J (2018). BRAF and AXL oncogenes drive RIPK3 expression loss in cancer. PLoS Biol 16, e2005756. 10.1371/journal.pbio.2005756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea E, Teruel MN, Quest AF, and Meyer T (1998). Green fluorescent protein (GFP)-tagged cysteine-rich domains from protein kinase C as fluorescent indicators for diacylglycerol signaling in living cells. J. Cell Biol. 140, 485–498. 10.1083/jcb.140.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco SL, Daniels BP, Yatim N, Messmer MN, Quarato G, Chen-Harris H, Cullen SP, Snyder AG, Ralli-Jain P, Frase S, et al. (2019). RIPK3 activation leads to cytokine synthesis that continues after loss of cell membrane integrity. Cell Rep 28, 2275–2287.e5. 10.1016/j.celrep.2019.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J, Xia S, Liu X, Lieberman J, and Wu H (2018). Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557, 62–67. 10.1038/s41586-018-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, and Broz P (2018). ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960. 10.1126/science.aar7607. [DOI] [PubMed] [Google Scholar]
- Samson AL, Zhang Y, Geoghegan ND, Gavin XJ, Davies KA, Mlodzianoski MJ, Whitehead LW, Frank D, Garnish SE, Fitzgibbon C, et al. (2020). MLKL trafficking and accumulation at the plasma membrane control the kinetics and threshold for necroptosis. Nat. Commun. 11, 3151. 10.1038/s41467-020-16887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer LL, Sreetama SC, Sharma N, Medikayala S, Brown KJ, Defour A, and Jaiswal JK (2014). Mechanism of Ca2+-triggered ESCRT assembly and regulation of cell membrane repair. Nat. Commun. 5, 5646. 10.1038/ncomms6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen PS, Yang X, DeCaen PG, Liu X, Bulkley D, Clapham DE, and Cao E (2016). The structure of the polycystic kidney disease channel PKD2 in lipid nanodiscs. Cell 167, 763–773.e11. 10.1016/j.cell.2016.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, and Shao F (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- Slowikowski K, Nguyen HN, Noss EH, Simmons DP, Mizoguchi F, Watts GFM, Gurish MF, Brenner MB, and Raychaudhuri S (2020). CUX1 and IκBζ (NFKBIZ) mediate the synergistic inflammatory response to TNF and IL-17A in stromal fibroblasts. Proc. Natl. Acad. Sci. USA 117, 5532–5541. 10.1073/pnas.1912702117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits GJ, Kapteyn JC, van den Ende H, and Klis FM (1999). Cell wall dynamics in yeast. Curr. Opin. Microbiol. 2, 348–352. 10.1016/s1369-5274(99)80061-7. [DOI] [PubMed] [Google Scholar]
- Soh J-W, and Weinstein IB (2003). Roles of specific isoforms of protein kinase C in the transcriptional control of cyclin D1 and related genes. J. Biol. Chem. 278, 34709–34716. 10.1074/jbc.M302016200. [DOI] [PubMed] [Google Scholar]
- Su Q, Hu F, Ge X, Lei J, Yu S, Wang T, Zhou Q, Mei C, and Shi Y (2018). Structure of the human PKD1-PKD2 complex. Science 361. 10.1126/science.aat9819. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, and Mesirov JP (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550. 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, and Wang X (2012). Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227. 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Thiery J, Walch M, Jensen DK, Martinvalet D, and Lieberman J (2010). Isolation of cytotoxic T cell and NK granules and purification of their effector proteins. Curr. Protoc. Cell Biol. Chapter 3, Unit3.37. 10.1002/0471143030.cb0337s47. [DOI] [PubMed] [Google Scholar]
- Vickers ER, Kasza A, Kurnaz IA, Seifert A, Zeef LA, O’Donnell A, Hayes A, and Sharrocks AD (2004). Ternary complex factor-serum response factor complex-regulated gene activity is required for cellular proliferation and inhibition of apoptotic cell death. Mol. Cell. Biol. 24, 10340–10351. 10.1128/MCB.24.23.10340-10351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sun L, Su L, Rizo J, Liu L, Wang L-F, Wang F-S, and Wang X (2014). Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 54, 133–146. 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Wu Y-T, Tan H-L, Huang Q, Sun X-J, Zhu X, and Shen H-M (2011). zVAD-induced necroptosis in L929 cells depends on autocrine production of TNFα mediated by the PKC-MAPKs-AP-1 pathway. Cell Death Differ 18, 26–37. 10.1038/cdd.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C, Green DR, Oberst A, and Albert ML (2015). RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8+ T cells. Science 350, 328–334. 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNAseq data for PM damage-induced responses generated in this study were uploaded to GEO: GSE166538.
No original code was developed in this study.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.