Abstract
Objective:
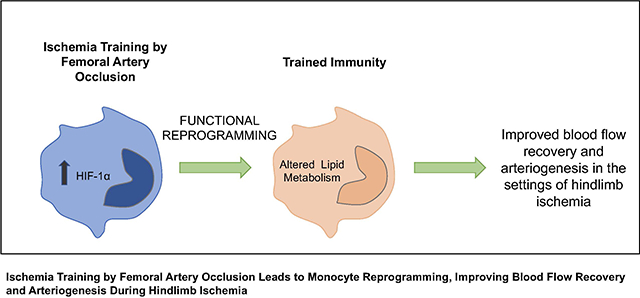
Monocytes, which play an important role in arteriogenesis, can build immunological memory by a functional reprogramming that modifies their response to a second challenge. This process, called “trained immunity,” is evoked by insults that shift monocyte metabolism, increasing hypoxia-inducible factor (HIF)-1α levels. Since ischemia enhances HIF-1α, we evaluate whether ischemia can lead to a functional reprogramming of monocytes, which would contribute to arteriogenesis after hindlimb ischemia.
Methods and Results:
Mice exposed to ischemia by 24h of femoral artery (FA) occlusion (24h trained) or sham were subjected to hindlimb ischemia one week later; the 24h trained mice showed significant improvement in blood flow recovery and arteriogenesis after hindlimb ischemia. Adoptive transfer using bone marrow-derived monocytes (BM-Mono) from 24h trained or sham donor mice, demonstrated that recipients subjected to hindlimb ischemia who received 24h ischemic-trained monocytes had remarkable blood flow recovery and arteriogenesis. Further, ischemic-trained BM-Mono had increased HIF-1α and GLUT-1 gene expression during FA occlusion. Circulating cytokines and GLUT-1 were also up-regulated during FA occlusion.Transcriptomic analysis and confirmatory qPCR performed in 24h trained and sham BM-Mono revealed that among the 15 top differentially expressed genes, four were involved in lipid metabolism in the ischemic-trained monocytes. Lipidomic analysis confirmed that ischemia training altered the cholesterol metabolism of these monocytes. Further, several histone-modifying epigenetic enzymes measured by qPCR were altered in mouse BM-Mono exposed to 24h hypoxia.
Conclusion:
Ischemia training in BM-Mono leads to a unique gene profile and improves blood flow and arteriogenesis after hindlimb ischemia.
Keywords: trained immunity, arteriogenesis, hindlimb ischemia, ischemia training, monocytes, Cellular Reprogramming, Ischemia, Vascular Biology, Epigenetics, Treatment, Peripheral Vascular Disease
Graphical Abstract
INTRODUCTION
Critical limb ischemia (CLI) is the advanced manifestation of peripheral arterial disease (PAD) and an underlying cause of ischemic rest pain, gangrene, and amputation1. The inflammatory process caused by limb ischemia is triggered by monocytes/macrophages and endothelial cells that release growth factors and cytokines as a physiological attempt to enhance neovascularization2,3. Monocytes are among the first cells to respond to ischemic muscle injury, infiltrating the tissue and differentiating into inflammatory and anti-inflammatory macrophages. In arteriogenesis, bone marrow-derived monocytes (BM-Mono) home to the collaterals area and act through paracrine effects, producing a large variety of metalloproteinases, vasoactive substances, chemokines, and growth factors, which facilitate the remodeling of collaterals2,4,5. However, often PAD/CLI patients do not develop enough limb neovascularization to achieve full recovery of the limb; to help those patients, we need new therapeutic approaches to alleviate this disease and its devastating consequences.
Recent observations have demonstrated that the innate immune response (including monocytes), thought to be rapid, non-specific, and unable to build immunological memory, can actually exert adaptive characteristics and build immunological memory6. This process, called “trained immunity”, is a long-term functional/epigenetic reprograming of the monocytes which is induced by endogenous or exogenous insults, modifying their response to the next challenge, even though they had already returned to a non-activated state6. Trained immunity is driven by a metabolism shift to glycolysis, and hypoxia inducible factor (HIF)-1α is one of the major contributors to this process6,7. It has been shown that HIF-1α inhibitors block monocytes from achieving trained immunity, and the induction of trained immunity by β-glucan is completely abolished in HIF-1α knockout mice. Recent reports have shown that in several cell types, HIF-1α can serve as both a direct and indirect regulator of several histone enzymes8–10.
Since the hypoxia/ischemia environment triggers the up-regulation of HIF-1α by blocking its ubiquitination/degradation process11–13, the goal of this study is to evaluate whether “ischemia training” can serve as a stimulus to improve monocyte response to neovascularization during the hindlimb ischemia process. We demonstrate that a single ischemia training insult created by unilateral femoral artery (FA) occlusion can be transmitted to BM-Mono, and these cells contribute to enhancing blood flow recovery as well as arteriogenesis in a mouse model of hindlimb ischemia. Moreover, BM-Mono present a unique gene expression profile with altered lipid metabolism genes. Further, our findings also show that hypoxia alters mouse BM-Mono’ histone-modifying epigenetic enzymes when the HIF-1α-target gene GLUT-1 is up-regulated.
METHODS
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals
All animal experiments were approved by the University of Miami Miller School of Medicine Institutional Animal Care and Use Committee and followed NIH guidelines. C57Bl6J male and female mice with an average age of 3 months were obtained from the Jackson Laboratory and housed in our animal facility.
Ischemia Training
Mice anesthetized with ketamine (100mg/kg) and xylazine (10mg/kg) solution were subjected to operative intervention to create a single insult of ischemia in unilateral limb. Exposure of the FA from left leg was done by performing an incision in the skin overlying the middle portion of the hindlimb and carefully dissecting the FA from the femoral vein and nerve. The FA was occluded distally at the level of the inguinal ligament and proximally at the level of the popliteal bifurcation. The 24h trained group was done by FA occlusion for 24 hours. Immediately post ligation, Laser Doppler Imaging (LDI) was performed to confirm the ischemia. In the next day, before opening the FA ligation, LDI was performed to confirm the maintenance of the ischemia, and after opening the FA, the LDI was performed again to confirm recovery of blood flow. In the sham group, the mice underwent the same procedure, but without FA occlusion.
Permanent Hindlimb Ischemia
FA occlusion was performed as described. Mice were anesthetized with ketamine and xylazine and the hindlimbs depilated. The left femoral artery was exposed through a 2mm incision without retraction and with minimal tissue disturbance. A ligature was placed distal to the origin of the lateral caudal femoral and superficial epigastric arteries and proximal to the genu artery (below the inguinal ligament). The femoral artery was transected between the sutures and separated 1–2 mm. The wound was irrigated with saline, closed, and one dose of analgesia of buprenorphine slow release was administered14.
Laser Doppler Imaging (LDI)
A LDI system (MoorLDI2-HR) was used to record serial blood flow measurements during the ischemia training as described above (ischemia training section) and during permanent hindlimb ischemia preoperatively, immediately post-operatively, and 3, 7, and 14 days after hindlimb ischemia surgery. Excessive hair was removed from the limb before imaging, and the mice were placed on a heating pad at 37 °C to minimize temperature variation. Blood flow was measured and quantified in the foot of the animals, and the ratio between ischemic and non-ischemic legs was performed to avoid variation of body temperature between the mice14.
Monocyte Adoptive Transfer
Monocytes were isolated from C57Bl6J` donor mice subjected or not to ischemia training (24h trained or sham). Two days or 7 days after the ischemia training was terminated, donor mice were sacrificed and the BM-Mono were isolated. For the adoptive transfer experiments, monocytes were isolated only from the bone marrow (BM) of the ischemic leg (left leg) or from both legs. Monocytes isolation was performed using the Monocytes Isolation Kit from Miltenyibiotec MACS (cat. no. 130–100-629) and the manufacturer’s instructions were followed. Recipient mice were C57Bl6J, subjected to permanent hindlimb ischemia one day prior to receiving 1×106 monocytes via tail vein injection. For the Lin−cells’ adoptive transfer, the remaining BM mononuclear cells in the Monocytes Isolation Kit, which were all mononuclear cells except for the monocytes, were injected using the same protocol mentioned above. Of note, the adoptive transfer was performed using a pool of male and female mice since sex separation would compromise the feasibility of the experiment due to the small number of cells per animal.
Immunohistochemistry (IHC)
Paraffin sections (5μ thick) of the thigh were stained with smooth muscle actin (SMA), a marker of smooth muscle cells, to obtain lumen diameter, at the same level, of the anterior and posterior gracilis muscle collaterals. The diameter of the collaterals was measured to quantify arteriogenesis. Briefly, after deparaffinization, antigens were retrieved from rehydrated sections using a Sodium citrate buffer (for SMA) for 20 min at 95oC before treatment with 3% hydrogen peroxide and TNB blocking solution (cat. no. FP1020; Perkin-Elmer, Waltham, MA). Slides were then incubated with Smooth Muscle Actin (SMA) antibody (Dako) overnight at 4οC. The next day, slides were incubated with anti-mouse biotinylated secondary antibody for 1h, followed by streptavidin/HRP for 30 minutes. Detection was performed using DAB kit, and slides were prepared by hematoxylin incubation and mounting media with coverslip.
BM-Mono qRT-PCR
To evaluate if the ischemia caused by FA occlusion can be transmitted to the BM-Mono, these cells were isolated from sham, 24h trained (FA was opened and blood flow restored for 2 days), and 24h ischemia groups (monocytes were isolated after 24h of FA occlusion when the FA still occluded). After BM-Mono isolation, using the same procedure mentioned above, total RNA was extracted using E.Z.N.A Total RNA Kit from Omega Bio-tek (cat. no. R6834–02), and equal amounts of RNA were reverse transcribed using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Gene expression of HIF-1α and its target GLUT-1 were measured by qRT-PCR, running in 20μL reactions in a 7300 Real-time PCR system machine using TaqMan Gene Expression master mix (Applied Biosystems), according to the manufacturer’s instructions. Data was expressed as relative fold change over the sham samples.
Mouse BM-Mono culture and qRT-PCR
After BM-Mono isolation using the same procedure mentioned above, these cells were cultured in RPMI-1640 medium, supplemented with 0.05mM of 2-mercaptoethanol and 10% fetal bovine serum. For our experiment, cells were cultured in normoxia (air, 95%; CO2, 5%) or hypoxia (O2, 1%; CO2, 5%) in the hypoxia chamber for 24h. After 24h, those in normoxia remained there for 4 additional days, while those in hypoxia were placed back under normoxia conditions for 4 days. Therefore, in this experiment we have the following groups: 24h normoxia, 24h hypoxia, 5d normoxia, and 4d after 24h hypoxia. Total RNA extraction and cDNA from these BM-Mono were performed as mentioned above. Gene expression of histone-modifying epigenetic enzymes (HDAC1, HDAC7, KDM1A, KDM3A, KDM3B, KDM4B, KDM4C) as well as GLUT-1 (to assure that the hypoxia chamber was leading to up-regulation of HIF-1α target gene) was measured by qPCR as mentioned above. Data was expressed as relative fold change over the 24h normoxia.
RNA Sequencing
Preparation and sequencing of RNA libraries was carried out in the John P. Hussman Institute for Human Genomics, Center for Genome Technology. Briefly, total RNA from 24h trained and sham BM-Mono (left and right legs) was quantified and qualified using the Agilent Bioanalyzer to have an RNA integrity score > 5. Then 200ng of total RNA was used as input for the NuGEN Universal Plus mRNA-Seq kit per the manufacturer’s instructions to create polyA-selected RNA sequencing libraries. Each sample was sequenced to more than 30 million raw single end reads on the Illumina NovaSeq 6000.
Differential Gene Expression Analysis
Sequencing data were processed with a bioinformatics pipeline including quality control, alignment to the mm10 mouse reference genome with STAR aligner v2.5.2a15, and gene quantification against the GENCODE vM25 annotation gene set. Count data was input into edgeR software16 for differential expression analysis. Counts were normalized using the trimmed mean of M-values method to account for compositional difference between the libraries17. Differential expression analysis between groups was performed using the exact test implemented in edgeR. Genes were considered to be statistically different with a false discovery rate p-value (FDR) ≤ 0.05 determined by applying the Benjamini-Hochberg multiplicity correction method.
Confirmatory qRT-PCR for Lipid Metabolism Genes
RNA isolation was performed as mentioned above from 24h trained and sham BM-Mono (left and right legs). Gene expression of Apolipoprotein E (ApoE), Lpin1, Squalene monooxygenase (SQLE), and 24-Dehydrocholesterol Reductase (Dhcr24) were measured by qRT-PCR, to confirm the RNA-seq data. Data was expressed as relative fold change over the sham samples.
Data Analysis
Statistical calculations were performed using GraphPad Prism version 9.0.1 (San Diego, CA). All values are expressed as mean ± standard error of mean (SEM) or median (95% CI). The data were tested for normality using either the Shapiro-Wilk test or QQ plot for samples that were too small. Mann-Whitney test was performed to compare two groups with sample size equal or less than 5 (Fig. 5 and IV and VI).The significance of LDI and arterogenesis data was determined using two-way analysis of variance (2-way ANOVA) with Šídák multiple comparisons (Fig. 1,2 and III). The Kruskal-Wallis test with Dunn’s posttest was performed for the qPCR data in Figs. 3 and 4. For the flow cytometry data, and gene expression of circulating GLUT-1, we used one-way analysis of variance (Brown-Forsythe ANOVA test) with Dunnett’s posttest (Fig. I and V). Adjusted P value <0.05 were considered statistically significant.
Figure 5. Confirmatory qPCR in Sham and 24h trained BM-Mono.
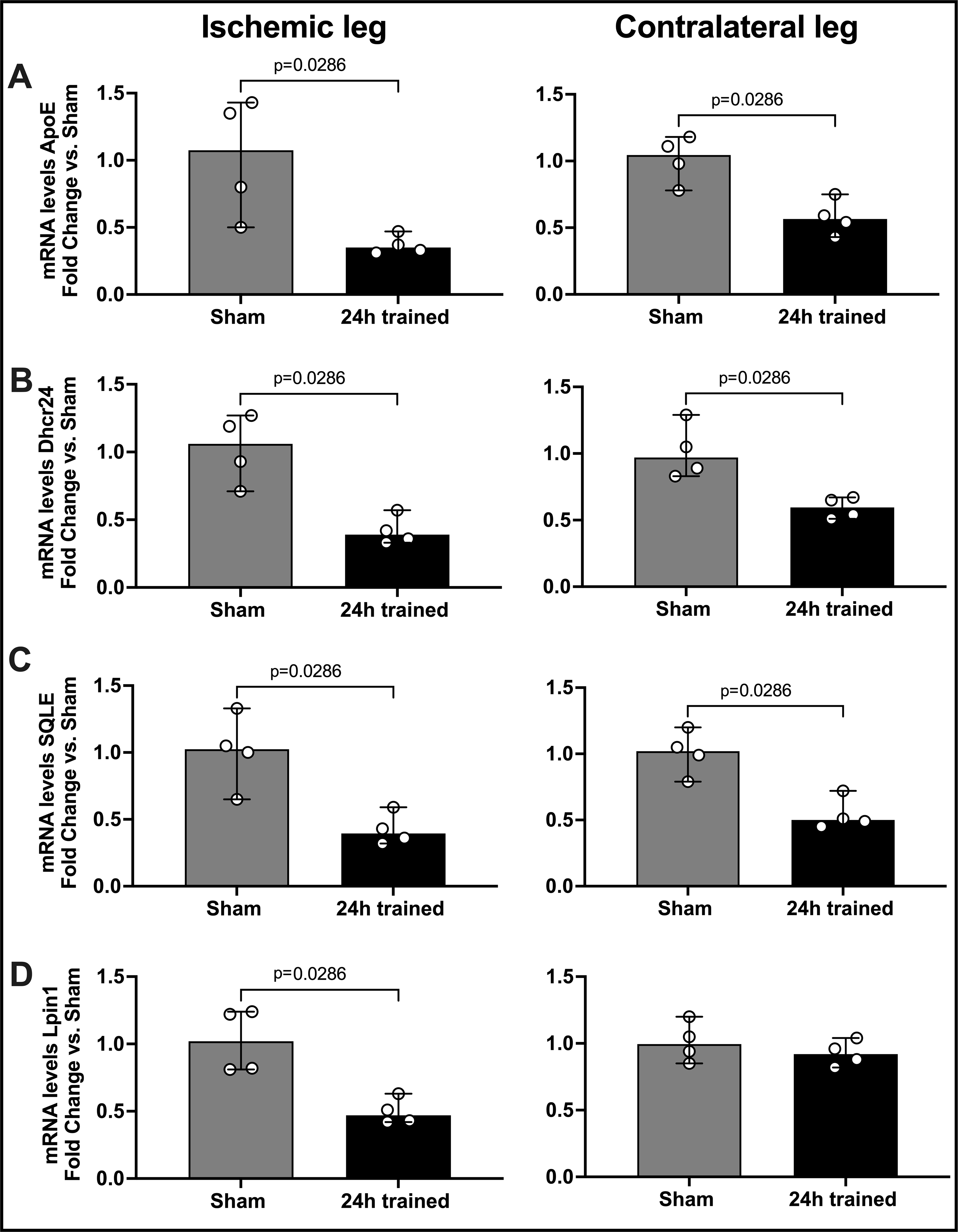
BM-Mono total RNA was isolated from sham and 24h trained groups (ischemic and contralateral leg) for gene expression quantification. Based on the transcriptomic analysis (Table S1 supplement demonstrated that 15 genes were significantly regulated in ischemic-trained BM-Mono with FDR<0.05), quantification of four genes involved in lipid metabolism, Apoe (A), Dhcr24 (B), SQLE (C), and Lpin1 (D), was performed. Here we demonstrate that Dhcr24, SQLE and Lpin1 were significantly down-regulated in the 24h trained BM-Mono from ischemic leg and three of them in the contralateral leg (median; 95% CI) by Mann-Whitney test.
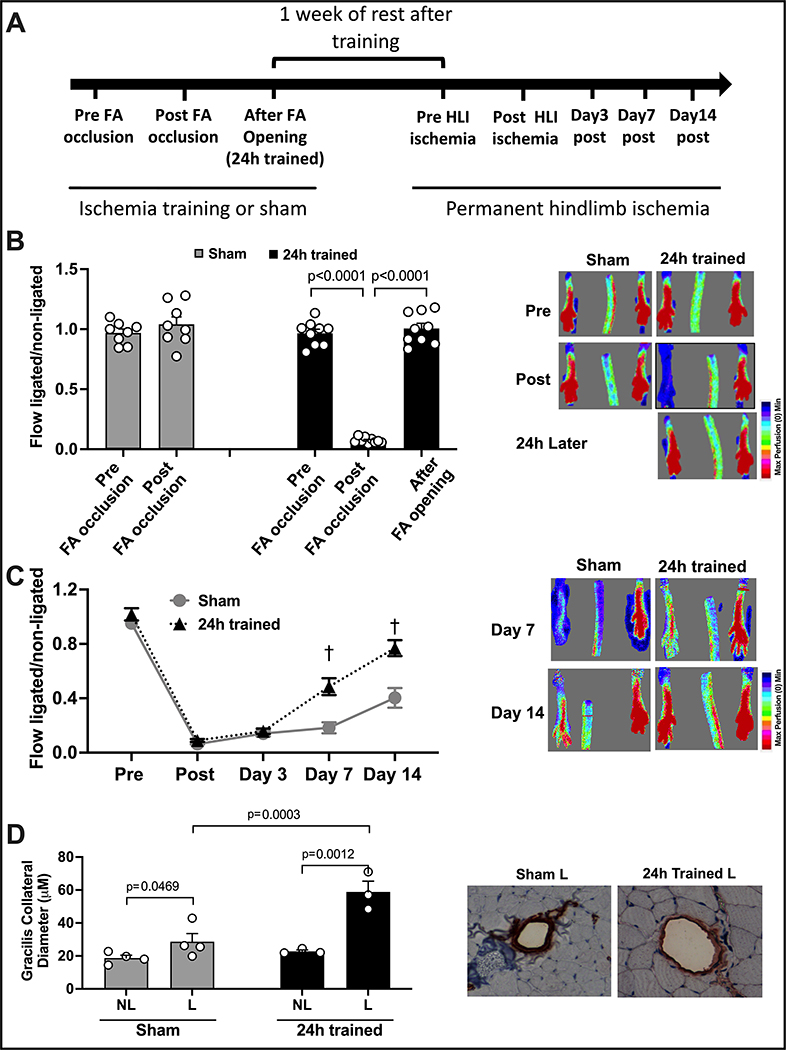
Figure 1. Ischemia Training by FA Occlusion Followed by Permanent Hindlimb Ischemia 1 Week Later.
(A) The timeline illustrates the experiments performed in this figure. (B) LDI demonstrates that blood flow is maintained after the dissection of the FA in the sham group, while in the 24h trained group, blood flow is significantly decreased after FA occlusion and completely restored immediately after FA opening. (C) LDI demonstrates that blood flow recovery after permanent hindlimb ischemia in the 24h trained group was significantly improved by days 7 and 14 (n=8). (D) Arteriogenesis of the gracillis collaterals showed a remarkable improvement in the 24h trained group. FA: femoral artery, HLI: hindlimb ischemia, NL: non-ligated, L: ligated. The data were analyzed by 2-way ANOVA, followed by Sidak multiple comparison tests. Errors bars represent SEM (†p<0.01 vs. same timepoint - Sham).
Figure 2. Adoptive Transfer of BM-Mono or Bone Marrow Mononuclear Lin− Cells.
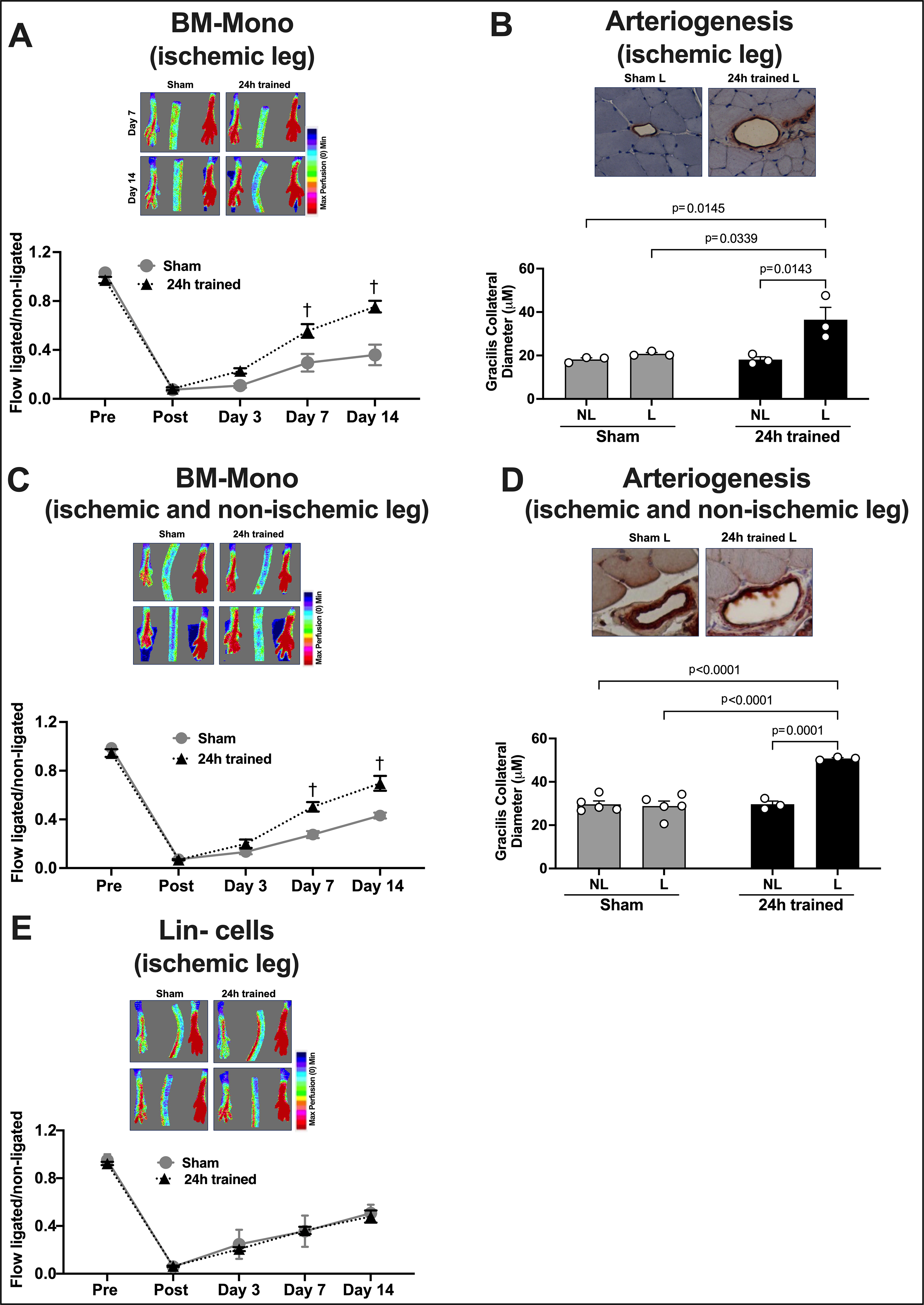
Cells from donor mice were injected systemically in recipient mice subjected to hindlimb ischemia the previous day. Monocyte adoptive transfer was performed using BM-Mono from the ischemic leg only (A) or from both legs (B). LDI demonstrates a significant improvement of blood flow recovery in recipient mice who received BM-Mono from the ischemic leg (n=10) (A) and from both legs (n=10) (C); however, no improvement in blood flow recovery was observed in recipient mice who received ischemic-trained Lin− cells (24h trained) compared to recipients with sham Lin− cells (n=9) (E). Remarkably effective arteriogenesis of gracillis collaterals was observed in recipient mice who received BM-Mono from the ischemic leg (B) and from both legs (D). NL: non-ligated, L: ligated. The data were analyzed by 2-way ANOVA, followed by Sidak multiple comparison tests. Errors bars represent SEM (†p<0.01 vs. same timepoint - Sham).
Figure 3. Gene Expression in Sham, 24h Ischemia, and 24h Trained Monocytes.
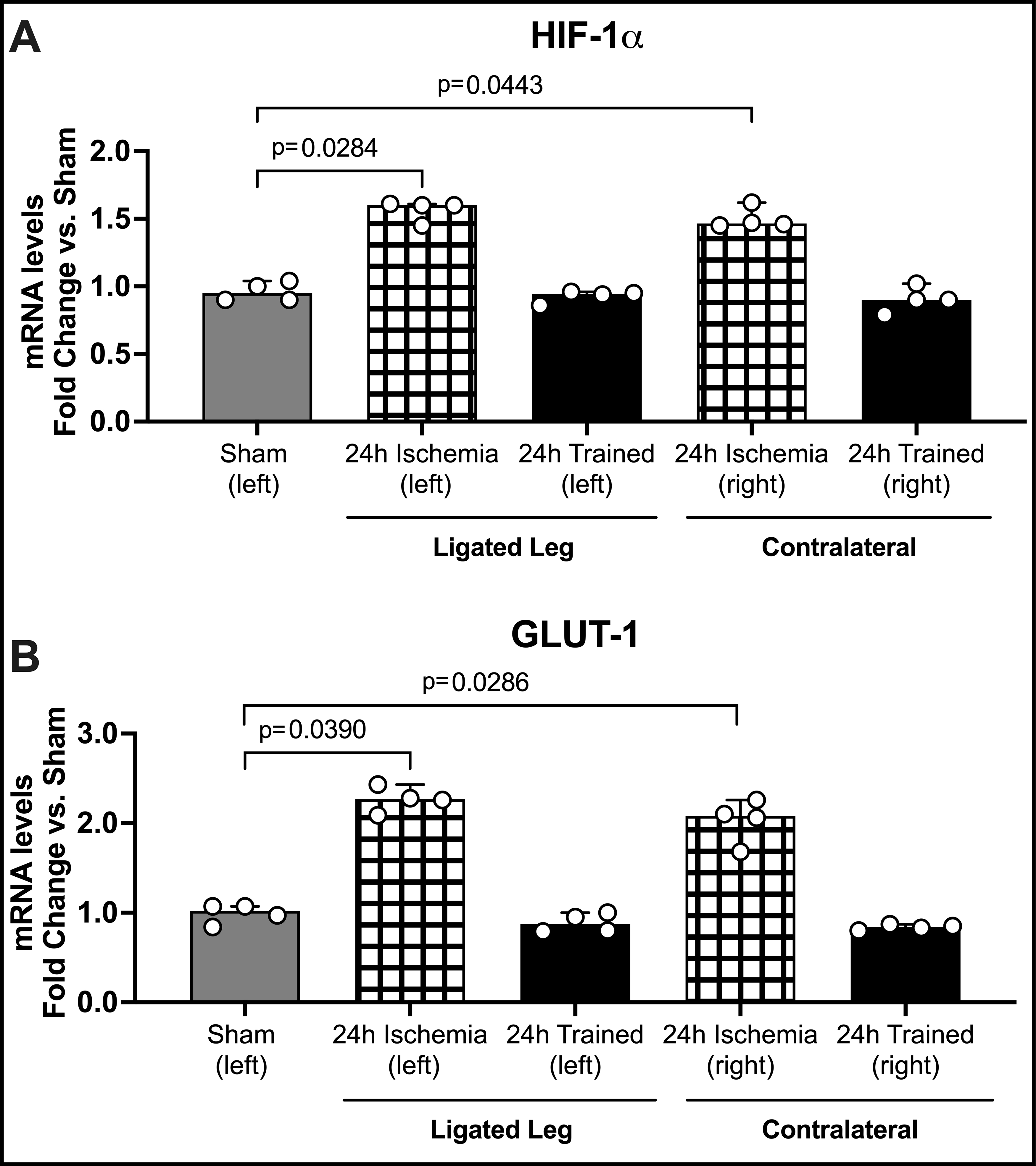
BM-Mono were isolated from 24h ischemia (when FA was still occluded after 24h of ligation); 24h trained (when FA was occluded for 24h but already opened for 2 days); and sham. For gene quantification (A) HIF-1α and (B) GLUT-1 qPCR was performed. Both genes were significantly up-regulated in the 24h ischemia group (median; 95% CI) by Kruskal-Wallis test followed by Dunn’s multiple comparisons test. Surprisingly, the same pattern occurred in the contralateral leg, suggesting a systemic effect of FA occlusion. GLUT-1, which is known to be targeted by HIF-1α, was more than two-fold up-regulated (± 0.13).
Figure 4. Gene Expression in Mouse BM-Mono Exposed to Hypoxia.
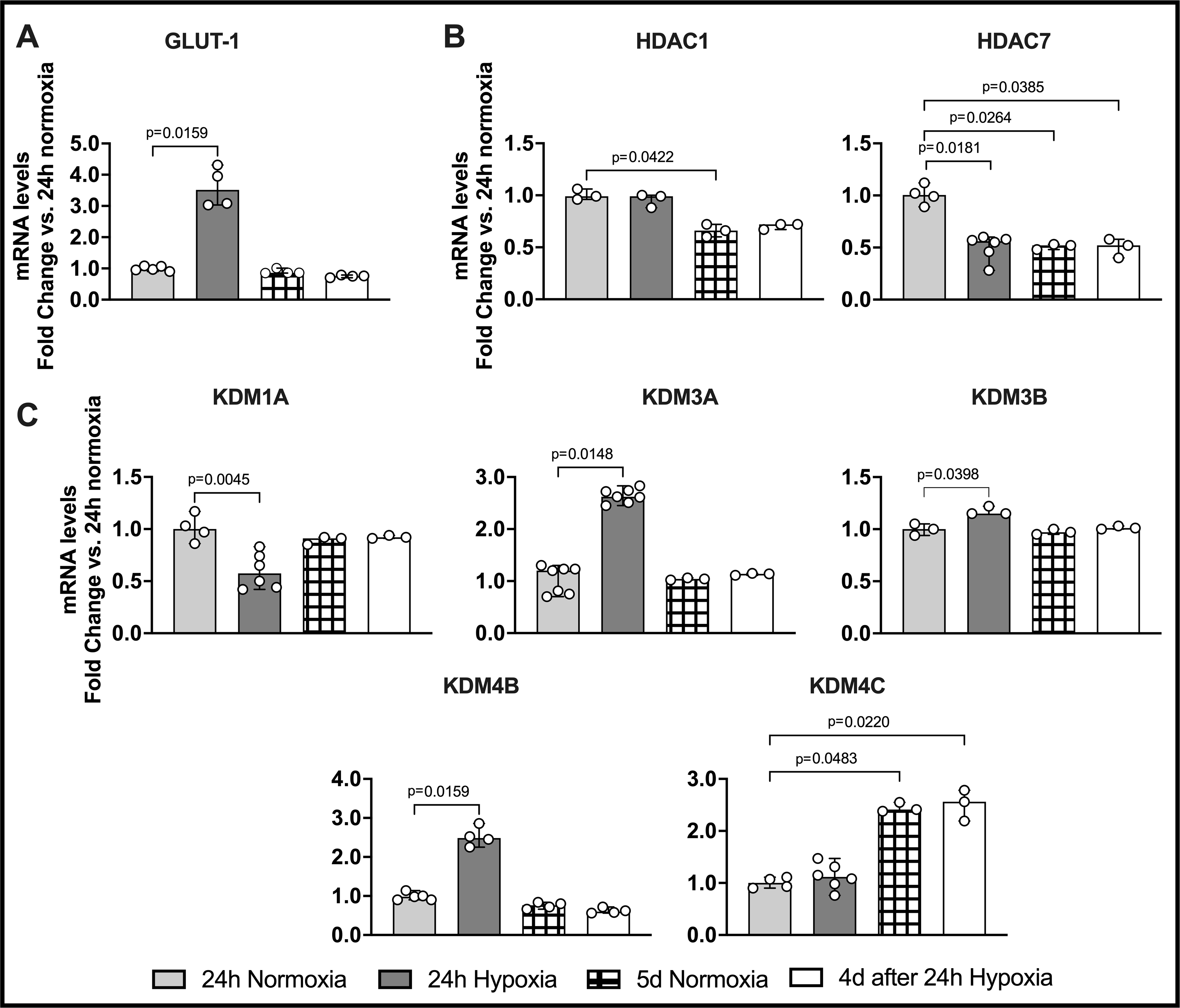
Mouse BM-Mono were exposed or not to 24h hypoxia followed by 4 days in normoxia. qPCR was performed to quantify the gene expression of GLUT-1 and several epigenetic enzymes. (A) GLUT-1 expression was up-regulated in 24h hypoxia. Histone-modifying epigenetic enzymes were significantly down-regulated (B) HDAC7 and (C) KDM1A, while others were up-regulated (KDM3A, KDM3B, and KDM4B) in 24h hypoxia as compared to 24h normoxia. Several histone-modifying epigenetic enzymes were also significantly regulated in 5d normoxia and 4d after 24h hypoxia groups. The data were analyzed by Kruskal-Wallis test followed by Dunn’s multiple comparisons test. Values are median and 95% CI.
RESULTS
Previous Insult of Ischemia Training Improves Blood Flow Recovery and Arteriogenesis After a Permanent Hindlimb Ischemia.
Ischemia training followed by permanent hindlimb ischemia -
The ischemia training insult was performed for 24h by FA occlusion (24h trained) or not (sham). After one week of resting (no procedure performed), the same animals were subjected to permanent hindlimb ischemia with occlusion and transection of the FA. A timeline with this experimental approach is illustrated in Figure 1A.
Blood flow recovery during and immediately after ischemia training -
During ischemia training, blood flow recovery in 24h trained and sham groups was measured confirming: a) the maintenance of the ischemia for 24h and the complete recovery of blood flow immediately after opening the ligation in the next day, and b) normal blood flow in the sham group after the procedure (Fig 1B, n=8 per group, p<0.01).
Blood flow recovery after permanent hindlimb ischemia -
Seven days after the ischemia insult (24h trained) or not (sham), mice were subjected to permanent hindlimb ischemia and blood flow recovery was measured before surgery; immediately post surgery; and 3, 7, and 14 days post surgery. There was no difference in blood flow analyses between the groups before ischemia, immediately post, or on day 3 after ischemia. However, on days 7 and 14 post ischemia, the 24h trained group demonstrated a significant improvement in blood flow recovery compared to sham. (Figure 1C, n=8 per group, p<0.05).
Arteriogenesis after permanent hindlimb ischemia -
Fifteen days after the permanent hindlimb ischemia, the mean diameter of gracilis collaterals vessels, located in the thigh muscle, was quantified by immunohistochemical staining of SMA in both legs. As shown in Figure 1D, the 24h trained group demonstrated a significant remodeling of the gracilis collaterals (n=4 per group, p<0.05), whereas the sham group displayed a small but significant remodeling of the collaterals (comparing non-ischemic with ischemic leg).
Adoptive Transfer of Ischemic-Trained BM-Mono Improves Blood Flow Recovery and Arteriogenesis in Recipient Mice Subjected to Hindlimb Ischemia.
Analysis of BM-Mono sub-population isolated and identification of these cells in recipient mice –
Flow cytometry demonstrated that ischemia training did not affect the sub-population of donor BM-Mono that were used for adoptive transfer. BM-Mono from ischemic (left) and non-ischemic (right) legs were analyzed, and there was no difference between the samples. Supplemental Figure S1 shows that BM-Mono from sham and 24h trained (left and right legs) were about 90% inflammatory (Ly6ChighCD11b+) and 10% patrollers (Ly6ClowCD11b+). BM-Mono from tomato donor mice were injected and these cells were identified in the hindlimb of recipient mice from both groups 24h after tail injection (Supplemental Figure S2).
Blood flow recovery after ischemic-trained BM-Mono adoptive transfer -
Donors’ BM-Mono were successfully isolated from the ischemic leg (left leg) or from both legs and injected in the recipient mice 48h or 7 days after the ischemia training termination. Recipient mice received 24h trained or sham BM-Mono one day after undergoing hindlimb ischemia, and the LDI was performed. There was no difference in blood flow analysis between the groups before ischemia and immediately post ischemia. However, on days 7 and 14 after surgery, the recipients who received 24h trained BM-Mono from ischemic leg (48h after ischemia termination) demonstrated a significant improvement in blood flow recovery (Figure 2A, n=11 per group p<0.001). Note that the findings in this BM-Mono adoptive transfer were similar to the one where ischemia insult (training) and permanent ischemia were performed in the same mouse, described above (Fig.1C). Surprisingly, the BM-Mono adoptive transfer using 24h trained BM-Mono from both legs showed a very similar improvement in blood flow recovery (Figure 2B n=8 per group p<0.01) as well as the BM-Mono adoptive transfer using 24h trained BM-Mono from ischemic leg isolated 7 days after ischemia training termination (Supplemental Figure S3A).
Arteriogenesis after ischemic-trained BM-Mono adoptive transfer -
Fifteen days after hindlimb ischemia, the mean diameter of gracilis collaterals vessels in the recipient mice, located in the thigh muscle, was quantified by immunohistochemical staining of SMA in both legs of recipient mice. As shown in Figure 2D, the recipients of 24h trained BM-Mono from the ischemic leg (48h after ischemia termination) demonstrated a remarkable improvement in arteriogenesis (n=4 per group, p<0.001), whereas recipients of sham BM-Mono displayed an ordinary remodeling of the collaterals (comparing non-ischemic with ischemic leg). Note that this findings of adoptive transfer were similar to the one where ischemia insult (training) and permanent ischemia were performed in the same mouse, described above (Fig.1C–D). Surprisingly, the BM-Mono adoptive transfer using 24h trained BM-Mono from both legs showed a similar improvement in arteriogenesis (Figure 2E n=3 per group p<0.001), as well as the BM-Mono adoptive transfer using 24h trained BM-Mono from ischemic leg isolated 7 days after the ischemia insult (Supplemental Figure S3B).
Ischemic-Trained Bone Marrow Mononuclear Lin− Cells’ Adoptive Transfer Did Not Improve Blood Flow Recovery in Recipient Mice.
Bone marrow mononuclear Lin- cells were isolated from the ischemic leg (left leg) of donor mice 48h after ischemia training termination in the 24h trained and sham groups. Recipient mice received trained or sham Lin− cells isolated one day after undergoing hindlimb ischemia, and LDI was performed. There was no difference in blood flow analysis between the groups in any of the time points, strongly suggesting that the effects of ischemia training in the BM are monocyte-specific (Figure 2C, n=9 per group).
Ischemia Training Increases HIF-1α and GLUT-1 Gene Expression in BM-Mono
We checked whether changes in gene expression occurred in monocytes during the ischemia process (before and after), and whether these changes could be seen in monocytes from both the ischemic and non-ischemic legs. Here we added a group called “24h ischemia” where the monocytes were isolated from the ligated (left) or contralateral (right) femoral and tibia bone marrow after 24h of FA occlusion but while the left artery was still occluded. This group differs from 24h trained monocytes because the mice were also subjected to 24h of FA occlusion, but the monocytes were isolated from the ligated and contralateral bone marrow 48h after the termination of the ischemia (FA opened for the last 2 days). The HIF-1α and GLUT-1 genes were chosen as markers because they are known to be upregulated in monocytes during glycolytic activation 18,19. Gene expression of HIF-1α and GLUT-1 were significantly greater in BM-Mono from the 24h ischemia group compared to sham and 24h trained groups (Figure 3, n=4 per group, p<0.001). While in the 24h ischemia group, the monocytes were isolated after 24h of ischemia, but when the FA was still occluded; in the 24h trained group the monocytes were isolated 2 days after the FA was opened (2 days after the ischemia was terminated). This second group was used in the adoptive transfer experiment because these cells were subjected to a functional reprogramming after ischemia, but they were not in ischemia anymore. These results strongly suggest that the ischemia insult caused by FA occlusion can be transmitted to the BM-Mono, and after the ischemia is ended, these genes, which are both markers of glycolysis metabolism, return to baseline values. Moreover, and quite interestingly, the effects of the unilateral FA occlusion have a systemic impact, since the up-regulation of these genes are also shown in the contralateral BM-Mono (right leg, Fig. 3). To support this finding we observed the same pattern described above in circulating erythrocytes. GLUT-1 gene expression was upregulated in the 24h ischemia group compared to 24h trained and sham groups. This data demonstrates that a single ischemia insult caused by unilateral FA occlusion can increase one of the HIF-1α major target genes in the circulation; however this change does not remain 48h after ischemia termination (Supplemental Figure S5).
Mouse Monocytes Exposed to 24h Hypoxia Have Differential Gene Expression of Epigenetic Enzymes and Increased GLUT-1 Gene Expression.
Mimicking the conditions of the in vivo animal model (normoxia, then hypoxia, then back to normoxia), we tested whether histone-modifying epigenetic enzymes could be regulated by hypoxia in bone marrow mouse monocytes in vitro. Mouse BM-Mono were isolated from C57Bl6J mice, and then cultured in normoxia (air, 95%; CO2, 5%) or hypoxia (O2, 1%; CO2, 5%) for 24h. Next, those in normoxia remained there for 4 additional days, while the ones in hypoxia were placed back under normoxia conditions for 4 days (Figure 4). The GLUT-1 gene expression was significantly increased in the hypoxic cells, which confirmed that these cells were exposed to hypoxia (Figure 4A, n=4, p<0.01). Interestingly, mouse BM-Mono exposed to 24h hypoxia demonstrated a significant, more than two-fold, increase in gene expression of histone demethylases KDM3A and KDM4B when compared to 24h normoxia. Moreover, 24h hypoxia showed almost two-fold significant decrease of histone demethylase KDM1A and histone deacetylase HDAC7 when compared to 24h normoxia. In addition, HDAC1 and KDM4C were significantly regulated in the 5d normoxia and 4d after 24h hypoxia groups, which may suggest that these enzymes are associated with senescence of the monocytes.
Ischemia Training Alters the Gene Profile of BM-Mono, Specifically Decreasing Genes Involved in Lipid Biosynthesis.
The transcriptomic analysis of BM-Mono from the 24h trained group (isolated 2 days after the ischemia training termination, as mentioned previously) and sham demonstrated the 15 genes that were most significantly altered in ischemic-trained BM-Mono with FDR<0.05 (Table S1 – Supplemental Material). Of these genes, almost one third are involved in lipid metabolism and were about two-fold down-regulated by ischemia training. These genes are ApoE, Dhcr24, SQLE, and Lpin1. Using a qPCR, the results of RNAseq were confirmed (Fig.5 A–D, n=4 per group, p<0.01). Moreover, and quite interestingly, the effects of ischemia training also altered these same genes involved in lipid metabolism in the BM-Mono from the contralateral leg; ApoE, Dhcr4, and SQLE were significantly downregulated in the 24h trained BM-Mono right leg (Figure 5A–D). This data also suggest a systemic effect of ischemia training in these donor mice. Of note, and supporting a potential systemic effect, the comparison between BM-Mono from ischemic and contratateral leg of the 24h trained group did not show differential regulation of a single gene when using the FDR<0.05 critiria (Data Set S2). To further assess this possibility we measured proinflammatory cytokines and chemokines in plasma samples of 24h trained and sham donor mice and demonstrated that an inflammatory cytokine, TNF-α, and inflammatory chemokines, IP-10 and MIP-1b, were upregulated in the 24h trained group, even 2 days after ischemia training had been ended (Supplemental Figure S4).
Ischemia Training Alters the Lipid Profile of BM-Mono, Specifically Increasing Desmosterol, Lanosterols and Others Oxysterols.
The lipidomic analysis of BM-Mono from the 24h trained group and sham demonstrated an interesting correlation between the transcriptomic data, specialty the down-regulation of Dhcr24 with the significant increase in lanosterol and desmosterol. Moreover, other oxysterols such as 24,25-epoxycholesterol, 27-hydroxycholesterol and cholestanol, as well as cholesterol were also up-regulated in the ischemic-trained compared to sham monocytes (Supplemental Figure S6). Since the transcriptomic data showed similar data between trained BM-Mono from right and leg, the lipidomic analysis was performed using monocytes from both legs comparing 24h trained and sham groups.
DISCUSSION
The estimated annual number of new PAD/CLI patients in the United States is around 160,00020, and despite the current surgical bypasses and endovascular procedures, 30% of patients are not eligible for or do not respond well to existing treatments, driving the need for novel therapeutic treatments21,22. Here, our study addresses the effects of ischemic-trained monocytes in a pre-clinical mouse model of hindlimb ischemia. We uncovered the consequences of a previous short ischemia insult in priming monocytes’ response to a permanent hindlimb ischemia. In this study, we found that 1) ischemic-trained BM-Mono improve blood flow recovery through remarkable arteriogenesis in a model of hindlimb ischemia, 2) ischemia training created by unilateral FA occlusion may lead to a systemic effect that can be transmitted to BM-Mono, increasing hypoxic/glycolytic markers in these cells, 3) ischemic-trained BM-Mono have a unique profile, with altered genes involved in lipid metabolism, and 4) exposure to 24h of hypoxia is sufficient to regulate histone-modifying epigenetic enzymes in mouse BM-Mono.
The presence of monocytes in the limb muscle after hindlimb ischemia and their contribution to blood flow recovery and arteriogenesis during this process have been previously reported2,3. Monocytes are among the first cells to arrive to the ischemic tissue adhering to the shear stress-activated endothelium via ICAM and VCAM and migrating into the perivascular space, to stimulate endothelial and smooth muscle cell proliferation, thereby promoting arteriogenesis23. Moreover, adoptive transfer using BM-derived or circulating monocytes in recipient mice subjected to hindlimb ischemia significantly improves blood flow recovery7,24. Interestingly, our findings demonstrate that the contribution of BM-Mono in hindlimb ischemia can be potentiated by a previous single ischemia insult created by unilateral FA occlusion. We have demonstrated the presence of the BM-Mono in the ischemic calf of recipient mice after adoptive transfer (Supplemental Figure S2); in addition, and most importantly, the ischemic-trained BM-Mono (from the ischemic leg or even from both legs), in the settings of hindlimb ischemia, significantly improved blood flow recovery and arteriogenesis in the recipient mice (Fig. 2). In contrast, the only report in the literature that used ischemia-exposed BM-Mono adoptive transfer (from the contralateral leg) in the setting of hindlimb ischemia did not find improvement in neovascularization due to an impairment in monocytes’ angiogenic capacity25. We suspect that this difference in results could be because we used ischemic-trained monocytes (that may undergo to trained immunity) and not monocytes conditioned to ischemia; in other words, our BM-Mono were exposed to ischemia, but returned to baseline condition (without ischemia) for 48 hours before isolation.
In fact, additional data supporting this idea of ischemia training leading to trained immunity can be found in the perpertuated effects of this ischemia training in the BM-Mono even 7 days after its termination (Supplemental Figure S3). The long-term functional outcome of monocytes and the greater systemic inflammatory response in the 24h trained donors (upregulation of inflammatory cytokines/chemokines - Supplemental Figure S4) are important characteristics of trained immunity26,27. Our data analysis assessing gene expression of hypoxic/glycolytic markers, normally up-regulated in ischemic monocytes18,19, corroborates this idea of ischemia training in the monocytes. Here we reveal that during FA occlusion, HIF-1α and GLUT-1 were up-regulated in BM-Mono from both legs; however these genes returned to baseline values in the ischemic-trained monocytes (24h trained group - Fig. 3). The ischemia insult ended immediately with the opening of the FA (LDI data - Fig 1B), and these ischemic-trained BM-Mono were maintained in normal condition for 2 days (or even 7 days - Supplemental Figure S3) until their isolation. The ischemic-trained monocytes suffer a functional (maybe epigenetic) reprogramming where they acquire a unique behavior, with a long-term effect, thereby improving their response during hindlimb ischemia.
The ischemia training created by unilateral FA occlusion generates a systemic beneficial effect in the BM-Mono from the contralateral leg. Adoptive transfer using 24h trained donor BM-Mono from both legs demonstrates a very similar improvement in blood flow recovery and arteriogenesis in recipient mice (Figure 2). Thus, the functional reprograming of BM-Mono has beneficial applications in limb neovascularization after hindlimb ischemia28. To corroborate our finding that suggests a systemic effect of a unilateral FA occlusion, we show that circulating inflammatory cytokines/chemokines are upregulated in donor mice even 2 days after ischemia training - at the time of adoptive transfer (Supplemental Figure S4). We believe that during FA occlusion, these and other inflammatory cytokines/chemokines could reach even higher levels29. Evidence has shown that unilateral FA occlusion generates systemic effects with significant augmentation of HIF-1α throughout the entire BM30; that leads to functional reprograming of bone marrow-derived cells28. Moreover, during the FA occlusion, we also show an enhancement in the levels of circulating GLUT-1 (Supplemental Figure S5) and the up-regulation of HIF-1α and GLUT-1 in the BM-Mono of the contralateral leg (Figure 3).
The major goal of a therapeutic strategy for PAD/CLI, as well as ischemic heart and brain diseases, is to increase blood flow and reduce ischemic areas1. Abundant evidence has established the favorable effects of remote ischemia preconditioning and classical ischemia preconditioning31,32, by decreasing the size of ischemic area33–37. However, our findings move towards a different direction; first because they are based specifically on BM-Mono and this ischemia training did not work with the remaining population of BM mononuclear cells (Figure 2C), second and most importantly, because the potential mechanism is driven by a gene and potential epigenetic reprograming of the monocytes. Remote ischemia preconditioning and ischemia preconditioning are both generated by sublethal ischemia; while in the former major ischemia will be created in a remote organ38, in the latter cells can be exposed to hypoxia and injected in the ischemic organ32; both events are performed to build ischemia tolerance for few hours/days32,39. Our adoptive transfer data used ischemic-trained monocytes isolated 2 or even 7 days after the ischemia insult had ended. Herein, we are evaluating a effect of ischemia training that perpetuates at least for a week and can have a long-term effect on the hindlimb ischemia scenario. Interestingly, the turnover of BM-Mono is less than 48h40,41, suggesting that at least part of the monocytes that were injected were not the ones exposed to the ischemia. This idea allows us to speculate that the effects of ischemia training in monocytes may pass to the next generation of cells. Moreover, our transcriptome data demonstrated that ischemia training modifies the gene expression profile of the monocytes 2 days after ischemia has ceased.
Our analysis shows that of the 15 genes altered after ischemia training, 4 of them are involved in lipid metabolism: 1) ApoE, which increases the cholesterol efflux in the cells and prevents monocytes/macrophages from becoming foam cells in the atherosclerosis scenario42, 2) SQLE, which encodes an enzyme that participates in cholesterol biosynthesis and is down-regulated during monocyte differentiation43, 3) Lpin1, which encodes Lpin, a phosphatidic acid phosphatase protein that catalyzes one of the last steps in triglyceride synthesis, and when it is down-regulated in monocytes-macrophages, contributes to an anti-inflammatory effect44, and 4) Dhcr24, which encodes an enzyme that participates in several reactions of cholesterol biosynthesis, including the transformation of desmosterol in cholesterol that, when down-regulated, accelerates inflammation resolution45. We confirmed the transcriptone data for these 4 genes by confirmatory qPCR (Fig. 5A–D). Interestingly, the ischemic-trained BM-Mono from the contralateral leg also show the same lipid/cholesterol metabolism gene regulation pattern, where three of these genes were significantly downregulated (Fig. 5A–D). Moreover, the transcriptome analysis comparing the ischemic-trained BM-Mono from the ischemic vs. contralateral leg did not identify differences in gene regulation with FDR<0.05. These results together also support our discussion above about the potential systemic effect caused by unilateral FA occlusion. The regulation of lipid/cholesterol metabolism in trained immunity has already been well documented in several studies: with the use of western diet46, in the transcriptome analysis of b-glucan-trained macrophages27, and with the identification of mevalonate (a cholesterol metabolite) as a crucial regulator in this process47. Moreover, liver X-receptor (LXR) and sterol regulatory element-binding protein (SREPB), are essencial transcription factors in cholesterol homeostasis and both regulate inflammatory response and epigenetic remodeling48,49. LXR is induced by intermediates downstream in the cholesterol synthesis pathway50,51; interestingly, our lipidomic analysis demonstrated that two of these intermediates oxysterols (oxygenated derivatives of cholesterol), 24,25 epoxycholesterol and 27- hydroxycholesterol which activates LXR52,53, are up-regulated in the ischemic-trained monocytes. Our lipidomic data also reveal that these trained monocytes have significant upregulation of desmosterol. These findings are supported by previous evidences where inhibition of Dhcr24 causes desmosterol accumulation, leading to LXR activation and suppression of SREPB, with consequent suppression of inflammatory responses45,54. Although our transcriptome data did not detect the suppression of SREPB in trained BM-Mono, the desmosterol upregulation (or accumulation) and Dhcr24 down-regulation could explaing the reduction of SQLE and Lpin genes, since both are activated by SREPB55–57. The interesting correlation in our transcriptomic and lipidomic data also demonstrated the effect of lower Dhcr24 expression with the up-regulation of lanosterol (Dhcr24 is responsible for catalyzing lanosterol in 24,25-dihydrolanosterol45,54), and the lower ApoE expression (which might compromise the cholesterol efflux42) with the up-regulation of cholesterol in these trained monocytes. However, the cellular levels of cholesterol is also attributed of few other biological processes such as biosysthesis, esterification and export58. In summary, although previous evidences of cholesterol metabolism in trained immunity were associated with the initial phase of the cholesterol pathway and have shown a pro-inflammatory profile of monocytes/macrophages in the atherosclerosis scenario47,48; our findings demonstrated the effects of ischemia training in the later phase of cholesterol pathway, suggesting that these cells were under trained immunity, but potentially inducing an anti-inflammatory phenotype54.
Trained immunity is a recently proposed concept; however, it has already been well established by several authors using different types of insult to study the epigenetic/functional reprogramming of monocytes6,59,60. This reprogramming is driven by a metabolism shift from oxidative phosphorylation towards glycolysis, and HIF-1α is a key player in this process61. As shown in Figure 3, HIF-1α and GLUT-1, which are markers of glycolysis, were up-regulated during 24h ischemia training. Trained immunity leads to changes in histone marks, which can be added or removed by specific histone-modifying epigenetic enzymes; and hypoxia/HIF-1α can modulate such enzymes8,10,62. Our in vitro experiment demonstrates that mouse BM-Mono exposed to 24h of hypoxia had enhanced levels of GLUT-1 and altered expression of histone-modifying epigenetic enzymes involved in methylation and acetylation. Specifically, lysine-specific demethylases KDM3A (also called JMJD1A) and KDM4B (also called JMJD2B) were up-regulated in hypoxic BM-Mono. In accordance with the literature, HIF-1α induces KDM3A, which is devoted to removing dimethyl marks on histone 3 lysine 9 (H3K9me1,2), and KDM4B, which targets H3K9me2,3 and H3K36me2,38,9,63–65. Although 24h of hypoxia has also significantly induced KDM3B, its upregulation is probably meaningless due to the very modest change in its expression compared to the 24h normoxia group. Our findings also demonstrate that another demethylase from the same family, KDM1A, was down-regulated by hypoxia. These findings are not uncommom; Liu et al. showed that in human umbilical vein cells, while several KDMs, including KDM3A and KDM4B, were up-regulated by hypoxia stimulus, others, such as KDM1A, were down-regulated by HIF-1α 66,67. Our data also revealed a significant down-regulation of the histone deacetylase HDAC7 in hypoxic BM-Mono. Interestingly, HDAC7 is up-regulated in inflammatory macrophages and interacts with HIF-1α, inducing expression of LPS- target genes68. Thus its down regulation may contribute to a less inflammatory profile of these monocytes. Most of the enzymes were only modulated in hypoxic monocytes, however; the significant changes of HDAC1, HDAC7, and KDM4C in 5d normoxia and 4d after 24h hypoxia groups could be related to the senescence of the cells since they were cultured for a longer time and there was no difference between these two groups.
In summary, we demonstrate that the contribution of BM-Mono against hindlimb ischemia can be potentiated by ischemia training using a previous single ischemia insult created by FA occlusion. Ischemic-trained BM-Mono improved revascularization of the ischemic limb through remarkably effective arteriogenesis. Further, ischemia training caused by FA occlusion may lead to a systemic effect transmitted to the BM and specifically affects monocytes. The functional reprogramming of these cells after ischemia training is associated with a unique gene expression and lipidomic profiles leading to altered cholesterol pathway. Finally, our findings open new avenues for using ischemic-trained monocytes as a potential therapeutic approach in the setting of PAD/CLI. Given the importance of this topic and the potential generation of trained immunity by ischemia training, our study reveals the need for further investigation towards the epigenetic profile of ischemic-trained monocytes and the lipid content of these cells, particularly in the clinical settings.
Supplementary Material
HIGHLIGHTS.
Ischemic-trained monocytes improve arteriogenesis during hindlimb ischemia
Bone marrow-derived monocytes can be trained through a single insult of ischemia by femoral artery occlusion
Ischemia training modifies cholesterol pathway in monocytes
Hypoxia alters histone-modifying epigenetic enzymes in mouse bone marrow monocytes
Unilateral femoral artery occlusion leads to a beneficial systemic effect that can be transmitted to bone marrow-derived monocytes from both legs
ACKNOWLEDGMENTS
We thank Dr. William Hulme, Director of Center for Genome Technology and April Mann, Director of Writing Center – University of Miami.
SOURCES OF FUNDING
This study was supported by National Institute of Health – NHLBI K01HL145359 to R.M.L.-S, and Department of Surgery, University of Miami.
Nonstandard Abbreviations and Acronyms
- PAD
peripheral arterial disease
- CLI
critical limb ischemia
- FA
femoral artery
- BM-Mono
bone marrow-derived monocytes
- LDI
laser doppler imaging
- SQLE
squalene monooxygenase
- FDR
false discovery rate
- Dhcr24
24-Dehydrocholesterol Reductase
- SREPB
sterol regulatory element-binding protein
- KDM
histone lysine demethylases
- HDAC
histone deacetylase
Footnotes
DISCLOSURES
None
SUPPLEMENTAL MATERIALS
Data Set S1 and S2
REFERENCES
- 1.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(1 SUPPL.). doi: 10.1016/j.jvs.2006.12.037 [DOI] [PubMed] [Google Scholar]
- 2.Fung E, Helisch A. Macrophages in collateral arteriogenesis. Front Physiol. 2012;3 SEP. doi: 10.3389/fphys.2012.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scholz D, Ziegelhoeffer T, Helisch A, et al. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. [DOI] [PubMed] [Google Scholar]
- 4.Stabile E, Susan Burnett M, Watkins C, et al. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. 2003;108:205–210. doi: 10.1161/01.CIR.0000079225.50817.71 [DOI] [PubMed] [Google Scholar]
- 5.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-Derived Stromal Cells Express Genes Encoding a Broad Spectrum of Arteriogenic Cytokines and Promote In Vitro and In Vivo Arteriogenesis Through Paracrine Mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC [DOI] [PubMed] [Google Scholar]
- 6.Netea MG, Joosten LAB, Latz E, et al. Trained immunity: A program of innate immune memory in health and disease. Science (80- ). 2016;352:427. doi: 10.1126/science.aaf1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capoccia BJ, Gregory AD, Link DC. Recruitment of the inflammatory subset of monocytes to sites of ischemia induces angiogenesis in a monocyte chemoattractant protein-1-dependent fashion. J Leukoc Biol. 2008;84:760–768. doi: 10.1189/jlb.1107756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieg AJ, Rankin EB, Chan D, Razorenova O, Fernandez S, Giaccia AJ. Regulation of the Histone Demethylase JMJD1A by Hypoxia-Inducible Factor 1α Enhances Hypoxic Gene Expression and Tumor Growth. Mol Cell Biol. 2010;30:344–353. doi: 10.1128/mcb.00444-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollard PJ, Loenarz C, Mole DR, et al. Regulation of Jumonji-domain-containing histone demethylases by hypoxia-inducible factor (HIF)-1α. Biochem J. 2008;416:387–394. doi: 10.1042/BJ20081238 [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Sun M, Yang H, et al. Hypoxia-inducible factor-1α mediates up-regulation of neprilysin by histone deacetylase-1 under hypoxia condition in neuroblastoma cells. J Neurochem. 2014;131:4–11. doi: 10.1111/jnc.12795 [DOI] [PubMed] [Google Scholar]
- 11.Huang LE, Willmore WG, Gu J, Goldberg MA, Bunn HF. Inhibition of hypoxia-inducible factor 1 activation by carbon monoxide and nitric oxide: Implications for oxygen sensing and signaling. J Biol Chem. 1999;274:9038–9044. doi: 10.1074/jbc.274.13.9038 [DOI] [PubMed] [Google Scholar]
- 12.Zhou B, Ge T, Zhou L, et al. Dimethyloxalyl Glycine Regulates the HIF-1 Signaling Pathway in Mesenchymal Stem Cells. Stem Cell Rev Reports. 2020;16. doi: 10.1007/s12015-019-09947-7 [DOI] [PubMed] [Google Scholar]
- 13.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol - Cell Physiol. 1996;271: C1172–80. doi: 10.1152/ajpcell.1996.271.4.c1172 [DOI] [PubMed] [Google Scholar]
- 14.Hernandez DR, Artiles A, Duque JC, et al. Loss of c-Kit function impairs arteriogenesis in a mouse model of hindlimb ischemia. Surg (United States). 2018;163:877–882. doi: 10.1016/j.surg.2017.10.052 [DOI] [PubMed] [Google Scholar]
- 15.Dobin A, Davis CA, Schlesinger F, et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26:139–140. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11. doi: 10.1186/gb-2010-11-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cramer Thorsten, Yamanishi Yuji, Clausen Björn E., Irmgard Förster RP, Mackman Nigel, Haase Volker H., Jaenisch Rudolf, Corr Maripat, Victor Nizet GS, Firestein, Gerber Hans-Peter, Ferrara Napoleone and RSJ. HIF-1 Is Essential for Myeloid Cell Inflammation. 2015;112:645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109 [DOI] [PubMed] [Google Scholar]
- 20.Agarwal S, Sud K, Shishehbor MH. Nationwide Trends of Hospital Admission and Outcomes among Critical Limb Ischemia Patients from 2003–2011. J Am Coll Cardiol. 2016;67:1901–1913. doi: 10.1016/j.jacc.2016.02.040 [DOI] [PubMed] [Google Scholar]
- 21.Dormandy J, Heeck L, Vig S. The fate of patients with critical leg ischemia. Semin Vasc Surg. 1999;12:142–147. [PubMed] [Google Scholar]
- 22.Lawall H, Bramlage P, Amann B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. J Vasc Surg. 2011;53:445–453. doi: 10.1016/j.jvs.2010.08.060 [DOI] [PubMed] [Google Scholar]
- 23.Hoefer IE, Van Royen N, Rectenwald JE, et al. Arteriogenesis proceeds via ICAM-1/Mac-1-mediated mechanisms. Circ Res. 2004;94:1179–1185. doi: 10.1161/01.RES.0000126922.18222.F0 [DOI] [PubMed] [Google Scholar]
- 24.Capoccia BJ, Shepherd RM, Link DC. G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood. 2006;108:2438–2445. doi: 10.1182/blood-2006-04-013755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregory AD, Capoccia BJ, Woloszynek JR, Link DC. Systemic levels of G-CSF and interleukin-6 determine the angiogenic potential of bone marrow resident monocytes. J Leukoc Biol. 2010;88:123–131. doi: 10.1189/jlb.0709499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saeed S, Quintin J, Kerstens HHD, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science (80- ). 2014;345. doi: 10.1126/science.1251086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.J K, J Q, F P, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109. doi: 10.1073/PNAS.1202870109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh PL, Rybalko V, Baker AB, Suggs LJ, Farrar RP. Recruitment and therapeutic application of macrophages in skeletal muscles after hind limb ischemia. J Vasc Surg. 2018;67:1908–1920.e1. doi: 10.1016/j.jvs.2017.04.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang GL, Chang DS, Sarkar R, Wang R, Messina LM. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J Vasc Surg. 2005;41:312–320. doi: 10.1016/j.jvs.2004.11.012 [DOI] [PubMed] [Google Scholar]
- 30.Urao N, McKinney RD, Fukai T, Ushio-Fukai M. NADPH oxidase 2 regulates bone marrow microenvironment following hindlimb ischemia: Role in reparative mobilization of progenitor cells. Stem Cells. 2012;30:923–934. doi: 10.1002/stem.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.CIR.74.5.1124 [DOI] [PubMed] [Google Scholar]
- 32.Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: An update. Nat Rev Cardiol. 2011;8:619–629. doi: 10.1038/nrcardio.2011.85 [DOI] [PubMed] [Google Scholar]
- 33.Kapur NK, Qiao X, Paruchuri V, et al. Mechanical Pre-Conditioning With Acute Circulatory Support Before Reperfusion Limits Infarct Size in Acute Myocardial Infarction. JACC Heart Fail. 2015;3:873–882. doi: 10.1016/j.jchf.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 34.Zhu XH, Yuan HJ, Wu YN, et al. Non-invasive limb ischemic pre-conditioning reduces oxidative stress and attenuates myocardium ischemia-reperfusion injury in diabetic rats. Free Radic Res. 2011;45:201–210. doi: 10.3109/10715762.2010.522576 [DOI] [PubMed] [Google Scholar]
- 35.Pilz PM, Hamza O, Gidlöf O, et al. Remote ischemic perconditioning attenuates adverse cardiac remodeling and preserves left ventricular function in a rat model of reperfused myocardial infarction. Int J Cardiol. 2019;285:72–79. doi: 10.1016/j.ijcard.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 36.Gaspar A, Lourenço AP, Pereira MÁ, et al. Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res Cardiol. 2018;113. doi: 10.1007/s00395-018-0672-3 [DOI] [PubMed] [Google Scholar]
- 37.Skyschally A, Kleinbongard P, Lieder H, et al. Humoral transfer and intramyocardial signal transduction of protection by remote ischemic perconditioning in pigs, rats, and mice. Am J Physiol - Hear Circ Physiol. 2018;315:H159–H172. doi: 10.1152/ajpheart.00152.2018 [DOI] [PubMed] [Google Scholar]
- 38.Birnbaum Y, Hale SL, Kloner RA. Ischemic Preconditioning at a Distance. Circ. 1997;96:1641–1646. 10.1161/01.CIR.96.5.1641. Accessed June 22, 2021. [DOI] [PubMed] [Google Scholar]
- 39.Healy DA, Walsh SR. Remote Preconditioning and Vascular Surgery: Challenges in Translation. J Cardiovasc Pharmacol Ther. 2017;22:316–320. doi: 10.1177/1074248417702892 [DOI] [PubMed] [Google Scholar]
- 40.Llewellyn RA, Thomas KS, Gutknecht MF, Bouton AH. The nonreceptor protein tyrosine kinase Pyk2 promotes the turnover of monocytes at steady state. J Leukoc Biol. 2017;102:1069–1080. doi: 10.1189/jlb.1a0217-063r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yona S, Kim KW, Wolf Y, et al. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu L, Dai Perrard X, Perrard JL, et al. Foamy monocytes form early and contribute to nascent atherosclerosis in mice with hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2015;35:1787–1797. doi: 10.1161/ATVBAHA.115.305609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ecker J, Liebisch G, Englmaier M, Grandl M, Robenek H, Schmitz G. Induction of fatty acid synthesis is a key requirement for phagocytic differentiation of human monocytes. Proc Natl Acad Sci U S A. 2010;107:7817–7822. doi: 10.1073/pnas.0912059107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meana C, Peña L, Lordén G, et al. Lipin-1 Integrates Lipid Synthesis with Proinflammatory Responses during TLR Activation in Macrophages. J Immunol. 2014;193:4614–4622. doi: 10.4049/jimmunol.1400238 [DOI] [PubMed] [Google Scholar]
- 45.Körner A, Zhou E, Müller C, et al. Inhibition of Δ24-dehydrocholesterol reductase activates pro-resolving lipid mediator biosynthesis and inflammation resolution. Proc Natl Acad Sci U S A. 2019;116:20623–20634. doi: 10.1073/pnas.1911992116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christ A, Günther P, Lauterbach MAR, et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell. 2018;172:162–175.e14. doi: 10.1016/j.cell.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bekkering S, Arts RJW, Novakovic B, et al. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell. 2018;172:135–146.e9. doi: 10.1016/J.CELL.2017.11.025/ATTACHMENT/CD16E7E1-A009-4F9A-8E0E-646462202D78/MMC4.PDF [DOI] [PubMed] [Google Scholar]
- 48.Van Tuijl J, Joosten LAB, Netea MG, Bekkering S, Riksen NP. Immunometabolism orchestrates training of innate immunity in atherosclerosis. Cardiovasc Res. 2019;115:1416–1424. doi: 10.1093/CVR/CVZ107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sohrabi Y, Sonntag GVH, Braun LC, et al. LXR Activation Induces a Proinflammatory Trained Innate Immunity-Phenotype in Human Monocytes. Front Immunol. 2020;11. doi: 10.3389/FIMMU.2020.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S, Lee J, Lee SK, Lee JW. Activating signal cointegrator-2 is an essential adaptor to recruit histone H3 lysine 4 methyltransferases MLL3 and MLL4 to the liver X receptors. Mol Endocrinol. 2008;22:1312–1319. doi: 10.1210/me.2008-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu H, Wu J, Yang M, et al. Involvement of liver X receptor alpha in histone modifications across the target fatty acid synthase gene. Lipids. 2012;47:249–257. doi: 10.1007/s11745-011-3635-0 [DOI] [PubMed] [Google Scholar]
- 52.Rowe AH, Argmann CA, Edwards JY, et al. Enhanced Synthesis of the Oxysterol 24(S),25-Epoxycholesterol in Macrophages by Inhibitors of 2,3-Oxidosqualene: Lanosterol Cyclase - A Novel Mechanism for the Attenuation of Foam Cell Formation. Circ Res. 2003;93:717–725. doi: 10.1161/01.RES.0000097606.43659.F4 [DOI] [PubMed] [Google Scholar]
- 53.Fu X, Menke JG, Chen Y, et al. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem. 2001;276:38378–38387. doi: 10.1074/JBC.M105805200 [DOI] [PubMed] [Google Scholar]
- 54.Spann NJ, Garmire LX, McDonald JG, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan JME, van der Stoel MM, van den Berg M, et al. The MARCH6-SQLE Axis Controls Endothelial Cholesterol Homeostasis and Angiogenic Sprouting. Cell Rep. 2020;32:107944. doi: 10.1016/j.celrep.2020.107944 [DOI] [PubMed] [Google Scholar]
- 56.Pittner RA, Fears R, Brindley DN. Interactions of insulin, glucagon and dexamethasone in controlling the activity of glycerol phosphate acyltransferase and the activity and subcellular distribution of phosphatidate phosphohydrolase in cultured rat hepatocytes. Biochem J. 1985;230:525–534. doi: 10.1042/bj2300525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu Z, Jiang Z, Zhou J, et al. Involvement of insulin resistance in the protective effect of metformin against alcoholic liver injury. Alcohol Clin Exp Res. 2014;38:1510–1519. doi: 10.1111/acer.12418 [DOI] [PubMed] [Google Scholar]
- 58.Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol 2019 214. 2019;21:225–245. doi: 10.1038/s41580-019-0190-7 [DOI] [PubMed] [Google Scholar]
- 59.van Splunter M, van Osch TLJ, Brugman S, et al. Induction of trained innate immunity in human monocytes by bovine milk and milk-derived immunoglobulin G. Nutrients. 2018;10. doi: 10.3390/nu10101378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Netea MG, Domínguez-Andrés J, Barreiro LB, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo W, Chang R, Zhong J, Pandey A, Semenza GL. Histone demethylase JMJD2C is a coactivator for hypoxia-inducible factor 1 that is required for breast cancer progression. Proc Natl Acad Sci U S A. 2012;109. doi: 10.1073/pnas.1217394109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Camuzi D, de Amorim Í, Ribeiro Pinto L, Oliveira Trivilin L, Mencalha A, Soares Lima S. Regulation Is in the Air: The Relationship between Hypoxia and Epigenetics in Cancer. Cells. 2019;8:300. doi: 10.3390/cells8040300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beyer S, Kristensen MM, Jensen KS, Johansen JV, Staller P. The histone demethylases JMJD1A and JMJD2B are transcriptional targets of hypoxia-inducible factor HIF. J Biol Chem. 2008;283:36542–36552. doi: 10.1074/jbc.M804578200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia O, Lemieux ME, Li W, et al. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci U S A. 2009;106:4260–4265. doi: 10.1073/pnas.0810067106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tausendschön M, Dehne N, Brüne B. Hypoxia causes epigenetic gene regulation in macrophages by attenuating Jumonji histone demethylase activity. Cytokine. 2011;53:256–262. doi: 10.1016/j.cyto.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 66.Chakraborty D, Cui W, Rosario GX, et al. HIF-KDM3A-MMP12 regulatory circuit ensures trophoblast plasticity and placental adaptations to hypoxia. Proc Natl Acad Sci U S A. 2016;113:E7212–E7221. doi: 10.1073/pnas.1612626113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu OHF, Kiema M, Beter M, Ylä-Herttuala S, Laakkonen JP, Kaikkonen MU. Hypoxia-mediated regulation of histone demethylases affects angiogenesis-associated functions in endothelial cells. Arterioscler Thromb Vasc Biol. 2020;40:2665–2677. doi: 10.1161/ATVBAHA.120.315214 [DOI] [PubMed] [Google Scholar]
- 68.Shakespear MR, Hohenhaus DM, Kelly GM, et al. Histone deacetylase 7 promotes toll-like receptor 4-dependent proinflammatory gene expression in macrophages. J Biol Chem. 2013;288:25362–25374. doi: 10.1074/jbc.M113.496281 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.