Abstract
The use of natural products has been shown to be a fruitful approach in the discovery of novel pharmaceuticals. In fact, many currently approved drugs originated from compounds that were first identified in nature. Chemical diversity of natural compounds cannot be matched by man-made libraries of chemically synthesized molecules. Many natural compounds interact with and modulate regulatory protein targets and can be considered evolutionarily-optimized drug-like molecules. Despite this, many pharmaceutical companies have reduced or eliminated their natural product discovery programs in the last two decades. Screening natural products for pharmacologically active compounds is a challenging task that requires high resource commitment. Novel approaches at the early stage of the drug discovery pipeline are needed to allow for rapid screening and identification of the most promising molecules. Here, we review the possible evolutionary roots for drug-like characteristics of numerous natural compounds. Since many of these compounds target evolutionarily conserved cellular signaling pathways, we propose novel, early-stage drug discovery approaches to identify drug candidates that can be used for the potential prevention and treatment of neurodegenerative diseases. Invertebrate in vivo animal models of neurodegenerative disease and innovative tools used within these models are proposed here as a screening funnel to identify new drug candidates and to shuttle these hits into further stages of the drug discovery pipeline.
Keywords: drug discovery, natural products, neurodegenerative disease, specialized metabolites, immobilized transmembrane proteins
Graphical Abstract

1. Introduction
Plants, bacteria, and fungi have historically been important and rich sources of biologically active compounds [1–4]. The following are just a few of the numerous natural compounds that have been incorporated into the repertoire of widely-used and FDA approved medicines: aspirin (acetylsalicylic acid), statins, morphine, codeine, paclitaxel, penicillin, artemisinin, reserpine, colchicine, and galantamine, among many others [4–7]. Plant-derived molecules have also been extensively utilized as templates for the development of medicines currently used for the management of neurodegenerative diseases, such as Alzheimer’s (AD) or Parkinson’s (PD) disease [4, 6, 7]. Galantamine and rivastigmine (the semisynthetic derivative of physostigmine) are natural alkaloids approved for the management of AD and are classified as acetylcholinesterase inhibitors, based on the outdated cholinergic hypothesis of AD [4, 8]. Other possible mechanisms of action for these two compounds include interactions with evolutionarily conserved cellular signaling pathways [4, 9]. Levodopa (L-DOPA), the number one drug used in the management of PD, was first identified in broad beans (Vicia faba) [10]. Current therapies for patients with PD attempt to both preserve and restore dopaminergic neurotransmission [6, 7]. Following enzymatically catalyzed decarboxylation, L-DOPA is converted to the neurotransmitter dopamine, the levels of which are known to decrease in PD patients. Using compounds that bind to and activate postsynaptic dopaminergic receptors is another approach aimed at increasing dopaminergic neurotransmission. Lisuride, pergolide, and bromocryptine, known dopamine-receptor agonists, are derivatives of alkaloids first isolated from rye ergot fungus (Claviceps purpurea) [11]. Apomorphine, a non-selective dopamine receptor agonist derived from morphine, is another example of a naturally-derived compound used in the management of PD [12].
Indisputably, natural compounds have a great potential to be developed into potent pharmaceuticals. However, in the era of automated high-throughput screening techniques, the pharmaceutical industry seems to favor the screening of large libraries of synthetic molecules [13]. Yet, the number of new drugs entering the market has been still on the decline. This is especially evident in the case of drugs used to prevent or treat neurodegenerative diseases [14]. One of the most significant challenges of drug discovery from natural products is the complexity of natural samples [5]. Extensive time and effort are needed to identify and isolate specialized metabolites that can be further tested for pharmacological activity. High-throughput screening (HTS) techniques designed to test extensive libraries of individual molecules are less suitable for analyzing complex natural matrices. Additional steps, following HTS, are needed to identify the molecule(s) responsible for the observed biological effects.
This complexity of natural samples has also resulted in an abundance of studies that focus only on assessing the activity of the entire extract without the further identification of the individual compounds responsible for the observed biological effects. The specialized metabolite profile of complex natural mixtures varies from batch to batch; therefore, it is important to either identify the molecules responsible for the biological effect or to assess the complete specialized metabolite profile that yielded the observed pharmacological activity.
The simplicity of certain preliminary screening assays that can be used to evaluate select biological activities has also resulted in an overabundance of studies that focus on the antioxidant or enzyme-inhibitory activity of the entire extract. For many years, the predominant hypothesis in the field of natural product discovery has promoted the idea that direct antioxidant activity of certain specialized metabolites is majorly responsible for the health-benefits that arise from certain physical activities and dietary choices [4, 9]. However, rigorous clinical trials have disproved the direct antioxidant activity hypothesis of natural products [4]. Here, we review early pipeline strategies that may speed up the drug discovery process and that are based on the idea that natural products are evolutionarily-optimized drug-like molecules.
One of the other serious bottlenecks in drug discovery from natural products is the lack of suitable tools allowing for the identification of biologically active specialized metabolites that are present in the full extract [5]. There is a dire need to develop novel approaches to speed up the discovery of pharmacologically active compounds present in complex natural samples.
One area of disease with a desperate need for new treatment options is age-associated neurodegeneration. Interestingly, numerous natural compounds have been found to extend lifespan, to improve healthspan, and to prevent the development of neurodegenerative diseases across multiple model organisms and are currently considered to be new drug leads in the field [1–4, 9]. The following are just a few examples of natural or nature-derived compounds that have been proven to extend and improve lifespan in numerous model organisms: rapamycin (produced by bacterium Streptomyces hygroscopicus), metformin (a derivative of a natural compound galegine, isolated from Galega officinalis), and several polyphenolic compounds: resveratrol, curcumin, fisetin or quercetin [6].
Epidemiological data strongly suggests that a diet rich in fruits, vegetables and herbs is associated with a lowered risk of developing chronic, age-associated diseases, including neurodegenerative disorders [15–25]. Most of these studies associate the beneficial effects of plant-rich diets with a certain class of abundant specialized plant metabolites called polyphenols, and, more specifically, the family of polyphenols called flavonoids [26, 27]. In drug discovery literature, these plant-derived metabolites have been often considered as invalid metabolic panaceas (IMPS) that lack the essential characteristics typically associated with valuable drug leads (e.g.: lack of well-defined targets, stability, bioavailability etc.) [28–30]. Additionally, most of these natural compounds have been observed to give false-positive results in high-throughput screening assays, and therefore have been classified as PAINS (pan-assay interference compounds) [31]. Therefore, polyphenols are usually excluded at the early stages of drug discovery programs from natural samples. Although polyphenols do not possess the typical characteristics associated with many drug leads, these abundant molecules may play an important role in biological systems, as is the case with many vitamins [30].
Many non-communicable diseases are associated with chronic inflammation, that has been involved in the pathogenesis of numerous diseases, including neurodegenerative disorders [32]. Interestingly, a diet rich in plants and omega-3 fatty acids has been shown to decrease the risk of developing chronic inflammatory diseases [32]. Omega-3 derived electrophilic molecules have been shown to be important and specialized pro-resolving mediators involved in the resolution of inflammation (catabasis) [32–37]. Numerous flavonoids and their metabolites, similarly to electrophilic lipids, contain structural elements that allow them to behave like soft electrophiles: namely, α,β-unsaturated carbonyl groups that allow them to undergo Michael additions with nucleophiles, such as cysteine thiols (Fig. 1) [35, 38, 39]. We propose that certain flavonoids, together with some essential fatty acids, may fulfil the role of essential soft electrophilic molecules that are required by biological systems to resolve inflammation [40]. The presence of a reactive electrophilic center is one of the factors associated with the high reactivity of these molecules in high-throughput screening assays. Therefore, it should be encouraged to test the biological effects of these molecules in the context of living organisms. This matter of investigation may serve to verify signal selectivity and reversibility of the reaction between plant-derived soft electrophiles and thiols.
Figure 1.

Examples of natural compounds possessing electrophilic centers (α,β-unsaturated carbonyl groups) that can react as Michael acceptors: (a) zerumbone (terpenoid), (b) α-santonin (terpenoid), (c) 13-EFOX-L2 (linoleic acid electrophilic oxo-derivative), (d) apigenin (flavone), and (e) nobiletin (flavone).
This review focuses on presenting the pharmacological potential of evolutionarily-optimized drug-like natural compounds. Frequent problems in the drug discovery process from natural samples are considered and novel solutions are proposed. In the following subsections, we consider why plants, bacteria, and fungi have evolved to produce such complex molecules. Further, we review modern screening and early drug discovery pipeline approaches used in the identification of new cures derived from nature. Finally, we present the use of simple in vivo invertebrate models for the identification of neuroprotective natural compounds. Modern screening approaches, together with in vivo phenotypic assays, may serve as a screening funnel at the beginning of the drug discovery pipeline from natural products. Compounds identified in these steps may then progress to the next stages of the pipeline: validation in mammalian models.
This article focuses on the characteristics of drug discovery for the prevention and/or treatment of neurodegenerative diseases.
2. Natural products as a source of new drug hits and leads – an evolutionary perspective
Biochemical communication evolved as a simple method of information exchange between different organisms that occupied the same or overlapping ecological niches. Immobile organisms, such as plants, synthesize a great variety of specialized metabolites in response to numerous abiotic and biotic signals from the environment as an effective way to defend themselves from environmental insults [1–4, 6, 9, 41]. Many specialized metabolites produced by microorganisms and plants evolved to interact with other organisms and animals such as humans. Interestingly, the chemical structures of certain signaling molecules are conserved between microorganisms, plants, and animals [42]. This is particularly evident for terpenoids, which are synthesized by all known organisms on Earth through the mevalonate pathway (Fig. 2) [43]. It has been hypothesized that terpenoids evolved as signaling molecules before life became multicellular. The terpenoid theory of the origin of life postulates that the first cellular membranes were terpenoid-based structures, rather than phospholipid bilayers [44]. Numerous terpenoid signaling molecules might have evolved from compounds that initially played structural roles as a form of communication between unicellular forms and the surrounding environment [44]. Mevinolin, more commonly known as lovastatin, is an example of a natural compound produced by the fungus Aspergillus terreus that inhibits one of the enzymes within the mevalonate pathway, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase [45]. High evolutionary conservation of the mevalonate pathway resulted in the successful use of lovastatin as a cholesterol-reducing medication in humans. Similarly to certain polyphenols, numerous terpenoids have also been also shown to possess anti-inflammatory activity [35]. This activity has been observed in compounds that possess electrophilic centers (e.g., α,β-unsaturated carbonyl groups) that can react as Michael acceptors. Examples of these compounds include zerumbone, helenalin, α-santonin, and citral, among many others (Fig. 1) [35, 36, 38].
Figure 2.

Biosynthesis of terpenoids via mevalonate (MVA) and non-mevalonate pathway (MEP) in plants. Reused from Yang et al. [43] in accordance with the Creative Commons Attribution (CC BY) license.
Polyphenolic compounds are, without a doubt, the most intensely studied group of specialized plant metabolites. Polyphenols have been proposed to be responsible for the health-promoting effects of a diet rich in vegetables, fruits, and herbs [26, 27, 46]. Studies focused on the direct antioxidant activity of polyphenols have dominated scientific literature for many years. However, multiple rigorous clinical trials have disproved the direct antioxidant activity hypothesis of polyphenolic compounds [4].
Emerging data suggest that plant polyphenols may interact with and modulate evolutionarily conserved cellular signaling pathways [2, 4]. Howitz and Sinclair proposed that polyphenols evolved as signaling molecules in a common unicellular ancestor of animals and plants [2]. Polyphenols are ubiquitous molecules in plants and have the ability to play different roles, such as antimicrobial or antifeedant activity [47]. Some polyphenols, such as salicylates, play an important role in controlling the plant’s response to numerous biotic and abiotic stressors [48]. Interestingly, the same molecules may regulate the synthesis of proinflammatory substances in animals [33]. A comparison of oxylipin pathways illuminates the evolutionary conservation of biochemical molecules between plants and animals (Fig. 3) [48]. Therefore, polyphenols may have evolved as chemical cues that play a crucial role in the stress response in both plants and animals [47, 48]. It has been recently shown in a Drosophila model that salicylates increase the synthesis of electrophilic pro-resolving lipids involved in the resolution of inflammation [33]. Future research should focus on better understanding whether natural polyphenols act directly as electrophilic pro-resolving molecules or if they upregulate the synthesis of these physiological electrophilic pro-resolving mediators.
Figure 3.

The comparison between the plant jasmonate hormones and orthologous mammalian eicosanoids. Reproduced with permission from [48].
During evolution, the animal phyla might have lost the ability to synthesize polyphenolic compounds in the same way that humans have lost their ability to synthesize ascorbic acid or certain amino acids [2, 47]. However, animal cells still possess protein targets that respond to polyphenolic compounds. It has, furthermore, been shown that numerous polyphenols can interact with and modify the activity of various animal enzymes and transmembrane proteins in vivo [2, 4, 49]. The ability of polyphenols to interact with multiple endogenous targets has resulted in their classification as pan-assay interference compounds (PAINS) and their removal from the serious consideration as potential drug leads [31]. However, what is currently considered to be a weakness of these polyphenolic compounds may in fact be a potential stress response mechanism, via the evolutionarily conservation of biochemical molecules between organisms[2].
Throughout evolution, plants acquired the ability to synthesize molecules to protect themselves from herbivory or omnivorous predation [4, 41]. Some plant species developed biosynthetic pathways that allow them to transform compounds that might have primarily evolved as signaling molecules (terpenoids and polyphenols) into noxious phytochemicals [4, 9, 41, 50, 51]. However, another class of bitter-tasting molecules called alkaloids evolved as the most effective natural pesticide, deterring insects, as well as other herbivores and omnivores [4, 9]. Alkaloids evolved to target very specific groups of proteins, which allow them to confer biological activity at low concentrations. The majority of currently approved drugs first identified in nature are alkaloids. Examples of these include morphine, codeine, ephedrine, galantamine, quinidine, atropine, scopolamine, and papaverine, among many others [4]. Animals feeding on plants evolved warning systems to protect them from the poisonous effects of alkaloids [9]. Alkaloids evoke a bitter taste that serves as a warning to limit the intake of plants containing these chemicals. By trial and error and the observation of other animals, humans learned which plants were safe to eat and which provide health benefits. Traditional medicine approaches are oftentimes based on this knowledge acquired through generations by close observations of the surrounding environment.
Biologically active specialized metabolites are present in very complex matrices that makes the identification of these molecules challenging. The following section discusses the most common problems encountered when working with natural samples.
3. Common obstacles in drug discovery from natural matrices
One of the most challenging obstacles in drug discovery from natural products stems from the complexity of natural samples [5, 52]. The drug discovery workflow from natural matrices usually starts with testing extracts or extract fractions to identify those characterized with biological activity. The phytochemical profile of natural samples varies from batch to batch; therefore, to assure study reproducibility, a phytochemical profile of the tested samples should be provided. Alternatively, the individual compounds responsible for biological effects should be identified, isolated, and their activity confirmed. One of the major challenges in screening complex mixtures for drug leads is the lack of efficient tools to identify and isolate biologically active compounds [5]. Traditional screening methods often require the isolation of individual compounds from mixtures through chemical separation, followed by dereplication and analysis. These processes are typically time consuming, labor intensive, and costly [53]. High throughput screening techniques mainly focus on synthetic libraries of individual compounds [13]. For mixtures, laborious isolation and characterization are needed to identify the pharmacologically active molecules. The most commonly practiced approach focuses on the isolation of individual compounds followed by testing them in their purified forms. This usually leads to isolation of the most abundant novel compounds or already well-characterized molecules.
There is a critical need to develop novel drug discovery approaches to speed up the process of identification of pharmacologically active compounds present in complex samples. Modern isolation techniques, including high speed countercurrent chromatography, have been introduced to speed up the process of the isolation of targeted metabolites [54–56]. Fractions obtained with the use of these methods increases the chance for the identification of biologically active natural compounds. Several other screening approaches that may be used at the beginning of the drug discovery pipeline are reviewed in Section 4.
The biological activity of isolated compounds does not always match the activity of the entire extract [57]. This discrepancy can often be attributed to the complexity of extracts where numerous compounds exert synergistic and/or antagonistic effects. If both the extracts and individual compounds are tested in in vitro assays, the difference may stem from the fact that compounds other than the isolated one might interact with the target (i.e., an enzyme). In the case of phenotypic assays, multiple compounds may interact with numerous regulatory proteins, resulting in biological activity different from that observed for purified molecules. Another important issue related to the assessment of the biological activity of natural compounds is the purity of the isolated compounds. Oftentimes, the observed biological effects are the result of other compounds present in the isolated sample [58, 59].
Natural compounds have also been characterized by pleiotropic effects, because of their ability to interact with multiple regulatory molecules in biological systems [2, 30, 49]. This has led to the classification of many of these molecules as invalid/improbable/interfering metabolic panaceas (IMPS) and pan-assay interference compounds (PAINS) [28–31]. The predominant paradigm in drug discovery focuses on designing exquisitely selective ligands in an attempt to reduce off-target effects [30]. However, multiple diseases are associated with the dysfunction of various biochemical processes that are controlled by networks of regulatory molecules [7].
Although considered negative, pleiotropic effects exerted by natural compounds may stem from their evolutionary potential to act as signaling and regulatory molecules [2, 4, 49]. For example, many studies that have attempted to develop polyphenolic compounds into drugs have been unsuccessful, due to the inability of the polyphenols to act as selective ligands at desired biological targets [30]. This behavior may be compared to the biological activity of certain essential nutrients, such as vitamins, that interact with and modify multiple targets. Flavonoids, for example, have been previously called vitamin P; however, their vitamin-like effects have not been proven [30]. As previously mentioned, certain flavonoids may potentially play roles similar to that of essential fatty acids (previously referred to as vitamin F) in the regulation of inflammation by acting as essential electrophilic molecules. Future research should explore whether certain natural polyphenols, together with omega-3 fatty acid derivatives, act as essential electrophilic molecules involved in important nucleophilic addition reactions. Dietary phytochemicals have been considered to fall into the category of nutritional dark matter, an underrecognized class of small molecules that may play an important role in human nutrition beyond traditional nutrients [60]. Future research should consider the possibility that ubiquitous specialized metabolites may possibly play important roles as essential nutrients.
It must also be noted that, in many cases, the reported biological activity of natural molecules is only tested in in vitro assays, without further verification in vivo. This may be very problematic for molecules such as polyphenols which contain highly reactive groups, such as α,β-unsaturated carbonyls or hydrogen-bond forming hydroxyl groups. These molecules may be tagged as reactive when tested in in vitro assays that contain isolated proteins or cells. Additionally, polyphenols may react with other molecules present in the assay mixture, resulting in false positive results. To better understand the biological potential of these molecules, it is important to have them tested in the context of a living organism [6, 7]. Additionally, in vitro assays often use high concentrations of the investigated molecules that may activate stress response pathways in the cells used in these assays. Activation of these pathways may mask the actual pharmacological potential that the investigated compounds may exert in vivo.
There is a need to use simple and high-throughput invertebrate drug screening assays at the beginning of the drug discovery pipeline in order to to verify the activity of natural compounds in the context of a living organism at physiologically relevant doses. The use of simple, efficient, and high-throughput Drosophila and C. elegans assays in the identification of natural neuroprotective compounds is reviewed in Section 5.
4. Novel screening solutions to speed up the identification of bioactive compounds interacting with regulatory proteins
Different screening approaches have been proposed to speed up the identification of biologically active specialized metabolites present in complex matrices [5, 52]. Previously published papers have thoroughly reviewed techniques most commonly used in the drug discovery process [5, 52]. Here, we discuss the general principles of several screening techniques that can be used in the facilitation of the identification of biologically active compounds present in complex natural mixtures. Since numerous drugs used in the treatment of neurological disorders target transmembrane proteins, special emphasis is placed on approaches utilizing immobilized transmembrane proteins.
One of the first screening approaches used in drug discovery from complex natural samples involve either the use of filtration techniques or cytoplasmic proteins [5, 52]. Detailed descriptions of these techniques, along with several examples of their use, can be found elsewhere [5, 52, 61–65].
In the ultrafiltration approach, semi-permeable membranes are applied to identify small molecules interacting with cytoplasmic proteins [63]. The targeted protein is incubated with the studied mixture, then followed by the ultrafiltration step. The complex formed between the cytosolic protein and the biologically active compounds do not permeate through the membrane, allowing for the identification of compounds interacting with the protein. Ultrafiltration requires relatively high concentrations of the targeted protein, as well as the use of approaches that allow for the discrimination between specific and nonspecific interactions between protein target and small molecules. Despite the aforementioned difficulties, several successful applications of ultrafiltration have been reported. For more details regarding this technique and for relevant examples, please refer to other review articles [5, 52].
The immobilization of cytosolic proteins is another example of an approach developed to speed up the identification of pharmacologically active metabolites [5, 52, 66–75]. First, the cytosolic protein is attached to the surface of a column adsorbent (bioaffinity chromatography) or magnetic bead surface (ligand fishing). In this approach, the targeted protein is immobilized through chemical bonds and the protein C or N terminus. Since the immobilization process involves the use of covalent bonds, one of the crucial elements of the experimental procedure is to determine the activity of the protein after the immobilization process. This procedure is not useful for proteins whose activity is substantially altered by the immobilization procedure. Several successful applications of the immobilized cytosolic proteins have been reported in the identification of natural products modifying protein activity [5, 66–75]. To avoid the alteration of cytosolic protein activity, several approaches that do not involve the immobilization step have been proposed. One of the approaches involves the incubation of the targeted protein in a reaction coil positioned past the chromatographic column [65]. Following the separation step on a chromatographic column, the compounds are then directed into the reaction coil, where they are allowed to interact with the investigated protein. The interaction between cytosolic proteins and small molecules is detected based on the formation of a new product, inhibition of the enzymatic reaction, or decreased fluorescence.
While the development of novel screening techniques has been relatively successful for cytosolic proteins, more than half of the drug discovery programs target transmembrane proteins. However, the immobilization of transmembrane proteins can be even more challenging, as the activity of these targets depends also on preserving subtle interactions between these proteins and lipid bilayer. Several analytical approaches have been proposed, including cellular membrane affinity chromatography (CMAC) (Fig. 4), cell membrane coated (encapsulated) magnetic nanoparticles (Fig. 5), and carbon nanotubes modified with magnetic nanoparticles, which allow for the preservation of proteins in their natural bilayer environment [5, 52, 76, 77].
Figure 4.

Figure (a) Fully assembled cell membrane affinity chromatography column after packing IAM particles with the immobilized cell membrane fragments into a glass column; (b) Frontal chromatograms of (a) increasing concentrations of [3H]-epibatidine (A =240 pM, B=150 pM, C=100 pM, D=80 pM and E=60 pM) on the α3β4α5 nicotinic receptor column. Panel (b) reproduced with permission from [76].
Figure 5.
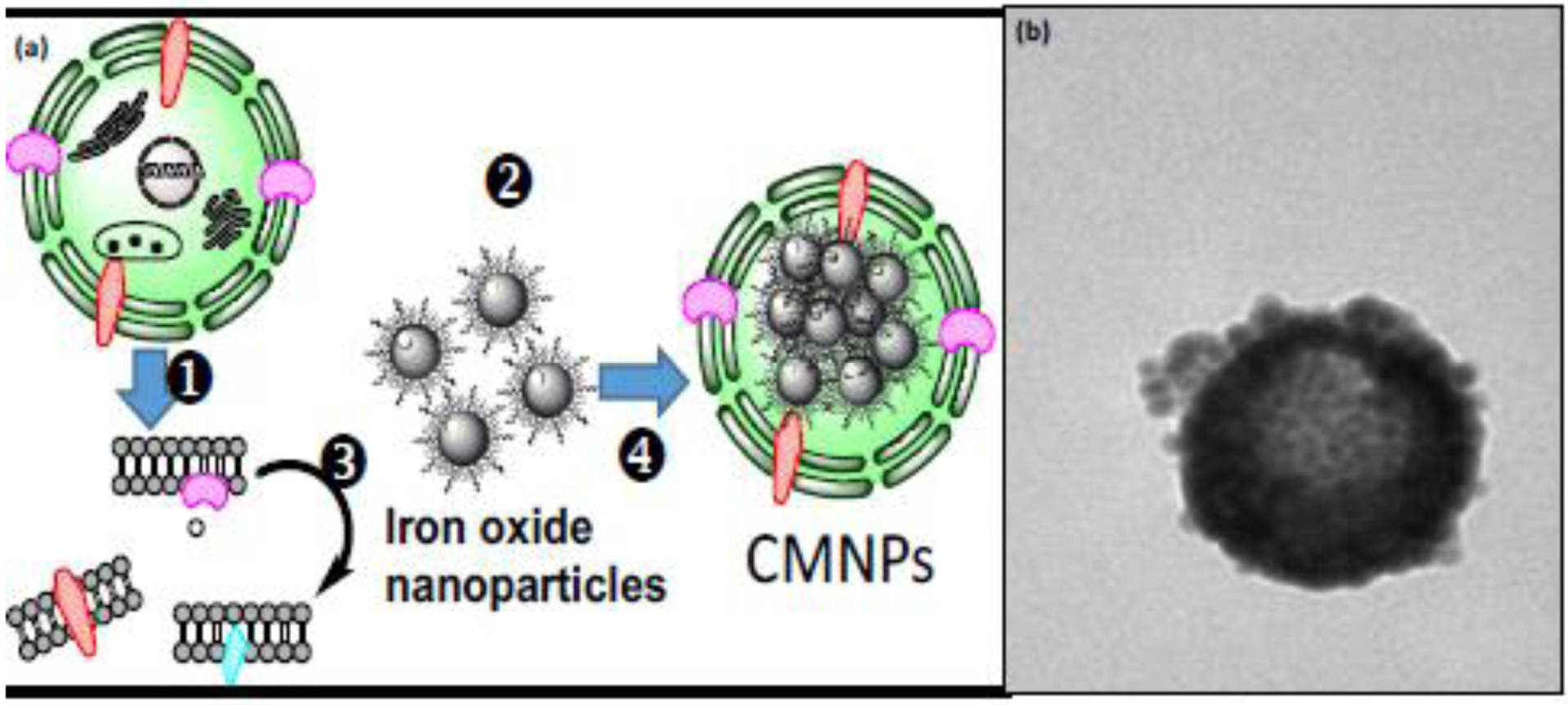
(a) Overview of cell membrane encapsulated nanoparticles formation: (1) cells expressing targeted proteins, (2) iron oxide nanoparticles, (3) cell membrane fragments obtained after cell lysis, and (4) cell membrane encapsulated nanoparticles; (b) typical transmission electron miscroscope image of cell membrane encapsulated nanoparticles. Panel (a) reproduced with permission from [77].
Cell membrane fragments can be immobilized on a solid surface and subsequently used to screen complex matrices for compounds interacting specifically with the transmembrane targets. In all cases, the first step involves acquiring cell membrane fragments with the studied transmembrane protein. Cell lines stably overexpressing the proteins are usually used, although the application of human and animal tissues have also been reported. Cell membranes are harvested after cell lysis and the removal of cellular organelles using centrifugation at different speeds. Cell membrane fragments are subsequently immobilized on the surface ofartificial membrane stationary phase particles (CMAC preparation) or used to encapsulate iron oxide nanoparticles or carbon nanotubes. Transmembrane receptors are immobilized without the formation of covalent bonds and still submerge into the lipid bilayer. The adsorption of cell membrane fragments without using covalent bonds excludes any unwanted modification of the functionality of the transmembrane proteins.
Different classes of transmembrane proteins have been reported to be successfully immobilized using CMAC technology, including ligand-gated ion channels, GPCRs, and protein transporters [5, 78–84]. Recently developed nanoparticle encapsulation technology has been proven useful in the immobilization of ligand-gated ion channels and tyrosine kinase receptors and applied in the screening of complex mixtures for new ligands binding to nicotinic receptors, tropomyosin receptor kinase B, and epidermal growth factor receptors [52, 77, 85]. Discerning between specific and nonspecific interactions is of crucial importance when using both CMAC and encapsulated nanoparticle technology. To rule out nonspecific interactions, negative control CMAC columns and cell membrane encapsulated nanoparticles are prepared using parental cell lines without the targeted protein. Small molecules that interact with the columns or the cell membrane coated nanoparticles that express the targeted protein, but that don’t interact with the negative control columns/nanoparticles, are the molecules specifically interacting with the targeted protein. The identification of compounds that specifically interact with the studied target allows researchers to focus the isolation process only on those compounds and, therefore, speeds up the drug discovery process.
The activation of neurotrophic and neurogenic factors, as well as signaling pathways, such as brain-derived neurotrophic factor (BDNF) and neurotrophic tyrosine kinase receptor 2 (TrkB), has been gaining much attention in the possible prevention and treatment of numerous neurological disorders, including major depressive disorder and neurodegenerative diseases [4, 86]. It has been recently shown that major antidepressant drugs act by directly binding to TrkB neurotrophin receptors [86]. Certain natural compounds have also been previously shown to bind to TrkB, to mimic the effects of BDNF, and toactivate the BDNF and TrkB signaling pathway [85, 87]. TrkB neurotrophin receptors can be immobilized on the surface of chemically modified nanoparticles or artificial cell membrane fragments and used to screen natural samples, as was recently shown for gotu kola plant extracts [85]. Additionally, these immobilization techniques can also be utilized to study the interactions between the isolated compounds and the immobilized receptor [5, 76]. It has been shown that TrkB signaling is linked to cholesterol levels and that cholesterol regulates TrkB signaling [86]. Our group has also recently been exploring the use of CMAC technology in assessing different levels of cholesterol on the interaction between immobilized TrkB neurotrophin receptors and natural compound ligands (unpublished data).
For more details regarding the preparation of CMAC columns and cell membrane encapsulated nanoparticles, please refer to previously published literature [5, 52].
One of the critical decisions to make before using any of the presented screening techniques is to choose the plant species to be tested [88]. Some approaches commonly used in ethnopharmacology is either consulting historical documents that report the use of plant species as therapeutics or interviewing informants that may possess generational knowledge of a plant’s medicinal abilities [89]. This approach lies at the foundation of one of the most recent and successful discoveries in natural product history: artemisinin, a potent antimalarial drug discovered by Dr. Youyou Tu. Dr. Tu consulted historical Traditional Chinese Medicine texts to identify plants that were used in the treatment of malaria and fever. This led to her eventual isolation of artemisinin, a potent antiprotozoal sesquiterpene lactone [89].
Although effective in the identification of compounds interacting with regulatory proteins, there is a chance that the screening assays may not be translatable or congruent with biological activity in vivo. Therefore, in order to develop and maintain a sustainable drug discovery pipeline, we argue that it is important to combine these approaches with in vivo screening. We propose the use of simple model organisms, such as D. melanogaster or C. elegans, at the beginning of the drug discovery pipeline combined with the use of natural product mixtures. Below, we present examples of high-throughput animal models of neurodegenerative diseases used to identify extracts with potential neuroprotective effects. Complex natural mixtures that prevent and/or treat neurodegenerative disorders can then be further screened for the compounds responsible for observed effects.
5. High-throughput invertebrate animal models for the identification of neuroprotective compounds
As discussed, working your way down the drug discovery pipeline can be a long and arduous process. Screening thousands of potential drug candidates can be time-consuming and costly, and often leads to uncertain success. There are many ways in which one can speed up the process of drug discovery while still ensuring valuable data is being collected, but choosing the right model organism to begin this process is of the utmost importance. The use of mammalian models tends to be extremely financially straining; rodents also have a long lifespan and relatively slow reproductive rates, making data collection take much longer than would be necessary in small invertebrate models, such as D. melanogaster or C. elegans. Therefore, choosing a small invertebrate model organism for large-scale screening of potential drug leads is an indisputable way to accelerate the discovery of novel pharmaceuticals.
5.1. Drosophila models of neurodegenerative diseases
Drosophila melanogaster, commonly known as the fruit fly, has been a powerful tool in genetics research and modeling human disease [90–93]. The completion of the Drosophila genome sequencing in 2000 revealed a high degree of conserved molecular pathways between the fly and human, with an estimated of 75% of known human disease-linked genes being conserved in the fly [94, 95]. The annotated genome sequence of Drosophila is readily accessible through the online database “Flybase”. The presence of evolutionarily conserved genes with similar molecular functions makes Drosophila an ideal model to unravel the pathogenic mechanisms of human disease, including neurodegenerative diseases.
Advantages:
Neurodegenerative diseases are mostly progressive disorders of the nervous system. These diseases pose a massive economic burden, due to the rise in the aging population. In recent years, several disease-associated genes have been identified in familial cases of Alzheimer’s (AD), Parkinson’s (PD), Huntington’s disease (HD), leading to the development of animal models to study the disease pathogenesis for therapeutic intervention [93]. The availability of these animal models helps to gain insight into disease mechanisms, since most human studies involve analysis of postmortem tissues that fail to reflect early molecular changes during the pre-symptomatic stage. However, the use of rodent models during the early stages of the drug discovery pipeline is relatively costly, time-consuming, and poses ethical issues. Furthermore, in vitro screening in cell culture models often fails to mirror pathogenic events in the context of the whole organism. Drosophila offers several advantages over traditional rodent models, due to the rapid generation cycle, shorter life span, and lower maintenance cost [96]. The shorter lifespan allows for faster mating and generation of a larger number of progeny in a shorter period of time, thereby reducing the overall experimental cost. Although there are differences in terms of the gross anatomical structure of the brain between humans and flies, several disease-associated genes and pathways are conserved. In addition, the simplicity of the Drosophila nervous system allows researchers to dissect the molecular function of target genes involved in the pathogenic mechanisms underlying neurodegeneration (Fig. 6).
Figure 6.

Drosophila models of neurodegenerative diseases in the identification of neuroprotective compounds from natural products.
Screening platform for therapeutics:
The availability of different genetic tools for targeting in vivo gene manipulation makes Drosophila a lucrative model for screening natural products against neurodegenerative diseases like AD, PD and HD for therapeutic intervention. Several gene manipulation approaches have been successfully applied to generate transgenic fly models of neurodegenerative diseases that are readily available at the Bloomington Stock Center (http://fly.bio.indiana.edu/). For example, the GAL4-UAS binary system was first described by Brand and Perrimon, which allows for the tissue or neuronal subtype-specific expression of dominant mutants of genes, including α-synuclein-A30P or LRRK2-G2019S to model disease phenotype in transgenic flies [97, 98]. RNA interference (RNAi) constructs can also be used with GAL4 drivers for targeted gene deletions in specific cells using the GAL4-UAS system [99]. Furthermore, specific neurons can also be labeled using fluorescent markers like green fluorescent proteins (GFP) that can be visualized using microscopy techniques [100]. Both forward and reverse genetic screens have been applied in fly models to unravel gene targets and molecular mechanisms underlying disease pathology in several neurodegenerative diseases [96]. In AD, excessive accumulation of amyloid-beta (Aβ) peptides is thought to induce neuronal dysfunction, and transgenic fly models of AD are generated that encode human amyloid precursor protein (APP) and mimic human disease pathology [101]. Drosophila models of AD provide a powerful toolkit to investigate not only the underlying pathogenic events during disease progression but also to screen and identify pharmacological modifiers of the disease phenotype [102]. Similarly, fly models of PD exhibit similar disease phenotypes, including loss of dopaminergic (DA) neurons, mobility defects, oxidative stress, mitochondrial dysfunction, and protein aggregation [103, 104]. Moreover, Drosophila models have linked α-synuclein with tau, thereby suggesting highly conserved pathways between human and fly models in PD pathogenesis [105]. Transgenic flies overexpressing the human mutant Huntingtin (HTT) gene has been shown to recapitulate human disease phenotypes, including protein aggregation, neurodegeneration, and a reduced lifespan [106]. Aging remains the primary risk factor for the majority of neurodegenerative disorders, including AD and PD [107]. Interestingly, Drosophila models serve as an excellent platform for age-related studies due to short lifespan, which makes it attractive for both life and health span studies [108, 109]. Drosophila models have been routinely used to screen and identify secondary plant metabolites that confer life span extension and improve overall health status. For example, several recent studies have employed Drosophila models to study the effects of both single compounds, such as the flavanol epicatechin and the flavonol fisetin, as well as complex extracts like cocoa powder, green tea extract, Ludwigia octovalvis extract, apple polyphenols, and black tea extract on lifespan and healthy aging. Another recent study identified the geroprotective effects of certain flavonoids like naringin, luteolin, chrysin in both Drosophila and C.elegans models along with underlying molecular mechanisms [46, 110]. Our lab has recently used the environmental toxin-induced PD model in flies to identify a polymethoxyflavonoid, Gardenin A, that confers neuroprotection by regulating the neuroinflammatory and cellular death responses [7]. Collectively, these neurodegenerative models provide an ideal in vivo platform for evaluating the efficacy and safety profile of drug candidates in the context of whole live animals [111].
Phenotypic screening tools:
Drosophila models of neurodegenerative disease also offer several advantages as a screening platform for new drug discovery, due to the easily observable phenotypes associated with a specific disease condition [112, 113]. For example, most neurodegenerative diseases generally exhibit age-dependent slowness or loss of locomotor ability. PD is a movement disorder characterized by rigidity, tremor, mobility defects, and postural instability. The negative geotaxis climbing assay is a fast and reliable way to monitor locomotion defects in flies based on their natural tendency to climb up against gravity [114]. Simple feeding assays can be employed to monitor the therapeutic effect of natural products by either mixing in the food or filter paper saturated with sucrose [115]. The toxicity levels can be assessed by survival assays to obtain optimal drug concentrations that can rescue the disease phenotype. Survival assays can also be used to examine the effect of potential drug candidates on lifespan and developmental defects. The loss of a specific subset of neurons is one of the most prominent features of neurodegenerative diseases, which can be easily monitored by microscopy techniques using fluorescent markers to tag specific neuronal subsets. For example, the loss of dopaminergic neurons and neuronal degeneration in the hippocampus have been observed in transgenic fly models of PD and AD, respectively, and can be used as a screening tool to monitor neuronal health for novel therapeutics [101, 103]. Eye degeneration in transgenic fly models can also be used as a tool for pharmacological screening [92]. For example, the GAL4-UAS system-driven overexpression of the Aβ and tau or the α-syn transgene involved in AD and PD, respectively, induces apoptotic eye degeneration, leading to reduced eye size and ocular deformity [116]. Dementia and learning impairment are the characteristic symptoms of AD, which can be assessed in transgenic fly models by courtship behavior and olfaction tests [117]. The availability of powerful genetics tools and relatively simple phenotypic assays, along with their low maintenance and experimental costs, make Drosophila a versatile in vivo platform for screening the therapeutic potential of natural products [118].
Limitations:
There are certain drawbacks of using Drosophila models to study neurodegenerative disease. Fly models don’t allow the researcher to accurately predict the dosage of drugs that is directly applicable to humans and should, therefore, be validated in mammalian models. In terms of drug testing, it is important to be aware of the differences in the physiology of the blood-brain barrier between mammals and invertebrates that might have differential effects on permeability and toxicity. However, the basis of neuronal transmission appears to be regulated by similar mechanisms in flies and humans [119]. Although fruit flies lack an adaptive immune system which might lead to differential immune responses in the context of certain neurological disorders [120], it serves as an ideal model to study innate immune responses associated with neurodegenerative diseases, due to the highly conserved mechanistic pathways between flies and humans. Flies also do not express certain pathogenic factors (e.g., α-Syn) that are important in human disorders. However, the vast availability of genetic tools, including the Gal4/UAS system and evolutionarily conserved molecular pathways, make it easier to model and study human diseases in flies effectively. Despite these limitations, the incorporation of invertebrate models such as Drosophila during the initial stages of drug screening have the ability to accelerate the discovery of positive hits in the context of a whole organism at a reasonable cost and leading to subsequent validation in mammalian models.
5.2. C. elegans models of neurodegenerative diseases
Caenorhabditis elegans is a small roundworm—about 1 mm in length at maturity. The legacy of C. elegans began when Dr. Sydney Brenner published a paper on its genetics. He was determined to establish this nematode as a model organism in research within cell and molecular biology by publishing a paper on its genetics [121]. He was also particularly interested in neuronal development, and his efforts eventually earned him the Nobel Prize in Physiology or Medicine in 2002. Since that time, C. elegans has become the only organism in history to have a completed diagram of neuronal circuitry [122], as well as the first multicellular organism to have its genome fully sequenced. This genomic sequencing, as well as other screenings, have led to the discovery that approximately 38% of genes in worms have a functional human ortholog—a necessary attribute in studying neurodegenerative disease [123]. Furthermore, visualization of the expression patterns of specific genes in live animals, such as worms, was made possible in 1994 with Dr. Martin Chalfie’s introduction of green fluorescent protein (GFP) reporter technology [124]. In recent history, C. elegans as a model organism has grown to become an essential tool in many research laboratories—used to gain a greater understanding of the molecular mechanisms of many diseases.
Advantages:
C. elegans is easily cultured in the lab because of its minute size, short lifespan, and rapid reproductive rates. It is non-parasitic, and its transparent anatomy makes it a very useful organism for genetic screening. The majority of the animals within the species are hermaphroditic [125], with about one in every thousand being male. These males can be induced to become more abundant in subsequent generations by methods such as heat shock, and they possess a specialized projection on their tails which is used strategically for mating [126]. This trait makes crossing strains in this organism a straightforward task. C. elegans is also one of the simplest organisms with a well characterized nervous system. In the hermaphrodite, this system is composed of a total of 302 neurons [127]. These animals are also capable of being frozen for long-term storage and are viable when thawed, making it possible to create a “worm library” containing a multitude of different strains which is useable for years [128].
Screening platform for therapeutics:
Genetic manipulation is relatively simple in C. elegans. This makes studying the effects of differential gene expression in regards to neurodegeneration and the application of potential drug candidates a valuable tool in these worms. One of the ways in which this process is done is by a gene knock-down technology called RNA interference (RNAi)—a process first discovered in C. elegans itself. This strategy involves first delivering double-stranded RNA (dsRNA) to the worms by methods such as soaking in a concentrated buffer, feeding of bacteria expressing dsRNA, or injection [129]. It has also been found that worms neuronally expressing SID-1, a transmembrane protein, have an increased response to being fed dsRNA [130]. This dsRNA delivery leads to the specific degradation of the corresponding mRNA, effectively knocking down the gene of interest and its expression with the nematode [129]. Fortunately, RNAi feeding libraries are commercially available—two of which being the Ahringer and the Vidal library. These libraries contain clones of the vast majority of all C. elegans genes, making the likelihood that one’s gene of interest is available to be knocked down with RNAi quite probable. The availability of this technology and its use in any larval stage of development in C. elegans makes it possible to strategically knock down a variety of genes in the worms, as well as to study their effects in regards to neurodegenerative diseases, such as AD, PD, or HD, as well as any potential compounds being tested to treat them.
Another advantageous tool used when screening a variety of drug candidates to combat neurodegenerative disease within C. elegans is the use of transgenic animals that either overexpress or ectopically express certain genes. The gene of interest is generally associated with a certain fluorescent marker, such as GFP or mCherry (a monomeric red fluorescent protein) [131]. These animals can be synthesized using DNA transformation by microinjection. This involves injecting manipulated genetic material into the gonads of hermaphrodites at the L4 stage of development. This genetic material then enters the developing oocytes, where it will be expressed with variable probability in the F1 generation. After successful microinjection, the use of a co-injection marker allows for worms expressing the mutation to be specifically picked under a fluorescent dissecting scope. Furthermore, the modulation of gene expression within these transgenic animals is typically achieved by promoter-driven expression. It is possible to direct the selected transgene to be expressed within the neuronal subtype associated with specific neurodegenerative disease pathology [132]. For example, Alzheimer’s disease can be characterized by the presence of amyloid-beta (Aβ) plaques and hyperphosphorylated tau aggregates called neurofibrillary tangles [133]. It has also been shown that the glutamatergic neurons within the hippocampus are the subtype primarily affected by these Aβ-aggregates [134]. Therefore, when studying Alzheimer’s disease in C. elegans, one might use a transgenic strain which expresses Aβ strictly within the glutamatergic neurons. Using this combination of both amyloid-beta and GFP being expressed within the glutamatergic neurons, one can rapidly and effectively study the effects of Alzheimer’s disease within live animals, as well as screen a multitude of compounds for their potential efficacy in fighting this disease. This method is also translatable to a wide variety of neurodegenerative diseases.
Phenotypic screening tools:
There are a wide variety of assays that can be used in order to study the effects of various compounds on neurodegenerative disease and aging in C. elegans (Fig. 7). Neuronal subtypes expressing a fluorescent marker allow for the scoring of neuronal health within the worms at various days post-hatching. Pharyngeal pumping assays can be used to measure either the frequency or amplitude of pharynx contractions. These parameters can be used as indicators of neuronal health. Using an eyelash pick, soft-touch assays can be performed on the worms in order to assay mechanosensation. This is done by gently stroking the worm at specific areas of both the head and tail, in order to stimulate six precise sensory neurons and assay their health. A healthy worm should respond to the touch by moving either forward or backward in response to it [135]. Furthermore, dopaminergic-mediated basal slowing responses within the worms can also be used to study diseases like Parkinson’s. This mechanosensory response is described as the natural slowing in locomotion that occurs whenever a healthy worm encounters food. This response is dopamine-dependent and therefore can be used to study the neurodegeneration that occurs within Parkinson’s Disease [136]. Lifespan assays, as a method of detecting changes in the aging process, have also been extensively used in C. elegans to assay various gene knockdowns and potential drug candidates [137]. In fact, both the natural products baicalein [138] and quercetin [46] have been shown to increase both stress resistance and lifespan in C. elegans using an assay such as this. Even memory, by the use of habituation assays, can be analyzed within these extremely flexible animals. These assays an include a variety of both visual and touch stimuli [139]. These are just a handful of the assays out of the very large toolbox that C. elegans researchers have to draw from. As evidenced above, C. elegans is an incomparable resource to be used within the first steps of the drug discovery pipeline.
Figure 7.

C. elegans models of neurodegenerative diseases in the identification of neuroprotective compounds from natural products.
Limitations:
As with any disease model, there are certain drawbacks to the use of C. elegans as a model organism in the drug discovery pipeline. While the minute size of the worm leads to ease of handling and storage, this small stature can also be a limitation when it comes to biochemical analysis. Traditionally, this means that whole worm extracts must be analyzed rather than singular animals. This can cause uncertainty when attempting to isolate a tissue-specific signal. As with Drosophila, compound testing in worms also cannot give the researcher an accurate prediction of the concentration or dosage of the compound that will be beneficial in humans. Therefore, both the safety and efficacy of these compounds must be further verified by the use of mammalian models. C. elegans also lacks the complex body plan and organ structure seen in higher mammals, including a lack of most internal organs, a circulatory system, skeletal elements, and defined fat cells. This can cause concern over the translatability of pharmacological data from worms to humans. Despite these limitations, the utilization of a C. elegans model of disease within the introductory phases of the drug discovery process is a distinguished way to facilitate the screening of potential drug leads with rapid turnaround rates and at a low cost to the researcher.
6. Conclusions
Specialized metabolites synthesized by plants, bacteria, and fungi play an important role in the process of discovery of novel drug hits and leads. To overcome the common problems encountered when working with natural products, novel approaches are proposed to be introduced at the beginning of the drug discovery pipeline. The combination of bioaffinity based screening assays along with high-throughput in vivo invertebrate animal models is recommended as a screening funnel in both building and maintaining a sustainable drug discovery pipeline for neurodegenerative disease. This concept can be easily optimized with other disease models by using properly designed bioaffinity assays and appropriate invertebrate animal models. This screening funnel facilitates the identification of biologically active molecules that interact with regulatory proteins and provides the opportunity to verify the activity of the identified compounds in the context of a living organism. While mostly pursued as potential drug candidates, certain specialized metabolites characterized with pleiotropic effects may better fall into the category of essential nutrients. Future research should consider the possibility that certain abundant dietary phytochemicals lacking drug-lead characteristics may play an essential role in biological systems, as is the case with other essential nutrients such as vitamins or fatty acids. Discovery of pharmacologically active compounds via the use of natural products is both a historical and promising source of successful drug leads.
Highlights.
Specialized metabolites can be considered as evolutionarily-optimized drug-like molecules.
Novel screening approaches speed up the identification of pharmacologically active compounds.
Biological activity of phytochemicals should be tested early in the context of a living organism.
7. Acknowledgements
This work was supported by the National Center for Complimentary and Integrative Medicine of the National Institutes of Health under award number 1R41AT011716-01; NSF-CBET 1915873; Merrymac-McKcinley Foundation Award; American of Pharmacognosy Research Starter Grant and Regis Technologies grant to L.C.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authorship contribution statement
Urmila Maitra – Conceptualization; Writing- Original draft preparation; Writing- Reviewing and Editing;
Cayman Stephen – Conceptualization; Writing- Original draft preparation; Writing- Reviewing and Editing;
Lukasz Ciesla – Conceptualization; Writing- Original draft preparation; Writing- Reviewing and Editing; Project administration; Funding acquisition.
Declaration of Competing Interest
Lukasz Ciesla collaborates with Regis Technologies, the provider of the IAM.PC.DD2 particles utilized in the development of CMAC technology.
8. References
- [1].Lamming DW, Wood JG, Sinclair DA, Small molecules that regulate lifespan: evidence for xenohormesis, Mol Microbiol 53(4) (2004) 1003–9. [DOI] [PubMed] [Google Scholar]
- [2].Howitz KT, Sinclair DA, Xenohormesis: sensing the chemical cues of other species, Cell 133(3) (2008) 387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baur JA, Sinclair DA, What is Xenohormesis?, Am J Pharmacol Toxicol 3(1) (2008) 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee J, Jo DG, Park D, Chung HY, Mattson MP, Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: focus on the nervous system, Pharmacol Rev 66(3) (2014) 815–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ciesla L, Moaddel R, Comparison of analytical techniques for the identification of bioactive compounds from natural products, Nat Prod Rep 33(10) (2016) 1131–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maitra U, Ciesla L, Using Drosophila as a platform for drug discovery from natural products in Parkinson’s disease, Medchemcomm 10(6) (2019) 867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maitra U, Harding T, Liang Q, Ciesla L, GardeninA confers neuroprotection against environmental toxin in a Drosophila model of Parkinson’s disease, Commun Biol 4(1) (2021) 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu PP, Xie Y, Meng XY, Kang JS, History and progress of hypotheses and clinical trials for Alzheimer’s disease, Signal Transduct Target Ther 4 (2019) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mattson MP, What Doesn"T Kill You, Sci Am 313(1) (2015) 40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hornykiewicz O, L-DOPA: from a biologically inactive amino acid to a successful therapeutic agent, Amino Acids 23(1–3) (2002) 65–70. [DOI] [PubMed] [Google Scholar]
- [11].Hoyer D, Targeting the 5-HT system: Potential side effects, Neuropharmacology 179 (2020) 108233. [DOI] [PubMed] [Google Scholar]
- [12].Borgemeester RW, Lees AJ, van Laar T, Parkinson’s disease, visual hallucinations and apomorphine: A review of the available evidence, Parkinsonism Relat Disord 27 (2016) 35–40. [DOI] [PubMed] [Google Scholar]
- [13].Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, Rollinger JM, Schuster D, Breuss JM, Bochkov V, Mihovilovic MD, Kopp B, Bauer R, Dirsch VM, Stuppner H, Discovery and resupply of pharmacologically active plant-derived natural products: A review, Biotechnol Adv 33(8) (2015) 1582–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Munafo A, Burgaletto C, Di Benedetto G, Di Mauro M, Di Mauro R, Bernardini R, Cantarella G, Repositioning of Immunomodulators: A Ray of Hope for Alzheimer’s Disease?, Front Neurosci 14 (2020) 614643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fitzgerald KN, Hodges R, Hanes D, Stack E, Cheishvili D, Szyf M, Henkel J, Twedt MW, Giannopoulou D, Herdell J, Logan S, Bradley R, Potential reversal of epigenetic age using a diet and lifestyle intervention: a pilot randomized clinical trial, Aging (Albany NY) 13(7) (2021) 9419–9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao L, Lee JY, Hwang DH, Inhibition of pattern recognition receptor-mediated inflammation by bioactive phytochemicals, Nutr Rev 69(6) (2011) 310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Corbi G, Conti V, Davinelli S, Scapagnini G, Filippelli A, Ferrara N, Dietary Phytochemicals in Neuroimmunoaging: A New Therapeutic Possibility for Humans?, Front Pharmacol 7 (2016) 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Davinelli S, Maes M, Corbi G, Zarrelli A, Willcox DC, Scapagnini G, Dietary phytochemicals and neuro-inflammaging: from mechanistic insights to translational challenges, Immun Ageing 13 (2016) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ostan R, Lanzarini C, Pini E, Scurti M, Vianello D, Bertarelli C, Fabbri C, Izzi M, Palmas G, Biondi F, Martucci M, Bellavista E, Salvioli S, Capri M, Franceschi C, Santoro A, Inflammaging and cancer: a challenge for the Mediterranean diet, Nutrients 7(4) (2015) 2589–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Martucci M, Ostan R, Biondi F, Bellavista E, Fabbri C, Bertarelli C, Salvioli S, Capri M, Franceschi C, Santoro A, Mediterranean diet and inflammaging within the hormesis paradigm, Nutr Rev 75(6) (2017) 442–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Szarc vel Szic K, Declerck K, Vidakovic M, Vanden Berghe W, From inflammaging to healthy aging by dietary lifestyle choices: is epigenetics the key to personalized nutrition?, Clin Epigenetics 7 (2015) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dean E, Gormsen Hansen R, Prescribing optimal nutrition and physical activity as “first-line” interventions for best practice management of chronic low-grade inflammation associated with osteoarthritis: evidence synthesis, Arthritis 2012 (2012) 560634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ruiz-Nunez B, Pruimboom L, Dijck-Brouwer DA, Muskiet FA, Lifestyle and nutritional imbalances associated with Western diseases: causes and consequences of chronic systemic low-grade inflammation in an evolutionary context, J Nutr Biochem 24(7) (2013) 1183–201. [DOI] [PubMed] [Google Scholar]
- [24].Agarwal P, Wang Y, Buchman AS, Holland TM, Bennett DA, Morris MC, MIND Diet Associated with Reduced Incidence and Delayed Progression of ParkinsonismA in Old Age, J Nutr Health Aging 22(10) (2018) 1211–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT, MIND diet associated with reduced incidence of Alzheimer’s disease, Alzheimers Dement 11(9) (2015) 1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bondonno NP, Dalgaard F, Kyro C, Murray K, Bondonno CP, Lewis JR, Croft KD, Gislason G, Scalbert A, Cassidy A, Tjonneland A, Overvad K, Hodgson JM, Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort, Nat Commun 10(1) (2019) 3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yeh TS, Yuan C, Ascherio A, Rosner B, Willett W, Blacker D, Long-term Dietary Flavonoid Intake and Subjective Cognitive Decline in US Men and Women, Neurology (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bisson J, McAlpine JB, Friesen JB, Chen SN, Graham J, Pauli GF, Can Invalid Bioactives Undermine Natural Product-Based Drug Discovery?, J Med Chem 59(5) (2016) 1671–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA, The Essential Medicinal Chemistry of Curcumin, J Med Chem 60(5) (2017) 1620–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Seigler J.B.F. David S., Bisson Jonathan, Graham James G., Bedran-Russo Ana, McAlpine James B., and Pauli Guido F., Vitamin P– Do Certain Polyphenolic IMPS Have a Vital Function?, 2020. [DOI] [PMC free article] [PubMed]
- [31].Baell J, Walters MA, Chemistry: Chemical con artists foil drug discovery, Nature 513(7519) (2014) 481–3. [DOI] [PubMed] [Google Scholar]
- [32].Basil MC, Levy BD, Specialized pro-resolving mediators: endogenous regulators of infection and inflammation, Nat Rev Immunol 16(1) (2016) 51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Panettieri S, Paddibhatla I, Chou J, Rajwani R, Moore RS, Goncharuk T, John G, Govind S, Discovery of aspirin-triggered eicosanoid-like mediators in a Drosophila metainflammation blood tumor model, J Cell Sci 133(5) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schopfer FJ, Cipollina C, Freeman BA, Formation and signaling actions of electrophilic lipids, Chem Rev 111(10) (2011) 5997–6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Arshad L, Jantan I, Bukhari SN, Haque MA, Immunosuppressive Effects of Natural alpha,beta-Unsaturated Carbonyl-Based Compounds, and Their Analogs and Derivatives, on Immune Cells: A Review, Front Pharmacol 8 (2017) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jackson PA, Widen JC, Harki DA, Brummond KM, Covalent Modifiers: A Chemical Perspective on the Reactivity of alpha,beta-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions, J Med Chem 60(3) (2017) 839–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Serhan CN, Pro-resolving lipid mediators are leads for resolution physiology, Nature 510(7503) (2014) 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Poganik JR, Aye Y, Electrophile Signaling and Emerging Immuno- and Neuro-modulatory Electrophilic Pharmaceuticals, Front Aging Neurosci 12 (2020) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rucker H, Al-Rifai N, Rascle A, Gottfried E, Brodziak-Jarosz L, Gerhauser C, Dick TP, Amslinger S, Enhancing the anti-inflammatory activity of chalcones by tuning the Michael acceptor site, Org Biomol Chem 13(10) (2015) 3040–7. [DOI] [PubMed] [Google Scholar]
- [40].LoPachin RM, Geohagen BC, Nordstroem LU, Mechanisms of soft and hard electrophile toxicities, Toxicology 418 (2019) 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mattson MP, Cheng A, Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses, Trends Neurosci 29(11) (2006) 632–9. [DOI] [PubMed] [Google Scholar]
- [42].Kushiro T, Nambara E, McCourt P, Hormone evolution: The key to signalling, Nature 422(6928) (2003) 122. [DOI] [PubMed] [Google Scholar]
- [43].Yang D, Du X, Liang X, Han R, Liang Z, Liu Y, Liu F, Zhao J, Different roles of the mevalonate and methylerythritol phosphate pathways in cell growth and tanshinone production of Salvia miltiorrhiza hairy roots, PLoS One 7(11) (2012) e46797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ourisson G, Nakatani Y, The terpenoid theory of the origin of cellular life: the evolution of terpenoids to cholesterol, Chem Biol 1(1) (1994) 11–23. [DOI] [PubMed] [Google Scholar]
- [45].Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E, Patchett A, Monaghan R, Currie S, Stapley E, Albers-Schonberg G, Hensens O, Hirshfield J, Hoogsteen K, Liesch J, Springer J, Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent, Proc Natl Acad Sci U S A 77(7) (1980) 3957–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pallauf K, Duckstein N, Rimbach G, A literature review of flavonoids and lifespan in model organisms, Proc Nutr Soc 76(2) (2017) 145–162. [DOI] [PubMed] [Google Scholar]
- [47].Stafford HA, Flavonoid evolution: an enzymic approach, Plant Physiol 96(3) (1991) 680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kennedy DO, Polyphenols and the human brain: plant “secondary metabolite” ecologic roles and endogenous signaling functions drive benefits, Adv Nutr 5(5) (2014) 515–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Barrajon-Catalan E, Herranz-Lopez M, Joven J, Segura-Carretero A, Alonso-Villaverde C, Menendez JA, Micol V, Molecular promiscuity of plant polyphenols in the management of age-related diseases: far beyond their antioxidant properties, Adv Exp Med Biol 824 (2014) 141–59. [DOI] [PubMed] [Google Scholar]
- [50].Mattson MP, Son TG, Camandola S, Viewpoint: mechanisms of action and therapeutic potential of neurohormetic phytochemicals, Dose Response 5(3) (2007) 174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Murugaiyah V, Mattson MP, Neurohormetic phytochemicals: An evolutionary-bioenergetic perspective, Neurochem Int 89 (2015) 271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hou X, Sun M, Bao T, Xie X, Wei F, Wang S, Recent advances in screening active components from natural products based on bioaffinity techniques, Acta Pharm Sin B 10(10) (2020) 1800–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Harvey AL, Edrada-Ebel R, Quinn RJ, The re-emergence of natural products for drug discovery in the genomics era, Nat Rev Drug Discov 14(2) (2015) 111–29. [DOI] [PubMed] [Google Scholar]
- [54].Skalicka-Wozniak K, Garrard I, Counter-current chromatography for the separation of terpenoids: a comprehensive review with respect to the solvent systems employed, Phytochem Rev 13 (2014) 547–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang QW, Lin LG, Ye WC, Techniques for extraction and isolation of natural products: a comprehensive review, Chin Med 13 (2018) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bucar F, Wube A, Schmid M, Natural product isolation--how to get from biological material to pure compounds, Nat Prod Rep 30(4) (2013) 525–45. [DOI] [PubMed] [Google Scholar]
- [57].Caesar LK, Cech NB, Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2, Nat Prod Rep 36(6) (2019) 869–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pauli GF, Chen SN, Simmler C, Lankin DC, Godecke T, Jaki BU, Friesen JB, McAlpine JB, Napolitano JG, Importance of purity evaluation and the potential of quantitative (1)H NMR as a purity assay, J Med Chem 57(22) (2014) 9220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jaki BU, Franzblau SG, Chadwick LR, Lankin DC, Zhang F, Wang Y, Pauli GF, Purity-activity relationships of natural products: the case of anti-TB active ursolic acid, J Nat Prod 71(10) (2008) 1742–8. [DOI] [PubMed] [Google Scholar]
- [60].Bland JS, The Dark Matter of Nutrition: Dietary Signals Beyond Traditional Nutrients, Integr Med (Encinitas) 18(2) (2019) 12–15. [PMC free article] [PubMed] [Google Scholar]
- [61].van Breemen RB, Tao Y, Li W, Cyclooxygenase-2 inhibitors in ginger (Zingiber officinale), Fitoterapia 82(1) (2011) 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Liu J, Burdette JE, Xu H, Gu C, van Breemen RB, Bhat KP, Booth N, Constantinou AI, Pezzuto JM, Fong HH, Farnsworth NR, Bolton JL, Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms, J Agric Food Chem 49(5) (2001) 2472–9. [DOI] [PubMed] [Google Scholar]
- [63].Yang Z, Zhang Y, Sun L, Wang Y, Gao X, Cheng Y, An ultrafiltration high-performance liquid chromatography coupled with diode array detector and mass spectrometry approach for screening and characterising tyrosinase inhibitors from mulberry leaves, Anal Chim Acta 719 (2012) 87–95. [DOI] [PubMed] [Google Scholar]
- [64].Li H, Song F, Xing J, Tsao R, Liu Z, Liu S, Screening and structural characterization of alpha-glucosidase inhibitors from hawthorn leaf flavonoids extract by ultrafiltration LC-DAD-MS(n) and SORI-CID FTICR MS, J Am Soc Mass Spectrom 20(8) (2009) 1496–503. [DOI] [PubMed] [Google Scholar]
- [65].Potterat O, Hamburger M, Concepts and technologies for tracking bioactive compounds in natural product extracts: generation of libraries, and hyphenation of analytical processes with bioassays, Nat Prod Rep 30(4) (2013) 546–64. [DOI] [PubMed] [Google Scholar]
- [66].Moaddel R, Marszall MP, Bighi F, Yang Q, Duan X, Wainer IW, Automated ligand fishing using human serum albumin-coated magnetic beads, Anal Chem 79(14) (2007) 5414–7. [DOI] [PubMed] [Google Scholar]
- [67].Marszall MP, Moaddel R, Kole S, Gandhari M, Bernier M, Wainer IW, Ligand and protein fishing with heat shock protein 90 coated magnetic beads, Anal Chem 80(19) (2008) 7571–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sanghvi M, Moaddel R, Wainer IW, The development and characterization of protein-based stationary phases for studying drug-protein and protein-protein interactions, J Chromatogr A 1218(49) (2011) 8791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lourenco Vanzolini K, Jiang Z, Zhang X, Vieira LC, Correa AG, Cardoso CL, Cass QB, Moaddel R, Acetylcholinesterase immobilized capillary reactors coupled to protein coated magnetic beads: a new tool for plant extract ligand screening, Talanta 116 (2013) 647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Singh N, Ravichandran S, Spelman K, Fugmann SD, Moaddel R, The identification of a novel SIRT6 modulator from Trigonella foenum-graecum using ligand fishing with protein coated magnetic beads, J Chromatogr B Analyt Technol Biomed Life Sci 968 (2014) 105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wubshet SG, Brighente IM, Moaddel R, Staerk D, Magnetic Ligand Fishing as a Targeting Tool for HPLC-HRMS-SPE-NMR: alpha-Glucosidase Inhibitory Ligands and Alkylresorcinol Glycosides from Eugenia catharinae, J Nat Prod 78(11) (2015) 2657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].de Moraes MC, Santos JB, Dos Anjos DM, Rangel LP, Vieira TC, Moaddel R, da Silva JL, Prion protein-coated magnetic beads: synthesis, characterization and development of a new ligands screening method, J Chromatogr A 1379 (2015) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Marszall MP, Sroka WD, Sikora A, Chelminiak D, Ziegler-Borowska M, Siodmiak T, Moaddel R, Ligand fishing using new chitosan based functionalized Androgen Receptor magnetic particles, J Pharm Biomed Anal 127 (2016) 129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhao YM, Wang LH, Luo SF, Wang QQ, Moaddel R, Zhang TT, Jiang ZJ, Magnetic beads-based neuraminidase enzyme microreactor as a drug discovery tool for screening inhibitors from compound libraries and fishing ligands from natural products, J Chromatogr A 1568 (2018) 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhang Y, Wang Q, Liu R, Zhou H, Crommen J, Moaddel R, Jiang Z, Zhang T, Rapid screening and identification of monoamine oxidase-A inhibitors from Corydalis Rhizome using enzyme-immobilized magnetic beads based method, J Chromatogr A 1592 (2019) 1–8. [DOI] [PubMed] [Google Scholar]
- [76].Ciesla L, Okine M, Rosenberg A, Dossou KSS, Toll L, Wainer IW, Moaddel R, Development and characterization of the alpha3beta4alpha5 nicotinic receptor cellular membrane affinity chromatography column and its application for on line screening of plant extracts, J Chromatogr A 1431 (2016) 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sherwood J, Sowell J, Beyer N, Irvin J, Stephen C, Antone AJ, Bao Y, Ciesla LM, Cell-membrane coated iron oxide nanoparticles for isolation and specific identification of drug leads from complex matrices, Nanoscale 11(13) (2019) 6352–6359. [DOI] [PubMed] [Google Scholar]
- [78].Kitabatake T, Moaddel R, Cole R, Gandhari M, Frazier C, Hartenstein J, Rosenberg A, Bernier M, Wainer IW, Characterization of a multiple ligand-gated ion channel cellular membrane affinity chromatography column and identification of endogenously expressed receptors in astrocytoma cell lines, Anal Chem 80(22) (2008) 8673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Maciuk A, Moaddel R, Haginaka J, Wainer IW, Screening of tobacco smoke condensate for nicotinic acetylcholine receptor ligands using cellular membrane affinity chromatography columns and missing peak chromatography, J Pharm Biomed Anal 48(2) (2008) 238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Moaddel R, Oliveira RV, Kimura T, Hyppolite P, Juhaszova M, Xiao Y, Kellar KJ, Bernier M, Wainer IW, Initial synthesis and characterization of an alpha7 nicotinic receptor cellular membrane affinity chromatography column: effect of receptor subtype and cell type, Anal Chem 80(1) (2008) 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bhatia PA, Moaddel R, Wainer IW, The synthesis and characterization of cellular membrane affinity chromatography columns for the study of human multidrug resistant proteins MRP1, MRP2 and human breast cancer resistant protein BCRP using membranes obtained from Spodoptera frugiperda (Sf9) insect cells, Talanta 81(4–5) (2010) 1477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Moaddel R, Bighi F, Yamaguchi R, Patel S, Ravichandran S, Wainer IW, Stereoselective binding of chiral ligands to single nucleotide polymorphisms of the human organic cation transporter-1 determined using cellular membrane affinity chromatography, Anal Biochem 401(1) (2010) 148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Moaddel R, Musyimi HK, Sanghvi M, Bashore C, Frazier CR, Khadeer M, Bhatia P, Wainer IW, Synthesis and characterization of a cellular membrane affinity chromatography column containing histamine 1 and P2Y(1) receptors: a multiple G-protein coupled receptor column, J Pharm Biomed Anal 52(3) (2010) 416–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Habicht KL, Singh NS, Khadeer MA, Shimmo R, Wainer IW, Moaddel R, Characterization of a multiple endogenously expressed adenosine triphosphate-binding cassette transporters using nuclear and cellular membrane affinity chromatography columns, J Chromatogr A 1339 (2014) 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Arituluk JHZC, Adhikari B, Steltzner J, Mansur S, Ahirwar P, Velu SE, Gray NE, Ciesla* LM, and Bao Y, Identification of TrkB Binders from Complex Matrices Using a Magnetic Drug Screening Nanoplatform, ACS Applied Bio Materials 4(8) (2021) 6244–6255. [DOI] [PubMed] [Google Scholar]
- [86].Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, Biojone C, Cannarozzo C, Sahu MP, Kaurinkoski K, Brunello CA, Steinzeig A, Winkel F, Patil S, Vestring S, Serchov T, Diniz C, Laukkanen L, Cardon I, Antila H, Rog T, Piepponen TP, Bramham CR, Normann C, Lauri SE, Saarma M, Vattulainen I, Castren E, Antidepressant drugs act by directly binding to TRKB neurotrophin receptors, Cell 184(5) (2021) 1299–1313 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Chen C, Wang Z, Zhang Z, Liu X, Kang SS, Zhang Y, Ye K, The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer’s disease, Proc Natl Acad Sci U S A 115(3) (2018) 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kellogg JJ, Paine MF, McCune JS, Oberlies NH, Cech NB, Selection and characterization of botanical natural products for research studies: a NaPDI center recommended approach, Nat Prod Rep 36(8) (2019) 1196–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tu Y, The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine, Nat Med 17(10) (2011) 1217–20. [DOI] [PubMed] [Google Scholar]
- [90].Bilen J, Bonini N, Drosophila as a model for human neurodegenerative disease, Annu Rev Genet. 39 (2005) 153–71. [DOI] [PubMed] [Google Scholar]
- [91].Bonini N, Fortini M, Human neurodegenerative disease modeling using Drosophila., Annu Rev Neurosci. 26 (2003) 627–56. [DOI] [PubMed] [Google Scholar]
- [92].Pandey UB, Nichols CD, Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery, Pharmacol Rev 63(2) (2011) 411–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].McGurk l., Berson A, Bonini N, Drosophila as an In Vivo Model for Human Neurodegenerative Disease, GENETICS 201(2) (2015) 377–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Reiter LT, Potocki L, Chien S, Gribskov M, Bier E, A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster, Genome Res 11(6) (2001) 1114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rubin GM, Yandell MD, Wortman JR, Gabor Miklos GL, Nelson CR, Hariharan IK, Fortini ME, Li PW, Apweiler R, Fleischmann W, Cherry JM, Henikoff S, Skupski MP, Misra S, Ashburner M, Birney E, Boguski MS, Brody T, Brokstein P, Celniker SE, Chervitz SA, Coates D, Cravchik A, Gabrielian A, Galle RF, Gelbart WM, George RA, Goldstein LS, Gong F, Guan P, Harris NL, Hay BA, Hoskins RA, Li J, Li Z, Hynes RO, Jones SJ, Kuehl PM, Lemaitre B, Littleton JT, Morrison DK, Mungall C, O'Farrell PH, Pickeral OK, Shue C, Vosshall LB, Zhang J, Zhao Q, Zheng XH, Lewis S, Comparative genomics of the eukaryotes, Science 287(5461) (2000) 2204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hales K, Korey C, Larracuente A, Roberts D, Genetics on the Fly: A Primer on the Drosophila Model System, Genetics 201(3) (2015) 815–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Brand AH, Perrimon N, Targeted gene expression as a means of altering cell fates and generating dominant phenotypes, Development 118(2) (1993) 401–15. [DOI] [PubMed] [Google Scholar]
- [98].Feany M, Bender W, A Drosophila model of Parkinson’s disease, Nature 404(6776) (2000) 394–398. [DOI] [PubMed] [Google Scholar]
- [99].Venken KJ BH, Transgenesis upgrades for Drosophila melanogaster, Development 134(20) (2007) 3571–3584. [DOI] [PubMed] [Google Scholar]
- [100].Xie T, Ho MCW, Liu Q, Horiuchi W, Lin CC, Task D, Luan H, White BH, Potter CJ, Wu MN, A Genetic Toolkit for Dissecting Dopamine Circuit Function in Drosophila, Cell Rep 23(2) (2018) 652–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wang J, Gu BJ, Masters CL, Wang YJ, A systemic view of Alzheimer disease - insights from amyloid-beta metabolism beyond the brain, Nat Rev Neurol 13(10) (2017) 612–623. [DOI] [PubMed] [Google Scholar]
- [102].Prussing K, Voigt A, Schulz JB, Drosophila melanogaster as a model organism for Alzheimer’s disease, Mol Neurodegener 8 (2013) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Whitworth AJ, Drosophila models of Parkinson’s disease, Adv Genet 73 (2011) 1–50. [DOI] [PubMed] [Google Scholar]
- [104].Xiong Y, Yu J, Modeling Parkinson’s Disease in Drosophila: What Have We Learned for Dominant Traits?, Front Neurol 9 (2018) 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Roy B, Jackson GR, Interactions between Tau and alpha-synuclein augment neurotoxicity in a Drosophila model of Parkinson’s disease, Hum Mol Genet 23(11) (2014) 3008–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lewis EA, Smith GA, Using Drosophila models of Huntington’s disease as a translatable tool, J Neurosci Methods 265 (2016) 89–98. [DOI] [PubMed] [Google Scholar]
- [107].Tanner CM, Epidemiology of Parkinson’s disease, Neurol Clin 10(2) (1992) 317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Brandt A, Vilcinskas A, The Fruit Fly Drosophila melanogaster as a Model for Aging Research, Adv Biochem Eng Biotechnol 135 (2013) 63–77. [DOI] [PubMed] [Google Scholar]
- [109].Piper MDW, Partridge L, Drosophila as a model for ageing, Biochim Biophys Acta Mol Basis Dis 1864(9 Pt A) (2018) 2707–2717. [DOI] [PubMed] [Google Scholar]
- [110].Lashmanova E, Zemskaya N, Proshkina E, Kudryavtseva A, Volosnikova M, Marusich E, Leonov S, Zhavoronkov A, Moskalev A, The Evaluation of Geroprotective Effects of Selected Flavonoids in Drosophila melanogaster and Caenorhabditis elegans, Front Pharmacol 8 (2017) 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cha SJ, Do HA, Choi HJ, Lee M, Kim K, The Drosophila Model: Exploring Novel Therapeutic Compounds against Neurodegenerative Diseases, Antioxidants (Basel) 8(12) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Giacomotto J, Segalat L, High-throughput screening and small animal models, where are we?, Br J Pharmacol 160(2) (2010) 204–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Maitra U, Ciesla L, Using Drosophila as a platform for drug discovery from natural products in Parkinson’s disease, Med. Chem. Comm (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Gargano J, Martin I, Bhandari P, Grotewiel M, Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila, Exp Gerontol 40(5) (2005) 386–95. [DOI] [PubMed] [Google Scholar]
- [115].Ja W, Carvalho G, Mak E, de la Rosa N, Fang A, Liong J, Brummel T, Benzer S, Prandiology of Drosophila and the CAFE assay, Proc Natl Acad Sci U S A 104(20) (2007) 8253–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lim JK, Li QX, He Z, Vingrys AJ, Wong VH, Currier N, Mullen J, Bui BV, Nguyen CT, The Eye As a Biomarker for Alzheimer’s Disease, Front Neurosci 10 (2016) 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Iijima-Ando K, Iijima K, Transgenic Drosophila models of Alzheimer’s disease and tauopathies, Brain Struct Funct 214(2–3) (2010) 245–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Fernandez-Hernandez I, Scheenaard E, Pollarolo G, Gonzalez C, The translational relevance of Drosophila in drug discovery, EMBO Rep 17(4) (2016) 471–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Limmer S, Weiler A, Volkenhoff A, Babatz F, Klambt C, The Drosophila blood-brain barrier: development and function of a glial endothelium, Front Neurosci 8 (2014) 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Fabian DK, Fuentealba M, Donertas HM, Partridge L, Thornton JM, Functional conservation in genes and pathways linking ageing and immunity, Immun Ageing 18(1) (2021) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Brenner S, The genetics of Caenorhabditis elegans, Genetics 77(1) (1974) 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].White JG, Southgate E, Thomson JN, Brenner S, The structure of the nervous system of the nematode Caenorhabditis elegans, Philos Trans R Soc Lond B Biol Sci 314(1165) (1986) 1–340. [DOI] [PubMed] [Google Scholar]
- [123].Shaye DD, Greenwald I, OrthoList: a compendium of C. elegans genes with human orthologs, PLoS One 6(5) (2011) e20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC, Green fluorescent protein as a marker for gene expression, Science 263(5148) (1994) 802–5. [DOI] [PubMed] [Google Scholar]
- [125].Kaletta T, Hengartner MO, Finding function in novel targets: C. elegans as a model organism, Nat Rev Drug Discov 5(5) (2006) 387–98. [DOI] [PubMed] [Google Scholar]
- [126].Nguyen CQ, Hall DH, Yang Y, Fitch DH, Morphogenesis of the Caenorhabditis elegans male tail tip, Dev Biol 207(1) (1999) 86–106. [DOI] [PubMed] [Google Scholar]
- [127].Hobert O, Neurogenesis in the nematode Caenorhabditis elegans, WormBook (2010) 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Stiernagle T.J.C.e., Maintenance of C. elegans, 2 (1999) 51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Grishok A, RNAi mechanisms in Caenorhabditis elegans, FEBS Lett 579(26) (2005) 5932–9. [DOI] [PubMed] [Google Scholar]
- [130].Calixto A, Chelur D, Topalidou I, Chen X, Chalfie M, Enhanced neuronal RNAi in C. elegans using SID-1, Nat Methods 7(7) (2010) 554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Link CD, elegans C models of age-associated neurodegenerative diseases: lessons from transgenic worm models of Alzheimer’s disease, Exp Gerontol 41(10) (2006) 1007–13. [DOI] [PubMed] [Google Scholar]
- [132].Berkowitz LA, Knight AL, Caldwell GA, Caldwell KA, Generation of stable transgenic C. elegans using microinjection, J Vis Exp (18) (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Bloom GS, Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis, JAMA Neurol 71(4) (2014) 505–8. [DOI] [PubMed] [Google Scholar]
- [134].Crimins JL, Pooler A, Polydoro M, Luebke JI, Spires-Jones TL, The intersection of amyloid beta and tau in glutamatergic synaptic dysfunction and collapse in Alzheimer’s disease, Ageing Res Rev 12(3) (2013) 757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Chalfie M, Hart AC, Rankin CH, Goodman MB, Assaying mechanosensation, WormBook (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Schmidt E, Seifert M, Baumeister R, Caenorhabditis elegans as a model system for Parkinson’s disease, Neurodegener Dis 4(2–3) (2007) 199–217. [DOI] [PubMed] [Google Scholar]
- [137].Sutphin GL, Kaeberlein M, Measuring Caenorhabditis elegans life span on solid media, J Vis Exp (27) (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Havermann S, Humpf HU, Watjen W, Baicalein modulates stress-resistance and life span in C. elegans via SKN-1 but not DAF-16, Fitoterapia 113 (2016) 123–7. [DOI] [PubMed] [Google Scholar]
- [139].Sugi T, Ohtani Y, Kumiya Y, Igarashi R, Shirakawa M, High-throughput optical quantification of mechanosensory habituation reveals neurons encoding memory in Caenorhabditis elegans, Proc Natl Acad Sci U S A 111(48) (2014) 17236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


