Abstract
Emotions are time-varying internal states that promote survival in the face of dynamic environments and shifting homeostatic needs. Research in non-human organisms has recently afforded specific insights into the neural mechanisms that support the emergence, persistence, and decay of affective states. Concurrently, a separate affective neuroscience literature has begun to dissect the neural bases of affective dynamics in humans. However, the circuit-level mechanisms identified in animals lack a clear mapping to the human neuroscience literature. As a result, critical questions pertaining to the neural bases of affective dynamics in humans remain unanswered. To address these shortcomings, the present review integrates findings from humans and non-human organisms to highlight the neural mechanisms that govern the temporal features of emotional states. Using the theory of affective chronometry as an organizing framework, we describe the specific neural mechanisms and modulatory factors that arbitrate the rise-time, intensity, and duration of emotional states.
Keywords: affective dynamics, affective chronometry, Emotion, neural mechanisms, Neurobiology, Neuroimaging
1. Introduction
1.1. Defining Emotion
There are multiple definitions of emotion. Anderson and Adolphs (2014) argue that emotion “constitutes an internal, central (as in central nervous system) state, which is triggered by specific stimuli (extrinsic or intrinsic to the organism).” These emotion states then give rise to an array of behavioral, cognitive, somatic and physiological responses (Anderson & Adolphs, 2014). Others state that an organism’s emotional experience is constructed and resultant from an array of behavioral, cognitive, somatic and physiological inputs (Barrett et al., 2007). Still others use the term affect in a similar manner to Adolphs and Anderson’s emotion. For example, Bliss-Moreau (2017) suggests that affect is “a global state characterized by valence and arousal…Stimuli are said to have affective value when they can perturb an individual’s allostasis, creating an affective state. The perturbation of allostasis is therefore the mechanism that produces affect. Thus, affective value is a barometer indexing an organism’s idiographic relationship to the environment. Critically, affect allows for efficient navigation of the environment in order to meet survival needs by signaling which stimuli and con-specifics may be harmful or beneficial.” (pg. 185, Bliss-Moreau, 2017). Indeed, there remains considerable controversy surrounding the precise definition of “emotion” (Adolphs et al., 2019).
Throughout this manuscript, we define emotion similarly to Anderson and Adolph’s definition. We discuss emotion states as time-varying internal states that are triggered by stimuli appraised as having some value or relevance for the organism. In addition, these states can be elicited by either extrinsic or intrinsic events. As Anderson and Adolphs suggest in their working definition, emotions yield a range of flexible subjective, behavioral, and physiological outputs (Anderson & Adolphs, 2014; Frijda, 1988; Scherer, 2005).
We also differentiate emotions from prolonged “mood states.” There are two main ways that can be used to distinguish emotions from mood states. First, compared with moods, emotions tend to be responses to “concrete events, objects, and situations” (Mulligan & Scherer, 2012). Relatedly, emotions are briefer than mood states. Enduring moods generally emerge from one or more independent emotional episodes and may not have a single precipitant (Mulligan & Scherer, 2012).
In summary, we assert that emotions are central nervous system states that are linked to an array of behavioral, cognitive, somatic and physiological responses evolved to meet survival needs. Defining emotions this way enables affective scientists to operationalize emotions as “states expressed by observable behaviors” (Adolphs et al., 2019). As discussed in the sections that follow, this working definition facilitates cohesive cross-species investigations of the neural mechanisms that support affective dynamics.
1.2. Function of Emotion
Emotions are considered to be adaptive for survival, as they prime approach towards and avoidance of appetitive or aversive stimuli, respectively (Carver & White, 1994; Olds & Olds, 1963). Across species, these adaptive responses can range from observable behaviors in animals to internal subjective feeling states in humans (Lazarus, 1991). For example, reflexive startle responses in rodents constitute simple and observable behavioral outputs from neural emotion states that ultimately aid survival (Landis and Hunt, 1939; Anderson & Adolphs, 2014). In humans, similar reflexive startle responses, such as eye blinks, are associated with emotion states (Hamm, 2015). Moreover, complex emotional responses, such as subjective feelings in humans, have likely emerged via progressive evolutionary modification of the neural circuits that govern simple reflexes in lower species (Tucker et al., 2000). Indeed, reflexive startle and other adaptive behavioral responses in animals are widely referred to as ‘emotion primitives’ (Panksepp, 2004), as they represent evolutionary antecedents to human emotional responses.
Though human emotional states and non-human instinctual behaviors used to operationalize affective states in animal research may differ in complexity, emotional states across all species exhibit functional and dynamic similarities. For example, organisms as simple as fruit flies (D. melanogaster) exhibit stronger and more persistent avoidance behaviors as the intensity of threatening stimuli increases (Gibson, 2015; Jung, 2020). Similarly, in humans, aversive stimuli of increasing intensity elicit more robust conditioned startle responses over time (Turpin et al., 2003). These dynamics are also present in humans’ subjective reporting of emotion, as naturalistic work suggests that intense, unexpected outcomes elicit stronger and more enduring perturbations to self-reported positive and negative emotion (Shepperd & McNulty, 2002; Villano et al., 2020).
Regardless of the complexity of the emotional response, both animal and human emotional responses to motivationally salient stimuli are similarly beholden to modification through learning and experience. Across phylogeny, organisms as simple as sea slugs and insects exhibit conditioned behavioral responses to aversive stimuli (Castellucci et al., 1970; Tully & Quinn, 1985). Likewise, human emotional responses to even neutral stimuli vary as a function of learning and experience (Dolan, 2002; Labar et al., 1995). Therefore, animal models may yield insights into the mechanisms through which factors such as learning and experience modulate the dynamics of human emotion states.
Across species, emotion states are inherently dynamic responses that vary around one’s “baseline” state, or homeostatic set point. Shifts away from a homeostatic baseline can be evoked by stimuli that are unexpected or novel and appraised as appetitive or aversive. As stimuli in the environment change, an organism’s attention and behavioral responses must adapt accordingly. Thus, emotional states are initiated, persist, and decay relative to the organism’s evolving external or internal homeostatic environment (Heller, 2018). All emotional states naturally decay over time and regress to the organism’s baseline, homeostatic state. However, some emotional states may also persist, even long after an emotion-evoking stimulus dissipates.
Fundamentally, such emotional dynamics emerge from patterns of neuronal activity (Anderson & Adolphs, 2014; LeDoux, 2000; Panksepp, 2011). Neural activity underlies central internal states that yield physiological, behavioral, and cognitive processes associated with emotion. Therefore, efforts to characterize the nature and dynamics of emotions will benefit from an improved characterization of their underlying neural circuits. Across species, these emotional dynamics depend on several evolutionarily conserved neural circuit motifs.
First, emotional states endure through persistent neural activity (Major & Tank, 2004). While varying emotional responses may depend on distinct neural circuits, the persistence of activity within these circuits ultimately dictates the duration of an emotional response.
Second, different emotional states may arise from overlapping regions or circuits. Animal models suggest that distinct brain regions or subnuclei within the same region can support opposing emotional responses (Berridge, 2019). Thus, specific patterns of neural activity between regions can also explain variability in the dynamics and content of emotional states. These patterns may include within-region synchronous activity or phasic entrainment between regions. As discussed below, patterns of coordinated activity support the transmission of information throughout the brain, and as a result, the emergence of emotional states.
Third, the dynamics of emotional states are mediated differentially by distinct neurotransmitters. Neurotransmitters alter the properties, firing patterns, and behavioral outputs of neural circuits (Marder, 2012). Thus, a single population of neurons might contribute to two entirely different emotional responses depending on neurotransmitter type and concentration (Flavell et al., 2013; Marder, 2012). Broadly, neurotransmitter activity enables coordinated, global shifts from baseline states to states associated with emotional responding.
1.3. Affective chronometry
The model of affective chronometry provides a guiding theoretical framework to characterize the temporal features of emotional responses (Davidson, 1998; Solomon & Corbit, 1974; Tomkins, 1978). The three primary parameters on which the emotional time course varies are: 1) rise-time, or the latency between stimulus onset and the initial peak of an emotional response, 2) amplitude, or the peak of an emotional response, and 3) duration, or the length of time before an emotional response returns to baseline. As has been suggested, emotional time-courses are not simple and may have multiple peaks (Verduyn et al., 2009). Such circumstances would certainly affect the duration and may simultaneously impact the rise-time or intensity parameters. The time course of any emotional response can be described by the combination of these three parameters (Figure 1).
Figure 1. Graphical representation of the three affective chronometry parameters: rise-time, intensity, and duration.
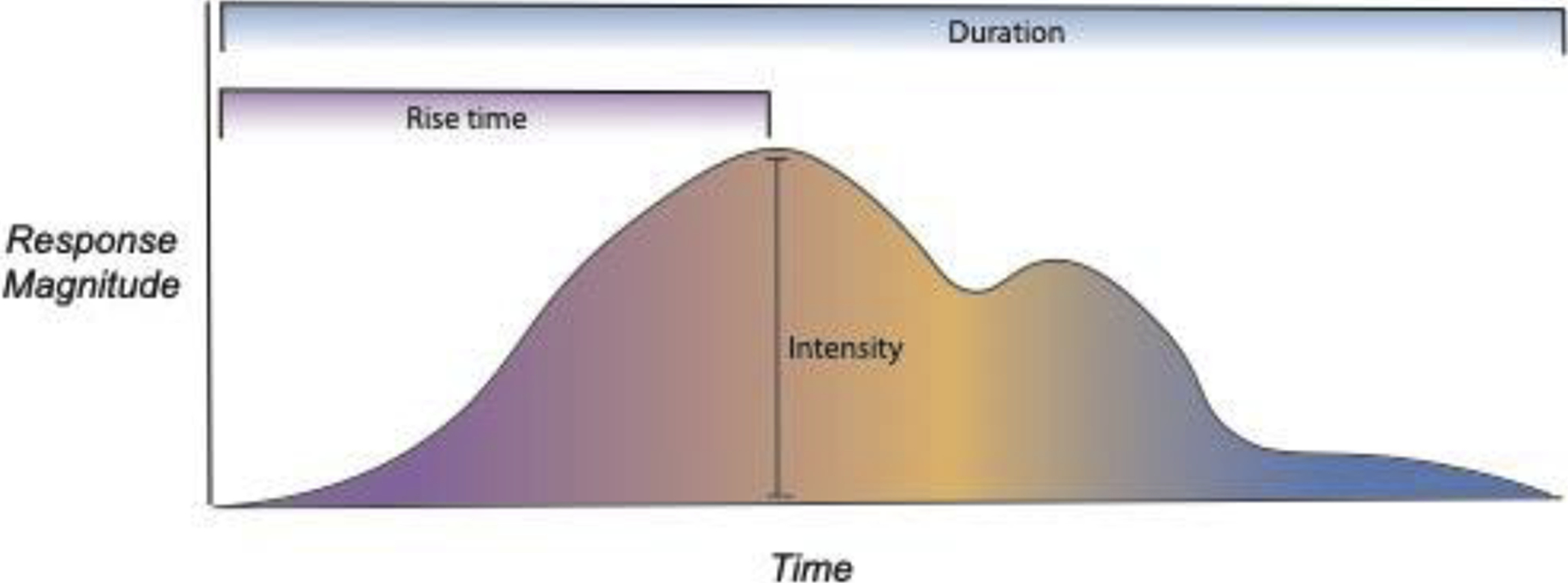
The three affective chronometry parameters collectively describe the time course of an emotional response. While these parameters are theoretically independent and are governed by partially separable neural mechanisms, they nevertheless influence each other. For instance, a more intense emotional perturbation may involve a longer rise-time and take longer to decay, thus increasing the duration of the episode.
In the following sections we describe how neural circuits supporting emotion give rise to the rise-time, amplitude, and duration of emotion. These emotional dynamic parameters are interrelated as are the neurobiological process that underlie them As such, established neural circuits involved in emotional responses, including the midbrain periaqueductal grey (PAG), sensory regions, thalamus, amygdala, bed nucleus of the stria terminalis (BNST), nucleus accumbens (NAcc) and the striatum, hippocampus, orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), insular cortex, and the medial prefrontal cortex (mPFC), among others, contribute to the temporal features of affect. Below, we draw from both animal and human research to highlight the circuits relevant to each parameter of emotional dynamics and describe stimulus and contextual properties that modulate these parameters (for a brief overview of how neural circuits and affective states are measured, see Appendix A).
2. Parameter 1 : Rise-time
Conceptually, rise-time refers to the time for an affective response to reach its initial peak following the onset of a stimulus (Davidson 1998). For neural and physiological signals that follow waveforms (e.g., skin conductance, EMG, EEG, and fMRI BOLD signal), rise-time can be operationalized as the latency to the wave’s peak (see “Rise Time” in Figure 1). Another way to operationalize rise-time is via the onset of affective behaviors, such as reaction time in humans (e.g. ,pressing a button). Similar metrics have been used to index the emotional states of animals, such as the speed of movement in environments with rewarding or aversive stimuli (Roelofs et al., 2016). For example, after mice housed in standard shoebox cages with minimal enrichment are introduced to a larger, complex environments, decreased approach latency when voluntarily re-entering the complex environment is suggested to index how rewarding the animals initially found it (Ratuski et al., 2021). These latency metrics highlight the many component cognitive and motor processes that may contribute to affective rise-time (Nguyen et al., 2020).
It is important to note that affective rise-time relies on multiple information processing components, including stimulus detection, (re)orienting, attentional allocation, and stimulus valuation. Determining unequivocal boundaries between processes such as stimulus detection and valuation is a theoretical and empirical challenge and frequently not possible. Some argue that the lines between distinctive cognitive and affective processes are in fact illusory and should be removed altogether (Pessoa, 2019). As one source of support for this idea, evidence suggests that perception of emotional expressions in conspecifics occurs in parallel with the encoding of the face expressing that emotion (Eimer & Holmes, 2002). Moreover, putatively “sensory” regions are embedded with emotion schemas: patterns of visual cortex activity encode rich, category-specific visual features that can be reliably mapped to distinct emotions (Kragel & LaBar, 2016). Thus, the following discussion of rise-time necessarily involves the neural systems involved in these various processes and how they may contribute to affective rise-time.
2.1. Critical Circuits
The neural systems subserving affective rise-time involve interactions between the thalamus and cortical sensory regions, which detect stimuli that are salient and goal-relevant. Faster detection of, and orienting to, a stimulus, such as a fearful versus neutral faces, can hasten the rise-time of an affective response to that stimulus (Kanske et al., 2011; Mavratzakis et al., 2016). This sensory information originates from primary sensory cortical regions (e.g., visual, auditory cortices) and then flows through modality-specific cascades toward higher association regions (McDonald, 1998; Mesulam, 1998). These association regions not only receive information via this cortical cascade, but they also have bidirectional connections with the thalamus and amygdala (McDonald, 1998).
The potential role of the cholinergic system in affective rise-time
These sensory processing regions, along with many others, are influenced by the cholinergic system (Woolf, 1991). Acetylcholine (ACh) is a neurotransmitter that modulates attention, perceptual discrimination, memory and more (Picciotto et al., 2012). With projections to a wide range of cortical and subcortical structures, cholinergic signals originate from brainstem nuclei (laterodorsal tegmental nucleus and peduculopontine tegmentum) and the basal forebrain (Woolf, 1991; Figure 2). From these areas, the cholinergic system operates both in a diffuse, slower, neuromodulatory fashion as well as a more spatially specific, phasic, neurotransmitter fashion (Sarter et al., 2016; Zaborszky et al., 2015), both of which appear to support attention and detection.
Figure 2. Cortical, subcortical, and cholinergic systems that support affective rise-time.
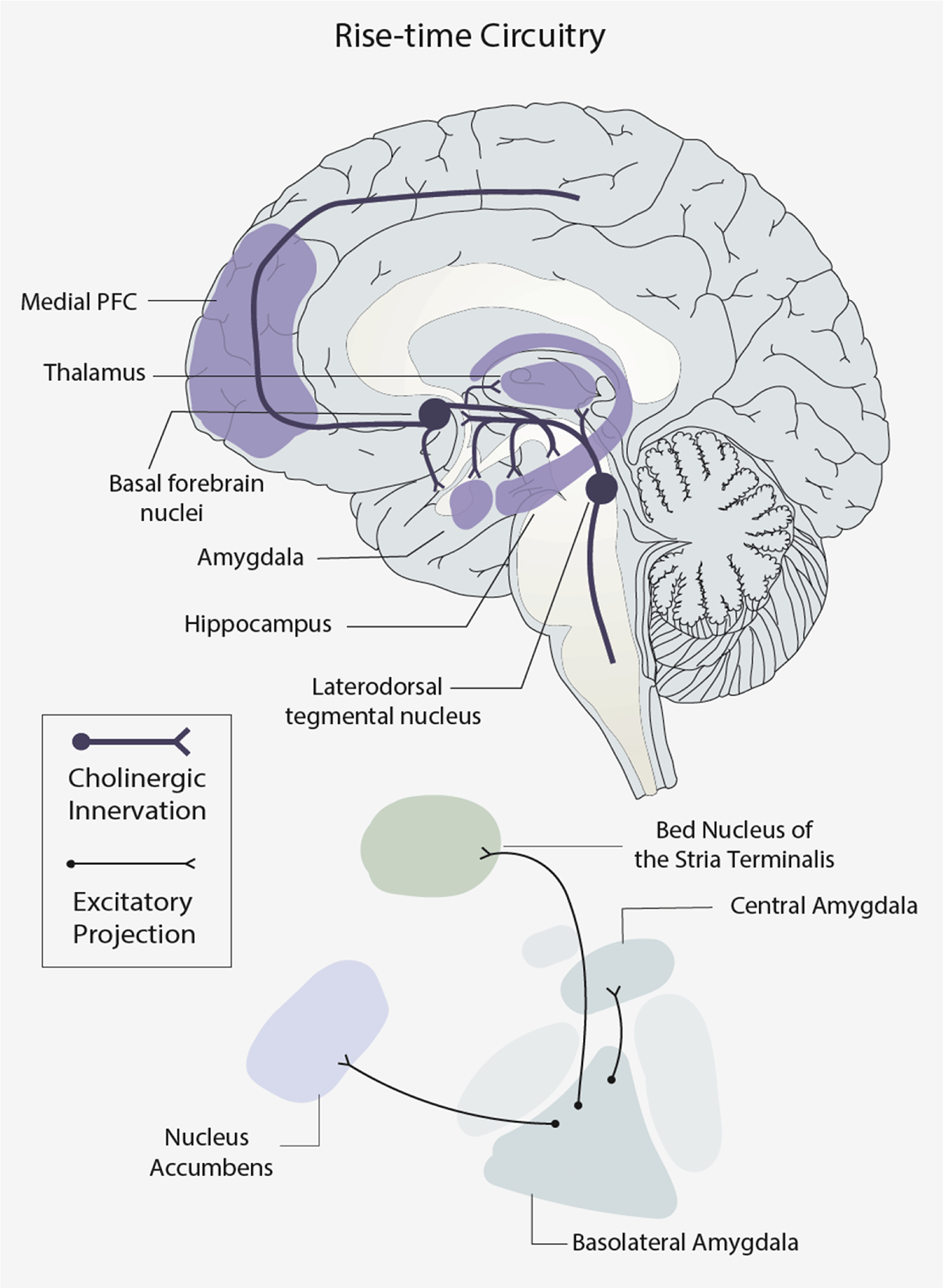
Top: a mid-sagittal view of medial brain regions and the cholinergic system. Bottom: a simplified, circuit diagram of nuclei of the amygdala, and some of their connections with the Bed Nucleus of the Stria Terminalis and the Nucleus Accumbens. Bold lines illustrate cholinergic innervations and thin lines illustrate excitatory subcortical neuronal projections. For each connection, a circle denotes the origin, and a fork denotes the destination of that connection.
Both slow and phasic cholinergic contributions improve attentional, perceptual, and behavioral processes that support salience detection, which can ultimately hasten affective rise time. For example, long-lasting increases of ACh in the mPFC is related to improved performance during a range of perceptual tasks tapping sustained attention, cue detection, and signal detection tasks (Kim et al., 2017; Passetti et al., 2000). ACh release in the cortex also signals novel stimuli in the environment (Miranda et al., 2000; Rangel-Gomez & Meeter, 2016). Beyond the frontal cortex, thalamic cholinergic innervation also predicts better signal detection performance in humans (Kim et al., 2017). Importantly, faster response speeds across multiple tasks of attention and visuo-spatial detection have been linked with a polymorphism of the gene encoding ACh receptors (Schneider et al., 2015), highlighting the potential role of the cholinergic system in the speed of cognitive and affective processes.
Faster, phasic ACh release lays the foundation for “perceptual” rise-time by facilitating shifts in attention and behavior towards salient and goal-relevant sensory inputs, ultimately aiding affective rise time too. (Sarter et al., 2014). In rats, fast ACh release during a cue-detection task occurs specifically on cue trials that are preceded by non-cue trials (Howe et al., 2013), suggesting that fast Ach release facilitates goal shifts, and initiation of behavioral responses (Howe et al., 2013; Sarter et al., 2014). This phasic ACh activity also increases sensitivity to thalamic inputs enhancing the signal-to-noise ratio of incoming information. The speed and accuracy of responses to salient sensory inputs in “cold” or non-emotional contexts are also important for affective rise time.
In fact, the initiation of aversive emotion states is linked to the cholinergic system. The ascending mesolimbic cholinergic system (AMCS) begins within the laterodorsal tegmental nucleus and terminates in the forebrain and thalamic areas (Wang & Morales, 2009). Evidence of a role for the AMCS in affective rise time comes from animal research on fear vocalizations, fundamental manifestations of affective states in cats, monkeys, rats, and more (Brudzynski, 2014; Burgdorf et al., 2020). Specifically, stimulation of the AMCS results in the onset of dose-dependent aversive states in these animals that are quantified by the magnitude and number of fear vocalizations (Brudzynski, 2014). The cholinergic system, including but not limited to the AMCS, influences the detection, attention, valuation, and initiation of expressions that affective rise time relies upon.
The role of subcortical systems in affective rise-time
While the amygdala is relevant for affective chronometry broadly, it is particularly well-positioned to influence rise time as it receives sensory cues, imbues them with value, and propagates this information to downstream regions. Neurons in the basolateral amygdala (BLA), which includes the lateral and basal nuclei (Sah et al., 2003), drive emotional learning (Quirk et al., 1995). This has been demonstrated through threat and reward conditioning paradigms (Johansen et al., 2010; Namubri et al., 2015), and from lesion studies of the lateral amygdala in animals and humans (LeDoux et al., 1990; Klumpers et al., 2015).
The learning process is enhanced by Ach, which could hasten affective rise time (Crouse et al., 2020). Specifically, stimulation of cholinergic input to the BLA in rodents increased firing of BLA principal neurons and enhanced glutamatergic synaptic transmission (Crouse et al., 2020). Moreover, reducing ACh in the BLA during training reduced the acquisition of conditioned fear behaviors. Intensifying the firing and transmission in the amygdala during learning could lead to faster affective rise-time.
BLA neuronal populations differentially encode fear and reward associations (Beyeler et al., 2018; Paton et al., 2006), which engage distinct downstream pathways. BLA neurons encoding an appetitive stimulus propagate signals to the NAcc, which drives reward-seeking behavior (Stuber et al., 2011). In contrast, neurons encoding an aversive stimulus propagate signals to the central nucleus of the amygdala (CeA; Namubri et al., 2015) and the neighboring BNST (Fox & Shackman, 2019; Kim et al., 2013; Figure 2). For instance, when BLA neurons signal an aversive conditioned auditory stimulus to the CeA, a subset of lateral CeA neurons become excited while a separate subset becomes inhibited (Ciocchi et al., 2010). The inhibition of that subset of neurons results in increased activity in CeM output neurons, resulting in threat-conditioned action (Fadok et al., 2018). Relatedly, BLA projections to the BNST also promote emotional behaviors (Davis et al., 2010; Fox & Shackman, 2019). These findings highlight the importance of BLA connections with other regions to translate stimulus value into motivated behavioral responses.
Conditioning may increase the speed and efficiency of these subcortical circuits, potentially generating a faster time-to-peak. In particular, there is evidence that learning increases circuit efficiency. Tye and colleagues (2008) conducted a reward conditioning experiment in rodents and found that greater task efficiency, defined as the number of rewards earned divided by the number of cues, was linked to a higher proportion of cue-responsive amygdala neurons and stronger amygdala-thalamic connections. Additional work has shown that stimulus valence alters glutamatergic synaptic strength of BLA efferents, such that BLA-CeA synapses are weakened during reward conditioning and BLA-NAcc synapses are weakened during fear conditioning (Namburi et al., 2015). Whether this circuit streamlining yields faster affective rise-time is yet to be determined and may be a fruitful future line of research.
Human neuroimaging research also highlights subcortical contributions to affective rise time. The time-to-peak for the BOLD signal serves as one metric of rise-time. For instance, young adults born with an “inhibited temperament” (a predisposition to be weary of new people, places, or things) display more rapid amygdala responses to novel compared with familiar faces (Blackford et al., 2009). This effect emerged despite no differences in the magnitude of peak amygdala response. The authors posit that faster rise-time for the amygdala response to novel faces is likely due to a bias for detecting novelty or threat and faster engagement of downstream limbic processes (Blackford et al., 2009). Similarly, faster amygdala rise-time has been found in individuals with spider phobia compared to healthy controls when viewing images of spiders (Larson et al., 2006).
The role of the subcortical-cortical interactions in affective rise-time
Affective rise-time may also be determined, in part, by the mPFC, where information from the BLA, the hippocampus, and other subcortical and cortical regions converge. Using contextual information, the mPFC can modify the expectedness and value of a stimulus based on the appropriateness of the stimulus and context for the organism’s current state and environment (Grace & Rosenkranz, 2002; Calhoon & Tye, 2015). This may also impact affective rise-time. For example, Mattavelli and colleagues (2011) measured response time when classifying happy and angry faces following a congruent or incongruent priming cue (i.e., the word happy or angry). They found that during congruent trials, when subjects were contextually primed, response times were longer when a transcranial magnetic stimulation pulse disrupted the mPFC (Mattavelli et al., 2011). This suggests that the mPFC can streamline affective responses based on environmental cues and context.
Studies measuring the time-to-peak of electrodermal skin conductance responses (SCR), an index of sympathetic nervous system activity, implicate the ACC as an additional hub coordinating the speed of responses to emotional stimuli (Neafsey, 1990; Vetrugno et al., 2003). For instance, electrical stimulation of the ACC modulates the SCR (Fredrikson et al., 1998). SCR rise-time also predicts emotional arousal above and beyond SCR amplitude (Jindrová et al., 2020). Nuanced patterns of activation within the ACC appear to differentiate SCR and heart rate, another sympathetic nervous system response to affective stimuli (Eisenbarth et al., 2016). These findings highlight the ACC as one potential cortical mediator of dynamic, affect-related biological responding across measurement modalities.
Beyond any single region, the coordination and synchrony of neural activity between the mPFC, amygdala, and hippocampus during affective processing facilitates information transfer and thus the affective rise-time (Buzsáki et al., 2012; Pape et al., 2005; Paz et al., 2008; Seidenbecher et al., 2003). With regards to affective responding, synchrony between the hippocampus and the BLA is increased in response to the conditioned stimulus after threat conditioning (Seidenbecher et al., 2003). Moreover, mPFC-originating theta rhythms can also promote safety behaviors after extinction (Lesting et al., 2013). Specific to affective rise-time, Karalis and colleagues (2016) demonstrated that prefrontal-amygdala synchrony predicted the onset of freezing behavior of rodents during threat conditioning. These studies demonstrate that dynamic shifts in oscillatory synchrony among regions shape the unfolding of emotion states.
2.2. Modulators: What influences rise-time?
There are a number of stimulus and contextual properties that can influence neural circuits involved in emotion and the affective rise-time. We posit that rise-time is likely to be faster for stimuli that are emotionally in line with expectations given the current environment (congruent), salient, or unambiguous. Importantly, these features may correlate differently with one another across contexts. For example, stimuli that are salient may be unexpected or incongruent. Therefore, while we discuss the literature that supports these modulators of rise-time, we acknowledge that more work is needed to determine when, how, and for whom these modulators are prioritized.
Congruence.
Emotional rise-time is faster for highly congruent affective material (John et al., 2016; Dzafic et al., 2016). As a predictive organ, the brain is constantly forming expectations about what is likely to occur (Clark, 2013). As such, it is often functionally adaptive and efficient to bias the appraisal of stimuli as threatening in aversive contexts, or conversely, to prioritize the appraisal of stimuli as rewarding in appetitive contexts. Prioritizing congruent information, streamlining emotional rise-time, can be achieved through the biasing of sensory systems (Pourtois et al., 2013). This biasing is supported, in part, by amygdala influence on gating mechanisms in the thalamus (John et al., 2016).
The amygdala can bias attention toward emotionally relevant information in the environment through its connection with the thalamus. While the amygdala receives projections from the thalamus, it also sends excitatory projections back to the thalamic reticular nucleus (TRN), which inhibits the other thalamic nuclei (Pinault, 2004). Specifically, the TRN exerts inhibitory control over the sensory thalamus, which promotes competitive gating of thalamo-cortical signals. The TRN is considered a hub of the attentional system (McAlonan et al., 2006) and this amygdala-TRN connection suggests that emotionally relevant information can influence ongoing attentional and sensory processing (John et al., 2016). Excitatory amygdala projections to the TRN can directly influence this attentional controller, biasing attention toward emotionally relevant information and suppressing competing sensory signals (John et al., 2016). Tuning attention to emotionally relevant and congruent sensory information can speed the rise-time of emotional responses.
One demonstration of the modulation of rise-time by congruence in humans comes from a structural and functional MRI study employing a dynamic emotion perception task (Dzafic et al., 2019). Similar to any Stroop effect, participants classified video clips as happy or angry, each of which was preceded with a “happy” or “angry” cue containing a still-image and the corresponding emotion word. These cues were either congruent or incongruent with the subsequent video (Dzafic et al., 2019). During congruent trials, a faster behavioral response time to angry stimuli was associated with greater recruitment and connectivity within an amygdala-limbic functional network. This network consisted of the right amygdala, hippocampus, mammillary bodies, caudate, and subgenual anterior cingulate cortex (Dzafic et al., 2019). This finding supports the amygdala as a key for affective rise-time and emotional-congruence as a modulator that can hasten rise-time, including the speed of behavioral responses.
Salience
Rise-time is also likely faster for perceptually salient information. Salient stimuli (novel or unexpected stimuli) are often prioritized, therefore shortening the rise-time for affective responses. In the visual system, salient stimuli are those that “appear to an observer to stand out relative to their neighboring parts” (p.185, Borji & Itti, 2010). These “stand-out” stimuli or features can be conceptualized as spatial or temporal prediction errors—deviations from what is anticipated. This definition of salience stands in contrast to the argument that stimulus “congruency” facilitates rise-time. However, it may be that either congruency or incongruency (salience) promotes affective rise-time, an apparent quadratic effect.
Many visual salience models account for the rapidity of attention and orienting by analyzing populations of neurons in the visual cortex. According to some models, salient stand-out stimuli are encoded in various maps specific to perceptual features, such as luminance contrast, edges, color, and motion (Borji & Itti, 2010; Itti et al., 1998). Other models of saliency maps suggest that multiple feature maps are not required, but rather, firing rates across V1 neurons, regardless of feature tuning, yield a single saliency map (Li, 2002). Still other models focus on subcortical structures that may encode salience, such as the superior colliculus (White et al., 2017). According to most of these computational models, bottom-up visual saliency maps interact with top-down priority maps, perhaps emanating from medial prefrontal cortical regions, to encode the behavioral relevance of stimuli, to guide attention and behavior (Fecteau & Munoz, 2006; Tanner & Itti, 2019).
Emotional faces are one example of salient stimuli that modulate the neural processes underlying affective rise-time in humans. In support of this, humans exhibit faster detection of emotional faces, particularly angry faces, compared with neutral faces (Öhman et al., 2010). For decades, affective neuroscientists have proposed that fast threat detection is possible through a “fast path” or “low road” route to the amygdala that bypasses cortical regions (Garrido et al., 2012; LeDoux, 1996). Through the fast path, the amygdala receives sensory information subcortically through the pulvinar nucleus of the thalamus, prior to reaching the sensory cortex (LeDoux, 1996; Méndez-Bértolo et al., 2016; Morris et al., 1999). From an evolutionary perspective, this fast path exists to prioritize responding to salient survival-related stimuli.
Recent evidence in humans further indicates that emotional faces may be sufficiently salient to reach the amygdala through this fast path (McFadyen, 2019; McFadyen et al., 2017; Méndez-Bértolo et al., 2016). Specifically, an intracranial electrophysiological study demonstrated that low spatial frequency fearful faces, which appear as blurred versions of the normal faces that maintain the original brightness and contrast, elicited amygdala activity approximately 100ms faster than in ventral visual cortex (Méndez-Bértolo et al., 2016). However, additional MEG work has demonstrated that the fast path amygdala responses may extended to faces regardless of spatial frequency or emotional expression (McFayden et al., 2017). Additional work exploring which types of stimuli and information are prioritized in this fast path will help inform salient features that may modulate affective rise-time.
Ambiguity
Rise-time is faster for non-ambiguous stimuli (Neta et al., 2009). Features of emotionally ambiguous stimuli, (e.g., a surprised facial expression or a woman crying in a wedding dress) convey subtle, inconclusive, or even conflicting information regarding valence. Such stimuli may be open to multiple interpretations and may require additional contextual information to be appraised. In contrast to controlled experiments that employ clearly-valenced and unequivocal stimuli, real-world affective events can be ambiguous. More elaborated and prolonged processing is required to resolve ambiguity and determine stimulus value.
Resolving ambiguity requires PFC and other cortical input to integrate additional contextual information (Bublatzky et al., 2020; Stujenske et al., 2020). Using MEG in humans, Bublatzky et al. presented morphed, difficult-to-recognize emotional faces during both contextual threat and safety. When subjects classified ambiguous fearful expressions under contextual threat or when subjects classified ambiguous happy faces under contextual safety, there was an amplification of early activity in centro-parietal (between 63–127 ms) and prefrontal regions (between 103–157 ms; Bublatzky et al., 2020). This double dissociation highlights the role of cortical regions in contextualizing and evaluating nuanced, ambiguous stimuli. This example also reiterates the influence of context on stimulus appraisal, as ambiguous stimuli are more likely to be appraised in congruence with the context.
Another way that cortical regions in general, and the mPFC in particular, help resolve ambiguity is through generalization of previous emotional learning to the current ambiguous stimulus. Generalization requires that organisms determine whether unconditioned stimuli that are similar to, yet distinct from, conditioned stimuli are nonetheless relevant for survival (e.g., predict threat; Asok et al., 2019). In mice, for instance, stimulating prelimbic cortical (analogous to mPFC in humans) inputs to the BLA enhanced stimulus discrimination, meaning mPFC stimulation reduced the probability of threat generalization to ambiguous, non-threatening stimuli (Stujenske et al., 2020). Overall, cortex-dependent processing and contextualizing that resolves ambiguity could, in turn, modulate affective rise-time in response to emotionally ambiguous stimuli.
3. Parameter 2: Intensity
Conceptually, the intensity of an emotional response refers to the absolute value of the maximal magnitude or peak of that response (Davidson, 1998; see “Intensity” in Figure 1). Terms such as the “amplitude” (Russell, 1980) or “scalability” (Anderson & Adolphs, 2014) of an emotion share conceptual overlap with our use of the term intensity. Similar to rise-time, intensity can be quantified by the amplitude of a waveform in physiological signals, such as SCR and fMRI. In humans, subjective “feeling ratings” can also serve as a metric of emotional intensity, which is often considered to vary continuously (e.g., from low to high intensity). In non-human animals, an emotion’s intensity can be inferred by certain facial expressions and behaviors within an organism (Dolensek et al., 2020). Importantly, these behavioral outputs associated with increasing intensity need not increase monotonically. For instance, under increasingly proximal threats, rodent behavior shifts from passive observation, to freezing, ultimately to defensive attack (Blanchard et al., 1990; Fanselow & Lester, 1988; Mobbs et al., 2007). These quantifiable shifts in behavior depend on measurable shifts in stimulus proximity, and likely, increases in affective intensity. Moreover, such increases in an emotional intensity are impacted by rise-time and can subsequently influence duration.
3.1. Critical Circuits
The neural circuits involved in an emotion’s intensity are deeply intertwined with those influencing its rise-time and duration. The intensity of an emotional response can be modulated via activity in brainstem, subcortical/limbic, as well as cortical regions (Figure 3). Panksepp and others have argued that low levels of stimulation of the most evolutionarily old and conserved brain regions, including the periaqueductal gray (PAG) of the midbrain, causes robust, reliable, and specific emotional responses (Pankesepp, 2004). One reason for this assertion is that the level of electrical stimulation needed to cause affective reactions is far lower and far more specific in the evolutionarily old midbrain than in regions that emerged more recently in phylogeny (even compared to the amygdala and NAcc, let alone the cerebral cortex). This may be due in part to the fact that the PAG has more direct connectivity with output effector regions. Furthermore, stimulation of midbrain subnuclei appears to cause affective reactions regardless of context. Critically, the intensity of affective reaction scales with the magnitude of stimulation.
Figure 3. Affective intensity depends on distributed networks of cortical, subcortical, and brainstem regions.
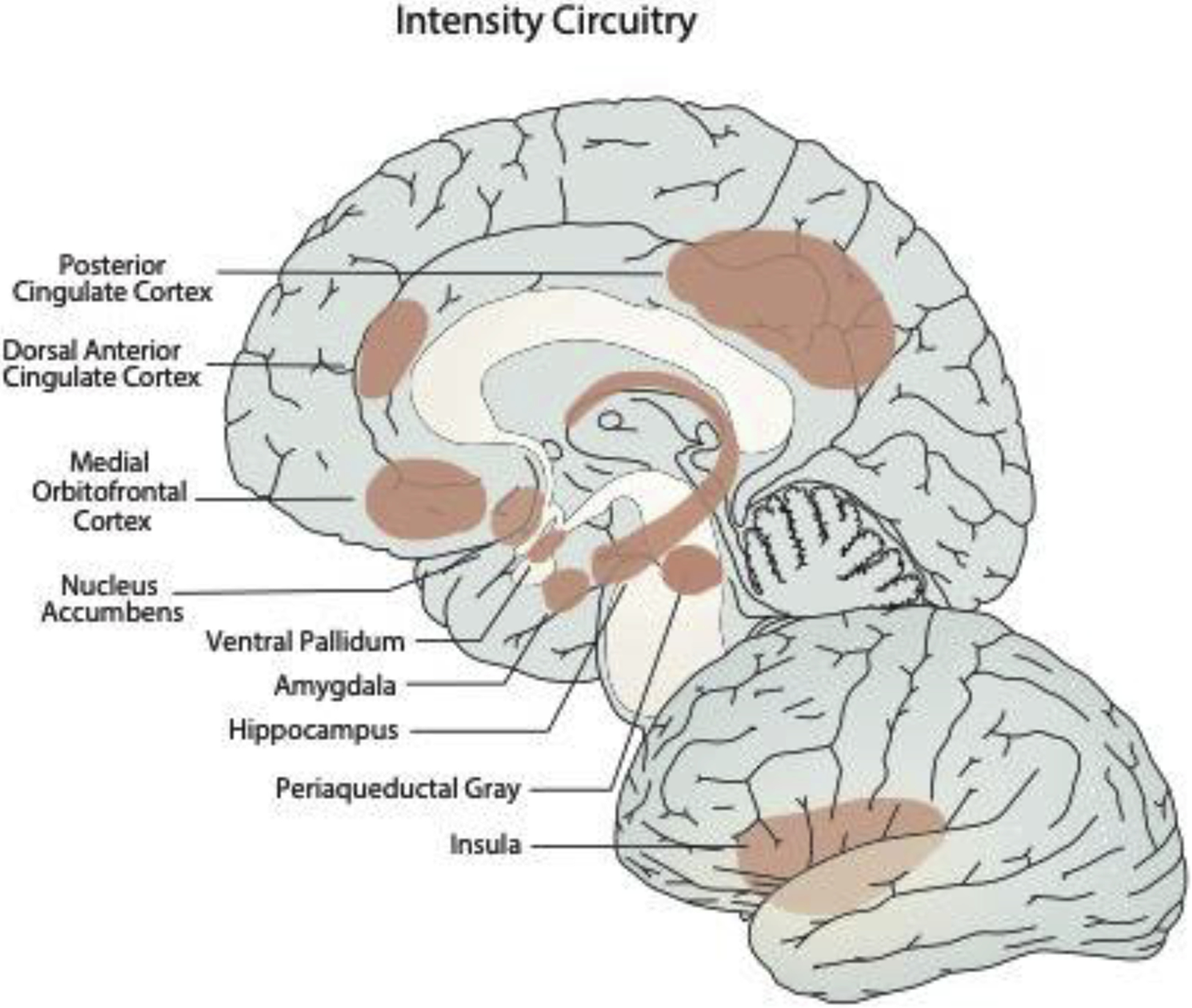
Medial and lateral views depicting distributed brain regions that support affective intensity.
However, evolutionarily old brain regions, such as the PAG, do not alone drive an emotion’s intensity. More recently evolved associative learning structures, including the amygdala, hippocampus, NAcc, ventral pallidum, as well as the association cortex (Mesulam, 1998) are anatomically connected. These regions encode and represent the life-history of the organism and provide the essential context about what to expect and how to make sense of incoming information. The interaction between these regions determines the intensity of the emotional response and behavioral output.
Empirical neuroimaging research with humans highlights the subcortical-cortical interactions that govern emotional intensity. For instance, fMRI work using machine learning indicates that increasing subjective ratings of negative emotion in response to affective images are predicted by patterns of cortical and subcortical brain responses, including the amygdala, insula, PAG, and posterior cingulate cortex, among others (Chang et al., 2015). Moreover, simultaneously acquired fMRI and objective facial EMG from the corrugator (‘frowning’ muscle) during a similar picture viewing task demonstrates that both lower vmPFC activity and higher amygdala activity predicted higher intensity of corrugator activity (Heller et al., 2014). Finally, concurrent fMRI and EEG recordings have probed the neural structures that give rise to the late positive potential (LPP; Sabatinelli et al., 2007; Liu et al., 2012), an event-related potential seen at 300–400ms whose amplitude varies with emotional intensity (Cuthbert et al., 2000; Schupp et al., 2000; Keil et al., 2002). In particular, trial-by-trial correlations between LPP amplitude and BOLD signal determined that the amygdala, insula, prefrontal, and visual cortices were encoding emotional intensity during positive- and negative-valenced picture viewing (Liu et al., 2012; Sabatinelli et al., 2007). Interestingly, the authors found valence effects, such that the modulation of the LPP during positive images, not negative images, was related to bilateral amygdalae activity whereas LPP modulation during negative images was related to insula and adjacent temporal and ventrolateral PFC activities (Liu et al., 2012).
Direct manipulations of cortical regions, such as the insular cortex, in animals and humans lead to changes in emotional intensity. For example, neuronal activity within the insula correlate with rodent facial expressions that reflect not only specific emotional states, but also their intensity (Dolensek et al., 2020). Specifically, the authors used a classifier to successfully distinguish emotional states in rodents (i.e. pain, fear, malaise, reward, etc.) based on their facial expression. The degree to which these emotion-driven facial patterns resembled a prototypical expression tracked with the intensity of the stimulus; for example, higher concentrations of rewarding sucrose compared with lower concentrations led to facial expressions that most closely matched the prototypical pleasure expression (Dolensek et al., 2020). Finally, optogenetics were used to manipulate the insular cortex and identify neurons in the posterior and anterior insular cortex that evoke prototypical disgust and pleasure expressions, respectively, which scale with emotional intensity (Dolensek et al., 2020). This animal research is echoed intracranial electrical stimulation research in humans, which demonstrates that a higher degree of electrical stimulation delivered to the insula, ACC, and OFC lead to more intense emotional experiences (Yih et al., 2019).
Intensity of hedonic processing, specifically, depends on a distributed circuit including cortical and subcortical regions. Human neuroimaging studies report that the OFC and insula encode the pleasantness of foods (Kringelbach et al., 2012; Small et al., 2001). For example, cortical activity robustly tracks the pleasantness of food and the intensity of this pleasure and the concomitant cortical activity decline as individuals become satiated (Kringelbach & Rolls, 2004). This evidence is supported by complementary decision-making work indicating that the value of a stimulus is encoded in a variety of cortical (mPFC/OFC) and subcortical (NAcc) regions (Knutson & Cooper, 2005). Indeed, these brain regions, including ventral striatum, not only encode the subjective value of stimuli, but also predict within-task ratings of the momentary positive emotion intensity (Rutledge et al., 2014).
3.2. Modulators: What influences intensity?
Stimulus Proximity
As noted above, the proximity of an affective stimulus modulates the intensity of the emotional reaction and the accompanying behavior. Fanselow and Lester advanced the “Threat Imminence Continuum” model (Fanselow & Lester, 1988) of shifts in behavior based primarily on studies in rodents. This model posits that threat-states change depending on the proximity of the threat (undetected, detected but far away, or close enough to immediately attack). Mobbs and others have applied this framework to create an fMRI paradigm where the subject actively evades an artificial predator that can chase, capture, and shock the subject. Mobbs and colleagues showed that as an artificial predator came closer to the subject, activity shifted from primarily cortical (e.g., vmPFC) to midbrain (PAG) regions (Mobbs et al., 2007). Mobbs has replicated and extended this work using more realistic stimuli (Mobbs et al., 2010). For instance, when a tarantula was placed progressively closer to the subject’s foot, they found that closeness was associated with greater activity in the PAG, amygdala, and BNST. Conversely, distance was associated with greater OFC activity. Pessoa and colleagues have used a similar paradigm of increasing threat proximity and found that threat proximity is similarly related to amygdala and salience network activity (Meyer et al., 2018). Together, these studies suggest that higher forebrain areas are involved in slower, deliberate responses to distant or potential threats, whereas the midbrain and perhaps the amygdala mediate fast, “hard-wired,” defensive reactions to imminent danger (Mobbs & Kim, 2015).
Stimulus Repetition
The repeated presentation of identical or similar stimuli (i.e., sharing several perceptual or categorical properties) leads to habituation (Thompson, 2009). Habituation is a learning process by which the presence of behaviors associated with emotion become attenuated. Neuronal habituation to repeated presentation of similar stimuli occurs throughout the brain, from the brainstem up to the cortex. The rate of habituation is typically a negative exponential function of the number of stimulus presentations. Habituation occurs in almost all readouts of emotion, including physiology (Davis, 1934), facial reactivity (e.g., startle: Prosser & Hunter, 1936), and exploration of novel contexts (Welker, 1961)). Habituation is a type of short-term memory and indicates that more novel stimuli (i.e., stimuli of uncertain value) can often evoke stronger affective reactions than recently encountered stimuli of known value. Moreover, the capacity for stimulus adaptation (i.e., habituation) is present throughout the brain – brainstem regions drive core emotional responses (not necessarily subjective human ‘feelings’), as well as evolutionarily newer regions (Sokolov, 1963). Such a conceptual orientation is central to more modern theories of “the predictive brain” (e.g., Friston et al., 2009).
Neurochemical modulators of hedonic processing
Rodent models of hedonic processing provide insights into the circuits and neurochemical systems influencing emotional intensity. Berridge and his colleagues have mapped the neural circuits involved in hedonic processing (Berridge & Kringelbach, 2015). They have identified distinguishable neural processes underlying the incentive salience (‘wanting’) and hedonic (‘liking’) processing of rewards. Moreover, their work has linked neuronal mechanisms of hedonic processing with objective affective behaviors during ingestion of pleasant stimuli. These objective reward-related behaviors, including lip licking and tongue protrusions, are readily observable in rodents, nonhuman primates, and human infants alike.
Neurochemical signals in subcortical regions modulate the intensity of these objective affective behaviors in rodents (Berridge, 2019). Stimulation of opioid, orexin, and endocannabinoid systems within either the NAcc shell or the ventral pallidum increase the frequency of objective liking behaviors. This suggests that enhanced signaling in these systems may increase the intensity of the subjective emotional hedonic experience (Berridge, 2019; Smith et al., 2011).
Additionally, these effects appear to be specific to opioid, orexin, and endocannabinoid systems, as dopamine agonist microinjections into the same regions did not similarly modulate hedonic responses (Castro & Berridge, 2014).
Moreover, the NAcc and ventral pallidum receive input from cortical sites, notably the prefrontal cortex and insula, which can also amplify hedonic reactions. To that end, Castro and Berridge extended earlier findings by injecting the mu-opioid agonist DAMGO and the neuropeptide orexin into orbitofrontal and insular regions to map their effects on the intensity of hedonic responses. In small (6–8mm3) hedonic hotspots in the anterior OFC and posterior insula, they found that mu-opioid or orexin microinjections amplified the hedonic impact of sweetness, expressed as a nearly 300% increase in behavioral “liking” reactions to the sucrose taste. Further supporting the role of these cortical regions in amplifying hedonic processing, stimulation of both the anterior OFC and posterior insula increased activity throughout the broader hedonic circuit, including in the NAcc and ventral pallidum (Castro & Berridge, 2017). These data suggest that there exist specific neurochemical modulators of the intensity of emotional responding within specific neuronal circuits supporting affective processing.
4. Parameter 3: Duration
Conceptually, the duration of an emotional response refers to the time that elapses between the start and end of the response. Here, the end of an emotional response is defined by an individual’s shift to either a different emotional state, or return to a baseline state - that is, the internal state that preceded the onset of the emotion. An enduring emotion may similarly be construed as a lasting perturbation. Enduring emotional responses are marked by lasting shifts in physiology, behavior, and cognition. Thus, the duration of an emotional response can be inferred via the duration of objective physiological and behavioral states in addition to subjective self-report.
4.1. Critical Circuits
After an emotional response begins, its duration depends on the persistence of activity in the underlying neural circuitry. This applies to all domains of emotional responses, including physiological, behavioral, and cognitive states (Major & Tank, 2004). In the sections that follow, we describe 1) how neuromodulators drive enduring emotional responses by sustaining neural activity in emotion-encoding circuits, 2) how persistent patterns of emotion-related network activity contribute to the duration of emotional responses, and 3) how evolutionarily conserved subcortical circuits that support the selection and maintenance of behavioral states also influence the duration of emotional states.
Role of neuromodulation in maintenance of persistent states
Emotional states emerge and persist through the action of a subfamilies of modulatory chemicals known as neuromodulators (dopamine, serotonin, acetylcholine, and norepinephrine; Fellous, 1999) and neuropeptides (Bos et al., 2012). Neuromodulatory neurotransmitters originate in distinct brainstem nuclei that project broadly to cortical and subcortical regions. Neuromodulatory neurotransmitters are small molecules, synthesized in axon terminals within district brainstem nuclei that project broadly to cortical and subcortical regions. Unlike neuromodulators, neuropeptides are larger molecules that are synthesized within the neurocyton (i.e., cell body) and distributed throughout the brain and viscera, where they exert broad effects on the regulation of homeostasis, behavior, and mental states. Via their wide distribution throughout the nervous system, neuromodulatory neurotransmitters and neuropeptides enable global shifts in neural activity. In contrast to the fast and transient action of the excitatory and inhibitory neurotransmitters (glutamate and GABA), neuromodulators and neuropeptides exert slow-acting modulatory effects on synaptic transmission (Avery & Krichmar, 2017), and thus induce persistent neural and behavioral states (Flavell et al., 2013; Lee & Dan, 2012). Critically, neuromodulatory activity enables organisms to adjust their behavior in response to dynamic environments and changing homeostatic needs (Pool & Scott, 2014).
The persistence of neuromodulatory neurotransmitter activity plays a phylogenetically conserved role in the dynamics of internal emotional states. As threats and rewards manifest or an organism’s needs change, neuromodulators fundamentally reconfigure neural circuits and their outputs (Marder, 2012). Through these context-dependent actions, neuromodulators enable flexible shifts in organisms’ behavioral state. Critically, the duration of these states is determined in part by the rate at which neuromodulators are cleared from neuronal synapses (Gibson et al., 2015). In humans, drugs that regulate the concentration and efficacy of neuromodulators at the synapse are widely prescribed to treat psychiatric disorders such as depression, OCD, anxiety, ADHD, and psychotic disorders. Interestingly, the pathology of each of these disorders involves persistence of some internal state; be it an affective state in depression, a behavioral state in ADHD, or a cognitive state in psychosis.
Studies employing simple organisms with well-characterized neuronal connectomes clearly demonstrate that neuromodulators and neuropeptides control the duration of persistent states that may be considered ‘affective’ (Lee & Dan, 2012) via modulation of network activity. For example, in C. elegans, opposing neurotransmitters and neuropeptides (serotonin and pigment-dispersing factor [PDF]) recruit neurons into opposing, bi-stable networks. These opposing networks drive opposing behaviors, such as roaming and dwelling (Ji et al., 2020). Similarly in C. elegans, the neuromodulatory neurotransmitter dopamine exhibits dissociable, state-dependent effects on the duration of egg-laying behavior (Cermak et al., 2020). In larval zebrafish, dopaminergic activity reduces the susceptibility of persistent neural states to potential distractors by increasing the gain (i.e., relative strength) of relevant neural signals (Randlett et al., 2019). These examples demonstrate that neuromodulatory signaling enables an internal state (i.e., a response) to persist for as long as the stimulus that evoked it remains salient (Likhtik & Johansen, 2019).
Importantly, opposing neuromodulators such as serotonin and PDF compete for behavioral control in a “winner takes all” manner. Thus, the relative balance of neuromodulators and neuropeptides may ultimately dictate the duration of behavioral states. While some might contend that behavioral states such as roaming and quiescence are instinctual and far-removed from human affective states, there is substantial evidence that the neuromodulatory signaling motifs responsible for behavioral state transitions in simple organisms are phylogenetically conserved in higher species (Katz & Lillvis, 2014) and roaming has been linked to affective states in humans (Heller et al., 2020). Indeed, in mammals, non-human primates, and humans, there is substantial evidence that neuromodulatory systems influence synaptic transmission in neocortical regions that govern the dynamics of complex cognitive and affective states (Berridge & Waterhouse, 2003; Castro-Alamancos & Gulati, 2014; Moran et al., 2013; Shine et al., 2019).
In addition to driving behavioral state duration via competition with neuromodulatory neurotransmitters, emerging work suggests that neuropeptides alone govern the dynamics of behavioral states by altering the way neuronal populations respond to stimuli (Marder, 2012). In C. elegans, the neuropeptide FMRMamide enhances the persistence of locomotive behavioral responses to food in an aversive environment (Laurent et al., 2015). In this example, neuropeptide signaling drives persistent alterations in neural activity, which in turn yields a persistent shift in behavior. In other model organisms such as Drosophila, the neuropeptide dNPF (an analog of human neuropeptide Y) modulates the retrieval of memories for salient appetitive and noxious stimuli (Krashes et al., 2009). Further studies demonstrate that neuropeptide-mediated retrieval of appetitive memories governs the duration of motivational and roaming states involved in approach and avoidance behavior (Yamazoe-Umemoto et al., 2015). In another notable example using rodents, administration of the neuropeptide corticotropin releasing hormone (CRH) yields a persistent enhancement of acoustic startle responses and enduring facilitation of associative learning (Servatius et al., 2005). Here, the neuropeptide CRH is theorized to alter the activity of subcortical structures involved in learning and conditioning (i.e., CeA, hippocampus), which in turn increases the persistence of learned behavioral responses to aversive stimuli.
One particular neuromodulator, dopamine, influences the duration of internal emotional states by, 1) reducing a state’s susceptibility to distractors (Jacob et al., 2016), and 2) increasing the signal-to-noise ratio of stimulus representations in neural activity (Vander Weele et al., 2018). Dopamine influences behavioral impulsivity and the persistence of internal states through its effects on dopamine receptor-expressing neurons in the striatum - a region involved in the selection and maintenance of behavioral states (Graybiel, 1998). Specifically, dopaminergic activity modulates the stability of neuronal UP and DOWN states (Gruber et al., 2006), which refer to shifts in the thresholds at which neuronal hyperpolarization and depolarization occur (Major & Tank, 2004). Through these shifts in conductance thresholds, neuromodulators confer striatal UP and DOWN states with reduced susceptibility to distractors (Gruber et al., 2006). Thus, in motivationally relevant contexts, dopamine-mediated activity in striatal medium spiny neurons enables representations of salient information to persist in downstream cortical targets (this corticostriatal gating mechanism is described in greater detail below). In line with dopamine-mediated neural persistence, reduced availability of D2 and D3 dopamine receptors in the ventral striatum is associated with increased impulsive behavior in rodents (Barlow et al., 2018). Moreover, in humans, reduced structural coherence of dopaminergic projections from the ventral tegmental area to the ventral striatum predict greater impulsivity, and thus impaired behavioral persistence (MacNiven et al., 2020). Impulsive behavior may indicate impaired persistence in neural activity perhaps due to increased susceptibility to distractors (Barlow et al., 2018).
In addition to dopamine, serotonin also modulates the duration of emotional states. This is accomplished via the effects of serotonin on circuits involved in action selection. To illustrate, in zebrafish, inescapable aversive behavioral challenges elicit enduring states of neural activity in the ventral habenula. Inhibitory projections from the ventral habenula then suppress downstream serotonergic neurons in the dorsal raphe nucleus. The resulting reduction in global serotonergic signaling prompts a shift in the organism’s behavioral state, from an active to a sustained passive coping strategy. This is consistent with learned helplessness behavior in animal models of depression. Here, the shift to a passive behavioral state is supported by the phylogenetically conserved, inhibitory effect of serotonin on excitatory projections from the basal ganglia to the habenula (Shabel et al., 2012). Via these projections, reduced serotonergic activity and resultant habenula hyperactivity increase the signal-to-noise ratio of the aversive stimulus representation, which ultimately generates an enduring, passive behavioral state (Andalman et al., 2019).
Role of persistent activity in Amygdala – Hippocampus – mPFC network in emotion duration
Across species, the duration of an emotional or behavioral state is also linked to the persistence of neural activity in emotion-encoding subcortical regions such as the amygdala (Kennedy et al., 2020). For instance, enduring emotions, such as anxiety, are thought to be supported by enduring neural signals in emotion-encoding regions such as the amygdala and BNST (Lee et al., 2017; Waugh et al., 2015). Conversely, transient emotional states of the same valence, such as fear, are generated by short-lived signals in the same regions (Lee et al., 2017). Human fMRI studies have revealed a similar relationship between the duration of subjective emotion and the duration of neural signals in emotion-encoding regions such as the amygdala, thalamus, and midbrain (Waugh et al., 2016). However, the duration of emotional responses is not only determined by these subcortical structures, but also by synchrony within a frontolimbic network comprised of the amygdala, hippocampus, and mPFC.
Oscillatory synchronization between the amygdala and connected regions contributes to the duration of emotional states. One particularly important region in this network is the mPFC, which maintains neural representations of affective stimuli even after they dissipate (Bliss-Moreau & Rudebeck, 2020; Powell & Ginsberg, 2005). Moreover, persistent oscillatory entrainment between the mPFC, amygdala, and hippocampus support enduring freezing responses to conditioned fear stimuli in rodents (Seidenbecher et al., 2003). In particular, the duration and power of 4 Hz oscillatory synchronization between the mPFC and BLA predicts the duration of freezing behavior in rodents when exposed to aversive conditioned stimuli (Karalis et al., 2016). Even after stimuli are no longer present, enduring stimulus representations in the mPFC are transmitted to the BLA to produce these behavioral responses. In related rodent threat-conditioning paradigms, fear memory retrieval and behavioral responses are supported by phase-correlated interactions between mPFC neurons and the BLA (Bocchio et al., 2017). However, the functional role of oscillatory entrainment extends beyond fear conditioning. For example, entrainment within this circuit further transmits state-dependent contextual information to the striatum during behavioral decision-making. As detailed below, this information biases the duration of emotional states (Sharpe et al., 2019).
Role of Cortico-striatal-thalamo-cortical circuitry in neural states and state transitions
An emerging literature posits that corticostriatal circuitry involved in motor planning and action selection (Gurney et al., 2015; Redgrave et al., 2011) maintains persistent behavioral, cognitive, and emotional states (Awh & Vogel, 2008; McNab & Klingberg, 2008; O’Reilly & Frank, 2006). Though primarily implicated in action planning and execution, evolutionary accounts suggest that the basal ganglia has undergone exaptation across phylogeny (Stephenson-Jones et al., 2011). Thus, corticostriatal circuits which govern the selection and duration of behavioral states in lower species are theorized to modulate the duration of emotional states in higher organisms (Pierce & Peron, 2020). In support of this perspective, recent neural evidence from humans suggests that basal ganglia circuitry plays a key role in governing the onset and duration of emotional responses (Ory et al., 2017; Peron et al., 2013)
The cortico-striatal-thalamo-cortical (CSTC) circuit receives multiple sources of information from the cortex and gates relevant neural signals into behavior (Figure 4). This is accomplished via the CSTC circuit’s recurrent, closed loop architecture. In this circuit, multiple sources of information are routed from distinct cortical regions to the basal ganglia, through the thalamus, and back to the cortex (Alexander et al., 1986). The CSTC circuit’s primary mechanism of action is inhibitory, serving to gate simultaneously competing cortical inputs (e.g., competing motor programs) into stable behavioral outputs (e.g., motor actions). Specifically, when a behavioral state is no longer adaptive for an organism, the basal ganglia shift the behavioral state by inhibiting its current output while simultaneously releasing a more optimal behavior from inhibition. Similarly, the basal ganglia may also hierarchically control the input, output, and maintenance of information in frontal cortical regions involved in higher-level cognition and planning (Chatham & Badre, 2015). Thus, the basal ganglia arbitrate the duration of an organism’s behavioral or cognitive state depending on a state’s current value to the organism’s survival.
Figure 4. Distinct motor and limbic corticostriatal thalamocortical (CSTC) loops support the maintenance of persistent behavioral and affective states.
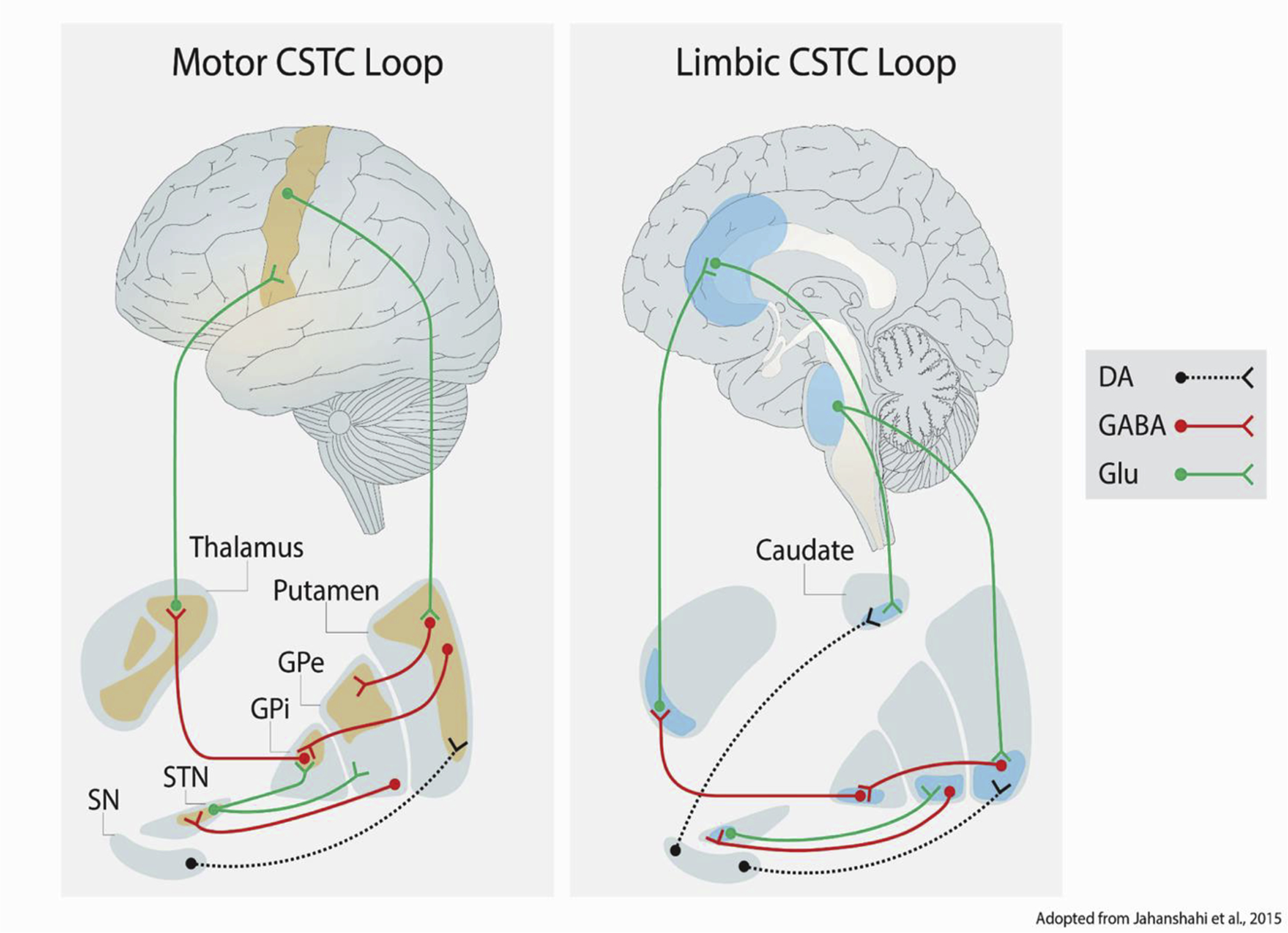
Neuromodulatory connections are depicted with dotted lines, while solid lines denote excitatory or inhibitory connections. For each connection, a circle denotes the origin, and a fork denotes the destination of that connection. Key: GPe = globus pallidus (external); GPi = globus pallidus (internal); STN = subthalamic nucleus; SN = substantia nigra; DA = dopamine; Glu = glutamate.
CSTC circuit loops and the values encoded in their inputs ultimately govern whether internal states persist or are interrupted by competing signals. Indeed, the duration of behavioral states depends on an organism’s current goals (Hubbard et al., 2020) and the relative values of its behavioral options in the current state (Daw et al., 2006). These values, which dictate the duration of behavioral, cognitive, and emotional responses, are encoded in cortico-striatal projections from the OFC, the ventromedial prefrontal cortex (vmPFC), and the dorsolateral prefrontal cortex (dlPFC) to the striatum (Sharpe et al., 2019). Within these cortical regions, distinct neural mechanisms convey value signals to the striatum. These value signals in turn govern the duration of behavioral states. For example, the OFC transmits value judgments through its projections to cholinergic interneurons in the striatum (Wilson et al., 2014). Additionally, context and salience-encoding limbic inputs to prefrontal and striatal regions influence behavioral state durations (Barlow et al., 2018; see “Subcortical regions…” in Modulators section). In summary, functionally diverse inputs to the striatum encode the salience of sensory information and the relative value of simultaneously competing cognitive and behavioral states. Ultimately, these values govern the duration of emotional states through inputs to CSTC loops.
4.2. Modulators: What influences duration?
Intensity of the stimulus or emotional response
The duration of an emotional response depends in part on the amplitude of the emotional response (Waugh et al., 2010). First, the intensity of an initial emotional reaction predicts its duration in subjective reports of emotional experience (Frijda et al., 1991). Objective assays of animal behavior replicate this effect. For example, in Drosophila, the intensity of a threatening stimulus predicts a more persistent behavioral response (Gibson et al., 2015). In this case, one or more neurons in the model organism cumulatively integrate signals from sensory neurons that encode the threatening stimulus. As time passes, accumulating sensory information in these so-called “leaky integrator” neurons decays at a constant rate. Thus, in circumstances where an organism faces repeated or high-amplitude sensory inputs, the accumulation of sensory information can outpace the decay rate. Within leaky integrator neurons, asymmetry in the rates of signal accumulation versus signal decay yields persistent activity, which can allow a behavioral response to persist even after stimuli dissipate. Convergent evidence suggests that persistent integrator neuron activity is necessary and sufficient to drive enduring behavioral responses in Drosophila (Jung et al., 2020). In sum, the response characteristics of integrator neurons (i.e., accumulation and slow decay of sensory signals) translate intense and persistent stimuli into more potent and lasting behavioral outputs.
Subcortical regions influence emotion duration
Neural activity in subcortical (i.e., limbic) regions influences the duration of emotional states via interactions with CSTC circuitry. Through their projections to CSTC circuits, limbic regions may initiate cortical state transitions or bias the probabilities of subsequent state shifts. As a result, limbic inputs to the CSTC circuitry may influence the persistence of neural and behavioral states that underlie emotional responding. This is accomplished in part through limbic influence over the striatal-substantia nigra-thalamo-cortical path (Aoki et al., 2019). Through this interaction, neuronal activity in limbic regions can unilaterally suppress thalamo-cortical output from the CSTC loop. This enables salient sensory inputs to perturb persistent states in CSTC-mediated neural activity. Functionally, this circuit may reduce the duration of an internal emotional state by terminating it altogether.
Bottom-up, neuromodulatory influence in the striatum can also bias the duration of behavioral states. In particular, aberrant dopaminergic signaling in CSTC circuits reduces the duration of behavioral states via impulsive behavioral responding. In both animal and human models, reduced density of D2 and D3 dopamine receptor subtypes seems to underlie natural variability in behavioral impulsivity (Buckholtz et al., 2010). Here, as in earlier examples, dopaminergic activity affords behavioral states greater resistance to potential distractors. This influence of dopamine on behavior may be explained by either the intrinsic effects of dopamine on the action selection machinery in the basal ganglia, or by dopaminergic effects on the signal-to-noise ratio of projections to CSTC circuits originating in the amygdala and the OFC.
Top-down influences from limbic and frontal regions may also influence the duration of emotional states by biasing the probability of state transitions. Indeed, lesions in limbic and orbitofrontal regions of the brain yield changes in CSTC signaling and increases in impulsive behavior (Barlow et al., 2018; Mobini et al., 2002). Additionally, increased functional connectivity within limbic networks, and reduced connectivity between frontal networks both contribute to impulsive behavior (Barlow et al., 2018). However, it remains unclear whether these top-down effects are independent of the bottom-up neuromodulatory influences of dopamine in the striatum (Dalley & Robbins, 2017). Emerging evidence from rodents suggests that the amygdala may indeed play an explicit role in coding real-time changes in behavioral states. Indeed, distinct neuronal subpopulations in the BLA encode behavioral state transitions (e.g., exploring vs. freezing) through slow-oscillating, attractor-like dynamics (Gründemann et al., 2019). State encoding in the amygdala may serve to integrate affective information into thalamocortical state representations via its vast cortical and subcortical connections. While this mechanism requires further investigation, it represents one additional process through which subcortical regions might influence state duration.
Frontoamygdalar circuits support the regulation of emotional states
The duration of an emotional response also depends on whether affectively salient stimuli are gated into conscious awareness (see rise time section). In conjunction with the mechanisms described by Mitchell & Greening (2012), persistent activity in frontal regions modulates amygdala activity (Inagaki et al., 2019), which gates sensory information in and out of conscious awareness. This is related to the well-established notion that frontoamygdalar connectivity supports the regulation, maintenance, and suppression of internal emotional states (Davidson, 2002). In another example of this phenomena, successful fear suppression is mediated via functional connectivity between the perigenual PFC and the amygdala, while unsuccessful suppression is marked by increased functional connectivity between the amygdala and regions in the ventral visual stream (Amting et al., 2010). This suggests that successful emotion regulation, which can shorten an emotional episode, is characterized in part by frontal influence over amygdala activity, and gating of information flow between the amygdala and sensory centers.
Conversely, inhibition of the lateral PFC (implicated in top-down control over emotional states) via transcranial magnetic stimulation increases the influence of affective information from previous experiences on future decision-making (Lapate et al., 2017). Here, a paucity of frontal control over emotion-encoding regions in the brain generates an emotional “spillover” effect that serves to lengthen the duration of aversive emotional states in the brain. Relatedly, the aforementioned study by Waugh and colleagues (2016) demonstrates that human emotions endure via persistent neural activity in key medial frontal, limbic, and midbrain regions; and effortful emotion regulation serves to shorten the persistence of activity within these regions. Finally, additional evidence from human fMRI studies suggests that dissociable projections from the ventrolateral PFC to the striatum and the amygdala mediate successes and failures in emotion regulation, respectively (Wager et al., 2008). Taken together, these findings suggest that the persistence of activity between prefrontal regions and emotion-encoding regions mediates the duration of emotional states.
5. Conclusion
Here, we have highlighted several neural mechanisms supporting the rise-time, intensity, and duration of emotional experiences. Critically, the different neural, peripheral, subjective, and behavioral measures of emotional responding unfold along unique timescales. For example, emotional responses captured by EEG occur more quickly than responses captured by skin conductance. Further, not only do the different indicators of emotional responses occur on different timescales, but their respective time courses can be relatively independent from each other. For example, the chronometry of biological measures may not always reflect the time course of subjective emotional experience or behavior (Mauss et al., 2005). Resolving the disparate time scales of emotional responding and connecting neural, physiological, behavioral, and subjective emotion signals remain a central challenge of emotion research (Davidson, 2015). It is critical to determine how neural firing, unfolding over milliseconds is linked to subjective feeling states which, from one’s introspection, last substantially longer.
Another issue concerns the similarity in time course and neural circuitry underlying processing of valence (Davidson, 2015). In general, it appears that negative emotional responses may persist longer than positive emotional responses (Villano et al., 2020; but see Verduyn et al., 2009), and proximal threats may be processed more rapidly than rewards (Vaish et al., 2008). While there is significant overlap in the neural circuits supporting processing of appetitive and aversive states, there appear to be localized “modules” as well as context-dependent “modes” within (and across) overlapping distributed networks that support valence specific processes (Berridge, 2019). Thus, regions, such as the amygdala or nucleus accumbens may support appetitive or aversive states depending on a variety of time-varying factors including, output targets of the region, homeostatic needs, context, and prior experience (Tye, 2018). This remains an active area of research aimed at capturing the nuanced relationship between the valence systems, their underlying circuitry, and their chronometry.
Comparing affective dynamics not only across units of measurement, timescales, and valence, but also across species with nervous systems of varying complexity requires continued attention. One avenue for future cross-species research is harnessing representational analytic methods (Diedrichsen & Kriegeskorte, 2017), which compare neural representations in terms of distance in geometric space rather than brain or voxel space. Further translational work is necessary to understand how fundamental differences in nervous system complexity across phylogeny might limit our conclusions from animal models of affective dynamics.
In this article, we have highlighted similarities between humans and other organisms’ emotion systems, however, critical differences must be accounted for. For example, in lower organisms, the temporal dynamics of behavioral operationalizations of emotion depend largely on the presence of salient, external stimuli. In contrast, human emotional responses not only vary as a function of immediate threats (Mobbs et al., 2007), but also intrinsic, mental concepts (Barrett, 2017; Mulligan & Scherer, 2012). Specifically, neocortical association areas also enable humans to ascribe value and respond emotionally to abstract stimuli, such as memories for past events (Dolan et al., 2000; Panksepp, 2007). Thus, human emotion responses may persist for longer and are less contingent on environmental stimuli than the reflexive and instinctive responses in certain other animals (i.e., emotion primitives).
Though not addressed explicitly in the present article, our review of the neural circuits supporting emotional dynamics suggests directions for clinical research and treatment. Exploration of the neural circuits linked to emotional dynamics may explain certain psychiatric phenomena, including prolonged dysphoric states in depression. For example, as described above, a ruminative cognitive-affective state may become habitual and persistent via entrainment into cortico-striatal-thalamo-cortical loops as a result of learning. Further research into the rise-time, intensity, and duration of emotion states will inform our understanding of the biological underpinnings of both individual symptoms and higher-order symptom dimensions in transdiagnostic models of psychopathology.
The neurobiological mechanisms underlying abnormal affective dynamics may also suggest novel risk factors for psychopathology, including so-called transdiagnostic risk factors that cut across disorders. Indeed, neural dynamics highlighted in this review predict risk for psychopathology. For example, amygdala activation rise-time in response to novel faces is faster for those with inhibited temperament (Kagan, 1997; Blackford at al., 2009), a well-studied risk factor for anxiety and depression (Shackman et al., 2016; Clauss & Blackford, 2012; Conway, et al., 2016). Therefore, individual differences in amygdala BOLD signal dynamics may be useful in predicting risk for psychiatric disorders.
In addition to refining models of psychopathology and their risk factors, the neural mechanisms of affective dynamics may also inform treatment. It is our hope that mapping the neural circuits governing affective dynamics will guide the development of novel interventions that target neural mechanisms that contribute to aberrant affective dynamics characteristic of psychopathology. Critically, the neural mechanisms highlighted here may contribute to ‘precision medicine’ (Serretti, 2018), which emphasizes individual variability, rather than “one-size-fits-all”, when selecting interventions for psychiatric disorders. While this work is nascent, recent advances have identified chronometric features of neural responses that predict treatment responses. For example, sustained amygdala responses following putatively neutral stimuli predicts poorer responses to attention bias modification in anxiety disorders (Woody et al., 2019). Machine learning algorithms integrating individual differences in affective processing biases have also shown considerable promise in informing treatment selection for subtypes of anxiety and depressive disorders (Browning et al., 2021). At present, however, precision medicine approaches for disorder subtyping or treatment selection in psychiatry often neglect affective chronometry and the related neural systems. As this research develops, the neural mechanisms highlighted in this review may better inform clinical decision-making in the treatment of psychiatric disorders.
The ways in which our affective experiences unfold over time is critical to who we are. However, there is an emerging set of non-human animal work and human imaging that provide insight to the neural mechanisms supporting the rise-time, intensity, and duration of emotional experiences. Linking these literatures can foster a deeper understanding of the neural mechanisms driving affective behaviors and how individual differences emerge.
Highlights:
Affective states are understood to emerge and persist through patterns of neural activity
Human and animal neuroscience research on how the brain directs the unfolding of emotion over time are scarcely integrated
We highlight the specific neural mechanisms and modulatory factors that arbitrate the rise-time, intensity, and duration of emotional states
Funding support:
Louisiana Board of Regents through the Board of Regents Support Fund (LEQSF(2018-21)-RD-A-17) and the National Institute of Mental Health of the National Institutes of Health under award number R01MH122561 to JPF, and 1R21MH125311 and a John Templeton Foundation grant to ASH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A.
To describe neural mechanisms that underlie the three temporal features defined by affective chronometry, it is necessary to introduce the dynamic features that comprise an emotional response. These signals include central nervous system activity, peripheral physiology, overt behaviors, and subjective ‘feelings’. We briefly describe each of these below.
Neural signals.
A variety of neural mechanisms generate emotional responses in animals and humans. We briefly review the methods used to measure neural signals described here.
Recordings of neural activity can establish correlative links between brain and behavior. Electrophysiological measures, including extra- and intra-cellular recordings, capture neural activity on the scale of milliseconds. Extracellular recordings measure the local field potential (LFP) or summed electrical changes near intracranial electrodes (Buzsáki et al., 2012; Csicsvari et al., 2003). They also capture high frequency ‘spikes’ reflecting synchronous neuronal firing. In contrast, intracellular electrodes record electrical activity within a single cell, providing richer information about the mechanisms of spikes (Henze et al., 2000).
In animal models, neural signals can be manipulated to establish causal links. Manipulations include creating lesions or injecting agonist/antagonist compounds. A more recent method of manipulating neuronal activity via optogenetics, which involves engineering cells to be excited or inhibited by specific frequencies of light (for a review see Fenno et al., 2011). These manipulations allow scientists to causally connect neural dynamics across milliseconds and seconds to behavior.
Compared with animal research, measures of neural activity in humans are often more indirect, less precise, and less invasive. Measurement of electrophysiological activity are obtained using electro (EEG) or magneto (MEG) which record electrical and magnetic signals from the scalp. Although these methods record cortical electrical brain activity with high temporal resolution, it is challenging to pinpoint electrical changes generated by deeper brain regions, such as the amygdala (Buzsáki et al., 2012; Darvas et al., 2004; Hansen et al., 2010). Relative to EEG and MEG, functional magnetic resonance imaging (fMRI) provides superior spatial resolution by imaging the whole brain using magnetic field gradients. fMRI tracks changes in oxygenated blood flow, reflecting metabolic responses to neuronal activity (Poldrack et al., 2011), but the temporal resolution is less precise than EEG or MEG.
Peripheral physiological signals.
Peripheral physiological responses to affective events also indicate the organism’s state. Triggered by the central nervous system, these peripheral indicators reflect sympathetic or parasympathetic responses to affective stimuli (Bradley & Lang, 2000). For example, pupillary dilation and constriction are modulated following affective stimuli (Maffei & Angrilli, 2019). Changes in heart rate and heart rate variability are also linked to emotional responses (Mauss & Robinson, 2009). The Galvanic Skin Response (GSR) measures sweat excretion (sympathetic nervous system activity) in response to arousing stimuli (Lang et al., 1993; Mauss & Robinson, 2009), as does respiration rate (Homma & Masaoka, 2008). Emotional responses can also be measured via facial electromyography (EMG), an objective measure of facial muscle activity. Emotional expressions, such as frowning and smiling, evoke EMG activity which track the valence and intensity of affective reactions (Cacioppo et al., 1986).
Subjective emotional signals.
Another indicator of an emotional episode is an individual’s description of the feelings comprising the experience. Subjective descriptions of emotion can be obtained retrospectively or during the emotional episode. This is typically accomplished with laboratory-based self-report surveys or via experience sampling methods (Shiffman et al., 2008). Subjective reports of emotional episodes are often structured with questions that assess unique emotions, such as “fear,” “happiness,” or “frustration.” Alternatively, self-report instruments may ask an individual to average across core dimensions of their emotional experience such as valence (i.e., positive vs negative) and arousal (i.e., high vs. low) of an emotion (Russell, 1980).
Behavioral signals.
Behavioral responses to stimuli can yield insight into an organism’s central emotional state. Using observable behavior as a proxy for emotional state enables researchers to infer the neural mechanisms driving affective behaviors across species.
Across species, objective behavioral states such as feeding, grooming, and freezing can be viewed as expressions of “emotion primitives” (Panksepp, 2004). The neural mechanisms that subserve these affective behaviors in lower organisms are conserved through evolution and can be construed as the “building blocks” for human emotional responding (Anderson & Adolphs, 2014). For example, the neural mechanisms that drive threat-related freezing responses in rodents are similar to those that support fearful states in humans (Calhoon & Tye, 2015). Viewing animal behaviors as emerging from central states allows researchers to connect the type and timing of behavioral responses to the valence and dynamics of the underlying state.
Aversive emotional states can be inferred from animal behaviors across phyla. Behaviors that involve avoidance of a threatening stimulus or situation, such as freezing, hiding, or fleeing a threat, are thought to indicate aversive emotional states. For instance, in simple model organisms such as the roundworm C. elegans, an aversive state can be inferred from stereotyped escape behaviors (Cermak et al., 2020; Leung et al., 2016). In rodents, compulsive self-grooming in response to an aversive stimulus may indicate an anxious emotional response, akin to human self-soothing responses to stress (Spruijt et al., 1992). Distress vocalizations in cats (Brudzynski et al., 1982), rats (Brudzynski, 1994), and monkeys (Lemasson et al., 2012), are all thought to express and communicate emotional states of varying intensity and duration.
Aversive emotional states may also be inferred from the timing of behaviors. In humans, faster reaction times to aversive stimuli may indicate that an individual’s response was primed by a latent aversive emotional state (Neta & Tong, 2016). Similarly, in non-human species, the affective structure of an organism’s environment can be inferred from the amount of time that the organism spends pausing in place before making a behavioral choice (Redish, 2016). Additionally, some objective behaviors can be used as proxies for appetitive states. For example, environmental exploration, mating, and feeding behaviors all indicate that an organism has evaluated their environment as sufficiently safe to explore or exploit potentially rewarding opportunities (McNaughton & Corr, 2018).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari A, Topiwala MA, & Gordon JA (2011). Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron, 71(5), 898–910.( 10.1016/j.neuron.2011.07.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Mlodinow L, & Barrett LF (2019). What is an emotion? Current Biology, 29(20), R1060–R1064. 10.1016/j.cub.2019.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, & Strick PL (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9(1), 357–381. [DOI] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, & Nicola SM (2008). Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron, 59(4), 648–661. 10.1016/j.neuron.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amting JM, Greening SG, & Mitchell DGV (2010). Multiple Mechanisms of Consciousness: The Neural Correlates of Emotional Awareness. Journal of Neuroscience, 30(30), 10039–10047. 10.1523/JNEUROSCI.6434-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andalman AS, Burns VM, Lovett-Barron M, Broxton M, Poole B, Yang SJ, Grosenick L, Lerner TN, Chen R, Benster T, Mourrain P, Levoy M, Rajan K, & Deisseroth K (2019). Neuronal Dynamics Regulating Brain and Behavioral State Transitions. Cell, 177(4), 970–985. e20. 10.1016/j.cell.2019.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, & Adolphs R (2014). A Framework for Studying Emotions across Species. Cell, 157(1), 187–200. 10.1016/j.cell.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S, Smith JB, Li H, Yan X, Igarashi M, Coulon P, Wickens JR, Ruigrok TJ, & Jin X (2019). An open cortico-basal ganglia loop allows limbic control over motor output via the nigrothalamic pathway. ELife, 8, e49995. 10.7554/eLife.49995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A, Kandel ER, & Rayman JB (2019). The neurobiology of fear generalization. Frontiers in Behavioral Neuroscience, 12, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, & Vogel EK (2008). The bouncer in the brain. Nature Neuroscience, 11(1), 5–6. 10.1038/nn0108-5 [DOI] [PubMed] [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, Glasser MF, Curtiss S, Dixit S, & Feldt C (2013). Function in the human connectome: Task-fMRI and individual differences in behavior. Neuroimage, 80, 169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow RL, Gorges M, Wearn A, Niessen HG, Kassubek J, Dalley JW, & Pekcec A (2018). Ventral striatal D2/3 receptor availability is associated with impulsive choice behavior as well as limbic corticostriatal connectivity. International Journal of Neuropsychopharmacology, 21(7), 705–715. 10.1093/ijnp/pyy030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF (2017). The theory of constructed emotion: An active inference account of interoception and categorization. Social Cognitive and Affective Neuroscience, 12(1), 1–23. 10.1093/scan/nsw154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, & Gross JJ (2007). The experience of emotion. Annu. Rev. Psychol, 58, 373–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2019). Affective valence in the brain: Modules or modes? Nature Reviews Neuroscience, 20(4), 225–234. 10.1038/s41583-019-0122-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, & Kringelbach ML (2013). Neuroscience of affect: brain mechanisms of pleasure and displeasure. Current opinion in neurobiology, 23(3), 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, & Kringelbach ML (2015). Pleasure systems in the brain. Neuron, 86(3), 646–664. 10.1016/j.neuron.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A, Chang C-J, Silvestre M, Lévêque C, Namburi P, Wildes CP, & Tye KM (2018). Organization of valence-encoding and projection-defined neurons in the basolateral amygdala. Cell Reports, 22(4), 905–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Shelton RC, & Zald DH (2009). Amygdala temporal dynamics: Temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neuroscience, 10(1), 145. 10.1186/1471-2202-10-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Rodgers J, & Weiss SM (1990). The characterization and modelling of antipredator defensive behavior. Neuroscience & Biobehavioral Reviews, 14(4), 463–472. 10.1016/S0149-7634(05)80069-7 [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E (2017). Constructing nonhuman animal emotion | Elsevier Enhanced Reader. 10.1016/j.copsyc.2017.07.011 [DOI] [PubMed]
- Bliss-Moreau E, & Rudebeck PH (2021). Animal models of human mood. Neuroscience & Biobehavioral Reviews, 120, 574–582. 10.1016/j.neubiorev.2020.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio M, Nabavi S, & Capogna M (2017). Synaptic Plasticity, Engrams, and Network Oscillations in Amygdala Circuits for Storage and Retrieval of Emotional Memories. Neuron, 94(4), 731–743. 10.1016/j.neuron.2017.03.022 [DOI] [PubMed] [Google Scholar]
- Borji A, & Itti L (2010). State-of-the-Art in visual attention Modeling. [DOI] [PubMed]
- Bradley MM, & Lang PJ (2000). Measuring emotion: Behavior, feeling, and physiology. In Cognitive neuroscience of emotion (pp. 242–276). Oxford University Press. [Google Scholar]
- Browning M, Bilderbeck AC, Dias R, Dourish CT, Kingslake J, Deckert J, … & Dawson GR (2021). The clinical effectiveness of using a predictive algorithm to guide antidepressant treatment in primary care (PReDicT): an open-label, randomised controlled trial. Neuropsychopharmacology, 46(7), 1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM (2014). The Ascending Mesolimbic Cholinergic System—A Specific Division of the Reticular Activating System Involved in the Initiation of Negative Emotional States. Journal of Molecular Neuroscience, 53(3), 436–445. 10.1007/s12031-013-0179-1 [DOI] [PubMed] [Google Scholar]
- Bublatzky F, Kavcıoğlu F, Guerra P, Doll S, & Junghöfer M (2020). Contextual information resolves uncertainty about ambiguous facial emotions: Behavioral and magnetoencephalographic correlates. NeuroImage, 215, 116814. 10.1016/j.neuroimage.2020.116814 [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, & Zald DH (2010). Dopaminergic network differences in human impulsivity. Science (New York, N.Y.), 329(5991), 532. 10.1126/science.1185778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Brudzynski S, & Moskal J (2020). Using rat ultrasonic vocalization to study the neurobiology of emotion: From basic science to the development of novel therapeutics for affective disorders. Current Opinion in Neurobiology, 60, 192–200. 10.1016/j.conb.2019.12.008 [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, & Quirk GJ (2009). Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. Journal of Neuroscience, 29(26), 8474–8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, & Koch C (2012). The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nature Reviews Neuroscience, 13(6), 407–420. 10.1038/nrn3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Petty RE, Losch ME, & Kim HS (1986). Electromyographic activity over facial muscle regions can differentiate the valence and intensity of affective reactions. Journal of Personality and Social Psychology, 50(2), 260. [DOI] [PubMed] [Google Scholar]
- Calhoon GG, & Tye KM (2015). Resolving the neural circuits of anxiety. Nature Neuroscience, 18(10), 1394–1404. 10.1038/nn.4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67(2), 319–333. 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- Castellucci V, Pinsker H, Kupfermann I, & Kandel ER (1970). Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science (New York, N.Y.), 167(3926), 1745–1748. 10.1126/science.167.3926.1745 [DOI] [PubMed] [Google Scholar]
- Castro DC, & Berridge KC (2014). Opioid Hedonic Hotspot in Nucleus Accumbens Shell: Mu, Delta, and Kappa Maps for Enhancement of Sweetness “Liking” and “Wanting.” Journal of Neuroscience, 34(12), 4239–4250. 10.1523/JNEUROSCI.445813.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, & Berridge KC (2017). Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proceedings of the National Academy of Sciences, 114(43), E9125–E9134. 10.1073/pnas.1705753114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak N, Stephanie KY, Clark R, Huang Y-C, Baskoylu SN, & Flavell SW (2020). Whole-organism behavioral profiling reveals a role for dopamine in state-dependent motor program coupling in C. elegans. Elife, 9, e57093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Gianaros PJ, Manuck SB, Krishnan A, & Wager TD (2015). A Sensitive and Specific Neural Signature for Picture-Induced Negative Affect. PLOS Biology, 13(6), e1002180. 10.1371/journal.pbio.1002180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, & Badre D (2015). Multiple gates on working memory. Current Opinion in Behavioral Sciences, 1, 23–31. 10.1016/j.cobeha.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorev E, Epsztein J, Houweling AR, Lee AK, & Brecht M (2009). Electrophysiological recordings from behaving animals—Going beyond spikes. Current Opinion in Neurobiology, 19(5), 513–519. 10.1016/j.conb.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, & Stadler MB (2010). Encoding of conditioned fear in central amygdala inhibitory circuits. Nature, 468(7321), 277–282. [DOI] [PubMed] [Google Scholar]
- Clark A (2013). Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behavioral and Brain Sciences, 36(3), 181–204. 10.1017/S0140525X12000477 [DOI] [PubMed] [Google Scholar]
- Clauss JA, & Blackford JU (2012). Behavioral inhibition and risk for developing social anxiety disorder: A meta-analytic study. Journal of the American Academy of Child and Adolescent Psychiatry, 51(10), 1066–1075. e1. 10.1016/j.jaac.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CC, Zinbarg RE, Mineka S, & Craske MG (2017). Core dimensions of anxiety and depression change independently during adolescence. Journal of abnormal psychology, 126(2), 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Correia SS, & Goosens KA (2016). Input-specific contributions to valence processing in the amygdala. Learning & Memory, 23(10), 534–543. 10.1101/lm.037887.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Henze DA, Jamieson B, Harris KD, Sirota A, Barthó P, Wise KD, & Buzsáki G (2003). Massively parallel recording of unit and local field potentials with silicon-based electrodes. Journal of Neurophysiology, 90(2), 1314–1323. 10.1152/jn.00116.2003 [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, & Lang PJ (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. [DOI] [PubMed] [Google Scholar]
- Dalley JW, & Robbins TW (2017). Fractionating impulsivity: Neuropsychiatric implications. Nature Reviews. Neuroscience, 18(3), 158–171. 10.1038/nrn.2017.8 [DOI] [PubMed] [Google Scholar]
- Darvas F, Pantazis D, Kucukaltun-Yildirim E, & Leahy RM (2004). Mapping human brain function with MEG and EEG: Methods and validation. NeuroImage, 23, S289–S299. 10.1016/j.neuroimage.2004.07.014 [DOI] [PubMed] [Google Scholar]
- Davidson RJ (1998). Affective style and affective disorders: Perspectives from affective neuroscience. Cognition & Emotion, 12(3), 307–330. [Google Scholar]
- Davidson RJ (2002). Anxiety and affective style: Role of prefrontal cortex and amygdala. Biological Psychiatry, 51(1), 68–80. 10.1016/S0006-3223(01)01328-2 [DOI] [PubMed] [Google Scholar]
- Davidson RJ (2015). Comment: Affective Chronometry Has Come of Age. Emotion Review, 7(4), 368–370. 10.1177/1754073915590844 [DOI] [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2010). Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology, 35(1), 105–135. 10.1038/npp.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Niv Y, & Dayan P (2006). Actions, Policies, Values, and the Basal Ganglia. Recent Breakthroughs in Basal Ganglia Research, 10(9), 1214–1221. [Google Scholar]
- Diedrichsen J, & Kriegeskorte N (2017). Representational models: A common framework for understanding encoding, pattern-component, and representational-similarity analysis. PLOS Computational Biology, 13(4), e1005508. 10.1371/journal.pcbi.1005508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ (2002). Emotion, Cognition, and Behavior. Science, 298(5596), 1191–1194. 10.1126/science.1076358 [DOI] [PubMed] [Google Scholar]
- Dolensek N, Gehrlach DA, Klein AS, & Gogolla N (2020). Facial expressions of emotion states and their neuronal correlates in mice. Science, 368(6486), 89–94. 10.1126/science.aaz9468 [DOI] [PubMed] [Google Scholar]
- Dzafic I, Oestreich L, Martin AK, Mowry B, & Burianová H (2019). Stria terminalis, amygdala, and temporoparietal junction networks facilitate efficient emotion processing under expectations. Human Brain Mapping, 40(18), 5382–5396. 10.1002/hbm.24779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, & Holmes A (2002). An ERP study on the time course of emotional face processing. NeuroReport, 13(4), 427–431. [DOI] [PubMed] [Google Scholar]
- Eisenbarth H, Chang LJ, & Wager TD (2016). Multivariate Brain Prediction of Heart Rate and Skin Conductance Responses to Social Threat. Journal of Neuroscience, 36(47), 11987–11998. 10.1523/JNEUROSCI.3672-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Markovic M, Tovote P, & Lüthi A (2018). New perspectives on central amygdala function. Current Opinion in Neurobiology, 49, 141–147. 10.1016/j.conb.2018.02.009 [DOI] [PubMed] [Google Scholar]
- Fanselow MS, & Lester LS (1988). A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In Evolution and learning (pp. 185–212). Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Fecteau JH, & Munoz DP (2006). Salience, relevance, and firing: A priority map for target selection. Trends in Cognitive Sciences, 10(8), 382–390. 10.1016/j.tics.2006.06.011 [DOI] [PubMed] [Google Scholar]
- Fellous J-M (1999). Neuromodulatory Basis of Emotion. The Neuroscientist, 5(5), 283–294. 10.1177/107385849900500514 [DOI] [Google Scholar]
- Fenno L, Yizhar O, & Deisseroth K (2011). The Development and Application of Optogenetics. Annual Review of Neuroscience, 34(1), 389–412. 10.1146/annurev-neuro-061010-113817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, & Bargmann CI (2013). Serotonin and the Neuropeptide PDF Initiate and Extend Opposing Behavioral States in C. elegans. Cell, 154(5), 1023–1035. 10.1016/j.cell.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, & Shackman AJ (2019). The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neuroscience Letters, 693, 58–67. 10.1016/j.neulet.2017.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrikson M, Furmark T, Olsson MT, Fischer H, Andersson J, & Långström B (1998). Functional neuroanatomical correlates of electrodermal activity: A positron emission tomographic study. Psychophysiology, 35(2), 179–185. [PubMed] [Google Scholar]
- Frijda Nico H. (1988). The laws of emotion. American Psychologist, 43(5), 349–358. 10.1037/0003-066X.43.5.349 [DOI] [PubMed] [Google Scholar]
- Frijda NH, Kuipers P, & Ter Schure E (1989). Relations among emotion, appraisal, and emotional action readiness. Journal of personality and social psychology, 57(2), 212. [Google Scholar]
- Frijda NH, Mesquita B, Sonnemans J, & Van Goozen S (1991). The duration of affective phenomena or emotions, sentiments and passions (pp. 187–225). Wiley. https://lirias.kuleuven.be/retrieve/311014 [Google Scholar]
- Friston KJ, Daunizeau J, & Kiebel SJ (2009). Reinforcement Learning or Active Inference? PLOS ONE, 4(7), e6421. 10.1371/journal.pone.0006421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Barnes GR, Sahani M, & Dolan RJ (2012). Functional Evidence for a Dual Route to Amygdala. Current Biology, 22(2), 129–134. 10.1016/j.cub.2011.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson WT, Gonzalez CR, Fernandez C, Ramasamy L, Tabachnik T, Du RR, Felsen PD, Maire MR, Perona P, & Anderson DJ (2015). Behavioral responses to a repetitive visual threat stimulus express a persistent state of defensive arousal in Drosophila. Current Biology: CB, 25(11), 1401–1415. 10.1016/j.cub.2015.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, & Maren S (2001). Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learning & Memory (Cold Spring Harbor, N.Y.), 8(3), 148–155. 10.1101/lm.37601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, & Rosenkranz JA (2002). Regulation of conditioned responses of basolateral amygdala neurons. Physiology & Behavior, 77(4–5), 489–493. [DOI] [PubMed] [Google Scholar]
- Graybiel AM (1998). The basal ganglia and chunking of action repertoires. Neurobiology of Learning and Memory, 70(1–2), 119–136. 10.1006/nlme.1998.3843 [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Dayan P, Gutkin BS, & Solla SA (2006). Dopamine modulation in the basal ganglia locks the gate to working memory. Journal of Computational Neuroscience, 20(2), 153. 10.1007/s10827-005-5705-x [DOI] [PubMed] [Google Scholar]
- Gründemann J, Bitterman Y, Lu T, Krabbe S, Grewe BF, Schnitzer MJ, & Lüthi A(2019). Amygdala ensembles encode behavioral states. Science (New York, N.Y.), 364(6437). 10.1126/science.aav8736 [DOI] [PubMed] [Google Scholar]
- Gurney KN, Humphries MD, & Redgrave P (2015). A New Framework for Cortico-Striatal Plasticity: Behavioural Theory Meets In Vitro Data at the Reinforcement-Action Interface. PLoS Biology, 13(1), e1002034. 10.1371/journal.pbio.1002034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guru A, Post RJ, Ho Y-Y, & Warden MR (2015). Making Sense of Optogenetics. International Journal of Neuropsychopharmacology, 18(11). 10.1093/ijnp/pyv079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm A (2015). Fear-Potentiated Startle. International Encyclopedia of the Social & Behavioral Sciences, 8, 860–867. 10.1016/B978-0-08-097086-8.55023-5 [DOI] [Google Scholar]
- Hansen P, Kringelbach M, & Salmelin R (2010). MEG: An Introduction to Methods. Oxford University Press. [Google Scholar]
- Heller A (2018). Emotions aren’t maladaptive. In The nature of emotion: Fundamental questions (2nd ed.). Oxford University Press. [Google Scholar]
- Heller AS, Lapate RC, Mayer KE, & Davidson RJ (2014). The face of negative affect: Trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. Journal of Cognitive Neuroscience, 26(9), 2102–2110. 10.1162/jocn_a_00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Shi TC, Ezie CC, Reneau TR, Baez LM, Gibbons CJ, & Hartley CA (2020). Association between real-world experiential diversity and positive affect relates to hippocampal–striatal functional connectivity. Nature neuroscience, 23(7), 800–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, & Buzsáki G (2000). Intracellular Features Predicted by Extracellular Recordings in the Hippocampus In Vivo. Journal of Neurophysiology, 84(1), 390–400. 10.1152/jn.2000.84.1.390 [DOI] [PubMed] [Google Scholar]
- Homma I, & Masaoka Y (2008). Breathing rhythms and emotions. Experimental Physiology, 93(9), 1011–1021. [DOI] [PubMed] [Google Scholar]
- Howe WM, Berry AS, Francois J, Gilmour G, Carp JM, Tricklebank M, Lustig C, & Sarter M (2013). Prefrontal Cholinergic Mechanisms Instigating Shifts from Monitoring for Cues to Cue-Guided Performance: Converging Electrochemical and fMRI Evidence from Rats and Humans. Journal of Neuroscience, 33(20), 8742–8752. 10.1523/JNEUROSCI.5809-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard NA, Romeo RR, Grotzinger H, Giebler M, Imhof A, Bauer CCC, & Gabrieli JDE (2020). Reward-Sensitive Basal Ganglia Stabilize the Maintenance of Goal-Relevant Neural Patterns in Adolescents. Journal of Cognitive Neuroscience, 32(8), 1508–1524. 10.1162/jocn_a_01572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HK, Fontolan L, Romani S, & Svoboda K (2019). Discrete attractor dynamics underlies persistent activity in the frontal cortex. Nature, 566(7743), 212–217. 10.1038/s41586-019-0919-7 [DOI] [PubMed] [Google Scholar]
- Inman CS, Bijanki KR, Bass DI, Gross RE, Hamann S, & Willie JT (2020). Human amygdala stimulation effects on emotion physiology and emotional experience. Neuropsychologia, 145, 106722. 10.1016/j.neuropsychologia.2018.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, Koch C, & Niebur E (1998). A model of saliency-based visual attention for rapid scene analysis. IEEE Transactions on Pattern Analysis and Machine Intelligence, 20(11), 1254–1259. 10.1109/34.730558 [DOI] [Google Scholar]
- Jacob SN, Stalter M, & Nieder A (2016). Cell-type-specific modulation of targets and distractors by dopamine D1 receptors in primate prefrontal cortex. Nature Communications, 7(1), 13218. 10.1038/ncomms13218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji N, Madan GK, Fabre GI, Dayan A, Baker CM, Nwabudike I, & Flavell SW (2020). A neural circuit for flexible control of persistent behavioral states. BioRxiv, 2020.02.04.934547. 10.1101/2020.02.04.934547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindrová M, Kocourek M, & Telenský P (2020). Skin conductance rise time and amplitude discern between different degrees of emotional arousal induced by affective pictures presented on a computer screen (p. 2020.05.12.090829). 10.1101/2020.05.12.090829 [DOI]
- Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, & LeDoux JE (2010). Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proceedings of the National Academy of Sciences, 107(28), 12692–12697. 10.1073/pnas.1002418107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John YJ, Zikopoulos B, Bullock D, & Barbas H (2016). The Emotional Gatekeeper: A Computational Model of Attentional Selection and Suppression through the Pathway from the Amygdala to the Inhibitory Thalamic Reticular Nucleus. PLOS Computational Biology, 12(2), e1004722. 10.1371/journal.pcbi.1004722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Kennedy A, Chiu H, Mohammad F, Claridge-Chang A, & Anderson DJ (2020). Neurons that Function within an Integrator to Promote a Persistent Behavioral State in Drosophila. Neuron, 105(2), 322–333. e5. 10.1016/j.neuron.2019.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Plitschka J, & Kotz SA (2011). Attentional orienting towards emotion: P2 and N400 ERP effects. Neuropsychologia, 49(11), 3121–3129. 10.1016/j.neuropsychologia.2011.07.022 [DOI] [PubMed] [Google Scholar]
- Karalis N, Dejean C, Chaudun F, Khoder S, Rozeske RR, Wurtz H, Bagur S, Benchenane K, Sirota A, Courtin J, & Herry C (2016). 4-Hz oscillations synchronize prefrontal–amygdala circuits during fear behavior. Nature Neuroscience, 19(4), 605–612. 10.1038/nn.4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, & Lang PJ (2002). Large-scale neural correlates of affective picture processing. Psychophysiology, 39(5), 641–649. [DOI] [PubMed] [Google Scholar]
- Kennedy A, Kunwar PS, Li L, Stagkourakis S, Wagenaar DA, & Anderson DJ (2020). Stimulus-specific hypothalamic encoding of a persistent defensive state. Nature, 586(7831), 730–734. https://doi.org/10/41586_2020_2728_MOESM2_ESM.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlstrom JF, Eich E, Sandbrand D, & Tobias BA (1999). Emotion and memory: Implications for self-report. In The science of self-report (pp. 93–112). Psychology Press. [Google Scholar]
- Kim JJ, & Jung MW (2006). Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience & Biobehavioral Reviews, 30(2), 188–202. 10.1016/j.neubiorev.2005.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, & Deisseroth K (2013). Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature, 496(7444), 219–223. 10.1038/nature12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Müller MLTM, Bohnen NI, Sarter M, & Lustig C (2017). Thalamic cholinergic innervation makes a specific bottom-up contribution to signal detection: Evidence from Parkinson’s disease patients with defined cholinergic losses. NeuroImage, 149, 295–304. 10.1016/j.neuroimage.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschstein T, & Köhling R (2009). What is the Source of the EEG? Clinical EEG and Neuroscience, 40(3), 146–149. 10.1177/155005940904000305 [DOI] [PubMed] [Google Scholar]
- Klumpers F, Morgan B, Terburg D, Stein DJ, & van Honk J (2015). Impaired acquisition of classically conditioned fear-potentiated startle reflexes in humans with focal bilateral basolateral amygdala damage. Social Cognitive and Affective Neuroscience, 10(9), 1161–1168. 10.1093/scan/nsu164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, & Cooper JC (2005). Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology, 18(4), 411–417. 10.1097/01.wco.0000173463.24758.f6 [DOI] [PubMed] [Google Scholar]
- Kragel PA, & LaBar KS (2016). Decoding the Nature of Emotion in the Brain. Trends in Cognitive Sciences, 20(6), 444–455. 10.1016/j.tics.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Stein A, & van Hartevelt TJ (2012). The functional human neuroanatomy of food pleasure cycles. Physiology & Behavior, 106(3), 307–316. 10.1016/j.physbeh.2012.03.023 [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, & Phelps EA (1995). Impaired fear conditioning following unilateral temporal lobectomy in humans. Journal of Neuroscience, 15(10), 6846–6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis C, & Hunt W (1939). The startle pattern. Farrar & Rinehart. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, & Hamm AO (1993). Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology, 30(3), 261–273. [DOI] [PubMed] [Google Scholar]
- Lapate RC, Samaha J, Rokers B, Hamzah H, Postle BR, & Davidson RJ (2017). Inhibition of Lateral Prefrontal Cortex Produces Emotionally Biased First Impressions: A Transcranial Magnetic Stimulation and Electroencephalography Study. Psychological Science, 28(7), 942–953. 10.1177/0956797617699837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson CL, Schaefer HS, Siegle GJ, Jackson CAB, Anderle MJ, & Davidson RJ (2006). Fear Is Fast in Phobic Individuals: Amygdala Activation in Response to Fear-Relevant Stimuli. Biological Psychiatry, 60(4), 410–417. 10.1016/j.biopsych.2006.03.079 [DOI] [PubMed] [Google Scholar]
- Lazarus RS (1991). Cognition and motivation in emotion. American Psychologist, 46(4), 352–367. 10.1037/0003-066X.46.4.352 [DOI] [PubMed] [Google Scholar]
- Ledoux J (1998). The Emotional Brain: The Mysterious Underpinnings of Emotional Life. Simon & Schuster. https://books.google.com/books?id=7EJN5I8sk2wC [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, & Romanski LM (1990). The lateral amygdaloid nucleus: Sensory interface of the amygdala in fear conditioning. Journal of Neuroscience, 10(4), 1062–1069. 10.1523/JNEUROSCI.10-04-01062.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux Joseph E. (2000). Emotion Circuits in the Brain. Annual Review of Neuroscience, 23(1), 155–184. 10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- Lee AK, & Brecht M (2018). Elucidating Neuronal Mechanisms Using Intracellular Recordings during Behavior. Trends in Neurosciences, 41(6), 385–403. 10.1016/j.tins.2018.03.014 [DOI] [PubMed] [Google Scholar]
- Lee SC, Amir A, Haufler D, & Pare D (2017). Differential Recruitment of Competing Valence-Related Amygdala Networks during Anxiety. Neuron, 96(1), 81–88. e5. 10.1016/j.neuron.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-H, & Dan Y (2012). Neuromodulation of brain states. Neuron, 76(1), 209–222. 10.1016/j.neuron.2012.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesting J, Daldrup T, Narayanan V, Himpe C, Seidenbecher T, & Pape H-C (2013). Directional theta coherence in prefrontal cortical to amygdalo-hippocampal pathways signals fear extinction. PloS One, 8(10), e77707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, & Pape H-C (2011). Patterns of Coupled Theta Activity in Amygdala-Hippocampal-Prefrontal Cortical Circuits during Fear Extinction. PLoS ONE, 6(6), e21714. 10.1371/journal.pone.0021714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K, Mohammadi A, Ryu WS, & Nemenman I (2016). Stereotypical Escape Behavior in Caenorhabditis elegans Allows Quantification of Effective Heat Stimulus Level. PLOS Computational Biology, 12(12), e1005262. 10.1371/journal.pcbi.1005262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z (2002). A saliency map in primary visual cortex. Trends in Cognitive Sciences, 6(1), 9–16. [DOI] [PubMed] [Google Scholar]
- Likhtik E, & Johansen JP (2019). Neuromodulation in circuits of aversive emotional learning. Nature Neuroscience, 22(10), 1586–1597. 10.1038/s41593-019-0503-3 [DOI] [PubMed] [Google Scholar]
- Likhtik E, & Paz R (2020). Chapter 21—Maladaptive learning and the amygdala—Prefrontal circuit. In Chen A (Ed.), Stress Resilience (pp. 323–348). Academic Press. 10.1016/B978-0-12-813983-7.00021-5 [DOI] [Google Scholar]
- Liu Y, Huang H, McGinnis-Deweese M, Keil A, & Ding M (2012). Neural Substrate of the Late Positive Potential in Emotional Processing. Journal of Neuroscience, 32(42), 14563–14572. 10.1523/JNEUROSCI.3109-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, & Lee AK (2012). Intracellular recording in behaving animals. Current Opinion in Neurobiology, 22(1), 34–44. 10.1016/j.conb.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNiven KH, Leong JK, & Knutson B (2020). Medial forebrain bundle structure is linked to human impulsivity. Science Advances, 6(38), eaba4788. 10.1126/sciadv.aba4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, & Angrilli A (2019). Spontaneous blink rate as an index of attention and emotion during film clips viewing. Physiology & Behavior, 204, 256–263. 10.1016/j.physbeh.2019.02.037 [DOI] [PubMed] [Google Scholar]
- Major G, & Tank D (2004). Persistent neural activity: Prevalence and mechanisms. Current Opinion in Neurobiology, 14(6), 675–684. 10.1016/j.conb.2004.10.017 [DOI] [PubMed] [Google Scholar]
- Marder E (2012). Neuromodulation of neuronal circuits: Back to the future. Neuron, 76(1), 1–11. 10.1016/j.neuron.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattavelli G, Cattaneo Z, & Papagno C (2011). Transcranial magnetic stimulation of medial prefrontal cortex modulates face expressions processing in a priming task. Neuropsychologia, 49(5), 992–998. 10.1016/j.neuropsychologia.2011.01.038 [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, & Gross JJ (2005). The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion, 5(2), 175. [DOI] [PubMed] [Google Scholar]
- Mauss IB, & Robinson MD (2009). Measures of emotion: A review. Cognition & Emotion, 23(2), 209–237. 10.1080/02699930802204677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavratzakis A, Herbert C, & Walla P (2016). Emotional facial expressions evoke faster orienting responses, but weaker emotional responses at neural and behavioural levels compared to scenes: A simultaneous EEG and facial EMG study. NeuroImage, 124, 931–946. 10.1016/j.neuroimage.2015.09.065 [DOI] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, & Wurtz RH (2006). Attentional modulation of thalamic reticular neurons. Journal of Neuroscience, 26(16), 4444–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ (1998). Cortical pathways to the mammalian amygdala. Progress in Neurobiology, 55(3), 257–332. 10.1016/S0301-0082(98)00003-3 [DOI] [PubMed] [Google Scholar]
- McFadyen J (2019). Investigating the Subcortical Route to the Amygdala Across Species and in Disordered Fear Responses. Journal of Experimental Neuroscience, 13. 10.1177/1179069519846445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadyen J, Mermillod M, Mattingley JB, Halász V, & Garrido MI (2017). A Rapid Subcortical Amygdala Route for Faces Irrespective of Spatial Frequency and Emotion. The Journal of Neuroscience, 37(14), 3864–3874. 10.1523/JNEUROSCI.3525-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, & Klingberg T (2008). Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience, 11(1), 103–107. 10.1038/nn2024 [DOI] [PubMed] [Google Scholar]
- McNaughton N, & Corr PJ (2018). Survival circuits and risk assessment. Current Opinion in Behavioral Sciences, 24, 14–20. 10.1016/j.cobeha.2018.01.018 [DOI] [Google Scholar]
- Méndez-Bértolo C, Moratti S, Toledano R, Lopez-Sosa F, Martínez-Alvarez R, Mah YH, Vuilleumier P, Gil-Nagel A, & Strange BA (2016). A fast pathway for fear in human amygdala. Nature Neuroscience, 19(8), 1041–1049. 10.1038/nn.4324 [DOI] [PubMed] [Google Scholar]
- Mesulam MM (1998). From sensation to cognition. Brain: A Journal of Neurology, 121(6), 1013–1052. 10.1093/brain/121.6.1013 [DOI] [PubMed] [Google Scholar]
- Meyer C, Padmala S, & Pessoa L (2019). Dynamic threat processing. Journal of Cognitive Neuroscience, 31(4), 522–542. 10.1162/jocn_a_01363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, & Quirk GJ (2002). Neurons in medial prefrontal cortex signal memory for fear extinction. Nature, 420(6911), 70–74. [DOI] [PubMed] [Google Scholar]
- Miranda MI, Ramıŕez-Lugo L, & Bermúdez-Rattoni F (2000). Cortical cholinergic activity is related to the novelty of the stimulus. Brain Research, 882(1), 230–235. 10.1016/S0926-6410(00)00050-1 [DOI] [PubMed] [Google Scholar]
- Mitchell DGV, & Greening SG (2012). Conscious Perception of Emotional Stimuli: Brain Mechanisms. The Neuroscientist, 18(4), 386–398. 10.1177/1073858411416515 [DOI] [PubMed] [Google Scholar]
- Mobbs D, & Kim JJ (2015). Neuroethological studies of fear, anxiety, and risky decision-making in rodents and humans. Current Opinion in Behavioral Sciences, 5, 8–15. 10.1016/j.cobeha.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, & Frith CD (2007). When Fear Is Near: Threat Imminence Elicits Prefrontal-Periaqueductal Gray Shifts in Humans. Science, 317(5841), 1079–1083. 10.1126/science.1144298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, & Dalgleish T (2010). Neural activity associated with monitoring the oscillating threat value of a tarantula. Proceedings of the National Academy of Sciences of the United States of America, 107(47), 20582–20586. 10.1073/pnas.1009076107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JFW, & Anderson IM (2002). Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology, 160(3), 290–298. 10.1007/s00213-001-0983-0 [DOI] [PubMed] [Google Scholar]
- Morris JS, Öhman A, & Dolan RJ (1999). A subcortical pathway to the right amygdala mediating “unseen” fear. Proceedings of the National Academy of Sciences, 96(4), 1680–1685. 10.1073/pnas.96.4.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan K, & Scherer KR (2012). Toward a Working Definition of Emotion. Emotion Review, 4(4), 345–357. 10.1177/1754073912445818 [DOI] [Google Scholar]
- Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Holden SS, Mertens KL, Anahtar M, Felix-Ortiz AC, Wickersham IR, Gray JM, & Tye KM (2015). A Circuit Mechanism for Differentiating Positive and Negative Associations. Nature, 520(7549), 675–678. 10.1038/nature14366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey EJ (1990). Prefrontal autonomic control in the rat: Anatomical and electrophysiological observations. Prog Brain Res, 85, 147–166. [DOI] [PubMed] [Google Scholar]
- Neta M, & Tong TT (2016). Don’t like what you see? Give it time: Longer reaction times associated with increased positive affect. Emotion, 16(5), 730–739. 10.1037/emo0000181 [DOI] [PubMed] [Google Scholar]
- Nguyen H, Guo C, & Homberg J (2020). Cognitive Bias Under Adverse and Rewarding Conditions: A Systematic Review of Rodent Studies. Frontiers in Behavioral Neuroscience, 14, 14. 10.3389/fnbeh.2020.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A, Juth P, & Lundqvist D (2010). Finding the face in a crowd: Relationships between distractor redundancy, target emotion, and target gender. Cognition and Emotion, 24(7), 1216–1228. 10.1080/02699930903166882 [DOI] [Google Scholar]
- Olds ME, & Olds J (1963). Approach-avoidance analysis of rat diencephalon. The Journal of Comparative Neurology, 120(2), 259–295. 10.1002/cne.901200206 [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, & Frank MJ (2006). Making working memory work: A computational model of learning in the prefrontal cortex and basal ganglia. Neural Computation, 18(2), 283–328. 10.1162/089976606775093909 [DOI] [PubMed] [Google Scholar]
- Panksepp J (2004). Affective neuroscience: The foundations of human and animal emotions. Oxford university press. [Google Scholar]
- Panksepp J (2007). Neurologizing the Psychology of Affects: How Appraisal-Based Constructivism and Basic Emotion Theory Can Coexist. Perspectives on Psychological Science, 2(3), 281–296. 10.1111/j.1745-6916.2007.00045.x [DOI] [PubMed] [Google Scholar]
- Panksepp J (2011). The basic emotional circuits of mammalian brains: Do animals have affective lives? Neuroscience and Biobehavioral Reviews, 35(9), 1791–1804. 10.1016/j.neubiorev.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Pape HC, Narayanan RT, Smid J, Stork O, & Seidenbecher T (2005). Theta activity in neurons and networks of the amygdala related to long-term fear memory. Hippocampus, 15(7), 874–880. [DOI] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, O’Connell MT, Everitt BJ, & Robbins TW (2000). Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. European Journal of Neuroscience, 12(8), 3051–3058. 10.1046/j.1460-9568.2000.00183.x [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, & Salzman CD (2006). The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature, 439(7078), 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Bauer EP, & Paré D (2008). Theta synchronizes the activity of medial prefrontal neurons during learning. Learning & Memory, 15(7), 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L (2008). On the relationship between emotion and cognition. Nature Reviews Neuroscience, 9(2), 148–158. 10.1038/nrn2317 [DOI] [PubMed] [Google Scholar]
- Pessoa L (2019). Embracing integration and complexity: Placing emotion within a science of brain and behaviour. Cognition & Emotion, 33(1), 55–60. 10.1080/02699931.2018.1520079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratuski AS, Makowska IJ, Dvorack KR, & Weary DM (2021). Using approach latency and anticipatory behaviour to assess whether voluntary playpen access is rewarding to laboratory mice. Scientific Reports, 11(1), 18683. 10.1038/s41598-021-98356-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA (2006). Emotion and cognition: Insights from studies of the human amygdala. Annu. Rev. Psychol, 57, 27–53. [DOI] [PubMed] [Google Scholar]
- Phillips RG, & LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106(2), 274. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, & Mineur YS (2012). Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron, 76(1), 116–129. 10.1016/j.neuron.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D (2004). The thalamic reticular nucleus: Structure, function and concept. Brain Research Reviews, 46(1), 1–31. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Nichols T, & Mumford J (2011). Handbook of Functional MRI Data Analysis. Cambridge University Press. 10.1017/CBO9780511895029 [DOI] [Google Scholar]
- Pool AH, & Scott K (2014). Feeding regulation in Drosophila. Current Opinion in Neurobiology, 29, 57–63. 10.1016/j.conb.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Schettino A, & Vuilleumier P (2013). Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biological Psychology, 92(3), 492–512. 10.1016/j.biopsycho.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Powell DA, & Ginsberg JP (2005). Single unit activity in the medial prefrontal cortex during pavlovian heart rate conditioning: Effects of peripheral autonomic blockade. Neurobiology of Learning and Memory, 84(3), 200–213. 10.1016/j.nlm.2005.08.001 [DOI] [PubMed] [Google Scholar]
- Prosser CL, & Hunter WS (1936). The extinction of startle responses and spinal reflexes in the white rat. American Journal of Physiology-Legacy Content, 117(4), 609–618. 10.1152/ajplegacy.1936.117.4.609 [DOI] [Google Scholar]
- Quirk GJ, Repa C, & LeDoux JE (1995). Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: Parallel recordings in the freely behaving rat. Neuron, 15(5), 1029–1039. 10.1016/0896-6273(95)90092-6 [DOI] [PubMed] [Google Scholar]
- Randlett O, Haesemeyer M, Forkin G, Shoenhard H, Schier AF, Engert F, & Granato M (2019). Distributed Plasticity Drives Visual Habituation Learning in Larval Zebrafish. Current Biology: CB, 29(8), 1337–1345. e4. 10.1016/j.cub.2019.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Gomez M, & Meeter M (2016). Neurotransmitters and Novelty: A Systematic Review. Journal of Psychopharmacology, 30(1), 3–12. 10.1177/0269881115612238 [DOI] [PubMed] [Google Scholar]
- Ratuski AS, Makowska IJ, Dvorack KR, & Weary DM (2021). Using approach latency and anticipatory behaviour to assess whether voluntary playpen access is rewarding to laboratory mice. Scientific Reports, 11(1), 18683. 10.1038/s41598-021-98356-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Vautrelle N, & Reynolds JNJ (2011). Functional properties of the basal ganglia’s re-entrant loop architecture: Selection and reinforcement. Neuroscience, 198, 138–151. 10.1016/j.neuroscience.2011.07.060 [DOI] [PubMed] [Google Scholar]
- Redish AD (2016). Vicarious trial and error. Nature Reviews Neuroscience, 17(3), 147. 10.1038/nrn.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs S, Boleij H, Nordquist RE, & van der Staay FJ (2016). Making Decisions under Ambiguity: Judgment Bias Tasks for Assessing Emotional State in Animals. Frontiers in Behavioral Neuroscience, 10, 119. 10.3389/fnbeh.2016.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, & Grace AA (2003). The Prefrontal Cortex Regulates Lateral Amygdala Neuronal Plasticity and Responses to Previously Conditioned Stimuli. The Journal of Neuroscience, 23(35), 11054–11064. 10.1523/JNEUROSCI.23-35-11054.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, & Wager TD (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–156. 10.1016/j.tics.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA (1980). A circumplex model of affect. Journal of Personality and Social Psychology, 39(6), 1161–1178. 10.1037/h0077714 [DOI] [Google Scholar]
- Rutledge RB, Skandali N, Dayan P, & Dolan RJ (2014). A computational and neural model of momentary subjective well-being. Proceedings of the National Academy of Sciences, 111(33), 12252–12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, & Bradley MM (2007). Emotional perception: Correlation of functional MRI and event-related potentials. Cerebral Cortex, 17(5), 1085–1091. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber EL, Lopez de Armentia M, & Power J (2003). The amygdaloid complex: Anatomy and physiology. Physiological Reviews, 83(3), 803–834. [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Berry AS, Gritton H, Howe WM, & Parikh V (2016). What do phasic cholinergic signals do? Neurobiology of Learning and Memory, 130, 135–141. 10.1016/j.nlm.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer KR (2005). What are emotions? And how can they be measured? Social Science Information, 44(4), 695–729. 10.1177/0539018405058216 [DOI] [Google Scholar]
- Scherer KR (2011). On the rationality of emotions: or, When are emotions rational?. Social Science Information, 50(3–4), 330–350. [Google Scholar]
- Schneider KK, Schote AB, Meyer J, Markett S, Reuter M, & Frings C (2015). Individual response speed is modulated by variants of the gene encoding the alpha 4 subunit of the nicotinic acetylcholine receptor (CHRNA4). Behavioural Brain Research, 284, 11–18. 10.1016/j.bbr.2015.01.041 [DOI] [PubMed] [Google Scholar]
- Schregardus DS, Pieneman AW, Ter Maat A, Jansen RF, Brouwer TJF, & Gahr ML (2006). A lightweight telemetry system for recording neuronal activity in freely behaving small animals. Journal of Neuroscience Methods, 155(1), 62–71. 10.1016/j.jneumeth.2005.12.028 [DOI] [PubMed] [Google Scholar]
- Schultz W (2000). Multiple reward signals in the brain. Nature Reviews Neuroscience, 1(3), 199–207. 10.1038/35044563 [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, & Lang PJ (2000). Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology, 37(2), 257–261. [PubMed] [Google Scholar]
- Schwarz DA, Lebedev MA, Hanson TL, Dimitrov DF, Lehew G, Meloy J, Rajangam S, Subramanian V, Ifft PJ, Li Z, Ramakrishnan A, Tate A, Zhuang K, & Nicolelis MAL (2014). Chronic, Wireless Recordings of Large Scale Brain Activity in Freely Moving Rhesus Monkeys. Nature Methods, 11(6), 670–676. 10.1038/nmeth.2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, & Pape H-C (2003). Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science, 301(5634), 846–850. [DOI] [PubMed] [Google Scholar]
- Selden NR, Everitt BJ, Jarrard LE, & Robbins TW (1991). Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience, 42(2), 335–350. 10.1016/0306-4522(91)90379-3 [DOI] [PubMed] [Google Scholar]
- Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Gründemann J, Fadok JP, Müller C, Letzkus JJ, & Lüthi A (2014). Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron, 81(2), 428–437. [DOI] [PubMed] [Google Scholar]
- Serretti A (2018). The Present and Future of Precision Medicine in Psychiatry: Focus on Clinical Psychopharmacology of Antidepressants. Clinical Psychopharmacology and Neuroscience, 16(1), 1–6. 10.9758/cpn.2018.16.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Trias A, Murphy RT, & Malinow R (2012). Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron, 74(3), 475–481. 10.1016/j.neuron.2012.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Tromp DPM, Stockbridge MD, Kaplan CM, Tillman RM, & Fox AS (2016). Dispositional negativity: An integrative psychological and neurobiological perspective. Psychological Bulletin, 142(12), 1275–1314. 10.1037/bul0000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe MJ, Stalnaker T, Schuck NW, Killcross S, Schoenbaum G, & Niv Y (2019). An Integrated Model of Action Selection: Distinct Modes of Cortical Control of Striatal Decision Making. Annual Review of Psychology, 70(1), 53–76. 10.1146/annurev-psych-010418-102824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepperd JA, & McNulty JK (2002). The Affective Consequences of Expected and Unexpected Outcomes. Psychological Science, 13(1), 85–88. 10.1111/1467-9280.00416 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, & Hufford MR (2008). Ecological Momentary Assessment. Annual Review of Clinical Psychology, 4(1), 1–32. 10.1146/annurev.clinpsy.3.022806.091415 [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, & Jones-Gotman M (2001). Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain: A Journal of Neurology, 124(Pt 9), 1720–1733. 10.1093/brain/124.9.1720 [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, & Aldridge JW (2011). Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proceedings of the National Academy of Sciences, 108(27), E255–E264. 10.1073/pnas.1101920108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, Duff E, Feinberg DA, Griffanti L, & Harms MP (2013). Resting-state fMRI in the human connectome project. Neuroimage, 80, 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN (1963). Higher Nervous Functions: The Orienting Reflex. Annual Review of Physiology, 25(1), 545–580. 10.1146/annurev.ph.25.030163.002553 [DOI] [PubMed] [Google Scholar]
- Solomon RL, & Corbit JD (1974). An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychological Review, 81(2), 119–145. 10.1037/h0036128 [DOI] [PubMed] [Google Scholar]
- Sparta DR, Smithuis J, Stamatakis AM, Jennings JH, Kantak PA, Ung RL, & Stuber GD (2014). Inhibition of projections from the basolateral amygdala to the entorhinal cortex disrupts the acquisition of contextual fear. Frontiers in Behavioral Neuroscience, 8, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt BM, Van Hooff JA, & Gispen WH (1992). Ethology and neurobiology of grooming behavior. Physiological Reviews, 72(3), 825–852. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, & Bonci A (2011). Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature, 475(7356), 377–380. 10.1038/nature10194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stujenske JM, O’Neill PK, Nahmoud I, Goldberg S, Diaz L, Labkovich M, Hardin W, Bolkan SS, Reardon TR, Spellman TJ, Salzman CD, Gordon JA, & Likhtik E (2020). Prelimbic-dependent activation of amygdala somatostatin interneurons signals nonaversive cues to promote discrimination [Preprint]. Neuroscience. 10.1101/2020.06.23.156018 [DOI] [Google Scholar]
- Stujenske Joseph M., Likhtik E, A.Topiwala M, & Gordon JA (2014). Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron, 83(4), 919–933. 10.1016/j.neuron.2014.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J, & Itti L (2019). A top-down saliency model with goal relevance. Journal of Vision, 19(1), 11–11. 10.1167/19.1.11 [DOI] [PubMed] [Google Scholar]
- Thompson RF (2009). Habituation: A History. Neurobiology of Learning and Memory, 92(2), 127–134. 10.1016/j.nlm.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman EC (1939). Prediction of vicarious trial and error by means of the schematic sowbug. Psychological Review, 46(4), 318. [Google Scholar]
- Tomkins SS (1978). Script theory: Differential magnification of affects. Nebraska Symposium on Motivation. Nebraska Symposium on Motivation, 26, 201–236. [PubMed] [Google Scholar]
- Tsvetkov E, Carlezon WA Jr, Benes FM, Kandel ER, & Bolshakov VY (2002). Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron, 34(2), 289–300. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Derryberry D, & Luu P (2000). Anatomy and physiology of human emotion: Vertical integration of brainstem, limbic, and cortical systems. In Borod JC (Ed.), The neuropsychology of emotion (pp. 56–79). Oxford University Press. [Google Scholar]
- Tully T, & Quinn WG (1985). Classical conditioning and retention in normal and mutant Drosophila melanogaster. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology, 157(2), 263–277. 10.1007/BF01350033 [DOI] [PubMed] [Google Scholar]
- Tye KM (2018). Neural Circuit Motifs in Valence Processing. Neuron, 100(2), 436–452. 10.1016/j.neuron.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Stuber GD, de Ridder B, Bonci A, & Janak PH (2008). Rapid strengthening of thalamo-amygdala synapses mediates cue–reward learning. Nature, 453(7199), 1253–1257. 10.1038/nature06963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaish A, Grossmann T, & Woodward A (2008). Not all emotions are created equal: The negativity bias in social-emotional development. Psychological Bulletin, 134(3), 383–403. 10.1037/0033-2909.134.3.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Weele CM, Siciliano CA, Matthews GA, Namburi P, Izadmehr EM, Espinel IC, Nieh EH, Schut EHS, Padilla-Coreano N, Burgos-Robles A, Chang C-J, Kimchi EY, Beyeler A, Wichmann R, Wildes CP, & Tye KM (2018). Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature, 563(7731), 397–401. 10.1038/s41586-018-0682-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verduyn P, Van Mechelen I, Tuerlinckx F, Meers K, & Van Coillie H (2009). Intensity profiles of emotional experience over time. Cognition and Emotion, 23(7), 1427–1443. 10.1080/02699930902949031 [DOI] [Google Scholar]
- Vetrugno R, Liguori R, Cortelli P, & Montagna P (2003). Sympathetic skin response: Basic mechanisms and clinical applications. Clinical Autonomic Research, 13(4), 256–270. 10.1007/s10286-003-0107-5 [DOI] [PubMed] [Google Scholar]
- Villano WJ, Otto AR, Ezie CE, Gillis R, & Heller AS (2020). Temporal dynamics of real-world emotion are more strongly linked to prediction error than outcome. Journal of Experimental Psychology: General. [DOI] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, & Fink GR (2014). Dorsal and Ventral Attention Systems. The Neuroscientist, 20(2), 150–159. 10.1177/1073858413494269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, & Ochsner KN (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–1050. 10.1016/j.neuron.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, & Morales M (2009). Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. The European Journal of Neuroscience, 29(2), 340–358. 10.1111/j.1460-9568.2008.06576.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Hamilton JP, & Gotlib IH (2010). The Neural Temporal Dynamics of the Intensity of Emotional Experience. NeuroImage, 49(2), 1699–1707. 10.1016/j.neuroimage.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Shing EZ, & Avery BM (2015). Temporal Dynamics of Emotional Processing in the Brain. Emotion Review, 7(4), 323–329. 10.1177/1754073915590615 [DOI] [Google Scholar]
- Waugh CE, Zarolia P, Mauss IB, Lumian DS, Ford BQ, Davis TS, Ciesielski BG, Sams KV, & McRae K (2016). Emotion regulation changes the duration of the BOLD response to emotional stimuli. Social Cognitive and Affective Neuroscience, 11(10), 1550–1559. 10.1093/scan/nsw067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker W (1961). An analysis of exploratory and play behavior in animals. Functions of Varied Experience. [Google Scholar]
- White BJ, Berg DJ, Kan JY, Marino RA, Itti L, & Munoz DP (2017). Superior colliculus neurons encode a visual saliency map during free viewing of natural dynamic video. Nature Communications, 8(1), 14263. 10.1038/ncomms14263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Takahashi YK, Schoenbaum G, & Niv Y (2014). Orbitofrontal cortex as a cognitive map of task space. Neuron, 81(2), 267–279. 10.1016/j.neuron.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody ML, Yang JO, Cummings L, Gilchrist D, Graur S, Siegle GJ, & Price RB (2019). Protracted amygdalar response predicts efficacy of a computer-based intervention targeting attentional patterns in transdiagnostic clinical anxiety. Translational Psychiatry, 9(1). 10.1038/s41398-019-0458-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ (1991). Cholinergic systems in mammalian brain and spinal cord. Progress in Neurobiology, 37(6), 475–524. 10.1016/0301-0082(91)90006-m [DOI] [PubMed] [Google Scholar]
- Yih J, Beam DE, Fox KCR, & Parvizi J (2019). Intensity of affective experience is modulated by magnitude of intracranial electrical stimulation in human orbitofrontal, cingulate and insular cortices. Social Cognitive and Affective Neuroscience, 14(4), 339–351. 10.1093/scan/nsz015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Duque A, Gielow M, Gombkoto P, Nadasdy Z, & Somogyi J (2015). Organization of the Basal Forebrain Cholinergic Projection System: Specific or Diffuse? The Rat Nervous System: Fourth Edition, 491–507. 10.1016/B978-0-12-374245-2.00019-X [DOI] [Google Scholar]


