INTRODUCTION:
Esophageal adenocarcinoma (EAC) is a lethal cancer with increasing incidence and mortality rates over the last several decades; incidence rising seven-fold in the U.S. from 1975 to 2016.1 As many as 40% of patients with Barrett’s-associated EACs present with advanced disease with a dismal 5-year survival rate. Several factors contribute to identification at an advanced stage, including the limited effectiveness of current screening and surveillance strategies.2
Similar to post-colonoscopy colorectal cancer,3 Barrett’s Esophagus (BE)-associated high-grade dysplasia (HGD) and EAC can be diagnosed before the next recommended endoscopic evaluation after prior endoscopy that was negative for HGD or EAC.2 Meta-analyses and cohort studies suggest that a high proportion of HGD or EAC are missed within the first year following the index endoscopy that diagnosed BE.4 Several factors may contribute to missed lesions, including lack of adherence to the Seattle biopsy protocol, missed dysplasia due to limited mucosal sampling with standard biopsies, inadequate BE segment inspection time (similar to colorectal cancer screening), inability to recognize subtle findings of early neoplasia and reliance on observer dependent histologic classification of dysplasia.5, 6 These shortcomings highlight the importance of optimizing endoscopy quality to reduce the incidence and mortality associated with EAC. An American Gastroenterological Association Clinical Practice Update on post-endoscopy EAC (PEEC) identified several knowledge gaps, including the need for consensus standardization of PEEC terminology and calculation.2 To address these gaps, an international working group [28% females, mean years in clinical practice 17.7 (standard deviation 17.1)] on post-endoscopy HGD and EAC was established to achieve the following aims using the RAND/University of California, Los Angeles Appropriateness Methodology: (i) standardize terminology and definitions, (ii) identify and assess potential explanations, (iii) establish a conceptual framework for future research, (iv) introduce post-endoscopy HGD and EAC as performance measures to facilitate benchmarking and comparisons between healthcare settings and (v) provide best practice advice on reducing the rate of post-endoscopy EAC and HGD in clinical practice. Detailed methodology is reported in the Supplementary Text.
Statements and Evidence Summary
The final statements that met the appropriateness criteria are listed in Table 1.
Table 1:
Appropriate statements determined using the RAND/University of California, Los Angeles Appropriateness Method with median score, and number of experts in each category range
| Consensus Statements | Median Score | # of Experts 1-3 Range (Inappropriate) (n, %) | # of Experts 4-6 Range (Uncertain) (n, %) | # of Experts 7-9 Range (Appropriate) (n, %) | MAD-M Score |
|---|---|---|---|---|---|
| Terminology and Definitions | |||||
| 1) Post-endoscopy esophageal neoplasia (PEEN) is the preferred term for high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC) detected before the next recommended surveillance endoscopy in a patient with non-dysplastic Barrett’s esophagus (NDBE) | 8 | 1 (4) | 2 (8) | 22 (88) | 1.1 |
| 2) Post-endoscopy esophageal adenocarcinoma (PEEC) is the preferred term for esophageal adenocarcinoma (EAC) detected before the next recommended surveillance endoscopy in a patient with NDBE | 8 | 3 (12) | 2 (8) | 20 (80) | 1.3 |
| 3) The time interval for which the occurrence of PEEN/PEEC applies is between 6 months and 3 years following screening or surveillance endoscopy | 7 | 1 (4) | 7 (28) | 17 (68) | 1.2 |
| Potential Explanations | |||||
| 4) The potential explanations for PEEN/PEEC include missed HGD/EAC and rapidly progressive EAC | 8 | 1 (4) | 2 (8) | 22 (88) | 0.9 |
| Quality Review of PEEN/PEEC cases | |||||
| 5) Endoscopy practices can consider reviewing PEEN/PEEC cases to understand contributing factors and areas of improvement | 8 | 0 (0) | 2 (8) | 23 (92) | 1 |
| 6) To facilitate the use of a common language when categorizing PEEN/PEEC cases according to their most plausible explanations, we suggest the following categories be used: a. Possible missed visible lesion, prior examination adequate b. Possible missed visible lesion, prior examination inadequate c. Detected visible lesion, no or inadequate sampling with targeted biopsies d. Detected visible lesion, incomplete resection of previously identified lesion e. Prior examination adequate and clinically indicated follow-up not recommended f. Prior examination inadequate and clinically indicated follow-up not recommended g. Prior examination adequate and failure of patient to follow-up on a recommended surveillance endoscopy interval. |
7 | 1 (4) | 3 (12) | 21 (84) | 1 |
| Best Practice Advice to Reduce PEEN/PEEC | |||||
| 7) Endoscopists should define the extent of BE using a standardized grading system documenting the circumferential and maximal extent of the columnar lined esophagus (Prague classification) with a clear description of landmarks and characteristics of visible lesions, when present | 8 | 0 (0) | 1 (4) | 24 (96) | 0.6 |
| 8) Screening and surveillance endoscopy for BE should be performed using high-definition white light endoscopy (HD-WLE) and chromoendoscopy (traditional or virtual) | 8 | 0 (0) | 2 (8) | 23 (92) | 0.7 |
| 9) Endoscopists should spend adequate time inspecting the BE segment | 9 | 0 (0) | 0 (0) | 25 (100) | 0.4 |
| 10) In patients undergoing screening or surveillance endoscopy for BE, endoscopists should obtain biopsies using the Seattle biopsy protocol (4-quadrant biopsies at least every 2 cm and additional targeted biopsies or resection or outlining a plan for resection for any visible lesions) | 8 | 0 (0) | 1 (4) | 24 (96) | 0.6 |
Terminology and Definitions
The group agreed to define two terms for BE-related neoplasia following index endoscopy: 1) include EAC only (PEEC) and 2) include both HGD and EAC (post-endoscopy esophageal neoplasia [PEEN]). Inclusion of HGD in the PEEN definition was driven by the fact that the goal of endoscopic screening and surveillance programs, as recommended by professional societies, is detection of HGD and early EAC.5, 7, 8 Further, up to 40% of patients with HGD have prevalent EAC (majority diagnosed with stage I or stage IIa), many of which have an actionable recommendation for endoscopic eradication therapy, underscoring the importance of capturing this population.9,7, 10 On the other hand, using EAC is a more clinically meaningful singular endpoint (similar to post-colonoscopy colorectal cancer), is most impactful and is the most serious missed lesion. In addition, the potential for different phenotypes of EAC11 raise the possibility that some subset of interval HGD may represent truly incident, rapidly growing neoplasia, especially when these lesions are detected near the end of a 3-5 year surveillance interval. Inclusion of LGD was considered inappropriate due to interobserver variability among pathologists, variable natural history outcomes, the need for risk stratification to determine ideal candidates likely to benefit from endoscopic eradication therapy compared with surveillance.12
The time interval of 6 months to 3 years following screening or surveillance endoscopy was rated as an appropriate window for occurrence of PEEN/PEEC. A minimum of six months was recommended to document healing and exclude BE in patients with erosive esophagitis. An upper limit of three years was selected, as data from a national benchmarking quality registry suggests that endoscopists recommend a 3-year interval more commonly than a 5-year interval for endoscopic surveillance of non-dysplastic BE (NDBE) patients.13 Lesions detected beyond this window are likely incident rather than missed at the index endoscopy.
Two analyses were conducted to provide contemporary estimates of PEEN/PEEC. In a retrospective U.S. cohort study performed among 50,817 individuals diagnosed with incident BE and 366 with EAC; EAC was classified as prevalent, PEEC and incident EAC, in 67.2%, 13.7% and 19.1% cases, respectively.14 In other words, the magnitude of PEEC approached that of incident cancer. An updated systematic review and meta-analysis of 145,726 patients revealed a PEEC and PEEN rate of 21% (95% CI 13-31) and 26% (95% CI 19-34), respectively. Interestingly, meta-regression analysis demonstrated a strong inverse association between PEEC and incident EAC, suggesting that measures which augment neoplasia detection will reduce the subsequent incidence of EAC detected in surveillance.4
Standardize Potential Explanations
While most cases of PEEN/PEEC may be attributed to missed HGD/EAC, rapidly progressive cancer due to accelerated pathways of neoplasia may also exist.11, 15 The limited data on the natural history of progression of NDBE to HGD limits our ability to estimate these proportions. Intuitively, the shorter the interval between the endoscopic finding of HGD or EAC and the endoscopy finding of NDBE, the greater the likelihood that the neoplasia was missed on the screening or surveillance endoscopy. As with post-colonoscopy colorectal cancer, investigators need to operationalize definitions and attempt to identify time intervals that distinguish missed from incident PEEN/PEEC. Further, BE-associated neoplasia may be diagnosed after successful endoscopic eradication therapy. The panelists acknowledged several factors that contribute to the finding of neoplasia after complete eradication of intestinal metaplasia including missed resectable lesions, incomplete resection or ablation, failure to complete recommended follow-up treatments, and failure to achieve complete eradication of intestinal metaplasia, among others.2, 16 Given the increasing use of endoscopic eradication therapy in patients with BE-associated neoplasia, quality indicators for endoscopic eradication therapy have recently been developed using an evidence-based approach endorsed by gastroenterology societies.17
Consensus Statements for Reducing Rates of PEEN/PEEC
Classification practices are surrogates for performance of a high-quality endoscopy exam. The Prague classification system (www.iwgco.net) is a validated grading system associated with high interobserver agreement among expert and non-expert endoscopists to document the circumferential and maximal extent of the BE segment.18 The Paris classification is a standardized morphology categorization, recommended by the US Multi-Society Task Force on colorectal cancer, and can provide relevant information regarding the risk of a lesion harboring submucosal invasion. Endoscopists are encouraged to use the Prague and Paris classification in descriptive terms for BE-associated lesions.3 Nevertheless, future studies are needed to assess whether use of these classifications leads to reduced rates of PEEN/PEEC.
Consistent with recent guidelines,5 the panelists rated the statement on the use of chromoendoscopy (virtual or traditional) in addition to high-definition white light endoscopy during screening and surveillance endoscopy for BE patients as appropriate. In an updated meta-analysis that included 504 patients, chromoendoscopy with high-definition white light endoscopy was associated with a higher detection rate of HGD/EAC compared to high-definition white light endoscopy alone [14.7% vs. 10.1%, relative risk: 1.44] (Supplementary Table 1, Appendix 5). There was low certainty of evidence due to risk of bias and imprecision associated with a low event rate. Available data suggest comparable rates of dysplasia detection between virtual and traditional chromoendoscopy techniques. Virtual chromoendoscopy (narrow band imaging) is available in most endoscopes and thus require no additional costs. Problems associated with dye-based chromoendoscopy include the need for dye spraying equipment, additional time required, cumbersome nature of the procedure, difficulty in achieving uniform coating of the mucosal surface with the dye, and inability to detect superficial vascular patterns.5 Incorporation of training in virtual and traditional chromoendoscopy during fellowship and training programs for the practicing endoscopists will be important for widespread routine implementation in clinical practice.
Although data are limited there was agreement that adequate inspection time would potentially increase detection of BE-associated neoplasia.19 Future studies need to define the optimal inspection time per cm of the BE segment. While the panel purposefully did not provide a time period comprising an adequate exam due to lack of data on this issue, the European societies recommend a procedure time of ≥ 7 minutes for upper endoscopy and inspection time of ≥1 minute/cm of the circumferential extent of the Barrett’s mucosa.20
With regards to evidence supporting the routine use of the Seattle biopsy protocol in patients undergoing screening or surveillance for BE, data from two observational studies that included 506 patients demonstrated that the use of the Seattle biopsy protocol was associated with a higher dysplasia detection rate (19.1% vs. 2.6%, relative risk 2.75) (Supplementary Table 2). Selection bias and imprecision and indirectness of evidence (since detection of dysplasia was used as a surrogate for PEEN/PEEC) limited interpretation. Endoscopists may avoid biopsy of a visible lesion with referral for endoscopic resection. Several studies have consistently demonstrated suboptimal adherence rates to the Seattle biopsy protocol.6 Using a community-based pathology database, Abrams et al reported that the odds of detecting dysplasia significantly decreased with non-adherence to the Seattle biopsy protocol [odds ratio: 0.53, 95% CI 0.35-0.82).21 A recent analysis using a national quality benchmarking registry that included 58,709 endoscopies showed that nearly 20% of endoscopies were not adherent to the Seattle biopsy protocol, and that endoscopists were less adherent with increasing BE length; with the odds of non-adherence increasing by 31% with every 1-cm increase in length.6
The panelists also acknowledged the significant interobserver variability in the interpretation of dysplasia among pathologists and the importance of high-quality expert pathology review in the diagnosis of dysplasia.5, 10 An accurate diagnosis of dysplasia is critical for clinical decision making and risk stratification, including the selection of endoscopic eradication therapy vs. intensive surveillance. Guidelines recommend that dysplasia of any grade be confirmed by a second pathologist with expertise in gastroenterology pathology.7, 10
Qualitative Review of Post-Endoscopy Esophageal Neoplasia Cases
Services and individual endoscopists are encouraged to review PEEN/PEEC cases periodically and identify areas for improvement. We provide a consensus-based categorization construct and acknowledge that this construct will need to be validated in future studies. An adequate examination was defined as one that met the following prerequisites: documentation of landmarks, use of high-definition white light endoscopy and chromoendoscopy and complete sampling using the Seattle biopsy protocol. Similar to the disclaimer provided by the World Endoscopy Organization for post-colonoscopy colorectal cancer, the panelists recommend using the above construct of explanations to facilitate quality assurance but not be used to support medico-legal decision making or to define accountability at an individual level.3 An essential theme of this project is development of consistent strategies to optimize detection of PEEN and PEEC, not to develop metrics for punitive use.
AREAS OF UNCERTAINTY AND FUTURE DIRECTIONS
In addition to the research directives outlined in the above section, the panelists also discussed and voted on statements for which there was not sufficient agreement (Supplementary Table 3) but consensus that some of these statements were important for future discussion and resolution (Appendix 6). Panelists discussed that operationalizing PEEN/PEEC as a quality indicator for providers may be challenging given the low number of endoscopies performed for BE screening and surveillance and low annual number of incident EAC cases. Future efforts will need to focus on standardizing how PEEN/PEEC rates should be calculated in an automated fashion and establishing a minimum performance standard.
The panelists acknowledged that the field would benefit from the BE equivalent of the adenoma detection rate, used to measure quality in colorectal cancer screening. Neoplasia detection rate, defined as the prevalence of HGD or EAC among BE patients at index upper endoscopy has also been proposed as a quality indicator in the management of BE.22 Nevertheless, significant work is needed to establish if neoplasia detection rate can be adopted as a quality indicator to reliably differentiate between endoscopists (or endoscopy centers) with discordant detection rates of PEEN and PEEC, enable accountability between providers, and demonstrably improve the quality of care provided to patients. To be considered a high-value quality indicator, neoplasia detection rate must be shown to correlate with important clinical outcomes such as interval development of EAC and/or mortality. In addition, while benchmarking thresholds need to be defined, it is imperative that these thresholds account for the different rates of neoplasia in the underlying population undergoing an upper endoscopy. This will most likely require risk-adjustment based on well-established risk factors for the development and progression in BE.
The routine use of wide-area transepithelial sampling in reducing rates of PEEN/PEEC in clinical practice was voted as uncertain. An updated systematic review and meta-analysis of the use of wide-area transepithelial sampling in BE screening/surveillance in 13,950 patients demonstrated an increase in detection of dysplasia among patients undergoing sampling using wide-area transepithelial sampling and forceps biopsies compared with forceps biopsies alone (3.2% vs. 1.2%, relative risk 2.13) (Supplementary Table 4). The certainty of evidence was reduced due to risk of bias (evidence largely driven by observational data), inconsistency (high heterogeneity, variability in detection rates of dysplasia), indirectness (detection of dysplasia was deemed as a surrogate for PEEN/PEEC) and uncertainty interpreting dysplasia diagnosed alone with wide-area transepithelial sampling. No study yet has evaluated the addition of wide-area transepithelial sampling to forceps biopsies for detection of dysplasia during Barrett’s surveillance when forceps biopsies are guided both by white light and chromoendoscopy. There are also limited data on longitudinal follow up of patients with non-dysplastic biopsies but positive wide-area transepithelial sampling analyses to fully understand the prognostic significance of these discrepant findings. Inherent in this need is the evaluation of other proposed sampling or diagnostic techniques to predict development of neoplasia, such as TissueCypher or more generic approaches including identification of additional methylation or other biomarkers.23
Efforts will also be needed to determine if implementation of further BE training leads to increased detection of PEEN/PEEC and a reduction in cancer mortality. It is critical that Barrett’s training courses designed to enhance detection of PEEN/PEEC undergo internal and external validation to achieve this goal. The “Barrett’s Oesophagus-Related Neoplasia” web-based training course is an available validated training course that primarily focuses on improving early neoplasia detection.24 This tool can be accessed at www.iwgco.net, www.ueg.eu, or www.best-academia.eu. No comprehensive training course is currently available that covers all facets of best practices in BE and this deficit is the focus of ongoing research. Similarly, pathologist training may be needed to improve interobserver agreement when assessing Barrett’s dysplasia. One study from the Netherlands demonstrated that benchmarks defined previously by a group of core expert BE pathologists was validated by demonstrating consistency and training potential when enlisting other core pathologists to the group of BE experts.25 Finally, a checklist for future manuscripts on this topic is proposed (Appendix 7).
CONCLUSIONS
Using an evidence-based consensus process, an international multidisciplinary panel provided comprehensive guidance on standardizing the definition and etiology for PEEN/PEEC, potential explanations and a template for the performance and improvement of high-quality endoscopy to reduce rates of PEEN/PEEC in clinical practice.
Supplementary Material
Figure 1:
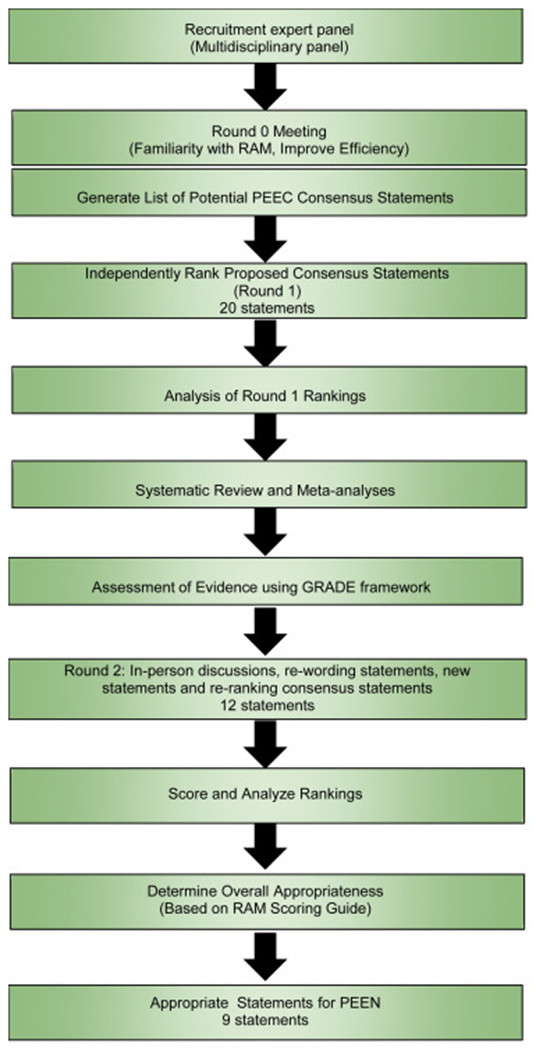
Study design used to develop consensus statements
Role of the funding source:
SW supported by U34DK124174, RY is supported by NIH K23 DK125266, SS is supported by K23DK117058 and R03DK129631
Conflicts of Interest:
SW: Consultant for Medtronic, Boston Scientific, Exact Sciences, Interpace; Advisory Board for Cernostics, Research Support: Lucid, Ambu, RY: Institutional Consulting Agreement: Medtronic, Ironwood Pharmaceuticals, Diversatek; Consultant: Phathom Pharmaceuticals; Research support: Ironwood Pharmaceuticals; Advisory Board with Stock Options: RJS Mediagnostix, JB: Consultancy for Aqua Medical, DyaMx Inc., Digma Medical, Medtronic. Research support for IRB approved studies through unrestricted grants from: Aqua Medical, Medtronic, Pentax/C2-therapeutics, Erbe, Olympus Endoscopy, Fuji-film endoscopy, Cernostics, CDx diagnostics, Lucid Medical, NinePoint Medical, VK: Exact Advisory Board, Medtronic Consulting, GWF: Research Support: Interpace, Lucid, Consultant: CDx, Cernostics, Interpace, Lucid, Phathom Pharmaceuticals, RCF: Named on patents related to Cytosponge and related assays that have been licensed by the Medical Research Council to Medtronic. RCF has undertaken consulting work for Medtronic, AC: Founder and consultant – Lucid Diagnostics, Consultant for Cernostics and CDx; research support through Pentax, SS: Consultant for Phathom Pharmaceuticals, Interpace Diagnostics, Ironwood Pharmaceuticals, ISOThrive, Cernostics, SK: Consultant for Boston Scientific, PGI: Research Funding from Exact Sciences, Pentax, Consultant for Medtronic, Symple Surgical and Ambu, NJS: Research funding from Medtronic, Steris, Pentax, CDx Diagnostics, Interpace Diagnostics, and Lucid Medical; Consultant for Cernostics, Phathom Pharmaceuticals, Exact Sciences, and Cook Medical, JK: None relevant to current work, MS: Consultant for CDx, Steris Endoscopy, PavMed/Lucid Diagnostics – Consultant, Advisory Board Member, RM: Consultant for Boston Scientific, Medtronic, Interpace, Medivators, Honoraria from Torax Medical, Stockholder – Capsovision, Research Support from Medtronic, Boston Scientific, MIC: Research Support from Pentax Medical Corporation, Endogastric Solutions, Lucid, Royalties from UptoDate, DK: Advisory Board Member for Celgene and Shire, TS, MH, DC, SS, JK, JI, FS, JHR, EM, RH: None relevant to current work
ABBREVIATIONS:
- BE
Barrett’s esophagus
- CI
confidence interval
- EAC
esophageal adenocarcinoma
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HGD
high-grade dysplasia
- LGD
low-grade dysplasia
- NDBE
non-dysplastic Barrett’s esophagus
- PEEC
Post-endoscopy esophageal adenocarcinoma
- PEEN
Post-endoscopy esophageal neoplasia
Appendix
POST-ENDOSCOPY ESOPHAGEAL NEOPLASIA IN BARRETT’S ESOPHAGUS: CONSENSUS STATEMENTS FROM AN INTERNATIONAL EXPERT PANEL
Sachin Wani MD,1 Rena Yadlapati MD, MSHS,2 Siddharth Singh MD, MS,2 Tarek Sawas MD, MPH3, David A. Katzka MD4 on behalf of the Post-Endoscopy Esophageal Neoplasia Expert Consensus Panel
1. Division of Gastroenterology and Hepatology, University of Colorado Anschutz Medical Campus, Aurora, Colorado
2. Division of Gastroenterology and Hepatology, University of California, San Diego, San Diego, California
3. Division of Gastroenterology and Hepatology, University of Texas Southwestern Medical Center, Dallas, Texas
4. Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota
Footnotes
Data Transparency Statement: Deidentified individual participant data that underlie the reported results will be made available 3 months after publication for a period of 5 years after the publication date. Proposals for access should be sent to corresponding author.
Preprint Server: None
REFERENCES
- 1.Kolb JM, Han S, Scott FI, et al. Early-Onset Esophageal Adenocarcinoma Presents With Advanced-Stage Disease But Has Improved Survival Compared With Older Individuals. Gastroenterology 2020;159:2238–2240 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wani S, Gyawali CP, Katzka DA. AGA Clinical Practice Update on Reducing Rates of Post-Endoscopy Esophageal Adenocarcinoma: Commentary. Gastroenterology 2020;159:1533–1537. [DOI] [PubMed] [Google Scholar]
- 3.Rutter MD, Beintaris I, Valori R, et al. World Endoscopy Organization Consensus Statements on Post-Colonoscopy and Post-Imaging Colorectal Cancer. Gastroenterology 2018;155:909–925 e3. [DOI] [PubMed] [Google Scholar]
- 4.Sawas T, Majzoub AM, Haddad J, et al. Magnitude and Time-trend analysis of Post-Endoscopy Esophageal Adenocarcinoma: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asge Standards Of Practice C, Qumseya B, Sultan S, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc 2019;90:335–359 e2. [DOI] [PubMed] [Google Scholar]
- 6.Wani S, Williams JL, Komanduri S, et al. Endoscopists systematically undersample patients with long-segment Barrett’s esophagus: an analysis of biopsy sampling practices from a quality improvement registry. Gastrointest Endosc 2019;90:732–741 e3. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol 2016;111:30–50; quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Gastroenterological A, Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084–91. [DOI] [PubMed] [Google Scholar]
- 9.Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high-grade dysplasia in Barrett’s esophagus overestimated? Clin Gastroenterol Hepatol 2008;6:159–64. [DOI] [PubMed] [Google Scholar]
- 10.Standards of Practice C, Wani S, Qumseya B, et al. Endoscopic eradication therapy for patients with Barrett’s esophagus-associated dysplasia and intramucosal cancer. Gastrointest Endosc 2018;87:907–931 e9. [DOI] [PubMed] [Google Scholar]
- 11.Sawas T, Killcoyne S, Iyer PG, et al. Identification of Prognostic Phenotypes of Esophageal Adenocarcinoma in 2 Independent Cohorts. Gastroenterology 2018;155:1720–1728 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wani S, Rubenstein JH, Vieth M, et al. Diagnosis and Management of Low-Grade Dysplasia in Barrett’s Esophagus: Expert Review From the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology 2016;151:822–835. [DOI] [PubMed] [Google Scholar]
- 13.Wani S, Williams JL, Komanduri S, et al. Over-Utilization of Repeat Upper Endoscopy in Patients with Non-dysplastic Barrett’s Esophagus: A Quality Registry Study. Am J Gastroenterol 2019;114:1256–1264. [DOI] [PubMed] [Google Scholar]
- 14.Vajravelu RK, Kolb JM, Thanawala SU, et al. Characterization of Prevalent, Post-Endoscopy, and Incident Esophageal Cancer in the United States: A Large Retrospective Cohort Study. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jammula S, Katz-Summercorn AC, Li X, et al. Identification of Subtypes of Barrett’s Esophagus and Esophageal Adenocarcinoma Based on DNA Methylation Profiles and Integration of Transcriptome and Genome Data. Gastroenterology 2020;158:1682–1697 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawas T, Alsawas M, Bazerbachi F, et al. Persistent intestinal metaplasia after endoscopic eradication therapy of neoplastic Barrett’s esophagus increases the risk of dysplasia recurrence: meta-analysis. Gastrointest Endosc 2019;89:913–925 e6. [DOI] [PubMed] [Google Scholar]
- 17.Wani S, Muthusamy VR, Shaheen NJ, et al. Development of Quality Indicators for Endoscopic Eradication Therapies in Barrett’s Esophagus: The TREAT-BE (Treatment With Resection and Endoscopic Ablation Techniques for Barrett’s Esophagus) Consortium. Am J Gastroenterol 2017;112:1032–1048. [DOI] [PubMed] [Google Scholar]
- 18.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392–9. [DOI] [PubMed] [Google Scholar]
- 19.Gupta N, Gaddam S, Wani SB, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc 2012;76:531–8. [DOI] [PubMed] [Google Scholar]
- 20.Bisschops R, Areia M, Coron E, et al. Performance measures for upper gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy 2016;48:843–64. [DOI] [PubMed] [Google Scholar]
- 21.Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol 2009;7:736–42; quiz 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parasa S, Desai M, Vittal A, et al. Estimating neoplasia detection rate (NDR) in patients with Barrett’s oesophagus based on index endoscopy: a systematic review and meta-analysis. Gut 2019;68:2122–2128. [DOI] [PubMed] [Google Scholar]
- 23.Davison JM, Goldblum J, Grewal US, et al. Independent Blinded Validation of a Tissue Systems Pathology Test to Predict Progression in Patients With Barrett’s Esophagus. Am J Gastroenterol 2020;115:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergman J, de Groof AJ, Pech O, et al. An Interactive Web-Based Educational Tool Improves Detection and Delineation of Barrett’s Esophagus-Related Neoplasia. Gastroenterology 2019;156:1299–1308 e3. [DOI] [PubMed] [Google Scholar]
- 25.van der Wel MJ, Klaver E, Duits LC, et al. Adherence to pre-set benchmark quality criteria to qualify as expert assessor of dysplasia in Barrett’s esophagus biopsies - towards digital review of Barrett’s esophagus. United European Gastroenterol J 2019;7:889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


