Introduction
Cutaneous lupus erythematosus (CLE) is an autoimmune disorder with variable skin manifestations that can occur with or without systemic lupus erythematosus (SLE). While topical agents such as corticosteroids and calcineurin inhibitors for single lesions and systemic agents such as antimalarials, immunomodulators, and immunosuppressants for multiple lesions have demonstrated effectiveness in the treatment of CLE, options are limited when these modalities fail. The limited access to quinacrine in recent years has restricted antimalarial combination use, emphasizing the need for alternative therapies. Recommended second and third-line systemic treatments for CLE include methotrexate, mycophenolate mofetil, dapsone, systemic retinoids, and thalidomide.1 However, the use of thalidomide is limited by cost, the risk of thromboembolic events, and the high incidence of peripheral neuropathy.2 Lenalidomide is not often readily available for non-oncologic use. Therefore, there is an unmet need for novel effective therapies in the treatment of CLE.
Janus kinases (JAKs) are receptor-bound tyrosine kinases that mediate the action of various inflammatory cytokines through intracellular signaling via transducing signal transducer and activator of transcription proteins. Many cytokines implicated in lupus pathogenesis depend on JAK signaling to exert their intracellular action. In particular, interferon-associated JAK activation is thought to play a key role in CLE lesions; a recent study showed a significant upregulation of JAK signaling in cutaneous lesions of lupus.3 Therefore, JAK inhibitors represent a compelling option for the treatment of CLE. Tofacitinib, a pan-JAK inhibitor with relative selectivity for JAK 1/3, has demonstrated promising results in the treatment of cutaneous features of SLE in a recent study.4 In another study, 67% of patients with SLE showed resolution of their arthritis or rash while on baricitinib 4 mg daily, a JAK 1/2 inhibitor, although improvement was not significant at a dose of 2 mg daily.5 In both studies, the subtype of CLE was not defined, as improvement was measured using the Systemic Lupus Erythematosus Disease Activity Index 2000 scale, which is not specific to skin disease.
In this article, we describe the efficacy of tofacitinib in 3 patients with CLE, using the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI), a scale that has been validated for the assessment of CLE.6
Methods
We identified all patients with CLE who were prescribed tofacitinib at the Skin Care Center. Improvement was evaluated using the CLASI score and clinical information in the patient's chart. Other endpoints included adverse events and the need for concurrent therapies. The subtype of CLE was assessed using information available in the charts. The cutaneous involvement in the first and second patients was evaluated using photographs and teleconsultation due to restrictions associated with the COVID-19 pandemic. Although in-person visits were offered, both patients declined. Patients were instructed to have a third party take photographs of all skin-involved areas. We ensured that all anatomical locations not included in the photographs were devoid of CLE involvement by questioning the patient. If there was any doubt, the patient was asked to send additional pictures. CLASI score items referring to mucous membrane involvement, dyspigmentation, and alopecia were determined by verbal questioning. It should be noted that the use of photographs to calculate CLASI scores has not yet been validated, thus caution should be exercised when interpreting the results.
Results
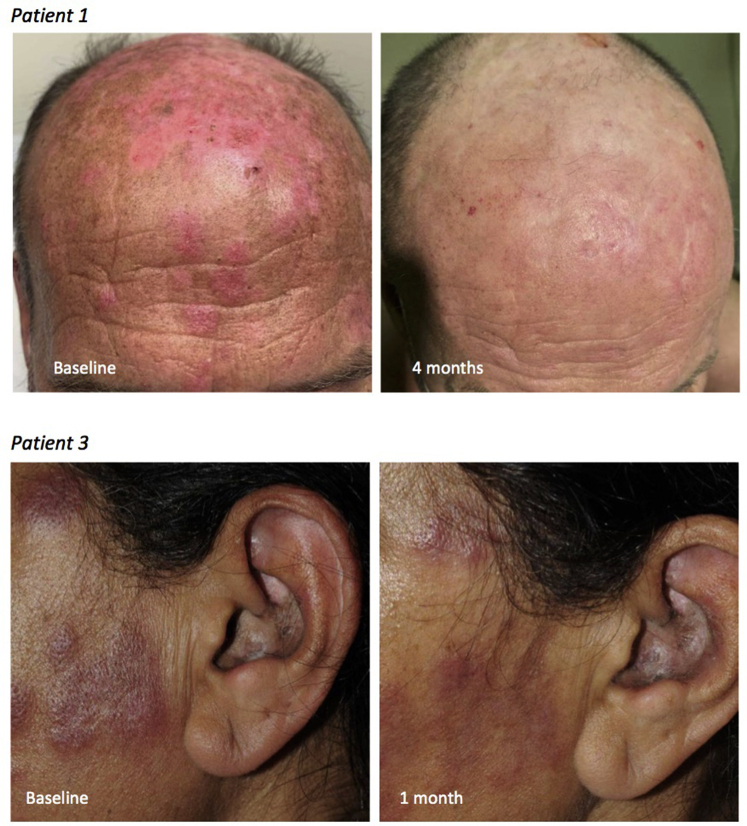
The diagnosis of CLE was based on clinical, histopathologic, and laboratory evidence. CLE subtype was assessed clinically, according to Gilliam's classification.7 Three patients with CLE treated with tofacitinib were identified (Table I). All of them had biopsy-confirmed CLE. They had multiple skin lesions and had failed at least 2 second or third-line systemic agents, in combination with antimalarial therapy and topical therapy, including potent corticosteroids and/or calcineurin inhibitors. The dose of tofacitinib was 5 mg twice daily for all patients. The first patient, a 51-year-old man with rheumatoid arthritis, had severe discoid lupus (Fig 1, upper panel), Raynaud syndrome, and fingertip ulcerations. He had been on hydroxychloroquine 400 mg daily for one year, and mycophenolate mofetil 1 g twice daily and prednisone 5 mg daily for 6 months for the treatment of his arthritis. Because his cutaneous lupus remained active, tofacitinib was added to the existing treatment. After 4 months, his CLASI score had decreased from 28 to 8, although he had ongoing digital involvement. The second patient was a 75-year-old woman with subacute CLE and Sjögren syndrome. She had failed multiple prior systemic treatments, had been maintained on hydroxychloroquine 200 mg daily for 2 years, and tofacitinib was added. She improved from a CLASI 21 to CLASI 0 in 5 months. It should be noted that the first and second patients were both assessed via telemedicine. The third patient was a 50-year-old woman with SLE, discoid lupus, and tumid lupus. Antimalarial therapy had been discontinued due to retinal toxicity. She had been on prednisone 10 mg daily for 3 months prior to addition of tofacitinib. Her CLASI improved from 23 to 16 in 1 month, but because of ongoing facial involvement (Fig 1, lower panel), the patient wished to switch therapy. Tofacitinib was well tolerated in all 3 patients. Laboratory parameters were evaluated monthly for 2 months after the initiation of tofacitinib, then every 2 months. Only the first patient had changes in his baseline parameters, his white blood cell count dropping from 4 × 109/L to 2.6 × 109/L, remaining stable afterward.
Table I.
Clinical summaries of patients treated with tofacitinib
| Patient No./Age/sex | Disease duration | Clinical presentation | Previous treatments | Concurrent treatments | Duration of tofacitinib treatment | CLASI activity |
Clinical outcome | |
|---|---|---|---|---|---|---|---|---|
| Before | After | |||||||
| 1/50s/M | 2 years | Discoid lupus Raynaud syndrome and fingertip ulcerations | HCQ MTX MMF Prednisone |
MMF 2 g/d HCQ 400 mg/d 5/7 days and 200 mg/d 2/7 days Prednisone 5 mg/d |
4 months | 28∗ | 8∗ | Improvement in discoid lupus Ongoing Raynaud syndrome despite treatment |
| 2/70s/F | 8 years | SCLE Sjögren syndrome |
HCQ CQ Quinacrine CsA MMF Acitretin |
HCQ 200 mg/d | 7 months | 21∗ | 0∗ | Resolution of all cutaneous lesions of lupus |
| 3/50s/F | 11 years | Discoid lupus Tumid lupus ACLE SLE |
HCQ CQ Quinacrine MTX AZA MMF Prednisone |
Prednisone 10 mg/d | 1 month | 23 | 16 | Effectiveness on limbs but ongoing facial involvement, tofacitinib switched to MMF and prednisone 20 mg/d after 1 month |
ACLE, Acute cutaneous lupus erythematosus; AZA, azathioprine; CLASI, cutaneous lupus erythematosus disease area and severity index; CsA, cyclosporine; CQ, chloroquine; HCQ, hydroxychloroquine; MTX, methotrexate; MMF, mycophenolate mofetil; SCLE, subacute cutaneous lupus erythematosus; SLE, systemic lupus erythematosus.
CLASI scores from patients 1 and 2 were calculated using patient-supplied pictures.
Fig 1.
Clinical images of patients 1 and 3 before and after tofacitinib treatment for cutaneous lupus erythematosus.
Discussion
Two studies have described the use of JAK inhibitors in the treatment of CLE, using the SLE Disease Activity Index 2000 as the main end point.4,5 JAK inhibitors have not specifically been studied for the treatment of skin involvement. In this study, we measured CLASI scores, which monitor skin disease activity on a more continuous scale than the SLE Disease Activity Index 2000. In the 3 patients studied, CLASI improvement was more than 4 points, which is the number required for clinically significant improvement.8 Reported side effects from tofacitinib include infections, cytopenia, thrombocytosis, and elevated liver enzymes, none of which were observed in the patients presented. Potentially increased rates of venous thromboembolic disease and malignancy are additional concerns. The main limitation of this study is the use of photographs and telemedicine to derive CLASI scores in 2 of the patients, a method that has not been validated and could lead to potential bias. Other limitations include the retrospective design, the small number of patients, the short follow-up, and the concurrent use of other treatments. Although our results are encouraging, larger studies are needed to assess the effectiveness of JAK inhibitors in CLE.
Conflicts of interest
None disclosed.
Acknowledgments
EB is a Immuno-dermatology Fellow partially supported by a fellowship from Abbvie; JPD is a Senior Scientist at the BC Children's Hospital Research Institute.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Kuhn A., Aberer E., Bata-Csörgő Z., et al. S2k guideline for treatment of cutaneous lupus erythematosus–guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV) J Eur Acad Dermatol Venereol. 2017;31(3):389–404. doi: 10.1111/jdv.14053. [DOI] [PubMed] [Google Scholar]
- 2.Cuadrado M.J., Karim Y., Sanna G., Smith E., Khamashta M.A., Hughes G.R.V. Thalidomide for the treatment of resistant cutaneous lupus: efficacy and safety of different therapeutic regimens. Am J Med. 2005;118(3):246–250. doi: 10.1016/j.amjmed.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Fetter T., Smith P., Guel T., Braegelmann C., Bieber T., Wenzel J. Selective Janus kinase 1 inhibition is a promising therapeutic approach for lupus erythematosus skin lesions. Front Immunol. 2020;11:344. doi: 10.3389/fimmu.2020.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.You H., Zhang G., Wang Q., et al. Successful treatment of arthritis and rash with tofacitinib in systemic lupus erythematosus: the experience from a single centre. Ann Rheum Dis. 2019;78(10):1441–1443. doi: 10.1136/annrheumdis-2019-215455. [DOI] [PubMed] [Google Scholar]
- 5.Wallace D.J., Furie R.A., Tanaka Y., et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10143):222–231. doi: 10.1016/S0140-6736(18)31363-1. doi: 10.1016/S0140-6736(18)31363-1. Erratum in: Lancet. 2018;392 (10146):476. [DOI] [PubMed] [Google Scholar]
- 6.Chakka S., Krain R.L., Concha J.S.S., Chong B.F., Merola J.F., Werth V.P. The CLASI, a validated tool for the evaluation of skin disease in lupus erythematosus: a narrative review. Ann Transl Med. 2021;9(5):431. doi: 10.21037/atm-20-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilliam J.N., Sontheimer R.D. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol. 1981;4(4):471–475. doi: 10.1016/s0190-9622(81)80261-7. [DOI] [PubMed] [Google Scholar]
- 8.Klein R., Moghadam-Kia S., LoMonico J., et al. Development of the CLASI as a tool to measure disease severity and responsiveness to therapy in cutaneous lupus erythematosus. Arch Dermatol. 2011;147(2):203–208. doi: 10.1001/archdermatol.2010.435. [DOI] [PMC free article] [PubMed] [Google Scholar]