Abstract
Antibiotic-loaded acrylic cement beads have a large surface area and excellent sustained-release properties. However, there are some difficulty manufacturing reasonably sized beads and their careful handling. We developed a self-made cement bead maker using a mold of a sphere with a diameter of 8 and 10 mm with a 2-mm-diameter connecting hole. With this instrument, approximately 32 beads can be made from 40 g of bone cement. We clinically applied this technique to 11 cases of periprosthetic joint infection. There was no recurrence of infection noted. The advantages of this device were that it was possible to adjust the combination of antimicrobial agents and that the shape was uniform and easy to handle during surgery.
Keywords: Antibiotic-loaded acrylic cement, Cement beads, Periprosthetic joint infection
Introduction
Treatment of infections, especially after arthroplasty, is difficult. Antibiotic-loaded acrylic cement can be used to deliver antimicrobial agents locally to the source of infection for a prolonged period. Its efficacy was first reported by Buchholz and Engelbrecht in 1970 [1]; since then, the experimental and clinical data have been validated, and it is still one of the standard treatments for periprosthetic joint infection. There are two main forms of bone cement currently used in the treatment of periprosthetic joint infection: spacers and beads, these are used according to the requirements. Cement beads have a large surface area and excellent sustained-release properties, and their small volume allows them to be placed in small spaces. However, there are some problems, such as the difficulty manufacturing reasonably sized beads and their careful handling. In some countries, cement beads containing antimicrobial agents are sold as ready-made products(Septopal; Merck, Darmstadt, Germany); however, these are not approved in Japan and therefore cannot be used. In this surgical technique report, we developed a new device for making cement beads and attempted its clinical application. In this article, we describe our results and discuss future prospects.
Surgical technique
We developed a cement bead maker (Pearl Maker) in collaboration with Iso Medical Systems Inc., Tokyo, Japan. The machine consists of a resin mold, a core rod, and a clamp (Fig. 1). The mold is shaped like a sphere with a diameter of 10 mm, and each sphere has a 2-mm-diameter connecting hole. First, both molds were coated with bone cement without clamping, after which the core rod was placed in the center hole of one mold. In this state, the molds were clamped together. This method resulted in a 10-mm-diameter spherical cement bead with a 2-mm hole in the center (Fig. 1). After the surface was trimmed with a luer, the beads were connected to a necklace with a thread or soft steel wire. We used the thick and strong No. 5 ETHIBOND (Johnson & Johnson, New Brunswick, NJ) because it is easier to handle. What strengthens this method is waiting for the bone cement to set sufficiently before removing the clamp and the core rod. The curing time of the beads depends on the type of bone cement, the type and content of the antimicrobial agent, and the room temperature of the operating room, but it is not very different from the curing time of ordinary cement. The beads are trimmed and threaded, and it takes about 10 minutes for one surgen to make 32 beads at a time. Once the device is set, we can let go and allow the cement to cure completely before starting the next operation. If we work by hand, we have to make beads at a time until it sets. The irreplaceable advantage of this system is that we can start the next operation at our own time.
Figure 1.
Cement beads maker (Pearl Maker). (a) Unassembled devices that consist of a mold, core rod, and clamp. (b) Mold size options. Molds of 8 × 4 rows of 8-mm and 10-mm diameter. (c) A picture showing assembly with cement, pressing the mold between the clamps. (d) Opened after hardening (prototype mold). (e) Finished spherical bead of 10-mm diameter with 2-mm internal hole. (f) Assembled beads with suture.
In previous experiments, 0.8 g of cement was required per 10-mm-diameter spherical cement bead. At a 10% concentration of antibiotics, each bead would contain about 0.1 g of antibiotic. The initial product was able to make 16 beads at a time, 8 × 2 rows, in one bead maker. Using two sets of these, it was possible to make at least 32 beads with 40 g of cement. To produce a large amount of bone cement without wasting it, we created an 8 × 4 bead maker and an 8-mm diameter to facilitate the application of bone cement (Fig. 1). The beads can be placed in narrow spaces, such as the medullary cavity. In combination with the conventional beads having a diameter of 10 mm, these beads can be filled densely into the dead space and can easily function as a spacer.
The product was launched in October 2014 and has been used for the treatment of periprosthetic joint infections after arthroplasty, including hemiarthroplasty and other infections. Eleven cases of periprosthetic joint infection after arthroplasty, that were amenable to follow-up for at least 6 months after the final surgery, were included in the study (Table 1). The age, sex, laterality, follow-up period, initial arthroplasty-caused infection, causal bacteria, and used antibiotics powder were as described in Table 1. The choice of antimicrobial agent was based on the susceptibility of the patient in cases where the causative organism was detected and on the experience and preference of the surgeon in the other cases. The viscosity of the bone cement used was medium (Endurance; Depuy Synthes). The concentration of the antimicrobial agents was adjusted depending on the case so that the total weight of the antimicrobial agents was approximately 5% to 10% of the polymer of the bone cement. The surgeon had the right to decide on the type and amount.
Table 1.
Case list.
| # | Age | Sex | Follow-up (mo) | Side | Initial arthroplasty | Comorbidity | Causal bacteria | Antibiotics (g/cement 40 g) | Mold use | Waiting period (d) | Two-stage surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77 | M | 8a | R | Total knee arthroplasty | Renal cell cancer | Staphylococcus epidermidis | AMK (1.6), TEIC (1.6) | Yes | 141 | Revision |
| 2 | 71 | F | 36a | L | Hemiarthroplasty | Bladder cancer, osteomyelitis pubis | Negative | AMK (1.5), VCM (1) | Yes | 106 | Removal |
| 3 | 68 | M | 73 | R | Total knee arthroplasty | Hypertension | Staphylococcus epidermidis | AMK (1), VCM (2), TEIC (1) | Yes | 106 | Revision |
| 4 | 76 | F | 67 | L | Total knee arthroplasty | Hypertension | Negative | AMK (2), TEIC (2) | Yes | 154b | Revision |
| 5 | 80 | M | 54 | R | Total knee arthroplasty | Cholecystitis | MRSA | AMK (2), TEIC (1.1) | No | 184 | Revision |
| 6 | 70 | F | 38 | L | Total hip arthroplasty | Hypertension, hyperuricaemia | Negative | AMK (1.5), VCM (1.5) | Yes | 30 | Revision |
| 7 | 81 | F | 27 | L | Total hip arthroplasty | Hypertension | α-Streptococcus | AMK (1.6), VCM (2) | No | 60b | Removal |
| 8 | 77 | F | 17 | R | Hemiarthroplasty | Hypertension, dyslipidemia | Staphylococcus epidermidis | AMK (2), VCM (1) | No | 38 | Revision |
| 9 | 75 | F | 17 | L | Total elbow arthroplasty | Rheumatoid arthritis | MSSA | AMK (4) | No | 43 | Revision |
| 10 | 66 | M | 13 | L | Total hip arthroplasty | Hepatitis C | Staphylococcus epidermidis | AMK (1.2), VCM (2) | No | 40 | Revision |
| 11 | 57 | M | 8 | L | Total hip arthroplasty | Hyperuricaemia | MRSE | AMK (1.2), VCM (2) | No | 28 | Revision |
AMK, amikacin; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; MRSE, methicillin-resistant Staphylococcus epidermidis; TEIC, teicoplanin;VCM, vancomycin.
Death in progress.
Replacement in progress.
During surgery, the infectious lesion was thoroughly dissected, and spacer molds (StageOne Knee Spacer Molds 60 mm; Zimmer Biomet, Warsaw, IN) were placed in combination with a commercially available spacer mold, in all but one case (case 3), after total knee arthroplasty. In two cases involving the hip (cases 2 and 6), hemispherical spacers were inserted into the acetabulum and used in combination with the beads. The bone marrow cavity was filled with cement beads as much as possible, and the surgery was completed. As a postoperative treatment, the patient was unloaded with non-weight-bearing in principle because of the possibility of damage to the inserted beads and spacers.
The average waiting period before the second-stage surgery was 85 days (range, 28–184 days) (Table 1; Figure 2, Figure 3, Figure 4, Figure 5). Two cases (cases 4 and 7) had spacer mold dislocation, which was replaced in the course of the waiting period for the two-stage surgery. There were no cases of bead-specific adverse events. In all 11 cases, the beads were removed without any damage to the beads themselves, and the threads were not broken. The beads are radiopaque, and postoperative radiographs showed that they had been completely removed. In addition, there were no abnormalities in blood tests that might have been caused by the implantation of cement-containing antimicrobial agents. Comparing the duration of implantation in the first and second halves, the average duration was 138 days in the first five cases and 40 days in the sixth and the subsequent cases. As a bipartite operation, a revision was performed in eight patients. In particular, all four cases after knee arthroplasty were revised. In one case after hemiarthroplasty and one case after total hip arthroplasty, the spacer and bead were removed, and resection arthroplasty was performed instead of revision in consideration of age, comorbidity, and activities of daily living (ADLs). No bacteria were detected in any of the specimens collected intraoperatively during the two-stage surgery. There were no cases of recurrence of local infection after cement bead implantation, including after the two-stage surgery. Two patients with malignant tumor (cases 1 and 2) died of cancer, but there was no recurrence of infection before their deaths.
Figure 2.
Case 3: a 73-year-old man, infected loosening after right knee joint revision surgery. (a) At the time of the initial examination, loosening of the implant after revision knee arthroplasty. (b) After insertion of a combination of beads and handmade spacers. (c) After two-stage surgery.
Figure 3.
Case 9: a 77-year-old woman with chronic infection after hemiarthroplasty in migration of the bipolar femoral head into the pelvis. (a) At the time of our initial examination, the bipolar head had migrated into the pelvis, and the leg was shortened. (b) A fracture occurred at the time of stem removal, and a wire ring was added for bead insertion. (c) Acetabular reconstruction with allogeneic bone grafting and femoral revision with a proximal femoral endoprosthesis.
Figure 4.
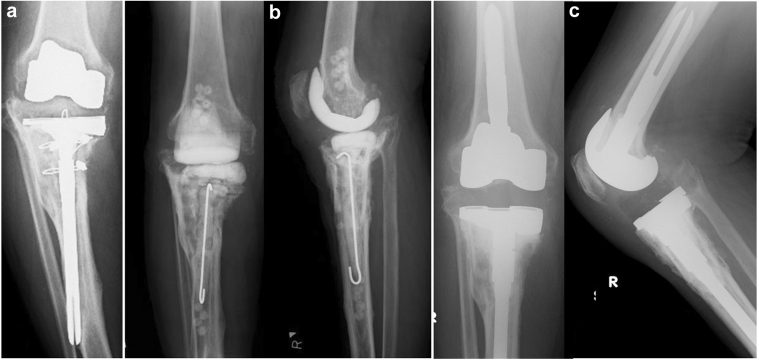
Case 10: a 75-year-old female, infected loosening after left elbow arthroplasty. (a) Preoperatively, findings of implant loosening. (b) Cement beads were inserted into the medullary cavity. (c) Revision surgery with internal fixation was performed.
Figure 5.
Case 11: a 66-year-old man with dislocation of the left acetabular component due to infectious loosening. (a) Preoperatively, the acetabular component was displaced and showed bone loss in the upper part of the acetabular. (b) An 8-mm-diameter bead was inserted into the large dead space in the proximal femur and acetabulum. (c) Acetabular reconstruction was performed with allogeneic bone grafting for the bone defect.
Informed consent was obtained from all patients, who approved the use and publication of their data. This retrospective study was approved by our institution’s ethics review board.
Discussion
Arthroplasty is an important procedure used by orthopedic surgeons to dramatically improve the ADL and quatity of life of patients with arthropathy. However, once an infection occurs, it is difficult to treat and significantly reduces the patient’s ADL and quatity of life. Early diagnosis and treatment are fundamental for the effective treatment of periprosthetic joint infection. It is necessary to remove the implant and thoroughly debride the infected and necrotic bone and soft tissue. From the viewpoint of drug delivery systems, it is important to keep the antimicrobial agent at a concentration higher than the minimum inhibitory concentration for a prolonged period. Therefore, it is important to apply topical antimicrobial agents. This is why antibiotic-loaded acrylic cement is an ideal treatment method for infected lesions with poor local migration of antimicrobial agents.
The efficacy of antibiotic-loaded acrylic cement was first reported in 1970 by Buchholz and Engelbrecht in Germany [1]. Various studies have shown that it could increase the local concentration of antimicrobial agents and could be administered for a prolonged period with fewer side effects than systemic administration [[2], [3], [4], [5]]. In Germany, a commercial gentamicin-containing cement (Refobacin-Palacos; Heraeus, Hanau, Germany) was used in the 1970s for patients with osteomyelitis and other diseases. However, simply implanting antibiotic-loaded acrylic cement did not improve clinical outcomes because of insufficient dissolution and sustained release. Therefore, Klemm used hand-made cement beads to increase the surface area and achieved good results [6]. Recently, treatment targets have been expanded to include osteomyelitis, postarthroplasty infections, and diabetic foot infections [[7], [8], [9], [10], [11]]. In the past, antibiotic-loaded acrylic cement has been produced in a variety of ways, such as by stringing together spherical beads using nonabsorbable thread or soft steel wire or by wrapping them around the steel wire. Gentamicin-containing cement beads (Septopal, Merck, Darmstadt, Germany) are commercially available in some countries, but they are not approved for use in Japan. Even in countries where they are approved, they are expensive and pose a problem in terms of the medical economy [10,12,13].
One of the advantages of this self-made cement bead maker is that the combination of antimicrobial agents can be adjusted. It has been reported that mixing two antimicrobial agents increases the elution rate of each because of their interaction [14]. In terms of size and shape, hand-made devices can be difficult to insert and remove from closed spaces, such as the bone marrow cavity, owing to their uneven size. Therefore, it is advantageous to use beads that are small, uniform in size, and spherical in shape. In addition, the beads themselves have holes, which allow them to be threaded onto a thread to be used as a necklace. This allows the number of beads to be adjusted according to the size of the space, and it also allows for a high degree of flexibility in the positioning of the beads during surgery. It should also be noted that the pores increase the surface area, which is advantageous for the dissolution and sustained release of the antimicrobial agents. Furthermore, the use of 8-mm-diameter molds makes it possible to place the device in narrow spaces such as the spinal cavity. In combination with conventional beads of 10-mm diameter, the beads can be filled more tightly in the dead space, and their function as spacers is easier to obtain.
The duration of implantation was longer than 4 months, partly because of the use of cement molds and spacers in the early cases and partly by the soft-tissue recovery. No optimal time has been established for the waiting period before the two-stage surgery, and the surgery should be performed when the treating medical team feels that the infection is under control [15]. Therefore, considering the limitation of the sustained release period of antimicrobial agents, we changed our policy and made it moving onto the two-stage surgery as soon as possible after the infection was controlled based on the clinical symptoms and blood test results. As a result, the average implantation period after the sixth case was reduced to 40 days, which is less than one-third of the previous period. In the present study, no disadvantages of shortening the implantation period were found, and we plan to further clarify this by investigating more cases in the future. In addition, this series was all used for chronic infections. Although we have no experience in the early postoperative stage of acute infection, we would like to try to apply it to acute periprosthetic joint infection.
A similar product to our device has been reported [16], which seems to be based on the Septopal chain (Merck, Darmstadt, Germany) described previously. It is similar to our device in that antimicrobial agents can be freely selected, but because the beads themselves are fixed to the thread, there is no freedom to adjust the number and placement of beads as described previously. It has not been commercialized, and the details are unknown. Calcium sulfate as a resorbable slow-drug-release carrier instead of bone cement is also available (STIMULAN; Biocomposites, Wilmington, NC). However, this is also not available in Japan. It is true that such a product is very attractive because it does not require removal. Although there are concerns about the strength of the product, we have no experience with it, but we think it is a very attractive tool.
The limitation of this surgical technique report is that it focused on showing the method using the bead maker and its clinical results, and not the efficacy of this method against the infection. Another issue was that the waiting period before the two-stage surgery differed between the first and second halves of the cases, and there was no uniformity in the type and use of antimicrobial agents. In addition, the follow-up period is still inadequate in some cases, and long-term follow-up is required in the future.
Acknowledgment
The authors would like to thank Editage (www.editage.com) for English language editing.
Footnotes
No author associated with this paper has disclosed any potential or pertinent conflicts which may be perceived to have impending conflict with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2021.10.021.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Appendix A. Supplementary data
References
- 1.Buchholz H.W., Engelbrecht H. Depot effects of various antibiotics mixed with palacos resins. Der Chirurg; Z alle Gebiete der operativen Medizen. 1970;41(11):511. [PubMed] [Google Scholar]
- 2.Wahlig H., Dingeldein E., Bergmann R., Reuss K. The release of gentamicin from polymethylmethacrylate beads. An experimental and pharmacokinetic study. J Bone Jt Surg Br Vol. 1978;60-B(2):270. doi: 10.1302/0301-620X.60B2.659478. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz H.W., Elson R.A., Engelbrecht E., Lodenkämper H., Röttger J., Siegel A. Management of deep infection of total hip replacement. J Bone Jt Surg Br Vol. 1981;63-B(3):342. doi: 10.1302/0301-620X.63B3.7021561. [DOI] [PubMed] [Google Scholar]
- 4.Henry S.L., Galloway K.P. Local antibacterial therapy for the management of orthopaedic infections. Pharmacokinetic considerations. Clin Pharmacokinet. 1995;29(1):36. doi: 10.2165/00003088-199529010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Neut D., van de Belt H., van Horn J.R., van der Mei H.C., Busscher H.J. Residual gentamicin-release from antibiotic-loaded polymethylmethacrylate beads after 5 years of implantation. Biomaterials. 2003;24(10):1829. doi: 10.1016/s0142-9612(02)00614-2. [DOI] [PubMed] [Google Scholar]
- 6.Klemm K.W. Antibiotic bead chains. Clin Orthopaedics Relat Res. 1993;295(295):63. [PubMed] [Google Scholar]
- 7.Boda A. Two cases of tuberculous caverna of the greater trochanter filled with gentamycin-PMMA-beads (septopal chain). A new field of application. Arch Orthopaedic Traumatic Surg Archiv Orthopadische Unfall-Chirurgie. 1982;101(1):67. doi: 10.1007/BF00455661. [DOI] [PubMed] [Google Scholar]
- 8.Calhoun J.H., Klemm K., Anger D.M., Mader J.T. Use of antibiotic-PMMA beads in the ischemic foot. Orthopedics. 1994;17(5):453. doi: 10.3928/0147-7447-19940501-12. [DOI] [PubMed] [Google Scholar]
- 9.Klemm K. The use of antibiotic-containing bead chains in the treatment of chronic bone infections. Clin Microbiol Infect. 2001;7(1):28. doi: 10.1046/j.1469-0691.2001.00186.x. [DOI] [PubMed] [Google Scholar]
- 10.Mohanty S.P., Kumar M.N., Murthy N.S. Use of antibiotic-loaded polymethyl methacrylate beads in the management of musculoskeletal sepsis--a retrospective study. J Orthopaedic Surg (Hong Kong) 2003;11(1):73. doi: 10.1177/230949900301100115. [DOI] [PubMed] [Google Scholar]
- 11.Geurts J.A., Janssen D.M., Kessels A.G., Walenkamp G.H. Good results in postoperative and hematogenous deep infections of 89 stable total hip and knee replacements with retention of prosthesis and local antibiotics. Acta Orthopaedica. 2013;84(6):509. doi: 10.3109/17453674.2013.858288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasyid H.N., van der Mei H.C., Frijlink H.W., et al. Concepts for increasing gentamicin release from handmade bone cement beads. Acta Orthopaedica. 2009;80(5):508. doi: 10.3109/17453670903389782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walenkamp G.H. Self-mixed antibiotic bone cement: western countries learn from developing countries. Acta Orthopaedica. 2009;80(5):505. doi: 10.3109/17453670903317122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penner M.J., Masri B.A., Duncan C.P. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11(8):939. doi: 10.1016/s0883-5403(96)80135-5. [DOI] [PubMed] [Google Scholar]
- 15.Aalirezaie A., Bauer T.W., Fayaz H., et al. Hip and knee section, diagnosis, reimplantation: proceedings of international consensus on orthopedic infections. J Arthroplasty. 2019;34(2s):S369. doi: 10.1016/j.arth.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Qi B., Ju W., Yu T., Wang T., Zhao Y., Sun D. A new device for efficient preparation of standard antibiotic bead chains and customized antibiotic delivery. Pharmazie. 2016;71(2):65. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.