Abstract
Bone biopsy is still the gold standard tool to evaluate either trabecular or cortical bone, though the quantitative computed tomography of the vertebrae (QCT), a non-invasive technique, could be useful to evaluate bone structure in patients with chronic kidney disease (CKD). Cortical bone microstructure derangements have been associated with poor outcomes in the general population. An association between trabecular bone density, assessed by QCT, and bone volume and microarchitecture by histomorphometry, has been previously documented. This relationship has not yet been fully evaluated in cortical bone in the CKD scenario. The aim of this study was to evaluate the relationship among vertebrae density measured by QCT, structural histomorphometric parameters of cortical bone and biochemical and hormonal data in 50 CKD stage 2-5ND patients. This was a post hoc analysis of a cross-sectional study where cortical porosity and cortical thickness were analyzed in undecalcified bone samples from the iliac crest. The cortical bone density was obtained by QCT from the thoracic vertebrae. The patients were 52 ± 10 years, 68% men, 30% diabetes and the estimated glomerular filtration rate 34 ± 16 mL/min/1.73 m2. Cortical porosity was 4.6% (3.6; 6.6) and cortical thickness was 578.4 ± 151.8 μm, while cortical bone density was 149.2 ± 58.3 HU. Cortical density correlated with cortical thickness (p = 0.001) but not with cortical porosity (p = 0.30). Higher porosity was associated with older age (p = 0.02), higher levels of PTH (p = 0.04) and lower renal function (p = 0.03), while smaller thickness was associated with higher levels of PTH (p = 0.02). Lower density was associated with older age (p = 0.02) and higher levels of PTH (p = 0.01). In conclusion, cortical bone density measured by QCT was able to mirror the cortical thickness of bone biopsy in pre-dialysis CKD patients. In addition, PTH action on cortical bone can be already seen in this population.
Keywords: Cortical bone, Bone QCT, Bone histomorphometry, Parathyroid-related disorders, Chronic kidney disease
1. Introduction
Renal osteodystrophy (ROD) is currently defined as an alteration of bone morphology in patients with chronic kidney disease (CKD), which is quantifiable by bone histomorphometry. It is the skeletal component of the systemic disorder of chronic kidney disease-mineral and bone disorder (CKD-MBD) (Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group, 2009). Most studies in ROD have been focused on the trabecular bone. This type of bone tissue is known to be metabolically active and plays an important role in the mineral homeostasis. On the other hand, the cortical bone is compact in structure and characterized to be denser, less porous and metabolically active than trabecular bone, essentially carrying out a mechanical function. Recently, more attention has been given to evaluating cortical bone in the CKD scenario. Cortical bone microstructure derangements, characterized by increased porosity and decreased thickness, have been shown to increase the fracture risk in CKD patients (Carvalho et al., 2015; Trombetti et al., 2013).
Although bone biopsy is the gold-standard method to evaluate ROD, noninvasive, less expensive and more accessible image methods have been used to analyze cortical bone density and microstructure (Jamal et al., 2006; Nickolas et al., 2010). Quantitative computed tomography (QCT) of the vertebrae is effective for evaluating trabecular bone (Carvalho et al., 2013). Our group has demonstrated an association between trabecular bone density, assessed by QCT, and bone volume and microarchitecture by histomorphometry, in hemodialysis patients (Carvalho et al., 2013). This relationship has not yet been fully evaluated in cortical bone. Furthermore, studies on the association between the cortical bone and biochemical and hormonal parameters related to CKD-MBD are scarce. Thus, we aimed to evaluate the cortical bone regarding histomorphometric parameters, computed tomography image, and serum bone markers of pre-dialysis CKD patients.
2. Methods
2.1. Patients and study protocol
This is a post hoc analysis of a cross-sectional study of 50 asymptomatic adult CKD stage 2–5 patients (Tomiyama et al., 2010). They had been followed by a nephrologist for at least 3 months, had not been receiving any phosphate binders, vitamin D analogs, bisphosphonates or corticosteroids, and had no evidence of inflammatory, neoplastic or infectious disease.
The selected patients had undergone clinical and physical evaluation, laboratory tests, quantitative computed tomography of the vertebrae (QCT), and undecalcified bone biopsy, performed within 30 days after selection.
This study was reviewed and approved by the Ethics Advisory Committee of the Federal University of São Paulo (n. 1855693) and all patients had signed an informed consent form.
2.2. Vertebral cortical bone density
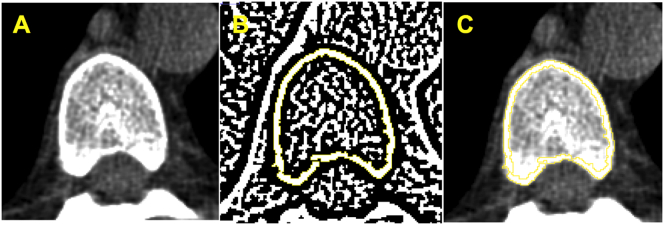
Vertebral computed tomography images previously obtained were re-evaluated to analyze cortical bone using the Image J software (Image J 1.49v, National Institutes of Health, Bethesda, Maryland, USA, 1997–2016). A DICOM image (16 bits) was selected from the vertebral body in the axial section at the level of the aortic root (Fig. 1A). This image was converted into an 8-bit image which allowed the transformation into a binary image and generation of a cortical mask through the automatic delineation of the cortical bone layer (Fig. 1B), using the threshold function with Niblack algorithm and radius 4. The generated cortical mask was overlapped on the original image (DICOM 16 bits) and the cortical bone density was automatically measured (Fig. 1C). The software provides three values, as minimum, medium and maximum values, in Hounsfield Units (HU), of the cortical bone area. The minimum value was considered for analyses. The cortical images were evaluated by 2 observers and inter-observer variability was <1%. In addition, the Bland-Altman Plot showed a good concordance between both observers without systematic (p = 0.27) or proportional bias (p = 0.20). The data from only one observer were used for the analyses.
Fig. 1.
Cortical vertebral tomography methodology.
(A) Axial vertebral image selection.
(B) Binary image and generation of a cortical mask through the automatic delineation of cortical bone layer performed by Image J software®.
(C) Overlapped cortical mask on the original image followed by automatic cortical density measurement.
2.3. Bone biopsy
Embedded toluidine blue-stained slides from iliac crest undecalcified bone samples were analyzed by light microscopy evaluating the histomorphometric cortical parameters. For the present study both, internal and external, cortical bone layers had their outer limits drawn. The histomorphometric parameters averaged across the entire cortices were obtained by the semi-automatic Osteomeasure software (Osteometrics, Inc., Atlanta, GA, USA). Histomorphometric parameters of cortical bone were those suggested by the American Society of Bone and Mineral Research (Dempster et al., 2013; Parfitt et al., 1987), including cortical thickness (Ct.Th; μm) and porosity (Ct.Po; %). These parameters were expressed by the mean value of the cortical layers (internal and external). The histomorphometric analysis was carried out by two independent examiners and the inter-observer variability was <1%. Therefore, the data from only one observer were used for the analyses.
2.4. Laboratory tests
Laboratory analyses included: creatinine, bicarbonate, ionized calcium, phosphorus, alkaline phosphatase, intact parathyroid hormone (iPTH), 25-hydroxyvitamin D (25(OH)D), 1,25-dihydroxy vitamin D (1,25(OH)2D), fibroblast growth factor 23 (FGF23) (ELISA Kainos Laboratories, Tokyo, Japan; reference value 28.9 ± 1.1 pg/mL) and klotho (ELISA assay, IBL America, MN, USA; reference value 239–1266 pg/mL). The estimated glomerular filtration rate (eGFR) was calculated by the CKD-EPI equation (Levey et al., 2009).
2.5. Statistical analysis
Mean and standard deviation, median and interquartile range or frequencies were calculated for all variables. The Kolmogorov-Smirnov statistical test was used to investigate the data distribution. Univariate associations were analyzed by Pearson's test using log transformation. Comparisons of continuous variables between groups were performed using Student's t-test and the Mann-Whitney U test, for normal and skewed data, respectively. Anova test followed by Bonferroni's test was performed to evaluate the relationship between the laboratory tests and the tertiles of the dependent variables (Ct.Po, Ct.Th and cortical density). A generalized logistic regression model for polytomous responses, adjusted for renal function, was performed to evaluate the relationship between them. P-values < 0.05 were considered statistically significant. All statistical analysis was performed using SPSS for Windows (SPSS 18.0, Chicago, IL, USA) and STATA 12 (College Station, TX, USA).
3. Results
The demographic, clinical and laboratory data of the 50 patients enrolled in the study are shown in Table 1. The patients were predominantly middle-aged males, and 27 (54%) were obese (BMI ≥ 25 kg/m2). Hypertension and diabetes were the main causes of CKD. According to the CKD classification (Levey et al., 2005), 5 patients (10%) were in stage 2, 7 (14%) in stage 3a, 17 (34%) in stage 3b, 17 (34%) in stage 4 and 4 (8%) in stage 5. Serum bicarbonate and bone mineral parameters were within the normal range, except for iPTH and FGF23, which is higher, and for klotho which is lower than the reference values.
Table 1.
Demographic and clinical characteristics (n = 50).
| Age (years) | 52 ± 10 |
| Male n (%) | 34 (68) |
| CKD etiology n (%) | |
| Hypertension | 20 (40) |
| Diabetes | 15 (30) |
| Others | 15 (30) |
| BMI (kg/m2) | 26.2 ± 4.3 |
| eGFR (mL/min/1.73m2) | 34 ± 16 |
| Bicarbonate (mEq/L) | 24 ± 3 |
| FGF23 (pg/mL) | 49.6 (25.6;83.8) |
| Klotho (pg/mL) | 57.2 (17.4;88.8) |
| Ionized calcium (mmol/L) | 1.30 ± 0.06 |
| Phosphorus (mg/dL) | 3.8 ± 0.7 |
| Alkaline phosphatase (U/L) | 116 (71.5;160.5) |
| iPTH (pg/mL) | 83 (53.5;167.5) |
| 25(OH)D (ng/mL) | 30.8 ± 10.1 |
| 1,25(OH)2D (pg/mL) | 35.8 (30;47.5) |
Mean and standard deviation and median (interquartile); CKD = chronic kidney disease; BMI = body mass index; eGFR = estimated glomerular filtration rate; FGF23 = fibroblast growth factor 23 and iPTH = intact parathyroid hormone.
Bone histomorphometry showed that Ct.Po was 4.6% (3.6; 6.6), and Ct.Th was 578.4 ± 151.8 μm. The cortical bone density was 149.2 ± 58.3 HU. There was a positive correlation between the vertebral cortical bone density and Ct.Th (Fig. 2A). No correlations between the vertebral cortical bone density and Ct.Po or between Ct.Po and Ct.Th were observed (Fig. 2B and C).
Fig. 2.
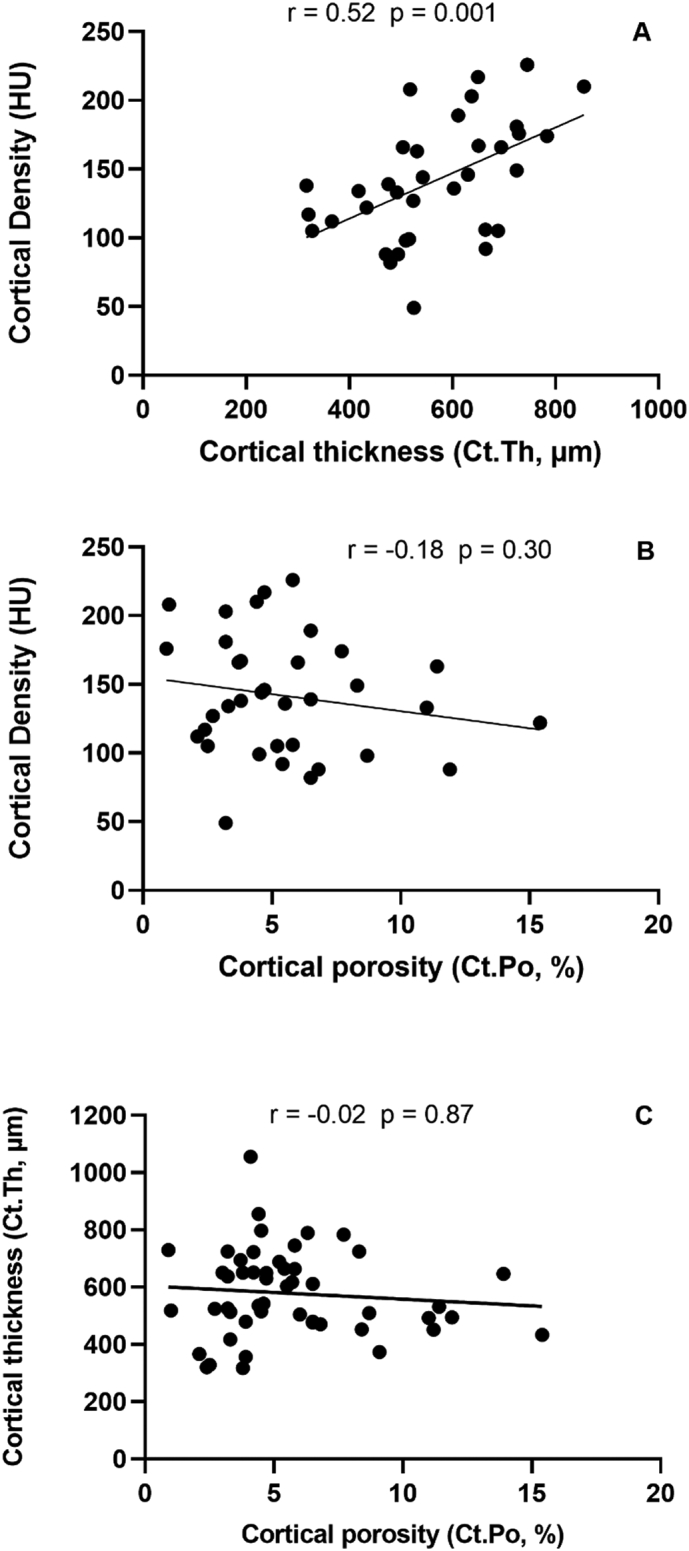
Correlations between cortical density and cortical thickness (A), cortical density and cortical porosity (B) and cortical thickness and cortical porosity (C).
In the univariate analysis, Ct.Po was positively correlated with age (r = 0.32, p = 0.02) and negatively correlated with eGFR (r = −0.31, p = 0.03), while Ct.Th was positively correlated with ionized calcium (r = 0.27, p = 0.02). Cortical density was negatively correlated with age (r = −0.52, p = 0.02), phosphorus (r = −0.59, p < 0.01) and iPTH (r = −0.42, p = 0.01). There were no differences between gender regarding the Ct.Po (p = 0.76), Ct.Th (p = 0.15) and cortical density (p = 0.38).
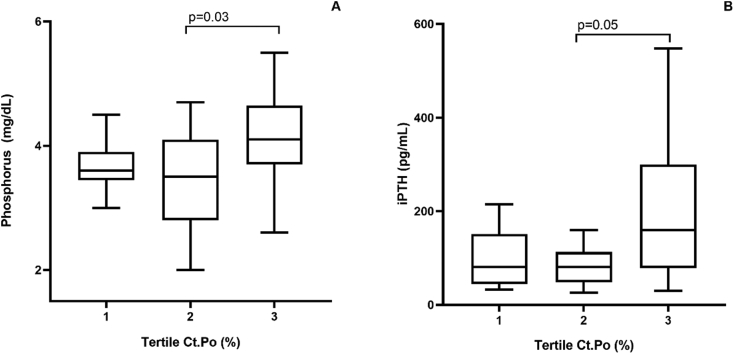
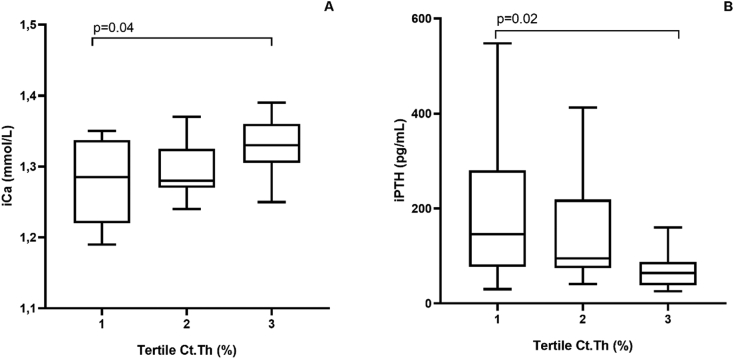
The relationship between the tertiles (tertiles 1, 2 and 3, shown in increasing order of magnitude) of the cortical bone variables (Ct.Po, Ct.Th and density) and the clinical and laboratory parameters was analyzed. Considering the Ct.Po, phosphorus was significantly higher in the 3rd, when compared to the 2nd tertile (p = 0.03; Fig. 3A). On the other hand, iPTH tended to be higher in the 3rd, when compared to the 2nd tertile (p = 0.05; Fig. 3B). The multivariate analysis did not identify any independent variables associated with Ct.Po (Table 2). Regarding the Ct.Th, calcium was significantly higher in the 3rd, when compared to the 1st tertile (p = 0.04; Fig. 4A). On the other hand, iPTH was lower in the 3rd compared to the 1st tertile (p = 0.02; Fig. 4B). The multivariate analysis did not identify any independent variables associated with Ct.Th (Table 2). Considering the cortical density, age (p = 0.01; Fig. 5A) and phosphorus (p = 0.01; Fig. 5B) were significantly lower, when compared the 3rd with the 1st tertile and iPTH was significantly lower, when compared the 3rd with the 2nd tertile (p = 0.04; Fig. 5C). The multivariate analysis identified phosphorus and age as the independent variables associated with cortical density (Table 2).
Fig. 3.
Relationship between cortical porosity (Ct.Po) tertiles and phosphorus (A) and iPTH (B).
Table 2.
Multivariate analysis of cortical bone parameters.
| Ct.Po | Tertile - (ref. = 1) |
||||
|---|---|---|---|---|---|
| Tertile 2 |
Tertile 3 |
p value | |||
| Odds ratio (CI95%) | p | Odds ratio (CI95%) | p | ||
| eGFR (mL/min/1.73m2) | 0.98 (0.92–1.04) | 0.65 | 1.00 (0.94–1.06) | 0.86 | 0.90 |
| Phosphorus (mg/dL) | 0.65 (0.19–2.22) | 0.50 | 1.60 (0.44–5.77) | 0.47 | 0.44 |
| iPTH (pg/mL) | 1.00 (0.99–1.00) | 0.96 | 1.00 (0.99–1.01) | 0.27 | 0.47 |
| Ct.Th | Tertile - (ref. = 3) |
||||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | ||||
| eGFR (mL/min/1.73m2) | 1.00 (0.95–1.06) | 0.55 | 0.98 (0.93–1.04) | 0.69 | 0.83 |
| Ionized calcium (mmol/L) × 100 | 0.86 (0.72–1.03) | 0.11 | 0.88 (0.74–1.05) | 0.17 | 0.25 |
| iPTH (pg/mL) | 1.00 (0.99–1.02) | 0.54 | 1.00 (0.99–1.01) | 0.56 | 0.81 |
| Density | Tertile - (ref. = 3) |
||||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | ||||
| Age (years) | 1.20 (1.02–1.42) | 0.02 | 1.05 (0.91–1.18) | 0.49 | 0.07 |
| eGFR (mL/min/1.73m2) | 1.05 (0.95–1.04) | 0.30 | 1.01 (0.91–1.18) | 0.81 | 0.49 |
| Phosphorus (mg/dL) | 19.88 (1.44–274.77) | 0.02 | 5.99 (0.65–54.61) | 0.11 | 0.08 |
| iPTH (pg/mL) | 1.01 (0.98–1.04) | 0.23 | 1.01 (0.99–1.04) | 0.15 | 0.35 |
eGFR = estimated glomerular filtration rate and iPTH = intact parathyroid hormone.
Fig. 4.
Relationship between cortical thickness tertiles (Ct.Th) and calcium (A) and iPTH (B).
Fig. 5.
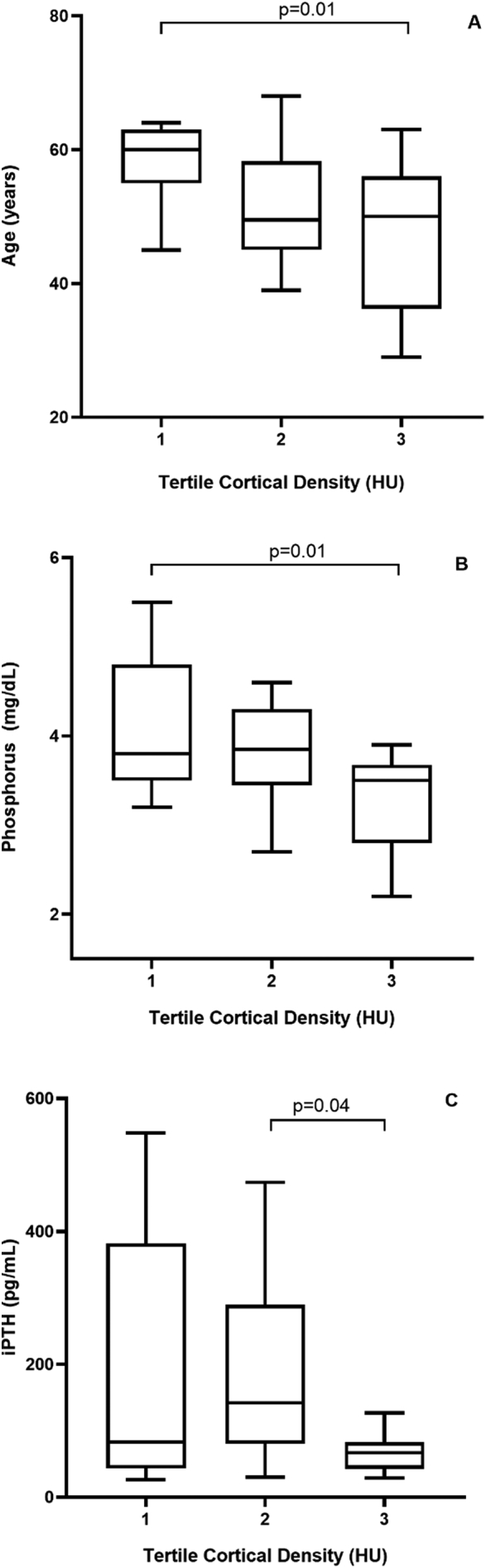
Relationship between cortical density tertiles and age (A), phosphorus (B), iPTH (C).
4. Discussion
In this study, we could demonstrate an association between cortical density evaluated by QCT and cortical thickness (Ct.Th) by bone histomorphometry in CKD stages 2-5ND patients. Additionally, we were able to identify associations between the parameters of cortical bone with some clinical, biochemical and hormonal variables in this population.
The bone biopsy followed by histomorphometry remains the gold-standard method to evaluate ROD. Efforts have been made to use non-invasive methods, whether biochemical or imaging, to analyze bone density and microstructure (Sprague et al., 2016; Bousson et al., 2000; Rantalainen et al., 2011; Ostertag et al., 2014; Cohen et al., 2010; Lehmann et al., 2008 Oct). Cortical other than trabecular bone is scarcely studied in the setting of ROD (Rantalainen et al., 2011; Marques et al., 2017). To our knowledge, the present study is the first one that evaluated the relationship between cortical bone density assessed by QCT and histomorphometric structural parameters by bone biopsy. It is noteworthy, however, that a study with a similar design, although evaluating the trabecular bone of hemodialysis patients, has already been published by our group (Carvalho et al., 2013). In that study, we demonstrated an association between trabecular bone density, assessed by QCT, and bone volume and microarchitecture parameters by histomorphometry in those patients (Carvalho et al., 2013).
While an association between cortical bone density and cortical thickness could be observed, the same relationship with cortical porosity was not seen, as expected. One reason for that negative result could rely on the technical limitations of QCT when it is used to evaluate the cortical porosity. This imaging method measures cortical porosity indirectly and assumes that the measurements of the density may reflect those of the porosity (Ahmed et al., 2015). Moreover, QCT has a resolution limitation in the order of 250 μm or larger (Cooper et al., 2016), while in normal conditions more than 60% of intracortical pores are smaller than 100 μm diameter (Zebaze and Seeman, 2015). Interestingly, our CKD patients exhibit a cortical porosity (4.6%) similar to those reported in healthy (1.9–10%) and post-mortem subjects without medical conditions that could affect bone metabolism (4.4%) (Malluche et al., 2011; Tong et al., 2017). Studies that included populations with greater porosity showed that QCT was able to reflect the porosity directly measured by imaging methods, such as microradiographies or HR-pQCT. Bousson et al., evaluating microradiographies of ex-vivo bone samples with cortical porosity ranging from 3 to 16%, observed that QCT was successful in detecting density changes (Bousson et al., 2000). Similarly, Ahmed et al., in a sample with cortical porosity bigger than 15%, found a strong correlation between the cortical density measured by QCT and the porosity measured by HR-pQCT (Ahmed et al., 2015). Therefore, the normal cortical porosity observed in the present study could have reduced the effectiveness of QCT to mirror the cortical porosity measured by bone biopsy in this population. We have also to consider the technical limitations of the bone biopsy. Although bone biopsy is considered the gold standard for determination of bone mineralization and turnover, it is probably not the best method to measure the bone structure (Ott, 2018). Bone biopsy provides only a two-dimensional areal view of the three-dimensional mineralized bone mass. Sharma et al., studying cortical bone in CKD patients, reported a greater cortical porosity in micro-CT than that observed on bone biopsy, besides they did not find any correlation between micro-CT and histomorphometric parameters. This difference was assigned to the limited portion of cortex assessed in 2D by histomorphometry versus the entire cortex assessed in 3D by micro-CT (Sharma et al., 2018). It is important to mention that biopsy evaluation of cortical bone is limited to the size and location of the bone sample, restraining its representativeness of the overall cortical bone mass (Ott, 2018). Bone biopsies are currently performed on the iliac crest because it is a safe and convenient anatomic location. A recent study that compared the bone histomorphometry from different bone sites, revealed the heterogeneity of cortical porosity among them (Tong et al., 2017). The iliac crest is not weight-bearing compared to the axial skeleton, such as the spine (Ott, 2018). This could have interfered with our results, when we compared the cortical layer of the iliac bone (biopsy) with the vertebra (QCT).
A second analysis of this study was to evaluate the relationship between the parameters of mineral metabolism and those of the cortical bone, measured by QCT and bone biopsy. Among the hormonal alterations of the CKD-MBD, the elevation of PTH and its association with high turnover bone disease stand out (Sprague et al., 2016). This type of ROD presents a fast and highly disorganized bone formation and resorption, leading to the derangement of trabecular bone. This histological pattern invades the cortical bone, enlarges the Haversian and Volkmann canals, resulting in the “trabecularization” of the cortex, with increased cortical porosity (Sharma et al., 2018). Indeed, Marques et al. observed a relationship between PTH and cortical porosity in a hemodialysis population with an increased rate of high turnover bone disease (Marques et al., 2017). Another prospective study, with patients on hemodialysis, demonstrated that the elevation of PTH in patients with low turnover bone disease was associated with the increase of cortical porosity (Araujo et al., October 2016). As already mentioned, cortical porosity was normal in this present cohort, which could be related to low turnover bone disease. In fact, as already published by ours and others, adynamic bone disease has been shown to be highly prevalent in CKD 2-5ND population (Tomiyama et al., 2010; Torres et al., May 1995; Carvalho et al., 1998; Lobão et al., 2004; Hernandez et al., 1994). One could speculate that the differences in cortical bone between high and low turnover bone diseases are associated with high and low levels of PTH, respectively. Interestingly, in the present study, in a scenario with low turnover bone disease (trabecular bone), normal porosity (cortical bone), and slightly high levels of PTH, we could demonstrate an association between increasing PTH levels and higher grades of porosity and, lower grades of thickness and density of cortical bone. These findings may indicate an early action of PTH on cortical bone metabolism, which may already influence the cortical porosity in predialysis CKD patients. Therefore, we can hypothesize that the levels of PTH in our patients are not high enough yet to cause an increase in the porosity of the cortical bone above its normal range.
Besides PTH, other variables of mineral metabolism, like calcium and phosphorus, were associated with cortical bone parameters. As these parameters are strictly linked within the calcium-PTH-axis, it is difficult to attribute to them a role independent of PTH in the cortical bone (Kumar, 1991; Kestenbaum and Houillier, 2018). Other variables that correlated with cortical porosity and density were age and renal function. These results are in agreement with others, showing the relationship of increased porosity with aging, as well as with the progression of CKD (Brockstedt et al., 1993; Melsen et al., 1978; Runesson et al., 2019; Jadoul et al., 2006).
This study has some limitations. One of them is the fact of being a post hoc analysis of a cross-sectional study and having used a new imaging method with no defined reference values yet. In addition, QCT of the cortical bone was evaluated at a site other than that of the bone biopsy.
In conclusion, cortical bone density measured by QCT was able to mirror the cortical thickness of bone biopsy in pre-dialysis CKD patients. In addition, PTH action on cortical bone can be already seen in this population.
CRediT authorship contribution statement
Amandha L. Bittencourt: Conceptualization, Methodology, Validation, Software, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization. Maria Eugênia F. Canziani: Conceptualization, Methodology, Validation, Software, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. Larissa D.B.R. Costa: Conceptualization, Methodology, Validation, Software, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization. Carlos E. Rochitte: Conceptualization, Methodology, Validation, Software, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization. Aluizio B. Carvalho: Conceptualization, Methodology, Validation, Software, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration.
Declaration of competing interest
None.
Acknowledgments
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) Hospital do Rim – Fundação Oswaldo Ramos (São Paulo, Brazil).
References
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD– MBD) Kidney Int. 2009;76(Suppl. 113):1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- Carvalho C.G., Pereira R.C., Gales B., Salusky I.B., Wesseling-Perry K. Cortical and trabecular bone in pediatric end-stage kidney disease. Pediatr. Nephrol. 2015;30:497–502. doi: 10.1007/s00467-014-2942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetti A., Soermann C., Chevalley T., Van Rietbergen B., Herrmann P., Martin P.Y., et al. Alterations of bone microstructure and strength in end-stage renal failure. Osteoporos. Int. 2013;24:1721–1732. doi: 10.1007/s00198-012-2133-4. [DOI] [PubMed] [Google Scholar]
- Jamal S.A., Gilbert J., Gordon C., Bauer D.C. Cortical pQCT measures are associated with fractures in dialysis patients. J. Bone Miner. Res. 2006;21:543–548. doi: 10.1359/jbmr.060105. [DOI] [PubMed] [Google Scholar]
- Nickolas T.L., Stein E., Cohen A., Thomas V., Staron R.B., McMahon D.J., et al. Bone mass and microarchitecture in CKD patients with fractures. J. Bone Miner. Res. 2010;21:1371–1380. doi: 10.1681/ASN.2009121208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A.B., Carneiro R., Leme G.M., Rochitte C.E., Santos R.D., Miname M.H., et al. Vertebral bone density by quantitative computed tomography mirrors bone structure histomorphometric parameters in hemodialysis patients. J. Bone Miner. Res. 2013;31(5):551–555. doi: 10.1007/s00774-013-0442-0. [DOI] [PubMed] [Google Scholar]
- Tomiyama C., Carvalho A.B., Higa A., Jorgetti V., Draibe S.A., Canziani M.E.F. Coronary calcification is associated with lower bone formation rate in CKD patients not yet in dialysis treatment. J. Bone Miner. Res. 2010;25(3):499–504. doi: 10.1359/jbmr.090735. [DOI] [PubMed] [Google Scholar]
- Dempster D.W., Compston J.E., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR histomorphometry nomenclature committee. J. Bone Miner. Res. 2013;28(1):1–16. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt A.M., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J. Bone Miner. Res. 1987;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Levey A.S., Stevens L.A., Schmid C.H., Zhang Y., Castro A.F., III, Feldman H.I., et al. A new equation to estimate glomerular filtration rate. Ann Inter Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A.S., Eckardt K.U., Tsukamoto Y., Levin A., Coresh J., Rossert J., et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO) Kidney Int. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- Sprague S.M., Bellorin-Font E., Jorgetti V., Carvalho A.B., Malluche H.H., Ferreira A., D'Haese P.C., Drüeke T.B., Du H., Manley T., Rojas E., Moe S.M. Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am. J. Kidney Dis. 2016;67(4):559–566. doi: 10.1053/j.ajkd.2015.06.023. [DOI] [PubMed] [Google Scholar]
- Bousson V., Bergot C., Meunier A., Barbot F., Parlier-Cuau C., Laval-Jeantet A.M., et al. CT of the middiaphyseal femur: cortical bone mineral density and relation to porosity. Radiology. 2000;217:179–187. doi: 10.1148/radiology.217.1.r00se11179. [DOI] [PubMed] [Google Scholar]
- Rantalainen T., Nikander R., Heinonen A., Daly R.M., Sievänen H. An open source approach for regional cortical bone mineral density analysis. J. Musculoskelet. Neuronal Interact. 2011;11(3):243–248. http://hdl.handle.net/10536/DRO/DU:30036200 [PubMed] [Google Scholar]
- Ostertag A., Peyrin F., Fernandez S., Laredo J.D., de Vernejoul M.C., Chappard C. Cortical measurements of the tibia from high resolution peripheral quantitative computed tomography images: a comparison with synchrotron radiation micro-computed tomography. Bone. 2014;63C:7–14. doi: 10.1016/j.bone.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Cohen A., Dempster D.W., Müller R., Guo X.E., Nickolas T.L., Liu S., et al. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos. Int. 2010;21(2):263–273. doi: 10.1007/s00198-009-0945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann G., Ott U., Kammerer D., Schutze J., Wolf G. Bone histomorphometry and biochemical markers of bone turnover in patients with chronic kidney disease stages 3–5. Clin. Nephrol. 2008 Oct;70(4):296–305. doi: 10.5414/cnp70296. [DOI] [PubMed] [Google Scholar]
- Marques I.D.B., Araújo M.J.C.L.N., Graciolli F.G., Reis L.M., Pereira R.M., Custódio M.R., et al. Biopsy vs. Peripheral computed tomography to assess bone disease in CKD patients on dialysis: differences and similarities. Osteoporos. Int. 2017;28(1675–83) doi: 10.1007/s00198-017-3956-9. [DOI] [PubMed] [Google Scholar]
- Ahmed L.A., Shigdel R., Joakimesen R.M., Eldevik O.P., Eriksen E.F., Ghasem-Zadeh A., et al. Measurement of cortical porosity of the proximal femur improves identification of women with nonvertebral fragility fractures. Osteoporos. Int. 2015;26:2137–2146. doi: 10.1007/s00198-015-3118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D.M.L., Kawalilak C.E., Harrison K., Johnston B.D., Johnston J.D. Cortical bone porosity: what is it, why is it important, and how can we detect it? Curr. Osteoporos. Rep. 2016;14:187–198. doi: 10.1007/s11914-016-0319-y. [DOI] [PubMed] [Google Scholar]
- Zebaze R., Seeman E. Cortical bone: a challenging geography. J. Bone Miner. Res. 2015;30:46–54. doi: 10.1002/jbmr.2419. [DOI] [PubMed] [Google Scholar]
- Malluche H.H., Mawad H.W., Monier-Faugere M.C. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J. Bone Miner. Res. 2011;26:1368–1376. doi: 10.1002/jbmr.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X., Burton I., Jurvelin J.K., Isaksson H., Kröger H. Iliac crest histomorphometry and skeletal heterogeneity in men. Bone Rep. 2017;6:9–16. doi: 10.1016/j.bonr.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott S. Cortical or trabecular bone: what’s the difference? Am. J. Nephrol. 2018;47:373–375. doi: 10.1159/000489672. [DOI] [PubMed] [Google Scholar]
- Sharma A.K., Toussanint N.D., Masterson R., Holt S.G., Rajapakse C.S., Ebeling P.R., et al. Deterioration of cortical bone microarchitecture: critical component of renal osteodystrophy evaluation. Am. J. Nephrol. 2018;47:376–384. doi: 10.1159/000489671. [DOI] [PubMed] [Google Scholar]
- Araujo M.C.L.N., Karohl C., Elias A., Rosilene M., Barreto F.C., Barreto D.V., Canziani M.E.F., Carvalho A.B., Jorgetti V., Moyses R.M.A. The pitfall of treating low bone turnover: effects on cortical porosity. Bone. October 2016;91:75–80. doi: 10.1016/j.bone.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Torres A., Lorenzo V., Hernández D., Rodríguez J.C., Concepción M.T., Rodríguez A.P., Hernández A., de Bonis E., Darias E., González-Posada J.M. Bone disease in predialysis, hemodialysis, and CAPD patients: evidence of a better bone response to PTH. Kidney Int. May 1995;47(5):1434–1442. doi: 10.1038/ki.1995.201. [DOI] [PubMed] [Google Scholar]
- Carvalho A.B., Lobao R.R., Cuppari L., Draibe S.A., Ajzen H. Does hypophosphatemia induce hypoparathyroidism in pre-dialysis patients? Nephrol. Dial. Transplant. 1998;13(Suppl. 3):12–14. doi: 10.1093/ndt/13.suppl_3.12. [DOI] [PubMed] [Google Scholar]
- Lobão R., Carvalho A.B., Cuppari L., Ventura R., Lazaretti-Castro M., Jorgetti V., et al. High prevalence of low bone mineral density in pre-dialysis chronic kidney disease patients: bone histomorphometric analysis. Clin. Nephrol. 2004;62(6):432–439. doi: 10.5414/cnp62432. [DOI] [PubMed] [Google Scholar]
- Hernandez D., Concepcion M.T., Lorenzo V., Martinez M.E., Rodriguez A.De, Bonis E., et al. Adynamic bone disease with negative aluminium staining in predialysis patients: prevalence and evolution after maintenance dialysis. Nephrol. Dial. Transplant. 1994;9(517–23) doi: 10.1093/ndt/9.5.517. [DOI] [PubMed] [Google Scholar]
- Kumar R. Vitamin D and calcium transport. Kidney Int. 1991;40:1177–1189. doi: 10.1038/ki.1991.332. [DOI] [PubMed] [Google Scholar]
- Kestenbaum B., Houillier P. 6ª edition. Elsevier; 2018. Comprehensive Clinical Nephrology. Chapter 10: Disorders of Calcium, Phosphate, and Magnesium Metabolism. [Google Scholar]
- Brockstedt H., Kassem M., Eriksen E.F., et al. Age- and sex related changes in iliac cortical bone mass and remodeling. Bone. 1993;14:681–691. doi: 10.1016/8756-3282(93)90092-o. [DOI] [PubMed] [Google Scholar]
- Melsen F., Melsen B., Mosekilde L., et al. Histomorphometric analysis of normal bone from the iliac crest. Acta Pathol. Microbiol. Scand. 1978;86:70–81. doi: 10.1111/j.1699-0463.1978.tb02014.x. [DOI] [PubMed] [Google Scholar]
- Runesson B., Trevisan M., Iseri K., et al. Fractures and their sequelae in non-dialysis-dependent chronic kidney disease: the Stockholm creatinine measurements project. Nephrol. Dial. Transplant. 2019 doi: 10.1093/ndt/gfz142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadoul M., Albert J.M., Akiba T., et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int. 2006;70:1358–1366. doi: 10.1038/sj.ki.5001754. [DOI] [PubMed] [Google Scholar]