Abstract
Aim
This study explored how body habitus in the paediatric population might potentially affect the use of one-third external anterior-posterior (APD) diameter when compared to age-appropriate absolute chest compression depth targets. It also explored how body habitus could potentially affect the relationship between one-third external and internal APD (compressible space) and if body habitus indices were independent predictors of internal APD at the lower half of the sternum.
Methods
This was a secondary analysis of a retrospective study of chest computed tomography (CT) scans of infants and children (>24-hours-of-life to less-than-18-years-old) from 2005 to 2017. Patients’ scan images were reviewed for internal and external APDs at the mid-point of the lower half of the sternum. Body habitus and epidemiological data were extracted from the electronic medical records.
Results
Chest CT scans of 193 infants and 398 children were evaluated. There was poor concordance between one-third external APD measurements and age-specific absolute chest compression depth targets, especially in infants and overweight/obese adolescents. There was a co-dependent relationship between one-third external APD and internal APD measurements. Overweight/obese children’s and adolescents’ internal and external APDs were significant different from the normal/underweight groups. Body-mass-index (BMI) of children and adolescents (p = 0.009), but not weight-for-length (WFL) of infants (p = 0.511), was an independent predictor of internal APD at the compression landmark.
Conclusion
This study demonstrated correlations between external and internal APDs which were affected by BMI but not WFL (infants). Clinical studies are needed to validate current chest compression guidelines especially for infants and overweight/obese adolescents.
(250 words)
Abbreviations: APD, anterior-posterior diameter; BMI, body mass index; CT, computed tomography; CI, confidence interval; SD, standard deviation; WFL, weight-for-length; WHO, World Health Organisation
Keywords: Paediatrics, Anthropometry, Cardiopulmonary resuscitation, Chest compressions, Computed tomography
Introduction
Chest size and cavity dimensions have clinical implications in resuscitation, especially for chest compression and respiratory function. The internal anterior-posterior diameter (APD) at the site of chest compression represents the “available space” for the heart to be compressed between the sternum and vertebral body. If chest compressions, based on current paediatric guidelines, are too shallow or too deep (relative to the internal cavity size, i.e., internal APD), this will result in either inadequate blood pressure generated or potential harm, respectively. Current paediatric resuscitation guidelines generally recommend chest compression depth targets of: i) relative compression depth of at least one-third external APD, and ii) absolute chest compression depth of approximately 40 mm in infants, approximately 50 mm in younger children, and 50–60 mm in adolescents with pubertal signs.1., 2., 3., 4., 5. Age is currently the only patient variable considered in current chest compression depth recommendations.1., 2., 3., 4., 5.
Chest dimensions are inherently affected by a child’s growth. Intuitively, external chest dimensions are affected by body habitus. However, it is unknown how internal chest cavity size may by be affected by body habitus, and other demographic factors such as race. An adult computed tomography (CT) study reported that body-mass-index (BMI) could affect the adequacy of chest compression and that obesity was associated with the need for deeper compressions due to relatively larger internal chest cavity sizes.6 The findings may also be relevant in paediatrics, but not previously reported. This may be a significant issue as childhood obesity is becoming increasing more prevalent worldwide.7 Although obesity/overweight has been associated with worse survival outcomes in paediatric in-hospital cardiac arrest,8 there are currently no paediatric studies to our knowledge, to inform if and how body habitus would affect external APD (use of one-third APD as a compression depth target) and internal chest cavity sizes at the chest compression landmark in infants, children, and adolescents, and if these factors, in turn, should be considered during chest compressions.
Using Chest CT imaging of a heterogenous, Asian, paediatric population, our study’s aims were to investigate i) effects of paediatric body habitus on the concordance of relative (one-third external APD) versus age-specific absolute chest compression depth targets (approximately 40 mm for infants, approximately 50 mm for children, and 50–60 mm for adolescents), ii) effects of body habitus on the relationship between the internal APD at the compression landmark (potential space for cardiac compression to occur) and one-third external APD (relative chest compression depth target), and iii) if paediatric body habitus was an independent predictor of internal APD at the lower half of the sternum.
Based on our current paediatric chest compression depth target guidelines, we hypothesised that in relation to body habitus, i) there would not be significant differences in the use of one-third external APD and age-specific absolute chest compression depth targets, ii) the internal APDs of normal weight infants, children, and adolescents would not be significantly different from underweight and overweight/obese counterparts, and iii) within and across age groups, these age- and gender- specific body habitus indices were not independent predictors of internal chest cavity dimensions at the lower half of the sternum.
Methods
Setting
This study was a secondary study of a prior publication on the potential for over-compression in paediatric population using retrospective, quantitative database of paediatric patients below 18-years of age who had chest CT scans performed for medical purposes in KK Women’s and Children’s Hospital in Singapore from January 2005 to December 2017.9 There was Institutional Review Board approval with waiver of informed consent (Reference Number 2017/3131). We used a large database of consecutive paediatric chest CTs as there were no prior studies in our local (Singapore) or international paediatric populations that studied the associations of body habitus with internal chest cavity dimensions at chest compression landmark (lower half of the sternum).
Inclusion and exclusion criteria
All chest CT scans of consecutive patients below 18-years of age from 1 January 2005 to 31 December 2017 were obtained via the institutional electronic database records with measurements done using built-in Vue Motion Image Viewer® were included in the study.
Patients whose weight and height/length were not available within 2 weeks of the CT scans were excluded.
Repeat chest CT scans of patients were excluded from the study if they were performed within two months of the index Chest CT or within same hospitalisation period (whichever was longer). Chest CT studies were also evaluated and excluded if they had pathologies which may affect measurements. These included studies with significant chest wall deformities, significant plural or intrathoracic pathologies, and technical limitations (inability to identify chest borders or landmarks).
Data collection and analysis
For infants, body habitus in infants was determined by using gender-specific weight-for-length (WFL) growth charts using World Health Organisation (WHO) standards.10., 11. Age- and gender-specific body-mass index [BMI = weight (kg)/height (m2] categories for children and adolescents were obtained using Singapore’s age- and gender-specific BMI growth charts.12 We used cut-offs of <5th centile to define paediatric population as underweight, 5th to 85th centile as normal weight, >85th to 95th centile as overweight, and >95th centile as obesity.11 The patient’s demographics such as age, gender, race, weight, and length/height were extracted from electronic databases based on the date of CT scans conducted (within 2 weeks).
Paediatric chest compression guidelines recommend depth targets of at least one-third external APD or approximately 40 mm in infants (<1 year-old), approximately 50 mm in prepubertal children and 50–60 mm in adolescents with pubertal signs.1., 2., 3., 4., 5. As we were not able to determine pubertal status (specifically males) from our database, we prospectively divided the children ≥ 1 year-old into two age groups: children 1–12 years old, and adolescents more than 12-years-old.
Chest CT scans of consecutive patients from 1 January 2005 to 31 December 2017 were obtained via the institutional electronic database records with measurements done using built-in Vue Motion Image Viewer®.
Details of the study’s design and methodology for collecting the chest CT scans are detailed in our primary study.8 Chest wall and cavity dimensions were obtained at mid-point of the lower half of the sternum. Axial and sagittal views of each CT scan were used to identify this measurement point and measurements were performed on the axial images. These sites were checked independently by at least 2 investigators who were guided by a senior radiologist on taking the measurements for data collection and were audited for consistency and reproducibility of radiological measurements (external APD and internal APD) as detailed in our primary paper.9
Statistical analysis
Demographic and clinical characteristics were summarised by mean (standard deviation) and frequency (percentage) where appropriate. Means of internal and one-third external APD between body habitus groups were compared by independent two-sample t test. Patients whose one-third external APD were concordant with age-specific absolute chest compression depth targets (40 mm ± 10% in infants, 50 mm ± 10% in children 1–12 years-old and 50–60 mm in adolescents > 12 years-old) were summarised by frequency (percentage) according to body habitus category. Percentage of concordance was subsequently compared between underweight, overweight/obese, and normal weight groups by Chi-square or Fisher’s exact test. Relationship between internal APD and one-third external APD at the lower half of the sternum was studied by multiple linear regression, with adjustment for gender-specific weight-for-length for infants and age- and gender-specific BMI for children and adolescents. The interaction between body habitus categories and internal APD was also tested to evaluate the effect modification of body habitus on internal APD in relation to one-third external APD. Association between patient characteristics and internal APD was studied by simple and multiple linear regression. All statistical analyses were conducted by using Stata/SE 17.0 (StataCorp LLC, USA) with two-sided tests of a 5% significance level.
Results
In this study, a total of 978 chest CT scans (700 scans of children and adolescents 1 to <18-years-old, and 278 scans of infants) were reviewed. 85 scans for infants and 302 scans for children and adolescents were rejected due to chest wall deformities (pectus excavatum, pectus carinatum, and thoracic masses), or due to inability to identify chest borders or landmarks on the CT scans, or did not have recent weight or length/height documented within 2 weeks of the chest CT scans.
Demographic characteristics
A total of 591 chest CT scans of 193 scans for infants, and 398 children and adolescents 1 to <18-years-old) were included for analysis. Patient characteristics such as weight, length (infants)/height (children and adolescents), and gender-specific WFL (infants)/BMI (children and adolescents), and other demographic data (age, gender, and race) are described in Table 1. The studied infant and child populations both approximated normal distribution.
Table 1.
Demographic characteristics of patients.
| Patient characteristics | Infants (<1 year) (n = 193) | Children (≥1 year) (n = 398) |
|---|---|---|
| Age | ||
| Age (years) for children, mean (SD, range) | 8.55 (4.94, 1–17) | |
| Age distribution for children, n (%) | ||
| 1–12 years | 265 (66.6) | |
| > 12 to <18 years | 133 (33.4) | |
| Gender, n (%) | ||
| Female | 86 (44.6) | 197 (49.5) |
| Male | 107 (55.4) | 201 (50.5) |
| Race, n (%) | ||
| Chinese | 96 (49.7) | 253 (63.6) |
| Malay | 43 (22.3) | 72 (18.1) |
| Indian | 23 (11.9) | 34 (8.5) |
| Others | 31 (16.1) | 39 (9.8) |
| Weight (kg), mean (SD) | 5.22 (2.21) | 29.63 (16.94) |
| Length/Height (meters), mean (SD) | 0.58 (0.09) | 1.26 (0.30) |
| Gender specific weight-for-length for infants, n (%) | ||
| < 5th percentile | 28 (14.5) | |
| 5th − 95th percentile | 151 (78.2) | |
| > 95th percentile | 14 (7.3) | |
| Age and gender specific BMI for children, n (%) | ||
| Underweight (<5th percentile) | 78 (19.6) | |
| Normal (5th –< 85th percentile) | 258 (64.8) | |
| Overweight (85th –< 95th percentile) | 39 (9.8) | |
| Obese (≥95th percentile) | 23 (5.8) |
Abbreviations: BMI, body mass index; SD, standard deviation.
Concordance between relative and absolute chest compression depth targets by body habitus
The mean infants’ one-third external APD was 33.96 mm (SD 4.70), and there was no significant difference between WFL categories. Overall, only 32.6% of the infant cohort’s one-third external APD was “approximately 40 mm” (in the 36 mm to 44 mm range), and this was similar for all WFL categories. (Table 2)
Table 2.
Concordance of one-third external APD measurements with absolute chest compression depth targets by body habitus.
| 1/3 external APD (mm) |
Patients whose 1/3 external APD were concordant with absolute chest compression depth targets (4 cm ± 10% in infants, 5 cm ± 10% in children, 5–6 cm in adolescents) |
||||||
|---|---|---|---|---|---|---|---|
| Age group and body habitus | n (%) | Mean (SD) | Difference (95% CI) | p | n (%) | Difference$ (95% CI) | p |
| Infants (<1 year) | 193 | 33.96 (4.70) | – | – | 63 (32.6) | – | – |
| WFL | |||||||
| 5th − 95th percentile | 151 (78.2) | 34.24 (4.62) | Ref | – | 52 (34.4) | Ref | – |
| < 5th percentile | 28 (14.5) | 32.40 (5.16) | −1.85 (−3.76 to 0.06) | 0.058 | 7 (25.0) | −9.4 (−27.2 to 8.3) | 0.329 |
| > 95th percentile | 14 (7.3) | 34.04 (4.30) | −0.20 (−2.74 to 2.33) | 0.875 | 4 (28.6) | −5.9 (−30.7 to 19.0) | 0.774 |
| Children (1–12 years) | 265 | 47.24 (6.88) | – | – | 119 (44.9) | – | – |
| BMI | |||||||
| Normal (5-<85th percentile) | 166 (62.6) | 46.58 (6.06) | Ref | – | 76 (45.8) | Ref | – |
| Underweight (<5th percentile) | 55 (20.8) | 45.15 (4.64) | −1.42 (−2.97 to 0.13) | 0.072 | 21 (38.2) | −7.6 (–22.5 to 7.3)) | 0.325 |
| Overweight/Obese (≥85th percentile) | 44 (16.6) | 52.33 (9.43) | 5.76 (2.75–8.76) | < 0.001 | 22 (50.0) | 4.2 (−12.4 to 20.8) | 0.618 |
| Adolescents (>12 years) | 133 | 60.94 (7.10) | – | – | 55 (41.4) | – | – |
| BMI | |||||||
| Normal (5–<85th percentile) | 92 (69.2) | 60.55 (4.83) | Ref | – | 38 (41.3) | Ref | – |
| Underweight (<5th percentile) | 23 (17.3) | 54.35 (6.21) | −6.20 (−8.57 to −3.83) | < 0.001 | 16 (69.6) | 28.3 (6.9–49.6) | 0.015 |
| Overweight/Obese (≥85th percentile) | 18 (13.5) | 71.35 (6.47) | 10.80 (8.18–13.42) | < 0.001 | 1 (5.6) | −35.7 (−50.4 to −21.1) | 0.004 |
$ Difference in percentage of concordance.
Abbreviations: APD, anterior-posterior diameter; BMI, body mass index; CI, confidence interval; SD, standard deviation; WFL, weight-for-length.
For children 1–12 years old, there was significant difference between the mean one-third external APDs of overweight/obese children 52.33 mm (SD 9.43) compared to the normal 46.58 mm (SD 6.06) and underweight 45.15 mm (SD 4.64) cohorts, p < 0.001. Overall, 44.9% of the cohort’s one-third external APD was “approximately 50 mm” (in the 45 mm to 55 mm range), and this was not significantly different between the BMI categories.
There was a difference of 17.00 mm in the mean one-third external APDs between the underweight [54.35 mm (SD 6.21)] and overweight/obese [71.35 mm (SD 6.47)] adolescents > 12 years old. The majority of overweight/obese adolescents were above the upper limit of adult chest compression depth target (60 mm).
The concordance between the one-third external APD and age-specific chest compression targets can be visualised in Fig. 1.
Fig. 1.
Box plot of one-third external APD according to age and body habitus category.
Correlations of body habitus with chest dimensions
The correlations of internal and one-third external APDs by age and body habitus were demonstrated in Table 3.
Table 3.
Correlations of internal and one-third external APD measurements by body habitus.
| Internal APD (mm) |
1/3 external APD (mm) |
For every 10 mm increase in internal APD, change in 1/3 external APD (mm) (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Age group and body habitus | n (%) | Mean (SD) | Difference$ (95% CI) | p | Mean (SD) | Difference$ (95% CI) | p | |
| Infants (<1 year) | 193 | 57.54 (8.13) | – | – | 33.96 (4.70) | – | – | |
| WFL | ||||||||
| 5th − 95th percentile | 151 (78.2) | 57.75 (8.24) | Ref | – | 34.24 (4.62) | Ref | – | 4.85 (4.40–5.29) |
| < 5th percentile | 28 (14.5) | 55.95 (8.03) | −1.80 (−5.13 to 1.53) | 0.288 | 32.40 (5.16) | −1.85 (−3.76 to 0.06) | 0.058 | 4.85 (4.40–5.29) |
| > 95th percentile | 14 (7.3) | 58.50 (7.19) | 0.75 (−3.75 to 5.25) | 0.741 | 34.04 (4.30) | −0.20 (−2.74 to 2.33) | 0.875 | 4.85 (4.40–5.29) |
| Children (1–12 years) | 265 | 75.15 (10.96) | – | – | 47.24 (6.88) | – | – | |
| BMI | ||||||||
| Normal (5–<85th percentile) | 166 (62.6) | 74.17 (10.20) | Ref | – | 46.58 (6.06) | Ref | – | 5.25 (4.79–5.71) |
| Underweight (<5th percentile) | 55 (20.8) | 74.83 (10.06) | 0.66 (−2.46 to 3.77) | 0.679 | 45.15 (4.64) | −1.42 (−2.97 to 0.13) | 0.072 | 4.06 (3.24–4.88) |
| Overweight/Obese (≥85th percentile) | 44 (16.6) | 79.25 (13.80) | 5.08 (0.62–9.54) | 0.026 | 52.33 (9.43) | 5.76 (2.75–8.76) | < 0.001 | 6.02 (5.35–6.69) |
| Adolescents (>12 years) | 133 | 91.85 (12.21) | – | – | 60.94 (7.10) | – | – | |
| BMI | ||||||||
| Normal (5–<85th percentile) | 92 (69.2) | 92.18 (11.26) | Ref | – | 60.55 (4.83) | Ref | – | 2.83 (2.17–3.49) |
| Underweight (<5th percentile) | 23 (17.3) | 83.29 (12.71) | −8.90 (−14.23 to −3.56) | 0.001 | 54.35 (6.21) | −6.20 (−8.57 to −3.83) | < 0.001 | 2.83 (2.17–3.49) |
| Overweight/Obese (≥85th percentile) | 18 (13.5) | 101.09 (8.88) | 8.91 (3.33–14.49) | 0.002 | 71.35 (6.47) | 10.80 (8.18–13.42) | < 0.001 | 2.83 (2.17–3.49) |
$ Difference between mean measurements compared to normal body habitus group
Abbreviations: APD, anterior-posterior diameter; BMI, body mass index; CI, confidence interval; SD, standard deviation; WFL, weight-for-length.
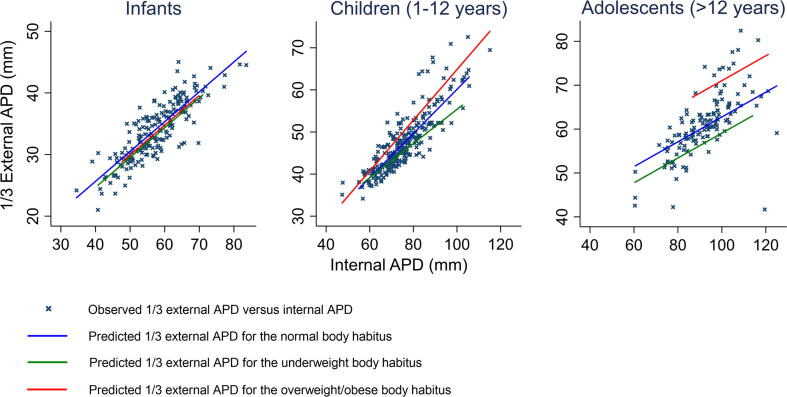
For infants, there were no significant difference between the internal and one-third external diameters across body habitus categories. Overweight/obese children’s and adolescents’ internal and one-third external APDs were significantly bigger than their underweight and normal BMI counterparts. However, the internal and external APDs of underweight adolescents, but not underweight children, were significantly different from their normal BMI counterparts. There was a co-dependent relationship between external (and hence one-third external APD) and internal APDs across all age groups and body habitus indices. (Table 3 and Fig. 2)
Fig. 2.
Scatter plot of one-third external APD against internal APD for infants, children (1–12 years) and adolescents (>12 years).
Body habitus as independent predictors of internal anterior-posterior diameters (APD) at the lower half of the sternum
Gender-specific WFL of infants was not significantly associated with internal APD in both unadjusted (p = 0.507) and adjusted analyses (0.511). (Supplementary Table 1) Similarly, race was not significantly associated with internal APD in the unadjusted (p = 0.710) and adjusted analyses (p = 0.713).
In children and adolescents ≥ 1 to < 18 years-old, age, race, and age- and gender specific-BMI were found to have significant associations with internal APD in unadjusted analysis but only BMI remained significant in the adjusted analyses (Table 4).
Table 4.
Association between patient characteristics and internal APD at lower half of the sternum for children 1 to < 18 years-old by linear regression.
| Patient characteristics | Univariate |
Multivariate for age and gender specific BMI |
||
|---|---|---|---|---|
| Unadjusted b-estimate (95% CI) | p | Adjusted b-estimate (95% CI) | p | |
| Age | ||||
| 1–12 years | Ref | – | ||
| > 12 years | 16.70 (14.32–19.08) | < 0.001 | ||
| Gender | ||||
| Female | Ref | – | ||
| Male | 1.91 (−0.81 to 4.64) | 0.169 | ||
| Race | 0.029 | 0.113 | ||
| Chinese | Ref | – | Ref | – |
| Malay | −1.31 (−4.91 to 2.30) | 0.477 | −1.24 (−4.83 to 2.34) | 0.496 |
| Indian | 6.35 (1.42–11.29) | 0.012 | 5.10 (0.12–10.08) | 0.045 |
| Others | −2.44 (−7.08 to 2.21) | 0.303 | −2.00 (−6.62 to 2.61) | 0.394 |
| Age and gender specific BMI | 0.005 | 0.009 | ||
| Normal (5–<85th percentile) | Ref | – | Ref | – |
| Underweight (<5th percentile) | −3.27 (−6.75 to 0.20) | 0.065 | −3.02 (−6.49 to 0.46) | 0.089 |
| Overweight/Obese (≥85th percentile) | 5.81 (1.19–10.43) | 0.014 | 4.25 (0.39–8.10) | 0.031 |
Abbreviations: APD, anterior-posterior diameter; BMI, body mass index; CI, confidence interval.
Subgroup analysis was done to investigate the BMI correlations between those in the 1-to-12 years old and those above 12-years-old. (Supplementary Table 2) Similarly, age- and gender-specific BMI remained as an independent predictor of internal APD at the lower half of the sternum in the adjusted analyses for the age subgroups of 1-to-12-years-old (p = 0.038) and > 12-years-old p < 0.001). Race was not an independent predictor of internal APD for children and adolescents for the age subgroups of 1-to-12-years-old (p = 0.292) and >12-years-old p = 0.284).
Discussion
Since the introduction of CPR, the guidelines on the chest compression depth have been evaluated and reviewed based on experts’ consensus, body measurements from computed tomography (CT) chest, extrapolation from adult data, and observational paediatric studies.17., 18., 1., 2., 3., 4., 5. The two observational paediatric studies17., 18. that informed current absolute paediatric chest compression depth target recommendations, were based on single-sensor cardiopulmonary resuscitation (CPR) quality monitoring devices that could have potentially over-estimated absolute compression depths. Due to the limitations of available technology, single-sensors used in these two studies to obtain absolute compression depth could not correct for mattress deflection, and patient movement during CPR. Based on the available evidence, it was thus assumed that the use of relative or absolute chest compression depth targets were both appropriate and were likely to be similar. Our study, however, suggested otherwise.(Table 2 and Fig. 1)
There were also minor variations in the chest compression depth target recommendations across resuscitation councils. Current International Liaison Committee on Resuscitation, American Heart Association, and European Resuscitation Council paediatric compression guidelines recommend chest compressions to be rendered “at least” one-third external chest depth or age-specific chest compression depth targets, while other councils (Singapore, Japan, Australian and New Zealand Committee on Resuscitation) recommend chest compressions to be “approximately” one-third external APD.9., 3., 4., 5.. Similarly, most resuscitation councils recommend age-specific chest compression depth targets of approximately 40 mm in infants, approximately 50 mm in younger children, and 50–60 mm in adolescents and adults. Our study demonstrated that the majority of paediatric patients’ one-third external APD were not concordant with age-specific chest compression depth targets, especially for most infants and nearly all overweight/obese adolescents.
For our infant population, the mean one-third external APD was less than 40 mm (current absolute chest compression target for infants), irrespective of body habitus (gender-specific WFL). WFL also did not appear to influence one-third external APD or internal APD. This could suggest that absolute chest compression targets may be useful in infants as body habitus did not appear to influence internal APD. However, it is unknown if “approximately 40 mm” is the optimal absolute chest compression depth target, especially since there were no prior clinical studies that validated this. Our primary study suggested that simulated chest compressions ≥ 40 mm and beyond one-third external APD would result in potential over-compression in a significant proportion of the infant cohort.9 The current chest compression depth targets of approximately 40 mm compared to relative chest compression depths (approximately one-third external APD) should be clinically validated as they were found to be significantly discordant in our study.
Only 38% (underweight) to 50% (overweight/obese) of the children’s mean one-third external APD were concordant with “50 ± 10 % mm”. The internal and one-third external APDs of overweight/obese children 1–12 years old were significantly different from the underweight and normal weight groups but the clinical significance of this is unknown. However, most of the overweight/obese adolescents > 12 years olds’ one-third external APD were beyond the 60 mm chest compression limit.
Internal APD below the chest compression landmark is the potential space for cardiac compression to occur. Under- or over-compression are influenced by chest compression depth achieved in relation to internal APD. We found that internal and external (hence one-third external) APDs were co-dependent across all age groups and body habitus categories.(Table 3 and Fig. 2) This is not surprising as external APD is a composite of internal APD and anterior and posterior chest wall (External APD = internal APD + anterior and posterior chest wall thickness). What was interesting was that our study found that age- and gender-specific BMI was an independent predictor of internal APD at the chest compression landmark. (Table 4 and Supplementary Table 2) This suggested that BMI does not affect the chest wall thickness alone (fat, muscles) but also internal APD. It would appear that BMI of children and adolescents could potentially influence the optimal chest compression depth especially for overweight/obese paediatric patients especially adolescents.
Underweight adolescents, however, had significantly smaller internal APD; especially when compared to overweight/obese adolescents (mean difference of 17.80 mm).(Table 3) In contrast, the absolute chest compression depth target for adolescents has a more limited 10 mm difference (50–60 mm), which may not consider these differences in mean internal APD (potential space for heart to be compressed) at the extremes of BMI.
The co-dependence of external APD (and correspondingly, one-third external APD) and internal APD may suggest that the use of relative chest compression depth may potentially “adjust” for the differences in the body habitus, especially for overweight/obese adolescents. (Table 3 and Fig. 2) However, it is unknown if one-third external APD is appropriate in the adolescent age group. While there have been published radiological studies, albeit limited, in the adult population, on how body habitus may potentially affect chest compressions, this was only study, to our knowledge, that looked into this in an heterogenous Asian paediatric population.6., 13. Lee et al 2019, reported in a technically similar adult chest CT study, that recommended chest compression depth targets of 50–60 mm may be inadequate for obese adults.6
While we could not derive what the optimal paediatric chest compression depth was, based on the limitations of a radiological study, our findings suggested that high BMI may potentially affect optimal chest compression depth in paediatric cardiac arrest patients. Specifically, paediatric populations with high BMI, especially the overweight/obese adolescents, may require deeper chest compressions, not just because of their large chest wall sizes, but also because they had a relatively larger internal chest cavity size at their compression site at the lower half of the sternum. Current paediatric chest compression depth targets using absolute depth recommendations do not consider this.
However, for infants, chest cavity sizes may be fairly similar irrespective of gender-specific WFL categories. Weight-for-length (WFL) is traditionally used in infants to evaluate body habitus, growth, and body composition.9., 10., 11. However, there have been recent observations which suggested that infant BMI correlated well with WFL but may also be a better body habitus indicator for infants9., 10., 11., 12., 14., 15., 16.. However, the use of BMI for infants as a validated body habitus index has not been universally endorsed and accepted, thus we did not specifically evaluate infant BMI in this study.
There were concerns that race could be a potential factor in considering the external validity of optimal paediatric chest compression depth targets which were based mainly on North American studies.17., 18. However, we have demonstrated that at least within a multi-racial Asian population, race was not an independent predictor of internal APD at the lower half of the sternum, after adjusting for BMI. We were not able to find other published paediatric literature on racial correlations of internal APD.
In the adult cardiac arrest studies, observational studies have reported conflicting data on how body habitus, specifically BMI (both underweight and obesity) might affect survival and neurological outcomes.19., 20., 21., 22., 23., 24., 25., 26. In the paediatric population, there was only one published observational study in 2010 which suggested that obesity was associated with poorer prognosis in cardiac arrest patients.8 Our study may suggest a potential basis for this but we recognise that body habitus may affect not only chest compression effectiveness but there may be associated pre-morbid associations and post cardiac arrest processes which might be associated with extremes of BMI.
We provided evidence on the poor concordance of one-third external APD and age-specific chest compression depth across all age and body habitus categories. We also demonstrated that there was co-dependent relationship between internal and external APDs. Finally, we showed how body habitus indices such as age- and gender- specific BMI of children and adolescents, and indices that do not such as WFL in infants, were independently predictive of internal chest cavity size at the chest compression landmark and may suggest that this may be a potential consideration that could influence chest compressions’ adequacy in paediatrics. It remains speculative if this has any significant clinical implications, specifically if current absolute chest compression depth targets may result in potential over-compression in a significant proportion of infants, and potential under-compression in overweight/obese adolescents. These may be areas for future clinical research, specifically, to validate current recommendations for chest approximately 40 mm in infants and if relative chest compression depth (compared to absolute chest depth targets) should be preferred chest compression depth target, especially for overweight/obese adolescents.
Limitations
For older children who are co-operative, adolescents or adults, chest CT scans are usually performed with the arms raised at end-inspiration. Adult patients undergoing CT scans often have arms raised at end-inspiration and there could be differences between the chest dimension measured during inspiration and expiration.27 This would be in contrast to patients undergoing cardiopulmonary resuscitation who have arms lowered and placed at the sides of the torso. However, infants, younger children, and critically ill and injured older children or adolescents (who are unable to follow instructions), may also have their arms by their side and in variable states of respiration. Furthermore, chest dimensions variations at the chest compression landmark at the end-inspiration and end-expiration tend to be smaller due to corresponding smaller tidal volumes in the paediatric population, especially for the infants and younger children. In our institution, we routinely use low tidal volumes (6–8 ml/kg) as part of our lung protection strategy for all intubated patients. Intubated patients were also likely have had their CT imaging done in varying phase of the respiratory cycle during scans and may not be end-inspiratory. In paediatric cardiac arrest, post-advanced airway placement, CPR is performed throughout the respiratory cycle. Of note, paediatric cardiac arrest victims whose arms are by their side have been reported to have approximately 5–10 compressions delivered during exhalation compared to inhalation.28 The findings our study would be applicable as paediatric patients in cardiac arrest may have had advanced airway placement (supraglottic devices or endotracheal intubation) with chest compressions rendered in varying phases of ventilation during chest compressions. Thus, the paediatric chest CT scans performed in our study were less likely to be consistently hyperinflated than reported in adult radiological studies.27
The chest CT of our study population usually had underlying medical conditions, hence their growth might have been affected causing their chest dimensions to be smaller than the chest dimensions in healthy children. However, ill patients are also more likely to receive chest compressions than the healthy population, thus our findings may still be relevant and generalisable.
The sample size of obese patients was limited and may require larger cohorts to validate the findings of our study. Further to these, our studied population was Singaporean and there may be limitations to its external validity in other international paediatric populations.
Conclusions
There was poor concordance between one-third external APD and age-specific chest compression depth across all age and body habitus categories, especially infants and overweight/obese adolescents. Overweight/obese children and adolescents with high BMIs had significantly larger internal APDs and one-third external APDs. As BMI in children and adolescents was an independent predictor of internal APD which determines optimal chest compression depth, clinical research is needed to evaluate and validate current chest compression depth target recommendations, especially for overweight and obese adolescents.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2022.100202.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Atkins D.L., Berger S., Duff J.P., et al. Part 11: Pediatric Basic Life Support and Cardiopulmonary Resuscitation Quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S519–S525. doi: 10.1161/CIR.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 2.Maconochie I.K., Bingham R., Eich C., et al. Paediatric life support section Collaborators. European Resuscitation Council Guidelines for Resuscitation 2015: Section 6. Paediatric life support. Resuscitation. 2015;95:223–248. doi: 10.1016/j.resuscitation.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 3.Topjian A.A., Raymond T.T., Atkins D., et al. Pediatric Basic and Advanced Life Support Collaborators. Part 4: Pediatric Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:S469–S523. doi: 10.1161/CIR.0000000000000901. [DOI] [PubMed] [Google Scholar]
- 4.Van de Voorde P., Turner N.M., Djakow J., et al. European Resuscitation Council Guidelines 2021: Paediatric Life Support. Resuscitation. 2021;161:327–387. doi: 10.1016/j.resuscitation.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Maconochie I.K., Aickin R., Hazinski M.F., et al. Pediatric Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2020;142:S140–S184. doi: 10.1161/CIR.0000000000000894. [DOI] [PubMed] [Google Scholar]
- 6.Lee H., Oh J., Lee J., et al. Retrospective Study Using Computed Tomography to Compare Sufficient Chest Compression Depth for Cardiopulmonary Resuscitation in Obese Patients. J Am Heart Assoc. 2019;8:e013948. doi: 10.1161/JAHA.119.013948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Cesare M., Sorić M., Bovet P., et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 2019;17:212. doi: 10.1186/s12916-019-1449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivasan V., Nadkarni V.M., Helfaer M.A., Carey S.M., Berg R.A. American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Childhood obesity and survival after in-hospital pediatric cardiopulmonary resuscitation. Pediatrics. 2010;125:e481–e488. doi: 10.1542/peds.2009-1324. [DOI] [PubMed] [Google Scholar]
- 9.Ong G.Y., Ang A.J.F., Aurangzeb A.S.O., et al. What is the potential for over-compression using current paediatric chest compression guidelines? A chest computed tomography study. Resusc Plus. 2021;27:100112. doi: 10.1016/j.resplu.2021.100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization Child Growth Standards – Weight-for-length. (Last accessed on 10th October 2021, at: https://www.who.int/tools/child-growth-standards/standards/weight-for-length-height).
- 11.Aris I.M., Rifas-Shiman S.L., Li L.J., et al. Association of Weight for Length vs Body Mass Index During the First 2 Years of Life With Cardiometabolic Risk in Early Adolescence. JAMA Netw Open. 2018;1:e182460. doi: 10.1001/jamanetworkopen.2018.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y.S., Biddle S., Chan M.F., et al. Health Promotion Board-Ministry of Health Clinical Practice Guidelines: Obesity. Singapore Med J. 2016;57:292–300. doi: 10.11622/smedj.2016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Secombe P., Sutherland R., Johnson R. Body mass index and thoracic subcutaneous adipose tissue depth: possible implications for adequacy of chest compressions. BMC Res Notes. 2017;10:575. doi: 10.1186/s13104-017-2918-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furlong K.R., Anderson L.N., Kang H., et al. BMI-for-Age and Weight-for-Length in Children 0 to 2 Years. Pediatrics. 2016;138:e20153809. doi: 10.1542/peds.2015-3809. [DOI] [PubMed] [Google Scholar]
- 15.Roy S.M., Fields D.A., Mitchell J.A., et al. Body Mass Index Is a Better Indicator of Body Composition than Weight-for-Length at Age 1 Month. J Pediatr. 2019;204 doi: 10.1016/j.jpeds.2018.08.007. 77.e1–83.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo J.G., Daniels S.R. Assessment of Body Mass Index in Infancy: It Is Time to Revise Our Guidelines. J Pediatr. 2019;204:10–11. doi: 10.1016/j.jpeds.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Sutton R.M., French B., Niles D.E., et al. 2010 American Heart Association Recommended Compression Depths During Paediatric In-hospital Resuscitations are Associated with Survival. Resuscitation. 2014;85:1179–1184. doi: 10.1016/j.resuscitation.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutton R.M., Case E., Brown S.P., et al. A quantitative analysis of out-of-hospital paediatric and adolescent resuscitation quality–A report from the ROC epistry-cardiac arrest. Resuscitation. 2015;93:150–157. doi: 10.1016/j.resuscitation.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C.T., Lin M.C., Lee Y.J., et al. Association between body mass index and clinical outcomes in out-of-hospital cardiac arrest survivors treated with targeted temperature management. J Chin Med Assoc. 2021;84:504–509. doi: 10.1097/JCMA.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 20.Sung C.W., Huang C.H., Chen W.J., et al. Obesity is associated with poor prognosis in cardiogenic arrest survivors receiving coronary angiography. J Formos Med Assoc. 2020;119:861–868. doi: 10.1016/j.jfma.2019.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Lee H., Oh J., Kang H., et al. Korean Cardiac Arrest Research Consortium (KoCARC) Investigators. Association between the body mass index and outcomes of patients resuscitated from out-of-hospital cardiac arrest: a prospective multicentre registry study. Scand J Trauma Resusc Emerg Med. 2021;29:24. doi: 10.1186/s13049-021-00837-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S.E., Kim H.H., Chae M.K., Park E.J., Choi S. Predictive Value of Estimated Lean Body Mass for Neurological Outcomes after Out-of-Hospital Cardiac Arrest. J Clin Med. 2020;10:71. doi: 10.3390/jcm10010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C.H., Chang W.T., Huang C.H., et al. Associations between Central Obesity and Outcomes of Adult In-hospital Cardiac Arrest: A Retrospective Cohort Study. Sci Rep. 2020;10:4604. doi: 10.1038/s41598-020-61426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matinrazm S., Ladejobi A., Pasupula D.K., et al. Effect of body mass index on survival after sudden cardiac arrest. Clin Cardiol. 2018;41:46–50. doi: 10.1002/clc.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y., Huang L., Zhang L., Yu H., Liu B. Association between body mass index and clinical outcomes of patients after cardiac arrest and resuscitation: A meta-analysis. Am J Emerg Med. 2018;36:1270–1279. doi: 10.1016/j.ajem.2018.03.079. [DOI] [PubMed] [Google Scholar]
- 26.Kakavas S., Georgiopoulos G., Oikonomou D., et al. The impact of body mass index on post resuscitation survival after cardiac arrest: A meta-analysis. Clin Nutr ESPEN. 2018;24:47–53. doi: 10.1016/j.clnesp.2018.01.071. [DOI] [PubMed] [Google Scholar]
- 27.Nozoe M., Mase K., Tsutou A. Regional chest wall volume changes during various breathing maneuvers in normal men. J Jpn Phys Ther Assoc. 2011;14:12–18. doi: 10.1298/jjpta.Vol14_002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niles D.E., Duval-Arnould J., Skellett S., et al. paediatric Resuscitation Quality (pediRES-Q) Collaborative Investigators. Characterization of Paediatric In-Hospital Cardiopulmonary Resuscitation Quality Metrics Across an International Resuscitation Collaborative. Pediatr Crit Care Med. 2018;19:421–432. doi: 10.1097/PCC.0000000000001520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.