Abstract
Objectives
Faecal immunochemical tests (FIT) for the detection of haemoglobin are widely used in the diagnostic pathway of colorectal cancer (CRC) in patients presenting with lower abdominal symptoms. Several quantitative immunochemical tests are available, however only a few tests are available on high throughput automated analyzers. Here we report the analytical and clinical evaluation of the Sentinel-FOB Gold assay on the Roche Cobas 8000 automated system.
Design
and Methods: The assessment of the analytical performance of the assay on the Roche Cobas analyzer comprised determination of imprecision, accuracy, limit of detection, linearity, carryover and prozone effect. In the clinical evaluation part of the study 163 patients presenting for coloscopy with lower abdominal symptoms were included in the study. Diagnostic accuracy and optimal cutoff of the FIT-assay were determined by comparing faecal haemoglobin (f-Hb) concentrations with coloscopy findings as reference.
Results
We confirmed good analytical performance of the assay on the Roche Cobas system. Patients with CRC had significant higher f-Hb concentrations than patients with non-advanced adenomas and patients with normal findings by coloscopy. When applying a cutoff of 10 μg/g the sensitivity and specificity were 96.2% and 51.8%, respectively for CRC and 84.6% and 57.7% for CRC and advanced adenoma.
Conclusion
The Sentinel-FOB Gold assay is appropriate for application on the automated Roche Cobas 8000 analyzer. The assay shows good analytical performance on the system. With regard to early detection of significant colorectal diseases a cutoff of 10 μg/g seems to be optimal.
Keywords: Colorectal cancer, Evaluation, Faecal haemoglobin, Faecal immunochemical test
Highlights
-
•
The SENTIFIT-FOB Gold assay for the quantitation of haemoglobin in faeces was evaluated on the widely used automated Roche Cobas 8000 system.
-
•
Good analytical and clinical performance were confirmed.
-
•
The diagnostic accuracy was assessed and the optimal cutoff for use in symptomatic patients was determined.
1. Introduction
Colorectal cancer (CRC) is the third most common cancer in men and women and has the second highest mortality rate worldwide [1]. In Norway, it is the second highest frequent cancer diagnosis after prostate cancer in men and breast cancer in women, with more than 4400 new individuals every year [2]. Most of the new CRC cases are found in patients presenting with symptoms in primary health care, such as changes in bowel habit and red blood in the faeces [3]. Unfortunately many patients do not have any specific clinical symptoms in spite of having a CRC, especially in early stages of the disease. Symptoms like iron deficiency anemia and abdominal pain are indicative but not specific for CRC, and can be a sign of benign diseases, too. Therefore, laboratory tests for detection of haemoglobin in faeces (f-Hb) are recommended as an aid to guide referral to secondary care in symptomatic patients [4,5].
Faecal immunochemical tests (FIT) for detection and quantification of f-Hb are well established and have replaced traditional guaiac-based tests in national screening programmes in most European countries [6]. The first study of diagnostic accuracy of FIT in the assessment of patients presenting with lower abdominal symptoms was conducted by McDonald et al. [7]. On the basis of available evidence FIT tests are the best currrently available non-invasive tests for rule-in of CRC and rule-out significant colon diseases in patients presenting in primary care with lower abdominal symptoms [[8], [9], [10]]. Quantitative FIT give a numerical result and allow to adjust for the optimal cutoff.
Our hospital in Western Norway serves 200 000 inhabitants. A quantitative FIT was introduced in 2019 and is used to triage referral to secondary care. The f-Hb concentrations are being used by gastroenterologists to assess the urgency of coloscopy.
Several quantitative FIT tests for detection of f-Hb are available, mostly on small and less automated analyzers.
For most of the assays, studies of diagnostic accuracy and clinical utility are lacking. The 2017 NICE guidelines recommend three quantitative FIT tests to guide referral to secondary care for suspected CRC in symptomatic patients without rectal bleeding [5]. The recommended assays are designed for application on manufacturers benchtop analyzers.
Recently the Sentinel-FOB Gold test was evaluated on a SENTIFIT 270 analyzer [11,12]. To our knowledge this benchtop-analyzer is rarely available in routine clinical chemical laboratories. Therefore, application of the Sentinel-FOB Gold test on an widely used automated high throughput analyzer will be more efficient and more attractive for many laboratories. Analyzers and assays from different manufacturers perform different and thoroughly evaluation is necessary prior to use.
Here we report the analytical and diagnostic evaluation of the Sentinel-FOB Gold assay on the Roche Cobas 8000 system. The aim of the study was to assess the analytical performance and to assess the diagnostic accuracy of the assay to find the optimal cutoff for use in patients with suspected CRC in primary care.
2. Materials and methods
2.1. Study design and patients
The study was performed between January 2020 and February 2021 at the Ålesund hospital, Norway. In the clinical evaluation part of the study 163 symptomatic patients over 18 years referred to coloscopy for assessment of lower abdominal symptoms were included. Invitation to coloscopy together with a collection device and detailed instructions for sampling of faeces was sent by mail 2-4 weeks before meeting for coloscopy. Patients were asked to collect a faeces sample 3-7 days before coloscopy. There were no dieatary restrictions before sampling. Medication such as anticoagulants were not discontinued for diagnostic coloscopy according to the BSG and ESGE guidelines [13].
All individuals participating in the study signed an informed consent agreement. The study was conducted in accordance with the hospitals ethic guidelines.
2.2. Samples and analyzer
Faecal samples were collected in FOB Gold sample tubes (Sysmex, Oslo, Norway). The sample devices include 1.7 mL stabilizing buffer to prevent degradation of haemoglobin. For sampling participants were instructed to insert the collection picker in two different places and to scrape the surface of the sample in a cross-wise motion. After sampling, tubes were stored refrigerated until hospital admisson. Samples were collected continuously during the study period, centrifuged according to manufacturers instructions and analyzed in batch at the end of the day. The tubes were placed manually onto the analyzer.
Samples were analyzed with the Sentinel-FOB Gold test (Sentinel Diagnostics, Milano, Italy, distributed by Sysmex, Oslo, Norway), a latex agglutination immunoturbidimetric assay for quantitation of haemoglobin in faeces. The assay was applied on an open channel on the Roche Cobas 8000 c702 automated analyzer (Roche Diagnostics, Oslo, Norway) using manufactures application protocol. The time of analysis on the Roche Cobas 8000 system is 10 min. For calibration FOB Gold NG Wide calibrator (ref. nr. 1156009, Sysmex, Oslo, Norway) was used. A conversion factor of 0.17 recommended by the manufacturer was applied to convert the haemoglobin concentration in the buffer (μg/L buffer) to haemoglobin concentration i faeces (μg/g faeces). The manufacturer states that the measuring range of the assay is 2-170 μg/g. Samples with haemoglobin concentrations higher than the upper limit of the measuring range were diluted and reanalyzed. Our laboratory used a cutoff of 10 μg/g [5].
The laboratory is accredited according to EN ISO 15189:2014. The analytical performance characteristics of the method were monitored daily using quality control samples and biannually using the RfB proficiency testing scheme (Referenzinstitut für Bioanalytik, Bonn, Germany).
2.3. Analytical imprecision, accuracy and limit of detection
Between-run imprecision was evaluated using control samples (Sysmex, Oslo, Norway) with haemoglobin concentrations at 17 μg/g and 57 μg/g and a patient sample pool at 19 μg/g. Control samples were stored at 2-8 °C and analyzed once a day on 26 consecutive days using separate aliquots. Mean concentration, standarddeviation (SD), coefficient of variation (CV%) and bias in relation to manufactures target were calculated for assessing analytical variation and accuracy.
The patient sample pool was stored at 2-8 °C and analyzed once a day on 20 consecutive days.
Within-run imprecision was assessed by measuring 20 replicates of two patient sample pools in one run. Mean concentration, SD and CV% were calculated.
Limit of detection (LoD) was determined by using both the measured limit of blank (LoB) and test replicates of a sample containing a low concentration of haemoglobin [14]. The highest concentration standard was diluted with buffer to a final concentration of 6 μg/g and analyzed with 20 replicates. In addition, a patient sample with low f-Hb concentration was analyzed with 20 replicates. Mean and SD were calculated. LoB was estimated by measuring 20 replicates of buffer and calculated as LoB = mean + 1.645 * SD (buffer). LoD was calculated as LoD = LoB +1.645 * SD (low conc. sample) for both the buffer spiked with standard and the patient sample [15].
2.4. Linearity, carryover and prozone effect
Linearity was assessed by polynomial regression using diluted samples of 5 different concentrations [16]. A patient sample pool with a high f-Hb concentration was diluted with a patient sample pool with a low F-Hb concentration generating five different concentrations in equidistant intervals covering a concentration intervall of 3-195 μg/g. Each sample was analyzed in duplicate. A p-value <0.05 was judged to be statistically significant.
Carryover was assed using two patient samples: one patient sample with a low f-Hb concentration of 3 μg/g and one patient sample with a very high f-Hb concentration (1700 μg/g). The low concentration sample was divided into 10 aliquots (L) and the high concentration sample was devided into 5 aliquots (H). All samples were analyzed in one run in the following order: L L L L L H L H L H L H L H L. The mean of the first five low concentration aliquots was compared with the mean of the five low concentration aliquots analyzed directly after the high concentration aliquots using Students t-test. Maximal allowable deviation was defined as twice the standard deviation of the first five low concentration measurements [11].
The presence of prozone effect was tested using five samples containing extremely high Hb-concentrations (272-3000 μg/g). The samples were prepared by adding different volumes of a haemoglobin lysate stock solution into manufacturers sample buffer generating five samples with different f-Hb concentrations. The haemoglobin concentration of the lysate stock solution was determined on a Symex XN-2000 hematology analyzer.
2.5. Endoscopy
All endoscopic findings were categorized. If polyps were present, sites were recorded and polyps removed. Polyps were examined histologically and the size and type of each polyp were recorded according to the European Society of Gastrointestinal Endoscopy guidelines [17]. Coloscopy findings were categorized as carcinoma (CRC), advanced ademona (AA), non-advanced adenoma (NAA), inflammatory bowel disease (IBD) and minor findings (other). Advanced adenoma was defined according to at least one of the following: ≥10 mm diameter, high grade dysplasia, tubullovillous or villous growth pattern, ≥3 adenomas, serrated polyps with dysplasia, or serrated polyps ≥10 mm diameter. Non-advanced adenoma was defined as 1-2 tubular adenomas with low grade dysplasia and <10 mm diameter. Minor findings are hemorrhoids, diverticulitis and bleeding of unknown reasons and were grouped as « other». Patients with no findings in coloscopy were classified as normal.
2.6. Statistics
Using a cutoff of 10 μg/g patients were classified as positive or negative based on f-Hb concentration. The distributions of f-Hb concentrations were positively skewed in all groups. Differences between groups’ f-Hb concentrations were assessed using Kruskal Wallis rank sum test. Significance values were adjusted by post hoc Bonferroni correction for multiple tests. A two-tailed p-value of <0.05 was judged to be statistically significant. Sensitivity, specificity, negative predictive values and positive predictive values at different cutoffs were calculated for the «CRC » group and the «CRC + AA » group. Results below the LoD were replaced with a value equal to halv the LoD [18].
Statistical analyses were performed using IBM SPSS Statistis software (version 27, IBM Corp), Analyze-it for Excel (Analyze-it Software Ltd., Leeds, UK) and Sigmaplot software (Sigmaplot Software Inc., San Jose, CA, USA).
3. Results
3.1. Analytical evaluation
The Sentinel-FOB Gold assay showed an imprecision with a total coefficient of variation (CV%) <10% at all concentrations (mean ± SD) of quality control material and patient sample pools analyzed. At average f-Hb concentrations of 24 ± 0.8 μg/g and 58 ± 2.3 μg/g in patient sample pools the within-run imprecision (repeatability) was 3.4% and 3.9%, respectively. The between-run imprecision (reproduceability) in the patient sample pool at f-Hb concentration of 24 μg/g was 2.8%. Using control samples at average haemoglobin concentrations of 16 ± 1.0 μg/g and 58 ± 5.0 μg/g between-run imprecision was 6.1% and 8.6%, respectively.
The accuracy of the assay was performed by comparison of the mean quality control sample haemoglobin concentration with the target specified by the manufacturer. The deviation of the mean from the specified target concentration was <4% in both control samples.
The LoB and LoD were estimated to 1 μg/g and 2 μg/g, respectively when using buffer spiked with standard. Using a real patient sample with low f-Hb concentration the LoD was 2 μg/g.
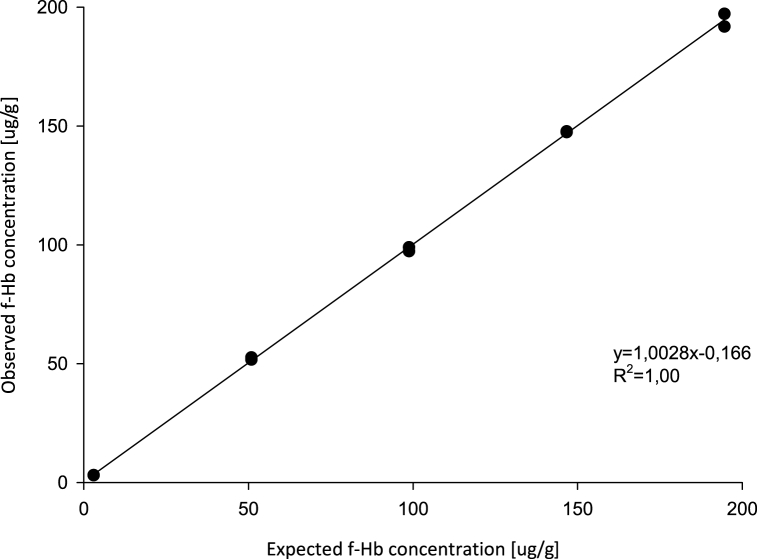
Visual inspection of the linearity plot showed good linearity in the measuring range 3-170 μg/g (Fig. 1). Polynomial regression analysis showed no statistically significant nonlinear terms of higher order. The recovery of the f-Hb concentrations was between 97.6% (minimum) and 100.7% (maximum).
Fig. 1.
Linear regression of observed and expected f-Hb concentration after dilution.
Carryover was assessed by analyzing aliquots of a low and a high f-Hb concentration sample in a defined order. No significant difference between the mean of the low f-Hb concentrations (12 ± 0.5 μg/g) and the mean of the low f-Hb concentrations after high concentration samples (12 ± 0.3 μg/g) was observed. All low f-Hb concentrations after high concentration samples fullfill the performance specifications of maximum allowable deviation (12 ± 1.0 μg/g).
The Sentinel-FOB Gold assay showed no prozone effect up to a f-Hb concentration of 3000 μg/g. No numerical results were reported. The user was alerted and advised to dilute the samples.
3.2. Clinical performance
Of the 163 patients referred to coloscopy 36 (22.1%) had no findings and 127 (77.9%) pathologic findings by coloscopy. 104 patients (63.8%) had f-Hb concentraions above the cutoff of 10 μg/g and 59 patients (36.2%) had f-Hb concentrations below the LoD of the assay.
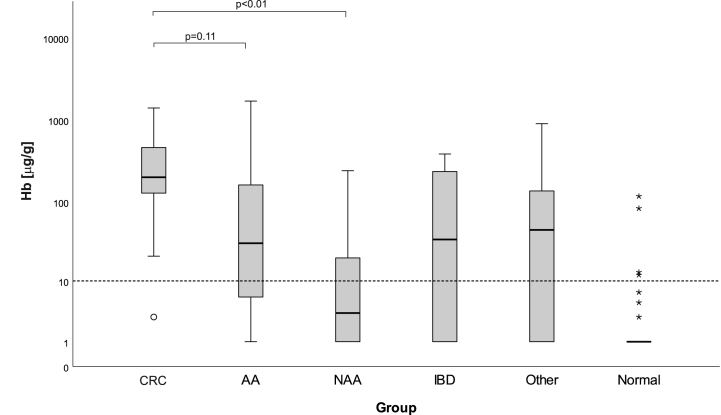
Using coloscopy as gold standard patients were classified into six groups: 26 (15.9%) patients had CRC, 26 (15.9%) had AA, 24 (14.7%) had NAA, 16 (9.8%) had IBD, 35 (21.4%) had other findings and 36 (22.1%) had no findings (normal). Patients with detected CRC had a median f-Hb concentration of 200 μg/g (Table 1). The median f-Hb concentration of patients classified as normal by coloscopy was below the LoD of the assay. Patients with CRC and AA had significant higher haemoglobin concentrations than patients classified as normal. For the cancer group a significant difference in median f-Hb concentration was found in relation to NAA, but no significant difference was found between CRC and AA. Fig. 2 shows the median and interquartile range of f-Hb concentration in the different groups. Using a cutoff of 10 μg/g four patients with no finding by coloscopy were classified as « positive » by quantitative FIT assay. One patient with CRC was missed because of a f-Hb concentration below 10 μg/g which was classified as « negative».
Table 1.
Faecal hemoglobin concentration [μg/g] according to coloscopy finding.
| Group | Number | Median [μg/g] | IQR [μg/g] | p-value |
|---|---|---|---|---|
| CRC | 26 | 200.0 | 123-473 | <0.01 |
| AA | 26 | 31.5 | 6-177 | <0.01 |
| NAA | 24 | 3.5 | 1-21 | 1.00 |
| IBD | 16 | 35.5 | 1-257 | 0.02 |
| Other | 35 | 45.5 | 1-136 | <0.01 |
| Normal | 36 | 1.0 | <LoD |
A p-value <0.05 is considered statistically significant. P-values are shown in relation to « normal». IQR, Interquartile range; CRC, colorectal cancer; AA, advanced adenoma; NAA, non-advanced adenoma; IBD, inflammatory bowel disease.
Fig. 2.
Faecal haemoglobin concentration in relation to coloscopy finding (CRC, colorectal cancer; AA, advanced adenoma; NAA, non-advanced adenoma; IBD, inflammatory bowel disease). 163 patients were included. The dashed line represents a cutoff of 10 mg/g. Outlier <3 x IQR (Interquartile range) are shown as circle and >3 x IRQ as asterisk.
The diagnostic accuracy of the Sentinel-FOB Gold test on the Roche Cobas 8000 system was evaluated by comparing f-Hb concentrations with coloscopy findings as gold standard. CRC was defined as the target condition and sensitivity and specificity calculated at different cutoffs of haemoglobin concentration (Table 2). The prevalence of CRC, diagnosed by coloscopy was 16.0%. The optimal test performance comprising of maximal sensitivity and acceptable specificity was found at a cutoff of 20 μg/g. Sensitivity and specificity were estimated to 96.2% (95% CI 81.1-99.3) and 60.6% (95% CI 52.2-68.4), respectively. Using 10 μg/g cutoff as recommended by the 2017 NICE guidelines results in a specificity of 51.8% (95% CI 43.5-60.0). Expanding the target condition from CRC only to CRC + AA resulted in decreased sensitivity and increased specificity. The prevalence in expanded target condition increased to 32.0%. Applying a cutoff of 10 μg/g to the expanded target condition resulted in 84.6% sensitivity (95% CI 72.5-92.0) and 57.7% specificity (95% CI 48.4-66.4).
Table 2.
Diagnostic accuracy of the SENTIFIT-FOB Gold assay at different cutoffs.
| Coloscopy finding | Cutoff [μg Hb/g] | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) |
|---|---|---|---|---|---|
| CRC | 10 | 96.2 (81.1-99.3) | 51.8 (43.5-60.0) | 27.5 (23.9-31.5) | 98.6 (91.1-99.8) |
| 15 | 96.2 (81.1-99.3) | 57.7 (49.3-65.6) | 30.2 (26.0-34.8) | 98.8 (92.0-99.8) | |
| 20 | 96.2 (81.1-99.3) | 60.6 (52.2-68.4) | 31.6 (27.1-36.6) | 98.8 (92.4-99.8) | |
| 30 | 92.3 (75.9-97.9) | 67.2 (58.9-74.5) | 34.9 (29.1-41.1) | 97.9 (92.3-99.4) | |
| 40 | 88.5 (71.0-96.0) | 69.3 (61.2-76.4) | 35.5 (29.2-42.3) | 96.9 (91.5-98.8) | |
| 50 | 88.5 (71.0-96.0) | 72.3 (64.2-79.1) | 37.8 (31.0-45.2) | 97.0 (91.9-99.0) | |
| 100 | 80.8 (62.1-91.5) | 75.9 (68.1-82.3) | 39.0 (31.0-47.6) | 95.4 (90.4-97.9) | |
| 150 | 57.7 (38.9-74.5) | 83.2 (76.1-88.5) | 39.6 (28.5-51.8) | 91.2 (86.8-94.2) | |
| CRC + AA | 10 | 84.6 (72.5-92.0) | 57.7 (48.4-66.4) | 48.5 (42.4-54.6) | 88.8 (80.5-93.9) |
| 15 | 82.7 (70.3-90.6) | 64.0 (54.7-72.3) | 51.9 (45.0-58.8) | 88.7 (81.0-93.5) | |
| 20 | 78.8 (66.0-87.8) | 65.8 (56.5-73.9) | 51.9 (44.7-59.2) | 86.9 (79.4-91.9) | |
| 30 | 71.2 (57.7-81.7) | 71.2 (62.1-78.8) | 53.7 (45.3-62.0) | 84.0 (77.1-89.1) | |
| 40 | 65.4 (51.8-76.8) | 72.1 (63.1-79.6) | 52.4 (43.5-61.2) | 81.6 (75.0-86.7) | |
| 50 | 61.5 (48.0-73.5) | 73.9 (65.0-81.1) | 52.6 (43.1-61.8) | 80.3 (74,0-85.4) | |
| 100 | 57.7 (44.2-70.1) | 78.4 (69.8-85.0) | 55.7 (45.1-65.7) | 79.7 (73.9-84.6) | |
| 150 | 44.2 (31.6-57.7) | 86.5 (78.9-91.6) | 60.6 (46.8-73.0) | 76.7 (71.9-80.9) |
Sensitivity, specificity, PPV (positive predictive value) and NPV (negative predictive value) are shown as percentages with 95% CI. CRC (colorectal cancer), AA (advanced adenoma).
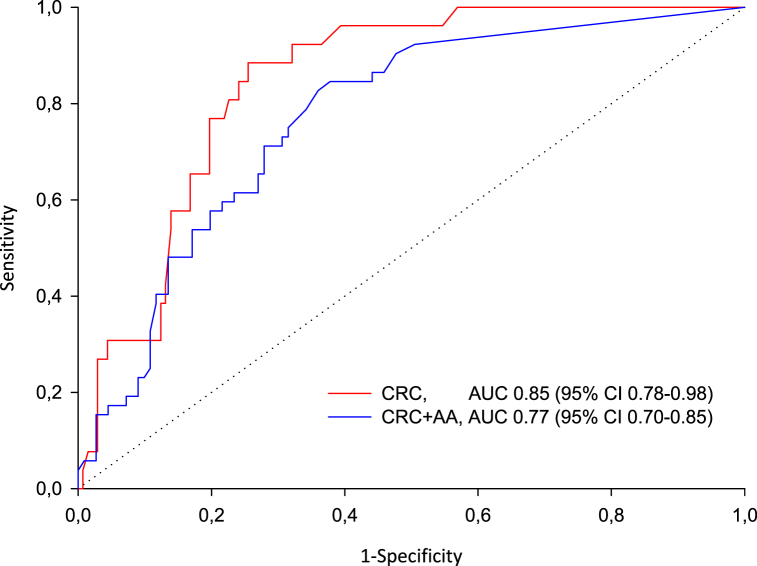
ROC curve analysis showed an AUC for detection of CRC of 0.85 (95% CI 0.78-0.98) and for detection of CRC + AA of 0.77 (95% CI 0.70-0.85), (Fig. 3).
Fig. 3.
Receiver operating curve for detection of colorectal cancer (red) and colorectal cancer and advanced adenom (blue). CRC (colorectal cancer), AA (advanced adenoma).
4. Discussion
To our knowledge, this is the first report on the use of the Sentinel-FOB-Gold assay on the automated Roche Cobas 8000 system. Quantitative FIT have different characteristics on different analytical platforms due to different analytical performance of the automated systems. It is therefore important that new combinations of assay and analytical platform be subject to evaluation.
Here we report the results of a performance evaluation study of the Sentinel-FOB Gold immunochemical test on an automated Roche Cobas 8000 system. The evaluation comprises an analytical and a clinical evaluation of performance. The main finding of the analytical evaluation was that the Sentinel-FOB Gold assay applied on an automated Roche Cobas system satisfies the current recommended analytical performance specifications for FIT-assays [19]. Nevertheless, our data show that the application of the assay on the automated Cobas system does not fullfill the specifications for imprecision claimed by the manufacturer of the assay (CV ≤ 5.0%). This is most probably due to that manufacturers validate methods under defined conditions which often do not agree with evaluation in routine laboratories.
Our results of within-run and between-run imprecision on the Roche Cobas 8000 system agree well with results found on the SENTIFIT 270 analyzer using control samples and haemoglobin lysate diluted in manufactures’ buffer [11,12]. In contrast to other published studies we used a faecal patient sample pool in addition to control samples for examination of both within-run and between-run imprecision. Using real sample matrix in assay evaluation better reflects real assay performance.
One should be aware that assessment of imprecision in this way does not reflect preanalytical variation. It has been shown that standardized sampling of faeces for FIT analysis is critical due to several sources of variation. The amount of faeces taken by the collection picker of the device can vary or samples could be homogenized insufficiently after insertion of the collection picker into the buffer. This affects the f-Hb concentration in the sample [20,21]. Assuming that the samples for assessing the analytical imprecision in our study each time would have been taken by using the collection picker of the device before analyzing, the calculated analytical imprecision would have been higher.
For evaluation of the accuracy quality control samples with assigned values provided by the manufacturer were used. One should keep in mind that internal quality control (IQC) samples are not independent and that comparison with the assigned values is not a true reflection of accuracy. To our knowledge, third-party IQC material is not available, yet. Quality control material used in external quality assurance (EQA) schemes on FIT is artificial stool material with method specific assigned values for f-Hb. Therefore, using samples from EQA schemes are of limited value, too when assessing true accuracy. Due to the lack of a reference method and certified reference material no independent assessment of true accuracy was possible. However, the Working Group on FIT of the Scientific Division of the IFCC has adressed this issue [22].
We found the LoB and LoD to be higher than specified by the manufacturer. The results agree almost with the specifications for the SENTIFIT 270 analyzer. Using a real faecal sample obtained from a patient presenting with lower abdominal symptoms better reflects the LoD due to the heterogenous mixture of a patient sample [19]. Nevertheless, we confirm that the coefficient of variation at the LoD is <10%. At the LoD the Sentinel-FOB Gold assay complies with the recommended analytical performance specifications for the limit of quantitation [19].
We confirmed good linearity of the assay in the measuring range and no carryover when analyzing samples with high f-Hb concentrations. No prozone effect was detectable up to f-Hb concentrations of 3000 μg/g. However, prozone effect at higher concentrations cannot be excluded.
In the clinical evaluation part of the study we found that 26 patients had CRC. These patients should have been detected by FIT. One patient with CRC would not have been detected because of a f-Hb concentration below the cutoff of 10 μg/g. One explanation could be that the tumor was in a non-bleeding phase at the time of sampling. Nevertheless, other reasons of false negative results such as localization or growing pattern of the tumor are possible, too.
Patients with CRC or AA have significant higher haemoglobin concentrations than patients without coloscopy finding. These results are in agreement with previous studies [11,23]. Auge et al. found higher median f-Hb concentrations for CRC (637 μg/g) and lower median concentrations for AA (3 μg/g). When using a cutoff of 10 μg/g more than 50% of the patients with AA would have been referred to coloscopy according to our findings. The explanation of the differences in median f-Hb concentration of patients with AA in different studies is probably the heterogenity of bleeding pattern in symptomatic patients. Faecal samples are unique in composition and degradation rates. Further, violation of faecal sampling instrucions can affect haemoglobin measurement [24]. In patients with NAA we found a median f-Hb concentration of 3.5 μg/g, in agreement with previous studies [11,23].
Our findings of median f-Hb concentration for patients with normal coloscopy agree well with other studies. Four patients with no findings by coloscopy had f-Hb concentrations over 10 μg/g, presumably due to causes other than colorectal disease. One of the four patients used Non-steroidal anti-inflammatory drugs which are known to increase the risk of bleeding in gastrointestinal tractus [25].
We assessed the 10 μg/g cutoff recommended by the 2017 NICE guidelines [5]. The estimated sensitivity of 96.2% for the condition CRC is in good agreement with other studies investigating both the same assay and the widely used OC-sensor assay (Eiken Chemical Ltd., Japan) [8,11]. A metaanalysis conducted by Stonestreet et al. included five studies in symptomatic populations using the OC-sensor system. They reported a pooled sensitivity and specificity of 93.0% and 87.0% for CRC using a similar cutoff f-Hb concentration (range 10 and 15 μg/g) [26]. A few studies had been published so far examining the diagnostic accuracy of the quantitative HM-JACKarc automated FIT assay (Kyowa-Medex Ltd., Japan). Godber et al. reported a significant lower sensitivity of 68.9% and a specificity of 80.2% using a cutoff of 10 μg/g [23]. Further, Chapman et al. [27] reported a sensitivity of 84% and a specificity of 78% in patients with increased risk of CRC.
On the other hand we found a lower specificity of 51.8% for CRC compared to other studies using the same assay as well as the OC Sensor and HM-JACKarc assay [11,23,26]. According to British guidelines on endoscopy in patients on anticoaguglation we have not excluded patients on anticoagulation while these patients were excluded in some other studies. Usage of direct anticoagulants is increasing and this could in part explain the lower specificity in our study.
When using a cutoff of 20 μg/g the specificity for CRC increased to 60.6%. Cubiella et al. found a specificity for CRC in symptomatic patients of 77.4% when using a similar cutoff of 17 μg/g [9]. They used the OC-Sensor assay which is known to give higher positive rates at cutoff >15 μg/g compared to the Sentinel-FOB Gold assay [28]. Because of high risk for AA to develop to cancer early detection is desirable. Therefore a cutoff of 10 μg/g is most appropriate for early detection of significant colorectal disease, despite of the lower specificity. A limitation of our study is that the analyses were based on a rather low number of participants which leads to broader 95% CIs.
In symptomatic patients rule out of significant colorectal disease is important. The negative predictive value for exclusion of CRC and CRC + AA using cutoffs between 40 μg/g and 10 μg/g ranged between 96.9% and 98.8% and between 81.6% and 88.8%, respectively. This is in good agreement with results from Auge et al. who reported negative predictive values ranging between 99.5% and 99.7% for CRC and 88.7% and 90.2% for CRC + AA for the assay applied on the SENTIFIT 270 analyzer [11]. One should keep in mind that the predictive values are highly influenced by the prevanlence of the disease.
5. Conclusion
The Sentinel-FOB Gold assay for quantification of haemoglobin in faeces applied on the automated Roche Cobas 8000 analyzer shows adequate analytical and clinical performace which agrees well with the performance of the assay on the SENTIFIT-270 analyzer. This enables effective and efficient use of a quantitative FIT on a high-throughput analyzer in large routine clinical chemistry laboratories.
In patients presenting with lower abdominal symptoms a cutoff of 10 μg/g seems to be optimal for referral to secondary care and provides a reliable prediction of the absence of CRC and advanced adenoma.
Author statement
Lutz Schwettmann: Conceptualization, Project administration, Methodology, Formal analysis, Writing-Original Draft, Visualization, Supervision.
Astrid Lied: Validation.
Ragnar Eriksen: Investigation, Resources, Data Curation, Writing-Review & Editing.
Declaration of competing interest
The authors state no conflict of interest.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021:1–41. doi: 10.3322/caac.21660. 0. [DOI] [PubMed] [Google Scholar]
- 2.Helsedirektoratet. Nasjonalt Handlingsprogram Med Retningslinjer for Diagnostikk, Behandling Og Oppfølging Av Kreft I Tykktarm Og Endetarm. 2021. www.helsedirektoratet.no IS-2971. Available from: (norwegian) [Google Scholar]
- 3.National Bowel Cancer Audit Report. 2020. www.nboca.org.uk Available from: [Google Scholar]
- 4.Strachan J.A., Mowat C. The use of faecal haemoglobin in deciding which patients presenting to primary care require further investigation (and how quickly)-the FIT approach. EJIFCC. 2021;32:52–60. [PMC free article] [PubMed] [Google Scholar]
- 5.NICE Diagnostic guidance . 2017. Quantitative Faecal Immun9ological Tests to Guide Referral for Colorectal Cancer in Primary Care.www.nice.org.uk DG30, Available from: [Google Scholar]
- 6.Navarro M., Nicolas A., Ferrandez A., Lanas A. Colorectal cancer population screening programs worldwide in 2016: an update. World J. Gastroenterol. 2017;23:3632–3642. doi: 10.3748/wjg.v23.i20.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald P.J., Digby J., Innes C., Strachan J.A., Carey F.A., Steele R.J.C., et al. Low faecal haemoglobin concentration potentially rules out significant colorectal disease. Colorectal Dis. 2013;15(3):e151–e159. doi: 10.1111/codi.12087. [DOI] [PubMed] [Google Scholar]
- 8.Westwood M., Lang S., Armstrong N., van Tourenhout S., Cubiella J., Stirk L., et al. Faecal immunological tests (FIT) can help to rule out colorectal cancer in patients presenting in primary care with lower abdominal symptoms: a systematic review conducted to inform new NICE DG30 diagnostic guidance. BMC Med. 2017;15:189. doi: 10.1186/s12916-017-0944-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cubiella J., Salve M., Diaz-Ondina M., Vega P., Alves M.T., Iglesias F., et al. Diagnostic accuracy of the faecal immunochemical test for colorectal cancer in symptomatic patients: comparison with NICE and SIGN referral criteria. Colorectal Dis. 2014;16:O273–O282. doi: 10.1111/codi.12569. [DOI] [PubMed] [Google Scholar]
- 10.Mowat C., Digby J., Strachan J.A., Wilson R., Carey F.A., Fraser C.G., et al. Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut. 2016;65:1463–1469. doi: 10.1136/gutjnl-2015-309579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auge J.M., Rodriguez C., Espanyol O., Rivero L., Sandalinas S., Grau J., et al. An evaluation of the SENTIFIT 270 analyser for quantitaion of faecal haemoglobin in the investigation of patients with suspected colorectal cancer. Clin. Chem. Lab. Med. 2018;56:625–633. doi: 10.1515/cclm-2017-0605. [DOI] [PubMed] [Google Scholar]
- 12.Piggott C., Carroll M.R.R., John C., O'Driscoll S., Benton S.A. Analytical evaluation of four faecal immunochemistry tests for haemoglobin. Clin. Chem. Lab. Med. 2021;59:173–178. doi: 10.1515/cclm-2020-0251. [DOI] [PubMed] [Google Scholar]
- 13.Veitch A.M., Vanbiervliet G., Gershlick A.H., Boustiere C., Baglin T.P., Smith L.A., et al. Endoscopy in patients on antiplatelet og anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut. 2016;65:374–389. doi: 10.1136/gutjnl-2015-311110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute . second ed. CLSI document 17-A2; Wayne PA, USA: 2012. Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures. Approved Guideline. [Google Scholar]
- 15.Armbruster D.A., Pry T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008;29:S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 16.NCCLS Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach; Approved Guideline. NCCLS Document EP6-A; 2003. [Google Scholar]
- 17.Hassan C., Quintero E., Dumonceau J.M., Regula J., Brando C., Caussade S., et al. Post-polypectomy colonoscopy surveillance: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2013;45:842–851. doi: 10.1055/s-0033-1344548. [DOI] [PubMed] [Google Scholar]
- 18.EPA Guidance for data quality assessment. In: Practical Methods for Data Analysis EPA QA/G-9 July 2000.
- 19.Fraser C.G., Benton S.C. Detection capability of quantitative faecal immunochemical tests for haemoglobin (FIT) and reporting of low faecal haemoglobin concentrations. Clin. Chem. Lab. Med. 2019;57:611–616. doi: 10.1515/cclm-2018-0464. [DOI] [PubMed] [Google Scholar]
- 20.Gies A., Gruner L.F., Schrotz-King P., Brenner H. Effect of imperfect compliance with instructions for fecal sample collection on diagnostic performance of 9 fecal immunochemical tests. Clin. Gastroenterol. Hepatol. 2019;17(9):1829–1839. doi: 10.1016/j.cgh.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 21.James T., Nicholson B.D., Marr R., Paddon M., East J.E., Justice S. Faecal immunochemical testing (FIT): sources of result variation base don three years of routine testing of symptomatic patients in English primary care. Br. J. Biomed. Sci. 2021;19:1–7. doi: 10.1080/09674845.2021.1896204. Mar. [DOI] [PubMed] [Google Scholar]
- 22.Benton S.C., IFCC-FIT Working Group (FIT-WG) 2017. IFCC E-News; pp. 16–17.http://www.ifcc.org/media/461890/IFCCeNewsJune2017. Assesed 11 June 2021 [Google Scholar]
- 23.Godber I.M., Todd L., Fraser C.G., MacDonald L.R., Younes H.B. Use of faecal immunochemical test for haemoglobin can aid in the investigation of patients with lower abdominal symptoms. Clin. Chem. Lab. Med. 2016;54:595–602. doi: 10.1515/cclm-2015-0617. [DOI] [PubMed] [Google Scholar]
- 24.Benton S., Symonds E., Djedovic N., Jones S., Deprez L., Kocna P., et al. International federation of clinical chemistry faecal immunochemical test working group (IFCC FIT-WG). Faecal immunochemical tests for haemoglobin: analytical challenges and potential solutions. Clin. Chim. Acta. 2021;517:60–65. doi: 10.1016/j.cca.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Laine L., Curtis S.P., Langman M., Jensen D.M., Cryer B., Kaur A., et al. Lower gastrointestinal events in a double-blind trial of the cyclo-oxygenase-2 selective inhibitor etoricoxib and the traditional nonsteroidal antiinflammatory drug diclofenac. Gastroenterology. 2008;15:1517–1525. doi: 10.1053/j.gastro.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 26.Stonestreet J., Chandrapalan S., Woolley D., Uthman O., Arasaradnam R.P. Systematic review and meta-analysis: diagnostic accuracy of faecal immunochemical testing for haemoglobin (FIT) in detecting colorectal cancer for both symptomatic and screening population. Acta gastroenterol Belg. 2019;82(2):291–299. [PubMed] [Google Scholar]
- 27.Chapman C.J., Banerjea A., Humes D.J., Allen J., Oliver S., Ford A., et al. Choice of faecal immunochemical test matters: comparison of OC-Sensor and HM-JACKarc, in the assessment of patients at high risk of colorectal cancer. Clin. Chem. Lab Med. 2021;59(4):721–728. doi: 10.1515/cclm-2020-1170. [DOI] [PubMed] [Google Scholar]
- 28.De Clerk C.M., Wieten E., Lansdorp-Vogelaar I., Bossuyt P.M., Spaander M.C.W., Dekker E. Performance of two immunochemical tests for the detection of advances neoplasia at different positivity thresholds: a cross-sectional study of the Dutch national colorectal cancer screening programme. Lancet Gastroenterol Hepatol. 2019;4:111–118. doi: 10.1016/S2468-1253(18)30319-4. [DOI] [PubMed] [Google Scholar]