Summary
Primary immune thrombocytopenia is an autoimmune disease associated with a reduced peripheral blood platelet count. The phenotype is variable with some patients suffering no bleeding whilst others have severe bleeding which may be fatal. Variability in clinical behaviour and treatment responses reflects its complex underlying pathophysiology. Historically the management has relied heavily on immune suppression. Recent studies have shown that the older empirical immune suppressants fail to alter the natural history of the disease and are associated with a poor quality of life for patients. Newer treatments, such as the thrombopoietin receptor agonists, have transformed ITP care. They have high efficacy, are well tolerated and improve patients’ quality of life. A greater understanding of the underlying pathophysiology of this disorder has helped develop a number of new targeted therapies. These include inhibitors of the neonatal Fc receptor inhibitors, Bruton tyrosine kinase and complement pathway. Here we discuss the mechanisms underlying ITP and the new approach to ITP care.
Keywords: immune thrombocytopenia, immune suppression, thrombopoietin receptor agonists
Introduction
Autoimmune disease places a significant burden on the healthcare system affecting between 3-9% of the population.1 According to the British Society of Immunology, £13bn is spent on treating autoimmune diseases in the UK (www.immology.org). Despite advances in management, the morbidity and mortality remain high for many of these disorders.
Primary immune thrombocytopenia is an organ-specific autoimmune disease characterised by a reduced peripheral blood platelet count.2 Symptoms and signs include fatigue in addition to dry or wet purpura. Many patients have few or only mild symptoms but severe and life-threatening bleeding may occur. Somewhat frustratingly, even today, ITP remains a diagnosis of exclusion; there should be no detectable underlying cause for the thrombocytopenia found on investigation.3 ITP in which there is a detectable underlying cause is termed secondary ITP but we will not discuss this here.
The reduced peripheral blood platelet count is a result of a combination of premature platelet destruction4 and a relative inadequacy of platelet production.5 In addition to antibody-mediated platelet destruction, which has been recognised since the 1950s,4 other mechanisms are clearly involved. These include T-cell mediated apoptosis of megakaryocytes, inhibition of platelet production and T cell destruction of platelets.6 The underlying pathophysiology is better understood today and has led to the development of new treatments including the TPO-RAs, syk inhibitor, Fcγ receptor (FcγR) inhibition, and other treatments.
ITP Pathophysiology
It has been 70 years since the famous Harrington-Hollingsworth experiment was performed showing that plasma from patients with ITP caused significant thrombocytopenia when infused into healthy volunteers.4,7 It now appears that B and T cell defects are a central feature of ITP pathophysiology (Figure 1) and the most compelling evidence is that platelet autoimmunity is caused by a failure of self-tolerance mechanisms.8, 9, 10 A summary of these mechanisms will be discussed.
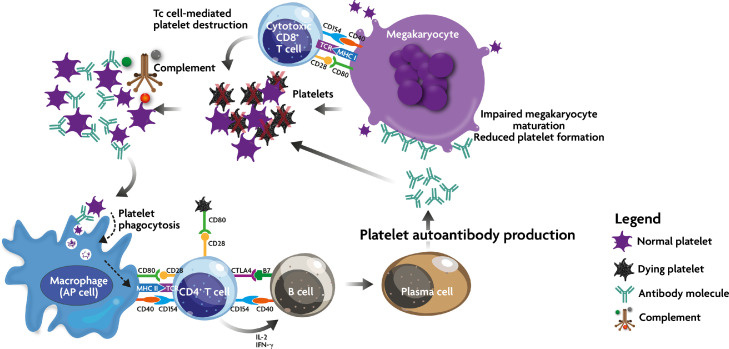
Figure 1.
Immune effector mechanism in ITP. Due to a breakdown in self-tolerance, APC (including megakaryocytes) process and present platelet autoantigens to autoreactive T cells, which then begin a cascade of events including stimulation of autoantibody production and cytotoxic T cell activation. These two mechanisms lead to peripheral platelet destruction and megakaryocyte inhibition in the bone marrow. In addition, autoantibody-opsonized platelets may come under the attack of the complement cascade.
Defects in antigen presenting cells
Essentially all IgG responses, including autoimmune IgG production in ITP, are initiated by T helper cells that recognize their cognate peptide antigens in association with major histocompatibility complexes (MHC) on antigen presenting cells (APC).11, 12, 13 APC are a diverse set of cells which include dendritic cells (DC), macrophages and in some instances, B cells. Recent studies have suggested that even platelets and their parental cells, the megakaryocytes, can act as antigen presenting cells.14, 15, 16 DCs are the most potent professional APC within the innate immune system17 and many reports have demonstrated their impairment in ITP.13,18,19 For instance, Catani et al.18 demonstrated that DCs had the ability to stimulate autoreactive T cell proliferation upon platelet challenge in vitro and this was attributed to elevated CD86 expression on the DC surface. Furthermore, low numbers of plasmacytoid DCs (pDCs) were observed in patients with primary ITP and those affected by secondary ITP associated with Helicobacter pylori infection, and these low numbers significantly correlated with low platelet counts.20 It was suggested that a lack of type 1 interferon secreted by the pDCs may play a role modulating activated autoreactive T cells in ITP.20 Perhaps more important were the observations of Catani et al18 who showed that DC-associated indoleamine 2,3-dioxygenase 1 (IDO1) was reduced in patients with ITP and this was thought to impair the differentiation of regulatory T cells (Tregs) which contributes to the observed Treg deficiency in ITP (Figure 2). As DC are the most efficient of all APC populations, it is not surprising that their abnormalities play an important role in ITP T cell pathogenic mechanisms, and molecules directed at correcting DC defects may be an attractive avenue for ITP therapy. With regards to macrophages, it is well known that they are the primary phagocyte responsible for splenic platelet destruction in ITP. In addition, these cells, particularly during states of inflammation e.g. bacterial infections, can be activated by inflammatory cytokines such as IFN-γ to up-regulate MHC class II molecules and enhance the antigen presenting capability of the cells. This may lead to a double edge sword in ITP where the macrophage not only destroy autoantibody-opsonized platelets, but also processes and present platelet autoantigens to autoreactive T helper cells. Such as scenario may contribute a continuous autoantigen feedback loop, which needs to be broken by immunosuppressive therapy.
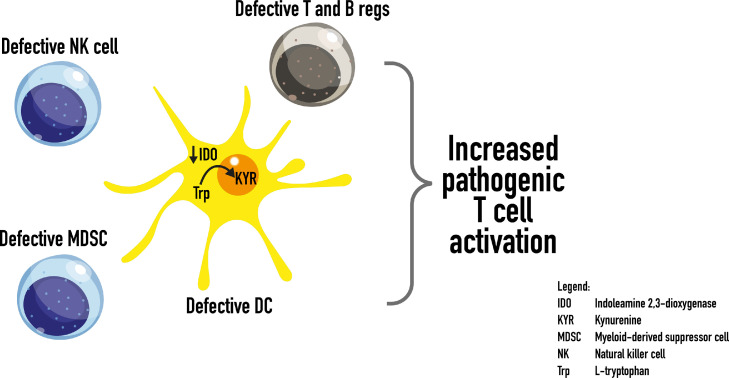
Figure 2.
Potential mechanisms of self-tolerance breakdown in ITP. Dendritic cell abnormalities may play a central role in inhibiting Treg and Breg suppressive activities while stimulating autoreactive effector mechanisms (Figure 1). In addition, defects in NK cells and MDSCs may also contribute to autoimmune generation.
Soluble factors
Patients with ITP also have numerous abnormalities associated with soluble immune mediators such as cytokines/chemokines and complement proteins. In the early 1970s, one of the first observations of cytokine impairment in ITP was the excessive in vitro production of macrophage inhibition factor (MIF) by lymphocytes from patients with ITP.21 This pioneering study may have been one of the first descriptions of a T cell abnormality in ITP as MIF is primarily a T cell-derived factor, although other cell types have now been shown to harbour this preformed cytokine.22 Subsequently, many publications of other cytokine abnormalities have been reported which have culminated in the broadly accepted dogma that active ITP is a manifestation of Th1 and Th17 cytokines most probably due to a lack of Treg suppression of autoimmunity.9,10,23,24 The inverse relationship between Th1/Th17 cytokines and lowered Treg activity in ITP is striking and, of interest, the common factor associated with reversing this relationship is the increase in platelet counts (mass) in patients after treatment with a wide variety of different therapies.25 This is not a surprising finding as platelets contain high amounts of transforming growth factor (TGF)-β, a major molecular switch to induce Treg production.26, 27, 28, 29, 30, 31 With respect to complement, the IgG autoantibodies formed in ITP have the ability to fix complement and complement components are found on the platelet surface.32,33 Both these processes can contribute to platelet destruction in ITP. Despite these observations, little research has been performed on serum complement in ITP and limited data exist on whether complement levels play a significant role in disease pathophysiology. In spite of this, however, therapeutics targeting complement such as the C1 esterase inhibitor sutimlimab are currently being studied in the treatment of ITP.34,35
NK & T lymphocyte dysregulation
Natural killer (NK) cells are a cytotoxic lymphocyte of the innate immune system that have rapid responses against virally-infected host cells and are activated by tumour formation.36,37 They have a unique ability to recognize and kill target cells in the absence of antibodies and/or MHC expression which allows them to act quickly on abnormal cells. Early studies suggested that although NK cell numbers in the peripheral blood of patients with ITP were relatively normal, their ability to kill K562 erythroleukaemic target cells in vitro was significantly suppressed.38 Subsequently, Ebbo et al39 demonstrated that NK cytotoxicity was also suppressed in spleen cells from patients with ITP. More recently, El-Rashedi et al40 examined NK cells in children with ITP and concluded that childhood ITP is associated with an increase in cytotoxic T lymphocytes, but a decrease in peripheral blood NK cells. The reasons for these observations are unclear, particularly since patients with ITP have elevated levels of interferon-γ which is predominantly produced by NK cells.24,41 However, NK cells are known inhibitors of B cell differentiation and affinity maturation42,43 and thus, it may be that their suppressed activity can influence autoantibody production in ITP (Figure 2).
Perhaps the first paper to suggest that autoantibody production in patients with ITP is not autonomous, but under the control of T cell regulation, was proposed by Hymes et al.44 This early study suggested that a defective CD8+ T cell suppression was responsible for the excessive production of anti-platelet autoantibodies. It then took almost 27 years for the notion that lack of T cell suppression was apparent in ITP when Liu et al9,45 demonstrated that patients with ITP have defective and reduced numbers of CD4+ T regulatory cells. Thus began an impressive output of papers all showing defective Tregs in ITP and this laid the foundation that ITP is due to a lack of peripheral T cell tolerance mechanisms.8,9
Other defects in cells involved in immune responses
In addition to Treg abnormalities in ITP, several other myeloid and lymphoid abnormalities have been documented that probably also play a role in the pathogenesis of the disorder. For example, Li et al46 elegantly demonstrated that CD19(+)CD24(hi)CD38(hi) B regulatory cells (Bregs) are reduced in number and defective in ITP, characterized by deficient production of the anti-inflammatory cytokine IL-10. This leads to the inhibition of monocyte TNF-α expression,47,48 but more intriguingly, when thrombopoietin (TPO) therapy increased platelet counts in the patients with ITP, it also increased Breg numbers and function, just as observations showing increases in platelet counts following TPO therapy rescues the Treg deficiency in ITP"49 (Figure 2). Thus, the compromised Breg compartment causes significant immune dysregulation in ITP and like Tregs, this cell population may be an efficacious target for therapy. Alternatively, in contrast to the low numbers of Breg in the periphery, Aslam et al50 found Bregs to be significantly elevated in the spleens of patients with ITP suggesting that peripheral reductions of Breg numbers, at least, may be due to sequestration of these potent immunoregulatory cells. On the other hand, myeloid-derived suppressor cells (MDSCs) have also been shown to be abnormal in patients with ITP. MDSCs are a heterogeneous population of myeloid progenitor cells that have been shown to be potent regulators of adaptive immunity51 having the ability to inhibit T cell proliferation by starving the cells of nutrients required.52 Hou et al53 demonstrated that MSDC were deficient in patients with active ITP and that high dose dexamethasone treatment rescued the MDSC numbers and restored Treg function (Figure 2). This work suggests a necessary role for MDSC in the pathogenesis and corticosteroid management of ITP and analogous results with respect to MSDC numbers were observed in spleen cell cultures from ITP patients treated with intravenous immunoglobulin (IVIg).54
Given the wealth of information now known about the pathophysiology of ITP, there are several potential immune mechanisms that may be targeted by therapy. In particular, active ITP is the result of a lack of immune tolerance due to faulty Tregs and any therapeutic designed to elevate Tregs will ultimately raise platelet counts in patients. These aspects are summarized in Figure 2. It is clear, however, that more basic and clinical research is required to unfold the precise mechanisms responsible for immune dysregulation in ITP.
Treatment goals for ITP
The goals of ITP management have not been clearly defined although the most recent International Consensus Report3 attempts to better define these. The primary focus of management is the prevention of bleeding and patient safety by elevating the patient's platelet count up to 20-30 × 109/L. The goal of “safety” rather than normalization of the platelet count was outlined many years ago by Karpatkin.55 Until quite recently there were few formally approved treatments for ITP. The most common treatment modality that has been used in ITP is immune suppression. This has been achieved using corticosteroids, immune suppressants such as azathioprine, mycophenolate, cyclosporin A56 and rituximab,57 in addition to chemotherapy agents,58 the attenuated androgen danazol,59 and dapsone, an antibacterial.60 These drugs are of variable efficacy, often accompanied by significant unwanted effects and their benefits to the patients are questionable. Patients may feel more unwell with the treatment than from the disease itself.
We will discuss the new era of ITP therapy here, beginning with the second generation thrombopoietin receptor agonists (TPO-RAs), before examining the other recently approved treatment, fostamatinib. A number of other agents are in clinical development including neonatal Fc receptor inhibitors, Bruton tyrosine kinase inhibitors, and anti-complement drugs. Table 1 summarises the recent treatments designed for primary ITP.
Table 1.
Recent treatments designed for primary ITP.
| Drug | Description | Mechanism of action |
|---|---|---|
| Romiplostim68, 69, 70, 71 | Peptibody TPO-RA | Stimulates the JAK-STAT pathway. Megakaryocyte proliferation, maturation and platelet production |
| Eltrombopag72,73 | Small molecule TPO-RA | Stimulates the JAK-STAT pathway. Megakaryocyte proliferation, maturation and platelet production |
| Avatrombopag74 | Small molecule TPO-RA | Stimulates the JAK-STAT pathway. Megakaryocyte proliferation, maturation and platelet production |
| Fostamatinib81,82 | syk inhibitor | Decreases antibody-dependent phagocytosis of platelets |
| Efgartigimod84 | Anti-FcRn | Decreases the half-life of IgG, reduces plasma IgG both normal and pathogenic |
| Rozanolixizumab90 | Anti-FcRn | Decreases the half-life of IgG, reduces plasma IgG both normal and pathogenic |
| Rilzabrutinib86,92 | BTKI | Inhibits Fcγ signal transduction, decreases platelet phagocytosis and autoantibody production |
| Sutimlimab35 | Anti-C1s | Decreases complement-dependent cytotoxicity thereby reducing platelet destruction |
Abbreviations used: TPO-RA, thrombopoietin receptor agonist; FcRn, neonatal Fc receptor; JAK, Janus kinase; STAT, signal transducer and activator of transcription; BTKI, Bruton kinase inhibitor.
Models of treatment
Many of the treatments used in autoimmune disease achieve few lasting remissions. The focus for many autoimmune diseases has shifted towards improving symptoms and health-related quality of life. The same is true for ITP where quality of life has been recognised as a major treatment goal for this disease.3
The treatment models in current medical practice include: the infectious disease model, oncologic disease model, metabolic disease model, and transplant rejection model. Over many decades, ITP has been managed using the transplant rejection model in which suppression of the immune system has been used to ameliorate the disease symptoms. It is clear from many published studies, and the recent I-WISh study,61 that this model seldom induces remissions and has serious adverse effects on morbidity or mortality, resulting in an overall poor quality of life for patients with ITP. Given that ITP is a “benign” disorder, it is disappointing that treatments offer few benefits to patients whilst, at the same time, badly affecting their health-related quality of life.
Shift in emphasis away from immune suppression
Over the past few years there has been a gradual shift away from immune suppression as a means of treating ITP. The recent COVID-19 pandemic has made immune suppression very unattractive as a modality of treatment since our focus has been on minimising patient risk from COVID.62 The TPO-RAs have filled this gap and in England and Wales, as well as other territories (Interim Clinical Commissioning Policy, NHS(E) 30 July, 2021, Version 4), TPO-RAs are being used first line (off-label) in order to avoid the use of immune suppression. This is a major paradigm shift for the management of ITP. Attempts to alter the standard first line treatment of ITP have included the addition of mycophenolate to corticosteroids upfront for newly-diagnosed adults.63 However, although there were fewer treatment failures in the mycophenolate arm, the quality-of-life was lower in this group. For this reason, in addition to the avoidance of immune suppression during the current pandemic, few patients have actually received mycophenolate upfront.
Thrombopoietin (TPO) mimetic agents
In order to move away from immune suppression, the first-generation TPO-mimetic drugs were developed in the 1990s including recombinant human TPO (rHu-TPO) and recombinant human megakaryocyte growth and development factor (rHu-MGDF).64 By stimulating the TPO receptor these agents raised the patients’ platelet counts. However, although these TPO mimetics had good efficacy in ITP, antibodies were generated against the drugs in some patients.65 Because the TPO mimetics were based on native endogenous human TPO, the antibodies against drugs were cross-reactive with native TPO resulting in profound thrombocytopenia. Development of the first generation TPO mimetics was therefore abandoned.
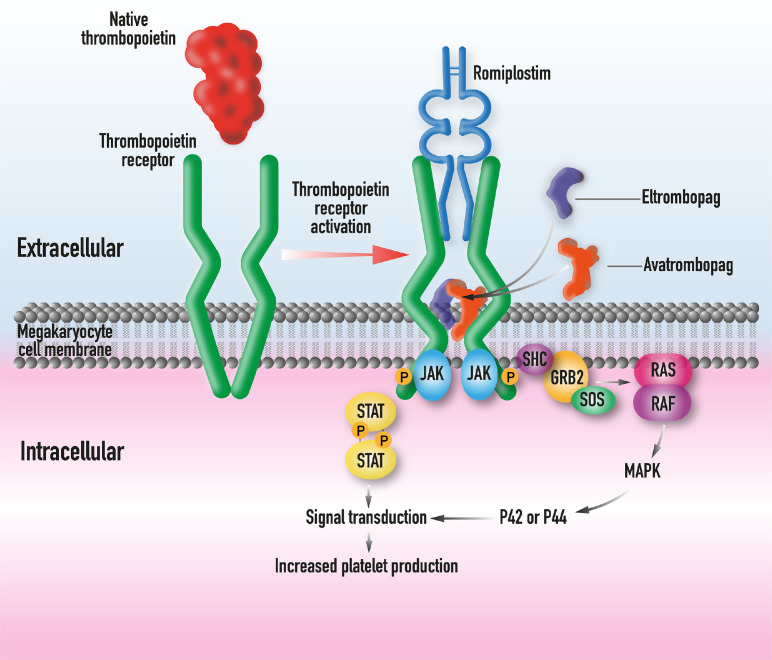
Over the last 20 years there has been significant drug development for ITP, beginning with the second generation thrombopoietin receptor agonists (TPO-RAs).66,67 This generation of TPO-RAs bears no resemblance to native thrombopoietin and therefore any antibodies directed against drug should not cross-react with the patient's native thrombopoietin. Romiplostim is a large peptibody molecule which binds to the same site as native TPO on the extracellular portion of the TPO receptor68, 69, 70, 71 (Figure 3). Eltrombopag is a small hydrazone molecule that binds to the transmembrane portion of the TPO receptor72,73 (Figure 3). Both drugs were launched in 2008 and have been used with great success in primary immune thrombocytopenia as second-line agents. Their efficacy is high at around 80%.66 More recently, another small molecule TPO receptor agonist has been developed, namely avatrombopag.74 This molecule binds to the same site as eltrombopag and stimulates the same pathway resulting in megakaryocyte proliferation and platelet production. All three approved TPO-RAs bind to the TPO receptor and stimulate the same pathway, shown in Figure 1. These new TPO mimetics are well tolerated by patients and have avoided many patients being exposed to immune suppression or splenectomy.67
Figure 3.
Native TPO binds to the extracellular domain of the TPO receptor (left). After a change in configuration the JAK-STAT pathway is activated. TPO-RAs mimic native TPO by binding to the extracellular domain (romiplostim) or transmembrane region (eltrombopag and avatrombopag) of the TPO receptor.
Although the TPO-RAs were regarded largely as “palliative” therapy (effective when the patient was taking the TPO-RA) somewhat unexpectedly, around one third of patients on TPO-RAs are able to be weaned slowly off the TPO-RA and maintain a safe or normal platelet count off all treatment. This is observed even in heavily-pretreated patients.75, 76, 77, 78, 79 Although more work needs to be carried out to determine why these patients are in a treatment-free “remission” it is possible that immune tolerance has been restored. The TPO-RAs appear to have activity at the stem cell level, as demonstrated with the use of eltrombopag for the treatment of severe aplastic anaemia, restoring haemoglobin levels and white cell counts.80
The TPO-RAs have therefore dramatically changed the ITP management landscape. Fewer patients require immune suppression which, during a pandemic, is advantageous. However, the TPO-RAs are not effective in all patients; some patients cannot tolerate them or there may be contraindications to their use. Clearly other treatment classes are required for these patients in order to maintain a safe platelet count.
Inhibition of FcγR platelet destruction: syk inhibition
Another novel agent for ITP is fostamatinib, a small molecule syk inhibitor. This oral agent acts by inhibiting syk and prevents platelet breakdown by interfering with FcγR-mediated destruction of opsonised platelets81,82 (Figure 4). Published studies have confirmed a response rate of 43% in patients who were previously heavily pretreated. The durable response rate was reported at 18%.81 This rate may be higher if fostamatinib is used earlier. Adverse events reported in the studies include hypertension in 28%, and diarrhoea in 31% of the subjects, respectively. These adverse events were predominantly mild to moderate.
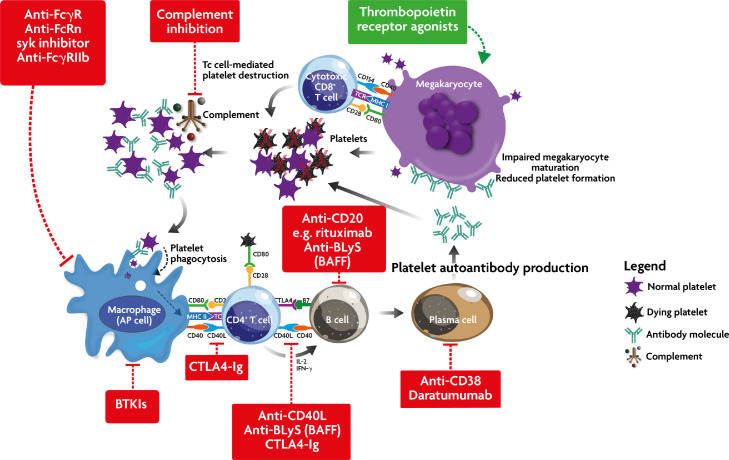
Figure 4.
Simplified schema of ITP pathogenesis showing the sites of action of new therapies for ITP. Stimulatory drugs are in green and inhibitory agents are shown in red. BTKIs, Bruton tyrosine kinase inhibitors; CTLA4-Ig, Cytotoxic T Lymphocyte Antigen 4 immunoglobulin G1 fusion protein; Anti-BLys, B lymphocyte stimulator; BAFF, B cell Activating Factor.
So, currently there are two new approved classes of therapy for ITP, namely the TPO-RAs and a syk inhibitor. But yet further classes of therapy are in advanced stages of clinical development including drugs active against the neonatal Fc receptor (anti-FcRn),83,85 Bruton tyrosine kinase inhibitors86 and complement inhibitors.87,88 We will discuss these briefly here.
Treatments in clinical development
Anti-FcRn
The neonatal Fc receptor modulates the half-life of IgG and albumin. It does this by binding to IgG and releasing it from endothelial endosomes. As well as recycling normal IgG, the FcRn also recycles pathologic IgG.89 Blocking the FcRn causes IgG breakdown within the endosomes.85 Two molecules are currently in advanced phase clinical development: rozanolixizumab (UCB)90 and efgartigimod (argenx)84 (Figure 4).
In phase 2 studies, both efgartigimod and rozanolixizumab were well tolerated and responses of 38% (efgartigimod) and 50% (rozanolixizumab) were seen.84,90 Phase 3 studies of both agents are ongoing. Data thus far would appear to indicate that although the IgG levels fall this does not appear to increase the risk of infection.
Bruton tyrosine kinase inhibitors (BTKIs)
Bruton tyrosine kinase, like syk, is involved in Fcγ signalling and is another potential target for ITP therapy (Figure 4). BTK is necessary for B cell development, function and antibody production. Several BTKIs have been developed for diseases such as chronic lymphocytic leukaemia.91 However, BTKIs have been reported to inhibit platelet aggregation which may result in bleeding, which is a concern in ITP. Although this was reported with ibrutinib it is not the case with rilzabrutinib.86
Results from a recent Phase 2 study of rilzabrutinib in adult patients with relapsed or refractory ITP have been published.92 Fifty nine adults, who had at least one prior response to treatment and a platelet count ≤30 × 109/L, were given oral rilzabrutinib at 200 or 400mg daily or 300 or 400mg twice daily. The highest response rate was seen in the 400mg twice daily group. Of 44 patients on this dose 39% achieved the primary endpoint (two or more platelet counts ≥50 × 109/L without the use of rescue therapy). Of the 33 patients who completed the study for more than 12 weeks, 17 of these (52%) responded. Of the responders half of these (50%) achieved a platelet count ≥30 × 109/L by day 8. Rilzabrutinib was well tolerated with adverse events of Grade 1 or 2 only.
The complement system
Complement is believed to be involved in the pathogenesis of ITP for some patients. However, the contribution made by complement to ITP is not clearly understood. Early phase studies with sutimlimab, a monoclonal IgG C1s inhibitor, have been conducted. The patients treated were adults with ITP for >1 year and an inadequate response to ≥2 prior treatments. A total of 12 patients were treated of which 42% responded (platelet count ≥ 50 × 109/L). Four patients (33%) achieved a platelet count of ≥ 50 × 109/L for ≥70% of their study visits. One third of patients responded in two days or less. No treatment-related adverse events were noted35 (Figure 4). Another complement inhibitor, iptacopan, a selective Factor B inhibitor, is currently undergoing Phase 2 clinical trials in ITP.
Other treatments in development
A number of other molecules are being developed. This includes other TPO receptor agonists lusutrombopag and heterombopag, as well as an anti-CD38 molecule (daratumumab), and others (Figure 4). Anti-CD38 treatment may be useful for some with refractory ITP which may be caused by long-lived plasma cells.93 The proteasome inhibitor, bortezomib, has been used anecdotally in refractory ITP with variable results. However, this agent is not currently licensed for this indication.
Conclusions
ITP has a complex and ill-defined pathophysiology. Basic science and the need to avoid the less effective immune suppressants, has led to the development of new more targeted treatments for ITP, starting with the second generation TPO-RAs and now include syk inhibition with other classes of treatment in clinical development. The introduction of these novel therapies has allowed us to use less immune suppression in ITP management. Over the last decade there has been a shift away from immune suppression for the treatment of ITP. However, the COVID-19 pandemic has forced our hand in speeding up this move towards less immune suppressive treatments. As a result of this, the patients’ quality-of-life has improved since drugs like TPO receptor agonists have high efficacy and are well tolerated. Once the drugs undergoing development are approved it is likely that there will be a much reduced reliance on the older empirical immune suppressive therapies. These new drugs also appear to offer patients a much higher chance of a treatment-free sustained response than with the older immune suppressants. Some of these “remissions” may be cures but we need much longer follow-up of the patients and good basic science studies.
Outstanding questions
Primary ITP is now recognized as an autoimmune syndrome with several types of underlying mechanisms. Unfortunately there are no specific assays to distinguish such differing pathogeneses and for this reason all patients with ITP are treated the same; phenotypically they look similar. Treatment until recently has been unsatisfactory and empirical but over the last 10 years or so patients have been treated with agents that have undergone randomized trials and are fully approved for primary ITP. But there is still great uncertainty in terms of which patients to treat, with which therapy and for how long. Overtreatment remains a problem for patients with ITP and health-related quality of life suffers as a result. We need better stratification of patients in order to avoid treatment in those who do not require this, whilst directing treatment at patients who will benefit. We have few licensed therapies currently but the list is growing with a number of very promising treatments undergoing clinical trials.
Search strategy and selection criteria
Data for this review were collected using PubMed searches between 1990-2021, using the terms “immune thrombocytopenia”, “idiopathic thrombocytopenic purpura”, “ITP”, “pathogenesis”, “pathophysiology”, “treatment”, “management”, “therapy”, “advances”, “novel therapies”.
Declaration of interests
DP has received research support and honoraria from Amgen, Novartis, SOBI, UCB, argenx, Rigel and Chugai and has acted as a consultant for UCB, argenx, MedImmune and Ono; he also serves on a DSMB for an investigator-led study of rituximab in ITP and has received a basic shares package from previous employment by GlaxoSmithKline. JWS has received honoraria from Amgen, Novartis, and UCB and has acted as a consultant for Amgen, Novartis, Argenx and UCB.
Acknowledgments
Acknowledgements
None. No funding was received for this review.
Contributors
Drew Provan: performed literature search, drafted 50% of the manuscript and drew the figures, proofread the entire manuscript. John W Semple: performed literature search, drafted 50% of the manuscript, helped design the figures, proofread the entire manuscript. Both authors read and approved the final version of the manuscript.
Contributor Information
Drew Provan, Email: a.b.provan@qmul.ac.uk.
John W. Semple, Email: john_w.semple@med.lu.se.
References
- 1.Hayter SM, Cook MC. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev. 2012;11:754–765. doi: 10.1016/j.autrev.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- 3.Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3:3780–3817. doi: 10.1182/bloodadvances.2019000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrington WJ, Minnich V, Hollingsworth JW, Moore CV. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. Journal of Laboratory and Clinical Medicine. 1951;38:1–10. [PubMed] [Google Scholar]
- 5.Gernsheimer T, Stratton J, Ballem PJ, Slichter SJ. Mechanisms of response to treatment in autoimmune thrombocytopenic purpura. N Engl J Med. 1989;320:974–980. doi: 10.1056/NEJM198904133201505. [DOI] [PubMed] [Google Scholar]
- 6.Olsson B, Andersson PO, Jernas M, et al. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9:1123–1124. doi: 10.1038/nm921. [DOI] [PubMed] [Google Scholar]
- 7.Semple JW, Freedman J. Abnormal cellular immune mechanisms associated with autoimmune thrombocytopenia. Transfus Med Rev. 1995;9:327–338. doi: 10.1016/s0887-7963(05)80080-x. [DOI] [PubMed] [Google Scholar]
- 8.Semple JW, Rebetz J, Maouia A, Kapur R. An update on the pathophysiology of immune thrombocytopenia. Curr Opin Hematol. 2020;27:423–429. doi: 10.1097/MOH.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 9.Audia S, Mahévas M, Nivet M, Ouandji S, Ciudad M, Bonnotte B. Immune Thrombocytopenia: Recent Advances in Pathogenesis and Treatments. HemaSphere. 2021;5:e574. doi: 10.1097/HS9.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swinkels M, Rijkers M, Voorberg J, Vidarsson G, Leebeek FWG, Jansen AJG. Emerging Concepts in Immune Thrombocytopenia. Front Immunol. 2018;9:880. doi: 10.3389/fimmu.2018.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morante-Palacios O, Fondelli F, Ballestar E, Martínez-Cáceres EM. Tolerogenic Dendritic Cells in Autoimmunity and Inflammatory Diseases. Trends in immunology. 2021;42:59–75. doi: 10.1016/j.it.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Kissel K, Berber S, Nockher A, Santoso S, Bein G, Hackstein H. Human platelets target dendritic cell differentiation and production of proinflammatory cytokines. Transfusion. 2006;46:818–827. doi: 10.1111/j.1537-2995.2006.00802.x. [DOI] [PubMed] [Google Scholar]
- 13.Catani L, Fagioli ME, Tazzari PL, et al. Dendritic cells of immune thrombocytopenic purpura (ITP) show increased capacity to present apoptotic platelets to T lymphocytes. Exp Hematol. 2006;34:879–887. doi: 10.1016/j.exphem.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Chapman LM, Aggrey AA, Field DJ, et al. Platelets Present Antigen in the Context of MHC Class I. The Journal of Immunology. 2012;189:916–923. doi: 10.4049/jimmunol.1200580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zufferey A, Speck ER, Machlus KR, et al. Mature murine megakaryocytes present antigen-MHC class I molecules to T cells and transfer them to platelets. Blood advances. 2017;1:1773–1785. doi: 10.1182/bloodadvances.2017007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcoux G, Laroche A, Hasse S, et al. Platelet EVs contain an active proteasome involved in protein processing for antigen presentation via MHC-I molecules. Blood. 2021 doi: 10.1182/blood.2020009957. [DOI] [PubMed] [Google Scholar]
- 17.Zanna MY, Yasmin AR, Omar AR, et al. Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species. International journal of molecular sciences. 2021:22. doi: 10.3390/ijms22158044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catani L, Sollazzo D, Trabanelli S, et al. Decreased expression of indoleamine 2,3-dioxygenase 1 in dendritic cells contributes to impaired regulatory T cell development in immune thrombocytopenia. Ann Hematol. 2013;92:67–78. doi: 10.1007/s00277-012-1556-5. [DOI] [PubMed] [Google Scholar]
- 19.Xu SQ, Wang CY, Zhu XJ, et al. Decreased indoleamine 2,3-dioxygenase expression in dendritic cells and role of indoleamine 2,3-dioxygenase-expressing dendritic cells in immune thrombocytopenia. Ann Hematol. 2012;91:1623–1631. doi: 10.1007/s00277-012-1451-0. [DOI] [PubMed] [Google Scholar]
- 20.Saito A, Yokohama A, Osaki Y, et al. Circulating plasmacytoid dendritic cells in patients with primary and Helicobacter pylori-associated immune thrombocytopenia. Eur J Haematol. 2012;88:340–349. doi: 10.1111/j.1600-0609.2011.01745.x. [DOI] [PubMed] [Google Scholar]
- 21.Clancy R. Cellular immunity to autologous platelets and serum-blocking factors in idiopathic thrombocytopenic purpura. Lancet. 1972;1:6–9. doi: 10.1016/s0140-6736(72)90003-7. [DOI] [PubMed] [Google Scholar]
- 22.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zufferey A, Kapur R, Semple JW. Pathogenesis and Therapeutic Mechanisms in Immune Thrombocytopenia (ITP) J Clin Med. 2017;6 [Google Scholar]
- 24.Semple JW, Milev Y, Cosgrave D, et al. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87:4245–4254. [PubMed] [Google Scholar]
- 25.Semple JW, Kapur R. Platelet immunology from the inside out. ISBT Science Series. 2020 [Google Scholar]
- 26.Karolczak K, Watala C. Blood Platelets as an Important but Underrated Circulating Source of TGFβ. International journal of molecular sciences. 2021:22. doi: 10.3390/ijms22094492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JD, Chang TK, Lin HK, Huang FL, Wang CJ, Lee HJ. Reduced expression of transforming growth factor-β1 and correlated elevation of interleukin-17 and interferon-γ in pediatric patients with chronic primary immune thrombocytopenia (ITP) Pediatr Blood Cancer. 2011;57:636–640. doi: 10.1002/pbc.22984. [DOI] [PubMed] [Google Scholar]
- 28.Andersson PO, Olsson A, Wadenvik H. Reduced transforming growth factor-beta1 production by mononuclear cells from patients with active chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2002;116:862–867. doi: 10.1046/j.0007-1048.2002.03345.x. [DOI] [PubMed] [Google Scholar]
- 29.Andersson PO, Stockelberg D, Jacobsson S, Wadenvik H. A transforming growth factor-beta1-mediated bystander immune suppression could be associated with remission of chronic idiopathic thrombocytopenic purpura. Ann Hematol. 2000;79:507–513. doi: 10.1007/s002770000177. [DOI] [PubMed] [Google Scholar]
- 30.Panitsas FP, Theodoropoulou M, Kouraklis A, et al. Adult chronic idiopathic thrombocytopenic purpura (ITP) is the manifestation of a type-1 polarized immune response. Blood. 2004;103:2645–2647. doi: 10.1182/blood-2003-07-2268. [DOI] [PubMed] [Google Scholar]
- 31.Roncarolo MG, Gregori S, Bacchetta R, Battaglia M, Gagliani N. The Biology of T Regulatory Type 1 Cells and Their Therapeutic Application in Immune-Mediated Diseases. Immunity. 2018;49:1004–1019. doi: 10.1016/j.immuni.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Najaoui A, Bakchoul T, Stoy J, et al. Autoantibody-mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP) Eur J Haematol. 2012;88:167–174. doi: 10.1111/j.1600-0609.2011.01718.x. [DOI] [PubMed] [Google Scholar]
- 33.Kurata Y, Curd JG, Tamerius JD, McMillan R. Platelet-associated complement in chronic ITP. Br J Haematol. 1985;60:723–733. doi: 10.1111/j.1365-2141.1985.tb07477.x. [DOI] [PubMed] [Google Scholar]
- 34.Cheloff AZ, Kuter DJ, Al-Samkari H. Serum Complement Levels in Immune Thrombocytopenia: Characterization and Relation to Clinical Features. Blood. 2019;134:1087. doi: 10.1002/rth2.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broome CM, Röth A, Kuter DJ, et al. Long-Term Safety and Efficacy of Sutimlimab in Patients with Chronic Immune Thrombocytopenia. Blood. 2020;136:14–15. [Google Scholar]
- 36.Pross HF, Jondal M. Cytotoxic lymphocytes from normal donors. A functional marker of human non-T lymphocytes. Clin Exp Immunol. 1975;21:226–235. [PMC free article] [PubMed] [Google Scholar]
- 37.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 38.Semple JW, Bruce S, Freedman J. Suppressed natural killer cell activity in patients with chronic autoimmune thrombocytopenic purpura. Am J Hematol. 1991;37:258–262. doi: 10.1002/ajh.2830370409. [DOI] [PubMed] [Google Scholar]
- 39.Ebbo M, Audonnet S, Grados A, et al. NK cell compartment in the peripheral blood and spleen in adult patients with primary immune thrombocytopenia. Clinical immunology (Orlando, Fla) 2017;177:18–28. doi: 10.1016/j.clim.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 40.El-Rashedi FH, El-Hawy MA, Helwa MA, SS Abd-Allah. Study of CD4 +, CD8 +, and natural killer cells (CD16 +, CD56 +) in children with immune thrombocytopenic purpura. Hematology/oncology and stem cell therapy. 2017;10:8–14. doi: 10.1016/j.hemonc.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Paolini R, Bernardini G, Molfetta R, Santoni A. NK cells and interferons. Cytokine Growth Factor Rev. 2015;26:113–120. doi: 10.1016/j.cytogfr.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Cook KD, Kline HC, Whitmire JK. NK cells inhibit humoral immunity by reducing the abundance of CD4+ T follicular helper cells during a chronic virus infection. J Leukoc Biol. 2015;98:153–162. doi: 10.1189/jlb.4HI1214-594R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rydyznski CE, Cranert SA, Zhou JQ, et al. Affinity Maturation Is Impaired by Natural Killer Cell Suppression of Germinal Centers. Cell Rep. 2018;24:3367–3373. doi: 10.1016/j.celrep.2018.08.075. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hymes KB, Karpatkin S. In vitro suppressor T lymphocyte dysfunction in autoimmune thrombocytopenic purpura associated with a complement-fixing antibody. Br J Haematol. 1990;74:330–335. doi: 10.1111/j.1365-2141.1990.tb02591.x. [DOI] [PubMed] [Google Scholar]
- 45.Liu B, Zhao H, Poon MC, et al. Abnormality of CD4(+)CD25(+) regulatory T cells in idiopathic thrombocytopenic purpura. Eur J Haematol. 2007;78:139–143. doi: 10.1111/j.1600-0609.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Zhong H, Bao W, et al. Defective regulatory B-cell compartment in patients with immune thrombocytopenia. Blood. 2012;120:3318–3325. doi: 10.1182/blood-2012-05-432575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denys A, Udalova IA, Smith C, et al. Evidence for a dual mechanism for IL-10 suppression of TNF-alpha production that does not involve inhibition of p38 mitogen-activated protein kinase or NF-kappa B in primary human macrophages. J Immunol. 2002;168:4837–4845. doi: 10.4049/jimmunol.168.10.4837. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong L, Jordan N, Millar A. Interleukin 10 (IL-10) regulation of tumour necrosis factor alpha (TNF-alpha) from human alveolar macrophages and peripheral blood monocytes. Thorax. 1996;51:143–149. doi: 10.1136/thx.51.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bao W, Bussel JB, Heck S, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116:4639–4645. doi: 10.1182/blood-2010-04-281717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aslam R, Segel GB, Burack R, et al. Splenic lymphocyte subtypes in immune thrombocytopenia: increased presence of a subtype of B-regulatory cells. Br J Haematol. 2016;173:159–160. doi: 10.1111/bjh.13567. [DOI] [PubMed] [Google Scholar]
- 51.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Highfill SL, Rodriguez PC, Zhou Q, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hou Y, Feng Q, Xu M, et al. High-dose dexamethasone corrects impaired myeloid-derived suppressor cell function via Ets1 in immune thrombocytopenia. Blood. 2016;127:1587–1597. doi: 10.1182/blood-2015-10-674531. [DOI] [PubMed] [Google Scholar]
- 54.Aslam R, Burack WR, Segel GB, McVey M, Spence SA, Semple JW. Intravenous immunoglobulin treatment of spleen cells from patients with immune thrombocytopenia significantly increases the percentage of myeloid-derived suppressor cells. Br J Haematol. 2017 doi: 10.1111/bjh.14542. [DOI] [PubMed] [Google Scholar]
- 55.Karpatkin S. Autoimmune thrombocytopenic purpura. Blood. 1980;56:329–343. [PubMed] [Google Scholar]
- 56.Stasi R, Provan D. Management of immune thrombocytopenic purpura in adults. Mayo Clin Proc. 2004;79:504–522. doi: 10.4065/79.4.504. [DOI] [PubMed] [Google Scholar]
- 57.Chugh S, Darvish-Kazem S, Lim W, et al. Rituximab plus standard of care for treatment of primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2015;2:e75–e81. doi: 10.1016/S2352-3026(15)00003-4. [DOI] [PubMed] [Google Scholar]
- 58.Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 59.Ahn YS, Harrington WJ, Simon SR, Mylvaganam R, Pall LM, So AG. Danazol for the treatment of idiopathic thrombocytopenic purpura. N Engl J Med. 1983;308:1396–1399. doi: 10.1056/NEJM198306093082306. [DOI] [PubMed] [Google Scholar]
- 60.Godeau B, Durand JM, Roudot-Thoraval F, et al. Dapsone for chronic autoimmune thrombocytopenic purpura: a report of 66 cases. Br J Haematol. 1997;97:336–339. doi: 10.1046/j.1365-2141.1997.412687.x. [DOI] [PubMed] [Google Scholar]
- 61.Cooper N, Kruse A, Kruse C, et al. Immune thrombocytopenia (ITP) World Impact Survey (I-WISh): Impact of ITP on health-related quality of life. Am J Hematol. 2021;96:199–207. doi: 10.1002/ajh.26036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavord S, Thachil J, Hunt BJ, et al. Practical guidance for the management of adults with immune thrombocytopenia during the COVID-19 pandemic. Br J Haematol. 2020;189:1038–1043. doi: 10.1111/bjh.16775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bradbury CA, Pell J, Hill Q, et al. Mycophenolate Mofetil for First-Line Treatment of Immune Thrombocytopenia. N Engl J Med. 2021;385:885–895. doi: 10.1056/NEJMoa2100596. [DOI] [PubMed] [Google Scholar]
- 64.Kuter DJ. New drugs for familiar therapeutic targets: thrombopoietin receptor agonists and immune thrombocytopenic purpura. Eur J Haematol Suppl. 2008:9–18. doi: 10.1111/j.1600-0609.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 65.Li J, Yang C, Xia Y, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98:3241–3248. doi: 10.1182/blood.v98.12.3241. [DOI] [PubMed] [Google Scholar]
- 66.Ghanima W, Cooper N, Rodeghiero F, Godeau B, Bussel JB. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019;104:1112–1123. doi: 10.3324/haematol.2018.212845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al-Samkari H, Kuter DJ. Optimal use of thrombopoietin receptor agonists in immune thrombocytopenia. Ther Adv Hematol. 2019;10 doi: 10.1177/2040620719841735. 2040620719841735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annu Rev Med. 2009;60:193–206. doi: 10.1146/annurev.med.60.042307.181154. [DOI] [PubMed] [Google Scholar]
- 69.Kuter DJ, Rummel M, Boccia R, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363:1889–1899. doi: 10.1056/NEJMoa1002625. [DOI] [PubMed] [Google Scholar]
- 70.Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013;98:10–23. doi: 10.1007/s12185-013-1382-0. [DOI] [PubMed] [Google Scholar]
- 71.Kuter DJ, Newland A, Chong BH, et al. Romiplostim in adult patients with newly diagnosed or persistent immune thrombocytopenia (ITP) for up to 1 year and in those with chronic ITP for more than 1 year: a subgroup analysis of integrated data from completed romiplostim studies. Br J Haematol. 2019;185:503–513. doi: 10.1111/bjh.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bussel JB, Pinheiro MP. Eltrombopag. Cancer Treat Res. 2011;157:289–303. doi: 10.1007/978-1-4419-7073-2_17. [DOI] [PubMed] [Google Scholar]
- 73.Erickson-Miller CL, Delorme E, Tian SS, et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009;27:424–430. doi: 10.1634/stemcells.2008-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jurczak W, Chojnowski K, Mayer J, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol. 2018;183:479–490. doi: 10.1111/bjh.15573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cooper N, Hill QA, Grainger J, et al. Tapering and Discontinuation of Thrombopoietin Receptor Agonist Therapy in Patients with Immune Thrombocytopenia: Results from a Modified Delphi Panel. Acta Haematol. 2021:1–9. doi: 10.1159/000510676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaja F, Carpenedo M, Baratè C, et al. Tapering and discontinuation of thrombopoietin receptor agonists in immune thrombocytopenia: Real-world recommendations. Blood Rev. 2020;41 doi: 10.1016/j.blre.2019.100647. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez-Lopez TJ, Sanchez-Gonzalez B, Pascual C, et al. Sustained response after discontinuation of short-and medium-term treatment with eltrombopag in patients with immune thrombocytopenia. Platelets. 2015;26:83–86. doi: 10.3109/09537104.2013.870987. [DOI] [PubMed] [Google Scholar]
- 78.Provan D, Taylor L, Nandigham R, Doobaree U, Kalkur P, Newland AC. Sustained Responses following Treatment with Romiplostim in Immune Thrombocytopenia: A Single-Centre Experience. Journal of Hematology & Thromboembolic Diseases. 2014;2:147–149. [Google Scholar]
- 79.Ghadaki B, Nazi I, Kelton JG, et al. Sustained remissions of immune thrombocytopenia associated with the use of thrombopoietin receptor agonists. Transfusion. 2013;53:2807–2812. doi: 10.1111/trf.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scheinberg P. Activity of eltrombopag in severe aplastic anemia. Blood Adv. 2018;2:3054–3062. doi: 10.1182/bloodadvances.2018020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: Results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93:921–930. doi: 10.1002/ajh.25125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bussel JB, Arnold DM, Boxer MA, et al. Long-term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program. Am J Hematol. 2019;94:546–553. doi: 10.1002/ajh.25444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jaime-Pérez JC, Ramos-Dávila EM, Meléndez-Flores JD, Gómez-De León A, Gómez-Almaguer D. Insights on chronic immune thrombocytopenia pathogenesis: A bench to bedside update. Blood Rev. 2021 doi: 10.1016/j.blre.2021.100827. [DOI] [PubMed] [Google Scholar]
- 84.Newland AC, Sánchez-González B, Rejtő L, et al. Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia. Am J Hematol. 2020;95:178–187. doi: 10.1002/ajh.25680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zuercher AW, Spirig R, Morelli AB, Rowe T, Käsermann F. Next-generation Fc receptor-targeting biologics for autoimmune diseases. Autoimmun Rev. 2019 doi: 10.1016/j.autrev.2019.102366. [DOI] [PubMed] [Google Scholar]
- 86.Langrish CL, Bradshaw JM, Francesco MR, et al. Preclinical Efficacy and Anti-Inflammatory Mechanisms of Action of the Bruton Tyrosine Kinase Inhibitor Rilzabrutinib for Immune-Mediated Disease. J Immunol. 2021;206:1454–1468. doi: 10.4049/jimmunol.2001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gavriilaki E, Peffault de Latour R, Risitano AM. Advancing therapeutic complement inhibition in hematologic diseases: PNH and beyond. Blood. 2021 doi: 10.1182/blood.2021012860. blood.2021012860. [DOI] [PubMed] [Google Scholar]
- 88.Cheloff AZ, Kuter DJ, Al-Samkari H. Serum complement levels in immune thrombocytopenia: Characterization and relation to clinical features. Res Pract Thromb Haemost. 2020;4:807–812. doi: 10.1002/rth2.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ulrichts P, Guglietta A, Dreier T, et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest. 2018;128:4372–4386. doi: 10.1172/JCI97911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robak T, Kaźmierczak M, Jarque I, et al. Phase 2 multiple-dose study of an FcRn inhibitor, rozanolixizumab, in patients with primary immune thrombocytopenia. Blood Adv. 2020;4:4136–4146. doi: 10.1182/bloodadvances.2020002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lasica M, Tam CS. Bruton Tyrosine Kinase Inhibitors in Chronic Lymphocytic Leukemia: Beyond Ibrutinib. Hematol Oncol Clin North Am. 2021;35:761–773. doi: 10.1016/j.hoc.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 92.Kuter D, Tzvetkov NE, Kaplan M, Z Mayer J, Choi P. Immune Thrombocytopenia: Extended Follow-up and Long-term Analyses with Optimal Dose. International Society on Thrombosis and Haemostasis Virtual Congress 2021. 2021. Phase I/II Ongoing Study of Rilzabrutinib, an Oral Bruton Tyrosine Kinase Inhibitor. OC 72.2. [Google Scholar]
- 93.Crickx E, Audia S, Robbins A, et al. Daratumumab, an original approach for treating multi-refractory autoimmune cytopenia. Haematologica. 2021 doi: 10.3324/haematol.2021.279232. [DOI] [PMC free article] [PubMed] [Google Scholar]