Abstract
Tuberculosis (TB) remains a major infectious disease across the globe. With increasing TB infections and a rise in multi-drug resistance, rapid diagnostic modalities are required to achieve TB control. Radiological investigations and microbiological tests (microscopic examination, cartridge-based nucleic acid amplification tests, and cultures) are most commonly used to diagnose TB. Histopathological/cytopathological examinations are also required for an accurate diagnosis in many patients.
The causative agent, Mycobacterium tuberculosis (Mtb), is known to circumvent the host's immune system. Circulating microRNAs (miRNAs) play a crucial role in biological pathways and can be used as a potential biomarker to detect tuberculosis. miRNAs are small non-coding RNAs and negatively regulate gene expression during post-transcriptional regulation. The differential expression of miRNAs in multiple clinical samples in tuberculosis patients may be helpful as potential disease biomarkers. This review summarizes the literature on miRNAs in various clinical samples as biomarkers for TB diagnosis.
Keywords: microRNAs, Profiling, Diagnostic biomarkers, Tuberculosis
Abbreviation list
- Mtb
Mycobacterium tuberculosis
- PCR
Polymerase chain reaction
- miRNA
micro- Ribonucleic Acid
- CT
Computed Tomography
- IFN-γ
Interferon-gamma
- SIRT-1
NAD-dependent deacetylase sirtuin-1
- BAL
Bronchoalveolar lavage
- EBB
Endobronchial biopsy
- TBLB
Transbronchial lung biopsy
- EBUS-TBNA
Endobronchial ultrasound guided transbronchial needle aspiration
- EBC
Exhaled breath condensate
- PBMC
peripheral blood mononuclear cell
- NFAT5
nuclear factor of activated T-cells 5
- NFkB
Nuclear factor k beta
- CXCL2
C-X-C Motif Chemokine Ligand 2
- CCL3
C-C Motif Chemokine Ligand 3
- IL
Interleukin
- TGF-β
Transforming growth factor-beta
- TLR/MyD88
toll-like receptor/myeloid differentiation factor 88
- TLR
Toll like receptor
- NK cell
Natural killer cell
- IGF
Insulin growth factor
- TNF
Tumour necrosis factor
- TRAF 3
TNF receptor-associated factor 3
- LPS
Lipopolysaccharide.
1. Introduction
Due to tuberculosis, more than 1 million people die every year in low-income and middle-income countries [1]. According to current reports, one-third of the world population is latently infected with tuberculosis [2]. However, only 5–10% of infected people develop active tuberculosis in their lifetime [3]. The causative bacillus, i.e. Mycobacterium tuberculosis (Mtb), commonly infects the respiratory system by inhalation and reaches the alveolar space [4]. Further, mycobacteria undergo phagocytosis by the alveolar macrophages and attribute to granuloma formation. Another report suggests that Mtb can modulate cellular processes like cytokine production and phagolysosome maturation in macrophages and dendritic cells [5]. Understanding the pathogenesis is crucial to the success of tuberculosis control programs.
For TB control, accurate and rapid modalities for the initial diagnosis are required. However, the standard methods to detect tuberculosis involve the growth of microorganisms in a selective medium that requires 3–12 weeks [6]. Smear examinations are also used to detect tuberculosis, but sputum smears have low sensitivity [7]. Although the PCR and immunological tests based evaluation of tuberculosis are rapid diagnostic methods [8], false-positive and negative results make them unreliable. Other reliable diagnosis modalities include demonstrating granulomatous inflammation in the affected organs and a compatible clinico-radiological profile [9]. The various modalities for tissue sampling in intrathoracic TB include endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), bronchoalveolar lavage (BAL), endobronchial biopsy (EBB), transbronchial lung biopsy (TBLB), or percutaneous sampling modalities [10]. These are invasive diagnostic modalities that require super-speciality facilities and expertise. To improve non-invasive and early diagnosis, the global scientific community continuously researches new diagnostic modalities.
Accumulating evidence suggests that most of the cell-mediated immune responses are controlled by microRNAs (miRNAs), and the modulation of miRNA expression associated with these biological processes is one of the crucial strategies to prevent the spread of infection [[11], [12], [13], [14], [15], [16]]. MiRNAs are small, single-stranded, non-coding RNAs that bind to the specific gene to modulate its expression [17,18]. These involve multiple cellular processes, such as cell cycle control, apoptosis, and several developmental and physiological processes [19]. Alteration of miRNAs expressions and gene expressions is associated with multiple respiratory diseases' pathogenesis.
Several studies have suggested the role of circulating miRNAs in patients with pulmonary tuberculosis and identified miRNA signatures that could discriminate between patients with active tuberculosis and healthy controls or latent tuberculosis [20]. Currently, global TB research has focused on the importance of miRNAs in the pathogenesis of tuberculosis. Several researchers have explored the role of thousands of miRNA effects in pathogenesis and attempted to identify new biomarkers for diagnosing tuberculosis in different patients' samples [[20], [21], [22]]. Given the importance of miRNAs as potential biomarkers for detecting tuberculosis, we have reviewed the literature on identifying miRNAs in different sample types and the role of the identified miRNAs in Mtb infected patients.
This review article summarizes recently discovered miRNAs isolated from different clinical samples in patients with tuberculosis. We have also highlighted their role in the pathogenesis of tuberculosis and their potential as TB biomarkers. This review may help understand how these small molecules play a crucial role in TB pathogenesis and immune response and using them as potential biomarkers to detect tuberculosis.
2. Methods
2.1. Inclusion criteria
Both PubMed and EMBASE electronic databases were screened for relevant studies up to November 2021. Abstracts and full texts were reviewed for information regarding eligibility criteria, excluding abstract-only studies and studies not in English.
2.2. Search strategy
A systematic search of the PubMed and EMBASE databases was performed to extract relevant studies using the following search terms: [(micro RNA OR Mirna OR snRNA) AND (tuberculosis OR tb OR tuberculous OR tubercular)]. The “Zotero” reference manager was used to create a database, and the electronic searches were added to the database. The full text of the qualifying articles was retrieved and studied in detail. The following information was extracted: (a) publication details (authors, year of publication), (b) miRNAs in inflammation and pathogenesis, and (c) miRNAs identified in different clinical samples.
2.3. Method of the review
Two reviewers independently screened citations and abstracts to identify articles potentially meeting the inclusion criteria. Full-text versions were retrieved and individually screened by two reviewers to determine whether they met the inclusion criteria. Disagreements in inclusion criteria were resolved through discussion with a third reviewer.
3. Results
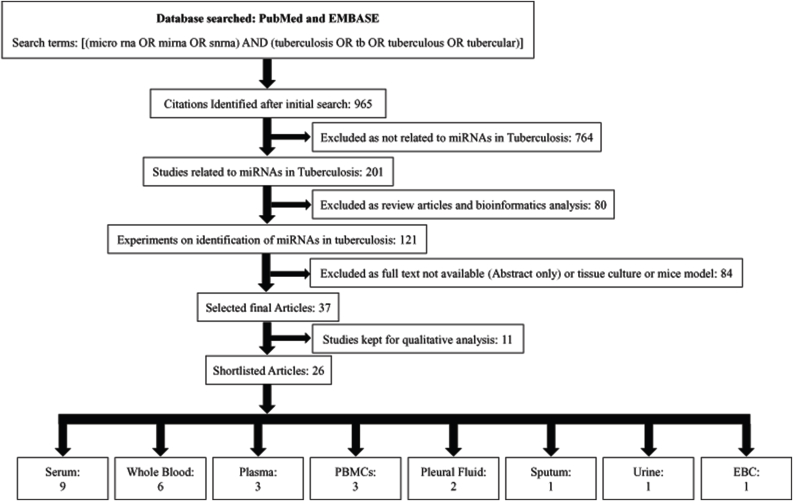
The initial search revealed a total of 965 studies, of which 764 were excluded as those studies were not related to miRNAs in tuberculosis (Fig. 1). We excluded 80 other articles by screening titles and abstracts as they were review articles and bioinformatics analysis. Further, 84 articles were excluded because of the unavailability of full text (abstract only), were cell culture studies, or mice models of tuberculosis infections. Thirty-seven studies were included in the qualitative analysis as these studies described the differential expression of miRNAs in various clinical samples of tuberculosis patients. We further shortlisted 26 articles focusing on miRNAs profiling and divided them into eight sub-groups based on the clinical samples used for miRNAs study in tuberculosis patients. A flow diagram of study screening and selection is shown in Fig. 1.
Fig. 1.
Methodology of a systematic review of the literature regarding miRNAs in Tuberculosis.
3.1. Identification of miRNAs in different clinical samples
Screening methods for tuberculosis based on disease biomarkers may help detect subjects in the early stages of the disease. In the last few decades, several studies have been published to identify miRNAs in tuberculosis clinical samples like whole blood, plasma, serum, peripheral blood mononuclear cells (PBMCs), sputum, Pleural fluid, Urine, and Exhaled Breath Condensates (EBCs) collected from patients with active pulmonary tuberculosis. These studies employed unbiased methods like miRNAs microarray analysis or next-generation sequencing techniques or selecting inflammatory and apoptosis-associated candidate miRNAs profiling. The RNA isolation method is crucial in all these techniques, influencing the results. Accumulating evidence suggests that miRNAs are abundantly present in exosomes. Hence, few studies have focused on exosome-vesicles associated with miRNAs isolated from any clinical samples. The identification of miRNAs from clinical samples in active tuberculosis are summarized in Table 1.
Table 1.
Identified miRNAs in clinical samples by profiling and their roles in pathology.
| S. No | Sample Type | Technique Used | microRNAs | Result | Function of miRNAs | Year | References |
|---|---|---|---|---|---|---|---|
| 1 | Serum | miRNAs Profiling Assay | miR-361-5p, miR-889, miR-576-3p | miR-361-5p, miR-889, miR-576-3p were up regulated in tuberculosis. |
miR-361-5p targets SP-1 transcription factor, a key signaling pathway for IL-10. miR-889 are associated with respiratory system development. miR-576-3p involved in immune system development. |
2012 | [82] |
| miRCURY LNA array | miR-3125, miR-93∗, and miR-29a | miR-3125 was found to be down regulated miR-93∗, and miR-29a were up regulated |
miR-93∗ involves in promoting tumor growth by targeting the tumor suppressor gene Fus1 and integrin- 8. miR-29a is a regulator of Wnt signaling pathway genes and lipid metabolism. | 2011 | [83] | ||
| TaqMan miRNA array | miR-1249, miR-1178, miR-668, miR-220b, let-7i∗, miR-941, miR-212, miR-28-3p, miR-620, miR-532-5p, miR-17, miR-151-5p, miR-20b, miR-369-3p, miR-573, miR-1263, miR103, miR-182, miR-196b, miR-130b∗, miR-1284, let-7b | miR-1249, miR-1178, miR-668 were up regulated. miR-220b, let-7i∗, miR-941, miR-212, miR-28-3p, miR-620, miR-532-5p, miR-17, miR-151-5p, miR-20b, miR-369-3p, miR-573, miR-1263, miR-103, miR-182, miR-196b, miR-130b∗, miR-1284, let-7b were down regulated. |
miR-1249, let-7i, miR-28-3p, miR-103, involve in glucose metabolism. miR-1178, miR-668, miR-220b, miR-941, miR-151-5p, miR-573, miR-1263, let-7b play role in inflammation. miR-212 regulates the expression of SIRT-1 gene expression. miR-620, miR-532-5p, miR-17, miR-20b, miR-369-3p, miR-182, miR-196b, miR-130b, miR-1284 regulate cancer cell proliferation. |
2015 | [84] | ||
| Solexa Sequencing | miR-17-5p, miR-20b-5p, and miR-423-5p | miR-17-5p, miR-20b-5p, and miR-423-5p were up-regulated | miR-17-5p and miR-423-5p regulate cell proliferation. miR-20b-5p regulates wound healing process. |
2019 | [58] | ||
| Solexa Sequencing | miR-196b, miR-516b, miR-486-5p and miR-376c | miR-196b, miR-516b, miR-486-5p and miR-376c up regulated | miR-196b inhibits proliferation and induces apoptosis. miR-516b, miR-486-5p and miR-376c target nuclear factor of activated T-cells 5 and regulate inflammation. |
2014 | [43] | ||
| TaqMan Low Density Array (TLDA) | let-7e, miR146a, miR-148a, miR-16, miR-192, miR-193a-5p, miR-25, miR-365, miR-451, miR-532-5p, miR-590-5p, miR-660, miR-885-5p, miR-223∗, miR-30e | Differentially expressed in serum samples | Let7e, miR-146a, miR-16, miR-223 modulates inflammation. miR-148a regulates B cell tolerance and autoimmunity. miR-192, miR193a-5p, miR-25, miR-365, miR-451, miR-532-5p, miR-590-5p, miR-660, miR-885-5p, miR-30e regulate cancer cell proliferation and invasion. |
2013 | [20] | ||
| Solexa Sequencing | miR-21-5p, miR-92a-3p, and miR-148b-3p, miR-16 | miR-21-5p, miR-92a-3p, and miR-148b-3p, miR-16 were up regulated in tuberculosis | miR-21-5p, miR-148b-3p regulates cancer cell proliferation. miR-92a-3p regulates p38 MAPK/NFκB pathway. miR-16 plays role as an anti-inflammatory miRNA. |
2017 | [33] | ||
| Solexa Sequencing | miR-378, miR-483-5p, miR-22, miR-29c, miR-320b and miR-101 | miR-378, miR-483-5p, miR-22, miR-29c were up regulated and miR-320b and miR-101 were down regulated in tuberculosis | miR-378, miR-483-5p, miR-22, miR-29c, miR-320b, miR-101 regulates cancer cell proliferation. | 2013 | [51] | ||
| Small RNA transcriptome profiles | let-7e-5p, let-7d-5p, miR-450a-5p, miR-140-5p, miR-1246, miR-2110, miR-370-3p, miR-28-3p, and miR-193b-5p | let-7e-5p, let-7d-5p, miR-450a-5p, miR-140-5p were up regulated in latent tuberculosis infection and miR-1246, miR-2110, miR-370-3p, miR-28-3p, and miR-193b-5p were up regulated in active tuberculosis infection. | Let7e-5p plays role in immune response in TB infection. miR-450a-5p inhibits autophagy, miR-140-5p regulates T cell differentiation. miR-1246, miR-2110 regulate cancer cell proliferation. miR-370-3p targets KDR/AKT signalling pathway gene and inhibits vascular smooth muscle cell proliferation. miR-28-3p regulates B cell lymphomas. miR-193b-3p regulates TGF-β gene. |
2019 | [40] | ||
| 2 | Whole Blood | Micro array | hsa-miR-150, hsa-miR-21, hsa-miR-29c and hsa-miR-194 | miR-150 was down regulated and miR-21, miR-29c, miR-194 were up regulated in tuberculosis | miR-150 negatively regulates natural killer cells. miR-21 promotes fibrosis. miR-29c targets IFN-γ and regulates immune cells. miR-194 inhibits innate antiviral immunity. |
2015 | [21] |
| Micro array | miR-1, miR-155, miR-31, miR-146a, miR-10a, miR-125b, miR-150 and miR-29 | miR-1, miR-155, miR-31, miR-146a, miR-10a, miR-125b, miR-150 were down regulated and miR-29 was up regulated | miR-1 regulate cancer cell proliferation. miR-155 regulates the adaptive immune response. miR-31 regulates lipid metabolism. miR-146a regulates IL-6 production and inflammation. miR-10 regulates TGF-β signaling pathway genes. miR-125b regulates EMT. miR-150 negatively regulates natural killer cells. miR-29c targets IFN-γ and regulates immune cells. |
2016 | [31] | ||
| Micro array | miR-182, miR-355, miR-15b∗, miR-340, and miR-144 | miR-182, miR-355, miR-15b∗, miR-340 were down regulated and miR-144 up regulated | miR-182, miR-355, miR-15b, miR-340, miR-144 regulate cancer cell proliferation. | 2012 | [85] | ||
| TaqMan miRNAs assay | miR-26a, miR-29a, and miR-142- 3p | miR-26a, miR-29a, and miR-142- 3p were down regulated | miR-26a regulates cancer cell proliferation. miR-29a is a regulator of Wnt signaling pathway genes and lipid metabolism. miR-142-3p regulates mesenchymal cells differentiation and proliferation. |
2013 | [50] | ||
| Small RNA sequencing | miR-1197, miR-1268b, miR-1275, miR-1299, miR-146a-3p, miR-150-3p, miR-150-5p, miR-30a-3p, miR-3135a, miR-342-3p, miR-3679-5p, miR-4467, miR-449a, miR-451b, miR-493-3p, miR-503-5p, miR-618, miR-629-5p, miR-6513-5p, miR-6734-3p, miR-6774-3p, miR-6802-3p, miR-708-3p, miR-708-5p, miR-874-3p, miR-874-5p, miR-876-3p, miR-877-3p, miR-92a-1-5p, miR-941 | miR-1197, miR-1299, miR-30a-3p, miR-3135a, miR-4467, miR-449a, miR-451b, miR-493-3p, miR-503-5p, miR-629-5p, miR-6513-5p, and miR-941 were up regulated. miR-1268b, miR-1275, miR-146a-3p, miR-150-3p, miR-150-5p, miR-342-3p, miR-3679-5p, miR-618, miR-6734-3p, miR-6774-3p, miR-6802-3p, miR-708-3p, miR-708-5p, miR-874-3p, miR-874-5p, miR-876-3p, miR-877-3p, miR-92a-1-5p were down regulated |
miR-1197, miR-1268b, miR-1299, miR-30a-3p, miR-342-3p, miR-449a, miR-451b, miR-493-3p, miR503-5p, miR-629-5p, miR-6734, miR-708, miR-874, miR-876, miR-92a-5p regulate cancer cell proliferation. miR-1275 regulates adipogenesis. miR-146a regulates IL-6 production and inflammation. miR-150 negatively regulates natural killer cells. miR-618 can regulate TIMP-1 expression which regulates MMP9 expression. miR-877-3p targets SMAD7 and regulates myofibroblast differentiation. |
2018 | [51] | ||
| GeneChip miRNAs array | miR-20a, miR-20b, miR-26a, miR-106a, miR-191, miR-486, miR-3128, miR-1468, miR-3201, miR-8084 | miR-20a, miR-20b, miR-26a, miR-106a, miR-191, miR-486 were up regulated and miR-3128, miR-1468, miR-3201, miR-8084 were down regulated | miR-20 helps in the cardiac development. miR-26a helps the cellular development, growth, apoptosis and metastasis. miR-106a, miR-191, miR-486, miR-1468 regulate cancer cell proliferation. |
2019 | [44] | ||
| 3 | Plasma | miRNAs micro array | let-7b-5p, let-7d-5p, let-7g-5p, miR-30b-5p, and miR-331- 3p | let-7b-5p, let-7d-5p, let-7g-5p, miR-30b-5p, and miR-331- 3p were up regulated in active tuberculosis | Let-7 miRNAs family involves in many inflammatory pathways by targeting various genes. miR-30b-3p, miR-331-3p regulates cancer cell proliferation. | 2016 | [41] |
| Real time PCR array | miR-21-5p, miR-99b-5p, miR-29a-5p, miR-223-5p, miR-221-3p, miR-146a-5p, miR-26a-5p, miR-28-5p, miR-133a, and miR-652-3p | miR-99b-5p, miR-29a-5p, miR-223-5p, miR-221-3p and miR-28-5p were up regulated and miR-21-5p, miR-146a-5p, miR-26a-5p, miR-133a, and miR-652- 3p were down regulated in tuberculosis | miR-21 targets TGB-β signaling pathway genes and regulates fibrosis. miR-99b-5p, miR-29a-5p, miR-223-5p, miR-221-3p, miR-146a-5p, miR-28-5p, regulate cancer cell proliferation. miR-26a-5p targets ULK1 and regulates cardiac fibroblast collagen expression. miR-133a plays role in cardiac remodeling. miR-652-3p regulates endothelial cell proliferation. |
2018 | [47] | ||
| Solexa Sequencing | miR-769-5p, miR-320a, miR-22-3p | miR-769-5p, miR-320a, miR-22-3p were down regulated | miR-769-5p involves in the prognosis of pancreatic cancer and non-small cell lung cancer miR-320a involves in the modulation of cytokine production, cell proliferation, migration and invasion. miR-22-3p involves in the DNA damage machinery. |
2017 | [86] | ||
| 4 | PBMCs | miRNA micro array | miR-200c, miR-193a-3p, miR-595, miR-432, miR-155, miR-9, miR-141, miR-32, miR-29b, miR-152, miR-144 | miR-200c, miR-193a-3p, miR-595, miR-432, miR-155 and miR-9 were up regulated and miR-141, miR-32, miR-29b, miR-152, miR-144 were down regulated | miR-200c, miR-193a-3p, miR-432, miR-141, miR-152 regulate cancer cell proliferation. miR-595 targets SOX7 and promotes glioblastoma cell proliferation. miR-9 targets AKT/GSK3β signaling pathway genes. miR-32 and miR-144 target PTEN expression. miR-29b regulates the proliferation and apoptosis of pulmonary artery smooth muscle cells. miR-155 regulates the adaptive immune response. |
2012 | [29] |
| miRNA micro array | miR-424 and miR-365 | miR-424 and miR-365 were up regulated in tuberculosis | miR-424 and miR-365 regulate cancer cell proliferation. | 2011 | [22] | ||
| Small RNA sequencing | miR-23a-5p, miR-183-5p, miR-193a-5p, miR-941, miR-16-1-3p | miR-23a-5p, miR-183-5p, miR-193a-5p, and miR-941 were up regulated miR-16-1-3p was found to be down regulated |
miR-23a-5p involves in the autophagy pathway. miR-183-5p promotes cell proliferation and migration. miR-193a-5p involves in proliferation and migration in cancer. miR-941 targets WNT16 to enhances osteoclastogenesis. |
2020 | [87] | ||
| 5 | Pleural Fluid | Small RNA sequencing | miR-3615, miR-320c, miR-205-5p, miR-218-5p, miR-135a-5p, miR-200a-5p, miR-320b, and miR-378i | miR-320c, miR-205-5p, miR-135a-5p, miR-200a-5p were up regulated and miR-3615, miR-205-5p, miR-320b, and miR-378i were down regulated in tuberculosis | miR-320c regulates inflammation. miR205-5p targets SMAD2 and regulates extracellular production. miR-135a-5p regulates adipogenesis. miR-200a-5p regulates myocardial necroptosis. miR-320c targets c-Myc and regulates cell proliferation. |
2016 | [36] |
| Small RNA deep sequencing | miR-205-5p, miR-200c-3p, miR-429, miR-200b-3, miR-200a-3p, miR-203a-3p, miR-141-3p, miR-148a-3p, miR-451a, miR-150-5p | miR-205-5p, miR-200c-3p, miR-429, miR-200b-3, miR-200a-3p, miR-203a-3p, miR-141-3p, miR-148a-3p, miR-451a, miR-150-5p were up regulated in tuberculosis | miR-200 family members targets ZEB1/ZEB2 and regulates EMT. miR-429, miR-203a-3p, miR-451a, miR-150-5p regulate cancer cell proliferation. miR-141-3p regulates mesenchymal stem cells. miR-141a-3p regulates angiogenesis. |
2017 | [88] | ||
| 6 | Sputum | Exiqon miRCURYTM LNA arrays | miR-19b-2∗, miR-3179 and miR-147 | miR-3179 and miR-147 were up regulated and miR-19b-2∗ was down regulated | miR-147 suppresses the expression of TNF-α and IL-6 and play as an anti-inflammatory miRNA. | 2012 | [49] |
| 7 | Urine | Real time PCR | miR-625-3p and miR-155 | miR-625-3p and miR-155 were up regulated in tuberculosis | miR-625-3p regulates cancer cell proliferation. miR-155 regulates the adaptive immune response. |
2018 | [30] |
| 8 | EBC | miRNome array | miR-649, miR-1264, miR-2861, miR-574-5p, miR-453 | miR-649, miR-1264, miR-2861, miR-574-5p, miR-453 were up regulated | miR-1264 targets DNA methyltransferase-1 and regulates its expression. miR-2281 and miR-574-5p regulate cancer cell proliferation. |
2013 | [89] |
3.2. Common miRNAs identified in multiple clinical samples or multiple reports
By establishing differential expression, miRNAs can be used as potential biomarkers in different clinical samples from active tuberculosis patients. A biomarker indicates a normal biological process or a pathogenic process [23]. miRNAs are involved in many pathological processes and can be measured in various clinical samples. In the era of precision medicine, the identification and understanding of the function of specific miRNAs in clinical samples will play an important role. In recent studies, co-regulatory networks of miRNAs and their targeted transcription factor genes were analyzed in various clinical samples of tuberculosis patients [24,25]. Other studies have identified differential expression of miRNAs in patients with tuberculosis compared with healthy controls [26]. These studies may help us discover potential miRNAs as tuberculosis biomarkers. This review has screened published research articles that identified differentially expressed miRNAs in different clinical samples and shortlisted common miRNAs reported in more than one clinical sample in tuberculosis patients. The shortlisted common miRNAs are miR-155, miR-16, miR-200, Let-7, miR-486, miR-223, miR-99, miR-29, miR-21, miR-193, miR-365, miR-30, miR-20b, miR-146a, miR-31, miR-150 (Table 2).
Table 2.
Differential expression of miRNAs in multiple clinical samples.
| S. No | miRNAs | Remarks | Sample type | References |
|---|---|---|---|---|
| 1 | miR-155 | Up regulated | Serum | [27] |
| Plasma | [28] | |||
| PBMCs | [29] | |||
| Urine | [30] | |||
| 2 | miR-16 | Up regulated | Serum | [20,32,90] |
| Plasma | [34] | |||
| 3 | miR-200 | Up regulated | PBMCs | [29] |
| Pleural fluid | [36,88] | |||
| 4 | Let-7 family | Up regulated | Serum | [20,40] |
| Plasma | [41] | |||
| 5 | miR-486 | Up regulated | Serum | [43] |
| Whole Blood | [44] | |||
| 6 | miR-223 | Up regulated | Serum | [20] |
| Plasma | [47] | |||
| 7 | miR-99 | Up regulated | Serum | [27] |
| Plasma | [47] | |||
| 8 | miR-29 | Up regulated | Serum | [27,91] |
| Whole Blood | [21] | |||
| Plasma | [34,47] | |||
| 9 | miR-21 | Up regulated | Serum | [27,90] |
| Whole Blood | [21] | |||
| Plasma | [28] | |||
| 10 | miR-193 | Up regulated | Serum | [20] |
| PBMCs | [29] | |||
| 11 | miR-365 | Up regulated | Serum | [20] |
| PBMCs | [22] | |||
| 12 | miR-30 | Up regulated | Serum | [20] |
| Whole Blood | [92] | |||
| Plasma | [41] | |||
| 13 | miR-20 | Up regulated | Serum | [58] |
| Whole Blood | [44] | |||
| 14 | miR-146 | Down regulated | Serum | [20] |
| Whole Blood | [31,92] | |||
| Plasma | [47] | |||
| 15 | miR-31 | Down regulated | Whole Blood | [31] |
| PBMCs | [60] | |||
| 16 | miR-150 | Down regulated | Whole Blood | [21,31,92] |
miR-155 is one of the extensively studied miRNAs found to be up-regulated in serum, plasma, PBMCs, and urine samples of tuberculosis patients [[27], [28], [29], [30]]. However, two reports found decreased levels of miR-155 in plasma and whole blood of tuberculosis patients [31,32]. miR-155 promotes the survival of tuberculosis-specific T cells and affects the adaptive immune response. Another report has suggested that serum miR-155 levels negatively regulated the TB-suppressing activity of natural killer cells. miR-16 was found to be elevated in both plasma and serum samples of tuberculosis patients [20,[32], [33], [34]]. According to a report, the levels of miR-16 decreased after anti-tuberculosis treatment [32]. However, miR-16 has been shown as a tumour suppressor miRNA for chronic lymphocyte leukaemia [35]. miR-200 was found to be elevated in pleural fluid collected from tuberculosis patients published in two different articles [29,36,37]. miR-200 family members could regulate mesenchymal to epithelial transition by regulating the expression of ZEB1/ZEB2 (zinc-finger- and homeobox-containing transcriptional regulator delta-crystalline enhancer-binding factor) and Smad-interacting protein 1 [38]. Another report suggests that miR-200 can inhibit adenocarcinoma [39]. Let-7 was found to be up-regulated both in serum and plasma samples of tuberculosis patients [20,40,41]. The let-7 family was involved in the pathogenesis by targeting various vital genes. Another report suggested that nuclear factor k beta (NF-kB) inhibits the let-7 family through activation of Lin28B, and the let7 family directly inhibited the expression of interleukin 6 (IL6) [42]. miR-486 was found to be up-regulated in whole blood and serum samples collected from tuberculosis patients [43,44]. miR-486 may regulate the nuclear factor of activated T-cells 5 (NFAT5) pathway genes [43]. miR-223 was found to be up-regulated in serum and plasma samples of tuberculosis patients compared with healthy controls [20,45]. miR-223 may regulate chemo-attractants including CXCL2, CCL3, and IL-6 in myeloid cells and control leukocyte chemotaxis [46]. miR-99b was elevated in serum, and whole blood samples of tuberculosis patients [27,47]. It was also found to be down-regulated in plasma after the anti-tuberculosis treatment. miR-29a was found to be up-regulated in serum and plasma samples of tuberculosis patients [21,27,[47], [48], [49]]. Interestingly, miR-29a was down-regulated in plasma samples after the treatment of tuberculosis. However, in another report, the levels of miR-29a were found down-regulated in whole blood samples [50]. miR-21 was found to be elevated in serum, whole blood and plasma samples collected from tuberculosis patients compared with healthy controls [21,27,28,51]. mir-21 targets TGF-β signalling pathway genes and regulates the expression [52]. It plays a crucial role in pulmonary fibrosis [53]. However, another report found the down-regulation of miR-21 in plasma samples [54]. miR-193 was up-regulated in PBMCs and serum samples in tuberculosis patients [20,29]. miR-193 plays an essential role in cancer cell proliferation [55]. miR-365 was up-regulated in serum and PBMCs collected from tuberculosis patients [20,22]. miR-365 regulates IL-6 production in macrophages, leading to the immune response in pulmonary tuberculosis [56]. miR-30 was found elevated in plasma, serum, and whole blood samples of tuberculosis patients [20,41,51]. During tuberculosis activation, miR-30 may suppress toll-like receptor/myeloid differentiation factor 88 (TLR/MyD88) activation and cytokine production [57]. miR-20b was found to be up-regulated in serum and whole blood samples collected from patients with tuberculosis [44,58]. However, published evidence suggested that miR-20b alleviates the inflammatory response in the tuberculosis mice model [59]. Another report showed the down-regulation of miR-20b in serum samples collected from tuberculosis patients [54]. miR-146a is also extensively studied miRNAs found to be down-regulated in plasma and whole blood samples collected from tuberculosis patients [20,31,47,51]. miR-31 was found to be down-regulated in whole blood and PBMCs samples collected from patients with tuberculosis [31,60]. According to another report, miR-31 was up-regulated in asthma patients [61]. miR-150 was down-regulated in the whole blood sample collected from patients with tuberculosis [21,31]. The report suggests that miR-150 plays an important role in B cell development [62]. However, the identified miRNAs are yet to be validated in all the clinical samples independently.
The discrepancies in the expression of miR-155, miR-29a, miR-21, and miR-20b in different clinical samples might be because of many reasons those influence the outcome of miRNA analysis [[63], [64], [65]]. These may include differences in the time of sample collection, comorbidities, and heterogeneity in the population studied. Other than this, the normalization methods used by different reports are different, and the effect of drug treatment on miRNA response is still unclear. Hence, a different set of subjects may give different expression patterns. Furthermore, different acquisition platforms and data-analysis pipelines used for miRNA quantification can influence the study outcome.
To overcome these issues, we reviewed articles showing the role of these miRNAs in the pathogenesis of tuberculosis. These miRNAs showed the potential role associated with the expression pattern reported in maximum articles. Hence, we precisely focused on the expression pattern of miRNAs reported in maximum articles.
3.3. miRNAs as potential biomarkers of tuberculosis
The human genome may encode more than two thousand functional miRNAs, which regulate most protein-coding genes. Interestingly, one miRNA can target multiple genes, and multiple miRNAs can regulate one gene [66]. According to various reports, miRNAs can regulate gene expression involved in the innate or adaptive immune pathways upon Mtb infection in the host [26]. Hence, knowing their association with any immune response, specific miRNAs can be used as a potential diagnostic or prognostic biomarker for tuberculosis. We reviewed the role of the miRNAs in the pathogenesis of tuberculosis. The shortlisted miRNAs including miR-155, miR-146a, miR-21 and miR-9, miR-125b, miR-26-5p, miR-132-3p can regulate innate immune cell activation ([26,54]). Interestingly, miR-155, miR-146a, and miR-21 have been identified in three or more clinical samples collected from tuberculosis patients. However, the rest of the miRNAs were identified in at least one clinical sample. miRNAs including miR-21-5p, miR-146a-5p, miR-20b-5p, miR-223-3p, miR-27b-3p, miR-99b-5p, miR-125-5p, miR-142-3p, miR-144, miR-27a are involved in the regulation of inflammation. MiR-21, miR-146, miR-20, miR-223, and miR-99 were identified in two or more clinical samples.
Recent studies have explored the role and regulation of other pathological processes (including autophagy and apoptosis) involved during TB infection. miRNAs including miR-155, miR-27a, miR-889, miR-106a, miR-125, miR-142-3p, miR-17, miR-144-3p, miR-20a, miR-23a-5p, miR-26a regulate the activation of autophagy to support the intracellular survival of Mtb. MiR-155 and miR-20 were identified in two or more clinical samples collected from tuberculosis patients. The rest of the miRNAs were identified in at least one clinical sample. Similarly, miRNAs including miR-155, miR-20a-5p, miR-21, Let-7e, miR-29a, miR-27b, miR-145 regulate apoptosis during Mtb infection. miR-27 and miR-145 were identified in at least one clinical sample, but the rest of the miRNAs were identified in two or more clinical samples. The summary of the targeted genes of the identified miRNAs and their roles in the pathogenesis of tuberculosis is mentioned in Table 3.
Table 3.
Summary of the role of miRNAs in the pathogenesis of tuberculosis.
| S. No | Function | miRNAs | Targeted Gene | References |
|---|---|---|---|---|
| 1 | Innate Immune response | miR-155 | SHIP1 and TAB2 | [93] |
| miR-146a | IRAK-1/TRAF-6 | [94] | ||
| miR-21 | PDCD4 | [95] | ||
| miR-9 | NFkB1 | [96] | ||
| miR-125b | ERK1 | [97] | ||
| miR-26-5p | KLF4 | [98] | ||
| miR-132-3p | TLR | [99] | ||
| 2 | Regulation of inflammation | miR-21-5p | TLR4 | [100] |
| miR-146a-5p | TRAF-6 | [101] | ||
| miR-20b-5p | NLRP3 | [102] | ||
| miR-223-3p | NFIA | [103] | ||
| miR-27b-3p | Bag2 | [104] | ||
| miR-99b-5p | TNF-α and TNFRSF-4 | [105] | ||
| miR-125-5p | TNF-α | [106] | ||
| miR-142-3p | N-Wasp | [107] | ||
| miR-144 | INF-γ and TNF-α | [81] | ||
| miR-27a | IRAK4 | [108] | ||
| 3 | Autophagy | miR-155 | Rheb | [109] |
| miR-27a | Cacna2d3 | [110] | ||
| miR-889 | TWEAK | [111] | ||
| miR-106a | ULK1, ATG7, ATG16L1 | [112] | ||
| miR-125 | DRAM2 | [113] | ||
| miR-142-3p | ATG16L1 | [114] | ||
| miR-17 | ATG7 | [115] | ||
| miR-144-3p | ATG4a | [116] | ||
| miR-20a | ATG7 and ATG16L1 | [117] | ||
| miR-23a-5p | TLR2/MyD88/NF-κB | [118] | ||
| miR-26a | KLF4 | [98] | ||
| 4 | Apoptosis | miR-155 | FOXO3 | [119] |
| miR-20a-5p | JNK2 | [120] | ||
| miR-27b | Bag2 | [104] | ||
| miR-145 | cIAP1 | [121] | ||
| Let-7e | Caspase 3 | [122] | ||
| miR-29a | Caspase 7 | [123] | ||
| miR-21 | PI3/AKT/NFkB | [124] |
4. Discussion
Accumulating evidence suggests that the differential expression of miRNAs may represent the differential pathogenesis of tuberculosis in different cell types. For example, in Mtb, infected macrophages derived from mouse bone marrow showed up-regulation of miR-155 and down-regulated in the infected macrophages derived from peripheral blood mononuclear cells (PBMCs) [15]. Upon Mtb infection, the expression of miR-155 get induced in murine derived macrophages and augment TLR signalling, thereby providing an environment for Mtb multiplication [67]. This promotes Mtb-specific T cells' survival and enables adaptive immune response. However, serum miR-155 levels show negative regulation of the TB-suppressing activity of NK cells [68]. miR-155 binds indirectly regulates TNF-α production [69]. Overexpression of let-7e, miR-29a, and miR-886-5p was found in Mtb infected macrophages derived from human monocyte [70]. Interestingly, target prediction analysis reveals that important apoptosis mediator caspases 3 and 7 are potential targets of let-7e and miR-29a. miRNAs profiling in Mtb infected human macrophages showed downregulation of miR-155, miR-146a, miR-145, miR-222, miR-27a, and miR-27b [71,72]. miR-145 is a pro-apoptotic miRNA found to be down-regulated, in line with a suppressed virulent Mtb strain to induce apoptosis [73]. miR-222, miR-27a, and miR-27b play crucial roles in controlling inflammatory response and lipid metabolism. Mtb was infected in human monocytes, and a miRNA expression profile was performed, where nine miRNAs including miR-30a, miR-30e, miR-155, miR-1275, miR-3665, miR-3178, miR-4484, miR-4668-5p, and miR-4497 were found to be differentially expressed [74]. miR-30a is activated by the TLR pathway and regulates inflammation and islet function by targeting interleukin one alpha (IL-1α) [57]. Another report showed that miR-30e inhibits apoptosis and promotes hepatocytes proliferation [75]. miR-1275 negatively regulates the expression of insulin growth factor (IGF) pathway associated genes and helps in the modulation of cell differentiation, proliferation, and apoptosis [76]. The IGF associated genes have been associated with LPS induced NFκB activation and pro-inflammatory cytokines production in human macrophages [77]. miR-3178 is known to target TNF receptor-associated factor 3 (TRAF 3) genes and ameliorates inflammation in gastric epithelial cells [78]. Bioinformatics analysis of miR-4484 suggests that the microRNA targets TGF-β and other Wnt/β-catenin signalling pathway-associated genes regulates apoptosis and is involved in collagen regulation related genes [79]. Another miRNA, miR-4668-5p, also targets TGF-β pathway-related genes and is involved in allergic diseases [80]. Other reports suggest that miR-144 expresses in T cells of active tuberculosis patients [81]. The up-regulation of miR-144 reduces T cell proliferation and inhibits the secretion of INF-γ and TNF-α.
Many research groups are identifying the detailed role of these differentially expressed miRNAs in the pathogenesis of tuberculosis. These studies may provide a deep understanding of tuberculosis pathogenesis and provide a good foundation for developing reliable biomarkers for the diagnosis.
However, the expression pattern of miRNAs ultimately depends on the study design. One miRNA may be differentially expressed in one study set, and the other studies may have used the same miRNA as a housekeeping gene. Similarly, one miRNA may be differentially expressed in certain disease conditions, and the same miRNA may also have shown differential expression in healthy controls in other studies.
The current article has focused on differentially expressed miRNAs in more than one clinical sample with the same expression pattern. However, a larger population size is required in different studies to establish miRNAs as potential biomarkers for tuberculosis. The diagnostic odds ratio of two oppositely expressed miRNAs may provide more accurate results as a diagnostic marker.
5. Conclusion
Tuberculosis remains one of the deadliest infectious diseases in the world. The causative agent, Mycobacterium tuberculosis, regulates the host defence system to survive and replicates through various pathways. In recent studies, the role of miRNAs in the innate and adaptive immune responses upon Mtb infection has been explored. miRNAs are involved in many pathways to alter immune responses by regulating various gene expressions in the host cell. However, there are some limitations in this regard. miRNAs are not entirely gene-specific or disease-specific. Apart from this, innate and adaptive immune response, autophagy and apoptosis are common phenomena in different inflammatory diseases. Same miRNAs can be involved in the pathogenesis of such inflammatory diseases, and it is challenging to separate tuberculosis from other inflammatory diseases based on the miRNAs signatures. Hence, multiple studies have focused on the signatures of shortlisted miRNAs and their expression associated with the pathogenesis of tuberculosis. With this aim, we have reviewed research articles published on differentially expressed miRNAs in clinical samples collected from tuberculosis patients and shortlisted miRNAs consistently shown in more than one clinical sample. This could help in identifying candidate miRNAs for tuberculosis detection.
In this review, we have found common miRNAs shown in various clinical samples in multiple studies. miRNA analysis was performed in the whole blood, serum, plasma, PBMCs, pleural fluid, sputum, urine, and EBC. Most miRNAs are detected in blood samples, which can easily be accessible from tuberculosis patients. Further, these miRNAs are also studied in the pathogenesis pathways of tuberculosis, such as innate and adaptive immune response, autophagy, and apoptosis. However, exploring the specific role of these miRNAs in pathogenesis is ongoing. Moreover, the identified miRNAs need to be validated in more extensive studies using in vitro and in vivo models. As the interest in this field increases, it is essential to identify other miRNAs biomarkers and a specific source of miRNAs in tuberculosis.
Declaration of competing interest
The author has no conflicts of interest to declare.
CRediT authorship contribution statement
Bijay Pattnaik: Writing the original draft, methodology, formal analysis, review & editing, Niharika Patnaik: Writing - review & editing, Saurabh Mittal: Supervision, Conceptualization, Methodology, Formal analysis, Resources, Anant Mohan, Anurag Agrawal, Randeep Guleria: Funding acquisition, Investigation, Project administration, Writing – original Writing - review & editing, Karan Madan: Supervision, Conceptualization, Methodology, Formal analysis, Resources.
Funding
None.
Financial disclosures
None of the authors have any financial disclosures.
Declaration of interest statement
None.
Acknowledgement
None.
References
- 1.Su Y., Garcia Baena I., Harle A.C., Crosby S.W., Micah A.E., Siroka A., et al. Tracking total spending on tuberculosis by source and function in 135 low-income and middle-income countries, 2000–17: a financial modelling study. Lancet Infect. Dis. 2020;20(8):929–942. doi: 10.1016/S1473-3099(20)30124-9. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen A., Mathiasen V.D., Schön T., Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur. Respir. J. 2019;54(3):1–14. doi: 10.1183/13993003.00655-2019. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 3.Ahmad S. New approaches in the diagnosis and treatment of latent tuberculosis infection. Respir. Res. 2010;11:1–17. doi: 10.1186/1465-9921-11-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chai Q., Lu Z., Liu C.H. Host defense mechanisms against Mycobacterium tuberculosis. Cell Mol. Life Sci. 2020;77(10):1859–1878. doi: 10.1007/s00018-019-03353-5. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariotti S., Pardini M., Gagliardi M.C., Teloni R., Giannoni F., Fraziano M., et al. Dormant Mycobacterium tuberculosis fails to block phagosome maturation and shows unexpected capacity to stimulate specific human T lymphocytes. J. Immunol. 2013;191(1):274–282. doi: 10.4049/jimmunol.1202900. [DOI] [PubMed] [Google Scholar]
- 6.Rohner P., Ninet B., Metral C., Emler S., Auckenthaler R. Evaluation of the MB/BacT system and comparison to the BACTEC 460 system and solid media for isolation of mycobacteria from clinical specimens. J. Clin. Microbiol. 1997;35(12):3127–3131. doi: 10.1128/jcm.35.12.3127-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geleta D.A., Megerssa Y.C., Gudeta A.N., Akalu G.T., Debele M.T., Tulu K.D. Xpert MTB/RIF assay for diagnosis of pulmonary tuberculosis in sputum specimens in remote health care facility Clinical microbiology and vaccines. BMC Microbiol. 2015;15(1):1–6. doi: 10.1186/s12866-015-0566-6. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Y., Yin L., Tang S., Zhang H., Lan J. Application of molecular, microbiological, and immunological tests for the diagnosis of bone and joint tuberculosis. J. Clin. Lab. Anal. 2018;32(2):1–7. doi: 10.1002/jcla.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortaz E., Masjedi M.R., Abedini A., Matroodi S., Kiani A., Soroush D., et al. Common features of tuberculosis and sarcoidosis. Int. J. Mycobacteriol. 2016;5(Suppl 1):S240–s241. doi: 10.1016/j.ijmyco.2016.09.031. 2017/01/04. [DOI] [PubMed] [Google Scholar]
- 10.Gupta N., Singh G., Rana M. Histopathological yield in different types of bronchoscopic biopsies in proven cases of pulmonary tuberculosis. Indian J. Pathol. Microbiol. 2015;58(4):439–442. doi: 10.4103/0377-4929.168881. [DOI] [PubMed] [Google Scholar]
- 11.Yang T., Ge B. miRNAs in immune responses to Mycobacterium tuberculosis infection. Cancer Lett. 2018;431:22–30. doi: 10.1016/j.canlet.2018.05.028. 2018/05/29. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal R.G., Sharma P., Nyati K.K. microRNAs in mycobacterial infection: modulation of host immune response and apoptotic pathways. Immune Netw. 2019;19(5):e30. doi: 10.4110/in.2019.19.e30. 2019/11/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueberberg B., Kohns M., Mayatepek E., Jacobsen M. Are microRNAs suitable biomarkers of immunity to tuberculosis? Mol. Cell Pediatr. 2014;1(1):8. doi: 10.1186/s40348-014-0008-9. 2015/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma F., Xu S., Liu X., Zhang Q., Xu X., Liu M., et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat. Immunol. 2011;12(9):861–869. doi: 10.1038/ni.2073. 2011/07/26. [DOI] [PubMed] [Google Scholar]
- 15.Rothchild A.C., Sissons J.R., Shafiani S., Plaisier C., Min D., Mai D., et al. MiR-155-regulated molecular network orchestrates cell fate in the innate and adaptive immune response to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 2016;113(41):E6172–e6181. doi: 10.1073/pnas.1608255113. 2016/09/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J.K., Kim T.S., Basu J., Jo E.K. MicroRNA in innate immunity and autophagy during mycobacterial infection. Cell Microbiol. 2017;19(1) doi: 10.1111/cmi.12687. 2016/10/30. [DOI] [PubMed] [Google Scholar]
- 17.Saliminejad K., Khorram Khorshid H.R., Soleymani Fard S., Ghaffari S.H. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019;234(5):5451–5465. doi: 10.1002/jcp.27486. [DOI] [PubMed] [Google Scholar]
- 18.Ying S.Y., Chang D.C., Lin S.L. The MicroRNA (miRNA): overview of the RNA genes that modulate gene function. Mol. Biotechnol. 2008;38(3):257–268. doi: 10.1007/s12033-007-9013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivey K.N., Srivastava D. microRNAs as developmental regulators. Cold Spring Harbor Perspect. Biol. 2015;7(7):1–9. doi: 10.1101/cshperspect.a008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miotto P., Mwangoka G., Valente I.C., Norbis L., Sotgiu G., Bosu R., et al. miRNA signatures in sera of patients with active pulmonary tuberculosis. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0080149. 2013/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latorre I., Leidinger P., Backes C., Domínguez J., de Souza-Galvão M.L., Maldonado J., et al. A novel whole-blood miRNA signature for a rapid diagnosis of pulmonary tuberculosis. Eur. Respir. J. 2015;45(4):1173–1176. doi: 10.1183/09031936.00221514. 2015/02/07. [DOI] [PubMed] [Google Scholar]
- 22.Wang C., Yang S., Sun G., Tang X., Lu S., Neyrolles O., et al. Comparative miRNA expression profiles in individuals with latent and active tuberculosis. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0025832. 2011/10/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aronson J.K., Ferner R.E. Biomarkers—a general review. Curr. Protoc. Pharmacol. 2017;2017(March):9.23.1–9.23.17. doi: 10.1002/cpph.19. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y., Zhang Y., Yu H., Tian R., Wang G., Li F. Identification of unique key genes and miRNAs in latent tuberculosis infection by network analysis. Mol. Immunol. 2019;112:103–114. doi: 10.1016/j.molimm.2019.04.032. 2019/05/15. [DOI] [PubMed] [Google Scholar]
- 25.Wu L.S., Lee S.W., Huang K.Y., Lee T.Y., Hsu P.W., Weng J.T. Systematic expression profiling analysis identifies specific microRNA-gene interactions that may differentiate between active and latent tuberculosis infection. BioMed Res. Int. 2014;2014:895179. doi: 10.1155/2014/895179. 2014/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabir N., Hussain T., Shah S.Z.A., Peramo A., Zhao D., Zhou X. miRNAs in tuberculosis: new avenues for diagnosis and host-directed therapy. Front. Microbiol. 2018;9:602. doi: 10.3389/fmicb.2018.00602. 2018/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yareta J., Galarza M., Capristano S., Pellón O., Sánchez C., Ballon J., et al. Differential expression of circulating micro-RNAs in patients with active and latent tuberculosis. Rev. Peru. Med. Exp. Salud Pública. 2020;37(1):51–56. doi: 10.17843/rpmesp.2020.371.4468. 2020/06/11. [DOI] [PubMed] [Google Scholar]
- 28.K M., S S S.M. Expression levels of candidate circulating microRNAs in pediatric tuberculosis. Pathog. Glob. Health. 2020;114(5):262–270. doi: 10.1080/20477724.2020.1761140. 2020/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J., Lu C., Diao N., Zhang S., Wang S., Wang F., et al. Analysis of microRNA expression profiling identifies miR-155 and miR-155∗ as potential diagnostic markers for active tuberculosis: a preliminary study. Hum. Immunol. 2012;73(1):31–37. doi: 10.1016/j.humimm.2011.10.003. 2011/11/01. [DOI] [PubMed] [Google Scholar]
- 30.Wang J., Zhu X., Xiong X., Ge P., Liu H., Ren N., et al. Identification of potential urine proteins and microRNA biomarkers for the diagnosis of pulmonary tuberculosis patients. Emerg. Microb. Infect. 2018;7(1):63. doi: 10.1038/s41426-018-0066-5. 2018/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou M., Yu G., Yang X., Zhu C., Zhang Z., Zhan X. Circulating microRNAs as biomarkers for the early diagnosis of childhood tuberculosis infection. Mol. Med. Rep. 2016;13(6):4620–4626. doi: 10.3892/mmr.2016.5097. 2016/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagh V., Urhekar A., Modi D. Levels of microRNA miR-16 and miR-155 are altered in serum of patients with tuberculosis and associate with responses to therapy. Tuberc. 2017;102:24–30. doi: 10.1016/j.tube.2016.10.007. 2017/01/08. [DOI] [PubMed] [Google Scholar]
- 33.Wang C., Yang S., Liu C.M., Jiang T.T., Chen Z.L., Tu H.H., et al. Screening and identification of four serum miRNAs as novel potential biomarkers for cured pulmonary tuberculosis. Tuberc. 2018;108:26–34. doi: 10.1016/j.tube.2017.08.010. 2018/03/11. [DOI] [PubMed] [Google Scholar]
- 34.Yi Z., Gao K., Li R., Fu Y. Dysregulated circRNAs in plasma from active tuberculosis patients. J. Cell Mol. Med. 2018;22(9):4076–4084. doi: 10.1111/jcmm.13684. 2018/07/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen T.M., Xiao Q., Wang X.J., Wang Z.Q., Hu J.W., Zhang Z., et al. miR-16 regulates proliferation and invasion of lung cancer cells via the ERK/MAPK signaling pathway by targeted inhibition of MAPK kinase 1 (MEK1) J. Int. Med. Res. 2019;47(10):5194–5204. doi: 10.1177/0300060519856505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin J., Wang Y., Zou Y.Q., Chen X., Huang B., Liu J., et al. Differential miRNA expression in pleural effusions derived from extracellular vesicles of patients with lung cancer, pulmonary tuberculosis, or pneumonia. Tumour Biol. 2016 doi: 10.1007/s13277-016-5410-6. 2016/10/16. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y., Xu Y.M., Zou Y.Q., Lin J., Huang B., Liu J., et al. Identification of differential expressed PE exosomal miRNA in lung adenocarcinoma, tuberculosis, and other benign lesions. Med. Plus. 2017;96(44) doi: 10.1097/MD.0000000000008361. 2017/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan T., Dominguez C.X., Amezquita R.A., Laidlaw B.J., Cheng J., Henao-Mejia J., et al. ZEB1, ZEB2, and the miR-200 family form a counterregulatory network to regulate CD8+ T cell fates. J. Exp. Med. 2018;215(4):1153–1168. doi: 10.1084/jem.20171352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roybal J.D., Zang Y., Ahn Y.H., Yang Y., Gibbons D.L., Baird B.N., et al. miR-200 inhibits lung adenocarcinoma cell invasion and metastasis by targeting Flt1/VEGFR1. Mol. Cancer Res. 2011;9(1):25–35. doi: 10.1158/1541-7786.MCR-10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyu L., Zhang X., Li C., Yang T., Wang J., Pan L., et al. Small RNA profiles of serum exosomes derived from individuals with latent and active tuberculosis. Front. Microbiol. 2019;10:1174. doi: 10.3389/fmicb.2019.01174. 2019/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xin H., Yang Y., Liu J., Li X., Li M., Feng B., et al. Association between tuberculosis and circulating microRNA hsa-let-7b and hsa-miR-30b: a pilot study in a Chinese population. Tuberc. 2016;99:63–69. doi: 10.1016/j.tube.2016.04.005. 2016/07/28. [DOI] [PubMed] [Google Scholar]
- 42.Iliopoulos D., Hirsch H.A., Struhl K. An epigenetic switch involving NF-κB, Lin28, let-7 microRNA, and.pdf. 2010;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H., Sun Z., Wei W., Liu Z., Fleming J., Zhang S., et al. Identification of serum microRNA biomarkers for tuberculosis using RNA-seq. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088909. 2014/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu X., Liao S., Bai H., Wu L., Wang M., Wu Q., et al. Integrating exosomal microRNAs and electronic health data improved tuberculosis diagnosis. EBioMedicine. 2019;40:564–573. doi: 10.1016/j.ebiom.2019.01.023. 2019/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barry S.E., Ellis M., Yang Y., Guan G., Wang X., Britton W.J., et al. Identification of a plasma microRNA profile in untreated pulmonary tuberculosis patients that is modulated by anti-mycobacterial therapy. J. Infect. 2018;77(4):341–348. doi: 10.1016/j.jinf.2018.03.006. 2018/05/11. [DOI] [PubMed] [Google Scholar]
- 46.Dorhoi A., Iannaccone M., Farinacci M., Faé K.C., Schreiber J., Moura-Alves P., et al. MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J. Clin. Invest. 2013;123(11):4836–4848. doi: 10.1172/JCI67604. 2013/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barry S.E., Ellis M., Yang Y., Guan G., Wang X., Britton W.J., et al. Identification of a plasma microrna profile in pulmonary tuberculosis patients that is modulated by anti-microbial therapy. Respirology. 2017;22:48. doi: 10.1016/j.jinf.2018.03.006. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L617841309 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 48.Fu Y., Wang J., Qiao J., Yi Z. Signature of circular RNAs in peripheral blood mononuclear cells from patients with active tuberculosis. J. Cell Mol. Med. 2019;23(3):1917–1925. doi: 10.1111/jcmm.14093. 2018/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yi Z., Fu Y., Ji R., Li R., Guan Z. Altered microRNA signatures in sputum of patients with active pulmonary tuberculosis. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0043184. 2012/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleinsteuber K., Heesch K., Schattling S., Kohns M., Sander-Jülch C., Walzl G., et al. Decreased expression of miR-21, miR-26a, miR-29a, and miR-142-3p in CD4+ T cells and peripheral blood from tuberculosis patients. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0061609. 2013/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X., Guo J., Fan S., Li Y., Wei L., Yang X., et al. Screening and identification of six serum microRNAs as novel potential combination biomarkers for pulmonary tuberculosis diagnosis. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0081076. 2013/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W., Liu R., Su Y., Li H., Xie W., Ning B. MicroRNA-21-5p mediates TGF-β-regulated fibrogenic activation of spinal fibroblasts and the formation of fibrotic scars after spinal cord injury. Int. J. Biol. Sci. 2018;14(2):178–188. doi: 10.7150/ijbs.24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu G., Friggeri A., Yang Y., Milosevic J., Ding Q., Thannickal V.J., et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010;207(8):1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinigaglia A., Peta E., Riccetti S., Venkateswaran S., Manganelli R., Barzon L. Tuberculosis-associated MicroRNAs: from pathogenesis to disease biomarkers. Cells. 2020;9(10):1–23. doi: 10.3390/cells9102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y., Xu X., Xu X., Li S., Liang Z., Hu Z., et al. MicroRNA-193a-3p inhibits cell proliferation in prostate cancer by targeting cyclin D1. Oncol. Lett. 2017;14(5):5121–5128. doi: 10.3892/ol.2017.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song Q., Li H., Shao H., Li C., Lu X. MicroRNA-365 in macrophages regulates Mycobacterium tuberculosis-induced active pulmonary tuberculosis via interleukin-6. Int. J. Clin. Exp. Med. 2015;8(9):15458–15465. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L606756398 [Internet] Available from: [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Y., Sun Q., Dai L. Immune regulation of miR-30 on the Mycobacterium tuberculosis-induced TLR/Myd88 signaling pathway in THP-1 cells. Exp. Ther. Med. 2017;14(4):3299–3303. doi: 10.3892/etm.2017.4872. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L618103326 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tu H., Yang S., Jiang T., Wei L., Shi L., Liu C., et al. Elevated pulmonary tuberculosis biomarker miR-423-5p plays critical role in the occurrence of active TB by inhibiting autophagosome-lysosome fusion. Emerg. Microb. Infect. 2019;8(1):448–460. doi: 10.1080/22221751.2019.1590129. 2019/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lou J., Wang Y., Zhang Z., Qiu W. MiR-20b inhibits mycobacterium tuberculosis induced inflammation in the lung of mice through targeting NLRP3. Exp. Cell Res. 2017;358(2):120–128. doi: 10.1016/j.yexcr.2017.06.007. 2017/06/14. [DOI] [PubMed] [Google Scholar]
- 60.Wang J.X., Xu J., Han Y.F., Zhu Y.B., Zhang W.J. Diagnostic values of microRNA-31 in peripheral blood mononuclear cells for pediatric pulmonary tuberculosis in Chinese patients. Genet. Mol. Res. 2015;14(4):17235–17243. doi: 10.4238/2015.December.16.23. 2015/12/19. [DOI] [PubMed] [Google Scholar]
- 61.Shi Z.G., Sun Y., Wang K.S., Jia J.D., Yang J., Li Y.N. Effects of mir-26a/mir-146a/miR-31 on airway inflammation of asthma mice and asthma children. Eur. Rev. Med. Pharmacol. Sci. 2019;23(12):5432–5440. doi: 10.26355/eurrev_201906_18212. [DOI] [PubMed] [Google Scholar]
- 62.Zhou B., Wang S., Mayr C., Bartel D.P., Lodish H.F. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely (Proceedings of the National Academy of Sciences of the United States of America (2007) 104, 17, (7080-7085) DOI: 10.1073/pnas.0702409104) Proc. Natl. Acad. Sci. U. S. A. 2008;105(46):18071. doi: 10.1073/pnas.0702409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mompeón A., Ortega-Paz L., Vidal-Gómez X., Costa T.J., Pérez-Cremades D., Garcia-Blas S., et al. Disparate miRNA expression in serum and plasma of patients with acute myocardial infarction: a systematic and paired comparative analysis. Sci. Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-61507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moldovan L., Batte K.E., Trgovcich J., Wisler J., Marsh C.B., Piper M. Methodological challenges in utilizing miRNAs as circulating biomarkers. J. Cell Mol. Med. 2014;18(3):371–390. doi: 10.1111/jcmm.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glinge C., Clauss S., Boddum K., Jabbari R., Jabbari J., Risgaard B., et al. Stability of circulating blood-based microRNAs-Pre-Analytic methodological considerations. PLoS One. 2017;12(2):1–16. doi: 10.1371/journal.pone.0167969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu P., Wu Q., Yu J., Rao Y., Kou Z., Fang G., et al. A systematic way to infer the regulation relations of miRNAs on target genes and critical miRNAs in cancers. Front. Genet. 2020;11(March):1–13. doi: 10.3389/fgene.2020.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar R., Halder P., Sahu S.K., Kumar M., Kumari M., Jana K., et al. Identification of a novel role of ESAT-6-dependent miR-155 induction during infection of macrophages with Mycobacterium tuberculosis. Cell Microbiol. 2012;14(10):1620–1631. doi: 10.1111/j.1462-5822.2012.01827.x. 2012/06/21. [DOI] [PubMed] [Google Scholar]
- 68.Zhang C., Xi X., Wang Q., Jiao J., Zhang L., Zhao H., et al. The association between serum miR-155 and natural killer cells from tuberculosis patients. Int. J. Clin. Exp. Med. 2015;8(6):9168–9172. 2015/08/27. [PMC free article] [PubMed] [Google Scholar]
- 69.Li X., Kong D., Chen H., Liu S., Hu H., Wu T., et al. MiR-155 acts as an anti-inflammatory factor in atherosclerosis-Associated foam cell formation by repressing calcium-regulated heat stable protein 1. Sci. Rep. 2016;6(February):1–11. doi: 10.1038/srep21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharbati J., Lewin A., Kutz-Lohroff B., Kamal E., Einspanier R., Sharbati S. Integrated microRNA-mRNA-analysis of human monocyte derived macrophages upon Mycobacterium avium subsp. hominissuis infection. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0020258. 2011/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McGregor R.A., Choi M.S. microRNAs in the regulation of adipogenesis and obesity. Curr. Mol. Med. 2011;11(4):304–316. doi: 10.2174/156652411795677990. http://www.ncbi.nlm.nih.gov/pubmed/21506921%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3267163 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Graff J.W., Dickson A.M., Clay G., McCaffrey A.P., Wilson M.E. Identifying functional microRNAs in macrophages with polarized phenotypes. J. Biol. Chem. 2012;287(26):21816–21825. doi: 10.1074/jbc.M111.327031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spizzo R., Nicoloso M.S., Lupini L., Lu Y., Fogarty J., Rossi S., et al. MiR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-α in human breast cancer cells. Cell Death Differ. 2010;17(2):246–254. doi: 10.1038/cdd.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Das K., Saikolappan S., Dhandayuthapani S. Differential expression of miRNAs by macrophages infected with virulent and avirulent Mycobacterium tuberculosis. Tuberc. 2013;93(Suppl):S47–50. doi: 10.1016/S1472-9792(13)70010-6. 2014/01/07. [DOI] [PubMed] [Google Scholar]
- 75.Ling L., Zhang S.H., Zhi L Da, Li H., Wen Q.K., Li G., et al. MicroRNA-30e promotes hepatocyte proliferation and inhibits apoptosis in cecal ligation and puncture-induced sepsis through the JAK/STAT signaling pathway by binding to FOSL2. Biomed. Pharmacother. 2018;104(2):411–419. doi: 10.1016/j.biopha.2018.05.042. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 76.Fawzy I.O., Hamza M.T., Hosny K.A., Esmat G., El Tayebi H.M., Abdelaziz A.I. MiR-1275: a single microRNA that targets the three IGF2-mRNA-binding proteins hindering tumor growth in hepatocellular carcinoma. FEBS Lett. 2015;589(17):2257–2265. doi: 10.1016/j.febslet.2015.06.038. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 77.Schakman O., Dehoux M., Bouchuari S., Delaere S., Lause P., Decroly N., et al. Role of IGF-I and the TNFα/NF-κB pathway in the induction of muscle atrogenes by acute inflammation. Am. J. Physiol. Endocrinol. Metab. 2012;303(6) doi: 10.1152/ajpendo.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zou M., Wang F., Jiang A., Xia A., Kong S., Gong C., et al. MicroRNA-3178 ameliorates inflammation and gastric carcinogenesis promoted by helicobacter pylori new toxin, Tip-α, by targeting TRAF3. Helicobacter. 2017;22(2):1–14. doi: 10.1111/hel.12348. [DOI] [PubMed] [Google Scholar]
- 79.Zhu Z., Qi Y., Fan H., Cui L., Shi Z. Systematic identification and bioinformatic analysis of MicroRNAs in response to infections of coxsackievirus A16 and enterovirus 71. BioMed Res. Int. 2016 doi: 10.1155/2016/4302470. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhardwaj N., Sena M., Ghaffari G., Ishmael F. MiR-4668 as a novel potential biomarker for eosinophilic esophagitis. Allergy Rhinol. 2020;11 doi: 10.1177/2152656720953378. 215265672095337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y., Wang X., Jiang J., Cao Z., Yang B., Cheng X. Modulation of T cell cytokine production by miR-144∗ with elevated expression in patients with pulmonary tuberculosis. Mol. Immunol. 2011;48(9–10):1084–1090. doi: 10.1016/j.molimm.2011.02.001. 2011/03/04. [DOI] [PubMed] [Google Scholar]
- 82.Qi Y., Cui L., Ge Y., Shi Z., Zhao K., Guo X., et al. Altered serum microRNAs as biomarkers for the early diagnosis of pulmonary tuberculosis infection. BMC Infect. Dis. 2012;12:384. doi: 10.1186/1471-2334-12-384. 2013/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fu Y., Yi Z., Wu X., Li J., Xu F. Circulating microRNAs in patients with active pulmonary tuberculosis. J. Clin. Microbiol. 2011;49(12):4246–4251. doi: 10.1128/JCM.05459-11. 2011/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu Z., Zhou A., Ni J., Zhang Q., Wang Y., Lu J., et al. Differential expression of miRNAs and their relation to active tuberculosis. Tuberc. 2015;95(4):395–403. doi: 10.1016/j.tube.2015.02.043. 2015/05/06. [DOI] [PubMed] [Google Scholar]
- 85.Maertzdorf J., Weiner J., 3rd, Mollenkopf H.J., Bauer T., Prasse A., Müller-Quernheim J., et al. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc. Natl. Acad. Sci. U. S. A. 2012;109(20):7853–7858. doi: 10.1073/pnas.1121072109. 2012/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cui J.Y., Liang H.W., Pan X.L., Li D., Jiao N., Liu Y.H., et al. Characterization of a novel panel of plasma microRNAs that discriminates between Mycobacterium tuberculosis infection and healthy individuals. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184113. 2017/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cao D., Wang J., Ji Z., Shangguan Y., Guo W., Feng X., et al. Profiling the mRNA and miRNA in peripheral blood mononuclear cells in subjects with active tuberculosis. Infect. Drug Resist. 2020;13:4223–4234. doi: 10.2147/IDR.S278705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y., Xu Y.M., Zou Y.Q., Lin J., Huang B., Liu J., et al. Identification of differential expressed PE exosomal miRNA in lung adenocarcinoma, tuberculosis, and other benign lesions. Med (United States) 2017;96(44) doi: 10.1097/MD.0000000000008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sinha A., Yadav A.K., Chakraborty S., Kabra S.K., Lodha R., Kumar M., et al. Exosome-enclosed microRNAs in exhaled breath hold potential for biomarker discovery in patients with pulmonary diseases. J. Allergy Clin. Immunol. 2013;132(1) doi: 10.1016/j.jaci.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 90.Wang C., Liu C.M., Wei L.L., Shi L.Y., Pan Z.F., Mao L.G., et al. A group of novel serum diagnostic biomarkers for multidrug-resistant tuberculosis by iTRAQ-2D LC-MS/MS and solexa sequencing. Int. J. Biol. Sci. 2016;12(2):246–256. doi: 10.7150/ijbs.13805. 2016/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fu Y., Yi Z., Wu X., Li J., Xu F. Circulating microRNAs in patients with active pulmonary tuberculosis. J. Clin. Microbiol. 2011;49(12):4246–4251. doi: 10.1128/JCM.05459-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X., Zhu M., Yang R., Zhao W., Hu X., Gan J. Identification and comparison of novel circular RNAs with associated co-expression and competing endogenous RNA networks in pulmonary tuberculosis. Oncotarget. 2017;8(69):113571–113582. doi: 10.18632/oncotarget.22710. 2018/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCoy C.E., Sheedy F.J., Qualls J.E., Doyle S.L., Quinn S.R., Murray P.J., et al. IL-10 inhibits miR-155 induction by toll-like receptors. J. Biol. Chem. 2010;285(27):20492–20498. doi: 10.1074/jbc.M110.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li S., Yue Y., Xu W., Xiong S. MicroRNA-146a represses mycobacteria-induced inflammatory response and facilitates bacterial replication via targeting IRAK-1 and TRAF-6. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sheedy F.J. Turning 21: induction of miR-21 as a key switch in the inflammatory response. Front. Immunol. 2015;6:19. doi: 10.3389/fimmu.2015.00019. 2015/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bazzoni F., Rossato M., Fabbri M., Gaudiosi D., Mirolo M., Mori L., et al. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc. Natl. Acad. Sci. U. S. A. 2009;106(13):5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee H.M., Kim T.S., Jo E.K. MiR-146 and miR-125 in the regulation of innate immunity and inflammation. BMB Rep. 2016;49(6):311–318. doi: 10.5483/BMBRep.2016.49.6.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sahu S.K., Kumar M., Chakraborty S., Banerjee S.K., Kumar R., Gupta P., et al. MicroRNA 26a (miR-26a)/KLF4 and CREB-C/EBPβ regulate innate immune signaling, the polarization of macrophages and the trafficking of Mycobacterium tuberculosis to lysosomes during infection. PLoS Pathog. 2017;13(5) doi: 10.1371/journal.ppat.1006410. 2017/05/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nahid M.A., Yao B., Dominguez-Gutierrez P.R., Kesavalu L., Satoh M., Chan E.K.L. Regulation of TLR2-mediated tolerance and cross-tolerance through IRAK4 modulation by miR-132 and miR-212. J. Immunol. 2013;190(3):1250–1263. doi: 10.4049/jimmunol.1103060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bayraktar R., Bertilaccio M.T.S., Calin G.A. The interaction between two worlds: MicroRNAs and Toll-like receptors. Front. Immunol. 2019;10(MAY):1–11. doi: 10.3389/fimmu.2019.01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yan F., Wufuer D., Wang J., Ding J. MicroRNA miR-146a-5p inhibits the inflammatory response and injury of airway epithelial cells via targeting TNF receptor-associated factor 6. Bioengineered. 2021;12(1):1916–1926. doi: 10.1080/21655979.2021.1927545. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao J., Wang H., Dong L., Sun S., Li L. MiRNA-20b inhibits cerebral ischemia-induced inflammation through targeting NLRP3. Int. J. Mol. Med. 2019;43(3):1167–1178. doi: 10.3892/ijmm.2018.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiao P., Wang X.P., Luoreng Z.M., Yang J., Jia L., Ma Y., et al. Mir-223: an effective regulator of immune cell differentiation and inflammation. Int. J. Biol. Sci. 2021;17(9):2308–2322. doi: 10.7150/ijbs.59876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liang S., Song Z., Wu Y., Gao Y., Gao M., Liu F., et al. MicroRNA-27b modulates inflammatory response and apoptosis during Mycobacterium tuberculosis infection. J. Immunol. 2018;200(10):3506–3518. doi: 10.4049/jimmunol.1701448. [DOI] [PubMed] [Google Scholar]
- 105.Singh Y., Kaul V., Mehra A., Chatterjee S., Tousif S., Dwivedi V.P., et al. Mycobacterium tuberculosis controls microRNA-99b (miR-99b) expression in infected murine dendritic cells to modulate host immunity. J. Biol. Chem. 2013;288(7):5056–5061. doi: 10.1074/jbc.C112.439778. 2012/12/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tili E., Michaille J.-J., Cimino A., Costinean S., Dumitru C.D., Adair B., et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. J. Immunol. 2007;179(8):5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 107.Bettencourt P., Marion S., Pires D., Santos L.F., Lastrucci C., Carmo N., et al. Actin-binding protein regulation by microRNAs as a novel microbial strategy to modulate phagocytosis by host cells: the case of N-Wasp and miR-142-3p. Front. Cell Infect. Microbiol. 2013;3:19. doi: 10.3389/fcimb.2013.00019. 2013/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lv Y ni, Ou-yang A jun, Fu L sheng. MicroRNA-27a negatively modulates the inflammatory response in lipopolysaccharide-stimulated microglia by targeting TLR4 and IRAK4. Cell. Mol. Neurobiol. 2017;37(2):195–210. doi: 10.1007/s10571-016-0361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J., Yang K., Zhou L., Minhaowu Wu Y., Zhu M., et al. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog. 2013;9(10) doi: 10.1371/journal.ppat.1003697. 2013/10/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu F., Chen J., Wang P., Li H., Zhou Y., Liu H., et al. MicroRNA-27a controls the intracellular survival of Mycobacterium tuberculosis by regulating calcium-associated autophagy. Nat. Commun. 2018;9(1):4295. doi: 10.1038/s41467-018-06836-4. 2018/10/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen D.Y., Chen Y.M., Lin C.F., Lo C.M., Liu H.J., Liao T.L. MicroRNA-889 inhibits autophagy to maintain mycobacterial survival in patients with latent tuberculosis infection by targeting TWEAK. mBio. 2020;11(1) doi: 10.1128/mBio.03045-19. 2020/01/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu K., Hong D., Zhang F., Li X., He M., Han X., et al. MicroRNA-106a inhibits autophagy process and antimicrobial responses by targeting ULK1, ATG7, and ATG16L1 during mycobacterial infection. Front. Immunol. 2021;11(January):1–13. doi: 10.3389/fimmu.2020.610021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bai S., Tian B., Li A., Yao Q., Zhang G., Li F. MicroRNA-125b promotes tumor growth and suppresses apoptosis by targeting DRAM2 in retinoblastoma. Eye. 2016;30(12):1630–1638. doi: 10.1038/eye.2016.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu Y., Gao J., Zhang S., Gu J., Lu H., Xia Y., et al. miR-142-3p regulates autophagy by targeting ATG16L1 in thymic-derived regulatory T cell (tTreg) Cell Death Dis. 2018;9(3):290. doi: 10.1038/s41419-018-0298-2. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Comincini S., Allavena G., Palumbo S., Morini M., Durando F., Angeletti F., et al. MicroRNA-17 regulates the expression of ATG7 and modulates the autophagy process, improving the sensitivity to temozolomide and low-dose ionizing radiation treatments in human glioblastoma cells. Cancer Biol. Ther. 2013;14(7):574–586. doi: 10.4161/cbt.24597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guo L., Zhou L., Gao Q., Zhang A., Wei J., Hong D., et al. MicroRNA-144-3p inhibits autophagy activation and enhances Bacillus Calmette-Guérin infection by targeting ATG4a in RAW264.7 macrophage cells. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0179772. 2017/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guo L., Zhao J., Qu Y., Yin R., Gao Q., Ding S., et al. microRNA-20a inhibits autophagic process by targeting ATG7 and ATG16L1 and favors mycobacterial survival in macrophage cells. Front. Cell Infect. Microbiol. 2016;6:134. doi: 10.3389/fcimb.2016.00134. 2016/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gu X., Gao Y., Mu D.G., Fu E.Q. MiR-23a-5p modulates mycobacterial survival and autophagy during mycobacterium tuberculosis infection through TLR2/MyD88/NF-κB pathway by targeting TLR2. Exp. Cell Res. 2017;354(2):71–77. doi: 10.1016/j.yexcr.2017.03.039. 2017/03/23. [DOI] [PubMed] [Google Scholar]
- 119.Huang J., Jiao J., Xu W., Zhao H., Zhang C., Shi Y., et al. MiR-155 is upregulated in patients with active tuberculosis and inhibits apoptosis of monocytes by targeting FOXO3. Mol. Med. Rep. 2015;12(5):7102–7108. doi: 10.3892/mmr.2015.4250. 2015/09/02. [DOI] [PubMed] [Google Scholar]
- 120.Zhang G., Liu X., Wang W., Cai Y., Li S., Chen Q., et al. Down-regulation of miR-20a-5p triggers cell apoptosis to facilitate mycobacterial clearance through targeting JNK2 in human macrophages. Cell Cycle. 2016;15(18):2527–2538. doi: 10.1080/15384101.2016.1215386. 2016/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng M., Wu Z., Wu A., Huang Z., He N., Xie X. MiR-145 promotes TNF-α-induced apoptosis by facilitating the formation of RIP1-FADDcaspase-8 complex in triple-negative breast cancer. Tumor Biol. 2016;37(7):8599–8607. doi: 10.1007/s13277-015-4631-4. [DOI] [PubMed] [Google Scholar]
- 122.Peng G., Yuan Y., He Q., Wu W., Luo B yan. MicroRNA let-7e regulates the expression of caspase-3 during apoptosis of PC12 cells following anoxia/reoxygenation injury. Brain Res. Bull. 2011;86(3–4):272–276. doi: 10.1016/j.brainresbull.2011.07.017. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 123.Mott J.L., Kobayashi S., Bronk S.F., Gores G.J. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26(42):6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou B., Wang D., Sun G., Mei F., Cui Y., Xu H. Effect of miR-21 on apoptosis in lung cancer cell through inhibiting the PI3K/Akt/NF-κB signaling pathway in vitro and in vivo. Cell. Physiol. Biochem. 2018;46(3):999–1008. doi: 10.1159/000488831. [DOI] [PubMed] [Google Scholar]