Abstract
The Mongolian gerbil model for Helicobacter pylori infection is an animal model that mimics human disease. We examined the serum immune response to H. pylori infection in gerbils by enzyme-linked immunosorbent assay (ELISA) and Western blotting, both with whole-cell (H. pylori) extracts. A total of 66 7-week-old specific-pathogen-free male gerbils were inoculated orogastrically with H. pylori strain ATCC 43504. Sera were collected 1, 2, 4, 8, 12, 26, 38, and 52 weeks after H. pylori inoculation. Sixty-nine noninfected gerbils and their sera were used as controls. The specificity of the ELISA was 95.7%. The frequency of seropositivity increased over time: 2 of 10 (20%), 7 of 10 (70%), and 7 of 7 (100%) samples of sera from inoculated gerbils were positive for H. pylori at 2, 4, and 8 weeks postinoculation, respectively. Western blot assays showed that the primary immunoglobulin G (IgG) response against low-molecular-mass (25-, 30-, and 20-kDa) proteins appeared after a lag period of 2 to 8 weeks after inoculation. Antibodies against 160-, 150-, 110-, 120-, 80-, 66-, and 63-kDa proteins were observed 12 weeks after inoculation. The early reactive 30-kDa protein was identified as a urease α subunit by N-terminal amino acid sequencing. After 26 weeks, two groups of animals could be distinguished: one group developed ulcers (n = 5), and the other developed hyperplastic polyps without ulcers (n = 19). Gerbils in the gastric ulcer group showed significantly higher serum anti-H. pylori IgG levels than did gerbils in the hyperplastic group (P = 0.001) as measured by ELISA. Furthermore, a higher proportion of animals developed antibodies to H. pylori proteins of 26, 25, and 20 kDa in the ulcer group than those animals with hyperplastic polyps (75 to 100% versus 17 to 50%) in Western blot assays. These results highlight the importance of the immune response of the host in the development of H. pylori-related gastric lesions.
Helicobacter pylori is the most important etiological agent of chronic active gastritis and peptic ulcer disease. H. pylori infection is also epidemiologically related to gastric carcinoma, and it has been classified as a group 1 carcinogen by the International Agency for Research on Cancer (21). Although all H. pylori-infected subjects have gastritis, in most cases the infection remains latent, with only a minority developing a symptomatic clinical disease such as peptic ulcer disease, gastric lymphoma, or adenocarcinoma. The risk factors for development of clinical diseases remain poorly understood.
A well-characterized animal model that mimics human H. pylori infection would significantly enhance the investigation of histopathogenic features of the interaction between the bacterium and the host's gastric mucosa. Hirayama et al. (17, 18) succeeded in establishing a Mongolian gerbil model that mimics human H. pylori infection. Ikeno et al. (20) reported the histological and histochemical characteristics of the gastric mucosa of normal and H. pylori-infected gerbils. H. pylori-infected gerbils developed gastritis, intestinal metaplasia, and gastric ulcers by 1 year after the experimental infection. More recently, Sugiyama et al. (30), Watanabe et al. (38), and Honda et al. (19) have shown that gastric carcinoma may also develop in H. pylori-infected Mongolian gerbils.
Understanding the serum immune response in this model might provide clues to the different outcomes of H. pylori infection. However, neither methods to measure serum anti-H. pylori antibody levels nor the time-dependent pattern of the serum antibody response to H. pylori in gerbils has been well described. The study reported here was undertaken with two aims: (i) to develop an enzyme-linked immunosorbent assay (ELISA) method to measure anti-H. pylori immunoglobulin G (IgG) levels in sera from H. pylori-infected gerbils and (ii) to identify the time-dependent antibody patterns in H. pylori-infected gerbils by using ELISA and Western blot assays.
MATERIALS AND METHODS
Preparation of horseradish peroxidase-conjugated anti-gerbil antibody.
Normal gerbil IgG was purified by protein A column chromatography (Seikagaku Kogyo, Tokyo, Japan), using the method of Ey et al. (14). New Zealand White rabbits were immunized subcutaneously several times with purified gerbil IgG containing complete Freund's adjuvant (Kanto Chemicals, Tokyo, Japan). Titers of immune sera were determined by the methods of Ouchterlony (29). A Fab′ fragment of rabbit anti-gerbil IgG conjugated to horseradish peroxidase (HRP) was prepared by the method of Ishikawa et al. (22). Briefly, the IgG fraction of immunized New Zealand White rabbit sera was separated by protein-A column chromatography. The IgG was digested by pepsin in 0.1 M acetate buffer (pH 4.5) at 37°C for 18 h, and the F(ab′)2 fragment was isolated by gel filtration (Ultrogel AcA-44; Pharmacia Biotech AB, Uppsala, Sweden). The Fab′ fragment was prepared by reducing the F(ab′)2 fragment by 0.01 M 2-mercaptoethyamine (pH 6.0) at 37°C for 90 min, followed by gel filtration (Sephadex G-25; Pharmacia Biotech AB). Fab′ fragments were mixed with N-succinimidyl-6-maleimidohexanoate–HRP complex (30°C, 60 min), and then the HRP-Fab′ fragments were purified by gel filtration on Ultrogel AcA-44. The conjugated material was dialyzed against phosphate-buffered saline (PBS) (pH 7.4) and then concentrated.
Animals.
Specific-pathogen-free 7-week-old male gerbils (MGS/Sea; Seac Yoshitomi, Fukuoka, Japan) were housed in an air-conditioned biohazard room for infection, with a 12-hour-light and 12-hour-dark cycle. They were given food (CE-2; Clea Japan, Inc., Tokyo, Japan) and water ad libitum.
Bacterial strain and inoculation.
H. pylori strain ATCC 43504 (American Type Culture Collection, Manassas, Va.) was grown in brucella broth (Becton Dickinson, Cockeysville, Md.) supplemented with 10% (vol/vol) horse serum and agitated at 35°C for 40 h in saturated humidity in the presence of 15% CO2. After a 24-h period of fasting, each animal was inoculated with a 0.5-ml inoculum of 109 CFU of H. pylori per ml intragastrically, using a feeding needle. Four hours after administration, animals were again allowed free access to water and food. H. pylori strain ATCC 43504 is CagA positive and produces VacA vacuolating cytotoxin.
Serum samples from Mongolian gerbils.
Before animals were sacrificed, blood samples were obtained from the orbital plexus using hematocrit tubes. Sera were obtained from 66 H. pylori-infected gerbils and 69 noninfected gerbils. That is, 5, 10, 10, 7, 10, 5, 4, and 15 serum samples were obtained at 1, 2, 4, 8, 12, 26, 38, and 52 weeks, respectively, after H. pylori inoculation. In addition, 11, 18, 18, 4, 11, 5, and 2 sera were obtained from gerbils in the noninfected group at ages 7, 10, 11, 15, 19, 33, and 59 weeks, respectively. Immediately after collection of the blood, gerbils were killed by cervical dislocation, and their stomachs were collected for microbiological and histological studies. The success of the experimental infection at each point was determined by the presence of positive results of culture and/or immunohistological staining.
ELISA.
Anti-H. pylori IgG values in sera from gerbils were determined by ELISA. The reference serum, which was pooled from sera of anti-H. pylori IgG-positive gerbils, was diluted serially from 1:100 to 1:3,200 with PBS (pH 7.4) containing 4% bovine serum albumin, and the amount of anti-H. pylori IgG corresponding to a 1:3,200 dilution was expressed as a reference value of a 1.0 arbitrary index (AI). Microwell strips coated with H. pylori antigens from a GAP-IgG kit (Biomerica, Newport Beach, Calif.) were used. The antigens in the GAP-IgG kit were acid extracts of organisms derived from the H. pylori ATCC 43504 strain. Aliquots of 100 μl of reference serum or 1:200 of diluted serum were added to the wells, and the plates were incubated for 1 h at room temperature. After washing was done, 100 μl of HRP-conjugated anti-gerbil IgG (diluted 1:1,500 in PBS containing 0.05% Tween 20 [PBS-T]) was added, and the plates were incubated for 30 min at room temperature. The plates were washed and incubated with 100 μl of substrate (0.35 mg of 3,3′,5,5′-tetramethylbenzidine per ml and 0.15 mg of H2O2 per ml) for 10 min. After stopping the reaction with 1 N HCl, the color was read at 450 nm. The serum anti-H. pylori IgG value was determined from a standard curve of calibrators (21, 23, 24, 30).
Antigen for Western blotting.
H. pylori cells of strain ATCC 43504 were harvested by centrifugation at 1,300 × g for 10 min at 4°C. The cells were suspended in washing buffer (1.0 M NaCl, 100 mM EGTA, 10 mM Tris-HCl [pH 8.0]) and washed twice with the same buffer. The washed cells were suspended in PBS (pH 7.4) and disrupted by sonication on ice by using a sonifier (Sonifier 250 D; Branson, Danbury, Conn.). The cell solubilisate was centrifuged at 12,000 × g for 20 min at 4°C, and the supernatant was dialyzed against PBS (pH 7.4) for 48 h. The suspension was stored at −80°C until used.
Western blotting.
The sonicated cell extract was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 8 to 16% polyacrylamide gels and transferred electrophoretically to nitrocellulose membranes (Toyo Roshi, Tokyo, Japan) at 0.8 mA/cm2 for 1 h at room temperature (Pharmacia LKB Biotechnology, Uppsala, Sweden). The blots were soaked in blocking solution (2% nonfat milk and 0.1% Tween 20 in PBS) for 1 h and then incubated for 1 h at room temperature with gerbil serum (1:100 to 1:4,000 diluted with blocking solution). After washing three times in PBS-T, the membranes were incubated for 1 h at room temperature with HRP-conjugated anti-gerbil IgG diluted 1:500 in blocking solution. After the washings in PBS-T, enhanced chemiluminescence (ECL) reagents (Amersham, Buckinghamshire, England) were used for detection of the Western blotting assay products. The ECL-detected blots were exposed to autoradiography film (Hyperfilm ECL; Amersham), and the film was developed using an X-ray film developer (Rendol; Fujifilm, Tokyo, Japan).
Two-dimensional gel electrophoresis.
Two-dimensional gel electrophoresis (GE) combining isoelectric focusing (IEF) in the first dimension and SDS-PAGE in the second dimension was performed. H. pylori ATCC 43504 (1010 cells) was solubilized in 5 ml of solubilization solution {8 M urea, 4% [wt/vol] 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, 100 mM dithiothreitol, 40 mM Tris-base, and 0.5% [vol/vol] Ampholine [pH 3.5 to 10; Pharmacia Biotech AB] [pH 9.5]}, vortexed, sonicated, and centrifuged at 12,000 × g for 20 min at 4°C. Following centrifugation, the supernatant was focused on 4% polyacrylamide gel containing 5% Ampholine (pH 3.5 to 10)–8 M urea–10% glycerol–0.06% ammonium persulfate–0.08% N,N,N′,N′-tetramethyl-ethylenediamine. IEF was performed under a constant current of 200 V for 16 h. A slice gel of IEF was then separated by SDS-PAGE (8 to 16% gel), blotting to polyvinylidene difluoride (PVDF) or nitrocellulose membrane, staining, and imaging as described above.
N-terminal amino acid sequences.
Coomassie blue-stained protein excised from a PVDF membrane was Edman sequenced for amino acid residues to provide an N-terminal amino acid sequence using a Procise 494 cLC (Applied Biosystems, Foster City, Calif.)
Statistical analysis.
Student's t test and Fisher's exact test were used to assess the significance of differences between means at each time point. The differences were considered to be significant when P was <0.05.
RESULTS
Macroscopic changes in H. pylori-inoculated gerbils.
Details of the histological changes in the gastric mucosa of H. pylori-inoculated gerbils have been described previously (20). Briefly described, time-dependent changes occurred throughout the gastric mucosa of all H. pylori-inoculated gerbils. There were no visible changes in the gastric mucosa of any noninfected gerbils or gerbils at 2 weeks postinfection. At 4 weeks after infection, the antral mucosa appeared slightly expanded and thickened and was covered by abundant mucus. Visible gastritis with edema and bleeding appeared at 12 weeks after inoculation. From week 26 through 52 postinoculation, two groups of animals could be distinguished; in one group the stomach showed ulcers, located distally close to the transitional zone between fundic and pyloric mucosa (ulcer group), and the other group showed many sessile hyperplastic polyps with occasional apical erosions but no ulcers (hyperplastic group). At 26, 38, and 52 weeks postinoculation, respectively, 2 of 5, 1 of 4, and 2 of 15 gerbils exhibited peptic ulcer.
Bacteriology.
Culturing of a piece of gastric mucosa (1 mm) obtained from the posterior wall of the antrum was done in samples from 60 of 66 experimentally H. pylori-inoculated gerbils. Culture examination showed no detectable H. pylori at 2 weeks postinoculation. H. pylori were cultured from the samples from 25 of 27 gerbils (93%) at 12 weeks and from the samples from 5 of 5 gerbils (100%) of the ulcer group and 4 of 13 gerbils (31%) of the hyperplastic group at 52 weeks. All gerbils in the ulcer group and in the hyperplastic group were H. pylori positive by histology, and the infection persisted up to the end of the experiment at 52 weeks.
Precision of ELISA.
The same day reproducibility was determined by making eight replicate measurements of three kinds of control sera on the same plate. Each control serum showed a mean of 0.65, 5.38, and 18.13 AI, and a coefficient of variation of 10.4, 6.7, and 6.4%, respectively. The between-day reproducibility was determined by making duplicate measurements of three kinds of control sera on each of 5 consecutive days. The control sera showed a mean of 0.23, 4.73, and 9.43 AI, and a coefficient of variation of 10.9, 9.4, and 9.5%, respectively. The detection limit of ELISA was 0.2 AI when it was defined as 2 standard deviations (SDs) above zero.
Cut-off values of anti-H. pylori IgG antibodies.
A total of 69 serum samples from noninfected gerbils aged 7 to 59 weeks showed a mean of 0.78 AI with an SD of 0.38 AI. The titer increased slightly with age (P < 0.001) such that the cutoff value of serum anti-H. pylori IgG antibodies for gerbils ≤15 weeks of age was defined as 1.37 AI and for gerbils >15 weeks of age was defined as 1.90 AI, based on the mean plus 2 SD. No noninfected gerbils showed positive serum results from culture or immunohistological staining. Sixty-six of 69 noninfected gerbils (95.7%) showed negative values, while three showed false-positive values; that is, specificity of this ELISA was 95.7%.
Change of serum anti-H. pylori IgG antibodies after inoculation.
A total of 66 serum samples of gerbils were obtained at 1, 2, 4, 8, 12, 26, 38, and 52 weeks after H. pylori inoculation. The primary IgG response appeared after a lag period of 2 to 8 weeks after inoculation. The changes in serum anti-H. pylori IgG antibodies detected by ELISA in infected groups are shown in Fig. 1. Sera from none of 5 (0%), 2 of 10 (20%), 7 of 10 (70%), and 7 of 7 (100%) gerbils showed positive anti-H. pylori IgG antibody levels at 1, 2, 4, and 8 weeks postinoculation, respectively. At 8 weeks after inoculation or later, 100% of gerbils had positive anti-H. pylori IgG levels.
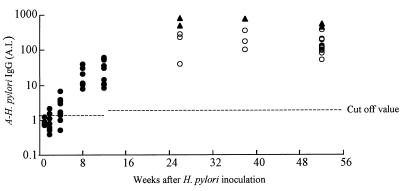
FIG. 1.
The change over 52 weeks in serum levels of anti-H. pylori IgG in gerbils after H. pylori inoculation. IgG levels were determined by ELISA. ●, serum antibody level of gerbils during first 12 weeks after inoculation. Mean antibody values (n = 5, 10, 10, 7, and 10) were 0.9, 0.9, 2.4, 21.5, and 31.9 AI, respectively. ▴, serum antibody level of ulcer group. Mean value and SD of the group were 605.1 and 159.4 AI (n = 2, 1, and 2) at 26, 38, and 52 weeks postinoculation, respectively. ○, serum antibody level of hyperplastic group. Mean value and SD of the group were 177.4 and 120.8 AI (n = 3, 3, and 13) at 26, 38, and 52 weeks postinoculation, respectively. For other details, see Results.
After 52 weeks, 5 of 24 gerbils exhibited gastric ulcers, and the others had gastric hyperplasia. The mean serum anti-H. pylori IgG value (±SD) of gerbils in the ulcer group was 605.1 ± 159.4 AI. The mean value for gerbils in the hyperplastic group was 177.4 ± 120.8 AI. The values in the ulcer group were significantly higher than those of the hyperplastic group (P = 0.001).
Western blotting.
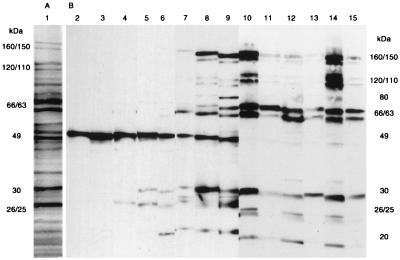
The major proteins of whole-cell sonicates were 160, 150, 120, 110, 80, 66, 63, 49, 35, 30, and 26 kDa in apparent molecular mass (Fig. 2A, lane 1). The first detectable IgG antibody of gerbils to H. pylori was observed against 25-kDa proteins and the earliest response in a gerbil was seen 2 weeks after inoculation (Fig. 2B, lane 4). This was followed by development of additional IgG antibody against a 30-kDa protein(s), then by antibody against a 20-kDa protein(s) (lanes 5 and 6). The reactive band against 49-kDa proteins was observed in both noninfected gerbils and H. pylori-infected gerbils (lanes 2 to 9) and was considered nonspecific. Additional antibodies against 160-kDa and 63-kDa proteins were followed by the appearance of antibodies against 150-, 120-, 110-, 80-, and 66-kDa proteins, which were observed 12 weeks after inoculation (lanes 7 to 9). At 26 weeks or later, the pattern of response differed between animals in the ulcer group (lanes 10, 12, and 14) and those in the hyperplastic group (lanes 11, 13, and 15). The antibodies against 25-, 26-, and 20-kDa proteins tended to be more frequent, and antibody bands against 120- and 110-kDa proteins tended to be both clear and strong in sera from the ulcer group compared to sera from those in the hyperplastic group.
FIG. 2.
(A) Lane 1, protein profile of whole-cell sonicates of H. pylori ATCC 43504. Proteins were separated on an SDS–8 to 16% PAGE gradient gel and stained with Coomassie brilliant blue R-250. (B) The time course of Western blotting patterns with sera from H. pylori-inoculated gerbils. Lane 2, serum from a noninoculated gerbil; lanes 3 to 15, sera from inoculated gerbils. Times after inoculation were as follows: lanes 3 and 4, 2 weeks; lanes 5 and 6, 4 weeks; lanes 7 and 8, 8 weeks; lane 9, 12 weeks; lanes 10 and 11, 26 weeks; lanes 12 and 13, 38 weeks; lanes 14 and 15, 52 weeks. Lanes 10, 12, and 14 show sera from the ulcer group; lanes 11, 13, and 15 show sera from the hyperplastic group. Sera were diluted as follows: lanes 2 to 6, 1:100; lanes 7 to 9, 1:1,000; lanes 10 to 15, 1:4,000. Molecular masses are indicated in kilodaltons.
We compared the frequency of the presence of immunoreactive bands among eight bands (160-, 150-, 120-, 110-, 30-, 26-, 25-, and 20-kDa proteins) between sera from gerbils of the ulcer group and the hyperplastic group (Table 1). The presence of a band at 26 and 25 kDa in the ulcer group tended to be more frequent than in the hyperplastic group, but the small sample size precluded determination of a significant relationship.
TABLE 1.
Frequencies of eight antibodies to H. pylori in sera from 16 Mongolian gerbils of ulcer group and hyperplastic group
| Immunoreactive band (kDa) | Reacting sera (%) froma
|
|
|---|---|---|
| Ulcer group (n = 4)b | Hyperplastic group (n = 12)c | |
| 160 | 100 | 92 |
| 150 | 75 | 75 |
| 120 | 75 | 75 |
| 110 | 75 | 83 |
| 30 | 100 | 92 |
| 26 | 100 | 33 |
| 25 | 75 | 17 |
| 20 | 100 | 50 |
Sera from gerbils of the ulcer group were diluted 1:4,000. Sera from gerbils of the hyperplastic group were diluted 1:1,250. Serum dilution of samples from the ulcer group was 3.2-fold greater than that of the hyperplastic group because the mean antibody-H. pylori IgG value of the ulcer group as measured by ELISA was about threefold greater than that of the hyperplastic group (605.1 AI versus 177.4 AI, respectively). The sera were taken at 26, 38, and 52 weeks after H. pylori inoculation.
One of five sera from the gerbils in the ulcer group was run short for Western blot analysis.
Seven of 19 sera of gerbils from the hyperplastic group were run short for Western blot analysis.
Characterization of the early reactive proteins.
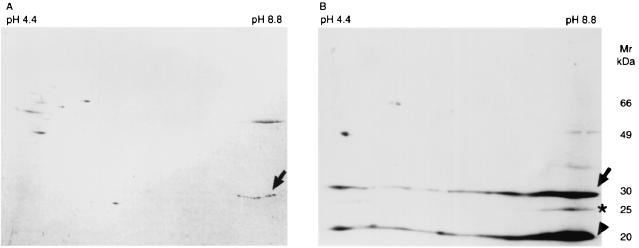
To determine the nature of the early reactive proteins, the cell extract was separated by two-dimensional GE, transferred to PVDF membrane, and then stained with Coomassie blue (Fig. 3A) or assayed by Western blotting with serum samples from gerbils taken at 5 weeks postinoculation (Fig. 3B). The isoelectric point (pI) of early reactive protein of 25 kDa was approximately 8.6 to 8.8 (Fig. 3B, asterisk). The horizontal streaking was observed against 20- and 30-kDa proteins (Fig 3B, arrow and arrowhead, respectively). The Coomassie-stained spots of 30-kDa (pI = 8.6 to 8.8) protein were in agreement on Western blotting spots (arrow). The N-terminal amino acid sequence of 30-kDa protein (pI = 8.6 to 8.8) revealed that the first eight amino acid sequences were MKLTPKEL. The sequence of the protein was identified as a urease α subunit of H. pylori by a search of the SWISS-PROT database (http://www.ebi.ac.uk/swissprot/).
FIG. 3.
(A) Protein spots of whole-cell sonicate of H. pylori ATCC 43504. Proteins were separated by two-dimensional GE: isoelectric focusing in the first dimension and SDS-PAGE in the second dimension. Proteins were transferred to PVDF membrane and then stained with Coomassie brilliant blue R-250. (B) Western blot result of the same preparation with serum from a gerbil taken 5 weeks after H. pylori inoculation. Serum was diluted 1:100. The pH is indicated at the top of the figure and molecular masses in kilodaltons are indicated on the right-hand side. Arrow, asterisk, and arrowhead indicate 30-, 25-, and 20-kDa proteins, respectively.
DISCUSSION
The time- and antigen-dependent immune response to H. pylori has not yet been described in humans, in part because the time of acquisition of the infection can not be identified in most individuals. We established an ELISA and Western blotting system for detecting serum immune response to H. pylori in gerbils and to study the time- and antigen-dependent immune response to H. pylori infection. We used the gerbil model, which mimics human H. pylori infection (17, 18, 20, 30), and newly prepared HRP-conjugated anti-gerbil IgG antibody to analyze the serum immune responses from the time of inoculation of H. pylori for periods up to 1 year after inoculation.
Western blotting allows for the analysis of the immune response to a number of defined antigens. Moreover, ECL Western blotting is a highly sensitive system (39) with at least 10-fold greater sensitivity than other nonradioactive or radioactive Western blotting systems (1). In this study, we found that the primary IgG antibody response was against low-molecular-mass proteins (25-, 30-, and 20-kDa proteins, in that order) and appeared after a lag period of 2 to 8 weeks. The lag period corresponded to the cellular events in the activation, proliferation, and differentiation of B cells to antibody-secreting cells (5). Vos et al. (36) reported humoral immune response to a thymus-independent (Escherichia coli lipopolysaccharide) and thymus-dependent (tetanus toxoid) antigen in 8-week-old male rats immunized intravenously. The IgG antibody response pattern of our study was similar to that of the response pattern to thymus-dependent antigen, but the lag period observed by Vos et al. was shorter (i.e., 5 to 20 days). One of the reasons for the difference in lag period may be the difference in immune pathway (i.e., mucosal immunity versus parenteral immunity). Takahashi et al. (32) reported an oral immune response when mice were administrated tetanus toxoid orally with E. coli heat-labile toxin as an adjuvant. Tetanus toxoid-specific IgG1 and IgG2b responses appeared by day 7, and peak titers were seen by day 14. On the other hand, IgG2a appeared by day 14 and gradually increased throughout the intervals assessed. Our study does not provide information regarding the specific antibodies against the IgG subclass of gerbils. There may be substantial differences in host immune responses. H. pylori is a mucosal pathogen that has the ability to colonize in host tissue, while tetanus toxoid does not. Further studies on the immune system of H. pylori infection are needed.
The first reactive antigens in the range of the 25-, 30-, and 20-kDa proteins in this study stimulated interest in the colonization of the bacterium and the host immune system. Binding and adhesion of the bacterium to the gastric mucosa are thought to be an important first step in bacterial colonization. A 30-kDa protein was identified as a urease α subunit of H. pylori. The horizontal streaking of this protein observed in Fig. 3B appears to be a result of incomplete focusing, which may indicate that the protein has become insoluble during the IEF run (16). Our data are similar to those of Dunn et al. (8), who reported that this urease subunit was not resolved by two-dimensional GE. H. pylori urease is a cell-bound enzyme and is composed of two protein subunits, with molecular masses of 66 kDa (pI = 5.93) and 30 kDa (12). Our results suggested that the urease α subunit may be involved early in colonization and in the host immune response. The amount of the 25-kDa protein (pI = 8.6 to 8.8) was too little to determine N-terminal amino acid sequences. The amount of a 20-kDa protein was also insufficient for N-terminal amino acid sequencing, and it was not well resolved by two-dimensional GE because there was also considerable horizontal streaking at the 20-kDa protein on the second-dimension gel. Sugiyama et al. (31) reported a unique antibody against 25-kDa antigen in sera from patients of chronic gastritis and gastric ulcer associated with H. pylori. Valkonen et al. (34) also reported 25-kDa outer membrane protein of H. pylori as N-acetylneuraminyllactose-specific laminin-binding protein. Evans et al. reported 20-kDa HpaA as an adhesin subunit (10, 11), and an immunoreactive species-specific 19-kDa outer membrane protein was reported by Drouet et al. (7), but it is as yet uncharacterized. Approximately 29- to 31-kDa urease subunit proteins, surface and outer membrane proteins of H. pylori such as 31- or 30-kDa proteins, and various reactive antigens of water-soluble proteins have been reported (3, 4, 6, 9, 12, 13, 15, 26). Results in different studies may depend on the methods of growing the bacteria and preparing the antigens and the details of the ELISA or Western blot. Data from humans suffer from this variability, and it is not possible to directly correlate the results between gerbils and humans. Some but not all of our results are in agreement with previous work with humans (2, 7, 25, 27). Mitchell et al. (25) reported that the presence of a band at 116, 89, and 35 kDa or of any two bands at 30, 26.5, and 19.5 kDa was a marker of H. pylori infection in humans. Nilsson et al. (27) reported that bands of 110 or 120 kDa and/or two of five low-molecular-mass proteins (26, 29, 30, 31, and 33 kDa) showed a strong correlation with H. pylori-positive patients. Evans et al. (13) reported a 150-kDa (15-kDa subunit) water-soluble antigen as a neutrophil-activating protein. Common reactive bands from low-molecular-mass proteins (about 20, 25 to 26, and 30 kDa) and from high-molecular-mass proteins (about 110 or 120 and 150 kDa) were found in both gerbils and humans. The time-dependent serum immune response to these common reactive antigens in humans is similar to that in gerbils. O'Toole et al. (28) isolated a 26-kDa species-specific protein that was shown to be antigenically unique to H. pylori. It is interesting that Wang et al. (37) reported that the 26-kDa protein, which was the same one reported by O'Toole et al. (28) was associated with gastric adenocarcinoma in humans.
From weeks 26 through 52 postinoculation, 5 of 24 gerbils (21%) developed peptic ulcers, while others did not develop serious disease. The remarkable feature of the host serum immune response of the ulcer group was significantly higher anti-H. pylori IgG levels than those of the hyperplastic group (P = 0.001). A tendency for a higher proportion of animals to develop antibody to H. pylori proteins of 26, 25, and 20 kDa as well as higher antibody titers against 110- or 120-kDa proteins in the ulcer group than in the hyperplastic group was also observed. The critical factors, either host, bacterial, or environmental, that influence the clinical manifestation of H. pylori infection are still being elucidated. This study focused on host factors, since all animals were infected with the same strain (ATCC 43504) of H. pylori. This strain is CagA and VacA positive and under the same experimental conditions, designed to control for bacterial and environmental factors, the animals had different outcomes. In addition, the different outcomes (ulcer versus hyperplastic polyps) were associated with different humoral immune responses, highlighting the importance of the immune response of the host in the development of H. pylori-related gastric lesions. The infection is not cleared by the humoral immune response, and the mechanisms and contribution of the activation of TH2 in the gerbil immune network must be studied. The 110- and 120-kDa proteins have been identified as CagA (33, 40), and the presence of anti-CagA has been associated with an increased risk of developing ulcers and gastric cancer (35). The gerbil model thus offers the opportunity to examine the outcome of H. pylori infection both from the perspective of the host and by altering the virulence of the infecting strain (e.g., by selective knock out of putative virulence genes such as cagA).
Although infection persisted in all gerbils, the number of animals with ulcers appeared to decrease over time with 2 of 5 (40%), 1 of 4 (25%), and 2 of 13 (13%) gerbils exhibiting ulcers at 26, 38, and 52 weeks postinoculation, respectively. Watanabe et al. (38) also reported the same tendency. Watanabe et al. used a gerbil model infected with the H. pylori TN2GF4 strain, which was isolated from a patient with gastric ulcer, and they found that 100, 100, 40, and 59% of the gerbils had ulcers at 26, 39, 52, and 62 weeks postinoculation, respectively. The apparent drop in the prevalence of ulcers could represent spontaneous healing of ulcers or progressive damage to the gastric mucosa such that the animals were unable to make sufficient acid to maintain the ulcers.
Clarification of antigens which are found to be involved in H. pylori infection in the gerbil model used in this study will promote our understanding of the mechanisms of H. pylori infection in gastric mucosa and the pathogenesis of H. pylori-related disease in gastric mucosa.
REFERENCES
- 1.Amersham Life Science Company. ECM Western blotting protocols. Buckinghamshire, England: Amersham International PLC; 1995. [Google Scholar]
- 2.Aucher P, Petit M L, Mannant P R, Pezennec L, Babin P, Fauchere J L. Use of immunoblot assay to define serum antibody patterns associated with Helicobacter pylori infection and with H. pylori-related ulcers. J Clin Microbiol. 1998;36:931–936. doi: 10.1128/jcm.36.4.931-936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bode G, Malfertheiner P, Lehnhardt G, Nilius M, Ditschuneit H. Ultrastructural localization of urease of Helicobacter pylori. Med Microbiol Immunol. 1993;182:233–242. doi: 10.1007/BF00579622. [DOI] [PubMed] [Google Scholar]
- 4.Bolin I, Lonroth H, Svennerholm A M. Identification of Helicobacter pylori by immunological dot blot method based on reaction of a species-specific monoclonal antibody with a surface-exposed protein. J Clin Microbiol. 1995;33:381–384. doi: 10.1128/jcm.33.2.381-384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bona C A, Bonilla F A. B cells and humoral immunity. In: Bona C A, editor. Textbook of immunology. 2nd ed. Amsterdam, The Netherlands: Harwood Academic Publishers; 1996. pp. 101–138. [Google Scholar]
- 6.Doig P, Trust T J. Identification of surface-exposed outer membrane antigens of Helicobacter pylori. Infect Immun. 1994;62:4526–4533. doi: 10.1128/iai.62.10.4526-4533.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drouet E B, Denoyel G A, Boude M, Wallano E, Andujar M, DeMontclos H P. Characterization of an immunoreactive species-specific 19-kilodalton outer membrane protein from Helicobacter pylori by using a monoclonal antibody. J Clin Microbiol. 1991;29:1620–1624. doi: 10.1128/jcm.29.8.1620-1624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn B E, Perez-Perez G I, Blaser M J. Two-dimensional gel electrophoresis and immunoblotting of Campylobacter pylori proteins. Infect Immun. 1989;57:1825–1833. doi: 10.1128/iai.57.6.1825-1833.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn B E, Vakil N B, Schneider B G, Miller M M, Zitzer J B, Peutz T, Phadnis S H. Localization of Helicobacter pylori urease and heat shock protein in human gastric biopsies. Infect Immun. 1997;65:1181–1188. doi: 10.1128/iai.65.4.1181-1188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans D G, Evans D J, Jr, Moulds J J, Graham D Y. N-Acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988;56:2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans D G, Karjalainen T K, Evans D J, Jr, Graham D Y, Lee C H. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993;175:674–683. doi: 10.1128/jb.175.3.674-683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans D J, Jr, Evans D G, Kirkpatrick S S, Graham D Y. Characterization of the Helicobacter pylori urease and purification of its subunits. Microb Pathog. 1991;10:15–26. doi: 10.1016/0882-4010(91)90062-f. [DOI] [PubMed] [Google Scholar]
- 13.Evans D J, Jr, Evans D G, Takamura T, Nakano H, Heather C L, Graham D Y, Granger D N, Kvietys P R. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213–2220. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ey P L, Prowse S J, Jenkin C R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978;15:429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- 15.Hawtin P R, Stacey A R, Newell D G. Investigation of the structure and localization of the urease of Helicobacter pylori using monoclonal antibodies. J Gen Microbiol. 1990;136:1995–2000. doi: 10.1099/00221287-136-10-1995. [DOI] [PubMed] [Google Scholar]
- 16.Herbert B R, Molloy M P, Gooley A A, Walsh B J, Bryson W G, Williams K L. Improved protein solubility in two-dimensional electrophoresis using tributyl phosphine as reducing agent. Electrophoresis. 1998;19:845–851. doi: 10.1002/elps.1150190540. [DOI] [PubMed] [Google Scholar]
- 17.Hirayama F, Takagi S, Kusuhara H, Iwao E, Yokoyama Y, Ikeda Y. Induction of gastric ulcer and intestinal metaplasia in Mongolian gerbils infected with Helicobacter pylori. J Gastroenterol. 1996;31:755–757. doi: 10.1007/BF02347631. [DOI] [PubMed] [Google Scholar]
- 18.Hirayama F, Takagi S, Yokoyama Y, Iwao E, Ikeda Y. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 1996;31:24–28. [PubMed] [Google Scholar]
- 19.Honda S, Fujioka T, Tokieda M, Satoh R, Nishizone A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 20.Ikeno T, Ota H, Sugiyama A, Ishida K, Katsuyama T, Genta R M, Kawasaki S. Helicobacter pylori-induced chronic active gastritis, intestinal metaplasia, and gastric ulcer in Mongolian gerbils. Am J Pathol. 1999;154:951–960. doi: 10.1016/S0002-9440(10)65343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, liver flukes and Helicobacter pylori: views and expert opinions of an IARC Working Group on the evaluation of carcinogenic risks to humans. Lyon, France: International Agency for Research on Cancer; 1994. Helicobacter pylori; pp. 177–240. [Google Scholar]
- 22.Ishikawa E, Imagawa M, Hashida S, Yoshitake S, Hamaguchi Y, Ueno T. Enzyme-labeling of antibodies and their fragments for enzyme immunoassay and immunohistochemical staining. J Immunoassay. 1983;4:209–327. doi: 10.1080/15321818308057011. [DOI] [PubMed] [Google Scholar]
- 23.Kumagai T, Malaty H M, Graham D Y, Hosogaya S, Misawa K, Furihata K, Ota H, Sei C, Tanaka E, Akamatsu T, Shimizu T, Kiyosawa K, Katsuyama T. Acquisition versus loss of Helicobacter pylori infection in Japan: results from an 8-year birth cohort study. J Infect Dis. 1998;178:717–721. doi: 10.1086/515376. [DOI] [PubMed] [Google Scholar]
- 24.Misawa K, Kumagai T, Shimizu T, Furihata K, Ota H, Akamatsu T, Katsuyama T. A new histological procedure for re-evaluation of the serological test for Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1998;17:14–19. doi: 10.1007/BF01584357. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell H M, Hazell S L, Li Y Y, Hu P J. Serological response to specific Helicobacter pylori antigens: antibody against CagA antigen is not predictive of gastric cancer in a developing country. Am J Gastroenterol. 1996;92:1785–1788. [PubMed] [Google Scholar]
- 26.Newell D G. Human serum antibody responses to the surface protein antigens of Campylobacter pyloridis. Serodiagn Immunother. 1987;1:209–217. [Google Scholar]
- 27.Nilsson I, Ljungh Å, Aleljung P, Wadstrom T. Immunoblot assay for serodiagnosis of Helicobacter pylori infection. J Clin Microbiol. 1997;35:427–432. doi: 10.1128/jcm.35.2.427-432.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Toole P W, Logan S M, Kostrzynska M, Wadstrom T, Trust T J. Isolation and biochemical and molecular analyses of a species-specific protein antigen from the gastric pathogen Helicobacter pylori. J Bacteriol. 1991;173:505–513. doi: 10.1128/jb.173.2.505-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouchterlony O. In vitro method for testing the toxin-producing capacity of diphtheria bacteria. Acta Pathol Microbiol Scand. 1948;25:186–191. doi: 10.1111/j.1699-0463.1948.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama A, Maruta F, Ikeno T, Ishida K, Kawasaki S, Katsuyama T, Shimizu N, Tatematsu M. Helicobacter pylori infection enhances N-methyl N-nitrosourea-induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res. 1998;58:2067–2069. [PubMed] [Google Scholar]
- 31.Sugiyama T, Imai K, Yoshida H, Takayama Y, Yabana T, Yokota K, Oguma K, Tachi A. A novel enzyme immunoassay for serodiagnosis of Helicobacter pylori infection. Gastroenterology. 1991;101:77–83. doi: 10.1016/0016-5085(91)90462-t. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi I, McGhee J R, Hamada S, Kiyono H. Oral immunization approaches for the prevention of gastrointestinal infections. In: Ernst P B, Michetti P, Smith P D, editors. The immunobiology of H. pylori: from pathogenesis to prevention. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 237–254. [Google Scholar]
- 33.Telford J, Ghiara L P, Dell'Orco M, Commanducci M, Burroni D, Bugnoli M, Tecce M F, Censini S, Covacci A, Xiang Z, Papini E, Montecucco C, Parente L, Rappuoli R. Gene structure of Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valkonen K H, Wadström T, Moran A P. Identification of the N-acetylneuraminyllactose-specific laminin-binding protein of Helicobacter pylori. Infect Immun. 1997;65:916–923. doi: 10.1128/iai.65.3.916-923.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Doorn L-J, Figueiredo C, Sanna R, Plaisier A, Schneeberger P, Boer W, Quint W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58–66. doi: 10.1016/s0016-5085(98)70365-8. [DOI] [PubMed] [Google Scholar]
- 36.Vos J G, Buys J, Beekhof P, Hagenaars A M. Quantification of total IgM and IgG and specific IgM and IgG to a thymus-independent (LPS) and a thymus-dependent (tetanus toxoid) antigen in the rat by enzyme-linked immunosorbent assay (ELISA) Ann N Y Acad Sci. 1979;320:518–534. [PubMed] [Google Scholar]
- 37.Wang J T, Chang C S, Lee C Z, Yang J C, Lin J T, Wang T H. Antibody to a Helicobacter pylori species specific antigen in patient with adenocarcinoma of the stomach. Biochem Biophys Res Commun. 1998;244:360–363. doi: 10.1006/bbrc.1998.8271. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 39.Whitehead T P, Kricka L J, Carter T J, Thorpe G H. Analytical luminescence: its potential in the clinical laboratory. Clin Chem. 1979;25:1531–1546. [PubMed] [Google Scholar]
- 40.Xiang Z, Censini S, Bayeli P F, Telford J T, Figura N, Rappuoli R, Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995;63:94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]