Abstract
Recently, a technique was described for amplification of Rhodococcus equi-specific chromosomal and vapA DNA from blood and tracheal wash fluids. It was hypothesized that this technique would be more sensitive than standard culture techniques or serology for diagnosis of R. equi pneumonia in foals. Tracheal wash fluid, nasal swabs, whole blood samples, and serum samples from 56 foals with pneumonia were analyzed. Final clinical diagnosis was determined by the attending clinician on the basis of final interpretation of all available information about each foal, including clinical presentation, diagnostic test results, response to therapy, and outcome. Clinical diagnosis was used as a final reference standard for calculation of sensitivity, specificity, and predictive values for PCR, serology using an agar gel immunodiffusion test, and tracheal wash fluid culture. PCR of tracheal wash fluid using primers that recognized the vapA virulence plasmid of R. equi had a diagnostic sensitivity of 100% and specificity of 90.6%. Sensitivity and specificity were 57.1 and 93.8%, respectively, for standard microbiologic culture of tracheal wash fluid and 62.5 and 75.9%, respectively, for serology. PCR of tracheal wash fluid is more sensitive and specific for diagnosis of R. equi pneumonia than are other available diagnostic tests.
Rhodococcus equi is a gram-positive, pleomorphic coccobacillus that causes pneumonia and enteritis in foals less than 6 months of age and has been associated with a variety of suppurative processes in immune-suppressed humans (19). The organism is worldwide in distribution and commonly isolated from soil and environmental samples (2, 4, 23). Strains of R. equi isolated from sick foals uniformly contain an 85- to 90-kb plasmid that carries the gene responsible for expression of a 15- to 17-kDa antigen of undetermined function (vapA) (24, 25). Environmental strains of R. equi not associated with equine disease do not contain this plasmid.
The onset of clinical signs of R. equi pneumonia in foals is often insidious, and the infection is not recognized until severe abscessation has occurred and prognosis is poor. Culture of the organism from tracheal wash (TW) fluid is currently considered the “gold standard” for diagnosis (7). However, it can be difficult to reliably grow R. equi from a single TW sample, possibly because of prior antibiotic administration (12, 14) or the presence of multiple pathogenic bacterial species (5, 21). Hillidge reported that only 7 of 11 foals (62%) with positive R. equi cultures at necropsy, and 57 of 89 foals (64%) with radiographic evidence of lung abscessation, yielded R. equi on culture of TW (10). Other studies reported a 100% positive result on cultures of TW fluid from foals later confirmed to have R. equi pneumonia at necropsy (14, 17).
Recently, a technique was described for amplification of R. equi-specific chromosomal and vapA DNA from blood and TW fluids (20). It was hypothesized that this technique would be more sensitive than standard culture techniques or serology for diagnosis of R. equi pneumonia in foals. TW fluid, nasal swabs, blood samples, and serum samples from 53 foals with pneumonia were analyzed in a comparison of the sensitivity and specificity of each type of diagnostic test.
MATERIALS AND METHODS
Sample collection and submission.
Clinical samples were obtained from 53 foals between 2 weeks and 8 months of age that had been referred to veterinary teaching hospitals at Washington State University, Oklahoma State University, and North Carolina State University or to a private referral hospital in California. For inclusion in the study, a foal was required to have clinical signs referable to the respiratory tract, (cough, nasal discharge, abnormal auscultation, fever, and/or rapid respiratory rate), and a TW aspiration had to be performed as part of the diagnostic evaluation. TW fluid was obtained via standard aseptic clinical techniques and aliquoted into evacuated glass tubes containing either no anticoagulant or EDTA as an anticoagulant. TW fluid samples for culture and PCR were required from each foal for inclusion in the study.
Serum, plasma, and nasal swab samples were not required for inclusion in the sample population but were submitted if available. Blood samples were obtained by jugular venipuncture after surgical scrub of the venipuncture site with povidone-iodine or chlorhexidine to minimize environmental contamination of the sample. For PCR analysis and serology, whole blood samples were collected into evacuated glass tubes containing either EDTA as an anticoagulant or no anticoagulant. For blood culture, samples were aseptically collected into glass tubes containing the appropriate culture media. Nasal swabs were obtained manually from the distal nares using a sterile culturette swab with PDC-300 Amies modified medium.
Clinical samples from foals in states other than Washington were shipped on ice by overnight carrier to Washington State University. Clumps of debris were removed from TW fluid by filtering the fluid through sterile cheesecloth. Filtered TW fluid, serum, and buffy coat samples for PCR or serologic assay were stored at −70°C until assayed. Culture specimens were plated as soon as possible after receipt.
Microbiologic culture.
TW fluid was plated directly on colistin-nalidixic acid agar (CNA) and Columbia agar base with 5% sheep red blood cells and incubated in 5% CO2 at 37°C. A MacConkey agar plate was also set and incubated at 37°C in air. Blood cultures in Isolator tubes were processed according to Isolator system protocols and plated onto four CNA plates incubated in 5% CO2 at 37°C. If conventional blood culture bottles were submitted, they were subcultured to CNA, if already incubated, and then vented and incubated aerobically. Suspect colonies were identified as R. equi as follows: catalase positive, mucoid (pink pigmented with time), no growth on MacConkey agar subcultures, nonfermenting, obligate aerobes, and CAMP positive with Corynebacterium pseudotuberculosis. Questionable isolates were identified with the API-Coryne test kit. In most cases TW fluid samples were also submitted for microbiologic culture and sensitivity to the diagnostic laboratory serving the clinic of origin. In some cases cultures were performed only at Washington State University or only at the laboratory of origin. Culture results for each foal were pooled and reported without regard to the laboratory performing the culture.
Nucleic acid preparation and amplification.
DNA was extracted from TW fluid, nasal swab, and buffy coat samples for nucleic acid amplification using a previously described technique, with slight modifications of centrifugation and incubation times (20). Samples consisted of approximately 0.5 to 1.0 ml of filtered TW fluid or buffy coat cells. Nasal swab samples were soaked in 1 ml of sterile phosphate-buffered saline for 2 to 3 min, and the swab was pressed against the side of the microcentrifuge tube to express fluid. Samples were centrifuged at 10,000 × g for 5 min. DNA extraction was performed as for TW fluid.
PCR was performed as previously described (20), but different virulence plasmid (VP) primers were used. Primer sequences are as follows: VP forward, GAG GGA TCC GGT TCT CGT AAC GCT ACA ATC; VP reverse, GGT TCG TCT TTC TGA AGG TT; 16S forward (20), TCG TCC GTG AAA ACT TGG G; and 16S reverse (20), CGA CCA CAA GGG GGC CGT. VP primers amplify a 301-bp segment of the gene coding for the virulence-associated antigen of R. equi. R. equi chromosomal DNA (16S) primers amplify a 441-bp segment of the 16S rRNA gene.
DNA amplification products were electrophoresed on an agarose gel containing ethidium bromide and visualized with a UV transilluminator. Molecular weights of the products were estimated by comparison to standard molecular weight markers. Negative controls included reaction mixtures without any DNA added and reaction mixtures without bacterial DNA added. Positive controls included high concentrations of R. equi total cellular DNA. Virulent (strain 238) and avirulent (ATCC 6939) strains of R. equi were maintained in broth culture for use as positive controls as previously described (20). Primers that amplified a region of the 16S rRNA gene of all bacteria (generic bacterial primers) were used to confirm the absence of reaction inhibitors in each sample.
Serologic assay.
Agar gel immunodiffusion (AGID) assays were performed by Veterinary Dynamics, Inc. Results were reported as negative, very weak, weak, 1+, 2+, 3+, or 4+. Samples that were negative, very weak, or weak were grouped as negative/low positive. Samples that were 1+ or greater were grouped as strong positive.
Statistical analysis.
TW fluid culture has historically been considered the reference standard for antemortem diagnosis of R. equi pneumonia. Therefore, test parameters for PCR, including sensitivity and specificity, were initially calculated using culture results as a reference standard. However, compared to postmortem results, antemortem TW fluid culture is inaccurate, with many false negatives. Therefore, after final data collection, clinicians were contacted and asked to declare each case in the study “R. equi pneumonia” or “not R. equi pneumonia.” This classification was based on the attending clinician's final interpretation of all available information, including clinical presentation, diagnostic test results (including PCR and culture), response to therapy, and outcome. Clinical diagnosis was used as a final reference standard for the calculation of sensitivity, specificity, and predictive values for PCR, AGID, and TW fluid culture.
The distribution of various parameters between R. equi-positive and R. equi-negative foals was compared using either a chi-square analysis of contingency tables or the Fisher exact test. Results were considered significant when P was <0.05.
RESULTS
Clinical summary.
Samples were collected from 53 foals: 16 in California, 15 in North Carolina, 13 in Oklahoma, 7 in Washington, and 2 in Georgia. Foals ranged from 2 weeks to 8 months of age. There were 23 quarter horse-type (quarter horse, paint, and Appaloosa), 13 Thoroughbred, 5 Tennessee walking horse, 3 Arabian, 3 Morgan, and 1 each saddlebred, Warmblood, draft horse, albino, and mixed light-breed foals. The mean age ± standard deviation for the 50 foals for which that information was available was 3.6 ± 1.7 months. There were 27 male and 26 female foals.
Culture results.
TW fluid cultures yielded bacterial growth for 48 of 51 foals (94.1%). (For four foals, TW fluid culture results were reported only as R. equi positive or R. equi negative, with no information regarding other bacterial isolates.) R. equi was isolated from the TW fluid of 10 of 53 foals (18.9%). Multiple bacterial species were isolated from the TW fluid of 32 of 49 foals (65.3%). A single bacterial species was isolated from 14 of 49 foals (28.6%). No bacteria were isolated from the TW fluid of 3 of 49 foals (6.1%). Table 1 shows the frequency of isolation of specific bacteria from the TW fluid of foals. Blood cultures were obtained from 30 foals; none were positive for R. equi.
TABLE 1.
Bacteria cultured from TW fluid of foals with pneumonia
| Bacterium | No. (%) with indicated bacteriuma
|
||
|---|---|---|---|
| VP-positive foals (n = 17) | VP-negative foals (n = 36) | All foals (n = 53) | |
| Streptococcus zooepidemicus | 6 (35.3) | 20 (55.6) | 26 (49.1) |
| R. equi | 9 (52.9)* | 1 (2.8)* | 10 (18.9) |
| Other streptococci | 3 (17.6) | 6 (16.7) | 9 (16.7) |
| Enterobacter sp. | 2 (11.8) | 6 (16.7) | 8 (15.1) |
| Pseudomonas sp. | 2 (11.8) | 5 (13.9) | 7 (13.2) |
| Escherichia coli | 3 (17.6) | 2 (5.6) | 5 (9.4) |
| Pasteurella or Actinobacillus sp. | 1 (5.9) | 4 (11.1) | 5 (9.4) |
| Bordetella bronchiseptica | 1 (5.9) | 3 (8.3) | 4 (7.5) |
| Corynebacterium sp. | 1 (5.9) | 3 (8.3) | 4 (7.5) |
| Klebsiella sp. | 0 (0.0) | 3 (8.3) | 3 (5.7) |
| Enterococcus sp. | 1 (5.9) | 2 (5.6) | 3 (5.7) |
| None isolated | 1 (5.9) | 2 (5.6) | 3 (5.7) |
| Not reported | 0 (0.0) | 2 (5.6) | 2 (3.8) |
VP positivity and negativity are defined by PCR results. ∗, difference between results for VP-positive foals and VP-negative foals is statistically significant (P < 0.0001).
PCR results.
Results of PCR of TW fluid with VP and 16S primers were compared between samples with no anticoagulant and samples anticoagulated with EDTA. The likelihood of a positive PCR result was not significantly different between the two sample types for either primer pair (Table 2). Agreement of PCR results between sample types was 81.4% for 16S primers and 92.5% for VP primers. Because TW fluid samples submitted for culture are typically submitted without anticoagulant, that sample type was considered the most practical and relevant for further analysis. Therefore, all further analysis of TW fluid PCR data is based only on results from samples that had no anticoagulant. Of the 10 foals with R. equi-positive TW fluid culture, 7 were classified as both VP and 16S positive by PCR, 2 were only VP positive, and 1 was only 16S positive.
TABLE 2.
PCR analysis of TW fluid samples using VP and 16S primers
| Primers | No. (%) of positive samples with:
|
|
|---|---|---|
| No anticoagulant (n = 53) | EDTA (n = 45) | |
| VP positive only | 5 (9.4) | 5 (11.1) |
| 16S positive only | 12 (22.6) | 10 (22.2) |
| VP and 16S positive | 12 (22.6) | 7 (15.5) |
| VP and 16S negative | 22 (41.5) | 22 (48.9) |
| Unreadablea | 2 (3.8) | 1 (2.2) |
“Unreadable” samples produced a heavy smear on the ethidium bromide-stained agarose gel.
Representative colonies of R. equi isolated from the TW fluid of eight foals were subcultured in Luria broth. After growth for 36 h, DNA was extracted and PCR was performed to confirm the presence of VP. Seven isolates were positive by PCR using both VP and 16S primers. The R. equi isolate from the foal with VP-negative TW fluid remained VP negative and 16S positive by PCR when a pure culture was analyzed. Plasmid analysis and Western immunoblotting using anti-vapA antibody confirmed the absence of VP in this isolate (data not shown).
PCR analysis of nasal swab samples was VP positive in 6 of 38 foals (15.8%) and 16S positive in 5 of 36 foals (13.9%). Nasal swabs were VP positive in 3 of 10 foals with VP-positive TW fluid (30%). Foals were not statistically more likely to have a VP-positive nasal swab if they had a VP-positive TW sample. Serum samples from 40 foals and EDTA-anticoagulated buffy coat cells from 43 foals were tested for R. equi by PCR using VP primers. Five serum samples (12.5%) and five buffy coat samples (11.6%) were positive using VP primers.
AGID results.
Of the 43 foals for which AGID results were available, 13 were considered strongly positive for antibody to R. equi (30.2%). Foals that were PCR positive with the VP primers were statistically more likely to have a strong positive AGID result than were foals that were PCR negative with the VP primers (P = 0.0056).
Diagnostic accuracy.
The sensitivity and specificity of PCR results were calculated using TW fluid culture as a reference standard for antemortem diagnosis (Table 3). However, it was obvious from the available data that there were culture-negative foals with classic clinical signs of R. equi infection that responded to antimicrobial therapy with erythromycin and rifampin. This was consistent with previous reports of a lack of sensitivity of TW fluid culture as an antemortem test for R. equi pneumonia in foals. Therefore, the primary care clinician for each foal was asked to retrospectively designate each case R. equi pneumonia or not R. equi pneumonia based on all available information: clinical presentation, radiographic and laboratory results, culture and PCR results, response to therapy, and necropsy results. This final clinical diagnosis was available for 46 foals and was in agreement with PCR (VP) results for 43 of 46 foals (93.5%). Final clinical diagnosis agreed with culture results for 40 of 46 foals (87.0%).
TABLE 3.
Accuracy of diagnostic tests for R. equi pneumonia
| Reference standard test | Diagnostic testa | Sensitivity (%) | Specificity (%) | Disease prevalenceb |
|---|---|---|---|---|
| TW fluid culture | PCR (VP) | 90.0 | 81.4 | 10/53 (18.9) |
| PCR (VP, 16S) | 70.0 | 88.4 | 10/53 (18.9) | |
| AGID | 75.0 | 80.0 | ||
| Final clinical diagnosis | TW fluid PCR (VP) | 100.0 | 90.6 | 14/46 (30.4) |
| TW fluid PCR (16S) | 78.6 | 68.8 | 14/46 (30.4) | |
| TW fluid PCR (VP, 16S) | 78.6 | 96.9 | 14/46 (30.4) | |
| TW fluid culture | 57.1 | 93.8 | 14/46 (30.4) | |
| AGID | 62.5 | 75.9 | 8/37 (21.6) | |
| Nasal swab PCR (VP) | 50.0 | 88.9 | 6/33 (18.2) | |
| Serum PCR (VP) | 12.5 | 88.9 | 8/35 (22.9) | |
| Buffy coat PCR (VP) | 11.1 | 86.7 | 9/39 (23.1) |
VP, PCR positive using VP primers; 16S, PCR positive using 16S primers.
Number of foals positive with the reference standard test divided by the total number of foals with results available for both the reference standard test and the diagnostic test being evaluated. (Values in parentheses are percentages.)
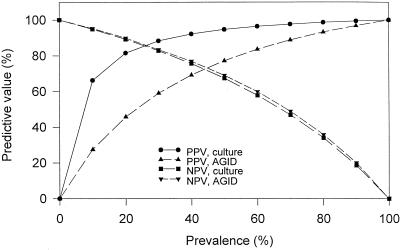
Using the final clinical diagnosis as the reference standard test, diagnostic accuracies (sensitivity and specificity) of TW fluid culture, TW fluid PCR with VP primers, nasal swab PCR with VP primers, serum PCR with VP primers, buffy coat PCR with VP primers, and serum AGID were calculated (Table 3). Using the final clinical diagnosis as the reference standard test, the positive and negative predictive values of TW fluid culture, TW fluid PCR with VP primers, and AGID were determined at prevalence rates from 0 to 100% (Fig. 1).
FIG. 1.
Predictive value of positive and negative tests (culture and AGID) at prevalence rates ranging from 0 to 100% using PCR with VP primers as the reference standard test. PPV, positive predictive value; NPV, negative predictive value.
DISCUSSION
Currently available diagnostic methods for R. equi pneumonia include culture and serology (1, 11, 21, 26, 27). However, the perceived lack of sensitivity of TW fluid culture (5, 7, 10, 14, 21) and the lack of specificity of serological techniques (6, 8, 9) have been problematic in the clinical setting. This is exacerbated by time delays in obtaining the results of these tests. A rapid, sensitive, and specific diagnostic test would be of benefit, especially in the diagnosis of cases on farms where R. equi is nonendemic. Nucleic acid amplification technology has been useful for rapid and accurate diagnosis of other types of bacterial infections of human beings and domestic animals, including horses (3, 13, 15, 18).
Assessment of the accuracy of a diagnostic test requires the availability of a valid reference standard test. Designation of such an antemortem reference standard test for R. equi is difficult. TW fluid culture, historically promoted as a gold standard test (7), can yield false-negative results, possibly because of prior antibiotic administration or overgrowth by multiple pathogenic bacterial species (5, 12, 14, 21). Hillidge reported that only 7 of 11 foals (62%) with positive R. equi cultures at necropsy and 57 of 89 foals (64%) with radiographic evidence of lung abscessation yielded R. equi on culture of TW fluid (10).
Using culture as a reference standard test for the calculation of sensitivity and specificity of PCR as a diagnostic assay suggests that the PCR assay results in numerous false positives (Table 3). However, several of the foals that were culture negative and VP PCR positive were considered highly likely to have R. equi pneumonia, including one foal that had a previous TW fluid culture that was positive for R. equi. The foal was on antimicrobial therapy but obviously ill with fever, an increased white blood cell count and plasma fibrinogen concentration, and no radiographic evidence of resolution of pneumonia. The TW fluid obtained at the time of reevaluation was negative for R. equi by culture but strongly positive for R. equi by PCR with the VP and 16S primers. In addition to the false negatives described, culture of TW fluid resulted in one false positive in the detection of an isolate that did not contain the VP.
New diagnostic tests for infectious diseases that rely on nucleic acid amplification techniques can be difficult to assess statistically because of their marked increase in sensitivity over previously identified gold standard tests (16). Determining the true disease state of the patient can be difficult, and clinical correlation has been suggested as a reasonable alternative in this situation (16). Because of the previously acknowledged lack of accuracy of TW fluid culture, we chose to calculate test sensitivities and specificities using final clinical diagnosis as the reference standard test (Table 3). This reference standard may be biased because the clinicians knew the results of culture and PCR (but not serology) at the time that the designation of R. equi pneumonia or not R. equi pneumonia was made. However, the data suggest that the clinicians did not rely solely on any single diagnostic test to reach their final clinical diagnosis. Sufficient time elapsed between sample submission and the designation of final clinical diagnosis (approximately 6 months to 2 years) that clinicians had ample opportunity to fully evaluate the clinical progression of disease and recovery in each foal. Final diagnosis as made by a qualifed referral veterinary clinician, while potentially biased, was considered the most accurate antemortem diagnosis.
PCR may result in false positives due to the detection of very small numbers of organisms that are present as environmental contaminants. R. equi is a ubiquitous organism that is present in the soil of most horse farms and in the manure of many adult horses (2, 22, 23). Nasal swab samples were obtained from 33 foals included in this study and assayed by PCR for detection of VP or 16S DNA sequences. In most cases (27 of 33), these nasal swab specimens tested negative, suggesting that environmental contamination was not a major cause of PCR-positive TW fluid.
The predictive values of positive and negative tests vary depending on disease prevalence in a population. The predictive values for TW fluid culture, TW fluid PCR, and AGID, using final clinical diagnosis as a reference standard test, are modeled in Fig. 1. These results demonstrate the large numbers of false-negative results that can occur if culture is the only diagnostic test used. The choice of an appropriate diagnostic test or tests should depend on the presumed prevalence in the population and the potential impact of a false-positive or -negative result on clinical outcome.
In 11 of 14 foals considered highly likely to have R. equi pneumonia as the final clinical diagnosis, TW fluid was positive by PCR with both VP and 16S primers. Five foals that tested positive with VP primers tested negative with 16S primers. Two of these five foals were TW fluid culture positive, suggesting that PCR failed to detect 16S sequences.
TW fluid samples that were 16S positive but VP negative may have represented detection of environmental strains of R. equi that do not contain the vapA plasmid. In one of these cases, R. equi was cultured from the TW fluid and confirmed not to carry the vapA plasmid associated with virulence. Although both culture and PCR may detect environmental contaminants of R. equi in TW fluid, PCR has the ability to distinguish between virulent and avirulent strains. However, detection of vapA DNA in TW fluid does not confirm that the organism is present as a pathogen. It is possible that foals may have virulent or avirulent strains of R. equi present as contaminants in their airways that are not responsible for clinical signs of pneumonia. This may be more likely to occur on farms where R. equi problems are endemic.
The types of organisms cultured from foals with clinical signs of pneumonia were typical of those reported in other studies (11). Most foals (32 of 49) had multiple organisms cultured. Foals with R. equi were just as likely to have multiple organisms cultured from their TW fluid as were foals that were R. equi culture negative.
Serologic assays for R. equi are not considered highly useful for diagnosis of individual foals (7). In this study, strongly positive AGID results were statistically more likely to occur in foals that had R. equi pneumonia. However, two culture-positive and PCR-positive foals were AGID negative, and strongly positive AGID results did occur in some foals that were culture and PCR negative for R. equi. One of these AGID-negative foals came from a farm where R. equi problems were endemic and had received hyperimmune plasma soon after birth. Serological tests are probably most useful as herd screening tests.
These results underscore the lack of sensitivity of standard culture and serological techniques for the diagnosis of R. equi pneumonia. PCR of TW fluid was a sensitive and specific diagnostic test for R. equi. TW fluid samples are most likely to be collected from pneumonic foals that have been nonresponsive to empirical antimicrobial therapy. In these situations, it is clinically important to rule out the presence of virulent R. equi in the foal. A negative PCR of TW fluid using VP primers is extremely reliable in this situation. None of the foals that were 16S positive and VP negative by PCR of TW fluid were considered to have R. equi pneumonia as their final clinical diagnosis; hence, PCR-negative foals are unlikely to have R. equi pneumonia.
VP primers alone would be appropriate for clinical diagnosis. PCR should always be used in conjunction with standard culture because of the probability of the presence of multiple bacterial pathogens. PCR may be useful to rule out R. equi pneumonia in culture-negative foals that have failed to improve with standard antimicrobial therapy and have clinical signs consistent with R. equi. It may also be useful in monitoring response to therapy and deciding when to quit therapy in foals that are confirmed to have R. equi pneumonia. In these foals, follow-up TW fluid cultures are often negative because of antimicrobial use. PCR has the added advantage of producing results more quickly than do standard culture techniques, and it may be useful in cases where rapid diagnosis is critical. As with any diagnostic technique, results should always be interpreted in light of the individual foal's history and clinical signs.
ACKNOWLEDGMENTS
This work was supported by U.S. Department of Agriculture Formula Funds and the State of Washington.
We thank Tressa Hochstatter for expert technical assistance. We thank Marianela Lopez and Steven Hines for plasmid analysis. We thank Tina Kemper, Barry Grant, Amelia Woolums, and Dianne McFarlane for submission of samples and case information for this project. Serologic assays (AGID) were kindly performed by Veterinary Dynamics, Inc.
REFERENCES
- 1.Anzai T, Wada R, Nakanishi A, Kamada M, Takai S, Shindo Y, Tsubaki S. Comparison of tracheal aspiration with other tests for diagnosis of Rhodococcus equi pneumonia in foals. Vet Microbiol. 1997;56:335–345. doi: 10.1016/s0378-1135(97)00100-4. [DOI] [PubMed] [Google Scholar]
- 2.Barton M D, Hughes K L. Ecology of Rhodococcus equi. Vet Microbiol. 1984;9:65–76. doi: 10.1016/0378-1135(84)90079-8. [DOI] [PubMed] [Google Scholar]
- 3.Cohen N D, Neibergs H L, Wallis D E, Simpson R B, McGruder E D, Hargis B M. Genus specific detection of salmonellae in equine feces by use of the polymerase chain reaction. Am J Vet Res. 1994;55:1049–1054. [PubMed] [Google Scholar]
- 4.Debey M C, Bailie W E. Rhodococcus equi in fecal and environmental samples from Kansas horse farms. Vet Microbiol. 1987;14:251–257. doi: 10.1016/0378-1135(87)90112-x. [DOI] [PubMed] [Google Scholar]
- 5.Falcon J, Smith B P, O'Brien T R, Carlson G P, Biberstein E. Clinical and radiographic findings in Corynebacterium equi pneumonia of foals. J Am Vet Med Assoc. 1985;186:593–599. [PubMed] [Google Scholar]
- 6.Gaskin J M, King R R, Lane T J, Mayhew I J, Brewer B D. Serological detection of Rhodococcus equi infection of foals. Proc Am Coll Vet Intern Med. 1990;8:581–584. [Google Scholar]
- 7.Giguere S, Prescott J F. Clinical manifestations, diagnosis, treatment, and prevention of Rhodococcus equi infections in foals. Vet Microbiol. 1997;56:313–334. doi: 10.1016/s0378-1135(97)00099-0. [DOI] [PubMed] [Google Scholar]
- 8.Higuchi T, Hashikura S, Gojo C, Inui T, Satoh S, Yoshida M, Ishiyama T, Yamada H, Takai S. Clinical evaluation of the serodiagnostic value of enzyme-linked immunosorbent assay for Rhodococcus equi infection in foals. Equine Vet J. 1997;29:274–278. doi: 10.1111/j.2042-3306.1997.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 9.Higuchi T, Taharaguchi S, Hashikura S, Hagiwara S, Gojo C, Satoh S, Yoshida M, Takai S. Physical and serologic examinations of foals at 30 and 45 days of age for early diagnosis of Rhodococcus equi infection on endemically infected farms. J Am Vet Med Assoc. 1998;212:976–981. [PubMed] [Google Scholar]
- 10.Hillidge C J. Use of erythromycin-rifampin combination in treatment of Rhodococcus equi pneumonia. Vet Microbiol. 1987;14:215–224. doi: 10.1016/0378-1135(87)90121-0. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman A M, Viel L, Prescott J F, Rosendal S, Thorsen J. Association of microbiologic flora with clinical, endoscopic, and pulmonary cytologic findings in foals with distal respiratory tract infection. Am J Vet Res. 1993;54:1615–1622. [PubMed] [Google Scholar]
- 12.Hondalus M K, Mosser D M. Survival and replication of Rhodococcus equi in macrophages. Infect Immun. 1994;62:4167–4175. doi: 10.1128/iai.62.10.4167-4175.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman A C, Greene C E, McGraw R A. Optimization of polymerase chain reaction for the detection of Borrelia burgdorferi in biologic specimens. J Vet Diagn Investig. 1993;5:558–564. doi: 10.1177/104063879300500408. [DOI] [PubMed] [Google Scholar]
- 14.Lavoie J P, Fiset L, Laverty S. Review of 40 cases of lung abscesses in foals and adult horses. Equine Vet J. 1994;26:348–352. doi: 10.1111/j.2042-3306.1994.tb04401.x. [DOI] [PubMed] [Google Scholar]
- 15.MacPherson J M, Gajadhar A A. Sensitive and specific polymerase chain reaction detection of Toxoplasma gondii for veterinary and medical diagnosis. Can J Vet Res. 1993;57:45–48. [PMC free article] [PubMed] [Google Scholar]
- 16.McAdam A J. Discrepant analysis: How can we test a test? J Clin Microbiol. 2000;38:2027–2029. doi: 10.1128/jcm.38.6.2027-2029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller N S, Madigan J E. Methods of implementation of an immunoprophylaxis program for the prevention of Rhodococcus equi pneumonia: Results of a 5-year field study. Proc Am Assoc Equine Pract. 1992;38:193–201. [Google Scholar]
- 18.Nagai S, Someno S, Yagihashi T. Differentiation of toxigenic from nontoxigenic isolates of Pasteurella multocida by PCR. J Clin Microbiol. 1994;32:1004–1010. doi: 10.1128/jcm.32.4.1004-1010.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prescott J F. Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev. 1991;4:20–34. doi: 10.1128/cmr.4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sellon D C, Walker K, Suyemoto M, Altier C. Nucleic acid amplification for rapid detection of Rhodococcus equi in equine blood and tracheal wash fluids. Am J Vet Res. 1997;58:1232–1237. [PubMed] [Google Scholar]
- 21.Sweeney C R, Sweeney R W, Divers T J. Rhodococcus equi pneumonia in 48 foals: Response to antimicrobial therapy. Vet Microbiol. 1987;14:329–336. doi: 10.1016/0378-1135(87)90120-9. [DOI] [PubMed] [Google Scholar]
- 22.Takai S, Fukunaga N, Ochiai S, Sakai T, Sasaki Y, Tsubaki S. Isolation of virulent and intermediately virulent Rhodococcus equi from soil and sand on parks and yards in Japan. J Vet Med Sci. 1996;58:669–672. doi: 10.1292/jvms.58.669. [DOI] [PubMed] [Google Scholar]
- 23.Takai S, Ohbushi S, Koike K, Tsubaki S, Oishi H, Kamada M. Prevalence of virulent Rhodococcus equi in isolates from soil and feces of horses from horse-breeding farms with and without endemic infections. J Clin Microbiol. 1991;29:2887–2889. doi: 10.1128/jcm.29.12.2887-2889.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takai S, Sekizaki T, Ozawa T, Sugawara T, Watanabe Y, Tsubaki S. Association between a large plasmid and 15- to 17-kilodalton antigens in virulent Rhodococcus equi. Infect Immun. 1991;59:4056–4060. doi: 10.1128/iai.59.11.4056-4060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takai S, Watanabe Y, Ikeda T, Ozawa T, Matsukura S, Tamada Y, Tsubaki S, Sekizaki T. Virulence-associated plasmids in Rhodococcus equi. J Clin Microbiol. 1993;31:1726–1729. doi: 10.1128/jcm.31.7.1726-1729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivrette S L. Rhodococcus equi pneumonia in foals. Vet Med. 1992;87:144–149. [Google Scholar]
- 27.Woolcock J B, Mutimer N M, Bowles P M. The immunological response of foals to R. equi: a review. Vet Microbiol. 1987;14:215–224. doi: 10.1016/0378-1135(87)90108-8. [DOI] [PubMed] [Google Scholar]