Abstract
Background
Most cancer-related deaths result from disseminated diseases that develop resistance to anticancer treatments. Inappropriate communication in this challenging situation may result in unmet patient information and support needs. Patient communication aids such as question prompt lists (QPLs) may help.
Objective
This study aims to develop and pilot-test a specific QPL in the following two contrasting clinical contexts in France after cancer resistance has developed: triple-negative and luminal B metastatic breast cancer (MBC) and metastatic uveal melanoma (MUM).
Methods
A sequential study design with a mixed methods collaborative approach will be applied. The first step aims to build a specific QPL. Step 1a will explore oncologist-patient communication issues from oncology professionals’ interviews (n=20 approximately). Step 1b will appraise information and support needs experienced by patients with MBC or MUM both quantitatively (n=80) and qualitatively (n=40 approximately). These data will be used to develop and pilot-test a QPL specific to patients with cancer experiencing initial or acquired resistance to treatment. We expect to obtain a core QPL that comprises questions and concerns commonly expressed by patients with resistant cancer and is complemented by specific issues for either MBC or MUM cancer sites. In step 1c, 2 focus groups of patients with any type of metastatic cancer (n=4) and health care professionals (n=4) will be conducted to revise the content of a preliminary QPL and elaborate an acceptable and feasible clinical implementation. In step 1d, the content of the QPL version 1 and implementation guidance will be validated using a Delphi process. Step 2 will pilot-test the QPL version 1 in real practice with patients with MBC or MUM (n=80). Clinical utility will be assessed by comparing responses to questionnaires administered in step 1b (QPL-naive historical control group) and step 2 (QPL intervention group).
Results
This study received grants in March and December 2019 and was approved by the French national ethics committee in July 2019. As of October 2021, interviews with oncology professionals have been conducted and analyzed (N=26 to reach saturation), and 39 and 27 patients with MBC and MUM, respectively, have been recruited.
Conclusions
A clinically and culturally tailored QPL is expected to facilitate patients’ participation in consultations, improve oncologists’ responses to patients’ information and support needs, and thus foster patients’ psychological adjustment to the diagnosis and follow-up of cancer resistance to treatment.
Trial Registration
ClinicalTrials.gov NCT04118062; http://clinicaltrials.gov/ct2/show/NCT04118062
International Registered Report Identifier (IRRID)
DERR1-10.2196/26414
Keywords: cancer resistance, physician-patient communication, question prompt list, patient participation, collaborative research, mixed methods
Introduction
Background
Most advanced cancers eventually develop resistance to anticancer therapies, ultimately leading to the progression of the disease, symptom burden, and death [1]. Resistance may occur very early in the course of disease (often called primary resistance) or, in most instances, is acquired under long-term treatment exposure after a favorable initial response (secondary resistance).
Cancer progression, resulting from resistance to therapy, often indicates the onset of an advanced disease that will not be cured, although life expectancy may still be months or years with adequate treatment. This clinical situation represents a critical moment in the course of cancer care. Oncologist-patient communication is then particularly challenging. When resistance occurs, patient information on the severity of the disease, its prognosis, and the therapeutic options must be conducted with utmost subtlety. Patients are informed about alternative cancer treatments, expectations about their effectiveness, and side effects. This information is anxiety provoking, often eliciting the need for emotional support.
Information on prognosis and treatment outcomes is important for achieving a shared perception of the disease status and treatment goals between patients and oncologists [2-4]. A general population survey performed in 7 European countries indicates that 73% of citizens prefer to be informed in case of a poor disease prognosis (≤1 year to live) [5]. In the advanced cancer setting, most patients want a realistic understanding of their current condition and life expectancy; however, not all wish to receive exact or definitive time frames [2,6-9]. Oncologists have difficulty appraising patients’ information preferences [10]. Information on prognosis is often lacking [11], and patients do not understand that the treatment provided is not likely to cure their cancer [12].
In addition, patients with advanced cancer do not participate as much as they wish during consultations [8,13]. Possible explanatory factors include patients forgetting questions, doubting the legitimacy of asking, expressing concerns indirectly, fear of the possible pejorative answer, and a lack of physicians encouraging their questions [14-16]. Patients may also present different needs and expectations, which depend on the time in their disease course [17] or factors such as their attentional coping style or sociodemographic background [10,18,19]. Discordance between the patient’s and oncologist’s perception of treatment aims and the disease timescale may result in medical decisions that do not align with life goals that are important to patients [20,21]. This leads to patients’ greater psychological distress [22].
Patient-centered communication is the cornerstone of high care quality [23]. This allows physicians to better respond to patients’ information and support needs. To this end, guidelines to improve communication skills of health care professionals are available in many countries [24-28]. Effective physician-patient communication may decrease patients’ anxiety, sustain hope [29], and increase satisfaction with care [30-32]. Patient-focused communication aids have also been developed to complement physician communication skills training [18,30-32]. These interventions are designed to enhance patients’ participation in the consultation and thus may increase physicians’ awareness and timely accommodation of their needs and expectations [33-36].
Question Prompt Lists
Among patient-focused communication aids, question prompt lists (QPLs) [37-43] may help patients express their information and support needs according to their wishes. A QPL includes a structured list of questions given to the patient before the consultation. This intervention drives patients to more frequently ask questions and express concerns, especially regarding disease prognosis, and may enhance patients’ recall of information and satisfaction with care [38,39,41,42,44-48].
Most QPLs that are already available address early cancers [39,49] or palliative care [41,50-52], specific clinical situations (eg, early breast cancer [53], esophageal cancer [54,55], and myelodysplastic syndrome [56]), care circumstances (eg, clinical trials [57,58]), and populations (eg, older patients with cancer [58] or cultures or ethnicity [53,59])
A QPL for oncology consultations taking place during the first treatment lines after cancer resistance has developed seems to be lacking. Such a QPL is expected to contain questions or concerns related to the following issues: disease severity, extent of spread, future course, medical tests, treatment options, course of symptoms and side effects [60], likelihood of cure, primary goal of cancer care, expected treatment effectiveness and life expectancy [61], psychological needs [62], self-management [63], and cancer care provision and organization [64]. A QPL designed for advanced cancer care in Australia and adapted in the United States [37] and in the Netherlands [18] will serve as a basis.
In Western countries, a shift has been witnessed in models of care from a paternalistic to a patient-centered approach, tailoring information to patient preferences and wishes [65]. However, for example, little information exists on French patients’ wishes to receive information on prognosis [56] and on the concordance between oncologists’ information provision and the expectations of patients with cancer. In a previous study with patients receiving palliative care, we observed that addressing disease prognosis was seen as particularly difficult for clinicians [66]. The cultural adaptation of a QPL may be necessary not only in terms of content but also in terms of implementation modalities. Theoretically, QPLs are simple and cost-effective; however, their acceptability and implementation feasibility should also be verified cross-culturally. Therefore, optimal modalities and procedures of applying this tool in real oncology practice in France will also be explored and delineated. It is possible that a patient coaching or education group intervention may be needed as a complement.
Clinical Setting
The QPL will be developed in the following two clinical contexts, which are prone to cancer resistance, and contrasted in terms of epidemiology, life expectancy, long-term treatment options, and expected effectiveness: (1) triple-negative and luminal B metastatic breast cancer (MBC) and (2) metastatic uveal melanoma (MUM), both within the first 3 lines of anticancer treatment after treatment resistance has occurred.
Triple-negative and luminal B MBC represents approximately 15% and 50% of all breast cancer cases, respectively [67], and recurs with distant metastasis in approximately 30% of early-stage patients [68]. MBC is an incurable disease, with a median overall survival of 3 years and a 5-year survival rate of 25% [69]. Patients with MBC are generally followed by their oncologist for years, punctuated by occurrences of treatment resistance for which a new treatment regimen may be offered. This implies successive disclosures of the progressing disease and discussions of alternative treatment regimens [70].
Uveal melanoma is a rare cancer [71]. Up to 50% of patients with uveal melanoma develop metastases, mostly in the liver, within a median time of 2 to 3 years [72]. Once metastasis occurs, the median overall survival ranges from 9 to 12 months because of the lack of effective treatment options [73]. Most patients with MUM are referred to a medical oncologist after a multidisciplinary tumor board meeting. Medical oncologists then inform patients about metastatic progression and possible treatment alternatives. The dismal prognosis and rapid evolution of the disease are challenging aspects in the communication between oncologists and patients. A discrepancy in knowledge appears when the medical oncologist is aware of the patient’s poor prognosis while the patient is still in good shape and does not expect such quick, fatal outcomes.
Objective
We propose an original design for the development of a complex intervention based on the Medical Research Council framework [74].
The specific aims are (1) to develop the content of a QPL for adults diagnosed with resistant cancer, who are still eligible for disease-targeted treatment, and (2) to pilot-test the implementation of this intervention in oncology consultations in a French cancer setting. During this process, the implementation will be prepared by investigating potential obstacles and facilitation strategies from the outset.
We expect to obtain a core QPL comprising questions and concerns that patients in the treatment resistance context might generally want to express at the oncology consultation. A common core QPL will be complemented with subsections containing specific issues for either the triple-negative and luminal B MBC or the MUM cancer sites.
Methods
Patient and Public Involvement
This psychosocial study is embedded in an overall medical research program performed within the Institut Curie Integrated Cancer Research Site—Site de Recherche Intégrée sur le Cancer. In France, this framework is equivalent to a comprehensive cancer center and has been labeled by the French National Cancer Institute. This research program deals with treatment resistance in triple-negative and luminal B MBC and MUM. A patient and partner representative committee was created with the objective of fostering public debate and integrating patients’ voices into research development, conduct, and dissemination. This committee was involved in the choice of the psychosocial study research question. Outcome measures were selected according to priorities, experience, and preferences. They found it particularly relevant and timely to address communication difficulties between the oncologist and the patient when treatment resistance occurs. Since 2017, their involvement has taken place at regular steering committees and brainstorming meetings. They provided feedback on this study protocol; the information and consent forms; and the content, format, and burden of the questionnaires.
Study Design
As shown in Figure 1 and Table 1, we will adopt a sequential design and mixed methods approach [75,76], evolving in successive steps and building on qualitative (individual and focus group interviews) and quantitative (standardized questionnaires, acceptability assessment, and Delphi process) data collection and analysis. It will involve the collaboration of adult patients with MBC or MUM, patients with any type of metastatic cancer (ie, expert patient, defined as “individuals with an experience of cancer diagnosis & treatment and educated knowledge of their disease and treatment trajectory” [77,78]), oncologists, supportive care specialists, cancer care administrators, and laboratory researchers dealing with cancer resistance [79].
Figure 1.
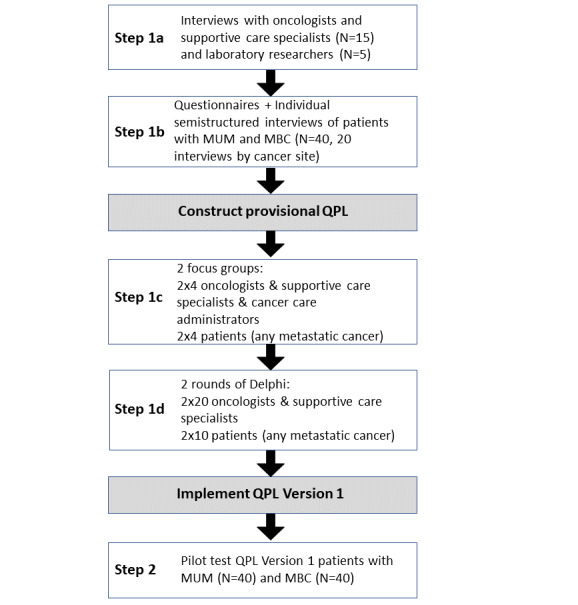
Sequential study design. MBC: metastatic breast cancer; MUM: metastatic uveal melanoma; QPL: question prompt list.
Table 1.
Sequential collaborative mixed methods approach.
| Steps | Aim | Method | Sources or population | |
| Step 1: QPLa development | To develop a QPL for the treatment of resistant cancer in 2 contrasting clinical contexts: MBCb (triple-negative and luminal B) and MUMc |
|
Professionals and patients | |
|
|
Step 1a | To explore oncologist-patient communication issues (ie, difficulties, obstacles, and strategies) in the context of cancer resistance |
|
Professionals, that is, oncology physicians, supportive care specialists, and laboratory researchers (from bench to bedside) |
|
|
Step 1b | To explore communication difficulties and information and support needs that are experienced by patients with resistant cancer after an oncology consultation to initiate or follow a new disease-specific treatment after cancer resistance has developed |
|
Patients with MBC (triple-negative and luminal B) or MUM within the first 3 lines after first cancer resistanced |
|
|
Step 1c | To revise the content of a QPL developed for patients with advanced cancer and elaborate an acceptable and feasible clinical implementation, potentially facilitated by a coaching intervention |
|
Professionals and patients (any type of metastatic cancer) |
|
|
Step 1d | To validate the content of a QPL preliminary version adapted to cancer resistance and the MBC (triple-negative and luminal B) and MUM contexts (version 1) and the implementation guidelines |
|
Professionals and patients (any type of metastatic cancer) |
| Step 2: QPL pilot testing | To pilot-test a QPL version 1: acceptability, feasibility, and potential clinical utility (effects) |
|
Patients with MBC (triple-negative and luminal B) or MUM within the first 3 lines after first cancer resistance | |
aQPL: question prompt list.
bMBC: metastatic breast cancer.
cMUM: metastatic uveal melanoma.
dPatients in step 1b comprise a historical control group to which the patients provided with the preliminary question prompt list in step 2 will be contrasted.
Study Assessment and Procedures
The approval for the study was obtained from the French national ethics committee (ID-RCB: 2019-A01713-54). All patients will provide signed informed consent. The study, for which the acronym is HECTOR (Helping Patients Communicate With Oncologists When Cancer Treatment Resistance Occurs), is registered as NCT04118062 in ClinicalTrials.gov.
Step 1: QPL Development and Implementation Guidelines
Overview
Step 1 focuses on determining the QPL content, structure, and format, as well as the clinical implementation modalities. This step will allow the definition of the clinical setting of cancer resistance and the factors that may facilitate or impede patients’ participation in oncology consultation. We aim to define a print brochure, along with a website and mobile app, offering a QPL perceived as attractive and satisfactory by patients and clinicians and that may be successfully used in the clinic (Table 1).
Interviews with patients and professionals will explore patient-oncologist communication issues in the context of cancer resistance. Moreover, study participants will be prompted to consider the relevance of a communication aid in the form of a QPL and complement this tool with patient coaching (ie, an intervention involving an interaction with a health professional or peer-support patient) and to prepare the operational implementation of this intervention in routine practice [80,81].
Step 1a: Oncologist-Patient Communication in the Clinical Management of Resistant Cancer
Individual semistructured interviews with oncologists, supportive care specialists, and laboratory researchers selected according to the inclusion and exclusion criteria listed in Textbox 1 started in July 2019.
Professionals and patients’ eligibility criteria included at each step of the study.
Inclusion criteria
Professionals
Oncology physicians (medical oncologist, oncology surgeon, radiation oncologist, and supportive care specialist), oncology laboratory researchers, and cancer care administrators
Dealing with cancer resistance in solid malignancies
Patients with metastatic breast cancer or metastatic uveal melanoma (MUM)
Aged ≥18 years
Diagnosed with resistant cancer
Metastatic triple-negative or luminal B breast cancer or MUM
Within the first 3 treatment lines after cancer resistance has developed
Informed of cancer diagnostic and treatment resistance
Patients with any type of metastatic cancer
Aged ≥18 years
With any type of cancer diagnosis
Exclusion criteria
Professionals
Physicians or laboratory researchers not involved in cancer resistance research
Patients with metastatic breast cancer or MUM
Unable to complete surveys in French
Patients with any type of metastatic cancer
Unable to participate in a group interview or complete a questionnaire survey because of physical, cognitive, or linguistic (French language) barriers
A purposeful sample is planned to allow the obtaining of a variety of perspectives on the clinical situation of cancer resistance [82]. Snowball sampling (ie, inclusion by approaching further participants through initial ones) will identify participants in different oncology hospitals or departments (France) from participants initially interviewed in 1 cancer center (Institut Curie, Paris).
Laboratory researchers will be solicited because of their outlook and up-to-date knowledge of cancer resistance research; they will be susceptible to anticipate new therapeutic regimens and their clinical translations.
An interview guide comprising open-ended questions will explore the following three central themes: (1) the definition of drug resistance and cancer resistance; (2) perceptions of oncologist-patient communication when the patient is diagnosed with resistant cancer, initial or acquired, after 1 or several treatment regimens; and (3) perceptions of oncologist-patient communication difficulties, obstacles, and facilitating strategies in the clinical context. The expected sample size based on data saturation is approximately 20 participants (Table 2) [83]. The duration of each interview is estimated to be between 30 and 45 minutes.
Table 2.
Sample size by study population.
| Population and data collection | Cancer resistance to treatment (before consultation) | |||
| Historical control (step 1; N=80) | QPLa (step 2; N=80) | |||
| Number of patients | ||||
| MUMb within the first 3 treatment lines after cancer resistance has developed | ||||
| Questionnaires | 40 | 40 | ||
| Individual interviews | 20 | —c | ||
| Triple-negative and luminal B MBCd within the first 3 treatment lines after cancer resistance has developed | ||||
| Questionnaires | 40 | 40 | ||
| Individual interviews | 20 | — | ||
| Number of professionals | ||||
| Oncologists or supportive care specialists or cancer care administrators | ||||
| Individual interviews | 15 | — | ||
| 2 focus groups | 8e | — | ||
| 2-round Delphi | 40f | — | ||
| Laboratory researchers | ||||
| Individual interviews | 5 | — | ||
| Number of patients with any type of cancer diagnosis | ||||
| Any cancer | ||||
| 2 focus groups | 8e | — | ||
| 2-round Delphi | 20g | — | ||
aQPL: question prompt list.
bMUM: metastatic uveal melanoma.
cNot available (no individual interview in step 2).
dMBC: metastatic breast cancer.
en=2×4.
fn=2×20.
gn=2×10.
Step 1b: Patients’ Communication Experience and Information and Support Needs in the Oncology Consultation Dealing With Treatment Resistance
Overview
A cross-sectional assessment of patients’ communication needs in the context of cancer resistance will then be performed. Patient enrollment started in January 2021 and will take place over 1 year.
Consecutive patients will be identified via lists of oncology consultation agendas in an oncology center (Institut Curie, Paris). If they respond to the inclusion and exclusion criteria listed in Textbox 1, they will be invited to participate in the study.
Patients’ sociodemographic data (age, gender, educational level, and marital, parental, and professional status) and clinical data (date of initial diagnosis, disease recurrence and metastatic occurrence, stage, previous and current disease-targeted treatments, and supportive care interventions) will be collected from patients or medical records to describe the study population.
Qualitative Assessment
Individual semistructured interviews will be proposed to a random subsample of these patients to specify in greater depth and detail the nature and temporality of communication experience, difficulties, and needs when confronted with cancer resistance.
On the basis of data saturation [83,84], an expected number of approximately 20 patients per cancer site will be interviewed no later than 1 month after they complete the questionnaires (Table 2).
The interview guide, comprising open-ended questions, will explore the following three central themes: (1) patients’ actual experience of communication with the oncologist; (2) their expectations, preferences, met and unmet information, and support needs (retrospectively and prospectively); and (3) their opinions about a specific QPL to help them communicate with oncologists. Individual interviews are estimated to last between 30 and 45 minutes.
Quantitative Assessment
Standardized questionnaires will be completed by patients to describe their unmet information and support needs while facing cancer resistance in the MBC and MUM settings. These questionnaires will also be used to obtain preliminary data, albeit limited to the potential clinical usefulness of the designed QPL. This will be performed by comparing responses from patients at step 1b (QPL-naive group) with clinically similar patients at step 2 (QPL intervention group).
As information needs may depend on patients’ characteristics [10,18], these outcomes will be assessed according to patients’ sociodemographic (ie, age, gender, educational level, and marital status) and psychological correlates (ie, beliefs, preference for information, level of distress, and coping strategies). To address these outcomes and correlates, patients will be invited to complete standardized questionnaires, as detailed in Table 3.
Table 3.
Standardized questionnaires.
| Measures of QPLa potential clinical benefits | Factors assessed | ||
| Outcomes | |||
|
|
1 item of the PTPQb [61] |
|
|
|
|
EORTC QLQ-INFO25c information about the disease, medical tests, and treatments scales (items 31-43) and satisfaction with information items (52-55) [60] |
|
|
|
|
SCNS-SF34d, Psychological (items 6-14 and 17) and Care and Support needs (items 18-22) [85] |
|
|
| Correlates | |||
|
|
12 items of the PTPQ [61] |
|
|
|
|
HADSe [86] |
|
|
|
|
Brief COPEf [87] |
|
|
aQPL: question prompt list.
bPTPQ: Prognosis and Treatment Perceptions Questionnaire.
cEORTC QLQ-INFO25: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Information Module.
dSCNS-SF34: Supportive Care Needs Survey–Short Form.
eHADS: Hospital Anxiety and Depression Scale.
fCOPE: Coping Orientation to Problems Experienced.
Patients’ perception of prognosis and treatment will be assessed using the Prognosis and Treatment Perception Questionnaire (PTPQ) [61]. The PTPQ assesses beliefs regarding (1) the likelihood of cure, (2) the importance and helpfulness of knowing about prognosis, (3) the primary goal of cancer care, (4) preference for information about treatment, and (5) satisfaction with the quality of information received about prognosis and treatment (Table 3). This questionnaire has been translated into French and pilot-tested according to the European Organization for Research and Treatment of Cancer Quality of Life Group guidelines [88].
The perception of information received about the disease, medical tests and treatments, and satisfaction with overall medical information will be measured by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Information Module (EORTC QLQ-INFO25) scale [60]. The perception of unmet psychological (eg, anxiety, fear of cancer spreading, and uncertainty) and care and support needs (eg, reassurance, sensitivity to patients’ feelings, and emotional needs) will be measured by the Supportive Care Needs Survey–Short Form (SCNS-SF34) [85].
Additional patient information will include coping strategies (usual strategies when facing stressful life events) and distress (during the past week), as measured by the Brief Coping Orientation to Problems Experienced [87] and the Hospital Anxiety and Depression Scale [86] questionnaires, respectively.
Questionnaires will be completed within 2 weeks of the consultation; if not completed within this time-lapse, 1 reminder will be made by telephone. Questionnaires not returned after 1 month will be considered missing.
As indicated in Table 2, a sample size of 80 patients (40 patients by tumor site) is planned. Sample sizes up to 40 per group are expected to provide estimates that are precise enough to assess the feasibility of QPL use and obtain preliminary clinical data for further randomized controlled trials of this tool [89].
Step 1c: Content and Implementation of the QPL in Routine Practice
From steps 1a and 1b, a core QPL comprising issues (ie, questions and concerns) commonly expressed by patients with resistant cancer, complemented by specific issues for either MBC or MUM cancer sites, will be developed. Issues to compose this QPL will be selected based on descriptive analyses of patients’ responses to relevant items of the PTPQ, EORTC QLQ-INFO25, and SNCS-SF34 questionnaires. For example, if ≥50% of patients report that they received little or no information about the spread of their illness from the EORTC QLQ-INFO25 and are dissatisfied with the information provided, this issue will be prioritized while composing the QPL. Other issues will be similarly selected based on responses to items of the SCNS-SF34 (50% of patients reporting medium or high unmet needs) or the PTPQ (50% reporting that the quality of information on treatment options received from the oncologist was fair or poor). These quantitative data will be considered in conjunction with the qualitative interview data. A thematic content analysis will help identify issues that patients would like to address more frequently during oncology consultations dealing with cancer resistance.
Focus groups will be implemented to discuss the provisional QPL. On the basis of research on sample size calculation for the content analysis of qualitative interviews [83], we aim to conduct 2 focus groups of approximately 8 different participants each (patients with any type of metastatic cancer, oncologists, supportive care specialists, and cancer care administrators from various oncology centers or departments in France), approached through snowball purposive sampling. Participants will be identified from contacts of expert patients (patient university, Institut Curie Site de Recherche Intégrée sur le Cancer patient and partner representatives, and cancer patient associations) and oncology professionals (UNICANCER oncology professionals’ network and French Association for Supportive Care—Association Francophone pour Soins Oncologiques de Support).
The group interview guide will address the following two central themes: (1) the appropriateness (adequacy, relevance, and importance for treatment resistance in oncology generally and in triple-negative and luminal B MBC and MUM specifically) and acceptability (satisfaction, anxiety-provoking, intrusiveness, irrelevance, and incompleteness) of the QPL content (questions, concerns or emotions, and narratives or testimonies), structure (logical order, length, and complexity), and format (paper, website, and app) and (2) the feasibility (obstacles and facilitating strategies such as complementary coaching, formal implementation blueprint, educational materials, and audit and feedback [90]) and optimal circumstances and procedures (when: timing of provision or access; where: hospital or home; how: text or video; who: coach or health educator expertise) for implementing the QPL in real-world clinical practice and ensuring its adoption and sustainability. The internationally designed QPL for patients with advanced cancer has been revised and further developed for the cancer resistance context by keeping, removing, or adding items according to their relevance and importance generally for treatment resistance in oncology and specifically for triple-negative and luminal B MBC and MUM.
Each focus group will be conducted via Microsoft Teams videoconference to facilitate participation and will last an estimated 90 to 120 minutes.
Step 1d: Content Validation With the Delphi Process
To facilitate the collection of individuals’ feedback on the provisional QPL version, a 2-round web-based Delphi process [91] will be performed involving participants through snowball purposive sampling. Participants in the focus groups will be offered the opportunity to participate in the consensus method. Other eligible participants (see criteria in Textbox 1) will be solicited according to the same recruitment methodology as for the focus groups.
The Delphi survey assesses (1) each QPL item (instructions, questions, concerns or emotions, and narratives or testimonies) in terms of content appropriateness, formulation clarity, structure, format, and acceptability on a 5-point Likert agreement scale and (2) the feasibility of a complementary coaching intervention and implementation guidance.
The procedure comprises successive evaluations according to the following steps:
Participants will indicate their level of agreement on the relevance and clarity of each proposed item of the QPL (ie, instruction sentences and questions or concerns) and implementation guidance (ie, ideas or sentences) using a 5-point Likert scale (ranging from 1=strongly disagree to 5=strongly agree). The overall length and clarity of the tool and guidance will also be assessed.
Participants will comment and propose changes or additions to the QPL and guidance if required based on the following questions addressed to patients (professionals):
What would you (your patients) have liked to ask?
What questions do you (your patients) often not ask, that you (I) wish you (they) would ask?
The research team will analyze the data obtained. It will then identify the issues on which there is consensus and make possible modifications and additions based on the participants’ comments.
Following the first evaluation, the modified items will be submitted to a second evaluation by participants who will be invited to rate each question, concern, or sentence on a 5-point Likert scale, and responses will be rated as essential or important; validation will be determined by an a priori threshold of ≥4.0.
Following the second evaluation, QPL version 1 and guidance will be validated in its final version.
A total of 2 rounds of Delphi surveys administered via REDCap (Research Electronic Data Capture; Vanderbilt University) software will be performed, including 30 participants responding to the first survey on a first QPL version and then the second survey on a QPL version revised from the initial survey responses and comments.
Step 2: QPL and Implementation Pilot Testing
The second step of the study pilot tests the QPL version 1 for its acceptability, feasibility, potential clinical utility, and sustainability. Consecutive patients (n=80) responding to the same eligibility criteria as in step 1b will be recruited. They will receive the QPL version 1 to prepare their subsequent oncology consultations, either a consultation during which the diagnosis of cancer resistance is communicated or in the course of a new disease-targeted treatment follow-up consultation. They will be invited to complete the same standardized questionnaires as in step 1b.
Additional questions will address the QPL acceptability in terms of uptake (the QPL has been read before the consultation), use (QPL items have been raised during the consultation), and patients’ and clinicians’ perceived helpfulness and satisfaction with this tool and its use in clinical practice.
Data Analysis
Qualitative Data
All interviews will be audiotaped, transcribed, and identified using an alphanumeric log. A thematic analysis will be conducted using the RQDA (R package for the qualitative data analysis) software (version 2.15.2; 2012-10-26). A total of 2 junior (JT and AR) postdoctoral health psychologists and 1 senior (AB) postdoctoral health psychologist will code the transcripts. The analysis of thematic content will allow both the frequencies of responses for each category to appear and the meaning of the responses associated with each category to emerge [92,93]. A coding grid will be constructed from 2 complementary processes; (1) a pre-established code based on the research objectives and the semidirected interview guide will be elaborated to create broad coding categories and subcategories, and (2) a third of the interviews will be coded, using an emergent coding method, to test and modify the grid. Following an iterative process, several rounds of analysis will be conducted to stabilize the coding sheet. Finally, double interjudge and intrajudge coding will be conducted to ensure the reliability and independence of coding.
A similar content analysis will be performed for focus group interviews, with coding based on themes related to the content, format, and clinical implementation guidance of the QPL.
Quantitative Data
Statistical analyses will be conducted using SPSS software (version 27.0; IBM Corp). Standardized questionnaires used in steps 1b and 2, and the Delphi surveys, will be analyzed descriptively in terms of missing data, response frequency, mean and SD, median, and range.
In step 1b, responses to items of the PTPQ, EORTC QLQ-INFO25, and SCNS-SF34 will determine the prevalence of communication needs in the cancer resistance setting and thus help prioritize communication issues to compose the QPL.
The step 1b group (QPL naive) will comprise a historical control of patients from step 2 (QPL intervention group). Quantitative data collected from standardized questionnaires at step 1b will be compared with the data collected at step 2. Patients will be consecutively included in steps 1b and 2; therefore, they are expected to be clinically similar; however, sociodemographic and clinical data between these groups will be compared to check their similarity. Bivariate analyses of outcome measures will be performed to preliminarily assess the potential clinical usefulness of the QPL. Bivariate analyses will also be performed to explore patients’ satisfaction with information and support needs in relation to their sociodemographic and psychological characteristics (distress and coping strategies) in step 2 samples, overall and by cancer site.
Results
This study received grants from the Ile-de-France CancerôPole (2019-1-EMERG-14-ICH-1; March 2019) and from the Fondation de France (2019 number 00101610; December 2019) and was approved by the French national ethics committee in July 2019. As of December 2020, to reach data saturation, 26 oncology professionals’ interviews have been conducted and analyzed. As of October 31, 2021, a total of 40 and 31 patients with MBC and MUM have been recruited, 20 and 20 have been interviewed, and 39 and 28 have completed questionnaires, respectively.
Discussion
Principal Findings
This protocol describes a study using an innovative, sequential, mixed methods approach and involving patients as well as oncology professionals to collaboratively develop a QPL for cancer resistance in the French cultural context.
This study will be undertaken in 2 clinical settings prone to cancer resistance and contrasted in terms of epidemiology, life expectancy, long-term treatment options, and expected effectiveness. The resulting QPL is expected to comprise core issues related to the cancer resistance context to which specific issues will be added if needed, according to the tumor site. The core QPL is expected to be applicable in other advanced cancer contexts.
QPLs seem effective in raising patients’ asking questions [38,42,94]; however, a complementary coaching intervention may be needed to further patients’ support [95,96,97]. Coaching is the provision of nondirective support by an individual (either in-person or remotely eg, by telephone or the internet) [98]. It is expected to help patients assess their information needs (ie, about treatment options such as disease-targeted treatment [standard or experimental], best supportive care, watchful waiting, and their benefits and harms) [18], prepare and rehearse questions [33], express their concerns or emotions [99], and prioritize issues to discuss during the consultation.
This study’s sequential collaborative mixed methods approach is innovative; the following methodological aspects are expected to be fruitful. First, a triangulation of perspectives is foreseen as it involves patients, clinicians, and researchers, and thus a better grasp of the specific realities of oncology consultation communication in resistant cancer care.
Second, the quantitative and qualitative data collection approaches are complementary, and the results will be sequentially and iteratively integrated. In-depth interviews with different protagonists who are experts in cancer resistance at their own level are expected to increase the appropriateness and acceptability of the tool. Considering the modalities and procedures of tool implementation from the outset is also meant to promote its adoption in routine practice. Focus group discussions and exploratory quantitative analyses will help decide for whom, when, and how the QPL will be implemented in clinical routine.
Third, the quantitative assessment allows the assessment of the specific information and support needs that are experienced when faced with cancer resistance. These data may be compared between 2 groups: before (step 1b) versus after (step 2) the availability of the QPL. This will offer initial information on the clinical utility of the tool tailored to cancer resistance in the French cultural context.
Owing to consecutive sampling, information will be available about the number of patients willing to use the QPL and the number of oncologists who will engage in its use during consultations [92]. Furthermore, the resulting QPL will be pilot-tested on outcomes such as patients’ beliefs about primary cancer treatment goals or satisfaction with the information provided about prognosis and treatment, which are important [93] and previously lacking effect measurement of QPL use [2].
Finally, focus group discussions will specifically elicit collaborative work. The reactions and proposals of various appropriate persons [100] will help prepare the modalities of QPL use in routine practice and promote its long-term adoption [101], that is, the tool use in real-world clinical practice [102]. Moreover, the Delphi process aims to reach consensus among patients and oncology professionals on a QPL version 1 and explicit guidance for clinical implementation [103,104]. We anticipate that these persons will have diverging views and opinions about the tool [91]; thus, a consensus will have to be developed.
This protocol has several limitations. The methodology described herein is an innovative but long process. It may not be systematically applied as a new clinical context in need of a QPL. However, this study offers the opportunity to reveal unnecessary steps and thus indicate an optimized process for future research.
We focus on the communication between oncologists and patients; however, other oncology professionals such as nurses or psychologists may also play an important role in responding to patients’ information and support needs; therefore, further research is needed to address their specific role in addressing these needs. In addition, patients’ relatives may also present their specific information and support needs and may interact with the patient when facing this information. The QPL may also be useful to them, as such or adapted, and this must be evaluated.
The assessment of patients at step 1b and step 2 of this study is cross-sectional; therefore, we also need to consider that information is not collected on the same individual patients over their evolving needs and information processing.
Dissemination
A clinically and culturally specific QPL complemented, if necessary, by a coaching intervention is expected to facilitate patients’ participation in oncology consultations to improve oncologists’ responses to their information and support needs. This tool allows patients to control the provided information according to their wishes and thus respects their potential ambivalence and need for hope. Digital health interventions provide patients with evidence-based interventions through software apps. The availability of QPLs through these technologies is increasing [55,105,106]. Digital health interventions are easily accessible to patients from their homes through mobile devices or websites. Studies suggest that they are cost-effective, increase uptake by patients and clinicians, and provide clinical benefits [107]. Therefore, this French-adapted QPL will be available not only in paper form (brochure) but also as an app (MyCurie app) and on the institutional website. It will contain information about the purpose, interests, and modalities of use of the brochure or web document. Moreover, studies have shown the importance of clinicians’ endorsement of the tool, encouraging its use by patients; therefore, particular attention will be given to an implementation guide that recommends communication about this tool during the consultation. Patient and partner representatives of the Institut Curie will be invited to attend the local public and scientific conferences planned to communicate about this study. They will be solicited for their feedback on articles to disseminate results nationally and internationally through popular or scientific journals.
Conclusions
This research proposes an original methodology to adapt and further develop a QPL for patients with resistant cancer and enable its implementation in the French cultural context. It is expected to facilitate patients’ expression of questions, concerns, and emotions and, in that way, improve oncologists’ responses to their information and support needs. Clinically, this study will also improve the understanding of patients’ and clinicians’ experiences, difficulties, obstacles, and strategies in discussing prognosis and treatment options in the first anticancer treatment lines after cancer resistance has developed. Methodologically, it will be possible to infer an efficient method for designing and guiding the implementation of communication aids for patients with advanced cancer.
Acknowledgments
The authors are grateful to Sergio Roman-Roman for his major role in funding this work through the Site de Recherche Intégrée sur le Cancer label, which has designated the Institut Curie an integrated cancer research site. The authors would also like to thank Françoise Apiou, Stéphanie Dogniaux, Laure Guéroult-Accolas, Patrice Marvanne, and Fabrizia Stavru, who are involved in the overall study process as patient and partner representatives of the Institut Curie. The authors would also like to thank Léonore Robieux, Leila El Mellah, and Mathilde Torsdorf for their help with the design and initial draft of this research project.
Abbreviations
- EORTC QLQ-INFO25
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Information Module
- HECTOR
Helping Patients Communicate With Oncologists When Cancer Treatment Resistance Occurs
- MBC
metastatic breast cancer
- MUM
metastatic uveal melanoma
- PTPQ
Prognosis and Treatment Perception Questionnaire
- QPL
question prompt list
- REDCap
Research Electronic Data Capture; Vanderbilt University
- SCNS-SF34
Supportive Care Needs Survey–Short Form
Footnotes
Authors' Contributions: AB, SD, EH, and ES conceived the original concept of the study and the intervention. AB, AR, SD, LDK, JT, and ES drafted the protocol. AR will perform data collection, individual and group interviews, data management, and analyses. AS will supervise statistical analyses. CB, PC, JYP, SPN, and MJR will support the intervention development and pilot testing. All authors contributed to the scientific design of the study and the protocol development, are involved in the implementation of the project, and have read and approved the final manuscript.
Conflicts of Interest: None declared.
References
- 1.Nikolaou M, Pavlopoulou A, Georgakilas AG, Kyrodimos E. The challenge of drug resistance in cancer treatment: a current overview. Clin Exp Metastasis. 2018 Apr;35(4):309–18. doi: 10.1007/s10585-018-9903-0.10.1007/s10585-018-9903-0 [DOI] [PubMed] [Google Scholar]
- 2.Selim S, Kunkel E, Wegier P, Tanuseputro P, Downar J, Isenberg SR, Li A, Kyeremanteng K, Manuel D, Kobewka DM. A systematic review of interventions aiming to improve communication of prognosis to adult patients. Patient Educ Couns. 2020 Aug;103(8):1467–97. doi: 10.1016/j.pec.2020.02.029.S0738-3991(20)30100-2 [DOI] [PubMed] [Google Scholar]
- 3.Elwyn G, Stiel M, Durand M, Boivin J. The design of patient decision support interventions: addressing the theory-practice gap. J Eval Clin Pract. 2011 Aug;17(4):565–74. doi: 10.1111/j.1365-2753.2010.01517.x.JEP1517 [DOI] [PubMed] [Google Scholar]
- 4.Vasista A, Stockler MR, Martin A, Lawrence NJ, Kiely BE. Communicating prognostic information: what do oncologists think patients with incurable cancer should be told? Intern Med J. 2020 Dec;50(12):1492–9. doi: 10.1111/imj.14739. [DOI] [PubMed] [Google Scholar]
- 5.Harding R, Simms V, Calanzani N, Higginson IJ, Hall S, Gysels M, Meñaca A, Bausewein C, Deliens L, Ferreira P, Toscani F, Daveson BA, Ceulemans L, Gomes B, PRISMA If you had less than a year to live, would you want to know? A seven-country European population survey of public preferences for disclosure of poor prognosis. Psychooncology. 2013 Oct;22(10):2298–305. doi: 10.1002/pon.3283. [DOI] [PubMed] [Google Scholar]
- 6.Butow PN, Clayton JM, Epstein RM. Prognostic awareness in adult oncology and palliative care. J Clin Oncol. 2020 Mar 20;38(9):877–84. doi: 10.1200/jco.18.02112. [DOI] [PubMed] [Google Scholar]
- 7.Hagerty RG, Butow PN, Ellis PM, Lobb EA, Pendlebury SC, Leighl N, MacLeod C, Mac Leod C, Tattersall MH. Communicating with realism and hope: incurable cancer patients' views on the disclosure of prognosis. J Clin Oncol. 2005 Feb 20;23(6):1278–88. doi: 10.1200/JCO.2005.11.138.23/6/1278 [DOI] [PubMed] [Google Scholar]
- 8.Johnson M, Tod AM, Brummell S, Collins K. Prognostic communication in cancer: a critical interpretive synthesis of the literature. Eur J Oncol Nurs. 2015 Oct;19(5):554–67. doi: 10.1016/j.ejon.2015.03.001.S1462-3889(15)00044-7 [DOI] [PubMed] [Google Scholar]
- 9.Henselmans I, Smets E, Han P, de Haes H, Laarhoven H. How long do I have? Observational study on communication about life expectancy with advanced cancer patients. Patient Educ Couns. 2017 Oct;100(10):1820–7. doi: 10.1016/j.pec.2017.05.012.S0738-3991(17)30284-7 [DOI] [PubMed] [Google Scholar]
- 10.Elkin EB, Kim SH, Casper ES, Kissane DW, Schrag D. Desire for information and involvement in treatment decisions: elderly cancer patients' preferences and their physicians' perceptions. J Clin Oncol. 2007 Nov 20;25(33):5275–80. doi: 10.1200/JCO.2007.11.1922.25/33/5275 [DOI] [PubMed] [Google Scholar]
- 11.Jenkins V, Solis-Trapala I, Langridge C, Catt S, Talbot DC, Fallowfield LJ. What oncologists believe they said and what patients believe they heard: an analysis of phase I trial discussions. J Clin Oncol. 2011 Jan 01;29(1):61–8. doi: 10.1200/JCO.2010.30.0814.JCO.2010.30.0814 [DOI] [PubMed] [Google Scholar]
- 12.Weeks JC, Catalano PJ, Cronin A, Finkelman MD, Mack JW, Keating NL, Schrag D. Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012 Oct 25;367(17):1616–25. doi: 10.1056/NEJMoa1204410. http://europepmc.org/abstract/MED/23094723 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Street RJ, Gordon H, Ward M, Krupat E, Kravitz RL. Patient participation in medical consultations: why some patients are more involved than others. Med Care. 2005 Oct;43(10):960–9. doi: 10.1097/01.mlr.0000178172.40344.70.00005650-200510000-00003 [DOI] [PubMed] [Google Scholar]
- 14.Clayton JM, Natalia C, Butow PN, Simpson JM, O'Brien AM, Devine R, Tattersall MH. Physician endorsement alone may not enhance question-asking by advanced cancer patients during consultations about palliative care. Support Care Cancer. 2012 Jul 26;20(7):1457–64. doi: 10.1007/s00520-011-1229-2. [DOI] [PubMed] [Google Scholar]
- 15.Dang BN, Westbrook RA, Njue SM, Giordano TP. Building trust and rapport early in the new doctor-patient relationship: a longitudinal qualitative study. BMC Med Educ. 2017 Feb 02;17(1):32. doi: 10.1186/s12909-017-0868-5. https://bmcmededuc.biomedcentral.com/articles/10.1186/s12909-017-0868-5 .10.1186/s12909-017-0868-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beach WA, Dozier DM. Fears, uncertainties, and hopes: patient-initiated actions and doctors' responses during oncology interviews. J Health Commun. 2015;20(11):1243–54. doi: 10.1080/10810730.2015.1018644. http://europepmc.org/abstract/MED/26134261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niranjan SJ, Turkman Y, Williams BR, Williams CP, Halilova KI, Smith T, Knight SJ, Bhatia S, Rocque GB. “I'd want to know, because a year's not a long time to prepare for a death”: role of prognostic information in shared decision making among women with metastatic breast cancer. J Palliative Med. 2020 Jul 01;23(7):937–43. doi: 10.1089/jpm.2019.0457. [DOI] [PubMed] [Google Scholar]
- 18.Henselmans I, Brugel SD, de Haes HC, Wolvetang KJ, de Vries LM, Pieterse AH, Baas-Thijssen MC, de Vos FY, van Laarhoven HW, Smets EM. Promoting shared decision making in advanced cancer: development and piloting of a patient communication aid. Patient Educ Couns. 2019 May;102(5):916–23. doi: 10.1016/j.pec.2018.12.018.S0738-3991(18)30412-9 [DOI] [PubMed] [Google Scholar]
- 19.Leonhardt M, Aschenbrenner K, Kreis ME, Lauscher JC. Does migrant background predict to what extent colorectal cancer patients want to be informed about their life expectancy? - a cross-sectional analysis. Int J Equity Health. 2019 Dec 05;18(1):192. doi: 10.1186/s12939-019-1105-0. https://equityhealthj.biomedcentral.com/articles/10.1186/s12939-019-1105-0 .10.1186/s12939-019-1105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douglas SL, Daly BJ, Meropol NJ, Lipson AR. Patient-physician discordance in goals of care for patients with advanced cancer. Curr Oncol. 2019 Dec;26(6):370–9. doi: 10.3747/co.26.5431. https://www.mdpi.com/resolver?pii=conc-26-370 .conc-26-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernacki RE, Block SD, American College of Physicians High Value Care Task Force Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014 Dec;174(12):1994–2003. doi: 10.1001/jamainternmed.2014.5271.1916912 [DOI] [PubMed] [Google Scholar]
- 22.El-Jawahri A, Forst D, Fenech A, Brenner KO, Jankowski AL, Waldman L, Sereno I, Nipp R, Greer JA, Traeger L, Jackson V, Temel J. Relationship between perceptions of treatment goals and psychological distress in patients with advanced cancer. J Natl Compr Canc Netw. 2020 Jul;18(7):849–55. doi: 10.6004/jnccn.2019.7525.jnccn19231 [DOI] [PubMed] [Google Scholar]
- 23.Levinson W. Patient-centred communication: a sophisticated procedure. BMJ Qual Saf. 2011 Oct;20(10):823–5. doi: 10.1136/bmjqs-2011-000323.bmjqs-2011-000323 [DOI] [PubMed] [Google Scholar]
- 24.Girgis A, Sanson-Fisher RW. Breaking bad news: consensus guidelines for medical practitioners. J Clin Oncol. 1995 Sep;13(9):2449–56. doi: 10.1200/JCO.1995.13.9.2449. [DOI] [PubMed] [Google Scholar]
- 25.Clayton JM, Hancock KM, Butow PN, Tattersall MH, Currow DC, Australiannew Zealand Expert Advisory Group. Adler J, Aranda S, Auret K, Boyle F, Britton A, Chye R, Clark K, Davidson P, Davis JM, das A, Graham S, Hardy J, Introna K, Kearsley J, Kerridge I, Kristjanson L, Martin P, McBride A, Meller A, Mitchell G, Moore A, Noble B, Olver I, Parker S, Peters M, Saul P, Stewart C, Swinburne L, Tobin B, Tuckwell K, Yates P, Australasian Society of HIV Medicine. AustralianNew Zealand Society of Palliative Medicine. Australasian Chapter of Palliative Medicine. Royal Australasian College of Physicians. Australian College of RuralRemote Medicine. Australian General Practice Network. Australian Society of Geriatric Medicine. Cancer Voices Australia. Cardiac Society of AustraliaNew Zealand. Clinical Oncological Soceity of Australia. Motor Neurone Disease Association of Australia. Palliative Care Australia. Palliative Care Nurses Australia. Royal Australian College of General Practitioners. Royal College of Nursing‚ Australia. Thoracic Society of AustraliaNew Zealand Clinical practice guidelines for communicating prognosis and end-of-life issues with adults in the advanced stages of a life-limiting illness, and their caregivers. Med J Aust. 2007 Jun 18;186(S12):77–105. doi: 10.5694/j.1326-5377.2007.tb01100.x.cla11246_fm [DOI] [PubMed] [Google Scholar]
- 26.Gilligan T, Coyle N, Frankel RM, Berry DL, Bohlke K, Epstein RM, Finlay E, Jackson VA, Lathan CS, Loprinzi CL, Nguyen LH, Seigel C, Baile WF. Patient-clinician communication: American Society of Clinical Oncology Consensus Guideline. J Clin Oncol. 2017 Nov 01;35(31):3618–32. doi: 10.1200/JCO.2017.75.2311. [DOI] [PubMed] [Google Scholar]
- 27.Libert Y, Peternelj L, Bragard I, Liénard A, Merckaert I, Reynaert C, Razavi D. Communication about uncertainty and hope: a randomized controlled trial assessing the efficacy of a communication skills training program for physicians caring for cancer patients. BMC Cancer. 2017 Jul 10;17(1):476. doi: 10.1186/s12885-017-3437-8. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-017-3437-8 .10.1186/s12885-017-3437-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stiefel F, Kiss A, Salmon P, Peters S, Razavi D, Cervantes A, Margulies A, Bourquin C, participants Training in communication of oncology clinicians: a position paper based on the third consensus meeting among European experts in 2018. Ann Oncol. 2018 Oct 01;29(10):2033–6. doi: 10.1093/annonc/mdy343. https://linkinghub.elsevier.com/retrieve/pii/S0923-7534(19)34215-2 .S0923-7534(19)34215-2 [DOI] [PubMed] [Google Scholar]
- 29.Clayton JM, Hancock K, Parker S, Butow PN, Walder S, Carrick S, Currow D, Ghersi D, Glare P, Hagerty R, Olver IN, Tattersall MH. Sustaining hope when communicating with terminally ill patients and their families: a systematic review. Psychooncology. 2008 Jul;17(7):641–59. doi: 10.1002/pon.1288. [DOI] [PubMed] [Google Scholar]
- 30.Walczak A, Butow PN, Davidson PM, Bellemore FA, Tattersall MH, Clayton JM, Young J, Mazer B, Ladwig S, Epstein RM. Patient perspectives regarding communication about prognosis and end-of-life issues: how can it be optimised? Patient Educ Couns. 2013 Mar;90(3):307–14. doi: 10.1016/j.pec.2011.08.009.S0738-3991(11)00458-7 [DOI] [PubMed] [Google Scholar]
- 31.Parker PA, Davison BJ, Tishelman C, Brundage MD, SCRN Communication Team What do we know about facilitating patient communication in the cancer care setting? Psychooncology. 2005 Oct;14(10):848–59. doi: 10.1002/pon.946. [DOI] [PubMed] [Google Scholar]
- 32.Haywood K, Marshall S, Fitzpatrick R. Patient participation in the consultation process: a structured review of intervention strategies. Patient Educ Couns. 2006 Oct;63(1-2):12–23. doi: 10.1016/j.pec.2005.10.005.S0738-3991(05)00314-9 [DOI] [PubMed] [Google Scholar]
- 33.Cegala DJ, Chisolm DJ, Nwomeh BC. A communication skills intervention for parents of pediatric surgery patients. Patient Educ Couns. 2013 Oct;93(1):34–9. doi: 10.1016/j.pec.2013.03.015.S0738-3991(13)00124-9 [DOI] [PubMed] [Google Scholar]
- 34.Street RL, Slee C, Kalauokalani DK, Dean DE, Tancredi DJ, Kravitz RL. Improving physician-patient communication about cancer pain with a tailored education-coaching intervention. Patient Educ Couns. 2010 Jul;80(1):42–7. doi: 10.1016/j.pec.2009.10.009. http://europepmc.org/abstract/MED/19962845 .S0738-3991(09)00499-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henselmans I, de Haes HC, Smets EM. Enhancing patient participation in oncology consultations: a best evidence synthesis of patient-targeted interventions. Psychooncology. 2013 May 14;22(5):961–77. doi: 10.1002/pon.3099. [DOI] [PubMed] [Google Scholar]
- 36.Epstein RM, Duberstein PR, Fenton JJ, Fiscella K, Hoerger M, Tancredi DJ, Xing G, Gramling R, Mohile S, Franks P, Kaesberg P, Plumb S, Cipri CS, Street RL, Shields CG, Back AL, Butow P, Walczak A, Tattersall M, Venuti A, Sullivan P, Robinson M, Hoh B, Lewis L, Kravitz RL. Effect of a patient-centered communication intervention on oncologist-patient communication, quality of life, and health care utilization in advanced cancer: the VOICE randomized clinical trial. JAMA Oncol. 2017 Jan 01;3(1):92–100. doi: 10.1001/jamaoncol.2016.4373. http://europepmc.org/abstract/MED/27612178 .2551984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walczak A, Mazer B, Butow PN, Tattersall MH, Clayton JM, Davidson PM, Young J, Ladwig S, Epstein RM. A question prompt list for patients with advanced cancer in the final year of life: development and cross-cultural evaluation. Palliat Med. 2013 Sep;27(8):779–88. doi: 10.1177/0269216313483659.0269216313483659 [DOI] [PubMed] [Google Scholar]
- 38.Brandes K, Linn AJ, Butow PN, van Weert JC. The characteristics and effectiveness of Question Prompt List interventions in oncology: a systematic review of the literature. Psychooncology. 2015 Mar;24(3):245–52. doi: 10.1002/pon.3637. [DOI] [PubMed] [Google Scholar]
- 39.Butow P, Dunn S, Tattersall M, Jones Q. Patient participation in the cancer consultation: evaluation of a question prompt sheet. Ann Oncol. 1994 Mar;5(3):199–204. doi: 10.1093/oxfordjournals.annonc.a058793. https://linkinghub.elsevier.com/retrieve/pii/S0923-7534(19)63009-7 .S0923-7534(19)63009-7 [DOI] [PubMed] [Google Scholar]
- 40.Brown RF, Bylund CL, Li Y, Edgerson S, Butow P. Testing the utility of a cancer clinical trial specific Question Prompt List (QPL-CT) during oncology consultations. Patient Educ Couns. 2012 Aug;88(2):311–7. doi: 10.1016/j.pec.2012.02.009. http://europepmc.org/abstract/MED/22390854 .S0738-3991(12)00082-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clayton J, Butow P, Tattersall M, Chye R, Noel M, Davis JM, Glare P. Asking questions can help: development and preliminary evaluation of a question prompt list for palliative care patients. Br J Cancer. 2003 Dec 01;89(11):2069–77. doi: 10.1038/sj.bjc.6601380. http://europepmc.org/abstract/MED/14647140 .6601380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keinki C, Momberg A, Clauß K, Bozkurt G, Hertel E, Freuding M, Josfeld L, Huebner J. Effect of question prompt lists for cancer patients on communication and mental health outcomes-A systematic review. Patient Educ Couns. 2021 Jun;104(6):1335–46. doi: 10.1016/j.pec.2021.01.012.S0738-3991(21)00039-2 [DOI] [PubMed] [Google Scholar]
- 43.Zetzl T, Mann D, Gruner S, Schuler M, Jentschke E, Neuderth S, Roch C, van Oorschot B. Question prompts to empower cancer patients: results of a randomized controlled trial. Support Care Cancer. 2020 Jun 10;28(6):2571–9. doi: 10.1007/s00520-019-05036-0.10.1007/s00520-019-05036-0 [DOI] [PubMed] [Google Scholar]
- 44.Glynne-Jones R, Ostler P, Lumley-Graybow S, Chait I, Hughes R, Grainger J, Leverton T. Can I look at my list? An evaluation of a 'prompt sheet' within an oncology outpatient clinic. Clin Oncol (R Coll Radiol) 2006 Jun;18(5):395–400. doi: 10.1016/j.clon.2006.01.005.S0936-6555(06)00021-5 [DOI] [PubMed] [Google Scholar]
- 45.Walczak A, Butow PN, Bu S, Clayton JM. A systematic review of evidence for end-of-life communication interventions: who do they target, how are they structured and do they work? Patient Educ Couns. 2016 Jan;99(1):3–16. doi: 10.1016/j.pec.2015.08.017.S0738-3991(15)30054-9 [DOI] [PubMed] [Google Scholar]
- 46.Miller N, Rogers S. A review of question prompt lists used in the oncology setting with comparison to the Patient Concerns Inventory. Eur J Cancer Care (Engl) 2018 Jan;27(1):e12489. doi: 10.1111/ecc.12489. [DOI] [PubMed] [Google Scholar]
- 47.Berger Z, Tung M, Yesantharao P, Zhou A, Blackford A, Smith TJ, Snyder C. Feasibility and perception of a question prompt list in outpatient cancer care. J Patient Rep Outcomes. 2019 Aug 15;3(1):53. doi: 10.1186/s41687-019-0145-y. doi: 10.1186/s41687-019-0145-y.10.1186/s41687-019-0145-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Licqurish SM, Cook OY, Pattuwage LP, Saunders C, Jefford M, Koczwara B, Johnson CE, Emery JD. Tools to facilitate communication during physician-patient consultations in cancer care: an overview of systematic reviews. CA Cancer J Clin. 2019 Nov;69(6):497–520. doi: 10.3322/caac.21573. doi: 10.3322/caac.21573. [DOI] [PubMed] [Google Scholar]
- 49.Dimoska A, Butow PN, Dent E, Arnold B, Brown RF, Tattersall MH. An examination of the initial cancer consultation of medical and radiation oncologists using the Cancode interaction analysis system. Br J Cancer. 2008 May 06;98(9):1508–14. doi: 10.1038/sj.bjc.6604348. http://europepmc.org/abstract/MED/18454160 .6604348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeh JC, Cheng MJ, Chung CH, Smith TJ. Using a question prompt list as a communication aid in advanced cancer care. J Oncol Pract. 2014 May;10(3):137–41. doi: 10.1200/JOP.2013.001295. http://europepmc.org/abstract/MED/24594680 .JOP.2013.001295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arthur J, Yennurajalingam S, Williams J, Tanco K, Liu D, Stephen S, Bruera E. Development of a question prompt sheet for cancer patients receiving outpatient palliative care. J Palliat Med. 2016 Aug;19(8):883–7. doi: 10.1089/jpm.2015.0545. http://europepmc.org/abstract/MED/27175461 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouleuc C, Savignoni A, Chevrier M, Renault-Tessier E, Burnod A, Chvetzoff G, Poulain P, Copel L, Cottu P, Pierga J, Brédart A, Dolbeault S. A question prompt list for advanced cancer patients promoting advance care planning: a French randomized trial. J Pain Symptom Manage. 2021 Feb;61(2):331–41. doi: 10.1016/j.jpainsymman.2020.07.026. https://linkinghub.elsevier.com/retrieve/pii/S0885-3924(20)30637-0 .S0885-3924(20)30637-0 [DOI] [PubMed] [Google Scholar]
- 53.Caminiti C, Diodati F, Filiberti S, Marcomini B, Annunziata MA, Ollari M, Passalacqua R. Cross-cultural adaptation and patients' judgments of a question prompt list for Italian-speaking cancer patients. BMC Health Serv Res. 2010 Jan 15;10:16. doi: 10.1186/1472-6963-10-16. https://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-10-16 .1472-6963-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smets E, van Heijl M, van Wijngaarden AK, Henselmans I, van Berge Henegouwen MI. Addressing patients' information needs: a first evaluation of a question prompt sheet in the pretreatment consultation for patients with esophageal cancer. Dis Esophagus. 2012 Aug;25(6):512–9. doi: 10.1111/j.1442-2050.2011.01274.x. [DOI] [PubMed] [Google Scholar]
- 55.Jacobs M, Henselmans I, Arts DL, Ten Koppel M, Gisbertz SS, Lagarde SM, van Berge Henegouwen MI, Sprangers MA, de Haes HC, Smets EM. Development and feasibility of a web-based question prompt sheet to support information provision of health-related quality of life topics after oesophageal cancer surgery. Eur J Cancer Care (Engl) 2018 Jan;27(1):12593. doi: 10.1111/ecc.12593. [DOI] [PubMed] [Google Scholar]
- 56.Mancini J, Butow PN, Julian-Reynier C, Dring R, Festy P, Fenaux P, Vey N. Question prompt list responds to information needs of myelodysplastic syndromes patients and caregivers. Leuk Res. 2015 Jun;39(6):599–605. doi: 10.1016/j.leukres.2015.03.011.S0145-2126(15)00081-8 [DOI] [PubMed] [Google Scholar]
- 57.Brown RF, Shuk E, Leighl N, Butow P, Ostroff J, Edgerson S, Tattersall M. Enhancing decision making about participation in cancer clinical trials: development of a question prompt list. Support Care Cancer. 2011 Aug;19(8):1227–38. doi: 10.1007/s00520-010-0942-6. http://europepmc.org/abstract/MED/20593202 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Weert JC, Jansen J, Spreeuwenberg PM, van Dulmen S, Bensing JM. Effects of communication skills training and a Question Prompt Sheet to improve communication with older cancer patients: a randomized controlled trial. Crit Rev Oncol Hematol. 2011 Oct;80(1):145–59. doi: 10.1016/j.critrevonc.2010.10.010.S1040-8428(10)00246-5 [DOI] [PubMed] [Google Scholar]
- 59.Amundsen A, Ervik B, Butow P, Tattersall MH, Bergvik S, Sørlie T, Nordøy T. Adapting an Australian question prompt list in oncology to a Norwegian setting-a combined method approach. Support Care Cancer. 2017 Jan;25(1):51–8. doi: 10.1007/s00520-016-3380-2.10.1007/s00520-016-3380-2 [DOI] [PubMed] [Google Scholar]
- 60.Arraras JI, Greimel E, Sezer O, Chie W, Bergenmar M, Costantini A, Young T, Vlasic KK, Velikova G. An international validation study of the EORTC QLQ-INFO25 questionnaire: an instrument to assess the information given to cancer patients. Eur J Cancer. 2010 Oct;46(15):2726–38. doi: 10.1016/j.ejca.2010.06.118.S0959-8049(10)00634-9 [DOI] [PubMed] [Google Scholar]
- 61.El-Jawahri A, Traeger L, Park ER, Greer JA, Pirl WF, Lennes IT, Jackson VA, Gallagher ER, Temel JS. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer. 2014 Jan 15;120(2):278–85. doi: 10.1002/cncr.28369. doi: 10.1002/cncr.28369. [DOI] [PubMed] [Google Scholar]
- 62.Wang T, Molassiotis A, Chung BP, Tan J. Unmet care needs of advanced cancer patients and their informal caregivers: a systematic review. BMC Palliat Care. 2018 Jul 23;17(1):96. doi: 10.1186/s12904-018-0346-9. https://bmcpalliatcare.biomedcentral.com/articles/10.1186/s12904-018-0346-9 .10.1186/s12904-018-0346-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teo I, Krishnan A, Lee GL. Psychosocial interventions for advanced cancer patients: a systematic review. Psychooncology. 2019 Jul;28(7):1394–407. doi: 10.1002/pon.5103. [DOI] [PubMed] [Google Scholar]
- 64.Brédart A, Kop J, Efficace F, Beaudeau A, Brito T, Dolbeault S, Aaronson N, EORTC Quality of Life Group Quality of care in the oncology outpatient setting from patients' perspective: a systematic review of questionnaires' content and psychometric performance. Psychooncology. 2015 Apr;24(4):382–94. doi: 10.1002/pon.3661. [DOI] [PubMed] [Google Scholar]
- 65.Arraras JI, Wintner LM, Sztankay M, Tomaszewski KA, Hofmeister D, Costantini A, Bredart A, Young T, Kuljanic K, Tomaszewska IM, Kontogianni M, Chie W, Kulis D, Greimel E, Conducted on behalf of the EORTC Quality of Life Group EORTC QLQ-COMU26: a questionnaire for the assessment of communication between patients and professionals. Phase III of the module development in ten countries. Support Care Cancer. 2017 May;25(5):1485–94. doi: 10.1007/s00520-016-3536-0.10.1007/s00520-016-3536-0 [DOI] [PubMed] [Google Scholar]
- 67.Fouquet C, Brédart A, Bouleuc C. [French adaptation of a question prompt list for cancer patients and their carers in supportive and palliative care] Bull Cancer. 2012 Jun;99(6):693–701. doi: 10.1684/bdc.2012.1593. http://www.jle.com/medline.md?issn=0007-4551&vol=99&iss=6&page=693 .S0007-4551(15)30436-7 [DOI] [PubMed] [Google Scholar]
- 67.Poortmans P. De-escalating and escalating radiation therapy in the management of early breast cancer. Breast. 2017 Mar;32(Supplement 1):2–3. doi: 10.1016/s0960-9776(17)30058-9. [DOI] [Google Scholar]
- 68.Gonzalez-Angulo A, Morales-Vasquez F, Hortobagyi G. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 69.Deluche E, Antoine A, Bachelot T, Robain M, Perol D, Delaloge S. A response letter to comments on "Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008-2016". Eur J Cancer. 2020 Nov;140:165–6. doi: 10.1016/j.ejca.2020.09.005.S0959-8049(20)30490-1 [DOI] [PubMed] [Google Scholar]
- 70.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro M, André F, Barrios C, Bergh J, Bhattacharyya G, Biganzoli L, Boyle F, Cardoso M, Carey L, Cortés J, El Saghir N, Elzayat M, Eniu A, Fallowfield L, Francis P, Gelmon K, Gligorov J, Haidinger R, Harbeck N, Hu X, Kaufman B, Kaur R, Kiely B, Kim S, Lin N, Mertz S, Neciosup S, Offersen B, Ohno S, Pagani O, Prat A, Penault-Llorca F, Rugo H, Sledge G, Thomssen C, Vorobiof D, Wiseman T, Xu B, Norton L, Costa A, Winer E. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020 Dec;31(12):1623–49. doi: 10.1016/j.annonc.2020.09.010. https://linkinghub.elsevier.com/retrieve/pii/S0923-7534(20)42460-3 .S0923-7534(20)42460-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mathis T, Cassoux N, Tardy M, Piperno S, Gastaud L, Dendale R, Maschi C, Nguyen A, Meyer L, Bonnin N, Baillif S, Tick S, Mouriaux F, Jaspart F, Dellis J, Rosier L, Desjardins L, Herault J, Caujolle JP, Thariat J. [Management of uveal melanomas, guidelines for oncologists] Bull Cancer. 2018 Oct;105(10):967–80. doi: 10.1016/j.bulcan.2018.07.011.S0007-4551(18)30232-7 [DOI] [PubMed] [Google Scholar]
- 72.Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004 Apr;111(4):816–21. doi: 10.1016/j.ophtha.2003.11.001.S0161-6420(03)01626-9 [DOI] [PubMed] [Google Scholar]
- 73.Mariani P, Almubarak M, Kollen M, Wagner M, Plancher C, Audollent R, Piperno-Neumann S, Cassoux N, Servois V. Radiofrequency ablation and surgical resection of liver metastases from uveal melanoma. Eur J Surg Oncol. 2016 May;42(5):706–12. doi: 10.1016/j.ejso.2016.02.019.S0748-7983(16)00114-1 [DOI] [PubMed] [Google Scholar]
- 74.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Int J Nurs Stud. 2013 May;50(5):587–92. doi: 10.1016/j.ijnurstu.2012.09.010.S0020-7489(12)00306-9 [DOI] [PubMed] [Google Scholar]
- 75.Creswell JD, Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches 5th Edition. Thousand Oaks, California: SAGE Publications; 2018. [Google Scholar]
- 76.Östlund U, Kidd L, Wengström Y, Rowa-Dewar N. Combining qualitative and quantitative research within mixed method research designs: a methodological review. Int J Nurs Stud. 2011 Mar;48(3):369–83. doi: 10.1016/j.ijnurstu.2010.10.005. http://europepmc.org/abstract/MED/21084086 .S0020-7489(10)00363-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarradon-Eck A. Cancer(s) et psy(s) France: Érès; 2019. Le patient contemporain. [Google Scholar]
- 78.Epstein S. The construction of lay expertise: AIDS activism and the forging of credibility in the reform of clinical trials. Sci Technol Human Values. 1995;20(4):408–37. doi: 10.1177/016224399502000402. [DOI] [PubMed] [Google Scholar]
- 79.Abelson J, Li K, Wilson G, Shields K, Schneider C, Boesveld S. Supporting quality public and patient engagement in health system organizations: development and usability testing of the Public and Patient Engagement Evaluation Tool. Health Expect. 2016 Aug;19(4):817–27. doi: 10.1111/hex.12378. doi: 10.1111/hex.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palinkas LA. Qualitative and mixed methods in mental health services and implementation research. J Clin Child Adolesc Psychol. 2014;43(6):851–61. doi: 10.1080/15374416.2014.910791. http://europepmc.org/abstract/MED/25350675 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown CH, Curran G, Palinkas LA, Aarons GA, Wells KB, Jones L, Collins LM, Duan N, Mittman BS, Wallace A, Tabak RG, Ducharme L, Chambers DA, Neta G, Wiley T, Landsverk J, Cheung K, Cruden G. An overview of research and evaluation designs for dissemination and implementation. Annu Rev Public Health. 2017 Mar 20;38:1–22. doi: 10.1146/annurev-publhealth-031816-044215. http://europepmc.org/abstract/MED/28384085 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015 Sep;42(5):533–44. doi: 10.1007/s10488-013-0528-y. http://europepmc.org/abstract/MED/24193818 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moser A, Korstjens I. Series: practical guidance to qualitative research. Part 3: sampling, data collection and analysis. Eur J Gen Pract. 2018 Dec;24(1):9–18. doi: 10.1080/13814788.2017.1375091. http://europepmc.org/abstract/MED/29199486 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morse JM. "Data were saturated . . . ". Qual Health Res. 2015 May;25(5):587–8. doi: 10.1177/1049732315576699.25/5/587 [DOI] [PubMed] [Google Scholar]
- 85.Brédart A, Kop J, Griesser A, Zaman K, Panes-Ruedin B, Jeanneret W, Delaloye J, Zimmers S, Jacob A, Berthet V, Fiszer C, Dolbeault S. Validation of the 34-item Supportive Care Needs Survey and 8-item breast module French versions (SCNS-SF34-Fr and SCNS-BR8-Fr) in breast cancer patients. Eur J Cancer Care (Engl) 2012 Jul;21(4):450–9. doi: 10.1111/j.1365-2354.2012.01356.x. [DOI] [PubMed] [Google Scholar]
- 86.Razavi D, Delvaux N, Farvacques C, Robaye E. Screening for adjustment disorders and major depressive disorders in cancer in-patients. Br J Psychiatry. 1990 Jan;156:79–83. doi: 10.1192/bjp.156.1.79.S0007125000060530 [DOI] [PubMed] [Google Scholar]
- 87.Muller L, Spitz E. [Multidimensional assessment of coping: validation of the Brief COPE among French population] Encephale. 2003;29(6):507–18.MDOI-ENC-12-2003-29-6-0013-7006-101019-ART5 [PubMed] [Google Scholar]
- 88.Kuliś D, Whittaker C, Greimel E, Bottomley A, Koller M. EORTC quality of life group translation procedure. EORTC. 2017. [2021-12-27]. https://www.eortc.org/app/uploads/sites/2/2018/02/translation_manual_2017.pdf . [DOI] [PubMed]
- 89.Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008 Apr;31(2):180–91. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- 90.Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, Proctor EK, Kirchner JE. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015 Feb 12;10:21. doi: 10.1186/s13012-015-0209-1. https://implementationscience.biomedcentral.com/articles/10.1186/s13012-015-0209-1 .10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000 Oct;32(4):1008–15.jan1567 [PubMed] [Google Scholar]
- 92.Thewes B, Rietjens JA, van den Berg SW, Compen FR, Abrahams H, Poort H, van de Wal M, Schellekens MP, Peters ME, Speckens AE, Knoop H, Prins JB. One way or another: the opportunities and pitfalls of self-referral and consecutive sampling as recruitment strategies for psycho-oncology intervention trials. Psychooncology. 2018 Aug;27(8):2056–9. doi: 10.1002/pon.4780. http://europepmc.org/abstract/MED/29808508 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Haes H, Bensing J. Endpoints in medical communication research, proposing a framework of functions and outcomes. Patient Educ Couns. 2009 Mar;74(3):287–94. doi: 10.1016/j.pec.2008.12.006.S0738-3991(08)00648-4 [DOI] [PubMed] [Google Scholar]
- 94.Sansoni JE, Grootemaat P, Duncan C. Question Prompt Lists in health consultations: a review. Patient Educ Couns. 2015 Jun 03; doi: 10.1016/j.pec.2015.05.015.S0738-3991(15)00258-X [DOI] [PubMed] [Google Scholar]
- 95.Eggly S, Hamel LM, Foster TS, Albrecht TL, Chapman R, Harper FW, Thompson H, Griggs JJ, Gonzalez R, Berry-Bobovski L, Tkatch R, Simon M, Shields A, Gadgeel S, Loutfi R, Ali H, Wollner I, Penner LA. Randomized trial of a question prompt list to increase patient active participation during interactions with black patients and their oncologists. Patient Educ Couns. 2017 May;100(5):818–26. doi: 10.1016/j.pec.2016.12.026. http://europepmc.org/abstract/MED/28073615 .S0738-3991(16)30579-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rodenbach RA, Brandes K, Fiscella K, Kravitz RL, Butow PN, Walczak A, Duberstein PR, Sullivan P, Hoh B, Xing G, Plumb S, Epstein RM. Promoting end-of-life discussions in advanced cancer: effects of patient coaching and question prompt lists. J Clin Oncol. 2017 Mar 10;35(8):842–51. doi: 10.1200/jco.2016.68.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walczak A, Butow PN, Tattersall MH, Davidson PM, Young J, Epstein RM, Costa DS, Clayton JM. Encouraging early discussion of life expectancy and end-of-life care: a randomised controlled trial of a nurse-led communication support program for patients and caregivers. Int J Nurs Stud. 2017 Feb;67:31–40. doi: 10.1016/j.ijnurstu.2016.10.008.S0020-7489(16)30198-5 [DOI] [PubMed] [Google Scholar]
- 98.Stacey D, Kryworuchko J, Belkora J, Davison BJ, Durand M, Eden KB, Hoffman AS, Koerner M, Légaré F, Loiselle M, Street RL. Coaching and guidance with patient decision aids: a review of theoretical and empirical evidence. BMC Med Inform Decis Mak. 2013;13 Suppl 2:S11. doi: 10.1186/1472-6947-13-S2-S11. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/1472-6947-13-S2-S11 .1472-6947-13-S2-S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Porter LS, Pollak KI, Farrell D, Cooper M, Arnold RM, Jeffreys AS, Tulsky JA. Development and implementation of an online program to improve how patients communicate emotional concerns to their oncology providers. Support Care Cancer. 2015 Oct;23(10):2907–16. doi: 10.1007/s00520-015-2656-2. http://europepmc.org/abstract/MED/25701437 .10.1007/s00520-015-2656-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duggleby W. What about focus group interaction data? Qual Health Res. 2005 Jul;15(6):832–40. doi: 10.1177/1049732304273916.15/6/832 [DOI] [PubMed] [Google Scholar]
- 101.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009 Aug 07;4:50. doi: 10.1186/1748-5908-4-50. https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-4-50 .1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shepherd HL, Geerligs L, Butow P, Masya L, Shaw J, Price M, Dhillon HM, Hack TF, Girgis A, Luckett T, Lovell M, Kelly B, Beale P, Grimison P, Shaw T, Viney R, Rankin NM. The elusive search for success: defining and measuring implementation outcomes in a real-world hospital trial. Front Public Health. 2019;7:293. doi: 10.3389/fpubh.2019.00293. doi: 10.3389/fpubh.2019.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arthur J, Yennu S, Zapata KP, Cantu H, Wu J, Liu D, Bruera E. Perception of helpfulness of a question prompt sheet among cancer patients attending outpatient palliative care. J Pain Symptom Manage. 2017 Jan;53(1):124–30. doi: 10.1016/j.jpainsymman.2016.08.017. https://linkinghub.elsevier.com/retrieve/pii/S0885-3924(16)30367-0 .S0885-3924(16)30367-0 [DOI] [PubMed] [Google Scholar]
- 104.Brandes K, van Weert JC. Implementing consultation audio-recordings and question prompt lists into routine cancer care: how can we address healthcare providers' barriers? Patient Educ Couns. 2017 Jun;100(6):1029–30. doi: 10.1016/j.pec.2017.04.002.S0738-3991(17)30216-1 [DOI] [PubMed] [Google Scholar]
- 105.Wolderslund M, Kofoed P, Holst R, Axboe M, Ammentorp J. Digital audio recordings improve the outcomes of patient consultations: a randomised cluster trial. Patient Educ Couns. 2017 Feb;100(2):242–9. doi: 10.1016/j.pec.2016.08.029.S0738-3991(16)30405-0 [DOI] [PubMed] [Google Scholar]
- 106.Yeganeh L, Johnston-Ataata K, Vincent AJ, Flore J, Kokanović R, Teede H, Boyle JA. Co-designing an early menopause digital resource: model for interdisciplinary knowledge translation. Semin Reprod Med. 2020 Sep;38(4-05):315–22. doi: 10.1055/s-0041-1726273. [DOI] [PubMed] [Google Scholar]
- 107.Aapro M, Bossi P, Dasari A, Fallowfield L, Gascón P, Geller M, Jordan K, Kim J, Martin K, Porzig S. Digital health for optimal supportive care in oncology: benefits, limits, and future perspectives. Support Care Cancer. 2020 Oct;28(10):4589–612. doi: 10.1007/s00520-020-05539-1. http://europepmc.org/abstract/MED/32533435 .10.1007/s00520-020-05539-1 [DOI] [PMC free article] [PubMed] [Google Scholar]


