Abstract
Following an approach used to specifically identify polioviruses and enterovirus 71, we have developed reverse transcriptase (RT) PCR primers containing mixed-base residues or deoxyinosine at positions of codon degeneracy. These primers permit specific RT-PCR amplification of echovirus 30 (E30) sequences by targeting sites that encode conserved amino acid motifs within the major capsid protein, VP1. All 221 E30 strains tested, isolated in 16 countries over a 44-year period, yielded the predicted 158-bp PCR product. No specific products were obtained by PCR assays containing templates from any of the other 63 EV serotypes. Inosine-containing degenerate primers may be widely applicable to the identification of echovirus serotypes by PCR.
Enteroviruses (EVs) are responsible for 30,000 to 50,000 hospitalizations for aseptic meningitis per year in the United States. Echovirus type 30 (E30) is one of the most frequently isolated EVs in the United States, comprising 6.8% of all reported EVs isolated from 1970 to 1983 (30) and 9.5% of EVs isolated from 1993 to 1996 (4). In 1998, E30 accounted for 42% of all U.S. EV isolations reported to the Centers for Disease Control and Prevention by state and territorial public health laboratories (20). A rapid method for determination of the serotype of the viral agent responsible for community-wide outbreaks of viral meningitis would be of great value to public health laboratories and would be applicable to epidemiologic studies as well. Antigenic variation among isolates of the E30 serotype may cause difficulties in identification of E30 isolates by the traditional neutralization assay (7, 31). On the basis of complete VP1 sequences for 136 E30 isolates, we previously identified four distinct E30 genetic groups (20). The genetic variation generally correlated with the observed antigenic diversity.
The application of PCR assays has improved the speed and accuracy of general EV detection (27, 28), but widely used molecular biology-based methods are unable to identify the EV serotype. Recent advances in diagnostic PCR have resulted in the ability to detect not only specific groups of viruses within the family Picornaviridae (11, 24, 28, 32) but also specific serotypes within this group of viruses (3, 10). The molecular typing of polioviruses was achieved by designing degenerate inosine-containing primers that targeted uniquely conserved amino acid motifs within the VP1 capsid protein. VP1 has been shown to be the major surface-accessible protein on the mature picornavirus virion (1, 9, 14, 26), and VP1 contributes to all three of the major neutralization sites that have been identified on the poliovirus surface (15, 18).
Sequencing and phylogenetic reconstructions have shown that VP1 contains serotype-specific genetic information that can be used for EV identification and evolutionary studies (19, 21, 22). Since these studies revealed that the EV VP1 sequence correlated with the serotype as defined by neutralization, it was clear that it should be possible to generally extend the molecular serotyping concept to other EVs by using serotype-specific primers targeted to any given serotype. In the present study, we have identified VP1 amino acid residues that are unique to E30 and show that inosine-containing degenerate primers targeted to this region amplified 221 E30 isolates representing isolates with the widest possible range of temporal, geographic, and genetic diversities.
MATERIAL AND METHODS
Viruses.
The E30 strains selected for analysis were temporally, geographically, and genotypically diverse; strains isolated from 1956 to 1999 in 16 countries on six continents were included (Table 1). Viruses were isolated from original clinical specimens and propagated in cell culture by standard methods (8). Isolates were typed by neutralization assay with standard antiserum pools (17). Complete VP1 sequences were determined (20), and the results of antigenic typing were confirmed by comparison of the VP1 sequences with a database of EV prototype strain sequences, as described previously (19, 21).
TABLE 1.
E30 isolates tested by RT-PCR with primers 120S-47A and/or 157S-47A
| Country | Year(s) | Genogroup(s)a | No. of isolates testedb |
|---|---|---|---|
| Argentina | 1998 | 4b | 1 |
| Australia | 1956–1997 | 1, 2, 3, 4a, 4b | 20 |
| Canada | 1985–1997 | 4a, 4b | 15 |
| Chile | 1993–1994 | 4b | 2 |
| Colombia | 1994–1995 | 1, 4b | 2 |
| Germany | 1988–1996 | 4b | 3 |
| Israel | 1999 | 1, 4b | 9 |
| Kuwait | 1999 | 4b | 2 |
| Mexico | 1992 | 4b | 1 |
| The Netherlands | 1999 | 1 | 4 |
| Philippines | 1995 | Not assigned | 1 |
| Poland | 1999 | 4b | 4 |
| Puerto Rico | 1961 | 1 | 1 |
| Trinidad and Tobago | 1983 | 4a | 1 |
| Tunisia | 1998–1999 | 4b | 2 |
| Turkey | 1999 | 4b | 2 |
| United Kingdom | 1959 | 2 | 1 |
| United States | 1957–1998 | 1, 2, 3, 4a, 4b | 150 |
| Total | 1956–1999 | 1, 2, 3, 4a, 4b | 221 |
E30 genogroups are those described previously (20).
The 158-bp E30-specific product was amplified from all E30 isolates tested.
Design and synthesis of E30-specific oligonucleotide primers.
The deduced VP1 amino acid sequences (292 amino acids) of E30 reference strains were aligned to facilitate identification of E30-specific amino acid motifs for the synthesis of degenerate primers (see Fig. 1). Synthetic oligodeoxynucleotide primers 120S (5′-GACCCIGARIRIGCIYTNAA; E30 VP1 nucleotides [nt] 4 to 23), 157S (5′-GACCCIGAkIRIGCIpTNARYA-3′; E30 VP1 nt 4 to 25), and 47A (5′-TKIACRTGICKIGTYTGCAT-3′; E30 VP1 nt 162 to 143) were prepared, purified, and analyzed as described previously (33). Degenerate nucleotide sites are indicated by standard ambiguity codes as follows: K = G or T; N = A, C, G, or T; R = A or G; Y = C or T; and I =deoxyinosine. To reduce the number of unique primer species for primer 157S, the pyrimidine and purine base analogs dP and dK, respectively, were incorporated at positions that include either T or C residues or the A or G residues, respectively (12) (k = purine base analog dk and p = pyrimidine base analog dP; Glen Research, Sterling, Va.).
FIG. 1.
(A) Partial VP1 amino acid alignment for three E30 reference strains and several related nonpoliovirus EVs. The E30 strains tested included Bastianni (E30B; E30 prototype strain, New York, 1958), Frater (E30F; Scotland, 1959), and Giles (E30G; Minnesota, 1960) (24). Sites that are identical in sequence to that of E30 Bastianni are indicated in the alignment by a period. Hyphens indicate gaps inserted to create the alignment. Motifs targeted by RT-PCR primers are boxed. CA, coxsackievirus type A; CB, coxsackievirus type B. (B) Sequences of degenerate primers 120S, 157S, and 47A. The E30 amino acids used to design the primers are shown above the primer sequences.
PCR amplification and analysis.
In vitro amplification by PCR was performed by a modification of methods described previously (10, 33). Amplification reactions were carried out in 50-μl reaction mixtures containing 1 μl of each individual virus cell culture lysate in 67 mM Tris-HCl (pH 8.8), 17 mM NH4SO4, 6 μM EDTA, 2 mM MgCl2, 1 mM 2-mercaptoethanol, 80 pmol of each degenerate primer, each deoxynucleoside triphosphate (Pharmacia Biotech, Piscataway, N.J.) at a concentration of 100 μM, 5 U of placental RNase inhibitor (Roche Molecular Biochemicals, Indianapolis, Ind.), 1.5 U of avian myeloblastosis virus reverse transcriptase (RT; Roche Molecular Biochemicals), and 1.25 U of Taq DNA polymerase (Roche Molecular Biochemicals). The reaction mixtures were prepared (excluding the RNase inhibitor, avian myeloblastosis virus RT, and Taq DNA polymerase), overlaid with mineral oil, heated for 5 min at 95°C to release the virion RNA, and chilled on ice. The excluded components were then added, and the samples were incubated at 42°C for 30 min before 30 cycles of programmed amplification (94°C for 1 min, 42°C for 1 min, and 60°C for 1 min). Conditions for polyacrylamide gel electrophoresis and detection of amplified products by ethidium bromide staining were as described previously (10, 33).
RESULTS
Previous studies have shown that it is possible to design serotype-specific PCR primers for the three poliovirus serotypes and for EV type 71 (EV71) by targeting serotype-specific amino acid motifs in VP1 and using mixed-base residues and deoxyinosine at positions of codon degeneracy (3, 10). To ensure efficient synthesis of first-strand cDNA, the antisense primer was targeted to an amino acid motif that is highly conserved among all EVs (Fig. 1A) (22). The motif MQTRHVV was present in 154 of 155 E30 isolates sequenced (20). One E30 strain, isolated in Mexico in 1992, had the motif MQTRHVI. Because the nucleotide difference is near the 5′ end of the primer, the sequence difference would not be expected to influence amplification, and this isolate was efficiently amplified (data not shown).
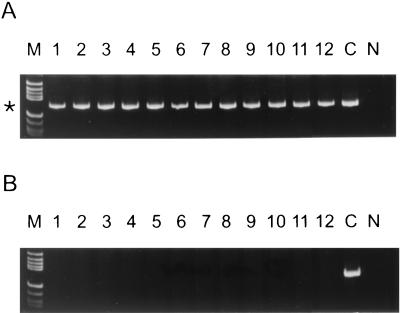
A conserved VP1 amino acid motif near the amino terminus of VP1, DPEGALN, was identified in the sequences of three E30 reference strains (Fig. 1A). This amino acid motif was not found in the VP1 capsid sequences of any of the other strains of the 63 EV serotypes tested (Fig. 1) (22). Examination of additional E30 VP1 sequences determined in our laboratory indicated that this motif was highly conserved among all members of the serotype but that most isolates had the motif DPESALN (20). Deoxyinosine-containing degenerate PCR primers (primers 120S and 157S) were designed to recognize this site (Fig. 1B). The sequence IRI was used to account for the presence of both Gly (codon GGN) and Ser residues (codons TCN and AGY) at residue 4. A mixture of all four nucleotides instead of inosine was used at the third position of the Leu codon in primers 120S and 157S because inosine near the 3′ end of a primer tends to destabilize primer-template annealing (D. Kilpatrick, unpublished data). After extensive testing of primer pair 120S-47A, we discovered that one of the older isolates (isolate ID76-8267) contained a G residue at the 3′ end of the 120S primer rather than the A residue found in all 220 other sequenced E30 isolates and failed to amplify as a result. To account for this difference, a degeneracy (R) was introduced at this site and the primer was extended by two nucleotides at the 3′ end, resulting in primer 157S (Fig. 1B). The extended nucleotides, Y and A, were present in the sequences of all E30 isolates. The purine (dK) and pyrimidine (dP) analogs were used in primer 157S at positions 7 and 14 from the 3′ end in order to reduce the number of primer species. The purine analog, dK, base pairs with both C and T, whereas the pyrimidine analog, dP, base pairs with both A and G. The new primer pair, 157S-47A, successfully amplified ID76-8267. A total of 221 E30 isolates, representing all previously defined genogroups (20), were tested with the primer pairs 120S-47A and/or 157S-47A (Table 1). A 158-bp product was amplified from all E30 templates (Table 1 and Fig. 2). Figure 2A shows the 157S-47A PCR results for representatives of each genogroup: genogroup 1, lanes 1 to 4; genogroup 2, lanes 5 and 6; genogroup 3, lanes 7 and 8; genogroup 4a, lanes 9 and 10; and genogroup 4b, lanes 11 and 12. No 157S-47A PCR product was produced from any of the other isolates of the 63 EV serotypes tested (Fig. 2B and data not shown).
FIG. 2.
RT-PCR amplification of E30 isolates and non-E30 echovirus prototype strains with primer pair 157S-47A. The position of the 158-bp E30-specific product is indicated by an asterisk. Lanes N, negative control; lanes C, positive control (E30 Frater); lanes M, molecular weight marker V (Boehringer Mannheim). (A) Lanes 1 to 12, E30 isolates AUS56-3999, MI67-0258, ID76-8267, COL95-6669, NV76-8213, NC81-3092, CA67-T673125, AUS73-120112, TRT83-4794, PQ88-8331, DEU96-2308, and SD98-2730, respectively. (B) Lanes 1 to 12, E6, E7, E8, E9, E12, E13, E14, E16, E21, E25, E29, and E32, respectively.
DISCUSSION
The 64 human EV serotypes have traditionally been typed by an antibody neutralization test (5, 6, 16), often with intersecting pools of antisera to reduce the total number of tests needed to identify a large number of serotypes. However, neutralization typing poses a number of difficulties. The neutralization test is labor-intensive and time-consuming and may fail due to antigenic drift, recombination, or the presence of virus mixtures. As a result of a high mutation rate and antigenic drift, antisera prepared against older prototype strains may not efficiently neutralize currently circulating strains. Antigenic variation among E30 isolates is well-documented and may result in the failure of antigenic typing methods (7, 31).
In recent years, rapid EV detection has been made possible by the molecular biology-based techniques of RT-PCR and sequencing (19, 21, 25, 27, 28, 34), but conventional PCR detection does not permit identification of the serotype. Previous molecular biology-based methods for EV detection primarily targeted highly conserved sequences in either the 5′ noncoding region or 3Dpol. Because of the high frequency of recombination among EV isolates, there is little correlation between the EV serotype and the sequences of either the 5′ noncoding region (2, 13) or the nonstructural genes (29). Sequences near the VP4-VP2 junction also appear to be unsuitable for typing (23). Analysis of the VP1 sequences of all 64 EV prototype strains tested revealed a very high degree of correlation between nucleotide sequences and serotypes (22) and led to the development of a method for the typing of EV isolates by comparison of partial VP1 sequences to the complete VP1 sequence database (19, 21). Sequencing isolates with generic VP1 primers that amplify all EV serotypes works very well (19), but it adds an additional step and the technology is often not available in the clinical laboratory. Serotype-specific PCR would speed the identification of EV isolates and make a rapid molecular biology-based typing method available to a wider range of laboratories.
The key to the development of a serotype-specific PCR assay is the identification of a unique amino acid motif within a protein that is involved in conferring serotype specificity and then targeting the sequence encoding that motif by using degenerate primers. One of the challenges in the design of serotype-specific PCR primers for EV detection is how to account for the high degree of intraserotypic nucleotide sequence diversity due to the high mutation rate of these viruses. Degenerate inosine-containing primers were developed to overcome such nucleotide diversity and were used to target uniquely conserved amino acid motifs to specifically amplify each of the three poliovirus serotypes (11) or to generically amplify all polioviruses (10). We used a similar approach to design PCR primers to specifically amplify EV71 isolates (3). The E30-specific PCR test described in this report was developed with the complete VP1 sequences of E30 strains isolated in geographically dispersed regions of the United States and nine other countries between 1956 and 1999 (20). This E30-specific PCR test has been used to identify E30 isolates associated with viral meningitis cases in Brazil (E. Da Silva, personal communication), Israel, and the United States. These E30-specific primers should be useful for the quick identification of community-wide outbreaks of E30 meningitis and will provide a valuable research tool for epidemiologic studies in the future.
ACKNOWLEDGMENTS
We greatly appreciate the contributions of E30 strains by D. Cisterna, Buenos Aires, Argentina; M. Kennett, Melbourne, Victoria, Australia; M. Carpenter, Halifax, Nova Scotia, Canada; N. Pelaéz and J. Boshell, Bogotá, Colombia; E. Schreier, Berlin, Germany; L. Shulman, Tel-Hashomer, Israel; S. al-Mufti, Kuwait City, Kuwait; H. van der Avoort and A. Ras, Bilthoven, The Netherlands; H. Triki, Tunis, Tunisia; D. Schnurr, Berkeley, California; and M. A. Patterson and S. Neill, Austin, Tex.
REFERENCES
- 1.Archarya R, Fry E, Stuart D, Fox G, Rowlands D J, Brown F. The three-dimensional structure of foot and mouth disease virus at 2.9 Å resolution. Nature. 1989;338:709–713. doi: 10.1038/337709a0. [DOI] [PubMed] [Google Scholar]
- 2.Bailly J L, Borman A M, Peigue-Lafeuille H, Kean K M. Natural isolates of ECHO virus type 25 with extensive variations in IRES sequences and different translational efficiencies. Virology. 1996;215:83–96. doi: 10.1006/viro.1996.0009. [DOI] [PubMed] [Google Scholar]
- 3.Brown B A, Kilpatrick D R, Oberste M S, Pallansch M A. Serotype-specific identification of enterovirus 71 by PCR. J Clin Virol. 1999;16:107–112. doi: 10.1016/s1386-6532(00)00065-2. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Nonpolio enterovirus surveillance—United States, 1993–1996. Morbid Mortal Wkly Rep. 1997;46:748–750. [PubMed] [Google Scholar]
- 5.Committee on Enteroviruses. Classification of human enteroviruses. Virology. 1962;16:501–504. [Google Scholar]
- 6.Committee on the Enteroviruses. The enteroviruses. Am J Public Health. 1957;47:1556–1566. [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan I B. A comparative study of 63 strains of ECHO virus type 30. Arch Gesamte Virusforsch. 1968;25:93–104. doi: 10.1007/BF01243094. [DOI] [PubMed] [Google Scholar]
- 8.Grandien M, Forsgren M, Ehrnst A. Enteroviruses and reoviruses. In: Lennette E H, Schmidt N J, editors. Diagnostic procedures for viral, rickettsial, and chlamydial infections. 6th ed. Washington, D.C.: American Public Health Association; 1989. pp. 513–569. [Google Scholar]
- 9.Hogle J M, Chow M, Filman D J. Three-dimensional structure of poliovirus at 2.9 Å resolution. Science. 1985;229:1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- 10.Kilpatrick D R, Nottay B, Yang C F, Yang S J, Da Silva E, Penaranda S, Pallansch M, Kew O. Serotype-specific identification of polioviruses by PCR using primers containing mixed-base or deoxyinosine residues at positions of codon degeneracy. J Clin Microbiol. 1998;36:352–357. doi: 10.1128/jcm.36.2.352-357.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilpatrick D R, Nottay B, Yang C F, Yang S J, Mulders M N, Holloway B P, Pallansch M A, Kew O M. Group-specific identification of polioviruses by PCR using primers containing mixed-base or deoxyinosine residue at positions of codon degeneracy. J Clin Microbiol. 1996;34:2990–2996. doi: 10.1128/jcm.34.12.2990-2996.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong Thoo Lin P, Brown D M. Oligonucleotides containing degenerate bases: synthesis and uses. Methods Mol Biol. 1994;26:187–206. doi: 10.1007/978-1-59259-513-6_7. [DOI] [PubMed] [Google Scholar]
- 13.Kopecka H, Brown B, Pallansch M A. Genotypic variation in coxsackievirus B5 isolates from three different outbreaks in the United States. Virus Res. 1995;38:125–136. doi: 10.1016/0168-1702(95)00055-u. [DOI] [PubMed] [Google Scholar]
- 14.Luo M, Vriend G, Kamer G, Minor I, Arnold E, Rossman M G, Boege U, Scraba D G, Duke G M, Palmenberg A C. The atomic structure of mengovirus at 3.0Å resolution. Science. 1987;235:182–191. doi: 10.1126/science.3026048. [DOI] [PubMed] [Google Scholar]
- 15.Mateu M G. Antibody recognition of picornaviruses and escape from neutralization. Virus Res. 1995;38:1–24. doi: 10.1016/0168-1702(95)00048-u. [DOI] [PubMed] [Google Scholar]
- 16.Melnick J L. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields B N, Knipe D M, Howley P M, Channock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 655–712. [Google Scholar]
- 17.Melnick J L, Rennick V, Hampil B, Schmidt N J, Ho H H. Lyophilized combination pools of enterovirus equine antisera: preparation and test procedures for the identification of field strains of 42 enteroviruses. Bull W H O. 1973;48:263–268. [PMC free article] [PubMed] [Google Scholar]
- 18.Minor P D. Antigenic structure of picornaviruses. Curr Top Microbiol Immunol. 1990;161:121–154. doi: 10.1007/978-3-642-75602-3_5. [DOI] [PubMed] [Google Scholar]
- 19.Oberste M S, Maher K, Flemister M R, Marchetti G, Kilpatrick D R, Pallansch M A. Comparison of classic and molecular approaches for the identification of “untypeable” enteroviruses. J Clin Microbiol. 2000;38:1170–1174. doi: 10.1128/jcm.38.3.1170-1174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberste M S, Maher K, Kennett M L, Campbell J J, Carpenter M S, Schnurr D, Pallansch M A. Molecular epidemiology and genetic diversity of echovirus type 30 (E30): genotype correlates with temporal dynamics of E30 isolation. J Clin Microbiol. 1999;37:3928–3933. doi: 10.1128/jcm.37.12.3928-3933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberste M S, Maher K, Kilpatrick D R, Flemister M R, Brown B A, Pallansch M A. Typing of human enteroviruses by partial sequencing of VP1. J Clin Microbiol. 1999;37:1288–1293. doi: 10.1128/jcm.37.5.1288-1293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberste M S, Maher K, Kilpatrick D R, Pallansch M A. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol. 1999;73:1941–1948. doi: 10.1128/jvi.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberste M S, Maher K, Pallansch M A. Molecular phylogeny of all human enterovirus serotypes based on comparison of sequences at the 5′ end of the region encoding VP2. Virus Res. 1998;58:35–43. doi: 10.1016/s0168-1702(98)00101-4. [DOI] [PubMed] [Google Scholar]
- 24.Oberste M S, Maher K, Pallansch M A. Specific detection of echoviruses 22 and 23 in cell culture supernatants by RT-PCR. J Med Virol. 1999;58:178–181. doi: 10.1002/(sici)1096-9071(199906)58:2<178::aid-jmv13>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Read S J, Kurtz J B. Laboratory diagnosis of common viral infections of the central nervous system by using a single multiplex PCR screening assay. J Clin Microbiol. 1999;37:1352–1355. doi: 10.1128/jcm.37.5.1352-1355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossman M G, Arnold A, Erickson J W, Frankenberger E A, Griffith J P, Hecht H J, Johnson J E, Kamer G, Luo M, Mosser A G, Rueckert R R, Sherry B, Vriend G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985;317:145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- 27.Rotbart H A, Ahmed A, Hickey S, Dagan R, McCracken G H, Jr, Whitley R J, Modlin J F, Cascino M, O'Connell J F, Menegus M A, Blum D. Diagnosis of enterovirus infection by polymerase chain reaction of multiple specimen types. Pediatt Infect Dis J. 1997;16:409–411. doi: 10.1097/00006454-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Rotbart H A, Romero J R. Laboratory diagnosis of enteroviral infections. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C.: ASM Press; 1995. pp. 401–418. [Google Scholar]
- 29.Santti J, Hyypiä T, Kinnunen L, Salminen M. Evidence of recombination among enteroviruses. J Virol. 1999;73:8741–8749. doi: 10.1128/jvi.73.10.8741-8749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strikas R A, Anderson L J, Parker R A. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970–1983. J Infect Dis. 1986;153:346–351. doi: 10.1093/infdis/153.2.346. [DOI] [PubMed] [Google Scholar]
- 31.Wenner H A, Harmon P, Behbehani A M, Rouhandeh H, Kamitsuka P S. The antigenic heterogeneity of type 30 echoviruses. Am J Epidemiol. 1967;85:240–249. doi: 10.1093/oxfordjournals.aje.a120687. [DOI] [PubMed] [Google Scholar]
- 32.Yang C-F, De L, Yang S-J, Ruiz Gómez J, Ramiro Cruz J, Holloway B P, Pallansch M A, Kew O M. Genotype-specific in vitro amplification of sequences of the wild type 3 polioviruses from Mexico and Guatemala. Virus Res. 1992;24:277–296. doi: 10.1016/0168-1702(92)90124-r. [DOI] [PubMed] [Google Scholar]
- 33.Yang C-F, De L, Holloway B P, Pallansch M A, Kew O M. Detection and identification of vaccine-related polioviruses by the polymerase chain reaction. Virus Res. 1991;20:159–179. doi: 10.1016/0168-1702(91)90107-7. [DOI] [PubMed] [Google Scholar]
- 34.Yerly S, Gervaix A, Simonet V, Caflisch M, Perrin L, Wunderli W. Rapid and sensitive detection of enteroviruses in specimens from patients with aseptic meningitis. J Clin Microbiol. 1996;34:199–201. doi: 10.1128/jcm.34.1.199-201.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]