Key Points
Question
Are plaque location and vessel geometry determined by coronary computed tomographic angiography (CCTA) associated with future acute coronary syndrome (ACS)–causing culprit lesions?
Findings
Of 548 coronary lesions in 116 patients with incident ACS after CCTA in this nested case-control study, lesions with a short distance from the ostium or located within vessel bifurcations or tortuous segments were more likely to develop into culprit lesions. A greater number of these adverse geometric characteristics were exhibited by culprit lesion precursors compared with the highest-grade stenosis lesions in 116 matched controls.
Meaning
These findings suggest that CCTA-derived adverse geometric characteristics of coronary plaques are associated with progression to ACS-causing culprit lesions.
This cohort study assesses the association of adverse geometric characteristics of coronary lesions with location and vessel geometry on coronary computed tomographic angiography.
Abstract
Importance
Distinct plaque locations and vessel geometric features predispose to altered coronary flow hemodynamics. The association between these lesion-level characteristics assessed by coronary computed tomographic angiography (CCTA) and risk of future acute coronary syndrome (ACS) is unknown.
Objective
To examine whether CCTA-derived adverse geometric characteristics (AGCs) of coronary lesions describing location and vessel geometry add to plaque morphology and burden for identifying culprit lesion precursors associated with future ACS.
Design, Setting, and Participants
This substudy of ICONIC (Incident Coronary Syndromes Identified by Computed Tomography), a multicenter nested case-control cohort study, included patients with ACS and a culprit lesion precursor identified on baseline CCTA (n = 116) and propensity score–matched non-ACS controls (n = 116). Data were collected from July 20, 2012, to April 30, 2017, and analyzed from October 1, 2020, to October 31, 2021.
Exposures
Coronary lesions were evaluated for the following 3 AGCs: (1) distance from the coronary ostium to lesion; (2) location at vessel bifurcations; and (3) vessel tortuosity, defined as the presence of 1 bend of greater than 90° or 3 curves of 45° to 90° using a 3-point angle within the lesion.
Main Outcomes and Measures
Association between lesion-level AGCs and risk of future ACS-causing culprit lesions.
Results
Of 548 lesions, 116 culprit lesion precursors were identified in 116 patients (80 [69.0%] men; mean [SD], age 62.7 [11.5] years). Compared with nonculprit lesions, culprit lesion precursors had a shorter distance from the ostium (median, 35.1 [IQR, 23.6-48.4] mm vs 44.5 [IQR, 28.2-70.8] mm), more frequently localized to bifurcations (85 [73.3%] vs 168 [38.9%]), and had more tortuous vessel segments (5 [4.3%] vs 6 [1.4%]; all P < .05). In multivariable Cox regression analysis, an increasing number of AGCs was associated with a greater risk of future culprit lesions (hazard ratio [HR] for 1 AGC, 2.90 [95% CI, 1.38-6.08]; P = .005; HR for ≥2 AGCs, 6.84 [95% CI, 3.33-14.04]; P < .001). Adverse geometric characteristics provided incremental discriminatory value for culprit lesion precursors when added to a model containing stenosis severity, adverse morphological plaque characteristics, and quantitative plaque characteristics (area under the curve, 0.766 [95% CI, 0.718-0.814] vs 0.733 [95% CI, 0.685-0.782]). In per-patient comparison, patients with ACS had a higher frequency of lesions with adverse plaque characteristics, AGCs, or both compared with control patients (≥2 adverse plaque characteristics, 70 [60.3%] vs 50 [43.1%]; ≥2 AGCs, 92 [79.3%] vs 60 [51.7%]; ≥2 of both, 37 [31.9%] vs 20 [17.2%]; all P < .05).
Conclusions and Relevance
These findings support the concept that CCTA-derived AGCs capturing lesion location and vessel geometry are associated with risk of future ACS-causing culprit lesions. Adverse geometric characteristics may provide additive prognostic information beyond plaque assessment in CCTA.
Introduction
Histopathologic and intracoronary imaging studies show acute coronary syndrome (ACS) to result from atherosclerotic plaque erosion or rupture, most frequently in the setting of thin-cap fibroatheromas.1,2,3 Coronary computed tomographic angiography (CCTA) enables the noninvasive assessment of coronary atherosclerosis, with disruption-prone vulnerable plaques exhibiting qualitative morphological adverse plaque characteristics such as positive remodeling, low attenuation plaque, spotty calcification, and napkin-ring sign.4,5,6,7 Furthermore, the burden of noncalcified plaque quantified from CCTA has been shown to be associated with patient-level and plaque-specific risk of future ACS.8,9
The risk of plaque disruption is a function of both intrinsic plaque vulnerability and extrinsic hemodynamic forces at the plaque surface,10 and evidence suggests that distinct plaque locations and vessel geometric features predispose to altered coronary flow hemodynamics and endothelial shear stress.11,12,13,14,15 In addition to evaluation of plaque morphology and burden, CCTA permits the accurate determination of plaque distribution and vessel curvature. In this subanalysis of patients with incident ACS from the ICONIC (Incident Coronary Syndromes Identified by Computed Tomography) study, we sought to characterize adverse geometric characteristics (AGCs) of coronary lesions capturing location and vessel geometry on baseline CCTA. We hypothesized that AGCs are independently associated with future ACS-causing culprit lesions beyond current qualitative and quantitative plaque assessment in CCTA.
Methods
Study Population
This nested case-control study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The ICONIC study16 was a nested case-control study within the prospective CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry of 23 251 consecutive patients undergoing baseline CCTA for suspected coronary artery disease. The ICONIC study design has been previously described,9 and each participating institution received local review board or ethics board approval. Written or oral patient informed consent or a waiver of consent was obtained according to site-specific regulations. Among 23 251 patients in the CONFIRM registry, 234 with ACS events were propensity matched 1:1 with controls with no events. Standardized demographical and clinical patient information were collected prospectively at each study site; race and ethnicity data were collected because coronary artery plaque characteristics and cardiovascular disease-associated morbidity and mortality vary widely across different ethnic groups. Among 234 patients with ACS, we excluded 118 in whom culprit lesion adjudication was not feasible for the following reasons: invasive coronary angiography was not performed at the time of ACS (n = 32); invasive coronary angiography images were not available (n = 26); no culprit lesion could be determined on invasive coronary angiography (n = 14); culprit lesions on invasive coronary angiography aligned with normal CCTA segments (n = 21); culprit lesions on CCTA could not be measured owing to artifact or small vessel size (n = 12); culprit lesions aligned to total occluded segments (n = 5); and CCTA images were not available for secondary review (n = 8) (eFigure 1 in the Supplement). The final study population included 116 patients with subsequent ACS and 116 matched non-ACS controls. Data were collected from July 20, 2012, to April 30, 2017.
ACS and Culprit Lesion Adjudication
Acute coronary syndrome events were adjudicated by the clinical and data coordinating center at the Dalio Institute of Cardiovascular Imaging, blinded to CCTA findings. Cardiac biomarker measurements and electrocardiograms were used to adjudicate ACS events.17 Invasive coronary angiography images at the time of ACS event were transmitted to independently masked readers at the core laboratory. The Society of Cardiovascular Computed Tomography 18-segment model18 was applied to invasive coronary angiography for the evaluation of coronary vessels to align with the same model used for CCTA analysis. Using the ROMICAT (Rule Out Myocardial Infarction by Computer Assisted Tomography) definition,19 a single lesion with significant stenosis was considered the culprit lesion. In patients with ACS with a single significant stenosis, this lesion was considered the culprit lesion. In patients with ACS with 2 or more significant lesions, the culprit lesion was defined using invasive coronary angiography and electrocardiographic findings.19,20 All other lesions were deemed nonculprits.
CCTA Analysis
All CCTA images were acquired using at least 64-detector row scanners in accordance with Society of Cardiovascular Computed Tomography guidelines.9 Using CCTA analysis software Autoplaque, version 2.5 (Cedars-Sinai Medical Center), all coronary segments of 2 mm or more according to the 18-segment Society of Cardiovascular Computed Tomography model were evaluated by 2 level III experienced readers (D.H. and A.L.) blinded to clinical and invasive coronary angiography data. Diameter stenosis was visually graded according to the guidelines.21 Positive remodeling was defined by a vessel remodeling index of greater than 1.1.4 Low attenuation plaque was defined by the presence of any voxel of less than 30 Hounsfield units.22 Spotty calcification was defined as calcification of less than 3 mm in any direction within a plaque.7 Napkin-ring sign was defined as noncalcified plaque with a central area of hypoattenuation surrounded by a peripheral rim of hyperattenuation.6
Using the same software, all coronary lesions were evaluated for the following 3 AGCs: location at bifurcation, distance from ostium to lesion, and vessel tortuosity (eFigure 2 in the Supplement). Bifurcation was defined as the presence of a side branch origin within the lesion. Vessel tortuosity was defined as the presence of 1 bend of greater than 90° or 3 curves of 45° to 90° using a 3-point angle within the lesion (eFigure 3 in the Supplement).9,23 Vessel angle measurements were repeated in 30 randomly selected lesions by 2 independent readers (D.H. and K.K.) to evaluate interobserver reproducibility. The intraclass correlation coefficient for lesion curve measurement was 0.91 (95% CI, 0.82-0.96; P < .001). The distance (in mm) from the coronary ostium to lesion (site of maximal diameter stenosis) was measured for each individual plaque. Using the median distance from coronary ostium to lesion as a cutoff, lesions were categorized as having a short (<39 mm for left coronary artery and <60 mm for right coronary artery) or long distance from the ostium for binary assessment.
The volumes of total plaque, calcified plaque (≥350 Hounsfield units), fibrous plaque (131-350 Hounsfield units), fibrofatty plaque (31-130 Hounsfield units), and necrotic core (−30 to 30 Hounsfield units) were quantified semiautomatically (MEDIS Qangio CT, version 2.1.9.1 [Medis Medical Imaging Systems]) as described previously.9 The respective plaque burdens were calculated as plaque volume divided by vessel volume of the lesion multiplied by 100 (in percentage).
Statistical Analysis
Data were analyzed from October 1, 2020, to October 31, 2021. At the patient level, 116 patients with ACS were compared with 116 matched controls. The distribution and mean propensity score were compared between patients and matched controls to ensure the matching remained balanced after exclusion of 118 pairs for the culprit lesion assessment. At the lesion level, 116 culprit lesion precursors were compared with (1) 432 nonculprit lesions in patients with ACS and (2) 116 highest-grade stenosis in control patients. Continuous variables are reported as mean (SD) or median (IQR), as appropriate. Categorical variables are reported as number (percentage). Comparisons of continuous variables and categorical variables between culprit lesion precursors and nonculprit lesions were performed using the paired or unpaired t test, Mann-Whitney test, or χ2 test as appropriate. The number of adverse plaque characteristics was categorized as 0, 1, or 2 or more,22 and the number of AGCs was categorized as 0, 1, or 2 or more. Kaplan-Meier analysis with log rank test was used to assess the risk of future culprit lesions. Cox proportional hazards regression analysis was used to determine the risk of future culprit lesions, with adjustment for diameter stenosis, number of adverse plaque characteristics, and quantitative plaque characteristics, which included plaque area at the maximal stenosis section, minimum lumen area, total plaque volume, noncalcified plaque volume, and necrotic core volume. Shared frailty models were used in the Cox proportional hazards regression analysis to adjust for within-group dependence. For comparisons between patients with ACS and controls, clustered sandwich estimator models were used to account for the clustering within matched sets. Receiver operating characteristic curve analysis was applied to assess the incremental value of CCTA-determined plaque features for discriminating culprit lesion, using 3 nested models: (1) diameter stenosis plus adverse plaque characteristics; (2) diameter stenosis plus adverse plaque characteristics plus quantitative plaque characteristics; and (3) diameter stenosis plus adverse plaque characteristics plus quantitative plaque characteristics plus AGCs. Internal validation was performed by correcting for optimism using 1000 bootstrap resampling method.24 The optimism-adjusted area under the receiver operating characteristic curve (AUC) was used to measure the discriminatory ability of the models for identifying culprit lesion precursors. Statistical analyses were performed using Stata, version 14 (StataCorp LLC). Two-sided P < .05 indicated statistical significance.
Results
Baseline Clinical Characteristics of Patients With ACS vs Controls
Baseline clinical and imaging characteristics between patients with ACS and controls are shown in Table 1 and eTable 1 in the Supplement. Of the 116 patients with ACS, mean (SD) age was 62.7 (11.5) years; 80 patients (69.0%) were men and 36 (31.0%) were women. The median time from CCTA to the first ACS event was 29 (IQR, 5-386) days. Twenty-one patients (18.1%) had ST-elevation myocardial infarction, 50 (43.1%) had non-ST elevation myocardial infarction, and 42 (36.2%) had unstable angina.
Table 1. Baseline Clinical and Imaging Characteristics Between Patients With ACS and Controls.
| Characteristic | Patient groupa | P value | |
|---|---|---|---|
| ACS (n = 116) | Controls (n = 116) | ||
| Baseline clinical characteristics included in propensity score | |||
| Age, mean (SD), y | 62.7 (11.5) | 64.6 (9.7) | .19 |
| Sex | |||
| Men | 80 (69.0) | 70 (68.3) | .87 |
| Women | 36 (31.0) | 46 (39.7) | |
| Risk factors | |||
| Hypertension | 72 (62.1) | 66 (56.9) | .42 |
| Diabetes | 26 (22.4) | 35 (30.2) | .18 |
| Dyslipidemia | 67 (57.8) | 61 (52.6) | .39 |
| Family history of premature CAD | 49 (42.2) | 38 (32.8) | .11 |
| Current smoker | 29 (25.0) | 23 (19.8) | .34 |
| CAD severity (site read) | |||
| Normal | 5 (4.3) | 9 (7.7) | .31 |
| Nonobstructive | 44 (37.9) | 49 (42.2) | |
| 1-Vessel obstructive | 19 (16.4) | 24 (20.7) | |
| 2-Vessel obstructive | 29 (25.0) | 23 (19.8) | |
| 3-Vessel/left main | 19 (16.4) | 11 (9.5) | |
| Propensity score, mean (SD) | 0.08 (0.04) | 0.08 (0.04) | .99 |
| CCTA characteristics per patient | |||
| Stenosis, mean (SD), % | |||
| Diameter | 45.4 (15.9) | 35.6 (19.1) | <.001 |
| Area | 67.6 (18.0) | 54.9 (25.7) | <.001 |
| Minimum luminal, mean (SD) | |||
| Area, mm2 | 2.1 (1.5) | 2.4 (1.7) | .07 |
| Diameter, mm | 1.3 (0.4) | 1.4 (0.5) | .06 |
| Total plaque volume, median (IQR), mm3 | 296.1 (118.3-480.7) | 226.8 (85.4-407.1) | .13 |
| Calcified | 73.5 (21.4-181.0) | 59.2 (11.3-155.6) | .91 |
| Fibrous | 131.8 (57.2-200.6) | 92.8 (31.8-158.5) | .01 |
| Fibrofatty | 35.7 (12.2-82.7) | 28.2 (4.2-58.6) | .02 |
| Necrotic core | 2.0 (0.1-7.3) | 1.2 (0-5.1) | .12 |
| Noncalcified | 180.4 (73.3-306.9) | 145.8 (57.2-231.6) | .008 |
| Total plaque burden, median (IQR), % | 11.1 (5.7-19.6) | 10.4 (3.3-18.1) | .13 |
| Calcified | 3.7 (0.8-7.0) | 2.7 (0.6-6.7) | .63 |
| Fibrous | 5.2 (2.5-9.1) | 4.0 (1.2-7.2) | .02 |
| Fibrofatty | 1.5 (0.5-3.4) | 1.2 (0.1-2.3) | .09 |
| Necrotic core | 0.1 (0-0.3) | 0.1 (0-0.2) | .36 |
| Noncalcified plaque | 7.4 (3.3-15.6) | 5.9 (2.1-9.7) | .03 |
| Maximum cross-sectional plaque burden, mean (SD), % | 70.8 (16.6) | 59.7 (26.9) | <.001 |
| Diffuseness, % | 28.6 (15.7-42.9) | 22.1 (9.8-35.5) | .003 |
| No. of total lesions, mean (SD) | 4.7 (2.4) | 4.1 (2.5) | .06 |
| Adverse plaque characteristics | |||
| Any positive remodeling present | 109 (94.0) | 95 (81.9) | .005 |
| Any low attenuation plaque present | 63 (54.3) | 38 (32.7) | .001 |
| Any spotty calcification present | 40 (34.5) | 24 (20.7) | .02 |
| Any napkin-ring sign present | 19 (16.4) | 5 (4.3) | .003 |
| Lesion with ≥2 APCs | 70 (60.3) | 50 (43.1) | .009 |
| No. of lesions, mean (SD) | |||
| With APCs | 3.8 (2.2) | 3.1 (2.3) | .02 |
| With ≥2 APCs | 1.1 (1.2) | 0.7 (0.1) | .009 |
| Adverse geometric characteristics | |||
| Lesion with short distance from ostium | 110 (94.8) | 100 (86.2) | .03 |
| Lesion at bifurcation | 112 (96.5) | 77 (66.4) | <.001 |
| Lesion at tortuous segment | 8 (6.9) | 4 (3.4) | .24 |
| Lesion with ≥2 AGCs | 92 (79.3) | 60 (51.7) | .04 |
| No. of lesion, mean (SD) | |||
| With AGCs | 3.4 (1.6) | 2.9 (2.0) | .02 |
| With ≥2 AGCs | 1.2 (0.9) | 0.8 (0.9) | .001 |
| APCs and AGCs | |||
| Lesion with both APCs and AGCs | 109 (94.0) | 90 (77.6) | <.001 |
| No. of lesions with both APCs and AGCs, mean (SD) | 2.9 (1.5) | 2.2 (1.7) | .003 |
| Lesions with both ≥2 APCs and ≥2 AGCs | 37 (31.9) | 20 (17.2) | <.001 |
| No. of lesions with both ≥2 APCs and ≥2 AGCs, mean (SD) | 0.4 (0.6) | 0.2 (0.5) | .03 |
| CCTA characteristics per lesionb | |||
| Diameter stenosis, % | |||
| 1-24 | 18 (15.5) | 32 (27.6) | .07 |
| 25-49 | 37 (31.9) | 40 (34.5) | |
| 50-69 | 36 (31.0) | 24 (20.7) | |
| ≥70 | 25 (21.5) | 20 (17.2) | |
| APCs | |||
| Positive remodeling | 98 (84.5) | 54 (46.5) | <.001 |
| Low attenuation plaque | 41 (35.3) | 21 (18.1) | .003 |
| Spotty calcification | 21 (18.1) | 8 (6.9) | .01 |
| Napkin-ring sign | 13 (11.2) | 6 (5.2) | .09 |
| AGCs | |||
| Lesion with short distance from ostium | 74 (63.8) | 48 (41.4) | .001 |
| Lesion at bifurcation | 85 (73.3) | 40 (34.5) | <.001 |
| Lesion at tortuous segment | 5 (4.3) | 3 (2.6) | .47 |
| Quantitative plaque volume and burden, median (IQR) | |||
| Total plaque volume, mm3 | 94.9 (29.1-205.0) | 21.5 (6.1-3.8) | <.001 |
| Calcified volume | 19.3 (2.2-78.6) | 3.8 (0-24.8) | .02 |
| Fibrous volume | 35.2 (13.0-93.3) | 7.0 (1.7-31.7) | <.001 |
| Fibrofatty volume | 9.5 (1.3-32.6) | 1.7 (0-9.9) | .001 |
| Necrotic core volume | 0.1 (0-2.7) | 0 (0-0.6) | .06 |
| Noncalcified volume | 42.4 (21.0-131.9) | 10.6 (2.2-45.2) | <.001 |
| Total plaque burden, % | 31.8 (20.1-42.2) | 24.5 (11.6-37.3) | .002 |
| Calcified burden | 8.1 (1.6-17.1) | 6.8 (0-13.3) | .31 |
| Fibrous burden | 13.8 (7.9-19.2) | 9.5 (2.8-15.5) | <.001 |
| Fibrofatty burden | 3.6 (0.6-9.2) | 1.4 (0-6.7) | .008 |
| Necrotic core burden | 0.1 (0-0.6) | 0 (0-0.4) | .02 |
| Noncalcified plaque burden | 20.3 (10.5-28.3) | 13.9 (3.6-24.0) | .003 |
Abbreviations: ACS, acute coronary syndrome; AGC, adverse geometric characteristic; APC, adverse plaque characteristic; CAD, coronary artery disease; CCTA, coronary computed tomography angiography.
Unless otherwise indicated, data are expressed as number (%) of patients.
Culprit lesions in patients with ACS vs maximum stenosed lesion in controls.
Baseline clinical characteristics used for propensity score matching did not differ significantly between the 116 patients with ACS and 116 matched controls (Table 1). The AUC of the propensity score model was 0.506 (95% CI, 0.432-0.581). There was no significant difference in mean propensity scores between included cases and controls (0.08 [0.04] vs 0.08 [0.04]; P = .91) (eFigure 4 in the Supplement).
CCTA-Based Plaque Characteristics in Culprit vs Nonculprit Lesions
Of the total 548 lesions in 116 patients, we identified 116 lesions as culprit lesion precursors associated with future ACS. Compared with 432 nonculprit lesions, culprit lesion precursors had a greater-diameter stenosis (eg, ≥70%, 25 [21.5%] vs 30 [6.9%]; P < .001 for trend) and a higher prevalence of all 4 adverse plaque characteristics (eg, any adverse plaque characteristic, 108 [93.1%] vs 331 [76.6%]; P < .001) (Table 2). Culprit lesion precursors exhibited higher median volumes and burdens of total plaque (94.9 [IQR, 29.1-205.0] vs 23.9 [IQR, 9.4-65.9] mm3 and 31.8% [IQR, 20.1%-42.2%] vs 19.9% [IQR, 11.9%-30.9%], respectively), calcified plaque (19.3 [IQR, 2.2-78.6] vs 5.8 [IQR, 1.3-19.7] mm3 and 8.1% [IQR, 1.6%-17.1%] vs 5.3% [IQR, 1.5%-12.0%], respectively), fibrous plaque (35.2 [IQR, 13.0-93.3] vs 11.1 [IQR, 4.6-29.5] mm3 and 13.8% [IQR, 7.9%-19.2%] vs 9.2% [IQR, 5.5%-14.3%], respectively), fibrofatty plaque (9.5 [IRQ, 1.3-32.6] vs 1.7 [IQR, 0.1-8.3] mm3 and 3.6% [IQR, 0.6%-9.2%] vs 1.2% [IQR, 0.1%-4.2%], respectively), and necrotic core (0.1 [IQR, 0-2.7] vs 0 [IQR, 0-0.3] mm3 and 0.1% [IQR, 0-0.6%] vs 0 [IQR, 0-0.1%], respectively) when compared with nonculprit lesions (all P < .05). With respect to AGCs, culprit lesion precursors had a shorter medium ostium to lesion distance (overall, 35.1 [IQR, 23.6-48.4] mm vs 44.5 [IQR, 28.2-70.8] mm [P < .001]; left coronary artery, 34.9 [IQR, 24.5-47.7] vs 39.9 [IQR, 26.1-54.5] mm [P = .02]; right coronary artery, 36.2 [IQR, 20-54.8] vs 65.9 [IQR, 30.4-103.8] mm [P < .001]) and more frequently localized to bifurcations (85 [73.3%] vs 168 [38.9%]) or tortuous vessel segments (5 [4.3%] vs 6 [1.4%]) (both P < .05) compared with nonculprit lesions.
Table 2. CCTA Characteristics of Culprit and Nonculprit Lesions in Patients With ACS.
| Characteristic | Lesiona | P value | |
|---|---|---|---|
| Culprit (n = 116) | Nonculprit (n = 432) | ||
| Stenosis grade | |||
| Diameter stenosis, % | |||
| 1-24 | 18 (15.5) | 164 (38.0) | <.001 |
| 25-49 | 37 (31.9) | 179 (41.4) | |
| 50-69 | 36 (31.0) | 59 (13.7) | |
| ≥70 | 25 (21.5) | 30 (6.9) | |
| Coronary vessel distribution | |||
| Left anterior descending | 64 (55.2) | 161 (37.3) | .002 |
| Left circumflex | 19 (16.4) | 114 (26.4) | |
| Right coronary artery | 33 (28.4) | 157 (36.3) | |
| Adverse plaque characteristics | |||
| Positive remodeling | 98 (84.5) | 317 (73.4) | .01 |
| Low attenuation plaque | 41 (35.3) | 56 (13.0) | <.001 |
| Spotty calcification | 21 (18.1) | 46 (10.6) | .03 |
| Napkin-ring sign | 13 (11.2) | 7 (1.6) | <.001 |
| Any APC | 108 (93.1) | 331 (76.6) | <.001 |
| ≥2 APCs | 45 (38.8) | 78 (18.1) | <.001 |
| AGCs | |||
| Distance from ostium, median (IQR), mm | 35.1 (23.6-48.4) | 44.5 (28.2-70.8) | <.001 |
| Short distance | 74 (63.8) | 198 (45.8) | .001 |
| Bifurcation | 85 (73.3) | 168 (38.9) | <.001 |
| Tortuosity | 5 (4.3) | 6 (1.4) | .046 |
| Any AGC | 106 (91.4) | 283 (65.5) | <.001 |
| ≥2 AGCs | 59 (50.9) | 90 (20.8) | <.001 |
| Both APCs and AGCs | 98 (84.5) | 222 (51.4) | <.001 |
| ≥2 APCs and ≥2 AGCs | 27 (23.3) | 21 (4.9) | <.001 |
| Quantitative plaque volume and burden, median (IQR) | |||
| Total plaque volume, mm3 | 94.9 (29.1-205.0) | 23.9 (9.4-65.9) | <.001 |
| Calcified | 19.3 (2.2-78.6) | 5.8 (1.3-19.7) | <.001 |
| Fibrous | 35.2 (13.0-93.3) | 11.1 (4.6-29.5) | <.001 |
| Fibrofatty | 9.5 (1.3-32.6) | 1.7 (0.1-8.3) | <.001 |
| Necrotic core | 0.1 (0-2.7) | 0 (0-0.3) | <.001 |
| Noncalcified | 42.4 (21.0-131.9) | 14.7 (5.5-40.5) | <.001 |
| Total plaque burden, % | 31.8 (20.1-42.2) | 19.9 (11.9-30.9) | <.001 |
| Calcified | 8.1 (1.6-17.1) | 5.3 (1.5-12.0) | .04 |
| Fibrous | 13.8 (7.9-19.2) | 9.2 (5.5-14.3) | <.001 |
| Fibrofatty | 3.6 (0.6-9.2) | 1.2 (0.1-4.2) | <.001 |
| Necrotic core | 0.1 (0-0.6) | 0 (0-0.1) | <.001 |
| Noncalcified plaque | 20.3 (10.5-28.3) | 11.7 (6.4-19.6) | <.001 |
Abbreviations: ACS, acute coronary syndrome; AGC, adverse geometric characteristics; APC, adverse plaque characteristics; CCTA, coronary computed tomography angiography.
Unless otherwise indicated, data are expressed as number (%) of lesions.
Association of AGCs With Future Culprit Lesions
An increasing number of AGCs was associated a higher prevalence of low attenuation plaque (0 AGCs, 13 of 151 [8.6%]; 1 AGC, 46 of 262 [17.5%]; ≥2 AGCs, 29 of 135 [21.5%]; P = .008) and napkin-ring sign (0 AGCs, 0; 1 AGC, 9 of 262 [3.4%]; ≥2 AGCs, 11 of 135 [8.1%]; P = .001) as well as a trend toward a greater-diameter stenosis (eg, ≥70%: 0 AGCs, 12 of 151 [7.9%]; 1 AGC, 25 of 262 [9.5%]; ≥2 AGCs, 18 of 135 [13.3%]; P = .07) (eTable 2 in the Supplement). Lesions without AGCs had a lower prevalence of positive remodeling (117 of 151 [77.5%]) and spotty calcification (16 of 151 [10.6%]) compared with lesions with 1 (215 of 262 [82.1%] and 43 of 262 [16.0%], respectively) or at least 2 AGCs (109 of 135 [80.7%] and 20 of 135 [14.8%], respectively). There was a graded increase in the quantitative volumes and burdens of all plaque components with an increasing number of AGCs (eg, total plaque volume, 15.5 [IQR, 6.9-30.8] vs 88.3 [IQR, 26.2-154.9] mm3; P < .001).
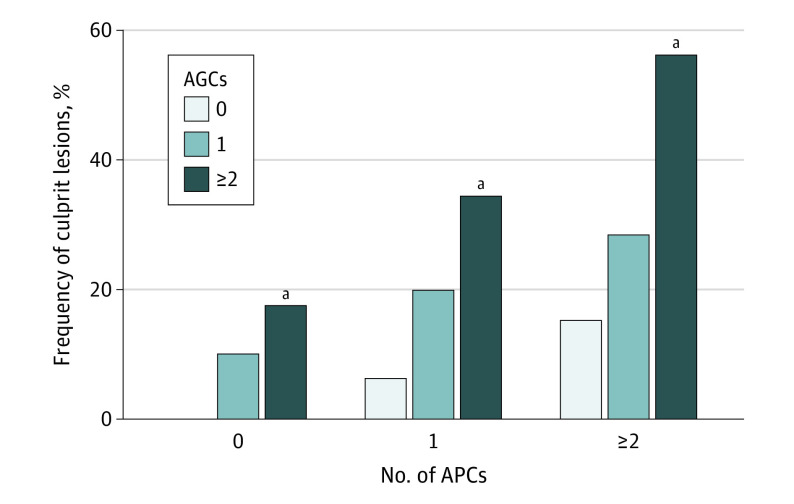
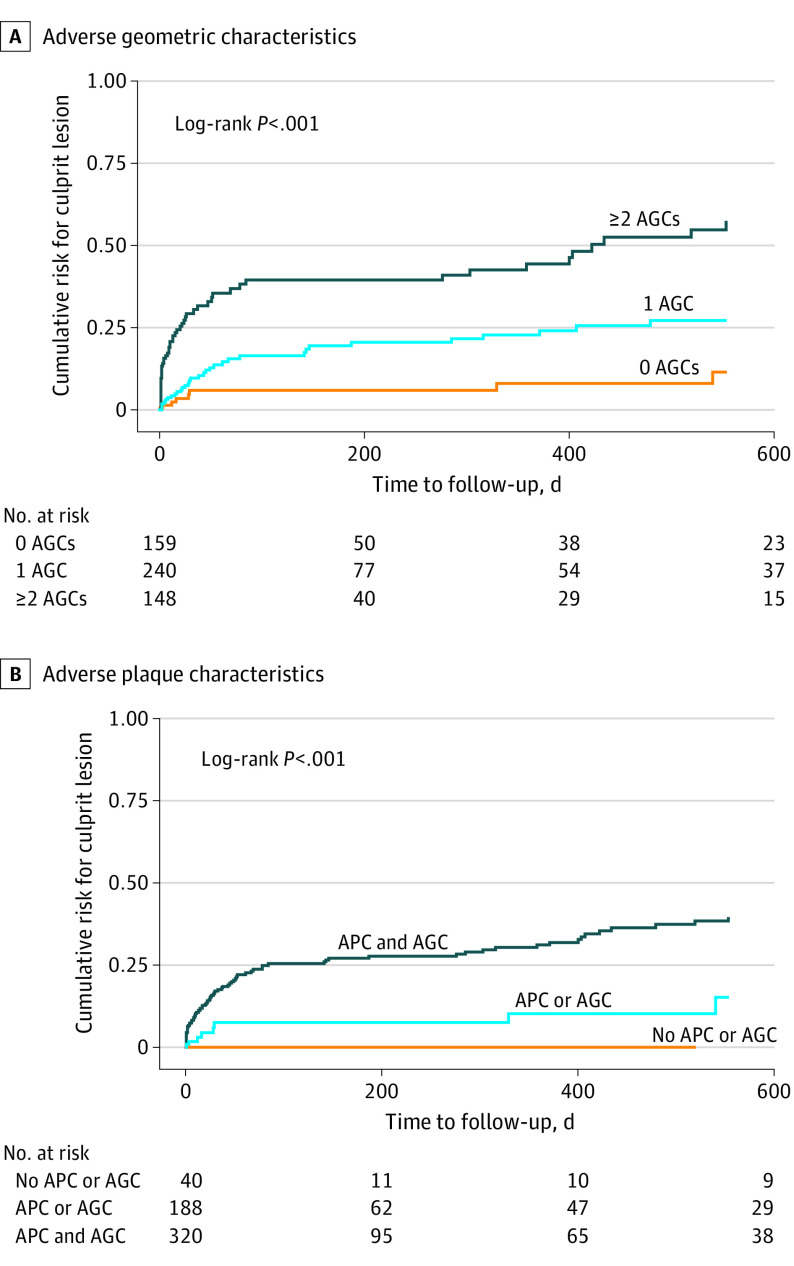
The prevalence of culprit lesion precursors increased with the number of AGCs, a trend that was more pronounced with an increasing frequency of adverse plaque characteristics (Figure 1). Among 40 lesions containing neither adverse plaque characteristics nor AGCs, none progressed to future culprit lesions. By contrast, more than half (27 of 48 [56.3%]) of lesions with at least 2 adverse plaque characteristics and at least 2 AGCs were culprit lesion precursors. On Kaplan-Meier analysis, the cumulative risk of plaques developing into culprit lesions increased with the number of AGCs (log-rank P < .001) (Figure 2A). Plaques exhibiting both adverse plaque characteristics and AGCs had the greatest probability of becoming culprit lesions compared to plaques with either adverse plaque characteristics or AGCs or plaques with no adverse plaque characteristics or AGCs (log-rank P < .001) (Figure 2B).
Figure 1. Frequency of Culprit Lesions According to Number of Adverse Plaque Characteristics (APCs) and Adverse Geometric Characteristics (AGCs).
The prevalence of culprit lesion precursors increased with an increasing number of APCs and AGCs. Lesions containing neither APCs nor AGCs did not develop into culprit lesions.
aP < .001 compared with 0 AGCs.
Figure 2. Kaplan-Meier Curve for Culprit Lesion Risk According to Number of Adverse Geometric Characteristics (AGCs) and Presence of Adverse Plaque Characteristics (APCs) and/or AGCs.
A, The risk of plaques developing into culprit lesions increased with the number of AGCs. B, Plaques with both APCs and AGCs had the greatest probability of becoming culprit lesions compared to plaques with either APCs or AGCs alone, or plaques with neither APCs nor AGCs.
In multivariable Cox proportional hazards regression analysis, AGCs were independently associated with increased risk of future culprit lesions after adjustment for diameter stenosis, number of adverse plaque characteristics, and quantitative plaque characteristics (eTable 3 in the Supplement). Compared with nonculprit lesions, culprit lesion precursors had an elevated hazard for a short distance from the ostium (hazard ratio [HR], 2.71 [95% CI, 1.73-4.24]; P < .001), bifurcation location (HR, 2.14 [95% CI, 1.39-3.29]; P < .001), and tortuosity (HR, 2.31 [95% CI, 1.01-5.27]; P = .04). An increasing number of AGCs was associated with greater risk of future culprit lesions, with 1 and at least 2 AGCs conferring a 2.9-fold (HR, 2.90 [95% CI, 1.38-6.08]; P = .005) and 7-fold (HR, 6.84 [95% CI, 3.33-14.04]; P < .001) risk, respectively.
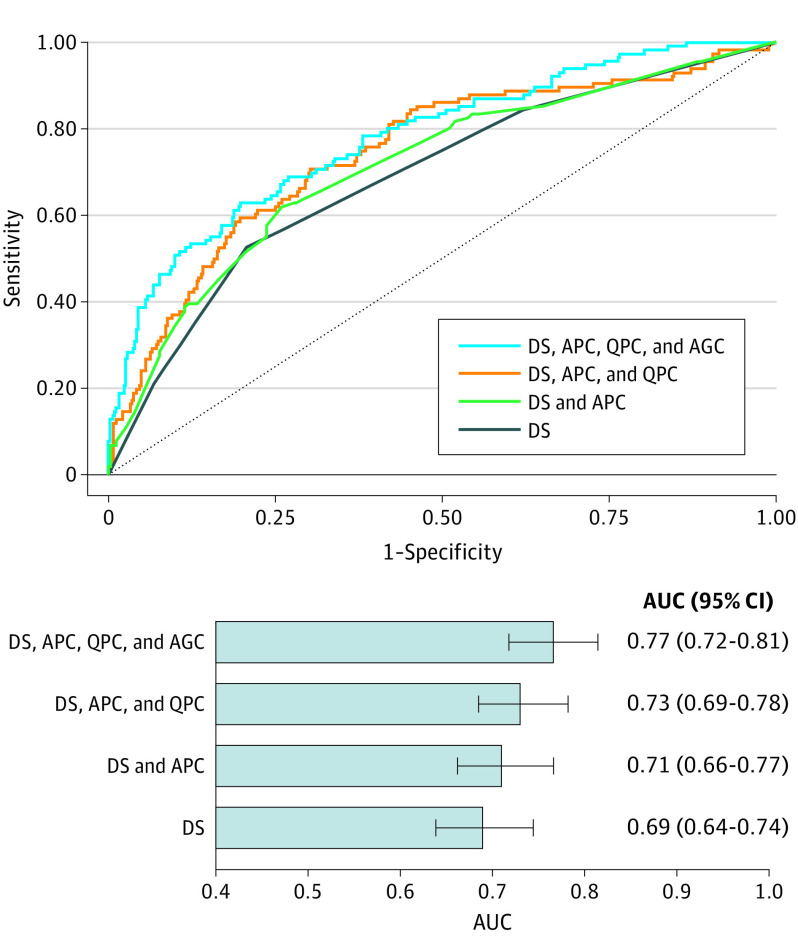
On receiver operating characteristic curve analysis, a model containing diameter stenosis, adverse plaque characteristics, and quantitative plaque characteristics (model 2) had a higher AUC for discriminating culprit lesion precursors compared with a model with diameter stenosis and adverse plaque characteristics (model 1) (AUC, 0.733 [95% CI, 0.685-0.782] vs 0.712 [95% CI, 0.662-0.766]). The addition of AGCs to model 2 provided further incremental discriminatory value (AUC, 0.766 [95% CI, 0.718-0.814]) (Figure 3).
Figure 3. Performance of Plaque Characteristics for Identifying Future Culprit Lesions.
Receiver operator characteristic curves show the discriminatory value of plaque characteristics for identifying future culprit lesions. The addition of adverse geometric characteristics (AGCs; including positive remodeling, low attenuation plaque, spotty calcification, and napkin-ring sign) to a model containing diameter stenosis (DS), adverse plaque characteristics (APCs; including ostium to lesion distance, bifurcation, and tortuosity), and quantitative plaque characteristics (QPCs; including plaque area at maximal stenosis section, minimum lumen area, total plaque, noncalcified plaque, and necrotic core volume) yielded a higher area under the curve (AUC).
Adverse Geometric Characteristics in Patients With ACS vs Controls
Compared with controls, patients with ACS had a higher diameter and area of stenosis and noncalcified plaque volume and burden (Table 1). Total plaque volume and calcified plaque volume did not differ between groups. Lesions in patients with ACS more frequently exhibited adverse plaque characteristics (eg, positive remodeling, 109 [94.0%] vs 95 [81.9%]; P = .005), a short distance from ostium (110 [94.8%] vs 100 [86.2%]; P = .03), bifurcation location (112 [96.5%] vs 77 [66.4%]; P < .001), or a combination of both adverse plaque characteristics and AGCs (109 [94.0%] vs 90 [77.6%]; P < .001) compared with lesions in controls. The prevalence of lesions in tortuous segments did not differ significantly between patients with ACS and controls (8 [6.9%] vs 4 [3.4%]; P = .24). The total mean number of lesions with any AGC (3.4 [1.6] vs 2.9 [2.0]; P = .02) or at least 2 AGCs (1.2 [0.9] vs 0.8 [0.9]; P = .001) was also significantly higher in patients with ACS than in controls. Patients with ACS had a higher frequency of lesions with adverse plaque characteristics, AGCs, or both compared with control patients (≥2 adverse plaque characteristics, 70 [60.3%] vs 50 [43.1%]; ≥2 AGCs, 92 [79.3%] vs 60 [51.7%]; ≥2 of both, 37 [31.9%] vs 20 [17.2%]; all P < .05).
Compared with the highest-grade stenosis lesion in controls, culprit lesion precursors exhibited a higher prevalence of adverse plaque characteristics (eg, positive remodeling, 98 [84.5%] vs 317 [73.4%]; P = .01) and greater total and noncalcified plaque volumes and burdens (total plaque volume and burden, 94.9 [IQR, 29.1-205.0] vs 21.5 [IQR, 6.1-3.8] mm3 and 31.8% [IQR, 20.1%- 42.2%] vs 24.5% [IQR, 11.6%-37.3%], respectively; noncalcified plaque volume and burden, 42.4 [IQR, 21.0-131.9] vs 10.6 [IQR, 2.2-45.2] mm3 and 20.3% [IQR, 10.5%-28.3%] vs 13.9% [IQR, 3.6%-24.0%], respectively; all P < .01) (Table 1). Culprit precursor lesions more frequently had a short distance from the ostium (74 [63.8%] vs 48 [41.4%]; P = .001) and localized to bifurcations (85 [73.3%] vs 40 [34.5%]; P < .001). The prevalence of vessel tortuosity did not differ significantly between culprit lesion precursors and the highest-grade stenosis control lesions (5 [4.3%] vs 3 [2.6%]; P = .47). In multivariable Cox proportional hazards regression, a short distance from the ostium (HR, 1.55 [95% CI, 1.02-2.33]; P = .03) and bifurcation location (HR, 2.63 [95% CI, 1.77-3.92]; P < .001) conferred an increased risk of future culprit lesions (eTable 4 in the Supplement). An increasing number of AGCs was associated with greater risk of future culprit lesions, with 1 and at least 2 AGCs conferring a 2.5-fold (HR, 2.53 [95% CI, 1.24-5.15]; P = .01) and 4.4-fold (HR, 4.43 [95% CI, 2.17-9.05]; P < .001) risk, respectively.
Discussion
This study examined the association of CCTA-derived AGCs (plaque location and vessel geometry) with culprit lesion precursors. Our primary findings included the following: (1) plaques located at bifurcations, tortuous vessel segments, or closer to the coronary ostium had a greater risk of developing into culprit lesions, independently of diameter stenosis, adverse plaque characteristics, and quantitative plaque characteristics; (2) among plaques without adverse plaque characteristics and AGCs (n = 40), none developed into culprit lesions; and (3) AGCs provided incremental discriminatory value for identifying culprit lesion precursors when added to a model containing diameter stenosis, adverse plaque characteristics, and quantitative plaque characteristics.
Prior reports demonstrating the predictive value of CCTA-based coronary plaque assessment for subsequent ACS have focused on morphological plaque characteristics.5,7,8,9,25 It is well established that CCTA-verified qualitative adverse plaque characteristics confer an increased risk of future coronary events.5,7,26 Beyond this, quantitative plaque measurements have independent predictive value for subsequent ACS in patients with stable coronary artery disease.8,9 The recent ICONIC study showed quantitative plaque burden and composition to be associated with future ACS at both the per-patient and per-lesion level9,27; however, the potential influence of plaque location and vessel geometry on outcomes was not examined. Coronary computed tomographic angiography enables precise 3-dimensional reconstruction of the entire coronary tree and hence the accurate and reproducible assessment of vessel angulation, tortuosity, and bifurcation points.28,29 Although patient-based prognostic CCTA indexes such as the computed tomography–Leaman score30 and modified Duke coronary artery disease index31 assign greater weighting to lesions in proximal coronary segments, they do not incorporate geometric vessel features. We demonstrate that assessment of plaque location and vessel geometry provides incremental value for identifying future ACS-causing culprit lesions over and above plaque morphological features and quantitative plaque characteristics. Although prior reports have examined AGCs at the time of ACS31,32,33 or identified adverse plaque characteristics from antecedent CCTA scans in patients with ACS, our study is the first, to our knowledge, to demonstrate the prognostic significance of both AGCs and adverse plaque characteristics in patients with stable disease who experience future ACS.
Mechanistically, thrombotic occlusions leading to ACS have a propensity to occur in the proximal third of the coronary arteries as demonstrated in invasive coronary imaging studies.32,34 Plaque distance from the coronary ostium has been shown to be independently associated with plaque lipid content33,35 and plaque rupture.36 Moreover, the presence of angulation on a lesion and bifurcations occurring before or after a lesion confer an increased risk of plaque rupture.34,36 Coronary plaques undergo constant evolution, including subclinical plaque rupture and healing, and the surrounding arterial wall also undergoes constant remodeling.1 These AGCs affect local hemodynamic factors such as wall shear stress (WSS) that play a pivotal role in coronary plaque progression and subsequent plaque disruption.37,38,39,40 Several experimental and histopathologic studies have shown that coronary atherogenesis preferentially involves the outer walls of vessel bifurcations and inner walls of regions of high curvature in the coronary arterial tree.41,42 In these geometrically predisposed locations, WSS is significantly lower and exhibits directional changes and flow separation, features absent from regions of the coronary tree that are generally spared from atherosclerosis.43,44 Furthermore, increased WSS and axial stress induced by the pressure gradient across a stenosis act directly on plaques to increase their risk of rupture.45 Thus, biomechanical stresses are closely associated with plaque location and vessel geometry. Coronary computed tomographic angiography–derived AGCs as proposed by the present study represent simple anatomical parameters that may capture the effect of local hemodynamic forces in noninvasive plaque-based risk assessment.
Although recent advances in CCTA permit the modeling of computational fluid dynamics and calculation of hemodynamic parameters such as WSS,46,47 such techniques require specialized software and are computationally expensive, limiting their clinical application. Herein, we present 3 AGCs that can be readily obtained from CCTA images using a combination of visual assessment and simple distance and angle measurements. We show these AGCs to provide incremental and complementary prognostic information to standard clinical CCTA evaluation of stenosis severity and adverse plaque characteristics. Hence, comprehensive plaque assessment, including lesion location and vessel geometry, may enhance the ability of CCTA to identify future ACS-causing culprit lesions and risk stratify patients with stable coronary artery disease.
Limitations
This study has several limitations. The post hoc nested case-control design of the ICONIC study may be susceptible to selection bias as well as unmeasured confounding factors, which may limit the generalizability of our findings. Future studies will need to externally validate these findings in larger, unselected cohorts. We did not assess emerging software-based hemodynamic parameters such as WSS, and hence were unable to directly explore the association between biomechanical stresses and AGCs in the present analysis. The low prevalence of lesions located in tortuous vessel segments (<5%) suggests that our study may have been underpowered to detect any significant between-patient differences for this AGC. Patients who died without a confirmatory finding of ACS were excluded from the ICONIC study, and thus our findings are limited to culprit lesions associated with nonfatal ACS. The extent of missing data in this analysis should be noted. Among 234 patients with ACS and 234 controls in the ICONIC study, we only included 116 patients with ACS with identifiable culprit lesion precursors and their 116 paired controls. This may have resulted in potential selection bias and unbalanced propensity matching in the remaining case-control pairs. Although clinical characteristics did not differ significantly between included vs excluded patients, included patients had significantly higher CCTA-derived volumes and burdens of all plaque components and a higher degree of stenosis compared to excluded patients (eTable 5 in the Supplement). Thus, the present study findings might be limited to patients with high cardiovascular risk and extensive coronary atherosclerosis at baseline. Further studies are required to confirm the association of AGCs with future ACS risk in low- and intermediate-risk populations.
Conclusions
The findings of this study support the concept that CCTA-derived AGCs capturing lesion location and vessel geometry are associated with the risk of future ACS-causing culprit lesions. Adverse geometric characteristics can be obtained from standard CCTA images and may provide additive prognostic information beyond plaque assessment in CCTA.
eTable 1. Additional Patient Characteristics Between ACS vs Control
eTable 2. CCTA Lesion Characteristics According to the Number of AGCs in Patients With ACS
eTable 3. Univariable and Multivariable Cox Proportional Hazards Regression Analysis of AGCs for Risk of Culprit Lesions
eTable 4. Multivariable Cox Proportional Hazards Regression Analysis of AGCs for Risk of Culprit Lesions (Patients With ACS vs Controls)
eTable 5. Clinical and CCTA Characteristics in Analyzed (n = 116) and Excluded (n=118) Patients With ACS From the ICONIC Study
eFigure 1. Patient Selection and Study Design
eFigure 2. Illustrations of Adverse Geometric Characteristics
eFigure 3. Lesion Curve Assessment Using Semiautomated Software
eFigure 4. Propensity Score Distribution of the 116 Case and 116 Control Pairs
References
- 1.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852-1866. doi: 10.1161/CIRCRESAHA.114.302721 [DOI] [PubMed] [Google Scholar]
- 2.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349(24):2316-2325. doi: 10.1056/NEJMoa035655 [DOI] [PubMed] [Google Scholar]
- 3.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20(5):1262-1275. doi: 10.1161/01.ATV.20.5.1262 [DOI] [PubMed] [Google Scholar]
- 4.Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50(4):319-326. doi: 10.1016/j.jacc.2007.03.044 [DOI] [PubMed] [Google Scholar]
- 5.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54(1):49-57. doi: 10.1016/j.jacc.2009.02.068 [DOI] [PubMed] [Google Scholar]
- 6.Maurovich-Horvat P, Hoffmann U, Vorpahl M, Nakano M, Virmani R, Alkadhi H. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc Imaging. 2010;3(4):440-444. doi: 10.1016/j.jcmg.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 7.Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014;64(7):684-692. doi: 10.1016/j.jacc.2014.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams MC, Kwiecinski J, Doris M, et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation. 2020;141(18):1452-1462. doi: 10.1161/CIRCULATIONAHA.119.044720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang HJ, Lin FY, Lee SE, et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol. 2018;71(22):2511-2522. doi: 10.1016/j.jacc.2018.02.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657-671. doi: 10.1161/01.CIR.92.3.657 [DOI] [PubMed] [Google Scholar]
- 11.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24(1):12-22. doi: 10.1161/01.ATV.0000105054.43931.f0 [DOI] [PubMed] [Google Scholar]
- 12.Kwak BR, Bäck M, Bochaton-Piallat ML, et al. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J. 2014;35(43):3013-3020, 3020a-3020d. doi: 10.1093/eurheartj/ehu353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morbiducci U, Kok AM, Kwak BR, Stone PH, Steinman DA, Wentzel JJ. Atherosclerosis at arterial bifurcations: evidence for the role of haemodynamics and geometry. Thromb Haemost. 2016;115(3):484-492. doi: 10.1160/th15-07-0597 [DOI] [PubMed] [Google Scholar]
- 14.Vorobtsova N, Chiastra C, Stremler MA, Sane DC, Migliavacca F, Vlachos P. Effects of vessel tortuosity on coronary hemodynamics: an idealized and patient-specific computational study. Ann Biomed Eng. 2016;44(7):2228-2239. doi: 10.1007/s10439-015-1492-3 [DOI] [PubMed] [Google Scholar]
- 15.Jackson ZS, Dajnowiec D, Gotlieb AI, Langille BL. Partial off-loading of longitudinal tension induces arterial tortuosity. Arterioscler Thromb Vasc Biol. 2005;25(5):957-962. doi: 10.1161/01.ATV.0000161277.46464.11 [DOI] [PubMed] [Google Scholar]
- 16.ClinicalTrials.gov . Incident Coronary Events Identified by Computed Tomography (ICONIC). NCT02959099. Accessed March 26, 2021. https://clinicaltrials.gov/ct2/show/NCT02959099
- 17.Mendis S, Thygesen K, Kuulasmaa K, et al. ; Writing group on behalf of the participating experts of the WHO consultation for revision of WHO definition of myocardial infarction . World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol. 2011;40(1):139-146. doi: 10.1093/ije/dyq165 [DOI] [PubMed] [Google Scholar]
- 18.Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8(5):342-358. doi: 10.1016/j.jcct.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann U, Moselewski F, Nieman K, et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47(8):1655-1662. doi: 10.1016/j.jacc.2006.01.041 [DOI] [PubMed] [Google Scholar]
- 20.Ferencik M, Schlett CL, Ghoshhajra BB, et al. A computed tomography-based coronary lesion score to predict acute coronary syndrome among patients with acute chest pain and significant coronary stenosis on coronary computed tomographic angiogram. Am J Cardiol. 2012;110(2):183-189. doi: 10.1016/j.amjcard.2012.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cury RC, Abbara S, Achenbach S, et al. ; Endorsed by the American College of Cardiology . CAD-RADS(TM) Coronary Artery Disease–Reporting and Data System: an expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2016;10(4):269-281. doi: 10.1016/j.jcct.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 22.Park HB, Heo R, Ó Hartaigh B, et al. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging. 2015;8(1):1-10. doi: 10.1016/j.jcmg.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(2):219-227. [PubMed] [Google Scholar]
- 24.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361-387. doi: [DOI] [PubMed] [Google Scholar]
- 25.Motoyama S, Ito H, Sarai M, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol. 2015;66(4):337-346. doi: 10.1016/j.jacc.2015.05.069 [DOI] [PubMed] [Google Scholar]
- 26.Williams MC, Moss AJ, Dweck M, et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART Study. J Am Coll Cardiol. 2019;73(3):291-301. doi: 10.1016/j.jacc.2018.10.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al’Aref SJ, Singh G, Choi JW, et al. A boosted ensemble algorithm for determination of plaque stability in high-risk patients on coronary CTA. JACC Cardiovasc Imaging. 2020;13(10):2162-2173. doi: 10.1016/j.jcmg.2020.03.025 [DOI] [PubMed] [Google Scholar]
- 28.Pflederer T, Ludwig J, Ropers D, Daniel WG, Achenbach S. Measurement of coronary artery bifurcation angles by multidetector computed tomography. Invest Radiol. 2006;41(11):793-798. doi: 10.1097/01.rli.0000239318.88270.9f [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulou SL, Girasis C, Dharampal A, et al. CT-SYNTAX score: a feasibility and reproducibility study. JACC Cardiovasc Imaging. 2013;6(3):413-415. doi: 10.1016/j.jcmg.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 30.Mushtaq S, De Araujo Gonçalves P, Garcia-Garcia HM, et al. Long-term prognostic effect of coronary atherosclerotic burden: validation of the computed tomography–Leaman score. Circ Cardiovasc Imaging. 2015;8(2):e002332-e002332. doi: 10.1161/CIRCIMAGING.114.002332 [DOI] [PubMed] [Google Scholar]
- 31.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161-1170. doi: 10.1016/j.jacc.2007.03.067 [DOI] [PubMed] [Google Scholar]
- 32.Wang JC, Normand SL, Mauri L, Kuntz RE. Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation. 2004;110(3):278-284. doi: 10.1161/01.CIR.0000135468.67850.F4 [DOI] [PubMed] [Google Scholar]
- 33.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8)(suppl):C13-C18. doi: 10.1016/j.jacc.2005.10.065 [DOI] [PubMed] [Google Scholar]
- 34.Hong MK, Mintz GS, Lee CW, et al. The site of plaque rupture in native coronary arteries: a three-vessel intravascular ultrasound analysis. J Am Coll Cardiol. 2005;46(2):261-265. doi: 10.1016/j.jacc.2005.03.067 [DOI] [PubMed] [Google Scholar]
- 35.Valgimigli M, Rodriguez-Granillo GA, Garcia-Garcia HM, et al. Distance from the ostium as an independent determinant of coronary plaque composition in vivo: an intravascular ultrasound study based radiofrequency data analysis in humans. Eur Heart J. 2006;27(6):655-663. doi: 10.1093/eurheartj/ehi716 [DOI] [PubMed] [Google Scholar]
- 36.Katritsis DG, Efstathopoulos EP, Pantos J, et al. Anatomic characteristics of culprit sites in acute coronary syndromes. J Interv Cardiol. 2008;21(2):140-150. doi: 10.1111/j.1540-8183.2007.00339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stone PH, Saito S, Takahashi S, et al. ; PREDICTION Investigators . Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation. 2012;126(2):172-181. doi: 10.1161/CIRCULATIONAHA.112.096438 [DOI] [PubMed] [Google Scholar]
- 38.Choi G, Lee JM, Kim HJ, et al. Coronary artery axial plaque stress and its relationship with lesion geometry: application of computational fluid dynamics to coronary CT angiography. JACC Cardiovasc Imaging. 2015;8(10):1156-1166. doi: 10.1016/j.jcmg.2015.04.024 [DOI] [PubMed] [Google Scholar]
- 39.Costopoulos C, Timmins LH, Huang Y, et al. Impact of combined plaque structural stress and wall shear stress on coronary plaque progression, regression, and changes in composition. Eur Heart J. 2019;40(18):1411-1422. doi: 10.1093/eurheartj/ehz132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JM, Choi G, Koo BK, et al. Identification of high-risk plaques destined to cause acute coronary syndrome using coronary computed tomographic angiography and computational fluid dynamics. JACC Cardiovasc Imaging. 2019;12(6):1032-1043. doi: 10.1016/j.jcmg.2018.01.023 [DOI] [PubMed] [Google Scholar]
- 41.Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990;66(4):1045-1066. doi: 10.1161/01.RES.66.4.1045 [DOI] [PubMed] [Google Scholar]
- 42.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75(3):519-560. doi: 10.1152/physrev.1995.75.3.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Resnick N, Yahav H, Shay-Salit A, et al. Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol. 2003;81(3):177-199. doi: 10.1016/S0079-6107(02)00052-4 [DOI] [PubMed] [Google Scholar]
- 44.Pantos J, Efstathopoulos E, Katritsis DG. Vascular wall shear stress in clinical practice. Curr Vasc Pharmacol. 2007;5(2):113-119. doi: 10.2174/157016107780368253 [DOI] [PubMed] [Google Scholar]
- 45.Thondapu V, Bourantas CV, Foin N, Jang IK, Serruys PW, Barlis P. Biomechanical stress in coronary atherosclerosis: emerging insights from computational modelling. Eur Heart J. 2017;38(2):81-92. [DOI] [PubMed] [Google Scholar]
- 46.Han D, Starikov A, Ó Hartaigh B, et al. Relationship between endothelial wall shear stress and high-risk atherosclerotic plaque characteristics for identification of coronary lesions that cause ischemia: a direct comparison with fractional flow reserve. J Am Heart Assoc. 2016;5(12):e004186. doi: 10.1161/JAHA.116.004186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park JB, Choi G, Chun EJ, et al. Computational fluid dynamic measures of wall shear stress are related to coronary lesion characteristics. Heart. 2016;102(20):1655-1661. doi: 10.1136/heartjnl-2016-309299 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Additional Patient Characteristics Between ACS vs Control
eTable 2. CCTA Lesion Characteristics According to the Number of AGCs in Patients With ACS
eTable 3. Univariable and Multivariable Cox Proportional Hazards Regression Analysis of AGCs for Risk of Culprit Lesions
eTable 4. Multivariable Cox Proportional Hazards Regression Analysis of AGCs for Risk of Culprit Lesions (Patients With ACS vs Controls)
eTable 5. Clinical and CCTA Characteristics in Analyzed (n = 116) and Excluded (n=118) Patients With ACS From the ICONIC Study
eFigure 1. Patient Selection and Study Design
eFigure 2. Illustrations of Adverse Geometric Characteristics
eFigure 3. Lesion Curve Assessment Using Semiautomated Software
eFigure 4. Propensity Score Distribution of the 116 Case and 116 Control Pairs