Abstract
There is an ever-growing need for new cancer diagnostic approaches that provide earlier diagnosis as well as richer diagnostic, prognostic, and resistance information. Extracellular vesicles (EVs) recovered from a liquid biopsy have paradigm-shifting potential to offer earlier and more complete diagnostic information in the form of a minimally invasive liquid biopsy. However, much remains unknown about EVs, and current analytical approaches are unable to provide precise information about the contents and source of EVs. New approaches have emerged to analyze EVs at the single particle level, providing the opportunity to study biogenesis, correlate markers for higher specificity, and connect EV cargo with the source or destination. In this critical review we describe and analyze methods for single EV analysis that have emerged over the last five years. In addition, we note that current methods are limited in their adoption due to cost and complexity and we offer opportunities for the research community to address this challenge
1. Extracellular vesicles are a rich source of cancer biomarkers
While it is widely recognized that early diagnosis of cancer can save a tremendous number of lives, standard diagnostic methods continue to rely on palpations, symptoms, imaging, and tissue biopsies. However, symptoms can be confounding, imaging resolution is not sufficient for early diagnosis, and biopsies are invasive and, in the case of some tissues, impractical or impossible. Despite numerous advances in cancer treatment, cancer death rates continue to remain relatively flat [1] due to the lack of early diagnostic approaches to identify cancer while it is treatable, to identify the most appropriate treatment for the specific cancer, and to monitor cancer for treatment-induced changes. To finally make progress toward a significant reduction in the number of cancer deaths, it is necessary to develop new approaches for diagnostics that enable early detection and that provide a detailed signature of the cancer without the need for invasive biopsies.
Over the last decade, the liquid biopsy has been increasingly explored for early cancer diagnosis and as a source for richer information than current methods. A liquid biopsy is a patient sample drawn from biological fluids (blood, saliva, urine, cerebrospinal fluid, etc.) that provides similar diagnostic and prognostic information as a solid-tissue-based biopsy [2]. Components within liquid biopsies that may serve as biomarkers include proteins, cell-free DNA, circulating tumor cells, platelets, and extracellular vesicles [3]. Studies have shown that liquid biopsies may provide information beyond an initial diagnosis, including the dynamics and heterogeneity of tumors, the emergence of therapy resistance, and disease recurrence [4]. Moreover, by utilizing methods in artificial intelligence to analyze the information derived from liquid biopsy samples, a single sample may be analyzed for dozens of biomarkers in search of a wide range of cancers [5].
Potentially the richest biomarker space in the liquid biopsy paradigm is extracellular vesicles (EVs), as their distribution throughout the body plays an active role in the initiation and the spread of cancer. EVs are sub-micron particles with a lipid bilayer membrane that contain membrane proteins and nucleic acids from the host cell. They include three types of particles: (1) exosomes, 40 – 120 nm particles generated by intra-luminal budding of multivesicular bodies, (2) microvesicles, 50 – 1000 nm particles generated by budding outward from the cell surface, and (3) apoptotic bodies, 500 – 2000 nm particles generated by blebbing of an apoptotic cell membrane [6]. Because EVs contain their components within a protective lipid bilayer, they are a highly effective means of cell-cell molecular transport through the body’s circulation. EVs deliver cargo to recipient cells by merging their membrane contents into the cell plasma membrane and delivering the encapsulated components, which include transcription factors, oncogenes, microRNAs (miRNAs), and mRNAs [6]. Notably, the transport of mRNA and miRNA via EVs enables a cell to induce and regulate transcription in a distant cell [7]. The differences between exosomes and microvesicles are exhibited in their cargo, as the larger size of microvesicles implies significantly more cargo (a 5X increase in radius implies a 25X larger surface area and a 125X larger volume). Additionally, because microvesicles are generated by budding, they tend to better represent the originating cell.
EVs are an excellent source for diagnostic biomarkers because of the profound role that they play in cancer. In fact, they may serve as early diagnostic markers as well as markers for disease state and resistance because of their involvement at every stage and in every aspect of cancer biology. EVs have been shown to enable immune escape, to promote tumor growth, to stimulate angiogenesis, to drive matrix remodeling, to generate the formation of the pre-metastatic niche, and to promote metastasis [6-9].
Through multiple mechanisms, EVs promote tumor initiation and metastasis by immunomodulation. It has been demonstrated that cancer exosomes can decrease immune cell proliferation and maturation [8-13], enabling tumor cells to evade an immune response. In addition, reports show that exosome signaling activates tumor-associated macrophages, which are known to create a tumor permissive and promoting niche [14,15]. Similarly, EVs promote the development of a tumor-supportive microenvironment. Specifically, exosomes have been shown to activate fibroblasts, which secrete growth factors, chemokines, and ECM components [16]. Exosomes have also been shown to promote angiogenesis, as hypoxic conditions promote exosomal communications with endothelial cells to induce new blood vessel growth [17,18].
EVs have also been shown to promote the metastatic spread of cancer to distant sites in the body. In fact, EVs have been shown to play a role in every step of the complicated multi-step process of cancer metastasis. Microvesicles released by macrophages have been shown to increase the invasiveness of cancer cell lines via miRNAs [19]. Exosomes have been shown to promote degradation of the local extracellular matrix by promoting matrix metalloproteases (MMPs) [20,21], which enables tumor cells to escape the primary tumor. Reports have also demonstrated that EVs can enable tumor cells to enter and leave circulation by promoting vascular leakiness [18,22,23]. Finally, EVs from a primary tumor are known to prepare distant sites for metastasis by creating a pre-metastatic niche [24-26], which includes cell recruitment [27], vascularization, and ECM deposition.
Because EVs have numerous roles in the initiation and spread of cancer, it is unsurprising that EVs represent a rich set of potential cancer biomarkers, including proteins and nucleic acids. Recent reviews on EVs as cancer biomarkers have compiled extensive lists of biomarker molecules that are reported to have been found in EVs [28-31]. Table S1 in the Electronic Supplementary Material (ESI) combines the markers reported by Abhange et al. and Zhou et al. [28,29] and lists the cancer type associated with the marker and how the marker can be used (e.g., diagnosis, prognosis, etc.). It is noteworthy that some of markers from EVs reported in the table are markers for multiple types of cancer. For example, EpCAM is a reported marker for breast cancer [32-34], ovarian cancer [35-41], and prostate cancer [42,43], among others, while miR-21 is a reported marker for breast cancer [44,45], lung cancer [46], and pancreatic neoplasm [47], among others. Thus, while reports of single biomarkers are promising, it is likely that a panel of EV-based biomarkers will be necessary for both sensitive and specific cancer diagnosis, as well as information for prognosis and treatment efficacy [48]. As an example, Jakobsen et al. demonstrated the use of an immuno-array that identified 30 potential EV-based protein biomarkers for lung cancer, with a sensitivity of 75% and a specificity of 76% [49].
2. Single EV analysis methods will advance biomarker discovery and detection
While EVs clearly hold great promise as a source of biomarkers for cancer diagnosis and prognosis, significant challenges must be overcome before they can be broadly applied. In particular, there is high variability in the EV population generated by individual cells due to the stochastic nature of EV generation and the rarity of molecular markers within the nanometer sized particles [50-55]. In addition, a liquid biopsy sample contains EVs generated by cells all throughout the body, making it difficult to identify and study only those from a tumor [56-58]. Conventional methods for the analysis of EVs can only generate information on the bulk sample, thus making it challenging to collect information from specific EVs. Moreover, with bulk-sample-based approaches, it is impossible to establish correlations of biomarkers in EVs, such as whether a specific membrane protein and RNA sequence are correlated in a subpopulation of EVs; the membrane protein could suggest either the source cell or the destination cell for the RNA cargo. Establishing such multiplexed correlations will create diagnostic approaches with higher specificity and richer information than approaches based on a single biomarker. To achieve this, approaches that analyze EVs at the single particle level are necessary.
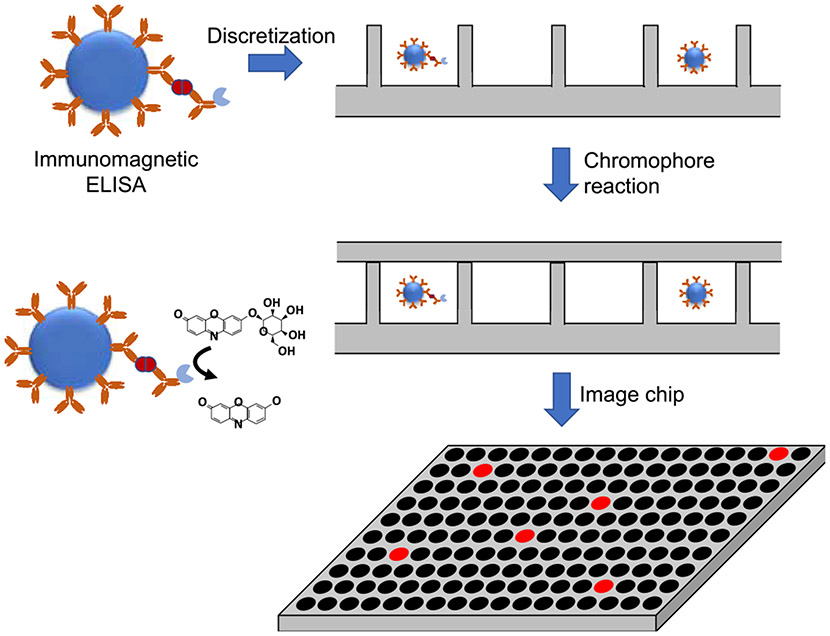
Digital analytical chemistry has rapidly emerged and has been commercialized over the last twenty years for both protein and nucleic acid biomarkers. In the case of protein biomarkers, David Walt pioneered single protein detection in which proteins are distributed across a high-density array of microscale wells, enabling detection of single protein molecules [59-61] (Fig. 1). First, protein markers are captured onto immunomagnetic beads, which are then “digitized,” meaning that they are distributed into an array of femtoliter- or picoliter-scale wells such that each well stochastically contains either one or zero bead(s) (or, more accurately, beads are distributed across the wells with a Poisson distribution with a mean of much less than one particle per well). Peroxidase labeled antibodies bound to the biomarker oxidize a redox active fluorophore in the reaction well, generating numerous fluorescent molecules for each biomarker protein. Because of the small volume of the reaction well, the fluorophores are confined to a small cross-section, thus increasing the optical intensity of the fluorescence. This enables detection of single protein molecules. The method has been commercialized as the Quanterix Simoa; reports in the literature demonstrate attomolar detection of specific proteins, orders of magnitude lower than an ELISA [62].
Fig. 1.
Digital ELISA. Protein biomarkers are captured and labeled with an immunoassay on magnetic beads. The sample is discretized into microwells with a fluorogenic substrate. Microwells that contain a protein biomarker molecule fluoresce, enabling counting.
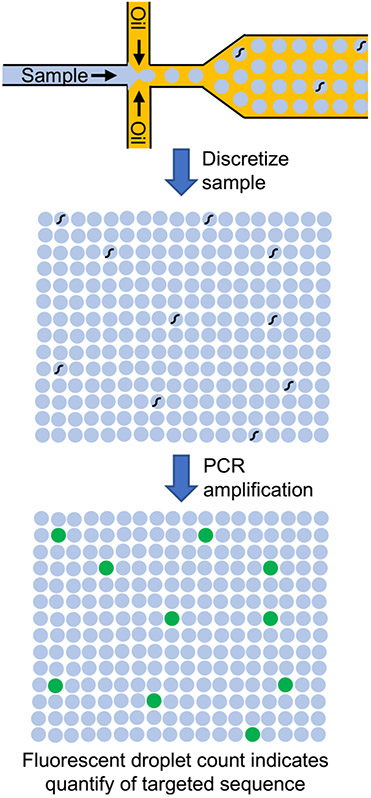
Digital confinement has also been applied for the detection of single nucleic acid molecules. In digital PCR (FIG. 2), a sample containing nucleic acids is segmented into small droplets primarily containing either zero copies or one copy of the nucleic acid due to stochastic confinement. The exact number of copies of the targeted sequence can then be determined by counting the number of fluorescent dots after amplification, as opposed to using a standard curve, which is typically inaccurate at low copy numbers. Digital microfluidic PCR first began to emerge a decade ago using chips that discretized the aqueous sample in oil using microfluidics [63,64]. One report has shown that up to one million droplets can be generated and analyzed [65], enabling a wide dynamic range of quantification. An alternative to droplet microfluidics for digitization is to use elastomeric valves to discretize the sample into tens of thousands of sample wells [66]. To date, digital droplet PCR (ddPCR) has been the most common approach and has been commercialized by multiple companies.
Fig. 2.
Droplet digital PCR. A sample containing DNA is discretized, along with PCR reagents, into aqueous droplets within an oil phase. The droplets are thermally cycled to enable PCR. Droplets that contained a targeted DNA sequence fluoresce.
Single EV analysis can draw inspiration from the progress on digital protein and nucleic acid analytics. In this review, we identify and describe the methods that have been reported for single EV analysis. The methods, which have all emerged within the last five years, include analysis of protein biomarkers, RNA biomarkers, and in some cases, both within the same EV. Single EV analysis is also discussed in other recent reviews, though generally these articles briefly discuss single EV analysis within a broader context or do not contain a critical analysis of the reported methods [67,68]. The review presented here offers more depth on the methods for single EV detection, alongside a critical analysis of each and opportunities for outstanding challenges in the field to be addressed. While this review demonstrates that significant progress has been made, further work is needed, in particular in the areas of multiplexing as well as in cost and ease of use to enable wide adoption. Nonetheless, we believe that the potential exists to begin applying the methods reported here to better understand the characteristics of EVs and their signatures.
3. Advances in methods for single EV analysis
3.1. Digital PCR
With minor adjustments, digital PCR has been used to amplify and identify the presence of RNA found in single extracellular vesicles. Currently, many applications involving digital PCR for EV analysis lyse the EVs and recover the nucleic acids prior to discretizing the sample into the droplets or wells [69-74]. Applications have targeted miRNA [71,74] (including miR-21, miR-16, miR-10b, and let7a, Table S1, ESI) and mRNA [70,73] (including cancer markers PIK3CA, KRAS, and BRAF, Table S1, ESI, and stroke markers vFOS, VCAN, PLBD1, MMP9, and CA4). Wijerathne et al. utilized ddPCR following microfluidic immuno-enrichment of CD-8(+) EVs, thus correlating the detected RNA sequences with the EV surface marker [73]. Commercial digital PCR systems have been typically used, including the Droplet Digital PCR System from BioRad or the QuantStudio 3D digital PCR system from ThermoFisher. However, because these reported methods first lysed EVs to release RNA into a single sample, they are essentially performing a population-based analysis on EV content as opposed to assessing contents one EV at a time.
One group has shown that EVs can be sequestered in the droplets prior to lysing, enabling the detection of nucleic acid biomarkers from a single EV [75]. Using the ddPCR system from BioRad to digitize EVs into droplets, Pasini et al. detected EGFR mutations (specifically L858R and T790M) in vesicular RNA. The authors argue that their EV-based RNA analysis is an improvement over the detection of circulating tumor DNA, demonstrating 90% sensitivity for the detection of mutations.
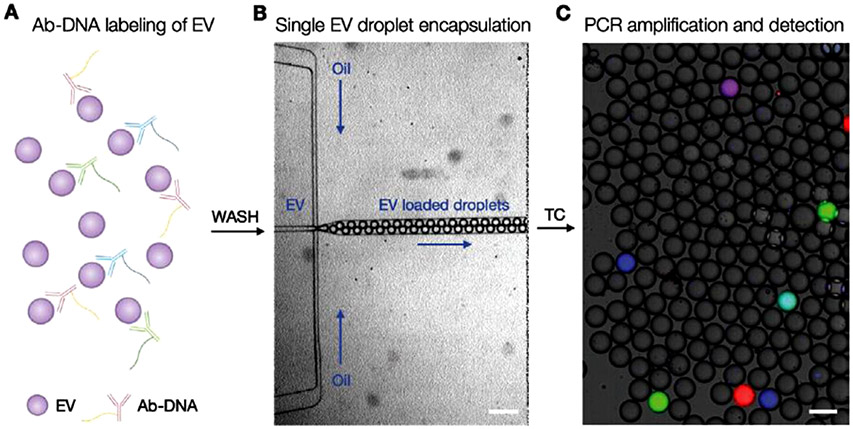
While digital PCR is intended for the amplification of nucleic acid sequences, and thus would seem to only be applicable for RNA cargo in EVs, Ko et al. leveraged the digitization and amplification power to detect membrane proteins on single EVs [50] (Fig. 3). EVs were labeled with DNA-tagged antibodies prior to encapsulation in the droplets. The authors constructed a custom droplet-generating system to introduce both the EVs and the PCR master mix into the droplets. A single antibody/probe combination was used to detect a single surface protein. To multiplex, different sequences were conjugated to differing antibodies, and multiple fluorescent probes were used to identify the specific sequences (and therefore the corresponding protein) present. As a proof of principle, the authors expanded the method to three targets to detect granzyme B, transcription factor 7, and PD-L1, three immune markers. This powerful approach is able to identify the presence of multiple coinciding membrane proteins on single EVs, thus providing richer data and more specific diagnostic information.
Fig. 3.
Digital PCR for the multiplexed detection of protein biomarkers on single EVs. (A) DNA-tagged antibodies bind to membrane proteins on the surface of EVs. (B) The EVs are then discretized using microfluidics. (C) Positive PCR of specific nucleic acid sequences is detected in the form of fluorescent droplets. Image reprinted from Ko et al. [50], copyrighted the authors and published by Wiley.
To further expand their single EV surface protein analysis, Ko et al. utilized DNA-barcoded beads [56]. The DNA sequence attached to the bead contains a bead barcode sequence, a unique molecular identifier, and a complement to the DNA that is tagged onto the antibody. The sequence on the antibody has a complementary sequence to the bead DNA, a barcode unique to the target of the antibody, and a T7 promoter region for RNA transcription. Labeled EVs, along with the DNA-bead complex, were sequestered into droplets for single EV analysis. For sequence detection following discretization, a DNA sequencing method was utilized. The sequencing begins with in vitro RNA transcription, followed by DNase treatment and RNA purification. Reverse transcription then converts the RNA to cDNA, which is then amplified by PCR for sequencing. Rather than a fluorescent readout as in their original protocol, this method relies on sequencing of the final product for identification, removing the limit placed on multiplexing by overlapping fluorescence emissions.
Tian et al. [76] demonstrated a digital detection method for the presence of EVs as well as for surface biomarkers found on those EVs. In this method, a biocompatible anchor molecule (BAM) is conjugated to DNA, then used to label EVs. The BAM inserts into the membrane and attaches the bound DNA to the EV. EVs are then labeled with DNA-tagged antibodies for human glypican 1 (GPC-1, Table S1, ESI). After labeling with both the BAM-DNA general exosome marker and the GPC-1 antibodies, the EVs are rinsed and excess DNA labels are removed. The EVs are then discretized into microchambers for single EV analysis. Isothermal amplification (instead of the thermal cycling used in PCR) was then used for the detection of both the BAM-DNA EV markers and the GPC-1 antibodies. A ROX probe was used for the BAM-DNA, while FAM was used for the GPC-1 antibody. Red fluorescence indicated the presence of an EV in the chamber, while red and green fluorescence indicated the presence of GPC-1. By imaging a large number of EVs, one could determine the percentage of EVs that contain the GPC-1 biomarker.
The use of digital PCR is enabling innovations and progress toward single EV analysis. In particular, discretization of EVs into droplets, followed by amplification of their RNA cargo, not only enables detection of RNA biomarkers with excellent sensitivity, but it also generates new information, such as the percentage of EVs that contain specific biomarkers. It is also possible to multiplex this method to show multiple RNA markers, however, the stochastic nature of the nanosized EVs means there is little RNA per EV, potentially making this less useful. The use of DNA-tagged antibodies in digital PCR elucidates the fraction of an EV population with specific protein biomarkers, as well as combinations of protein biomarkers on single EVs (replacing DNA-tagged antibodies with aptamers may simplify the assays, although aptamers typically have lower affinity than antibodies). In the future, combining the detection of RNA with DNA-tagged antibodies will enable the correlation of protein markers with RNA cargo, potentially enabling the determination of the source or destination of specific RNA sequences transported by EVs. Unfortunately, at the current time commercial ddPCR systems are expensive (hundreds of thousands of US dollars) while homemade systems are complicated to construct and use. These burdens significantly limit the deployment of ddPCR for more widespread use in EV analysis. Novel methods for discretization of EVs along with isothermal amplification methods are needed to enable the wider adoption of ddPCR for EV biology studies and biomarker discovery.
3.2. Digital ELISA
Just as digital PCR has been adapted for single EV analysis, the digital immunoassay methodology developed by Walt and colleagues has been applied for the detection of membrane proteins on single EVs. In a report by Liu et al., EVs are labeled with antibodies (as in a traditional ELISA assay) and are then sequestered into droplets using microfluidics for single-particle detection [77]. To show this, they used EVs from the MDA-MB-231 breast cancer cell line. EVs were captured with a CD63 antibody bound to a magnetic bead, with the ratio of EVs to beads such that a given bead will capture at most one EV (or more accurately, EVs will be distributed across the beads according to Poisson statistics in which the mean of EVs on each bead is much less than one). EVs were labeled with a biotinylated antibody for GPC-1 (Table S1, ESI), a breast cancer marker; the labeling antibody was complexed to β-galactosidase. The β-galactosidase catalyzes the reaction in which fluorescein-di-β-d-galactopyranoside, contained within each reaction droplet, produces fluorescence. Using this approach, the authors demonstrated the detection of EVs in plasma from cancer patients. While this method may be useful for single biomarker detection (and diagnostic approaches that require only a single membrane protein), the identification of multiple markers on single EVs is not yet possible with this approach.
3.3. Flow cytometry
Flow cytometry measures light scattering as single particles pass through a laser beam in sheath flow. Side scatter light gives an indication of the size of the particle, while forward scatter light indicates granularity or complexity. Specific molecules on a particle, often surface proteins, are detected with fluorescence via a fluorophore-tagged antibody bound to the target. Flow cytometry would appear to be ideal for single EV analysis, as information is collected from single particles. However, conventional flow cytometry optics and fluidics work for micron-scale particles, such as cells and microparticles, rather than the nano-sized EVs.
Shen et al. used a clever approach to overcome this limitation to enable EV analysis in conventional flow cytometers [78]. DNA aptamers were developed to form a hairpin structure that opens upon binding to the target (CD63, a common EV membrane protein, in this proof-of-principle experiment). Opening of this structure exposes the tail of the DNA sequence, which is complementary to a sequence on a second hairpin, extending the DNA further. A third hairpin is then used, with the second and third hairpins alternating sequentially to further lengthen the sequence (an amplification method referred to as hybridization chain reaction, HCR [79,80]). The non-aptamer hairpins were labeled with biotin, which was in turn bound to a streptavidin-conjugated fluorophore. The resulting particles were able to be analyzed with conventional flow cytometry instrumentation, indicating that the size enrichment due to the lengthened DNA sequences was sufficient. To multiplex this system, a second set of aptamer-hairpins and extension hairpins were designed against a human epidermal growth factor receptor 2 (HER2) target, a breast cancer marker (Table S1, ESI). The biotin-labeling of these hairpins was replaced with direct fluorophore tagging, while biotin-streptavidin fluorophore tagging was still used for CD63. The two different fluorophores were then simultaneously detected via flow cytometry. This method relies on the presence of a relatively high number of markers on a single EV, and thus may not be suitable for biomarkers with lower levels of targeted membrane protein biomarkers.
Welsh et al. artificially expanded the size of EVs by using nanoparticle tags [81]. Their “NanoTag” labels, ultimately chosen to be silver or gold nanoparticles, were selected and characterized to enable the detection of low copy numbers of markers on small particles, including EVs. The NanoTag labels are detectable through flow cytometry on their own, and the ratio of fluorescence at 488 and 561 nm observed. When attached to EVs stained with membrane-intercalating dye, the shift in this ratio of fluorescence of the NanoTags (either one or both) was observed, indicating the presence of EVs.
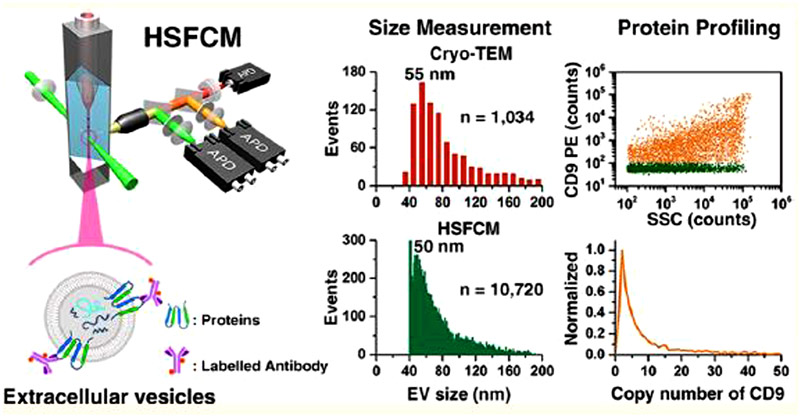
Others have advanced flow cytometry systems to enable EV analysis without artificially enlarging the vesicles. Tian et al. used flow cytometry instrumentation previously developed in the same group for the analysis of EVs [82,83] (Fig. 4). Their high-sensitivity flow cytometry instrument involves a lowered probing volume to reduce background signal. With their instrumentation, both fluorescence as well as side scatter can be detected, allowing for the analysis of both size and labeled biomarkers. For EV analysis, a third detector was added to the setup, enabling the detection and correlation of two biomarkers simultaneously, in addition to the sizing of the EVs through the side scatter light. To determine some of the subpopulations present in EVs, they analyzed the simultaneous presence of CD9/CD63, CD9/CD81, and CD63/CD81. This also demonstrates the multiplexing capability of this method. They then applied this to the detection of CD147 (Table S1, ESI), a protein shown to be overexpressed in some human tumors. The detection of CD147 from EVs collected from colorectal cancer cell lines and a normal colon fibroblast cell line, as well as EVs from human plasma in both cancer patients and healthy donors, showed an increase in CD147 in the EVs from cancer cells and patients over the normal cells and healthy patients.
Fig. 4.
Flow cytometry for the detection of membrane proteins on single EVs. Fluorophore-tagged antibodies label membrane proteins on the surface of EVs. As the EVs pass through a laser beam, scattered light is recorded for each particle, enabling multiple forms of analysis. Reprinted from [82] with permission from the American Chemistry Society, copyright 2018.
Both de Rond et al. and Arkesteijn et al. have shown improvements in the detection of side scatter and forward scatter light in flow cytometry instruments by optimizing both fluidics and optics [84,85]. Both groups use example analytes (polystyrene beads or microspheres), instead of directly analyzing EVs for optimization. Determination of parameters to allow detection of forward and side scatter from EVs, while not directly enabling detection of biomarkers, can allow for a more accurate count of total EVs and for subpopulations to be better understood.
A number of groups have attempted to optimize parameters used with imaging flow cytometry, which generates an image of the particle as it flows through the instrument, rather than only gathering data through photomultiplier tubes or other such detection systems. Ricklefs et al. optimized labeling and settings for imaging flow cytometry for the analysis of multiple biomarkers on a single EV [86], where previously it had been used for single-biomarker analysis. They illustrated this with the simultaneous detection of three tetraspanin markers commonly used to identify EVs, CD9, CD63, and CD81.
Görgens et al. similarly optimized EV labeling and instrumentation settings for the analysis of EVs with imaging flow cytometry [87]. To determine the best instrument settings for analysis of EVs, they used EVs expressing the fusion protein of CD63 and enhanced GFP (eGFP), CD63eGFP. A determination of masks to use in the data processing protocols was made by analyzing signals from increasing concentrations of EVs. These masks serve to eliminate signals from aggregates, leaving those that come from single EVs. Furthermore, CD63eGFP-EVs were identified from unprocessed cell cultures using the parameters previously determined. These parameters were used for the detection of CD63 on EVs via fluorescent-tagged antibodies, as well as multiplexed detection of CD9 and CD81.
Towards establishing a standardized protocol to enable replication and comparison of results between labs, Armitage et al. also optimized imaging flow cytometry parameters [88]. Specifically, they employed gating protocols and spot count in the methods to detect and remove swarm events, which they define to be two or more EVs illuminated simultaneously in the flow cytometer but which are detected as one event.
Millan et al. showed identification of two markers in single EVs from patient samples using imaging flow cytometry [89]. They detected GDF15, a driver of prostate cancer progression, and CD9, a common EV marker to validate that signals came from EVs. They indicate that while this method can and does show that the two proteins are present on a single EV, these EVs appear larger in the image than would be expected from TEM and NTA analysis on the same sample. They theorize that this artificially large particle image results from the presence of multiple antibody-fluorophores on a single EV, making it appear larger. While brightfield microscopy in the imaging or side scatter in the flow cytometry would enable a more accurate determination of size with this method, this is not yet feasible for particles as small as EVs, although progress is being made towards this goal as discussed above.
Although flow cytometry has typically been used to analyze surface proteins on EVs, RNA cargo also provides valuable information for biomarker detection. By detecting RNA with flow cytometry, de Oliveira et al. aimed to enable the study of EV heterogeneity to enable the detection of rare miRNA cargo [90]. They approached miRNA detection by flow cytometry by coupling molecular beacons to cell penetrating peptides to allow the beacons entry into EVs without lysing the EVs, thus enabling single EV analysis. These cell penetrating peptides are not well understood, but are short peptides known to cross biological membranes. The molecular beacons are composed of a sequence complementary to the miRNA of interest in a hairpin shape with a fluorophore and a quencher at either end. When bound to the miRNA, the hairpin opens and the fluorophore is far enough removed from the quencher to emit. Specifically, they showed a molecular beacon against miR-495 (Table S1, ESI), which is found in platelets and implicated in multiple cancers. They note, however, that low copy numbers of RNA will give a lower signal.
3.4. Nanoparticle tracking analysis
Nanoparticle tracking analysis (NTA) is one of the more common methods used in bulk analysis of EVs for the determination of concentration and size. These measurements are made by tracking individual particles and using Brownian motion to determine the radius of each individual particle. Thus, NTA is analyzing single EVs, although the information is often presented as an average in aggregate. Although EVs do not need to be fluorescently labeled for NTA particle assessment, some instruments are equipped with a fluorescence mode where such labels can be employed to detect specific biomarkers. More often, however, NTA is used in conjunction with other methods for confirmation of analysis or to give further information about a population of EVs.
Dragovic et al. reported the adaptation of the original nonfluorescent NTA to include fluorescence capabilities and thus to enable biomarker detection with immunolabeling [91]. Their proof-of-principle experiments involved labeling EVs from human plasma with NDOG2 antibodies, specific for placental vesicles, conjugated to quantum dots (QDs), and analyzing their labeling with NTA.
Thane, Davis, and Hoffman further demonstrated that immunolabeling EVs with QDs can overcome the issue of instability of fluorophores that has been shown in NTA [92]. The use of QDs, however, has proven challenging, with lowered antibody reactivity when directly conjugated to QDs, as well as the lack of cleanup protocols to remove unbound QDs from QD-labeled EVs. A number of indirect QD-labeling methods were attempted. Using CD9 as an example target, they determined that the binding of biotinylated antibodies to EVs with the subsequent addition of streptavidin QDs was most effective, as antibodies pre-conjugated to QDs had lowered reactivity with the target. They also report the use of biotinylated beads to remove excess streptavidin-QDs from solution but indicate that there is a substantial loss of EVs when using this method for QD removal and therefore it should only be used when the loss of EVs is acceptable. It is worth noting, as the authors did, that this method as proposed is not multiplexable, as it relies on the streptavidin-biotin reaction, which would ultimately result in indiscriminate tagging with QDs to any biotin-antibody-labeled target.
NTA is a relatively high-throughput method. Used in fluorescent mode, it can be a useful tool in single EV analysis for smaller-volume samples; it can determine the fraction of EVs with a particular surface marker (as labeled and unlabeled EVs are counted) and can correlate size to the presence of labeled biomarkers. However, to date, NTA is limited in multiplexing capability as compared to flow cytometry due to challenges with using fluorophores, though optical modifications to NTA instrumentation may enable the technique to leverage the multiplexing power of quantum dots.
3.5. Raman spectroscopy
Raman spectroscopy enables the identification of chemical composition by measuring the frequency scattering upon laser excitation; because particular chemical structures result in specific scattering frequencies, the measured spectrum indicates the precise molecular components within the laser excitation. Raman spectroscopy has been utilized to analyze components within single particles (e.g., cells) by trapping the particles with laser tweezers and collecting the scattered light with a Raman spectrometer [93-96]. This approach can be applied to EVs as well. Unique identification of specific protein or nucleic acid biomarkers is not possible with Raman spectroscopy as macromolecules are of similar composition. However, by using numerical analysis techniques, such as principal component analysis (PCA), on the measured spectra, researchers have been able to differentiate targeted EVs from non-targeted EVs.
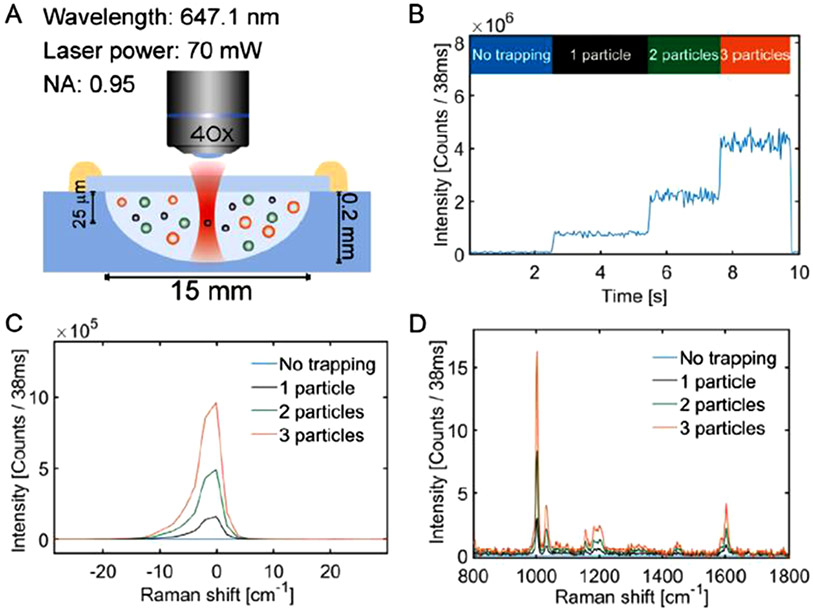
Enciso-Martinez et al. developed a method in which the presence of EVs in the trap is counted by using Rayleigh scattering [97] (Fig. 5). Upon detecting that a new particle has been trapped, the Raman spectrum collection is gated. At regular time intervals, the accumulated EVs are released from the laser trap to prevent the area from becoming too saturated. The authors used this method to distinguish tumor-derived EVs from red blood cell-derived EVs and lipoproteins of similar size by performing principal component analysis and identifying signatures found only in either group [98].
Fig. 5.
Raman spectroscopic analysis of EVs with laser trapping. (A) Laser tweezers trap EV. (B-C) Rayleigh scattering intensity indicates the trapping of a particle. (D) Raman spectra are utilized to analyze the captured EVs. Reprinted from [97] with permission from Elsevier, copyright 2020.
Kruglik et al. also demonstrated that Raman spectroscopy can distinguish differences in varying populations of EVs [99]. They studied EVs collected from rat hepatocytes, both treated and untreated with acetaminophen. The Raman spectra from different EVs from treated cells were found to be very similar, while the spectra from EVs from untreated cells had different characteristics.
Carney et al. combined fluorescence imaging with Raman spectroscopy to enable Raman analysis of a subpopulation of EVs [100]. Specifically, EVs were labeled with an anti-CD9 antibody tagged with FITC. These EVs were trapped with optical tweezers and the fluorescence was visualized with a microscope. Raman spectra of the CD9(+) EVs were then collected. They compared the spectra from the CD9(+) subpopulation to an unlabeled sample and found more heterogeneity in the unlabeled sample than those that were known to have even the one protein in common.
Another group combined brightfield microscopy, rather than fluorescence, with Raman analysis. Dai et al. studied EVs from three sources: a cancer cell line, a knockdown cancer cell line, and a normal cell line using this set up [101]. As in the previously discussed cases, they determined information about chemical groups from Raman spectroscopy. However, they also took brightfield microscopic images to determine size and refractive index of the trapped vesicles. With the information from the Raman spectra and the size, they performed principal component analysis and were able to separate the EVs into distinct subpopulations, which was not possible when the size was ignored.
While Raman spectroscopy cannot directly identify macromolecular biomarkers, labeling the biomarker with a tagged molecule that binds specifically (e.g., an antibody) works similarly to fluorescence spectroscopy methods. In this case, it is the Raman spectrum of the tag that is used to identify the presence of the biomarker. Carney et al. used this approach to show binding of a ligand LXY30 to an integrin overexpressed in tumor cells [102]. The LXY30 peptide was tagged with a Raman active molecule containing groups not typically found in EVs: a di-alkyne and an aromatic group. EVs collected from SKOV-3 ovarian tumor cells were assessed using optical trapping and Raman spectroscopy.
An alternative to laser trapping is the use of gold nanoholes, which similarly provide electromagnetic trapping. However, due to the strong optical enhancement of the gold nanostructures, the Raman signal is greatly enhanced; this phenomenon is known as surface enhanced Raman scattering (SERS). Culum et al. used this approach to distinguish between MSC EVs derived from pancreatic tissue and from bone marrow [103].
The reports summarized here demonstrate that Raman spectroscopy, when combined with electromagnetic trapping, can analyze single EVs, including the differentiation of certain populations of EVs. In addition, through the use of reporters with strong Raman scattering cross-section, biomarkers can be labeled and detected with Raman spectroscopy. However, in general, the methods are low throughput as compared to flow cytometry and will likely not yield rich information about arrays of possible biomarkers. Moreover, a combined Raman microscopy and laser tweezers system is costly and cumbersome, thus not providing advantages over other methods.
3.6. Microscopy
While all of the methods described above have used specialized techniques to view EVs, including molecular amplification, particle organization and movement by sheath flow, and laser trapping, it is possible to view EVs directly with fluorescence microscopy. Lee et al. have demonstrated a microfluidic chip-based method for single EV analysis using fluorescence microscopy (Fig. 6). EVs from a human glioblastoma model cell line were isolated, biotinylated, and immobilized within a chip by neutravidin [58]. The EVs were then stained with fluorescently-labeled antibodies for tetraspanin EV markers (CD9, CD63, and CD81) and/or glioblastoma protein markers (EGFRvIII and PDGFRα, see Table S1, ESI), then imaged with microscopy. Up to three markers can be labeled and imaged at one time with three different fluorophores. The method can be further multiplexed by quenching the fluorescence after imaging and re-labeling for other targets with repeated label-image-quench steps.
Fig. 6.
Imaging of single EVs immobilized within a microfluidic channel. EVs are captured on the channel surface and then labeled with fluorescently tagged antibodies. Reprinted from [58] with permission from the American Chemical Society, copyright 2018.
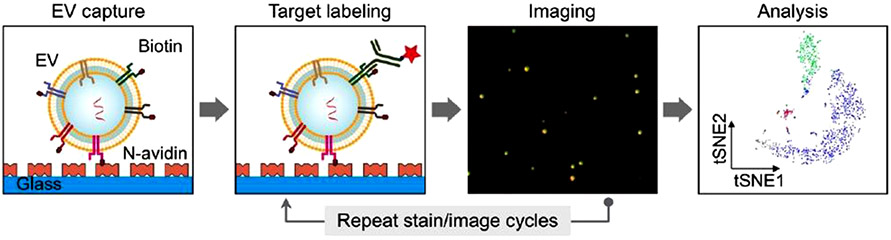
Zhou et al. have illustrated a method for simultaneous RNA and surface protein detection using total internal reflective fluorescence (TIRF) microscopy [57] (Fig. 7). In TIRF, the excitation light is coupled into the immobilization surface (typically glass), which acts as a waveguide. The evanescent field that extends slightly beyond the surface of the waveguide, where the imaging targets are immobilized, excites fluorophores within about 100 nm of the surface only; the scattered fluorescence light is collected using a microscope. With this approach, the authors developed a system called a High-throughput Nano-bio Chip Integrated System for Liquid Biopsy (HNCIB). For surface protein detection, fluorescently-labeled antibodies for CD9, CD63, and/or PD-L1 (Table S1, ESI) were used. For nucleic acid detection, the capture surface was functionalized with liposomes loaded with molecular beacon probes. The EVs merge with the liposomes, allowing the beacons to enter the EVs. They showed that both nucleic acids and proteins could be simultaneously detected by labeling both PD-L1 mRNA and PD-L1 protein in the same EVs. They also expanded this study to clinical samples. In a study that compared EVs from plasma of patients with lung cancer to those from healthy donors, miR-21 was detected at a higher level in the cancer patient EVs. The simultaneous detection of PD-L1 mRNA and protein was also performed for the two groups, with a higher level found for each in the cancer EVs.
Fig. 7.
Analysis of single EVs using TIRF. Membrane proteins are detected using fluorescently tagged antibodies. RNA sequences are detected using molecular beacon probes inside vesicles attached to the image surface; the vesicles merge with the EVs to insert the beacon probes. Image reprinted from Zhou et al. [57], copyrighted by the authors and published by AAAS.
Coupling surface plasmon resonance to microscopy can add information while also enhancing the fluorescence signal through plasmonic enhancement. Yang et al. reported the use of interferometric plasmonic microscopy (iPM) for the analysis of single EVs [104]. iPM records the adsorption of EVs onto a surface and is paired with fluorescence imaging to confirm that the signal in fact comes from EVs. They used a surface coated with CD63 antibodies to show the ability of iPM to study the binding dynamics of EVs. They observe a “hit-stay-run” pattern indicating a binding event, residence time, and dissociation.
Min et al. used a method they reported previously, nanoplasmonic exosome (nPLEX) [39], for multiplexed detection of surface biomarkers via fluorescently-labeled antibodies [105]. The antibodies are captured on a surface with gold nanoholes to enable plasmon-enhancement of the fluorescence. To illustrate this multiplexed method, they used EVs from glioblastoma cell lines that are labeled with antibodies for CD63, GAPDH, and EGFR, a marker that is frequently amplified in glioblastoma (Table S1, ESI). They showed that they were able to detect fluorescence from three channels, even with the low copy numbers of markers found on a single EV.
To enhance the fluorescence detection capabilities, Guo et al. used fluorophore-tagged DNA superstructures as labels to detect surface markers, including CD63, HER2, and EGFR, (Table S1, ESI) on EVs [106]. The EVs were labeled with an aptamer for the specific protein linked to an initiator for rolling circle amplification (RCA). RCA was then carried out, incorporating biotinylated dATP into the formed repeating sequence. The sequence is such that it folds into DNA “nanoflowers.” Streptavidin bound to a fluorophore was added to label the biotin-containing nanoflowers, while DiO dye was used to label the EV itself. The labeled EVs were imaged with confocal microscopy. Through this method, the percentage of EVs containing the marker of interest can be determined. Multiplexing with this method was performed by replacing the biotin-streptavidin labeling of the nanoflower with short fluorophore-tagged sequences complimentary to part of the repeating sequence formed by RCA.
While variations of microscopy have been demonstrated for the analysis of EVs at the single EV scale, microscopy remains a low throughput method as compared to some of the approaches described above. Even with automation, it cannot compare to digitized molecular assays or flow cytometry. It is expected that microscopy-based methods will continue to be utilized to study EVs, but not at the level of the higher throughput methods. It should be noted that while microscopy is a powerful tool for studying cells, including localizing intracellular components, it is more difficult to do so in vesicles. EVs are much smaller than cells, and thus it may be difficult to localize markers to the inside or outside of the vesicle. Further, with the lack of membrane channels and transporters that are abundant in cells, it is difficult to introduce fluorophores into EVs. The method demonstrated by Zhou et al. discussed above involves the merging of other small vesicles, loaded with molecular beacons, with the target vesicles [57]. Similarly, de Oliveira et al. used molecular beacons attached to cell penetrating peptides in their flow cytometric method [90]. These methods are useful for strategies such as molecular beacons, but are impractical for approaches such as immunostaining, where washing of unbound fluorophore cannot occur.
4. Comparison and outlook
The methods for single EV analysis described in Section 3 are summarized in Table 1 below. They are compared for their ability to detect protein and RNA biomarkers, the ability to detect multiple markers (which is critical for improving specificity), the degree of throughput, and the cost. In our assessment, digital PCR and flow cytometry appear to be the most likely candidates to emerge as the standards for single EV analysis, first for biomarker discovery, and then for diagnostics. This is based on their ability to simultaneously detect multiple markers on single EVs, including both proteins and RNA. At this point, flow cytometry appears more poised to move the field forward, as discretization of EVs prior to lysis and multiplexed analysis with digital PCR is currently rare in the literature.
Table 1.
Comparison of methods for single EV analysis.
| Method | Protein, RNA, both | Multiplexing | Throughput | Cost |
|---|---|---|---|---|
| Digital PCR | Both | Yes | Moderate | $ $ $ |
| Digital ELISA | Protein | No | Moderate | $ $ |
| Flow cytometry | Both | Yes | High | $ $ $ |
| NTA | Both | No | High | $ $ |
| Raman | None | No | Low | $ $ $ |
| Microscopy | Both | Yes | Low | $ $ |
At the current time, however, flow cytometry and digital PCR have limited penetration in research labs around the globe. This is primarily due to cost, as both technologies cost in the hundreds of thousands of US dollars. Meanwhile, homemade replicates are challenging to produce and complex to operate. Innovations in microfluidics may redirect this trend, however. Significant progress has been made on the implementation of flow cytometry in microfluidic chips [107,108], including imaging flow cytometry [109]. Commercialization and standardization of the technology may eliminate the need for large equipment purchases to analyze EVs with flow cytometry. With regards to digital PCR, the use of droplet-based discretization is complex and expensive to implement within an easy-to-use commercial system. Advances in microfluidics that enable low-cost self-discretization of samples [110,111] may hold the key to low-cost digital PCR systems. Advances that enable rapid droplet sorting may improve the capabilities of both technologies. Lim and Abate recently demonstrated a rapid microfluidic droplet sorting sample preparation chip for flow cytometry that could process 104 droplets per second [112].
As new systems emerge that enable widespread implementation of single EV analysis, we anticipate that research on EV-based biomarkers for liquid biopsy will intensify dramatically. Initially, the most critical advances will be related to the discovery of new biomarkers, or more likely, combinations of molecular markers that can provide highly specific diagnostic information. As new discoveries are made, the emphasis may begin to shift to the adaptation of EV analysis systems toward diagnostic applications. It is likely that EV-based diagnostics in liquid biopsies will suffer from the challenge of the needle-in-a-haystack problem, and thus high-throughput systems that can analyze EVs at the single EV level will be needed. To supplement this, we expect that the application of machine learning algorithms may be necessary to deduce diagnostic information from the wealth of data collected.
5. Conclusion
New approaches for early cancer diagnostics are necessary to change the continuing flat trajectory of annual cancer deaths. Extracellular vesicles within liquid biopsies may be the richest source of diagnostic information because of the significant role that they play in the initiation, growth, and spread of cancer. However, current population-based – i.e., bulk sample-based – methods fail to generate the necessary specificity of information for biomarker discovery. Analysis of EVs at the single particle level may hold the key to the establishment of relevant diagnostic biomarkers. Over the last decade, several new methods for single EV analysis have been reported in the literature, including digital assays, flow cytometry, nanoparticle tracking analysis, Raman spectroscopy, and microscopy-based methods. Digital PCR and flow cytometry provide high throughput readouts and are capable of multiplexing, including simultaneous detection of protein and RNA biomarkers. However, to date, both methods are costly. Further advances in these technologies may lead to wide adoption by the global research community, which is expected to accelerate the discovery of cancer biomarkers and to lead to new opportunities to diagnose cancer at earlier stages, to provide more accurate prognostic information, and to monitor the response of cancer to treatment.
Supplementary Material
Acknowledgments
The authors acknowledge funding from the National Institute of General Medical Sciences (R01AI166417) and the National Cancer Institute (T32CA154274).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.snr.2021.100052.
References
- [1].CDC, Cancer Statistics At a Glance. https://gis.cdc.gov/Cancer/USCS/#/AtAGlance/(accessed August 8, 2021).
- [2].Pantel K, Alix-Panabières C, Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res 73 (2013) 6384–6388, 10.1158/0008-5472.CAN-13-2030. [DOI] [PubMed] [Google Scholar]
- [3].Chi KR, The Tumour Trail Left in Blood, Nature 532 (2016) 569–571. [DOI] [PubMed] [Google Scholar]
- [4].Heitzer E, Haque IS, Roberts CES, Speicher MR, Current and future perspectives of liquid biopsies in genomics-driven oncology, Nat. Rev. Genet 20 (2019) 71–88, 10.1038/s41576-018-0071-5. [DOI] [PubMed] [Google Scholar]
- [5].Klein EA, Richards D, Cohn A, Tummala M, Lapham R, Cosgrove D, Chung G, Clement J, Gao J, Hunkapiller N, Jamshidi A, Kurtzman KN, Seiden MV, Swanton C, Liu MC, Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set, Ann. Oncol xxx (2021), 10.1016/j.annonc.2021.05.806. [DOI] [PubMed] [Google Scholar]
- [6].El Andaloussi S, Mäger I, Breakefield XO, Wood MJA, Extracellular vesicles: biology and emerging therapeutic opportunities, Nat. Rev. Drug Discov 12 (2013) 347–357, 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- [7].Colombo M, Raposo G, Théry C, Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles, Annu. Rev. Cell Dev. Biol 30 (2014) 255–289, 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- [8].Zhang HG, Grizzle WE, Exosomes and cancer: a newly described pathway of immune suppression, Clin. Cancer Res 17 (2011) 959–964, 10.1158/1078-0432.CCR-10-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ruivo CF, Adem B, Silva M, Melo SA, The biology of cancer exosomes: insights and new perspectives, Cancer Res 77 (2017) 6480–6488, 10.1158/0008-5472.CAN-17-0994. [DOI] [PubMed] [Google Scholar]
- [10].Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, Yang Y, Wang L, Cao X, Wang J, Activated T cell exosomes promote tumor invasion via fas signaling pathway, J. Immunol 188 (2012) 5954–5961, 10.4049/jimmunol.1103466. [DOI] [PubMed] [Google Scholar]
- [11].Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, Min WP, Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis, Blood Cells, Mol. Dis 35 (2005) 169–173, 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- [12].Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Kappes JC, Barnes S, Kimberly RP, Grizzle WE, Zhang H-G, Murine Mammary Carcinoma Exosomes Promote Tumor Growth by Suppression of NK Cell Function, J. Immunol 176 (2006) 1375–1385, 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- [13].Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, Grizzle WE, Falkson C, Zhang H-G, Tumor exosomes inhibit differentiation of bone marrow dendritic cells, J. Immunol 178 (2007) 6867–6875, 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- [14].Noy R, Pollard JW, Tumor-associated macrophages: from mechanisms to therapy, Immunity 41 (2014) 49–61, 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z, Huang W, Ngo V, Kortylewski M, Wang SE, Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κ B, Sci. Rep (2014) 4, 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Webber J, Steadman R, Mason MD, Tabi Z, Clayton A, Cancer exosomes trigger fibroblast to myofibroblast differentiation, Cancer Res 70 (2010) 9621–9630, 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- [17].Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain E, Bengzon J, Belting M, Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development, Proc. Natl. Acad. Sci. U.S.A 110 (2013) 7312–7317, 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, Hu J, Zhu X, Yang W, Liao W, Li G, Ding Y, Liang L, Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis, Nat. Commun 9 (2018), 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang M, Chen J, Yu B, Su F, Lin L, Liu Y, Huang J-D, Song E, Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells, Mol. Cancer 10 (2011) 6–10, http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L51634956 http://www.molecular-cancer.com/content/10/1/117 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM, Exosome secretion is enhanced by invadopodia and drives invasive behavior, Cell Rep 5 (2013) 1159–1168, 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mu W, Rana S, Zöller M, Host matrix modulation by tumor exosomes promotes motility and invasiveness, Neoplasia (United States) 15 (2013) 875–887, 10.1593/neo.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar CM, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D, Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET, Nat. Med 18 (2012) 883–891, 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O’Connor STF, Chin AR, Yen Y, Wang Y, Marcusson EG, Chu P, Wu J, Wu X, Li AX, Li Z, Gao H, Ren X, Boldin MP, Lin PC, Wang SE, Cancer-Secreted miR-105 destroys vascular endothelial barriers to promote metastasis, Cancer Cell 25 (2014) 501–515, 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wortzel I, Dror S, Kenific CM, Lyden D, Exosome-Mediated Metastasis: communication from a Distance, Dev. Cell 49 (2019) 347–360, 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- [25].Hood JL, San Roman S, Wickline SA, Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis, Cancer Res 71 (2011) 3792–3801, 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- [26].Li F, Tiede B, Massagué J, Kang Y, Beyond tumorigenesis: cancer stem cells in metastasis, Cell Res 17 (2007) 3–14, 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- [27].Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Jørgen Labori K, Kure EH, Grandgenett PM, Hollingsworth MA, De Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D, Tumour exosome integrins determine organotropic metastasis, Nature 527 (2015) 329–335, 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Abhange K, Makler A, Wen Y, Ramnauth N, Mao W, Asghar W, Wan Y, Small extracellular vesicles in cancer, Bioact. Mater 6 (2021) 3705–3743, 10.1016/j.bioactmat.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou S, Yang Y, Wu Y, Liu S, Review: multiplexed profiling of biomarkers in extracellular vesicles for cancer diagnosis and therapy monitoring, Anal. Chim. Acta 1175 (2021), 338633, 10.1016/j.aca.2021.338633. [DOI] [PubMed] [Google Scholar]
- [30].Conti I, Simioni C, Varano G, Brenna C, Costanzi E, Neri LM, MicroRNAs Patterns as Potential Tools for Diagnostic and Prognostic Follow-Up in Cancer Survivorship, Cells 10 (2021) 2069, 10.3390/cells10082069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chang YC, Chan MH, Li CH, Fang CY, Hsiao M, Chen CL, Exosomal components and modulators in colorectal cancer: novel diagnosis and prognosis biomarkers, Biomedicines 9 (2021) 1–18, 10.3390/biomedicines9080931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu C, Zhao J, Tian F, Chang J, Zhang W, Sun J, I-DNA- A nd, Aptamer-Mediated Sorting and Analysis of Extracellular Vesicles, J. Am. Chem. Soc 141 (2019) 3817–3821, 10.1021/jacs.9b00007. [DOI] [PubMed] [Google Scholar]
- [33].Chen C, Zong S, Liu Y, Wang Z, Zhang Y, Chen B, Cui Y, Profiling of Exosomal Biomarkers for Accurate Cancer Identification: combining DNA-PAINT with Machine- Learning-Based Classification, Small 15 (2019) 1–9, 10.1002/smll.201901014. [DOI] [PubMed] [Google Scholar]
- [34].Kwizera EA, O’Connor R, Vinduska V, Williams M, Butch ER, Snyder SE, Chen X, Huang X, Molecular detection and analysis of exosomes using surface-enhanced Raman scattering gold nanorods and a miniaturized device, Theranostics 8 (2018) 2722–2738, 10.7150/thno.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhang P, Zhou X, Zeng Y, Multiplexed immunophenotyping of circulating exosomes on nano-engineered ExoProfile chip towards early diagnosis of cancer, Chem. Sci 10 (2019) 5495–5504, 10.1039/c9sc00961b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang P, He M, Zeng Y, Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating, Lab Chip 16 (2016) 3033–3042, 10.1039/c6lc00279j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhao Z, Yang Y, Zeng Y, He M, A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis, Lab Chip 16 (2016) 489–496, 10.1039/c5lc01117e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Park J, Im H, Hong S, Castro CM, Weissleder R, Lee H, Analyses of Intravesicular Exosomal Proteins Using a Nano-Plasmonic System, ACS Photonics 5 (2018) 487–494, 10.1021/acsphotonics.7b00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Im H, Shao H, Il Park Y, Peterson VM, Castro CM, Weissleder R, Lee H, Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor, Nat. Biotechnol 32 (2014) 490–495, 10.1038/nbt.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jeong S, Park J, Pathania D, Castro CM, Weissleder R, Lee H, Integrated Magneto-Electrochemical Sensor for Exosome Analysis, ACS Nano 10 (2016) 1802–1809, 10.1021/acsnano.5b07584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhou S, Hu T, Zhang F, Tang D, Li D, Cao J, Wei W, Wu Y, Liu S, Integrated microfluidic device for accurate extracellular vesicle quantification and protein markers analysis directly from human whole blood, Anal. Chem 92 (2020) 1574–1581, 10.1021/acs.analchem.9b04852. [DOI] [PubMed] [Google Scholar]
- [42].Sunkara V, Kim CJ, Park J, Woo HK, Kim D, Ha HK, Kim MH, Son Y, Kim JR, Cho YK, Fully automated, label-free isolation of extracellular vesicles from whole blood for cancer diagnosis and monitoring, Theranostics 9 (2019) 1851–1863, 10.7150/thno.32438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jin D, Yang F, Zhang Y, Liu L, Zhou Y, Wang F, Zhang GJ, ExoAPP: exosome-Oriented, Aptamer Nanoprobe-Enabled Surface Proteins Profiling and Detection, Anal. Chem 90 (2018) 14402–14411, 10.1021/acs.analchem.8b03959. [DOI] [PubMed] [Google Scholar]
- [44].Wang H, He D, Wan K, Sheng X, Cheng H, Huang J, Zhou X, He X, Wang K, In situ multiplex detection of serum exosomal microRNAs using an all-in-one biosensor for breast cancer diagnosis, Analyst 145 (2020) 3289–3296, 10.1039/d0an00393j. [DOI] [PubMed] [Google Scholar]
- [45].Lee JH, Kim JA, Jeong S, Rhee WJ, Simultaneous and multiplexed detection of exosome microRNAs using molecular beacons, Biosens. Bioelectron 86 (2016) 202–210, 10.1016/j.bios.2016.06.058. [DOI] [PubMed] [Google Scholar]
- [46].Yang Y, Kannisto E, Yu G, Reid ME, Patnaik SK, Wu Y, An immuno-biochip selectively captures tumor-derived exosomes and detects Exosomal RNAs for cancer diagnosis, ACS Appl. Mater. Interfaces 10 (2018) 43375–43386, 10.1021/acsami.8b13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Goto T, Fujiya M, Konishi H, Sasajima J, Fujibayashi S, Hayashi A, Utsumi T, Sato H, Iwama T, Ijiri M, Sakatani A, Tanaka K, Nomura Y, Ueno N, Kashima S, Moriichi K, Mizukami Y, Kohgo Y, Okumura T, An elevated expression of serum exosomal microRNA-191, - 21, −451a of pancreatic neoplasm is considered to be efficient diagnostic marker, BMC Cancer 18 (2018) 1–11, 10.1186/s12885-018-4006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shao H, Chung J, Lee K, Balaj L, Min C, Carter BS, Hochberg FH, Breakefield XO, Lee H, Weissleder R, Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma, Nat. Commun 6 (2015) 1–9, 10.1038/ncomms7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jakobsen KR, Paulsen BS, Bæk R, Varming K, Sorensen BS, Jørgensen MM, Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma, J. Extracell. Vesicles 4 (2015) 1–10, 10.3402/jev.v4.26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ko J, Wang Y, Carlson JCT, Marquard A, Gungabeesoon J, Charest A, Weitz D, Pittet MJ, Weissleder R, Single extracellular vesicle protein analysis using immuno-droplet digital polymerase chain reaction amplification, Adv. Biosyst 4 (2020) 1–8, 10.1002/adbi.201900307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Contreras-Naranjo JC, Wu HJ, Ugaz VM, Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine, Lab Chip 17 (2017) 3558–3577, 10.1039/c7lc00592j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Torrano V, Royo F, Peinado H, Loizaga-Iriarte A, Unda M, Falcón-Perez JM, Carracedo A, Vesicle-MaNiA: extracellular vesicles in liquid biopsy and cancer, Curr. Opin. Pharmacol 29 (2016) 47–53, 10.1016/j.coph.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Palma J, Yaddanapudi SC, Pigati L, Havens MA, Jeong S, Weiner GA, Weimer KME, Stern B, Hastings ML, Duelli DM, MicroRNAs are exported from malignant cells in customized particles, Nucleic Acids Res 40 (2012) 9125–9138, 10.1093/nar/gks656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN, Pogosova-Agadjanyan EL, Morrissey C, Stirewalt DL, Hladik F, Yu EY, Higano CS, Tewari M, Quantitative and stoichiometric analysis of the microRNA content of exosomes, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 14888–14893, 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wei Z, Batagov AO, Schinelli S, Wang J, Wang Y, El Fatimy R, Rabinovsky R, Balaj L, Chen CC, Hochberg F, Carter B, Breakefield XO, Krichevsky AM, Coding and noncoding landscape of extracellular RNA released by human glioma stem cells, Nat. Commun 8 (2017), 10.1038/s41467-017-01196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ko J, Wang Y, Sheng K, Weitz DA, Weissleder R, Sequencing-based protein analysis of single extracellular vesicles, ACS Nano 15 (2021) 5631–5638, 10.1021/acsnano.1c00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhou J, Wu Z, Hu J, Yang D, Chen X, Wang Q, Liu J, Dou M, Peng W, Wu Y, Wang W, Xie C, Wang M, Song Y, Zeng H, Bai C, High-throughput single-EV liquid biopsy: rapid, simultaneous, and multiplexed detection of nucleic acids, proteins, and their combinations, Sci. Adv 6 (2020) 1–14, 10.1126/sciadv.abc1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lee K, Fraser K, Ghaddar B, Yang K, Kim E, Balaj L, Chiocca EA, Breakefield XO, Lee H, Weissleder R, Multiplexed profiling of single extracellular vesicles, ACS Nano 12 (2018) 494–503, 10.1021/acsnano.7b07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Walt DR, Protein measurements in microwells, Lab Chip 14 (2014) 3195–3200, 10.1039/c4lc00277f. [DOI] [PubMed] [Google Scholar]
- [60].Michael KL, Taylor LC, Schultz SL, Walt DR, Randomly ordered addressable high-density optical sensor arrays, Anal. Chem 70 (1998) 1242–1246, 10.1021/ac971343r. [DOI] [PubMed] [Google Scholar]
- [61].Blicharz TM, Siqueira WL, Helmerhorst EJ, Oppenheim FG, Wexler PJ, Little FF, Walt DR, Fiber-optic microsphere-based antibody array for the analysis of inflammatory cytokines in saliva, Anal. Chem 81 (2009) 2106–2114, 10.1021/ac802181j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L, Piech T, Patel PP, Chang L, Rivnak AJ, Ferrell EP, Randall JD, Provuncher GK, Walt DR, Duffy DC, Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations, Nat. Biotechnol 28 (2010) 595–599, 10.1038/nbt.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Beer NR, Hindson BJ, Wheeler EK, Hall SB, Rose KA, Kennedy IM, Colston BW, On-chip, real-time, single-copy polymerase chain reaction in picoliter droplets, 79 (2007) 8471–8475. [DOI] [PubMed] [Google Scholar]
- [64].Beer NR, Wheeler EK, Lee-houghton L, Watkins N, Nasarabadi S, Hebert N, Leung P, Arnold DW, Bailey CG, Colston BW, Reverse-Transcription PCR in Isolated Picoliter Droplets, 80 (2008) 1854–1858. [DOI] [PubMed] [Google Scholar]
- [65].Hatch AC, Fisher JS, Tovar AR, Hsieh AT, Lin R, Pentoney SL, Yang DL, Lee AP, 1-Million droplet array with wide-field fluorescence imaging for digital PCR, Lab Chip 11 (2011) 3838–3845, 10.1039/c1lc20561g. [DOI] [PubMed] [Google Scholar]
- [66].Ottesen E, Hong JW, Quake SR, Leadbetter JR, Microfluidic digital PCR enables multigene analysis of individual environmental bacteria, Science (80-.) 314 (2006) 1464–1467, 10.1126/science.1131370. [DOI] [PubMed] [Google Scholar]
- [67].Huang G, Lin G, Zhu Y, Duan W, Jin D, Emerging technologies for profiling extracellular vesicle heterogeneity, Lab Chip 20 (2020) 2423–2437, 10.1039/d0lc00431f. [DOI] [PubMed] [Google Scholar]
- [68].Bordanaba-Florit G, Royo F, Kruglik SG, Falcón-Pérez JM, Using single-vesicle technologies to unravel the heterogeneity of extracellular vesicles, Nat. Protoc 16 (2021) 3163–3185, 10.1038/s41596-021-00551-z. [DOI] [PubMed] [Google Scholar]
- [69].Vitale SR, Helmijr JA, Gerritsen M, Coban H, van Dessel LF, Beije N, van der Vlugt-Daane M, Vigneri P, Sieuwerts AM, Dits N, van Royen ME, Jenster G, Sleijfer S, Lolkema M, Martens JWM, Jansen MPHM, Detection of tumor-derived extracellular vesicles in plasma from patients with solid cancer, BMC Cancer 21 (2021) 1–17, 10.1186/s12885-021-08007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Laurenzana I, Trino S, Lamorte D, Girasole M, Dinarelli S, De Stradis A, Grieco V, Maietti M, Traficante A, Statuto T, Villani O, Musto P, Sgambato A, De Luca L, Caivano A, Analysis of amount, size, protein phenotype and molecular content of circulating extracellular vesicles identifies new biomarkers in multiple myeloma, Int. J. Nanomedicine 16 (2021) 3141–3160, 10.2147/IJN.S303391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Galbiati S, Damin F, Brambilla D, Ferraro L, Soriani N, Ferretti AM, Burgio V, Ronzoni M, Vago R, Sola L, Chiari M, Small evs-associated dna as complementary biomarker to circulating tumor dna in plasma of metastatic colorectal cancer patients, Pharmaceuticals 14 (2021) 1–13, 10.3390/ph14020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zocco D, Bernardi S, Novelli M, Astrua C, Fava P, Zarovni N, Carpi FM, Bianciardi L, Malavenda O, Quaglino P, Foroni C, Russo D, Chiesi A, Fierro MT, Isolation of extracellular vesicles improves the detection of mutant DNA from plasma of metastatic melanoma patients, Sci. Rep 10 (2020) 1–12, 10.1038/S41598-020-72834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wijerathne H, Witek MA, Jackson JM, Brown V, Hupert ML, Herrera K, Kramer C, Davidow AE, Li Y, Baird AE, Murphy MC, Soper SA, Affinity enrichment of extracellular vesicles from plasma reveals mRNA changes associated with acute ischemic stroke, Commun. Biol 3 (2020) 1–11, 10.1038/s42003-020-01336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Maas SLN, van Solinge TS, Schnoor R, Yekula A, Senders JT, de Vrij J, Robe P, Carter BS, Balaj L, Arkesteijn GJA, Nolte-’T Hoen ENM, Broekman MLD, Orally administered 5-aminolevulinic acid for isolation and characterization of circulating tumor-derived extracellular vesicles in glioblastoma patients, Cancers (Basel) 12 (2020) 1–16, 10.3390/cancers12113297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Pasini L, Notarangelo M, Vagheggini A, Burgio MA, Crinò L, Chiadini E, Prochowski AI, Delmonte A, Ulivi P, D’Agostino VG, Unveiling mutational dynamics in non-small cell lung cancer patients by quantitative EGFR profiling in vesicular RNA, Mol. Oncol (2021) 1–16, 10.1002/1878-0261.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tian Q, He C, Liu G, Zhao Y, Hui L, Mu Y, Tang R, Luo Y, Zheng S, Wang B, Nanoparticle counting by microscopic digital detection: selective quantitative analysis of exosomes via surface-anchored nucleic acid amplification, Anal. Chem 90 (2018) 6556–6562, 10.1021/acs.analchem.8b00189. [DOI] [PubMed] [Google Scholar]
- [77].Liu C, Xu X, Li B, Situ B, Pan W, Hu Y, An T, Yao S, Zheng L, Single-exosome-counting immunoassays for cancer diagnostics, Nano Lett 18 (2018) 4226–4232, 10.1021/acs.nanolett.8b01184. [DOI] [PubMed] [Google Scholar]
- [78].Shen W, Guo K, Adkins GB, Jiang Q, Liu Y, Sedano S, Duan Y, Yan W, Wang SE, Bergersen K, Worth D, Wilson EH, Zhong W, A single extracellular vesicle (EV) flow cytometry approach to reveal EV heterogeneity, Angew. Chemie - Int. Ed 57 (2018) 15675–15680, 10.1002/anie.201806901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Dirks RM, Pierce NA, Triggered amplification by hybridization chain reaction, Proc. Natl. Acad. Sci. U. S. A 101 (2004) 15275–15278, 10.1073/pnas.0407024101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Choi HMT, Beck VA, Pierce NA, Next-generation in situ hybridization chain reaction: higher gain, lower cost, greater durability, ACS Nano 8 (2014) 4284–4294, 10.1021/nn405717p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Welsh JA, Kepley J, Rosner A, Horak P, Berzofsky JA, Jones JC, Prospective use of high-refractive index materials for single molecule detection in flow cytometry, Sensors (Switzerland) 18 (2018) 1–11, 10.3390/S18082461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Tian Y, Ma L, Gong M, Su G, Zhu S, Zhang W, Wang S, Li Z, Chen C, Li L, Wu L, Yan X, Protein Profiling and Sizing of Extracellular Vesicles from Colorectal Cancer Patients via Flow Cytometry, ACS Nano 12 (2018) 671–680, 10.1021/acsnano.7b07782. [DOI] [PubMed] [Google Scholar]
- [83].Zhu S, Ma L, Wang S, Chen C, Zhang W, Yang L, Hang W, Nolan JP, Wu L, Yan X, Light-scattering detection below the level of single fluorescent molecules for high-resolution characterization of functional nanoparticles, ACS Nano 8 (2014) 10998–11006, 10.1021/nn505162u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].de Rond L, van der Pol E, Bloemen PR, Van Den Broeck T, Monheim L, Nieuwland R, van Leeuwen TG, Coumans FAW, A Systematic Approach to Improve Scatter Sensitivity of a Flow Cytometer for Detection of Extracellular Vesicles, Cytom. Part A 97 (2020) 582–591, 10.1002/cyto.a.23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Arkesteijn GJA, Lozano-Andrés E, Libregts SFWM, Wauben MHM, Improved Flow Cytometric Light Scatter Detection of Submicron-Sized Particles by Reduction of Optical Background Signals, Cytom. Part A 97 (2020) 610–619, 10.1002/cyto.a.24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ricklefs FL, Maire CL, Reimer R, Dührsen L, Kolbe K, Holz M, Schneider E, Rissiek A, Babayan A, Hille C, Pantel K, Krasemann S, Glatzel M, Heiland DH, Flitsch J, Martens T, Schmidt NO, Peine S, Breakefield XO, Lawler S, Chiocca EA, Fehse B, Giebel B, Görgens A, Westphal M, Lamszus K, Imaging flow cytometry facilitates multiparametric characterization of extracellular vesicles in malignant brain tumours, J. Extracell. Vesicles 8 (2019), 10.1080/20013078.2019.1588555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Görgens A, Bremer M, Ferrer-Tur R, Murke F, Tertel T, Horn PA, Thalmann S, Welsh JA, Probst C, Guerin C, Boulanger CM, Jones JC, Hanenberg H, Erdbrügger U, Lannigan J, Ricklefs FL, El-Andaloussi S, Giebel B, Optimisation of imaging flow cytometry for the analysis of single extracellular vesicles by using fluorescence-tagged vesicles as biological reference material, J. Extracell. Vesicles 8 (2019), 10.1080/20013078.2019.1587567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Armitage JD, Tan DBA, Cha L, Clark M, Gray ES, Fuller KA, Moodley YP, A standardised protocol for the evaluation of small extracellular vesicles in plasma by imaging flow cytometry, J. Immunol. Methods 468 (2019) 61–66, 10.1016/j.jim.2019.03.006. [DOI] [PubMed] [Google Scholar]
- [89].Millan C, Prause L, Vallmajo-Martin Q, Hensky N, Eberli D, Extracellular Vesicles from 3D Engineered Microtissues Harbor Disease-Related Cargo Absent in EVs from 2D Cultures, Adv. Healthc. Mater 2002067 (2021) 1–13, 10.1002/adhm.202002067. [DOI] [PubMed] [Google Scholar]
- [90].de Oliveira GP, Zigon E, Rogers G, Davodian D, Lu S, Jovanovic-Talisman T, Jones J, Tigges J, Tyagi S, Ghiran IC, Detection of Extracellular Vesicle RNA Using Molecular Beacons, IScience (2020) 23, 10.1016/j.isci.2019.100782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJP, Hole P, Carr B, Redman CWG, Harris AL, Dobson PJ, Harrison P, Sargent IL, Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis, nanomedicine nanotechnology, Biol. Med 7 (2011) 780–788, 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Thane KE, Davis AM, Hoffman AM, Improved methods for fluorescent labeling and detection of single extracellular vesicles using nanoparticle tracking analysis, Sci. Rep 9 (2019) 1–14, 10.1038/s41598-019-48181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Casabella S, Scully P, Goddard N, Gardner P, Automated analysis of single cells using Laser Tweezers Raman Spectroscopy, Analyst 141 (2016) 689–696, 10.1039/c5an01851j. [DOI] [PubMed] [Google Scholar]
- [94].Chan JW, Taylor DS, Zwerdling T, Lane SM, Ihara K, Huser T, Micro-Raman spectroscopy detects individual neoplastic and normal hematopoietic cells, Biophys. J 90 (2006) 648–656, 10.1529/biophysj.105.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wu MY, Ling DX, Ling L, Li W, Li YQ, Stable optical trapping and sensitive characterization of nanostructures using standing-wave Raman tweezers, Sci. Rep 7 (2017) 1–8, 10.1038/srep42930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chen D, Shelenkova L, Li Y, Kempf CR, Sabelnikov A, Laser tweezers Raman spectroscopy potential for studies of complex dynamic cellular processes: single cell bacterial lysis, Anal. Chem 81 (2009) 3227–3238, 10.1021/ac8023476. [DOI] [PubMed] [Google Scholar]
- [97].Enciso-Martinez A, van der Pol E, Lenferink ATM, Terstappen LWMM, van Leeuwen TG, Otto C, Synchronized Rayleigh and Raman scattering for the characterization of single optically trapped extracellular vesicles, Nanomedicine Nanotechnology, Biol. Med 24 (2020), 10.1016/j.nano.2019.102109. [DOI] [PubMed] [Google Scholar]
- [98].Enciso-Martinez A, Van Der Pol E, Hau CM, Nieuwland R, Van Leeuwen TG, Terstappen LWMM, Otto C, Label-free identification and chemical characterisation of single extracellular vesicles and lipoproteins by synchronous Rayleigh and Raman scattering, J. Extracell. Vesicles 9 (2020), 10.1080/20013078.2020.1730134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kruglik SG, Royo F, Guigner JM, Palomo L, Seksek O, Turpin PY, Tatischeff I, Falcón-Pérez JM, Raman tweezers microspectroscopy of: circa 100 nm extracellular vesicles, Nanoscale 11 (2019) 1661–1679, 10.1039/c8nr04677h. [DOI] [PubMed] [Google Scholar]
- [100].Carney RP, Hazari S, Colquhoun M, Tran D, Hwang B, Mulligan MS, Bryers JD, Girda E, Leiserowitz GS, Smith ZJ, Lam KS, Multispectral Optical Tweezers for Biochemical Fingerprinting of CD9-Positive Exosome Subpopulations, Anal. Chem 89 (2017) 5357–5363, 10.1021/acs.analchem.7b00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Dai Y, Bai S, Hu C, Chu K, Shen B, Smith ZJ, Combined Morpho-Chemical Profiling of Individual Extracellular Vesicles and Functional Nanoparticles without Labels, Anal. Chem 92 (2020) 5585–5594, 10.1021/acs.analchem.0c00607. [DOI] [PubMed] [Google Scholar]
- [102].Carney RP, Hazari S, Rojalin T, Knudson A, Gao T, Tang Y, Liu R, Viitala T, Yliperttula M, Lam KS, Targeting Tumor-Associated Exosomes with Integrin-Binding Peptides, Adv. Biosyst 1 (2017) 1–12, 10.1002/adbi.201600038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ćulum NM, Cooper TT, Bell GI, Hess DA, Lagugné-Labarthet F, Characterization of extracellular vesicles derived from mesenchymal stromal cells by surface-enhanced raman spectroscopy, Anal. Bioanal. Chem (2021), 10.1007/S00216-021-03464-8. [DOI] [PubMed] [Google Scholar]
- [104].Yang Y, Shen G, Wang H, Li H, Zhang T, Tao N, Ding X, Yu H, Interferometric plasmonic imaging and detection of single exosomes, Proc. Natl. Acad. Sci. U. S. A 115 (2018) 10275–10280, 10.1073/pnas.1804548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Min J, Son T, Hong JS, Cheah PS, Wegemann A, Murlidharan K, Weissleder R, Lee H, Im H, Plasmon-Enhanced Biosensing for Multiplexed Profiling of Extracellular Vesicles, Adv. Biosyst 4 (2020) 1–8, 10.1002/adbi.202000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Guo K, Li Z, Win A, Coreas R, Adkins GB, Cui X, Yan D, Cao M, Wang SE, Zhong W, Calibration-free analysis of surface proteins on single extracellular vesicles enabled by DNA nanostructure, Biosens. Bioelectron 192 (2021), 113502, 10.1016/j.bios.2021.113502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Gong Y, Fan N, Yang X, Peng B, Jiang H, New advances in microfluidic flow cytometry, Electrophoresis 40 (2019) 1212–1229, 10.1002/elps.201800298. [DOI] [PubMed] [Google Scholar]
- [108].Piyasena ME, Graves SW, The intersection of flow cytometry with microfluidics and microfabrication, Lab Chip 14 (2014) 1044–1059, 10.1039/c3lc51152a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Stavrakis S, Holzner G, Choo J, deMello A, High-throughput microfluidic imaging flow cytometry, Curr. Opin. Biotechnol 55 (2019) 36–43, 10.1016/j.copbio.2018.08.002. [DOI] [PubMed] [Google Scholar]
- [110].Ning Y, Cui X, Yang C, Jing F, Bian X, Yi L, Li G, A self-digitization chip integrated with hydration layer for low-cost and robust digital PCR, Anal. Chim. Acta 1055 (2019) 65–73, 10.1016/j.aca.2018.12.029. [DOI] [PubMed] [Google Scholar]
- [111].Sposito AJ, DeVoe DL, Staggered trap arrays for robust microfluidic sample digitization, Lab Chip 17 (2017) 4105–4112, 10.1039/c7lc00846e. [DOI] [PubMed] [Google Scholar]
- [112].Lim SW, Abate AR, Ultrahigh-throughput sorting of microfluidic drops with flow cytometry, Lab Chip 13 (2013) 4563–4572, 10.1039/c3lc50736j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.