Abstract
Clinical studies demonstrate alterations to immune measures in psychosis that can vary with illness stage and severity. For example, recent data show that changes to the JAK-STAT1 transcriptional signature, characteristic of an “M1“ proinflammatory monocyte and macrophages phenotype, are related to illness duration. While antipsychotics have demonstrated immunomodulatory properties, their effects on this important immune signaling pathway are unknown. The primary aims of this study were to determine the effects of risperidone, a commonly prescribed antipsychotic drug, on the JAK-STAT1 transcriptional signature. Selected measures of JAK-STAT1 signature gene expression in peripheral blood mononuclear cells (PBMCs) from a clinical sample with psychosis were compared to examine differences induced by risperidone treatment. Additionally, the direct effects of risperidone on the JAK-STAT1 signature were investigated using a THP-1 human monocyte and macrophage cell model. Comparisons within the clinical sample demonstrated that the JAK-STAT1 signature was elevated in PBMCs from participants treated with risperidone who had a longer illness duration compared to either untreated participants or those who were risperidone treated but had a shorter illness duration. Results of the in-vitro experiments showed a consistent potentiating effect of risperidone on expression of JAK-STAT1 signature genes in activated monocytes and monocyte-derived macrophages. Collectively these data indicate that risperidone may contribute to increases in expression of JAK-STAT1 signature genes in participants with a longer illness duration and may skew myeloid cells to a more proinflammatory phenotype.
Keywords: THP-1, schizophrenia, bipolar disorder, antipsychotic, risperidone, monocyte, macrophage, PBMC
1. Background
Individuals with psychotic disorders are frequently shown to have alterations to immune parameters both peripherally and centrally 1. While much of the literature posits an overall systemic inflammatory state in psychosis, recent results indicate a more nuanced alteration to immune parameters that are related to clinical characteristics such as illness stage and duration 2–4. For example, our recent results show that in participants who are earlier in illness, circulating peripheral blood mononuclear cells (PBMCs) exhibit a suppressed JAK-STAT1 transcriptional signature, which increases, or normalizes, over the course of the illness 2. A canonical function of IFN-γ mediated JAK-STAT1 signaling is activation of myeloid cells such as monocytes and macrophages to a classically activated proinflammatory phenotype, frequently termed “M1” 5–7. Changes to this “M1“-like transcriptional signature in circulating immune cells as a function of duration of psychotic illness indicates a shift in phenotype over the course of the illness that may be related to various endogenous or exogenous factors. These findings are in line with results from groups that found decreased or unchanged markers of immune activation early in psychotic illness, which were increased in those with chronic illness 3,4, in contrast to other findings that show increased cytokine levels at illness onset 8.
Monocytes and macrophages are equipped with a vast array of receptors that make them exceptionally sensitive to the environmental milieu, and cell phenotypes can ranges from those that are more proinflammatory in nature (“M1“-like) to those that are associated with anti-inflammatory and tissue remodeling functions (“M2“-like) 6. In-vitro, a classical “M1” proinflammatory phenotype is induced following JAK-STAT1 pathway activation by IFN-γ, often alongside an additional NF-κB activating stimulus such as the bacterial ligand LPS 5,7. Here, genes associated with the “M1” phenotype are poised or expressed, and genes associated with the “M2” phenotype are suppressed by epigenetic modifications 9. The “M2” phenotype is subdivided based on the polarizing stimulus and we focus here on the “M2“ ‘tolerized’ (“M2tol”) phenotype, also referred to as ‘endotoxin tolerance’, which is induced by high levels of the NF-κB activating endotoxin LPS 10,11. “M2tol“ cells are refractory to inflammatory stimuli, and expression of many “M1” characteristic genes are suppressed by repressive chromatin modifications, while expression of “M2” characteristic genes are elevated 10,12.
In addition to these well-studied polarizing stimuli, pharmacological agents can also influence monocyte and macrophage phenotypes 13. Antipsychotic drugs, the primary pharmacological treatment for psychosis, are well known to impact immune function 14–18. However, specific effects are not well characterized, and vary by study sample or model characteristics as well as the particular drug investigated. While monocytes and macrophages express cell surface receptors including dopamine and serotonin receptors and are responsive to antipsychotics in culture 17,19, the majority of clinical studies have focused on changes to overall serum cytokine levels, and thus little is known about more specific effects of antipsychotics on circulating immune cells.
Given that antipsychotic drugs clearly impact immune activity and the sensitivity of immune cells such as monocytes to the presence of modulating ligands, we hypothesized that treatment over the course of psychotic illness may contribute to changes in the “M1“-like JAK-STAT1 signature seen in circulating immune cells in relation to illness duration, and that this may occur via pharmacological effects on myeloid cell phenotype. This hypothesis was tested firstly by examining available in-vivo clinical data and secondly using a human monocyte cell line. JAK-STAT1 signature expression data in PBMCs was compared between a subsample of participants with psychosis treated with risperidone monotherapy (the most frequently prescribed antipsychotic drug in this clinical sample) and participants not treated with antipsychotic drugs, in relation to duration of illness.
Next, the THP-1 monocyte cell line was used to directly test the effect of risperidone on “M1” (JAK-STAT1) gene expression. An additional advantage of using this monocyte cell line is that these cells can be differentiated to a state more closely resembling tissue macrophages, which are rarely accessible in living subjects. Some tissue macrophages are derived from circulating monocytes; however, others are replenished by embryologically-derived local progenitors 20. With high relevance to neuropsychiatric illness, brain parenchymal and extra-parenchymal monocytes and macrophages are indispensable for many aspects of central nervous system function and can have detrimental or protective effects during injury or illness 21,22. Therefore, the THP-1 cell model was further used to determine the effects of risperidone on “M1” and “M2tol“ characteristic gene expression in differentiated and polarized monocyte-derived macrophages using established protocols and markers (Figure 1).
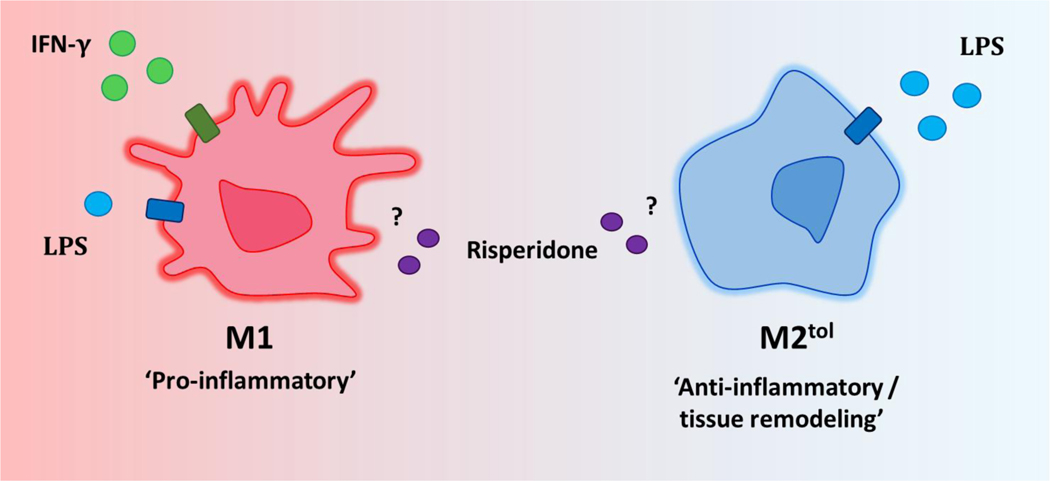
Figure 1.
“M1” and “M2tol“ phenotypes. Monocytes and macrophages display a spectrum of functional phenotypes, ranging from pro-inflammatory (“M1“) to anti-inflammatory and tissue remodeling (“M2“). “M1“ polarization is induced with IFN-γ and low-dose LPS. An “M2“ phenotype can be induced with multiple polarizing stimuli, one of which is high-dose LPS (“M2tol“). The effects of the presence of risperidone on “M1“ and “M2“ characteristic gene expression will be investigated in the present study.
2. Methods
2.1. Clinical sample characteristics
The clinical sample consisted of 22 participants with psychosis (schizophrenia and bipolar disorder with psychosis), comprised of 11 who were not being treated with antipsychotic mediation at the time of participation, and 11 who were being treated with risperidone monotherapy. Recruitment and diagnostic details for these participants are as reported for the larger sample 2. Information pertaining to antipsychotic medication treatment was collected from participants at study recruitment. Monotherapy was defined as participants who reported current treatment with only one antipsychotic medication. Demographic and clinical characteristics are outlined by antipsychotic status in Table 1. The sample was selected by matching risperidone treated participants optimally on mean and range of duration of illness with the untreated group (untreated M=17.82 years and range from illness onset to 42 years; risperidone monotherapy treated M=18.36 years and range from illness onset to 42 years). Here the terms ‘untreated’ and ‘treated’ are used to refer to treatment with antipsychotic medication, and do not include other types of medication.
Table 1.
Participant demographic and clinical characteristics.
| Untreated | Risperidone Treated | ||
|---|---|---|---|
| Total (n) | 11 | 11 | |
| Age (M ± SD) | 38.82 ± 13.91 | 43.27 ± 14.37 | |
| Female (n) | 5 | 6 | |
| Male (n) | 6 | 5 | |
| Race* | Caucasian, non-Hispanic (n) | 4 | 0 |
| Black, non-Hispanic (n) | 5 | 10 | |
| Asian or other Pacific Islander (n) | 0 | 1 | |
| Hispanic (n) | 2 | 0 | |
| BMI (M ± SD)* | 26.02 ± 5.51 | 34.05 ± 9.05 | |
| Illness Duration (M ± SD) | 17.82 ± 16.32 | 18.36 ± 15.28 | |
| Illness Status | Inpatient (n) | 5 | 3 |
| Outpatient (n) | 6 | 8 | |
| PANSS | Positive (M ± SD) | 23.81 ± 4.31 | 26.64 ± 5.33 |
| Negative (M ± SD) | 16.09 ± 7.35 | 20.82 ± 6.49 | |
| General (M ± SD) | 43.73 ± 7.46 | 43.45 ± 7.03 | |
| JAK-STAT1 Composite Score (M ± SD) | −1.35 ± 1.73 | .70 ± 3.15 | |
p<.05 comparison of untreated participants with risperidone treated participants. M=mean, SD=standard deviation.
The previously published JAK-STAT1 composite score was used as the measure of the JAK-STAT1 transcriptional signature for the current analysis 2. Specifically, mRNA expression of five genes (IFNG, CXCL10, IRF1, STAT1 and TLR4), measured in PBMCs from participants with psychosis and controls, was used as a signature of JAK-STAT1 pathway activation. The JAK-STAT1 composite score was computed by combining the standardized expression (z-score) of each gene 2,23.
Illness duration was used to further split participants into the following low and high illness duration groups: untreated low duration (n=5, M=2.20 years, range from illness onset to 5 years), untreated high duration (n=6, M=30.83, range from 19 to 42 years), risperidone treated low duration (n=6, M=6.33, range from illness onset to 11 years) and risperidone treated high duration (n=5, M=32.80, range from 21 to 42 years).
2.2. THP-1 Cell Culture and Macrophage Differentiation
Human monocytic THP-1 cells (ATCC TIB-202) were maintained at 37⁰C in a humidified incubator at 5% CO2. Cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 0.1 mg/ml l-glutamine and 50 U/mL each of penicillin and streptomycin (all Invitrogen). For all experiments, cells were plated at 106 cells per well using 6-well plates. Resting macrophages (M0) were generated by treating THP-1 monocytes with 15nM phorbol 12-myristate-13 acetate (PMA; Sigma) for 24 hours to induce macrophage differentiation, followed by 24 hours rest in fresh media 24.
2.3. Risperidone Treatment of THP-1 cells
For THP-1 monocyte experiments, cells were stimulated with 10ng/μL recombinant IFN-γ (BD Pharmingen) for 7 hours to activate the JAK-STAT1 pathway. 10μM risperidone (Sigma-Aldrich), or DMSO vehicle control, was added to the cell culture medium for the final hour. It has previously been shown that risperidone at this concentration is immunomodulatory but does not affect cell viability in human monocyte and murine macrophage cell lines, whereas higher concentrations of risperidone resulted in toxicity 17,18,25. For macrophage polarization, M0 cells were treated with 10ng/μL recombinant IFN-γ and low dose (1ng/mL) LPS (E. coli O111:B4; Sigma-Aldrich) (“M1“ phenotype) or a tolerizing dose (100ng/mL) of LPS (“M2tol“ phenotype) for 24 hours 10,26. Cells were treated with 10μM risperidone, or DMSO vehicle control, alongside these “M1“ and “M2tol“ polarizing stimuli. Cells were harvested, washed with phosphate buffered saline (Gibco), and pelleted by centrifugation prior to RNA extraction.
2.4. RNA Extraction and qRT-PCR of THP-1 cells
RNA extraction, cDNA synthesis and qRT-PCR for the cell culture experiments were carried out using previously described protocols 2. Three of the JAK-STAT1 signature genes used for the composite score, CXCL10, IRF1 and STAT1, were selected as “M1“ gene expression markers, and MT1E and MMP9 were selected as “M2“ gene expression markers based on previous publications 10.
2.5. Statistical Analyses
Statistical analyses were carried out using SPSS for the clinical sample and GraphPad Prism for cell culture experiments. Demographic differences and medication effects between groups and in relation to the JAK-STAT1 composite score in the clinical sample were assessed using chi-squared tests, independent samples t-tests and ANOVA with post-hoc Tukey’s. Bivariate correlations were carried out using Spearman’s rho. For the cell culture experiments, at least two biological and two technical replicates were used. Expression results for CXCL10 were calculated using 2^-Δct, and presented as relative expression, as this gene was not expressed under non-stimulated conditions. Expression results for IRF-1 and STAT1 were calculated using 2^-ΔΔct (fold change relative to the unstimulated condition). Differences between conditions were assessed using one- and two-sample t-tests and ANOVA. Tukey post-hoc tests were used for ANOVA that yielded significant results.
3. Results
3.1. Demographics
When comparing untreated participants with risperidone monotherapy treated participants there was no significant difference in age (t20=−.74, p=.47), sex (χ2(1)=.18, p=.67), illness duration (t20=−.08, p=.94), illness status (χ2(1)=.79, p=.38), or PANSS positive (t20=−1.36, p=.19), negative (t20=−1.60, p=.13), general (t20=−.09, p=.93) subscales (Table 1). Race (χ2(3)=8.67, p=.03) and BMI (t19=−2.42, p=.03) were unmatched between the two groups. However, there were no significant associations of the JAK-STAT1 composite score with either race (F(3,18)=1.23, p=.33) or BMI (r21=.03, p=.92), nor did these variables predict the JAK-STAT1 composite score in the larger sample 2.
3.2. Comparison of the JAK-STAT1 composite score in risperidone treated and untreated participants with psychosis
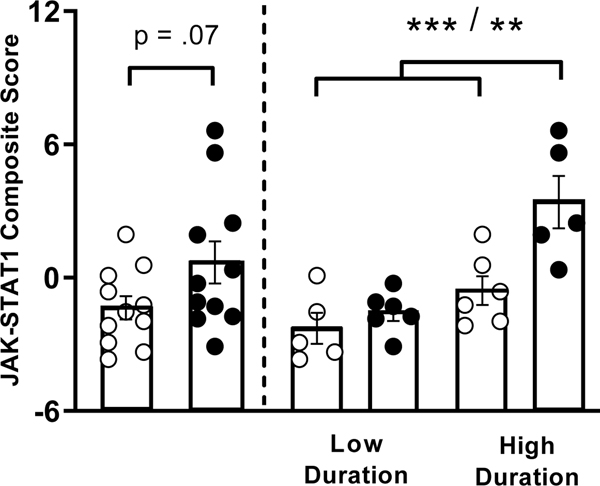
As antipsychotics have been shown to have immunomodulatory effects, it’s possible that treatment may contribute to changes seen to the “M1“-like (JAK-STAT1) signature in PBMCs from naturalistically treated participants with psychosis. A sample of participants with psychosis who were treated with risperidone monotherapy were compared with those not treated with antipsychotic medication. The mean JAK-STAT1 composite score was greater in the risperidone treated compared to untreated participants, and trended towards significance (t20=−1.89, p=.07; Figure 2).
Figure 2.
Left: Comparison of the JAK-STAT1 composite score (the normalized combined mRNA expression of IFNG, CXCL10, IRF1, STAT1 and TLR4) in all untreated (open circles) and risperidone treated (closed circles) participants with psychosis. Right: JAK-STAT1 composite score grouped by risperidone treatment and illness duration. ***p<.001, **p<.01. Error bars represent SEM.
3.3. Comparison of the JAK-STAT1 composite score in participants with psychosis in relation to risperidone treatment and illness duration
Given our previous data demonstrating that illness duration predicts the JAK-STAT1 composite score, untreated and risperidone treated participants were split into low and high illness duration groups for further analysis. Comparison of the JAK-STAT1 composite score between these groups revealed significant differences (F(3,18)=10.84, p<.001) (Figure 2). Post-hoc comparisons demonstrated that there was no difference in the JAK-STAT1 composite score between untreated participants with a low compared to high illness duration, or between untreated participants and treated participants both with a low illness duration. The treated participants with a high illness duration had an elevated JAK-STAT1 composite score compared to untreated participants with low illness duration (p<.001), untreated participants with a high illness duration (p=.007) and treated participants with a low illness duration (p=.001). Thus, risperidone treated participant PBMCs only demonstrated increased “M1“-like (JAK-STAT1) gene expression if they had a longer illness duration.
3.4. THP-1 cell model: morphology and “M1“ (JAK-STAT1) gene expression
To determine the direct effects of risperidone on “M1“ (JAK-STAT1) gene expression in monocytes and macrophages an in-vitro cellular assay was used. The expression of the “M1“ genes CXCL10, IRF1 and STAT1 were selected as readouts of activation of this pathway. Representative images, culture conditions and gene expression for each phenotype are presented in Figure 3. As expected, treatment of THP-1 monocytes with PMA induced morphological changes expected of transition to macrophages, including decreased proliferation, cell adherence and spreading 27 (Figure 3A). Furthermore, polarization with “M1“ and “M2“ stimuli resulted in morphological characteristics as previously reported 28. In undifferentiated THP-1 monocytes CXCL10 mRNA is not expressed, whereas IRF1 and STAT1 are constitutively expressed (Figure 3B). M0 showed similar expression of JAK-STAT1 signature genes. As expected, CXCL10, IRF1 and STAT1 were strongly induced in “M1“ macrophages. To confirm an “M2tol“ phenotype, the response to acute LPS challenge was compared before and after a 24 hour tolerizing dose of LPS. Acute LPS challenge alone resulted in increased expression of CXCL10 and IRF1 compared to baseline M0. Following “M2tol“ polarization with LPS, CXCL10 and IRF1 mRNA expression was decreased compared to the acute LPS stimulus. Furthermore, CXCL10 and IRF1 expression remained suppressed when “M2tol“ macrophages were challenged with acute LPS, demonstrating endotoxin tolerance 29. On the other hand, STAT1 expression was not responsive to acute LPS at M0 baseline and did not demonstrate tolerization.
Figure 3.
Cell culture and JAK-STAT1 gene expression. A) Phenotype, representative image and stimuli for each culture condition. B) JAK-STAT1 expression signature for each phenotype. In the “M2tol“ column gray bars indicate acute LPS challenge. L to R: M0 with acute LPS challenge; “M2tol“; “M2tol“ with acute LPS challenge. *p<.05. Error bars represent SEM.
3.5. THP-1 monocytes: Risperidone effects on “M1“ (JAK-STAT1) gene expression
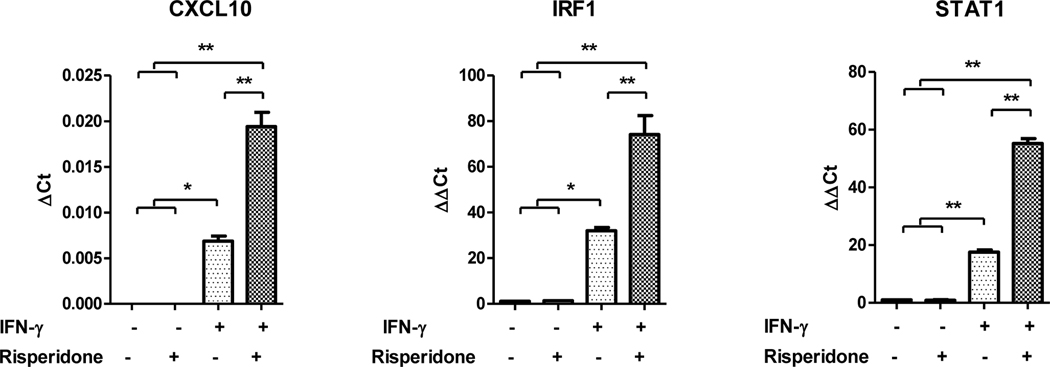
To test the effects of risperidone treatment on “M1“ (JAK-STAT1) gene expression in monocytes, THP-1 cells were stimulated with IFN-γ to activate the JAK-STAT1 pathway, followed by treatment with risperidone. As expected, IFN-γ alone increased expression of all three “M1“ genes (Figure 4). When cells were stimulated with IFN-γ and treated with risperidone, expression of all three genes was significantly increased compared to the IFN-γ stimulus alone. These data demonstrate that risperidone has a potentiating effect on “M1“ (JAK-STAT1) gene expression in THP-1 monocytes.
Figure 4.
Effects of risperidone on “M1“ (JAK-STAT1) gene expression in THP-1 monocytes. THP-1 monocytes were stimulated with IFN-γ for 7hours and treated with risperidone for the final hour. Risperidone treatment increased the expression of “M1“ genes in IFN-γ stimulated monocytes. *p<.05, **p<.01. Error bars represent SEM.
3.6. “M1“ macrophages: Risperidone effects on “M1“ (JAK-STAT1) gene expression
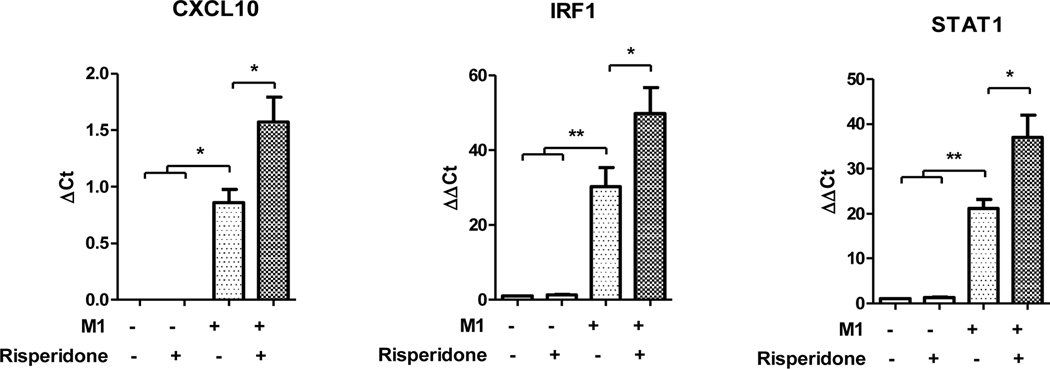
Next the effect of risperidone on “M1“ gene expression in the “M1“ polarizing condition was tested. M0 macrophages were polarized to “M1“ with and without concurrent risperidone treatment. In “M1“ macrophages expression of “M1“ (JAK-STAT1) genes were elevated, as expected (Figure 5). Risperidone alone did not have a significant effect on “M1“ (JAK-STAT1) gene expression in M0 macrophages. We found that concurrent treatment with “M1“ polarizing stimuli and risperidone resulted in significantly increased mRNA expression of CXCL10, IRF-1 and STAT1. Thus, as was the case in the THP-1 monocyte condition, risperidone potentiated expression of JAK-STAT1 gene expression and strengthened this measure of the “M1“ macrophage phenotype.
Figure 5.
Effects of risperidone on “M1“ (JAK-STAT1) gene expression during “M1“ polarization. M0 macrophages were polarized to “M1“ in the presence and absence of risperidone. Risperidone treatment increased the expression of JAK-STAT1 gene expression compared to the “M1“ alone. *p<.05, **p<.01. Error bars represent SEM.
3.7. “M2tol“ macrophages: Risperidone effects on “M1“ (JAK-STAT1) gene expression
The effects of risperidone on “M1“ gene expression in the “M2tol“ polarizing condition were assessed next. In this condition, expression of “M1“ (JAK-STAT1) genes was decreased, similar to the pattern of expression seen in the clinical data early in illness and with increased acuity. M0 macrophages were polarized to “M2tol“ with and without concurrent risperidone treatment. Once again, cells treated with risperidone showed increased expression of CXCL10, IRF1 and STAT1 (Figure 6). For CXCL10 and IRF1 this equated to blocking or reversal of the tolerization of these genes. However, in the case of STAT1, increased expression due to risperidone in the “M2tol“ polarizing condition occurs independent of prior tolerization.
Figure 6.
Effects of risperidone on JAK-STAT1 (“M1“) gene expression in the “M2tol“ condition. M0 macrophages were polarized to “M2tol“ in the presence and absence of risperidone. In the presence of risperidone, expression of all “M1“ genes are increased. *p<.05, **p<.01. Error bars represent SEM.
3.8. “M2tol“ macrophages: Risperidone effects on “M2tol“ gene expression
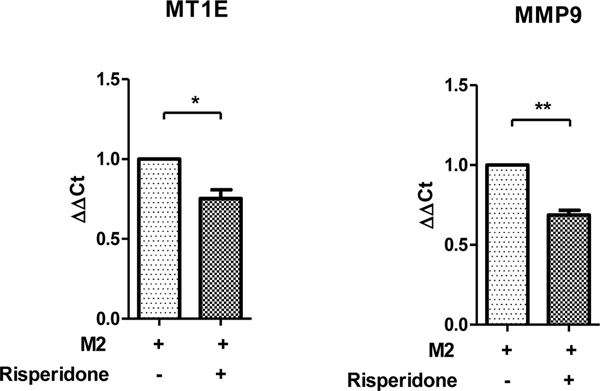
The data so far demonstrate that risperidone increases expression of “M1“ (JAK-STAT1) genes under both “M1“ and “M2tol“ polarizing conditions. Next, the effects of risperidone on two established markers of the “M2tol“ phenotype, MT1E and MMP9 10, were investigated in the “M2tol“ polarizing condition. Exposure to risperidone decreased expression of both “M2tol“ genes (Figure 7). Combined, these data suggest that risperidone skews these cells from an “M2tol“ to an “M1“ phenotype as expression of “M2tol“ genes are decreased and “M1“ genes are increased in the presence of this drug.
Figure 7.
Effects of risperidone on expression of “M2tol“ genes in the “M2tol“ condition. In the presence of risperidone, the expression of “M2tol“ genes MT1E and MMP2 are significantly decreased. *p<.05, **p<.01. Error bars represent SEM.
4. Discussion
Alterations to immune parameters in psychosis are not static, rather they appear to fluctuate in relation illness-related variables. This is perhaps not surprising given the plasticity of the immune system, and particularly cells of the myeloid lineage, to previous and current signals present in the tissue milieu, including pharmacological agents such as antipsychotic drugs. In this novel study, the potential contribution of risperidone to changes in the circulating immune cell JAK-STAT1 transcriptional signature was investigated by using available data from a naturalistically treated clinical sample. The effects of risperidone on “M1“ (JAK-STAT1) and “M2tol“ characteristic gene expression were further investigated using a human THP-1 monocyte/macrophage cell model.
In the psychosis clinical sample, our previous results showed that illness duration predicted the JAK-STAT1 transcriptional signature in PBMCs, which was overall decreased early in illness compared to non-psychiatric controls, and increased to control levels over the course of the illness 2. We hypothesized that antipsychotic treatment may contribute to these immune alterations, either by suppressing or potentiating this “M1“-like gene expression. In the present study, we find that the JAK-STAT1 transcriptional signature is significantly increased in PBMCs from risperidone treated participants, but specifically those who are later in illness, compared to all untreated participants as well as risperidone treated participants who are earlier in illness.
Next, we investigated the direct effects of risperidone in a human monocyte and monocyte derived-macrophage cell model. Risperidone consistently increased expression of the “M1“ (JAK-STAT1) genes CXCL10, IRF1 and STAT1 across different cellular differentiation states, including both monocytes and monocyte-derived macrophages, as well as in opposing polarizing conditions characterized by high (“M1“) or low (“M2tol“) expression of JAK-STAT1 signature genes. These data using this specific signature indicate that the presence of risperidone may skews cells away from an “M2“-like towards a more “M1“-like phenotype. This interpretation is further supported by the results showing that treatment with risperidone decreased expression of “M2tol“ genes MT1E and MMP9.
With regards to the alterations to the JAK-STAT1 transcriptional signature previously described in PBMCs from participants with psychosis, neither the clinical nor the in-vitro data indicate an inhibitory effect of risperidone treatment on “M1“ (JAK-STAT1) gene expression. It is therefore unlikely that the suppressed JAK-STAT1 signature seen earlier in illness is due to the effects of antipsychotic medication. Instead, the results indicate that risperidone may contribute to the increase in JAK-STAT1 signature gene expression observed over the course of the illness. While the in-vitro results were more straightforward, the clinical data showed that risperidone treatment is only associated with increased JAK-STAT1 signature gene expression in those with a longer illness duration. Possible explanations for the observation that risperidone treatment is not associated with increased expression of this signature in those earlier in illness are that the effects of risperidone treatment may only be manifest after long-term antipsychotic use, or that there are baseline/contextual difference between those earlier versus later in illness that influence the acute response to risperidone. Indeed, monocytes and macrophages are well known to exhibit immune memory (i.e. priming or sensitization) and for various combinations of stimuli to have synergistic or antagonistic effects on transcription and function 30. Interestingly, we have previously demonstrated that risperidone treatment can reverse transcriptionally restrictive heterochromatin at prototypical proinflammatory cytokine promoters 31, opening up the possibility that a similar mechanism may underlie the effects described here.
Progress in understanding the effects of pharmacotherapeutics prescribed for brain disorders on immune function is critical given that the immune system is increasingly being recognized for its role in neuropsychiatric illness 32. For example, circulating monocytes and monocyte-derived cells are shown to play roles in mediating the effects of stress and infection on psychiatrically relevant behaviors 33,34. Additionally, that risperidone may skew monocytes and macrophages from a more “M2“-like towards an “M1“-like phenotype is interesting with regards to the potential effects of psychotropic medications on myeloid cells in less accessible peripheral and central tissues including the brain parenchyma and its borders. Although there are important tissue specific differences, which appear to be largely driven by local microenvironmental signals 35, monocytes and macrophages in and around the CNS exhibit molecular similarities to the cells utilized in these experiments, and may be expected to respond similarly to pharmacological agents. Indeed, it was recently shown that long-term in-vivo treatment with antipsychotic medications in rodents resulted in increased microglial activation 36. Finally, the results described here when considered in relation to reports of increased immune activation markers in chronic psychosis but not early illness 3,4 indicate that changes to immune parameters over the course of illness may be related to environmental factors including pharmacotherapeutics.
Our study sample size was limited by the number of participants who are not prescribed or who are prescribed only one antipsychotic medication. Going forward larger numbers of participants are needed to investigate and confirm associations of antipsychotic treatment and cell-specific immune changes at different illness stages. Furthermore, testing these effects of risperidone using ex-vivo primary human monocytes and monocyte-derived macrophages would help to bridge the clinical and experimental data described here, though this is outside the scope of our current study. As genes for analyses were selected based on expression patterns demonstrated in primary monocytes/monocyte-derived macrophages under polarizing conditions 10,37–40, which were consistent with those in the THP-1 human monocyte cell line, we anticipate concordant risperidone effects. We selected the 10μM dose of risperidone to use for the in-vitro experiments based on previous literature demonstrating cell-type specific effects at the selected dose and toxicity at higher concentrations 17,18,25, including a previous publication in which we found similar effects on expression of proinflammatory immune genes in a human liposarcoma cell line31. However, the lack of a dose-response specific to the outcomes measured here is noted as an important limitation and should be addressed in future studies. These approaches make possible the detection of early shifts in immune signaling pathways that can be measured in peripheral blood samples that are routine procedures in the clinic. We also note here that further refinements of the signal can be achieved by selecting homogenous and specialized samples that can be readily isolated, such as CD14+ monocytes.
In conclusion, the present analysis highlights a consistent potentiating effect of risperidone on the M1-like JAK-STAT1 transcriptional signature in human immune cells both at the in-vitro cellular level and in naturalistically treated individuals with chronic psychosis, and highlights a potential proinflammatory phenotypic skewing of myeloid cells in the presence of this antipsychotic drug.
Highlights.
Risperidone treated subjects with chronic psychosis have increased “M1” gene expression
Risperidone increases “M1” gene expression in monocytes and macrophages
Risperidone decreases “M2” gene expression in macrophages
Acknowledgments
Funding: this work was supported in part by NIH grant R01MH094358 (RPS) and UL1TR002003 (JKM)
Footnotes
Declaration of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: Implications for pathophysiology and treatment. The Lancet Psychiatry. 2015;2(3):258–270. doi: 10.1016/S2215-0366(14)00122-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melbourne JK, Rosen C, Feiner B, Pang Y, Sharma RP. The JAK-STAT1 transcriptional signature in peripheral immune cells reveals alterations related to illness duration and acuity in psychosis. Brain Behav Immun. 2018. doi: 10.1016/j.bbi.2018.11.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frydecka D, Krzystek-Korpacka M, Lubeiro A, et al. Profiling inflammatory signatures of schizophrenia: A cross-sectional and meta-analysis study. Brain Behav Immun. 2018;71(April):28–36. doi: 10.1016/j.bbi.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 4.Dickerson F, Stallings C, Origoni A, et al. Inflammatory Markers in Recent Onset Psychosis and Chronic Schizophrenia. Schizophr Bull. 2016;42(1):134–141. doi: 10.1093/schbul/sbv108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray PJ, Allen JE, Biswas SK, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell AJ, Roediger B, Weninger W. Monocyte homeostasis and the plasticity of inflammatory monocytes. Cell Immunol. 2014;291(1–2):22–31. doi: 10.1016/j.cellimm.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 7.Ivashkiv LB. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol. 2018;18(9):545–558. doi: 10.1038/s41577-018-0029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. In: Molecular Psychiatry. Vol 21. Nature Publishing Group; 2016:1696–1709. doi: 10.1038/mp.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang K, Park SH, Chen J, Kang K, Ivashkiv LB. Interferon- g Represses M2 Gene Expression in Human Macrophages by Disassembling Enhancers Bound by the Transcription Factor MAF Article Interferon- g Represses M2 Gene Expression in Human Macrophages by Disassembling Enhancers Bound by the Transcription. Immunity. 2017;47(2):235–250.e4. doi: 10.1016/j.immuni.2017.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pena OM, Pistolic J, Raj D, Fjell CD, Hancock REW. Endotoxin Tolerance Represents a Distinctive State of Alternative Polarization (M2) in Human Mononuclear Cells. J Immunol. 2011;186(12):7243–7254. doi: 10.4049/jimmunol.1001952 [DOI] [PubMed] [Google Scholar]
- 11.Porta C, Rimoldi M, Raes G, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor B. Proc Natl Acad Sci. 2009;106(35):14978–14983. doi: 10.1073/pnas.0809784106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7147):972–978. doi: 10.1038/nature05836 [DOI] [PubMed] [Google Scholar]
- 13.Mantovani A, Vecchi A, Allavena P. Pharmacological modulation of monocytes and macrophages. Curr Opin Pharmacol. 2014;17:38–44. doi: 10.1016/j.coph.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 14.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Witte L, Tomasik J, Schwarz E, et al. Cytokine alterations in fi rst-episode schizophrenia patients before and after antipsychotic treatment. Schizophr Res. 2014;154:23–29. doi: 10.1016/j.schres.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 16.Melbourne JK, Feiner B, Rosen C, Sharma RP. Targeting the Immune System With Pharmacotherapy in Schizophrenia. Curr Treat Options Psychiatry. 2017;4:139–151. doi: 10.1007/s40501-017-0114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M-L, Wu S, Tsai T-C, Wang L-K, Tsai F-M. Regulation of macrophage immune responses by antipsychotic drugs. Immunopharmacol Immunotoxicol. 2013;35(2):573–580. doi: 10.3109/08923973.2013.828744 [DOI] [PubMed] [Google Scholar]
- 18.Da Cruz Jung IE, Machado AK, Da Cruz IBM, et al. Haloperidol and Risperidone at high concentrations activate an in vitro inflammatory response of RAW 264.7 macrophage cells by induction of apoptosis and modification of cytokine levels. Psychopharmacology (Berl). 2016;233(9):1715–1723. doi: 10.1007/s00213-015-4079-7 [DOI] [PubMed] [Google Scholar]
- 19.Gaskill PJ, Carvallo L, Eugenin EA, Berman JW. Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse. J Neuroinflammation. 2012;9(1):704. doi: 10.1186/1742-2094-9-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Italiani P, Boraschi D. From monocytes to M1 / M2 macrophages : phenotypical vs. functional differentiation. Front Immunol. 2014;5(October):1–22. doi: 10.3389/fimmu.2014.00514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20(2). doi: 10.1038/nn.4418 [DOI] [PubMed] [Google Scholar]
- 22.Herz J, Filiano AJ, Smith A, Yogev N, Kipnis J. Myeloid Cells in the Central Nervous System. Immunity. 2017;46(6):943–956. doi: 10.1016/j.immuni.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song M, Lin F, Ward SE, Fine JP, Hill C. Composite Variables: When and How. Nurs Res. 2013;62(1):45–49. doi: 10.1097/NNR.0b013e3182741948.Composite [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genin M, Clement F, Fattaccioli A, Raes M, Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer. 2015;15(1):577. doi: 10.1186/s12885-015-1546-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt A, Kreig J, Clement H, Schulz E, Vedder H, Heiser P. Effects of quetiapine, risperidone, 9-hydroxyrisperidone and ziprasidone on the survival of human neuronal and immune cells in vitro. J Psychopharmacol. 2010;24(3):349–354. [DOI] [PubMed] [Google Scholar]
- 26.Murphy M, Xiong Y, Pattabiraman G, Qiu F, Medvedev AE. Pellino-1 positively regulates Toll-like receptor (TLR) 2 and TLR4 signaling and is suppressed upon induction of endotoxin tolerance. J Biol Chem. 2015;290(31):19218–19232. doi: 10.1074/jbc.M115.640128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richter E, Ventz K, Harms M, Mostertz J, Hochgräfe F. Induction of Macrophage Function in Human THP-1 Cells Is Associated with Rewiring of MAPK Signaling and Activation of MAP3K7 ( TAK1 ) Protein Kinase. 2016;4(March):1–15. doi: 10.3389/fcell.2016.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ploeger DTA, Hosper NA, Schipper M, Koerts JA, De Rond S, Bank RA. Cell plasticity in wound healing : paracrine factors of M1 / M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun Signal. 2013;11(29):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biswas SK, Lopez-Collazo E. Endotoxin tolerance : new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30(10):475–487. doi: 10.1016/j.it.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 30.Glass CK, Natoli G. Molecular control of activation and priming in macrophages. Nat Immunol. 2015;17(1):26–33. doi: 10.1038/ni.3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feiner B, Chase KA, Melbourne JK, Rosen C, Sharma RP. Risperidone effects on heterochromatin: the role of kinase signaling. Clin Exp Immunol. 2019;196(1):67–75. doi: 10.1111/cei.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller AH, Haroon E, Felger JC. The Immunology of Behavior—Exploring the Role of the Immune System in Brain Health and Illness. Neuropsychopharmacology. 2017;42:1–4. doi: 10.1038/npp.2016.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber MD, Godbout JP, Sheridan JF. Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology. 2016;42(April):1–51. doi: 10.1038/npp.2016.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garré JM, Silva HM, Lafaille JJ, Yang G. CX3CR1+ monocytes modulate learning and learning-dependent dendritic spine remodeling via TNF-α. Nat Med. 2017;23(6):714–722. doi: 10.1038/nm.4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavin Y, Winter D, Blecher-Gonen R, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159(6):1312–1326. doi: 10.1016/j.cell.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cotel M-C, Lenartowicz EM, Natesan S, et al. Microglial activation in the rat brain following chronic antipsychotic treatment at clinically relevant doses. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol. 2015;25(11):2098–2107. doi: 10.1016/j.euroneuro.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 37.Kittan NA, Allen RM, Dhaliwal A, et al. Cytokine Induced Phenotypic and Epigenetic Signatures Are Key to Establishing Specific Macrophage Phenotypes. PLoS One. 2013;8(10):1–15. doi: 10.1371/journal.pone.0078045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waddell SJ, Popper SJ, Rubins KH, et al. Dissecting Interferon-Induced Transcriptional Programs in Human Peripheral Blood Cells. PLoS One. 2010;5(3):1–13. doi: 10.1371/journal.pone.0009753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional Profiling of the Human Monocyte-to-Macrophage Differentiation and Polarization: New Molecules and Patterns of Gene Expression. J Immunol. 2006;177(10):7303–7311. doi: 10.4049/jimmunol.177.10.7303 [DOI] [PubMed] [Google Scholar]
- 40.Qiao Y, Kang K, Giannopoulou E, et al. IFN- g Induces Histone 3 Lysine 27 Trimethylation in a Small Subset of Promoters to Stably Silence Gene Expression in Human Macrophages Report IFN- g Induces Histone 3 Lysine 27 Trimethylation in a Small Subset of Promoters to Stably Silence Gene Expressi. CellReports. 2016;16(12):3121–3129. doi: 10.1016/j.celrep.2016.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]