Abstract
The central gain model of hyperacusis proposes that loss of auditory input can result in maladaptive neuronal gain increases in the central auditory system, leading to the over-amplification of sound-evoked activity and excessive loudness perception. Despite the attractiveness of this model, and supporting evidence for it, a critical test of the central gain theory requires that changes in sound-evoked activity be explicitly linked to perceptual alterations of loudness. Here we combined an operant conditioning task that uses a subject’s reaction time to auditory stimuli to produce reliable measures of loudness growth with chronic electrophysiological recordings from the auditory cortex and inferior colliculus of awake, behaviorally-phenotyped animals. In this manner, we could directly correlate daily assessments of loudness perception with neurophysiological measures of sound encoding within the same animal. We validated this novel psychophysical-electrophysiological paradigm with a salicylate-induced model of hearing loss and hyperacusis, as high doses of sodium salicylate reliably induce temporary hearing loss, neural hyperactivity, and auditory perceptual disruptions like tinnitus and hyperacusis. Salicylate induced parallel changes to loudness growth and evoked response-intensity functions consistent with temporary hearing loss and hyperacusis. Most importantly, we found that salicylate-mediated changes in loudness growth and sound-evoked activity were correlated within individual animals. These results provide strong support for the central gain model of hyperacusis and demonstrate the utility of using an experimental design that allows for within-subject comparison of behavioral and electrophysiological measures, thereby making inter-subject variability a strength rather than a limitation.
Keywords: hyperacusis, central gain enhancement, sodium salicylate, auditory reaction time, local field potentials
INTRODUCTION
Hyperacusis is an auditory perceptual disorder in which moderate-intensity “everyday” sounds are perceived as intolerably loud, aversive, or painful (Baguley, 2003). Accumulating evidence has pointed to excessive central gain enhancement as a potential mechanism underlying loudness hyperacusis, a common and debilitating type of the disorder that is often associated with sensorineural hearing loss. The central auditory system employs a variety of adaptive gain control mechanisms to maintain hearing sensitivity in response to changes in auditory input (Chambers et al., 2016; Salvi et al., 2016; Dean et al., 2005; Robinson and McAlpine, 2009). According to the central gain model of hyperacusis, when these gain control mechanisms are dysregulated, hearing loss can cause a maladaptive over-amplification of sound-evoked activity along the central auditory pathway, leading to normal sounds being perceived as excessively loud (Noreña, 2011; Zeng, 2013; Auerbach et al., 2014). The attractiveness of this model is that it can account for major features of the disorder via plausible neuronal mechanisms, and there is a solid basis of supporting evidence (Auerbach et al., 2014). Validation of the central gain theory of hyperacusis, however, requires a more complete characterization of the relationship between central gain changes and loudness perception.
Human imaging studies have demonstrated increased sound-evoked activity across multiple auditory structures in hyperacusis patients compared to control subjects (Lockwood et al., 1998; Lanting et al., 2008; Melcher et al., 2009; Gu et al., 2010), consistent with the central gain model. Because these studies are in patients with established loudness tolerance issues, it is difficult to discern the dynamic relationship between the observed neurophysiological changes and hyperacusis development. Animal models provide an opportunity to examine hyperacusis pathogenesis in more detail, and numerous animal studies have indeed found evidence for central gain enhancement or sound-evoked hyperactivity following hearing loss induced by noise or ototoxic drugs (Auerbach et al., 2014). A majority of these studies, however, have relied on between-group comparisons using acute electrophysiological measurements from anesthetized subjects, often focusing on one specific auditory area. As such, few studies to date have been able to directly correlate central gain changes with perceptual alterations of loudness, which would provide a crucial test of the central gain theory.
Establishing a direct relationship between central gain enhancement and loudness hyperacusis has been limited by several factors. First, there is a need for objective behavioral readouts of loudness perception that can adequately account for both hearing loss and hyperacusis within individual animals. To address this gap, we have developed a Go/No-go operant conditioning task that can infer psychometric measures of loudness growth based on a subject’s reaction time (RT) to auditory stimuli presented over a wide range of intensities (Chen et al., 2015; Radziwon et al., 2017). RT-intensity (RT-I) measures are closely correlated with estimates of loudness growth and equal loudness contours in humans (Stebbins, 1966; Pfingst et al., 1975; Marshall and Brandt, 1980), and they recapitulate the basic features of loudness recruitment in both hearing impaired humans and animal models of hearing loss (Moody, 1973; Arieh and Marks, 2003; May et al., 2009). We have also observed hyperacusis-like changes to RT-I functions in rats treated with high-dose sodium salicylate (SS), a well-documented ototoxic drug that reliably induces temporary hearing loss (McFadden et al., 1984; Brennan et al., 1996; Radziwon et al., 2015), tinnitus (Turner and Parrish, 2008; Stolzberg et al., 2013); hyperacusis (Radziwon et al., 2017), and neural hyperactivity in animals (Sun et al., 2009; Stolzberg et al., 2012; Chen et al., 2014; Chen et al., 2015). Thus, RT-I functions are a validated behavioral readout of loudness growth, hearing loss, and hyperacusis.
Another major impediment to modeling hyperacusis in animals is accounting for the inherent variability in hyperacusis induction. While damage to the peripheral auditory system is the primary risk factor for the disorder, a majority of individuals with sensorineural hearing loss do not present with loudness tolerance issues (Tyler et al., 2014). Hearing loss is therefore a trigger but not a sufficient predictor for the development of hyperacusis. This variable presentation undoubtedly extends to animal models as well, and it is unlikely that all animals will respond to manipulations of auditory input in the same manner or be expected to consistently develop hyperacusis to the same degree, even in highly controlled experimental settings. Being able to account for this inter-subject variability is a crucial component of any potential hyperacusis model. While some animal studies of hearing loss have attempted to correlate sound-evoked neural hyperactivity with hyperacusis-like behavior at the group level (Sun et al., 2012; Chen et al., 2014; Hickox and Liberman, 2014), a more rigorous test of the central gain model would involve correlating electrophysiological and perceptual changes over time within the same animal in order to account for inter-subject variability. To this end, we have developed a combined psychophysical-electrophysiological approach to track changes in loudness perception and sound-evoked activity from the same experimental animals in a longitudinal manner.
To accomplish this, we chronically implanted electrodes in the auditory cortex (AC) and inferior colliculus (IC) of animals trained on our operant-based RT-I task. First, this allowed us to monitor sound-evoked activity from multiple auditory areas simultaneously in awake animals, thus bypassing the confounds of anesthesia. Moreover, by performing chronic electrophysiological recordings from behaviorally trained animals we were also able to directly correlate neurophysiological measures of sound encoding with daily behavioral assessment of loudness growth on a within-subject basis. To validate this approach and critically test the central gain model, we determined the effect of salicylate treatment on RT-I measures of loudness growth and sound-evoked neural input/output (I/O) functions collected from the same animals. We found that salicylate-induced changes to RT-I functions and sound evoked-activity co-varied within individual animals, providing crucial support for the central gain model and demonstrating the utility of this combined behavioral-electrophysiological paradigm.
EXPERIMENTAL PROCEDURES
Subjects
Eight adult (6–10 months old) Sprague–Dawley rats (Charles River Laboratories; 6 males, 2 females) were used for all behavioral and electrophysiological experiments. All procedures were approved by the Institutional Animal Care and Use Committee at the University at Buffalo. Rats were group housed except following recovery from surgery when they were housed separately for 2–3 days, and were kept on a 12-hour day/night cycle (lights on 6 am; lights off 6 pm). The rats were food restricted and kept at approximately 85% of their free-feeding weight during the course of the experiment. The animals had unrestricted access to water, except while participating in the experiment.
Behavioral training
Food restricted rats were trained in a Go/No-go operant conditioning paradigm to detect broadband noise bursts (1–42 kHz; 50 ms duration, 5 ms rise/fall time, cosine gated) presented at intensities ranging from 30 to 90 dB SPL in 10 dB steps (Fig. 1A). Rats were tested in an acoustically transparent acrylic cage (28 × 30 × 38 cm) located inside a sound attenuating chamber (76 × 71 × 71 cm) lined with 5-cm-thick sound attenuating foam (Illbruck, Inc., Minneapolis, MN, USA). The test cage was equipped with a speaker (FT28D Dome Tweeter, Fostex, Tokyo, Japan), feeder (Med Associates Model ENV-203 M, St. Albans, VT, USA), and nose-poke hole equipped with infrared sensors (Vulintus, Dallas, TX, USA). The behavior of the animals during test sessions was monitored by a digital camera (Fire-i Digital Camera, Unibrain, San Ramon, CA, USA). Tucker-Davis Technologies (TDT, Gainesville, FL) system-3 equipment using TDT RPvds software and custom MATLAB software (MathWorks, Natick, MA, USA) were used to control all aspects of the experiment as described previously (Stolzberg et al., 2011).
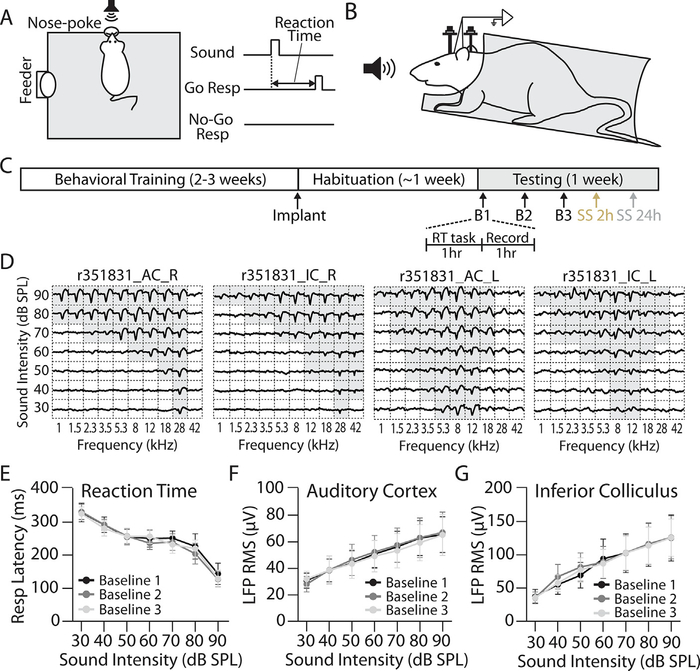
Fig. 1.
Experimental design. (A) Behavioral testing. Animals were trained on a Go/No-go operant conditioning task to detect broadband (1–42 kHz) noise bursts presented in pseudorandom order from 30 to 90 dB SPL. Reaction time (RT) was quantified as the latency between sound onset and removal of the rat’s nose from the nose-poke hole on Go trials. (B) Electrophysiological recordings. Behaviorally trained rats were bilaterally implanted with chronic tungsten microelectrodes in the AC and IC. Recordings were made from awake, head-fixed rats while passively listening to the same range of broadband noise bursts used for behavioral testing. (C) Behavioral-electrophysiological paradigm. Animals were tested daily to collect RT-I functions (see Methods and panel A.). Immediately following behavioral testing, auditory-evoked local field potentials (LFPs) were recorded from RT-I-trained rats (see Methods and panel B). Following 3 baseline LFP testing sessions (every other day over 5 days), rats were treated with sodium salicylate (SS; 200 mg/kg, i.p.) and then RT-I functions, followed by LFP input/output (I/O) functions, were collected beginning 2 h and 24 h post-SS. (D) Representative LFP tuning curves from the left (L) and right (R) AC/IC of an awake animal. Tone-evoked LFP responses were well-tuned for frequency and matched for characteristic frequency within but not between hemispheres. Scale for each division is 400 μV by 30 ms. (E) Mean (n = 8) of three baseline RT-I functions; note close overlap of three measurements. (F) Mean AC and (G) IC I/O functions (n = 8) collected concurrently from the same animals over the same 3 baseline days of testing as RT-I functions in (E). Note overlap and stable measures over all three AC and IC baseline test sessions. One-way repeated measures ANOVA found no significant effect of testing session for RT-I (F(2,18) = 0.037, p = 0.98, ƞ2 = 0.004), AC LFP I/O (F(2,18) = 0.053, p = 0.98, ƞ2 = 0.006), or IC LFP I/O (F(2,18) = 0.019, p = 0.99, ƞ2 = 0.002) functions. Data points represent mean ± SEM.
Details of our behavioral testing procedures have been described previously (Chen et al., 2014; Radziwon et al., 2015). Briefly, a rat initiated a trial by placing its nose in a nose-poke hole, which initiated a variable waiting interval ranging from 1 to 4 s. The rat had to maintain its position in the nose-poke hole until it heard a noise burst or the trial was aborted. In the Go condition, the target stimulus was the noise burst. If the rat detected this signal by removing its nose from the nose-poke hole within 2 s, a food reward (45 mg dustless rodent grain pellets, BioServ) was delivered and a hit was recorded. A miss was recorded if the rat failed to remove its nose from the nose-poke within the 2-s response interval. Approximately 30% of all trials were catch trials. These constituted the No-go part of the procedure and noise bursts were not presented during these trials. If the rat removed its nose during a catch trial, a false alarm was recorded and the rat received a 15-s timeout, during which the house light was turned off and the rat could not initiate another trial. However, if the rat maintained its position in the nose-poke during trials when the stimulus was absent, a correct rejection was recorded. No reinforcement was given for a correct rejection. Catch trials were necessary to ensure that the animals were under stimulus control during the course of the experiments.
The rats were first trained to detect a 60 dB SPL broadband noise burst. Rats were considered trained and under stimulus control if the following conditions were met each day over five consecutive days: >200 trials initiated, >90% hit rate, and <10% false alarm rate. Once the training criteria were met, suprathreshold reaction time-intensity (RT-I) functions were collected from the rats in response to noise bursts presented according to the psychophysical Method of Constant Stimuli (MOCS). Within each 10-trial block, seven predetermined target intensities (30–90 dB SPL in 10 dB steps) were presented in random order along with three catch trials. RTs were taken from the onset of the noise burst to the time the rat removed its nose from the nose-poke hole (Fig. 1A). Only RTs for hits (when the animal correctly detected the stimulus) were included in our analysis. The average RT at each intensity over the first 150–200 trials of each session (i.e. 15–20 repeats for each intensity) were used for data analysis, which we have previously shown is sufficient to produce reliable response measures (Radziwon et al., 2017).
Surgical procedures
Our surgical procedures were similar to those described in our recent publications (Sun et al., 2009; Stolzberg et al., 2013). Behaviorally trained rats were chronically implanted in the AC and IC with single tungsten microelectrodes (0.5–1.0 mΩ; FHC, Bowdoin, ME) using sterile surgical techniques. A surgical plane of anesthesia was induced with a cocktail of ketamine (70 mg/kg, i.p.) and xylazine (7 mg/kg, i.p.) supplemented with smaller doses (20/2 ketamine/xylazine mg/kg, i.p.) as needed to maintain a suitable plane of anesthesia. Body temperature was maintained at 37 °C using a homeothermic heating pad (Harvard Apparatus, Holliston, MA).
Anesthetized rats were fixed in a standard stereotaxic frame with blunted ear bars, and the scalp swabbed with alcohol and then povidone-iodine (Betadine). An incision was made along the scalp midline, connective tissue was removed by blunt dissection around the skull, and the top of the skull scraped with a scalpel blade to remove any remaining tissue and cleaned with hydrogen peroxide (3%). The muscle overlying the temporal bone was separated from the skull using blunt resection and gently extended away from the skull using surgical sutures attached to hemostats. Seven sterilized stainless steel bone screws were fixed to the surface of the skull; 2 in the left and right frontal bones over the olfactory bulb, 2 in the left and 2 in the right parietal bones, and 1 along the midline of the occipital bone over the cerebellum. A stainless steel threaded holding bar (1 cm long, 0.5 cm diameter) was attached via dental cement to the two head screws over the frontal bone and a stainless steel flathead rivet (1.5 cm length) was attached via dental cement to the head screw over the occipital bone to create a two-point attachment fixture for subsequent recordings from awake, head-fixed animals. A silver wire was attached to an additional head screw over the right frontal bone that served as a combined ground/reference. Rats were given post-surgical injections of carprofen (4 mg/kg) and sterile saline (2.0 mL) to reduce pain and dehydration, respectively. Animals were allowed to recover for at least 48 hours after the surgery before returning to behavioral testing.
Electrode implantation
Using appropriate stereotaxic coordinates (Paxinos and Watson, 2007), small craniotomies were made using a motorized drill with a carbide bit over the dorsal and lateral skull to gain access to the left/right IC and AC, respectively, leaving the dura mater intact. A single channel tungsten microelectrode (FHC, Catalog #: UEWSFE-SEBN1K), was slowly lowered via a hydraulic microdrive into the primary AC of the left hemisphere while presenting 70 dB broadband noise bursts (1–42 kHz, 50 ms, 5 ms rise/fall time, cosine gating) to the contralateral ear to confirm the electrode was in the appropriate location. Brief tone-evoked (50 ms tone bursts, 5 ms rise/fall time, cosine gating; 1–42 kHz, 10 logarithmic steps; 20–80 dB SPL, 10 dB steps; 5 repetitions) excitatory frequency receptive fields (FRFs) of multiunit clusters were determined from at least three locations along anterior-posterior axis of the AC in order to delineate the tonotopic gradient of primary AC. Following the final electrode placement, the cortex was covered with Kwik-Sil™ (World Precision Instruments; Sarasota, FL) to protect the exposed area and then the electrode was fixed in place with dental cement. A similar procedure was then used to implant a single tungsten electrode (FHC, Catalog #: UEWSGESEBN1K) into the left IC. In this case, multiunit FRFs were determined for at least 3 locations along the dorsal–ventral axis of the IC in order to determine the tonotopic gradient and confirm electrode location was in the central nucleus of the IC. This procedure was then repeated for electrodes implanted in the right AC and right IC. Care was taken to match characteristic frequency (CF) between AC and IC electrodes within a given hemisphere, but the electrodes in opposite hemispheres were not necessarily matched for CF in order to increase the number of frequency regions sampled (Fig. 1D). Across all animals, the CF of the implanted electrodes ranged from 8 to 36 kHz, with a majority (26/32 total electrodes) having a CF between 12–24 kHz. Following electrode fixation, the flexible gold pin electrode leads were fixed with cyanoacrylic glue and dental cement. A final layer of dental cement was used to complete the headcap. In order to reduce the immune response associated with electrode implantation and to increase the long-term viability of electrode recordings, rats were treated with minocycline in their home cage drinking water (100 mg/L) for 2 days pre- and 5 days post-implantation (Rennaker et al., 2007).
Acoustic stimulation
Broadband noise or tone bursts (1–42 kHz; 50 ms; 5 ms rise/fall cos2 gating) were generated using a TDT RX6–2 processor (~100 kHz sampling rate) and delivered through a dome tweeter speaker (FT28D, Fostex, Tokyo, Japan). Binaural sound stimulation was delivered free field with the speaker located 10 cm from the animal’s head, equidistant from each ear. Sound pressure levels were calibrated using a sound level meter (Larson Davis System 824;) equipped with a microphone (1/4″ free field microphone, model 2520, Larson-Davis, Depew, NY, USA) located at the position where the animal’s head would be in the behavioral apparatus or electrophysiology recording chamber (Fig. 1).
Electrophysiological recordings
Sound-evoked local field potentials (LFPs) were acquired from awake, head-fixed rats in a sound isolation booth using a Tucker-Davis System 3 processor (TDT, Alachua, FL). Signals were pre-amplified by an RA16PA and sampled at 25 kHz by an RX5 base station. Custom-written data acquisition and analysis software (MATLAB R2007b, MathWorks) were used to acquire the data as previously described (Stolzberg et al., 2011; Chen et al., 2012). LFPs were continuously acquired and digitally resampled at 610 Hz and bandpass filtered from 2–300 Hz. LFP responses were collected in response to broadband noise bursts (1–42 kHz; 50 ms duration, 5 ms rise/fall time, cosine gated) presented at intensities ranging from 30 to 90 dB SPL in 10 dB steps with each intensity presented in blocks of pseudorandom order (100 repetitions per intensity, 500 ms inter-stimulus interval). LFPs were averaged over 100 repetitions for each intensity. In order to characterize FRFs from awake animals, tone-evoked LFPs were also collected using tone bursts (50 ms duration, 5 ms rise/fall time, cosine gated) presented in 10 logarithmic frequency steps between 1 and 42 kHz with intensities from 30 to 90 dB SPL in 10-dB steps (30 repetitions per frequency/level, 300 ms inter-stimulus interval, pseudorandom order). Tone-evoked LFPs from awake animals were well-tuned for frequency and followed the expected tonotopic gradients in the AC and IC (Fig. 1D). Individual waveforms for each trial were manually inspected for movement artifacts and removed from analysis if necessary. Response magnitude was quantified by taking the root mean square of the average LFP waveform at each intensity over a 50-ms window beginning with stimulus onset.
Experimental design
During the training phase of behavioral testing, rats were habituated to the electrophysiological recording chamber and sound presentation for at least 1 week prior to electrode implantation. Once the rats were fully trained and exhibited stable baseline RT-I functions, electrode implantation was performed as described above. Following recovery from surgery, rats resumed daily behavioral testing in the RT paradigm. Immediately after behavioral testing, rats were transferred to the recording chamber situated in a sound isolation booth where they were gradually habituated to head-fixing over ~1 week. The recording chamber consisted of an opaque, narrow plastic tube that snuggly fit the rat with an opening for the head and a speaker (FT28D Dome Tweeter, Fostex, Tokyo, Japan) placed equidistant (10 cm) from each ear for binaural sound stimulation (Fig. 1B). The chamber was at a slight incline to allow animals to assume typical posture with their hind limbs rested and the rostral portion of the body slightly elevated. Two modified stereotaxic arms were used to hold the head in place at the front and rear headposts. Habituation to head-fixing followed a graded exposure technique (Schwarz et al., 2010). For the first several days, the animal was allowed to freely enter the recording apparatus and fixation was gradually introduced by gently holding the headpost by hand for increasing time intervals (5–30 s). Over the next several days, the animal was firmly held in place with a 2-point head-fixture (Fig. 1B), starting with 1 minute sessions and gradually increasing the duration. After each daily habituation session, the animal was immediately given a food pellet reward. During this habituation period, noise bursts of varying intensity (30–90 dB SPL) were gradually introduced as well. Animals were typically fully habituated to head-fixing and sound stimulation by one week (Fig. 1C).
Following habituation, animals continued to be tested daily in the RT-I paradigm (~1 h/animal/day). On most days, following behavioral testing the animal was immediately transferred to the recording chamber where sound-evoked responses were recorded from chronically implanted electrodes while animals were awake and passively listening (<1 h/animal/day). To ensure stability of behavioral and electrophysiological responses, RT-I measures were obtained daily for five consecutive days and electrophysiological recordings were obtained from at least three recording sessions spanning these five days (i.e. every other day over the five-day period) before salicylate administration (Fig. 1C). All animals exhibited stable RT-I functions over this time period. Only animals that exhibited stable LFP input/output (I/O) functions in both the AC and IC of at least one hemisphere over this baseline period were included in the data analysis. Six out of eight animals tested had stable AC and IC responses in both hemispheres, with the other two only having stable AC and IC responses across one hemisphere. One animal was excluded from analysis due to electrode failure. For animals with stable responses in both hemispheres, LFP response functions were averaged across hemispheres in order to facilitate comparison between behavioral and electrophysiological measures in a within-subject manner.
Salicylate administration
Following behavioral training, electrode implantation, and stable baseline testing, rats were administered sodium salicylate (SS, 200 mg/kg, dissolved in sterile saline) via intraperitoneal (i.p.) injection (Fig. 1C). These injections were administered 2 h before behavioral testing.
Data analysis
Group differences in RT-I, AC, and IC LFP I/O functions between baseline and post-SS administration were analyzed using 2-way repeated measures ANOVAs with sound level (30–90 dB SPL in 10 dB steps) and treatment (Baseline vs. Post-SS 2 h vs. Post-SS 24 h) as factors and Bonferroni post-hoc pairwise significance tests corrected for multiple comparisons (significance level p < 0.05). Changes to response gain for behavioral and electrophysiological growth functions following SS administration were quantified as follows. First, RT-I functions and AC/IC LFP I/O functions collected from each animal were fit by linear regression and the slope of the fitted line, representing the gains of the responses, were compared between pre- and post-treatments times. Because our analysis was restricted to suprathreshold responses (30–90 dB SPL) we found a linear regression fit the data better than a sigmoid or two-tailed, six parameter Gaussian function (Watkins and Barbour, 2008). The average coefficient of determination (r2) for each condition (Average Baseline, Post-SS 2 h, Post-SS 24 h) was as follows for RT-I, AC I/O, and IC I/O functions. Baseline: RT r2 = 0.82, AC r2 = 0.85, IC r2 = 0.96. Post-SS 2 h: RT r2 = 0.89, AC r2 = 0.90, IC r2 = 0.95. Post-SS 24 h: RT r2 = 0.84, AC r2 = 0.79, IC r2 = 0.91. Significant slope changes were determined using a two-tailed F-test against the null hypothesis that the slopes of the two lines (Baseline vs. Post-SS 2 h, Baseline vs. Post-SS 24 h, or Post-SS 2 h vs. Post-SS 24 h) were identical (significance level p < 0.05).
Our second method to quantify gain changes was to perform a linear transformation of the average baseline response (e.g., RT, AC LFP, or IC LFP amplitude) versus the response on any given day pre- or post-SS treatment. To do this, the average baseline response (RBaseline) at each intensity (30–90 dB SPL, 10 dB steps) was plotted against the response at the equivalent intensity on a given day (RDay). The resulting scatter plot indicates the difference in response magnitude at each intensity on a given day as compared to baseline (see Fig. 2D–F). The scatter plot was then fitted with a linear regression according the formula:
Fig. 2.
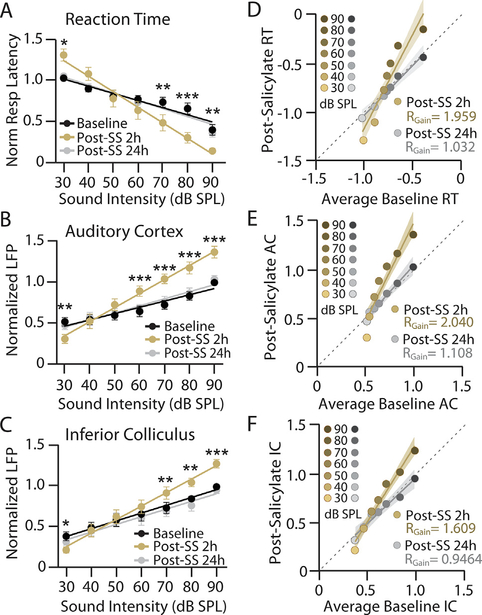
Salicylate induces time-dependent parallel changes to reaction time, cortical, and subcortical response-intensity functions in a manner consistent with temporary hearing loss, increased loudness perception and central gain enhancement. A-C: Mean (n = 8) (A) RT-I functions, (B) AC LFP I/O functions, and (C) IC LFP I/O functions from behaviorally trained rats with chronically implanted electrodes before and after sodium salicylate (SS) administration (200 mg.kg, i. p.); data shown before (black), 2 h post-SS (orange), and 24 h post-SS (gray). Baseline (black) represents average response across three baseline sessions. For ease of comparison, all responses were normalized to maximum average baseline response to account for between-subject differences in absolute RTs and AC/IC response size. Data points represent mean +/−SEM. Lines represent linear fit of RT, AC, and IC response functions, which were used to determine changes in response slope (see Table 1). Two-way repeated measure ANOVAs found significant main effects of SS treatment and sound intensity, as well as an interaction between the two, for RT (SS: F2,98 = 8.84, **p = 0.0003, ; Intensity: F6,98 = 28.82, ***p < 0.0001, ; interaction: F12,98 = 6.14, ***p < 0.0001, ), AC responses (SS: F2,98 = 35.64, ***p < 0.0001, ; Intensity: F6,98 = 22.03, ***p < 0.0001, ; interaction: F12,98 = 8.90, ***p < 0.0001, ) and IC responses (SS F2,98 = 25.18, ***p < 0.0001, ; Intensity: F6,98 = 17.83, ***p < 0.0001, ; interaction F12,98 = 4.67, ***p < 0.0001, 0.46). Post-hoc significance for intensity-dependent effects of SS at 2 h compared to baseline signified as: *p < 0.05, **p < 0.01, ***p < 0.0001. No significant effects of were found between baseline and 24 h post-SS. D-F: Scatter plots comparing the average normalized baseline response at each intensity against the normalized response 2 h post-SS (orange-brown) or 24 h post-SS (gray-black) of equivalent intensity for (D) RT, (E) AC LFP, and (F) IC LFP responses. The slope of the linear fit (RGain) provides an estimate of the change in response gain across conditions where RGain = 1 indicates a response growth rate matched to baseline levels while RGain > 1 and RGain < 1 indicate a multiplicative enhancement or divisive decrease in response growth rate compared to baseline, respectively. Shading represents 95% confidence interval of linear fit. A two-tailed F-test determined that RGain was significantly different from 1 at 2 h post-SS for RT-I, AC, and IC LFP functions (RT: RGain = 1.959, F(1,54) = 21.89, ***p < 0.0001, d = 3.31; AC: RGain = 2.040, F(1,54) = 31.84, ***p < 0.0001, d = 3.99; IC: RGain = 1.609, F(1,54) = 26.19, ***p < 0.0001, d = 3.62). A 24 h post-SS, no response gain significantly deviated from 1 (RT: RGain = 1.032, F(1,54) = 0.070, p = 0.792, d = 0.187; AC: RGain = 1.108, F(1,54) = 0.596, p = 0.444, d = 0.546; IC: RGain = 0.9464, F(1,54) = 0.190, p = 0.665, d = 0.308).
In this manner, the slope of the linear fit (RGain) describes the multiplicative (RGain > 1), divisive (RGain < 1), or negligible (RGain = 1) change in response growth rate (i.e., response gain) on any given testing session with respect to the baseline period. Significant gain changes were determined by: (1) a two-tailed F-test against the null hypothesis that the slope of the linear fit (RGain) was not significantly different from 1 (i.e., no change compared to baseline growth rate); or (2) a one-way repeated measures ANOVA to examine gain changes across conditions (Baseline 2, Baseline 3, Post-SS 2 h, Post-SS 24 h) with Tukey post-hoc test for pairwise significance corrected for multiple comparisons (significance level p < 0.05).
To quantify the relationship between changes to RT, AC and IC response gain following SS administration, RT response gain (RGain) was plotted against AC or IC RGain and a Pearson’s correlation coefficient (r2) was determined for each of these scatter plots. A linear regression was also performed to determine the correspondence between variables (a slope of 1 would indicate a perfect correspondence). Additionally, the Lin’s concordance coefficient (rc) was used to assess the degree of agreement between behavioral (X) and electrophysiological (Y) measures, calculated as follows:
This method measures how far the data deviate from the line of perfect concordance (i.e., 45° slope on a square scatter plot) and thus quantifies how closely two measures relate to each other in terms of magnitude (Lin, 1989). In order to quantify the relationship between electrophysiological and behavioral changes following SS administration in an intensity-dependent manner, the percent change in RT, as compared to the average baseline response, for each animal at each intensity was plotted against the percent change in AC or IC response. A Pearson’s correlation coefficient (r2) and a linear regression were determined for each of these scatter plots. All statistical analyses were performed using Graphpad Prism 5 or MATLAB (R2017b) software.
RESULTS
Stable behavioral and electrophysiological measures of loudness growth
Reaction time-intensity (RT-I) functions and LFP input/output (I/O) functions were collected from eight rats trained in a Go/No-go operant conditioning paradigm with electrodes chronically implanted in the primary AC and central nucleus of the IC (Fig. 1A–C). Fig. 1D shows representative LFP tuning curves from one animal. Tone-evoked LFPs were well-tuned for frequency, consistent with primary-like responses, and AC and IC electrodes were roughly matched for characteristic frequency within each hemisphere. The mean (±SEM, n = 8) baseline RT-I and LFP I/O functions to broadband noise burst (30–90 dB SPL) are shown in Fig. 1D–E. Mean RTs for the baseline testing sessions decreased from ~325 ms at 30 dB SPL to ~140 ms at 90 dB SPL. The mean amplitudes of the AC I/O functions measured across the same baseline testing sessions increased monotonically from ~30 μV at 30 dB SPL to ~70 μV at 90 dB SPL (Fig. 1E). Finally, mean amplitudes of the IC I/O functions collected from the same animals increased from ~40 μV at 30 dB SPL to ~125 μV at 90 dB SPL (Fig. 1F). No statistically significant differences in RT-I, AC, or IC I/O functions were observed across baseline sessions (Fig. 1, see legend for details on statistical comparisons). Taken together, these results indicate that behavioral and electrophysiological responses measured in this manner are extremely stable in the absence of any explicit manipulation.
Salicylate-induced hyperacusis and hyperactivity
Following baseline testing, animals were injected with 200 mg/kg SS (i.p.) and changes to RT and evoked responses were assessed 2 h post-SS treatment and again 24 h later. To facilitate analysis of the group data, RTs and AC/IC LFP amplitudes were normalized to their maximum average baseline values (Fig. 2). At 2 h post-SS (orange), RT-I functions and AC/IC I/O functions changed significantly from baseline, but the changes were in opposite directions at low versus high intensities (Fig. 2, see legend for details on statistical comparisons). For low-intensity noise bursts of 30 dB SPL, RTs were significantly slower than baseline (Fig. 2A) whereas AC and IC amplitudes were significantly smaller than baseline (Fig. 2B–C). The behavioral and electrophysiological changes observed at low-intensities are likely the result of cochlear hearing loss associated with SS-mediated ototoxicity (Yang et al., 2007; Chen et al., 2015). In contrast, at moderate-to-high intensities of 70–90 dB SPL, RTs were significantly faster than baseline and AC/IC amplitudes were significantly larger than baseline. The significantly shorter RTs at high intensities would indicate that these sounds are perceived as louder than normal (Marshall and Brandt, 1980), suggestive of hyperacusis (Lauer and Dooling, 2007). The significantly larger AC and IC amplitudes in the 70–90 dB range are evidence of enhanced central gain. Both the behavioral and electrophysiological changes that were readily apparent 2 h post-SS (Fig. 2A–C, orange) were fully reversed 24 h post-SS (Fig. 2A–C, gray), likely due to drug washout (Jastreboff et al., 1986; Boettcher et al., 1990).
Salicylate-induced gain modulation
The results in Fig. 2A–C demonstrate that behavioral measures of loudness growth and sound-evoked LFP responses are altered by SS in an intensity-dependent manner, with slower RTs and decreased LFPs at low intensities but faster RTs and enhanced LFPs at high intensities. In other words, the slope of the response functions became steeper, a hallmark of the central gain theory of hyperacusis (Zeng, 2013; Auerbach et al., 2014). Response gain, defined as the change in output (e.g. RT or LFP amplitude) per dB SPL, was measured pre- and post-SS using two approaches. In the first approach, RT-I and AC/IC LFP response functions were fit with linear regression and the slope (normalized response/dB) of the fit lines were used to determine how SS altered response gain (Fig. 2A–C, Table 1). At 2 h post-SS treatment, RT, AC, and IC response functions all had significantly greater slopes compared to baseline (see Table 1 for details on statistical comparisons). At 24 h post-SS, there was no significant difference in slope for any response functions compared to baseline (Table 1).
Table 1.
Salicylate-induced changes in the slope of behavioral and electrophysiological response functions. A linear regression was performed for RT-I, AC LFP, and IC LFP I/O functions for average baseline, Post-SS 2 h, and Post-SS 24 h conditions. The slope of the linear fit for RT, AC and IC response functions at each condition (Baseline, Post-SS 2 h, Post-SS 24 h) was used to quantify changes in response gain. Pairwise (Baseline vs. Post-SS 2 h, Baseline vs. Post-SS 24 h, or Post-SS 2 h vs. Post-SS 24 h) two-tailed F test against the null hypothesis that there was no difference in response slopes between conditions was used to determine significant changes to response slope for RT-I, AC LFP and IC LFP I/O functions. Bold p values represent significant differences.
| Response Slope (normalized response/dB SPL) |
Two-tailed F Tests |
||||||
|---|---|---|---|---|---|---|---|
| Baseline | Post-SS 2 h | Post-SS 24 h | Comparison | F (1,108) | d | p value | |
| RT | −0.009 ± 0.001 | −0.018 ± 0.002 | −0.009 ± 0.001 | Baseline vs. Post-SS 2 h | 28.91 | 3.80 | ***p < 0.0001 |
| r2 = 0.56 | r2 = 0.71 | r2 = 0.56 | |||||
| Baseline vs. Post-SS 24 h | 0.049 | 0.157 | p = 0.825 | ||||
| Post-SS 2 h vs. 24 h | 26.52 | 3.64 | ***p < 0.0001 | ||||
| AC | 0.008 ± 0.001 | 0.017 ± 0.001 | 0.009 ± 0.001 | Baseline vs. Post-SS 2 h | 34.83 | 4.17 | ***p < 0.0001 |
| r2 = 0.52 | r2 = 0.77 | r2 = 0.52 | |||||
| Baseline vs. Post-SS 24 h | 0.456 | 0.478 | p = 0.501 | ||||
| Post-SS 2 h vs. 24 h | 24.90 | 3.53 | ***p < 0.0001 | ||||
| IC | 0.010 ± 0.001 | 0.016 ± 0.001 | 0.009 ± 0.001 | Baseline vs. Post-SS 2 h | 14.65 | 2.71 | **p = 0.0002 |
| r2 = 0.59 | r2 = 0.74 | r2 = 0.52 | |||||
| Baseline vs. Post-SS 24 h | 0.069 | 0.19 | p = 0.793 | ||||
| Post-SS 2 h vs. 24 h | 15.26 | 2.76 | **p = 0.0002 | ||||
Salicylate-induced gain modulation was also quantified by transforming the average baseline RT/AC/IC intensity-response function to the equivalent response function post-SS treatment (see Methods). The resulting scatter plots indicate the change in response magnitude at each intensity for a given time point following SS treatment (Fig. 2D–F). For instance, Fig. 2D plots the average baseline RT for each intensity (x axis) against the RT for that given intensity at 2 h post-SS (y axis; orange to brown symbols) or 24 h post-SS (y axis; gray to black symbols). In this manner, any point falling below the 45° unity line (dashed line) indicates a decrease in response at that intensity following SS treatment. Conversely, any point above the 45° line indicates an increased response at that intensity following SS treatment. Similar scatter plots are shown for the AC and IC LFPs in Fig. 2E and F, respectively. Because of the inverse relationship between RT and sound intensity (i.e. RTs decrease with increasing sound intensity), we took the additive inverse of RT measures in order to facilitate comparison with evoked response functions. The scatter plots were then fit by linear regression for both the 2 h (orange line) and 24 h (gray line) post-SS data. The slope of the linear fit (RGain) for this plot describes the magnitude of gain modulation caused by SS treatment (Fig. 2D–F). If RGain > 1, this would indicate that SS treatment caused a multiplicative enhancement in response gain (RG +). If RGain < 1, this would indicate that SS treatment caused a divisive decrease in response gain (RG −). If RGain = 1, this would indicate that there was no change in response gain (RG ==), i.e. the response growth rate following SS treatment is the same as the baseline rate.
Response gain did not significantly deviate from 1 (i.e. RG ==) across baseline sessions for RT-I or AC/IC LFP I/O functions, confirming that baseline responses were indeed stable (Fig. 4A–C). However, at 2 h post-SS treatment, the slope of linear fit to the RT, AC, and IC functions were all significantly greater than 1 (RG +), indicative of increased response gain (Fig. 2D–F, see legend for details on statistical comparisons). Interestingly, we found a more robust gain increase in the AC than the IC (F(1,115) = 4.0445, *p = 0.0467, d = 1.42), suggesting there is a progressive amplification of auditory-evoked activity along the ascending auditory pathway. At 24 h post-SS, slopes for all response functions no longer significantly deviated from 1 (RG ==), indicating response growth rate had returned to baseline levels (Fig. 2D–F).
Fig. 4.
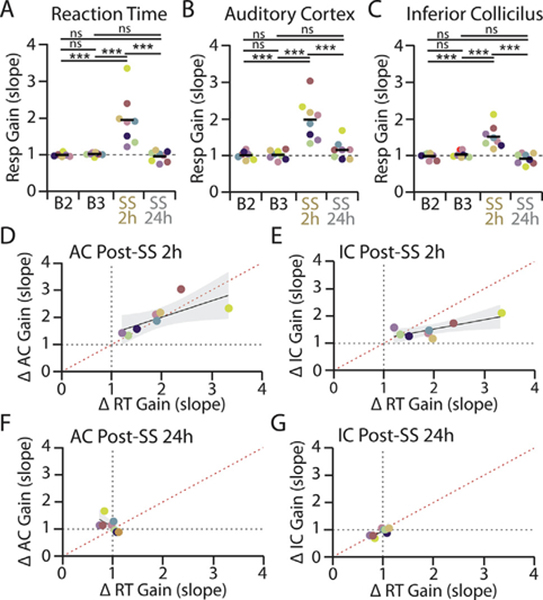
Salicylate-induced changes to reaction time and evoked response-intensity functions co-vary within individual animals. A-C: Response gain (RGain), as quantified in Fig. 2D–F, was determined for each animal (n = 8) over the final two baseline testing sessions (B2, B3), 2 h post-SS and 24 h post-SS for (A) RT, (B) AC, and (C) IC response functions. Each color corresponds to an individual animal. One-way repeated measures ANOVAs found a significant effect of SS treatment on RT (F(3,28) = 14.44, ***p < 0.0001, ƞ2 = 0.67), AC LFP (F(3,28) = 19.25, ***p < 0.0001, ƞ2 = 0.73) and IC LFP (F(3,28) = 15.20, ***p < 0.0001, ƞ2 = 0.68) response gains. Post-hoc significance ***p < 0.0001. There was also a significant increase in the variance of response gains following SS administration (RT: Bartlett’s statistic for equal variance = 58.7, *** p < 0.0001; AC: Bartlett’s statistic = 22.97, ***p < 0.0001; IC: Bartlett’s statistic = 15.62, **p = 0.0014). D-G: Relationship between changes to RT-I and evoked response following salicylate administrations. Correlation between the fold change in RT gain (RGain) versus (D) AC response gain or (E) IC response gain at 2 h post-SS. F-G: Correlation between the fold change in RT gain and (F) AC response gain or (G) IC response gain at 24 h post-SS. Each color corresponds to an individual animal as in panels A-C. Shading represents 95% confidence intervals of linear fit (black). Red dotted line corresponds to 45° unity line.
Within-subject relationship between RT, AC and IC gain changes
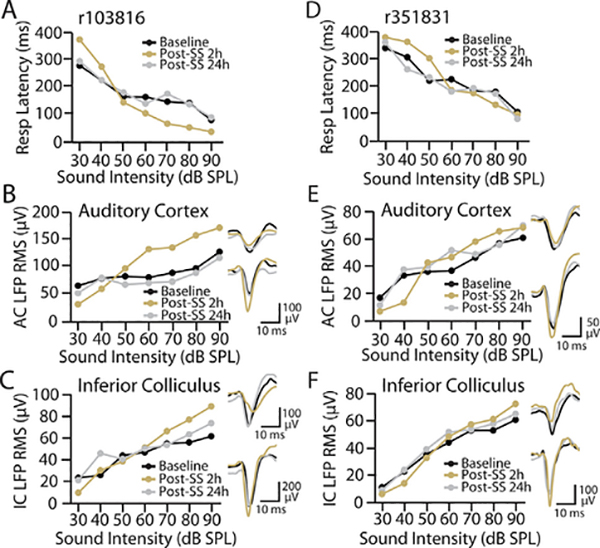
The mean data in Fig. 2 demonstrate that salicylate produced similar changes to response gain for RT and LFP I/O functions, suggesting that central gain enhancement is associated with altered loudness perception. However, from the grouped data it is unclear how closely these gain changes in RT and LFP responses correspond to each other within an individual animal. Fig. 3 shows the effects of SS treatment on behavioral and electrophysiological I/O functions for two animals. In an animal that had substantial alterations to its RT-I intensity function 2 h post-SS, there were also pronounced changes to its AC I/O function and to a slightly lesser extent its IC I/O function (Fig. 3A–C). Conversely, in an animal that exhibited only modest behavioral changes 2 h post-SS, relatively small alterations were also observed in the AC and IC I/O functions (Fig. 3D–F). In both animals, RT-I and AC/IC I/O functions returned to baseline levels at 24 h post-SS, consistent with the grouped data. These results suggest that, at least qualitatively, the behavioral and electrophysiological changes induced by salicylate appear to be highly correlated within an individual animal.
Fig. 3.
Individual variability in behavioral and electrophysiological response to salicylate administration. A-C: Example animal that exhibited significant changes to (A) RT, (B) AC, and (C) IC response functions 2 h post-SS (orange), which recovered to baseline (black) levels by 24 h post-SS (gray). D-F: Example animal that exhibited relatively mild alterations to (A) RT, (B) AC, and (C) IC response functions following salicylate administration. Waveforms represent LFP response from each animal in each auditory area to 30 dB SPL (top) or 90 dB SPL (bottom) noise bursts during baseline (black), 2 h post-SS (orange) or 24 h post-SS (gray).
To quantify the relationships between RT-I, AC and IC gain changes on a subject-by-subject basis, we computed the change in response gain (RGain) for the RT-I, AC and IC I/O functions as in Fig. 2D–F for each individual animal across baseline and post-SS testing sessions (Fig. 4A–C, each colored symbol represents a different animal). Consistent with the mean data (Fig. 2), a majority of the subjects exhibited a significant increase in response gain (RG +) for RT-I, AC and IC I/O functions at 2 h post-SS, with the gain increases being more pronounced for RT-I and AC I/O functions than IC I/O functions. For RT-I functions, 7/8 animals had a significant change in response gain 2 h post-SS treatment compared to baseline (significance level p < 0.05). For AC I/O functions, 6/8 animals had a significant increase in response gain 2 h post-SS treatment compared to baseline (significance level p < 0.05). For IC I/O functions, 5/8 animals had a significant change in response gain 2 h post-SS treatment compared to baseline (significance level p < 0.05). Individual response gains for RT-I, AC and IC I/O functions all fully recovered to baseline levels (RG ==) at 24 h post-SS (Fig. 4A–C). It is important to note that even though a majority of animals exhibited increased response gain following SS treatment, the magnitude of the gain changes varied greatly across animals. If the individual gain alterations to RT-I, AC and IC I/O functions were highly correlated within individual animals, this would be strong evidence for a direct relationship between gain enhancement and altered loudness perception.
To test this hypothesis, we quantified the relationship between SS-induced changes in RT-I, AC and IC I/O in two ways. First, the response gains (RGain values in Fig. 4A–C) at 2 h post-SS for individual AC I/O (Fig. 4D) or IC I/O functions (Fig. 4E) were plotted against that individual’s RT-I gain change. There was a significant positive correlation between individual AC and RT-I response gains (r2 = 0.726, *p = 0.0413) as well as between individual IC and RT-I response gains (r2 = 0.707, *p = 0.0498). While the correlation between these measures indicates they are related, it does not necessarily mean they are matched in terms of magnitude of change. If the RT-I gain changes were perfectly matched to the AC and IC gain changes, then all points on the scatter plots in Fig. 4D and 4E would lie on the red 45° line and the slope of the linear fit would equal 1. For the RT-I versus AC scatter plot data at 2 h post-SS, individual points were clustered around the 45° line and the slope of linear fit was significantly greater than 0 (slope = 0.6928; F(1,6) = 6.704, *p = 0.0413, d = 1.83) but not significantly different from 1 (F(1,6) = 3.162, p = 0.1257, d = 1.26). Furthermore, the value of Lin’s concordance coefficient (rc) for the AC-RT scatter plot, which measure how far the data points deviate from the 45 ° line (see Methods), was high and similar in value to the Pearson’s correlation coefficient (rc = 0.7104 vs. r2 = 0.726). These results demonstrate that AC and RT-I gain changes were not only correlated, but there was close concordance in the magnitude of these changes.
While there was a significant correlation between IC and RT gain changes, most data points for the RT-IC plot fell below the 45° line. Consistent with this, while the slope of linear fit was significantly greater than 0 (slope = 0.3392; F(1,6) = 8.410, *p = 0.0273, d = 2.05), it was also significantly less than 1 (F(1,6) = 31.91, **p = 0.0013, d = 3.99). Moreover, the concordance coefficient for the RT-IC scatter plot was much lower than the correlation coefficient (rc = 0.4093 vs. r2 = 0.707). Thus, while RT-I and IC gain changes were correlated, IC gain changes consistently underestimated the magnitude of behavioral RT-I gain changes. As expected, after SS washout, there was no observable relationship between RT-I and AC or IC gain changes at 24 h post-SS (Fig. 4F,G), when response functions were back to baseline levels (Fig. 2A–C). Together, the data indicate that SS-mediated changes to perceived loudness co-varies with AC and IC electrophysiological changes in terms of both magnitude and time course; however, the changes in AC responses were more closely correlated with behavioral RT-I functions than IC responses.
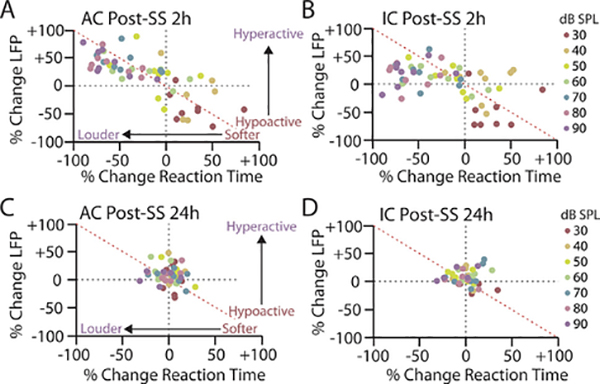
Response gain provides a simple metric for quantifying the relationship between behavioral and electrophysiological measures of loudness growth. However, an increase in the slope of a response function does not necessarily indicate that the response exceeds baseline levels at any point, particularly when this slope increase coincides with a subtractive shift in the response as well, as is the case with loudness growth following hearing loss. Consequently, an increase in response gain does not distinguish between loudness recruitment, where loudness/neural responses catch up but do not overshoot baseline responses, versus hyperacusis, where loudness/neural responses grow more rapidly and overshoot baseline. In order to address this issue on a subject-by-subject basis, we compared intensity-dependent changes in loudness (RT) observed 2 h and 24 h post-SS against intensity-dependent measures of neural activity in the AC and IC. To accomplish this, we plotted the percent change in RT relative to baseline against the percent change in AC (Fig. 5, left column) or IC (Fig. 5, right column) LFP magnitude for each animal at each intensity either 2 h (Fig. 5, top row) or 24 h (Fig. 5, bottom row) post-SS. At 2 h post-SS, there was a significant correlation between SS-mediated changes in behavioral RTs and electrophysiological response magnitudes for both AC (r2 = −0.7466, ***p < 0.0001) and IC (r2 = −0.5574, ***p < 0.0001) responses. The correlation coefficients between RT and neural activity are negative due to the inverse relationship between RT and sound intensity, i.e., as stimulus intensity increases RTs decrease (Fig. 5).
Fig. 5.
Relationship between salicylate-induced changes to reaction time and evoked response is intensity-dependent. A,B: Scatter plot of percent change in RT, as compared to the average baseline, for each animal at each intensity versus percent change in (A) AC or (B) IC LFP magnitude 2 h post-SS. C,D: Scatter plot of percent change in RT, as compared to the average baseline response, for each animal at each intensity versus percent change in (C) AC or (D) IC evoked response 24 h post-SS. Each color corresponds to a specific sound intensity. Each point signifies the relationship between behavioral and electrophysiological response magnitude at a specific intensity for a specific animals (n = 8).
The correspondence between SS-mediated changes in RT and AC amplitude at 2 h post-SS were striking (Fig. 5A). As RTs became faster (more negative) relative to baseline (i.e. sounds became louder) the AC amplitudes became larger (i.e. hyperactive); this typically occurred at high-intensities. Conversely, when RTs became slower (more positive) relative to baseline (i.e. sounds became softer), AC amplitudes became smaller (i.e. hypoactive); this typically occurred at very low intensities. In contrast, while there was a significant correlation between SS-mediated changes in RT and IC amplitude at 2 h post-SS (Fig. 5B), the correspondence between RT and IC responses varied as a function of sound intensity. There was a strong relationship between RT and IC response changes for low-intensity stimuli, similar to the AC, but IC response enhancement consistently underrepresented RT changes at moderate-to-high intensities, as indicated by the number of points below the red 4500b0030 line (Fig. 5B). Consequently, there was better concordance between AC and RT changes across intensities than those in the IC, as indicated by significantly greater negative slope of the linear fit of the AC-RT plot compared to the IC-RT plot (AC slope = −0.6944, IC slope = −0.4167; F(1,108) = 5.419, *p = 0.0218, d = 1.65). Once again, no significant relationship between RT-I and either AC or IC intensity-response functions were observed 24 h post-SS (Fig. 5C,D), reflecting the temporary nature of SS-induced alterations. These results indicate that decreased sensitivity to low-intensity sounds following SS administration, likely due to hearing loss, is well correlated with response changes in both the AC and IC. In contrast, increased loudness perception/hyperacusis-like behavior seen at moderate-to-high intensities is more closely related to cortical than subcortical response alterations.
DISCUSSION
This study used a combined psychophysical-electrophy siological paradigm to assess the relationship between hyperacusis and central gain enhancement on a subject-by-subject basis. First, we demonstrated that stable loudness growth functions and auditory-evoked I/O functions can be reliably obtained from the same animals across multiple testing sessions (Fig. 1). Next, we determined that salicylate induced parallel changes to RT-I and AC/IC I/O functions consistent with temporary hearing loss and hyperacusis (Figs. 2 and 3). Most importantly, we found that salicylate-mediated changes in loudness growth and sound-evoked activity were correlated within individual animals, suggesting that electrophysiological gain changes are directly related to altered loudness perception (Figs. 4 and 5). These results provide strong support for the central gain model of hyperacusis and demonstrate the utility of using an experimental design that allows for within-subject comparison of behavioral and electrophysiological alterations, thereby making inter-subject variability a strength rather than a limitation of the study.
Behavioral measures of hyperacusis
Development of animal models of hyperacusis has been hindered by a relative lack of objective behavioral measures of loudness. Here we demonstrate that an animal’s RT to a sound in a simple Go/No-Go operant conditioning paradigm is a reliable indicator of perceived loudness and can be used to assess abnormal loudness growth associated with hearing loss (Fig. 2), consistent with previous human and animal studies (Marshall and Brandt, 1980; Lauer and Dooling, 2007; Chen et al., 2015; Radziwon et al., 2017). One of the benefits of this task is that it requires the animal to make a perceptual decision, and RT measures may therefore be more closely related to the perceived loudness of a sound than a stereotyped reflexive response (Hayes et al., 2014). RT measures are also sensitive over a wide range of intensities, including low-intensity sounds, and can thus simultaneously account for hearing loss, loudness recruitment, and hyperacusis-like changes in loudness growth. It is important to note, however, that some forms of hyperacusis may not be associated with changes to loudness per se, but rather may arise from alterations to the affective qualities of sound (e.g. pain or avoidance hyperacusis) (Tyler et al., 2014), which are not likely to be captured in RT-based measures of loudness growth. Complementary behavioral tasks, such as avoidance paradigms for assessing sound tolerance levels (Flores et al., 2015; Manohar et al., 2017), can be used in future studies to distinguish between distinct forms of hyperacusis and how they may or may not relate to central gain enhancement.
Finally, a crucial component of this psychophysical paradigm is that RT-I functions are remarkably stable across testing sessions (Fig. 1D, Fig. 4A). This allows for longitudinal, within-subject characterization of perceived loudness changes before and after experimental manipulation. For instance, we not only demonstrated that RT-I functions were stable over a baseline period (Fig. 1D), and that SS treatment results in rapid changes to the gain of loudness growth functions, but that these response functions recover completely to baseline levels within 24 hours (Fig. 2A). This ability to reliably measure perceived loudness over a prolonged period within individual animals is an essential prerequisite for correlating perceptual changes with electrophysiological measures of sound encoding.
Salicylate-induced gain enhancement in the central auditory system
Salicylate has several well-characterized effects on sound-evoked activity that have been important for the development of the central gain theory. SS treatment is known to consistently reduce cochlear output but paradoxically enhance suprathreshold responses in central auditory structures (Stolzberg et al., 2012). Most previous studies of SS-mediated gain enhancement, however, have been performed in anesthetized animals, often focusing on alterations to single auditory nuclei. Anesthesia can have profound effects on sound-evoked activity (Thornton and Sharpe, 1998; Szalda and Burkard, 2005; Ros et al., 2017), and different types of anesthetics have been shown to directly influence the levels of central gain enhancement observed following salicylate treatment (Yang et al., 2006). It is also becoming increasingly clear that even simple elements of perception, such as the apparent loudness of a sound, are likely to involve dynamic activity across a distributed network of brain areas (Winer and Lee, 2007; Chen et al., 2015). Thus, in order to understand the neural correlates of loudness or hyperacusis, it may be imperative to monitor sound-evoked activity across auditory regions. Only a limited number of studies have characterized sound-evoked hyperactivity following SS treatment in awake animals, and these studies have focused solely on the AC (Yang et al., 2007; Noreña et al., 2010; Zhang et al., 2011). The present study is the first, to our knowledge, to assess SS-mediated gain changes from multiple levels of the auditory system simultaneously in awake, behaviorally phenotyped animals.
We found that salicylate decreased evoked responses to low-intensity sounds, suggestive of hearing loss/threshold shifts, but enhanced suprathreshold activity in the IC and AC, indicative of increased central gain. While sound-evoked responses to moderate-to-high intensity sound were increased in both the IC and AC (Fig. 2B,C), response gain was significantly greater in the AC than IC (Fig. 2E,F). Interestingly, previous results from anesthetized animals have suggested that SS-mediated hyperactivity emerges incrementally at ascending levels of the auditory system. While partial recovery of suprathreshold responses is seen as early as the cochlear nucleus, response amplitudes continue to gradually increase at more central auditory structures, culminating in robust hyperactivity of sound-evoked responses in the AC (Jiang et al., 2017). Thus, there appears to be a progressive amplification of sound-evoked responses at ascending auditory stations, suggesting that gain modulation may be simultaneously occurring at multiple levels of the central auditory system.
Relationship between central gain enhancement and hyperacusis
Many experimental manipulations that result in central gain enhancement, such as salicylate administration or acoustic trauma, are also often associated with tinnitus and/or hyperacusis-like behavior (Auerbach et al., 2014). At the group level, we observed parallel changes to loudness growth and AC/IC I/O functions following SS administration that were consistent with temporary hearing loss and hyperacusis. After SS treatment, animals exhibited slower than normal RTs and decreased response amplitudes for low-intensity sounds, reflecting decreased sensitivity to low-level sounds (i.e. hearing loss), but much faster than normal RTs and sound-evoked hyperactivity for moderate-to-high intensity sounds, indicative of increased loudness perception (i.e. hyperacusis) (Figs. 2 and 5). Our chronic recording paradigm also allowed us to track SS-mediated gain enhancement in a longitudinal manner. SS-mediated behavioral changes and gain enhancement were both rapid in onset, appearing 2 h post-administration, but completely back to baseline levels 24 h post-SS treatment (Fig. 2). These results show that SS-mediated central gain enhancement and hyperacusis-like changes to loudness perception are observed in the same group of animals in a temporally correlated manner. However, despite the temptation to speculate that sound-evoked hyperactivity is directly responsible for observed hyperacusis-like behavior, this only demonstrates that the two are both common consequences of hearing loss that can co-occur. An explicit test of the central gain model requires a formal correlational analysis between central gain changes and altered loudness growth on a subject-by-subject basis.
This is the first study, to our knowledge, that has directly correlated longitudinal changes in sound-evoked responses with behavioral measures of loudness perception in a within-subject manner. Importantly, we found that SS-mediated changes in RT and evoked response growth were correlated within individual animals, in terms of both magnitude and time-course (Figs. 4 and 5). This result indicates that neuronal gain enhancement is indeed an important mechanism underlying hyperacusis and aberrant loudness growth associated with hearing loss. While the covariation in cortical and behavioral response dynamics is a strong indication that central gain enhancement is directly linked to altered loudness perception, it is important to note that these results do not necessarily mean these two phenomena are causally related. Future experiments examining the effect of local manipulation of neuronal activity on RT-I functions before/after SS treatment or noise exposure would further substantiate the results from this study.
This study not only highlights the benefit of combining behavioral and electrophysiological measures from the same animals, but it also demonstrates the importance of characterizing neuronal changes from multiple brain areas simultaneously. We found a significant correlation between increased loudness growth and response gain enhancement in both the AC and IC. However, the relationship between these perceptual and electrophysiological changes was quantitatively and qualitatively different between cortical and subcortical structures. There was a stronger concordance between the magnitude of RT and AC response gain (Fig. 4D) than between RT and IC response gain (Fig. 4E), suggesting loudness perception was more closely correlated with cortical than subcortical response dynamics. Inspection of the data suggests that this was due to saturation of the IC amplitude changes, as IC response enhancement consistently under-represented decreases in RT (i.e. increased loudness) at moderate-to-high intensity sounds compared to AC response enhancement (Fig. 5). This is likely due to the cumulative nature of SS-mediated gain enhancement, as we showed that sound-evoked hyperactivity grew progressively stronger at ascending levels of the auditory system.
Taken together, our results demonstrate that hyperacusis development likely involves the aggregate effects of neuronal gain changes occurring simultaneously at multiple levels of the auditory system. Thus, rather than focusing on specific brain regions or auditory nuclei as generators of auditory perceptual disorders like hyperacusis, it may be more beneficial to view observed changes to sound encoding in the context of a broader neural network. In previous studies, we demonstrated that both salicylate treatment and noise exposure can also result in sound-evoked hyperactivity in many non-classical auditory areas, particularly limbic areas involved in emotion, arousal and assigning contextual salience to sensory stimuli (Chen et al., 2014; Chen et al., 2015; Chen et al., 2016; Chen et al., 2017). Future work must directly examine the relationship between gain changes across classical and non-classical auditory centers to determine how these together contribute to perceptual changes in loudness and sound tolerance following hearing loss.
Salicylate model of ototoxicity and auditory perceptual disorders
Salicylate is a well-characterized ototoxic drug that reliably induces temporary hearing loss and auditory perceptual disruptions like tinnitus, hyperacusis, and temporal processing deficits (McFadden et al., 1984; Stolzberg et al., 2012; Knipper et al., 2013). It is this consistency, coupled with its rapid and reversible effects, that has made salicylate such a useful experimental tool for investigating the neural correlates of auditory perceptual disorders. Indeed, there are several parallels to the neurophysiological alterations induced by SS treatment and noise trauma. For instance, salicylate and noise trauma result in a similar profile of progressive gain enhancement through the ascending auditory system, albeit with distinct time-courses (Noreña et al., 2010; Auerbach et al., 2014). Both manipulations also result in overlapping changes at the cellular and molecular level, such as decreased levels of synaptic inhibition, which has led to potential treatment strategies (Milbrandt et al., 2000; Sun et al., 2009; Lu et al., 2011; Yang et al., 2011; Wang et al., 2011). Thus, examination of salicylate-induced changes to auditory processing and evoked activity is a useful model for investigating the neural and biological mechanisms underlying hearing loss and auditory perceptual disorders. There are, however, some caveats to consider regarding the salicylate model, particularly in relation to hyperacusis.
Tinnitus and hyperacusis are highly comorbid disorders, with over 60% of tinnitus patients presenting with hyperacusis and, conversely, over 80% of hyperacusis subjects also experiencing chronic tinnitus (Anari et al., 1999; Dauman and Bouscau-Faure, 2005; Sztuka et al., 2010). One attractive aspect of the central gain model is that it can potentially account for both tinnitus and hyperacusis (Noreña, 2011; Zeng, 2013; Auerbach et al., 2014), providing a unifying pathophysiological framework for these highly related disorders. Consistent with this model, salicylate reliably induces neural hyperactivity, tinnitus and hyperacusis-like behavior in animal models, as demonstrated in this study and others (Stolzberg et al., 2012). However, while there is an extremely well-established relationship between salicylate and tinnitus in humans, the relationship between salicylate and hyperacusis is less clear. One reason for this may be that tinnitus is an all-or-none phenomenon that is readily apparent to individuals, while the changes to loudness tolerance associated with hyperacusis are graded and therefore often more subtle. Indeed, a recent study demonstrated that many tinnitus patients were unaware of their increased loudness sensitivity until explicitly tested (Gu et al., 2010). More studies directly assessing loudness tolerance in individuals treated with salicylate are therefore needed.
It is also possible that salicylate-induced tinnitus may directly influence auditory RTs. Interestingly, some studies have shown that auditory RTs are decreased in tinnitus patients (Goodwin and Johnson, 1980; Nieschalk et al., 1998). While it is conceivable that the presence of a tinnitus percept could modulate RT to certain sound stimuli (e.g., those near tinnitus frequency band), it is unlikely that this could account for the pronounced changes in RTs across the wide-range of intensities and frequencies that we have observed with salicylate here and in related studies (Radziwon et al., 2017). Moreover, none of the previous studies examining RT measures in individuals with tinnitus controlled for presence of hyperacusis. Because of the high comorbidity between these disorders, any observed changes in RT in tinnitus subjects could also be due to the presence of hyperacusis. Thus, the most parsimonious explanation for the data is that changes to auditory RTs reflect increased loudness growth that originates from salicylate-induced neuronal hyperexcitability.
A final caveat to consider when attempting to generalize these results is how closely the mechanisms of salicylate and noise-induced gain enhancement are related to each other. Recent evidence suggests that SS-mediated hearing loss and sound-evoked hyperactivity are likely due to independent effects of the drug on the cochlea and central auditory system, respectively, and that these alterations are at least in part dissociable (Sun et al., 2009). Thus, while noise-induced gain enhancement is thought to arise via homeostatic adaption to the loss of auditory input, SS-mediated gain enhancement is likely due to the combination of reduced cochlear input and direct modulation of neuronal excitability in the central auditory system. It is therefore of the utmost importance to determine if the correlation between central gain enhancement and altered loudness perception extends to noise-induced models of hyperacusis. In fact, as the effects of noise trauma are undoubtedly more variable in nature than salicylate ototoxicity (Noreña et al., 2010), the ability to correlate behavioral and electrophysiological changes on an individual animal basis are even more important when considering the role of central gain enhancement in hyperacusis associated with noise-induced hearing loss. That said, we found considerable variability in the magnitude of behavioral and electrophysiological changes following salicylate treatment as well (Figs. 3 and 4). The fact that such a substantial covariation was observed between these behavioral and electrophysiological measures in a salicylate model of hyperacusis is strong validation of our approach. This study therefore serves as a foundation for future studies using our psychophysical-electrophysiological paradigm to determine the relationship between central gain enhancement and noise-induced hyperacusis.
SUMMARY
It is becoming increasingly clear that central auditory plasticity is a critical component to the perceptual consequences of cochlear hearing impairment. Recent studies have demonstrated that compensatory central gain enhancement can restore sound detection thresholds in the face of even profound cochlear denervation (Lobarinas et al., 2013; Chambers et al., 2016; Salvi et al., 2016). This likely contributes to the phenomenon of “hidden hearing loss”, where individuals present with clinically normal hearing but nonetheless exhibit more subtle auditory perceptual disturbances as a result of significant damage to the inner hair cell/auditory nerve synapse (i.e., synaptopathy) (Kujawa and Liberman, 2009; Liberman, 2015). In addition to hidden hearing loss, excessive gain enhancement has long been speculated to be a potential mechanism underlying tinnitus and hyperacusis, two of the most common auditory perceptual disruptions associated with hearing loss. This study offers substantial evidence in support of the central gain model of hyperacusis, demonstrating that sound-evoked hyperactivity is directly correlated with increased loudness growth within individual animals. Moreover, this study provides a novel platform for explicitly linking changes in sound intensity coding with its perceptual consequences, laying the groundwork for future studies examining the mechanisms of hearing loss plasticity and hyperacusis.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Health to R.S. (R01DC014452) and B.D.A. (F32DC015160). We would like to thank Carol Altman for her excellent administrative support as well as Heather Bool, Li Li, and Anika French for their technical assistance in running the behavioral experiments.
REFERENCES
- Anari M, Axelsson A, Eliasson A, Magnusson L (1999) Hypersensitivity to sound–questionnaire data, audiometry and classification. Scand Audiol 28:219–230. 10.1080/010503999424653. [DOI] [PubMed] [Google Scholar]
- Arieh Y, Marks LE (2003) Time course of loudness recalibration: implications for loudness enhancement. J Acoust Soc Am 114:1550–1556. 10.1121/1.1603768. [DOI] [PubMed] [Google Scholar]
- Auerbach BD, Rodrigues PV, Salvi RJ (2014) Central gain control in tinnitus and hyperacusis. Front Neurol 5:206. 10.3389/fneur.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley DM (2003) Hyperacusis. J R Soc Med 96:582–585. 10.1177/0141076800309601203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher FA, Bancroft BR, Salvi RJ (1990) Concentration of salicylate in serum and perilymph of the chinchilla. Arch Otolaryngol Head Neck Surg 116:681–684. 10.1001/archotol.1990.01870060039005. [DOI] [PubMed] [Google Scholar]
- Brennan JF, Brown CA, Jastreboff PJ (1996) Salicylate-induced changes in auditory thresholds of adolescent and adult rats. Dev Psychobiol 29:69–86. . [DOI] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB (2016) Central gain restores auditory processing following near-complete cochlear denervation. Neuron 89:867–879. 10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Chen GD, Auerbach BD, Manohar S, Radziwon K, Salvi R (2017) Tinnitus and hyperacusis: contributions of paraflocculus, reticular formation and stress. Hear Res 349:208–222. 10.1016/j.heares.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-C, Li X, Liu L, Wang J, Lu C-Q, Yang M, Jiao Y, Zang F-C, Radziwon K, Chen G-D (2015) Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. eLife 4:e06576. 10.7554/eLife.06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Li X, Liu L, Wang J, Lu CQ, Yang M, Jiao Y, Zang FC, Radziwon K, Chen GD, Sun W, Krishnan Muthaiah VP, Salvi R, Teng GJ (2015) Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. eLife 4:e06576. 10.7554/eLife.06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Manohar S, Salvi R (2012) Amygdala hyperactivity and tonotopic shift after salicylate exposure. Brain Res 1485:63–76. 10.1016/j.brainres.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Radziwon KE, Kashanian N, Manohar S, Salvi R (2014) Salicylate-induced auditory perceptual disorders and plastic changes in nonclassical auditory centers in rats. Neural Plasticity 2014. 10.1155/2014/658741658741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G-D, Sheppard A, Salvi R (2016) Noise trauma induced plastic changes in brain regions outside the classical auditory pathway. Neuroscience 315:228–245. 10.1016/j.neuroscience.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauman R, Bouscau-Faure F (2005) Assessment and amelioration of hyperacusis in tinnitus patients. Acta Otolaryngol 125:503–509. 10.1080/00016480510027565. [DOI] [PubMed] [Google Scholar]
- Dean I, Harper NS, McAlpine D (2005) Neural population coding of sound level adapts to stimulus statistics. Nat Neurosci 8:1684–1689. 10.1038/nn1541. [DOI] [PubMed] [Google Scholar]
- Flores EN, Duggan A, Madathany T, Hogan AK, Marquez FG, Kumar G, Seal RP, Edwards RH, Liberman MC, Garcia-Anoveros J (2015) A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Curr Biol 25:606–612. 10.1016/j.cub.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin PE, Johnson RM (1980) A comparison of reaction times to tinnitus and nontinnitus frequencies. Ear Hear 1:148–155. [DOI] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR (2010) Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol 104:3361–3370. 10.1152/jn.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SH, Radziwon KE, Stolzberg DJ, Salvi RJ (2014) Behavioral models of tinnitus and hyperacusis in animals. Front Neurol 5:179. 10.3389/fneur.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC (2014) Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol 111:552–564. 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff PJ, Hansen R, Sasaki PG, Sasaki CT (1986) Differential uptake of salicylate in serum, cerebrospinal fluid, and perilymph. Arch Otolaryngol Head Neck Surg 112:1050–1053. 10.1001/archotol.1986.03780100038004. [DOI] [PubMed] [Google Scholar]
- Jiang C, Luo B, Manohar S, Chen GD, Salvi R (2017) Plastic changes along auditory pathway during salicylate-induced ototoxicity: hyperactivity and CF shifts. Hear Res 347:28–40. 10.1016/j.heares.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, Van Dijk P, Nunes I, Ruttiger L, Zimmermann U (2013) Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol 111:17–33. 10.1016/j.pneurobio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC (2009) Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 29:14077–14085. 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting CP, De Kleine E, Bartels H, Van Dijk P (2008) Functional imaging of unilateral tinnitus using fMRI. Acta Otolaryngol 128:415–421. 10.1080/00016480701793743. [DOI] [PubMed] [Google Scholar]
- Lauer A, Dooling R (2007) Evidence of hyperacusis in canaries with permanent hereditary high-frequency hearing loss. Sem Hear 28:319–326. 10.1055/s-2007-990718. [DOI] [Google Scholar]
- Liberman MC (2015) Hidden hearing loss. Sci Am 313:48–53. 10.1038/scientificamerican0815-48. [DOI] [PubMed] [Google Scholar]
- Lin LI (1989) A concordance correlation coefficient to evaluate reproducibility. Biometrics 45:255–268. 10.2307/2532051. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D (2013) Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res 302:113–120. 10.1016/j.heares.2013.03.012S0378-5955(13)00093-2 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW (1998) The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology 50:114–120. 10.1212/WNL.50.1.114. [DOI] [PubMed] [Google Scholar]
- Lu J, Lobarinas E, Deng A, Goodey R, Stolzberg D, Salvi RJ, Sun W (2011) GABAergic neural activity involved in salicylate-induced auditory cortex gain enhancement. Neuroscience 189:187–198. 10.1016/j.neuroscience.2011.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar S, Spoth J, Radziwon K, Auerbach BD, Salvi R (2017) Noise-induced hearing loss induces loudness intolerance in a rat Active Sound Avoidance Paradigm (ASAP). Hear Res 353:197–203. 10.1016/j.heares.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Brandt JF (1980) The relationship between loudness and reaction time in normal hearing listeners. Acta Otolaryngol 90:244–249. 10.3109/00016488009131721. [DOI] [PubMed] [Google Scholar]
- May BJ, Little N, Saylor S (2009) Loudness perception in the domestic cat: reaction time estimates of equal loudness contours and recruitment effects. J Assoc Res Otolaryngol 10:295–308. 10.1007/s10162-009-0157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Plattsmier HS, Pasanen EG (1984) Aspirin-induced hearing loss as a model of sensorineural hearing loss. Hear Res 16:251–260. 10.1016/0378-5955(84)90114-X. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Levine RA, Bergevin C, Norris B (2009) The auditory midbrain of people with tinnitus: abnormal sound-evoked activity revisited. Hear Res 257:63–74. 10.1016/j.heares.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM (2000) GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res 147:251–260. 10.1016/S0378-5955(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Moody DB (1973) Behavioral studies of noise-induced hearing loss in primates: loudness recruitment. Adv Otolaryngol 20:82–101. 10.1159/000393090. [DOI] [PubMed] [Google Scholar]
- Nieschalk M, Hustert B, Stoll W (1998) Auditory reaction times in patients with chronic tinnitus with normal hearing. Am J Otol 19:611–618. [PubMed] [Google Scholar]
- Noreña AJ (2011) An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci Biobehav Rev 35:1089–1109. 10.1016/j.neubiorev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Noreña AJ, Moffat G, Blanc JL, Pezard L, Cazals Y (2010) Neural changes in the auditory cortex of awake guinea pigs after two tinnitus inducers: salicylate and acoustic trauma. Neuroscience 166:1194–1209. 10.1016/j.neuroscience.2009.12.063. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. 6th ed. Amsterdam: Elsevier. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Hienze R, Kimm J, Miller J (1975) Reaction-time procedure for measuring hearing. I. Suprathreshold functions. J Acoust Soc Am 57:421–430. 10.1121/1.380465. [DOI] [PubMed] [Google Scholar]
- Robinson BL, McAlpine D (2009) Gain control mechanisms in the auditory pathway. Curr Opin Neurobiol 19:402–407. 10.1016/j.conb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Radziwon K, Holfoth D, Lindner J, Kaier-Green Z, Bowler R, Urban M, Salvi R (2017) Salicylate-induced hyperacusis in rats: dose- and frequency-dependent effects. Hear Res 350:133–138. 10.1016/j.heares.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwon KE, Stolzberg DJ, Urban ME, Bowler RA, Salvi RJ (2015) Salicylate-induced hearing loss and gap detection deficits in rats. Front Neurol 6:31. 10.3389/fneur.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennaker RL, Miller J, Tang H, Wilson DA (2007) Minocycline increases quality and longevity of chronic neural recordings. J Neural Eng 4:L1–L5. 10.1088/1741-2560/4/2/L01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros C, Soler C, Garcia de Carellan Mateo A (2017) Comparison of the brainstem auditory evoked responses during sevoflurane or alfaxalone anaesthesia in adult cats. Vet Anaesth Analg 44:1085–1090. 10.1016/j.vaa.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Salvi R, Sun W, Ding D, Chen GD, Lobarinas E, Wang J, Radziwon K, Auerbach BD (2016) Inner hair cell loss disrupts hearing and cochlear function leading to sensory deprivation and enhanced central auditory gain. Front Neurosci 10:621. 10.3389/fnins.2016.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz C, Hentschke H, Butovas S, Haiss F, Stuttgen MC, Gerdjikov TV, Bergner CG, Waiblinger C (2010) The head-fixed behaving rat–procedures and pitfalls. Somatosens Mot Res 27:131–148. 10.3109/08990220.2010.513111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins WC (1966) Auditory reaction time and the derivation of equal loudness contours for the monkey. J Exp Anal Behav 9:135–142. 10.1901/jeab.1966.9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzberg D, Chen GD, Allman BL, Salvi RJ (2011) Salicylate-induced peripheral auditory changes and tonotopic reorganization of auditory cortex. Neuroscience 180:157–164. 10.1016/j.neuroscience.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzberg D, Chrostowski M, Salvi RJ, Allman BL (2012) Intracortical circuits amplify sound-evoked activity in primary auditory cortex following systemic injection of salicylate in the rat. J Neurophysiol 108:200–214. 10.1152/jn.00946.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzberg D, Salvi RJ, Allman BL (2012) Salicylate toxicity model of tinnitus. Front Syst Neurosci 6. 10.3389/fnsys.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzberg D, Hayes SH, Kashanian N, Radziwon K, Salvi RJ, Allman BL (2013) A novel behavioral assay for the assessment of acute tinnitus in rats optimized for simultaneous recording of oscillatory neural activity. J Neurosci Methods 219:224–232. 10.1016/j.jneumeth.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ (2009) Salicylate increases the gain of the central auditory system. Neuroscience 159:325–334. 10.1016/j.neuroscience.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Deng A, Jayaram A, Gibson B (2012) Noise exposure enhances auditory cortex responses related to hyperacusis behavior. Brain Res 1485:108–116. 10.1016/j.brainres.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Szalda K, Burkard R (2005) The effects of nembutal anesthesia on the auditory steady-state response (ASSR) from the inferior colliculus and auditory cortex of the chinchilla. Hear Res 203:32–44. 10.1016/j.heares.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Sztuka A, Pospiech L, Gawron W, Dudek K (2010) DPOAE in estimation of the function of the cochlea in tinnitus patients with normal hearing. Auris Nasus Larynx 37:55–60. 10.1016/j.anl.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Thornton C, Sharpe RM (1998) Evoked responses in anaesthesia. Br J Anaesth 81:771–781. 10.1093/bja/81.5.771. [DOI] [PubMed] [Google Scholar]
- Turner JG, Parrish J (2008) Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am J Audiol 17:S185–S192. 10.1044/1059-08-89(2008/080006). [DOI] [PubMed] [Google Scholar]
- Tyler RS, Pienkowski M, Roncancio ER, Jun HJ, Brozoski T, Dauman N, Dauman N, Andersson G, Keiner AJ, Cacace AT, Martin N, Moore BC (2014) A review of hyperacusis and future directions: part I. Definitions and manifestations. Am J Audiol 23:402–419. 10.1044/2014_AJA-14-0010. [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Caspary DM (2011) Inhibitory neurotransmission in animal models of tinnitus: maladaptive plasticity. Hear Res 279:111–117. 10.1016/j.heares.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PV, Barbour DL (2008) Specialized neuronal adaptation for preserving input sensitivity. Nat Neurosci 11:1259–1261. 10.1038/nn.2201. [DOI] [PubMed] [Google Scholar]
- Winer JA, Lee CC (2007) The distributed auditory cortex. Hear Res 229:3–13. 10.1016/j.heares.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W (2006) Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res 226. 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang LY, Turner J, Stolzberg D, Salvi R, Sun W (2007) Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res 226:244–253. 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho S-J, Bao S (2011) Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci USA 108:14974–14979. 10.1073/pnas.1107998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG (2013) An active loudness model suggesting tinnitus as increased central noise and hyperacusis as increased nonlinear gain. Hear Res 295:172–179. 10.1016/j.heares.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yang P, Cao Y, Qin L, Sato Y (2011) Salicylate induced neural changes in the primary auditory cortex of awake cats. Neuroscience 172:232–245. 10.1016/j.neuroscience.2010.10.073. [DOI] [PubMed] [Google Scholar]