Abstract
Background and Objectives
To understand patterns of care and circumstances surrounding end of life in patients with intracranial gliomas.
Methods
We retrospectively analyzed end-of-life circumstances in patients with intracranial high-grade gliomas at Columbia University Irving Medical Center who died from January 2014 to February 2019, including cause of death, location of death, and implementation of comfort measures and resuscitative efforts.
Results
There were 152 patients (95 men, 57 women; median age at death 61.5 years, range 24–87 years) who died from January 2014 to February 2019 with adequate data surrounding end-of-life circumstances. Clinical tumor progression (n = 117, 77.0%) was the most common cause of death, with all patients transitioned to comfort measures. Other causes included, but were not limited to, infection (19, 12.5%); intratumoral hemorrhage (5, 3.3%); seizures (8, 5.3%); cerebral edema (4, 2.6%); pulmonary embolism (4, 2.6%); autonomic failure (2, 1.3%); and hemorrhagic shock (2, 1.3%). Multiple mortal events were identified in 10 (8.5%). Seventy-three patients (48.0%) died at home with hospice. Other locations were inpatient hospice (40, 26.3%); acute care hospital (34, 22.4%), including 27 (17.8%) with and 7 (4.6%) without comfort measures; skilled nursing facility (4, 3.3%), including 3 (2.0%) with and 1 (0.7%) without comfort measures; or religious facility (1, 0.7%) with comfort measures. Acute cardiac or pulmonary resuscitation was performed in 20 patients (13.2%).
Discussion
Clinical tumor progression was the most common (77.0%) cause of death, followed by infection (12.5%). Hospice or comfort measures were ultimately implemented in 94.7% of patients, although resuscitation was performed in 13.2%. Improved understanding of circumstances surrounding death, frequency of use of hospice services, and frequency of resuscitative efforts in patients with gliomas may allow physicians to more accurately discuss end-of-life expectations with patients and caregivers, facilitating informed care planning.
Glioblastoma is the most common type of primary brain tumor in adults and is nearly universally fatal despite advances in therapy.1,2 However, cause of death from glioblastoma and other high-grade gliomas depends on many factors,3 including comorbid conditions, and clinical events surrounding death are frequently complex. Palliative care improves symptoms and quality of life in patients with cancer, particularly when engaged early in the disease process.4-8 The types of symptoms (e.g., cognitive impairment, seizures, difficulty ambulating) that patients with gliomas experience as the disease becomes refractory to treatment can affect quality of life and functional independence and may lead to hospitalization.9-15 Accordingly, patients with gliomas and their caregivers have specific palliative care and psychosocial needs.16-22 The frequency and settings in which palliative care and hospice services are used vary greatly.23-28 Uncertainty surrounding end-of-life expectations, including whether death involves physical pain or suffering, may exacerbate anxiety among patients and caregivers, who often ask about end-of-life circumstances in the setting of disease progression. Therefore, we sought to characterize cause and location of death as well as frequency of use of palliative measures and resuscitative efforts among patients with gliomas at a single academic medical center in a systematic and comprehensive manner over a 5-year period.
Methods
We conducted a single-institution retrospective study of adults with intracranial high-grade gliomas. In more detail, the Herbert Irving Comprehensive Neurologic Cancer Center Database (principal investigator, F.M.I.) is an institutional research database that is manually and prospectively annotated with clinical details extracted from the medical record of patients followed at Columbia University Irving Medical Center for neuro-oncologic care (by A.E.J.-G.). Demographics, date of diagnosis, and date of death are routinely collected in all available cases (by A.E.J.-G.). This database was manually reviewed (by A.E.J.-G.) to identify cases fulfilling the following inclusion criteria: (1) diagnosis of intracranial high-grade glioma, (2) age at diagnosis of at least 18 years, and (3) date of death from January 2014 to February 2019. We (M.B.) also screened patients who were alive when the study was initiated in December 2017 for inclusion on death until February 2019. For additional details pertinent to the current research study, the medical record was manually reviewed retrospectively (by M.B.) to determine and collect circumstances surrounding death, specifically (1) cause of death, (2) location of death, (3) performance of resuscitation, and (4) implementation of hospice or other comfort measures. Caregivers were surveyed if necessary to supplement the information available in the medical record. Cases without available details regarding circumstances surrounding death (1–4 above) were excluded.
Cause of death was defined as the primary clinical circumstance(s) immediately preceding and contributing to death for all patients. Death was attributed to tumor progression in cases of neurologic decline attributable to tumor growth with or without corresponding imaging. Location was categorized as home, inpatient hospice facility, acute care hospital, skilled nursing facility, or religious facility. Implementation of hospice services or other comfort measures, resuscitation status before death, and whether cardiac or pulmonary resuscitation were performed at the end of life, in addition to other relevant clinical variables, were also recorded. We defined comfort measures as supportive medical care for the purpose of controlling end-of-life symptoms, including comfort measures through formal hospice programs and other non–hospice-based comfort measures.
We defined cardiac resuscitation as use of basic life support or advanced cardiac life support protocols in an effort to restore spontaneous circulation after cardiac arrest. We defined pulmonary resuscitation as endotracheal intubation. Descriptive statistics were used for analyses.
Standard Protocols, Approvals, Registrations, and Patient Consents
We received approval from the Human Research Protection Office and Institutional Review Board of Columbia University to conduct this study, to retrospectively review medical records, and to survey caregivers as needed after obtaining verbal informed consent.
Data Availability
Requests should be submitted to the corresponding author.
Results
Study Population
There were 213 patients identified with intracranial high-grade gliomas who died during the 5-year interval of study. In 61, data regarding end-of-life circumstances (cause of death, location of death, performance of resuscitation, or implementation of hospice or other comfort measures) were unavailable in the medical record or from caregivers. Therefore, 152 patients (95 men, 57 women) made up the study cohort, for whom median age at death was 61.5 years (range 24–87 years, Table 1); histology was glioblastoma in 145 (95.4%, Table 2).
Table 1.
Demographics (N = 152)
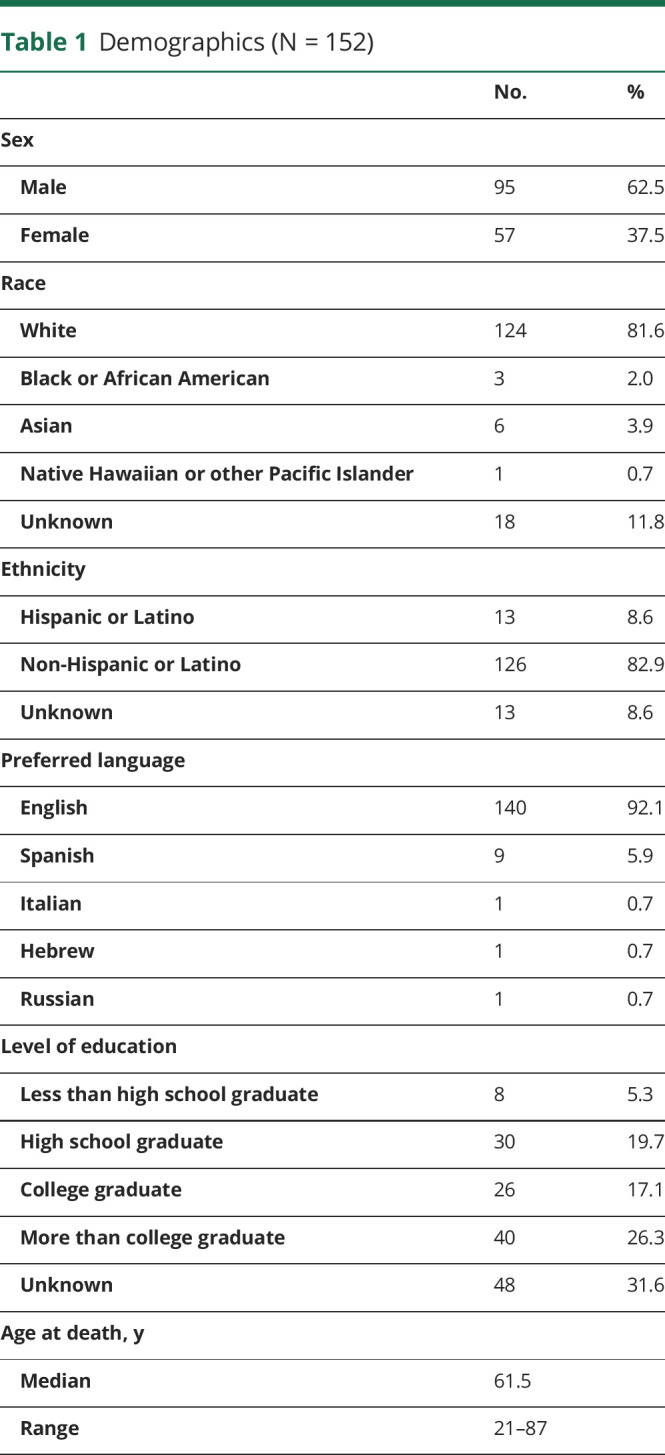
Table 2.
Diagnosis (N = 152)

Cause of Death
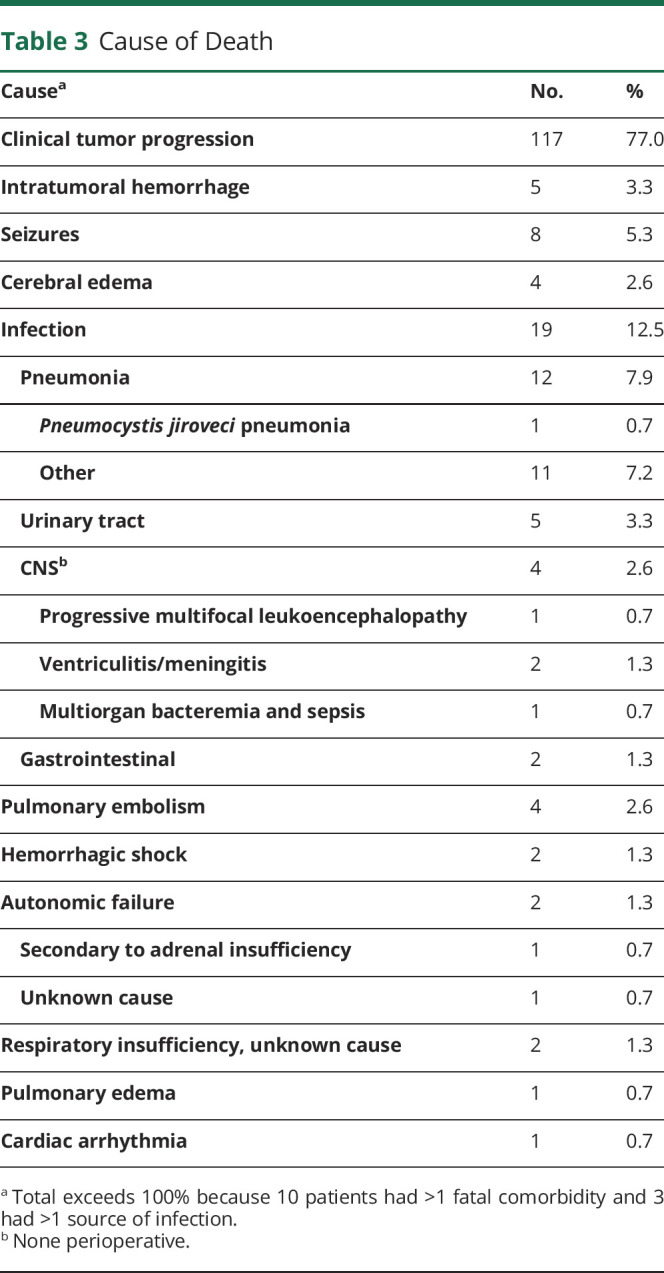
Clinical tumor progression was the cause of death in most patients (n = 177, 77.0%; Table 3). These patients were transitioned to formal hospice programs or other non–hospice-based comfort measures in the setting of neurologic decline. Fatal infection occurred in 19 (12.5%), most commonly pneumonia (n = 12, 7.9%), which occurred in the setting of aspiration in 8 (5.3%); 1 patient (0.7%) died of Pneumocystis jiroveci pneumonia. Five patients (3.3%) had intratumoral hemorrhage as a cause of death, 4 of whom were receiving therapeutic anticoagulation. Other infrequent mortal events are shown in Table 3. Multiple mortal events were identified in 10 (6.5%).
Table 3.
Cause of Death
Location of Death
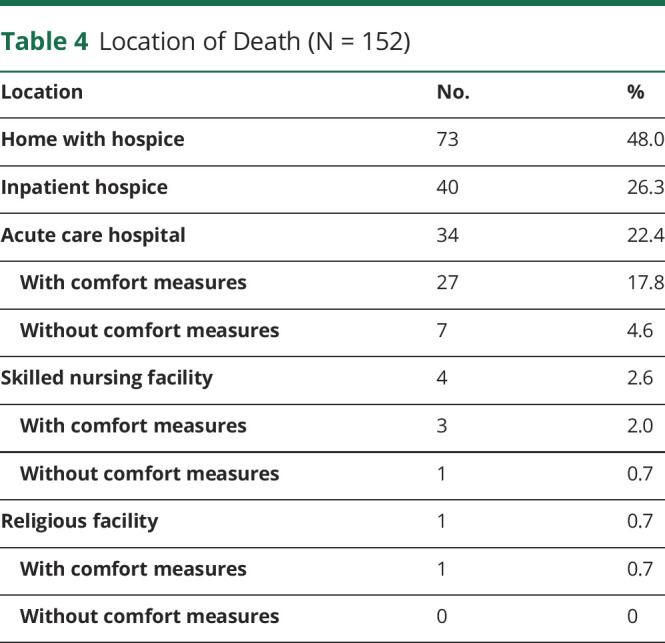
Nearly all patients (n = 144, 94.7%) died with hospice or other nonhospice comfort measures in place (Table 4). Most died at home (n = 73, 48.0%) or in an inpatient hospice facility (n = 40, 26.3%; Table 4). However, 34 (22.4%) died in an acute care hospital, 27 (17.8%) with comfort measures.
Table 4.
Location of Death (N = 152)
Resuscitation
Most patients (n = 132, 86.8%) did not undergo cardiac or pulmonary resuscitation (Table 5). Pulmonary resuscitation (without cardiac resuscitation) occurred in 15 patients (9.9%), 12 (7.9% overall) of whom were ultimately transitioned to hospice or other comfort measures before death, but the remainder (n = 3, 2.0% overall) died in acute care hospitals. One patient (0.7%) was intubated early in the disease course for unresponsiveness but was successfully extubated and received tumor-directed therapy; when clinical tumor progression occurred, a “do not resuscitate/do not intubate” (DNR/DNI) order was entered, and the patient died on home hospice. Five patients (3.3%) remained intubated and mechanically ventilated at the time of death.
Table 5.
Resuscitation (N = 152)
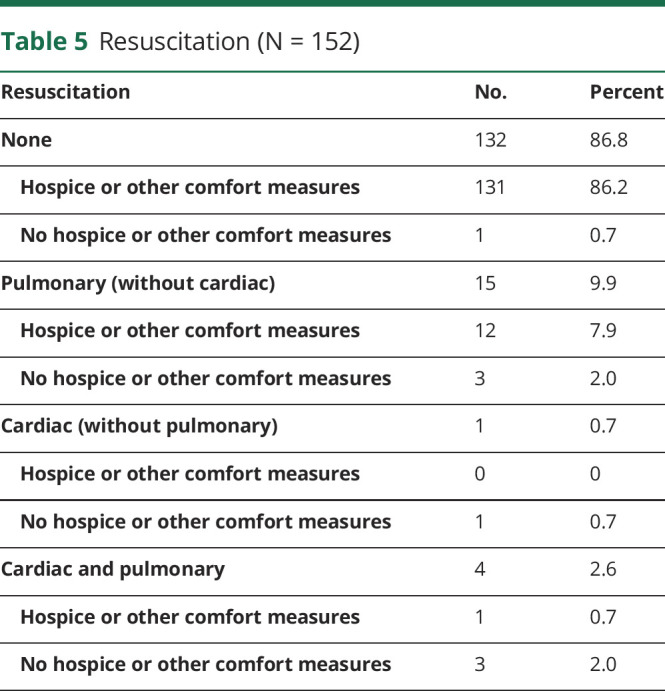
No order was obtained to avoid resuscitation for 1 patient (0.7%), resulting in suctioning and bag-mask ventilation at the end of life; caregivers ultimately refused intubation and chest compressions at the time of cardiopulmonary arrest. Unsuccessful cardiac resuscitation without intubation was performed in 1 (0.7%, Table 5).
Reasons for resuscitation varied. For example, cardiac or pulmonary resuscitation was performed in 6 patients (3.9%) because of religious concerns. Hope for longer survival to receive an additional therapy prompted resuscitation in 3 patients (2.0%). Rapid and unanticipated decline early in the disease course drove resuscitation in 4 patients (2.6%).
Only 68 patients (44.7%) had clear DNR/DNI documentation present in the medical record. In 18 (11.8%) other cases, caregivers clarified that DNR/DNI documentation was completed.
We identified 4 patients who died 1 to 3 months after diagnosis. Among them, 3 had comfort or hospice measures implemented. Tumor progression, respiratory failure, pneumonia, and malignant cerebral edema were the cause of death in 1 patient each. Resuscitation was performed in 3 (pulmonary in 2, cardiac in 1). The small sample size precluded formal statistical comparisons against other subgroups with longer survival.
Discussion
In our clinical experience, patients and caregivers often ask what to expect over the arc of the illness as gliomas worsen. We found that most (77.0%) died of (presumptive) tumor growth and that transition to hospice or other non–hospice-based comfort measures in the setting of resultant neurologic decline was possible. For example, nearly all patients (94.7%) died with hospice or other comfort measures in place, and 74.3% died either at home or as part of an inpatient hospice program. Only 13% underwent cardiac or pulmonary resuscitation. These observations suggest that death was natural and comfortable, but we did not formally assess levels of pain, symptom distress, or psycho-existential distress. We are unaware of similar studies.
Specific neurologic complications of tumor progression such as refractory seizures, painful intratumoral hemorrhage, and symptomatic cerebral edema were identified as infrequent clinical events leading to death compared with clinical tumor progression. Seizures are a known complication of gliomas, particularly during the last week to month of life, and often affect quality of life.10,12,14 Only 5.3% of patients in this study, however, had seizures at the end of life, perhaps due to adequate counseling for caregivers about seizure risk and aggressively treating epilepsy.
Approximately one-half (48.0%) of our patients died at home. Location of death is often very important to patients with cancer, including primary brain tumors. Dying in the preferred place of death improves quality of care.29,30 Patients often prefer to die at home; however, late hospitalization—at times including intensive care—and death in an acute care hospital occur commonly in patients with both systemic malignancies and primary brain tumors.29,31-34 In-hospital death, including death in the intensive care unit, is associated with poorer quality of death in patients with cancer and increased risk of psychological distress among caregivers.35 Patients with gliomas who receive palliative care are more likely to die outside the hospital setting, particularly if enrolled in hospice >7 days before death.36 In our study population, nearly one-fourth (22.4%) died in an acute care hospital. Further efforts to engage palliative services at home to reduce the need for inpatient support are warranted to improve both quality and cost-effectiveness of care at the end of life in patients with brain tumors.20,37
Although 86.8% of patients did not undergo acute cardiac or pulmonary resuscitation, only 45% had clear documentation of a DNR/DNI preference in the medical record, consistent with the literature suggesting that advanced directives are often poorly documented in patients with gliomas.29,34
More robust efforts to discuss and document resuscitation status in patients with gliomas are needed to increase preparedness for the end of life. In 3.9% of patients in our cohort, resuscitative efforts were requested by caregivers for religious reasons. Religious concerns in patients with cancer, including glioblastoma, can be associated with increased risk for late hospitalization and receipt of futile medical care near the time of death,33,38 even though patients with advanced cancer may perceive that their spiritual needs are not supported.39 Further investigation is warranted regarding care patterns in religiously observant patients and the required specific support.
There are several limitations to our study. As a result of the retrospective nature of the study, we were limited to extracting data from the clinical record as opposed to prospectively collecting prespecified points in a uniform manner. We ameliorated this by contacting caregivers, but recall bias could have influenced the supplemental information provided. Furthermore, while 213 patients with high-grade gliomas who died within the 5-year time frame of the study were identified, 61 were excluded due to insufficient available information from the medical record or caregivers regarding end-of-life circumstances. Thus, selection bias may have affected our observations of end-of-life outcomes, because only those patients who had adequate end-of-life data in the medical record or whose caregivers were able to provide supplemental information when needed were included. In addition, as a single-center study at a major urban academic center, our results may not be generalizable to other settings. Finally, we did not formally compare end-of-life circumstances by duration of survival.
Improved understanding of the most common clinical circumstances surrounding death and the frequency of hospice use, comfort care, and resuscitative efforts in patients with gliomas nearing death may ultimately allow physicians to provide more accurate expectations and options for end-of-life care, thus reducing anxiety and uncertainty about the end of life and permitting more thorough, informed care planning. Our observation that nearly all patients (94.7%) died with hospice or other comfort measures suggests that a peaceful death from glioma is possible; however, we did not formally measure comfort or pain. Because use of chemotherapy in the last months of life is associated with increased use of aggressive measures such as mechanical ventilation and cardiopulmonary resuscitation in patients with systemic malignancies,40 the field of neuro-oncology would also benefit from analyzing how circumstances surrounding death, including comfort, hospitalization, and resuscitation, are affected by continuation of aggressive disease-modifying therapy (such as surgery, radiotherapy, or chemotherapy) in the last months of life for patients with gliomas.
In conclusion, clinical tumor progression was the most common cause of death in our patient population, and most patients died at home with hospice or in an inpatient hospice facility. The majority of patients were placed on hospice or non–hospice-based comfort measures and were not resuscitated at the end of life. These results may inform end-of-life expectations by patients and caregivers, fostering care planning and emotional preparedness.
Acknowledgment
The authors thank outpatient clinical NPs Esther Kim, Amily Koshy, and Shannon Higgins; Dr. Laura Lennihan and the Neurology Graduate Service, especially NPs Susan Kreiner, Shawanda Patterson, Shinta Jose, and Hasratdeep “Noor” Tung; expert tumor neurosurgeons, particularly Jeffrey Bruce, Guy McKhann, and Michael Sisti; CNS radiation oncologists Tony Wang and Simon Cheng; Neuroradiology colleagues, especially Angela Lignelli-Dipple; and the Neuro-Oncology research team of the Herbert Irving Comprehensive Cancer Center. Their partnerships are crucial for compassionate and accessible care of our patients.
Glossary
- DNR/DNI
do not resuscitate/do not intubate
Appendix. Authors
Study Funding
The authors report no targeted funding specific to this study. Outside of the submitted work: The William Rhodes and Louise Tilzer-Rhodes Center for Glioblastoma at New York-Presbyterian Hospital provided partial support for M.B., C.D.B., F.M.I., T.N.K., M.R.W., Y.O., L.E.D., K.A.E., and A.B.L.; NIH/National Cancer Institute grants P30CA013696 and UG1CA189960 also supported A.B.L. in part; the Matheson Foundation supported Y.O. and L.E.D.; and the Herbert Irving Comprehensive Cancer Center supported L.E.D. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute/NIH or other funders. Publication costs were supported by The Michael Weiner Glioblastoma Research Into Treatment Fund.
Disclosure
The authors report no relevant disclosures regarding the submitted work. T.N. Kreisl is now an employee of Novartis. Go to Neurology.org/N for full disclosures.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 2.Stark AM, Nabavi A, Mehdorn HM, Blömer U. Glioblastoma multiforme-report of 267 cases treated at a single institution. Surg Neurol. 2005;63(2):162-169. [DOI] [PubMed] [Google Scholar]
- 3.Silbergeld DL, Rostomily RC, Alvord EC Jr. The cause of death in patients with glioblastoma is multifactorial: clinical factors and autopsy findings in 117 cases of supratentorial glioblastoma in adults. J Neurooncol. 1991;10(2):179-185. [DOI] [PubMed] [Google Scholar]
- 4.Amano K, Morita T, Tatara R, Katayama H, Uno T, Takagi I. Association between early palliative care referrals, inpatient hospice utilization, and aggressiveness of care at the end of life. J Palliat Med. 2015;18(3):270-273. [DOI] [PubMed] [Google Scholar]
- 5.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112. [DOI] [PubMed] [Google Scholar]
- 6.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880-887. [DOI] [PubMed] [Google Scholar]
- 7.Parikh RB, Kirch RA, Smith TJ, Temel JS. Early specialty palliative care: translating data in oncology into practice. N Engl J Med. 2013;369(24):2347-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walbert T. Palliative care, end-of-life care, and advance care planning in neuro-oncology. Continuum. 2017;23(6, Neuro-oncology):1709-1726. [DOI] [PubMed] [Google Scholar]
- 9.Gofton TE, Graber J, Carver A. Identifying the palliative care needs of patients living with cerebral tumors and metastases: a retrospective analysis. J Neurooncol. 2012;108(3):527-534. [DOI] [PubMed] [Google Scholar]
- 10.Oberndorfer S, Lindeck-Pozza E, Lahrmann H, Struhal W, Hitzenberger P, Grisold W. The end-of-life hospital setting in patients with glioblastoma. J Palliat Med. 2008;11(1):26-30. [DOI] [PubMed] [Google Scholar]
- 11.Pace A, Di Lorenzo C, Lorenzo CD, et al. End of life issues in brain tumor patients. J Neurooncol. 2009;91(1):39-43. [DOI] [PubMed] [Google Scholar]
- 12.Pace A, Villani V, Di Lorenzo C, et al. Epilepsy in the end-of-life phase in patients with high-grade gliomas. J Neurooncol. 2013;111(1):83-86. [DOI] [PubMed] [Google Scholar]
- 13.Sizoo EM, Koekkoek JA, Postma TJ, et al. Seizures in patients with high-grade glioma: a serious challenge in the end-of-life phase. BMJ Support Palliat Care. 2014;4(1):77-80. [DOI] [PubMed] [Google Scholar]
- 14.Koekkoek JAF, Dirven L, Reijneveld JC, et al. Epilepsy in the end of life phase of brain tumor patients: a systematic review. Neurooncol Pract. 2014;1(3):134-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sizoo EM, Braam L, Postma TJ, et al. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro Oncol. 2010;12(11):1162-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catt S, Chalmers A, Fallowfield L. Psychosocial and supportive-care needs in high-grade glioma. Lancet Oncol. 2008;9(9):884-891. [DOI] [PubMed] [Google Scholar]
- 17.Edelstein K, Coate L, Massey C, Jewitt NC, Mason WP, Devins GM. Illness intrusiveness and subjective well-being in patients with glioblastoma. J Neurooncol. 2016;126(1):127-135. [DOI] [PubMed] [Google Scholar]
- 18.Ford E, Catt S, Chalmers A, Fallowfield L. Systematic review of supportive care needs in patients with primary malignant brain tumors. Neuro Oncol. 2012;14(4):392-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostgathe C, Gaertner J, Kotterba M, et al. Differential palliative care issues in patients with primary and secondary brain tumours. Support Care Cancer. 2010;18(9):1157-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pompili A, Telera S, Villani V, Pace A. Home palliative care and end of life issues in glioblastoma multiforme: results and comments from a homogeneous cohort of patients. Neurosurg Focus. 2014;37(6):E5. [DOI] [PubMed] [Google Scholar]
- 21.Sizoo EM, Pasman HR, Dirven L, et al. The end-of-life phase of high-grade glioma patients: a systematic review. Support Care Cancer. 2014;22(3):847-857. [DOI] [PubMed] [Google Scholar]
- 22.Lin E, Rosenthal MA, Le BH, Eastman P. Neuro-oncology and palliative care: a challenging interface. Neuro Oncol. 2012;14(suppl 4):iv3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins A, Sundararajan V, Brand CA, et al. Clinical presentation and patterns of care for short-term survivors of malignant glioma. J Neurooncol. 2014;119(2):333-341. [DOI] [PubMed] [Google Scholar]
- 24.Diamond EL, Russell D, Kryza-Lacombe M, et al. Rates and risks for late referral to hospice in patients with primary malignant brain tumors. Neuro Oncol. 2016;18(1):78-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forst D, Adams E, Nipp R, et al. Hospice utilization in patients with malignant gliomas. Neuro Oncol. 2018;20(4):538-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemminger LE, Pittman CA, Korones DN, et al. Palliative and end-of-life care in glioblastoma: defining and measuring opportunities to improve care. Neurooncol Pract. 2017;4(3):182-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walbert T. Integration of palliative care into the neuro-oncology practice: patterns in the United States. Neurooncol Pract. 2014;1(1):3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walbert T, Puduvalli VK, Taphoorn MJB, Taylor AR, Jalali R. International patterns of palliative care in neuro-oncology: a survey of physician members of the Asian Society for Neuro-Oncology, the European Association of Neuro-Oncology, and the Society for Neuro-Oncology. Neurooncol Pract. 2015;2(2):62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koekkoek JA, Dirven L, Reijneveld JC, et al. End of life care in high-grade glioma patients in three European countries: a comparative study. J Neurooncol. 2014;120(2):303-310. [DOI] [PubMed] [Google Scholar]
- 30.Tang ST. When death is imminent: where terminally ill patients with cancer prefer to die and why. Cancer Nurs. 2003;26(3):245-251. [DOI] [PubMed] [Google Scholar]
- 31.Bostanci A, Horey D, Jackson K, et al. Insights into hospitalisation of advanced cancer patients: a study of medical records. Eur J Cancer Care (Engl). 2016;25(1):190-201. [DOI] [PubMed] [Google Scholar]
- 32.Bruera E, Russell N, Sweeney C, Fisch M, Palmer JL. Place of death and its predictors for local patients registered at a comprehensive cancer center. J Clin Oncol. 2002;20(8):2127-2133. [DOI] [PubMed] [Google Scholar]
- 33.Diamond EL, Panageas KS, Dallara A, et al. Frequency and predictors of acute hospitalization before death in patients with glioblastoma. J Pain Symptom Manage. 2017;53(2):257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuchinad KE, Strowd R, Evans A, Riley WA, Smith TJ. End of life care for glioblastoma patients at a large academic cancer center. J Neurooncol. 2017;134(1):75-81. [DOI] [PubMed] [Google Scholar]
- 35.Wright AA, Keating NL, Balboni TA, Matulonis UA, Block SD, Prigerson HG. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers' mental health. J Clin Oncol. 2010;28(29):4457-4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundararajan V, Bohensky MA, Moore G, et al. Mapping the patterns of care, the receipt of palliative care and the site of death for patients with malignant glioma. J Neurooncol. 2014;116(1):119-126. [DOI] [PubMed] [Google Scholar]
- 37.Pace A, Di Lorenzo C, Capon A, et al. Quality of care and rehospitalization rate in the last stage of disease in brain tumor patients assisted at home: a cost effectiveness study. J Palliat Med. 2012;15(2):225-227. [DOI] [PubMed] [Google Scholar]
- 38.Phelps AC, Maciejewski PK, Nilsson M, et al. Religious coping and use of intensive life-prolonging care near death in patients with advanced cancer. JAMA. 2009;301(11):1140-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balboni TA, Vanderwerker LC, Block SD, et al. Religiousness and spiritual support among advanced cancer patients and associations with end-of-life treatment preferences and quality of life. J Clin Oncol. 2007;25(5):555-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright AA, Zhang B, Keating NL, Weeks JC, Prigerson HG. Associations between palliative chemotherapy and adult cancer patients' end of life care and place of death: prospective cohort study. BMJ. 2014;348:g1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests should be submitted to the corresponding author.


