Abstract
Background and Objectives
Cerebral microbleeds (CMBs) are common in patients with acute ischemic stroke and are associated with increased risk of intracerebral hemorrhage (ICH) after intravenous thrombolysis. Whether CMBs modify the treatment effect of thrombolysis is unknown.
Methods
We performed a prespecified analysis of the prospective randomized controlled multicenter Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke (WAKE-UP) trial including patients with acute ischemic stroke with unknown time of symptom onset and diffusion-weighted imaging–fluid-attenuated inversion recovery mismatch on MRI receiving alteplase or placebo. Patients were screened and enrolled between September 2012 and June 2017 (with final follow-up in September 2017). Patients were randomized to treatment with IV thrombolysis with alteplase at 0.9 mg/kg body weight or placebo. CMB status (presence, number, and distribution) was assessed after study completion by 3 raters blinded to clinical information following a standardized protocol. Outcome measures were excellent functional outcome at 90 days, defined by modified Rankin Scale (mRS) score ≤1, and symptomatic ICH according to National Institutes of Neurological Disease and Stroke trial criteria 22 to 36 hours after treatment.
Results
Of 503 patients enrolled in the WAKE-UP trial, 459 (91.3%; 288 [63%] men) were available for analysis. Ninety-eight (21.4%) had at least 1 CMB on baseline imaging; 45 (9.8%) had exactly 1 CMB; 37 (8.1%) had 2 to 4 CMBs; and 16 (3.5%) had ≥5 CMBs. Presence of CMBs was associated with a nonsignificant increased risk of symptomatic ICH (11.2% vs 4.2%; adjusted odds ratio [OR] 2.32, 95% confidence interval [CI] 0.99–5.43, p = 0.052) but had no effect on functional outcome at 90 days (mRS score ≤1: 45.8% vs 50.7%; adjusted OR 0.99, 95% CI 0.59–1.64, p = 0.955). Patients receiving alteplase had better functional outcome (mRS score ≤1: 54.6% vs 44.6%, adjusted OR 1.61, 95% CI 1.07–2.43, p = 0.022) without evidence of heterogeneity in relation to CMB presence (p of the interactive term = 0.546). Results were similar for subpopulations with strictly lobar (presumed cerebral amyloid angiopathy related) or not strictly lobar CMB distribution.
Discussion
In the randomized-controlled WAKE-UP trial, we saw no evidence of reduced treatment effect of alteplase in patients with acute ischemic stroke with ≥1 CMBs. Additional studies are needed to determine the treatment effect of alteplase and its benefit-harm ratio in patients with a larger number of CMBs.
Trial Registration Information
ClinicalTrials.gov identifier NCT01525290; ClinicalTrialsRegister.EU identifier 2011-005906-32.
Classification of Evidence
This study provides Class II evidence that for patients with acute ischemic stroke with unknown time of onset and diffusion-weighted imaging–fluid-attenuated inversion recovery mismatch who received IV alteplase, CMBs are not significantly associated with functional outcome at 90 days.
Early treatment with IV thrombolysis with alteplase (recombinant tissue plasminogen activator) improves functional outcome in patients with acute ischemic stroke.1,2 Cerebral microbleeds (CMBs), small hypointense lesions that are visible on hemorrhage-sensitive MRI sequences, are present on pretreatment imaging in ≈15% to 38% of patients with ischemic stroke.3,4 CMBs, particularly if present in larger numbers, are associated with a significantly increased risk of postalteplase symptomatic intracerebral hemorrhage (sICH).4-6 Besides, the spatial distribution (i.e., the underlying vasculopathy7) of CMBs may influence the effects of CMBs on bleeding risk and functional outcome.8 To date, no randomized controlled trials of IV alteplase in patients with acute ischemic stroke stratified by CMB status on pretreatment baseline MRI have been conducted, and there are no studies describing the effect of CMB presence, burden, and spatial distribution or presumed pathophysiology on the risk of acute hemorrhagic complications after acute stroke without alteplase treatment. Thus, while the prognostic importance of CMBs in patients with acute stroke treated with alteplase is well documented, the effect of baseline CMB status on the treatment effect of alteplase compared to placebo is currently unknown. Reflecting this uncertainty, the American Heart Association/American Stroke Association guidelines state that treatment with IV alteplase in patients with CMBs may be associated with an increased risk of sICH and that the benefits of treatment are uncertain in patients who have previously had a high burden of CMBs demonstrated on MRI.3 On the other hand, an exploratory analysis of the New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial Versus ASA to Prevent Embolism in Embolic Stroke of Undetermined Source (NAVIGATE ESUS) trial that compared the direct oral anticoagulant rivaroxaban to the platelet inhibitor aspirin recently showed that CMBs in patients with ischemic stroke may well be a marker of worse clinical outcome in general without implying annulment of a beneficial effect of an intervention.9
All patients enrolled in the Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke (WAKE-UP),10 which demonstrated benefit of alteplase in imaging-selected patients with acute ischemic stroke, underwent pretreatment MRI showing an acute ischemic lesion. In this preplanned exploratory analysis of the WAKE-UP trial data, we aimed to investigate how presence, number, and spatial distribution of CMBs affect functional outcome and risk of hemorrhage after acute ischemic stroke and whether they modify the treatment effect of alteplase.
Methods
Study Design
We performed a preplanned post hoc analysis including patients enrolled in the WAKE-UP trial to provide Class II evidence for the question of whether CMBs are associated with functional outcome at 90 days in patients with acute ischemic stroke with unknown time of onset and diffusion-weighted imaging–fluid-attenuated inversion recovery mismatch who received IV alteplase. The WAKE-UP trial was a multicenter, 2-arm, double-blind, randomized placebo-controlled clinical trial that evaluated the efficacy and safety of IV thrombolysis with alteplase in imaging-selected patients with acute ischemic stroke and unknown time of symptom onset. The detailed protocol has been published previously with the main results.10 Briefly, patients 18 to 80 years of age with a diffusion-weighted imaging–fluid-attenuated inversion recovery mismatch on acute MRI indicating lesion age ≤4.5 hours11 were enrolled and randomized in a 1:1 ratio to treatment with either alteplase at a dose of 0.9 mg/kg body weight or placebo. Randomization was implemented by means of a web-based procedure with a permuted-block design according to trial center. Contraindications included dependency in daily living before the stroke incident and planned mechanical thrombectomy. Presence of CMBs was not considered a contraindication for participation in the trial; however, patients could be excluded from the trial at the discretion of the local investigators if CMB burden was considered to pose a significantly increased risk of severe thrombolysis-associated bleeding. Patients were recruited in 61 experienced stroke centers in Europe. Patients were screened and enrolled between September 2012 and June 2017. Enrollment was stopped on June 30, 2017, due to cessation of funding.
Assessment of CMBs
All patients enrolled in the WAKE-UP trial underwent acute MRI before randomization. For the current analysis, CMBs were defined as small (generally 2–5 mm in diameter but sometimes up to 10 mm) areas of signal void with associated blooming seen on T2*-weighted MRI or other sequences that are sensitive to susceptibility effects.12 Precise parameters of MRI sequences varied according to center and manufacturer: field strength was 1.5T or 3.0T; section thickness ranged from 1.5 to 7 mm; echo time ranged from 11 to 51 milliseconds; and repetition time ranged from 460 to 2,460 milliseconds. Three raters (L.S., T.B.B., J.V.) blinded to all clinical data independently assessed and counted the number of CMBs in separate subsamples of patients and classified their location as lobar, deep (i.e., in the basal ganglia/thalamus), or infratentorial (i.e., brainstem or cerebellar). Interrater reliability was assessed by calculating the Krippendorff α13 for a randomly selected subsample of 100 scans that were assessed independently by all 3 raters; results were interpreted according to recommendations.14 For further analysis, CMB number was discretized into bins: 0, 1, 2 to 4, and ≥5 CMBs.4
Outcome Parameters
Outcome parameters included the degree of disability and death, both 90 days after the stroke, and the occurrence of intracranial hemorrhage, assessed on follow-up imaging 22 to 36 hours after treatment. The degree of disability was quantified on the modified Rankin Scale (mRS); a score ≤1 was considered an excellent outcome, and improvement of at least 1 point was assessed in ordinal shift analysis. Regarding intracranial hemorrhage, endpoints included the incidence of sICH according to the protocols of the Safe Implementation of Thrombolysis in Stroke–Monitoring Study (SITS-MOST),15 European Cooperative Acute Stroke Study (ECASS) II,16 ECASS III,17 or National Institutes of Neurological Disease and Stroke (NINDS)18; hemorrhagic transformation (HT; including type 1 and 2 parenchymal hematoma [PH] and type 2 hemorrhagic infarction but not type 1 hemorrhagic infarction); and PH2 by itself. Multivariable regression analysis was restricted to sICH according to NINDS criteria to include sufficient numbers of events (see below). Imaging outcomes were ascertained by a central image reading and a safety adjudication committee within the WAKE-UP trial.
Statistical Analysis
Treatment outcomes were assessed in the intention-to-treat population for all patients with available information on clinical endpoints. Continuous variables are shown as median and interquartile range (IQR); proportions are shown as absolute numbers and percentage. Associations between presence and number of CMBs and baseline characteristics were assessed with χ2 and Wilcoxon rank-sum tests.
Associations between the presence of CMBs and binary outcome parameters were assessed with multivariable unconditional logistic regression models with a logit link function. For analyses using the ordinal mRS score as dependent variable, the proportional odds assumption was tested with a score test based on a χ2 distribution with 12 df. Because this assumption was not met, analyses were limited to the binary measure excellent functional outcome. For all regression analyses, independent variables included presence of CMBs (total number ≥1 vs 0), treatment group (alteplase vs placebo), the covariates NIH Stroke Scale sum score and age (selected in accordance with the study protocol of the main trial), and a facultative interactive term (presence of CMBs × treatment group). For sensitivity analyses, we also created models including variables that showed a significant univariate association with either presence or number of CMBs at the 0.05 significance level as additional covariates. These models led to the same conclusions as the primary models. Detailed results are presented in the online supplement (eTables 1 and 2, links.lww.com/WNL/B658).
The relationships between clinical outcome parameters and variables of interest are expressed as odds ratios (point estimates and 95% confidence intervals [CIs]).
For analyses involving the number of CMBs as an ordinal variable (0, 1, 2–4, ≥5) or the safety outcome parameters death and occurrence of PH2, the creation of robust multivariable models was not possible due to low event numbers (i.e., failure to obtain maximum likelihood estimates). In these cases, we provide a description of the outcome distributions and, with ordinal CMB categories, results from unadjusted nonparametric tests for linear trend (Mantel-Haenszel test for trend). All tests were performed with a 2-sided α level of 0.05 without adjustment for multiple testing. Analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC) and SPSS version 25 (SPSS Inc, Chicago, IL).
Standard Protocol Approvals, Registrations, and Patient Consents
For each study site, the competent authorities and the corresponding ethics committee approved the trial. Written informed consent was obtained from patients or their legal representatives according to national and local regulations. There was an exception from explicit informed consent in emergency circumstances in some countries. The WAKE-UP study was preregistered (ClinicalTrials.gov NCT01525290; EudraCT 2011-005906-32).
Data Availability
Individual patient data, after deidentification, will be shared with the Virtual International Stroke Trials Archive and be accessible for researchers according to the Virtual International Stroke Trials Archive rules.
Results
Baseline CMB Results
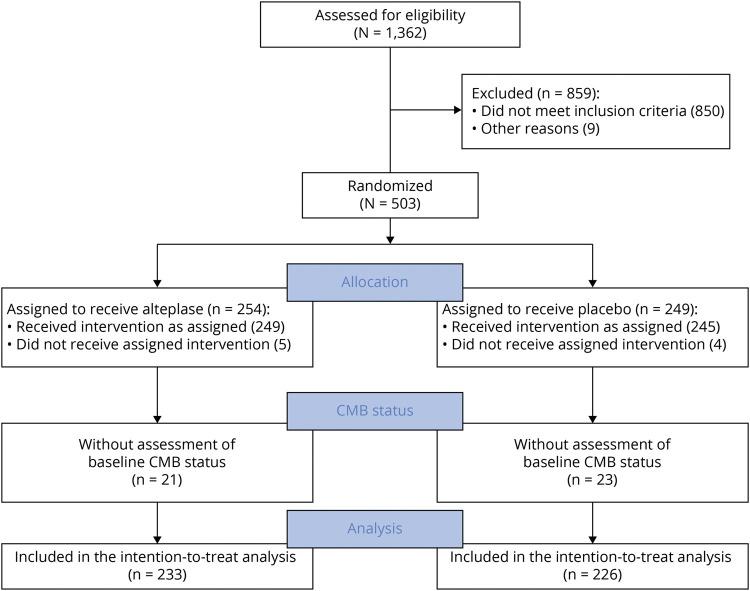
CMB status on baseline prerandomization MRI could be assessed for 459 of 503 (91.3%) patients enrolled in the WAKE-UP trial. In 23 patients (4.6%), image quality of gradient echo sequences did not allow a reliable assessment of CMBs; in 21 patients (4.2%), no gradient echo sequence at baseline was available for analysis. A flowchart is shown in Figure 1. The median number of CMBs across all patients was 0 (IQR 0–0, range 0–19). Of 459 patients included in the analysis, 98 (21.4%) had at least 1 CMB, 45 (9.8%) had exactly 1 CMB, 37 (8.1%) had 2 to 4 CMBs, and 16 (3.5%) had ≥5 CMBs. In 53 patients, CMBs were found exclusively in a lobar location; in 45 patients, CMBs were not strictly lobar (Table 1).
Figure 1. Flow Diagram.
CMB = cerebral microbleed.
Table 1.
Spatial Distribution of Cerebral Microbleeds
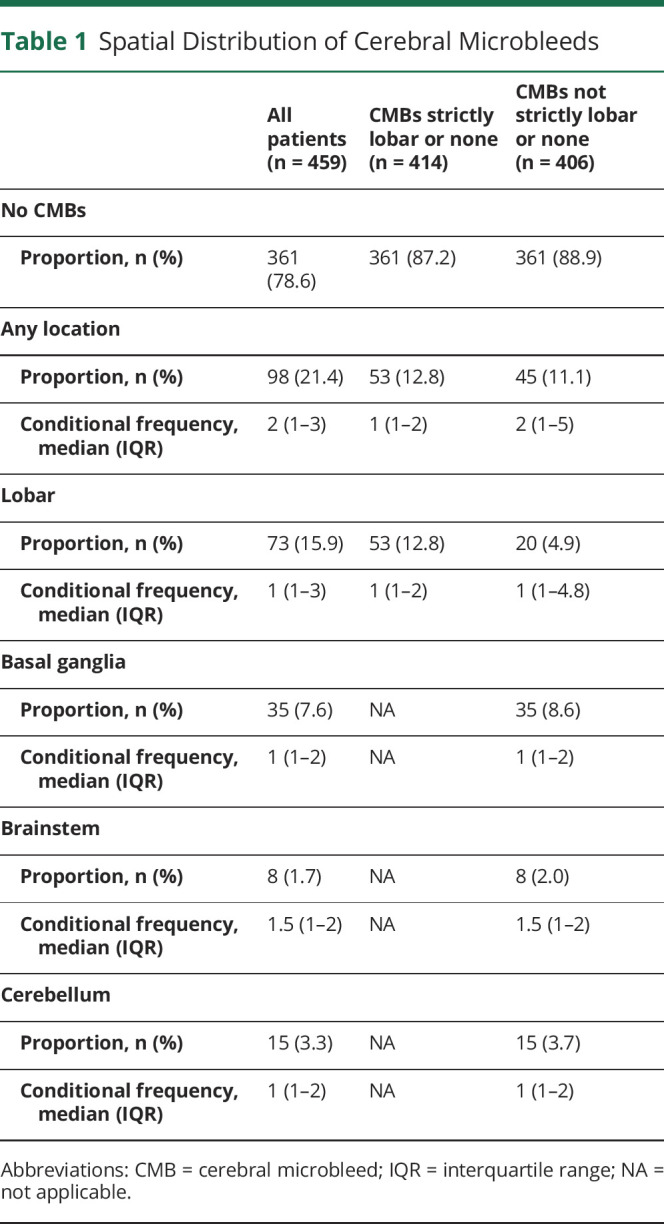
The interrater reliability between the 3 raters was very good or excellent (presence of CMBs, Krippendorff α = 0.71; total number of CMBs, α = 0.74; distribution of CMBs [strictly lobar or not strictly lobar], α = 0.85).
Baseline Characteristics of Patient Population
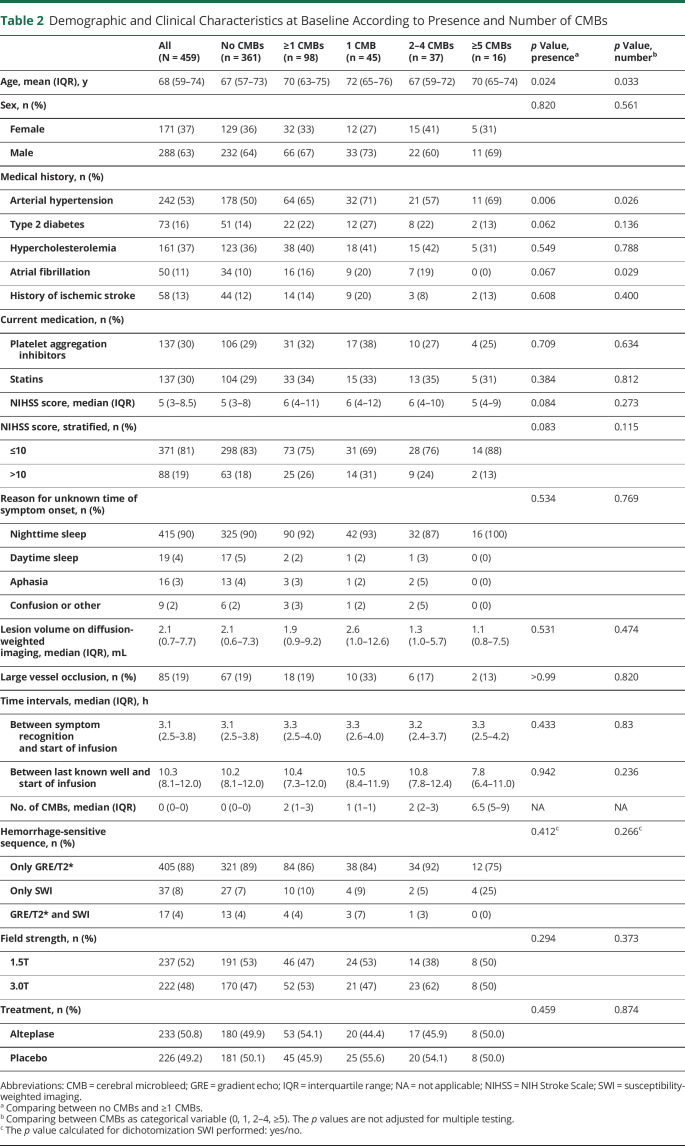
Demographic and clinical characteristics at baseline according to presence and number of CMBs are shown in Table 2. Patients with at least 1 CMB were on average older (median age 70 [IQR 63–75] vs 67 [IQR 57–73] years, p = 0.024) and more often had a diagnosis of arterial hypertension (65% vs 50%, p = 0.006). In addition, a trend for a between-group difference was observed for the frequency of type 2 diabetes (22% vs 14%, p = 0.062) and atrial fibrillation (16% vs 10%, p = 0.067) and baseline stroke symptom severity (median NIH Stroke Scale score 6 [IQR 4–11] vs 5 [3–8], p = 0.084). Remaining baseline characteristics, including the availability of susceptibility-weighted imaging, were not associated with presence or number of CMBs.
Table 2.
Demographic and Clinical Characteristics at Baseline According to Presence and Number of CMBs
Relationship Between Presence of CMBs and Functional Outcome and Safety Endpoints
The proportion of patients with excellent outcome (mRS score ≤1) at 90 days did not differ significantly in patients with at least 1 CMB and patients without CMBs (44 of 96 [45.8%] vs 179 of 353 [50.7%]; adjusted odds ratio 0.99, 95% CI 0.59–1.64, p = 0.955; eFigure 1, links.lww.com/WNL/B658).
Across all patients, incidence of any sICH according to NINDS criteria at follow-up imaging was numerically more than twice as frequent in patients with at least 1 CMB as compared to those with no CMBs without reaching the predefined statistical significance threshold (11 of 98 [11.2%] vs 15 of 361 [4.2%]; adjusted odds ratio 2.32, 95% CI 0.99–5.43, p = 0.052). The occurrence of HT did not differ between groups (21 of 95 [22.1%] vs 46 of 358 [12.8%]; adjusted odds ratio 1.57, 95% CI 0.84–2.95, p = 0.158). The magnitude of the relative increase of hemorrhagic complications in patients with at least 1 CMB was similar in the placebo and alteplase groups without evidence of heterogeneity between treatment groups (p for interaction = 0.522 [sICH] and 0.884 [HT]; eFigure 2, links.lww.com/WNL/B658).
Modification of Treatment Effect of Alteplase by Presence of CMBs
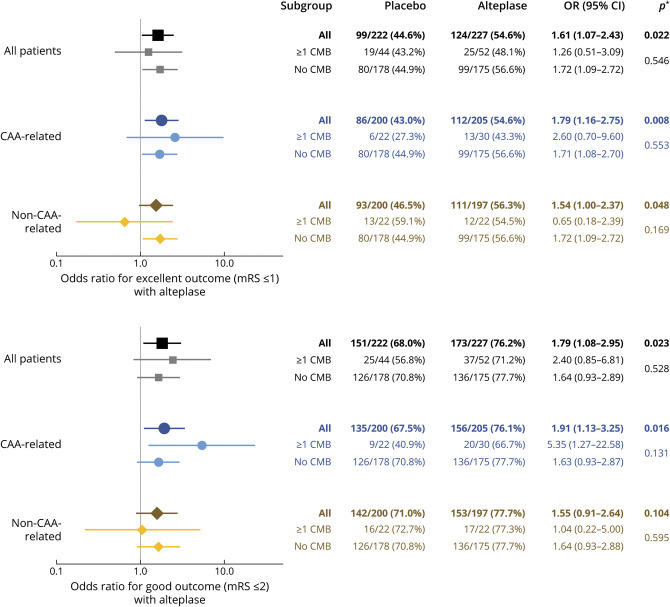
In analyses of the group of patients with strictly lobar CMBs or no CMBs, treatment with alteplase compared to placebo was associated with higher rates of excellent functional outcome (mRS score ≤1) at 90 days (112 of 205 [54.6%] vs 86 of 200 [43.0%], adjusted odds ratio 1.79, 95% CI 1.16–2.75, p = 0.008) without evidence of interaction between CMB presence and treatment group (adjusted odds ratioCMB 2.6, 95% CI 0.70–9.60; adjusted odds rationo CMB 1.71, 95% CI 1.08–2.70, p for interaction = 0.553). Similarly, we could not determine a modifying role of not strictly lobar CMBs on the treatment effect of alteplase (adjusted odds ratiocombined group 1.54, 95% CI 1.00–2.37, p = 0.048; adjusted odds ratioCMB 0.65, 95% CI 0.18–2.39; adjusted odds rationo CMB 1.72, 95% CI 1.09–2.72, p for interaction = 0.169; Figure 2).
Figure 2. Association Between Alteplase Treatment and Functional Outcome at 90 Days According to Presence and Spatial Distribution of CMBs.
Point estimates >1 indicate better functional outcome with alteplase treatment. *First p value per group corresponds to the hypothesis that across patients with any number of cerebral microbleeds (CMBs), the odds ratio (OR) is equal to 1 (no effect); the second p value corresponds to the hypothesis that within strata of patients with no CMBs and ≥1 CMBs, the OR is identical (no interaction). CAA = cerebral amyloid angiopathy; CI = confidence interval; mRS = modified Rankin Scale.
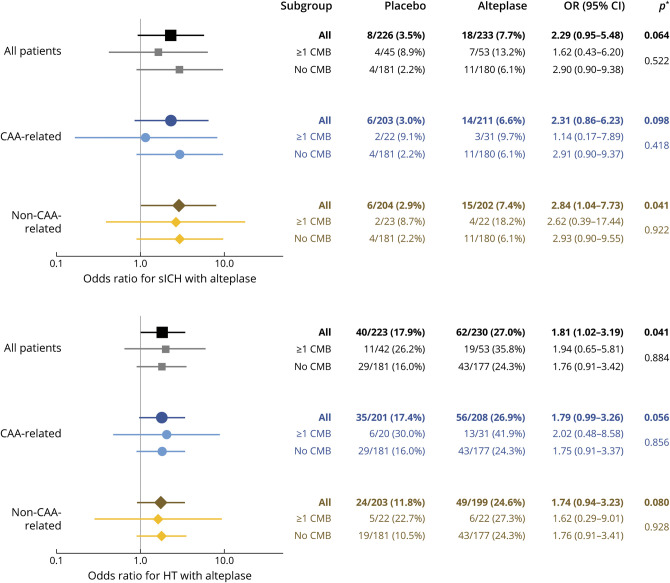
Treatment with alteplase compared to placebo was associated with a trend toward higher rates of sICH (18 of 233 [7.7%] vs 8 of 226 [3.5%], adjusted odds ratio 2.29, 95% CI 0.05–5.48, p = 0.064) and significantly higher rates of HT (62 of 230 [27.0%] vs 40 of 223 [17.9%], adjusted odd ratio 1.181, 95% CI 1.02–3.19, p = 0.041). Again, there was no evidence of heterogeneity with regard to the presence or spatial distribution of CMBs (strictly lobar vs not strictly lobar; all p for interaction >0.05; Figure 3).
Figure 3. Association Between Alteplase Treatment and Hemorrhagic Complications According to Presence and Spatial Distribution of CMBs.
Point estimates >1 indicate higher risk of hemorrhage with alteplase treatment. *First p value per group corresponds to the hypothesis that across patients with any number of cerebral microbleeds (CMBs), the odds ratio (OR) is equal to 1 (no effect); the second p value corresponds to the hypothesis that within strata of patients with no CMBs and ≥1 CMBs, the OR is identical (no interaction). CAA = cerebral amyloid angiopathy; CI = confidence interval; HT = hemorrhagic transformation; sICH = symptomatic intracranial hemorrhage.
Low-Frequency Safety Endpoints: PH2 and Death
For the safety endpoints PH2 and death and sICH according to SITS-MOST or ECASS II/III criteria, event numbers were too low to obtain reliable maximum likelihood estimates in adjusted regression models. PH2 and death appeared to be more frequent in patients treated with alteplase than in those receiving placebo (8 of 230 [3.5%, 95% CI 1.5%–6.7%] vs 1 of 223 [0.5%, 95% CI 0.0%–2.5%] and 9 of 176 [5.1%, 95% CI 2.4%–9.5%] vs 1 of 188 [0.5%, 95% CI 0.0%–2.9%], respectively). In addition, among patients treated with alteplase but not among patients receiving placebo, PH2 and death appeared to be more frequent in patients with at least 1 CMB than in those without CMBs (5 of 53 [9.4%, 95% CI 3.1%–20.7%] vs 3 of 177 [1.7%, 95% CI 0.4%–4.9%] and 4 of 43 [9.3%, 95% CI 2.6%–22.1%] vs 5 of 133 [3.8%, 95% CI 1.2%–8.6%], respectively; Figure 4). Data for sICH according to SITS-MOST or ECASS II/III criteria are presented in eFigure 3 (links.lww.com/WNL/B658).
Figure 4. Relative Frequencies of Low-Frequency Safety Endpoints by Treatment Group and Presence of CMBs.
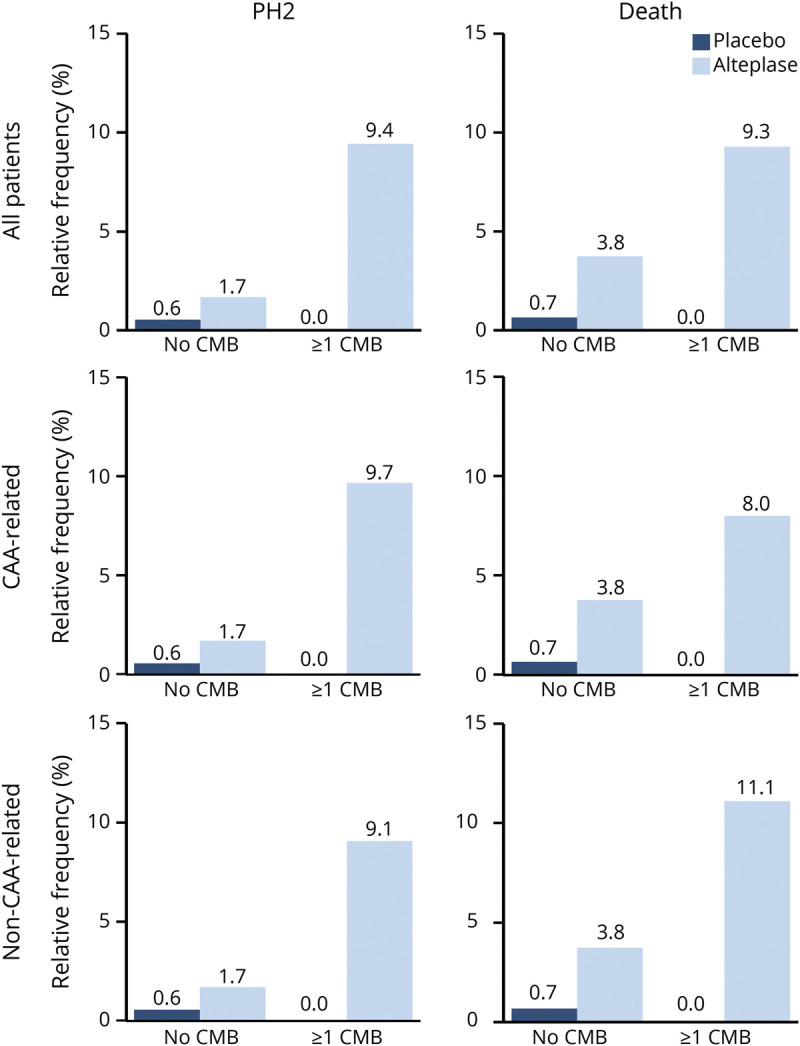
CAA = cerebral amyloid angiopathy; CMB = cerebral microbleed; PH2 = parenchymal hematoma type 2.
Ordinal CMB Count and Outcome
Among the combined group of patients treated with either placebo or alteplase, the rates of excellent functional outcome were similar for patients with 0, 1, 2 to 4, and ≥5 CMBs of any location (crude p for linear trend = 0.812 and 0.531, respectively; Figure 5, A and B). With regard to safety endpoints, sICH, HT, and PH2, but not death, appeared to be more frequent among patients with higher CMB number (crude p for linear trend = 0.013, 0.032, <0.001, and 0.686, respectively; Figure 5, C–F). Frequencies of sICH according to different definitions and subcategories of HT by baseline CMB status are shown in eTable 3 (links.lww.com/WNL/B658). Small sample sizes in groups with higher CMB burden prohibited further subgroup analyses by treatment allocation or spatial distribution, as well as adjustment for potential confounders.
Figure 5. Associations Between Number of CMBs and Functional and Safety Outcomes.
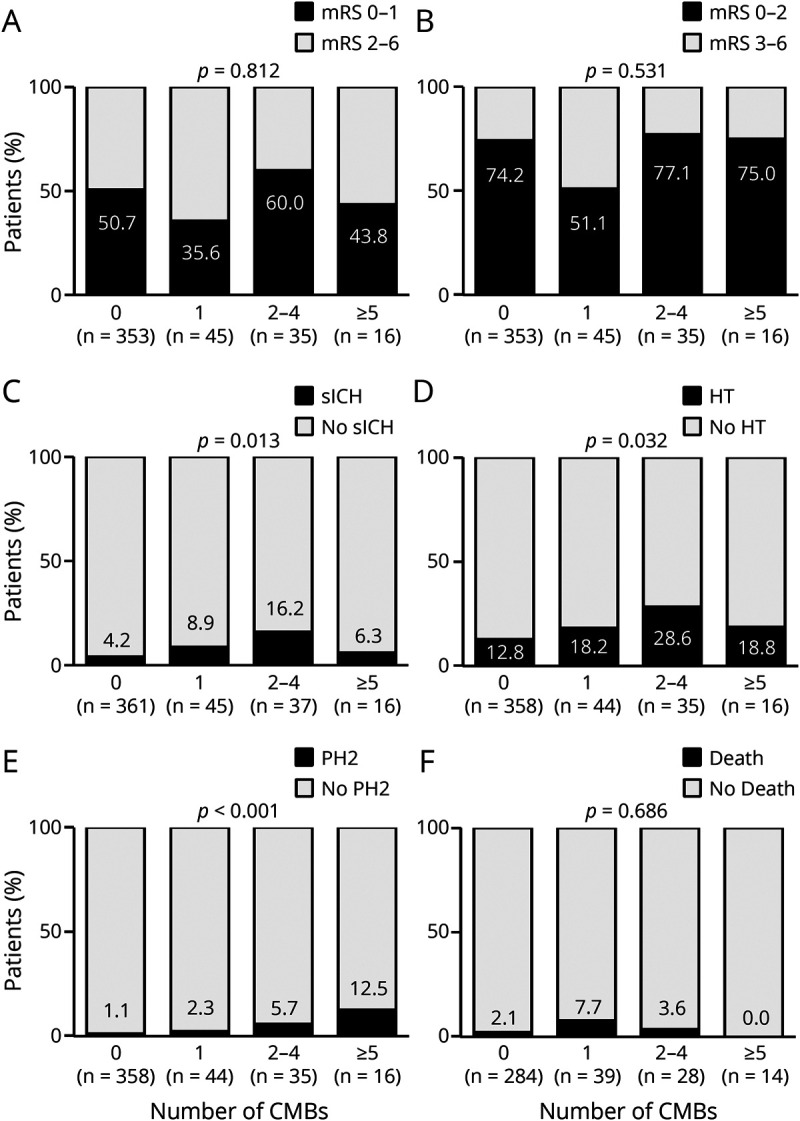
The p values for linear association between cerebral microbleed (CMB) number and outcome measure (linear trend) are derived from Mantel-Haenszel tests for trend. mRS = modified Rankin Scale; PH2 = parenchymal hemorrhage; sICH = symptomatic intracranial hemorrhage.
Discussion
In the current study, we analyzed data from the multicenter, double-blind, randomized WAKE-UP trial to investigate how presence, number, and spatial distribution of CMBs affect outcome after stroke and whether they may modify the treatment effect of alteplase. In line with results from the main trial,10 we found that treatment with alteplase significantly increased the odds of excellent functional outcome despite a trend toward higher rates of hemorrhagic complications. We found no evidence that the beneficial effects of alteplase treatment were different in patients with ≥1 CMBs and those without CMB.
We were able to assess the benefit and harm of IV alteplase in patients with acute ischemic stroke with known CMB burden quantified on pretreatment MRI in a randomized, double-blind, controlled trial. Previous observational studies that established the relationship between CMB status and increased risk of sICH after alteplase and poorer functional outcome at 90 days did not include a control group of untreated patients and therefore could not determine the isolated effect of CMBs on hemorrhagic complications and functional outcome.
In line with results from 2 recent meta-analyses,4,5 occurrence of sICH in our study population was numerically more frequent among patients with ≥1 CMBs than among patients without CMBs. However, this association between the presence of ≥1 CMB and hemorrhagic complications was equally present in patients receiving placebo and in patients receiving alteplase. As a consequence, our analysis yielded similar effect size estimates for the beneficial treatment effect of alteplase on 90-day functional outcome in patients with ≥1 CMBs and patients without CMBs, indicating that presence of CMBs on MRI should not deter physicians from treating eligible patients with thrombolysis. Only in patients with ≥1 not strictly lobar CMBs lay the point estimate of the treatment effect of alteplase for achieving excellent functional outcome (mRS score ≤1) <1; even without evidence of between-group heterogeneity, this could indicate a particular need for further studies in this patient group.
Beyond the presence of CMBs, patients with a higher number of CMBs might be particularly prone to hemorrhagic complications. Although having to be interpreted with caution due to small sample sizes, our results are consistent with previous studies that have suggested an association between the number of CMBs and higher risk of hemorrhagic complications and poor functional outcome.4,5,19 Similar to the models involving the presence of CMBs, however, there was no signal in our data indicating that the number of CMBs was related to functional outcome or death at 90 days.
Previous modeling work related to the treatment effect of alteplase indicated that alteplase treatment may be associated with more harm than benefit in some patients with a large number (>10) of CMBs.20 The population enrolled in the WAKE-UP trial and thereby available for the current analysis included very few patients with >10 CMBs. Our results therefore do not exclude the possibility that alteplase is of reduced benefit in some patients with high CMB burden. Additional trials involving a sufficiently large number of patients with a large number of CMBs would be needed to answer this question.
Strictly lobar CMBs may be a sign of underlying cerebral amyloid angiopathy.7 There is conflicting evidence for whether the presumed pathophysiology of CMBs as indicated by a strictly lobar location is of prognostic importance in assessment of the relationship between CMB status and outcome.4,8 We performed our analyses once for all patients and separately for patients with strictly lobar CMBs and not strictly lobar CMBs and found no evidence of heterogeneity with regard to presumed CMB pathophysiology.
Strengths of the current study include the availability for analysis of patients treated with alteplase and placebo, MRI-proven ischemic stroke in all patients, assessment of CMB burden before administration of the study drug, outcome ascertainment within the framework of a prospective randomized controlled trial, and the availability of medium term functional outcome data.
This study has some limitations. First, the number of patients with high or very high CMB burden was low, precluding adjusted analyses of CMB number, raising the possibility of type 2 errors and limiting generalizability to patients with high CMB burden. The endpoints PH2 and death could not be analyzed in adjusted regression models due to low numbers of events. Unadjusted analyses suggested that the presence of ≥1 CMB might increase the odds of these complications in patients treated with alteplase but not in patients receiving placebo. Further studies are required to confirm or refute these observations. Second, although the presence of CMBs on initial brain MRI was not a formal contraindication for participation in the WAKE-UP trial, patients with many CMBs might have been excluded from participation at the discretion of local investigators due to a perceived increased risk of severe thrombolysis-associated bleeding. This could have introduced an indication bias by which our analysis could have underestimated the true bleeding risk in the CMB group. A screening log was not maintained, so the exact number of patients excluded for this reason cannot be quantified. However, 21.4% of patients in our study had at least 1 and 3.5% had ≥5 CMBs, consistent with findings from previous observational studies,4 suggesting that preferential exclusion of patients with higher CMB burden did not play major role. Third, imaging protocols and hemorrhage-sensitive sequences were not standardized across study sites, which might have introduced additional heterogeneity; however, presence and total number of CMBs were not related to field strengths or the availability of susceptibility-weighted imaging. While such associations might have been expected on the basis of previous studies,12 the small number of patients receiving susceptibility-weighted imaging and the heterogeneity of T2* imaging protocols may have masked this effect in our study, highlighting the need for harmonized imaging protocols across study sites in future trials. Fourth, only a minority of patients in the WAKE-UP trial had high stroke symptom severity scores or proximal large vessel occlusions or were treated with mechanical thrombectomy; therefore, our results cannot be extended to these patient groups without further study.
This predefined post hoc analysis of the randomized controlled WAKE-UP trial data showed no evidence that treatment with alteplase is less effective or even causes harm in patients with acute ischemic stroke with ≥1 CMBs. The effect of CMBs on hemorrhagic complications was equally present in patients receiving placebo or alteplase. Our results corroborate findings from previous observational studies indicating that in otherwise eligible patients, IV thrombolysis should not be withheld on the basis of a small to moderate number of CMBs alone. The study was not powered to provide direct evidence of superiority of alteplase over placebo in patients with CMBs. Additional studies are needed to determine the treatment effect of alteplase and its benefit-harm ratio in patients with a larger number of CMBs.
Glossary
- CI
confidence interval
- CMB
cerebral microbleed
- ECASS
European Cooperative Acute Stroke Study
- HT
hemorrhagic transformation
- IQR
interquartile range
- mRS
modified Rankin Scale
- NAVIGATE ESUS
New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial Versus ASA to Prevent Embolism in Embolic Stroke of Undetermined Source
- NINDS
National Institutes of Neurological Disease and Stroke
- PH
parenchymal hematoma
- sICH
symptomatic intracerebral hemorrhage
- SITS-MOST
Safe Implementation of Thrombolysis in Stroke–Monitoring Study
- WAKE-UP
Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke
Appendix 1. Authors
Appendix 2. Coinvestigators

Footnotes
CME Course: NPub.org/cmelist
Study Funding
The WAKE-UP trial was funded by the European Union Seventh Framework Program (278276).
Disclosure
L. Schlemm is a participant in the Berlin Institute of Health-Charité Clinical Scientist Program funded by the Charité-Universitätsmedizin Berlin and the Berlin Institute of Health and reports lecture fees from Daiichi Sankyo, outside of the submitted work. T.B. Braemswig is a participant in the Berlin Institute of Health-Charité Clinical Scientist Program funded by the Charité-Universitätsmedizin Berlin and the Berlin Institute of Health. C.Z. Simonsen reports research grants from Novo Nordisk Foundation and Health Research Foundation of Central Denmark Region. J.B. Fiebach reports personal fees from Abbvie, AC Immune, Artemida, Bioclinica, Biogen, BMS, Brainomix, Cerevast, Daiichi-Sankyo, Eisai, Eli Lilly, Guerbet, Ionis Pharmaceuticals, Julius Clinical, jung diagnostics, Merck, Nicolab, and Tau Rx, all outside the submitted work. M. Endres received funding from DFG under Germany's Excellence Strategy–EXC-2049–390688087, BMBF, DZNE, DZHK, EU, Corona Foundation, and Fondation Leducq, and reports grants from Bayer and fees paid to the Charité from Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Amgen, GSK, Sanofi, Covidien, Novartis, and Pfizer, all outside the submitted work. R. Lemmens is a senior clinical investigator of FWO Flanders. C.H. Nolte reports research grants from the German Ministry of Research and Education, the German Center for Neurodegenerative Diseases, and the German Center for Cardiovascular Research, as well as speaker and/or consultation fees from Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer Pharma, Abbott, and W.L. Gore and Associates, all outside the submitted work. All other authors report no conflicts of interest. Go to Neurology.org/N for full disclosures.
References
- 1.Emberson J, Lees KR, Lyden P, et al. . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lees KR, Emberson J, Blackwell L, et al. . Effects of alteplase for acute stroke on the distribution of functional outcomes: a pooled analysis of 9 trials. Stroke. 2016;47(9):2373-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers WJ, Rabinstein AA, Ackerson T, et al. . Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. [DOI] [PubMed] [Google Scholar]
- 4.Charidimou A, Turc G, Oppenheim C, et al. . Microbleeds, cerebral hemorrhage, and functional outcome after stroke thrombolysis. Stroke. 2017;48(8):2084-2090. [DOI] [PubMed] [Google Scholar]
- 5.Tsivgoulis G, Zand R, Katsanos AH, et al. . Risk of symptomatic intracerebral hemorrhage after intravenous thrombolysis in patients with acute ischemic stroke and high cerebral microbleed burden: a meta-analysis. JAMA Neurol. 2016;73(6):675-683. [DOI] [PubMed] [Google Scholar]
- 6.Fiehler J, Albers GW, Boulanger JM, et al. . Bleeding risk analysis in stroke imaging before thromboLysis (BRASIL): pooled analysis of T2*-weighted magnetic resonance imaging data from 570 patients. Stroke. 2007;38(10):2738-2744. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg SM, Vernooij MW, Cordonnier C, et al. . Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi KH, Kim JH, Kang KW, et al. . Impact of microbleeds on outcome following recanalization in patients with acute ischemic stroke. Stroke. 2019;50(1):127-134. [DOI] [PubMed] [Google Scholar]
- 9.Shoamanesh A, Hart RG, Connolly SJ, et al. . Microbleeds and the effect of anticoagulation in patients with embolic stroke of undetermined source: an exploratory analysis of the NAVIGATE ESUS randomized clinical trial. JAMA Neurol. 2021;78(1):11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomalla G, Simonsen CZ, Boutitie F, et al. . MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611-622. [DOI] [PubMed] [Google Scholar]
- 11.Thomalla G, Cheng B, Ebinger M, et al. . DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol. 2011;10(11):978-986. [DOI] [PubMed] [Google Scholar]
- 12.Wardlaw JM, Smith EE, Biessels GJ, et al. . Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes AF, Krippendorff K. Answering the call for a standard reliability measure for coding data. Commun Methods Measures. 2007;1(1):77-89. [Google Scholar]
- 14.Regier DA, Narrow WE, Clarke DE, et al. . DSM-5 field trials in the United States and Canada, part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170(1):59-70. [DOI] [PubMed] [Google Scholar]
- 15.Wahlgren N, Ahmed N, Davalos A, et al. . Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275-282. [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Kaste M, Fieschi C, et al. . Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II): Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245-1251. [DOI] [PubMed] [Google Scholar]
- 17.Hacke W, Kaste M, Bluhmki E, et al. . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329. [DOI] [PubMed] [Google Scholar]
- 18.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587. [DOI] [PubMed] [Google Scholar]
- 19.Dannenberg S, Scheitz JF, Rozanski M, et al. . Number of cerebral microbleeds and risk of intracerebral hemorrhage after intravenous thrombolysis. Stroke. 2014;45(10):2900-2905. [DOI] [PubMed] [Google Scholar]
- 20.Schlemm L, Endres M, Werring DJ, Nolte CH. Benefit of intravenous thrombolysis in acute ischemic stroke patients with high cerebral microbleed burden. Stroke. 2020;51(1):232-239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual patient data, after deidentification, will be shared with the Virtual International Stroke Trials Archive and be accessible for researchers according to the Virtual International Stroke Trials Archive rules.