Abstract
Familial hypercholesterolemia (FH) is one of the most common and, therefore, important inherited disorders in preventive cardiology. This disease is mainly caused by a single pathogenic mutation in the low-density lipoprotein receptor or its associated genes. Moreover, it is correlated with a high risk of cardiovascular disease. However, the phenotype severity even in this monogenic disease significantly varies. Thus, the current study aimed to describe FH and its importance and the factors (inherited and acquired) contributing to differences in phenotype severity. Different lipid-modification therapies according to these factors can lead to individualized treatments, which are also essential in the general populations.
Keywords: Hyperlipoproteinemia type II, LDL receptors, Cardiovascular diseases
INTRODUCTION
Familial hypercholesterolemia (FH) is characterized by the clinical triad of primary low-density lipoprotein (LDL) hypercholesterolemia, tendon xanthomas, and premature coronary artery disease (CAD).1 This disease is mainly caused by pathogenic mutations in genes. These include the LDL receptor (LDLR), apolipoprotein B (APOB), proprotein convertase subtilisin/kexin type 9 (PCSK9), and LDL receptor adaptor protein 1 (LDLRAP1), which are associated with LDL metabolism.2 Based on the number of pathogenic FH mutation, FH is classified into heterozygous FH (HeFH) with a single FH mutation and homozygous FH (HoFH) caused by mutations of both copies of FH genes.3 The LDL cholesterol level in HeFH is about two times higher, and that in HoFH is four times higher than that of the general population.1 Thus, patients with HoFH exhibit more severe phenotypes than those with HeFH. However, the phenotype severity is significantly diverse even among patients with HeFH.4,5,6,7 This difference is attributed to several factors, which include inherited and acquired ones. The risk for CAD must be determined because of their extremely high risk at the first place. Now that there are different lipid-modification therapies, including the use of statins, ezetimibe, PCSK9 inhibitor, bempedoic acid, lomitapide, and evinacumab,8,9,10,11,12,13,14,15 the risk for CAD on top of FH should be determined in this personalized medicine era.16 Therefore, the current understanding of the different types of FH and the importance of genetic analysis for not only their diagnosis but also further risk stratification were discussed.
FH AND DEVELOPMENT OF CORONARY ATHEROSCLEROSIS
FH was initially described as xanthomatous disease and first documented approximately 100 years ago.17 In the 1903s, physicians and researchers discovered that it is an inherited disease.18 Since then, it was characterized by the clinical triad of primary LDL hypercholesterolemia, tendon xanthomas, and premature CAD. Further, in the 1970s, Brown and Goldstein discovered that it was primarily caused by genetic abnormalities in LDLR.1 More recently, studies have found that it was correlated with LDLR-associated genes including APOB, LDLRAP1, and PCSK9.2,19,20,21,22 Its prevalence in the general population is approximately 1 per 500 persons.23 However, another study showed that its prevalence was as high as 1 per 208 person based on genetic studies conducted in Hokuriku district in Japan.24 Based on reports published after ours, the prevalence of FH in the general population in the United States and Europe is similar.25,26 Recent meta-analyses have revealed that its prevalence rates are around 1 per 300 person in the general population, 1 per 31 among patients with CAD, and 1 per 15 among patients with premature CAD.27,28
Interestingly, this disorder uniquely exemplifies the causal association between LDL cholesterol and CAD because of the following facts: 1) patients with FH who have hypercholesterolemia since birth have a significantly higher risk for CAD than the general populations and their siblings who do not have mutations,29 2) the use of LDL cholesterol-lowering method can reduce the risk of CAD,30 3) The most frequent cause of death at earlier ages compared with non-FH among patients with untreated FH is CAD (Fig. 1),1 and 4) FH accounts for 3%–8% of all patients with acute coronary syndrome.31,32,33
Fig. 1. Cause of death and average life span of patients with HeFH.
(A) Cause of death. (B) Average life span.
HeFH, heterozygous familial hypercholesterolemia; CAD, coronary artery disease.
In 1970, Brown and Goldstein described the role of LDLR in LDL metabolism, and results showed that FH could be caused by a defect in this gene. Since its discovery, along with the development and standardization of genetic analysis, several different pathogenic mutations have been identified,34,35,36 which include those in the APOB and PCSK9 genes. Based on this concept, only one monogenic mutation causes the critical phenotype. Thus, both cascade screening and the assessment of segregation pattern are important. However, in a substantial proportion of patients with FH, no deleterious mutation in such genes has been found.37,38 Therefore, researchers have been assessing one or more novel genes associated with this disorder via comprehensive genetic analyses, including exome sequencing.39 However, there was no novel gene compatible with the concept of the monogenic type of FH, which is the standard type of FH, and the presence of pathogenic mutation in FH-gene has been associated with a higher risk of CAD, independent of LDL cholesterol level.40 Moreover, the presence of pathogenic mutation in FH-gene increased the risk of developing CAD in people other than those with FH, which include those with a family history of premature CAD/FH and Achilles tendon thickness.41 Collectively, these data indicate that the identification of monogenic FH could be quite useful for not only diagnosis but also further risk stratification.
PHENOTYPIC VARIATIONS OF FH AND SEVERE FH
FH is associated with an extremely high risk for CAD. However, there are substantial phenotypic variations in this simple familiar disorder. Some patients with FH present with severe CAD during early life. Meanwhile, other patients do not. In fact, there are several different types of clinical criteria for FH worldwide, which include the Dutch Lipid Clinic Network,42 Simon Broome,43 and Japan Atherosclerosis Society.44 However, none of these specifically defined severe FH. Under these circumstances, the International Atherosclerosis Society defines severe familial hypercholesterolemia and the implications for clinical management in 2016.45 Several factors can contribute to such phenotypic variations, or severity. These include the classical risk factors of coronary atherosclerosis, such as hypertension, diabetes, and smoking, which are not specific to FH. By contrast, the factors specific to FH associated with a more severe phenotype, such as Achilles tendon thickness and the presence of pathogenic mutation in FH-gene, should be identified.
PHENOTYPIC VARIATIONS OF FH
1. Achilles tendon thickness
There are several clinical factors correlated with the phenotypic severity of FH. For example, Achilles tendon thickness, which is quite a specific physical finding of FH, is associated with worse clinical outcomes among patients with extremely elevated LDL cholesterol levels.46 This phenomenon may be attributed to the fact that Achilles tendon thickness reflects not only the state of FH but also exposure to more elevated LDL cholesterol levels with inflammatory properties. Moreover, it is positively associated with aging only among patients with FH,47,48 and the association between Achilles tendon thickness and cholesterol year score is stronger than that of Achilles tendon thickness and simple aging.49 Collectively, these data indicate that patients with FH should be diagnosed before the occurrence of Achilles tendon thicken, even though this is one of the major diagnostic criteria of the disease. In addition to these specific physical findings, we showed that the presence of pathogenic mutation in FH-gene (e.g., LDLR and PCSK9) was significantly associated with a higher risk for CAD, independent of other conventional risk factors (Fig. 2). This likely reflects the fact that patients with FH mutation have true FH, and their LDL cholesterol level have been elevated since the fetal period.50
Fig. 2. Odds ratio for CAD.
Patients with hyper-LDL cholesterol levels (≥180 mg/dL) were divided into four groups based on the presence of pathogenic mutation in FH-gene and the clinical signs of FH.
CAD, coronary artery disease; LDL, low-density lipoprotein; FH, familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; LDLR, LDL receptor; PCSK9, proprotein convertase subtilisin/kexin type 9.
2. Assessment of coronary and carotid plaque in FH
Currently, we can assess the plaque burden of coronary artery invasively and less invasively. For example, intravascular ultrasonography (IVUS) has been a useful tool for assessing coronary artery plaque burden for not only patients with FH but also the non-FH general populations.51 A larger plaque burden has been associated with worse clinical outcomes.52 Thus, the assessment of plaque burden quantitatively may be a useful marker for risk stratification in patients with FH. Moreover, we can assess coronary plaque burden via computed tomography (CT) scan, which is a less invasive procedure. Coronary plaque burden assessed with CT scan were significantly associated with future cardiovascular events in FH.53 In addition to the assessments or coronary plaque burden, CT scan can be performed to assess coronary and aortic calcification quantitatively, which is also associated with future cardiovascular events.54,55,56 Moreover, we can now assess other important characteristics of coronary plaque, such as the presence of lipid-rich plaque and thin-cap fi1broatheroma, using optical coherence tomography.57,58 Both of them have been associated with worse clinical outcomes.
By contrast, we can assess their state of atherosclerosis non-invasively via carotid ultrasonography. It is quite helpful for this purpose because 1) it can be used to assess plaque burden in pediatric patients with FH, 2) plaque burden can be evaluated quantitatively, and 3) carotid plaque burden is associated with future cardiovascular events in patients with FH.59 Using these modalities, we showed that carotid plaque may start to develop at the age of 17 years in men and 26 years in women.59 Moreover, coronary plaque is commonly observed at an average age of 23 years in men and 34 years in women.60 Hence, individuals with FH must be diagnosed before those ages.
FACTORS CONTRIBUTING TO THE PHENOTYPIC VARIATIONS OF FH
1. Conventional and FH-specific factors associated with atherosclerosis
Other studies and ours have revealed that conventional risk factors for coronary atherosclerosis, such as hypertension, diabetes, and smoking, are also associated with disease severity even among patients with FH.61,62,63 Some reports have shown that lipoprotein (a) (Lp[a]) can exacerbate the phenotype.64,65 Notably, patients with FH have elevated Lp(a) levels,66 indicating that LDLR may be involved in the catabolism of Lp(a). All these factors are not specific to FH but are applicable to the general populations.
2. Timing of the initial therapy (cascade vs. proband)
With consideration of FH phenotype severity, the timing of diagnosis is quite important, and this might also be applicable in other cardio metabolic inherited diseases. For example, almost all patients with pediatric HeFH are asymptomatic, except for those with hyper-LDL cholesterolemia.67 Meanwhile, adult patients with HeFH are gradually developing carotid and coronary atherosclerosis (Fig. 3).59,60 Moreover, early diagnosis and LDL-lowering therapy at childhood can prevent atherosclerotic cardiovascular disease (ASCVD) events.68 In addition, in our study, we managed brothers with compound heterozygous FH. That is, the older brother who was initially treated at the age of 23 years exhibited repeated coronary events. Meanwhile, the younger brother who has been receiving treatment since the age of 15 years had been event-free for a long period, despite having similar LDL cholesterol levels caused by the same mutations (NM_000527.4 [LDLR]:c.2054C>T [p.Pro685Leu]/NM_000527.4[LDLR]:c.2431A>T [p.Lys811Ter]) (Fig. 4).69 The phenotypic difference between them clearly indicate that early intervention for LDL cholesterol can be quite beneficial even for extremely worse cases. In fact, we have shown that Achilles tendon thickness in HeFH was significantly associated with age.41 In addition, it is correlated with phenotypic severity.41 Accordingly, diagnosing patients with FH prior to Achilles tendon thickening may be quite beneficial to prevent ASCVD events.
Fig. 3. Coronary and carotid plaque score assessed via computed tomography scan or carotid ultrasonography.
Upper panel indicates coronary plaque score. The X-axis represents age and the Y-axis represents coronary plaque score. The blue section indicates men; the red section, women. Lower panel indicates carotid plaque score. The X-axis represents age and the Y-axis represents carotid plaque score. The blue section indicates men, the red section, women.
Fig. 4. Clinical course of brothers with compound heterozygous FH.
(A) Clinical course of the older brother: Image of the buttocks with a large xanthoma is shown in the left image. The clinical course is depicted in the middle image. The blue section indicates total cholesterol levels (mg/dL). Images obtained via intravascular ultrasonography are shown in the right images. (B) Clinical course of the younger brother: Image of the buttocks with a small xanthoma is shown in the left image. The clinical course is depicted in the middle image. The blue section indicates total cholesterol levels (mg/dL). Images obtained via intravascular ultrasonography are shown in the right images.
UAP, unstable angina pectoris; CAG, coronary angiogram; PCI, percutaneous coronary intervention.
In order to identify patients with FH at early phase of this disorder, there are two major methods that can effectively identify patients with FH. First is the universal screening at a certain age, which is a proposed screening method for FH.70,71 Second is the cascade screening for FH, which has been recommended by several organizations worldwide.72,73 In countries where dedicated cascade screening programs have been implemented, the number of patients with FH is significantly higher. For example, about 71% and 43% of patients were diagnosed with FH in the Netherlands and Norway, respectively.74 In any case, we strongly recommend to identify as many patients with FH. Then, early treatment should be started if possible.
3. Cholesterol year score
In recent years, the accumulation of exposure to cholesterol for a long period has been proposed to contribute the development of coronary atherosclerosis, and this considered an important concept.74 This is an idea based on the fact that LDL cholesterol is the causal factor for coronary atherosclerosis, and this concept is likely to explain the development of coronary atherosclerosis in HeFH and HoFH, including attenuation by LDL-lowering therapies. Typically, cholesterol-year-score is calculated as: LDL Cholesterol Max × (Age at Diagnosis/Statin Initiation) + LDL Cholesterol at Inclusion × (Age at Inclusion − Age at Diagnosis/Statin Initiation). In fact, we have shown that a cholesterol year score representing the accumulation of exposure to LDL cholesterol in HeFH is significantly associated with major cardiovascular events beyond their cross-sectional LDL cholesterol levels.49 In addition, it was significantly correlated with Achilles tendon thickness in HeFH, which was one of the established clinical signs of severe FH.49 Collectively, these data indicated that we need to the degree and duration of exposure to elevated LDL cholesterol.
4. Genetic backgrounds of FH (LDLR, PCSK9 E32K)
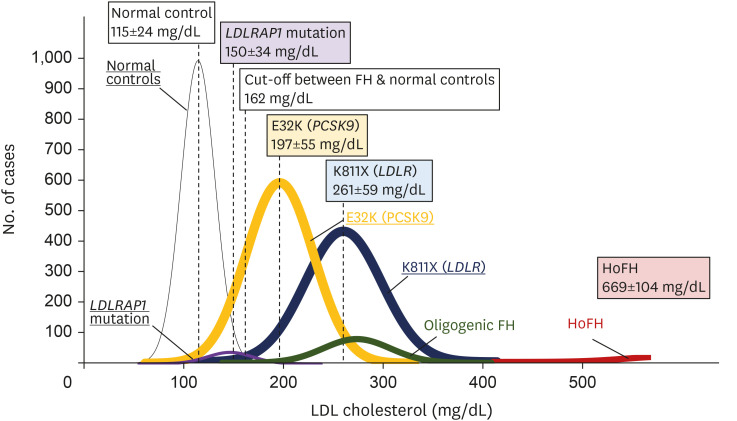
About 2,000 different types of mutations causes FH in LDLR alone.75 Even in Japan, we have shown that at least 132 different pathogenic mutations exist, and it is likely that there are several region-specific and/or private mutations across the country (Fig. 5) and worldwide.76,77 Based on the abovementioned information, the presence of pathogenic mutation in FH-gene (LDLR or PCSK9) is associated with a higher risk for CAD.41 However, accumulating evidence has shown differences in the effect of mutations (and their phenotypes) on LDL cholesterol levels among the different types of FH mutations. For example, the LDL cholesterol level of patients with HeFH caused by E32K (PCSK9) was lower than that in patients with HeFH caused by K811X (LDLR) (Fig. 6). Moreover, some studies have shown that there are significant differences in phenotypes (LDL cholesterol and coronary atherosclerosis) among the different types of mutations in LDLR, such as missense mutations and loss-of function mutations, including nonsense, frameshift, splice cite, and large insertion/deletion mutations.78 Thus, information regarding the pathogenicity of the genetic variations in FH must be further collected, and they can be classified according to their effects on LDL cholesterol and CAD.
Fig. 5. Pathogenic mutations in the LDLR gene found in Japanese patients with familial hypercholesterolemia.
The left pie chart shows the total mutation distribution. The right upper pie chart indicates the mutation distribution found in Kanazawa University. The right lower pie chart depicts the mutation distribution found in the National Cerebral and Cardiovascular Center.
LDLR, low-density lipoprotein receptor
Fig. 6. LDL cholesterol level distribution according to mutation status.
The X-axis represents LDL cholesterol levels (mg/dL); the Y-axis, the number of cases. The black section indicates normal controls (without FH mutation); purple, patients with a LDLRAP1 mutation; yellow, patients with E32K (PCSK9) mutation; blue, patients with K811X (LDLR) mutation; green, patients with oligogenic FH; and red, HoFH.
LDL, low-density lipoprotein; FH, familial hypercholesterolemia; LDLRAP1, LDL receptor adapter protein 1; PCSK9, proprotein convertase subtilisin/kexin type 9; LDLR, LDL receptor; HoFH, homozygous FH.
5. Monogenic, polygenic, and oligogenic FH
In addition to the so-called FH mutation, there are other classifications among patients with FH according to genetic backgrounds. One is monogenic FH, which is a conventional concept in which one rare deleterious pathogenic mutation causes an FH phenotype. However, Talmud et al. have discussed about polygenic FH, which is another interesting concept in which multiple common genetic variations can mimic FH phenotype.79 There may be substantial proportions of such patients80 simply because some individuals have millions of common genetic variations. However, their phenotypes are milder than those of the typical monogenic FH.81,82 By contrast, at least a portion of patients with monogenic FH also have rare and deleterious mutation(s) in the LDL-related genes, such as ATP-binding cassette sub-family G member 5 (ABCG5) and ATP-binding cassette sub-family G member 8 (ABCG8), leading to the exacerbation of their phenotype (Fig. 7).83 This is referred to as oligogenic FH. In fact, oligogenic FH is an unique status accompanied by mutations in related genes along with conventional FH gene mutations. ABCG5 and ABCG8 are cause sitosterolemia, which is a recessive disorder.84 The effect size of ABCG5 mutation may be smaller than that of LDLR mutation. Moreover, a single rare mutation in both genes can increase LDL cholesterol levels and the risk of CAD.85 Notably, the development of CAD can be explained by the high LDL cholesterol levels as they are linearly correlated.
Fig. 7. Concept of monogenic, polygenic, and oligogenic FH.
Upper panel: The circles indicate the effect on the phenotype. Lower panel: The X-axis represents LDL cholesterol level; the Y-axis, the incidence of FH in the general population. Typically, the frequency distribution is normal. However, monogenic, oligogenic, or polygenic mutations could cause an increase in the LDL cholesterol level above the threshold for FH.
FH, familial hypercholesterolemia; LDL, low-density lipoprotein; ABCG5 and ABCG8, adenosine triphosphate (ATP)–binding cassette sub-family G, members 5 and 8; APOE, apolipoprotein E; LDLR, LDL receptor; LDLRAP1, LDL receptor adapter protein 1; PCSK9, proprotein convertase subtilisin/kexin type 9; SORT1, sortilin 1.
6. Homozygous FH and its phenocopies
Homozygous FH is the most severe FH phenotype. Further, it is characterized by an extremely elevated LDL cholesterol level (>500 mg/dL), cutaneous xanthomas since childhood, premature CAD during childhood, and supravalvular aortic stenosis.3 Typically, homozygous FH refers to double pathogenic mutations in the FH genes (LDLR, PCSK9, APOB, or LDLRAP1). The severity of this condition can be assessed based on genes and/or mutation types. For example, the phenotypes of homozygous FH caused by loss-of-function mutations in LDLR are more severe than those caused by missense mutations in LDLR, PCSK9, or APOB. In addition, homozygous FH caused by LDLRAP1 mutations (so-called ARH) is characterized by a milder phenotype, probably because the function of their LDLR, particularly in the clearance of remnant lipoproteins, is not completely impaired.86,87 Thus, based on these findings, the genotypes of homozygous FH is helpful for not only diagnosis but also risk stratification.
Moreover, sitosterolemia is an important differential diagnosis of homozygous FH. This condition is caused by double pathogenic mutations in ABCG5 or ABCG8 and is extremely rare. However, the prevalence of sitosterolemia has been significantly higher than previously expected based on the prevalence of loss-of-function mutation in the general population in public database (gnomAD).88 Occasionally, HoFH is challenging to differentiate from sitosterolemia because both conditions have similar symptoms. These include elevated LDL cholesterol levels, cutaneous xanthomas since childhood, and premature CAD in childhood.89,90,91 We have managed worse cases of sitosterolemia with premature CAD, a case of a young lady (aged 25 years old) with myocardial infarction,89 and a boy (aged 15 years old) with ischemic heart failure.91 Both patients had cutaneous xanthomas and Achilles tendon thickness similar to HoFH. Notably, the prognosis and standard therapy for sitosterolemia differ from those of HoFH. For example, ezetimibe, rather than statins, are strongly recommended. Meanwhile, statins are less effective against HoFH because of their reduced LDLR activity. Moreover, dietary counseling is quite effective in reducing LDL cholesterol levels in sitosterolemia. Meanwhile, dietary counseling has almost no beneficial effect on HoFH.92 Accordingly, an accurate diagnosis which include differentiating HoFH from sitosterolemia, is important.
7. Common genetic variations associated with coronary atherosclerosis
Do common genetic variations have any effects on their phenotypes in FH? The answer is probably yes. Common genetic variations are associated with coronary atherosclerosis in the general populations.93 In particular, polygenic risk score (PRS) comprising multiple common genetic variations are attracting significant attention to date. Fahed et al.94 have shown that independent of other traditional risk factors, such PRS is also associated with CAD among patients with FH. Notably, the most common genetic variations included in this score are associated with hypertension, diabetes, inflammation, and other unknown factors, but not with lipid levels.94 Accordingly, assessing such risk caused by common genetic variations are useful for not only the general populations but also patients with FH regardless of genetic status.
PRINCIPLES OF INDIVIDUALIZED TREATMENT FOR PATIENTS WITH FH
As stated above, we need to identify patients with FH very early, ideally, before their Achilles tendon becomes thick, and then start to treat them. Secondly, we need to clarify the genetic backgrounds not only for FH-gene, but also other genes associated with CAD and lipids, including common genetic variations. These information can tell us clear diagnosis of them as well as responsiveness to lipid-lowering therapy.76 Thirdly, we need to assess phenotypic severity, including coronary and carotid atherosclerosis. Finally, we need to decide the intensity of therapies based on all information. The most intensive therapies, including LDL apheresis and PCSK9 inhibitor would be needed when patients diagnosed late who have high risk genetic variants and exhibit coronary and carotid artery stenoses.
CONCLUSION
FH should be diagnosed as early as possible. Then, patients must receive individualized treatment based on other clinical risk factors (FH-specific or not) and genetic backgrounds (rare and common). As FH is one of the most common cardio metabolic disorders, a full understanding of its associated factors will help improve prognosis, thereby reducing the burden of cardiovascular disease worldwide.
ACKNOWLEDGEMENTS
We would like to express special thanks to Ms. Yoko Iwauchi, Ms. Kazuko Honda, and Mr. Sachio Yamamoto (staffs of Kanazawa University) for their assistance.
Footnotes
Funding: None.
Conflict of Interest: The authors have no conflicts of interest to declare.
- Conceptualization: Tada H, Takamura M, Kawashiri M.
- Data curation: Tada H.
- Supervision: Tada H, Takamura M, Kawashiri M.
- Validation: Tada H, Takamura M, Kawashiri M.
- Visualization: Tada H.
- Writing - original draft: Tada H, Takamura M, Kawashiri M.
References
- 1.Mabuchi H. Half a century tales of familial hypercholesterolemia (FH) in Japan. J Atheroscler Thromb. 2017;24:189–207. doi: 10.5551/jat.RV16008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soutar AK, Naoumova RP. Mechanisms of disease: genetic causes of familial hypercholesterolemia. Nat Clin Pract Cardiovasc Med. 2007;4:214–225. doi: 10.1038/ncpcardio0836. [DOI] [PubMed] [Google Scholar]
- 3.Nohara A, Tada H, Ogura M, Okazaki S, Ono K, Shimano H, et al. Homozygous familial hypercholesterolemia. J Atheroscler Thromb. 2021;28:665–678. doi: 10.5551/jat.RV17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foody JM, Vishwanath R. Familial hypercholesterolemia/autosomal dominant hypercholesterolemia: Molecular defects, the LDL-C continuum, and gradients of phenotypic severity. J Clin Lipidol. 2016;10:970–986. doi: 10.1016/j.jacl.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Santos RD. Phenotype vs. genotype in severe familial hypercholesterolemia: what matters most for the clinician? Curr Opin Lipidol. 2017;28:130–135. doi: 10.1097/MOL.0000000000000391. [DOI] [PubMed] [Google Scholar]
- 6.Bourbon M, Alves AC, Alonso R, Mata N, Aguiar P, Padró T, et al. Mutational analysis and genotype-phenotype relation in familial hypercholesterolemia: the SAFEHEART registry. Atherosclerosis. 2017;262:8–13. doi: 10.1016/j.atherosclerosis.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Di Taranto MD, Giacobbe C, Fortunato G. Familial hypercholesterolemia: a complex genetic disease with variable phenotypes. Eur J Med Genet. 2020;63:103831. doi: 10.1016/j.ejmg.2019.103831. [DOI] [PubMed] [Google Scholar]
- 8.Gagné C, Gaudet D, Bruckert E, Ezetimibe Study Group Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation. 2002;105:2469–2475. doi: 10.1161/01.cir.0000018744.58460.62. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto A, Harada-Shiba M, Endo M, Kusakabe N, Tanioka T, Kato H, et al. The effect of ezetimibe on serum lipids and lipoproteins in patients with homozygous familial hypercholesterolemia undergoing LDL-apheresis therapy. Atherosclerosis. 2006;186:126–131. doi: 10.1016/j.atherosclerosis.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 10.Gotto AM, Jr, Moon JE. Pharmacotherapies for lipid modification: beyond the statins. Nat Rev Cardiol. 2013;10:560–570. doi: 10.1038/nrcardio.2013.117. [DOI] [PubMed] [Google Scholar]
- 11.Santos RD, Stein EA, Hovingh GK, Blom DJ, Soran H, Watts GF, et al. Long-term evolocumab in patients with familial hypercholesterolemia. J Am Coll Cardiol. 2020;75:565–574. doi: 10.1016/j.jacc.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Harada-Shiba M, Ikewaki K, Nohara A, Otsubo Y, Yanagi K, Yoshida M, et al. Efficacy and safety of lomitapide in Japanese patients with homozygous familial hypercholesterolemia. J Atheroscler Thromb. 2017;24:402–411. doi: 10.5551/jat.38216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nohara A, Otsubo Y, Yanagi K, Yoshida M, Ikewaki K, Harada-Shiba M, et al. Safety and efficacy of lomitapide in Japanese patients with homozygous familial hypercholesterolemia (HoFH): results from the AEGR-733-301 long-term extension study. J Atheroscler Thromb. 2019;26:368–377. doi: 10.5551/jat.45708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenson RS, Burgess LJ, Ebenbichler CF, Baum SJ, Stroes ES, Ali S, et al. Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med. 2020;383:2307–2319. doi: 10.1056/NEJMoa2031049. [DOI] [PubMed] [Google Scholar]
- 15.Agarwala A, Quispe R, Goldberg AC, Michos ED. Bempedoic acid for heterozygous familial hypercholesterolemia: from bench to bedside. Drug Des Devel Ther. 2021;15:1955–1963. doi: 10.2147/DDDT.S251865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tada H, Usui S, Sakata K, Takamura M, Kawashiri MA. Challenges of precision medicine for atherosclerotic cardiovascular disease based on human genome information. J Atheroscler Thromb. 2021;28:305–313. doi: 10.5551/jat.60087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fagge CH. Xanthomatous disease of the skin. I. General xanthelasma of vitiligoides. Trans Pathol Soc Lond. 1873;24:242–250. [Google Scholar]
- 18.Thannhauser SJ, Magendantz H. The different clinical groups of xanthomatous diseases: a clinical physiological study of 22 cases. Ann Intern Med. 1938;11:1162. [Google Scholar]
- 19.Hori M, Takahashi A, Son C, Ogura M, Harada-Shiba M. The first Japanese cases of familial hypercholesterolemia due to a known pathogenic APOB gene variant, c.10580 G>A: p.(Arg3527Gln) J Clin Lipidol. 2020;14:482–486. doi: 10.1016/j.jacl.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Harada-Shiba M, Takagi A, Miyamoto Y, Tsushima M, Ikeda Y, Yokoyama S, et al. Clinical features and genetic analysis of autosomal recessive hypercholesterolemia. J Clin Endocrinol Metab. 2003;88:2541–2547. doi: 10.1210/jc.2002-021487. [DOI] [PubMed] [Google Scholar]
- 21.Tada H, Kawashiri MA, Ikewaki K, Terao Y, Noguchi T, Nakanishi C, et al. Altered metabolism of low-density lipoprotein and very-low-density lipoprotein remnant in autosomal recessive hypercholesterolemia: results from stable isotope kinetic study in vivo. Circ Cardiovasc Genet. 2012;5:35–41. doi: 10.1161/CIRCGENETICS.111.960948. [DOI] [PubMed] [Google Scholar]
- 22.Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein JL, Hobbs HH, Brown MS. In: The metabolic and molecular bases of inherited disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. New York (NY): McGraw-Hill; 2001. Familial hypercholesterolemia; p. 2863e913. [Google Scholar]
- 24.Mabuchi H, Nohara A, Noguchi T, Kobayashi J, Kawashiri MA, Tada H, et al. Molecular genetic epidemiology of homozygous familial hypercholesterolemia in the Hokuriku district of Japan. Atherosclerosis. 2011;214:404–407. doi: 10.1016/j.atherosclerosis.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Benn M, Watts GF, Tybjærg-Hansen A, Nordestgaard BG. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J. 2016;37:1384–1394. doi: 10.1093/eurheartj/ehw028. [DOI] [PubMed] [Google Scholar]
- 26.de Ferranti SD, Rodday AM, Mendelson MM, Wong JB, Leslie LK, Sheldrick RC. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States national health and nutrition examination surveys (NHANES) Circulation. 2016;133:1067–1072. doi: 10.1161/CIRCULATIONAHA.115.018791. [DOI] [PubMed] [Google Scholar]
- 27.Beheshti SO, Madsen CM, Varbo A, Nordestgaard BG. Worldwide prevalence of familial hypercholesterolemia: meta-analyses of 11 million subjects. J Am Coll Cardiol. 2020;75:2553–2566. doi: 10.1016/j.jacc.2020.03.057. [DOI] [PubMed] [Google Scholar]
- 28.Hu P, Dharmayat KI, Stevens CA, Sharabiani MT, Jones RS, Watts GF, et al. Prevalence of familial hypercholesterolemia among the general population and patients with atherosclerotic cardiovascular disease: a systematic review and meta-analysis. Circulation. 2020;141:1742–1759. doi: 10.1161/CIRCULATIONAHA.119.044795. [DOI] [PubMed] [Google Scholar]
- 29.Paquette M, Baass A. Predicting cardiovascular disease in familial hypercholesterolemia. Curr Opin Lipidol. 2018;29:299–306. doi: 10.1097/MOL.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 30.Raal FJ, Hovingh GK, Catapano AL. Familial hypercholesterolemia treatments: guidelines and new therapies. Atherosclerosis. 2018;277:483–492. doi: 10.1016/j.atherosclerosis.2018.06.859. [DOI] [PubMed] [Google Scholar]
- 31.Samuel R, Birdsey G, Amerena J. Prevalence of familial hypercholesterolaemia in acute coronary syndrome patients in a large regional coronary care unit. Heart Lung Circ. 2021;30:730–733. doi: 10.1016/j.hlc.2020.09.929. [DOI] [PubMed] [Google Scholar]
- 32.Kheiri B, Simpson TF, Osman M, Balla S, Rahmouni H, Mehta A, et al. Familial hypercholesterolemia related admission for acute coronary syndrome in the United States: incidence, predictors, and outcomes. J Clin Lipidol. 2021;15:460–465. doi: 10.1016/j.jacl.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Takasaki A, Kurita T, Masuda J, Hoshino K, Seko T, Tanigawa T, et al. Prevalence and prognosis of familial hypercholesterolemia in patients with acute coronary syndrome in Mie Prefecture, Japan - report from Mie ACS registry. Circ J. 2020;85:9–18. doi: 10.1253/circj.CJ-20-0112. [DOI] [PubMed] [Google Scholar]
- 34.Shin DG, Han SM, Kim DI, Rhee MY, Lee BK, Ahn YK, et al. Clinical features of familial hypercholesterolemia in Korea: predictors of pathogenic mutations and coronary artery disease - A study supported by the Korean Society of Lipidology and Atherosclerosis. Atherosclerosis. 2015;243:53–58. doi: 10.1016/j.atherosclerosis.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 35.Pirillo A, Garlaschelli K, Arca M, Averna M, Bertolini S, Calandra S, et al. Spectrum of mutations in Italian patients with familial hypercholesterolemia: new results from the LIPIGEN study. Atheroscler Suppl. 2017;29:17–24. doi: 10.1016/j.atherosclerosissup.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Tada H, Hori M, Nomura A, Hosomichi K, Nohara A, Kawashiri MA, et al. A catalog of the pathogenic mutations of LDL receptor gene in Japanese familial hypercholesterolemia. J Clin Lipidol. 2020;14:346–351.e9. doi: 10.1016/j.jacl.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Taylor A, Wang D, Patel K, Whittall R, Wood G, Farrer M, et al. Mutation detection rate and spectrum in familial hypercholesterolaemia patients in the UK pilot cascade project. Clin Genet. 2010;77:572–580. doi: 10.1111/j.1399-0004.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 38.Reeskamp LF, Tromp TR, Defesche JC, Grefhorst A, Stroes ES, Hovingh GK, et al. Next-generation sequencing to confirm clinical familial hypercholesterolemia. Eur J Prev Cardiol. 2021;28:875–883. doi: 10.1093/eurjpc/zwaa451. [DOI] [PubMed] [Google Scholar]
- 39.Stitziel NO, Peloso GM, Abifadel M, Cefalu AB, Fouchier S, Motazacker MM, et al. Exome sequencing in suspected monogenic dyslipidemias. Circ Cardiovasc Genet. 2015;8:343–350. doi: 10.1161/CIRCGENETICS.114.000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khera AV, Won HH, Peloso GM, Lawson KS, Bartz TM, Deng X, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016;67:2578–2589. doi: 10.1016/j.jacc.2016.03.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H, Yamagishi M. Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur Heart J. 2017;38:1573–1579. doi: 10.1093/eurheartj/ehx004. [DOI] [PubMed] [Google Scholar]
- 42.Austin MA, Hutter CM, Zimmern RL, Humphries SE. Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol. 2004;160:407–420. doi: 10.1093/aje/kwh236. [DOI] [PubMed] [Google Scholar]
- 43.Scientific Steering Committee on behalf of the Simon Broome Register Group. Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ. 1991;303:893–896. doi: 10.1136/bmj.303.6807.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harada-Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T, Ogura M, et al. Guidelines for diagnosis and treatment of familial hypercholesterolemia 2017. J Atheroscler Thromb. 2018;25:751–770. doi: 10.5551/jat.CR003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos RD, Gidding SS, Hegele RA, Cuchel MA, Barter PJ, Watts GF, et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016;4:850–861. doi: 10.1016/S2213-8587(16)30041-9. [DOI] [PubMed] [Google Scholar]
- 46.Tada H, Okada H, Nomura A, Usui S, Sakata K, Nohara A, et al. Clinical diagnostic criteria of familial hypercholesterolemia - A comparison of the Japan Atherosclerosis Society and Dutch lipid clinic network criteria. Circ J. 2021;85:891–897. doi: 10.1253/circj.CJ-20-0901. [DOI] [PubMed] [Google Scholar]
- 47.Tada H, Okada H, Nomura A, Nohara A, Usui S, Sakata K, et al. A reassessment of the Japanese clinical diagnostic criteria of familial hypercholesterolemia in a hospital-based cohort using comprehensive genetic analysis. Pract Lab Med. 2020;22:e00180. doi: 10.1016/j.plabm.2020.e00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tada H, Hori M, Matsuki K, Ogura M, Nohara A, Kawashiri MA, et al. Achilles tendon thickness assessed by X-ray predicting a pathogenic mutation in familial hypercholesterolemia gene. J Atheroscler Thromb. 2021 doi: 10.5551/jat.62869. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tada H, Okada H, Nohara A, Yamagishi M, Takamura M, Kawashiri MA. Effect of cumulative exposure to low-density lipoprotein-cholesterol on cardiovascular events in patients with familial hypercholesterolemia. Circ J. 2021;85:2073–2078. doi: 10.1253/circj.CJ-21-0193. [DOI] [PubMed] [Google Scholar]
- 50.de Gennes JL, Daffos F, Dairou F, Forestier F, Capella-Pavlosky M, Truffert J, et al. Direct fetal blood examination for prenatal diagnosis of homozygous familial hypercholesterolemia. Arteriosclerosis. 1985;5:440–442. doi: 10.1161/01.atv.5.5.440. [DOI] [PubMed] [Google Scholar]
- 51.Daida H, Dohi T, Fukushima Y, Ohmura H, Miyauchi K. The goal of achieving atherosclerotic plaque regression with lipid-lowering therapy: insights from IVUS trials. J Atheroscler Thromb. 2019;26:592–600. doi: 10.5551/jat.48603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol. 2014;11:390–402. doi: 10.1038/nrcardio.2014.60. [DOI] [PubMed] [Google Scholar]
- 53.Pérez de Isla L, Alonso R, Gómez de Diego JJ, Muñiz-Grijalvo O, Díaz-Díaz JL, Zambón D, et al. Coronary plaque burden, plaque characterization and their prognostic implications in familial hypercholesterolemia: a computed tomographic angiography study. Atherosclerosis. 2021;317:52–58. doi: 10.1016/j.atherosclerosis.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Okada H, Tada H, Hayashi K, Kawashima H, Takata T, Sakata K, et al. Aortic root calcification score as an independent factor for predicting major adverse cardiac events in familial hypercholesterolemia. J Atheroscler Thromb. 2018;25:634–642. doi: 10.5551/jat.42705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miname MH, Bittencourt MS, Pereira AC, Jannes CE, Krieger JE, Nasir K, et al. Vascular age derived from coronary artery calcium score on the risk stratification of individuals with heterozygous familial hypercholesterolaemia. Eur Heart J Cardiovasc Imaging. 2020;21:251–257. doi: 10.1093/ehjci/jez280. [DOI] [PubMed] [Google Scholar]
- 56.Gallo A, Pérez de Isla L, Charrière S, Vimont A, Alonso R, Muñiz-Grijalvo O, et al. The added value of coronary calcium score in predicting cardiovascular events in familial hypercholesterolemia. JACC Cardiovasc Imaging. 2021;14:2414–2424. doi: 10.1016/j.jcmg.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Liu Z, Peng J, Wang S, Jiang T, Zhang W, Zhang C, et al. Percutaneous coronary intervention for a Chinese familial hypercholesterolemia homozygous under the guidance of optical coherence tomography. Atheroscler Suppl. 2019;36:19–23. doi: 10.1016/j.atherosclerosissup.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Katamine M, Minami Y, Hashimoto T, Ako J. Familial hypercholesterolemia and vulnerability of coronary plaque in patients with coronary artery disease. Pract Lab Med. 2021;24:e00202. doi: 10.1016/j.plabm.2021.e00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tada H, Kawashiri MA, Okada H, Nakahashi T, Sakata K, Nohara A, et al. Assessments of carotid artery plaque burden in patients with familial hypercholesterolemia. Am J Cardiol. 2017;120:1955–1960. doi: 10.1016/j.amjcard.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 60.Tada H, Kawashiri MA, Okada H, Teramoto R, Konno T, Yoshimuta T, et al. Assessment of coronary atherosclerosis in patients with familial hypercholesterolemia by coronary computed tomography angiography. Am J Cardiol. 2015;115:724–729. doi: 10.1016/j.amjcard.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 61.Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H, Yamagishi M. Assessment of arterial stiffness in patients with familial hypercholesterolemia. J Clin Lipidol. 2018;12:397–402.e2. doi: 10.1016/j.jacl.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Tada H, Kawashiri MA, Nohara A, Sakata K, Inazu A, Mabuchi H, et al. Remnant-like particles and coronary artery disease in familial hypercholesterolemia. Clin Chim Acta. 2018;482:120–123. doi: 10.1016/j.cca.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Gidding SS, Champagne MA, de Ferranti SD, Defesche J, Ito MK, Knowles JW, et al. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167–2192. doi: 10.1161/CIR.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 64.Li S, Wu NQ, Zhu CG, Zhang Y, Guo YL, Gao Y, et al. Significance of lipoprotein(a) levels in familial hypercholesterolemia and coronary artery disease. Atherosclerosis. 2017;260:67–74. doi: 10.1016/j.atherosclerosis.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 65.Vuorio A, Watts GF, Schneider WJ, Tsimikas S, Kovanen PT. Familial hypercholesterolemia and elevated lipoprotein(a): double heritable risk and new therapeutic opportunities. J Intern Med. 2020;287:2–18. doi: 10.1111/joim.12981. [DOI] [PubMed] [Google Scholar]
- 66.Tada H, Kawashiri MA, Yoshida T, Teramoto R, Nohara A, Konno T, et al. Lipoprotein(a) in familial hypercholesterolemia with proprotein convertase subtilisin/kexin Type 9 (PCSK9) gain-of-function mutations. Circ J. 2016;80:512–518. doi: 10.1253/circj.CJ-15-0999. [DOI] [PubMed] [Google Scholar]
- 67.Tada H, Takamura M, Kawashiri MA. Familial hypercholesterolemia: a narrative review on diagnosis and management strategies for children and adolescents. Vasc Health Risk Manag. 2021;17:59–67. doi: 10.2147/VHRM.S266249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luirink IK, Wiegman A, Kusters DM, Hof MH, Groothoff JW, de Groot E, et al. 20-year follow-up of statins in children with familial hypercholesterolemia. N Engl J Med. 2019;381:1547–1556. doi: 10.1056/NEJMoa1816454. [DOI] [PubMed] [Google Scholar]
- 69.Tada H, Usui S, Sakata K, Takamura M, Kawashiri MA. Low-density lipoprotein cholesterol level cannot be too low: considerations from clinical trials, human genetics, and biology. J Atheroscler Thromb. 2020;27:489–498. doi: 10.5551/jat.RV17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lozano P, Henrikson NB, Dunn J, Morrison CC, Nguyen M, Blasi PR, et al. Lipid screening in childhood and adolescence for detection of familial hypercholesterolemia: Evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:645–655. doi: 10.1001/jama.2016.6176. [DOI] [PubMed] [Google Scholar]
- 71.Groselj U, Kovac J, Sustar U, Mlinaric M, Fras Z, Podkrajsek KT, et al. Universal screening for familial hypercholesterolemia in children: the Slovenian model and literature review. Atherosclerosis. 2018;277:383–391. doi: 10.1016/j.atherosclerosis.2018.06.858. [DOI] [PubMed] [Google Scholar]
- 72.Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015;36:2425–2437. doi: 10.1093/eurheartj/ehv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tada H, Okada H, Nomura A, Nohara A, Yamagishi M, Takamura M, et al. Prognostic impact of cascade screening for familial hypercholesterolemia on cardiovascular events. J Clin Lipidol. 2021;15:358–365. doi: 10.1016/j.jacl.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 74.Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–3490a. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brautbar A, Leary E, Rasmussen K, Wilson DP, Steiner RD, Virani S. Genetics of familial hypercholesterolemia. Curr Atheroscler Rep. 2015;17:491. doi: 10.1007/s11883-015-0491-z. [DOI] [PubMed] [Google Scholar]
- 76.Kim H, Lee CJ, Pak H, Kim DI, Rhee MY, Lee BK, et al. GENetic characteristics and REsponse to lipid-lowering therapy in familial hypercholesterolemia: GENRE-FH study. Sci Rep. 2020;10:19336. doi: 10.1038/s41598-020-75901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee SH. Characteristics and vascular complications of familial hypercholesterolemia in Korea. J Atheroscler Thromb. 2016;23:532–538. doi: 10.5551/jat.34363. [DOI] [PubMed] [Google Scholar]
- 78.Santos PC, Pereira AC. Type of LDLR mutation and the pharmacogenetics of familial hypercholesterolemia treatment. Pharmacogenomics. 2015;16:1743–1750. doi: 10.2217/pgs.15.113. [DOI] [PubMed] [Google Scholar]
- 79.Talmud PJ, Shah S, Whittall R, Futema M, Howard P, Cooper JA, et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: a case-control study. Lancet. 2013;381:1293–1301. doi: 10.1016/S0140-6736(12)62127-8. [DOI] [PubMed] [Google Scholar]
- 80.Kwon M, Han SM, Kim DI, Rhee MY, Lee BK, Ahn YK, et al. Evaluation of polygenic cause in Korean patients with familial hypercholesterolemia - A study supported by Korean Society of Lipidology and Atherosclerosis. Atherosclerosis. 2015;242:8–12. doi: 10.1016/j.atherosclerosis.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 81.Wang J, Dron JS, Ban MR, Robinson JF, McIntyre AD, Alazzam M, et al. Polygenic versus monogenic causes of hypercholesterolemia ascertained clinically. Arterioscler Thromb Vasc Biol. 2016;36:2439–2445. doi: 10.1161/ATVBAHA.116.308027. [DOI] [PubMed] [Google Scholar]
- 82.Mariano C, Alves AC, Medeiros AM, Chora JR, Antunes M, Futema M, et al. The familial hypercholesterolaemia phenotype: monogenic familial hypercholesterolaemia, polygenic hypercholesterolaemia and other causes. Clin Genet. 2020;97:457–466. doi: 10.1111/cge.13697. [DOI] [PubMed] [Google Scholar]
- 83.Tada H, Kawashiri MA, Nomura A, Teramoto R, Hosomichi K, Nohara A, et al. Oligogenic familial hypercholesterolemia, LDL cholesterol, and coronary artery disease. J Clin Lipidol. 2018;12:1436–1444. doi: 10.1016/j.jacl.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 84.Tada H, Nomura A, Ogura M, Ikewaki K, Ishigaki Y, Inagaki K, et al. Diagnosis and management of sitosterolemia 2021. J Atheroscler Thromb. 2021;28:791–801. doi: 10.5551/jat.RV17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nomura A, Emdin CA, Won HH, Peloso GM, Natarajan P, Ardissino D, et al. Heterozygous ABCG5 gene deficiency and risk of coronary artery disease. Circ Genom Precis Med. 2020;13:417–423. doi: 10.1161/CIRCGEN.119.002871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tada H, Kawashiri MA, Tanaka A, Nakano T, Nakajima K, Inoue T, et al. Post-prandial remnant lipoprotein metabolism in autosomal recessive hypercholesterolaemia. Eur J Clin Invest. 2012;42:1094–1099. doi: 10.1111/j.1365-2362.2012.02700.x. [DOI] [PubMed] [Google Scholar]
- 87.Tada H, Kawashiri MA, Nohara A, Inazu A, Kobayashi J, Mabuchi H, et al. Autosomal recessive hypercholesterolemia: a mild phenotype of familial hypercholesterolemia: insight from the kinetic study using stable isotope and animal studies. J Atheroscler Thromb. 2015;22:1–9. doi: 10.5551/jat.27227. [DOI] [PubMed] [Google Scholar]
- 88.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawamura R, Saiki H, Tada H, Hata A. Acute myocardial infarction in a 25-year-old woman with sitosterolemia. J Clin Lipidol. 2018;12:246–249. doi: 10.1016/j.jacl.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 90.Tanaka H, Watanabe Y, Hirano S, Tada H, Nomura A, Kawashiri MA, et al. Sitosterolemia exhibiting severe hypercholesterolemia with tendon xanthomas due to compound heterozygous ABCG5 gene mutations treated with ezetimibe and alirocumab. Intern Med. 2020;59:3033–3037. doi: 10.2169/internalmedicine.3811-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamada Y, Sugi K, Gatate Y, Senbonmatsu T, Inoue I, Fukushima K, et al. Premature acute myocardial infarction in a young patient with sitosterolemia. CJC Open. 2021;3:1085–1088. doi: 10.1016/j.cjco.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tada H, Nohara A, Inazu A, Sakuma N, Mabuchi H, Kawashiri MA. Sitosterolemia, hypercholesterolemia, and coronary artery disease. J Atheroscler Thromb. 2018;25:783–789. doi: 10.5551/jat.RV17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tada H, Melander O, Louie JZ, Catanese JJ, Rowland CM, Devlin JJ, et al. Risk prediction by genetic risk scores for coronary heart disease is independent of self-reported family history. Eur Heart J. 2016;37:561–567. doi: 10.1093/eurheartj/ehv462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fahed AC, Wang M, Homburger JR, Patel AP, Bick AG, Neben CL, et al. Polygenic background modifies penetrance of monogenic variants for tier 1 genomic conditions. Nat Commun. 2020;11:3635. doi: 10.1038/s41467-020-17374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]