Version Changes
Revised. Amendments from Version 1
Structure
Paragraph sub-headings have been changes
In Dexamethasone subsection:
Details on study controls in the references dexamethasone studies have been added following comments from reviewer 1.
We have added a comment on CSF cytokines as a maker of cerebral inflammation following comments from reviewer 1.
The issue of dexamethasone dosage (and of rifampicin induction) and of the use of other corticosteroids has been commented on within this section.
In Aspirin subsection:
Further detail in anticipated anti-inflammatory effect of aspirin has been added at request of reviewer 1.
In Immunomodulatory subsection:
In table 2, the following references have been added to include additional information on adalimumab:
Adalimumab treatment may replace or enhance the activity of steroids in steroid-refractory tuberculous meningitis. Lee HS, Lee Y, Lee SO, Choi SH, Kim YS, Woo JH, Kim SH. J Infect Chemother. 2012 Aug;18(4):555-7 Adalimumab for Corticosteroid and Infliximab-Resistant Immune Reconstitution Inflammatory Syndrome in the Setting of TB/HIV Coinfection. Lwin N, Boyle M, Davis JS. Open Forum Infect Dis. 2018 Jan 30;5(2):ofy027 Chapter Future Pathways:
The subsection on statin therapy has been shortened.
Subsection on host response:
The discussion regarding the LTA4H gene and the response to dexamethasone has been expanded following comments from reviewer 1.
Abstract
A dysregulated host immune response significantly contributes to morbidity and mortality in tuberculous meningitis (TBM). Effective host directed therapies (HDTs) are critical to improve survival and clinical outcomes. Currently only one HDT, dexamethasone, is proven to improve mortality. However, there is no evidence dexamethasone reduces morbidity, how it reduces mortality is uncertain, and it has no proven benefit in HIV co-infected individuals. Further research on these aspects of its use, as well as alternative HDTs such as aspirin, thalidomide and other immunomodulatory drugs is needed. Based on new knowledge from pathogenesis studies, repurposed therapeutics which act upon small molecule drug targets may also have a role in TBM. Here we review existing literature investigating HDTs in TBM, and propose new rationale for the use of novel and repurposed drugs. We also discuss host variable responses and evidence to support a personalised approach to HDTs in TBM.
Keywords: Tuberculous Meningitis, Host Directed Therapies, Dexamethasone
Introduction
Clinical outcomes in tuberculous meningitis (TBM) depend upon both killing Mycobacterium tuberculosis (M.tb) and managing host inflammatory response. Antimicrobial drug therapy for TBM has been adapted from that used for pulmonary tuberculosis (TB); four drugs are given initially, with subsequent tapering to two or three drugs (in drug-susceptible TBM, dependent on local guidelines) for continuation of therapy up to one year. Yet the host immune response may be dysregulated, and contributes to the poor outcomes associated with TBM. Host directed therapies (HDT) seek to control this host response and reduce death and neurological injury.
The discovery and assessment of new therapeutics in TBM has been a neglected area; this includes the development of bespoke antitubercular drug regimens which account for differing ability of drugs to penetrate the central nervous system, and the design of HDTs which counter dysregulated immune responses to M.tb within the central nervous system. In fact, corticosteroids are the only widely used host directed therapy in TBM with any proven benefit in both adults 1 and children 2 . In adults, in particular, questions around their clinical use remain including whether they have a role in improving outcomes in HIV-associated TBM and the mechanisms by which they improve survival. Clinical trials to assess the efficacy of other HDTs including aspirin and thalidomide have been conducted, however there is not yet conclusive evidence to suggest when, with whom and at what dose they may be effective. New knowledge from studies uncovering mechanisms of inflammation and brain injury may also allow for a directed approach to modulating the host response. Similarly studies aiming to contribute knowledge of factors at play which influence variability in the host may lead us away from a ‘one size fits all’ therapeutic approach.
We review the evidence on currently used HDTs in TBM and suggest potential therapeutics based on pathogenesis studies and drawing from knowledge and experience in other forms of tuberculosis and neuroinflammatory conditions. We will review work which has contributed to our understanding of variation in host response and discuss how this knowledge might be harnessed to design a personalised approach to the use of HDT in TBM.
Existing Host Directed Therapies for Tuberculous Meningitis
Dexamethasone
Adjunctive corticosteroids reduce mortality from TBM, at least in the short term 1, 3, 4 . The mechanism through which corticosteroids confer clinical benefit is unclear, although reduction in intracerebral inflammation seems most likely. Glucocorticoids bind to and activate the glucocorticoid receptor of macrophages and other cells, interfering with inflammatory mediator transcription and expression 5 . Additional indirect genomic effects of inhibition of pro-inflammatory transcription factors such as activator protein-1, and non-genomic mechanisms further mediate glucocorticoid anti-inflammatory effects 6– 9 .
Murine studies suggest M.tb induces activation of the microglial NLRP3 inflammasome, a multimolecular immune complex of receptors and sensors that mediates innate immune responses and induces inflammation via pro-inflammatory caspases and cytokines; a process inhibited by dexamethasone 10, 11 . In TBM, pro-inflammatory cerebrospinal fluid (CSF) cytokine concentrations are acutely elevated, although therapeutically reducing these concentrations may not be clinically beneficial. In a study of 16 individuals with TBM in India, concentrations of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8, IL-10 were elevated in TBM vs. controls (10 non-neurological patients undergoing spinal anaesthesia due to obstructive uropathy), and declined during TB treatment, yet cytokine concentrations were not related to disease severity, brain magnetic resonance imaging (MRI) abnormalities or clinical outcome 12 . In a paediatric study (n=30), CSF TNF-α, IL-1β, and interferon (IFN)-gamma concentrations were elevated in acute TBM, but again did not correlate with disease severity, nor were they influenced by corticosteroid administration 13 . However in a large study of clinical and intracerebral inflammatory phenotype and 9-month survival in adults with TBM from Vietnam, multiple pro-inflammatory and anti-inflammatory CSF cytokines were significantly reduced in HIV uninfected individuals who died vs. in HIV uninfected who survived 14 . This effect (lower pro-inflammatory cytokines in individuals who died) was not seen in HIV co-infection.
In 545 Vietnamese individuals >14 years recruited to a randomized placebo-controlled trial of dexamethasone for TBM, dexamethasone was associated with a reduced risk of death (relative risk 0.69, p=0.01) 1 . In a representative subset of this study, dexamethasone did not significantly alter tested CSF cytokines (TNF-α, IL-1β, IL-6, IL-8, IL-10, IL-12) over time vs. placebo 15 . CSF concentrations of IL-6, IL-8, and IL-10 fell slowly after commencement of anti-TB chemotherapy, and TNF-α fell rapidly, all irrespective of dexamethasone treatment. In a subgroup of HIV uninfected adults (n=37), dexamethasone significantly reduced CSF matrix metalloproteinase-9 (MMP-9) in follow up samples taken after a median 5 days of treatment 16 . CSF cytokine concentration measurement is frequently used as a proxy for measurement for intracerebral inflammation. Further work is required to determine whether the protective effect of dexamethasone correlates with a measurable reduction in intracerebral inflammation.
International guidelines recommend adjunctive corticosteroids for TBM management 17 . Corticosteroid use in TBM is commonplace, dexamethasone is commonly used as it is affordable and widely available although the optimal corticosteroid preparation, dose, and route of administration are unknown 18 . Issues with prolonged intravenous therapy, access to intravenous therapy, and pill burden for oral therapy, all require consideration when designing clinical trials of new corticosteroid regimens. Whether beneficial therapeutic effects extend to HIV co-infected individuals is uncertain. In a HIV-positive subgroup (n=98) from a randomized trial of adjunctive corticosteroids for TBM in Vietnamese adults, dexamethasone was associated with a non-significant trend towards improved survival 1 . Subsequently, a study of adults with HIV-associated TBM showed global increase in pro-inflammatory cytokine concentrations, running counter to theory that immunosuppressed HIV co-infected individuals have lower intracerebral inflammation 14 . A multicentre randomized controlled trial of adjunctive corticosteroids for HIV co-infected adults with TBM is currently underway in Vietnam and Indonesia (NCT03092817) 19 .
Corticosteroids are frequently used to treat common neuro-complications of TBM; paradoxical reactions, and the immune reconstitution inflammatory syndrome (IRIS). Paradoxical neuro-inflammatory reactions, which occur despite appropriate anti-TB chemotherapy, may reflect host response to dead and dying bacteria 20 . TBM-IRIS is a common and often severe complication of starting anti-retroviral therapy (ART) in TBM, and is associated with high CSF neutrophil counts and a positive M. tuberculosis culture at presentation 21 . Interestingly, a CSF inflammatory process, specifically high neutrophils and high TNF-α in combination with low IFN-gamma, predicted later TBM-IRIS in a study of 34 individual with TBM in South Africa 21 . Inflammasome activation appears to be involved in the development of TBM-IRIS, with matrix metalloproteinase (MMP)-9 a possible mediator of brain tissue damage 11 . Whilst corticosteroids during the first 4 weeks after initiation of ART reduced TB-associated IRIS in HIV co-infected individuals in a trial in South Africa, individuals with TBM were excluded 22 . There are no randomized trials of corticosteroid therapy for TBM-IRIS, nor for paradoxical neurological reactions in HIV uninfected individuals. Table 1 summarises the current evidence for dexamethasone use in TBM, as well as for other host-directed therapies.
Table 1. Summary of clinical studies investigating the efficacy of dexamethasone, aspirin and thalidomide in TBM.
| Reference | Intervention
(drug, dose, duration) |
Study design | Population | Primary
outcome |
Key findings |
|---|---|---|---|---|---|
| Mai 27 | Aspirin 81 mg vs. 1000 mg vs. placebo for 60 days | RCT: double-blind,
Placebo controlled |
Adults
Non-HIV, Vietnam n = 120 |
Mortality or Stroke | No difference in 2-month mortality.
Subgroup analysis showed reduction in infarcts and death with aspirin 81 mg (15%) and 1000 mg (11%) compared to placebo (34%); p = 0.06 |
| Misra 34 | Aspirin 150mg vs. placebo | RCT: Placebo controlled | Adults
n=118 |
Mortality or Stroke | Decreased 3-month mortality (21.7%) vs placebo (43.4%); Odds Ratio = 3.17, 95%CI 1.21 - 8.31. Aspirin resulted in absolute risk reduction of stroke in 19.1% and significant reduction in mortality compared to placebo (21.7% vs 43.4%, p=0.02) |
| Misra 35 | Aspirin 150mg | Retrospective cohort | n=135 | Mortality | Non-statistical reduction in deaths (25%) at 3 months compared to standard TB treatment (17%). |
| Schoeman 36 | Aspirin 75mg or 100mg/kg | RCT | Children
n=146 |
No improved neurological or cognitive outcomes or survival with aspirin | |
| Schoeman 37 | Thalidomide 6mg/kg, 12mg/kg, or 24mg/kg | Dose escalating Pilot study | Children
n=15 |
Safety and tolerability | Reduce CSF TNF-α in children with stage 2 TBM |
| Schoeman 38 | Thalidomide 24mg/kg for 1month | RCT: Double blinded | Children
n=47 |
Discontinued prematurely due to side effects and deaths in thalidomide arm | |
| Thwaites 1 | Dexamethasone | RCT: Double-blind Placebo controlled | Adult
n=545 HIV and non-HIV |
Mortality | Reduced risk of death through 9 months (relative risk 0.69, p=0.01) with dexamethasone |
| Simmons 15 | Dexamethasone | RCT: Double-blind Placebo controlled | Adult
N=87 |
Dexamethasone did not significantly alter tested CSF cytokines (TNF-α, IL-1β, IL-6, IL-8, IL-10, IL-12) over time vs. placebo |
RCT = randomised clinical trial; IL = interleukin; TNF = tumor necrosis factor.
In childhood TBM, benefit from corticosteroids has been demonstrated in a number of studies 2, 23– 25 . Unlike in adults, improvement in disability, albeit moderate, is described 3 . Dosage and duration however is debated and in randomized trials dosage has varied between 1mg/kg and 4mg/kg daily, for 3-4 weeks. One trial compared three dosage regimens; 2 mg/kg/day over 4 weeks vs 4 mg/k/day over 1 week and 2 mg/k/day for the next 3 weeks vs 4 mg/kg/day over 4 weeks 26 . In each group the initial 4 weeks was following by 4 weeks of tapering. There was no difference in mortality between groups, however prolonged periods of higher dose prednisolone were associated with new onset optic neuropathy and hydrocephalus 26 . These findings highlight the delicate balance between moderating host immunity, and avoiding the occurrence of adverse events. Further studies are needed to identify ideal dosage regimen, as well as explore host variability in response to corticosteroids in childhood TBM.
Promising Host Directed Therapies for Tuberculous Meningitis
Aspirin
Cerebral infarction occurs in 25–71% of TBM cases 27, 28 , and stroke was associated with a two-fold increase in mortality in a recent meta-analysis 29 . The inflammatory state occurring in TBM contributes to the pathogenesis of stroke. A prospective study of 146 TBM patients demonstrated an acute phase inflammatory response with significantly elevated cytokines (e.g. IL-2, IL-4, IL-6, IL-1β, IFN-γ, TNF- α) in blood and CSF 30 . A hypercoagulable state was reflected by elevated protein C, factor VII, plasminogen activator inhibitor-1 and anticardiolipin antibodies, as well as decreased protein S in a case series of 16 children. Bleeding times were also markedly shorter and platelet counts remained markedly raised in this subgroup 31 . Hypercoagulability has been shown to occur in adults with pulmonary tuberculosis, and may also contribute to pathogenesis of stroke in TBM. Local intra- and extra-vascular factors contributes to TBM pathogenesis, most significantly in the form of cerebral vasculitis secondary to inflammatory infiltrates; initially believed to be directly due to tubercle bacilli implantation, now known to correlate with the inflammatory exudate in the basal cisterns and subarachnoid space 32, 33 . The significance of intravascular thrombosis is still unclear. While thrombosis might be common in the context of vasculitis, autopsies on TBM patients failed to demonstrate frequent arterial thrombosis 32 . Significant platelet dysfunction has also been demonstrated in TBM, manifesting as increased mean platelet volumes, platelet distribution width and platelet-large cell ratio 39 . These parameters are significantly associated with infarcts and suggests the use of antiplatelet agents in TBM 39 . Local intra- and extra-vascular factors contribute to TBM pathogenesis in the form of vasculitis due to bacilli infiltration. In an effort to reduce mortality and long-term neurological disability in TBM, aspirin is increasingly being studied due to its anti-inflammatory and inhibitory effects on platelet and thrombus production. In murine models, low dose aspirin (3 mg/kg) showed a systemic decrease in serum cytokines (e.g. TNF-α, IL-6, IL-1β) and late stage T cell responses in M.tb infection. Aspirin also enhances T helper cell 1 responses for eliminating bacilli from lungs 40 . Aspirin contributes to the resolution of inflammation by generating 15-epi-lipoxims, resolvins and protectins, recognized for their anti-inflammatory as well as pro-resolving characteristics 41 It is also well described how the drug inhibits pro-inflammatory prostanoid production via acetylation of COX 42 .
To date, three randomized controlled trials have investigated the role of aspirin in adult and paediatric TBM. In 118 adult TBM patients in India, aspirin resulted in absolute risk reduction of stroke in 19.1% and significant reduction in mortality compared to placebo (10 of 118 (21.7%) versus 23 of 118 (43.4%), p=0.02) 34 . A randomised controlled trial of TBM involving children in South Africa (n=146) could not establish improved neurological/cognitive outcomes or survival with ASA at doses of 75 mg (low dose) or 100 mg/kg/day (high dose) 36 . However, the developmental outcome of children on high dose ASA was similar to the placebo and low dose ASA groups, despite being younger of age and having higher baseline severity. This finding warrants further investigation of high-dose ASA in childhood TBM. A study of 120 Vietnamese adults with TBM demonstrated a reduction in death and new infarcts with the addition of 81 mg/day aspirin (8 of 36 or 22.2%) and 1000 mg/day aspirin (6 of 38 or 15.8%), versus placebo (11 of 38 or 28.9%) 27 . Aspirin was associated with dose-dependent inhibition of thromboxane A2 and upregulation of pro-resolving protectins in the CSF. Another retrospective study by Misra et al. in India failed to validate clinical benefit, showing an insignificant reduction in deaths with the addition of 150 mg aspirin as compared to standard anti-TB therapy 35 . However, 25% (11 of 135) of patients randomized to the aspirin arm had a complete recovery at 3 months versus 17.1% (7 of 135) in the standard treatment arm. In the three adult trials, corticosteroids were administered alone or in conjunction with aspirin with no adverse event signal found. None of these trials observed an increase in adverse events, but safety concerns with increasing doses of aspirin persist. Whilst these studies of adjunctive aspirin described varying results regarding morbidity and mortality, they paved the way for further large randomised controlled trials. The above described trials utilized a daily aspirin regimen duration or primary outcome measured at two to three months. A recent study utilized MRI to quantify baseline and follow up brain lesions in TB meningitis and found that 60% (n=48) of participants had the presence of acute infacts at enrolment, with only one new infact at follow up 2 months later 43 . This correlates to the acute phase in which most complications of TB meningitis occur and the window where meningeal inflammation and vasculitis needs to be treated. Phase 2 (NCT03927313) and 3 (NCT04145258) trials are currently underway to validate aspirin as a host-directed therapy. Given the insufficient evidence base, aspirin is not routinely used in most individuals with TBM.
Thalidomide
Thalidomide has a wide range of biological effects, due to its ability to interfere with the immune system, and depending on the cell type or pathway of activation. The inhibition of TNF-α, which is produced primarily by macrophages and monocytes, accounts for most of the immunological effects of the drug. TNF-α performs a delicate balancing act during host response to mycobacterium tuberculosis infection, whereby on the one hand it is mandatory for keeping infection under control, but on the other hand, if produced at too high levels it induces a hyperinflammatory state resulting in severe tissue damage. The potential of thalidomide to activate T-cells, resulting in elevated production of IL2, IFN and TNF-α, may potentially interfere with its anti-inflammatory properties 44 . In addition, thalidomide does not inhibit TNF-α produced by stimulated T-cells. The therapeutic effect of thalidomide therefore appears to be dose dependant since differing TNF-α concentrations will result in opposing physiological consequences.
Thalidomide has been shown to reduce CSF TNF-α experimentally in rabbits 45 as well as in children with UK Medical Research Council (MRC) grade 2 TBM in a dose-escalating pilot study 37 . However, a double-blind, randomized trial of high dose thalidomide treatment (24mg/kg/day for 1 month) in children with grade 2 and 3 TBM was discontinued due to side effects (skin rash, hepatitis, neutropenia or thrombocytopenia) and deaths in the thalidomide arm 38 .
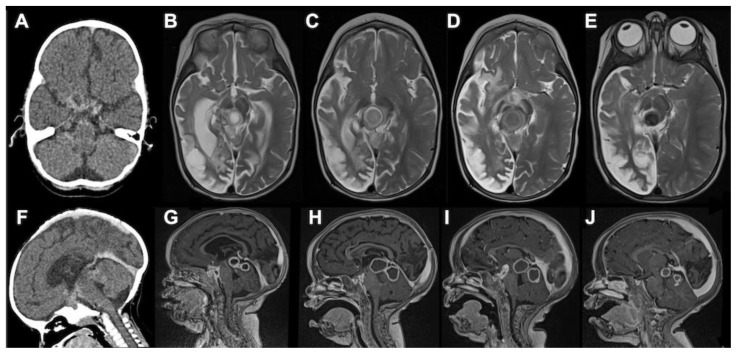
The anti-inflammatory benefits of thalidomide (e.g. improved resolution of basal enhancement and tuberculomas) noted in both the pilot and randomized trials have led to more targeted studies, albeit at a much reduced dosage (≤5 mg/kg/day). Additionally, adjunctive thalidomide has been shown to be particularly effective in observational studies involving tuberculous brain abscesses 46, 47 and blindness-related to optochiasmatic arachnoiditis 48, 49 . Adverse drug effects have been less of an issue in these situations. The life-threatening nature of these TBM sequelae as well as the anatomical location of the lesions, which precluded surgery, disqualified them from being included in trials. Nonetheless, the clinical improvements noted have been substantial. ( Figure 1).
Figure 1. CT axial, MRI T2 axial, CT sagittal and MRI T1 post-gadolinium sagittal images at 3–4 month intervals of a 16-month-old HIV-infected female with stage III TBM.
The initial CT axial and sagittal scans ( A, F) showed a large right sided middle cerebral artery infarction, hydrocephalus as well as multiple small rim-enhancing foci in the prepontine cisterns. After 3 months of anti-TB and 2 months of anti-retroviral therapy, they presented with a depressed level of consciousness. MRI T2 axial ( B) and MRI T1 post-gadolinium sagittal ( G) demonstrated multiple TB abscesses in the interpeduncular, prepontine and chiasmic cisterns (paradoxical HIV related TB IRIS) as well as right cerebral hemisphere spongiotic changes (old infaction). Thalidomide was initiated following a poor response to 1 week of high dose corticosteroids. This resulted in rapid improvement in the level of consciousness, gradual decrease in the size of the TB abscesses and loss of T2 signal (i.e. inflammation), which is a marker of cure as it represents gradual calcification. ( C– E & H– J).
When used, the duration of adjunctive thalidomide therapy should be guided by subsequent clinical and radiological responses. In TBM clinical improvement of mass lesions generally precedes radiological improvement due to a reduction in peri-lesional inflammation. Serial MRI T2-weighted studies have shown that evolution of the lesions from early stage “T2 bright” abscesses with oedema to “T2 black” represents a marker of cure 47 . Regression is associated with fibrosis, mineralization (calcification) and eventually disappearance, usually with no residual structural abnormalities. T2-black granulomas may however persist for years in asymptomatic children. In most cases, cure is achieved after less than 3 months of adjunctive thalidomide therapy.
It is the authors experience that adjunctive thalidomide warrants consideration in the following TBM-related conditions: corticosteroid-unresponsive optochiasmatic arachnoiditis resulting in visual impairment and/or optic disc pallor; enlarging TB abscess despite corticosteroid therapy (TB-IRIS); large TB abscess/tuberculomas in critical brain regions (i.e. brainstem) that is not amenable to surgical drainage and not responding to corticosteroids; large dural-based TB abscess resulting in epilepsia partialis.
TNF-α has been shown to exert deleterious effects on capillaries already sensitized by exposure to mycobacterial products. The endarteritis, coupled with raised intracranial pressure because of edema and obstructive hydrocephalus, often leads to cerebral ischaemia/infarction. The value of low-dose adjunctive thalidomide in modifying the progressive endarteritis is yet to be explored.
Immunomodulatory therapies
Modulation of cytokines known to contribute to pathology is a potential strategy to support host defenses or control deleterious inflammation in TBM. In TBM a number of pro-inflammatory cytokines are thought to play a role in pathogenesis, including IL-2, IL-6, IL-1β, IFN-γ and TNF- α 33 . However, like in other neuroinflammatory conditions where cytokines such as IFN-γ have opposing roles 50 , inhibition of these cytokines may not necessarily lead to improved outcomes and therefore caution must be exercised in exploring the potential drugs which inhibit these pro-inflammatory cytokines as candidate HDTs.
There are accumulating data on the role of the anti-TNF-α monoclonal antibodies infliximab and adalimumab and the soluble TNF-α receptor etanercept in TBM treatment. Although these agents are described as options for treating refractory paradoxical reactions involving the CNS 51– 54 , they may also be responsible for latent TB reactivation and dissemination to the CNS in those where the drug is used to treat autoimmune conditions 55 . Anakinra is a human interleukin-1 receptor antagonist that blocks the biological activity of natural IL-1 and may also have a role in TBM. Anakinra demonstrated efficacy in one case of life-threatening protracted paradoxical inflammation in CNS TB where high dose corticosteroids failed 56 . Other immunomodulatory agents of interest include canakinumab and tocilizumab, human monoclonal antibodies inhibiting IL-1 and IL-6 respectively. In TBM, vasculitis occurs due to the proximity of the progressive exudative meningitis to the basal subarachnoid cistern and the circle of Willis. Cyclophosphamide, an alkylating cytotoxic drug is an effective drug in the treatment of primary cerebral vasculitis. Two case reports have described clinical improvement with the use of cyclophosphamide in TBM associated cerebral vasculitis 57, 58 ; however, its role as an effective treatment in this context needs further investigation particularly due to concerns over its potential adverse activity as a potent immunosuppressive drug. Table 2 summarises cases within the published literature where these agents have been used in the context of TBM; however, pre-clinical and clinical studies to systematically investigate the therapeutic effectiveness is required before they can be used more widely as adjunctive therapies in TBM.
Table 2. Biologics and other immunomodulatory therapies in TBM; summary of published case reports.
| Reference | Drug | Dose | Mechanism | Clinical outcome |
|---|---|---|---|---|
| Blackmore 51 | Infliximab | 10mg/kg, three
doses at monthly intervals |
Anti-TNF | Given after 4 months due to ongoing clinical
deterioration, despite treatment with dexamethasone and cyclophosphamide; resulted in clinical improvement. |
| Jorge 52 | Infliximab | 10mg/kg, three
doses at monthly intervals |
Anti-TNF | Young adult with juvenile idiopathic arthritis, treated
with infliximab developed disseminated TB. With stopping of infliximab, neurological deterioration occurred with isolation of M.tb in CSF, with no improvement with corticosteroids. Infliximab re-initiation led to neurological improvement. |
| Molten 53 | Infliximab | Case 1: 10mg/kg,
three doses at monthly intervals Case 2: 5mg/kg, three doses at 6 week intervals |
Anti-TNF | Two cases describing paradoxical worsening
after initiation of TBM treatment, unresponsive to dexamethasone. In both cases, clinical improvement occurred following administration of infliximab. |
| Abo 54 | Infliximab | 5mg/kg, three doses
at weeks 1, 3 and 7 |
Anti-TNF | Paradoxical worsening (optochiasmatic arachnoiditis,
leading to loss of vision) on starting TB treatment in a 7 year old with TBM, despite dexamethasone. Clinical improvement occurred following infliximab administration. |
| Keeley 56 | Anakinra | 100 mg
subcutaneously daily |
Interleukin-1
receptor antagonist |
Two cases of steroid dependant neurotuberculosis
(paradoxical worsening when steroids stopping). In both cases, patients responded to anakinra therapy. |
| A. Gonzalez-
Duarte 58 |
Cyclophosphamide | 750mg/m
3 every
3 weeks |
Alkylating agent
of nitrogen mustard type.2 |
Clinical improvement |
| Celloti 57 | Cyclophosphamide | 750mg/m
3 every
3 weeks |
Alkylating agent
of nitrogen mustard type.2 |
Clinical improvement |
| Lee 72 | Adalimumab | 40mg SC, total 3
doses every 2 weeks |
Anti-TNF | Clinical improvement |
| Lwin 73 | Adalimumab | 40mg SC, every 2
weeks for 3 months |
Anti-TNF | Clinical improvement of TBM IRIS refractory to steroid
treatment |
TNF = tumor necrosis factor
Potential Pathways for Future Host Directed Therapies for Tuberculous Meningitis
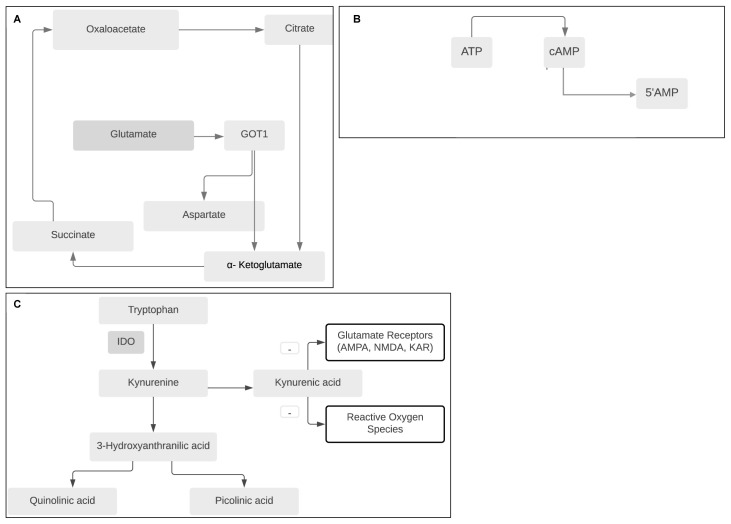
Although host directed therapies are in use, they are limited in either efficacy or availability. Therefore the quest for more effective therapeutics remains ongoing. Here we discuss potential therapies which target pathways highlighted in recent pathogenesis studies, or draw on insights from other forms of TB or inflammatory conditions with shared mechanisms of pathogenesis ( Figure 2).
Figure 2. Schematic of relevant biochemical pathways which, if targeted with future host directed therapies, may improve outcomes in TBM.
A: Drugs which reduce glutamate ‘glutamate grabbers’ by increasing breakdown of glutamate (either recombinant glutamic-oxaloacetic transaminase (GOT1), or others that mimic its action) may decrease neuro-excitotoxicity associated with brain injury in TBM. B: Phosphodiesterase inhibitors reduce breakdown of cAMP to 5’AMP leading to increased neuronal survival, immune modulation, and increase in axon plasticity and myelination. C: Interruption of the tryptophan pathway via modulation of Indoleamine 2,3-dioxygenase (IDO) activity may have neuroprotective effect, although more data to understand the role of downstream metabolites particularly the contribution of kyruneic acid in antagonism of glutamate receptors is needed.
Statin Therapy Pathways
HMG-CoA reductase inhibitors (‘statins’) are ubiquitously used in prevention and treatment of cardiovascular disease, but are also known to have immunomodulatory, anti‐inflammatory and anti‐oxidative properties. Several in vitro studies have demonstrated that statins enhance anti-inflammatory and inhibit pro-inflammatory functions in microglial cells and inhibit mechanisms involved in neurodegeneration 59– 62 . Anti-inflammatory properties may be due to modulation of isoprenylation 63 with downstream effects on inhibitory and stimulatory transcription pathways, or via allosteric inhibition of leucocyte function antigen (LFA)-1 integrin 64 which is involved in the transmigration of activated T cells through the blood brain barrier. Neuroprotective effects may be due to modulation of excitotoxicity, vascular function, angiogenesis, and/or reduced oxidative damage through nitric oxide stimulas 65, 66 . Importantly, some studies have shown increased neuronal death with higher concentrations of statins 67– 69 .
The potential of statins to effect CNS inflammation and neurodegeneration in other conditions are of interest given the shared mechanistic pathways in TBM. For example, animal models of multiple sclerosis (MS) show that statins skew immune responses towards an anti-inflammatory T-helper cell 2 response, inhibiting pro-inflammatory cytokines IL-2, IL-12 and IFN-γ 70 . Patients with secondary progressive MS benefited from statin therapy 71 with a phase 3 trial underway (NCT03387670). In a mouse model of traumatic brain injury, atorvastatin led to profound attenuation of T cell, neutrophil and natural killer cell invasion into the CNS, and reduction in production of pro-inflammatory cytokines (IFN-y and IL-6) and chemokines (CCL5 and CXCL10) 74 . In a double-blind randomised trial involving 36 patients with traumatic brain injury, rosuvastatin given for 10 days in the acute phase of injury significantly reduced TNF-α which correlated with a reduction in disability scores 75 . Other conditions where the role of statins has been explored include Alzheimer’s disease 76 , and Parkinson’s disease 77 . Further, statins may be associated with reduced risk of tuberculosis 78 . In a TB murine model, adjunctive simvastatin shortened time to culture clearance by 1 month, enhanced bacterial killing, and decreased culture-positive relapse and enhance bacterial killing 79– 81 . Clinical trials (NCT03456102, NCT04147286) will investigate the efficacy of statins in pulmonary tuberculosis. Given their potential use as an adjunctive TB therapy, their lipophilic properties allowing good penetration to the CNS, as well as their potential as an anti-inflammatory and neuroprotective agent, statins may have a role as a HDT in TBM; trials to explore this hypothesis are needed.
Glutamate ‘grabbing’ drugs
Excessive glutamate and neuro-excitotoxicity are thought to contribute to brain injury and cell death in TBM. In one study, RNA sequencing of whole blood and CSF from children with TBM demonstrated significant enrichment of transcripts associated with neural excitotoxicity predominantly driven by glutamate release, NMDA receptor binding and uptake 82 . This mechanism is thought to contribute to brain injury and cell death in other neurological conditions such as stroke, epilepsy, traumatic brain injury, Alzheimer’s and Huntington’s disease 83, 84 . Therapeutics which aim to reduce glutamate excitotoxicity either by i) modulating the downstream effects of glutamate via NMDA receptor binding or ii) reducing extracellular glutamate (e.g. glutamate ‘grabbing’) may have a role in the treatment of TBM. In acute stroke, a similar approach was taken however although animal studies were promising, randomised trials in humans assessing efficacy of NMDA antagonists largely failed 85– 87 . Therapeutics have been designed to reduce glutamate induced excitotoxicity by lowering blood glutamate concentration thus leading to a larger natural glutamate gradient between the brain and blood thereby facilitating the efflux of extracellular brain glutamate into the blood 88 . In an animal study riboflavin (vitamin B 2), selected for its ability to interact with Glutamate-Oxaloacetate transaminase (GOT) to significantly reduced blood glutamate levels compared to placebo ( Figure 2A) 89 . In a randomised trial, riboflavin was correlated with improvement of disability when given intravenously in adults with acute stroke 89 . A number of studies have explored the neuroprotective properties of riboflavin including in conditions such as migraine and Parkinson’s disease 90 . It is unclear whether drugs such as riboflavin, or others which reduce glutamate neuro-excitotoxicity, have a role as an adjunctive therapy to promote neuroprotection in TBM; however, given the emerging body of evidence which suggest involvement of the glutamate-glutamine pathway, this is a potential area of interest for future studies.
Tryptophan Pathway Drug Targets
Tryptophan is an essential amino acid which can either be converted to serotonin or oxidized kynurenines via indoleamine 2,3-dioxygenase (IDO1) ( Figure 2C). Further oxidization occurs to convert kynurenine to kynurenic acid, which has neuroprotective properties. Prior studies have shown that M.tb induces marked upregulation of IDO-1 expression in both human and murine macrophages in vitro 91 ; and that blockade of IDO activity reduces both clinical manifestations of TB as well as microbial and pathological correlates of the human TB syndrome in macaques 92 . In an observational cohort study of TBM, low CSF tryptophan levels were found in those who survived, compared to non-survivors or controls 93 . It is therefore unclear in TBM whether drugs which block IDO-1 such as indoximod, an immunometabolic adjuvant that is current under investigation in cancer therapy 94 , would cause benefit or harm. It is plausible that improved survival seen in those with low CSF tryptophan is due to increased availability of kynurenic acid which has neuroprotective action via glutamate receptors and reactive oxygen species. Further investigation into the influence of tryptophan and its downstream metabolites on pathogenesis in TBM is required in order to establish suitable targets along this pathway for HDTs.
Eicosanoid Modulating Drugs
Eicosanoids are arachidonic acid derived lipid mediators that trigger pro-and anti-inflammatory responses and include prostaglandins, resolvins, lipoxins, and leukotrienes which serve as signalling molecules, modulating inflammation and cell death in TB 95 . A delicate balance in eicosanoid levels is crucial for M.tb control and regulating the production of pro-inflammatory cytokines 96 .
Non-steroidal inflammatory drugs (NSAIDs), which exert their effects by inhibiting cyclooxygenase (COX) activity may lead to reduction of excessive inflammation in TBM. As discussed, aspirin, a non-selective COX inhibitor has been investigated in three trials in TBM with variable outcomes 27, 34, 36 . New generation NSAIDs with more selective inhibition of COX2 may have more favourable safety profiles. Phase 1 trials to assess the safety and bactericidal activity of celecoxib and etoricoxib in healthy volunteers with a view to developing these agents as HDTs for drug sensitive TB are currently underway (NCT02602509; NCT02503839). Although trials to further investigate the role of aspirin in TBM are underway, future research should consider the potential contribution of newer more selective COX2 inhibitors in TBM.
Phosphodiesterase Inhibitors
Phosphodiesterase inhibitors (PDE-i) are small-molecule inhibitors that reduce inflammation by increasing intracellular cyclic adenosine monophosphate and cyclic guanine monophosphate 97 ( Figure 2B). Phosphodiesterase 4 (PDE-4) inhibitors such as roflumilast have shown to be effective in the treatment of numerous inflammatory conditions including chronic obstructive inflammatory disease 98 . PDE-4 is expressed within the cortex and hippocampus and animal models suggest that inhibition of PDE-4 may have a beneficial role in CNS conditions where inflammation plays a role in pathogenesis 99– 103 . In animal models of pulmonary TB, inhibition of PDE-3 (cilostazol), PDE-4 (roflumilast) and PDE-5 (sildenafil) have all increased bacterial clearance and reduced pro-inflammatory cytokines which contributed to a reduction in neutrophil infiltration and lung pathology 104– 107 . The role of phosphodiesterase inhibitors has not been studied in TBM but the properties above make them intriguing candidates for adjunctive therapy in TBM.
Antiretroviral Therapy
Although not an HDT per se, the decision as to when antiretroviral therapy is started must consider the potential immunopathogenic complications as well as the benefit in preventing further opportunistic infection. Guidelines vary slightly regarding the timing of initiation of ART relative to initiation of anti-TB chemotherapy in those co-infected with TB and HIV. The 2010 World Health Organisation (WHO) ART guidelines recommend initiating ART within 8 weeks of anti-TB chemotherapy in all HIV-TB co-infected patients regardless of CD4 count 108 . The U.S. National Institutes of Health HIV guidelines recommend starting ART within 2 weeks of anti-TB chemotherapy for HIV-TB co-infected patients with CD4 cell counts <50 cells/ µL and within 8 weeks for CD4 counts >50 cells/µL 109 . In TBM there are unique considerations given the infection surrounds crucial structures (the brain and spinal cord) with a very limited ability to expand within the skull and spinal canal should excess inflammation occur. Inflammation occurring following the initiation of HIV therapy is known as immune reconstitution inflammatory syndrome (IRIS), which in the context of TBM is associated is frequent (up to 40%) and associated with high mortality (30%) 110, 111 .
In a randomised trial of testing immediate HIV therapy initiation (at time of initiating TB treatment) vs delayed (after 2 months) in TBM, immediate therapy was associated with significantly more grade 4 adverse events (n=102) than delayed HIV therapy (n=87; p=.04). This trial informed current consensus that ART initiation should be delayed by between 4-8 weeks after starting TBM therapy 112 . This approach hopes to strike a balance between the beneficial effects of ART (immune reconstitution, control of HIV, prevention of other opportunistic infections) and the potential harms of TB-IRIS.
Variable Host Responses and a Personalized Approach
Host immune response to M.tb in TBM is vital; although excessive inflammation leads to neurological damage. Polymorphisms in genes involved in immune response or signaling pathways can influence host inflammatory response, or susceptibility to TBM 113 .
A previous study in the zebrafish model showed that the leukotriene A4 hydrolase ( LTA4H) gene influenced the balance of pro and anti-inflammatory eicosanoids in response to M tuberculosis infection 114 . LTA4H catalyzes the final step in pro-inflammatory leukotriene B4 (LTB4) synthesis 114 , with LTB4 effects usually balanced by anti-inflammatory lipoxin A4 (LXA4), the two together ensuring an appropriate response to M. tuberculosis without excessive tissue damage 115 . A single nucleotide polymorphism (SNP) (rs17525495) in the promoter region of the LAT4H gene alters gene expression, and LTB4 LXA4 balance; low (CC) and high (TT) inflammatory states result from LTA4H allele homozygosity whereas an intermediate (CT) inflammatory state results from allele heterozygosity 114 . Both TT and CC inflammatory states were associated with increased death in a retrospective study of adults with TBM 116 . In this retrospective study adjunctive dexamethasone was associated with improved survival in the high inflammatory TT group, with the effect of dexamethasone unclear in the CC and CT groups 116 . In a subsequent study of 764 Vietnamese adults, ten CSF cytokines were measured of: TNF-α, IFN-γ, IL-1β, IL-2, IL4, IL-5, IL-6, IL-10, IL-12, IL-13 14 . In HIV-uninfected adults with TBM, pro-inflammatory IL-1β, IL-2, and IL-6 (but not TNF-α) were significantly associated with LTA4H genotype; low concentrations in CC genotype, intermediate concentrations in CT genotype, and high concentrations in TT genotype 14 . In HIV co-infected individuals with TBM, LTA4H genotype did not appear to influence survival, response to dexamethasone, or CSF cytokine profile 14 . Additionally LTA4H genotype did not influence survival in a study of HIV-uninfected Indonesian adults with TBM, all of whom received corticosteroids 117 . A LTA4H genotype stratified approach to adjunctive corticosteroid therapy in TBM is now being assessed in an ongoing randomized placebo-controlled LTA4H genotype stratified non-inferiority trial of HIV uninfected adults with TBM in Vietnam (NCT03100786) 118 . If benefits of adjunctive corticosteroids as a host directed therapy are shown to be limited to one or more LTA4H genotypes, this paves the way for personalized corticosteroid therapy in TBM. Such benefit related to LTA4H genotype may lead to innovation and development of affordable point of care tests, to enable implementation of LTA4H genotype testing into patient management.
Where variable host responses to M. tuberculosis increase intracerebral inflammation, or genetic polymorphisms lead to overexpression of a specific molecule or target, targeted personalized therapies may be beneficial. In a study of tryptophan genome wide SNP data we identified 11 quantitative trait loci associated with CSF tryptophan concentrations, and found that these quantitative trait loci were predictive of patient survival 19 . A SNP (rs17842268) in CD43, a surface glycoprotein, has been associated with more severe presentation, and decreased survival, in TBM 119 . Why SNPs in CD43 affect M tuberculosis susceptibility is uncertain, but CD43 has a role in regulating proinflammatory cytokines 119 , and theoretically anti-inflammatory therapies may be beneficial in such patients., evidence that patients with a dysregulated host immune response benefit from more, or different, host directed therapies is lacking.
Conclusions and Key Areas for Future Research
Host directed therapies are an evolving area of TBM research. We know that the inflammatory response in TBM contributes to poor outcomes. Further, we know that dexamethasone reduces death from TBM. What is unknown is how the drug works, who might benefit most from dexamethasone or whether other therapies should be given in addition to dexamethasone or in place of it in some scenarios. There may also be scenarios where dexamethasone is harmful. Important questions regarding the exact role of thalidomide and aspirin also remain. While in the case of the former, a narrow context in which the drug might be useful is becoming clearer, in the latter the optimal and safe dose of aspirin considering its antiplatelet and anti-inflammatory properties, is uncertain. Although the use of immunomodulatory therapies have been reported sporadically, often where corticosteroid treatments have failed, no clinical trials have been conducted to systematically assess their safety profile and efficacy.
Drug discovery depends on accurately identifying molecular targets which play crucial roles in disease biology, and which are amenable to modulation via biologics or small molecule drug therapeutics. In diseases with high global incidences such diabetes or hypertension, large scale data repositories are beginning to provide genetic insights to inform drug discovery and therefore change the direction of and speed at which novel and repurposed therapeutics become available 120 . In TBM we must work towards establishing similar repositories through international collaboration. However, the relatively low global incidence of the disease, and the challenging environments in which TBM most commonly occurs will make this a lengthy endeavour.
In the near future, we can focus on better understanding of key pathogenic processes underpinning inflammation and brain injury. For example, further understanding of the role and interaction of glutamate and tryptophan in brain injury may uncover targets for which existing drugs can be repurposed and novel therapeutics developed. The rational design of animal models to help inform which of these might deserve clinical trials in TBM is also key; although the rabbit model of TBM has been in use since the early 1900s 121 , further research is required to establish whether a more refined or alternative model could better recapitulate human disease. Genomic research to identify variation in host response will allow further refinement of therapeutic approaches based on factors at the individual patient and population level. While studies of LTA4H genotype have led the way in this area of TBM research, focus must now widen to include other pathways that are likely to vary between hosts. As we move forward with host-directed therapies for TBM we must remain cognisant of the characteristics of the hosts whose responses we are attempting to change. Whether these changes are obvious (e.g. HIV infection) or more opaque (e.g. unknown genetic polymorphisms) they must be considered with trial design so that we can understand as fully as possible, the role of these therapies in improving outcomes in TBM.
Funding Statement
This work was supported by the Wellcome Trust through a UCL Wellcome Trust PhD Programme for Clinicians Fellowship to AGD [175479]; support for GT and JD [110179]; a Wellcome International Intermediate Fellowship to NTTT [206724]; a Wellcome Clinical PhD Fellowship to FVC [210772]; support for RJW [104803; 203135] and funding for the Francis Crick Institute [FC0010218]. RL is supported by a MRC CDA Fellowship [MRC/R008922/1]. DRB is supported by the National Institutes of Neurological Diseases and Stroke of the National Institutes of Health [R01NS086312]. RJW receives support from Francis Crick Institute, which is funded by UKRI [FC0010218]; Wellcome [FC0010218] and CRUK [FC0010218]. He also receives support from Meningitis Now and NIH [R01AI45436]. NCB is supported by the National Institutes of Neurological Diseases and Stroke of the National Institutes of Health [K23NS110470].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. Thwaites GE, Nguyen DB, Nguyen HD, et al. : Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741–1751. 10.1056/NEJMoa040573 [DOI] [PubMed] [Google Scholar]
- 2. Schoeman JF, Van Zyl LE, Laubscher JA, et al. : Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics. 1997;99(2):226–231. 10.1542/peds.99.2.226 [DOI] [PubMed] [Google Scholar]
- 3. Prasad K, Singh MB, Ryan H: Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev. 2016;4(4):CD002244. 10.1002/14651858.CD002244.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torok ME, Nguyen DB, Tran TH, et al. : Dexamethasone and long-term outcome of tuberculous meningitis in Vietnamese adults and adolescents. PLoS One. 2011;6(12):e27821. 10.1371/journal.pone.0027821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abraham SM, Lawrence T, Kleiman A, et al. : Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203(8):1883–1889. 10.1084/jem.20060336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meintjes G, Scriven J, Marais S: Management of the immune reconstitution inflammatory syndrome. Curr HIV/AIDS Rep. 2012;9(3):238–250. 10.1007/s11904-012-0129-5 [DOI] [PubMed] [Google Scholar]
- 7. Newton R: Molecular mechanisms of glucocorticoid action: what is important? Thorax. 2000;55(7):603–613. 10.1136/thorax.55.7.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rhen T, Cidlowski JA: Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Eng J Med. 2005;353(16):1711–1723. 10.1056/NEJMra050541 [DOI] [PubMed] [Google Scholar]
- 9. Adcock IM: Glucocorticoid-regulated transcription factors. Pulm Pharmacol Ther. 2001;14(3):211–219. 10.1006/pupt.2001.0283 [DOI] [PubMed] [Google Scholar]
- 10. Lee HM, Kang J, Lee SJ, et al. : Microglial activation of the NLRP3 inflammasome by the priming signals derived from macrophages infected with mycobacteria. Glia. 2013;61(3):441–452. 10.1002/glia.22448 [DOI] [PubMed] [Google Scholar]
- 11. Marais S, Lai RPJ, Wilkinson KA, et al. : Inflammasome Activation Underlying Central Nervous System Deterioration in HIV-Associated Tuberculosis. J Infect Dis. 2017;215(5):677–686. 10.1093/infdis/jiw561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Misra UK, Kalita J, Srivastava R, et al. : A study of cytokines in tuberculous meningitis: clinical and MRI correlation. Neurosci Lett. 2010;483(1):6–10. 10.1016/j.neulet.2010.07.029 [DOI] [PubMed] [Google Scholar]
- 13. Donald PR, Van Toorn R: Use of corticosteroids in tuberculous meningitis. Lancet. 2016;387(10038):2585–2587. 10.1016/S0140-6736(16)30770-X [DOI] [PubMed] [Google Scholar]
- 14. Thuong NTT, Heemskerk D, Tram TTB, et al. : Leukotriene A4 Hydrolase Genotype and HIV Infection Influence Intracerebral Inflammation and Survival From Tuberculous Meningitis. J Infect Dis. 2017;215(7):1020–1028. 10.1093/infdis/jix050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simmons CP, Thwaites GE, Quyen NT, et al. : The clinical benefit of adjunctive dexamethasone in tuberculous meningitis is not associated with measurable attenuation of peripheral or local immune responses. J Immunol. 2005;175(1):579–590. 10.4049/jimmunol.175.1.579 [DOI] [PubMed] [Google Scholar]
- 16. Green JA, Tran CT, Farrar JJ, et al. : Dexamethasone, cerebrospinal fluid matrix metalloproteinase concentrations and clinical outcomes in tuberculous meningitis. PLoS One. 2009;4(9):e7277. 10.1371/journal.pone.0007277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thwaites G, Fisher M, Hemingway C, et al. : British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59(3):167–187. 10.1016/j.jinf.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 18. Donovan J, Figaji A, Imran D, et al. : The neurocritical care of tuberculous meningitis. Lancet Neurol. 2019;18(8):771–783. 10.1016/S1474-4422(19)30154-1 [DOI] [PubMed] [Google Scholar]
- 19. Donovan J, Phu NH, Mai NTH, et al. : Adjunctive dexamethasone for the treatment of HIV-infected adults with tuberculous meningitis (ACT HIV): Study protocol for a randomised controlled trial [version 2; peer review: 1 approved, 2 approved with reservations]. Wellcome Open Res. 2018;3:31. 10.12688/wellcomeopenres.14006.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thuong NTT, Thwaites GE: Treatment-Associated Inflammatory Deterioration in Tuberculous Meningitis: Unpicking the Paradox. J Infect Dis. 2017;215(5):665–667. 10.1093/infdis/jiw565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marais S, Meintjes G, Pepper DJ, et al. : Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2013;56(3):450–460. 10.1093/cid/cis899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meintjes G, Stek C, Blumenthal L, et al. : Prednisone for the Prevention of Paradoxical Tuberculosis-Associated IRIS. N Eng J Med. 2018;379(20):1915–1925. 10.1056/NEJMoa1800762 [DOI] [PubMed] [Google Scholar]
- 23. Girgis NI, Farid Z, Kilpatrick ME, et al. : Dexamethasone adjunctive treatment for tuberculous meningitis. Pediatr Infect Dis J. 1991;10(3):179–183. 10.1097/00006454-199103000-00002 [DOI] [PubMed] [Google Scholar]
- 24. O'Toole RD, Thornton GF, Mukherjee MK, et al. : Dexamethasone in tuberculous meningitis. Relationship of cerebrospinal fluid effects to therapeutic efficacy. Ann Intern Med. 1969;70(1):39–48. 10.7326/0003-4819-70-1-39 [DOI] [PubMed] [Google Scholar]
- 25. Kumarvelu S, Prasad K, Khosla A, et al. : Randomized controlled trial of dexamethasone in tuberculous meningitis. Tuber Lung Dis. 1994;75(3):203–207. 10.1016/0962-8479(94)90009-4 [DOI] [PubMed] [Google Scholar]
- 26. Shah I, Meshram L: High dose versus low dose steroids in children with tuberculous meningitis. J Clin Neurosci. 2014;21(5):761–764. 10.1016/j.jocn.2013.07.021 [DOI] [PubMed] [Google Scholar]
- 27. Mai NT, Dobbs N, Phu NH, et al. : A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults. eLife. 2018;7:e33478. 10.7554/eLife.33478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wasay M, Khan M, Farooq S, et al. : Frequency and Impact of Cerebral Infarctions in Patients With Tuberculous Meningitis. Stroke. 2018;49(10):2288–2293. 10.1161/STROKEAHA.118.021301 [DOI] [PubMed] [Google Scholar]
- 29. Wen L, Li M, Xu T, et al. : Clinical features, outcomes and prognostic factors of tuberculous meningitis in adults worldwide: systematic review and meta-analysis. J Neurol. 2019;266(12):3009–3021. 10.1007/s00415-019-09523-6 [DOI] [PubMed] [Google Scholar]
- 30. Sharma S, Goyal MK, Sharma K, et al. : Cytokines do play a role in pathogenesis of tuberculous meningitis: A prospective study from a tertiary care center in India. J Neurol Sci. 2017;379:131–136. 10.1016/j.jns.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 31. Schoeman J, Mansvelt E, Springer P, et al. : Coagulant and fibrinolytic status in tuberculous meningitis. Pediatr Infect Dis J. 2007;26(5):428–431. 10.1097/01.inf.0000261126.60283.cf [DOI] [PubMed] [Google Scholar]
- 32. Lammie GA, Hewlett RH, Schoeman JF, et al. : Tuberculous cerebrovascular disease: a review. J Infect. 2009;59(3):156–166. 10.1016/j.jinf.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 33. Davis AG, Rohlwink UK, Proust A, et al. : The pathogenesis of tuberculous meningitis. J Leukoc Biol. 2019;105(2):267–280. 10.1002/JLB.MR0318-102R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Misra UK, Kalita J, Nair PP: Role of aspirin in tuberculous meningitis: a randomized open label placebo controlled trial. J Neurol Sci. 2010;293(1–2):12–17. 10.1016/j.jns.2010.03.025 [DOI] [PubMed] [Google Scholar]
- 35. Misra UK, Kalita J, Sagar B, et al. : Does adjunctive corticosteroid and aspirin therapy improve the outcome of tuberculous meningitis? Neurol India. 2018;66(6):1672–1677. 10.4103/0028-3886.246278 [DOI] [PubMed] [Google Scholar]
- 36. Schoeman JF, van Rensburg AJ, Laubscher JA, et al. : The role of aspirin in childhood tuberculous meningitis. J Child Neurol. 2011;26(8):956–962. 10.1177/0883073811398132 [DOI] [PubMed] [Google Scholar]
- 37. Schoeman JF, Springer P, Ravenscroft A, et al. : Adjunctive thalidomide therapy of childhood tuberculous meningitis: possible anti-inflammatory role. J Child Neurol. 2000;15(8):497–503. 10.1177/088307380001500801 [DOI] [PubMed] [Google Scholar]
- 38. Schoeman JF, Springer P, van Rensburg AJ, et al. : Adjunctive thalidomide therapy for childhood tuberculous meningitis: results of a randomized study. J Child Neurol. 2004;19(4):250–257. 10.1177/088307380401900402 [DOI] [PubMed] [Google Scholar]
- 39. Verma R, Mahapatro S, Kumar A, et al. : Platelet dysfunction and coagulation assessment in patients of tuberculous meningitis. Neurol Sci. 2020;41(8):2103–2110. 10.1007/s10072-020-04299-4 [DOI] [PubMed] [Google Scholar]
- 40. Kroesen VM, Rodriguez-Martinez P, Garcia E, et al. : A Beneficial Effect of Low-Dose Aspirin in a Murine Model of Active Tuberculosis. Front Immunol. 2018;9:798. 10.3389/fimmu.2018.00798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. P.H.E: Tuberculosis in England: 2018.Public Health England, London,2018. [Google Scholar]
- 42. Vane JR, Botting RM: The mechanism of action of aspirin. Thromb Res. 2003;110(5–6):255–258. 10.1016/s0049-3848(03)00379-7 [DOI] [PubMed] [Google Scholar]
- 43. Dian S, Hermawan R, van Laarhoven A, et al. : Brain MRI findings in relation to clinical characteristics and outcome of tuberculous meningitis. PLoS One. 2020;15(11):e0241974. 10.1371/journal.pone.0241974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haslett PA, Corral LG, Albert M, et al. : Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med. 1998;187(11):1885–1892. 10.1084/jem.187.11.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tsenova L, Sokol K, Freedman VH, et al. : A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. J Infect Dis. 1998;177(6):1563–1572. 10.1086/515327 [DOI] [PubMed] [Google Scholar]
- 46. Schoeman JF, Fieggen G, Seller N, et al. : Intractable intracranial tuberculous infection responsive to thalidomide: report of four cases. J Child Neurol. 2006;21(4):301–308. 10.1177/08830738060210040801 [DOI] [PubMed] [Google Scholar]
- 47. van Toorn R, du Plessis AM, Schaaf HS, et al. : Clinicoradiologic response of neurologic tuberculous mass lesions in children treated with thalidomide. Pediatr Infect Dis J. 2015;34(2):214–218. 10.1097/INF.0000000000000539 [DOI] [PubMed] [Google Scholar]
- 48. Roberts MT, Mendelson M, Meyer P, et al. : The use of thalidomide in the treatment of intracranial tuberculomas in adults: two case reports. J Infect. 2003;47(3):251–255. 10.1016/s0163-4453(03)00077-x [DOI] [PubMed] [Google Scholar]
- 49. Schoeman JF, Andronikou S, Stefan DC, et al. : Tuberculous meningitis-related optic neuritis: recovery of vision with thalidomide in 4 consecutive cases. J Child Neurol. 2010;25(7):822–828. 10.1177/0883073809350507 [DOI] [PubMed] [Google Scholar]
- 50. Ottum PA, Arellano G, Reyes LI, et al. : Opposing Roles of Interferon-Gamma on Cells of the Central Nervous System in Autoimmune Neuroinflammation. Front Immunol. 2015;6:539. 10.3389/fimmu.2015.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blackmore TK, Manning L, Taylor WJ, et al. : Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis. 2008;47(10):e83–85. 10.1086/592695 [DOI] [PubMed] [Google Scholar]
- 52. Jorge JH, Graciela C, Pablo AP, et al. : A life-threatening central nervous system-tuberculosis inflammatory reaction nonresponsive to corticosteroids and successfully controlled by infliximab in a young patient with a variant of juvenile idiopathic arthritis. J Clin Rheumatol. 2012;18(4):189–191. 10.1097/RHU.0b013e318258b725 [DOI] [PubMed] [Google Scholar]
- 53. Molton JS, Huggan PJ, Archuleta S: Infliximab therapy in two cases of severe neurotuberculosis paradoxical reaction. Med J Aust. 2015;202(3):156–157. 10.5694/mja14.00716 [DOI] [PubMed] [Google Scholar]
- 54. Abo YN, Curtis N, Butters C, et al. : Successful Treatment of a Severe Vision-Threatening Paradoxical Tuberculous Reaction with Infliximab: First Pediatric Use. Pediatr Infect Dis J. 2020;39(4):e42–e45. 10.1097/INF.0000000000002578 [DOI] [PubMed] [Google Scholar]
- 55. Harris J, Keane J: How tumour necrosis factor blockers interfere with tuberculosis immunity. Clin Exp Immunol. 2010;161(1):1–9. 10.1111/j.1365-2249.2010.04146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Keeley AJ, Parkash V, Tunbridge A, et al. : Anakinra in the treatment of protracted paradoxical inflammatory reactions in HIV-associated tuberculosis in the United Kingdom: a report of two cases. Int J STD AIDS. 2020;31(8):808–812. 10.1177/0956462420915394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Celotti A, Vianello F, Sattin A, et al. : Cyclophosphamide immunomodulation of TB-associated cerebral vasculitis. Infect Dis (Lond). 2018;50(10):779–782. 10.1080/23744235.2018.1467038 [DOI] [PubMed] [Google Scholar]
- 58. Gonzalez-Duarte A, Higuera-Calleja J, Flores F, et al. : Cyclophosphamide treatment for unrelenting CNS vasculitis secondary to tuberculous meningitis. Neurology. 2012;78(16):1277–1278. 10.1212/WNL.0b013e318250d84a [DOI] [PubMed] [Google Scholar]
- 59. Kata D, Földesi I, Feher LZ, et al. : Rosuvastatin enhances anti-inflammatory and inhibits pro-inflammatory functions in cultured microglial cells. Neuroscience. 2016;314:47–63. 10.1016/j.neuroscience.2015.11.053 [DOI] [PubMed] [Google Scholar]
- 60. Churchward MA, Todd KG: Statin treatment affects cytokine release and phagocytic activity in primary cultured microglia through two separable mechanisms. Mol Brain. 2014;7:85. 10.1186/s13041-014-0085-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cordle A, Landreth G: 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors attenuate beta-amyloid-induced microglial inflammatory responses. J Neurosci. 2005;25(2):299–307. 10.1523/JNEUROSCI.2544-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McFarland AJ, Davey AK, McDermott CM, et al. : Differences in statin associated neuroprotection corresponds with either decreased production of IL-1β or TNF-α in an in vitro model of neuroinflammation-induced neurodegeneration. Toxicol Appl Pharmacol. 2018;344:56–73. 10.1016/j.taap.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 63. Waiczies S, Bendix I, Zipp F: Geranylgeranylation but not GTP-loading of Rho GTPases determines T cell function. Sci Signal. 2008;1(12):pt3. 10.1126/stke.112pt3 [DOI] [PubMed] [Google Scholar]
- 64. Weitz-Schmidt G, Welzenbach K, Brinkmann V, et al. : Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7(6):687–692. 10.1038/89058 [DOI] [PubMed] [Google Scholar]
- 65. Bösel J, Gandor F, Harms C, et al. : Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J Neurochem. 2005;92(6):1386–1398. 10.1111/j.1471-4159.2004.02980.x [DOI] [PubMed] [Google Scholar]
- 66. Ponce J, de la Ossa NP, Hurtado O, et al. : Simvastatin reduces the association of NMDA receptors to lipid rafts: a cholesterol-mediated effect in neuroprotection. Stroke. 2008;39(4):1269–1275. 10.1161/STROKEAHA.107.498923 [DOI] [PubMed] [Google Scholar]
- 67. Michikawa M, Yanagisawa K: Inhibition of cholesterol production but not of nonsterol isoprenoid products induces neuronal cell death. J Neurochem. 1999;72(6):2278–2285. 10.1046/j.1471-4159.1999.0722278.x [DOI] [PubMed] [Google Scholar]
- 68. Tanaka T, Tatsuno I, Uchida D, et al. : Geranylgeranyl-pyrophosphate, an isoprenoid of mevalonate cascade, is a critical compound for rat primary cultured cortical neurons to protect the cell death induced by 3-hydroxy-3-methylglutaryl-CoA reductase inhibition. J Neurosci. 2000;20(8):2852–2859. 10.1523/JNEUROSCI.20-08-02852.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schulz JG, Bösel J, Stoeckel M, et al. : HMG-CoA reductase inhibition causes neurite loss by interfering with geranylgeranylpyrophosphate synthesis. J Neurochem. 2004;89(1):24–32. 10.1046/j.1471-4159.2003.02305.x [DOI] [PubMed] [Google Scholar]
- 70. Youssef S, Stüve O, Patarroyo JC, et al. : The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84. 10.1038/nature01158 [DOI] [PubMed] [Google Scholar]
- 71. Chataway J, Schuerer N, Alsanousi A, et al. : Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383(9936):2213–2221. 10.1016/S0140-6736(13)62242-4 [DOI] [PubMed] [Google Scholar]
- 72. Lee HS, Lee Y, Lee SO, et al. : Adalimumab treatment may replace or enhance the activity of steroids in steroid-refractory tuberculous meningitis. J Infect Chemother. 2012;18(4):555–557. 10.1007/s10156-011-0334-y [DOI] [PubMed] [Google Scholar]
- 73. Lwin N, Boyle M, Davis JS: Adalimumab for Corticosteroid and Infliximab-Resistant Immune Reconstitution Inflammatory Syndrome in the Setting of TB/HIV Coinfection. Open Forum Infect Dis. 2018;5(2):ofy027. 10.1093/ofid/ofy027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xu X, Gao W, Cheng S, et al. : Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J Neuroinflammation. 2017;14(1):167. 10.1186/s12974-017-0934-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sánchez-Aguilar M, Tapia-Pérez JH, Sánchez-Rodríguez JJ, et al. : Effect of rosuvastatin on cytokines after traumatic head injury. J Neurosurg. 2013;118(3):669–675. 10.3171/2012.12.JNS121084 [DOI] [PubMed] [Google Scholar]
- 76. Jick H, Zornberg GL, Jick SS, et al. : Statins and the risk of dementia. Lancet. 2000;356(9242):1627–1631. 10.1016/s0140-6736(00)03155-x [DOI] [PubMed] [Google Scholar]
- 77. Yan J, Qiao L, Tian J, et al. : Effect of statins on Parkinson's disease: A systematic review and meta-analysis. Medicine (Baltimore). 2019;98(12):e14852. 10.1097/MD.0000000000014852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lai CC, Lee MTG, Lee SH, et al. : Statin treatment is associated with a decreased risk of active tuberculosis: an analysis of a nationally representative cohort. Thorax. 2016;71(7):646–651. 10.1136/thoraxjnl-2015-207052 [DOI] [PubMed] [Google Scholar]
- 79. Dutta NK, Bruiners N, Pinn ML, et al. : Statin adjunctive therapy shortens the duration of TB treatment in mice. J Antimicrob Chemother. 2016;71(6):1570–1577. 10.1093/jac/dkw014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Skerry C, Pinn ML, Bruiners N, et al. : Simvastatin increases the in vivo activity of the first-line tuberculosis regimen. J Antimicrob Chemother. 2014;69(9):2453–2457. 10.1093/jac/dku166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Parihar SP, Guler R, Khutlang R, et al. : Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J Infect Dis. 2014;209(5):754–763. 10.1093/infdis/jit550 [DOI] [PubMed] [Google Scholar]
- 82. Rohlwink UK, Figaji A, Wilkinson KA, et al. : Tuberculous meningitis in children is characterized by compartmentalized immune responses and neural excitotoxicity. Nat Commun. 2019;10(1):3767. 10.1038/s41467-019-11783-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Meldrum BS: Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S Suppl):1007S–1015S. 10.1093/jn/130.4.1007S [DOI] [PubMed] [Google Scholar]
- 84. Wang R, Reddy PH: Role of Glutamate and NMDA Receptors in Alzheimer's Disease. J Alzheimers Dis. 2017;57(4):1041–1048. 10.3233/JAD-160763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jia M, Njapo SAN, Rastogi V, et al. : Taming glutamate excitotoxicity: strategic pathway modulation for neuroprotection. CNS Drugs. 2015;29(2):153–162. 10.1007/s40263-015-0225-3 [DOI] [PubMed] [Google Scholar]
- 86. Kalia LV, Kalia SK, Salter MW: NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008;7(8):742–755. 10.1016/S1474-4422(08)70165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Grupke S, Hall J, Dobbs M, et al. : Understanding history, and not repeating it. Neuroprotection for acute ischemic stroke: from review to preview. Clin Neurol Neurosurg. 2015;129:1–9. 10.1016/j.clineuro.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 88. Castillo J, Loza MI, Mirelman D, et al. : A novel mechanism of neuroprotection: Blood glutamate grabber. J Cereb Blood Flow Metab. 2016;36(2):292–301. 10.1177/0271678X15606721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. da Silva-Candal A, Perez-Diaz A, Santamaria M, et al. : Clinical validation of blood/brain glutamate grabbing in acute ischemic stroke. Ann Neurol. 2018;84(2):260–273. 10.1002/ana.25286 [DOI] [PubMed] [Google Scholar]
- 90. Marashly ET, Bohlega SA: Riboflavin Has Neuroprotective Potential: Focus on Parkinson's Disease and Migraine. Front Neurol. 2017;8:333. 10.3389/fneur.2017.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Blumenthal A, Nagalingam G, Huch JH, et al. : M. tuberculosis induces potent activation of IDO-1, but this is not essential for the immunological control of infection. PLoS One. 2012;7(5):e37314. 10.1371/journal.pone.0037314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gautam US, Foreman TW, Bucsan AN, et al. : In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2018;115(1):E62–E71. 10.1073/pnas.1711373114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. van Laarhoven A, Dian S, Aguirre-Gamboa R, et al. : Cerebral tryptophan metabolism and outcome of tuberculous meningitis: an observational cohort study. Lancet Infect Dis. 2018;18(5):526–535. 10.1016/S1473-3099(18)30053-7 [DOI] [PubMed] [Google Scholar]
- 94. Fox E, Oliver T, Rowe M, et al. : Indoximod: An Immunometabolic Adjuvant That Empowers T Cell Activity in Cancer. Front Oncol. 2018;8:370. 10.3389/fonc.2018.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ricciotti E, FitzGerald GA: Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000. 10.1161/ATVBAHA.110.207449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Young C, Walzl G, Du Plessis N: Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol. 2020;13(2):190–204. 10.1038/s41385-019-0226-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Page CP, Spina D: Phosphodiesterase inhibitors in the treatment of inflammatory diseases. Handb Exp Pharmacol. 2011; (204):391–414. 10.1007/978-3-642-17969-3_17 [DOI] [PubMed] [Google Scholar]
- 98. Calverley PM, Rabe KF, Goehring UM, et al. : Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685–694. 10.1016/S0140-6736(09)61255-1 [DOI] [PubMed] [Google Scholar]
- 99. Schaal SM, Garg MS, Ghosh M, et al. : The therapeutic profile of rolipram, PDE target and mechanism of action as a neuroprotectant following spinal cord injury. PLoS One. 2012;7(9):e43634. 10.1371/journal.pone.0043634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Atkins CM, Oliva AA, Jr, Alonso OF, et al. : Modulation of the cAMP signaling pathway after traumatic brain injury. Exp Neurol. 2007;208(1):145–158. 10.1016/j.expneurol.2007.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gong B, Vitolo OV, Trinchese F, et al. : Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114(11):1624–1634. 10.1172/JCI22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gonzalez-Garcia C, Bravo B, Ballester A, et al. : Comparative assessment of PDE 4 and 7 inhibitors as therapeutic agents in experimental autoimmune encephalomyelitis. Br J Pharmacol. 2013;170(3):602–613. 10.1111/bph.12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wu Q, Qi L, Li H, et al. : Roflumilast Reduces Cerebral Inflammation in a Rat Model of Experimental Subarachnoid Hemorrhage. Inflammation. 2017;40(4):1245–1253. 10.1007/s10753-017-0567-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Subbian S, Tsenova L, O'Brien P, et al. : Phosphodiesterase-4 inhibition combined with isoniazid treatment of rabbits with pulmonary tuberculosis reduces macrophage activation and lung pathology. Am J Pathol. 2011;179(1):289–301. 10.1016/j.ajpath.2011.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Maiga MC, Ahidjo BA, Maiga M, et al. : Roflumilast, a Type 4 Phosphodiesterase Inhibitor, Shows Promising Adjunctive, Host-Directed Therapeutic Activity in a Mouse Model of Tuberculosis. Antimicrob Agents Chemother. 2015;59(12):7888–7890. 10.1128/AAC.02145-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Maiga M, Agarwal N, Ammerman NC, et al. : Successful shortening of tuberculosis treatment using adjuvant host-directed therapy with FDA-approved phosphodiesterase inhibitors in the mouse model. PLoS One. 2012;7(2):e30749. 10.1371/journal.pone.0030749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Konrad FM, Bury A, Schick MA, et al. : The unrecognized effects of phosphodiesterase 4 on epithelial cells in pulmonary inflammation. PLoS One. 2015;10(4):e0121725. 10.1371/journal.pone.0121725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. https://www.who.int/hiv/pub/arv/adult2010/en/ AtfHiiaaaRfaphar. [Google Scholar]
- 109. https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/27/tb-hiv GftUoAAiAaAwH. [Google Scholar]
- 110. Marais S, Pepper DJ, Schutz C, et al. : Presentation and outcome of tuberculous meningitis in a high HIV prevalence setting. PLoS One. 2011;6(5):e20077. 10.1371/journal.pone.0020077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pepper DJ, Marais S, Maartens G, et al. : Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis. 2009;48(11):e96–107. 10.1086/598988 [DOI] [PubMed] [Google Scholar]
- 112. https://sahivsoc.org/Files/2019%20ART%20Guideline%2028042020%20pdf.pdf. [Google Scholar]
- 113. Wilkinson RJ, Rohlwink U, Misra UK, et al. : Tuberculous meningitis. Nat Rev Neurol. 2017;13(10):581–598. 10.1038/nrneurol.2017.120 [DOI] [PubMed] [Google Scholar]
- 114. Tobin DM, Vary JC, Jr, Ray JP, et al. : The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140(5):717–730. 10.1016/j.cell.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hawn TR, Shah JA, Kalman D: New tricks for old dogs: countering antibiotic resistance in tuberculosis with host-directed therapeutics. Immunol Rev. 2015;264(1):344–362. 10.1111/imr.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tobin DM, Roca FJ, Oh SF, et al. : Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148(3):434–446. 10.1016/j.cell.2011.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. van Laarhoven A, Dian S, Ruesen C, et al. : Clinical Parameters, Routine Inflammatory Markers, and LTA4H Genotype as Predictors of Mortality Among 608 Patients With Tuberculous Meningitis in Indonesia. J Infect Dis. 2017;215(7):1029–1039. 10.1093/infdis/jix051 [DOI] [PubMed] [Google Scholar]
- 118. Donovan J, Phu NH, Thao LTP, et al. : Adjunctive dexamethasone for the treatment of HIV-uninfected adults with tuberculous meningitis stratified by Leukotriene A4 hydrolase genotype (LAST ACT): Study protocol for a randomised double blind placebo controlled non-inferiority trial [version 1; peer review: 2 approved]. Wellcome Open Res. 2018;3:32. 10.12688/wellcomeopenres.14007.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Campo M, Randhawa AK, Dunstan S, et al. : Common polymorphisms in the CD43 gene region are associated with tuberculosis disease and mortality. Am J Respir Cell Mol Biol. 2015;52(3):342–348. 10.1165/rcmb.2014-0114OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hingorani AD, Kuan V, Finan C, et al. : Improving the odds of drug development success through human genomics: modelling study. Sci Rep. 2019;9(1):18911. 10.1038/s41598-019-54849-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kasahara M: The Production of tuberculous meningitis in the rabbit and the changes in its cerebrospinal fluid. Am J Dis Child. 1924;27(5):428–432. 10.1001/archpedi.1924.01920110009002 [DOI] [Google Scholar]