Abstract
Orexin-A (OX-A) and orexin-B (OX-B) (hypocretin 1 and hypocretin 2) are synthesized in neurons of the perifornical, dorsomedial, lateral, and posterior hypothalamus. The locus coeruleus (LC) receives the densest extrahypothalamic projections of the orexin (OX) system. Recent evidence suggests that descending projections of the LC have a facilitatory role in the regulation of muscle tone. The pontine inhibitory area (PIA), located ventral to LC, receives a moderate OX projection and participates in the suppression of muscle tone in rapid-eye-movement sleep. We have examined the role of OX-A and -B in muscle-tone control using microinjections (0.1 μM to 1 mM, 0.2 μl) into the LC and PIA in decerebrate rats. OX-A and -B microinjections into the LC produced ipsi- or bilateral hindlimb muscle-tone facilitation. The activity of LC units was correlated with the extent of hindlimb muscle-tone facilitation after OX microinjections (100 μM, 1 μl) into fourth ventricle. Microinjections of OX-A and -B into the PIA produced muscle-tone inhibition. We did not observe any significant difference in the effect of OX-A and -B on muscle tone at either site. Our data suggest that OX release activates LC units and increases noradrenergic tonus in the CNS. Moreover, OX-A and -B may also regulate the activity of pontine cholinoceptive and cholinergic neurons participating in muscle-tone suppression. Loss of OX function may therefore disturb both facilitatory and inhibitory motor processes.
INTRODUCTION
Sakurai and co-authors (1998) identifity two novel neuropeptides, the orexins (OXs), which are synthesized in neurons of the lateral and posterior hypothalamus. These neuropeptides are identical to the hypocretins previously described by De Lecea and collaborators (1998). Axons of OX cells have a widespread distribution throughout the adult brain in areas outside the hypothalamus, including the cerebral cortex, medial portions of the thalamus, subfornical organ, area postrema, hippocampus, amygdala, indusium griseum, locus coeruleus (LC), and raphe nuclei (Date et al. 1999; De Lecea et al. 1998; Horvath et al. 1999; Lubkin and Stricker-Krongrad 1998; Nambu et al. 1999; Peyron et al. 1998). Initially it was proposed that the OXs participate in the control of feeding behavior as neuromodulators or neurotransmitters (Lubkin and Stricker-Krongrad 1998; Sweet et al. 1999; Takahashi et al. 1999), but this function has been questioned (Edwards et al. 1999; Ida et al. 1999; Taheri et al. 1999). Current evidence indicates that OX-A and -B are involved in the CNS regulation of endocrine and autonomic functions, glucose and energy homeostasis, reproduction, and the sleep-wakefulness cycle (Hagan et al. 1999; Kilduff and Peyron 2000; Lubkin and Stricker-Krongrad 1998; Moriguchi et al. 1999; Pu et al. 1998; Samson et al. 1999; Siegel 1999; Takahashi et al. 1999).
Cataplexy, the sudden loss of muscle tone occurring in narcoleptic humans and animals, is triggered by the sudden onset of certain strong emotions and by particular kinds of motor activity. A mutation in the hypocretin (orexin) receptor 2 gene is the genetic cause of canine narcolepsy (Lin et al. 1999). Most human narcoleptics have reduced levels of hypocretin-1 in their cerebrospinal fluid (Nishino et al. 2000). Mice with a null mutation of the prepro-orexin show aspects of narcolepsy (Chemelli et al. 1999). Wu and co-authors (1999) reported that LC units invariably cease their activity before and during cataplectic attacks in narcoleptic dogs. The LC has been shown to have a role in muscle-tone facilitation in studies in decerebrate cats (D’Ascanio et al. 1989; Stampacchia et al. 1987). The LC receives the densest extrahypothalamic projections of the OX system (Hagan et al. 1999; Horvath et al. 1999; Nambu et al. 1999; Peyron et al. 1998). OX-A and -B applications excite LC units in the in vitro slice preparation (Hagan et al. 1999; Horvath et al. 1999). These findings suggest that OX release in the LC could have a role in muscle-tone control.
The pontine inhibitory area (PIA) elicits muscle-tone suppression with cholinergic (George et al. 1964; Takakusaki et al. 1994) and glutamatergic (Lai and Siegel 1990a) stimulation. Lesions of this region produce rapid-eye-movement (REM) sleep without muscle atonia (Henley and Morrison 1974; Jouvet and Delorme 1965). The middle portion of the pontine reticular nucleus, oral part, and subcoeruleus region from which these effects were elicited, termed the PIA, has a low to moderate concentration of OX axons (Peyron et al. 1998). Thus OX release in the PIA might also affect muscle tone. The current study was undertaken to determine the effects of OX microinjections into the LC and PIA on the regulation of muscle tone and motor activity.
METHODS
All procedures were approved by the Animal Studies Committee of the Sepulveda Veterans Affairs Medical Center/UCLA in accordance with U.S. Public Health Service guidelines. Seventy-two Wistar rats (250–300 g) were operated on, and 48 of them showed muscle rigidity and were used in our experiments. We used decerebrate rats with postdecerebrate electromyographic (EMG) levels from 20 to 300 μV in our studies (Lai and Siegel 1988, 1990a,b, 1991). Animals with EMG level less than 20 μV or showing unilateral muscle rigidity were excluded from this study.
Surgery
Animals were anesthetized with halothane (Fluothane) followed by ketamine HCL (Ketalar, 70 mg/kg ip) for cannulation of the trachea and decerebration. Two holes (diameter, 2.0 mm) were symmetrically drilled over the skull above the LC nuclei and PIA (Hajnik et al. 2000) at 9.2 mm posterior to bregma and 1.3 mm lateral to midline for microinjections and LC unit recordings. Then two rectangular holes were cut in each parietal bone in preparation for decerebration. The transverse anterior and posterior borders of these holes were located 1 and 5 mm posterior to bregma with longitudinal sagittal borders 0.5 mm from midline. Precollicular-postmammillary decerebration was carried out 4.0–5.0 mm posterior to bregma using a stainless steel spatula, taking care not to injure the sagittal vein. Excess fluid was aspirated by syringe and absorbed with Gelfoam (Upjohn). Guide cannulae, 0.6-mm diam, were fixed on the skull bone for OX-A, OX-B, saline, and clonidine microinjections into the fourth ventricle. Coordinates of all structures were based on the rat brain atlas (Paxinos and Watson 1997).
Rectal temperature was continuously monitored with an electrical thermometer (Model BAT-12, Physitemp) and maintained between 37 and 38°C with a Heat Therapy Pump (Model NO. TP-500, Gaymar Industries). Experiments were begun when bilateral postdecerebrate muscle rigidity appeared (0.5–3 h).
Microinjections
The animals were divided into four groups: the first group (n = 17) was used for OX-A microinjections into the LC and PIA, the second group (n = 19) for OX-B microinjections into these structures, the third group (n = 4) for control saline microinjections into the LC and PIA, and the fourth group (n = 8) for saline, OX-A, OX-B, and clonidine microinjections into fourth ventricle during LC unit recording.
In each experiment, four injection sites (2 in the LC and 2 in the PIA) were sequentially tested each with one microinjection. Sites were tested in random order. Subsequent microinjections were performed when muscle tone returned to background. The microinjections into the LC and PIA consisted of 0.2 μl administrated at a rate 10 nl/s. OX-A and -B (Peptide Institute, Osaka, Japan) were dissolved in distilled water to create stock solution (1 mM). Stock solution was stored at 4°C for a maximum of 3 wk. Before experiments, stock solution was dissolved in 0.9% saline to get 0.1, 0.2, 1, and 100 μM concentrations of OX-A and -B.
The response of LC units to OX-A and -B microinjections (100 μM, 1 μl) and clonidine hydrochloride (Sigma) microinjections (20 mM, 1.0 μl, PH 4.0) was determined. Clonidine was dissolved in saline. The microinjections into the fourth ventricle were performed via guide cannulae with outside diameter of 0.6 mm. All injections were performed using a 1-μl Hamilton microsyringe and an injecting cannula with outside diameter of 0.25 mm. A previous study (Hajnik et al. 2000) has shown that the majority of inhibitory sites were located in the oral pontine reticular nucleus in the region rostral to the LC. Therefore the cannula did not damage LC units during microinjections performed into the ventral pons.
Stimulation and recording
Constant-current square-wave pulses (0.2 ms, 30 Hz, 50–100 μA, continuous stimulation for 5–10 s) were delivered using an S88 stimulator (Grass Instruments) coupled to a Grass SIU5 stimulus isolation unit. The areas of the pontine reticular formation producing hindlimb muscle-tone inhibition were located by stereotaxic coordinates (Hajnik et al. 2000) and confirmed with electrical stimulation via a tungsten monopolar microelectrode (A-M Systems). The LC was located by stereotaxic coordinates. OX-A, OX-B, or saline was microinjected into the identified areas.
EMGs were recorded from the neck (splenius muscle) and hindlimbs (gastrocnemius and tibialis anterior muscles) bilaterally with stainless steel wires and were amplified using a Grass polygraph (Model 78D). We chose these ankle extensors and flexors because LC electrical stimulation has been shown to excite spinal motoneurons innervating these muscles (Fung et al. 1987) and facilitate their muscle tone (Lai et al. 1989). Extracellular unit recordings were performed using tungsten monopolar microelectrodes (A-M Systems). Spikes were amplified with a Model 1700 A-M Systems amplifier. Unit pulses and EMGs were recorded on a PC using the 1401plus interface and Spike2 program (Cambridge Electronic Design, Cambridge, UK). The rate of digitization was 372 Hz for EMG and 21 kHz for unit activity.
Identification of LC cells
Cells were classified as noradrenergic using the criteria reported for LC cells in rats and cats (Aston-Jones and Bloom 1986; Foote et al. 1980; Guyenet 1980). These criteria include slow and regular firing, long-duration (>2 ms) action potentials, suppression of firing by intraventricular administration of the alpha2-agonist clonidine, fast activation followed by inhibition in response to a pinch applied to hindlimb paw, and histological location within the LC tyrosine hydroxylase-positive cell cluster.
Histology
Cathodal current (0.1–0.2 mA, 4–6 s) was passed through the microelectrodes at the end of each track. The location of recorded neurons was determined by using the track made by the microelectrode, depth of the marking lesion, and depth measurements on the microdrive. Rats were deeply anesthetized with pentobarbital (70 mg/kg ip) and perfused transcardially with 0.01 M phosphate-buffered saline (PBS, pH = 7.4) followed by 4% paraformaldehyde in 0.1 M PBS. Brains were removed, cut into 60-μm-thick sections, and immunostained for tyrosine hydroxylase (TH). Sections were rinsed three times in 0.05 M Trizma-buffered saline (pH = 7.4) followed by a 24-h incubation in a 1/400 dilution of primary mouse antiserum to TH (Chemicon, Temecula, CA) with a solution of 2% normal goat serum in 0.05 M Trizma-buffered saline (pH 7.4) at 4°C. This was followed by a 2-h incubation in a 1/200 dilution of biotinylated goat anti-mouse IgG (Vector, Burlingame, CA). The tissue was then incubated for 2 h with the avidin-biotin complex (Vector). Each incubation was followed by three rinses. Dilutions and rinses were prepared with 2% normal goat serum in 0.05 M Trizma-buffered saline. The sections were then treated with 0.05% solution of 3,3’ diaminobenzidine and 0.01% hydrogen peroxide. The electrode tracks and the TH-positive cells were identified on a Nikon microscope and plotted with a Neurolucida interface according to the rat brain atlas (Paxinos and Watson 1997).
Data analysis
The latency of muscle-tone changes was measured from the start of the microinjection to either a 30% increase or a 30% decrease in nonintegrated EMG amplitude compared with baseline. The duration was calculated from the time of onset of a sustained increase or decrease in muscle tone until the return to baseline levels. The latencies and durations were averaged for right and left hindlimbs for microinjections that produced bilateral muscle-tone variations. The latencies and durations of muscle-tone changes were analyzed by ANOVA. Unit firing rates were analyzed by Wilcoxon matched-pairs test. The average unit firing rates were calculated for 10 s before and after OX microinjections when sustained muscle-tone facilitation was observed.
Regression analysis was used for estimating the correlation between changes of LC unit firing rate and integrated ipsilateral gastrocnemius EMG. LC unit excitation and inhibition predominantly affect extensor tonus in the ipsilateral limbs (Andre et al. 1995; Pompeiano 1998). The changes of integrated EMG and firing rate were calculated in percent of baseline level. Digital EMG integration was performed with 10-s epochs. All average values are means ± SE.
RESULTS
OX-A and -B microinjections in the vicinity of LC
Thirty-four OX-A microinjections (0.2 μM to 1 mM, 0.2 μl) were performed in the vicinity of LC (Fig. 1) in 17 decerebrate rats. Sixteen microinjection sites located in the LC produced bilateral muscle-tone facilitation (Fig. 2A), and 12 sites located similarly evoked ipsilateral muscle-tone facilitation (Fig. 2B). Four OX-A microinjections into the subcoeruleus nucleus, alpha part (SubCA) inhibited muscle tone bilaterally (Fig. 2C) and two microinjections into the SubCA produced ipsilateral muscle-tone inhibition.
FIG. 1.
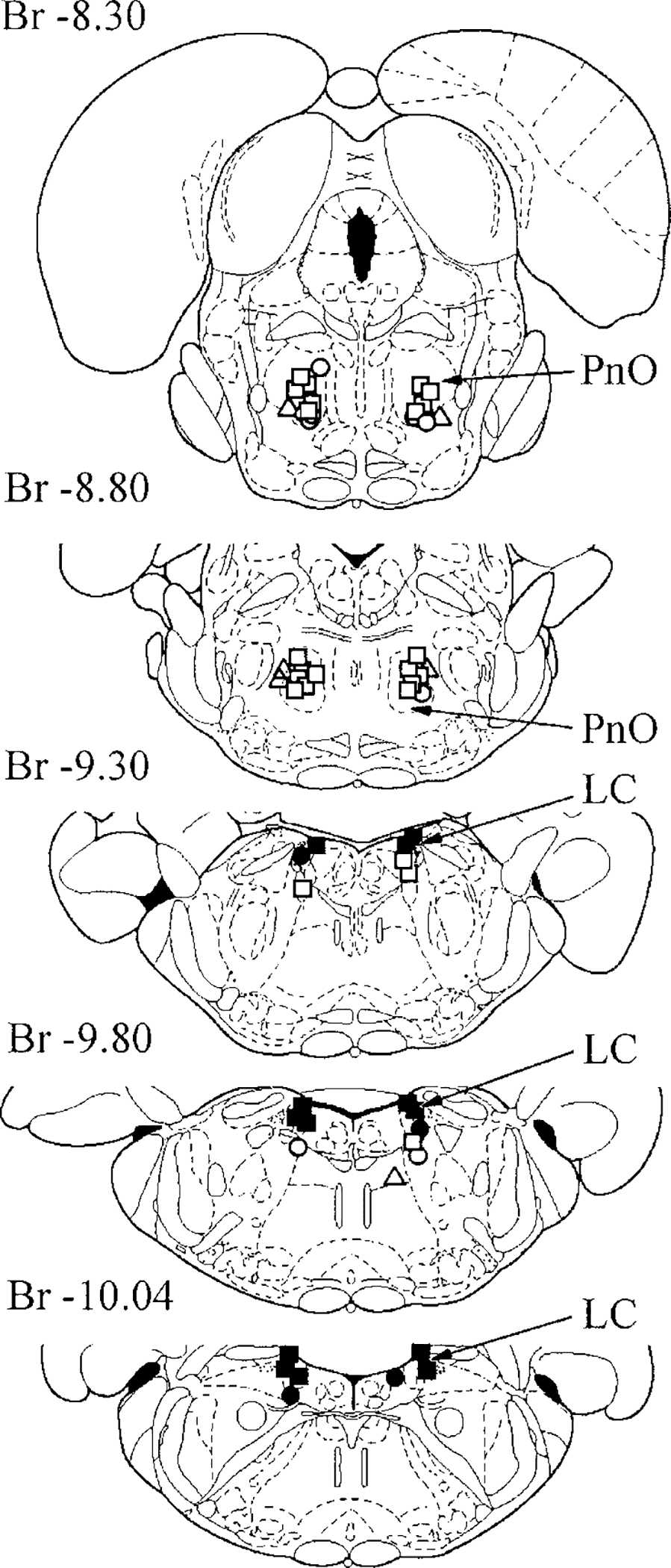
Location of orexin-A (OX-A) microinjection zones in the vicinity of locus coeruleus (LC) and pontine reticular formation inducing ipsilateral muscle-tone facilitation (○), bilateral muscle-tone facilitation (■), ipsilateral muscle-tone inhibition (○), bilateral muscle-tone inhibition (□), and contralateral muscle-tone inhibition (Δ) in decerebrate rats. PnO, pontine reticular nucleus, oral. Location of orexin-B (OX-B) microinjection zones in the vicinity of LC and pontine reticular formation was similar.
FIG. 2.
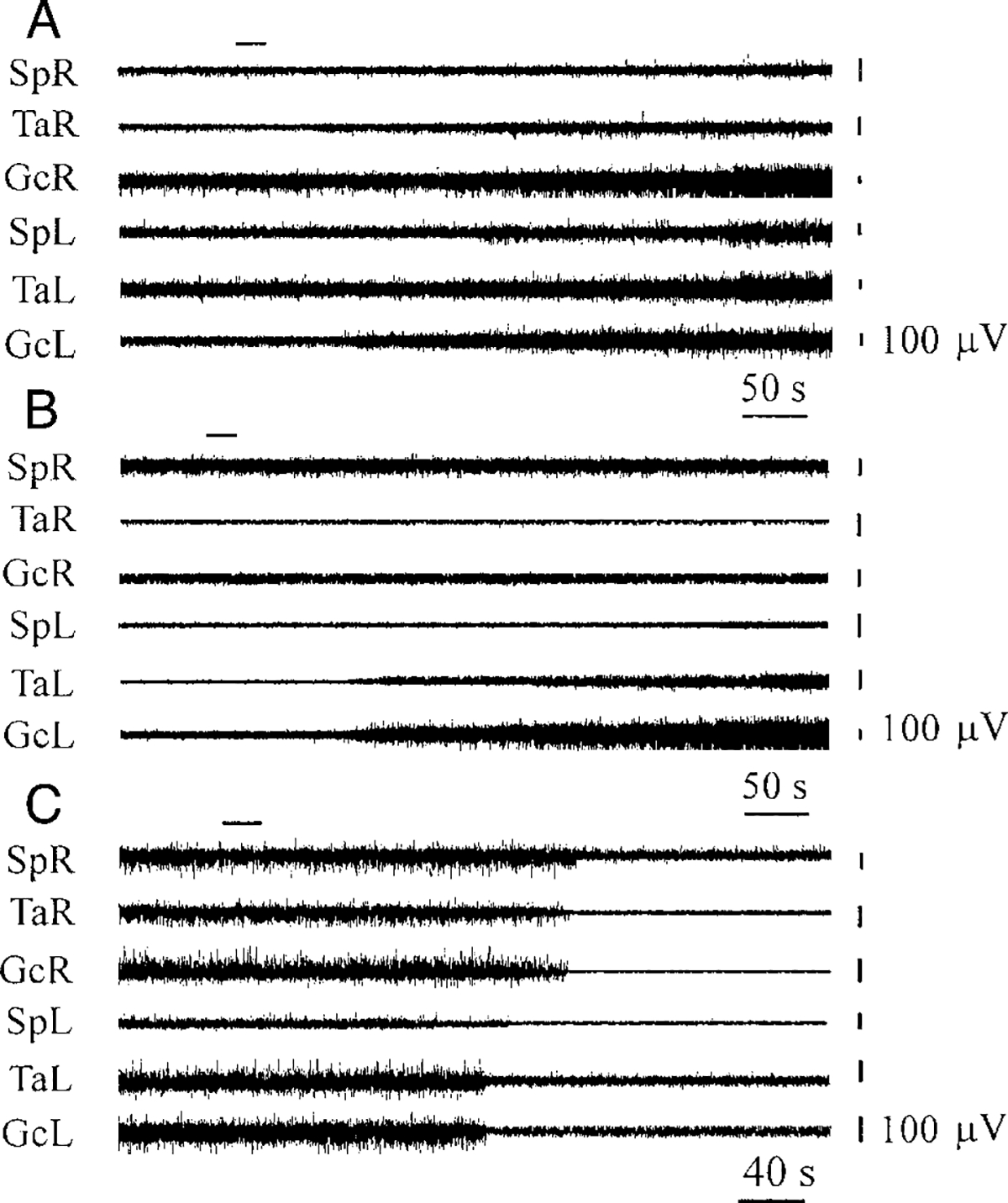
Bilateral muscle-tone facilitation (A), ipsilateral muscle-tone facilitation (B), and bilateral muscle-tone inhibition (C) after OX-A microinjections (A: 0.2 μM, B: 0.2μM, C: 1.0μM) in the vicinity of LC and subcoeruleus nucleus, alpha part (SubCA). SpR, SpL, electromyogram (EMG) of muscle splenius (right and left side); TaR, TaL, EMG of tibialis anterior muscle (right and left side); GcR, GcL, EMG of gastrocnemius muscle (right and left side). Top horizontal left bars indicate the time and duration of injections. Muscle-tone changes during OX-B microinjections in the vicinity of LC were similar.
Similar doses of OX-B were injected into 38 sites in the vicinity of LC in 19 decerebrate rats. The location of OX-B microinjection sites was similar to these represented in Fig. 1. Eighteen microinjections into the LC produced bilateral muscle-tone facilitation, and 11 sites evoked ipsilateral muscle-tone facilitation. Five OX-B microinjections into the SubCA decreased muscle tone bilaterally, two produced ipsilateral muscle-tone inhibition, and two microinjections were ineffective.
Latency and duration of muscle-tone facilitation
OX-A and -B microinjections in the vicinity of LC increased hindlimb muscle rigidity in a dose-dependent manner. The response thresholds were between 0.1 and 0.2 μM for OX-A and -B. The latency of muscle-tone facilitation (Fig. 3A) changed from 200 ± 13 s (n = 7, 0.2 μM) to 66 ± 8 s (n = 7, 1 mM) for OX-A and from 182 ± 17 s (n = 7, 0.2 μM) to 63 ± 7 s (n = 7, 1 mM) for OX-B. The duration of muscle-tone facilitation also had a dose-dependent time course (Fig. 3B) and ranged from 530 ± 67 s (n = 7, 0.2 μM) to 2211 ± 208 s (n = 7, 1 mM) for OX-A and from 491 ± 40 s (n = 7, 0.2 μM) to 2116 ± 285 s (n = 7, 1 mM) for OX-B. We did not observe significant differences between the action of equimolar concentrations of OX-A and -B on hindlimb muscle tone (for latency: F0.2 μM = 0.72, P > 0.5; F1 μM = 0.36, P > 0.75; F100 μM = 0.08, P > 0.85; F1 mM = 0.13, P > 0.75; df = 1, 12; for duration: F0.2 μM = 0.29, P > 0.75; F1 μM = 0.46, P > 0.75; F100 μM = 0.08, P > 0.8; F1 mM = 0.07, P > 0.8; df = 1, 12). Saline microinjections (n = 6) into the vicinity of LC in four control rats did not produce muscle-tone facilitation within the 1-h postinjection observation period.
FIG. 3.
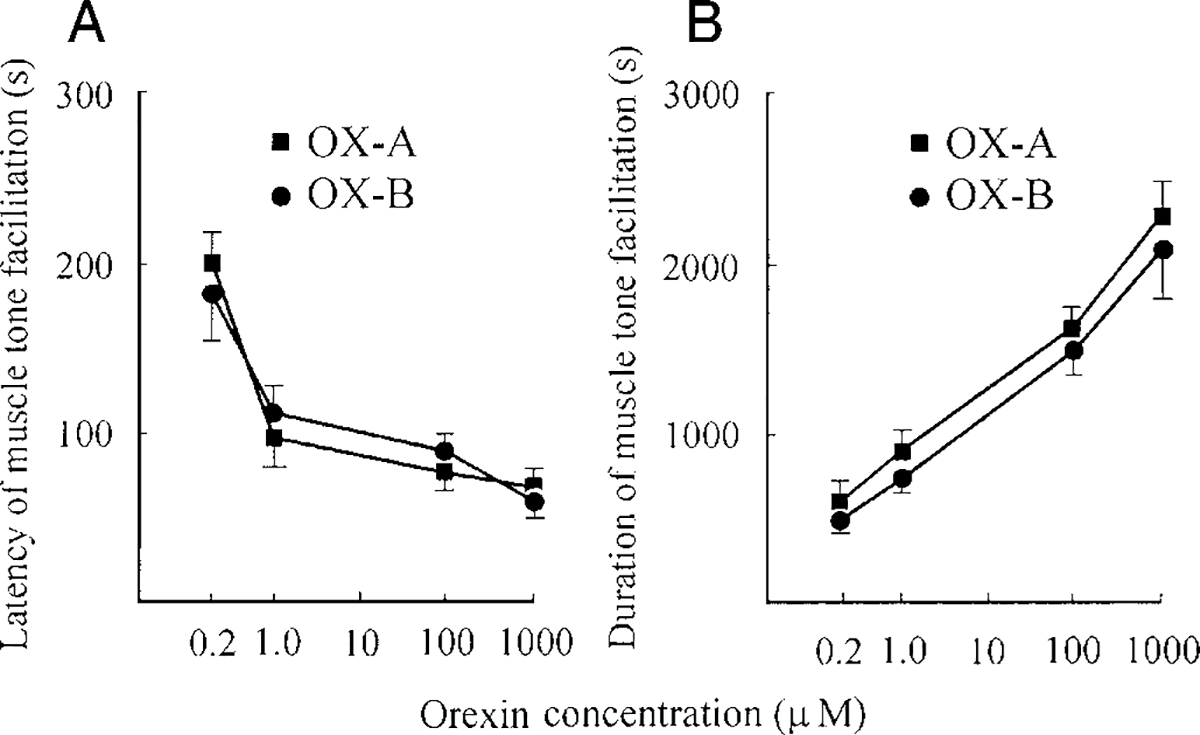
Latency (A) and duration (B) of muscle-tone facilitation after OX-A (■) and OX-B (○) microinjections in the vicinity of LC. The vertical bars represent SE (n = 7) for each point of the curve.
OX-A and -B microinjections into the PIA
Thirty-four OX-A microinjections (0.2 μM to 1 mM, 0.2 μl) were performed into the PIA sites (Fig. 1) identified previously by electrical stimulation (50–100 μA) in 17 rats. Twenty microinjection sites produced bilateral muscle-tone inhibition (Fig. 4A). Eight sites evoked ipsilateral hindlimb muscle-tone inhibition with contralateral muscle-tone facilitation (Fig. 4B), and six sites induced contralateral muscle-tone inhibition (Fig. 4C).
FIG. 4.
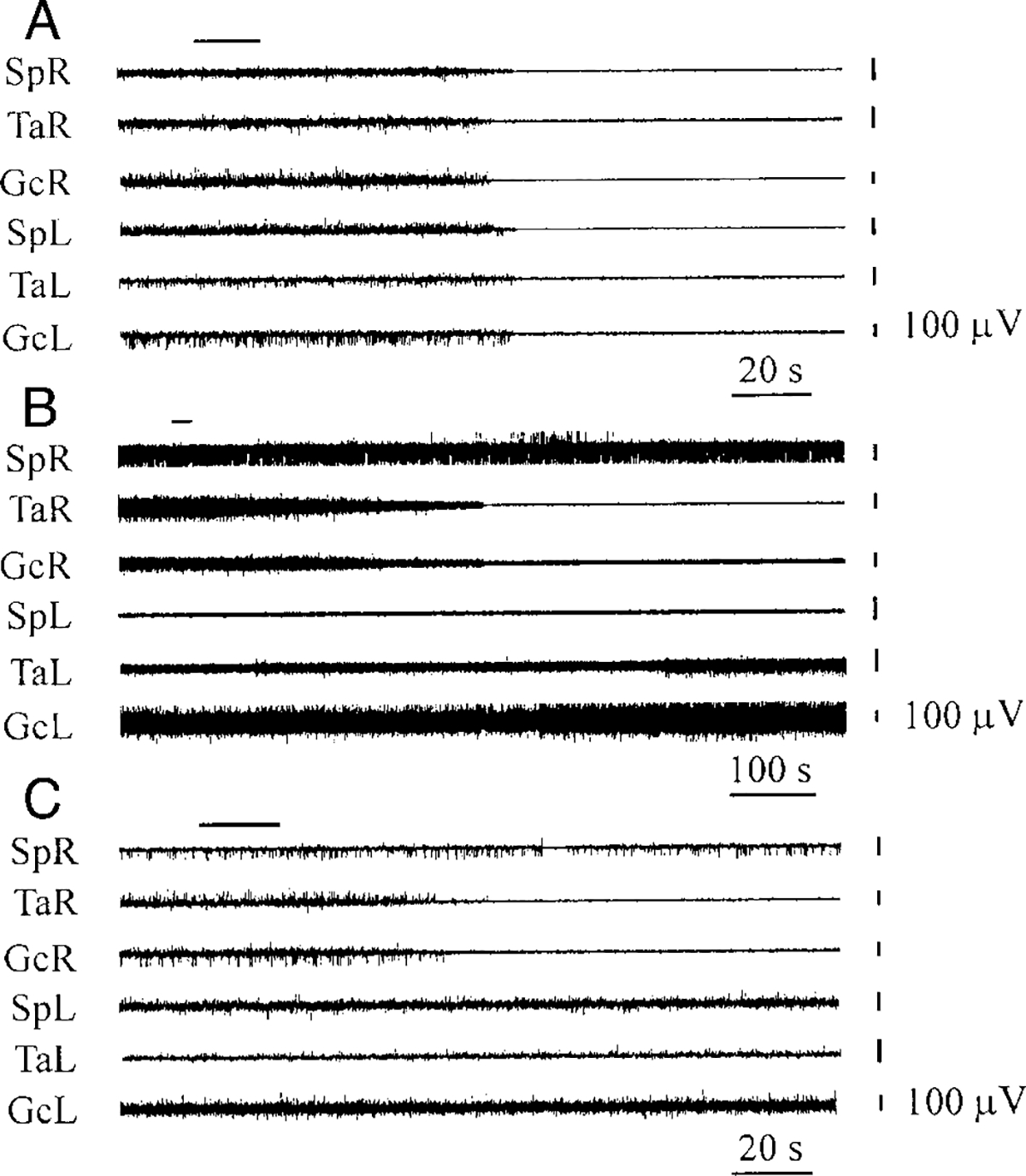
Bilateral hindlimb muscle-tone inhibition (A), ipsilateral hindlimb muscle-tone inhibition with contralateral muscle-tone facilitation (B), and contralateral muscle-tone inhibition (C) after OX-A microinjections (A: 1 μM, B: 0.2 μM, C: 1 μM) into pontine reticular formation. See abbreviations in Fig. 2. Top horizontal bars at the left indicate the time and duration of injections. Muscle-tone changes during OX-B microinjections in the pontine reticular formation were similar.
OX-B microinjections were performed into 38 identified inhibitory sites of the PIA in 19 rats. Twenty-two microinjection sites produced bilateral muscle-tone inhibition, 10 sites induced ipsilateral muscle-tone inhibition with contralateral muscle-tone facilitation, and 6 sites evoked contralateral muscle-tone inhibition.
Latency and duration of muscle-tone inhibition
The latency of bilateral muscle-tone inhibition produced by OX-A and -B microinjections had a dose-dependent time course and changed from 225 ± 28 s (n = 5, 0.2 μM) to 52 ± 8 s (n = 5, 1 mM) for OX-A and from 221 ± 32 s (n = 5,0.2 μM) to 57 ± 8 s (n = 5, 1 mM) for OX-B (Fig. 5A). The response thresholds for OX-A and -B were between 0.1 and 0.2 mM. The duration of muscle-tone inhibition (Fig. 5B) changed from 208 ± 15 s (n = 5, 0.2 μM) to 965 ± 87 s (n = 5, 1 mM) for OX-A and from 179 ± 19 s (n = 5, 0.2 μM) to 810 ± 54 s (n = 5, 1 mM) for OX-B. We did not observe significant differences between the action of equimolar concentrations of OX-A and -B in the PIA (for latency: F0.2 μM = 0.01, P. 0.9; F1 μM = 0.01, p > 0.9; F100 μM = 0.90, P > 0.5; F1 mM = 0.52, P > 0.5; df = 1, 8; for duration: F0.2 μM = 1.50, P. 0.5; F1 μM = 0.69, P > 0.5; F100 μM = 0.20, P > 0.75; F1 mM = 2.26, P > 0.25; df = 1, 8). Control microinjections of saline at the same sites (n = 6) did not produce hindlimb muscle-tone inhibition in three control rats.
FIG. 5.
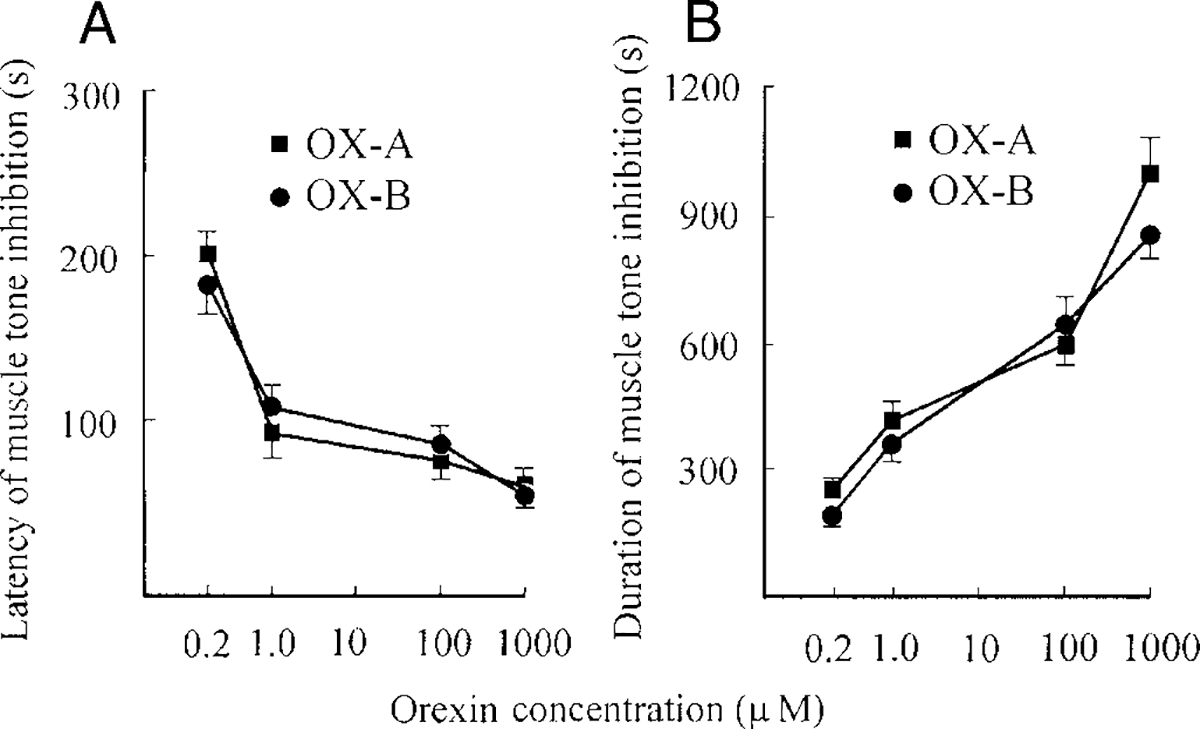
Latency (A) and duration (B) of muscle-tone inhibition after OX-A (■) and OX-B (○) microinjections into the pontine inhibitory area (PIA). The vertical bars represent the SE (n = 5) for each point of the curve.
LC unit activity after OX-A and -B microinjections into the fourth ventricle
Microinjections of OX-A and -B into the fourth ventricle between the LC nuclei produced muscle-tone facilitation in eight rats with postdecerebrate muscle-tone rigidity. We used these microinjections to determine if LC units participate in the muscle-tone facilitation induced by OX-A and -B.
Eighteen recorded neurons met the criteria for noradrenergic cells (Fig. 6). These neurons showed a regular (4.7 ± 2.0 spike/s, n = 18) firing rate when hindlimb rigidity was observed and had an average spike duration of 2.4 ± 0.1 ms (n = 18). Pinching of the hindlimb paw on either side evoked an initial (0.3–0.8 s) burst of activity following by inhibition (lasting 1–3 s). Immunohistochemistry confirmed that these cells were located in TH-positive regions. Clonidine injections in the fourth ventricle performed after OX-A and -B microinjections inhibited activity in all putative noradrenergic neurons and suppressed muscle tone. Complete inhibition of LC unit activity and muscle-tone suppression after clonidine microinjection occurred at average latencies 28.0 ± 1.7 s (n = 18) and 42.2 ± 3.9 s (n = 18), respectively. Unit activity and muscle tone returned in parallel to the baseline level by 52.9 ± 2.0 min (n = 18) after clonidine microinjections.
FIG. 6.
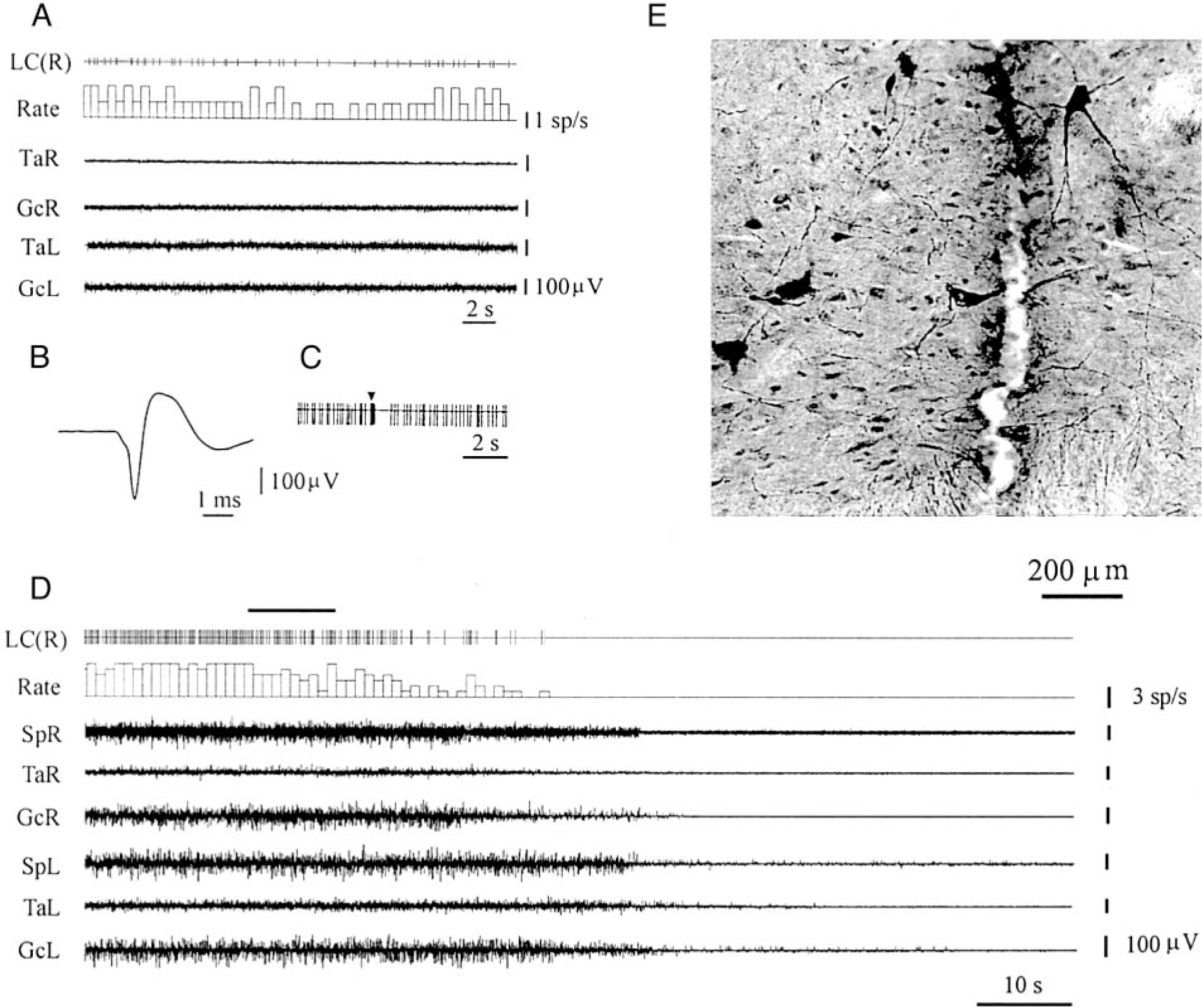
Electrophysiological and histological identification of noradrenergic LC neuron. A: slow and regular spontaneous firing during muscle rigidity. B: long duration of spike. C: fast activation followed by inhibition in response to a pinch stimulus applied to hindlimb paw. D: time course of LC unit and muscle-tone changes after clonidine microinjection in the 4th ventricle. E: histological location of tyrosine hydroxylase-positive cells near the recording track in the LC. LC(R), LC unit (right side). See other abbreviations in Fig. 2.
Ten LC units were observed during OX-A microinjections (100 μM, 1 μl) into the fourth ventricle. The discharge frequency of nine of these cells was increased by an average 187 ± 24% (n = 9, T = 0, P < 0.01) compared with the baseline and the magnitude of the increase was correlated with the size of EMG facilitation (Fig. 7A). Excitation of LC cells after OX-A microinjections preceded muscle-tone facilitation by an average 5.8 ± 0.9 s (n = 9). We used a regression analysis for estimating the numerical relationship between changes of integrated gastrocnemius EMG and ipsilateral LC unit firing rate after OX-A microinjections (Fig. 8A). The regression equation of EMG (%) on rate (%) after OX-A microinjections was EMG = 48.34 + 0.46Rate (SE = 26.09, 0.16; t = 2.80; P < 0.05; df = 14). The correlation coefficient between EMG and ipsilateral LC unit firing rate was 0.59 (n = 16, P < 0.05). One LC cell did not change firing frequency after OX-A microinjection.
FIG. 7.
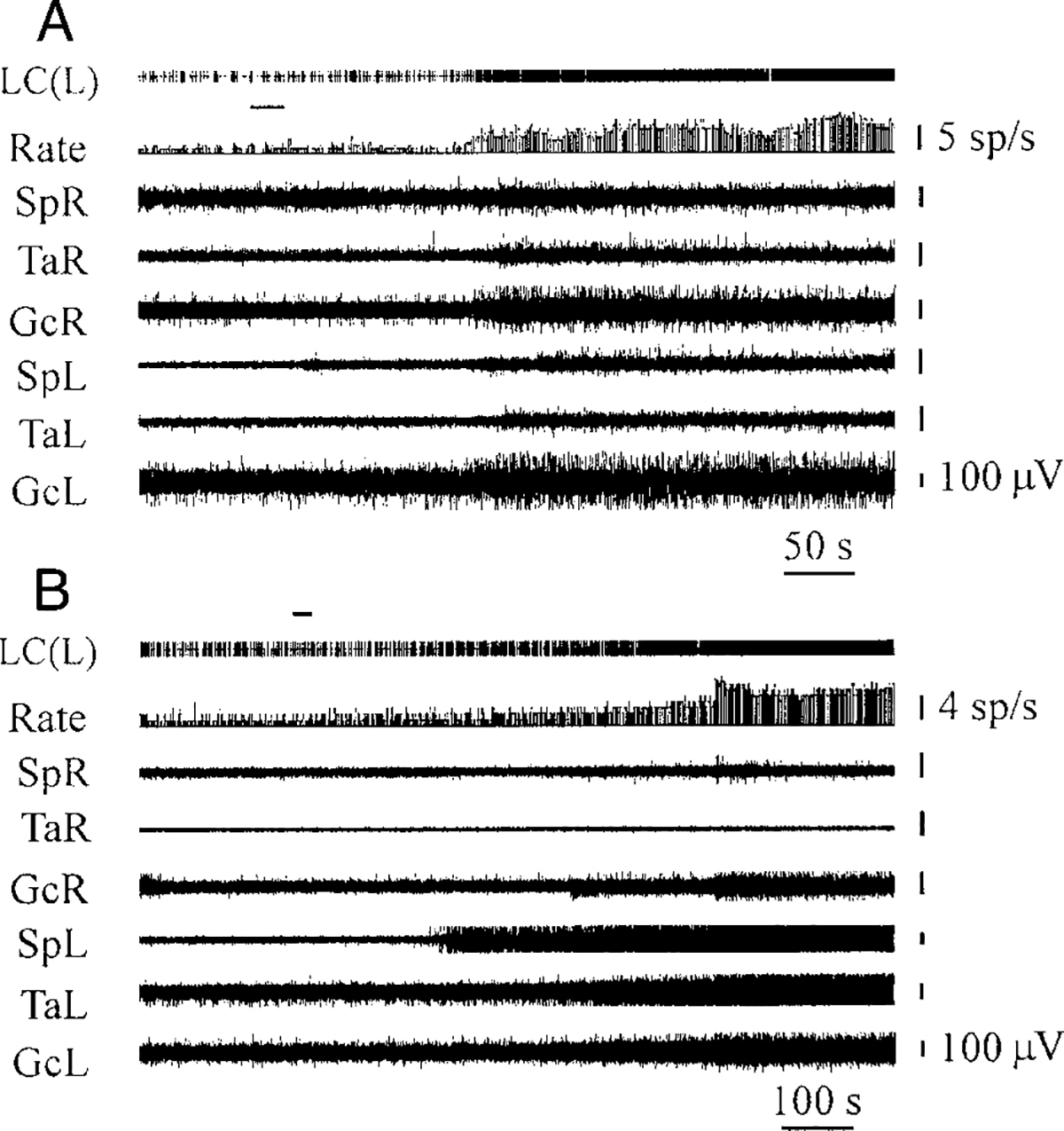
LC unit excitation and muscle-tone facilitation after OX-A (A) and OX-B (B) microinjections (100 μM) into the 4th ventricle. Top horizontal bars at the left indicate the time and duration of injections. LC(L), LC unit (left side). See other abbreviations in Fig. 2.
FIG. 8.
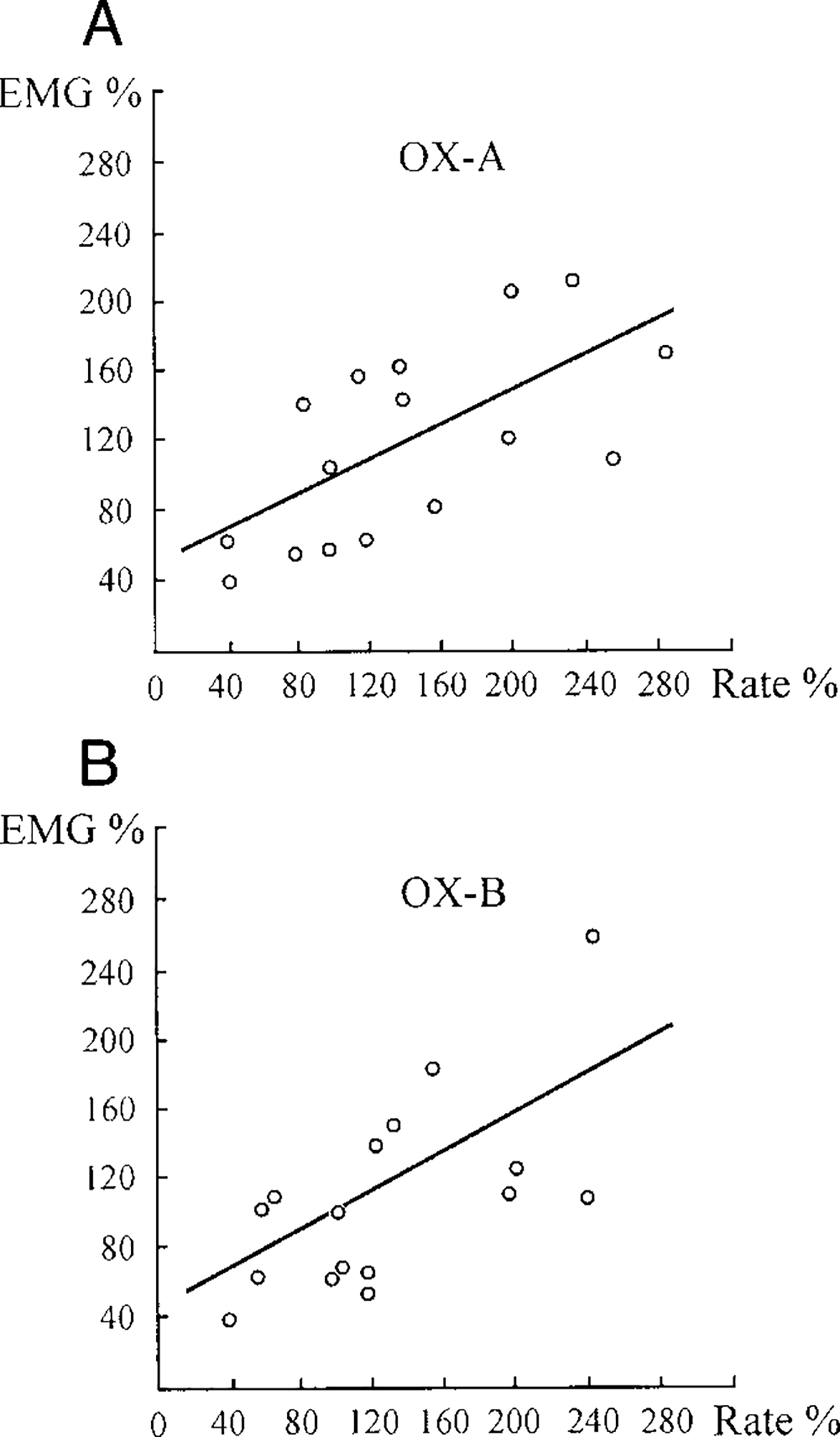
Regression diagrams showing relationship between changes of gastrocnemius EMG (EMG %) and ipsilateral LC unit firing rate (Rate %) after OX-A (A) and OX-B (B) microinjections into the 4th ventricle. O, experimental points (n = 16). For OX-A microinjections: EMG = 48.34 + 0.46Rate (SE = 26.09, 0.16; t = 2.80; P < 0.05; df = 14), R = 0.59 (n = 16, P < 0.05). For OX-B microinjections: EMG = 45.41 + 0.52Rate (SE = 26.59, 0.19; t = 2.83; P < 0.05; df = 14), R = 0.60 (n = 16, P < 0.05).
The discharge rate of eight LC units was analyzed during OX-B microinjections into the fourth ventricle. All cells were excited by OX-B microinjections (177 ± 13%, n = 8, T = 0, P < 0.01), and their activity correlated with hindlimb muscle-tone facilitation (Figs. 7B and 8B). The increased firing frequency of LC units preceded the muscle-tone increase after OX-B microinjections by an average 6.2 ± 1.0 s (n = 8). The regression equation of EMG (%) on rate (%) after OX-B microinjections was EMG = 45.41 + 0.52Rate (SE = 26.59, 0.19; t = 2.83; P < 0.05; df = 14) and the correlation coefficient between these variables was 0.60 (n = 16, P < 0.05).
DISCUSSION
We found that microinjections of OX-A and -B in the vicinity of LC increase LC unit activity and produce a correlated facilitation of muscle tone in decerebrate rats. No difference was observed in the effect of OX-A and -B on muscle tone and LC unit firing rate. Our data concerning excitatory OX influences on LC neurons are consistent with results obtained in rat brain slices. Intra- and extracellular recordings revealed that OX-A applications evoked depolarization of LC units and increased their firing frequency (Hagan et al. 1999). In a similar manner, hypocretin-2 (OX-B) applications depolarized all tested LC cells and increased their spontaneous rate. Membrane depolarization persisted in the presence of tetrodotoxin, indicating that the response to OX-B was synaptically mediated (Horvath et al. 1999; Ivanov and Aston-Jones 2000).
Trivedi and co-authors (1998) using in situ hybridization with receptor subtype-specific oligonucleotide probes reported that only OX1R were located in the LC. These receptors are relatively selective for OX-A, whereas OX2R is nonselective for OX-A and -B (Sakurai et al. 1998). OX-A fibers project widely in the brain stem, and the LC receives the densest OX-A innervation (Cutler et al. 1999; Hagan et al. 1999). These data were complemented by studies that revealed a widespread distribution of both OX-A and -B immunoreactive fibers in the rat brain and in the LC (Date et al. 1999). Horvath and co-authors (1999) using electron microscopy showed that all TH-positive cells in the LC receive asymmetrical (excitatory) synaptic contacts from multiple axons containing hypocretin-2. A specific radioimmunoassay performed for OX-A and -B showed that OX-B concentration was double OX-A level in the rat brain stem (Mitsuma et al. 1999, 2000).
Electrophysiological and pharmacological studies indicate that LC units with descending projections have a facilitatory action on spinal motoneurons (Andre et al. 1995; D’Ascanio et al. 1989; Fung and Barnes 1981; Fung et al. 1991, 1994; Rispoli et al. 1994). Moreover, noradrenergic LC neurons also may produce an indirect excitatory effect on spinal motoneurons by inhibition of pontine cholinergic and cholinoceptive neurons participating in descending muscle-tone suppression (Horn et al. 1987; Pompeiano et al. 1987). LC units reduce their activity during non-REM sleep and cease discharge for an extended period during REM sleep with its associated muscle atonia (Hobson et al. 1975; Jacobs 1986). It has been shown recently that a significant decrease of REM sleep occurred after OX-A microinjections into the LC in freely moving rats (Bourgin et al. 2000).
Wu et al. (1999) reported that LC neurons cease discharge immediately before and throughout cataplexy periods in canine narcoleptics. These results combined with our recent data showing a correlation between LC activity and muscle tone (Kiyashchenko et al. 1999; Mileykovskiy et al. 2000) indicate that LC activity contributes to the regulation of muscle tone across the sleep cycle and to the facilitation of motor activity during waking. The current results lead us to hypothesize that the major descending projections of the OX system to the LC facilitates muscle tone, perhaps especially during emotionally linked movements with correlated motor inhibition.
We have observed hindlimb muscle-tone inhibition as a result of OX-A and -B microinjections into the PIA. The PIA is part of an inhibitory brain stem-reticulospinal system hyperpolarizing spinal motoneurons (Jankowska et al. 1968; Siegel et al. 1991; Takakusaki et al. 1994). This pontine region participates in induction of REM-sleep atonia (Chase and Morales 1990; Lai and Siegel 1990a,b; Sakai et al. 1979) and causes cessation of discharge in LC neurons during electrical stimulation (Mileykovskiy et al. 2000). Neurons located in the mesencephalic locomotor region and related to muscle-tone facilitation (Garcia-Rill and Skinner 1988) are also inhibited by PIA electrical stimulation (Mileykovskiy et al. 2000). The PIA has a low OX fiber innervation (Nambu et al. 1999; Peyron et al. 1998). However, this region is a very effective inhibitor of muscle tone (Kohyama et al. 1998; Lai and Siegel 1990a,b; Siegel et al. 1983) and even a low level of OX innervation of the PIA may be sufficient to trigger potent reticulospinal inhibition.
We also propose that OXs may act on their presynaptic receptors located on glutamatergic axons and modulate brain stem neuron activity by presynaptic glutamatergic release as it was described for hypothalamic cells in addition to their direct postsynaptic action (Van den Pol et al. 1998). The LC receives glutamatergic inputs from nucleus paragigantocellularis (Ennis and Aston-Jones 1988). Ultrastructural and intracellular studies have indicated that glutamate may locally modulate activity in noradrenergic LC neurons through N-methyl-D-aspartate (NMDA) and non-NMDA receptors (Luque et al. 1995; Sengoku et al. 1999; Van Bockstaele and Colago 1996). The PIA receives dense glutamatergic projections from the mesencephalic reticular nucleus, the retrorubral nucleus, and the ventral portion of the paralemniscal tegmental field (Lai et al. 1993). Glutamate microinjections into the PIA evoked muscle-tone suppression in decerebrate cats (Lai and Siegel 1991). Ultrastructural studies of pontine nuclei revealed that asymmetric glutamatergic synaptic contacts are located on dendrites, neuronal somata and axons (Border and Mihailoff 1991). Our hypothesis that OXs may modulate brain stem neuron activity by presynaptic glutamatergic release is supported by data about the similar latency of muscle-tone facilitation and inhibition (50–120 s) after injections of small doses of glutamate agonists into the pontomedullary region (Lai and Siegel 1991). The latency of muscle-tone facilitation and LC unit firing rate increase (2–3 min) after OX microinjections into fourth ventricle is presumably related to the diffusion of drugs from the ventricle into the LC.
At present only two OX receptor types have been identified. However, the possibility that other OX receptor types exist has not yet been ruled out. Other receptors with a similar response to OX-A and -B could be responsible for the similar response profile to these ligands. Alternatively, the relative selectivity of these receptors may not be sufficient to discriminate between OX-A and -B when applied by microinjection.
We have recently shown that a massive loss of OX neurons is linked to narcolepsy (Thannical et al. 2000). The loss of OX input to the LC would reduce a major facilitatory drive to the LC. We hypothesize that a loss of this facilitatory input allows phasic motor inhibition elicited by emotion to cause a loss of muscle tone (cataplexy). In neurologically normal individuals, phasic OX release prevents these losses of tone.
The role of OX in the control of both LC and PIA suggests that a malfunction of this system can affect both motor facilitation and motor inhibition. In this regard, it is interesting to note that many narcoleptic patients not only experience sudden losses of muscle tone in waking (cataplexy) but also have a pathological absence of muscle atonia during REM sleep, producing the REM sleep behavior disorder (Schenck and Mahowald 1992). Malfunction of the OX system may be responsible for both of these symptoms of narcolepsy.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants HL-41370 and HL-60296 and by the Medical Research Service of the Department of Veterans Affairs.
REFERENCES
- Andre P, Horn E, and Pompeiano O Microinjections of GABAergic agents in the locus coeruleus modify the gain of vestibulospinal reflexes in decerebrate cats. Arch Ital Biol 133: 47–75, 1995. [PubMed] [Google Scholar]
- Aston-Jones G and Bloom FE Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in sleep-waking cycle. J Neurosci 1: 876–886, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border BG and Mihailoff GA Glutamate immunoreactivity in the rat basilar pons: light and electron microscopy reveals labeled boutons and cells of origin of afferent projections. Neuroscience 45: 47–61, 1991. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte, Criado JR, Sutcliffe JG, Henriksen SJ, and de Lecea L Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci 20: 7760–7765, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase MH and Morales FR The atonia and myoclonia of active (REM) sleep. Annu Rev Physiol 41: 557–584, 1990. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammelli T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, and Yanagisawa M Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98: 437–451, 1999. [DOI] [PubMed] [Google Scholar]
- Cutler DJ, Morris R, Sheridhar V, Wattam TA, Holmes S, Patel S,Arch JR, Wilson S, Buchingham RE, Evans ML, Leslie RA, and William SG Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides 20: 1455–1470, 1999. [DOI] [PubMed] [Google Scholar]
- D’Ascanio P, Horn E, Pompeiano O, and Stampacchia G Injections of beta-adrenergic substances in the locus coeruleus affect the gain of vestibulospinal reflexes in decerebrate cats. Arch Ital Biol 127: 187–218, 1989. [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, and Nakazato M Orexins, orexinergic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA 96: 748–753, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lecea L, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett FS II, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, and Sutcliffe JG The hypocretins: hypothalamic-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95: 322–327, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CM, Abusana S, Sunter D, Murphy KG, Ghatei MA, ANDBloom SR The effect of orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol 160: R7–R12, 1999. [DOI] [PubMed] [Google Scholar]
- Ennis M and Aston-Jones G Activation of locus coeruleus from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain. J Neurosci 8: 3644–3657, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, and Bloom FE Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA 77: 3033–3037, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ and Barnes CD Evidence of facilitatory coeruleospinal action in lumbar motoneurons of cats. Brain Res 216: 299–311, 1981. [DOI] [PubMed] [Google Scholar]
- Fung SJ and Barnes CD. Membrane excitability changes in hindlimb motoneurons induced by stimulation of the locus coeruleus in cats. Brain Res 402: 230–242, 1987. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Chan JY, Manzoni D, White SR, Lai YY, Strahlendorf HK, Zhuo H, Liu RH, Reddy VK, and Barnes CD Cotransmitter-mediated locus coeruleus action on motoneurons. Brain Res Bull 35: 423–432, 1994. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Manzoni D, Chan JY, Pompeiano O, and Barnes CD Locus coeruleus control of spinal motor output. Prog Brain Res 88: 395–409, 1991. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E and Skinner RD Modulation of rhythmic function in the posterior midbrain. Neuroscience 27: 639–654, 1988. [DOI] [PubMed] [Google Scholar]
- George R, Haslett WL, and Jenden DJ A cholinergic mechanism in the brainstem reticular formation: induction of paradoxical sleep. Int J Neuropharmacol 3: 541–552, 1964. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The coeruleospinal noradrenergic neurons: anatomical and electrophysiological studies in the rat. Brain Res 189: 121–133, 1980. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S,Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hather JP, Hather PD, Jones DNC, Smith MI, Piper DC, Hunter AJ, Porter RA, and Upton N Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Neurobiology 96: 10911–10916, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnik T, Lai YY, and Siegel JM Atonia related regions in the rodent pons and medulla. J Neurophysiol 84: 1942–1948, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley K and Morrison AR A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Acta Neurobiol Exp 34: 215–232, 1974. [PubMed] [Google Scholar]
- Hobson JC, Mccarley RW, and Wyzinski PW Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science 189: 55–58, 1975. [DOI] [PubMed] [Google Scholar]
- Horn ED, D’Ascanio P, Pompeiano O, and Stampacchia G Pontinereticular origin of cholinergic excitatory afferents to the locus coeruleus controlling the gain of vestibulospinal and cervicospinal reflexes in decerebrate cats. Arch Ital Biol 125: 273–304, 1987. [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, and van den Pol AN Hypocretin (orexin) activation and synaptic innervation of locus coeruleus noradrenergic system. J Comp Neurol 415: 145–159, 1999. [PubMed] [Google Scholar]
- Ida T, Nakahara K, Katayama T, Murakami N, and Nakazato M Effect of lateral cerebroventricular injection of appetite-stimulating neuropeptide, orexin and neuropeptide Y, on the various behavioral activities of rats. Brain Res 821: 526–529, 1999. [DOI] [PubMed] [Google Scholar]
- Ivanov AY and Aston-Jones G Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport 11: 1755–1758, 2000. [DOI] [PubMed] [Google Scholar]
- Jacobs BL. Single unit activity of locus coeruleus neurons in behaving animals. Prog Neurobiol 27: 184–194, 1986. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lund S, Lunberg A, and Pompeiano O Inhibitory effect evoked through ventral reticulospinal pathways. Arch Ital Biol 106: 124–140, 1968. [PubMed] [Google Scholar]
- Jouvet M and Delorme F Locus coeruleus et sommeil paradoxal. C R Soc Biol 159: 895–899, 1965. [Google Scholar]
- Kilduff TS and Peyron C The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci 23: 359–365, 2000. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Gulyani SA, and Siegel JM Locus coeruleus and dorsolateral pontine area unit activity during atonia induced by stimulation of the medial medulla in decerebrate rats. Soc Neurosci Abstr 25: 626, 1999. [Google Scholar]
- Kohyama J, Lai YY, and Siegel JM Inactivation of the pons blocks medullary-induced muscle tone suppression in the decerebrate cat. Sleep 21: 695–699, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Clements JR, and Siegel JM Glutamatergic and cholinergic projections to the pontine inhibitory area identified with horseradish peroxidase retrograde transport and immunohistochemistry. J Comp Neurol 336: 321–330, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY and Siegel JM Cardiovascular and muscle tone changes producedby microinjection of cholinergic and glutamatergic agonists in dorsolateral pons and medial medulla. Brain Res 514: 27–36, 1990a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY and Siegel JM Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation. J Neurosci 10: 2727–2734, 1990b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY and Siegel JM Medullary region mediating atonia. J Neurosci 8: 4790–4796, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY and Siegel JM Ponto-medullary glutamate receptors mediating locomotion and muscle tone suppression. J Neurosci 11: 2931–2937, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Strahlendorf HK, Fung SJ, and Barnes CD The action of two monoamines on spinal motoneurons from stimulation of the locus coeruleus in the cat. Brain Res 484: 268–272, 1989. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, and Mignot E The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98: 365–376, 1999. [DOI] [PubMed] [Google Scholar]
- Lubkin M and Stricker-Krongrad A Independent feeding and metabolic actions of orexins in mice. Biochem Biophys Res Commun 253: 241–245, 1998. [DOI] [PubMed] [Google Scholar]
- Luque JM, Malherbe P, and Richards JG Localization of NMDA receptor subunit mRNAs in the rat locus coeruleus. Brain Res Mol Brain Res 29: M224–232,1995. [DOI] [PubMed] [Google Scholar]
- Mitsuma T, Hirooka Y, Kayama M, Mori Y, Yokoi Y, Izumi M, Rhue N, Ping J, Adachi K, Ikai R, Kawai N, Nakayashiki A, and Nogimori T Radioimmunoassay for hypocretin-2. Endocrine Regul 33: 23–27, 1999. [PubMed] [Google Scholar]
- Mitsuma T, Hirooka Y, Kayama M, Mori Y, Yokoi Y, Rhue N, Ping J, Izumi M, Ikai R, Adachi K, and Nogimori T Radioimmunoassay for orexin A. Life Sci 66: 897–904, 2000. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Kodama T, Lai YY, and Siegel JM Activation of pontine and medullary motor inhibitory regions reduces discharge in neurons located in the locus coeruleus and the anatomical equivalent of the midbrain locomotor region. J Neurosci 20: 8551–8558, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Sakurai T, Nambu T, Yanagisawa M, and Goto K Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci Lett 264: 101–104,1999. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, and Goto K Distribution of orexin neurons in the adult rat brain. Brain Res 827: 243–260, 1999. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, and Mignot E Hypocretin (orexin) deficiency in human narcolepsy. Lancet 355: 39–40, 2000. [DOI] [PubMed] [Google Scholar]
- Paxinos G and Watson C The Rat Brain in Stereotaxic Coordinates. San Diego: Academic, 1997. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, and Kilduff TS Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano O Vasopressin in the locus coeruleus and dorsal pontine tegmentum affects posture and vestibulospinal reflexes. Prog Brain Res 119: 537–554,1998. [DOI] [PubMed] [Google Scholar]
- Pompeiano O, Stampacchia G, Horn E, and D’Ascanio P The role of the locus coeruleus in the gain regulation of vestibulospinal reflexes. Acta Otolaringol (Stockh) 103: 404–409, 1987. [PubMed] [Google Scholar]
- Pu S, Jain MR, Kalra PS, and Kalra SP Orexins, a novel family of hypothalamic neuropeptides, modulate pituitary luteinizing hormone secretion in an ovarian steroid-dependent manner. Regul Pept 78: 133–136, 1998. [DOI] [PubMed] [Google Scholar]
- Rispoli V, Lopilato R, Priolo E, David E, Marra R, and Nistico G Electrocortical desynchronization after microinfusion of kainic acid into the locus coeruleus in rats. Funct Neurol 9: 203–208, 1994. [PubMed] [Google Scholar]
- Sakai K, Sastre JP, Salvert D, Touret M, Tohyama M, and Jouvet M Tegmentoreticular projections with special references to the muscular atonia during paradoxical sleep in the cat: an HRP study. Brain Res 176: 233–254, 1979. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Williams SC, Richardso JA, Kozlovski GP, Wilson S, Arch JRS, Buchingam RE, Haynes AC, Carr SA, Annan RS, Mcnulty DE, Liu WS, Terrett JA, Elshourbag NA, Bergsma DJ, and Yanagisawa M Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 573–585, 1998. [DOI] [PubMed] [Google Scholar]
- Samson WK, Gosnell B, Chang JK, Resch ZT, and Murphy TC Cardiovascular regulatory actions of the orexins in brain. Brain Res 831: 248–253, 1999. [DOI] [PubMed] [Google Scholar]
- Schenck CH and Mahowald MW Motor dyscontrol in narcolepsy: rapid-eye-movement (REM) sleep without atonia and REM sleep behavior disorder. Ann Neurol 32: 3–10, 1992. [DOI] [PubMed] [Google Scholar]
- Sengoku A, Mori M, Okada Y, and Kamidono S Glutamate-induced currents in acutely dissociated guinea pig locus coeruleus neurons. Kobe J Med Sci 45: 85–92, 1999. [PubMed] [Google Scholar]
- Siegel JM. Narcolepsy: a key role for hypocretins. Cell 98: 409–412, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, Paul R, Shiromani P, Dement WC, Mignot E, and Chiu C Neuronal activity in narcolepsy: identification of cataplexy-related cells in medial medulla. Science 252: 1315–1318, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, and Tomaszewski KS Rostral brainstem contributes to medullary inhibition of muscle tone. Brain Res 268: 344–348, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampacchia G, Barnes CD, D’Ascanio P, and Pompeiano O Effects of microinjection of a cholinergic agonist into the locus coeruleus on gain of vestibulospinal reflexes in decerebrate cats. Arch Ital Biol 125: 107–138, 1987. [PubMed] [Google Scholar]
- Sweet DC, Levine AS, Billington CJ, and Kotz CM Feeding response to central orexins. Brain Res 821: 535–538, 1999. [DOI] [PubMed] [Google Scholar]
- Taheri S, Mahmoodi M, Opacka-Juffry J, Gnatei MA, and Bloom SR Distribution and quantification of immunoreactive orexin A in rat tissues. FEBS Lett 457: 157–161, 1999. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Okumura T, Yamada H, and Kohgo Y Stimulation of gastric acid secretion by centrally administered OX-A in conscious rats. Biochem Biophys Commun 254: 623–627, 1999. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Shimoda N, Matsuyama K, and Mori S Discharge properties of medullary reticulospinal neurons during postural changes induced by intrapontine injections of carbachol, atropine and serotonin and their functional linkages to hindlimb motoneurons in cats. Exp Brain Res 99: 361–374, 1994. [DOI] [PubMed] [Google Scholar]
- Thannical TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, and Siegel JM Reduced number of hypocretin neurons in human narcolepsy. Neuron 27: 469–474, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Hong Y, Macneil DJ, van der Ploeg LHT, and Guan X-M Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438: 71–75, 1998. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ and Colago EE Ultrastructural location of the kainate selective glutamate receptor in noradrenergic perikarya and dendrites of the nucleus locus coeruleus in the rat brain. Brain Res 732: 223–231, 1996. [DOI] [PubMed] [Google Scholar]
- Van den Pol A, Gao X-B, Obrietan K, Kilduff TS, and Belousov A Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci 18: 7962–7971, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MF, Gulyani SA, Yai E, Mignot E, Phan B, and Siegel JM Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience 91: 1389–1399, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


