Abstract
Understanding the evolution and function of the earliest immune responses following HIV infection could inform future vaccines and treatments, and provide insights for combating other viral infections. Recent prospective human studies in at-risk populations have afforded critical vantages into the earliest moments after HIV detection, revealing the onset of immune dysfunction within days-to-weeks after infection. Transcriptomic and proteomic studies highlight interconnected multicellular and multi-cytokine/chemokine responses prior to and following peak viremia. Treatment at onset of viremia mitigates peripheral T and B cell dysfunction, limits seroconversion, and enhances cellular antiviral immunity despite persistence of infection in lymphoid tissues. Further application of high-throughput genomic technologies to these samples, alongside targeted non-human primate studies, will elucidate immune response features to target in novel preventions and cures.
Introduction
More than four decades after the first cases of acquired immunodeficiency syndrome (AIDS), we still lack effective human immunodeficiency virus (HIV) vaccines and curative treatments. The vast majority of those infected require daily anti-HIV therapy to stave off disease, with long-term adherence and uninterrupted access to treatment remaining ongoing global challenges.
Much of what is known about HIV infection comes from studying human samples from chronic infection or utilizing non-human primate (NHP) models with natural or human engineered AIDS virus variants – simian immunodeficiency virus (SIV) and simian-human (SHIV), respectively (Estes et al., 2018; Garcia-Tellez et al., 2016). NHP models have demonstrated that features of initial pathology in the days immediately following infection can predict overall disease outcome and that tissue damage begins prior to the onset of plasma viremia (Evans and Silvestri, 2013; Policicchio et al., 2016). A more detailed understanding of host-pathogen interactions in humans during the entirety of the acute infection window, however, had been limited by the difficulty in screening and sampling at-risk populations during the earliest days following exposure, before peak viremia is achieved (McMichael et al., 2010). Characterizing acute HIV infection (AHI) in humans, especially relative to pre-infection, to identify responses linked to disease course is critical to inform future vaccines and therapeutics. More broadly, HIV could serve as a model for acute human viral infections in general (Hargreaves et al., 2020; Robb and Ananworanich, 2016), given its historied role in establishing many modern concepts in immunology (Abbott et al., 2018; Colomer-Lluch et al., 2018; Hughes and Andersson, 2015; Youngblood et al., 2012) and the native heterogeneity in disease course, ranging from progression to natural viral control (Walker and Yu, 2013).
In recent years, a combination of new technologies and the ability to perform longitudinal studies of uninfected persons in areas of high incidence have provided new insights regarding immune and viral dynamics from the onset of plasma viremia. One example is the Females Rising through Education, Support, and Health (FRESH) study in South Africa, in which uninfected 18-23 year old women at high risk of infection are monitored twice weekly as part of an HIV prevention and poverty alleviation project (Dong et al., 2018). Others include the RV217 and RV254 studies in Thailand (Ananworanich et al., 2017; Robb et al., 2016), which rely on screening of blood donations for persons who are HIV RNA and/or antigen positive and antibody negative. These peripheral blood mononuclear cell (PBMC) and plasma samples have provided some of the first insights into human immune responses immediately following detectible infection. Moreover, application of novel transcriptomic and proteomic technologies, including single-cell RNA sequencing (scRNA-seq) and single-cell mass cytometry (CyTOF) (Coindre et al., 2018; Kazer et al., 2020; Sannier et al., 2020), to these and other rare samples has begun to generate more comprehensive, longitudinal data from the AHI timeframe than ever before. Early treatment arms in these studies, where participants initiate antiretroviral therapy (ART) immediately following their first positive plasma viremia test, have also begun to contextualize the effects of limiting acute antigen exposure on cellular and molecular responses, pinpointing dysfunctional immune activity and highlighting the impact of modulating host-pathogen interactions during peak plasma viremia.
Here, we review emerging data on the evolution of AHI in humans from the time of onset of plasma viremia, with inclusion of relevant data from NHP models. Using studies spanning both hyperacute (prior to peak viremia; i.e., approximately less than one month following transmission) and late-acute infection (approximately one to six months post-transmission; see Figure 1a), we review the timing, identity, and function of the diverse immune responses induced. We also discuss the effects of early anti-retroviral therapy (ART) administration on these responses and highlight pertinent areas for future investigation, including cure research, as well as novel technological approaches that can be used to maximize the amount of information extracted from these precious primary human samples.
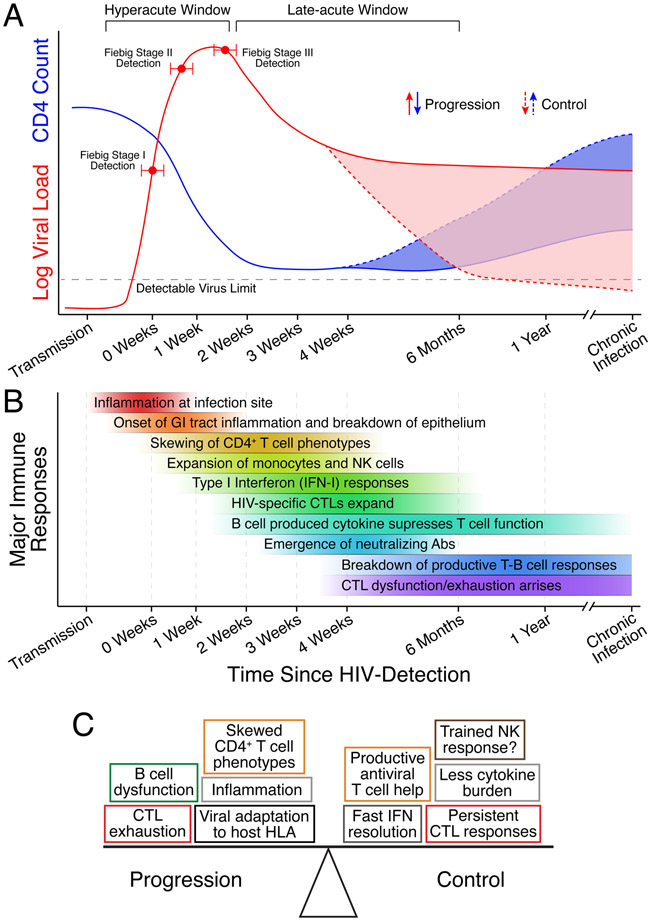
Figure 1. Acute HIV infection dynamics.
(A) Representative dynamics of plasma viral load and peripheral CD4+ T cell counts during acute HIV infection. Individuals that develop control of HIV without ART can maintain low chronic viral setpoint and reestablish pre-infection CD4+ T cell levels (Elite or Viremic Controllers). Individuals and animals can also maintain high chronic viral setpoint but also recover CD4+ T cell counts (Long-Term Non-Progressors [LTNP], and natural hosts, respectively). (B) Representative immune responses and their timing along the course of acute HIV infection. Without structured cohorts to reliably sample before Fiebig Stage I, the earliest responses are inferred from SIV/SHIV models and limited human studies. (C) Cellular and molecular factors in AHI that contribute to disease progression or control. The progression-control spectrum has been linked to the magnitude, timing, and function of several distinct immune responses. Box colors represent different cell types or cytokine responses.
Rapid systemic dissemination of infection and induction of pro-inflammatory responses upon infection
The very earliest mucosal events in HIV infection have largely been inferred from tissue studies of SIV infection in NHP. Studies of viral dynamics in models of mucosal challenge have demonstrated that intravaginal and intrarectal SIV infection lead to local viral replication within hours (Haase, 2011; Santos et al., 2011) and systemic spreading by 1-3 days in the majority of animals (Barouch et al., 2016; Deleage et al., 2019; Rabezanahary et al., 2020). This is accompanied by a robust systemic pro-inflammatory response with induction of innate immune activity including components of the inflammasome, which is thought to contribute to suppression of host cell antiviral restriction factors favoring viral replication (Barouch et al., 2016; Lu et al., 2014). The molecular and cellular events that enable and facilitate viral transmission through mucosal barriers have been reviewed recently elsewhere (Gonzalez et al., 2019).
Following initial infection and local tissue inflammation, both pro-inflammatory and antiviral cytokines and chemokines are robustly upregulated systemically before onset of plasma viremia and during viral expansion (Figure 1b) (Katsikis et al., 2011; Keating et al., 2016; McMichael et al., 2010; Muema et al., 2020; Stacey et al., 2009). Onset of plasma viremia in humans is characterized by increased levels of IFN-α, CXCL10 (formerly termed IP-10), IL-8, IL-10, IL-12 and CCL2 in the periphery (Muema et al., 2020; Stacey et al., 2009). Interspecies studies of pathogenic SIV infection (Rhesus Macaque; leads to AIDS) compared to non-pathogenic SIV infection (African Green Monkey; persistent viremia without immunodeficiency) highlight that the timing of the pro-inflammatory and antiviral responses in tissues and the periphery associate with disease progression (Bosinger and Utay, 2015; Bosinger et al., 2012). In non-pathogenic SIV infection, inflammatory responses quell within days post-infection and upregulated interferon stimulated genes (ISGs) return to baseline within 30 days post infection, in stark contrast to pathogenic infection, where these responses are prolonged for weeks and years, respectively (Bosinger and Utay, 2015), highlighting the need to better understand early events in AHI and how they correspond to long-term outcome. Relatedly, longitudinal multicellular single-cell RNA-sequencing (scRNA-seq) of FRESH study participants confirmed a ubiquitous ISG response to HIV in the hyperacute window (Kazer et al., 2020). In NHP, higher levels of cytokines like IFN-α, IFN-γ, IL-18, IL-1β, and others associated with pathogenic infection suggest that the extent of inflammatory and interferon (IFN)-related cytokine production during acute infection may contribute to long-term immunodeficiency (Keating et al., 2016) (see Box 1 for further discussion of pro-inflammatory and antiviral responses and insights from NHP models). A similar study in humans demonstrated significant correlations between peak levels of IFN-a and soluble IL-2R during hyperacute infection and peak viral load (Muema et al., 2020). Higher levels of peripheral CXCL10, which modulates immune responses by activation and recruitment of leukocytes, have also been shown to correlate with disease progression in humans (Jiao et al., 2012; Liovat et al., 2012). Thus, the timing and magnitude of pro-inflammatory and antiviral responses within tissues and the periphery may tip the balance of future disease progression or control (Figure 1c); similarly, in certain respects, NHP models can recapitulate critical features of human infection.
Box 1: Inflammation and Type-I IFN signaling in progressive and non-progressive SIV infection models.
Early events in the GI tract may be particularly revealing regarding the extent of local and peripheral cytokine response, since this is the site of the majority of lymphoid tissue in the body (Brenchley et al., 2006; Redd et al., 2009). Acute infection leads to dramatic depletion of both activated and resting CD4+ T cells in the gut, already evident within 4 days of experimental NHP infection (Li et al., 2005; Mattapallil et al., 2005). This massive cell death during hyperacute infection manifests chronic inflammation and permanent damage to the gut epithelial barrier, promoting microbial translocation and IL-17 production in the tissues (Klase et al., 2015; Klatt et al., 2010). SIV infection of African green monkeys (non-progressive infection), compared to Rhesus Macaques (progressive infection) revealed induction of inflammation-induced genes TNF-α and IL10 in peripheral blood mononuclear cells (PBMCs) and LN lymphocytes at 5 and 10 days post infection only in non-progressive infection, suggesting that early inflammation may reduce antiviral signaling and lead to further CD4+ T cell depletion. Indeed, characterization of the GI tract during hyperacute SIV infection in African green monkeys demonstrated reduced apoptosis of both enterocytes and lymphocytes despite transient increases in pro-inflammatory cytokines and chemokines in the plasma and increased T-cell activation in the blood (Raehtz et al., 2020). RNA-seq analysis confirmed limited transient antiviral and T-cell activation programming during the first two weeks of infection, but no genes associated with microbial translocation, which was corroborated by lipopolysaccharide immunohistochemistry. Thus, one path to reduce overall disease burden could be protection of the gut epithelium to resist infection and inflammatory signaling, potentially by modulating resident innate lymphoid cells (Shah et al., 2017), macrophages (Swan et al., 2017), or the epithelial cells themselves (Ordovas-Montanes et al., 2020).
Persistent Type-I IFN signaling in pathogenic SIV and HIV infection can lead to broad dysfunction in both innate and adaptive immunity during chronic infection (Bosinger and Utay, 2015; Cheng et al.; Soper et al., 2017; Wang et al., 2017; Zhen et al., 2017). Understanding how levels and timing of the initial inflammation at the site of infection, GI tract, and in the periphery impacts when and how antiviral Type-I IFN responses form and ultimately transition from productive to dysfunctional (Wang et al., 2017) is crucial to mitigate chronic inflammation and deter sustained Type-I IFN responses. Given the roles of tissue resident macrophages (Grainger et al., 2017), DCs (Sun et al., 2020), ILCs (Branzk et al., 2018), and epithelial cells themselves (Okumura and Takeda, 2017) in maintaining tissue homeostasis and propagating both inflammatory and Type-I IFN signaling in the GI tract, further work is needed to understand how their depletion or perturbation in AHI impacts outcome.
Alongside rapid viral spreading and inflammation following transmission, HIV integrates into the genome of CD4+ T cells that then transition into resting memory cells, creating a persistent viral reservoir (Davey et al., 1999). If ART is interrupted in HIV infected individuals, infection can resume from latent viruses hidden in these resting memory cells (Chun et al., 1997; Finzi et al., 1997; Wong et al., 1997). The viral reservoir is established within days after infection, as ART initiated at Fiebig Stage I still permits viral rebound after treatment interruption (Colby et al., 2018). Indeed, in a pathogenic SIV infection model, the viral reservoir is seeded before detectible plasma viremia; the reservoir is established in the GI tract no later than 3 days post infection (Cantero-Pérez et al., 2019; Whitney et al., 2014, 2018), and in splenic and mesenteric LNs as early as 4 days post infection, and is preferentially harbored in T follicular helper (Tfh) and T effector memory cells (Rabezanahary et al., 2020). Sequencing of viral RNA/DNA from the central nervous system of acutely infected individuals demonstrated distinct mutations from transmitter/founder viruses, providing further evidence for early establishment of viral sanctuaries, tissue sites that facilitate persistent viral replication despite successful ART treatment (Tovanabutra et al., 2019). NHP studies showing exclusion of CD8+ T cells from germinal centers (GCs) in lymph nodes (LNs) and secondary lymphoid organs implicate these structures as important viral reservoirs and potential viral sanctuaries that likely contributes to ongoing chronic inflammation (Fukazawa et al., 2015).
Innate immune cells respond during hyperacute infection and orchestrate the initial antiviral response
Innate immune cells are the first-line defense against previously unencountered foreign pathogens and subsequently instruct adaptive immunity (Altfeld and Gale Jr, 2015; Iwasaki and Medzhitov, 2015). Nevertheless, the roles of these cells throughout AHI are not completely understood. Here, we describe what is known about various innate immune cell subsets in hyperacute infection and highlight what remains to be elucidated.
Early work in NHP models pinpointed plasmacytoid dendritic cells (pDCs) as instigators of systemic antiviral Type-I IFN production following infection. After sensing viral RNA/DNA by TLR7 and TLR8, pDCs produce IFN-α and IFN-β in LNs and the GI tract (O’Brien et al., 2013). A treatment interruption study in ART-adherent individuals with undetectable viremia who began ART therapy at onset of plasma viremia demonstrated that pDCs upregulate migration and activation markers before detectible viremia but exhibit a transient decline (compared to prior to treatment) in their ability to produce IFN-α in vitro (Mitchell et al., 2020). Multicellular analysis in the FRESH study also showed that pDCs do not express Type-I IFN genes or Interferon Regulatory Factor 7 (IRF7) prior to peak viremia but upregulate ISGs and signaling factor genes, supporting a hypothesis that pDC function in the periphery may be to recruit other immune cells rather than to produce IFN-α/β (Kazer et al., 2020). Nevertheless, multiple other peripheral immune subsets upregulate IRF7 during hyperacute infection, suggesting that while pDCs likely play an important role in directing IFN signaling in LNs, both direct viral sensing and IFN-feedback from other cell types contribute to the overall peripheral response. Novel, low-input genomic technologies (Corces et al., 2017; Skene and Henikoff, 2017; Stubbington et al., 2017) will provide new opportunities to study pDCs from tissue biopsies, fine need aspirates, and small amounts of blood to better understand their compartment specific roles (see Box 2 for a brief description of these technologies and their utility in studying HIV).
Box 2: Novel “-omic” technologies and their utility in studying acute HIV infection.
With the advent of next generation sequencing and steady improvements in low-input and single-cell profiling technologies, high-content measurements of gene expression, protein expression, open chromatin, and other cellular features are becoming increasingly accessible and affording substantial insights into disease biology in heterogeneous tissue environments (Hartmann and Bendall, 2020; Shema et al., 2019; Stubbington et al., 2017). Critically, many of these assays can be used to study rare cell types from low-input samples like tissue biopsies, fine needle aspirates, and small amounts of blood. Below, we briefly describe some of these new technologies, their use thus far in HIV infection, and some of what we stand to learn through future applications. For a more extensive discussion of single-cell technologies that can be applied to study tissues in human disease, please see Slyper et al and Rozenblatt-Rosen et al. (Rozenblatt-Rosen et al., 2020; Slyper et al., 2020).
Single-cell RNA-sequencing (scRNA-seq):
High-throughput scRNA-seq methods, using droplets (Macosko et al., 2015; Zheng et al., 2017), picowells (Gierahn et al., 2017; Han et al., 2018), or split-pool approaches (Cao et al., 2017; Rosenberg et al., 2018), enable simultaneous whole transcriptome measurements of thousands of single cells. We have applied scRNA-seq to blood samples in AHI to discern multicellular responses and identify subsets of innate immune cells associated with viral control (Kazer et al., 2020). In chronic infection settings, heterogeneity in T cell phenotype has also been explored (Liu et al., 2020; Sannier et al., 2020). Use in LN and gut tissues may help discern response features associated with CTL exclusion from GCs or polyfunctionality and clonality (Azizi et al., 2018; Tu et al., 2019) of CD4+ T cells, as well as tissue-level adaptations to the temporary or permanent loss of direct viral targets or virally-/antiretrovirally-driven inflammation.
Single-cell Mass Cytometry (CYToF):
Multiplexed measurement of protein expression from single cells using mass cytometry (Atkuri et al., 2015) has expanded the range of traditional single-cell measurements like flow cytometry to over 50 simultaneous protein and RNA markers. Applied to measure NK cell diversity (Strauss-Albee et al., 2015) and CD4+ T cell infection susceptibility (Manganaro et al., 2018), its potential to understand changes in cellular protein expression during AHI is just emerging. Combined with imaging (Baharlou et al., 2019), it will be possible, for example, to discern cell-cell interactions in tissues with on-going HIV infection and validate the roles of DCs in orchestrating pro-inflammatory and antiviral signaling.
Assay for transposase-accessible chromatin by sequencing (ATAC-seq):
Technological advances allowing for low-input (Corces et al., 2017) and single-cell (Buenrostro et al., 2015; Cusanovich et al., 2015) measurements of chromatin accessibility have begun to link phenotype and gene expression to epigenetic state across immune cells in disease settings (see review by (Shema et al., 2019)). Thus far, ATAC-seq has been used to understand changes to chromatin accessibility in central memory and effector memory CD4+ T cells (Einkauf et al., 2019) and DCs (Johnson et al., 2018) from HIV-infected individuals. Further application to both innate and adaptive immune subsets before and throughout AHI, in the presence and absence of prophylactic interventions, will help determine how innate immune cells may acquire protective responses [e.g. trained immunity or memory; (Netea et al., 2020; Wang et al., 2020)] and ascertain when and where T cells begin to acquire features of exhaustion, among other insights.
Cleavage Under Targets (CUT) for chromatin profiling:
CUT&RUN for low-input samples (Skene and Henikoff, 2017) and now CUT&Tag for single-cell samples (Kaya-Okur et al., 2019) enable efficient, detailed profiling of diverse chromatin components like histone modifications and transcription factor binding sites. CUT&RUN demonstrated the role of TCF7 in generating memory NK cells during HIV infection (Wang et al., 2020), but its application to other cell types and specifically during AHI has yet to be accomplished. In addition to highlighting epigenetic marks associated with activation or silencing, this approach could help identify and validate transcription factors contributing to cellular dysfunction and nominate putative targets for restricting cytokine signaling.
Studies of peripheral cytokines upregulated in AHI [e.g. CXCL9, CXCL10, IL-10, etc.; (Jiao et al., 2012; Katsikis et al., 2011; Stacey et al., 2009)] implicate monocytes and macrophages as key players in orchestrating cellular trafficking and antiviral factor production. Comparison of complete blood counts pre-infection and at peak viremia in the FRESH study revealed expansion of monocytes following onset of plasma viremia (Muema et al., 2020). Transcriptional analysis showed that monocytes upregulate genes encoding many known pathogen recognition receptors (PRRs) and HIV-restriction factors during hyperacute infection (e.g. RIG-I, APOBEC3 family, LGALS3BP, etc.) (Kazer et al., 2020). Monocytes were also shown to upregulate CCL2 and CXCL10 within the first 4-6 days of Zika virus infection, implicating their importance in sensing and responding to viral pathogens more broadly (Michlmayr et al., 2020). Moreover, monocytes also persistently upregulate HLA-DR throughout hyperacute and late-acute infection (Chen et al., 2017; Liu et al., 2019), potentially contributing to prolonged antigenic stimulation of T cells and subsequent T cell exhaustion. Human beta defensin 1 was also demonstrated to be upregulated in circulating monocytes 3 months post infection (Corleis et al., 2017), suggesting that monocytes may encounter and recognize components of HIV directly in addition to responding to Type-I IFN and/or durable changes to myeloid progenitors may occur. Whether tissue resident macrophages play similar roles to peripheral monocytes as signal transducers in hyperacute infection, or potentially adopt more niche-specific roles (Guilliams et al., 2020; Lavin et al., 2015), needs to be further explored using NHP models and what limited tissue samples from humans are available.
Innate-like cytotoxic lymphocytes also expand and activate during hyperacute infection. Peripheral CD56dimCD16+ Natural Killer (NK) cells expand (Alter et al., 2005), activate (Naranbhai et al., 2013), and express cytolytic gene modules starting within 1-week post onset of plasma viremia (Kazer et al., 2020). Early proliferation of NK cells was also associated with subsequent viremia control in two FRESH study participants. Their role in tissues during AHI, however, is unclear. In a pathogenic vaginal SIV challenge model, NK cells were recruited to the female genital tract (FGT), albeit in small numbers, during the first week of infection, but lacked CD107a and Ki67 expression (Shang et al., 2014), indicating that they did not adhere to their traditional cytotoxic roles. Nevertheless, data in HIV models suggest that NK cells may be protective against infection. Treatment of healthy human NK cells with IL-15 superagonist in vitro before injection into humanized mice challenged with HIV inhibited infection and could directly suppress viremia in the spleen (Seay et al., 2015). Moreover, the demonstration of adaptive-like NK cells in SIV or SHIV infection restricted to Gag- and Env- cross-presenting DCs hints that these cells may play a more important role in acute infection than currently known, and could conceivably be primed to respond during acute HIV infection (Reeves et al., 2015). Indeed, Wang et al. confirmed this finding in humans, showing the expansion of CD94+ memory NK cells by TCF7 in HIV infection (Wang et al., 2020). With improved NK cell manipulation in NHP models, it will be essential to further test the potential for NK cell mediated protection during acute infection, especially before cytotoxic T lymphocyte (CTL) responses fully mature. Moreover, the realization of both adaptive and innate training in NK cells (Adams et al., 2016) makes them a potential vaccine target alongside T cells.
Both mucosal-associated invariant T (MAIT) and γδT cells change in frequency during hyperacute infection and potentially play cytolytic and regulatory roles following onset of plasma viremia. While initially shown to be depleted in late-acute infection (Cosgrove et al., 2013), a recent study demonstrated that MAIT cells actually expand and upregulate genes associated with IFN-γ production and innate mediated cytotoxic immunity during hyperacute viremia (Lal et al., 2020). Conversely, γδT cells, which are known to target conserved regions of bacteria present at mucosal surfaces, skew in proportion away from the traditionally cytotoxic subset (Vδ2) towards the tissue resident effector memory subset (Vδ1 in the periphery during AHI (Bhatnagar et al., 2017; Juno and Eriksson, 2019). In their review on the role of γδT cells throughout acute and chronic HIV infection, Juno and Eriksson indicate that the expansion of the Vδ1 subset is likely in response to increased exposure to antigens, suggesting that these cells may be responding to microbial translocation resulting from breakdown of mucosal barriers like the gut epithelium. Whether changes in MAIT and γδT cells directly affect disease trajectory in AHI through antiviral or other means is unknown.
Helper innate lymphoid cells (ILCs) are a recently described class of innate immune cells in mucosal tissues that play similar roles to T cells but are not engaged through an adaptive immune receptor (i.e., T-cell receptor), instead reacting to tissue perturbations independent of antigen stimulation (Eberl et al., 2015). Studies in HIV/SIV infection (Shah et al., 2017) and other disease settings (Ardain et al., 2019; Ebbo et al., 2017; Stehle et al., 2018) have implicated ILC2s, ILC3s, and ILC progenitors (ILCPs) in mounting productive [and sometimes deleterious (Pantazi and Powell, 2019)] immune responses upon pathogenic or inflammatory assault. Thus far, studies of these cells in AHI has been limited. Circulating ILC2s and ILC3s are irreversibly depleted during hyperacute HIV infection; transcriptionally, these cells exhibited gene programming associated with apoptosis and cell death, suggesting that they undergo cell death rather than migrating into tissues (Kløverpris et al., 2016), though the impact of their loss on disease progression is unclear. Confirming the depletion of ILCs during AHI, Wang and colleagues also tested their depletion in vitro, demonstrating that γ-chain cytokines (e.g. IL-2, IL-4, IL-15), which are known to be elevated during HIV-infection, restricted Lin−CD127+ ILC numbers through JAK3 signaling (Wang et al., 2020). This suggests that these cells are lost as bystanders in the pro-inflammatory and antiviral cytokine milieu at the onset of plasma viremia. While not yet measured in humans, SIV studies have shown depletion of ILC3s in the infant NHP gut (Hueber et al., 2020) as well as depletion and phenotypic skewing of NKp44+ ILCs during acute infection in adult gut (Li et al., 2014), and suggested protective qualities of ILC3s in adenovirus-SIV vaccination and mother-to-child transmission (Hueber et al., 2020; Rahman et al., 2019). Group 1 and 3 ILCs present in the GI tract during SIV infection potentially contribute to pro-inflammatory responses by producing higher levels of IFN-γ, TNF-α, and IL-22 during the breakdown of the epithelium (Cogswell et al., 2020); however, when these pro-inflammatory ILCs arise is unknown. Further work demonstrating functional roles for helper ILC subsets in T-cell rich tissues is necessary to determine their roles in hyperacute HIV infection and the potential of targeting these cells in vaccines and treatments.
In summary, both the myeloid and lymphocyte innate immune compartments respond robustly following onset of plasma viremia. While monocytes and pDCs clearly contribute to cell traffic signaling and amplify the Type-I IFN response (Figure 2), the immediate antiviral functions of these cells as well as innate lymphocytes are still unclear. Moreover, changes in myeloid subsets during other acute viral infections are increasingly being shown to be associated with disease outcomes (Arts et al., 2018; Heung and Hohl, 2019; Kwissa et al., 2014; Michlmayr et al., 2020). Vaccine studies demonstrating protection conferred by NK cells and ILCs (Gorini et al., 2020; Rahman et al., 2019) suggest that these cells likely play more direct antiviral roles in controlling infection spread following onset of plasma viremia than is currently understood. Critically, how the timing and the magnitude of the initial innate responses to HIV infection impact the development of productive T and B cell responses has yet to be adequately explored.
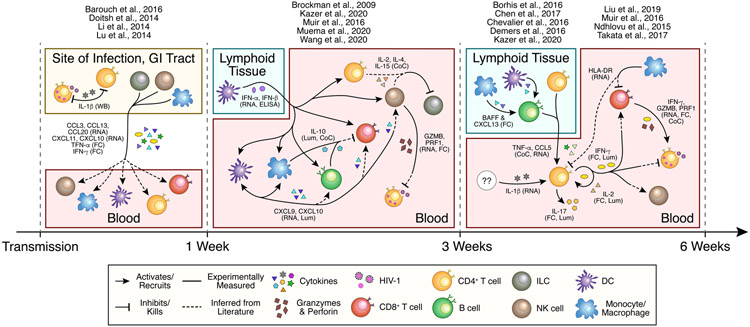
Figure 2. Known and inferred immune cell signaling during untreated acute HIV infection.
Understanding the interplay between distinct immune subsets in acute HIV infection is essential to disentangle the effects of key cytokines (e.g. Type I and II interferons, IL-10, IL-17, etc.) on each cell type. Moreover, realizing the compartment specific responses and their effects will allow for more targeted treatment. Experimentally measured interactions are depicted with solid arrows. Interactions for which only the signaler or receiver has been measured are shown by arrows with hybrid solid and dashed lines, which are inferred from literature on chronic infection or other models. The supporting assay for each interaction is listed with each cytokine or factor. GZMB = granzyme B; PRF1 = perforin; WB = Western Blot; RNA = RNA-sequencing; FC = Flow Cytometry; ELISA = Enzyme-linked Immunosorbent Assay; Lum = Luminex Multiplex Assay; CoC = in vitro coculture.
Early adaptive immune responses are dysfunctional
HIV-specific T and B cell responses arise in the days-to-weeks following peak plasma viremia and exert pressure on HIV forcing sequential immune escape mutations (Fischer et al., 2010). Both HIV-specific responses and non-HIV-specific lymphocyte responses, however, have been shown to exhibit early dysfunction, skewing in phenotype, and lack of reactivity starting in hyperacute infection (Figure 1b). Here we discuss both the productive and dysfunctional adaptive immune responses starting in hyperacute infection and throughout late-acute infection, and their potential impact on disease trajectory.
As the primary targets of HIV, CD4+ T lymphocytes are the first subset to demonstrate significant phenotypic changes during hyperacute infection. Gut homing CD4+ T cells expressing α4β7 (Arthos et al., 2008) are depleted in the GI mucosa starting at onset of plasma viremia, but are not depleted in the periphery until after peak viral load is achieved (Sivro et al., 2018). Another AHI cohort corroborated the targeted depletion of these cells, specifically T helper (Th) 17 and Th1 subsets (Lu et al., 2016b). Globally, peripheral CD4+ T cells were shown to upregulate genes associated with differentiation and downstream of pro-inflammatory cytokines IL-1β and TNF-α starting after peak viremia (Kazer et al., 2020). The phenotypes and roles of HIV-specific CD4+ T cells during AHI, however, are more diverse.
Despite the targeted loss of Th17 cells, HIV-specific CD4+ T cells produce IL-17, IFN-γ, and IL-2 during AHI (Yue et al., 2008) and in response to Gag p24 antigen ex vivo (Chevalier et al., 2016), indicating that at least a portion of Th cells are functional. However, IL-17 is not classically antiviral (Iwasaki and Medzhitov, 2015) and its production by HIV-specific CD4+ T cells may indicate skewing away from, or depletion of, the antiviral Th1 phenotype. The extent of cytokine production by HIV-specific CD4+ T cells was shown to be positively associated with the magnitude of peak viral load (Chevalier et al., 2016), though whether these cytokines effectively inhibit viral replication is unclear. In addition to higher frequencies of Gag-specific T cell responses in the late-acute widow (Schieffer et al., 2014), the frequency of cytolytic HIV-specific CD4+ T cells producing granzyme A during the hyperacute window also negatively correlated with viral load set point (Soghoian et al., 2012). These findings suggest that those CD4+ T cells that do exhibit Th1 activity may respond through diverse antiviral mechanisms variably over time. Notably, the extent of activation marker expression by memory CD4+ T cells in the periphery during late-acute infection correlated with higher CD4+ T cell counts 2 years post infection (Maenetje et al., 2010; Xia et al., 2018), indicating the importance of T cell help during AHI. However, others have shown that this association may be subset specific; Pušnik and colleagues demonstrated that frequencies of stem cell-like memory (CCR7+CD27+CD95+CD45RA+) CD4+ T cells, potentially induced by Fas upregulation, was associated with increased HIV replication and rapid disease progression (Pušnik et al., 2019). Re-polarization of Th17-like CD4+ T cells or redirection of pro-inflammatory signals during AHI may help prevent further T cell loss, misdirected T cell help, and dysfunction and promote proliferation of naïve T cells (Figure 1c).
T-B cell interactions are also inhibited during AHI, but not until late-acute infection. Investigation of Tfh cells, known to expand in chronic infection (Lindqvist et al., 2012; Roider et al., 2018), at 2 months revealed a shift in helper phenotype towards Tfh1 that negatively correlated with chronic viral set point and was predictive of p24-specific plasma IgG titers at 1 year after infection (Baiyegunhi et al., 2018). In vitro cocultures using primary cells from the RV254 study, however, demonstrated that the quality of Tfh-B cell reactions begin to deteriorate in late-acute infection, with poorer survival of resting memory B cells (CD21+CD27+) isolated from Fiebig Stage III compared to Stages I-II (i.e., earlier in infection trajectory, Figure 1a) (Muir et al., 2016). Moreover, these B cells showed skewing of cytokine production, with reduced IL-10 production and higher levels of CCL5 (RANTES) and TNF-α, highlighting B cells as a potential source of pro-inflammatory cytokines during both hyperacute and late-acute infection. Production of IL-10 by B cells during AHI (Liu et al., 2014) may have deleterious effects on early CTL responses, which are inhibited by IL-10 (Brockman et al., 2009). Secreted factors by other cells, especially macrophages and DCs, likely also influence T-B cell interactions and must be investigated further.
Relatedly, B-cell activating factor (BAFF), which has been shown to be produced by monocytes and pDCs during late-acute HIV infection (Borhis et al., 2016), has been posited to dysregulate Tfh-B cell responses in GCs (Borhis et al., 2017). Indeed, an anergic subset of CD21−CD27+IgD+ B cells (termed marginal zone B cells) was found to emerge in AHI and expand during chronic infection (Liechti et al., 2019). Treatment of SIV infected macaques throughout hyperacute infection with a BAFF antagonist, however, inhibited the proliferation of GC B cells, and delayed the upregulation of IFN-α and CXCL10 (Borhis et al., 2020), suggesting that levels of BAFF may need to be finely tuned to promote productive antibody responses. More generally, the extent of cytokine/chemokine production and signaling, rather than its presence or absence, likely tips the balance between disease and control (Figure 1c).
Peripheral B cells are activated starting in hyperacute infection and persist throughout AHI. Longitudinal sampling of acutely infected individuals in the FRESH study revealed a relative depletion of resting memory B cells from the blood during hyperacute infection alongside gradual increases in tissue-like and activated memory B cells and plasmablasts in the late-acute stage [also confirmed in other cohorts (Liechti et al., 2019; Muir et al., 2016)], consistent with increased levels of BAFF and CXCL13 in the plasma (Mabuka et al., 2017). Interestingly, levels of CXCL13 at several timepoints during AHI were predictive of the development of cross-neutralizing antibodies at 1 year[ (Mabuka et al., 2017), though the direct relationship or mechanism is unknown [NB the development of broadly neutralizing antibodies is complex and outside the scope of this review; see (Dashti et al., 2019; Sadanand et al., 2016; Wang and Zhang, 2020) for additional details]. The majority of antibodies targeting critical HIV proteins Gag, Pol, and Env produced throughout AHI are non-neutralizing (Kardava et al., 2014; Tomaras et al., 2008). Knox and colleagues, however, showed that T-bet+ B cells specifically contribute to effective HIV Env (i.e. binding protein) memory response (Knox et al., 2017), suggesting that targeted transcription factor induction may direct productive B cells. B cell mediated cytokine signaling, especially in the hyperacute infection window, must be explored further to ascertain their relative contribution to blocking successful T cell responses.
HIV-specific CD8+ T cells also activate following peak plasma viremia and are associated with enhanced control of viremia. Both the FRESH and RV254 studies have shown that HIV-specific CD8+ T cells expand in the periphery and upregulate cytotoxic effector molecules perforin and granzyme B within the first month of infection (Demers et al., 2016; Ndhlovu et al., 2015; Takata et al., 2017). The extent of this response has also been shown to associate with slower disease progression (Streeck et al., 2014), supporting the theory of protective HLA alleles against HIV (Figure 1c) (Carlson et al., 2015; Goulder and Walker, 2012). The magnitude of the activated (HLA-DR+CD38+) CD8+ cytotoxic lymphocyte (CTL) response also negatively correlated with viral load setpoint (Ndhlovu et al., 2015). Interestingly, activated HIV-specific CD8+ T cells were found to be present in cerebral spinal fluid (CSF) during AHI (Kessing et al., 2017). The ability of these cells to traffic to sites of viral persistence is likely a key factor; relatedly, SIV-specific CTLs are restricted from entry into germinal centers (GCs) in LN in both acute and chronic infection (Fukazawa et al., 2015; Li et al., 2019), indicating tissue/microenvironment specific control of their migration. The exclusion of CTLs from LN starting early in infection [though unclear in humans; (Petrovas et al., 2017)] may enhance early seeding of the reservoir in these tissues and must be further explored in HIV.
The first HIV-specific CTL responses appear to have a detectable antiviral effect, but this is not durable in most persons. CTLs sampled from five participants during late-acute infection and cocultured with primary CD4+ T cells infected with various transmitter/founder strains inhibited viral replication in vitro (Freel et al., 2012). However, when cocultured with target cells infected with HIV containing known early escape mutations, these cells relatively underperformed, suggesting that any given HIV-specific response may be limited in its usefulness over time. Indeed, recent work suggests that while CTLs can kill infected cells and induce escape mutations in HIV during late-acute infection, these cells lack cross-reactivity, are dysfunctional, and become exhausted and/or do not develop into long-term effector memory cells (Du et al., 2016; Ndhlovu et al., 2015; Takata et al., 2017). HIV-specific cells expressing CD38 but reduced levels of CD8 and CD27 within the hyperacute window were shown to have reduced efficacy in inhibiting HIV replication and made up to 40% of all HIV-specific cells (Eller et al., 2016). The expansion of these cells was associated with CD4+ T cell depletion, suggesting that a loss of CD4+ T cell help may lead to a dysfunctional CTL phenotype. Stimulating HLA-DR+CD38+ CTLs collected at various Fiebig Stages (I-III) ex vivo through the T cell receptor (TCR) revealed reduced IFN-γ, TNF-α, and IL-2 production from those sampled at Fiebig Stage III, indicating dysfunction begins within one-month post detection (Takata et al., 2017).
Impaired function of CTLs with constant antigen exposure, termed T-cell exhaustion, has been widely explored in chronic viral infections and cancer (McLane et al., 2019). Studies in acute SIV infection suggest that SIV-specific CTLs may be primed for exhaustion due to persistent high expression of PD-1 (Petrovas et al., 2007). In addition to reduced T cell help, several putative mechanisms of CTL dysfunction during AHI have been proposed including: (1) dysregulation of T-bet expression leading to lower levels of perforin (Demers et al., 2016), (2) apoptosis after activation without transition to effector memory (Ndhlovu et al., 2015), and (3) altered metabolism (Takata et al., 2017; Trautmann et al., 2012). These various dysfunctional states likely represent concerted CTL programming induced by changes in T cell help in addition to other immune signaling events. Whether dysfunction arises first in HIV-specific T cells, hindering helper and antibody responses through uncontrolled viremia, or improper help and cytokine production induce dysfunction in HIV-specific T cells, is unclear. Moreover, exacerbated viral replication in the absence of productive HIV-specific CD8+ T cell responses could further fuel functional changes to naïve T and B cells. Understanding precisely when, where, and how CTLs begin to fail is critical to develop novel interventions to prevent dysfunction during AHI (Figure 1b,c).
Collectively, the depletion and subsequent phenotype skewing of CD4+ T cells during hyperacute infection likely imparts lasting dysfunction on other adaptive lymphocytes, leading to skewed cytokine production in B cells and a pro-apoptotic phenotype in HIV-specific CD8+ T cells. Improper neutralization or killing of virally infected cells throughout AHI could lead to persistent antigen presentation, potentially by monocytes and DCs (Chen et al., 2017; Kazer et al., 2020; Liu et al., 2019), and thus contribute to early T cell exhaustion. Targeting differentiation and class-switch programming in CD4+ T cells and B cells, respectively, could enhance helper and antibody responses during AHI and promote effective CTL killing and memory generation.
Understanding multicellular immune responses throughout AHI
While recent cell-type-centric studies of various lymphocyte and myeloid cells have begun to highlight specific cell states, circulating factors, and PRRs as critical “lynch pins” to target during AHI, their relationships with one another are only beginning to be understood. We propose how the measured responses in various cell types may form both productive and dysfunctional immunity during untreated AHI, as depicted in Figure 2. Following inflammatory responses at mucosal tissue sites, peripheral myeloid cells expand and orchestrate a multicellular antiviral Type-I IFN signal to induce cell trafficking. These cells also prime B-T cell interactions through BAFF and CXCL13 production, likely in germinal centers. B cells produce pro-inflammatory cytokines, which may skew CD4+ T cell differentiation and helper function. Persistent B cell activation and uncontrolled viremia potentially inhibit neutralizing antibody production. Deficient T cell help also hinders effective and durable CD8+ T cell responses in the periphery, which in turn may allow for persistent antigenic stimulation and subsequent lymphocyte exhaustion throughout the body.
It is critical that future work incorporate longitudinal multicellular studies across tissue compartments to discern who, when, and where the balance is tipped between control and progression of viremia. In hyperacute infection, NK and MAIT cells exhibit direct antiviral activity through cytolytic killing whereas monocytes and DCs serve to recruit antiviral and inflammatory agents. Whether the loss of helper ILCs during the hyperacute window impacts disease trajectory must be further explored. Throughout the late-acute window, CD8+ T cells, CD4+ T cells, and B cells directly combat viremia through killing, IFN-γ secretion, and antibody production, respectively. IL-17 producing CD4+ T cells and activated monocytes, however, may hinder antiviral responses in this timeframe; these skewed responses may stem from pro-inflammatory signals produced in LNs and the GI tract. To understand the persistent role for various innate immune cell subsets in helping or impeding adaptive responses and/or directly fighting viremia, more granular characterization of these cells across tissue compartments will need to be performed, likely in NHP models given current standard of care (i.e., treatment at detection) and a dearth of samples. NHP models also provide opportunities to functionally test both already established cell-cell relationships, and their antiviral contributions, and those yet to be discovered.
Early initiation of ART drastically mitigates systemic and cellular immune responses during AHI in the periphery
In addition to providing insight into some of the earliest responses during untreated AHI, recent studies have provided invaluable opportunities to understand the impact of early treatment (at HIV detection, Fiebig Stage I-III) on both AHI trajectory and long-term disease progression (Ananworanich et al., 2017; Dong et al., 2018). Alongside results from NHP early treatment models and chronic infection studies, it is becoming clear that the timing of ART makes a significant difference in the dynamics and quality of antiviral immunity and disease trajectory in humans (Figure 3) (Crowell et al., 2016; Hellmuth et al., 2019; Ndhlovu et al., 2019; Schuetz et al., 2014). Critically, early treatment can be utilized as a control-of-sorts to compare against progressive infection, to identify the timing and quality of viral spread, and the impact of dysfunctional and/or overreactive immune responses during AHI. Moreover, it can be used to examine how different tissues dynamically adapt (or maladapt) to the loss of direct viral targets, potentially elevating risk for conditions associated with chronic inflammation (Deeks et al., 2013; Somsouk et al., 2015), as well as the ability of early treatment to reestablish homeostatic function across tissues.
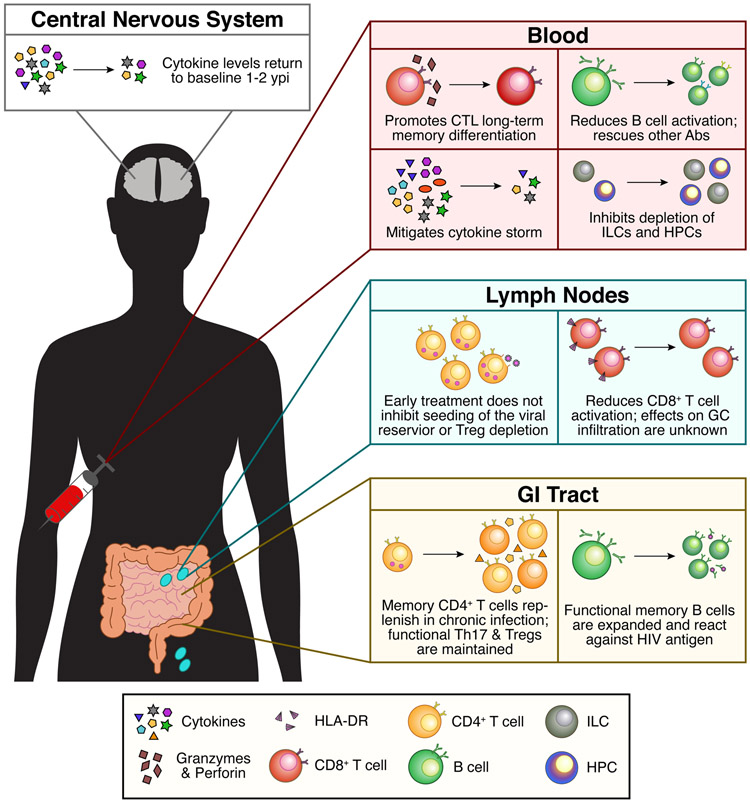
Figure 3. Effects of early ART administration during acute HIV infection on peripheral and tissue immune responses.
We are just beginning to understand the effects of early ART on AHI with improved NHP models and cohorts treating at HIV-detection throughout Fiebig Stages I-III. Overall, early ART mitigates peripheral cytokine production and adaptive immune cell dysfunction. Samples from early ART treated individuals in prospective cohorts provide a unique opportunity to explore similarities and differences in cellular response and phenotype across tissue compartments. Whether we can use our understanding of these improved antiviral responses to inform long-term cure strategies or vaccines is still unknown. Abs = antibodies; HPC = hematopoietic precursor cell.
Profiling of the viral reservoir in early treated individuals (ETIs; at onset of plasma viremia) has shown that immediate ART administration restricts the detected copies of HIV DNA (per million cells) in PBMCs, gut tissue, and LNs (Ananworanich et al., 2012; Crowell et al., 2016; Leyre et al., 2020). Long term, ETIs demonstrate lower levels of cell-associated HIV DNA 10 years after infection and faster decay of HIV infected cells in the periphery when compared to individuals treated during chronic infection (Buzon et al., 2014). An in-depth longitudinal study of 2 ETIs sampling multiple tissue sites starting from Fiebig Stage I showed that even near complete depletion of detectible HIV RNA or DNA from LN, bone marrow, and CSF through 2 years after infection in one individual (but not the other) still resulted in viral rebound after ceasing ART with HIV sequences matching those measured during AHI (Henrich et al., 2017). Given the direct association of granzyme B producing HIV-specific CTLs and reduced viral reservoir in early infection (Yue et al., 2017), treatments that facilitate CTL trafficking to lymphoid GCs in conjunction with ART may be required for HIV eradication. While early ART can mitigate the spread and magnitude of the viral reservoir, it cannot prevent seeding in humans. Thus, with current prophylactics, only pre-exposure prophylaxis, or post-exposure prophylaxis initiated within 72 hours post-infection (Barouch and Deeks, 2014), appear able to inhibit the establishment of the viral reservoir.
Given that ETIs exhibit decreased seroreactivity, and occasionally lack HIV-specific antigen and/or antibody in the periphery (Dong et al., 2018; Manak et al., 2019), the frequencies of other circulating factors like cytokines and chemokines may also be reduced or altered during AHI. Indeed, cytokine profiling in the FRESH study showed that pro-inflammatory and Type-I IFN cytokines in plasma stay closer to baseline levels in ETIs treated in Fiebig Stages I-II throughout the first month post-detection (Muema et al., 2020). ETIs in the FRESH and RV254/304 studies (Hellmuth et al., 2019; Sereti et al., 2017), however, show increased levels of CXCL13 and soluble CD14 ~36 weeks after infection and C-reactive protein, TNF, soluble IL-6R, soluble CD14, CXCL10, and CCL2 ~96 weeks after infection, compared to uninfected individuals. These persistent elevated cytokine levels suggest that even early ART administration cannot mitigate systemic changes to circulating immune factors. Moreover, increased levels of inflammatory cytokines TNF and IL-6R may reflect ongoing viral replication or tissue damage throughout the body despite mitigating systemic viral spread. Interestingly, Hellmuth and colleagues demonstrated that in the CSF, however, cytokine levels return to uninfected levels by 96 weeks (Hellmuth et al., 2019), indicating that timing of treatment also affects compartmentalization of the immune response.
Peripherally, early ART administration mitigates the emergence of dysfunctional adaptive immune cells and prevents the loss of innate lymphoid cells. ART initiation before 6 months after infection reduced peripheral CD8+ T cell counts closer to healthy levels 2 years post-infection compared to ART naïve individuals or those treated in chronic infection (Cao et al., 2016). Multiple studies demonstrated that HIV-specific CTLs in AHI from ETIs downregulate cytotoxic and activation markers and genes compared to untreated infection (Ndhlovu et al., 2019; Takata et al., 2017). Moreover, early treatment prevents apoptosis of these cells, and shifts their memory differentiation trajectory toward effector and central memory, resulting in longer lasting and more functional (IFN-γ and proliferation) CTLs. This more “normal” function may be imparted by HIV-specific CD4+ T helper cells, which demonstrate better proliferation capacity and IFN-γ production in ETIs, though the directionality of this relationship is unknown.
B cell responses are also markedly altered with early ART. B cell activation is mitigated, and resting memory cells are maintained near baseline levels (Mabuka et al., 2017; Moir et al., 2010; Muir et al., 2016), potentially due to reduced cytokine levels. Moreover, Moir et al. showed that the number of antibody secreting cells following influenza immunization are increased in ETIs compared to individuals who started ART during chronic infection, suggesting sustained improvement in B cell and GC function. Finally, ETIs maintain normal levels of ILCs and lymphocyte hematopoietic progenitor cells in the periphery, whereas these cells are depleted without treatment (Bordoni et al., 2018; Kløverpris et al., 2016). The contributions of the absence of virus/ongoing replication, reduced peripheral cytokine levels, or something yet to be described to the overall reconstitution of adaptive immune responses in ETIs is unclear. Understanding differences in innate immune subsets and antigen presenting cells, and their frequencies and durations of function, is critical to further contextualize these differences in adaptive immune function and antiviral activity.
Gut and lymphoid tissues are still impacted even with early ART initiation
ART administration studies in NHP models within hours to days following infection have established the near un-avoidable depletion of CD4+ T cells in the GI tract during acute SIV infection. While early ART does not prevent CD4+ T cell loss, central memory CD4+ T cells have been shown to repopulate to near pre-infection levels by 6 months post infection in the GI tract (George et al., 2005; Verhoeven et al., 2008), similar to natural SIV hosts that experience non-pathogenic infection. Limited sampling in early treated humans has also demonstrated recovery of CD4+ T cell numbers in the GI tract in this timeframe (Allers et al., 2016; Ananworanich et al., 2012), though the full extent of clonal diversity and polyfunctionality of these cells has not yet been determined. Th17 cells, however, are mostly preserved if treatment is started during Fiebig Stages I-II (Kök et al., 2015; Schuetz et al., 2014). Moreover, their cytokine production polyfunctionality remains intact (IL-17 and IL-22 subsets) only when treated this early. Deleage et al. also showed that neutrophil infiltration in the GI tract is reduced in ETIs compared to untreated individuals 24 weeks after ART initiation (Deleage et al., 2016). Immunohistochemistry revealed reduced proliferating Ki67+ cells when treated during Fiebig Stages I-II, but levels of TNF-α+ cells were similar between ETIs and untreated individuals. On the cellular level, frequencies of resting memory B cells and Tfh were preserved and expanded, respectively, in the gut compared to untreated individuals, suggesting improved GC function in secondary and tertiary lymphoid tissue sites in the GI tract (Planchais et al., 2018). Indeed, HIV Env gp140-reactive memory cells were shown to expand in ETIs. It is unclear, however, how maintaining Th17, Tfh, and resting memory B cells in the gut directly contributes to antiviral activity, impacts CTL function, or affects long-term disease outcome and to what degree early treatment can restore pre-treatment functionality and clonal diversity.
Progressive SIV models show establishment of viral reservoir and CD4+ T cell death, likely by inflammation-mediated pyroptosis (Doitsh et al., 2014), in lymphoid tissues as early as 4-6 days post infection (Lu et al., 2016a; Rabezanahary et al., 2020). ART initiation 5 weeks post SIV infection failed to mitigate Tfh and GC B cell proliferation and activation, likely facilitating ongoing viral infection during treatment (Hong et al., 2017). However, when ART was started at 4 days post-infection, mesenteric LNs contained lower frequencies of HLA-DR+ and CD39+ CD8+ T cells, but Tregs were still depleted relative to Th17 cells (Yero et al., 2019). This is in contrast to the blood, where frequencies of Tregs were restored and CTLA-4+PD-1− memory CD4+ T cells [known to contribute to viral persistence in SIV (McGary et al., 2017)] were diminished compared to untreated acute infection. Given the potential role of the LN in harboring ongoing HIV infection during ART (McManus et al., 2019), sampling this tissue during AHI could provide critical insight for developing novel treatments to eliminate HIV in both acute and chronic infection.
We summarize the known effects of early ART administration in acute HIV and SIV infection in Figure 3. It is critical to further distinguish molecular and cellular differences in the earliest stages of AHI, before complete suppression of viremia, between ETIs and untreated individuals. These earliest time points provide the best opportunity to discern how mitigating viremia, and potentially inflammation, affect the longevity and quality of both adaptive and innate immune responses, as well as tissue functionality. Moreover, linking these events to long term disease progression and comorbidities (e.g., inflammation) could inform clinical or cellular metrics and indicate alternative, or adjunctive, treatments to daily ART for life [e.g., therapeutic vaccines (Stephenson, 2018) or monoclonal broadly neutralizing antibody treatments (Ananworanich et al., 2015; Dashti et al., 2019)].
Discussion & Future Directions
Studies of acute HIV and SIV infection have been instrumental to determine how the quality and quantity of the immune response affects long term outcome and persistent disease. Moreover, these findings have highlighted how both adaptive and innate immune subsets develop dysfunctional phenotypes. However, the extent to which this dysfunction imparts subsequent disadvantages to other cell types, modulates overall tissue function, impacts antiviral activity, and the durability thereof without ongoing viral infection, are unknown. Further perturbations of putative cell-cell signaling events in NHP models and in vitro coculture settings will help determine which cell types and signals should be targeted by vaccines and/or treatments. Moreover, as multiple measurements accumulate from the same individuals in prospective and early infection studies, systems biology approaches using machine learning-based classifiers [like those successfully applied in systems serology vaccine settings (Chung and Alter, 2017)] may begin to causally link cellular and molecular features of the AHI response to disease outcome. More broadly, the extensive, valuable literature of AHI provides a guide and foil for investigating host responses to other acute viral infections, and demonstrates the need for longitudinal, early sampling to ascertain the phenotypes and dynamics of immune responses to target with treatments or therapeutics.
For HIV specifically, NHP models have been incredibly productive in depicting the roles of the antiviral and pro-inflammatory responses in the periphery, CD4+ T cell privileged tissues, and at sites of transmission (Box 1). As more data are generated from studies of AHI and the effects of early ART administration, re-purposing of these models to test human-infection-informed hypotheses may yield fruitful results to guide future vaccine and therapeutic efforts. Critically, innate immune subsets—especially NK cells, helper ILCs, monocytes, and macrophages—and their roles during acute infection are poorly described in SIV models, and must be further functionally characterized and subsequently perturbed to contextualize recent findings from hyperacute infection studies (Kazer et al., 2020; Kløverpris et al., 2016; Muema et al., 2020; Wang et al., 2020). Given the literature suggesting that HIV controllers have enhanced subsets of myeloid cells (Kazer et al., 2020; Martin-Gayo et al., 2015, 2018; Sáez-Cirión et al., 2011; Walker et al., 2015), changes to their phenotype during, or before, AHI may impart and/or reflect protective function in chronic infection. Indeed, a body of literature describing innate immune cell training in NK cells and macrophages in many infection and vaccine settings (Arts et al., 2018; Kaufmann et al., 2018; Netea et al., 2020) suggests that these cells may be potential targets alongside antibody- and T-cell-based vaccine approaches. With the advent of high-throughput clonotype sequencing, NHP models are also being used to study how T and B cell clones are shared across tissues and individuals and relate to disease progression (Price et al., 2009; Starke et al., 2020). While applied to chronic infection in humans (Costa et al., 2015; Meyer-Olson et al., 2010), this approach could further reveal the interplay of host-pathogen dynamics and link immune repertoire evolution to disease progression in future NHP and human acute infection studies.
With more advanced and sensitive tools to measure cellular state and phenotype from low-input human samples (Box 2) and multi-omic approaches [reviewed in (Chappell et al., 2018)], the community is better equipped than ever to thoroughly map immune responses throughout the earliest stages of acute HIV infection. Moreover, by applying them to both tissue samples (biopsies or fine-needle aspirates) and matching peripheral blood samples, we will begin to understand viral and immune dynamics in peripheral blood compared to different tissues. Critically, further investigation across tissues is needed to understand how the earliest host-pathogen interactions in CD4+ T cell rich tissues impact long-term disease outcome. Together, new genomic technologies, paired with more traditional approaches, have demonstrated their capacity to inform actionable hypotheses (Shalek and Benson, 2017; Shema et al., 2019), and will nominate a new set of vaccine and therapeutic strategies for not only HIV infection, but also other viral, bacterial and fungal infections moving forward.
Acknowledgements
We would like to thank M. Carrington for her insight and suggestions. This work was supported in part by the Beckman Young Investigator Program, a Sloan Fellowship in Chemistry, the NIH (5U24AI118672, 1R01AI138546, 1R01HL134539, 1R01DA046277), and the Bill and Melinda Gates Foundation to A.K.S, the NIH (UM1AI100663, UM1AI144462 and R37AI067073, R01A149704, R01A118574), the Richard and Lisa Witten Foundation, and the Bill and Melinda Gates Foundation to B.D.W., and the Hugh Hampton Young Memorial Fund Fellowship to S.W.K.
Footnotes
Declaration of Interests
A.K.S. reports compensation for consulting and/or SAB membership from Merck, Honeycomb Biotechnologies, Cellarity, Repertoire Immune Medicines, Orche Bio, and Dahlia Biosciences.
References
- Abbott RK, Lee JH, Menis S, Skog P, Rossi M, Ota T, Kulp DW, Bhullar D, Kalyuzhniy O, Havenar-Daughton C, et al. (2018). Precursor Frequency and Affinity Determine B Cell Competitive Fitness in Germinal Centers, Tested with Germline-Targeting HIV Vaccine Immunogens. Immunity 48, 133–146.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams NM, O’Sullivan TE, Geary CD, Karo JM, Amezquita RA, Joshi NS, Kaech SM, and Sun JC (2016). Natural Killer Cell Responses Redefine Immunological Memory. J. Immunol. Baltim. Md 1950 197, 2963–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers K, Puyskens A, Epple H-J, Schürmann D, Hofmann J, Moos V, and Schneider T (2016). The effect of timing of antiretroviral therapy on CD4+ T-cell reconstitution in the intestine of HIV-infected patients. Mucosal Immunol. 9, 265–274. [DOI] [PubMed] [Google Scholar]
- Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, Streeck H, Johnston MN, Staller KD, Zaman MT, et al. (2005). Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 106, 3366–3369. [DOI] [PubMed] [Google Scholar]
- Altfeld M, and Gale M Jr (2015). Innate immunity against HIV-1 infection. Nat. Immunol 16, 554–562. [DOI] [PubMed] [Google Scholar]
- Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, Souza M. de, Rerknimitr R, Dewar R, Marovich M, van Griensven F, Sekaly R, et al. (2012). Impact of Multi-Targeted Antiretroviral Treatment on Gut T Cell Depletion and HIV Reservoir Seeding during Acute HIV Infection. PLOS ONE 7, e33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananworanich J, McSteen B, and Robb ML (2015). Broadly neutralizing antibody and the HIV reservoir in acute HIV infection: a strategy toward HIV remission? Curr. Opin. HIV AIDS 10, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananworanich J, Eller LA, Pinyakorn S, Kroon E, Sriplenchan S, Fletcher JL, Suttichom D, Bryant C, Trichavaroj R, Dawson P, et al. (2017). Viral kinetics in untreated versus treated acute HIV infection in prospective cohort studies in Thailand. J. Int. AIDS Soc 20, 21652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardain A, Domingo-Gonzalez R, Das S, Kazer SW, Howard NC, Singh A, Ahmed M, Nhamoyebonde S, Rangel-Moreno J, Ogongo P, et al. (2019). Group 3 innate lymphoid cells mediate early protective immunity against tuberculosis. Nature 570, 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, et al. (2008). HIV-1 envelope protein binds to and signals through integrin α 4 β 7 , the gut mucosal homing receptor for peripheral T cells. Nat. Immunol 9, 301–309. [DOI] [PubMed] [Google Scholar]
- Arts RJW, Moorlag SJCFM, Novakovic B, Li Y, Wang S-Y, Oosting M, Kumar V, Xavier RJ, Wijmenga C, Joosten LAB, et al. (2018). BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 23, 89–100.e5. [DOI] [PubMed] [Google Scholar]
- Atkuri KR, Stevens JC, and Neubert H (2015). Mass Cytometry: A Highly Multiplexed Single-Cell Technology for Advancing Drug Development. Drug Metab. Dispos 43, 227–233. [DOI] [PubMed] [Google Scholar]
- Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M, et al. (2018). Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 174, 1293–1308.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharlou H, Canete NP, Cunningham AL, Harman AN, and Patrick E (2019). Mass Cytometry Imaging for the Study of Human Diseases-Applications and Data Analysis Strategies. Front. Immunol 10, 2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiyegunhi O, Ndlovu B, Ogunshola F, Ismail N, Walker BD, Ndung’u T, and Ndhlovu ZM (2018). Frequencies of Circulating Th1-Biased T Follicular Helper Cells in Acute HIV-1 Infection Correlate with the Development of HIV-Specific Antibody Responses and Lower Set Point Viral Load. J. Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, and Deeks SG (2014). Immunologic strategies for HIV-1 remission and eradication. Science 345, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Ghneim K, Bosche WJ, Li Y, Berkemeier B, Hull M, Bhattacharyya S, Cameron M, Liu J, Smith K, et al. (2016). Rapid Inflammasome Activation following Mucosal SIV Infection of Rhesus Monkeys. Cell 165, 656–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar N, Girard P-M, Lopez-Gonzalez M, Didier C, Collias L, Jung C, Bollens D, Duvivier C, Von Platen C, Scott-Algara D, et al. (2017). Potential Role of Vδ2+ γδ T Cells in Regulation of Immune Activation in Primary HIV Infection. Front. Immunol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoni V, Viola D, Sacchi A, Pinnetti C, Casetti R, Cimini E, Tumino N, Antinori A, Ammassari A, and Agrati C (2018). IL-18 and Stem Cell Factor affect hematopoietic progenitor cells in HIV-infected patients treated during primary HIV infection. Cytokine 103, 34–37. [DOI] [PubMed] [Google Scholar]
- Borhis G, Burelout C, Chaoul N, Smith N, Goujard C, Meyer L, Paul S, Saoudin H, Hosmalin A, Gilbert C, et al. (2016). Plasmacytoid dendritic cells and myeloid cells differently contribute to B-cell-activating factor belonging to the tumor necrosis factor superfamily overexpression during primary HIV infection. AIDS 30, 365–376. [DOI] [PubMed] [Google Scholar]
- Borhis G, Trovato M, Chaoul N, Ibrahim HM, and Richard Y (2017). B-Cell-Activating Factor and the B-Cell Compartment in HIV/SIV Infection. Front. Immunol 8, 1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borhis G, Trovato M, Ibrahim HM, Isnard S, Le Grand R, Bosquet N, and Richard Y (2020). Impact of BAFF Blockade on Inflammation, Germinal Center Reaction and Effector B-Cells During Acute SIV Infection. Front. Immunol 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosinger SE, and Utay NS (2015). Type I interferon: understanding its role in HIV pathogenesis and therapy. Curr. HIV/AIDS Rep 12, 41–53. [DOI] [PubMed] [Google Scholar]
- Bosinger SE, Jacquelin B, Benecke A, Silvestri G, and Müller-Trutwin M (2012). Systems biology of natural simian immunodeficiency virus infections. Curr. Opin. HIV AIDS 7, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzk N, Gronke K, and Diefenbach A (2018). Innate lymphoid cells, mediators of tissue homeostasis, adaptation and disease tolerance. Immunol. Rev 286, 86–101. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. (2006). Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med 12, 1365–1371. [DOI] [PubMed] [Google Scholar]
- Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le Gall S, Waring MT, Moss K, et al. (2009). IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114, 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Wu B, Litzenburger UM, Ruff D, Gonzales ML, Snyder MP, Chang HY, and Greenleaf WJ (2015). Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 523, 486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzon MJ, Martin-Gayo E, Pereyra F, Ouyang Z, Sun H, Li JZ, Piovoso M, Shaw A, Dalmau J, Zangger N, et al. (2014). Long-Term Antiretroviral Treatment Initiated at Primary HIV-1 Infection Affects the Size, Composition, and Decay Kinetics of the Reservoir of HIV-1-Infected CD4 T Cells. J. Virol 88, 10056–10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero-Pérez J, Grau-Expósito J, Serra-Peinado C, Rosero DA, Luque-Ballesteros L, Astorga-Gamaza A, Castellví J, Sanhueza T, Tapia G, Lloveras B, et al. (2019). Resident memory T cells are a cellular reservoir for HIV in the cervical mucosa. Nat. Commun 10, 4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Packer JS, Ramani V, Cusanovich DA, Huynh C, Daza R, Qiu X, Lee C, Furlan SN, Steemers FJ, et al. (2017). Comprehensive single-cell transcriptional profiling of a multicellular organism. Science 357, 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Mehraj V, Trottier B, Baril J-G, Leblanc R, Lebouche B, Cox J, Tremblay C, Lu W, Singer J, et al. (2016). Early Initiation Rather Than Prolonged Duration of Antiretroviral Therapy in HIV Infection Contributes to the Normalization of CD8 T-Cell Counts. Clin. Infect. Dis 62, 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Le AQ, Shahid A, and Brumme ZL (2015). HIV-1 adaptation to HLA: a window into virus–host immune interactions. Trends Microbiol. 23, 212–224. [DOI] [PubMed] [Google Scholar]
- Chappell L, Russell AJC, and Voet T (2018). Single-Cell (Multi)omics Technologies. Annu. Rev. Genomics Hum. Genet 19, 15–41. [DOI] [PubMed] [Google Scholar]
- Chen P, Su B, Zhang T, Zhu X, Xia W, Fu Y, Zhao G, Xia H, Dai L, Sun L, et al. (2017). Perturbations of Monocyte Subsets and Their Association with T Helper Cell Differentiation in Acute and Chronic HIV-1-Infected Patients. Front. Immunol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Yu H, Li G, Li F, Ma J, Li J, Chi L, Zhang L, and Su L Type I interferons suppress viral replication but contribute to T cell depletion and dysfunction during chronic HIV-1 infection. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier MF, Didier C, Girard P-M, Manea ME, Campa P, Barré-Sinoussi F, Scott-Algara D, and Weiss L (2016). CD4 T-Cell Responses in Primary HIV Infection: Interrelationship with Immune Activation and Virus Burden. Front. Immunol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, and Fauci AS (1997). Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A 94, 13193–13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AW, and Alter G (2017). Systems serology: profiling vaccine induced humoral immunity against HIV. Retrovirology 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell A, Ferguson N, and Barker E (2020). Presence of Inflammatory Group I and III Innate Lymphoid Cells in the Colon of Simian Immunodeficiency Virus-Infected Rhesus Macaques. J. Virol 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coindre S, Tchitchek N, Alaoui L, Vaslin B, Bourgeois C, Goujard C, Avettand-Fenoel V, Lecuroux C, Bruhns P, Le Grand R, et al. (2018). Mass Cytometry Analysis Reveals the Landscape and Dynamics of CD32a+ CD4+ T Cells From Early HIV Infection to Effective cART. Front. Immunol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby DJ, Trautmann L, Pinyakorn S, Leyre L, Pagliuzza A, Kroon E, Rolland M, Takata H, Buranapraditkun S, Intasan J, et al. (2018). Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat. Med 24, 923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer-Lluch M, Ruiz A, Moris A, and Prado JG (2018). Restriction Factors: From Intrinsic Viral Restriction to Shaping Cellular Immunity Against HIV-1. Front. Immunol 9, 2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces MR, Trevino AE, Hamilton EG, Greenside PG, Sinnott-Armstrong NA, Vesuna S, Satpathy AT, Rubin AJ, Montine KS, Wu B, et al. (2017). An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods 14, 959–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corleis B, Lisanti AC, Körner C, Schiff AE, Rosenberg ES, Allen TM, Altfeld M, and Kwon DS (2017). Early type I Interferon response induces upregulation of human β-defensin 1 during acute HIV-1 infection. PloS One 12, e0173161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove C, Ussher JE, Rauch A, Gärtner K, Kurioka A, Hühn MH, Adelmann K, Kang Y-H, Fergusson JR, Simmonds P, et al. (2013). Early and nonreversible decrease of CD161++ /MAIT cells in HIV infection. Blood 121, 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AI, Koning D, Ladell K, McLaren JE, Grady BPX, Schellens IMM, van Ham P, Nijhuis M, Borghans JAM, Keşmir C, et al. (2015). Complex T-cell receptor repertoire dynamics underlie the CD8+ T-cell response to HIV-1. J. Virol 89, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell TA, Fletcher JL, Sereti I, Pinyakorn S, Dewar R, Krebs SJ, Chomchey N, Rerknimitr R, Schuetz A, Michael NL, et al. (2016). Initiation of antiretroviral therapy before detection of colonic infiltration by HIV reduces viral reservoirs, inflammation and immune activation. J. Int. AIDS Soc 19, 21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusanovich DA, Daza R, Adey A, Pliner HA, Christiansen L, Gunderson KL, Steemers FJ, Trapnell C, and Shendure J (2015). Multiplex single-cell profiling of chromatin accessibility by combinatorial cellular indexing. Science 348, 910–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti A, DeVico AL, Lewis GK, and Sajadi MM (2019). Broadly Neutralizing Antibodies against HIV: Back to Blood. Trends Mol. Med 25, 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey RT, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, et al. (1999). HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. U. S. A 96, 15109–15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Tracy R, and Douek DC (2013). Systemic effects of inflammation on health during chronic HIV infection. Immunity 39, 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleage C, Schuetz A, Alvord WG, Johnston L, Hao X-P, Morcock DR, Rerknimitr R, Fletcher JLK, Puttamaswin S, Phanuphak N, et al. (2016). Impact of early cART in the gut during acute HIV infection. JCI Insight 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleage C, Immonen TT, Fennessey CM, Reynaldi A, Reid C, Newman L, Lipkey L, Schlub TE, Camus C, O’Brien S, et al. (2019). Defining early SIV replication and dissemination dynamics following vaginal transmission. Sci. Adv 5, eaav7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers KR, Makedonas G, Buggert M, Eller MA, Ratcliffe SJ, Goonetilleke N, Li CK, Eller LA, Rono K, Maganga L, et al. (2016). Temporal Dynamics of CD8+ T Cell Effector Responses during Primary HIV Infection. PLOS Pathog. 12, e1005805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsh G, Galloway NLK, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Muñoz-Arias I, et al. (2014). Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505, 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong KL, Moodley A, Kwon DS, Ghebremichael MS, Dong M, Ismail N, Ndhlovu ZM, Mabuka JM, Muema DM, Pretorius K, et al. (2018). Detection and treatment of Fiebig stage I HIV-1 infection in young at-risk women in South Africa: a prospective cohort study. Lancet HIV 5, e35–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du VY, Bansal A, Carlson J, Salazar-Gonzalez JF, Salazar MG, Ladell K, Gras S, Josephs TM, Heath SL, Price DA, et al. (2016). HIV-1-Specific CD8 T Cells Exhibit Limited Cross-Reactivity during Acute Infection. J. Immunol. Baltim. Md 1950 196, 3276–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbo M, Crinier A, Vély F, and Vivier E (2017). Innate lymphoid cells: major players in inflammatory diseases. Nat. Rev. Immunol 17, 665–678. [DOI] [PubMed] [Google Scholar]
- Eberl G, Colonna M, Santo JPD, and McKenzie ANJ (2015). Innate lymphoid cells: A new paradigm in immunology. Science 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einkauf KB, Lee GQ, Gao C, Sharaf R, Sun X, Hua S, Chen SMY, Jiang C, Lian X, Chowdhury FZ, et al. (2019). Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J. Clin. Invest 129, 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller MA, Goonetilleke N, Tassaneetrithep B, Eller LA, Costanzo MC, Johnson S, Betts MR, Krebs SJ, Slike BM, Nitayaphan S, et al. (2016). Expansion of Inefficient HIV-Specific CD8 T Cells during Acute Infection. J. Virol 90, 4005–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes JD, LeGrand R, and Petrovas C (2018). Visualizing the Immune System: Providing Key Insights into HIV/SIV Infections. Front. Immunol 9, 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DT, and Silvestri G (2013). Non-Human Primate Models in AIDS Research. Curr. Opin. HIV AIDS 8, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, et al. (1997). Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300. [DOI] [PubMed] [Google Scholar]
- Fischer W, Ganusov VV, Giorgi EE, Hraber PT, Keele BF, Leitner T, Han CS, Gleasner CD, Green L, Lo C-C, et al. (2010). Transmission of Single HIV-1 Genomes and Dynamics of Early Immune Escape Revealed by Ultra-Deep Sequencing. PLOS ONE 5, e12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freel SA, Picking RA, Ferrari G, Ding H, Ochsenbauer C, Kappes JC, Kirchherr JL, Soderberg KA, Weinhold KJ, Cunningham CK, et al. (2012). Initial HIV-1 Antigen-Specific CD8+ T Cells in Acute HIV-1 Infection Inhibit Transmitted/Founder Virus Replication. J. Virol 86, 6835–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, et al. (2015). B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med 21, 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Tellez T, Huot N, Ploquin MJ, Rascle P, Jacquelin B, and Müller-Trutwin M (2016). Non-human primates in HIV research: Achievements, limits and alternatives. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis 46, 324–332. [DOI] [PubMed] [Google Scholar]
- George MD, Reay E, Sankaran S, and Dandekar S (2005). Early Antiretroviral Therapy for Simian Immunodeficiency Virus Infection Leads to Mucosal CD4+ T-Cell Restoration and Enhanced Gene Expression Regulating Mucosal Repair and Regeneration. J. Virol 79, 2709–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierahn TM, Wadsworth MH, Hughes TK, Bryson BD, Butler A, Satija R, Fortune S, Love JC, and Shalek AK (2017). Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nat. Methods 14, 395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez SM, Aguilar-Jimenez W, Su R-C, and Rugeles MT (2019). Mucosa: Key Interactions Determining Sexual Transmission of the HIV Infection. Front. Immunol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini G, Fourati S, Vaccari M, Rahman MA, Gordon SN, Brown DR, Law L, Chang J, Green R, Barrenäs F, et al. (2020). Engagement of monocytes, NK cells, and CD4+ Th1 cells by ALVAC-SIV vaccination results in a decreased risk of SIVmac251 vaginal acquisition. PLOS Pathog. 16, e1008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder PJR, and Walker BD (2012). HIV and HLA class I: an evolving relationship. Immunity 37, 426–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger JR, Konkel JE, Zangerle-Murray T, and Shaw TN (2017). Macrophages in gastrointestinal homeostasis and inflammation. Pflugers Arch. 469, 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, Thierry GR, Bonnardel J, and Bajenoff M (2020). Establishment and Maintenance of the Macrophage Niche. Immunity 52, 434–451. [DOI] [PubMed] [Google Scholar]
- Haase AT (2011). Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu. Rev. Med 62, 127–139. [DOI] [PubMed] [Google Scholar]
- Han X, Wang R, Zhou Y, Fei L, Sun H, Lai S, Saadatpour A, Zhou Z, Chen H, Ye F, et al. (2018). Mapping the Mouse Cell Atlas by Microwell-Seq. Cell 172, 1091–1107.e17. [DOI] [PubMed] [Google Scholar]
- Hargreaves J, Davey C, Hargreaves J, Davey C, Auerbach J, Blanchard J, Bond V, Bonell C, Burgess R, Busza J, et al. (2020). Three lessons for the COVID-19 response from pandemic HIV. Lancet HIV 7, e309–e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann FJ, and Bendall SC (2020). Immune monitoring using mass cytometry and related high-dimensional imaging approaches. Nat. Rev. Rheumatol 16, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmuth J, Slike BM, Sacdalan C, Best J, Kroon E, Phanuphak N, Fletcher JLK, Prueksakaew P, Jagodzinski LL, Valcour V, et al. (2019). Very Early Initiation of Antiretroviral Therapy During Acute HIV Infection Is Associated With Normalized Levels of Immune Activation Markers in Cerebrospinal Fluid but Not in Plasma. J. Infect. Dis 220, 1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich TJ, Hatano H, Bacon O, Hogan LE, Rutishauser R, Hill A, Kearney MF, Anderson EM, Buchbinder SP, Cohen SE, et al. (2017). HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLOS Med. 14, e1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heung LJ, and Hohl TM (2019). Inflammatory monocytes are detrimental to the host immune response during acute infection with Cryptococcus neoformans. PLoS Pathog. 15, e1007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JJ, di Silveira ELV, Amancha PK, Byrareddy SN, Gumber S, Chang K-T, Ansari AA, and Villinger F (2017). Early initiation of antiretroviral treatment postSIV infection does not resolve lymphoid tissue activation. AIDS 31, 1819–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueber B, Curtis AD, Kroll K, Varner V, Jones R, Pathak S, Lifton M, Van Rompay KKA, De Paris K, and Reeves RK (2020). Functional Perturbation of Mucosal Group 3 Innate Lymphoid and Natural Killer Cells in Simian-Human Immunodeficiency Virus/Simian Immunodeficiency Virus-Infected Infant Rhesus Macaques. J. Virol 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D, and Andersson DI (2015). Evolutionary consequences of drug resistance: shared principles across diverse targets and organisms. Nat. Rev. Genet 16, 459–471. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, and Medzhitov R (2015). Control of adaptive immunity by the innate immune system. Nat. Immunol 16, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Zhang T, Wang R, Zhang H, Huang X, Yin J, Zhang L, Xu X, and Wu H (2012). Plasma IP-10 is associated with rapid disease progression in early HIV-1 infection. Viral Immunol. 25, 333–337. [DOI] [PubMed] [Google Scholar]
- Johnson JS, Lucas SY, Amon LM, Skelton S, Nazitto R, Carbonetti S, Sather DN, Littman DR, and Aderem A (2018). Reshaping of the Dendritic Cell Chromatin Landscape and Interferon Pathways During HIV Infection. Cell Host Microbe 23, 366–381.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juno JA, and Eriksson EM (2019). γδ T-cell responses during HIV infection and antiretroviral therapy. Clin. Transl. Immunol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardava L, Moir S, Shah N, Wang W, Wilson R, Buckner CM, Santich BH, Kim LJY, Spurlin EE, Nelson AK, et al. (2014). Abnormal B cell memory subsets dominate HIV-specific responses in infected individuals. J. Clin. Invest 124, 3252–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsikis PD, Mueller YM, and Villinger F (2011). The Cytokine Network of Acute HIV Infection: A Promising Target for Vaccines and Therapy to Reduce Viral Set-Point? PLOS Pathog. 7, e1002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, Tzelepis F, Pernet E, Dumaine A, Grenier J-C, et al. (2018). BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 172, 176–190.e19. [DOI] [PubMed] [Google Scholar]
- Kaya-Okur HS, Wu SJ, Codomo CA, Pledger ES, Bryson TD, Henikoff JG, Ahmad K, and Henikoff S (2019). CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun 10, 1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazer SW, Aicher TP, Muema DM, Carroll SL, Ordovas-Montanes J, Miao VN, Tu AA, Ziegler CGK, Nyquist SK, Wong EB, et al. (2020). Integrated single-cell analysis of multicellular immune dynamics during hyperacute HIV-1 infection. Nat. Med 26, 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating SM, Heitman JW, Wu S, Deng X, Stacey AR, Zahn RC, de la Rosa M, Finstad SL, Lifson JD, Piatak M, et al. (2016). Magnitude and Quality of Cytokine and Chemokine Storm during Acute Infection Distinguish Nonprogressive and Progressive Simian Immunodeficiency Virus Infections of Nonhuman Primates. J. Virol 90, 10339–10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing CF, Spudich S, Valcour V, Cartwright P, Chalermchai T, Fletcher JLK, Takata H, Nichols C, Josey BJ, Slike B, et al. (2017). High Number of Activated CD8+ T Cells Targeting HIV Antigens Are Present in Cerebrospinal Fluid in Acute HIV Infection. JAIDS J. Acquir. Immune Defic. Syndr 75, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klase Z, Ortiz A, Deleage C, Mudd JC, Quiñones M, Schwartzman E, Klatt NR, Canary L, Estes JD, and Brenchley JM (2015). Dysbiotic bacteria translocate in progressive SIV infection. Mucosal Immunol. 8, 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, Morcock D, McGinty JW, Lifson JD, Lafont BA, et al. (2010). Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol. 3, 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kløverpris HN, Kazer SW, Mjösberg J, Mabuka JM, Wellmann A, Ndhlovu Z, Yadon MC, Nhamoyebonde S, Muenchhoff M, Simoni Y, et al. (2016). Innate Lymphoid Cells Are Depleted Irreversibly during Acute HIV-1 Infection in the Absence of Viral Suppression. Immunity 44, 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JJ, Buggert M, Kardava L, Seaton KE, Eller MA, Canaday DH, Robb ML, Ostrowski MA, Deeks SG, Slifka MK, et al. (2017). T-bet+ B cells are induced by human viral infections and dominate the HIV gp140 response. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kök A, Hocqueloux L, Hocini H, Carrière M, Lefrou L, Guguin A, Tisserand P, Bonnabau H, Avettand-Fenoel V, Prazuck T, et al. (2015). Early initiation of combined antiretroviral therapy preserves immune function in the gut of HIV-infected patients. Mucosal Immunol. 8, 127–140. [DOI] [PubMed] [Google Scholar]
- Kwissa M, Nakaya HI, Onlamoon N, Wrammert J, Villinger F, Perng GC, Yoksan S, Pattanapanyasat K, Chokephaibulkit K, Ahmed R, et al. (2014). Dengue Virus Infection Induces Expansion of a CD14+CD16+ Monocyte Population that Stimulates Plasmablast Differentiation. Cell Host Microbe 16, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal KG, Kim D, Costanzo MC, Creegan M, Leeansyah E, Dias J, Paquin-Proulx D, Eller LA, Schuetz A, Phuang-ngern Y, et al. (2020). Dynamic MAIT cell response with progressively enhanced innateness during acute HIV-1 infection. Nat. Commun 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Mortha A, Rahman A, and Merad M (2015). Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol 15, 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyre L, Kroon E, Vandergeeten C, Sacdalan C, Colby DJ, Buranapraditkun S, Schuetz A, Chomchey N, de Souza M, Bakeman W, et al. (2020). Abundant HIV-infected cells in blood and tissues are rapidly cleared upon ART initiation during acute HIV infection. Sci. Transl. Med 12, eaav3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Richert-Spuhler LE, Evans TI, Gillis J, Connole M, Estes JD, Keele BF, Klatt NR, and Reeves RK (2014). Hypercytotoxicity and Rapid Loss of NKp44+ Innate Lymphoid Cells during Acute SIV Infection. PLOS Pathog. 10, e1004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Duan L, Estes JD, Ma Z-M, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, and Haase AT (2005). Peak SIV replication in resting memory CD4 + T cells depletes gut lamina propria CD4 + T cells. Nature 434, 1148–1152. [DOI] [PubMed] [Google Scholar]
- Li S, Folkvord JM, Kovacs KJ, Wagstaff RK, Mwakalundwa G, Rendahl AK, Rakasz EG, Connick E, and Skinner PJ (2019). Low levels of SIV-specific CD8+ T cells in germinal centers characterizes acute SIV infection. PLoS Pathog. 15, e1007311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti T, Kadelka C, Braun DL, Kuster H, Böni J, Robbiani M, Günthard HF, and Trkola A (2019). Widespread B cell perturbations in HIV-1 infection afflict naive and marginal zone B cells. J. Exp. Med 216, 2071–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, et al. (2012). Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J. Clin. Invest 122, 3271–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liovat A-S, Rey-Cuillé M-A, Lécuroux C, Jacquelin B, Girault I, Petitjean G, Zitoun Y, Venet A, Barré-Sinoussi F, Lebon P, et al. (2012). Acute Plasma Biomarkers of T Cell Activation Set-Point Levels and of Disease Progression in HIV-1 Infection. PLoS ONE 7, e46143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhan W, Kim CJ, Clayton K, Zhao H, Lee E, Cao JC, Ziegler B, Gregor A, Yue FY, et al. (2014). IL-10-Producing B Cells Are Induced Early in HIV-1 Infection and Suppress HIV-1-Specific T Cell Responses. PLoS ONE 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang Q, Chen P, Guo N, Song A, Huang X, Xia W, Li L, Moog C, Wu H, et al. (2019). Foxp3+Helios+ regulatory T cells are associated with monocyte subsets and their PD-1 expression during acute HIV-1 infection. BMC Immunol. 20, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Yeh Y-HJ, Varabyou A, Collora JA, Sherrill-Mix S, Talbot CC, Mehta S, Albrecht K, Hao H, Zhang H, et al. (2020). Single-cell transcriptional landscapes reveal HIV-1-driven aberrant host gene transcription as a potential therapeutic target. Sci. Transl. Med 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Ma F, Churbanov A, Wan Y, Li Y, Kang G, Yuan Z, Wang D, Zhang C, Xu J, et al. (2014). Virus-Host Mucosal Interactions During Early SIV Rectal Transmission. Virology 464–465, 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Demers AJ, Ma F, Kang G, Yuan Z, Wan Y, Li Y, Xu J, Lewis M, and Li Q (2016a). Next-Generation mRNA Sequencing Reveals Pyroptosis-Induced CD4+ T Cell Death in Early Simian Immunodeficiency Virus-Infected Lymphoid Tissues. J. Virol 90, 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Li Z, Li Q, Jiao Y, Ji Y, Zhang H, Liu Z, Li W, and Wu H (2016b). Preferential loss of gut-homing α4β7 CD4 + T cells and their circulating functional subsets in acute HIV-1 infection. Cell. Mol. Immunol 13, 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuka JM, Dugast A-S, Muema DM, Reddy T, Ramlakhan Y, Euler Z, Ismail N, Moodley A, Dong KL, Morris L, et al. (2017). Plasma CXCL13 but Not B Cell Frequencies in Acute HIV Infection Predicts Emergence of Cross-Neutralizing Antibodies. Front. Immunol 8, 1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, et al. (2015). Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 161, 1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenetje P, Riou C, Casazza JP, Ambrozak D, Hill B, Gray G, Koup RA, Bruyn G. de, and Gray CM (2010). A Steady State of CD4+ T Cell Memory Maturation and Activation Is Established during Primary Subtype C HIV-1 Infection. J. Immunol 184, 4926–4935. [DOI] [PubMed] [Google Scholar]
- Manak MM, Jagodzinski LL, Shutt A, Malia JA, Leos M, Ouellette J, Akapirat S, Colby DL, Phanuphak N, Eller LA, et al. (2019). Decreased Seroreactivity in Individuals Initiating Antiretroviral Therapy during Acute HIV Infection. J. Clin. Microbiol 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganaro L, Hong P, Hernandez MM, Argyle D, Mulder LCF, Potla U, Diaz-Griffero F, Lee B, Fernandez-Sesma A, and Simon V (2018). IL-15 regulates susceptibility of CD4+ T cells to HIV infection. Proc. Natl. Acad. Sci 115, E9659–E9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Gayo E, Buzon MJ, Ouyang Z, Hickman T, Cronin J, Pimenova D, Walker BD, Lichterfeld M, and Yu XG (2015). Potent Cell-Intrinsic Immune Responses in Dendritic Cells Facilitate HIV-1-Specific T Cell Immunity in HIV-1 Elite Controllers. PLoS Pathog. 11, e1004930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Gayo E, Cole MB, Kolb KE, Ouyang Z, Cronin J, Kazer SW, Ordovas-Montanes J, Lichterfeld M, Walker BD, Yosef N, et al. (2018). A Reproducibility-Based Computational Framework Identifies an Inducible, Enhanced Antiviral State in Dendritic Cells from HIV-1 Elite Controllers. Genome Biol. 19, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, and Roederer M (2005). Massive infection and loss of memory CD4 + T cells in multiple tissues during acute SIV infection. Nature 434, 1093–1097. [DOI] [PubMed] [Google Scholar]
- McGary CS, Deleage C, Harper J, Micci L, Ribeiro SP, Paganini S, Kuri-Cervantes L, Benne C, Ryan ES, Balderas R, et al. (2017). CTLA-4+PD-1− Memory CD4+ T Cells Critically Contribute to Viral Persistence in Antiretroviral Therapy-Suppressed, SIV-Infected Rhesus Macaques. Immunity 47, 776–788.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLane LM, Abdel-Hakeem MS, and Wherry EJ (2019). CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol 37, 457–495. [DOI] [PubMed] [Google Scholar]
- McManus WR, Bale MJ, Spindler J, Wiegand A, Musick A, Patro SC, Sobolewski MD, Musick VK, Anderson EM, Cyktor JC, et al. (2019). HIV-1 in lymph nodes is maintained by cellular proliferation during antiretroviral therapy. J. Clin. Invest 129, 4629–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, and Haynes BF (2010). The immune response during acute HIV-1 infection: clues for vaccine development. Nat. Rev. Immunol 10, 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Olson D, Simons BC, Conrad JA, Smith RM, Barnett L, Lorey SL, Duncan CB, Ramalingam R, and Kalams SA (2010). Clonal expansion and TCR-independent differentiation shape the HIV-specific CD8+ effector-memory T-cell repertoire in vivo. Blood 116, 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michlmayr D, Kim E-Y, Rahman AH, Raghunathan R, Kim-Schulze S, Che Y, Kalayci S, Gümüş ZH, Kuan G, Balmaseda A, et al. (2020). Comprehensive Immunoprofiling of Pediatric Zika Reveals Key Role for Monocytes in the Acute Phase and No Effect of Prior Dengue Virus Infection. Cell Rep. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JL, Takata H, Muir R, Colby DJ, Kroon E, Crowell TA, Sacdalan C, Pinyakorn S, Puttamaswin S, Benjapornpong K, et al. (2020). Plasmacytoid dendritic cells sense HIV replication before detectable viremia following treatment interruption. J. Clin. Invest 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S, Buckner CM, Ho J, Wang W, Chen J, Waldner AJ, Posada JG, Kardava L, O’Shea MA, Kottilil S, et al. (2010). B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood 116, 5571–5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muema DM, Akilimali NA, Ndumnego OC, Rasehlo SS, Durgiah R, Ojwach DBA, Ismail N, Dong M, Moodley A, Dong KL, et al. (2020). Association between the cytokine storm, immune cell dynamics, and viral replicative capacity in hyperacute HIV infection. BMC Med. 18, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir R, Metcalf T, Tardif V, Takata H, Phanuphak N, Kroon E, Colby DJ, Trichavaroj R, Valcour V, Robb ML, et al. (2016). Altered Memory Circulating T Follicular Helper-B Cell Interaction in Early Acute HIV Infection. PLOS Pathog. 12, e1005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranbhai V, Altfeld M, Karim SSA, Ndung’u T, Karim QA, and Carr WH (2013). Changes in Natural Killer Cell Activation and Function during Primary HIV-1 Infection. PLOS ONE 8, e53251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndhlovu ZM, Kamya P, Mewalal N, Kløverpris HN, Nkosi T, Pretorius K, Laher F, Ogunshola F, Chopera D, Shekhar K, et al. (2015). Magnitude and Kinetics of CD8+ T Cell Activation during Hyperacute HIV Infection Impact Viral Set Point. Immunity 43, 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndhlovu ZM, Kazer SW, Nkosi T, Ogunshola F, Muema DM, Anmole G, Swann SA, Moodley A, Dong K, Reddy T, et al. (2019). Augmentation of HIV-specific T cell function by immediate treatment of hyperacute HIV-1 infection. Sci. Transl. Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM, et al. (2020). Defining trained immunity and its role in health and disease. Nat. Rev. Immunol 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien M, Manches O, and Bhardwaj N (2013). Plasmacytoid Dendritic Cells in HIV Infection. Adv. Exp. Med. Biol 762, 71–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura R, and Takeda K (2017). Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med 49, e338–e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordovas-Montanes J, Beyaz S, Rakoff-Nahoum S, and Shalek AK (2020). Distribution and storage of inflammatory memory in barrier tissues. Nat. Rev. Immunol 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazi E, and Powell N (2019). Group 3 ILCs: Peacekeepers or Troublemakers? What’s Your Gut Telling You?! Front. Immunol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, Vaccari M, Tryniszewska E, Gostick E, Roederer M, et al. (2007). SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood 110, 928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C, Ferrando-Martinez S, Gerner MY, Casazza JP, Pegu A, Deleage C, Cooper A, Hataye J, Andrews S, Ambrozak D, et al. (2017). Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci. Transl. Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchais C, Hocqueloux L, Ibanez C, Gallien S, Copie C, Surenaud M, Kök A, Lorin V, Fusaro M, Delfau-Larue M-H, et al. (2018). Early Antiretroviral Therapy Preserves Functional Follicular Helper T and HIV-Specific B Cells in the Gut Mucosa of HIV-1–Infected Individuals. J. Immunol 200, 3519–3529. [DOI] [PubMed] [Google Scholar]
- Policicchio BB, Pandrea I, and Apetrei C (2016). Animal Models for HIV Cure Research. Front. Immunol 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DA, Asher TE, Wilson NA, Nason MC, Brenchley JM, Metzler IS, Venturi V, Gostick E, Chattopadhyay PK, Roederer M, et al. (2009). Public clonotype usage identifies protective Gag-specific CD8+ T cell responses in SIV infection. J. Exp. Med 206, 923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pušnik J, Eller MA, Tassaneetrithep B, Schultz BT, Eller LA, Nitayaphan S, Kosgei J, Maganga L, Kibuuka H, Alter G, et al. (2019). Expansion of Stem Cell-Like CD4+ Memory T Cells during Acute HIV-1 Infection Is Linked to Rapid Disease Progression. J. Virol 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabezanahary H, Moukambi F, Palesch D, Clain J, Racine G, Andreani G, Benmadid-Laktout G, Zghidi-Abouzid O, Soundaramourty C, Tremblay C, et al. (2020). Despite early antiretroviral therapy effector memory and follicular helper CD4 T cells are major reservoirs in visceral lymphoid tissues of SIV-infected macaques. Mucosal Immunol. 13, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehtz KD, Barrenäs F, Xu C, Busman-Sahay K, Valentine A, Law L, Ma D, Policicchio BB, Wijewardana V, Brocca-Cofano E, et al. (2020). African green monkeys avoid SIV disease progression by preventing intestinal dysfunction and maintaining mucosal barrier integrity. PLOS Pathog. 16, e1008333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MA, Ko E-J, Enyindah-Asonye G, Helmold Hait S, Hogge C, Hunegnaw R, Venzon DJ, Hoang T, and Robert-Guroff M (2019). Differential Effect of Mucosal NKp44+ Innate Lymphoid Cells and Δγ Cells on Simian Immunodeficiency Virus Infection Outcome in Rhesus Macaques. J. Immunol. Baltim. Md 1950 203, 2459–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd AD, Dabitao D, Bream JH, Charvat B, Laeyendecker O, Kiwanuka N, Lutalo T, Kigozi G, Tobian AAR, Gamiel J, et al. (2009). Microbial translocation, the innate cytokine response, and HIV-1 disease progression in Africa. Proc. Natl. Acad. Sci. U. S. A 106, 6718–6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RK, Li H, Jost S, Blass E, Li H, Schafer JL, Varner V, Manickam C, Eslamizar L, Altfeld M, et al. (2015). Antigen-specific NK cell memory in rhesus macaques. Nat. Immunol 16, 927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb M, and Ananworanich J (2016). Lessons from acute HIV infection. Curr. Opin. Hiv Aids 11, 555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb ML, Eller LA, Kibuuka H, Rono K, Maganga L, Nitayaphan S, Kroon E, Sawe FK, Sinei S, Sriplienchan S, et al. (2016). Prospective Study of Acute HIV-1 Infection in Adults in East Africa and Thailand. N. Engl. J. Med 374, 2120–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roider J, Maehara T, Ngoepe A, Ramsuran D, Muenchhoff M, Adland E, Aicher T, Kazer SW, Jooste P, Karim F, et al. (2018). High-Frequency, Functional HIV-Specific T-Follicular Helper and Regulatory Cells Are Present Within Germinal Centers in Children but Not Adults. Front. Immunol 9, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AB, Roco CM, Muscat RA, Kuchina A, Sample P, Yao Z, Graybuck LT, Peeler DJ, Mukherjee S, Chen W, et al. (2018). Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt-Rosen O, Regev A, Oberdoerffer P, Nawy T, Hupalowska A, Rood JE, Ashenberg O, Cerami E, Coffey RJ, Demir E, et al. (2020). The Human Tumor Atlas Network: Charting Tumor Transitions across Space and Time at Single-Cell Resolution. Cell 181, 236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanand S, Suscovich TJ, and Alter G (2016). Broadly Neutralizing Antibodies Against HIV: New Insights to Inform Vaccine Design. Annu. Rev. Med 67, 185–200. [DOI] [PubMed] [Google Scholar]
- Sáez-Cirión A, Hamimi C, Bergamaschi A, David A, Versmisse P, Mélard A, Boufassa F, Barré-Sinoussi F, Lambotte O, Rouzioux C, et al. (2011). Restriction of HIV-1 replication in macrophages and CD4+ T cells from HIV controllers. Blood 118, 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannier G, Dubé M, and Kaufmann DE (2020). Single-Cell Technologies Applied to HIV-1 Research: Reaching Maturity. Front. Microbiol 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos PR dos, Rancez M, Prétet J-L, Michel-Salzat A, Messent V, Bogdanova A, Couëdel-Courteille A, Souil E, Cheynier R, and Butor C (2011). Rapid Dissemination of SIV Follows Multisite Entry after Rectal Inoculation. PLOS ONE 6, e19493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieffer M, Jessen HK, Oster AF, Pissani F, Soghoian DZ, Lu R, Jessen AB, Zedlack C, Schultz BT, Davis I, et al. (2014). Induction of Gag-Specific CD4 T Cell Responses during Acute HIV Infection Is Associated with Improved Viral Control. J. Virol 88, 7357–7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz A, Deleage C, Sereti I, Rerknimitr R, Phanuphak N, Phuang-Ngern Y, Estes JD, Sandler NG, Sukhumvittaya S, Marovich M, et al. (2014). Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 10, e1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seay K, Church C, Zheng JH, Deneroff K, Ochsenbauer C, Kappes JC, Liu B, Jeng EK, Wong HC, and Goldstein H (2015). In Vivo Activation of Human NK Cells by Treatment with an Interleukin-15 Superagonist Potently Inhibits Acute In Vivo HIV-1 Infection in Humanized Mice. J. Virol 89, 6264–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereti I, Krebs SJ, Phanuphak N, Fletcher JL, Slike B, Pinyakorn S, O’Connell RJ, Rupert A, Chomont N, Valcour V, et al. (2017). Persistent, Albeit Reduced, Chronic Inflammation in Persons Starting Antiretroviral Therapy in Acute HIV Infection. Clin. Infect. Dis 64, 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SV, Manickam C, Ram DR, and Reeves RK (2017). Innate Lymphoid Cells in HIV/SIV Infections. Front. Immunol 8, 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalek AK, and Benson M (2017). Single-cell analyses to tailor treatments. Sci. Transl. Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L, Smith AJ, Duan L, Perkey KE, Qu L, Wietgrefe S, Zupancic M, Southern PJ, Masek-Hammerman K, Reeves RK, et al. (2014). NK Cell Responses to Simian Immunodeficiency Virus Vaginal Exposure in Naive and Vaccinated Rhesus Macaques. J. Immunol 193, 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema E, Bernstein BE, and Buenrostro JD (2019). Single-cell and single-molecule epigenomics to uncover genome regulation at unprecedented resolution. Nat. Genet 51, 19–25. [DOI] [PubMed] [Google Scholar]
- Sivro A, Schuetz A, Sheward D, Joag V, Yegorov S, Liebenberg LJ, Yende-Zuma N, Stalker A, Mwatelah RS, Selhorst P, et al. (2018). Integrin α4β7 expression on peripheral blood CD4+ T cells predicts HIV acquisition and disease progression outcomes. Sci. Transl. Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene PJ, and Henikoff S (2017). An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. ELife 6, e21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyper M, Porter CBM, Ashenberg O, Waldman J, Drokhlyansky E, Wakiro I, Smillie C, Smith-Rosario G, Wu J, Dionne D, et al. (2020). A single-cell and single-nucleus RNA-Seq toolbox for fresh and frozen human tumors. Nat. Med 26, 792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghoian DZ, Jessen H, Flanders M, Sierra-Davidson K, Cutler S, Pertel T, Ranasinghe S, Lindqvist M, Davis I, Lane K, et al. (2012). HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci. Transl. Med 4, 123ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsouk M, Estes JD, Deleage C, Dunham RM, Albright R, Inadomi JM, Martin JN, Deeks SG, McCune JM, and Hunt PW (2015). Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS Lond. Engl 29, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper A, Kimura I, Nagaoka S, Konno Y, Yamamoto K, Koyanagi Y, and Sato K (2017). Type I Interferon Responses by HIV-1 Infection: Association with Disease Progression and Control. Front. Immunol 8, 1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, et al. (2009). Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol 83, 3719–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke CE, Vinton CL, Ladell K, McLaren JE, Ortiz AM, Mudd JC, Flynn JK, Lai SH, Wu F, Hirsch VM, et al. (2020). SIV-specific CD8+ T cells are clonotypically distinct across lymphoid and mucosal tissues. J. Clin. Invest 130, 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle C, Hernández DC, and Romagnani C (2018). Innate lymphoid cells in lung infection and immunity. Immunol. Rev 286, 102–119. [DOI] [PubMed] [Google Scholar]
- Stephenson KE (2018). Therapeutic vaccination for HIV: hopes and challenges. Curr. Opin. HIV AIDS 13, 408–415. [DOI] [PubMed] [Google Scholar]
- Strauss-Albee DM, Fukuyama J, Liang EC, Yao Y, Jarrell JA, Drake AL, Kinuthia J, Montgomery RR, John-Stewart G, Holmes S, et al. (2015). Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci. Transl. Med 7, 297ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck H, Lu R, Beckwith N, Milazzo M, Liu M, Routy J-P, Little S, Jessen H, Kelleher AD, Hecht F, et al. (2014). Emergence of Individual HIV-Specific CD8 T Cell Responses during Primary HIV-1 Infection Can Determine Long-Term Disease Outcome. J. Virol 88, 12793–12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbington MJT, Rozenblatt-Rosen O, Regev A, and Teichmann SA (2017). Single-cell transcriptomics to explore the immune system in health and disease. Science 358, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Nguyen A, and Gommerman JL (2020). Dendritic Cell Subsets in Intestinal Immunity and Inflammation. J. Immunol. Baltim. Md 1950 204, 1075–1083. [DOI] [PubMed] [Google Scholar]
- Swan ZD, Bouwer AL, Wonderlich ER, and Barratt-Boyes SM (2017). Persistent accumulation of gut macrophages with impaired phagocytic function correlates with SIV disease progression in macaques. Eur. J. Immunol 47, 1925–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata H, Buranapraditkun S, Kessing C, Fletcher JLK, Muir R, Tardif V, Cartwright P, Vandergeeten C, Bakeman W, Nichols CN, et al. (2017). Delayed differentiation of potent effector CD8+ T cells reducing viremia and reservoir seeding in acute HIV infection. Sci. Transl. Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, et al. (2008). Initial B-Cell Responses to Transmitted Human Immunodeficiency Virus Type 1: Virion-Binding Immunoglobulin M (IgM) and IgG Antibodies Followed by Plasma Anti-gp41 Antibodies with Ineffective Control of Initial Viremia. J. Virol 82, 12449–12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovanabutra S, Sirijatuphat R, Pham PT, Bonar L, Harbolick EA, Bose M, Song H, Chang D, Oropeza C, O’Sullivan AM, et al. (2019). Deep Sequencing Reveals Central Nervous System Compartmentalization in Multiple Transmitted/Founder Virus Acute HIV-1 Infection. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann L, Mbitikon-Kobo F-M, Goulet J-P, Peretz Y, Shi Y, Van Grevenynghe J, Procopio FA, Boulassel MR, Routy J-P, Chomont N, et al. (2012). Profound metabolic, functional, and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. Blood 120, 3466–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu AA, Gierahn TM, Monian B, Morgan DM, Mehta NK, Ruiter B, Shreffler WG, Shalek AK, and Love JC (2019). TCR sequencing paired with massively parallel 3′ RNA-seq reveals clonotypic T cell signatures. Nat. Immunol 20, 1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven D, Sankaran S, Silvey M, and Dandekar S (2008). Antiviral Therapy during Primary Simian Immunodeficiency Virus Infection Fails To Prevent Acute Loss of CD4+ T Cells in Gut Mucosa but Enhances Their Rapid Restoration through Central Memory T Cells. J. Virol 82, 4016–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BD, and Yu XG (2013). Unravelling the mechanisms of durable control of HIV-1. Nat. Rev. Immunol 13, 487–498. [DOI] [PubMed] [Google Scholar]
- Walker WE, Kurscheid S, Joshi S, Lopez CA, Goh G, Choi M, Barakat L, Francis J, Fisher A, Kozal M, et al. (2015). Increased Levels of Macrophage Inflammatory Proteins Result in Resistance to R5-Tropic HIV-1 in a Subset of Elite Controllers. J. Virol 89, 5502–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, and Zhang L (2020). Broadly neutralizing antibodies and vaccine design against HIV-1 infection. Front. Med 14, 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Kang W, Zuo J, Kang W, and Sun Y (2017). The Significance of Type-I Interferons in the Pathogenesis and Therapy of Human Immunodeficiency Virus 1 Infection. Front. Immunol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lifshitz L, Gellatly K, Vinton CL, Busman-Sahay K, McCauley S, Vangala P, Kim K, Derr A, Jaiswal S, et al. (2020). HIV-1-induced cytokines deplete homeostatic innate lymphoid cells and expand TCF7 -dependent memory NK cells. Nat. Immunol 21, 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, et al. (2014). Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512, 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JB, Lim S-Y, Osuna CE, Kublin JL, Chen E, Yoon G, Liu P-T, Abbink P, Borducci EN, Hill A, et al. (2018). Prevention of SIVmac251 reservoir seeding in rhesus monkeys by early antiretroviral therapy. Nat. Commun 9, 5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK, Hezareh M, Günthard HF, Havlir DV, Ignacio CC, Spina CA, and Richman DD (1997). Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278, 1291–1295. [DOI] [PubMed] [Google Scholar]
- Xia H, Jiang W, Zhang X, Qin L, Su B, Li Z, Sun J, Zhang Y, Zhang T, Lu X, et al. (2018). Elevated Level of CD4+ T Cell Immune Activation in Acutely HIV-1-Infected Stage Associates With Increased IL-2 Production and Cycling Expression, and Subsequent CD4+ T Cell Preservation. Front. Immunol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yero A, Farnos O, Rabezanahary H, Racine G, Estaquier J, and Jenabian M-A (2019). Differential Dynamics of Regulatory T-Cell and Th17 Cell Balance in Mesenteric Lymph Nodes and Blood following Early Antiretroviral Initiation during Acute Simian Immunodeficiency Virus Infection. J. Virol 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B, Wherry EJ, and Ahmed R (2012). Acquired Transcriptional Programming in Functional and Exhausted Virus-specific CD8 T Cells. Curr. Opin. HIV AIDS 7, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue FY, Merchant A, Kovacs CM, Loutfy M, Persad D, and Ostrowski MA (2008). Virus-Specific Interleukin-17-Producing CD4+ T Cells Are Detectable in Early Human Immunodeficiency Virus Type 1 Infection. J. Virol 82, 6767–6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue FY, Cohen JC, Ho M, Rahman AKMN-U, Liu J, Mujib S, Saiyed A, Hundal S, Khozin A, Bonner P, et al. (2017). HIV-Specific Granzyme B-Secreting but Not Gamma Interferon-Secreting T Cells Are Associated with Reduced Viral Reservoirs in Early HIV Infection. J. Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen A, Rezek V, Youn C, Lam B, Chang N, Rick J, Carrillo M, Martin H, Kasparian S, Syed P, et al. (2017). Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J. Clin. Invest 127, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng GXY, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, Ziraldo SB, Wheeler TD, McDermott GP, Zhu J, et al. (2017). Massively parallel digital transcriptional profiling of single cells. Nat. Commun 8, 14049. [DOI] [PMC free article] [PubMed] [Google Scholar]