Abstract
BACKGROUND
Hepatic stellate cell (HSC) hyperactivation is a central link in liver fibrosis development. HSCs perform aerobic glycolysis to provide energy for their activation. Focal adhesion kinase (FAK) promotes aerobic glycolysis in cancer cells or fibroblasts, while FAK-related non-kinase (FRNK) inhibits FAK phosphorylation and biological functions.
AIM
To elucidate the effect of FRNK on liver fibrosis at the level of aerobic glycolytic metabolism in HSCs.
METHODS
Mouse liver fibrosis models were established by administering CCl4, and the effect of FRNK on the degree of liver fibrosis in the model was evaluated. Transforming growth factor-β1 was used to activate LX-2 cells. Tyrosine phosphorylation at position 397 (pY397-FAK) was detected to identify activated FAK, and the expression of the glycolysis-related proteins monocarboxylate transporter 1 (MCT-1) and enolase1 (ENO1) was assessed. Bioinformatics analysis was performed to predict putative binding sites for c-myc in the ENO1 promoter region, which were validated with chromatin immunoprecipitation (ChIP) and dual-luciferase reporter assays.
RESULTS
The pY397-FAK level was increased in human fibrotic liver tissue. FRNK knockout promoted liver fibrosis in mouse models. It also increased the activation, migration, proliferation and aerobic glycolysis of primary hepatic stellate cells (pHSCs) but inhibited pHSC apoptosis. Nevertheless, opposite trends for these phenomena were observed after exogenous FRNK treatment in LX-2 cells. Mechanistically, the FAK/Ras/c-myc/ENO1 pathway promoted aerobic glycolysis, which was inhibited by exogenous FRNK.
CONCLUSION
FRNK inhibits aerobic glycolysis in HSCs by inhibiting the FAK/Ras/c-myc/ENO1 pathway, thereby improving liver fibrosis. FRNK might be a potential target for liver fibrosis treatment.
Keywords: Liver fibrosis, Hepatic stellate cells, Focal adhesion kinase, Focal adhesion kinase-related non-kinase, Aerobic glycolysis, Enolase1
Core Tip: We show that focal adhesion kinase-related non-kinase (FRNK) limits hepatic stellate cell (HSC) activation, proliferation, and migration and promotes HSC apoptosis by inhibiting aerobic glycolysis, thereby ameliorating liver fibrosis. FRNK may represent a potential therapeutic candidate for liver fibrosis treatment.
INTRODUCTION
Long-term damage to liver function by hepatitis viruses, alcohol, and diet may cause chronic hepatic injuries leading to liver fibrosis and cirrhosis[1-4], which is characterized by the activation of hepatic stellate cells (HSCs) and their transformation into myofibroblasts[5]. This process continuously damages the liver and disrupts the balance of liver self-repair, causing increased cell proliferation and migration and a reduced apoptosis rate[6-8]. At present, the pathological changes associated with chronic liver injuries in individuals without cirrhosis can be reversed after removing the etiological agent, such as in a small proportion of patients with hepatitis B and alcoholic fatty liver disease and most patients with hepatitis C[1,9], but the remaining patients with hepatic fibrosis develop irreversible cirrhosis due to an inability to completely and effectively reverse the pathogenesis and a lack of effective antifibrotic drugs[10]. Therefore, studies of the treatment of liver fibrosis are particularly critical, among which the regulation of the relevant biological functions of HSCs is the most important antifibrotic approach[11,12].
Focal adhesion kinases (FAKs) are a class of nonreceptor cytosolic protein tyrosine kinases that belong to the protein tyrosine kinase superfamily[13,14]. FAK plays an important role in cellular signal transduction and enhances biological behaviors such as proliferation, migration, wound healing and angiogenesis in cells and tissues after integrating signals from integrins, growth factors and mechanical stimuli[15,16]. FAK binds to extracellular matrix (ECM) proteins through an accumulation of integrin receptors to form FAK dimers, which further induce tyrosine phosphorylation at position 397 (pY397-FAK); pY397-FAK regulates these biological functions in cells[17,18] and therefore plays an important role in a variety of malignant tumor cells[18,19]. FAK-related non-kinase (FRNK), which has a nucleotide sequence corresponding to the C-terminus of FAK but lacks the N-terminal functional site of FAK, is an independently expressed protein[20] with the main function of inhibiting FAK phosphorylation, thereby inversely regulating the function of FAK after cell activation[21,22]. FRNK negatively regulates FAK signaling axis function, thereby improving pulmonary fibrosis in an experimental mouse model[23].
FAK is also overexpressed in pancreatic ductal adenocarcinoma cells[24,25], promoting the conversion of pyruvate into lactate by increasing enolase1 (ENO1), pyruvate kinase 2, lactate dehydrogenase, and monocarboxylate transporter (MCT)-1 expression and lactate transport, enhancing aerobic glycolysis in cancer cells, and inhibiting mitochondrial oxidative phosphorylation in cancer cells[16]. This switch to aerobic glycolysis is an important mechanism by which tumor cells acquire energy[17,26], as shown by the fact that oxidative phosphorylation simultaneously provides energy to cells performing aerobic glycolysis even in the presence of sufficient oxygen and normal mitochondrial function[27,28]. HSCs also exhibit increased aerobic glycolysis, resulting in lactate accumulation and gluconeogenesis inhibition when they differentiate into myofibroblasts[8,28]. This phenomenon also occurs in individuals with congenital pulmonary fibrosis[29]. Therefore, the inhibition of FAK-related pathways by FRNK may reduce energy acquisition through aerobic glycolysis during HSC activation and could be used as a targeted therapy to ameliorate liver fibrosis. Nevertheless, few studies have focused on the physiological or pathological role of FRNK in obtaining energy during hepatic fibrosis, and its mechanism remains unclear.
In the present study, we first showed that FRNK was downregulated in human liver fibrotic tissues. Then, we verified that FRNK knockout in vivo and in vitro promoted aerobic glycolysis and hepatic fibrosis. Exogenous FRNK inhibited aerobic glycolysis by inhibiting the FAK/Ras/c-myc/ENO1 pathway, limiting HSC activation, migration, and proliferation and increasing apoptosis to ameliorate liver fibrosis. Together, these data provide a detailed mechanism through which FRNK functions and suggest that FRNK represents a potential target to inhibit aerobic glycolysis in HSCs and treat liver fibrosis.
MATERIALS AND METHODS
Human liver samples
Paraffin blocks of liver tissues from 15 patients with liver fibrosis were collected from the Department of Infectious Diseases, Affiliated Hospital of Guizhou Medical University (Guiyang, China) between March 2019 and September 2019; none of the patients had any other organ-specific or systemic diseases, and liver fibrosis was diagnosed by pathological biopsy. Fifteen healthy liver samples were obtained from distal hepatocarcinoma liver tissue without any abnormalities in specimens surgically resected from patients at the Department of Hepatobiliary Surgery, Affiliated Hospital of Guizhou Medical University. None of the aforementioned subjects had contraindications to liver biopsy, and the study was approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University (Approval 2018 Ethics Review No. 032) and conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from the patients.
Animals
FRNK knockout (FRNK-/-) mice were a gift from the Respiratory and Critical Care Medicine Center, School of Medicine, University of Alabama at Birmingham, Birmingham, AL, United States. All mouse interventions were approved by the Animal Care Committee (IACUC) of Guizhou Medical University (No. 1801109), and the methods and experimental procedures were performed in accordance with the relevant guidelines and regulations. Wild-type (WT) mice of the same genotype were used as controls, and all experimental mice were on the C57BL/6 background.
Mice were maintained under pathogen-free conditions at a controlled temperature (22 ± 2 °C) with a consistent photoperiod (12:12 h light-dark cycle); five mice were housed in each cage, with cages containing soft bedding. The mice were habituated to these conditions for 2 d before inclusion in an experiment. Healthy male mice (aged 8-11 wk, weighing 20 ± 3 g) were selected and intraperitoneally injected with 1.5 μL/g of a 10% Carbon tetrachloride (CCl4) in corn oil solution three times a week to establish a liver fibrosis model. Mice in the control group were injected with a 1.5 μL/g solution of corn oil three times a week. Livers were harvested at each time point, namely, 0, 2, 4 and 6 wk, for experiments. Six mice per group were used.
Reagents and antibodies
CCl4, corn oil, and OptiPrep were purchased from Sigma-Aldrich (St. Louis, MO, United States). Transforming growth factor-β1 (TGF-β1) was purchased from R&D Systems (Minneapolis, MN, United States). Primary antibodies specific for the following proteins were purchased from Abcam (Cambridge, United Kingdom): Desmin rabbit monoclonal antibodies (ab32362), FAK rabbit monoclonal antibodies (ab40794), ENO1 mouse monoclonal antibodies (ab190365), alpha SMA rabbit polyclonal antibodies (ab5694), k-ras rabbit monoclonal antibodies (ab275876) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) rabbit polyclonal antibodies (ab9485). Anti-c-myc (13987) rabbit monoclonal antibodies were purchased from Cell Signaling Technology (Shanghai, China). MCT-1 rabbit polyclonal antibodies (20139-1-AP) were purchased from Proteintech (Wuhan, China). pY397-FAK rabbit polyclonal antibodies (AF3398) were purchased form Affinity Bioscience (Cincinnati, OH, United States). All other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, United States) and Fisher Scientific (Waltham, MA, United States).
Immunohistochemistry (IHC), hematoxylin & eosin (H&E), Masson’s trichrome and Sirius Red staining and hydroxyproline assay
H&E staining kits, Masson’s trichrome staining solution and Sirius Red staining solution were purchased from Solarbio Biotechnology Co., Ltd. (Beijing, China) and used according to the manufacturer’s guidelines. A hydroxyproline assay was performed using a Nanjing Jiancheng Biotechnology (Nanjing, China) hydroxyproline kit. All kits were used according to the instructions for use. Liver samples were fixed with neutral buffered formalin and embedded in paraffin for IHC. Briefly, sections were incubated with the indicated antibodies. Horseradish peroxidase-conjugated antibodies were used as the secondary antibodies. Finally, a diaminobenzidine colorimetric reagent solution was applied, followed by hematoxylin counterstaining. The slides were then scanned, and representative images were acquired.
Cells and cell culture
LX-2 cells were purchased from Zhongqiao Xinzhou (Shanghai, China). Human HSCs (ZQ0026) and LX-2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS; Biological Industries, Kibbutz Beit-Haemek, Israel). Primary hepatic stellate cells (pHSCs) were extracted from C57BL/6 WT or FRNK-/- mice aged 8-11 wk, as previously described[30,31]. Briefly, the abdominal cavity was opened with a "cross" incision, an 18-gauge trocar was inserted from the left ventricle to inject the preperfusate, and the blood in the liver was flushed by exsanguination until the tissue turned yellow. Then, the preperfusate was replaced with pronase and collagenase for 15-20 min, and the liver was removed and washed with normal saline. The liver capsule and connective tissue were removed, fully digested with a digestion solution at 37 °C with shaking and ground to generate a single-cell suspension. The supernatant was discarded after centrifugation at 1500 rpm for 5 min, and the pellet was resuspended in D-Hank's solution. The hepatocytes were removed by centrifugation, and a gradient lymphocyte separation solution was directly added. HSCs were isolated in one step using monolayer gradient centrifugation, and the cells were washed twice with DMEM and cultured with DMEM containing 10% FBS. Cell survival was evaluated by performing trypan blue staining, and cell purity was identified by desmin immunocytochemical staining. All cells were cultured in an incubator containing 5% CO2 at 37 °C.
Recombinant FRNK adenoviral vector transfection and HSC activation
An adenovirus-mediated gene delivery system was used to effectively deliver the FRNK cDNA into HSCs. An adenoviral vector carrying the FRNK protein and green fluorescent protein (Ad-FRNK) as well as a green fluorescent protein-carrying adenovirus (Ad-GFP) were purchased from Jikai Gene (Shanghai, China). All transfections were performed according to the manufacturer's instructions, and cells in serum-free medium (DMEM with 1% BSA) were transfected with Ad-FRNK or the control vector (Ad-GFP) 24 h before TGF-β1 treatment. Twenty-four hours later, the cells were cultured with complete medium containing 2 ng/mL TGF-β1 and treated for 36 h[31].
Transwell, cell counting kit-8 (CCK-8) and flow cytometry assays
Transwell migration experiments used 8.0-μm pore size membranes (Corning, United States) according to the manufacturer's protocol. A total of 105 cells were seeded in the upper chamber of each well in 100 μL of serum-free medium, while 600 μL of complete medium was added to the lower chamber as a chemoattractant. After a 6-h incubation at 37 °C, the cells remaining on the upper surface of the membrane were removed with a cotton swab, and the cells on the lower surface of the membrane were considered migrated cells. After fixation with 4% paraformaldehyde and staining with a 0.1% crystal violet solution, images were acquired under an inverted microscope. Cell Counting Kit-8 (CCK-8) was purchased from Dojindo (Shanghai, China), and 104 cells (100 μL/well) were seeded in 96-well plates. After placing the culture plate in an incubator for preincubation (37 °C, 5% CO2), 10 μL of CCK-8 solution was added to each well, and then the culture plate was evaluated with a microplate reader to detect the absorbance at 450 nm. A total of 105 cells in each group were stained with an Annexin V-PE/7-AAD apoptosis kit (Hangzhou Lianke, Hangzhou, Zhejiang Province, China) according to the instructions for use, sorted with a flow cytometer (Beckman, United States) and analyzed using Flow Jo software (Tree Star); dead cells were excluded based on forward scatter and side scatter data.
Western blot analysis
Western blot analysis was performed as previously described[30]. Briefly, 1% NP-40-treated whole-liver tissue lysates or whole-cell lysates were used for Western blot analysis. Protein levels were quantified using a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA) after total protein extraction. Twenty milligrams of each protein sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). GAPDH was used as a loading control for all blots. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes, which were incubated with primary antibodies overnight at 4 °C. The next day, after an incubation with an appropriate secondary antibody, signals were generated with an electrochemiluminescence detection kit.
Glucose consumption, 2-NBDG uptake and lactate assays
The lactate level in culture medium was detected with the Lactate Colorimetric Assay Kit (BioVision, Milpitas, CA, United States) according to the manufacturer's instructions. The 2-NBDG Glucose Uptake Kit (BioVision, Milpitas, CA, United States) was used to detect the cellular uptake of glucose, and the Glucose Colorimetric Assay Kit (BioVision, Milpitas, CA, United States) was used to detect the glucose concentration in culture medium and thus measure the cellular consumption of glucose. The 2-NBDG Glucose Uptake Kit and the Glucose Colorimetric Assay Kit were used according to the manufacturer's protocol.
Chromatin immunoprecipitation (ChIP) assay
JASPAR (http://jaspar.genereg.net) and PROMO (http://alggen.lsi.upc.es) database analyses predicted two putative c-myc binding sites in the ENO1 promoter region. A total of 107 cells fixed with formaldehyde were collected in 500 μL of lysis buffer from the Magna ChIP HiSens Kit (Millipore, Bedford, Massachusetts, United States) according to the manufacturer's manual. Cells were then sonicated for 25 cycles with a 6-s power-on interval of 30 s and an intensity of 200 W. Afterward, the supernatant was diluted and thoroughly mixed with Protein A/G beads. Then, 5 μg of IgG or an anti-c-myc antibody was added and incubated with the mixture overnight at 4 °C. After washing the beads the next day, the mixture was incubated with elution buffer at 62 °C for 2 h and then 95 °C for 10 min. The eluted DNA was then purified and subjected to a PCR assay to assess the binding sequence. Specific primer sequences were used to perform PCR.
Dual-luciferase reporter assays
The effect of c-myc on the ENO1 promoter was determined by cotransfecting pcDNA-c-myc or pcDNA-vector (NC) into LX-2 cells with pGL3-based constructs containing an empty sequence (NC) or the WT or MT1/MT2 ENO1 promoter sequences, and Renilla luciferase reporter plasmids. Twenty-four hours after transfection, firefly and Renilla luciferase activities were measured with a luciferase reporter assay kit (Genomeditech, Shanghai, China). Fluorescence detection was performed according to the instructions of the instrument, the parameters were set, the measurement time was 10 s, and the measurement interval was 2 s. Each sample was added into a measuring tube in a total volume of 20 μL (the sample volume was consistent in each measurement), and then 20 μL of Firefly Luciferase Assay Reagent was added, mixed well 2-3 times (without vortexing), mixed well again and evaluated to determine relative light unit (RLU) 1. Cell lysis buffer was set as the blank control well. The tested samples were mixed with 20 μL of prepared Renilla Luciferase Assay working solution 2-3 times and mixed well before measuring RLU2. The measured RLU1 value was compared to the corresponding RLU2 value, and the resulting ratio determined the degree of reporter activation. The ratio of firefly luciferase activity to Renilla luciferase activity was calculated for each sample.
Statistical analysis
Data were statistically analyzed using GraphPad Prism 5.0 software, and a two-tailed Student's t test was used for comparisons between different groups. P < 0.05 was considered statistically significant. Data are presented as the mean ± SD.
RESULTS
The level of the pY397-FAK protein was increased while the level of the FRNK protein was decreased in human fibrotic liver tissue
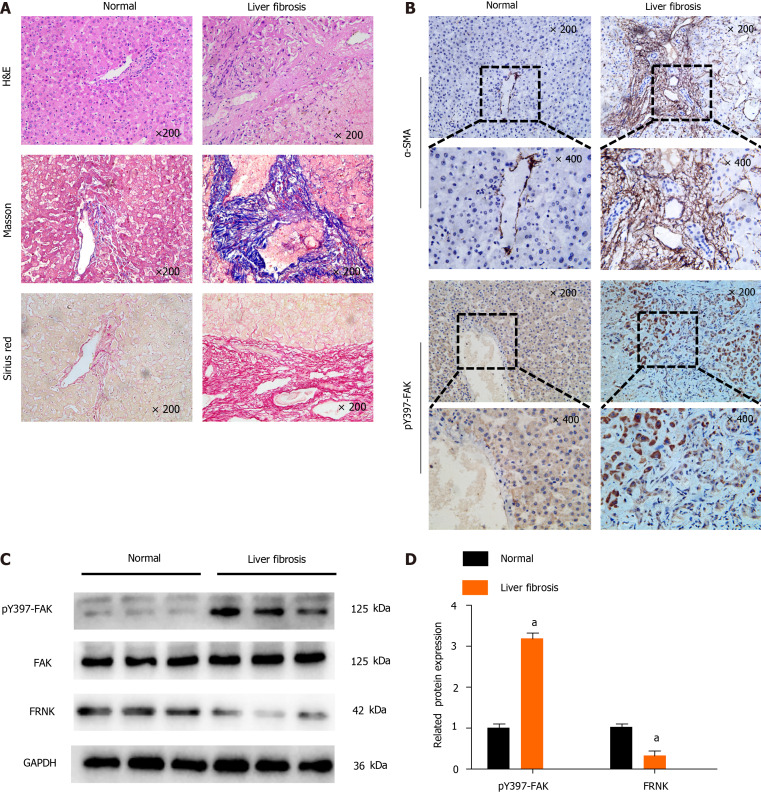
We first investigated the level of pY397-FAK in fibrotic liver tissue to explore the role of pY397-FAK in liver fibrosis. Compared with normal liver tissue, liver tissue samples from patients in the liver fibrosis group showed typical pathological features, including significant steatosis, inflammatory necrosis, significant collagen deposition, hepatic fibrosis and hepatocyte loosening. Masson’s trichrome staining showed less collagen deposition and a normal cell morphology in the normal group, while a large number of blue-stained collagen fibers was observed in the liver fibrosis group, and the tissue had accumulated a wide band of collagen fibers that extended into and was distributed in the hepatic lobules. Sirius Red staining showed less collagen deposition in normal subjects and a normal cell morphology but substantial red staining indicating collagen deposition in portal areas in fibrotic liver tissues. Notably, IHC showed higher α-smooth muscle actin (α-SMA) and pY397-FAK expression in fibrotic liver tissue than in normal liver tissues (Figure 1A and B). Western blot analysis showed higher levels of the pY397-FAK protein in fibrotic liver tissues than in normal liver tissues. Conversely, in fibrotic liver tissue, FRNK was expressed at lower levels than that in normal tissues (P < 0.05, Figure 1C and D). These results suggest that pY397-FAK protein expression is increased and FRNK protein expression is decreased in fibrotic liver tissue.
Figure 1.
Tyrosine phosphorylation at position 397 of FAK is upregulated, while FRNK expression is downregulated in human fibrotic liver tissue. A: H&E, Masson’s trichrome and Sirius Red staining were performed after liver biopsy to assess the tissues of normal subjects and patients with liver fibrosis under a light microscope at 200× magnification; B: Immunohistochemistry showed changes in the expression of α-smooth muscle actin (α-SMA) and tyrosine phosphorylation at position 397 of FAK(pY397-FAK) in the livers of normal subjects compared with patients with liver fibrosis under a light microscope at 200× or 400× magnification; C and D: Protein expression in biopsy tissues was analyzed using Western blotting. Representative results from three independent replicate assays are shown. aP < 0.05. Data are presented as the mean ± SD. FAK: Focal adhesion kinase; FRNK: Focal adhesion kinase-related non-kinase; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
Exacerbation of liver fibrosis and aerobic glycolysis in mice after FRNK knockout in vivo
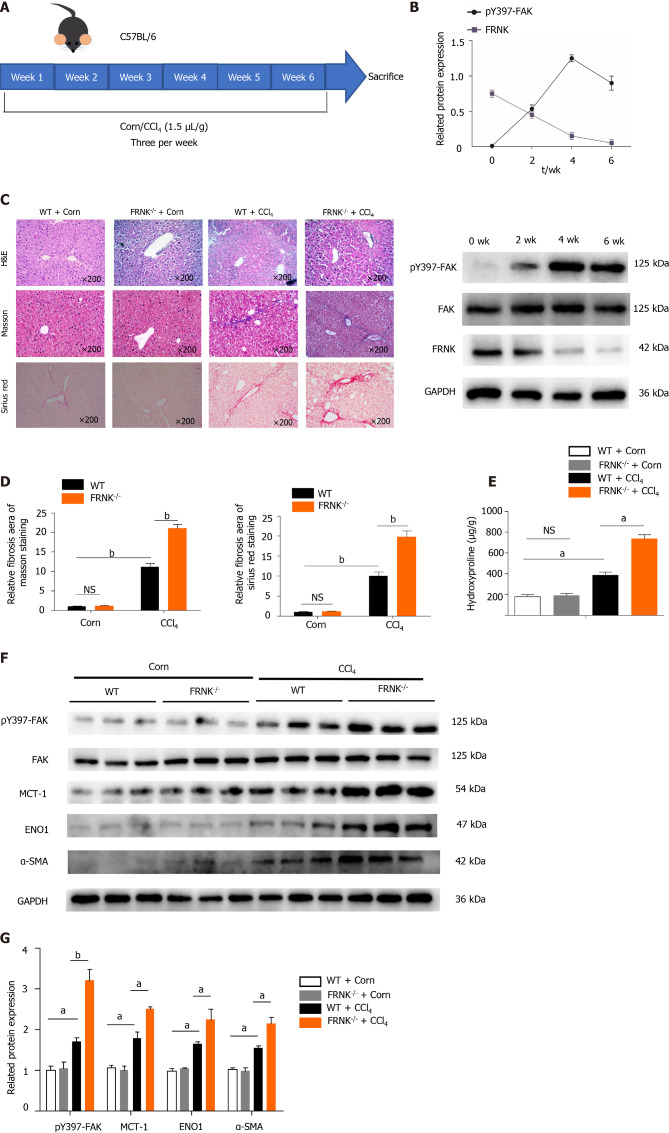
We established a fibrosis model by injecting CCl4 into WT mice and FRNK-/- mice. After two fortnights, the expression of the pY397-FAK protein peaked, while the FRNK protein was expressed at a low level (P < 0.05, Figure 2A and B). Therefore, mouse models with four weeks of injection were used in subsequent experiments. By performing H&E, Masson’s trichrome and Sirius Red staining, we found a greater liver fibrosis area and more extensive liver fibrosis in FRNK-/- mice than in WT mice after the CCl4 intervention (P < 0.05, Figure 2C and D), while the hydroxyproline content in FRNK-/- mice with fibrosis was greater than that in WT mice with fibrosis (P < 0.05, Figure 2E). Western blot analysis revealed higher levels of the pY397-FAK, MCT-1, ENO1 and α-SMA proteins in the liver tissues from FRNK-/- mice treated with CCl4 than in WT mice (P < 0.05, Figure 2F and G). Based on these results, FRNK-/- mice develop more severe liver fibrosis after the CCl4 intervention, along with increased expression of the aerobic glycolysis-related proteins MCT-1 and ENO1. It suggests that there may be more active aerobic glycolysis in the liver.
Figure 2.
Liver fibrosis in mice was aggravated after FRNK knockout. A and B: WT mice were modeled for 6 wk, pY397-FAK and FRNK protein expression levels in vivo was measured using Western blotting every fortnight; C and D: FRNK-/- and WT mice were used to establish a liver fibrosis model by administering CCl4 (1.5 μL/g), and liver tissues from these mice were stained with H&E, Masson’s trichrome, and Sirius Red after 4 wk and observed under a light microscope × 200 magnification. The relative fibrotic areas were analyzed; E: The hydroxyproline content in liver tissues from the liver fibrosis model was also measured; F and G: Western blotting was used to detect the relative expression of proteins in the liver fibrosis model established with FRNK-/- mice and WT mice. Representative results from three independent replicate assays are shown (n = 6). aP < 0.05 and bP < 0.01. Data are presented as the mean ± SD. MCT-1: Monocarboxylate transporter-1; ENO1: Enolase1.
FRNK knockout promotes liver fibrosis and aerobic glycolysis in vitro
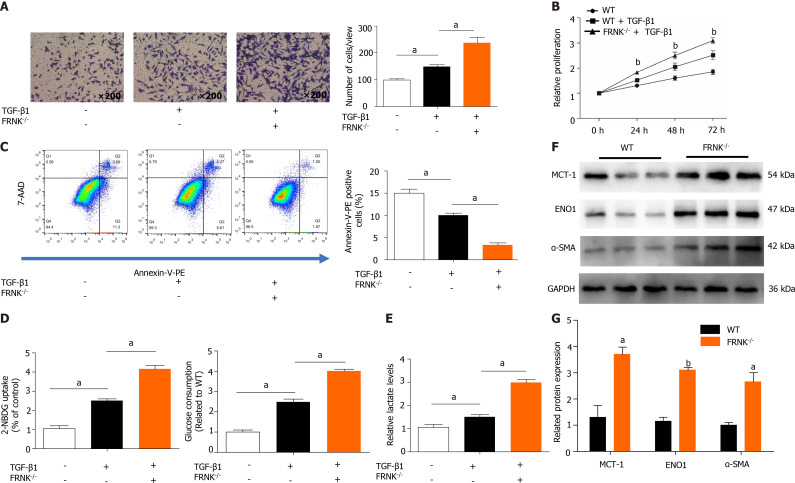
We extracted pHSCs from WT mice and FRNK-/- mice for in vitro experiments (Supplementary Figure 1). After 36 h of TGF-β1 treatment, the migration of pHSCs from the FRNK-/- groups in a Transwell chamber was increased (P < 0.05, Figure 3A). As indicated by the level of cell proliferation, pHSCs from the FRNK-/- group exhibited increased cellular activity (P < 0.05, Figure 3B). After adding TGF-β1 to pHSCs from FRNK-/- mice for 36 h, the apoptosis rate was lower than that of pHSCs from the control mice (P < 0.05, Figure 3C). Moreover, pHSCs from FRNK-/- mice showed increased glucose uptake and consumption compared with control pHSCs. Additionally, the lactate level in the medium of pHSCs from FRNK-/- mice was increased compared with the lactate level in the medium of pHSCs from the control group (P < 0.05, Figure 3D and E). Western blot analysis showed higher levels of the MCT-1, ENO1 and α-SMA proteins in the pHSCs from FRNK-/- mice was higher than in the pHSCs from WT mice (P < 0.05, Figure 3F and G). The above results illustrate that FRNK knockout in mice increases the activation, migration, and proliferation of pHSCs and attenuates pHSC apoptosis while enhancing their aerobic glycolytic capacity in vitro.
Figure 3.
Knockout of FRNK promotes liver fibrosis and aerobic glycolysis in vitro. A: After 36 h of culture with TGF-β1 (2 ng/mL), the migratory ability of primary hepatic stellate cells(pHSCs) was measured under a light microscope at × 200 magnification (105 cells per well); B: The proliferation of pHSCs was assessed with a CCK-8 assay; C: The apoptosis of pHSCs was analyzed using flow cytometry after 36 h of intervention; D and E: pHSCs cultured under the same intervention conditions were examined for glucose uptake and consumption, and lactate levels in the cell culture medium were also assessed; F and G: MCT-1, ENO1 and α-SMA levels in pHSCs were assessed using Western blotting. Representative results from three independent replicate assays are shown. aP < 0.05 and bP < 0.01 Results are presented as the mean ± SD.
Exogenous FRNK ameliorates experimental liver fibrosis and aerobic glycolysis in vitro
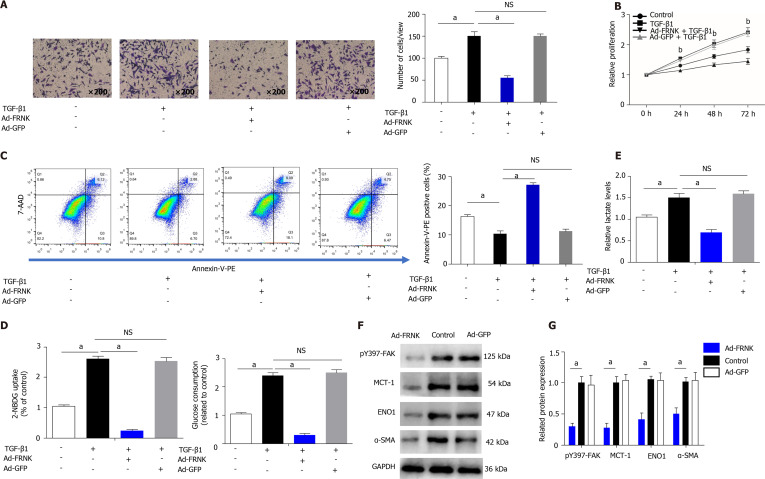
We transfected LX-2 cells with an adenovirus containing FRNK, induced the expression of the exogenous FRNK gene and incubated the cells with TGF-β1 for 36 h. We then performed Transwell, CCK-8 and flow cytometry assays with the transfected cells to evaluate migration, proliferation and apoptosis. The migration of LX-2 cells was inhibited and apoptosis was increased after the introduction of exogenous FRNK compared to the control treatment (P < 0.05, Figure 4A and C). In addition, proliferation was also inhibited (P < 0.01, Figure 4B). Based on these results, exogenous FRNK inhibits cell migration and proliferation and promotes apoptosis. The abilities of cells in the Ad-FRNK group to take up and consume glucose were reduced, and the lactate level in the cell culture medium was reduced (P < 0.05, Figure 4D and E). Subsequently, cellular proteins were extracted, and the relative levels of intracellular pY397-FAK, MCT-1, ENO1 and α-SMA proteins were detected by Western blotting. The relative expression of the aforementioned proteins in the Ad-FRNK group was lower than that in the control group (P < 0.05, Figure 4F and G). Thus, the introduction of exogenous FRNK into HSCs inhibits cell proliferation and migration and promotes apoptosis. It also inhibits cellular aerobic glycolysis and thus inhibits cellular energy generation in vitro.
Figure 4.
Exogenous FRNK ameliorates experimental liver fibrosis and aerobic glycolysis in vitro. LX-2 cells were transfected with a green fluorescent protein-carrying adenoviral vector (Ad-GFP) also encoding the FRNK gene. A: Cultured with TGF-β1 (2 ng/mL) for 36 h (105 cells per well). Migration was measured by analyzing cells under a light microscope × 200 magnification; B: The proliferation of LX-2 cells cultured under the intervention conditions is presented; C: The apoptosis of LX-2 cells was analyzed after 36 h of intervention using flow cytometry; D and E: LX-2 cells cultured under the same intervention conditions were examined for glucose consumption and uptake abilities, and lactate levels in the cell culture medium were also assessed; F and G: Levels of pY397-FAK, MCT-1, ENO1 and α-SMA in LX-2 cells were detected using Western blotting. Representative results from three independent replicate assays are shown . aP < 0.05 and bP < 0.01. Results are presented as the mean ± SD.
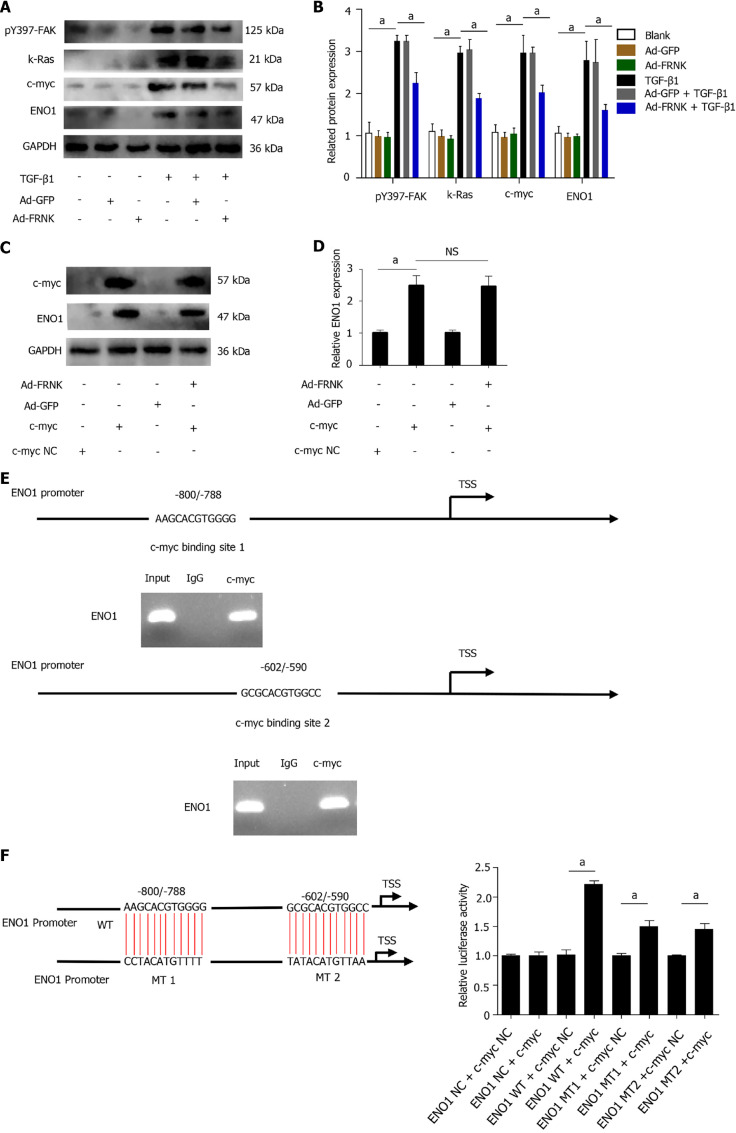
FRNK does not directly target ENO1
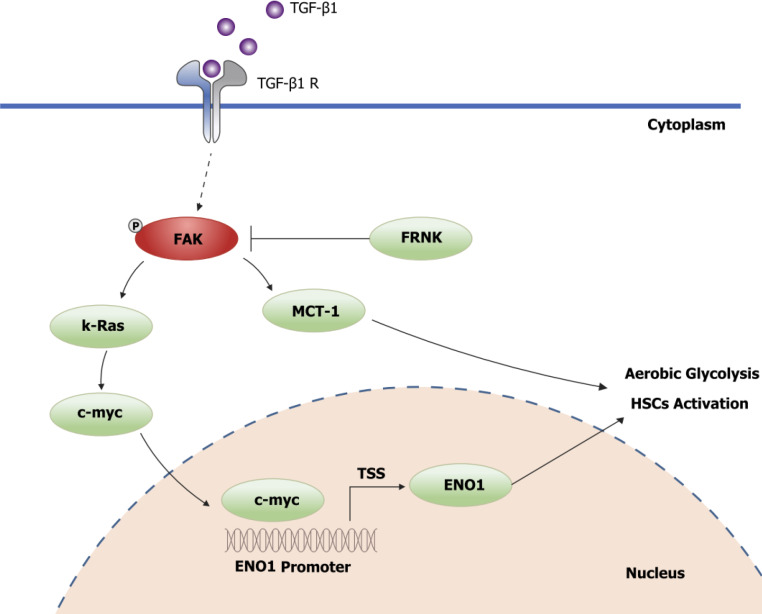
To explore the precise molecular mechanism by which FRNK regulates the ENO1 protein, TGF-β1 was used to stimulate LX-2 cells to activate FAK. The level of pY397-FAK was increased after stimulation with TGF-β1. The expression of the K-Ras, c-myc and ENO1 proteins downstream of FAK was also examined (P < 0.05, Figure 5A and B). While examining whether FRNK directly inhibits ENO1 protein expression, increased ENO1 protein expression was observed after introducing exogenous c-myc into LX-2 cells, but ENO1 protein expression was not reduced after the continued introduction of exogenous FRNK (P < 0.05, Figure 5C and D), suggesting that exogenous FRNK does not directly inhibit ENO1 protein expression to exert its biological function. As a method to investigate whether c-myc directly regulates ENO1, a bioinformatics analysis of the ENO1 promoter was performed to predict the putative binding site for c-myc in the ENO1 promoter, followed by ChIP and dual-luciferase reporter assays to verify that c-myc transcriptionally activates the ENO1 promoter (P < 0.05, Figure 5E and F). The results suggested that TGF-β1 activates FAK by inducing FAK phosphorylation at position 397. Then, pY397-FAK increased the expression of downstream K-Ras and c-myc proteins, followed by transcriptional activation of ENO1 expression by c-myc, promoting aerobic glycolysis and activation in HSCs. pY397-FAK and its biological functions were inhibited by the introduction of exogenous FRNK, limiting aerobic glycolysis and activation in HSCs and ameliorating liver fibrosis (Figure 6).
Figure 5.
FRNK does not directly target ENO1. A and B: Proteins were extracted from LX-2 cells, and relative levels of the pY397-FAK, K-Ras, c-myc and ENO1 proteins were determined; C and D: After transfection of LX-2 cells with pcDNA-c-myc or pcDNA-vector (NC), adenoviral vectors containing the FRNK gene (Ad-FRNK) or the negative control (Ad-GFP) were transfected, and the extracted protein was used to evaluate ENO1 expression through Western blotting; E: Schematic representation of the structure of the putative c-myc binding site in the human ENO1 promoter and chromatin immunoprecipitation (ChIP) assays with anti-c-myc or IgG; F: A dual-luciferase reporter assay showed the luciferase activity of WT, mutation (MT)1 and MT2 ENO1 promoters in LX-2 cells transfected with the c-myc or NC plasmid. Representative results from three independent replicate assays are shown. aP < 0.05 and bP < 0.01. Results are presented as the mean ± SD.
Figure 6.
Schematic diagram of FRNK inhibition of the FAK/Ras/c-myc/ENO1 pathway to ameliorate liver fibrosis.
DISCUSSION
The imbalance between liver injury and self-repair is the key to the development of liver fibrosis, and restoring the balance from an imbalanced state is a potential treatment for liver diseases. In the present study, we verified that FRNK alters the activation, proliferation, migration and apoptosis of HSCs by regulating aerobic glycolysis during liver fibrosis. FRNK inhibits aerobic glycolysis in HSCs by suppressing ENO1 activation through the FAK/Ras/c-myc/ENO1 pathway.
Early studies by our group verified that FAK plays important roles in the activation of HSCs and the development of liver fibrosis and that inhibition of FAK gene expression inhibits liver fibrogenesis[30]. Ding et al[23] verified that FRNK negatively regulates pulmonary fibrosis induced by FAK phosphorylation during pulmonary fibrosis. If FRNK inhibits the biological function of FAK in pulmonary fibrosis and uses a similar mechanism to repress liver fibrosis, it may represent a potential therapeutic target in liver fibrosis. Previous studies on FRNK have focused on the inhibition of the migratory function of vascular smooth muscle[32,33], combined with the presence of extracellular lactate accumulation during HSC activation[28,34] and FAK activation of aerobic glycolytic function in tumor cells[35-37]. FRNK may improve liver fibrosis by inhibiting aerobic glycolysis and inhibiting FAK activation in HSCs, but the mechanism by which FRNK exerts this effect remains unclear.
In the current study, we first observed increased expression of the pY397-FAK protein and decreased expression of the FRNK protein in tissues from patients with liver fibrosis (Figure 1). Subsequent experiments using CCl4 to replicate liver fibrosis in a mouse model yielded the same results (Figure 2B). Therefore, we speculated that a correlation between the occurrence of liver fibrosis and the downregulation of FRNK expression may exist and subsequently performed experiments in WT mice and FRNK-/- mice. After the CCl4 intervention, the degree of liver fibrosis in WT mice was lower than that in FRNK-/- mice (Figure 2C and D). The expression of the aerobic glycolysis-related proteins MCT-1 and ENO1 in the liver tissue of FRNK-/- mice was increased (Figure 2F and G), suggesting that FRNK gene deletion may promote intrahepatic aerobic glycolysis and aggravate the occurrence and development of liver fibrosis. We extracted pHSCs from WT mice and FRNK-/- mice for in vitro experiments to further explore the effect of FRNK on the biological functions of HSCs. After treatment with TGF-β1, the biological functions of pHSCs from FRNK-/- mice were more active than those of pHSCs from WT mice, as evidenced by the increased migration and proliferation and reduced apoptosis rate (Figure 3A-C). Furthermore, the uptake and consumption of glucose and extracellular lactate levels of FRNK-/- pHSCs were increased (Figure 3D and E), suggesting that HSCs lacking FRNK exhibited more active aerobic glycolysis, which supplied energy for their biological functions, such as activation, proliferation and migration. On the other hand, we transfected LX-2 cells with an adenovirus carrying the FRNK gene to verify whether exogenous FRNK is a promising therapeutic target in liver fibrosis. After FRNK overexpression, cell proliferation and migration decreased, while the percentage of apoptotic cells increased (Figure 4A-C). The uptake and utilization of glucose and the extracellular lactate level in LX-2 cells were also decreased (Figure 4D and E), and pY397-FAK expression in these cells was decreased (Figure 4F and G). These results further indicated that increasing exogenous FRNK expression prevented HSCs from obtaining energy through aerobic glycolysis and reduced cell activation and the energy supply required for a series of biological functions after activation, thereby inhibiting liver fibrosis. While investigating the mechanism by which FRNK regulates aerobic glycolysis in HSCs, we found that FAK is phosphorylated in LX-2 cells stimulated with TGF-β1 and that the downstream proteins K-Ras, c-myc, and ENO1 are activated. Following the introduction of FRNK, ENO1 protein expression was reduced (Figure 5A and B). We transfected both the c-myc and FRNK genes into LX-2 cells to determine whether FRNK directly inhibited ENO1 expression and found that ENO1 protein expression was not reduced upon increased exogenous FRNK expression (Figure 5C and D), thus suggesting that FRNK does not directly inhibit ENO1 protein expression. Therefore, we further hypothesized that c-myc directly alters ENO1 protein expression. Early studies revealed that c-myc is involved in the regulation of various biological functions, including metabolism, cell growth, cell cycle regulation and apoptosis[38,39], consistent with the results of our study. An increasing number of studies have shown that c-myc is involved in the regulation of promoters as a transcription factor. Hence, we predicted the putative c-myc binding site in the ENO1 promoter region by performing a bioinformatics analysis, followed by confirmation of our hypothesis that c-myc transcriptionally activates ENO1 and subsequently promotes liver fibrosis in HSCs by performing dual-luciferase reporter and ChIP assays (Figure 5E and F). Therefore, our study revealed that FRNK alleviated hepatic fibrosis via the FAK/Ras/c-myc/ENO1 pathway. The molecular mechanism by which FRNK regulates ENO1 and MCT-1 expression should be confirmed by conducting more complicated investigations in the future, and our group will be dedicated to studying this pathway.
CONCLUSION
In conclusion, this study is the first to reveal the effect of FRNK on liver fibrosis at the metabolic level. The experimental results suggest that the FAK/FRNK genes are potentially useful therapeutic targets in liver fibrosis and provide some rationale for the development of related drugs in the future.
ARTICLE HIGHLIGHTS
Research background
Hepatic stellate cell (HSC) hyperactivation is a central link in liver fibrosis development. HSCs perform aerobic glycolysis to provide energy for their activation.
Research motivation
Focal adhesion kinase (FAK) promotes aerobic glycolysis in cancer cells or fibroblasts, while FAK-related non-kinase (FRNK) inhibits FAK phosphorylation and biological functions.
Research objectives
To elucidate the effect of FRNK on liver fibrosis at the level of aerobic glycolytic metabolism in HSCs.
Research methods
Mouse liver fibrosis models were established by administering CCl4, and the effect of FRNK on the degree of liver fibrosis in the model was evaluated. Transforming growth factor-β1 was used to activate LX-2 cells. Tyrosine phosphorylation at position 397 (pY397-FAK) was detected to identify activated FAK, and the expression of the glycolysis-related proteins monocarboxylate transporter 1 (MCT-1) and enolase1 (ENO1) was assessed. Bioinformatics analysis was performed to predict putative binding sites for c-myc in the ENO1 promoter region, which were validated with chromatin immunoprecipitation (ChIP) and dual-luciferase reporter assays.
Research results
The pY397-FAK level was increased in human fibrotic liver tissue. FRNK knockout promoted liver fibrosis in mouse models. It also increased the activation, migration, proliferation and aerobic glycolysis of primary hepatic stellate cells (pHSCs) but inhibited pHSC apoptosis. Nevertheless, opposite trends for these phenomena were observed after exogenous FRNK treatment in LX-2 cells. Mechanistically, the FAK/Ras/c-myc/ENO1 pathway promoted aerobic glycolysis, which was inhibited by exogenous FRNK.
Research conclusions
FRNK inhibits aerobic glycolysis in HSCs by inhibiting the FAK/Ras/c-myc/ENO1 pathway, thereby improving liver fibrosis. FRNK might be a potential target for liver fibrosis treatment.
Research perspectives
The molecular mechanism by which FRNK regulates ENO1 and MCT-1 expression should be confirmed by conducting more complicated investigations in the future, and our group will be dedicated to studying this pathway.
ACKNOWLEDGEMENTS
The authors thank Professor Qiang Ding's laboratory at the University of Alabama at Birmingham, School of Medicine, Birmingham, AL, United States, for the gift of FRNK-/- mice and providing experimental technical guidance.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Institutional Review Board of the Affiliated Hospital of Guizhou Medical University (Approval 2018 Ethics Review No. 032).
Institutional animal care and use committee statement: Animal care and experimental procedures were authorized by the Animal Ethics Committee of Guizhou Medical University (No. 1801109).
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: July 14, 2021
First decision: October 3, 2021
Article in press: December 22, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morozov S, Truong NH S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
Contributor Information
Tao Huang, Department of Infectious Diseases, Affiliated Hospital of Guizhou Medical University, Guizhou Medical University, Guiyang 550004, Guizhou Province, China.
Yuan-Qing-Xiao Li, Department of Infectious Diseases, Affiliated Hospital of Guizhou Medical University, Guizhou Medical University, Guiyang 550004, Guizhou Province, China.
Ming-Yu Zhou, Department of Infectious Diseases, Affiliated Hospital of Guizhou Medical University, Guizhou Medical University, Guiyang 550004, Guizhou Province, China.
Rui-Han Hu, Department of Infectious Diseases, Affiliated Hospital of Guizhou Medical University, Guizhou Medical University, Guiyang 550004, Guizhou Province, China.
Gao-Liang Zou, Department of Infectious Diseases, Affiliated Hospital of Guizhou Medical University, Guizhou Medical University, Guiyang 550004, Guizhou Province, China.
Jian-Chao Li, Department of Infectious Diseases, Affiliated Hospital of Guizhou Medical University, Guizhou Medical University, Guiyang 550004, Guizhou Province, China.
Shu Feng, Department of Infectious Diseases, Affiliated Hospital of Guizhou Medical University, Guizhou Medical University, Guiyang 550004, Guizhou Province, China.
Yong-Mei Liu, Clinical Laboratory Center, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China.
Chang-Qin Xin, Department of Infectious Diseases, People’s Hospital of Weining Yi, Hui and Miao Autonomous County, Weining 553100, Guizhou Province, China.
Xue-Ke Zhao, Department of Infectious Diseases, Affiliated Hospital of Guizhou Medical University, Guizhou Medical University, Guiyang 550004, Guizhou Province, China. zhaoxueke1@163.com.
Data sharing statement
No additional data are available.
References
- 1.Testino G, Leone S, Fagoonee S, Pellicano R. Alcoholic liver fibrosis: detection and treatment. Minerva Med. 2018;109:457–471. doi: 10.23736/S0026-4806.18.05844-5. [DOI] [PubMed] [Google Scholar]
- 2.Stål P. Liver fibrosis in non-alcoholic fatty liver disease - diagnostic challenge with prognostic significance. World J Gastroenterol. 2015;21:11077–11087. doi: 10.3748/wjg.v21.i39.11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebastiani G, Gkouvatsos K, Pantopoulos K. Chronic hepatitis C and liver fibrosis. World J Gastroenterol. 2014;20:11033–11053. doi: 10.3748/wjg.v20.i32.11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015;22:512–518. doi: 10.1002/jhbp.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37–55. doi: 10.1016/j.mam.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campana L, Iredale JP. Regression of Liver Fibrosis. Semin Liver Dis. 2017;37:1–10. doi: 10.1055/s-0036-1597816. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 9.Rocco A, Compare D, Angrisani D, Sanduzzi Zamparelli M, Nardone G. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20:14652–14659. doi: 10.3748/wjg.v20.i40.14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018;68-69:435–451. doi: 10.1016/j.matbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 12.Dhar A, Sadiq F, Anstee QM, Levene AP, Goldin RD, Thursz MR. Thrombin and factor Xa link the coagulation system with liver fibrosis. BMC Gastroenterol. 2018;18:60. doi: 10.1186/s12876-018-0789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai IR, Chu PY, Lin HS, Liou JY, Jan YJ, Lee JC, Shen TL. Phosphorylation of focal adhesion kinase at Tyr397 in gastric carcinomas and its clinical significance. Am J Pathol. 2010;177:1629–1637. doi: 10.2353/ajpath.2010.100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapial Martínez P, López Navajas P, Lietha D. FAK Structure and Regulation by Membrane Interactions and Force in Focal Adhesions. Biomolecules. 2020;10 doi: 10.3390/biom10020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai M, Ohtake J, Ishikawa T, Tanemura K, Hoshino Y, Arima T, Sato E. Distribution and Y397 phosphorylation of focal adhesion kinase on follicular development in the mouse ovary. Cell Tissue Res. 2012;347:457–465. doi: 10.1007/s00441-011-1307-2. [DOI] [PubMed] [Google Scholar]
- 16.Mikuriya K, Kuramitsu Y, Ryozawa S, Fujimoto M, Mori S, Oka M, Hamano K, Okita K, Sakaida I, Nakamura K. Expression of glycolytic enzymes is increased in pancreatic cancerous tissues as evidenced by proteomic profiling by two-dimensional electrophoresis and liquid chromatography-mass spectrometry/mass spectrometry. Int J Oncol. 2007;30:849–855. [PubMed] [Google Scholar]
- 17.Li L, Liang Y, Kang L, Liu Y, Gao S, Chen S, Li Y, You W, Dong Q, Hong T, Yan Z, Jin S, Wang T, Zhao W, Mai H, Huang J, Han X, Ji Q, Song Q, Yang C, Zhao S, Xu X, Ye Q. Transcriptional Regulation of the Warburg Effect in Cancer by SIX1. Cancer Cell. 2018;33:368–385.e7. doi: 10.1016/j.ccell.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sayers RL, Sundberg-Smith LJ, Rojas M, Hayasaka H, Parsons JT, Mack CP, Taylor JM. FRNK expression promotes smooth muscle cell maturation during vascular development and after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28:2115–2122. doi: 10.1161/ATVBAHA.108.175455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidkamp MC, Bayer AL, Kalina JA, Eble DM, Samarel AM. GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes. Circ Res. 2002;90:1282–1289. doi: 10.1161/01.res.0000023201.41774.ea. [DOI] [PubMed] [Google Scholar]
- 22.Hayasaka H, Martin KH, Hershey ED, Parsons JT. Disruption of FRNK expression by gene targeting of the intronic promoter within the focal adhesion kinase gene. J Cell Biochem. 2007;102:947–954. doi: 10.1002/jcb.21329. [DOI] [PubMed] [Google Scholar]
- 23.Ding Q, Cai GQ, Hu M, Yang Y, Zheng A, Tang Q, Gladson CL, Hayasaka H, Wu H, You Z, Southern BD, Grove LM, Rahaman SO, Fang H, Olman MA. FAK-related nonkinase is a multifunctional negative regulator of pulmonary fibrosis. Am J Pathol. 2013;182:1572–1584. doi: 10.1016/j.ajpath.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang H, Liu X, Knolhoff BL, Hegde S, Lee KB, Jiang H, Fields RC, Pachter JA, Lim KH, DeNardo DG. Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut. 2020;69:122–132. doi: 10.1136/gutjnl-2018-317424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, Pachter JA, Wang-Gillam A, DeNardo DG. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22:851–860. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Ren B, Yang G, Wang H, Chen G, You L, Zhang T, Zhao Y. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol Life Sci. 2020;77:305–321. doi: 10.1007/s00018-019-03278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, Xie G, Moylan CA, Garibaldi F, Premont R, Suliman HB, Piantadosi CA, Diehl AM. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319–1329.e11. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z, Liu M, Li L, Chen L. Involvement of the Warburg effect in non-tumor diseases processes. J Cell Physiol. 2018;233:2839–2849. doi: 10.1002/jcp.25998. [DOI] [PubMed] [Google Scholar]
- 30.Zhao XK, Yu L, Cheng ML, Che P, Lu YY, Zhang Q, Mu M, Li H, Zhu LL, Zhu JJ, Hu M, Li P, Liang YD, Luo XH, Cheng YJ, Xu ZX, Ding Q. Focal Adhesion Kinase Regulates Hepatic Stellate Cell Activation and Liver Fibrosis. Sci Rep. 2017;7:4032. doi: 10.1038/s41598-017-04317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou GL, Zuo S, Lu S, Hu RH, Lu YY, Yang J, Deng KS, Wu YT, Mu M, Zhu JJ, Zeng JZ, Zhang BF, Wu X, Zhao XK, Li HY. Bone morphogenetic protein-7 represses hepatic stellate cell activation and liver fibrosis via regulation of TGF-β/Smad signaling pathway. World J Gastroenterol. 2019;25:4222–4234. doi: 10.3748/wjg.v25.i30.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koshman YE, Engman SJ, Kim T, Iyengar R, Henderson KK, Samarel AM. Role of FRNK tyrosine phosphorylation in vascular smooth muscle spreading and migration. Cardiovasc Res. 2010;85:571–581. doi: 10.1093/cvr/cvp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koshman YE, Kim T, Chu M, Engman SJ, Iyengar R, Robia SL, Samarel AM. FRNK inhibition of focal adhesion kinase-dependent signaling and migration in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2010;30:2226–2233. doi: 10.1161/ATVBAHA.110.212761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang YH, Israelsen WJ, Lee D, Yu VWC, Jeanson NT, Clish CB, Cantley LC, Vander Heiden MG, Scadden DT. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell. 2014;158:1309–1323. doi: 10.1016/j.cell.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demircioglu F, Wang J, Candido J, Costa ASH, Casado P, de Luxan Delgado B, Reynolds LE, Gomez-Escudero J, Newport E, Rajeeve V, Baker AM, Roy-Luzarraga M, Graham TA, Foster J, Wang Y, Campbell JJ, Singh R, Zhang P, Schall TJ, Balkwill FR, Sosabowski J, Cutillas PR, Frezza C, Sancho P, Hodivala-Dilke K. Cancer associated fibroblast FAK regulates malignant cell metabolism. Nat Commun. 2020;11:1290. doi: 10.1038/s41467-020-15104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao D, Qi Z, Pang Y, Li H, Xie H, Wu J, Huang Y, Zhu Y, Shen Y, Dai B, Hu X, Ye D, Wang Z. Retinoic Acid-Related Orphan Receptor C Regulates Proliferation, Glycolysis, and Chemoresistance via the PD-L1/ITGB6/STAT3 Signaling Axis in Bladder Cancer. Cancer Res. 2019;79:2604–2618. doi: 10.1158/0008-5472.CAN-18-3842. [DOI] [PubMed] [Google Scholar]
- 37.Siu MKY, Jiang YX, Wang JJ, Leung THY, Han CY, Tsang BK, Cheung ANY, Ngan HYS, Chan KKL. Hexokinase 2 Regulates Ovarian Cancer Cell Migration, Invasion and Stemness via FAK/ERK1/2/MMP9/NANOG/SOX9 Signaling Cascades. Cancers (Basel) 2019;11 doi: 10.3390/cancers11060813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo W, Chen J, Li L, Ren X, Cheng T, Lu S, Lawal RA, Nie Q, Zhang X, Hanotte O. c-Myc inhibits myoblast differentiation and promotes myoblast proliferation and muscle fibre hypertrophy by regulating the expression of its target genes, miRNAs and lincRNAs. Cell Death Differ. 2019;26:426–442. doi: 10.1038/s41418-018-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao ZD, Han L, Lee H, Zhuang L, Zhang Y, Baddour J, Nagrath D, Wood CG, Gu J, Wu X, Liang H, Gan B. Energy stress-induced lncRNA FILNC1 represses c-Myc-mediated energy metabolism and inhibits renal tumor development. Nat Commun. 2017;8:783. doi: 10.1038/s41467-017-00902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.